-
磷是生物体生长所必须的营养元素,也是影响水生生态系统的关键因素[1]。大量的磷排放到水体中会引起水体富营养化,对水生生态系统产生严重威胁[2]。此外,磷酸盐是一种不可再生资源,并且随着需求的增长,全球的磷资源可能在50~100 a内耗尽,对水体中的磷酸盐进行回收再利用是大势所趋[3]。传统的化学沉淀法、生物去除法、电化学处理法和离子交换法常用来去除水体中的磷酸盐,但其处理效果往往很难达到污水处理厂出水一级A标准(总磷质量浓度不超过0.5 mg·L−1),对低浓度磷酸盐的回收利用也存在一定困难,因此,新的磷回收技术值得深入研究[4]。
吸附法是近年来国内外对废水中磷酸盐的去除和回收研究最多的技术之一[5-6]。与其他技术相比,吸附法简单、快速、操作灵活、经济性高,可用于多种环境并取得良好的出水水质,具有较好的应用前景。在众多吸附剂中,锆(Zr)基氧化物由于其无毒、抗酸碱和氧化性能好、热稳定性高、在水中的溶解度低等特点,是一种很有潜力的水处理吸附剂,并被大量研究[7]。ZHANG等[8]通过共沉淀法制备了Zr-Fe双金属氧化物,其对磷的吸附量达到21.3 mg·g−1且具有磁性,能够采用磁分离法将材料分离。SU等制备的非晶ZrO2比表面积达到327 m2·g−1,且大部分的空隙为介孔,其对磷的吸附量达到32.29 mg·g−1[9]。ZHAO等[10]合成了胺化木质素负载的Zr(OH)4复合材料,吸附量达到54.7 mg·g−1,在酸性条件下表面的氨基质子化能促进磷酸盐的吸附。SHANG等[11]将水合氧化锆(HZO)负载到季胺基团改性的芦苇上,吸附量高达59.2 mg·g−1,带永久正电荷的季胺基团能够强化静电引力从而提高磷吸附量。以往的研究表明,负载活性组分为Zr4+、ZrO2或Zr(OH)4的Zr基吸附剂均具有很好的吸附性能,成为近年来水中磷酸盐回收利用的研究热点之一。此外,磁分离技术因其分离效率高、分离时间短、能耗低、选择性高等优点而引起人们越来越多的关注[12]。具有良好分离性能的磁性材料,在吸附领域的应用前景可观。因此,将新的Zr基活性组分负载到磁性Fe3O4纳米颗粒上以获得良好的吸附与磁分离能力,值得进一步研究。
本研究利用溶剂热法,将非晶(碳酸)氧化锆与Fe3O4进行掺杂,制备了磁性非晶(碳酸)氧化锆(MZCO)吸附材料;采用XRD、SEM、EDS、TEM、BET、Zeta电位等分析方法对MZCO的基本形态和物理化学性质进行了表征和分析;研究了MZCO吸附磷酸盐的等温线、动力学、pH、共存离子的影响特征,实际废水的吸附再生和吸附穿透曲线,并结合ATR-IR和XPS的表征结果揭示了MZCO吸附磷酸盐的相关机理。
-
纳米四氧化三铁(Fe3O4,200 nm)为分析纯,购于上海麦克林生化科技有限公司;氯化锆(ZrCl4)、乙二醇((CH2OH)2)均为分析纯,购于上海阿拉丁生化科技有限公司;聚乙二醇(PEG2 000)、无水乙酸钠(CH3COONa)、尿素(CH4N2O)、氢氧化钠(NaOH)、盐酸(HCl)、氯化钠(NaCl)、无水乙醇(C2H5OH)、磷酸二氢钾(KH2PO4)、酒石酸锑钾(C8H4K2O12Sb2)、抗坏血酸(C6H8O6)、钼酸铵((NH4)6Mo7O24·4H2O)、氯化钾(KCl)、碳酸氢钾(KHCO3)、硝酸钾(KNO3)、硫酸钾(K2SO4)、氯化钙(CaCl2)、氯化镁(MgCl2)、腐殖酸(HA)均为分析纯,购买于国药集团化学试剂有限公司。
实际水样为北京市某污水处理厂的二沉池出水,其COD和pH分别为4.8 mg·L−1和7.68,并含有0.034 mg·L−1 PO43−、14.2 mg·L−1 NO3−、143 mg·L−1 Cl−、85.26 mg·L−1 SO42−、90.7 mg·L−1 Ca2+、27.7 mg·L−1 Mg2+、109.51 mg·L−1 Na+、21.98 mg·L−1 K+等离子。该水样经过定性滤纸过滤后,调节磷酸盐浓度为5 mg·L−1,在4 ℃下储存备用。
-
本实验采用溶剂热法制备MZCO材料。首先称取一定摩尔质量的ZrCl4,在超声辅助下溶于乙二醇中形成澄清溶液。然后将0.385 g纳米Fe3O4(200 nm),7.2 g乙酸钠,2 g聚乙二醇(分子质量2 000 Da)和1 g尿素依次加入到该溶液中,剧烈搅拌30 min后,将所得混合溶液密封在100 mL四氟乙烯衬里的不锈钢高压釜中。将高压釜在200 ℃下加热12 h,然后冷却至室温。产物经纯水和乙醇洗涤数次,真空冷冻干燥,研磨后备用。
-
使用X射线衍射仪(XRD-7 000,Shimadzu,日本)表征材料晶体结构;使用场发射扫描电子显微镜(Merlin,Zeiss,德国)和高分辨率透射电子显微镜(SU-8010,Hitachi,美国)观察样品的形貌和元素分布;使用振动样品磁力计(703T,Lake Shore,美国)测试磁饱和强度;使用Zeta电位仪(Nano ZS90, Malvern Instruments)对不同pH下MZCO的表面电荷进行了表征;使用比表面积及孔径分析仪(ASAP2460,Mike,美国)测定MZCO比表面积;使用激光粒度仪(Mastersizer 2000,Malvern,英国)测定MZCO的粒径;使用电感耦合等离子体发射光谱仪(9800,Shimadzu,日本)测定金属含量;通过傅里叶变换红外光谱仪(ATR-IR,Vector 22,Bruker)表征吸附前后官能团变化;使用X光电子能谱仪(XPS,ESCALAB 250 XI,Thermo)对MZCO吸附前后化学结构以及官能团的变化进行表征,分析结果使用XPS峰拟合程序(XPS peak)进行分析。
-
在Zr投加量的优化实验中,磷酸根初始质量浓度为50 mg·L−1,投加量为0.3 g·L−1;在吸附等温线实验中,配制不同质量浓度(0~50 mg·L−1)的50 mL磷酸盐水样中,加入0.1 g·L−1吸附剂;在吸附动力学实验中,取1 L磷酸盐(30 mg·L−1)水样, 加入0.1 g·L−1吸附剂,间隔一定时间取样。在上述实验过程中,采用0.1 mol·L−1的HCl或NaOH溶液调节水样pH至6.8±0.1,吸附时间为24 h。在pH影响实验中,取50 mL磷酸盐水样(2 mg·L−1),加入0.1 g·L−1吸附剂,采用0.1 mol·L−1的HCl或NaOH溶液调节水样pH至不同初始值(2~11),并在振荡期间每隔4 h调节1次pH。在共存离子实验中,取50 mL磷酸盐水样(2 mg·L−1),按0.1 g·L−1投加量加入吸附剂,共存离子(Cl–、NO3–、SO42、HCO3−、Ca2+和Mg2+)和溶剂性有机物腐殖酸(HA)的质量浓度分别为10、50和100 mg·L−1。所有实验样品均过0.45 μm聚醚砜膜,然后将样品存放于(4±0.5) ℃冰箱内待测。采用钼酸铵分光光度法测定水样中磷浓度(以P计)。所有实验均重复3次。
吸附等温线涉及的Langmuir、Freundlich和Sips吸附等温模型方程如式(1)~式(3)所示。吸附动力学涉及的准一级(式(4))和准二级(式(5))吸附动力学模型方程如下。
式中:qe和qm分别为吸附平衡时的吸附量和最大吸附量,mg·g−1;KL(L·mg−1)、KF((mg·g−1)·( mg·L−1)−n)和Ks(L·g−1)分别为Langmuir、Freundlich和Sips方程模型常数;Ce为平衡质量浓度,mg·L−1;n为Freundlich吸附指数;αs为平衡常数;βs为吸附平衡分解常数(当βs=1时,Sips方程与Langmuir方程式相同);β为吸附能量的参数。
式中:qe和qt分别为平衡吸附量以及t时刻的吸附量,mg·g−1;t为吸附时间,min;k1为准一级动力学方程常数,min−1;k2为准一级动力学方程常数,g∙(mg·h)−1。
-
在再生液配比实验中,取一定质量的样品,使其在高浓度磷酸根(30 mg·L−1)溶液中吸附饱和,然后将吸附饱和的样品采用磁分离取出。分别采用不同NaOH/NaCl比的再生液对吸附后的MZCO进行再生,选取磷脱附量高和金属溶出低的再生液进行吸附再生实验。
在吸附再生实验中,取0.15 g的样品,在500 mL二沉池出水中(磷酸盐质量浓度为5 mg·L−1)中吸附12 h,然后采用磁分离法将吸附后的样品采用取出。使用3 mol·L−1的NaOH和1.5 mol·L−1的NaCl混合溶液进行脱附,磁力搅拌12 h后将样品磁分离,用去离子水洗涤烘干进行下一次吸附再生实验,共重复5次。
在动态吸附实验中,采用直径15 mm,高150 mm的玻璃吸附柱为实验容器,填充吸附剂为2.65 cm3(0.6 g)。使用二沉池出水(磷酸盐质量浓度为5 mg·L−1)作为原水,控制蠕动泵流速为1.5 mL·min−1,空床停留时间为1.8 min。磷酸根离子穿透质量浓度设定为0.1 mg·L−1。穿透完成后,使用3 mol·L−1的NaOH和1.5 mol·L−1的NaCl混合溶液对MZCO进行12 h脱附。脱附后的MZCO进行第2次吸附柱实验,实验条件与之前保持一致。
-
有研究表明,Zr基吸附材料中锆的比例是影响其对磷酸根吸附能力的关键[8]。不同Zr投加量产物MZCO的磷吸附量如图1所示。随着Zr投加量从5 mmol升高到30 mmol,产物磷吸附量先增后降,当Zr投加量达到25 mmol时,产物的吸附量最高达到69.5 mg·g−1。因此,选择合成条件中Zr投加量为25 mmol的合成产物进行下一步研究。
-
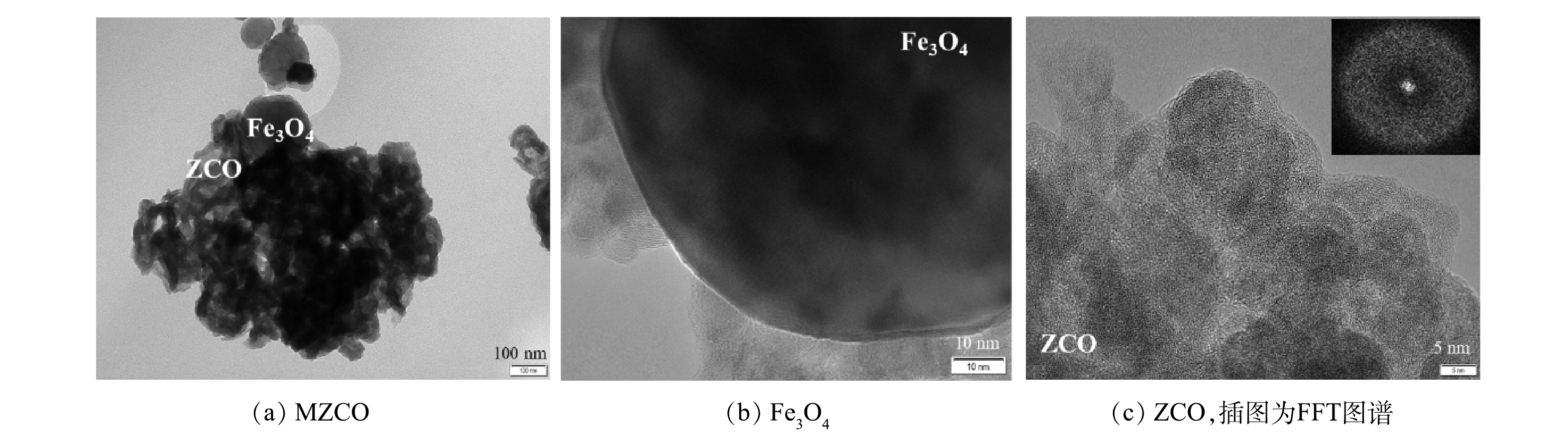
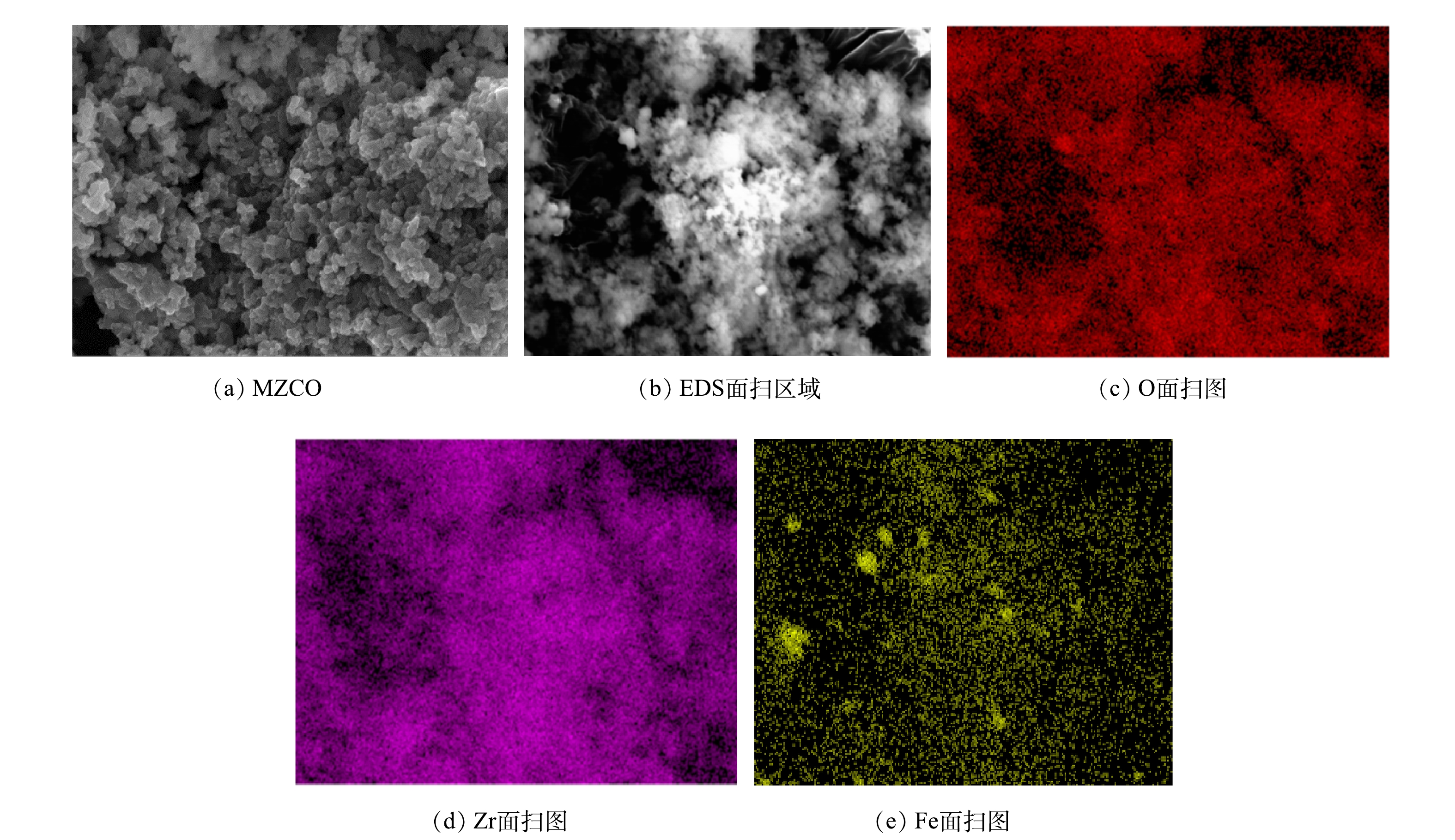
由图2可以看出,其衍射峰在36°出现的尖峰与标准的立方晶型Fe3O4(JCPDs 72-2303)能很好地匹配。这证明Fe3O4以晶体的形式存在于MZCO复合材料中。在以30°为中心出现的宽峰与ZrO2(JCPDs 79-1796)较为匹配,表明MZCO复合材料中存在大量非晶ZrO2成分。这也与之前的报道结果相一致[13]。此外,结合材料红外分析结果可知,材料表面还存在大量的CO32-基团。由图3(a)可以看到直径约为200 nm的Fe3O4颗粒的附着在非晶态ZCO表面上。在ZCO的快速傅里叶变换图像(图3(c))中观察到很宽的扩散环,表明ZCO组分具有非晶结构特征。以上结果表明,MZCO复合材料为Fe3O4负载的非晶态(碳酸)二氧化锆。由图4可以看出,MZCO具有粗糙的多孔结构,EDS光谱表明MZCO中的Zr、Fe和O在复合材料的表面均匀分布,没有发生元素分离或相分离。
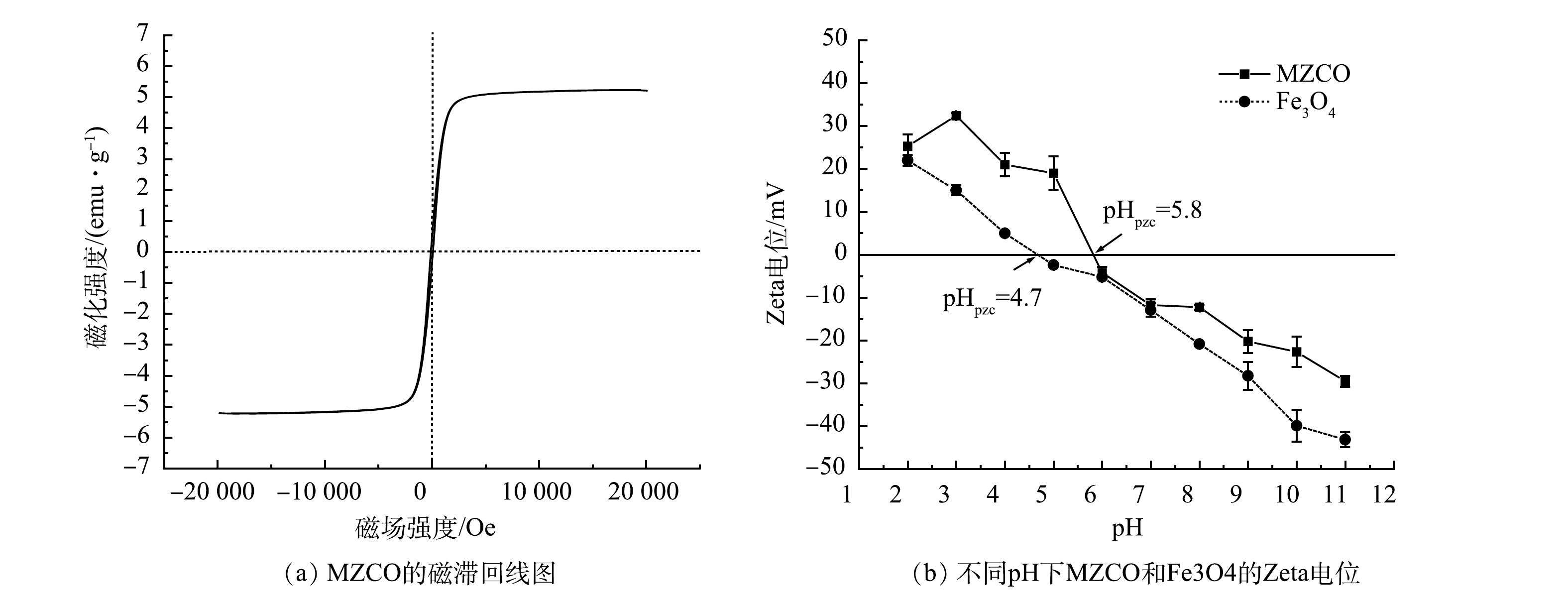
MZCO的磁滞曲线和Zeta电位如图5所示,其他相关物理化学性质见表1。MZCO的饱和磁化强度为5.22 emu·g−1,远低于纯Fe3O4(63.80 emu·g−1),这归因于无磁性ZCO与Fe3O4的掺杂。MZCO近似表现出超顺磁特性。这说明材料在无磁场的情况下可以较好地分散在溶液中;在有磁场的情况下可快速分离收集[14]。MZCO的等电点(pHpzc)为5.8,pH为3.0时Zeta电位最高为32.4 mV。ZCO的掺杂使得复合材料的表面具有更多的正电荷,从而增强了对磷酸盐的静电吸引。粒径分析结果表明MZCO的D50为10.12 μm,这说明该材料为是一种微米级材料。此外,MZCO的比表面积为478.28 m2·g−1,孔径为13.7 nm,孔体积为1.09 cm3·g−1,均远大于Fe3O4。这可能归因于多晶ZCO的引入,其具有较大的比表面积和孔隙参数[15]。MZCO中锆和铁的含量分别为431.0 mg·g−1和61.8 mg·g−1,较高的Zr含量和较大的比表面积,能够使表面Zr活性位点更多的暴露出来,从而获得较高的磷吸附容量。
-
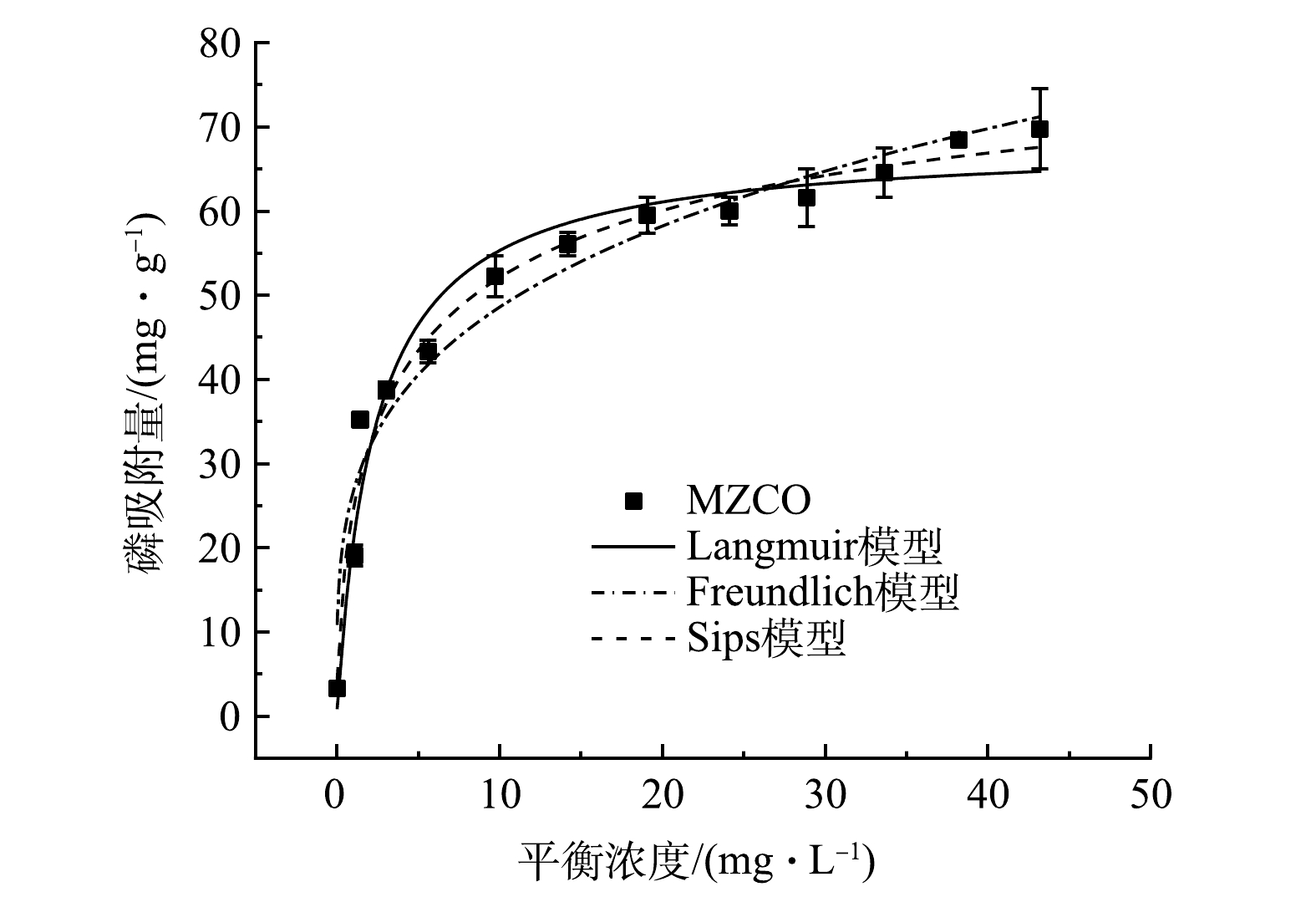
1)吸附等温线。MZCO在磷酸根初始质量浓度为0~50 mg·g−1内的吸附等温线如图6所示。随着磷酸盐平衡浓度的增长,MZCO对磷酸盐的吸附能力迅速增强,然后逐渐达到稳定值。吸附等温线数据使用Langmuir、Freundlich和Sips模型进行拟合,具体参数如表2所示。由Langmuir模型拟合的饱和吸附量为68.16 mg·g−1,略低于实际测出的最大吸附量69.75 mg·g−1。在3种模型中,Sips模型的拟合结果最佳(R2=0.98),表明MZCO吸附磷酸盐过程更加符合非均匀的多分子层吸附模型[16]。MZCO与其他Zr基吸附剂的磷酸盐吸附性能比较见表3。结果表明,大多数Zr基金属材料对磷酸盐的最大吸附能力均低于本研究合成的MZCO。这可能是因为MZCO中含有较多的Zr活性组分(43.1%)。此外,MZCO具有较高的比表面积(478.28 m2·g−1),这也有利于Zr活性位点的分散,从而更好的参与磷酸盐吸附。
2)吸附动力学。MZCO对磷酸根吸附动力学如图7所示。其磷酸盐吸附量在前120 min中迅速升高,在30、60和120 min的吸附量分别可以达到饱和吸附容量的60.3%、78.8%和87.2%。随后,磷酸盐吸附量在4 h后逐渐趋于稳定,并在6 h内达到平衡。吸附反应早期的快速吸附可能是由于MZCO表面上丰富的活性位点,能高效的吸附磷酸盐。此外良好的表面结构(比表面积、孔径和孔体积),有利于磷酸盐在材料表面的快速传质[25]。在随后的阶段,缓慢的吸附速率意味其主要吸附机制为颗粒内扩散[26]。使用准一级和准二级动力学模型对其动力学曲线进行拟合,相关拟合参数如表4所示。MZCO复合材料的吸附过程更符合准二级动力学模型(R2=0.95),表明其磷酸根的吸附过程主要以吸附活性位点与磷酸盐之间的化学吸附为主导[27-28]。
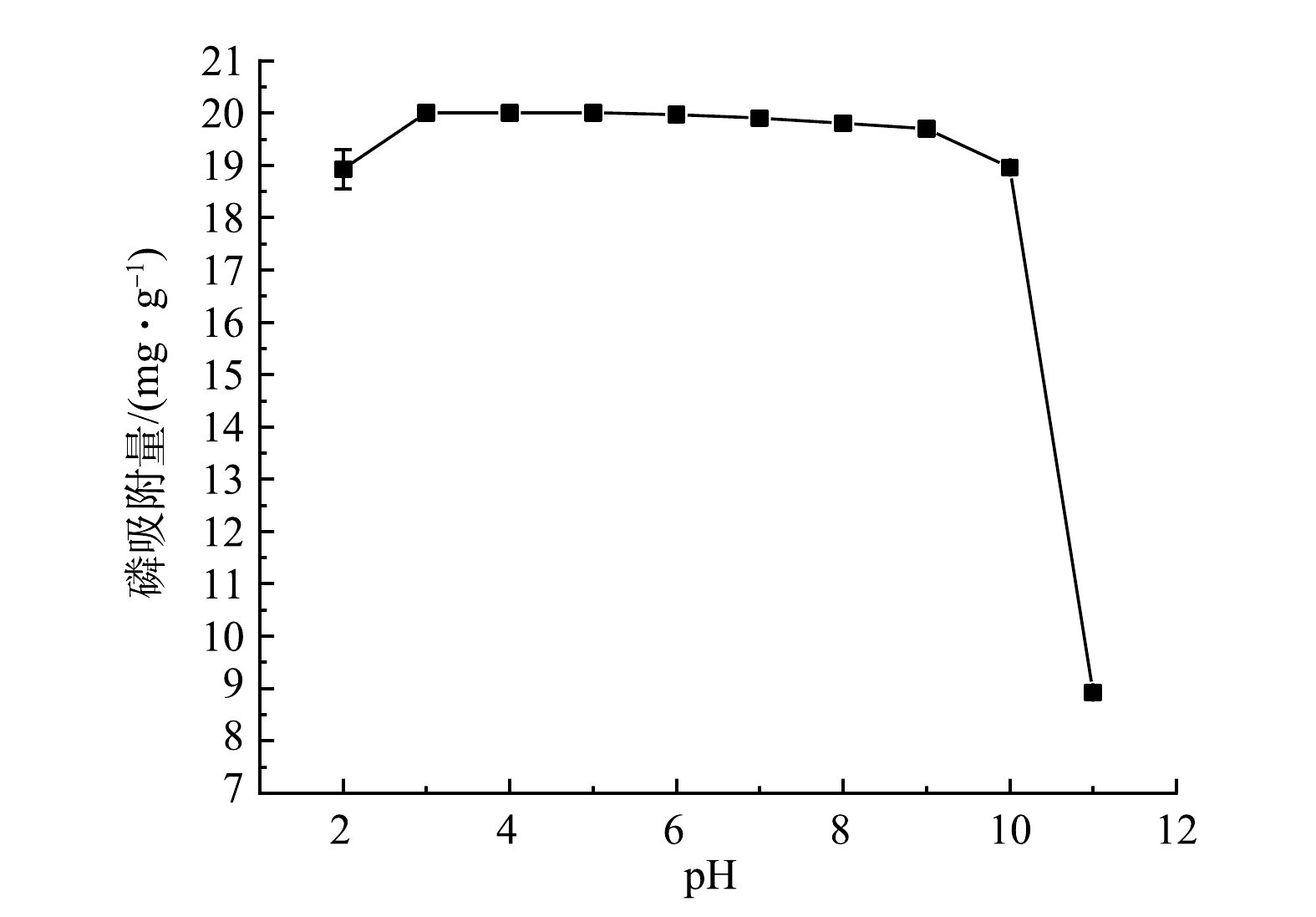
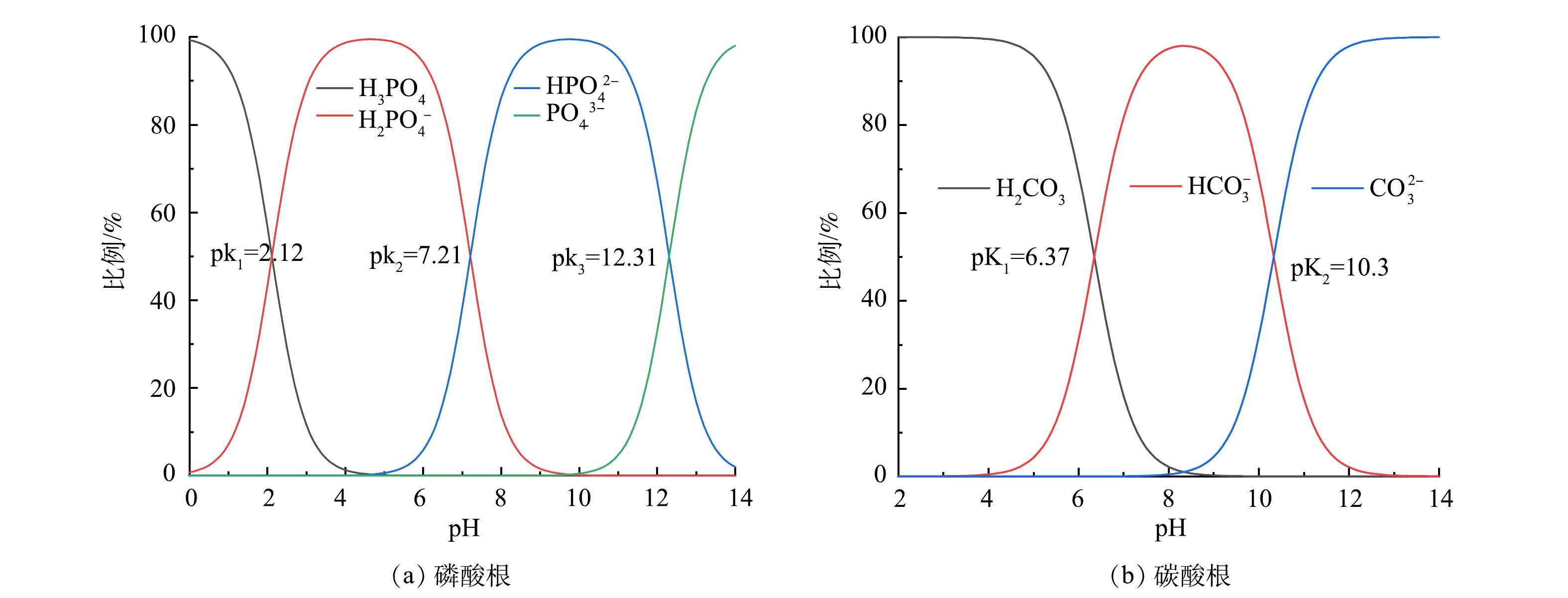
3) pH影响。由图8可见,随着pH由2.0增加到3.0,磷吸附量达到最高;随着pH增加到6.0,磷吸附量一直保持稳定。当pH在6.0~10.0内继续升高,MZCO的磷吸附量略微降低。当pH升高到11时,其磷吸附量降到最低。该结果表明MZCO在较宽的pH(2~10)范围内都具有很好的磷酸根去除能力。当pH为2.0~3.0时,磷酸盐主要以一价的H2PO4−形态存在,H3PO4含量较低(图8)。在酸性条件下,MZCO的表面质子化,Zeta电位在pH为3时达到32 mV(图5(b))。这说明表面带正电荷的MZCO可能通过静电吸引促进与H2PO4−的相互作用。此外,当pH增加到3.0时,MZCO没有Zr溶出(表5),这也有利于磷酸盐的吸附。在pH为3.0~5.8时,MZCO的表面质子化程度降低,Zeta电位逐渐下降,使得其与磷酸盐之间的静电引力降低,但由于MZCO较高的饱和吸附量,使得其在吸附低浓度磷酸盐(2 mg·L−1)时,依然能保持20 mg·g−1的吸附量。随着pH从6.0增加到10.0,MZCO表面的碳酸根主要以HCO3−的形式存在(图9),其可以与带负电的磷酸盐进行配体交换。二价的HPO42-逐渐占据主导地位,与H2PO4−相比,HPO42-拥有较高的吸附自由能而更难被吸附[29-30]。此外,在碱性条件下,OH−的浓度增强并与磷酸盐产生竞争吸附,MZCO表面去质子化程度加深,表面带负电荷的MZCO与磷酸盐产生静电排斥,这些原因共同造成了磷酸盐吸附能力的下降。之后,当pH进一步增加到11.0时,MZCO的吸附能力急剧下降。较强的静电排斥力和OH−竞争吸附共同导致磷酸盐吸附能力的降低[31]。
通过测定Zr和Fe元素在不同pH溶液中的溶出量,对MZCO的稳定性进行了评价。在pH为 5.0~9.0内,均未检测到Zr和Fe的金属溶出,MZCO这种较宽的pH适用性极大地扩展了其应用范围。
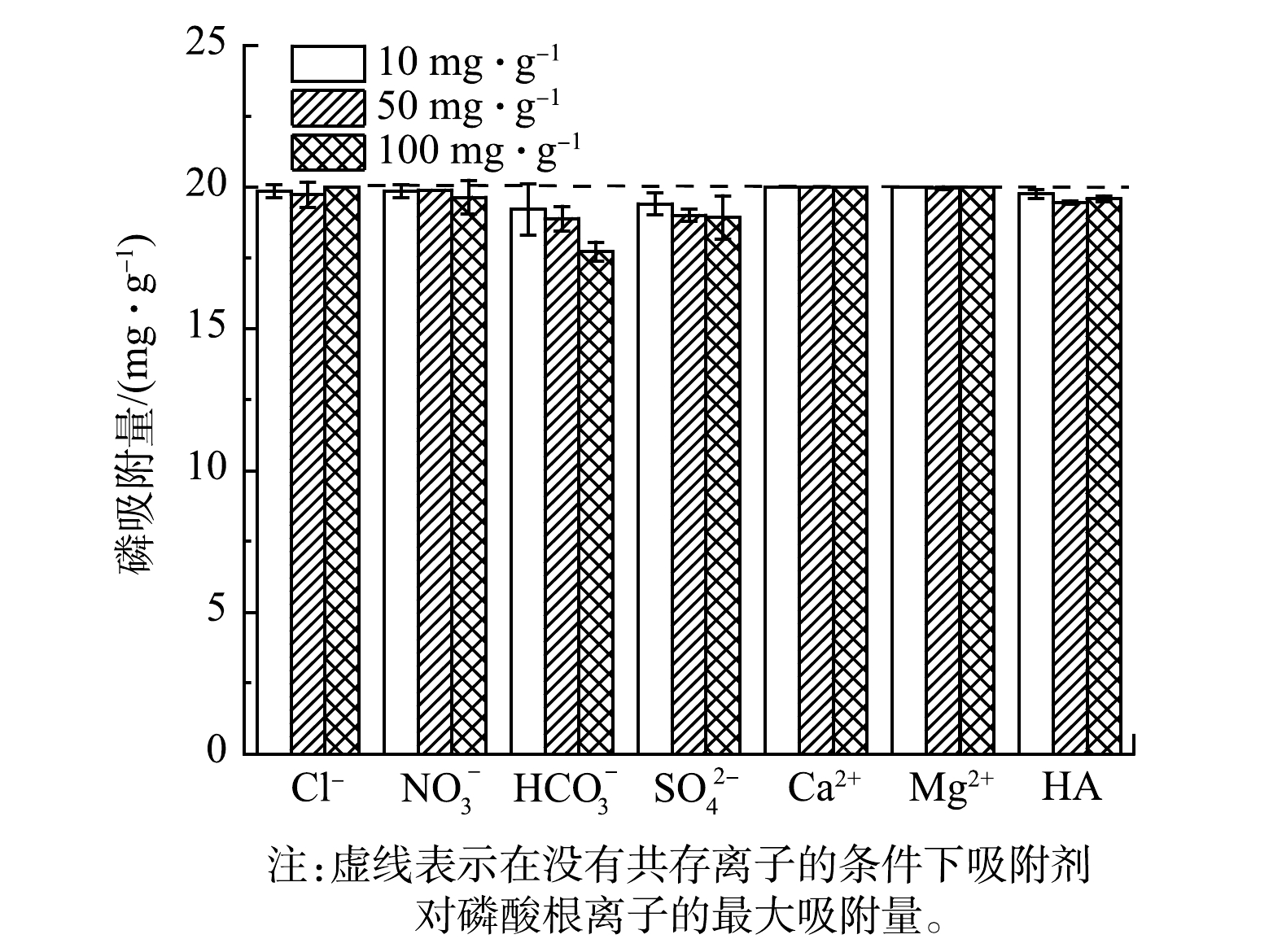
4)共存离子影响。在低浓度磷酸盐溶液中,3种不同浓度无机离子和HA对磷酸盐吸附的影响如图10所示。其中Ca2+、Mg2+、Cl–和NO3–对磷酸盐吸附的影响可忽略不计。SO42带有2个负电荷,与带有1个负电荷的H2PO4−相比,能与MZCO表面产生更强的静电吸引。此外,SO42的离子半径(0.230 nm)与H2PO4−的离子半径(0.238 nm)接近,更容易与H2PO4−竞争MZCO表面的金属活性位点[32]。HCO3−的存在,使得溶液pH升高,OH−浓度的升高和MZCO表面Zeta电位的降低,共同影响了磷酸盐的吸附。此外,HCO3−的大量存在还会影响MZCO表面碳酸盐的解离,从而影响其与溶液中磷酸盐的配体交换。HA作为高分子聚芳香族化合物,其表面具有—OH、—COOH等多种官能团且带负电荷,能与磷酸盐产生竞争吸附[33]。总体来看,即使在异常高浓度的竞争阴离子和溶解性有机物存在的情形下,MZCO复合材料依然可以有效去除磷酸盐,这有利于其在低浓度含磷废水磷回收中的实际应用。
-
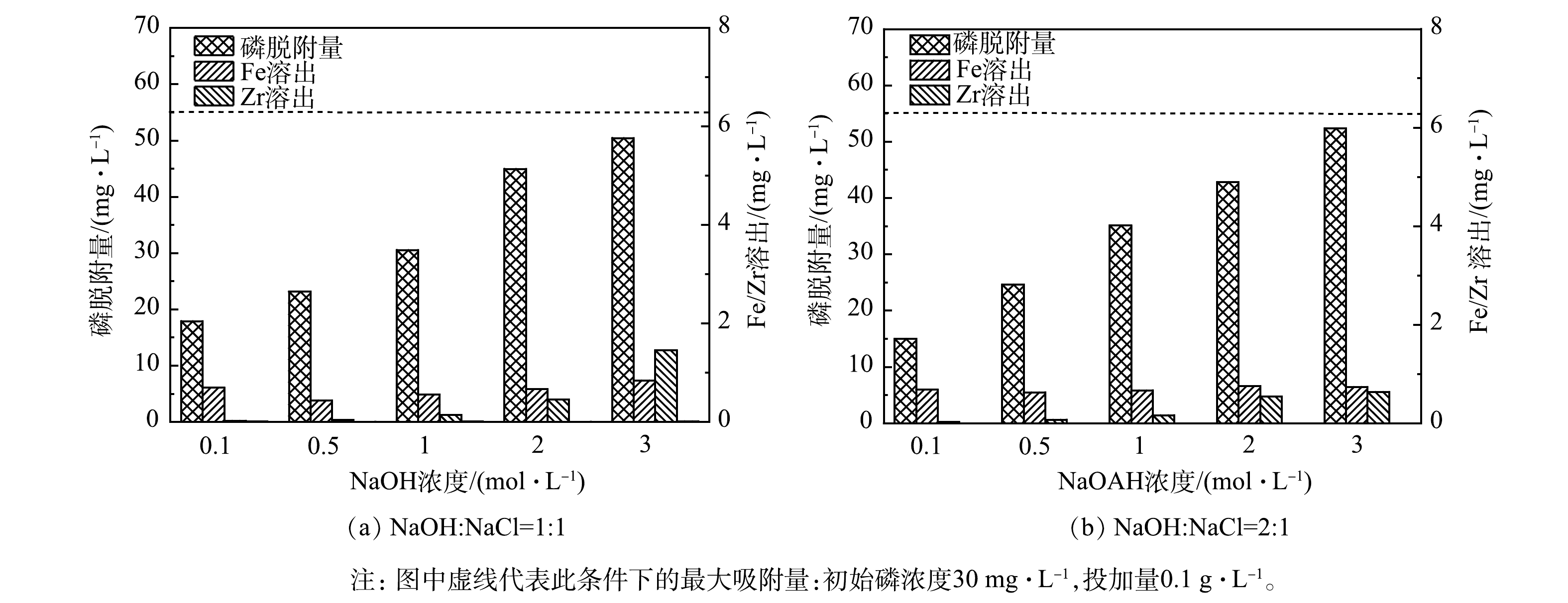
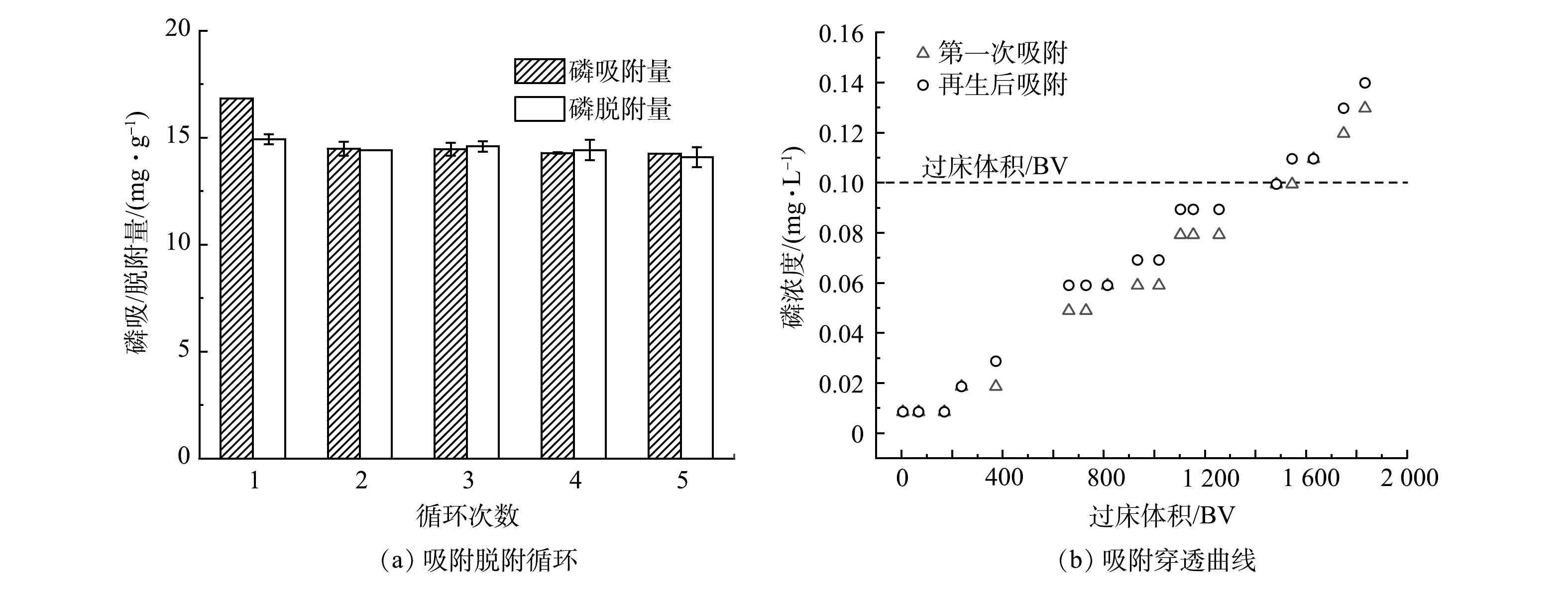
由图11可见,磷的脱附量随着NaOH和NaCl浓度的升高而增大,在3.0 mol·L−1 NaOH和1.5 mol·L−1 NaCl的浓度下,磷脱附量达到最大值,且Fe和Zr离子的溶出浓度较小。因此,选取此浓度的脱附液进行吸附再生实验。由图12可见,对于低浓度的实际水样,MZCO在5次吸附脱附后,吸附量可以保持为原吸附量的84.68%。脱附时Zr金属的溶出可能是其吸附量降低的主要原因。在柱实验中,根据地表水二类标准(GB 3838-2002),将穿透值设定为0.1 mg·L−1。在第1次吸附过程中,MZCO的穿透体积达到~1 500 BV(~34.05 mg·g−1),对吸附后的MZCO进行12 h的脱附再生后,再次吸附的穿透体积略微下降,保持在~1 400 BV(~32.9 mg·g−1)。以上实验结果均表明,MZCO是一种有效且稳定的除磷吸附剂。
-
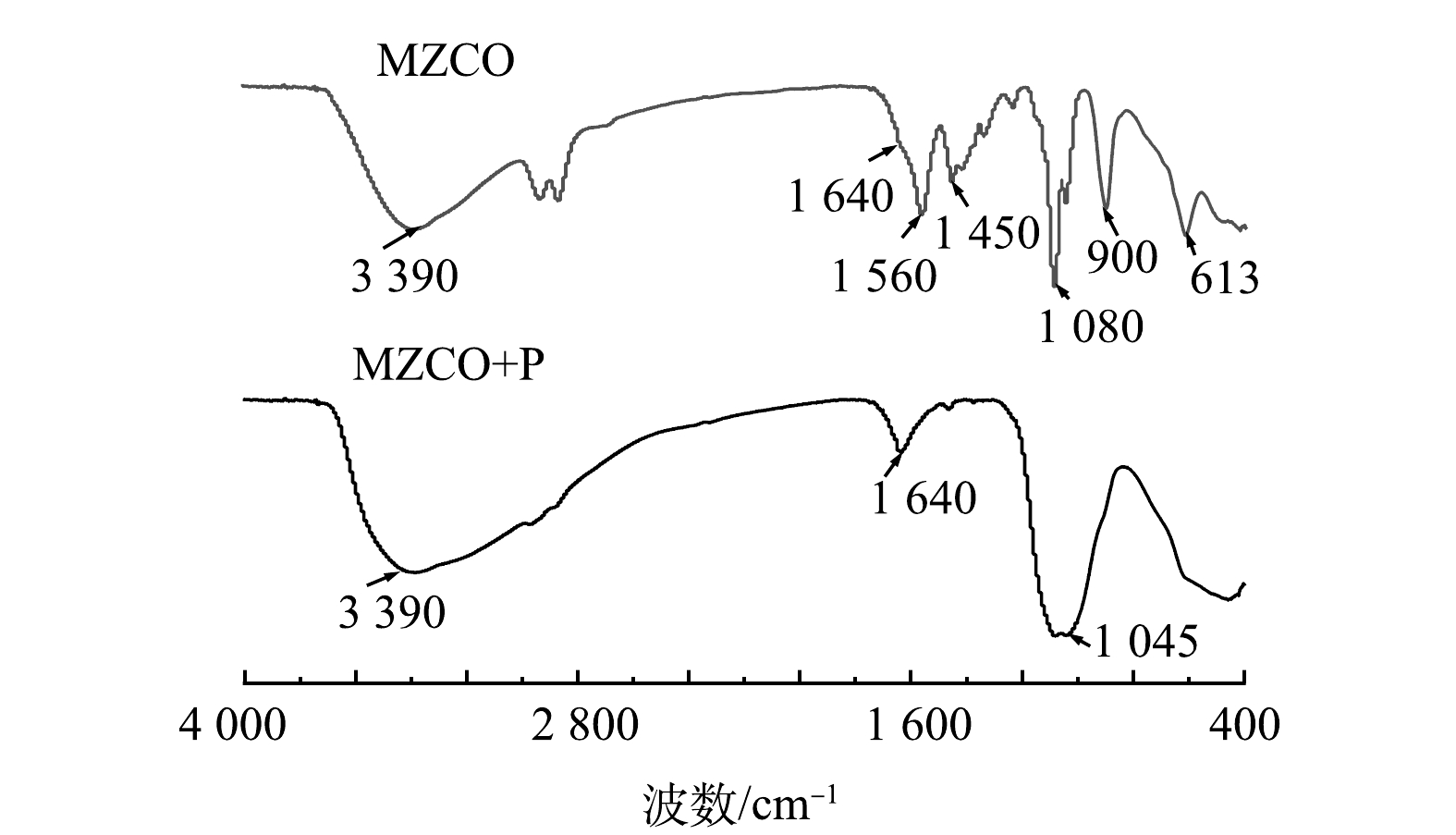
图13为吸附前后MZCO的红外谱图。吸附前,以3 390 cm−1为中心的宽峰和1 640 cm−1的峰分别属于物理吸附分子水或者材料表面—OH基团伸缩振动[9, 30],1 560 cm−1是Zr—OH基团的弯曲振动峰[34],1 450、1 080和900 cm−1是C—O的弯曲振动峰[35-36],613 cm−1的吸收峰归属于Fe—O键的特征峰[37]。吸附后,1 045 cm−1出现的新吸收峰归属于HPO42-或H2PO4−的ν3振动。与此同时,1 560 cm−1和1 450 cm−1的特征峰消失。这意味着HPO42-或H2PO4−通过与—OH和CO32-配体交换成功的吸附到MZCO表面[38]。此外,位于3 390 cm−1处的宽峰面积略微增大。这可能是由于磷酸盐吸附后在材料表面生成单齿的(ZrO)PO2(OH),使得表面—OH基团增多[39]。
通过XPS光谱分析MZCO吸附磷酸盐前后元素结合能的变化,可进一步探索吸附机制。MZCO吸附磷酸盐前后的XPS全谱图、Zr3d和O1s的精细谱图如图14所示。由图14(a)可见,结合能531.08、285.08和182.08 eV 的峰分别归属于O1s、C1s和Zr3d。在吸附磷酸盐后,在130.08 eV出现了P2p 的峰,表明磷酸盐成功被MZCO吸附。由图14(b)可见,磷酸盐吸附前后锆的结合能增加。这表明Zr也参与了磷酸盐的吸附。O1s的XPS光谱在磷酸盐吸附前后变化显著(图14(c))。将O1s拟合为4个峰:532.5、531.2、530.5和529.7 eV,其分别对应吸附水(H2O)、—OH、CO32-和O2−。吸附后,—OH和O2−所占比例由32.19%和27.72%升高到34.82%和44.37%,而CO32-所占比例由35.63%下降到16.03%(表5)。这与之前的研究结果一致[18]。这可能是由于材料表面的—OH和CO32-与磷酸盐发生配体交换作用,形成了单齿的(ZrO)PO2(OH),使得—OH比例略微升高,而CO32-比例有所下降。
-
1)利用溶剂热法成功合成了Fe3O4负载非晶态(碳酸)氧化锆复合材料,其中Fe3O4是磁性来源,而非晶态(碳酸)氧化锆是吸附磷的主要活性位点。
2) MZCO对磷的饱和吸附容量为68.16 mg·g−1,2 h内的磷吸附量可以达到饱和吸附容量的87.2%;在低浓度磷酸盐溶液中,MZCO除磷性能在pH为5~9内保持稳定,并且表现出良好的选择吸附性。对于低浓度实际水样,MZCO具有良好的重复利用性与实际应用潜力。
3)吸附过程符合非均匀的多分子层模型,且以化学吸附为主导;静电吸引作用和配体交换作用是MZCO吸附磷酸盐的主要机理。
Fe3O4负载非晶态(碳酸)氧化锆复合材料对磷的吸附性能及机理
Fe3O4 loaded amorphous zirconium (carbonate) oxides composite for phosphate adsorption: Performance, mechanism and treatment effect in real wastewater
-
摘要: 有效控制富营养化和磷酸盐回收是近年来日益受到重视的问题。为此,采用溶剂热法合成了一种去除水中磷酸盐的Fe3O4负载非晶态(碳酸)氧化锆复合材料(MZCO),通过X射线衍射仪(XRD)、场发射扫描电镜(SEM)、高分辨率透射电镜(TEM)、傅里叶变换红外光谱仪(ATR-IR)、X光电子能谱仪(XPS)等对其进行了结构表征、吸附性能评价和吸附机理的探究。结果表明:MZCO为Fe3O4负载非晶态(碳酸)氧化锆复合产物,其比表面积高达478.28 m2·g−1。MZCO对磷酸盐的饱和吸附容量为68.16 mg·g−1,在前120 min的吸附量可以达到该饱和吸附量的87.2%。对于低浓度(2 mg·L−1)磷酸盐溶液,MZCO在pH为5~9内能保持稳定的吸附性能,相应的吸附容量为20 mg·g−1左右,Mg2+、Ca2+ 、Cl–、NO3–、SO42–和HCO3−等共存离子和腐殖酸(HA)对磷酸盐吸附均无较大影响。对于低浓度实际废水(5 mg·L−1),经5次吸附再生后MZCO吸附量可以保持初始吸附量的84.68%;当穿透值为0.1 mg·L−1时,柱吸附量可达到~1 500 BV (~34.05 mg·g−1)。MZCO吸附磷酸盐的主要机制为静电吸引、—OH和CO32−与磷酸根之间的配体交换。Abstract: At present, the effective eutrophication control and phosphate recovery have attracted an increasing attention. In this study, a type of Fe3O4 loaded amorphous zirconium (carbonate) oxides composite (MZCO) was synthesized by solvothermal method to remove phosphate from water. XRD, SEM, TEM, AFT-IR and XPS were used to characterize the micro-morphology and physicochemical properties of MZCO, and evaluate its adsorption performance and investigate the adsorption mechanisms towards phosphate. The results showed that MZCO consisted of Fe3O4 loaded amorphous zirconium (carbonate) oxides and its large specific surface area was 478.28 m2·g−1. The adsorption capacity of MZCO towards phosphate was 68.16 mg·g−1, and within the first 120 min its adsorption amount could reach 87.2 % of this capacity. For the phosphate solution with an initial PO43−-P concentration of 2 mg·L−1, MZCO could maintain a stable adsorption amount of about 20 mg·g−1 over a wide pH range of 5.0~9.0. Moreover, MZCO showed a strong selectivity to low-concentration phosphate in the presence of competing ions (Cl−, NO3−, SO42−, HCO3−, Ca2+, and Mg2+) and humic acid (HA). After 5 cycles of adsorption-desorption in real wastewater samples, the adsorption amount of regenerated MZCO still remained 84.68% of its maximum value. The column adsorption capacity of MZCO could reach ~1500 BV (~34.05 mg·g−1) as P concentration decreased from 5 mg·L−1 to 0.1 mg·L−1. The mechanism of phosphate uptake onto MZCO could be mainly ascribed to electrostatic attraction and ligand exchanges between —OH and PO43-, CO32- and PO43-.
-
磷是生物体生长所必须的营养元素,也是影响水生生态系统的关键因素[1]。大量的磷排放到水体中会引起水体富营养化,对水生生态系统产生严重威胁[2]。此外,磷酸盐是一种不可再生资源,并且随着需求的增长,全球的磷资源可能在50~100 a内耗尽,对水体中的磷酸盐进行回收再利用是大势所趋[3]。传统的化学沉淀法、生物去除法、电化学处理法和离子交换法常用来去除水体中的磷酸盐,但其处理效果往往很难达到污水处理厂出水一级A标准(总磷质量浓度不超过0.5 mg·L−1),对低浓度磷酸盐的回收利用也存在一定困难,因此,新的磷回收技术值得深入研究[4]。
吸附法是近年来国内外对废水中磷酸盐的去除和回收研究最多的技术之一[5-6]。与其他技术相比,吸附法简单、快速、操作灵活、经济性高,可用于多种环境并取得良好的出水水质,具有较好的应用前景。在众多吸附剂中,锆(Zr)基氧化物由于其无毒、抗酸碱和氧化性能好、热稳定性高、在水中的溶解度低等特点,是一种很有潜力的水处理吸附剂,并被大量研究[7]。ZHANG等[8]通过共沉淀法制备了Zr-Fe双金属氧化物,其对磷的吸附量达到21.3 mg·g−1且具有磁性,能够采用磁分离法将材料分离。SU等制备的非晶ZrO2比表面积达到327 m2·g−1,且大部分的空隙为介孔,其对磷的吸附量达到32.29 mg·g−1[9]。ZHAO等[10]合成了胺化木质素负载的Zr(OH)4复合材料,吸附量达到54.7 mg·g−1,在酸性条件下表面的氨基质子化能促进磷酸盐的吸附。SHANG等[11]将水合氧化锆(HZO)负载到季胺基团改性的芦苇上,吸附量高达59.2 mg·g−1,带永久正电荷的季胺基团能够强化静电引力从而提高磷吸附量。以往的研究表明,负载活性组分为Zr4+、ZrO2或Zr(OH)4的Zr基吸附剂均具有很好的吸附性能,成为近年来水中磷酸盐回收利用的研究热点之一。此外,磁分离技术因其分离效率高、分离时间短、能耗低、选择性高等优点而引起人们越来越多的关注[12]。具有良好分离性能的磁性材料,在吸附领域的应用前景可观。因此,将新的Zr基活性组分负载到磁性Fe3O4纳米颗粒上以获得良好的吸附与磁分离能力,值得进一步研究。
本研究利用溶剂热法,将非晶(碳酸)氧化锆与Fe3O4进行掺杂,制备了磁性非晶(碳酸)氧化锆(MZCO)吸附材料;采用XRD、SEM、EDS、TEM、BET、Zeta电位等分析方法对MZCO的基本形态和物理化学性质进行了表征和分析;研究了MZCO吸附磷酸盐的等温线、动力学、pH、共存离子的影响特征,实际废水的吸附再生和吸附穿透曲线,并结合ATR-IR和XPS的表征结果揭示了MZCO吸附磷酸盐的相关机理。
1. 材料与方法
1.1 试剂与水样
纳米四氧化三铁(Fe3O4,200 nm)为分析纯,购于上海麦克林生化科技有限公司;氯化锆(ZrCl4)、乙二醇((CH2OH)2)均为分析纯,购于上海阿拉丁生化科技有限公司;聚乙二醇(PEG2 000)、无水乙酸钠(CH3COONa)、尿素(CH4N2O)、氢氧化钠(NaOH)、盐酸(HCl)、氯化钠(NaCl)、无水乙醇(C2H5OH)、磷酸二氢钾(KH2PO4)、酒石酸锑钾(C8H4K2O12Sb2)、抗坏血酸(C6H8O6)、钼酸铵((NH4)6Mo7O24·4H2O)、氯化钾(KCl)、碳酸氢钾(KHCO3)、硝酸钾(KNO3)、硫酸钾(K2SO4)、氯化钙(CaCl2)、氯化镁(MgCl2)、腐殖酸(HA)均为分析纯,购买于国药集团化学试剂有限公司。
实际水样为北京市某污水处理厂的二沉池出水,其COD和pH分别为4.8 mg·L−1和7.68,并含有0.034 mg·L−1 PO43−、14.2 mg·L−1 NO3−、143 mg·L−1 Cl−、85.26 mg·L−1 SO42−、90.7 mg·L−1 Ca2+、27.7 mg·L−1 Mg2+、109.51 mg·L−1 Na+、21.98 mg·L−1 K+等离子。该水样经过定性滤纸过滤后,调节磷酸盐浓度为5 mg·L−1,在4 ℃下储存备用。
1.2 材料制备
本实验采用溶剂热法制备MZCO材料。首先称取一定摩尔质量的ZrCl4,在超声辅助下溶于乙二醇中形成澄清溶液。然后将0.385 g纳米Fe3O4(200 nm),7.2 g乙酸钠,2 g聚乙二醇(分子质量2 000 Da)和1 g尿素依次加入到该溶液中,剧烈搅拌30 min后,将所得混合溶液密封在100 mL四氟乙烯衬里的不锈钢高压釜中。将高压釜在200 ℃下加热12 h,然后冷却至室温。产物经纯水和乙醇洗涤数次,真空冷冻干燥,研磨后备用。
1.3 样品表征
使用X射线衍射仪(XRD-7 000,Shimadzu,日本)表征材料晶体结构;使用场发射扫描电子显微镜(Merlin,Zeiss,德国)和高分辨率透射电子显微镜(SU-8010,Hitachi,美国)观察样品的形貌和元素分布;使用振动样品磁力计(703T,Lake Shore,美国)测试磁饱和强度;使用Zeta电位仪(Nano ZS90, Malvern Instruments)对不同pH下MZCO的表面电荷进行了表征;使用比表面积及孔径分析仪(ASAP2460,Mike,美国)测定MZCO比表面积;使用激光粒度仪(Mastersizer 2000,Malvern,英国)测定MZCO的粒径;使用电感耦合等离子体发射光谱仪(9800,Shimadzu,日本)测定金属含量;通过傅里叶变换红外光谱仪(ATR-IR,Vector 22,Bruker)表征吸附前后官能团变化;使用X光电子能谱仪(XPS,ESCALAB 250 XI,Thermo)对MZCO吸附前后化学结构以及官能团的变化进行表征,分析结果使用XPS峰拟合程序(XPS peak)进行分析。
1.4 静态吸附实验
在Zr投加量的优化实验中,磷酸根初始质量浓度为50 mg·L−1,投加量为0.3 g·L−1;在吸附等温线实验中,配制不同质量浓度(0~50 mg·L−1)的50 mL磷酸盐水样中,加入0.1 g·L−1吸附剂;在吸附动力学实验中,取1 L磷酸盐(30 mg·L−1)水样, 加入0.1 g·L−1吸附剂,间隔一定时间取样。在上述实验过程中,采用0.1 mol·L−1的HCl或NaOH溶液调节水样pH至6.8±0.1,吸附时间为24 h。在pH影响实验中,取50 mL磷酸盐水样(2 mg·L−1),加入0.1 g·L−1吸附剂,采用0.1 mol·L−1的HCl或NaOH溶液调节水样pH至不同初始值(2~11),并在振荡期间每隔4 h调节1次pH。在共存离子实验中,取50 mL磷酸盐水样(2 mg·L−1),按0.1 g·L−1投加量加入吸附剂,共存离子(Cl–、NO3–、SO42、HCO3−、Ca2+和Mg2+)和溶剂性有机物腐殖酸(HA)的质量浓度分别为10、50和100 mg·L−1。所有实验样品均过0.45 μm聚醚砜膜,然后将样品存放于(4±0.5) ℃冰箱内待测。采用钼酸铵分光光度法测定水样中磷浓度(以P计)。所有实验均重复3次。
吸附等温线涉及的Langmuir、Freundlich和Sips吸附等温模型方程如式(1)~式(3)所示。吸附动力学涉及的准一级(式(4))和准二级(式(5))吸附动力学模型方程如下。
stringUtils.convertMath(!{formula.content}) (1) stringUtils.convertMath(!{formula.content}) (2) stringUtils.convertMath(!{formula.content}) (3) 式中:qe和qm分别为吸附平衡时的吸附量和最大吸附量,mg·g−1;KL(L·mg−1)、KF((mg·g−1)·( mg·L−1)−n)和Ks(L·g−1)分别为Langmuir、Freundlich和Sips方程模型常数;Ce为平衡质量浓度,mg·L−1;n为Freundlich吸附指数;αs为平衡常数;βs为吸附平衡分解常数(当βs=1时,Sips方程与Langmuir方程式相同);β为吸附能量的参数。
stringUtils.convertMath(!{formula.content}) (4) stringUtils.convertMath(!{formula.content}) (5) 式中:qe和qt分别为平衡吸附量以及t时刻的吸附量,mg·g−1;t为吸附时间,min;k1为准一级动力学方程常数,min−1;k2为准一级动力学方程常数,g∙(mg·h)−1。
1.5 吸附再生和动态吸附柱实验
在再生液配比实验中,取一定质量的样品,使其在高浓度磷酸根(30 mg·L−1)溶液中吸附饱和,然后将吸附饱和的样品采用磁分离取出。分别采用不同NaOH/NaCl比的再生液对吸附后的MZCO进行再生,选取磷脱附量高和金属溶出低的再生液进行吸附再生实验。
在吸附再生实验中,取0.15 g的样品,在500 mL二沉池出水中(磷酸盐质量浓度为5 mg·L−1)中吸附12 h,然后采用磁分离法将吸附后的样品采用取出。使用3 mol·L−1的NaOH和1.5 mol·L−1的NaCl混合溶液进行脱附,磁力搅拌12 h后将样品磁分离,用去离子水洗涤烘干进行下一次吸附再生实验,共重复5次。
在动态吸附实验中,采用直径15 mm,高150 mm的玻璃吸附柱为实验容器,填充吸附剂为2.65 cm3(0.6 g)。使用二沉池出水(磷酸盐质量浓度为5 mg·L−1)作为原水,控制蠕动泵流速为1.5 mL·min−1,空床停留时间为1.8 min。磷酸根离子穿透质量浓度设定为0.1 mg·L−1。穿透完成后,使用3 mol·L−1的NaOH和1.5 mol·L−1的NaCl混合溶液对MZCO进行12 h脱附。脱附后的MZCO进行第2次吸附柱实验,实验条件与之前保持一致。
2. 结果与讨论
2.1 MZCO制备中Zr投加量的确定
有研究表明,Zr基吸附材料中锆的比例是影响其对磷酸根吸附能力的关键[8]。不同Zr投加量产物MZCO的磷吸附量如图1所示。随着Zr投加量从5 mmol升高到30 mmol,产物磷吸附量先增后降,当Zr投加量达到25 mmol时,产物的吸附量最高达到69.5 mg·g−1。因此,选择合成条件中Zr投加量为25 mmol的合成产物进行下一步研究。
2.2 MZCO的表征
由图2可以看出,其衍射峰在36°出现的尖峰与标准的立方晶型Fe3O4(JCPDs 72-2303)能很好地匹配。这证明Fe3O4以晶体的形式存在于MZCO复合材料中。在以30°为中心出现的宽峰与ZrO2(JCPDs 79-1796)较为匹配,表明MZCO复合材料中存在大量非晶ZrO2成分。这也与之前的报道结果相一致[13]。此外,结合材料红外分析结果可知,材料表面还存在大量的CO32-基团。由图3(a)可以看到直径约为200 nm的Fe3O4颗粒的附着在非晶态ZCO表面上。在ZCO的快速傅里叶变换图像(图3(c))中观察到很宽的扩散环,表明ZCO组分具有非晶结构特征。以上结果表明,MZCO复合材料为Fe3O4负载的非晶态(碳酸)二氧化锆。由图4可以看出,MZCO具有粗糙的多孔结构,EDS光谱表明MZCO中的Zr、Fe和O在复合材料的表面均匀分布,没有发生元素分离或相分离。
MZCO的磁滞曲线和Zeta电位如图5所示,其他相关物理化学性质见表1。MZCO的饱和磁化强度为5.22 emu·g−1,远低于纯Fe3O4(63.80 emu·g−1),这归因于无磁性ZCO与Fe3O4的掺杂。MZCO近似表现出超顺磁特性。这说明材料在无磁场的情况下可以较好地分散在溶液中;在有磁场的情况下可快速分离收集[14]。MZCO的等电点(pHpzc)为5.8,pH为3.0时Zeta电位最高为32.4 mV。ZCO的掺杂使得复合材料的表面具有更多的正电荷,从而增强了对磷酸盐的静电吸引。粒径分析结果表明MZCO的D50为10.12 μm,这说明该材料为是一种微米级材料。此外,MZCO的比表面积为478.28 m2·g−1,孔径为13.7 nm,孔体积为1.09 cm3·g−1,均远大于Fe3O4。这可能归因于多晶ZCO的引入,其具有较大的比表面积和孔隙参数[15]。MZCO中锆和铁的含量分别为431.0 mg·g−1和61.8 mg·g−1,较高的Zr含量和较大的比表面积,能够使表面Zr活性位点更多的暴露出来,从而获得较高的磷吸附容量。
表 1 MZCO的物理化学性质Table 1. General characteristics of MZCO吸附剂 形貌 外观 饱和磁化强度/(emu·g−1) 比表面积/(m2·g−1) 孔径/nm 孔体积/(cm3·g−1) 粒径/μm 金属含量/(mg·g−1) Fe3O4 粉末 黑色 63.80 27.48 5.84 0.07 0.39 — MZCO 粉末 灰色 5.22 478.28 13.7 1.09 10.12 61.2 (Fe)431.0 (Zr) 2.3 MZCO吸附性能评价
1)吸附等温线。MZCO在磷酸根初始质量浓度为0~50 mg·g−1内的吸附等温线如图6所示。随着磷酸盐平衡浓度的增长,MZCO对磷酸盐的吸附能力迅速增强,然后逐渐达到稳定值。吸附等温线数据使用Langmuir、Freundlich和Sips模型进行拟合,具体参数如表2所示。由Langmuir模型拟合的饱和吸附量为68.16 mg·g−1,略低于实际测出的最大吸附量69.75 mg·g−1。在3种模型中,Sips模型的拟合结果最佳(R2=0.98),表明MZCO吸附磷酸盐过程更加符合非均匀的多分子层吸附模型[16]。MZCO与其他Zr基吸附剂的磷酸盐吸附性能比较见表3。结果表明,大多数Zr基金属材料对磷酸盐的最大吸附能力均低于本研究合成的MZCO。这可能是因为MZCO中含有较多的Zr活性组分(43.1%)。此外,MZCO具有较高的比表面积(478.28 m2·g−1),这也有利于Zr活性位点的分散,从而更好的参与磷酸盐吸附。
表 2 MZCO吸附磷的3种吸附等温线拟合参数Table 2. Adsorption isotherm parameters for phosphorus adsorption onto MZCOLangmuir Freundlich Sips qm kL RL2 kF 1/n RF2 ks βs αs Rs2 68.16 0.42 0.96 26.60 0.26 0.95 33.86 0.59 0.39 0.98 表 3 锆基除磷吸附剂吸附量对比Table 3. Comparison of phosphorus adsorption capacity of different Zr-based materials吸附剂 活性组分/(mg·g−1) 活性物质 qm/(mg·g−1) 参考文献 Zr@蒙脱石 151 Zr4+ 15.5 [17] ZrO2@Fe3O4 260 ZrO2 15.98 [18] MIl-101@Zr 52.3 ZrO2 23.81 [19] ZrFe-活性炭纳米纤维 — Zr/Fe—OH 26.3 [20] am-ZrO2 — Zr—O 32.3 [9] Ws−N−Zr 193 HZO 31.9 [21] Zr-CS-SAC — Zr4+ 42.7 [22] La-Zr@Fe3O4 113.9 (La)154.6 (Zr) La(OH)3Zr(OH)4 49.3 [23] AL-DETA@Zr — Zr(OH)4 54.7 [10] ZrO2/SiO2纳米纤维膜 63.5 (Zr) ZrO2 57.58 [24] MZCO 431 (Zr) Zr—O/Zr—CO32- 68.16 本研究 2)吸附动力学。MZCO对磷酸根吸附动力学如图7所示。其磷酸盐吸附量在前120 min中迅速升高,在30、60和120 min的吸附量分别可以达到饱和吸附容量的60.3%、78.8%和87.2%。随后,磷酸盐吸附量在4 h后逐渐趋于稳定,并在6 h内达到平衡。吸附反应早期的快速吸附可能是由于MZCO表面上丰富的活性位点,能高效的吸附磷酸盐。此外良好的表面结构(比表面积、孔径和孔体积),有利于磷酸盐在材料表面的快速传质[25]。在随后的阶段,缓慢的吸附速率意味其主要吸附机制为颗粒内扩散[26]。使用准一级和准二级动力学模型对其动力学曲线进行拟合,相关拟合参数如表4所示。MZCO复合材料的吸附过程更符合准二级动力学模型(R2=0.95),表明其磷酸根的吸附过程主要以吸附活性位点与磷酸盐之间的化学吸附为主导[27-28]。
表 4 MZCO的磷吸附动力学模型参数Table 4. The kinetic model parameters for phosphorus adsorption onto MZCO准一级动力学 准二级动力学 qe/(mg·g−1) k1 R2 qe/(mg·g−1) K2 R2 51.28 0.036 72 0.85 53.88 0.001 05 0.95 3) pH影响。由图8可见,随着pH由2.0增加到3.0,磷吸附量达到最高;随着pH增加到6.0,磷吸附量一直保持稳定。当pH在6.0~10.0内继续升高,MZCO的磷吸附量略微降低。当pH升高到11时,其磷吸附量降到最低。该结果表明MZCO在较宽的pH(2~10)范围内都具有很好的磷酸根去除能力。当pH为2.0~3.0时,磷酸盐主要以一价的H2PO4−形态存在,H3PO4含量较低(图8)。在酸性条件下,MZCO的表面质子化,Zeta电位在pH为3时达到32 mV(图5(b))。这说明表面带正电荷的MZCO可能通过静电吸引促进与H2PO4−的相互作用。此外,当pH增加到3.0时,MZCO没有Zr溶出(表5),这也有利于磷酸盐的吸附。在pH为3.0~5.8时,MZCO的表面质子化程度降低,Zeta电位逐渐下降,使得其与磷酸盐之间的静电引力降低,但由于MZCO较高的饱和吸附量,使得其在吸附低浓度磷酸盐(2 mg·L−1)时,依然能保持20 mg·g−1的吸附量。随着pH从6.0增加到10.0,MZCO表面的碳酸根主要以HCO3−的形式存在(图9),其可以与带负电的磷酸盐进行配体交换。二价的HPO42-逐渐占据主导地位,与H2PO4−相比,HPO42-拥有较高的吸附自由能而更难被吸附[29-30]。此外,在碱性条件下,OH−的浓度增强并与磷酸盐产生竞争吸附,MZCO表面去质子化程度加深,表面带负电荷的MZCO与磷酸盐产生静电排斥,这些原因共同造成了磷酸盐吸附能力的下降。之后,当pH进一步增加到11.0时,MZCO的吸附能力急剧下降。较强的静电排斥力和OH−竞争吸附共同导致磷酸盐吸附能力的降低[31]。
表 5 MZCO吸附前后的O1s XPS分峰拟合数据Table 5. Peak fitting parameters for O1s XPS peak of MZCO before and after phosphate adsorption样品 峰 位置 面积 宽度 百分比/% MZCO H2O 532.5 9 711.97 1.79 4.44 —OH 531.2 70 418.1 1.94 32.19 CO32- 530.5 77 932.47 1.96 35.63 O2- 529.7 60 671.53 2.41 27.74 MZCO + P H2O 532.6 10 345.8 1 4.78 —OH 531.5 75 336.02 1.85 34.82 CO32- 531 34 685.04 1.27 16.03 O2- 530.4 96 018.32 2 44.37 通过测定Zr和Fe元素在不同pH溶液中的溶出量,对MZCO的稳定性进行了评价。在pH为 5.0~9.0内,均未检测到Zr和Fe的金属溶出,MZCO这种较宽的pH适用性极大地扩展了其应用范围。
4)共存离子影响。在低浓度磷酸盐溶液中,3种不同浓度无机离子和HA对磷酸盐吸附的影响如图10所示。其中Ca2+、Mg2+、Cl–和NO3–对磷酸盐吸附的影响可忽略不计。SO42带有2个负电荷,与带有1个负电荷的H2PO4−相比,能与MZCO表面产生更强的静电吸引。此外,SO42的离子半径(0.230 nm)与H2PO4−的离子半径(0.238 nm)接近,更容易与H2PO4−竞争MZCO表面的金属活性位点[32]。HCO3−的存在,使得溶液pH升高,OH−浓度的升高和MZCO表面Zeta电位的降低,共同影响了磷酸盐的吸附。此外,HCO3−的大量存在还会影响MZCO表面碳酸盐的解离,从而影响其与溶液中磷酸盐的配体交换。HA作为高分子聚芳香族化合物,其表面具有—OH、—COOH等多种官能团且带负电荷,能与磷酸盐产生竞争吸附[33]。总体来看,即使在异常高浓度的竞争阴离子和溶解性有机物存在的情形下,MZCO复合材料依然可以有效去除磷酸盐,这有利于其在低浓度含磷废水磷回收中的实际应用。
2.4 MZCO吸附再生与吸附柱实验
由图11可见,磷的脱附量随着NaOH和NaCl浓度的升高而增大,在3.0 mol·L−1 NaOH和1.5 mol·L−1 NaCl的浓度下,磷脱附量达到最大值,且Fe和Zr离子的溶出浓度较小。因此,选取此浓度的脱附液进行吸附再生实验。由图12可见,对于低浓度的实际水样,MZCO在5次吸附脱附后,吸附量可以保持为原吸附量的84.68%。脱附时Zr金属的溶出可能是其吸附量降低的主要原因。在柱实验中,根据地表水二类标准(GB 3838-2002),将穿透值设定为0.1 mg·L−1。在第1次吸附过程中,MZCO的穿透体积达到~1 500 BV(~34.05 mg·g−1),对吸附后的MZCO进行12 h的脱附再生后,再次吸附的穿透体积略微下降,保持在~1 400 BV(~32.9 mg·g−1)。以上实验结果均表明,MZCO是一种有效且稳定的除磷吸附剂。
2.5 MZCO吸附机理
图13为吸附前后MZCO的红外谱图。吸附前,以3 390 cm−1为中心的宽峰和1 640 cm−1的峰分别属于物理吸附分子水或者材料表面—OH基团伸缩振动[9, 30],1 560 cm−1是Zr—OH基团的弯曲振动峰[34],1 450、1 080和900 cm−1是C—O的弯曲振动峰[35-36],613 cm−1的吸收峰归属于Fe—O键的特征峰[37]。吸附后,1 045 cm−1出现的新吸收峰归属于HPO42-或H2PO4−的ν3振动。与此同时,1 560 cm−1和1 450 cm−1的特征峰消失。这意味着HPO42-或H2PO4−通过与—OH和CO32-配体交换成功的吸附到MZCO表面[38]。此外,位于3 390 cm−1处的宽峰面积略微增大。这可能是由于磷酸盐吸附后在材料表面生成单齿的(ZrO)PO2(OH),使得表面—OH基团增多[39]。
通过XPS光谱分析MZCO吸附磷酸盐前后元素结合能的变化,可进一步探索吸附机制。MZCO吸附磷酸盐前后的XPS全谱图、Zr3d和O1s的精细谱图如图14所示。由图14(a)可见,结合能531.08、285.08和182.08 eV 的峰分别归属于O1s、C1s和Zr3d。在吸附磷酸盐后,在130.08 eV出现了P2p 的峰,表明磷酸盐成功被MZCO吸附。由图14(b)可见,磷酸盐吸附前后锆的结合能增加。这表明Zr也参与了磷酸盐的吸附。O1s的XPS光谱在磷酸盐吸附前后变化显著(图14(c))。将O1s拟合为4个峰:532.5、531.2、530.5和529.7 eV,其分别对应吸附水(H2O)、—OH、CO32-和O2−。吸附后,—OH和O2−所占比例由32.19%和27.72%升高到34.82%和44.37%,而CO32-所占比例由35.63%下降到16.03%(表5)。这与之前的研究结果一致[18]。这可能是由于材料表面的—OH和CO32-与磷酸盐发生配体交换作用,形成了单齿的(ZrO)PO2(OH),使得—OH比例略微升高,而CO32-比例有所下降。
3. 结论
1)利用溶剂热法成功合成了Fe3O4负载非晶态(碳酸)氧化锆复合材料,其中Fe3O4是磁性来源,而非晶态(碳酸)氧化锆是吸附磷的主要活性位点。
2) MZCO对磷的饱和吸附容量为68.16 mg·g−1,2 h内的磷吸附量可以达到饱和吸附容量的87.2%;在低浓度磷酸盐溶液中,MZCO除磷性能在pH为5~9内保持稳定,并且表现出良好的选择吸附性。对于低浓度实际水样,MZCO具有良好的重复利用性与实际应用潜力。
3)吸附过程符合非均匀的多分子层模型,且以化学吸附为主导;静电吸引作用和配体交换作用是MZCO吸附磷酸盐的主要机理。
-
表 1 MZCO的物理化学性质
Table 1. General characteristics of MZCO
吸附剂 形貌 外观 饱和磁化强度/(emu·g−1) 比表面积/(m2·g−1) 孔径/nm 孔体积/(cm3·g−1) 粒径/μm 金属含量/(mg·g−1) Fe3O4 粉末 黑色 63.80 27.48 5.84 0.07 0.39 — MZCO 粉末 灰色 5.22 478.28 13.7 1.09 10.12 61.2 (Fe)431.0 (Zr) 表 2 MZCO吸附磷的3种吸附等温线拟合参数
Table 2. Adsorption isotherm parameters for phosphorus adsorption onto MZCO
Langmuir Freundlich Sips qm kL RL2 kF 1/n RF2 ks βs αs Rs2 68.16 0.42 0.96 26.60 0.26 0.95 33.86 0.59 0.39 0.98 表 3 锆基除磷吸附剂吸附量对比
Table 3. Comparison of phosphorus adsorption capacity of different Zr-based materials
吸附剂 活性组分/(mg·g−1) 活性物质 qm/(mg·g−1) 参考文献 Zr@蒙脱石 151 Zr4+ 15.5 [17] ZrO2@Fe3O4 260 ZrO2 15.98 [18] MIl-101@Zr 52.3 ZrO2 23.81 [19] ZrFe-活性炭纳米纤维 — Zr/Fe—OH 26.3 [20] am-ZrO2 — Zr—O 32.3 [9] Ws−N−Zr 193 HZO 31.9 [21] Zr-CS-SAC — Zr4+ 42.7 [22] La-Zr@Fe3O4 113.9 (La)154.6 (Zr) La(OH)3Zr(OH)4 49.3 [23] AL-DETA@Zr — Zr(OH)4 54.7 [10] ZrO2/SiO2纳米纤维膜 63.5 (Zr) ZrO2 57.58 [24] MZCO 431 (Zr) Zr—O/Zr—CO32- 68.16 本研究 表 4 MZCO的磷吸附动力学模型参数
Table 4. The kinetic model parameters for phosphorus adsorption onto MZCO
准一级动力学 准二级动力学 qe/(mg·g−1) k1 R2 qe/(mg·g−1) K2 R2 51.28 0.036 72 0.85 53.88 0.001 05 0.95 表 5 MZCO吸附前后的O1s XPS分峰拟合数据
Table 5. Peak fitting parameters for O1s XPS peak of MZCO before and after phosphate adsorption
样品 峰 位置 面积 宽度 百分比/% MZCO H2O 532.5 9 711.97 1.79 4.44 —OH 531.2 70 418.1 1.94 32.19 CO32- 530.5 77 932.47 1.96 35.63 O2- 529.7 60 671.53 2.41 27.74 MZCO + P H2O 532.6 10 345.8 1 4.78 —OH 531.5 75 336.02 1.85 34.82 CO32- 531 34 685.04 1.27 16.03 O2- 530.4 96 018.32 2 44.37 -
[1] ELSER J, BENNETT E. Phosphorus cycle: A broken biogeochemical cycle[J]. Nature, 2011, 478(7367): 29. doi: 10.1038/478029a [2] RUI X, ZHANG M, MORTIMER R, et al. Enhanced phosphorus locking by novel Lanthanum/Aluminum-hydroxide composite: Implication for eutrophication control[J]. Environmental Science & Technology, 2017, 51(6): 3418-3425. [3] DESMIDT E, GHYSELBRECHT K, ZHANG Y, et al. Global phosphorus scarcity and full-scale p-recovery techniques: A review[J]. Critical Reviews in Environmental Science & Technology, 2015, 45(4): 336-384. [4] ZHOU K, WU B, SU L, et al. Enhanced phosphate removal using nanostructured hydrated ferric-zirconium binary oxide confined in a polymeric anion exchanger[J]. Chemical Engineering Journal, 2018, 345(1): 640-647. [5] WAAJEN, GUIDO, VAN, et al. Management of eutrophication in Lake De Kuil (The Netherlands) using combined flocculant-Lanthanum modified bentonite treatment[J]. Water research, 2016, 97(15): 83-95. [6] 姜其, 岑渝华, 朱玥, 等. 树脂基水合氧化铈复合材料深度去除污水中磷酸盐[J]. 中国给水排水, 2021, 37(3): 99-103. [7] 魏婷, 牛丽君, 张光明, 等. 三元复合吸附剂Ce-Zr-Zn对水中低浓度磷的吸附性能及其机理[J]. 环境工程学报, 2021, 14(11): 2938-2945. [8] ZHANG C, LI Y, WANG F, et al. Performance of magnetic zirconium-iron oxide nanoparticle in the removal of phosphate from aqueous solution[J]. Applied Surface Science 2017, 396: 1783-1792. [9] SU Y, CUI H, LI Q, et al. Strong adsorption of phosphate by amorphous zirconium oxide nanoparticles[J]. Water Research, 2013, 47(14): 5018-5026. doi: 10.1016/j.watres.2013.05.044 [10] ZHAO Y, SHAN X, AN Q, et al. Interfacial integration of zirconium components with amino-modified lignin for selective and efficient phosphate capture[J]. Chemical Engineering Journal, 2020, 398(15): 125561. [11] SHANG Y, XU X, QI S, et al. Preferable uptake of phosphate by hydrous zirconium oxide nanoparticles embedded in quaternary-ammonium Chinese reed[J]. Journal of Colloid and Interface Science, 2017, 496: 118-129. doi: 10.1016/j.jcis.2017.02.019 [12] TANG S, LO I. Magnetic nanoparticles: Essential factors for sustainable environmental applications[J]. Water Research, 2013, 47(8): 2613-2632. doi: 10.1016/j.watres.2013.02.039 [13] WANG Z, XING M, FANG W, et al. One-step synthesis of magnetite core/zirconia shell nanocomposite for high efficiency removal of phosphate from water[J]. Applied Surface Science, 2016, 366: 67-77. doi: 10.1016/j.apsusc.2016.01.059 [14] LU A, SALABAS E, SCHUTH F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application[J]. Angewandte Chemie International Edition, 2007, 46(8): 1222-1244. doi: 10.1002/anie.200602866 [15] QI Z, JOSHI T, LIU R, et al. Synthesis of Ce(III)-doped Fe3O4 magnetic particles for efficient removal of antimony from aqueous solution[J]. Journal of Hazardous Materials, 2017, 329(5): 193-204. [16] 李俊仪, 王毅力. 磁性环氧丙基三甲基氯化铵-β-环糊精复合水凝胶对草甘膦的吸附性能[J]. 环境工程学报, 2021, 14(11): 2969-2979. [17] HUO J, MIN X, WANG Y. Zirconium-modified natural clays for phosphate removal: Effect of clay minerals[J]. Environmental Research, 2021, 194: 110685. doi: 10.1016/j.envres.2020.110685 [18] FANG L, WU B, LO I. Fabrication of Silica-free superparamagnetic ZrO2@Fe3O4 with enhanced phosphate recovery from sewage: Performance and adsorption mechanism[J]. Chemical Engineering Journal, 2017, 319: 258-267. doi: 10.1016/j.cej.2017.03.012 [19] LIU T, FENG J, WAN Y, et al. ZrO2 nanoparticles confined in metal organic frameworks for highly effective adsorption of phosphate[J]. Chemosphere, 2018, 210: 907-916. doi: 10.1016/j.chemosphere.2018.07.085 [20] XIONG W, TONG J, YANG Z, et al. Adsorption of phosphate from aqueous solution using iron-zirconium modified activated carbon nanofiber: Performance and mechanism[J]. Journal of Colloid & Interface Science, 2017, 493: 17-23. [21] QIU H, LIANG C, ZHANG X, et al. Fabrication of a biomass-based hydrous zirconium oxide nanocomposite for preferable phosphate removal and recovery[J]. ACS Applied Materials & Interfaces, 2015, 7: 20835-20844. [22] BANU H. T, KARTHIKEYAN P, MEENAKSHI S. Zr4+ ions embedded chitosan-soya bean husk activated bio-char composite beads for the recovery of nitrate and phosphate ions from aqueous solution[J]. International Journal of Biological Macromolecules, 2019, 130: 573-583. doi: 10.1016/j.ijbiomac.2019.02.100 [23] LIN X, XIE Y, LU H, et al. Facile preparation of dual La-Zr modified magnetite adsorbents for efficient and selective phosphorus recovery[J]. Chemical Engineering Journal, 2021, 431: 127530. [24] WANG X, DOU L, LI Z, et al. Flexible Hierarchical ZrO2 nanoparticle-embedded SiO2 nanofibrous membrane as a versatile tool for efficient removal of phosphate[J]. ACS Applied Materials & Interfaces, 2016, 8: 34668-34676. [25] YU J, XIANG C, ZHANG G, et al. Activation of lattice oxygen in LaFe (Oxy)hydroxides for efficient phosphorus removal[J]. Environmental Science & Technology, 2019, 53(15): 9073-9080. [26] LU J, LIU H, LIU R, et al. Adsorptive removal of phosphate by a nanostructured Fe-Al-Mn trimetal oxide adsorbent[J]. Powder Technology, 2013, 233: 146-154. doi: 10.1016/j.powtec.2012.08.024 [27] ZHANG Y, QIAN Y, Li W, et al. Fluoride uptake by three lanthanum based nanomaterials: Behavior and mechanism dependent upon lanthanum species[J]. Science of the Total Environment, 2019, 683(15): 609-616. [28] 常全超, 杜玉凤, 戴敏, 等. 太阳能热解制备生物炭及其对水中铜离子的吸附[J]. 环境工程学报, 2021, 14(11): 2946-2958. [29] CHUBAR N, KANIBOLOTSKYY V, STRELKO V, et al. Adsorption of phosphate ions on novel inorganic ion exchangers[J]. Colloids & Surfaces A Physicochemical & Engineering Aspects, 2005, 255(1/2/3): 55-63. [30] SHI W, FU W, JIANG W, et al. Enhanced phosphate removal by zeolite loaded with Mg-Al-La ternary (hydr)oxides from aqueous solutions: Performance and mechanism[J]. Chemical Engineering Journal, 2019, 357: 33-44. doi: 10.1016/j.cej.2018.08.003 [31] YU Z, ZHANG C, ZHENG Z, et al. Enhancing phosphate adsorption capacity of SDS-based magnetite by surface modification of citric acid[J]. Applied Surface Science, 2017, 403: 413-425. doi: 10.1016/j.apsusc.2017.01.163 [32] LI R, WANG J, ZHOU B, et al. Enhancing phosphate adsorption by Mg/Al layered double hydroxide functionalized biochar with different Mg/Al ratios[J]. Science of the Total Environment, 2016, 559: 121-129. doi: 10.1016/j.scitotenv.2016.03.151 [33] Li X, WANG Y, LI J, et al. Rapid and selective harvest of low-concentration phosphate by La(OH)3 loaded magnetic cationic hydrogel from aqueous solution: Surface migration of phosphate from —N+(CH3)3 to La(OH)3[J]. Science of the Total Environment, 2021, 800: 149418. doi: 10.1016/j.scitotenv.2021.149418 [34] XIANG C, WANG H, JI Q, et al. Tracking internal electron shuttle using X-ray spectroscopies in La/Zr hydroxide for reconciliation of charge-transfer interaction and coordination toward phosphate[J]. ACS applied materials & interfaces, 2019, 11(27): 24699. [35] ZHANG X, HE C, WANG L, et al. Non-isothermal kinetic analysis of thermal dehydration of La2(CO3)3·3.4H2O in air[J]. Transactions of Nonferrous Metals Society of China, 2014, 24(10): 3378-3385. doi: 10.1016/S1003-6326(14)63480-4 [36] JQ A, MSA A, YAN F, et al. One-pot hydrothermal synthesis of NaLa(CO3)2 decorated magnetic biochar for efficient phosphate removal from water: Kinetics, isotherms, thermodynamics, mechanisms and reusability exploration[J]. Chemical Engineering Journal, 2020, 394(15): 124915. [37] HAO H, WANG Y, SHI B. NaLa(CO3)2 hybridized with Fe3O4 for efficient phosphate removal: Synthesis and adsorption mechanistic study[J]. Water Research, 2019, 155: 1-11. doi: 10.1016/j.watres.2019.01.049 [38] XIE J, WANG Z, LU S, et al. Removal and recovery of phosphate from water by lanthanum hydroxide materials[J]. Chemical Engineering Journal, 2014, 254(15): 163-170. [39] WANG Z, FANG W, XING M, et al. A bench-scale study on the removal and recovery of phosphate by hydrous zirconia-coated magnetite nanoparticles[J]. Journal of Magnetism and Magnetic Materials, 2017, 424: 213-220. doi: 10.1016/j.jmmm.2016.10.067 -







 下载:
下载: