-
我国是全球最大的染料生产和消费国,染料产量约占全球70%,消费量约占全球55%。因此,我国印染废水产生量很大,据相关统计,纺织染料废水排放量每年超过2×109 t,约占全国废水排放的11%,已成为危害我国水生态安全的一大威胁[1-2]。亚甲基蓝作为一种阳离子染料被广泛应用于织物染色,亚甲基蓝染料不易降解,易引起人体皮肤过敏、呼吸困难、抽搐和角膜炎等症状[3-4],此外,亚甲基蓝废水可通过降低水体透明度、毒害水生生物进而破坏水体的自净功能[5-6]。
当前国内外多采用化学氧化法、吸附法、生化法或它们的优化组合方法对染料废水进行脱色处理[7-8]。其中,吸附法以其高效、操作简便、吸附剂类型多样等优势在染料废水处理中应用广泛[9-10]。活性炭作为常用吸附剂,在染料废水处理中存在运行成本高、难再生等问题[11];石墨烯[12]、膨润土[13]、珍珠岩[14]等吸附剂相对昂贵的价格限制了其应用;新型纳米材料及复合材料吸附剂存在水分散性能差等技术瓶颈[15-16]。基于上述原因,利用矿石、粉煤灰等廉价原料作吸附基质引起了广泛的兴趣[17-18]。钢渣是钢铁冶炼过程中产生的副产物之一,目前我国钢渣堆存量已达18×109 t,然而极低的综合利用率导致大量钢渣作为废弃物被填埋或堆放处理,这不仅占用土地资源,而且会造成土壤污染[11]。钢渣类型多样且多孔的结构特性使其作为吸附剂成为可能,已有研究证明,钢渣或改性钢渣在亚甲基蓝、直接大红4BE、碱性品红等染料废水处理中具有显著优势[19-20]。因此,以钢渣为吸附剂基质不仅可以达到染料废水脱色的目的,同时可以实现“以废治废”的环保理念。现有研究表明,原钢渣对亚甲基蓝染料废水的吸附效果较差,只有40%左右[21-22]。LDHs是主层板带正电荷、层间带有阴离子插层的层状双电子吸附剂,LDHs具有无毒无害、制备成本低、易生产、易再生以及经济效益高等优势,并且其独特的孔隙结构及较大的表面积使其在染料废水处理中具有一定的应用前景[23]。
目前,关于改性电炉原钢渣对亚甲基蓝吸附的研究鲜有报道。本研究旨在利用电炉钢渣为原料制备多元LDHs,并通过批式实验研究了不同环境因子对多元LDHs吸附亚甲基蓝的影响。采用XRD和BET手段对改性材料进行了表征分析。采用SEM和FT-IR技术及吸附动力学、吸附等温线和热力学方法探究了多元LDHs对亚甲基蓝染料废水的吸附过程及吸附机理。后续研究拟制备成吸附剂,应用于工业染料废水脱色环节或用作生态湿地填料去除水体色度。
-
实验试剂。冰乙酸(CH3COOH);氢氧化铝(Al(OH)3);氢氧化钠(NaOH);亚甲基蓝(C16H18CN3S·3H2O)。以上试剂均为分析纯并购于国药集团化学试剂有限公司。
实验仪器。电子天平(FA2204B,上海精密科学仪器有限公司);热鼓风干燥箱(101-1A,天津市泰斯特仪器有限公司);分光光度计(G10S UV-Vis,美国赛默飞世尔科技公司);高速离心机(TDZ4-WS,德国Sigma实验室离心机公司);恒温培养振荡器(ZWY-2102C,上海智城分析仪器制造有限公司);立式行星球磨机(XQM-0.4,长沙天创粉末技术有限公司)。
-
本实验中钢渣样品为采集于河北唐山某钢厂的电炉钢渣,经XRD检测,钢渣中主要含有CaCO3、SiO2、Fe2O3、Fe3O4、Ca2SiO4、Ca3SiO5、Ca(OH)2、Al2Si2O5(OH)2、FeCO3和Fe等物质。电炉钢渣经球磨机研磨后,筛滤得到粒径小于200 目的钢渣,置于水中浸泡60 min,弃去水溶液后于热鼓风干燥箱100 ℃烘干3 h,冷却后装入密封样品袋中保存备用。
参照陈艳[23]报道的合成路径制备多元LDHs。配置1 L 6 mol·L−1冰乙酸加入14.4 g电炉钢渣,90 ℃恒温水浴搅拌120 min,待冷却后过滤。取1.44 g固体氢氧化铝加入到过滤所得滤液中,加入与滤液等量的去离子水后用固体氢氧化钠调pH至12.0,然后放入聚四氟乙烯内衬的不锈钢水热合成釜中,在150 ℃下于烘箱中晶化16 h,自然冷却后离心、洗涤至pH为7.0并于80 ℃条件下干燥12 h,制备得到多元LDHs。
-
配制1 000 mg·L−1的亚甲基蓝贮备液,称取1.0 g亚甲基蓝染料定容至1 000 mL,调节pH至7.0,根据需求将贮备液分别稀释至10、50和100 mg·L−1。并于668 nm处测定不同浓度亚甲基蓝染料的OD值,绘制得到亚甲基蓝溶液的标准曲线y=0.117x+0.002 4 (y为OD668值,x为亚甲基蓝溶液质量浓度),R2=0.999 9。
-
采用批式实验分别验证不同环境因素对多元LDHs去除亚甲基蓝的影响。所有实验均于250 mL锥形瓶中进行。初始反应条件如下:反应体系为含有初始质量浓度为10 mg·L−1的亚甲基蓝100 mL溶液,pH为7.0,多元LDHs投加量为10 g·L−1,在25 ℃、150 r·min−1的温控振荡培养箱中,在120 min内每隔一定时间取样。水样经0.45 μm滤膜过滤后,利用绘制的亚甲基蓝标准曲线计算亚甲基蓝质量浓度。并分别根据式(1)和式(2)计算亚甲基蓝的去除率和吸附量。每个实验组均设置3个平行。
式中:R为钢渣对染料的去除率,%;qt为钢渣对染料的吸附量,mg·g−1;C0为吸附前染料的初始质量浓度,mg·L−1;Ct为吸附后染料的最终质量浓度,mg·L−1;V为水样体积,L;m为吸附剂投加质量,g。
吸附时间影响实验:采用初始反应体系分别验证电炉原钢渣及多元LDHs在120 min内对亚甲基蓝染料的去除效果。初始浓度影响实验:分别验证C0为10、50和100 mg·L−1时多元LDHs对亚甲基蓝溶液的去除效果。温度影响实验:分别于4、15、25和35 ℃条件下考察温度对多元LDHs吸附亚甲基蓝的影响。pH影响实验:在初始反应体系中考察不同初始pH(2.0、3.0、5.0、7.0、9.0及11.0)对多元LDHs吸附亚甲基蓝的影响。投加量影响实验:分别于初始反应体系中投加10、20和40 g·L−1的多元LDHs,考察吸附剂投加量对吸附效果的影响。
-
利用X射线衍射(XRD)对改性材料的成分进行分析;通过BET对比分析多元LDHs与原钢渣的比表面积及孔容情况;采用扫描电镜(SEM)分析多元LDHs去除亚甲基蓝前后的形貌变化;使用傅里叶变换红外光谱仪(FT-IR)观察吸附剂吸附前后表面官能团信息。
-
1)吸附动力学实验。为分析多元LDHs对亚甲基蓝的吸附机理,分别采用拟一级动力学方程、拟二级动力学方程、Elovich方程和颗粒内扩散方程对吸附数据进行拟合。配置亚甲基蓝C0为10 mg·L−1,pH为7.0,多元LDHs投加量10 g·L−1的100 mL反应体系,于25 ℃的条件下连续反应90 min,设置取样间隔为15 min,计算吸附量,并利用拟一级动力学方程(式(3))、拟二级动力学方程(式(4))、Elovich方程(式(5))、颗粒内扩散方程(式(6))[24]进行数据拟合。
式中:qt为t时的吸附量,mg·g−1;qe为吸附平衡时多元LDHs对染料的吸附量,mg·g−1;K1为拟一级动力学模型的常数,h−1;K2为拟二级动力学模型的常数,mg·(g·h1/2)−1;Kd为颗粒内扩散模型的常数,g·(mg·h)−1;A为Elovich动力学模型的常数;B为Elovich动力学模型的常数。
2)吸附等温线实验。分别配置亚甲基蓝C0为10、50和100 mg·L−1的反应体系,分别采用Freundlich(式(7))和Langmuir(式(8))模型对亚甲基蓝在多元LDHs上的吸附数据进行拟合。
式中:qe为平衡时吸附量,mg·g−1;Ce为平衡时吸附质的浓度,mg·L−1;KF为Freundlich模型的吸附参数,L·g−1;n为表面非均匀性因子,由qe与Ce1/n的截距和斜率获得。n的值表示吸附驱动力的大小或表面的不均匀性:当n=1时,吸附是线性的;当n<1时,是化学过程;当n>1时,是物理过程[24]。
式中:qe为平衡时吸附量,mg·g−1;Ce为平衡时吸附质剩余浓度,mg·L−1;qm为最大吸附量,mg·g−1;KL为与吸附能相关的Langmuir常数,L·mg−1。
3)吸附热力学实验。为进一步探究多元LDHs吸附亚甲基蓝过程中的能量变化,采用吸附热力学研究亚甲基蓝在多元LDHs上的吸附热力学参数。设置亚甲基蓝C0为10 mg·L−1、pH 7.0、多元LDHs投加量10 g·L−1的100 mL反应体系,反应时间120 min,分别在277.15、288.15、298.15和308.15 K下考察温度对多元LDHs吸附亚甲基蓝的影响。
热力学参数根据式(9)~(11)计算[25],通过阿伦尼乌斯方程(式(12))计算亚甲基蓝吸附在多元LDHs上的活化能。
式中:R为摩尔气体常数,8.314 J·(mol·K)−1;T为热力学温度,K;Kd为平衡常数;ΔH0为焓变,kJ·mol−1;ΔS0为熵变,J·(mol·K)−1;ΔG0为吉布斯自由能变化,kJ·mol−1;A为前因子;E为活化能,kJ·mol−1,依据活化能来判断物理吸附还是化学吸附。
-
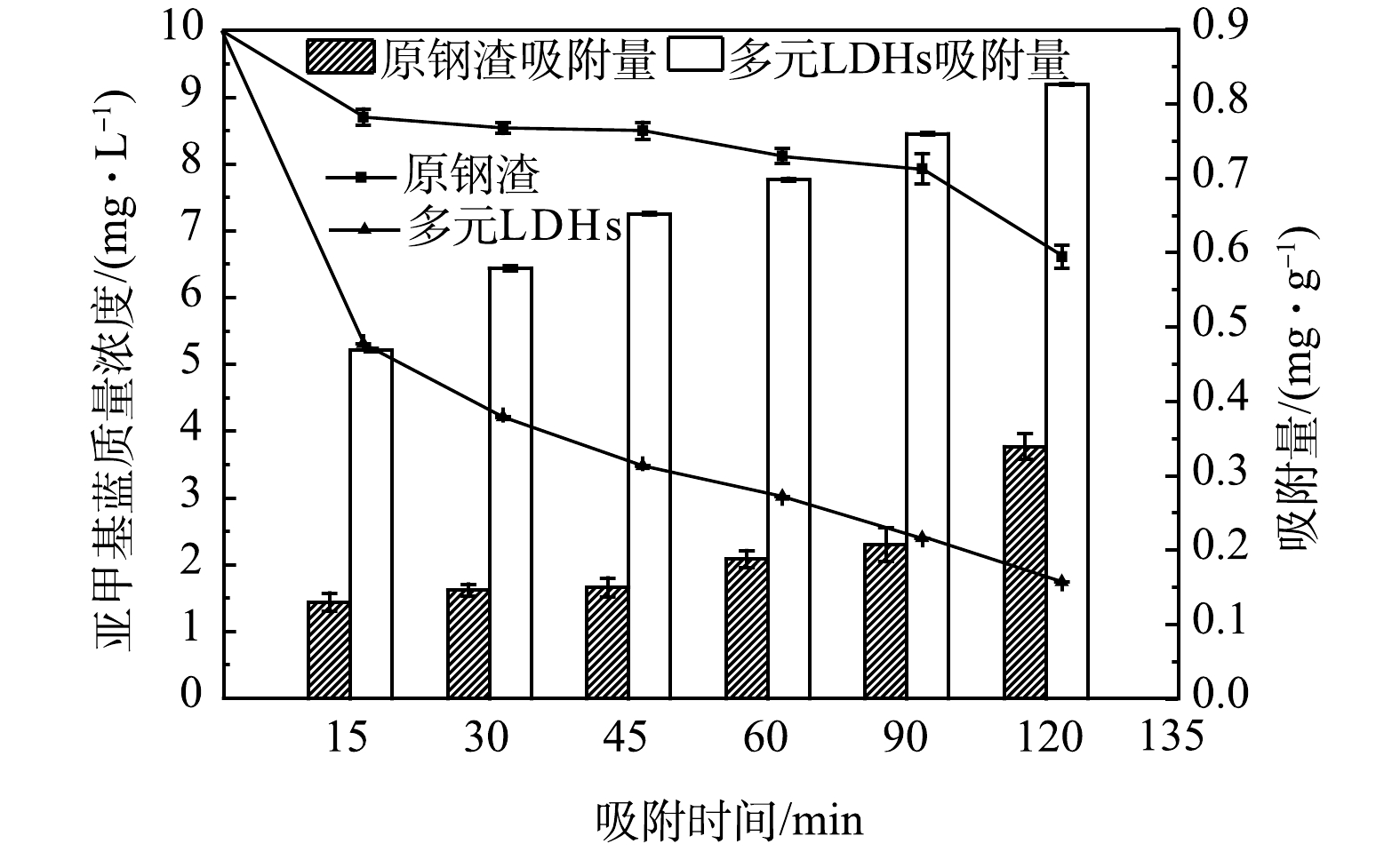
如图1所示,多元LDHs对亚甲基蓝的吸附效果显著优于原钢渣:0~15 min内多元LDHs和原钢渣对亚甲基蓝溶液吸附速率最快,分别为1.88 mg·h−1和0.52 mg·h−1;随着反应时间的延长,2种吸附剂对亚甲基蓝的吸附效果均呈上升趋势,120 min后原钢渣去除率达到最大为33.91%,多元LDHs最大去除率达82.60%。在90~120 min,原钢渣对亚甲基蓝的吸附速率加快。这主要是因为原钢渣对亚甲基蓝的吸附以物理吸附为主的同时辅以少量化学吸附,相对于物理吸附,化学吸附的速度较慢,化学吸附达到平衡的时间相对较长。因此,可能在90~120 min时才发生较显著的化学吸附。与原钢渣对比,在同等投加量的条件下,改性材料多元LDHs的去除效果更好。该结果与LE[19]的研究结果一致。这可能是由于将电炉钢渣改性为多元LDHs后,其比表面积变大,特殊的双电层结构使其更有利于对阳离子亚甲基蓝的吸附[26]。
-
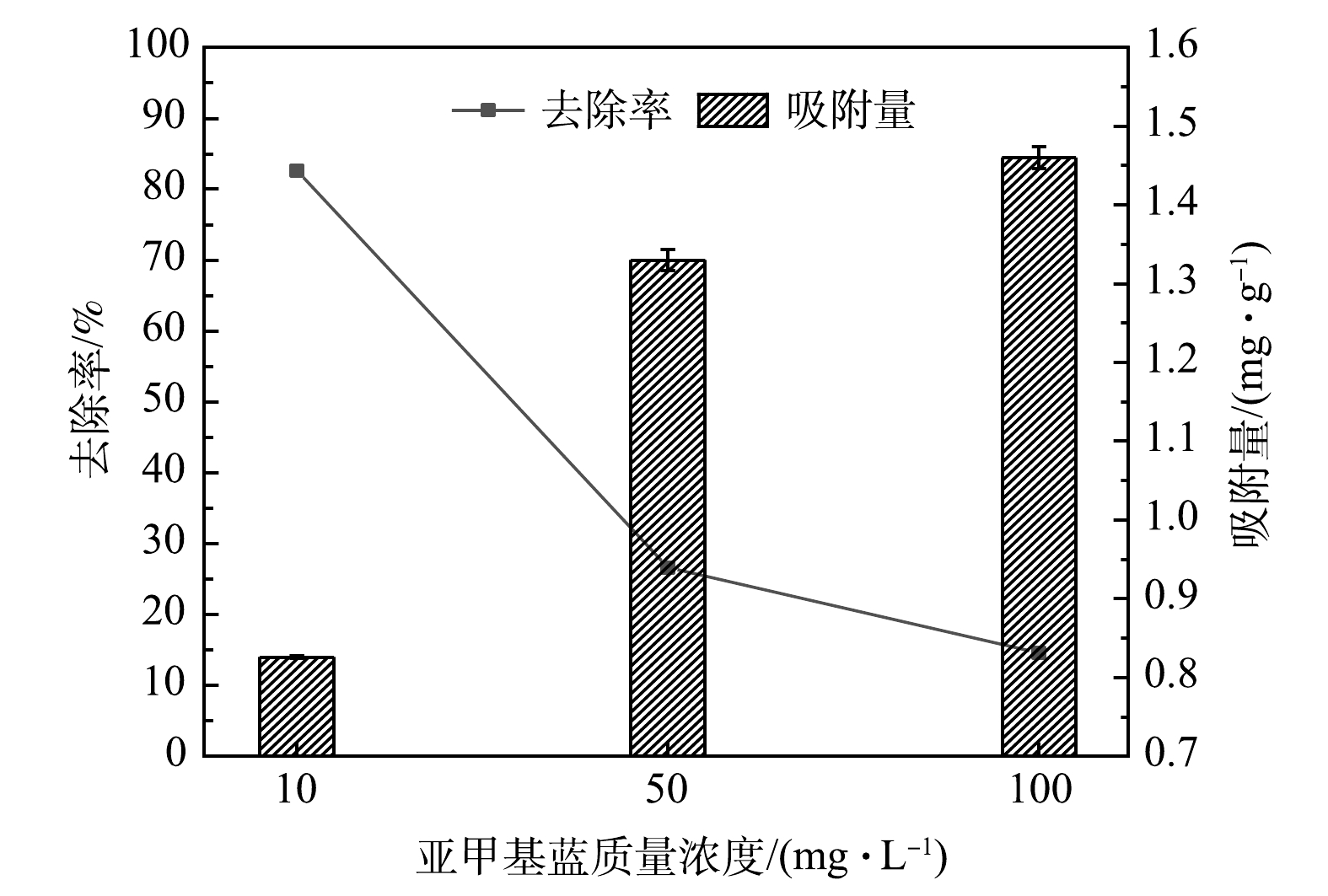
相较于电炉原钢渣,改性的多元LDHs对亚甲基蓝具有更强的吸附效果,但不同类型吸附剂对不同污染物具有不同的吸附饱和度[23]。多元LDHs对不同初始浓度亚甲基蓝的吸附效果如图2所示。当投加量为10 g·L−1时,多元LDHs对C0分别为10、50和100 mg·L−1的亚甲基蓝溶液的最大去除率分别为82.60%、26.60%及14.55%,最大吸附量分别为0.83、1.33及1.46 mg·g−1。经对比发现:多元LDHs对亚甲基蓝的去除率随着C0的增加而降低。这是因为:在较低的C0下吸附质与吸附剂上的吸附位点相结合的概率更高;而在较高的C0下,随着吸附反应的进行,多元LDHs上的吸附位点逐渐被饱和,抑制了对亚甲基蓝的吸附。等质量的多元LDHs对高浓度的亚甲基蓝具有更高的吸附量。这主要是由于吸附质浓度越高,与吸附剂活性位点产生作用的数量越多,吸附量就越大,而本实验制备的多元LDHs结构层间距增大、孔隙增多且吸附位点多等特征更有利于亚甲基蓝吸附[27]。
-
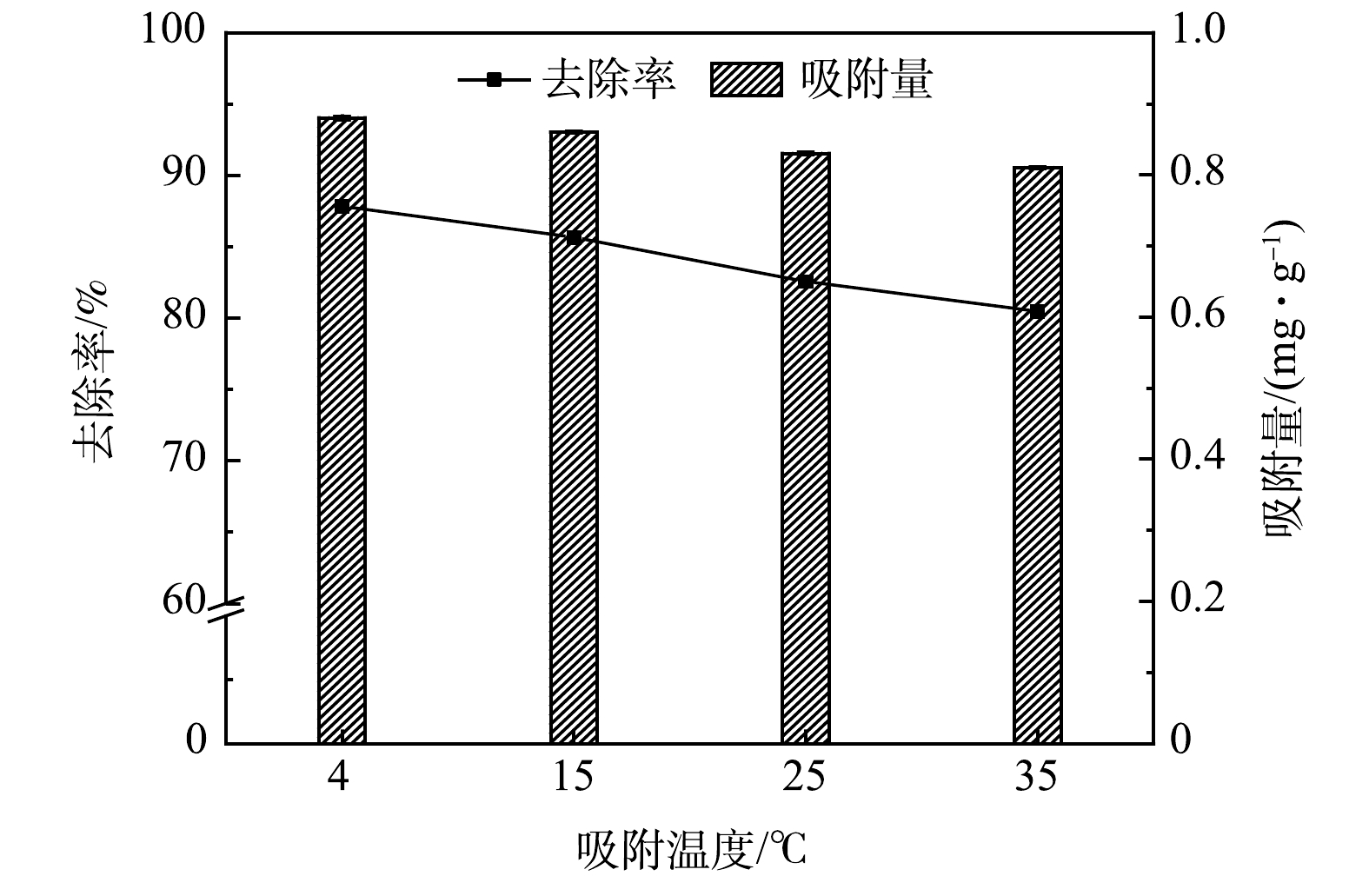
不同温度条件下多元LDHs对亚甲基蓝的吸附效果如图3所示。4 ℃的低温条件更有利于多元LDHs对亚甲基蓝溶液的去除,在反应120 min时,4、15、25及35 ℃实验组对亚甲基蓝的去除率分别为87.48%、85.65%、82.56%和80.48%。因此,较低的温度更有利于多元LDHs对亚甲基蓝的吸附,故推测该吸附过程是自发的放热反应[19]。相比于较高的温度条件,低温环境更有利于热量散发,进而有利于吸附反应的进行。
-
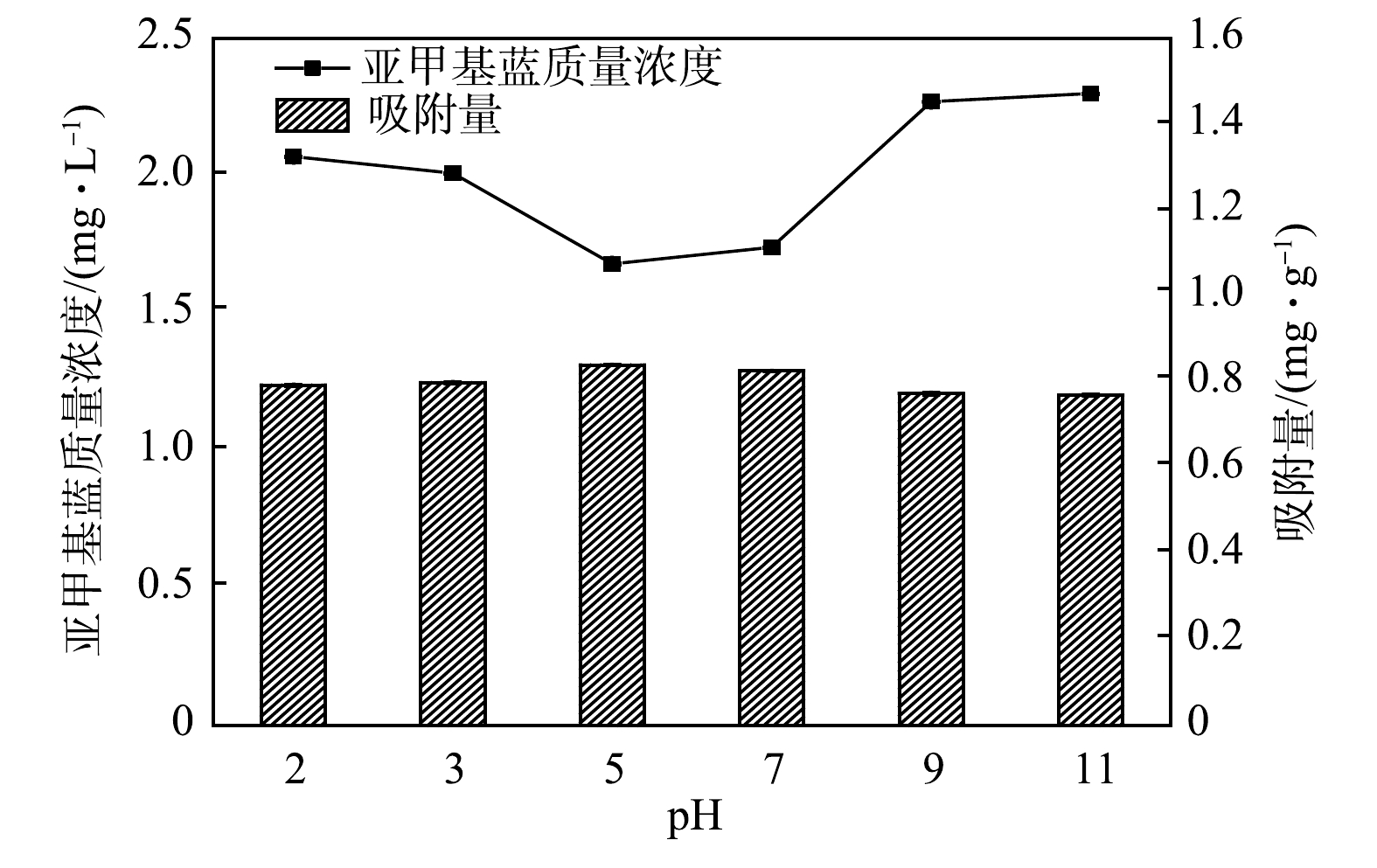
反应体系中初始pH通过影响表面电荷、电离度和吸附剂表面结合位点等进而影响吸附效果[28]。由图4可知,当反应时间为120 min时,在初始pH为2.0~11.0内,多元LDHs对亚甲基蓝的去除率为77.00%~83.20%。当pH为5.0时,去除率最高为83.20%;pH为7.0时,去除率为82.60%。过低或过高的pH环境均会对多元LDHs吸附亚甲基蓝产生抑制作用,分析原因:pH较低的酸性环境下,会使吸附剂表面带正电荷,进而与亚甲基蓝产生静电排斥降低吸附量,并且较低的pH可能导致Mg-Al LDHs结构溶解,进而降低对亚甲基蓝的吸附效果[29-30];pH较高的碱性环境下,会使得OH−吸附在吸附剂表面,一定程度会阻碍阳离子染料与多元LDHs层间阴离子发生相互作用而降低吸附效果[31]。所以,中性或者偏酸性环境更有利于吸附。
-
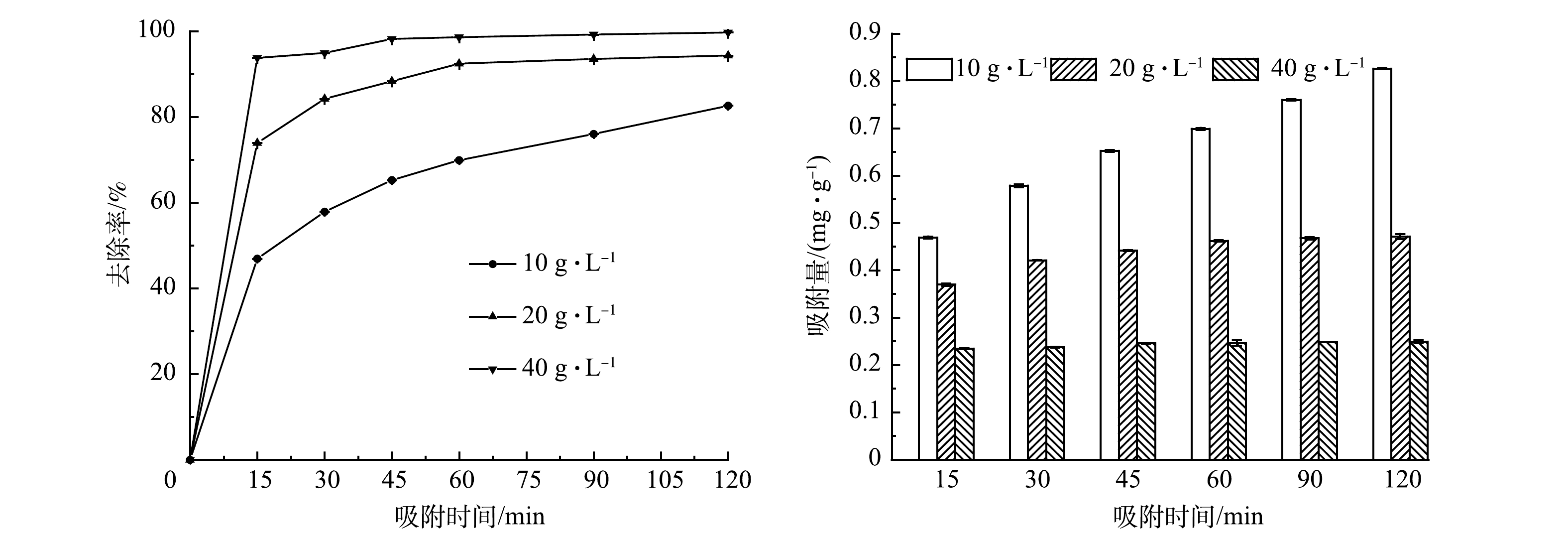
为确定多元LDHs的最优吸附剂用量,考察了多元LDHs投加量对初始质量浓度为10 mg·L−1亚甲基蓝溶液的吸附效果,以期为实际应用提供指导。由图5可以看出,当多元LDHs 投加量分别为10、20及40 g·L−1时,均在0~15 min内对亚甲基蓝吸附速率最快,分别为1.88、3.00和3.75 mg·h−1。这是因为:在吸附初期,多元LDHs存在大量的结合位点,使其吸附速率较快;在120 min时亚甲基蓝去除率分别达到82.60%、94.30%和99.70%。上述结果表明,多元LDHs投加量越大,可用于吸附的活性位点增加,吸附效果越好。本研究中,当多元LDHs投加量为40 g·L−1、反应时间大于90 min时,基本可实现对10 mg·L−1亚甲基蓝的完全脱色。
-
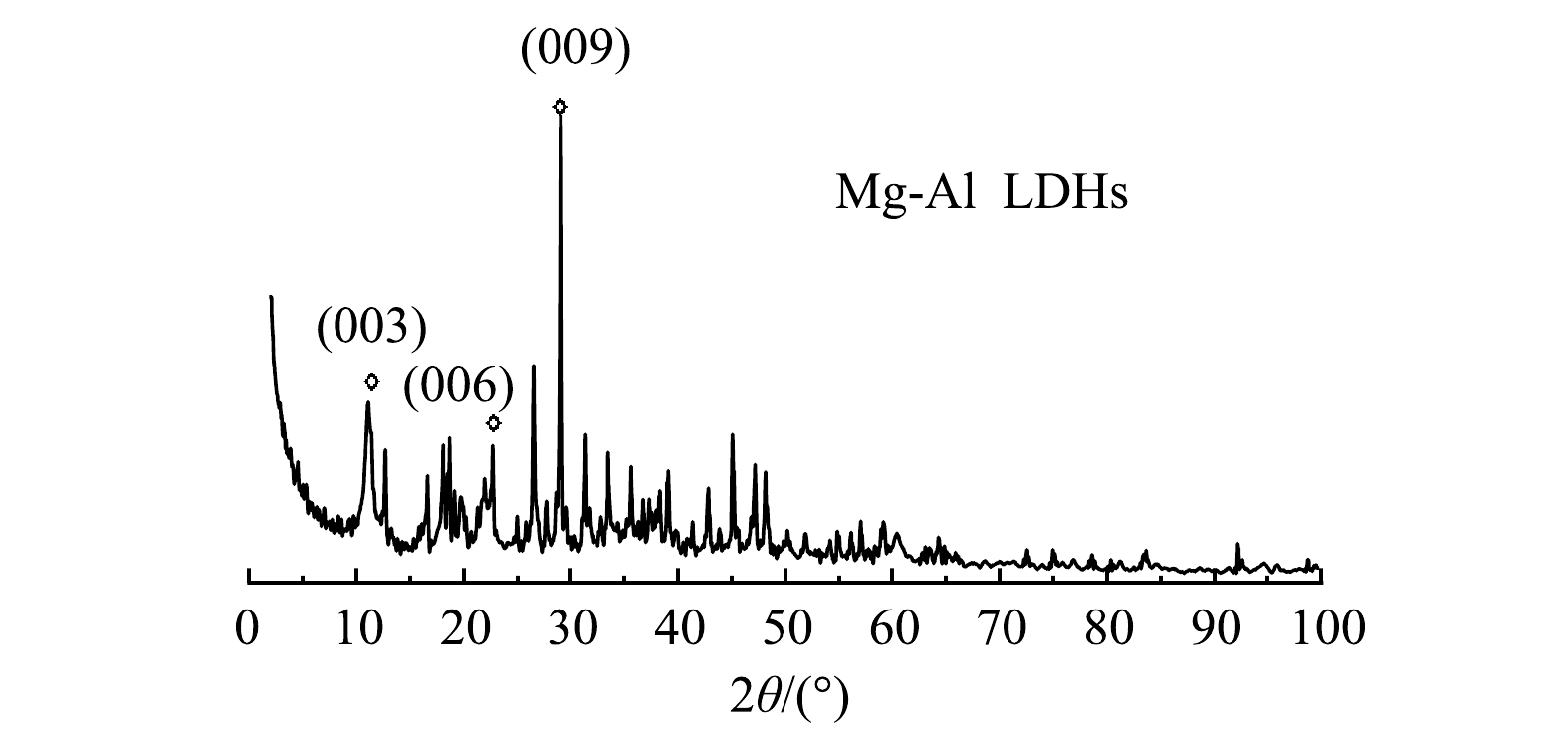
1) XRD分析。钢渣改性材料的XRD结构表征结果如图6所示。改性材料在(003)、(006)及(009)处出现了LDHs典型三晶面的特征衍射峰,表明有层状结构形成并且制备得到的材料是LDHs,但仍含有少部分杂质。
2) BET分析。对多元LDHs和原钢渣进行了BET表征分析。结果表明,多元LDHs和原钢渣的比表面积分别为209.177 m2·g−1和1.402 m2·g−1,多元LDHs的孔容约为原钢渣的74倍。以上结果说明改性所得多元LDHs的结构更有利于吸附的进行。
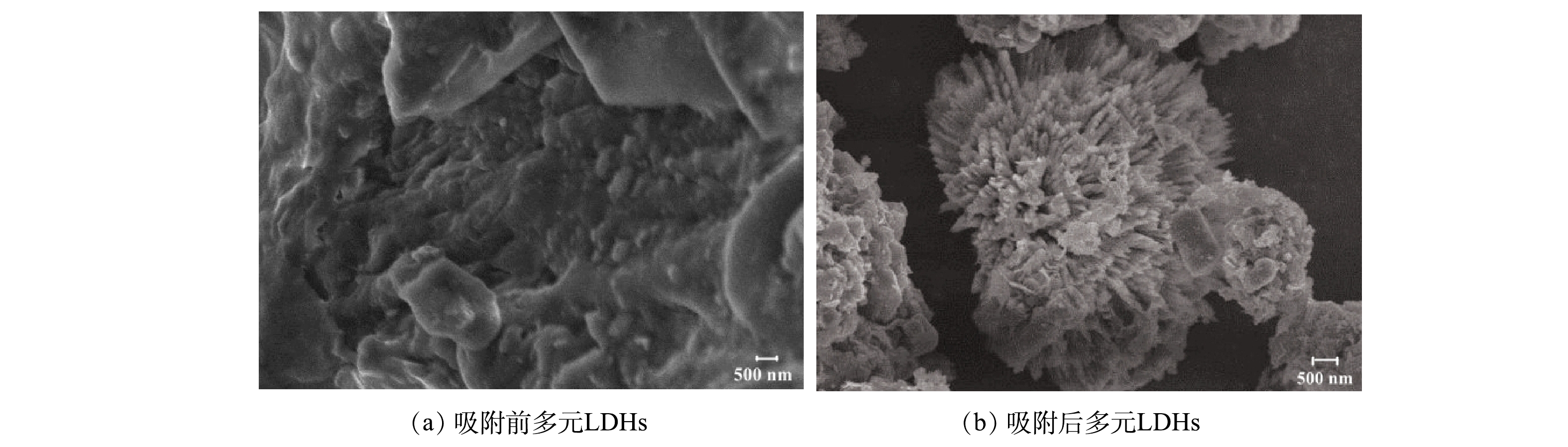
3) SEM分析。由图7(a)可见,吸附前多元LDHs表面相对规整,具有显著紧密的片层结构,较多的褶皱形貌特征使其具有较高的比表面积,更有利于吸附的发生;由图7(b)可见,吸附亚甲基蓝后多元LDHs表面形态变化明显,大量成团的刺状结晶沉淀紧密包裹在多元LDHs表面,表明亚甲基蓝染料分子吸附在多元LDHs上。
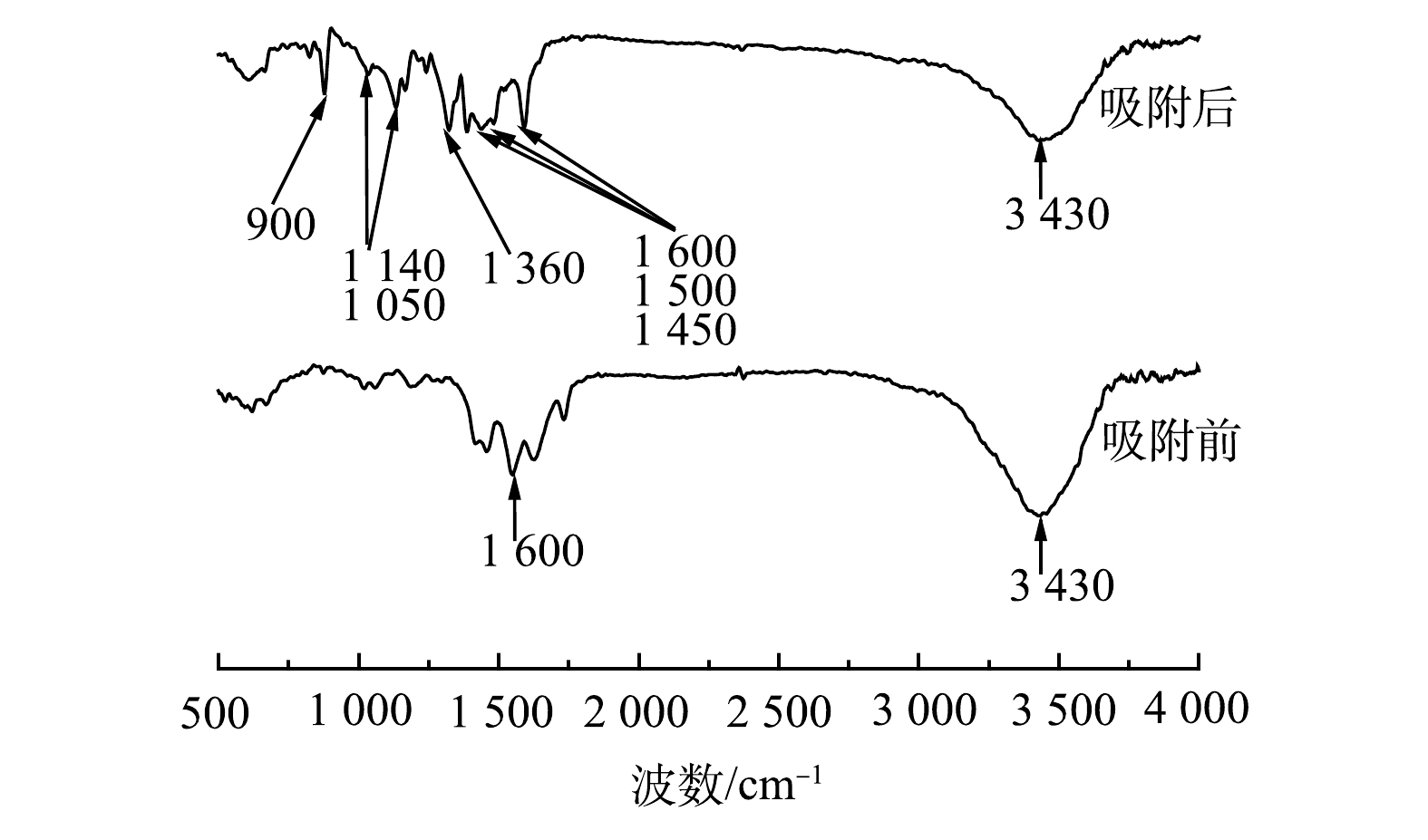
4) FT-IR分析。由图8可见,吸附前在3 430 cm−1处可观察到羟基(—OH)化合物的特征吸收峰以及在1 600 cm−1附近的R—OO—吸收峰,吸附后的主峰仍为3 430 cm−1附近的—OH特征吸收峰,但与吸附前相比,吸收峰强度明显降低,同时也观察到苯环骨架振动峰(1 600、1 500和1 450 cm−1)、—CH3对称变性振动峰(1 360 cm−1)、C—S键特征峰(1 140 cm−1和1 050 cm−1)和苯环表面骨架平面振动峰(900 cm−1)等亚甲基蓝含有官能团的特征峰。这说明亚甲基蓝已吸附到多元LDHs上。
-
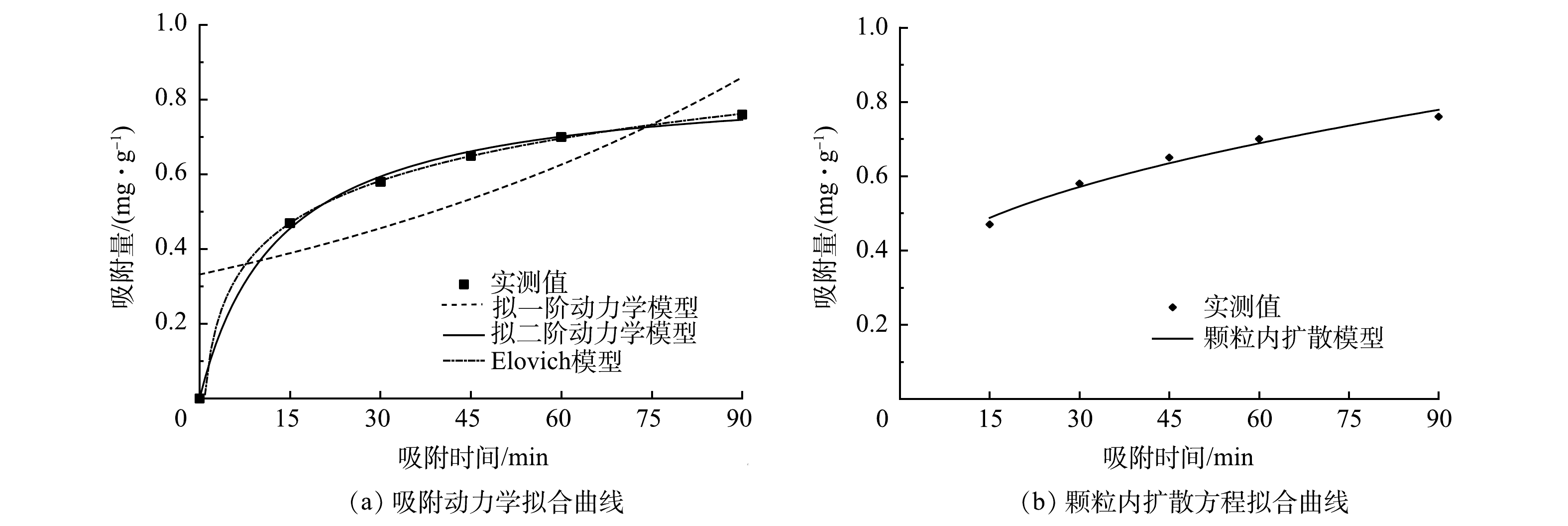
由图9(a)和表1可见,多元LDHs对亚甲基蓝的吸附动力学更符合拟二级动力学和Elovich动力学模型,其对应的R2均在0.99以上,因此,多元LDHs对亚甲基蓝的吸附为化学吸附[28]。如图9(b)所示,可决系数R2为0.970 4,Kd为0.051 8。可见,在吸附过程中膜扩散和颗粒内扩散过程同时发生,多元LDHs对亚甲基蓝的吸附受化学吸附控制,并可能通过表面交换反应发生,当多元LDHs表面活性位点被完全占据后,亚甲基蓝染料分子会扩散到吸附剂内部进行进一步吸附反应。
-
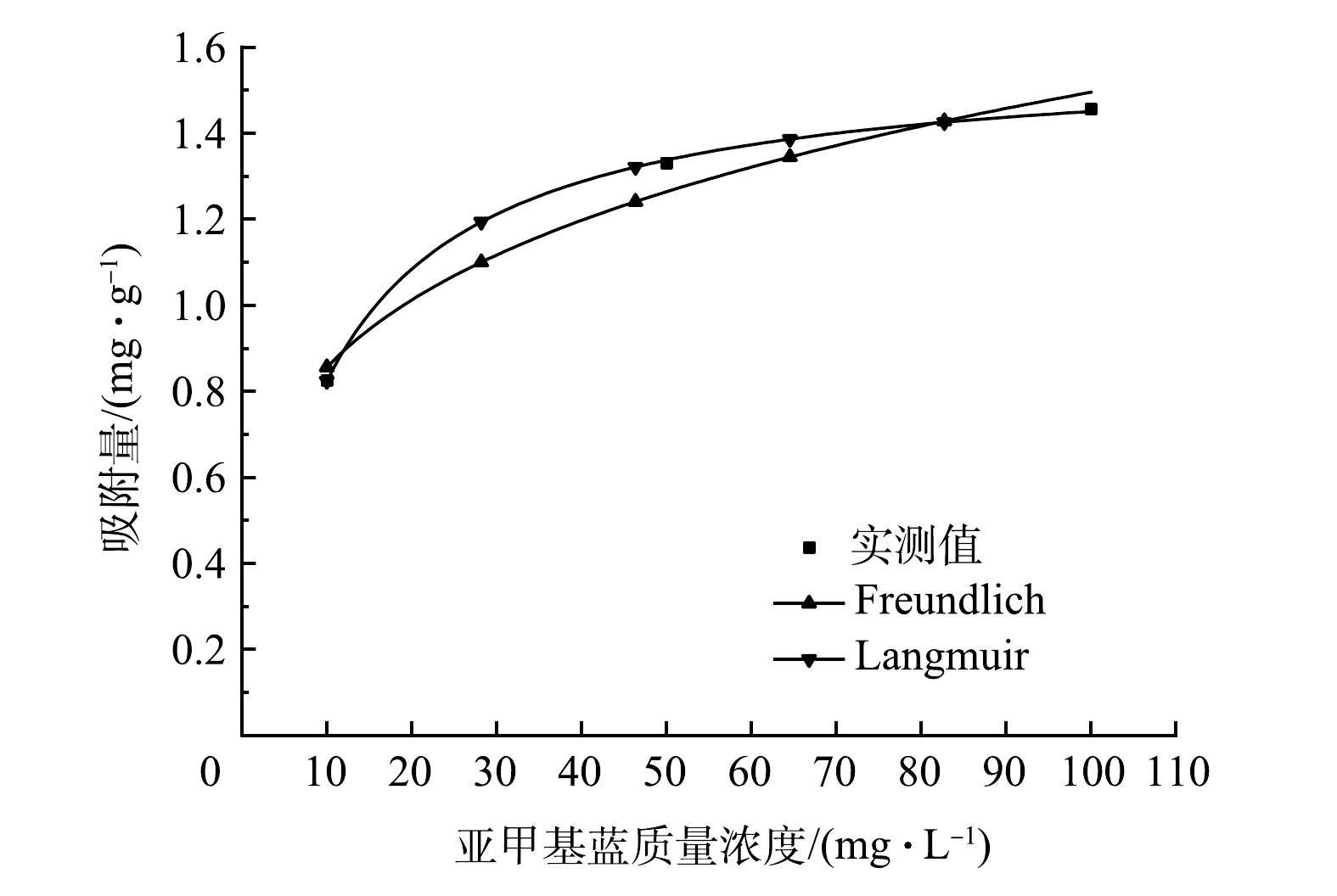
平衡等温线的确定对于分析吸附剂在固液两相间的分布规律,确定吸附量以及揭示吸附机理具有重要意义[32]。由图10亚甲基蓝在多元LDHs上的吸附等温线和表2等温线模型拟合参数可看出,Langmuir方程的R2(0.999 3)高于Freundlich方程的R2(0.937 8)。因此,选择Langmuir模型可以更好地描述亚甲基蓝在多元LDHs上的吸附行为,表明亚甲基蓝在多元LDHs表面上的吸附主要为均匀的单层吸附,并且化学吸附的可能性较大。
-
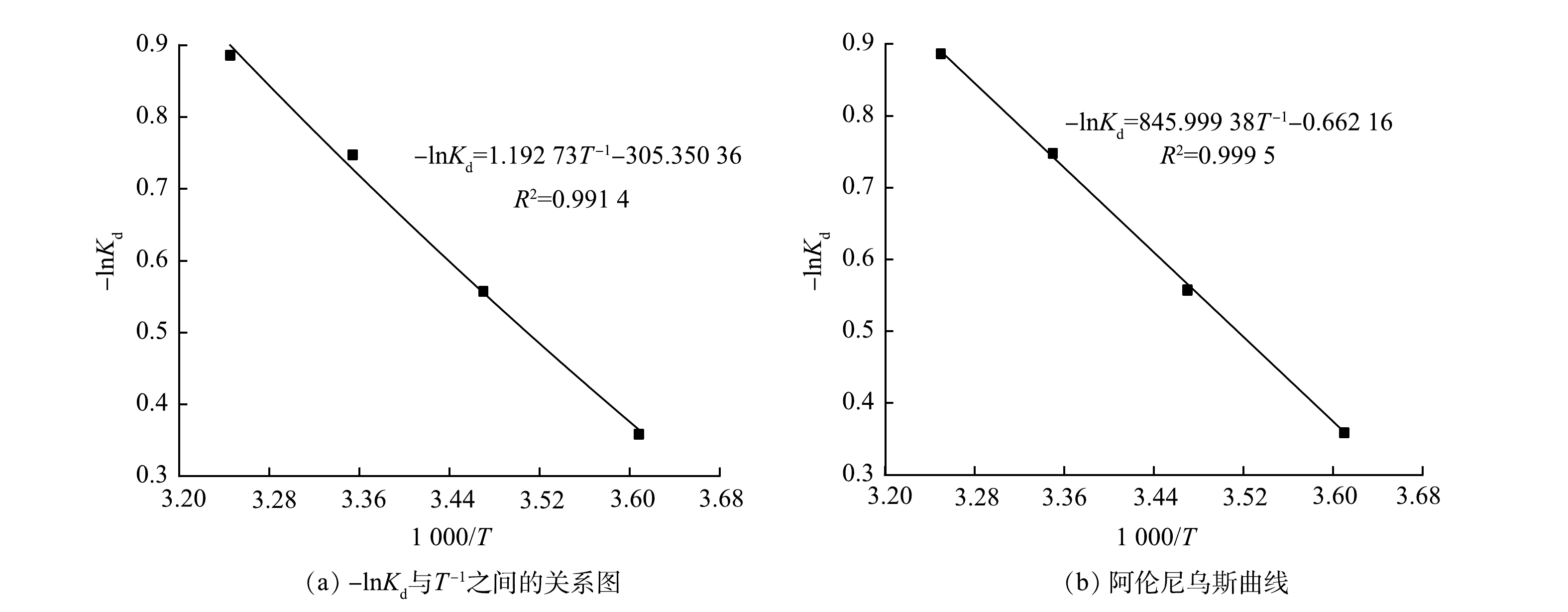
亚甲基蓝在多元LDHs上的吸附热力学曲线及热力学参数详见图11和表3。由图11(a) -lnKd与T−1的关系图可知,ΔG0随温度的升高而减小,且各温度下的ΔG0均为负值,表明多元LDHs对亚甲基蓝的吸附过程是自发进行的[33];亚甲基蓝在多元LDHs上的焓值ΔH0为负值,表明该反应是一个放热反应,该结论与第2.3节温度实验现象相一致。图11(b)为吸附活化能拟合线性图,计算得到E =101.756 kJ·mol−1,一般E在5~40 kJ·mol−1时为物理吸附,在40~800 kJ·mol−1为化学吸附[32],因此,多元LDHs对亚甲基蓝的吸附主要为化学吸附过程。
-
1)由炼钢废弃物电炉原钢渣改性获得的多元LDHs对亚甲基蓝的吸附效能显著优于原钢渣,吸附速率约是原钢渣的3.62倍,去除率约是原钢渣的2.44倍。
2)多元LDHs对亚甲基蓝吸附的最优反应条件为:亚甲基蓝初始浓度为10 mg·L−1、反应温度为4 ℃、pH为5.0、多元LDHs投加量为40 g·L−1,在120 min内可实现完全脱色。
3)多元LDHs的比表面积及孔容远高于原钢渣,其结构特征更有利于吸附发生;SEM及FT-IR表征结果证实亚甲基蓝成功附着到了多元LDHs上。
4)多元LDHs对亚甲基蓝的吸附更符合拟二级动力学方程、Elovich方程和颗粒内扩散方程,表明该过程是膜扩散和颗粒内扩散过程共存的、存在化学吸附的过程。多元LDHs对亚甲基蓝的吸附行为符合Langmuir吸附等温模型,表明该过程主要为均匀的单层吸附。吸附热力学参数表明,该吸附过程是一个自发、放热、化学吸附为主的过程。
改性电炉钢渣-多元LDHs对亚甲基蓝染的料脱色性能
Decolorization performance of methylene blue dye by the multi-LDHs of modified electric furnace steel slag
-
摘要: 研究了电炉钢渣改性材料——多元层状双金属氢氧化物(multivariate layered double hydroxide,多元LDHs)对亚甲基蓝染料的脱色性能。考察了亚甲基蓝染料初始质量浓度、吸附时间、吸附温度、pH和吸附剂投加量对吸附亚甲基蓝效果的影响。结果表明,当亚甲基蓝质量浓度为10 mg·L−1、pH为5.0、多元LDHs投加量为40 g·L−1时,多元LDHs对亚甲基蓝的吸附效果最佳,45 min内的去除率可达98.00%,120 min内基本完全去除。X射线衍射(XRD)表征结果表明,利用电炉原钢渣成功制备了多元LDHs;BET测试结果表明,多元LDHs的比表面积及孔容显著优于原钢渣。采用扫描电镜(SEM)和傅里叶变换红外光谱仪(FT-IR)技术对多元LDHs吸附亚甲基蓝前后形态特征以及关键官能团信息进行系统表征,结果表明,亚甲基蓝已成功吸附到多元LDHs上。吸附动力学研究表明,多元LDHs对亚甲基蓝的吸附过程中膜扩散和颗粒内扩散同时发生;吸附等温线拟合结果表明,该吸附过程符合Langmuir吸附等温模型;由热力学参数可知,多元LDHs对亚甲基蓝的去除为自发进行的、放热、以化学吸附为主的过程。以上研究结果可为电炉钢渣的资源化及其在亚甲基蓝废水处理中的应用提供参考。Abstract: In this study, the multivariate layered double hydroxide (multi-LDHs) material was prepared by modifying the electric furnace steel slag, and its decolorization properties of methylene blue dye was investigated. The effects of initial dye concentration, adsorption time, adsorption temperature, pH and multi-LDHs dosage on the adsorption of methylene blue solution onto multi-LDHs were investigated. The results indicated that under the optimal operating parameters of 10 mg·L−1 methylene blue, pH 5.0 and multi-LDHs dosage of 40 g·L−1, the maximum decolorization efficiency could reach 98% within 45 min and the dye was almost completely removed within 120 min. X-ray diffraction (XRD) results showed that the multi-LDHs have been successfully prepared from raw steel slag of electric furnace. BET test results demonstrated that the specific surface area and pore volume of multi-LDHs were significantly better than raw steel slag. The morphological characteristics and key functional groups of multi-LDHs pre-and post-adsorption of methylene blue were systematically characterized by scanning electron microscope (SEM) and Fourier transform infrared spectrometer (FT-IR), the results showed that methylene blue was successfully adsorbed on multi-LDHs. The adsorption kinetics confirmed that the membrane diffusion and intra-particle diffusion occurred simultaneously during the adsorption process of methylene blue by multi-LDHs. The fitting results of adsorption isotherm showed that the adsorption process conformed to the Langmuir isotherm model. The thermodynamic parameters indicated that methylene blue removal by multi-LDHs was spontaneous, exothermic and dominated by chemical adsorption. The above results can provide a reference for resource utilization of electric furnace slag and its application in methylene blue dye wastewater treatment.
-
表 1 多元LDHs对亚甲基蓝的吸附动力学参数
Table 1. Adsorption kinetic parameters of methylene blue by multi-LDHs
拟一级动力学 拟二级动力学 Elovich动力学 qe/(mg·g−1) K1/h−1 R2 qe/(mg·g−1) K2/h−1 R2 A B R2 0.332 0 0.010 6 0.474 8 0.855 7 0.088 5 0.999 9 0.026 7 0.163 5 0.997 6 表 2 吸附等温线参数
Table 2. Adsorption isotherm parameters
Freundlich Langmuir KF/(mg·g-1) n R2 qm/(mg·g-1) KL/(L·mg-1) R2 0.489 4 4.124 3 0.937 8 0.108 3 1.584 2 0.999 3 表 3 活化能及热力学参数
Table 3. Activation energy and thermodynamic parameters
T/K ΔG0/(kJ·mol−1) E/(kJ·mol−1) ΔS0/(J·(mol·K)−1) ΔH0/(kJ·mol−1) 277.15 −10.322 5 101.713 5 36.727 3 −0.143 5 288.15 −10.726 5 101.730 7 36.727 3 −0.143 5 298.15 −11.093 7 101.756 0 36.727 3 −0.143 5 308.15 −11.462 1 101.771 3 36.727 3 −0.143 5 -
[1] LIANG J, NING X, KONG M, et al. Elimination and ecotoxicity evaluation of phthalic acid esters from textile-dyeing wastewater[J]. Environmental Pollution, 2017, 231: 115-122. doi: 10.1016/j.envpol.2017.08.006 [2] PARROTT J L, BARTLETT A J, BALAKRISHNAN V K. Chronic toxicity of azo and anthracenedione dyes to embryo-larval fathead minnow[J]. Environmental Pollution, 2016, 210: 40-47. doi: 10.1016/j.envpol.2015.11.037 [3] SWAMY M M, NAGABHUSHANA B, KRISHNA R H, et al. Fast adsorptive removal of methylene blue dye from aqueous solution onto a wild carrot flower activated carbon: Isotherms and kinetics studies[J]. Water Treat, 2017, 71: 399-405. [4] CHENG S, ZHANG L, XIA H, et al. Adsorption behavior of methylene blue onto waste-derived adsorbent and exhaust gases recycling[J]. RSC Advances, 2017, 7(44): 27331-27341. doi: 10.1039/C7RA01482A [5] BAALAMURUGAN J, KUMAR V G, PRASAD B N, et al. Removal of cationic textile dye methylene blue (MB) using steel slag composite[J]. Rasayan Journal of Chemistry, 2020, 13: 1014-1021. doi: 10.31788/RJC.2020.1325715 [6] SELVAKUMAR S, MANIVASAGAN R, CHINNAPPAN K. Biodegradation and decolourization of textile dye wastewater using ganoderma lucidum[J]. 3 Biotech, 2013, 3(1): 71-79. doi: 10.1007/s13205-012-0073-5 [7] 隋智慧. 粉煤灰基混凝剂处理印染废水[J]. 印染, 2004, 30(20): 11-13. doi: 10.3321/j.issn:1000-4017.2004.20.004 [8] 白晓龙, 冯启言, 乔启成, 等. 酸性染料废水处理技术研究现状[J]. 印染助剂, 2020, 37(3): 16-19. doi: 10.3969/j.issn.1004-0439.2020.03.008 [9] GONZALEZ -ORTEGA M A, SEGURA I, CAVALARO S, et al. Radiological protection and mechanical properties of concretes with EAF steel slags[J]. Construction and Building Materials, 2014, 51: 432-438. doi: 10.1016/j.conbuildmat.2013.10.067 [10] 张峻搏. 钢渣对含磷废水及染料废水的吸附性能及应用研究[D]. 石家庄: 河北科技大学, 2020. [11] CHENG M, ZENG G, HUANG D, et al. High adsorption of methylene blue by salicylic acid-methanol modified steel converter slag and evaluation of its mechanism[J]. Journal of Colloid and Interface Science, 2018, 515: 232-239. doi: 10.1016/j.jcis.2018.01.008 [12] LIU T, LI Y, DU Q, et al. Adsorption of methylene blue from aqueous solution by graphene[J]. Colloids and Surfaces B:Biointerfaces, 2012, 90: 197-203. doi: 10.1016/j.colsurfb.2011.10.019 [13] HONG S, WEN C, HE J, et al. Adsorption thermodynamics of methylene blue onto bentonite[J]. Journal of Hazardous Materials, 2009, 167(1-3): 630-633. doi: 10.1016/j.jhazmat.2009.01.014 [14] DOGAN M, ALKAN M, ONGANER Y. Adsorption of methylene blue from aqueous solution onto perlite[J]. Water, Air, and Soil Pollution, 2000, 120(3): 229-248. [15] XU P, ZENG G M, HUANG D L, et al. Use of iron oxide nanomaterials in wastewater treatment: a review[J]. Science of the Total Environment, 2012, 424: 1-10. doi: 10.1016/j.scitotenv.2012.02.023 [16] YANG J M. A facile approach to fabricate an immobilized-phosphate zirconium-based metal-organic framework composite and its activity in the adsorption and separation of organic dyes[J]. Journal of Colloid and Interface Science, 2017, 505: 178-185. doi: 10.1016/j.jcis.2017.05.040 [17] 朱洪涛. 改性粉煤灰对活性艳兰染料吸附性能的研究[J]. 环境污染治理技术与设备, 2005, 6(3): 53-55. [18] 张勇, 吕瑞喜. 钢渣在污水治理中的应用[J]. 干旱环境监测, 2004, 18(4): 220-221. doi: 10.3969/j.issn.1007-1504.2004.04.008 [19] LE H S, TRAN X V, TRAN T, et al. Removal of nickel and methylene blue from aqueous solutions by steel slag as a low cost adsorbent[J]. Vietnam Journal of Science, Technology and Engineering, 2017, 59(4): 7-13. doi: 10.31276/VJSTE.59(4).07 [20] 赵艳锋, 王艳, 刘露. 钢渣处理碱性品红染料废水的实验研究[J]. 长春理工大学学报(自然科学版), 2011, 34(4): 91-93. [21] 胡恩柱, 李秋燕, 高涵, 等. 钢渣吸附去除水中亚甲基蓝[J]. 东北大学学报 (自然科学版), 2018, 39(4): 516. [22] 谢复青. 改性钢渣处理亚甲基蓝染料废水研究[J]. 针织工业, 2006(1): 68-70. doi: 10.3969/j.issn.1000-4033.2006.01.029 [23] 陈艳, 王丽秋, 王晨晔, 等. 以钢渣为原料合成层状双氢氧化物及其结构表征[J]. 化工学报, 2015, 66(12): 5149-5156. [24] TRAN H N, YOU S J, HOSSEINI-BANDEGHARAEI A, et al. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review[J]. Water Research, 2017: 120. [25] DAS B, MONDAL N, BHAUMIK R, et al. Insight into adsorption equilibrium, kinetics and thermodynamics of lead onto alluvial soil[J]. International Journal of Environmental Science and Technology, 2014, 11(4): 1101-1114. doi: 10.1007/s13762-013-0279-z [26] 王晨晔, 陈艳, 郭占成, 等. 以钢渣为原料合成Ca-Mg-Al-Fe层状双金属氢氧化物及其对甲基橙的吸附[J]. 过程工程学报, 2018, 18(3): 570-574. doi: 10.12034/j.issn.1009-606X.217335 [27] 王蒙, 詹旭, 杨龙, 等. MgZnAl-LDHs和MgZnAlFe-LDHs对磷酸盐的吸附[J]. 环境工程学报, 2021, 15(9): 2935-2943. doi: 10.12030/j.cjee.202106113 [28] ZHANG L, LI Y J, YAN X B. Diffusion model and empirical study of the multi-generation products based on competition[J]. Systems Engineering-Theory & Practice, 2008, 28(12): 84-92. [29] DE Sá F P, CUNHA B N, NUNES L M. Effect of pH on the adsorption of Sunset Yellow FCF food dye into a layered double hydroxide (CaAl-LDH-NO3)[J]. Chemical Engineering Journal, 2013, 215: 122-127. [30] LI Y, GAO B, WU T, et al. Adsorption properties of aluminum magnesium mixed hydroxide for the model anionic dye Reactive Brilliant Red K-2BP[J]. Journal of Hazardous Materials, 2009, 164(2-3): 1098-1104. doi: 10.1016/j.jhazmat.2008.09.009 [31] 陈艳. 以钢渣为原料合成层状双氢氧化物及其用于印染废水处理[D]. 秦皇岛: 燕山大学, 2016. [32] 张迎. 改性MOF材料光催化降解染料废水性能研究[D]. 石家庄: 河北科技大学, 2019. [33] SHENG G, WANG S, HU J, et al. Adsorption of pb (II) on diatomite as affected via aqueous solution chemistry and temperature[J]. Golloids and Surfaces A:Physicochemical and Engineering Aspects, 2009, 339(1/2/3): 159-166. -






 下载:
下载: