-
铬被广泛用于皮革、纺织、电镀和其他化学工业,当大量含铬废水排放到水体和土壤中时,会严重造成环境污染和危害人类健康[1]。铬主要以Cr(Ⅵ)(CrO42-、HCrO4−和Cr2O72-)和Cr(Ⅲ)的形式存在[2]。其中Cr(Ⅵ)毒性更强,加上其较好的水溶性会对水环境造成污染,可诱发癌症和呼吸系统疾病[3]。去除废水中Cr(Ⅵ)的方法包括生物化学法[4]、吸附法[5]、离子交换法[6]、液膜法[7]、膜过滤法[8]、电化学法和化学还原沉淀法[9]。其中,工业中常用的方法为化学还原沉淀法和吸附法。化学还原沉淀法通过加入化学试剂将 Cr(Ⅵ)还原为Cr(Ⅲ),然后调节pH使其在碱性条件下形成沉淀,进而分离沉淀去除Cr(Ⅵ)。这种处理手段能耗低、操作简便且工艺成熟,但其一般用于处理浓度较大的 Cr(Ⅵ)废水,而且需要多步处理才能达到出水标准,在处理的同时还会产生大量的污泥,易造成二次污染。吸附法主要是利用吸附剂的物理吸附和化学吸附性能去除水中Cr(Ⅵ),其吸附剂种类多、应用范围广、成本低、吸附容量大、处理效率高、可选择性地去除重金属,并且可以重复使用[10],所以利用吸附技术去除废水中的Cr(Ⅵ)的研究变得十分有意义。
氧化石墨烯(graphene oxide , GO)源自于石墨烯合成时衍生的中间产物,是一种新型的材料,含有原子级薄的单分子层,适宜大规模生产,并且具有高比表面积和低质量比容量[11]。此外,GO表面含有各种碳氧基团(C—OH)、环氧基(C—O—C)、羧基(COOH)和羰基(C=O),这些结合位点使GO成能与重金属结合[12],如Pb(Ⅱ)、Cd(Ⅱ)、Cu(Ⅱ)和Hg(Ⅱ)。然而GO具有易聚集性,含氧官能团数量有限且没有选择性,不足以有效去除水溶液中的重金属离子[13],对Cr(Ⅵ)的吸附效果并不好。因此,通过改性剂的搭载制备功能化的GO材料,提高对重金属的吸附性能与选择性,才能提升材料的实际应用性。
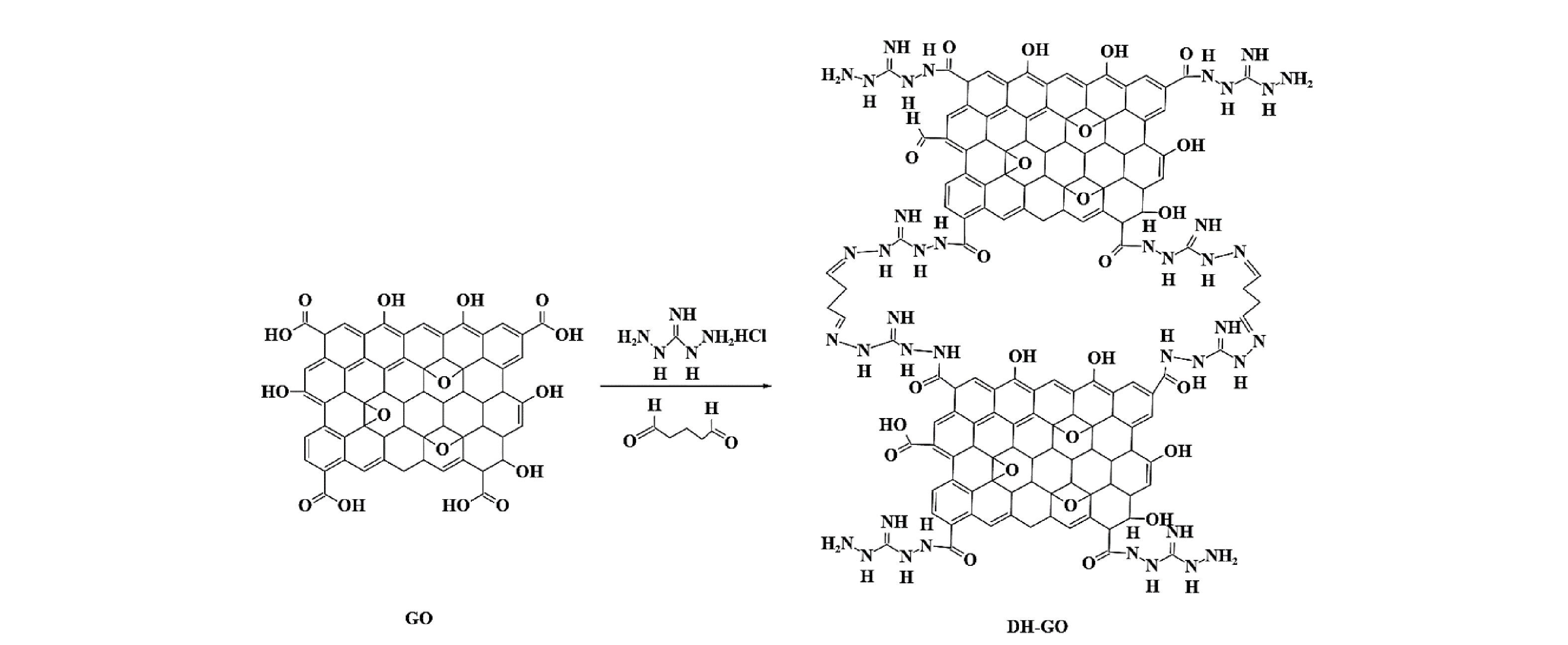
众多研究发现,含氨基基团的改性材料对Cr(Ⅵ)有较强的去除能力,其中伯胺或者仲胺基 ,在酸性条件下容易质子化,从而通过静电引力结合铬酸根,达到净化水质的效果[14]。KU等[15]以乙二胺为改性剂,通过有机转化反应和水热法合成了氨基功能化氧化石墨烯复合材料(NH2-GO/ZnO-ZnFe2O4),在pH为4时,对Cr(Ⅵ)的最大吸附容量为109.89 mg·g−1。GONG等[16]以乙二胺盐酸盐作为改性剂,采用水热法制备了改性氧化石墨烯(AGO),在酸性水体中下,AGO主要通过静电引力作用去除Cr(Ⅵ),同时伴有着部分的Cr(Ⅵ)还原。KIM等[17]用聚乙烯亚胺为改性剂制备了一种光活性功能化氧化石墨烯(GO@PEI),能高效吸附/光还原Cr(Ⅵ)。二氨基胍盐酸盐(diaminoguanidine hydrochloride, DH)具有低廉低毒、结构简单的特点,含有大量的—NH2和—NH基团,对Cr(Ⅵ)有较好的吸附能力[18],然而并未见利用DH改性GO材料去除Cr(Ⅵ)的研究,因此,本研究以DH改性GO材料为主体。
本研究以GO为前驱体、戊二醛为交联剂、DH为改性剂,采用一步合成法制备了氨基改性氧化石墨烯(DH-GO),探讨了不同条件下DH-GO的制备工艺并优化了相关参数;使用扫描电镜(SEM)、红外光谱(FTIR)、X射线衍射(XRD)、拉曼光谱(Raman)、X射线光电子能谱仪(XPS)等表征手段分析了DH-GO的表面形貌、化学官能团、晶型及结构组成特征;探讨了溶液初始pH、投加量、吸附时间、离子强度等对Cr(Ⅵ)去除效果的影响,并采用吸附动力学和等温线模型探明了吸附特性,揭示了DH-GO对水溶液中Cr(Ⅵ)的去除机理。本研究可为含Cr(Ⅵ)废水处理技术选择提供参考。
-
实验试剂:工业级单层氧化石墨烯(GO),二氨基胍盐酸盐(diaminoguanidine hydrochloride)、戊二醛(glutaraldehyde)为优级纯,重铬酸钾(K2Cr2O7),硫酸(H2SO4)、磷酸(H3PO4)和丙酮(CH3COCH3)等试剂均为分析纯,实验用水为超纯水。
实验仪器:集热式恒温加热磁力搅拌器(DF-101S,上海力辰邦西仪器科技有限公司)、水浴恒温振荡器(SHA-B,上海力辰仪器科技有限公司)、真空冷冻干燥机(LGJ-18,北京松源华兴科技发展有限公司)、紫外可见分光光度计(UV-1800PC,日本岛津公司)、高分辨场发射扫描电镜(Sigma 300,德国蔡司公司)、激光拉曼光谱仪(InVia Reflex,英国雷尼绍公司)、傅里叶变换红外光谱仪(IRTracer-100型,日本岛津公司)、X射线衍射(Rigaku D/MAX 2500 V,日本理学公司)、高灵敏Zeta电位分析仪(NANO ZS90 型,英国马尔文仪器设备有限公司)、X射线光电子能谱仪(Thermo Scientific K-Alpha Nexsa 型,美国赛默飞世尔科技公司)。
-
材料制备路线如图1所示。将0.3 g GO加入75 ml超纯水中,超声分散、磁力搅拌30 min形成均相的4 g·L−1 GO分散液;将1.5 g DH溶解于75 ml超纯水中,再加入到GO分散液中,并加入300 uL戊二醛,用NaOH溶液调节pH=10,随后水浴加热35 ℃振荡2.5 h,待混合液冷却至室温后抽滤产物,最后将产物真空冷冻干燥48 h。
-
批量吸附实验步骤如下:首先称取一定质量的 DH-GO加入含Cr(Ⅵ)的溶液中,采用1 mol·L−1的NaOH、HNO3调节反应溶液pH至2,吸附12 h后,溶液经0.45 μm滤膜过滤。采用二苯碳酰二肼分光光度法(GBT 7466-87)测定Cr(Ⅵ)的初始和剩余质量浓度。Cr(Ⅵ)的吸附容量和去除率分别根据式(1)和式(2)进行计算。采用伪一级动力学(式(3))和伪二级动力学(式(4))模拟DH-GO的吸附过程。吸附剂的吸附行为利用Freundlich和Langmuir等温模型进行解释,如式(5)~式(7)所示。以百里酚兰为指示剂、以盐酸标准溶液进行酸碱滴定,氨基的含量根据式(8)进行计算。
式中:C0和Ce分别为Cr(Ⅵ)的初始和剩余质量浓度,mg·L−1;qe为Cr(Ⅵ)的吸附容量,mg·g−1;Re 为去除率,%;V为Cr(Ⅵ)溶液的体积,L;m为DH-GO的投加质量,g。
式中:qe和qt分别为平衡时和t时刻吸附剂的吸附容量,mg·g−1;k1和k2分别为伪一级和伪二级动力学吸附速率常数,min−1。
式中:qm为拟合得出的单层饱和吸附容量或最大吸附容量,mg·g−1;KL为Langmuir常数,L·mg−1;RL为分离常数;KF为Freundlich常数,mg·L−1;n为Freundlich系数。
式中:m为DH-GO的投加质量,g;c为盐酸浓度,mol·L−1;V为消耗盐酸溶液的体积,mL;V0为空白消耗盐酸溶液的体积,mL;w为氨基含量,mmol·g−1。
采用扫描电子显微镜(SEM)观察GO和DH-GO的表面形态结构;利用X射线衍射光谱仪(XRD)观察其晶型变化;利用傅利叶红外光谱(FTIR)分析其化学键和官能团种类;利用拉曼光谱分析(Raman)分析其分子结构变化;利用X射线光电子能谱仪(XPS)分析其化学元素和形态;利用Zeta电位分析仪测定其不同pH下表面电位值。
-
1) DH-GO的制备和优化。本研究采用单一变量法分别依次优化了改性时间、温度、DH的投加量(m(GO∶DH))和pH这4个关键参数,结果如图4所示。在改性时间的优化实验(图2(a))中,DH-GO对Cr(Ⅵ)的吸附2 h内基本达到平衡;而在2~10 h时,吸附容量增加的幅度并不明显。为保证充分改性和考虑时间成本,选择2.5 h为最佳反应时间。在优化改性温度的实验(图2(b))中,随着温度的升高,吸附容量反而下降。在30~40 ℃时,吸附容量最高且较为接近;当温度大于40 ℃后,吸附容量明显下降。据此可推测,温度升高,分子热运动过于剧烈,不利于DH接近并与氧化石墨烯的表面羧基结合反应,故选择35 ℃为最佳反应温度。
随着DH投加量(图2(c))从质量比1∶0.5增加到1∶4时,吸附容量从99.73 mg·g−1提升至156.17 mg·g−1,显著提高;但当质量比大于1:4时,Cr(Ⅵ)的吸附容量增长缓慢,基本趋于平稳。这是由于DH的用量在一定范围内的增加,可提高材料表面的氨基负载量,但超过一定量后,氧化石墨烯表面的羧基等可结合位点已经饱和,不再接受DH的负载。在兼顾吸附容量与经济效益的前提下,故选择 m(GO∶DH)=1∶5 作为最优的投加量。制备溶液的pH对材料吸附效果的影响如图2(d)所示。可见,随着pH从2增加到10时,吸附容量显著升高,且在pH=10时达到峰值;但pH超过10时,吸附容量下降。这一现象可追溯为:在碱性条件下,氧化石墨烯单层片状结构充满了负电荷,具有较大的静电排斥力,缓解了氧化石墨烯片层间的团聚,增加了改性剂进入片层与GO表面官能团的接触概率,提升了DH对GO的改性程度,但碱性过强(pH>10),DH的结构会受到一定破坏,导致DH-GO的吸附性能下降。综上所述,DH-GO的最佳制备条件为:改性时间2.5 h、改性温度35 ℃、m(GO∶DH)=1∶5、pH=10。
-
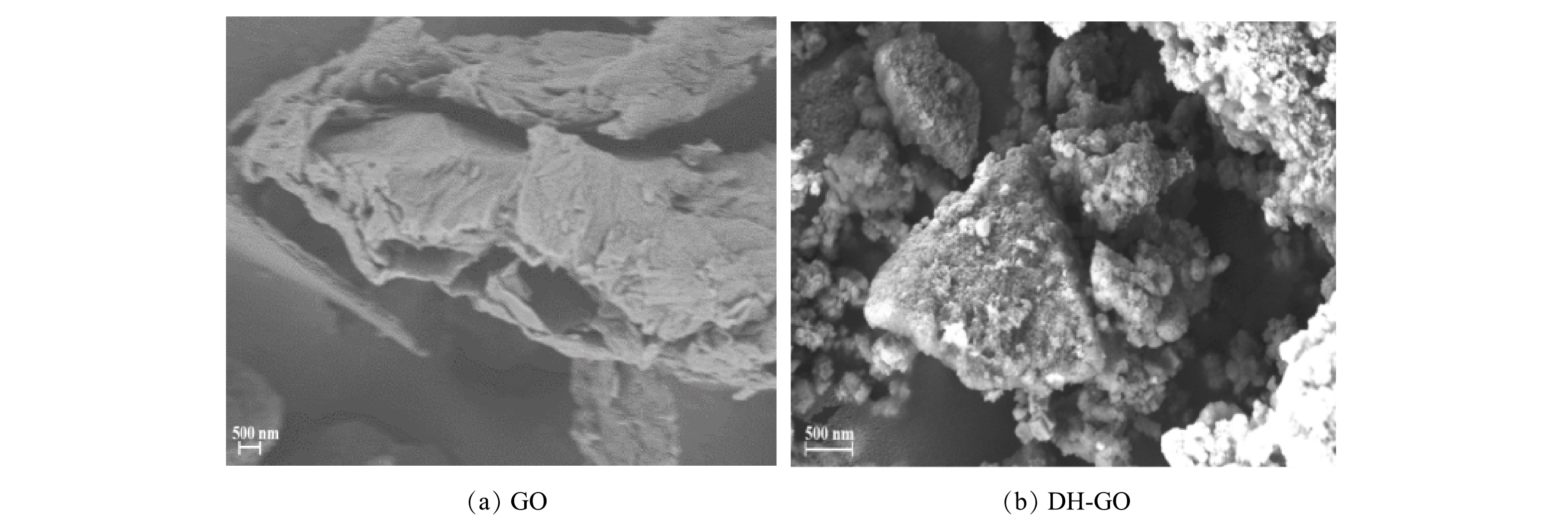
1) SEM表征及分析。如图3所示,原始的GO存在部分剥离的层状堆叠,粗糙、褶皱的片状结构。经 DH改性后,表面仍保持了原本GO的片层结构,但褶皱程度和粗糙程度加深,并负载了许多粗糙和不规则的颗粒,具备了更多的活性位点。
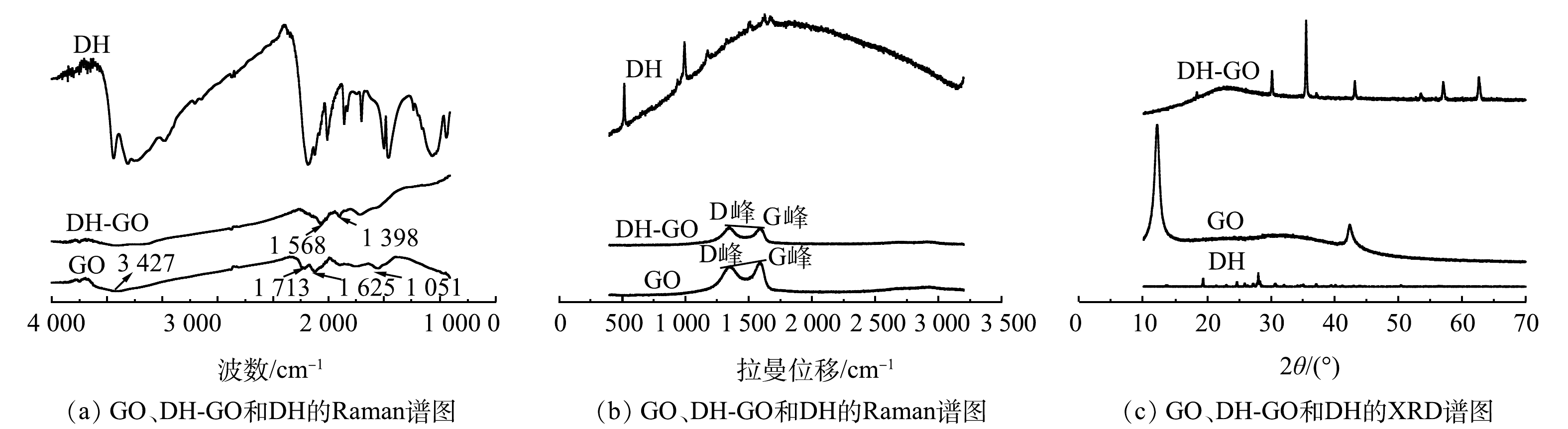
2) FTIR表征及分析。在GO改性前后的的红外谱图(图4(a))中,均发现了3 427、1 713、1 625和1 051 cm−1有明显的吸收峰,可推测分别是因为—OH的不对称伸缩振动峰、羧基中的C=O双键的伸缩振动、芳香环上的C=C双键伸缩、环氧基团C—O—C伸缩振动峰的特征吸收产生的。上述结果说明GO上具有丰富的羧基、环氧基、羟基等官能团,并与已有报道的结果相符合[19]。改性前后各官能团的位置虽然发生了一定偏移,但主要的特征峰依然存在,证明改性并没有破坏氧化石墨烯基本结构。此外,DH-GO在1 568 cm−1处出现了亚胺伸缩振动的新峰,表明了DH的成功负载[20]。另外,羧基振动吸收峰明显减弱,环氧基团的振动吸收峰减弱,暗示着氨基主要与羧基、少部分环氧基发生了酰胺化反应、亲核取代反应,而成功地将DH引入到GO中。
3) Raman表征及分析。氧化石墨烯材料的结构特性通常用拉曼光谱图(图4(b))研究,谱图的内部缺陷(D峰)、sp2杂化的碳原子的平面振动峰(G峰)可以为改性前后的GO提供重要的信息。图中D峰和G峰分别位1 361 cm−1和1 588 cm−1处。一般来说,如果ID/IG的比值越高,就表明材料的结构越无序度增加,更有利于污染物的去除。GO的ID/IG比值明显地低于DH-GO。这说明DH经化学修饰成功接枝到了GO片层后,增加了改性材料的表面缺陷,增强了无序度,更有有利于吸附反应的发生和持续进行。
4) XRD表征及分析。XRD谱图(图4(c))主要表征材料的晶型结构的变化,在2θ为12.07°、42.36°出现了原始GO的特征峰[21]。然而,改性后的DH-GO在12.07°处的特征峰几乎消失,但42.36°依然存在。这说明改性过程中氧化石墨层晶体结构被破环,间距变大,规则幅度下降,结晶程度减弱。此外,DH-GO的谱图中在30.24°、35.56°、53.56°、57.06°、62.57°处出现了新的一系列衍射峰。这可能是由于DH改性GO后,其表面出现了丰富的小颗粒,产生了新的衍射峰。
-
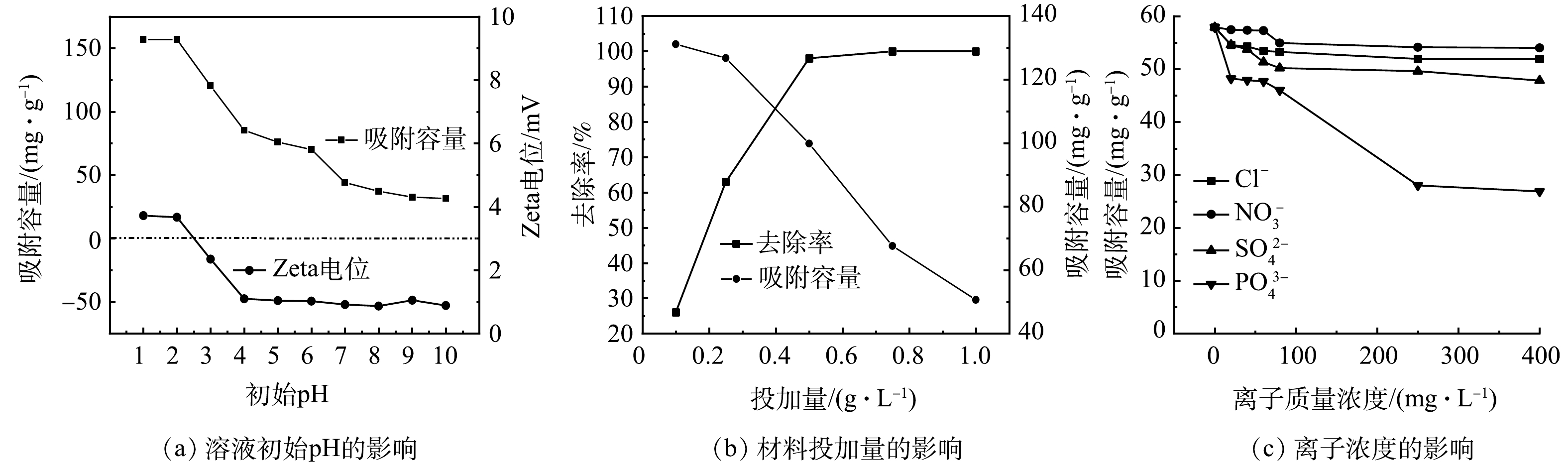
1)初始pH对Cr(Ⅵ)吸附的影响。不同pH下DH-GO对Cr(Ⅵ)的吸附效果和Zeta电位变化如图5(a)所示。随着pH增大,DH-GO对Cr(Ⅵ)的吸附容量逐渐降低,这与其Zeta电位的变化趋势一致。此结果与不同pH下Cr(Ⅵ)的存在形态和DH-GO的带电状态相关,在水环境中,Cr(Ⅵ)通常以HCrO4−、CrO42-和Cr2O72-的形态存在[22]。当在pH=2时,Zeta电位接近正电荷峰值,DH-GO表面上的—NH2和—NH更容易发生质子化,形成带正电的位点(—NH3+和—NH2+),并与阴离子形态存在的Cr(Ⅵ)产生静电作用,对Cr(Ⅵ)的去除率也达到最高。但随着pH的增大,溶液中质子化的氨基官能团随之减少,OH−的含量增加,也会占据部分吸附位点,这就导致DH-GO对Cr(Ⅵ)的吸附容量急剧下降。Zeta电位的测定结果显示改性材料的零点电位在2.50,这进一步证明改性基团的成功负载和和低pH下质子化的程度较高,有利于吸附。因此,后续的吸附实验中将溶液的初始浓度pH设置为2.0。
2) DH-GO投加量对Cr(Ⅵ)吸附的影响。吸附剂DH-GO的投加量决定了含Cr(Ⅵ)废水处理工艺的成本,在Cr(Ⅵ)浓度为50 mg·L−1时,考察了DH-GO投加量为0.1~1 g·L−1时Cr(Ⅵ)对吸附效果的影响,结果见图5(b)。随着DH-GO投加量的增大,Cr(Ⅵ)的去除率迅速增加,且在投加量不低于0.5 g·L−1时稳定在98.40%~99.97%。相反,Cr(Ⅵ)的吸附容量随吸附剂投加量的增大有较为明显的下降趋势。这主要是由于吸附剂用量的增加,可接受Cr(Ⅵ)的吸附位点虽然相应地增多,但是溶液中Cr(Ⅵ)的总量固定,故每单位质量可吸收Cr(Ⅵ)的质量减少。综合考虑吸附容量,去除率和经济效益等因素,选择0.5 g·L−1作为该实验的最优投加量。
3)离子浓度对Cr(Ⅵ)吸附效果的影响。电镀、制革、颜料、冶金、纺织等行业生产中排放的废水中,存在很多阴离子,由于静电作用或其他去除机制的作用,溶液中的阴离子浓度会影响吸附剂对Cr(Ⅵ)的吸附性能。本研究考察了Cl−、NO3−、SO42-、PO43- 4种常见的阴离子对 Cr(Ⅵ)吸附效果的影响。如图5(c)所示,4种阴离子对Cr(Ⅵ)吸附效果的影响大小为NO3−<Cl−<SO42-<PO43-。其中,SO42-和PO43-对Cr(Ⅵ)吸附的影响较大,特别当PO43-的浓度达到80 mg·L−1后,吸附剂的吸附容量快速降低。这是因为Cr(Ⅵ)在pH=2的溶液中主要以HCrO4−和CrO42-的形态存在,NO3− 和Cl−作为单价阴离子,HCrO4−和CrO42-和单价阴离子与DH-GO材料表面的吸附位点存在竞争但作用较小;而SO42-和PO43-作为多价阴离子,同等情况下,会通过静电吸引作用下结合更多的活性的吸附位点,导致吸附容量下降得更多。除此之外,PO43-作为多元弱酸根,即使在低pH下,也有部分PO43-极易水解为HPO42-和OH−离子,导致溶液的pH随着PO43-的浓度变大而升高,使吸附容量下降低得最多。
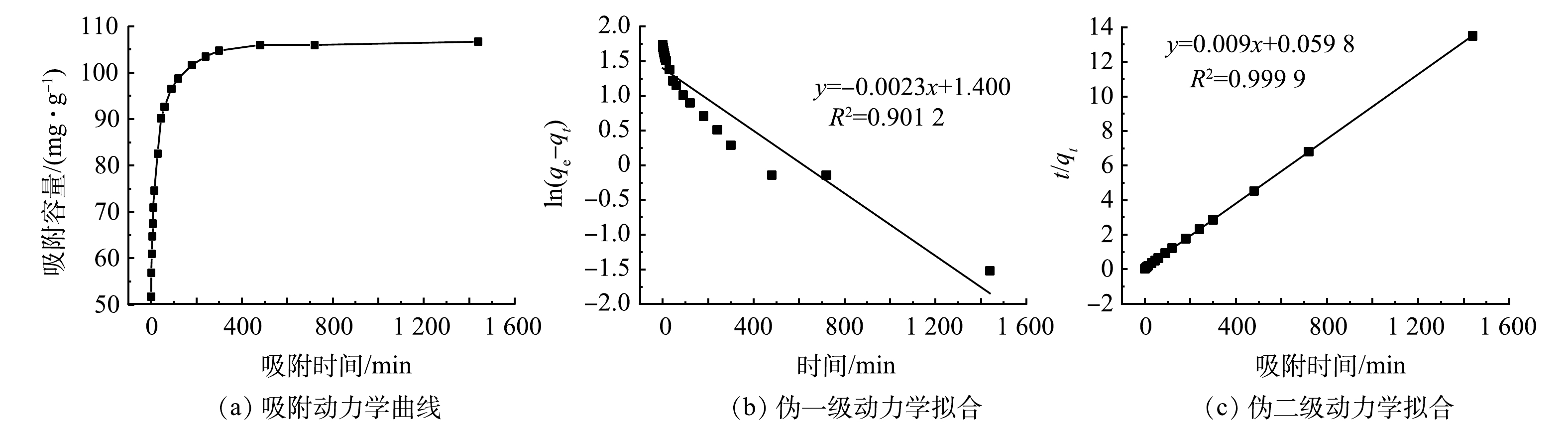
4)吸附动力学研究。吸附时间对Cr(Ⅵ)去除效果的影响如图6(a)所示。随着吸附时间的增加,对Cr(Ⅵ)的去除率先迅速增加后趋于稳定。吸附时间在最初的15 min内,Cr(Ⅵ)的去除速率较快,大约180 min时达到吸附平衡状态,去除率稳定在95%以上。使用实验数据进行动力学拟合的结果见图6(b)和图6(c)。伪二级动力学的可决系数(R2=0.999 9)明显高于伪一级动力学的可决系数(R2=0.901 2),且伪二级动力学模型算得的平衡吸附量(qe,cal=111.11 mg·g−1)与实际结果(qe,exp=106.68 mg·g−1)更接近。因此,DH-GO吸附Cr(Ⅵ)过程以化学吸附为主[23]。
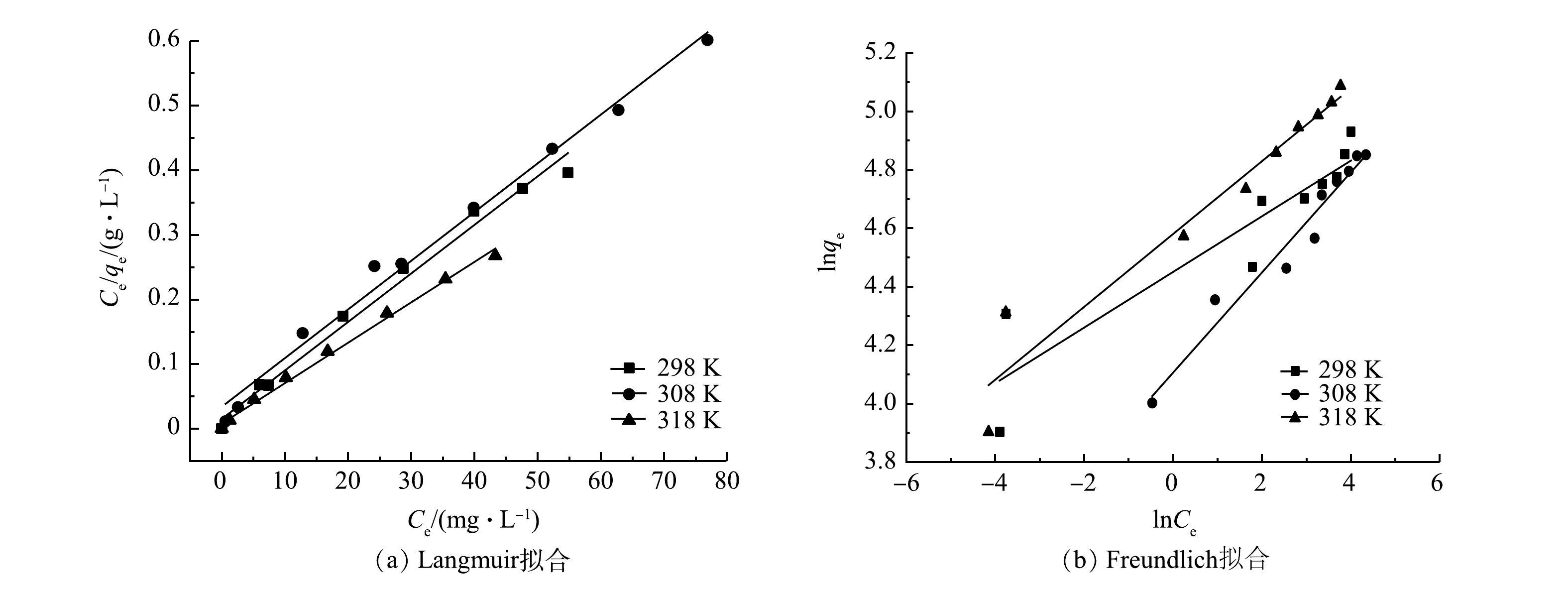
5)吸附等温线研究。以25、35、45 ℃,不同Cr(Ⅵ)浓度的吸附实验数据进行线性拟合,得出的结果如图7所示,对应的相关参数见表1。Langmuir等温方程可决系数整体上高于Freundlich等温方程的。表明Langmuir等温模型能够更好地描述DH-GO对Cr(Ⅵ)的吸附过程,吸附为单层吸附为主导,且25、35、45 ℃下的拟合的最大吸附容量分别为133.16、132.63、160.00 mg·g−1。可见,温度的升高可促进吸附容量的提升,说明吸附过程是吸热反应。
6)氨基含量的测定。DH-GO上的氨基负载含量对吸附Cr(Ⅵ)的性能有直接的影响。通常带有氨基基团的物质的具有一定碱的特性,可与酸发生反应。根据此原理,经过计算后得出氨基含量为0.118 5 mmol·g−1,表明改性后成功实现了氨基负载。
7)吸附剂的循环再生研究。本实验采用100 mg·L−1的Cr(Ⅵ)溶液进行吸附,然后用2 mol·L−1 NaOH作为洗脱剂,考察吸附剂的循环使用性能。如图8所示,经过4次循环再生后,DH-GO的吸附容量由49.59 mg·g−1下降到44.19 mg·g−1,仅有10%的下降。这证明了DH-GO具有良好的再生性和可重复使用性,增强了改性材料的实际应用性。脱附的机理推测为:在2 mol·L−1 NaOH的碱性条件下,丰富的OH−削弱了DH-GO与Cr(Ⅵ)之间的静电吸附作用,使Cr(Ⅵ)以铬盐的形式脱去;而且OH−可占用DH-GO上的吸附位点,使得Cr(Ⅵ)难以接近并吸附。
8) DH-GO与同类吸附材料吸附容量的比较。表2比较了DH-GO与近期文献中其他同类吸附材料对Cr(Ⅵ) 的吸附容量。很显然,本研究所制备的DH-GO的吸附容量优于已有的同类GO材料,是聚乙烯亚胺改性GO三维复合材料的4倍左右,可与聚间苯二胺接枝的氧化石墨烯相媲美。此外,DH-GO采用的更简单的一步合成法和低廉低毒的改性剂,与同类的GO基材料相比,更具有实际应用潜力。
-
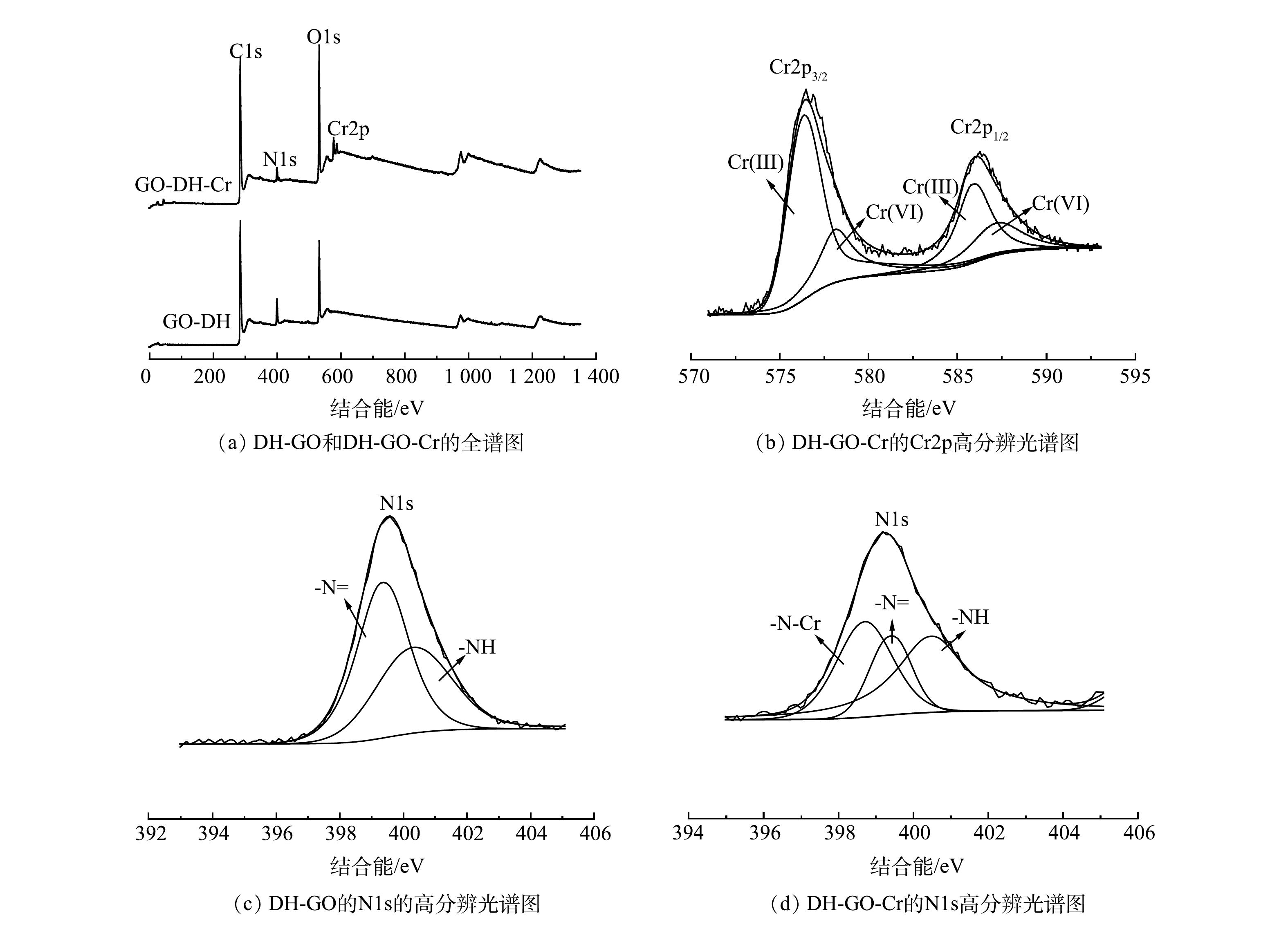
X射线能谱(XPS)分析结果如图9所示。在DH-GO和DH-GO-Cr的全扫谱图(图9(a))可以看出,在285、400和530 eV分别观察到 C1s、N1s 和 O1s,表明DH-GO中存在 C、N和O元素。与DH-GO相比,DH-GO-Cr在576.37 eV和586.13 eV附近出现了新峰,可归因于Cr2p3/2和Cr2p1/2。这表明Cr(Ⅵ)成功地吸附在DH-GO上。从Cr2p的高分辨率光谱图(图9(b))可看出,578.24 eV (Cr2p3/2)和587.4 eV(Cr2p1/2)处的峰对应于Cr(Ⅵ),而576.4 eV (Cr2p3/2)和585.96 eV(Cr2p1/2) 处的峰对应于Cr(Ⅲ),证明吸附Cr(Ⅵ)后DH-GO表面上既存在Cr(Ⅵ)也存在Cr(Ⅲ)[28]。从吸附前后的N1s的高分辨率光谱图(图9(c)和图9(d))中可看出,DH-GO的N 1s处的=N—和—NH分别位于399.4 eV和400.36 eV,DH-GO-Cr的处的=N—、—NH、—N-Cr三个峰分别位于399.2、399.8和398.7 eV[29]。同时,在吸附Cr(Ⅵ)前后,N1s由399.57 eV降至399.24 eV,这进一步说明氨基和Cr(Ⅵ)发生了反应。
基于上述结果,DH-GO对Cr(Ⅵ)的吸附机理可描述为:在25 ℃和pH为2时,DH-GO表面上的—NH2, —NH和—OH和易质子化形成—NH3+、—NH2+、—OH2+和—COOH2+,使其表面带有正电荷基团[30]。此时,根据Cr(Ⅵ)的形态分布,主要以HCrO4−(含量90%以上)和CrO42-的形态存在[31],带负电的Cr(Ⅵ)会通过静电吸引作用被DH-GO吸附。根据DH-GO和GO的实际吸附容量(分别为132.38 mg·g−1和9.04 mg·g−1)可推测,Cr(Ⅵ)的去除主要通过静电作用,少部分Cr(Ⅵ)的去除可能是与改性材料中羧基、羟基反应及其比表面能产生的物理吸附作用所致[32]。同时,由于羟基和胺基团的还原性,一些Cr(Ⅵ)会被还原为Cr(Ⅲ)[33]。
-
1)在所制备的氨基功能化氧化石墨烯(DH-GO)中,氨基主要与羧基发生了酰胺化反应、亲核取代反应将DH引入到GO上,然后细小有晶型的颗粒大量沉积于GO表面,增加了其粗糙度和表面能。
2)当pH为2时,Cr(Ⅵ)吸附容量最大,吸附在480 min内可达到平衡,单层的化学吸附占主导。25 °C时,Langmuir拟合的理论最大吸附容量为133.16 mg·g−1,优于其他改性的GO基材料。高浓度的NO3−和Cl−对吸附的影响甚微,但高浓度的SO42-和PO43-会有一定的抑制作用,主要是因为竞争吸附。
3)氨基改性基团可成功负载在氧化石墨烯上,表面上的氨基官能团质子化后,与带负电的Cr(Ⅵ)产生静电吸引;同时,羧基和羟基的结合作用、比表面能带来的物理吸附也是另一贡献者。
4) DH-GO经过4次再生循环后,其吸附容量仅下降10%,仍保持着良好的吸附性能。
氨基功能氧化石墨烯的制备(DH-GO)及其对废水中Cr(VI) 的去除效果
Preparation of amino functional graphene oxide (DH-GO) and its performance on Cr(VI) removal from wastewater
-
摘要: 本研究通过一步合成法,以二氨基胍盐酸盐(DH)为改性剂,以戊二醛为交联剂,制备了氨基功能化氧化石墨烯(DH-GO)。利用单一变量法优化制备条件后,得到最优参数为:改性时间2.5 h,温度35 ℃,pH=10,m(GO∶DH)=1∶5。通过红外和拉曼光谱、扫描电镜等手段对DH-GO进行了形貌的表征和结构的分析,发现改性材料表面因氨基的引入,导致ID/IG比值变大,表面负载了许多不规则晶状颗粒。批量吸附实验结果表明:pH对材料的性能影响大,这与材料表面的带电情况、Cr(Ⅵ)的主要存在形态相关;当pH为2时,对Cr(Ⅵ)的吸附容量达到最大。吸附在480 min内可达到平衡,符合拟二级动力学、Langmuir等温模型,主要为单层吸热的化学反应,据此可推测25 ℃时理论最大吸附容量为133.16 mg·g−1。高浓度的
NO−3 和Cl−并不干扰吸附的进行,但高浓度的SO2−4 和PO3−4 对吸附会有一定的抑制作用。4次循环再生后DH-GO的吸附容量下降了约10%,仍保持着良好的再生性能。吸附机理主要是氨基官能团与水中的H+结合并质子化后,与带负电的Cr(Ⅵ)产生静电吸引和反应,同时,羧基和羟基的结合作用、比表面能带来的物理吸附也是另一贡献者。以上研究结果可为GO改性材料应用于含Cr(Ⅵ)废水处理提供参考。Abstract: The amino-functionalized GO (DH-GO) was prepared by one-step method using diaminoguanidine hydrochloride (DH) as the modifier and glutaraldehyde as the crosslinking agent in this study. After being optimized with the single variable method, the preparation parameters were selected as follows: the modification time of 2.5 h, 35℃, pH 10, m(GO∶DH)=1∶5. The morphology and structure was characterized by Infrared and Raman spectroscopy, Scanning electron microscopy, which indicated that amino groups introduction caused the increase of ID /IG value, and many irregular crystalline particles were loaded on the surface. The batch experiments showed that pH had a significant influence on the adsorption capacity of material, which was related to the surface charge of DH-GO and the main existing forms of Cr(VI). At pH 2, the Cr(VI) adsorption capacity of DH-GO reached its peak value. The adsorption reached the equilibrium within 480 min, the process was well fitted with the pseudo-second-order dynamics model and Langmuir isotherm model, indicating the monolayer endothermic chemical reaction occurred, and the maximum adsorption capacity at 25℃ was theoretically estimated to be 133.16mg·g−1. NO3− and Cl− at high concentrations had slight interference with the adsorption process, while SO42− and PO43− at high contents had the inhibitory effect to some extent. The adsorption capacity of DH-GO after four cycles of regeneration decreased by about 10%, and maintained a good regeneration performance. The adsorption mechanism was mainly proposed that amino functional groups was firstly protonated by the H+ in the solution at pH 2, and then electrostatically attracted and reacted with negatively charged Cr(VI). The bonding of Cr(VI) to carboxyl and hydroxyl groups, and physical adsorption resulting from the high surface area also contributed to adsorption. This is an attempt to modify GO with amino groups, the findings could provide a reference for the application of the modified GO-based materials in Cr(VI) wastewater treatment.-
Key words:
- graphene oxide /
- amino functionalization /
- Cr(VI) removal /
- adsorption mechanism
-
表 1 DH-GO吸附Cr(VI)的吸附等温线模型参数
Table 1. Parameters of the adsorption isotherms for Cr(VI) adsorption on DH-GO
T/K Langmuir Freundlich qm/(mg·g−1) KL/(mg·L−1) RL R12 n KF R22 298 133.16 0.502 7 0.015 6~0.073 0 0.987 8 1.05 85.62 0.861 3 308 132.63 0.223 4 0.034 6~0.151 9 0.990 3 5.84 60.34 0.961 3 318 160.00 0.799 2 0.009 1~0.167 2 0.994 8 8.03 96.54 0.941 7 -
[1] KUMAR K A S, JIANG S, TSENG W, et al. Effective adsorption of chromium(vi)/Cr(iii) from aqueous solution using ionic liquid functionalized multiwalled carbon nanotubes as a super sorbent[J]. Journal of Materials Chemistry A, 2015, 3(13): 7044-7057. [2] GENG J, YIN Y, LIANG Q, et al. Polyethyleneimine cross-linked graphene oxide for removing hazardous hexavalent chromium: Adsorption performance and mechanism[J]. Chemical Engineering Journal, 2019, 361: 1497-1510. doi: 10.1016/j.cej.2018.10.141 [3] NAUSHAD M, KHAN A M, ALOTHMAN A Z, et al. Adsorption of methylene blue on chemically modified pine nut shells in single and binary systems: Isotherms, kinetics, and thermodynamic studies[J]. Desalination and Water Treatment, 2016, 57(34): 15848-15861. doi: 10.1080/19443994.2015.1074121 [4] JACOB J. M, KARTHI C, SARATALE R. G, et al. Biological approaches to tackle heavy metal pollution: A survey of literature[J]. Journal of Environmental Managemen, 2018, 217: 56-70. doi: 10.1016/j.jenvman.2018.03.077 [5] BARCZAK M, MICHALAK-ZWIERZ K, GDULA K, et al. Ordered mesoporous carbons as effective sorbents for removal of heavy metal ions[J]. Microporous and Mesoporous Materials, 2015, 211: 162-173. [6] FANG L, LI L, QU Z, et al. A novel method for the sequential removal and separation of multiple heavy metals from wastewater[J]. Journal of Hazardous Materials, 2018, 342: 617-624. [7] JEAN E, VILLEMIN D, HLAIBI M, et al. Heavy metal ions extraction using new supported liquid membranes containing ionic liquid as carrier[J]. 2018, 201: 1-9. [8] ABDULLAH N, YUSOF N, LAU W. J, et al. Recent trends of heavy metal removal from water/wastewater by membrane technologies[j]. Journal of Industrial and Engineering Chemistry, 2019, 76: 17-38. [9] HUANG L, ZHOU P, QUAN X, et al. Removal of binary Cr(VI) and Cd(II) from the catholyte of MFCs and determining their fate in EAB using fluorescence probes[J]. Bioelectrochemistry, 2018, 122: 61-68. [10] YU S, YIN L, PANG H, et al. Constructing sphere-like cobalt-molybdenum-nickel ternary hydroxide and calcined ternary oxide nanocomposites for efficient removal of U(VI) from aqueous solutions[J]. Chemical Engineering Journal, 2018, 352: 360-370. [11] LI F, JIANG X, ZHAO J, et al. Graphene oxide: A promising nanomaterial for energy and environmental applications[J]. Advanced Materials, 2015, 16: 488-515. [12] LI X, ZHOU H, WU W, et al. Studies of heavy metal ion adsorption on chitosan/sulfydryl-functionalized graphene oxide composites[J]. Journal of Colloid and Interface Science, 2015, 448: 389-397. [13] CHEN L, ZHAO D, CHEN S, et al. One-step fabrication of amino functionalized magnetic graphene oxide composite for uranium(VI) removal[J]. Journal of Colloid and Interface Science, 2016, 472: 99-107. [14] SHI Y, ZHANG T, Ren H, et al. Polyethylene imine modified hydrochar adsorption for chromium (VI) and nickel (II) removal from aqueous solution[J]. Bioresource Technology, 2018, 247: 370-379. [15] SAHOO S. K, HOTA G. Amine-Functionalized GO decorated with ZnO-ZnFe2O4 nanomaterials for remediation of Cr(VI) from water[J]. ACS Applied Nano Materials, 2019, 2(2): 983-996. [16] 龚秀文, 薛秀玲, 陈小奕, 等. 氨基化氧化石墨烯(AGO)的制备及其对六价铬的吸附机制[J]. 环境化学, 2021, 40(3): 851-858. doi: 10.7524/j.issn.0254-6108.2019102703 [17] KIM C, AN S, LEE J, et al. Photoactive Polyethylenimine-coated graphene oxide composites for enhanced Cr(VI) reduction and recovery[J]. ACS Applied Materials & Interfaces, 2021, 13(24): 28027-28035. [18] HSINI A, NACIRI Y, BENAFQIR M, et al. Facile synthesis and characterization of a novel 1, 2, 4, 5-benzene tetracarboxylic acid doped polyaniline@zinc phosphate nanocomposite for highly efficient removal of hazardous hexavalent chromium ions from water[J]. Journal of Colloid and Interface Science, 2021, 585: 560-573. [19] KONG D, HE L, LI H, et al. Preparation and characterization of graphene oxide/chitosan composite aerogel with high adsorption performance for Cr(VI) by a new crosslinking route[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 625: 126832. [20] KHATRI P. K, CHOUDHARY S, SINGH R, et al. Grafting of a rhenium-oxo complex on Schiff base functionalized graphene oxide: An efficient catalyst for the oxidation of amines[J]. Dalton Trans, 2014, 43(21): 8054-8061. [21] 赵博涵, 林海英, 冯庆革, 等. L-半胱氨酸改性氧化石墨烯去除水中Hg(Ⅱ)[J]. 应用化工. 2021, 50(4): 991-996. [22] 闫帅欣, 王方, 王中良. 氧化石墨烯对水环境中金属离子的吸附作用研究进展[J]. 环境化学, 2018, 37(5): 1089-1098. [23] 陈芳妮, 孙晓君, 魏金枝, 等. 磁性三乙烯四胺氧化石墨烯对Cu^2+的吸附行为[J]. 化工学报. 2016, 5(5): 1949-1956. [24] 王丽平, 张明瑜, 黄慧, 等. 三维GO/PEI复合材料的制备及其对六价铬的吸附[J]. 炭素技术. 2016, 35(5): 28-33. [25] WANG Q, FENG T, XIONG S. Preparation of GO-based iron-containing and adsorption of Cr in water[J]. Materials Science and Engineering, 2019, 472(1): 12085. [26] 涂敏, 匡尹杰. 聚间苯二胺接枝氧化石墨烯的制备及对Cr(Ⅵ)吸附性能研究[J]. 化工新型材料. 2020, 48(12): 185-190. [27] WU L, QIN Z, YU F, et al. Graphene oxide cross-linked chitosan nanocomposite adsorbents for the removal of Cr (VI) from aqueous environments[J]. Desalination and Water Treatment, 2017, 72: 300-307. [28] FU R B, ZHANG X, XU Z, et al. Fast and highly efficient removal of chromium (VI) using humus-supported nanoscale zero-valent iron: Influencing factors, kinetics and mechanism[J]. Separation and Purification Technology, 2017, 174: 362-371. [29] LI R, AN Q D, MAO B Q, et al. PDA-meditated green synthesis of amino-modified, multifunctional magnetic hollow composites for Cr(VI) efficient removal[J]. Journal of the Taiwan Institute of Chemical Engineers, 2017, 80: 596-606. [30] XING H T, Chen J. H, Sun X, et al. NH2-rich polymer/graphene oxide use as a novel adsorbent for removal of Cu(II) from aqueous solution[J]. Chemical Engineering Journal, 2015, 263: 280-289. [31] ZHANG Q, LI Y, YANG Q, et al. Distinguished Cr(VI) capture with rapid and superior capability using polydopamine microsphere: Behavior and mechanism[J]. Journal of Hazardous Materials, 2018, 342: 732-740. [32] 彭鑫, 王静蕾, 常金明, 等. 基于壳聚糖的吸附材料在六价铬吸附中的应用[J]. 高分子材料科学与工程. 2021, 37(6): 181-190. [33] LIANG X T, FAN X Y, LI R M , et al. Efficient removal of Cr(VI) from water by quaternized chitin/branched polyethylenimine biosorbent with hierarchical pore structure[J]. Bioresource Technology, 2017: 178-184. -









 下载:
下载: