-
我国农田重金属污染问题十分突出,成为影响农产品质量的重要因素,耕地重金属超标点位数达19.4%,其中镉污染最为严重[1]。当前我国农田污染治理的工程应用中,以重金属钝化技术的应用最为广泛,这一技术具有操作和使用的便利性,但不能清除土壤中的重金属,只能使之无效化,一旦土壤环境受到扰动,重金属易于再活化。相对于这类化学或物理修复技术,植物修复技术则能清除土壤中的重金属,其具有成本低、土壤扰动少、环境友好以及无二次污染等优势[2]。然而,植物修复会产生大量含重金属的生物质,如何处置这些生物质,成为该项技术获得推广应用的关键所在。
近些年,国内外学者陆续开展了重金属富集植物生物质的处置技术研究[3-4],主要有焚烧法[5]、热解法[6]、压缩填埋法[7]、液相萃取法[8]、堆肥法[9]等。当前研究较多的焚烧、热解等处理技术,存在成本高、产品中仍含有大量重金属、环境二次污染等问题[10],焚烧与热解法一般还需前期烘、晒干过程,耗时长、能耗高等问题也是不利因素。相对地,液相萃取技术具有一定的应用前景,即将含重金属的生物质通过机械破碎,加入特定的提取剂进行萃取,再进行固液分离,实现对生物质所含重金属的脱除,液相中的重金属则易于通过化学沉淀或吸附方法而分离。液相萃取技术具有设备要求低、易于现场实施的优点,是一项具有实际应用潜力的技术。然而,目前这项技术未见深入的研究。龙葵[11](Solanum nigrum L.)和伴矿景天[12](Sedum plumbizincicola)是Cd超富集植物,具有修复重金属污染土壤的潜力和应用前景,当前已在一定范围内进行了推广应用,在修复过程中产生了大量含重金属的生物质,如何处置这些有毒副产品成为植物修复技术工程应用的一个制约因素。由于金属具有酸溶性特征,尤其易溶于强酸,金属还易于与络合剂形成可溶性络合物,因而,无机强酸和强络合剂具有从生物材料中萃取重金属的潜力。从萃取废液中去除重金属则可借鉴工业废水处理技术,原理上主要是通过碱化沉淀或物理吸附而实现。因此,本文按此技术思路,研究了龙葵和伴矿景天生物质中重金属的液相萃取技术和废液处置技术,以期为植物修复技术的工程应用提供配套支撑。
全文HTML
-
龙葵种子购自辽宁省辽阳市园艺花卉研究所,伴矿景天幼苗取自广东省韶关市某重金属污染修复基地。供试土壤采自广东省韶关市马坝镇耕地(0~20 cm)。土壤基本理化性质如下:pH为5.29,有机质含量为26.8 g∙kg−1,全N含量为1.57 g∙kg−1,全P含量为3.09 g∙kg−1,CEC为7.48 cmol∙kg−1,Cd全量为2.50 mg∙kg−1,有效态Cd为1.54 mg∙kg−1。
盐酸(HCl,GR)和硫酸(H2SO4,GR)购于广州化学试剂厂;硝酸(HNO3,GR)购于西格玛奥尔德里奇(上海)贸易有限公司;柠檬酸(Citric Acid,GR)、氯化钙(CaCl2,GR)和乙二胺四乙酸二钠(EDTA,GR)均购于上海麦克林生化科技有限公司。氢氧化钾(KOH,GR)和碳酸钾(K2CO3,GR)购自上海麦克林生化科技有限公司;絮凝剂聚合氯化铝(PAC,AR)购于上海阿拉丁生化科技股份有限公司。孔径为0.4 nm分子筛(4Å分子筛,325目),购自上海有新分子筛有限公司。不锈钢粉碎机(拜杰,BJ-150,25 000 r∙min−1)和不锈钢搅拌机(Midea,WBL25B26,15 000~18 000 r∙min−1)分别购买自德清拜杰电器有限公司与美的集团。
-
1)修复植物种植。龙葵和伴矿景天是通过盆栽实验种植所得,种植时间为2019年3月1日至2019年6月8日。选用PVC塑料花盆(29 cm×23 cm×19 cm)种植,每盆8 kg土。龙葵采用种子直播的方式进行种植,伴矿景天采用幼苗(长势较为一致)移栽的方式进行种植,最终定植每盆3株,定植生长60 d后收获。实验期间实行正常水肥与除虫除草管理,根据盆中土壤缺水状况,不定期利用自来水进行土壤水分补偿,使土壤含水量维持在田间持水量的70%。
2)植物生物质预处理。收获后的龙葵(分为茎与叶)和伴矿景天(茎与叶合并)生物质,利用去离子水充分清洗干净后晾干表面水分,再分别利用不锈钢粉碎机和搅拌机进行3~5 min破碎处理。破碎后的龙葵新鲜茎和叶采用密封袋(PE)封装并保存于4 ℃冰箱待用,伴矿景天破碎后的匀浆采用聚乙烯(PE)塑料瓶封装并保存于4 ℃冰箱备用。
3)液相萃取实验。龙葵茎、叶和伴矿景天生物质的含水率分别为78.3%、61.5%和96.0%,对应Cd质量分数分别为5.51、19.3和10.6 mg∙kg−1(鲜质量)。按植物干质量(S)∶提取剂(L)=1∶20的比例进行萃取,分别称取破碎后的4.61 g龙葵茎(Cd总质量为25.4 μg)、2.60 g龙葵叶(Cd总质量为50.1 μg)和25.3 g伴矿景天(Cd总质量为268.1 μg)于50 mL离心管中,加入20 mL不同提取剂,利用往复振荡机在室温(25~30 ℃)下以220 r∙min−1萃取24 h,然后以4 700 r∙min−1高速离心10 min,过0.45 μm滤膜,滤液放入冰箱4 ℃保存待测。每个处理设置3个重复。萃取残渣利用去离子水清洗2遍,于75 ℃烘箱中烘干至恒重待测。具体液相萃取所选用的提取剂与浓度见表1。
4)萃取废液中Cd去除实验。使用K2CO3与KOH碱化沉淀和4Å分子筛物理吸附的方法进行Cd去除。碳酸钾处理采用1 mol∙L−1 K2CO3,调节溶液pH至10[13],并加入0.3%聚合氯化铝[14](PAC)进行充分的絮凝沉淀;氢氧化钾处理采用1 mol∙L−1 KOH调节溶液pH至11[15],并加入0.3%聚合氯化铝[14](PAC)进行充分的絮凝沉淀。4Å分子筛吸附处理[16]先用1 mol∙L−1 NaOH/HCl调节溶液pH至6.5,然后加入2.5 g∙L−1分子筛,在(30±1) ℃条件下振荡90 min。上述处理经过充分反应后,以4 700 r∙min−1高速离心10 min,用0.22 μm滤膜过滤并于4 ℃冰箱保存待测。每个处理均设置3个重复。
5)植物及萃取残渣中镉形态分析。对龙葵原样茎、叶及去离子水、T4、T9萃取残渣,测定了Cd形态,Cd的形态采用化学试剂逐步提取法进行分析[17]:(a) 80%乙醇,提取了硝酸盐/亚硝酸盐、氯化物和氨基苯酚镉等无机Cd,即为80%乙醇提取态Cd;(b) 去离子水,提取了水溶性Cd、Cd-有机酸络合物和Cd(H2PO4)2,即为去离子水提取态Cd;(c) 1 mol∙L−1氯化钠,提取了果胶和蛋白质结合态Cd,即为氯化钠提取态Cd;(d) 2%醋酸,提取了未溶解的镉磷酸盐,包括CdHPO4和Cd3(PO4)3,即为醋酸提取态Cd;(e) 0.6 mol∙L−1盐酸,提取了草酸镉,即为盐酸提取态Cd;(f) 最后剩余残渣内的Cd为残渣态Cd。
-
供试土壤中全量Cd的测定先采用浓硝酸-浓盐酸-氢氟酸(v∶v∶v=6∶3∶2)进行微波消解(Multiwave Pro,AntonPaar,Austria)[18],再用1%硝酸溶液定容至50 mL,过滤待测。供试土壤中的有效态Cd采用0.10 mol∙L−1 CaCl2进行提取与测定[19]。植物含水率的测定采用烘干法[20]进行。植物样品、残渣态Cd以及萃取残渣中Cd含量的测定先采用浓硝酸进行微波消解[18],然后用1%硝酸溶液定容至50 mL,过滤待测。消解过程与分析测定过程设置了标准物质(GBW(E)100357)、空白和重复进行质量控制。液相萃取、植物Cd形态提取以及Cd去除后萃取废液中Cd含量的测定先采用浓硝酸-高氯酸(v∶v=3∶1)进行电热板消解[21],最后用1%硝酸溶液定容至50 mL并过滤待测。上述样品的Cd含量利用火焰原子吸收分光光度计(AAS,contrAA800,Analytikjena,Germany)与电感耦合等离子体质谱分析仪(ICP-MS,7700X,Agilent,USA)进行分析测定。各参数具体计算方法如式(1)~式(3)所示。
式中:M为植物的含水率;W1为植物的鲜质量,g;W2为植物的干质量,g。
式中:E为镉的萃取率;C1为萃取废液中Cd的质量浓度,mg∙L−1;V为萃取废液的体积,L;C2为萃取生物质中Cd的质量分数,mg∙kg−1;W3为萃取生物质的质量,kg。
式中:R为萃取渣、液中Cd的回收率,%;C1为萃取废液中Cd的质量浓度,mg∙L−1;V为萃取废液的体积,L;C3为萃取残渣中Cd的质量分数,mg∙kg−1;W4为萃取残渣的质量,kg;C2为萃取生物质中Cd的质量分数,mg∙kg−1;W3为萃取生物质的质量,kg。
所有数据的统计分析均采用SPSS 26.0软件进行单因素方差分析,使用Tukey检验法(α=0.05)做多重比较分析,数据表达为平均值±标准误(n=3),图形绘制采用OriginPro2020软件绘图。
1.1. 实验材料与样品处置设备
1.2. 实验方法
1.3. 分析方法
-
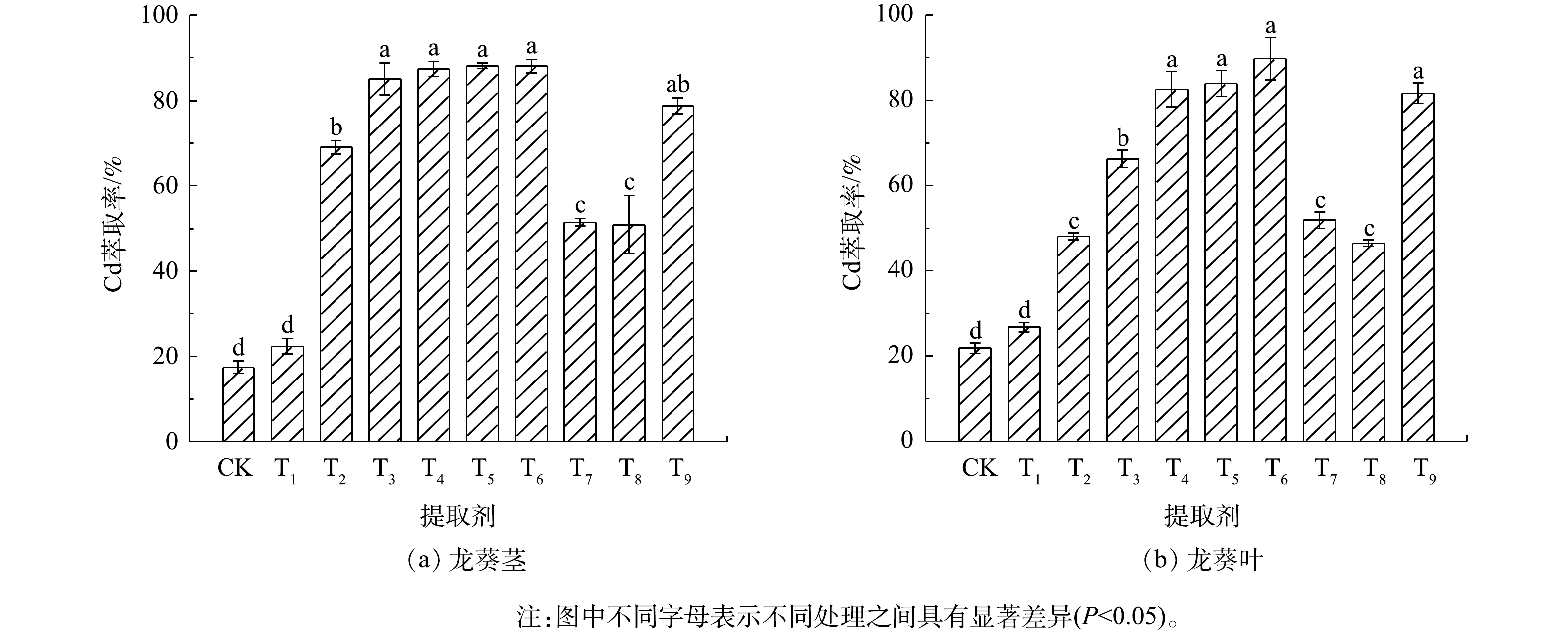
不同提取剂对龙葵茎和叶中Cd的萃取效果见图1。龙葵茎中Cd萃取效果最好的提取剂为0.10 mol∙L−1盐酸(T3)、0.25 mol∙L−1盐酸(T4)、0.25 mol∙L−1硝酸(T5)和0.25 mol∙L−1硫酸(T6),对应的Cd萃取率分别为85.1%、87.4%、88.2%和88.1%,差异不显著(P>0.05)。0.10 mol∙L−1 EDTA对Cd的萃取率为78.7%,其余几种提取剂对龙葵茎中Cd的萃取率均低于70%,其中0.01 mol∙L−1盐酸萃取Cd的效果最差。龙葵叶中萃取Cd的最优提取剂为0.25 mol∙L−1硫酸(T6),Cd萃取率达89.8%;4种浓度的盐酸中,0.25 mol∙L−1盐酸(T4)对龙葵叶中Cd的萃取效果最好(82.6%);0.25 mol∙L−1硝酸(T5)和0.10 mol∙L−1 EDTA(T9)分别可萃取龙葵叶中84.0%和81.7%的Cd,但以上几种提取剂的Cd萃取效果的差异不显著(P>0.05);其余几种提取剂对龙葵叶中Cd的萃取效果与茎中的趋势相似。
表2为不同提取剂对龙葵茎和叶中Cd萃取后的残渣、废液中Cd含量情况。龙葵茎的萃取废液中Cd质量浓度为0.22~1.12 mg∙L−1,龙葵叶的萃取废液中Cd质量浓度为0.55~2.25 mg∙L−1;相应地,龙葵茎和叶的萃取残渣中Cd的质量分数分别为0.51~4.23 mg∙kg−1和1.69~18.5 mg∙kg−1。龙葵茎和叶的萃取废液中Cd的总质量分别为4.43~22.4 μg和11.0~45.0 μg,而残渣中Cd的总质量分别为2.33~19.5 μg和4.40~36.4 μg。龙葵茎中Cd回收率在82.5%~106%,龙葵叶中Cd回收率在94.6%~122%,说明液相萃取技术对龙葵生物质中Cd的萃取效果较好。
图2结果是沿着a~f的先后提取次序测定的Cd形态。由图2可知,乙醇提取态Cd极低,经EDTA处理的龙葵有相对较高的乙醇提取态Cd(图2(a))。去离子水提取态Cd以原样最高(图2(b)),经HCl和EDTA处理的龙葵含有极低的水溶态Cd。氯化钠提取态Cd的质量分数最高(图2(c)),尤其是原样以及去离子水萃取后的残渣,具有最高质量分数的氯化钠提取态Cd,茎中为3.05~3.32 mg∙kg−1,叶中为7.41~7.63 mg∙kg−1,但经HCl与EDTA萃取过的残渣,氯化钠提取态Cd质量分数较低,茎中为0.19~0.69 mg∙kg−1,叶中为1.21~2.18 mg∙kg−1。醋酸提取态Cd(图2(d))及盐酸提取态Cd(图2(e))是各类形态Cd中分别位列第2和3形态,均以原样最高,其次为去离子水萃取残渣,HCl与EDTA萃取后的残渣,2类形态Cd的质量分数均不高。最后是残渣态Cd(图2(f)),这部分形态Cd经过不同萃取处理后均大幅降低,最高质量分数为0.13 mg∙kg−1。
由上述研究结果可知,以盐酸作为提取剂时,随着盐酸浓度增加,Cd的萃取率提高,残渣Cd的质量分数降低,0.25 mol∙L−1盐酸对Cd的萃取效果最佳,这与YUAN等[22]研究发现油菜饼粕中Cd随着盐酸浓度增加而减少的规律相一致。在不同种类酸萃取下,同一浓度(0.25 mol∙L−1)的硝酸、硫酸和盐酸对Cd的萃取效果相近。这可能是与3种提取剂的pH相近有关,三者的pH分别为0.22、0.19和0.23,在低pH下,酸对重金属的浸提效果主要取决于质子运动[23]。在本研究中,0.10 mol∙L−1 EDTA对植物体中Cd的萃取效果稍弱于0.25 mol∙L−1的盐酸、硝酸和硫酸,但差异不显著(P>0.05),这与GITIPOUR等[24]研究发现0.30 mol∙L−1盐酸与0.10 mol∙L−1 EDTA淋洗土壤中重金属Cd的去除效果相当的结果一致。
WEI等[25]和LI等[26]发现,龙葵茎和叶中Cd的亚细胞分布组成以可溶组分与细胞壁结合组分为主;将Cd结合在细胞壁部分是植物对Cd的重要解毒机制之一,细胞壁作为重金属进入植物细胞中的第一道防线,主要由纤维素、半纤维素、果胶、蛋白质等组成,其为重金属离子的螯合提供了大量的结合位点[27]。在本研究中,龙葵茎和叶中Cd的形态主要是氯化钠提取态(图2),其中的Cd主要结合在果胶和蛋白质中。0.25 mol∙L−1盐酸、0.25 mol∙L−1硝酸和0.25 mol∙L−1硫酸能有效萃取龙葵茎和叶中Cd的原因可能是,酸处理导致生物质细胞壁结构的破坏并释放出由多糖与蛋白质结合的Cd2+,H+与Cd2+进行置换。WU等[28]的研究结果表明,利用硫酸预处理Cd富集油菜生物质可以破坏细胞壁结构并将多糖等结合的Cd2+释放出来。液泡区隔化作用是修复植物对重金属耐受与解毒的重要机制之一[29]。有研究[30]表明,植物细胞可溶组分主要由液泡组成,酸处理破坏了液泡结构,从而释放出修复植物细胞中的可溶性组分Cd。0.25 mol∙L−1的不同酸萃取富集植物时,低pH条件可能也增强了植物生物质细胞表面Cd的解吸以及一些离散金属化合物的溶解。0.10 mol∙L−1 EDTA能有效萃取龙葵茎和叶中Cd的原因可能是,相对于细胞壁中蛋白质等提供的螯合位点,EDTA具有更强的螯合能力,从而将修复植物细胞壁上的Cd螯合下来。而盐酸浓度增加引起了Cd的萃取率升高、残渣Cd的含量降低,其原因在于盐酸对龙葵植物细胞壁结构的破坏程度以及细胞壁多糖与蛋白质的提取强度均随着其浓度的增加而增大,进而促进龙葵生物质中细胞壁结合Cd的释放。有研究[31]表明,在利用不同浓度的盐酸预处理水稻秸秆时,提高酸度有助于纤维素间糖苷键的破坏并促进酶解产糖效果。生物质细胞的渗透压也可能随着盐酸浓度的增加而逐渐改变,导致液泡内可溶性组分Cd的外溢逐渐加剧;此外,随着浓度的提高,盐酸溶液的pH也逐渐降低,H+的有效作用浓度逐渐增加,这些方面的改变都会促使龙葵茎和叶中Cd的萃取率的提升以及残渣的Cd含量的下降。
在本研究中,不同提取剂对龙葵茎和叶中Cd的萃取率也略有不同。首先,这可能与龙葵茎和叶中Cd含量的差异有关,龙葵茎部Cd的质量分数为5.51 mg∙kg−1,而龙葵叶部Cd的质量分数为19.3 mg∙kg−1,是茎部Cd含量的3~4倍,这也决定了同一提取剂对于不同部位Cd的萃取效果的差异。其次,同一提取剂对龙葵生物质中Cd的不同萃取率也可能是由龙葵茎和叶中Cd的亚细胞分布组成差异、Cd结合部位的性质差异以及Cd的化学形态差异所导致[25-26]。此外,由于植物体不同部位的木质纤维素组成以及细胞壁结构存在差异,比如茎秆一般粗纤维较多,木质化程度高[32],这造成了提取剂对于茎和叶中细胞壁多聚物的降解以及多糖与蛋白质结合Cd的提取的差别,从而导致同一提取剂对龙葵茎和叶中Cd的萃取效果有所不同。
不同化学形态Cd的迁移性和毒性不同,其活性从乙醇提取态到残渣态逐渐降低[17]。在本研究中,龙葵茎和叶中的Cd主要以氯化钠提取态Cd为主(图2)。龙葵茎和叶在经过0.25 mol∙L−1盐酸和0.10 mol∙L−1 EDTA萃取后,除了乙醇提取态以及去离子水提取态等活性Cd外,氯化钠提取态等惰性Cd均显著减少(图2(c)),这也是HCl和EDTA为何能高效萃取龙葵中Cd的印证。相反,去离子水对龙葵体内主要的氯化钠提取态Cd无明显萃取作用。经0.25 mol∙L−1盐酸萃取后,生物质中剩余的Cd主要是以氯化钠提取态为主的惰性Cd;而龙葵经0.10 mol∙L−1 EDTA萃取后,生物质中除了主要的氯化钠提取态Cd以外,还有较多高活性的乙醇提取态Cd和去离子水提取态Cd。这可能是因为EDTA萃取后导致修复植物生物质中不同形态Cd的再分配[33]。此结果表明,经过0.10 mol∙L−1 EDTA萃取后的龙葵生物质相较于0.25 mol∙L−1盐酸处理后的生物质具有的潜在风险更大。
-
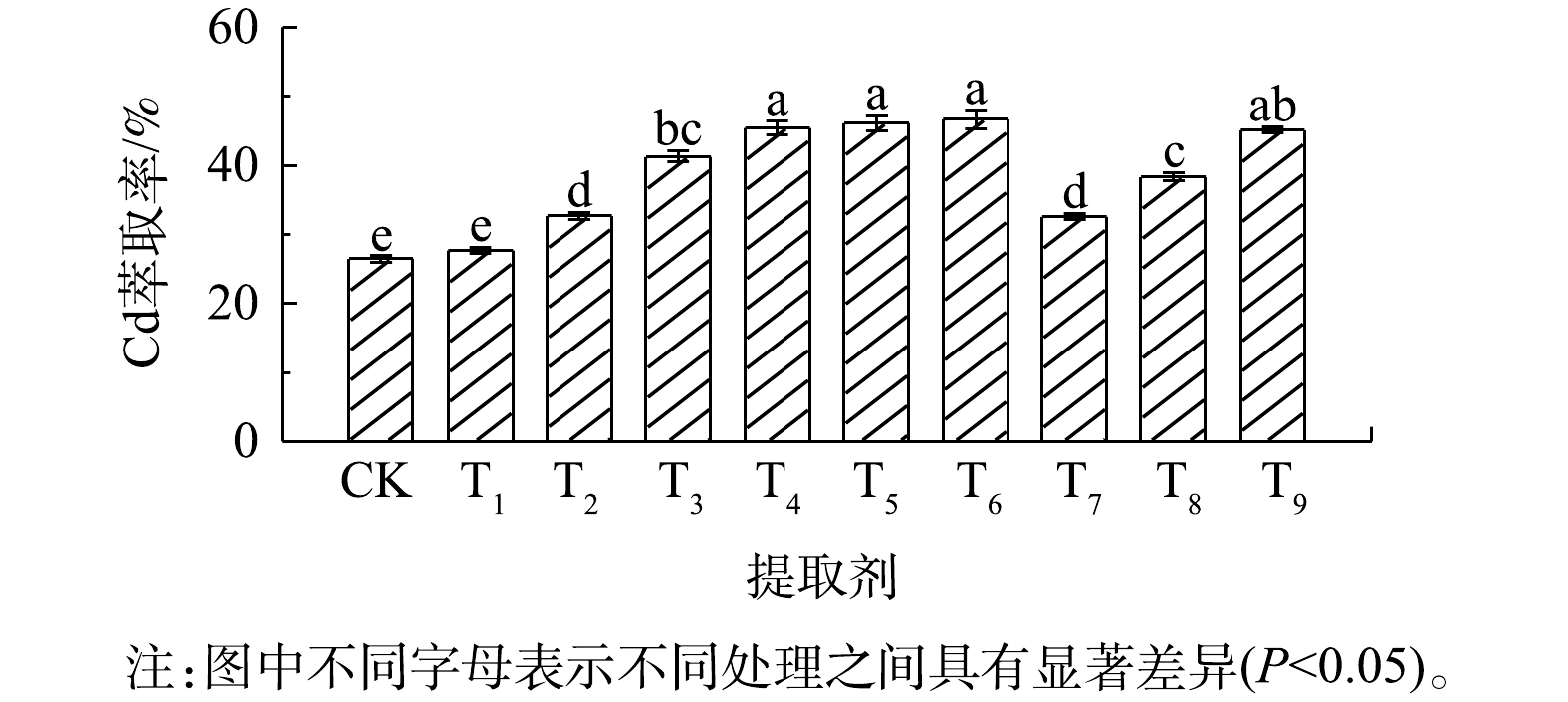
10种不同提取剂对伴矿景天生物质中Cd的萃取率均在50%以下(图3)。其中,0.25 mol∙L−1盐酸(T4)、0.25 mol∙L−1硝酸(T5)、0.25 mol∙L−1硫酸(T6)和0.10 mol∙L−1 EDTA(T9)的Cd萃取效果最佳,Cd的萃取率为45.1%~46.6%;其余几种提取剂对Cd的萃取率均显著(P<0.05)低于以上4种提取剂。
表3为不同提取剂对伴矿景天中Cd萃取后的残渣、废液中Cd含量情况。伴矿景天的萃取废液中Cd质量浓度为3.54~6.25 mg∙L−1,所得萃取残渣中Cd质量分数为3.22~8.79 mg∙kg−1;相应地,萃取废液中Cd的总质量为70.8~125 μg,萃取残渣中Cd的总质量为81.3~222 μg。不同提取剂萃取伴矿景天时,Cd回收率在76.4%~109%,同样说明液相萃取法对伴矿景天中Cd的萃取效果较好。
由以上结果可看出,同一提取剂对不同植物体在Cd的萃取效果上表现差异较大。在本研究中,不同提取剂萃取伴矿景天中Cd的萃取率(<50%)远远低于龙葵中Cd的萃取率,这可能跟伴矿景天本身高含水率的特性有关。在本研究中,伴矿景天的含水率高达96.0%,且直接将其破碎成匀浆,水分含量高、重金属浓度大,该状态下致使研究中选用的较低浓度提取剂的有效浓度大大降低,从而使得不同提取剂对伴矿景天中Cd的萃取作用大幅减弱。该研究结果与SONG等[34]的研究结果不一致。SONG等[34]发现,采用0.6 mol∙L−1盐酸振荡浸提伴矿景天(S∶L=1∶25,气浴30 ℃),并以3 500 r∙min−1离心45 min,Cd萃取率达95%以上。由此可见,液相萃取效果还取决于其他因素(固液比、萃取温度和时间、植物体本身特性),对于如何提高液相萃取法对伴矿景天中Cd的萃取效果值得进一步探究。
-
萃取废液中重金属的去除是含重金属生物质处置的一个重要环节,选取了0.25 mol∙L−1 HCl和0.10 mol∙L−1 EDTA萃取龙葵后所得萃取废液(合并龙葵茎和叶的萃取废液)为对象,以去离子水萃取废液作为对照,实验碳酸钾、氢氧化钾和4Å分子筛3种萃取废液镉清除方法,这些材料具有吸附或沉淀溶液中镉的潜力。由表4可知,不同处理方法对不同萃取废液中Cd的去除效果有所差异。在去离子水萃取废液中(CK,低浓度Cd),K2CO3、KOH和4Å分子筛的Cd去除效果表现为4Å分子筛(88.4%)>KOH(88.2%)>K2CO3(82.0%),处理间不存在显著差异(P>0.05)。3种处理后溶液中Cd的含量均低于0.10 mg∙L−1;溶液pH均由酸性(pH=5.81)变为中性至偏碱性,分别为8.53(K2CO3)、7.60(KOH)和8.71(4Å分子筛)。0.25 mol∙L−1盐酸萃取液(0.25 mol∙L−1 HCl)中Cd在K2CO3、KOH和4Å分子筛处理下的去除情况表现为4Å分子筛(91.9%)>K2CO3(86.1%)>KOH(81.1%),处理间具有显著性差异(P<0.05);处理后溶液pH均从强酸性(pH=0.64)变为弱碱性,分别为9.23(K2CO3)、8.01(KOH)和8.24(4Å分子筛)。对于0.10 mol∙L−1 EDTA萃取废液(0.10 mol∙L−1 EDTA),K2CO3、KOH和4Å分子筛均对其中Cd的去除作用不大;经过3种方法处理后,溶液pH由强酸性(pH=3.88)变为中性至碱性,分别为9.93(K2CO3)、10.44(KOH)和7.14(4Å分子筛)。进一步检测处理后的废液发现,经4Å分子筛处理后,0.25 mol∙L−1盐酸萃取废液中Cd的含量达污水排放标准(GB 8978-1996,Cd≤0.10 mg∙L−1),pH达污水排放标准(GB 8978-1996,6≤pH≤9)。
本研究中选取的3种不同处理剂对不同萃取废液中Cd的去除效果各有差异。对于低浓度Cd萃取废液(CK)和高浓度Cd萃取废液(0.25 mol∙L−1 HCl),4Å分子筛的Cd去除效果显著优于其余2种处理剂,说明4Å分子筛的物理吸附去除Cd的效果比K2CO3和KOH的化学沉淀去除Cd的效果更好。采用化学方法处理不同Cd萃取废液时,表现为K2CO3优于KOH。而对于0.10 mol∙L−1 EDTA萃取废液中的Cd,3种措施均无明显的去除效果。
在重金属废水处理中,化学沉淀法是最为常见且应用最多的方式,由于其成本低、操作简单方便,在工业上得到广泛应用,比如利用生石灰、NaOH等进行重金属污水沉淀净化[35]。许多重金属碳酸盐的溶解度介于重金属氢氧化物和重金属硫化物的溶解度[36],PATTERSON等[13]发现,当采用碳酸钠沉淀废水中的Cd时,在pH为8.4时沉淀的Cd相当于采用氢氧化钠在pH为10.4下所得量,且前者处理废液的过滤速度是后者的2倍,同时碳酸镉沉淀的密度更大,减少了重金属废水处理后的沉淀体积。这与本研究中采用碳酸钾(pH为10)去除Cd的效果较氢氧化钾(pH为11)更佳的结果相一致,这可能是因为碳酸镉沉淀比氢氧化镉沉淀更稳定,氢氧化物沉淀法常因溶液中Al、Zn等两性氢氧化物金属离子的存在而影响总体的沉淀效果[37]。在化学沉淀过程中,氰化物以及螯合剂如EDTA或者NTA都会对重金属的去除形成干扰[38],这可能也是在0.10 mol∙L−1 EDTA萃取废液中利用K2CO3与KOH去除Cd作用极小的原因所在。
传统的化学沉淀、絮凝浮选等方法会产生大量的化学污泥,二次处理成本较高,因此,采用吸附材料诸如活性炭、生物质改性炭、分子筛等脱除污水中重金属并将其再生更具经济高效性[39]。4Å分子筛具有较强吸附去除废水中Cd的能力,有研究[16]表明,4Å分子筛可吸附初始Cd质量浓度为20 mg∙L−1废水中98.2%的Cd;4Å分子筛去除废水中Cd2+的主要机理是离子交换吸附[40-41]。在本研究中,4Å分子筛在低、高浓度萃取废液(CK和0.25 mol∙L−1 HCl)中均表现出较好的Cd去除性能,而0.10 mol∙L−1 EDTA萃取废液中的Cd主要是以EDTA-Cd的形式存在,EDTA配位体的螯合干扰可能影响了Cd2+与4Å分子筛上的离子进行交换吸附,导致分子筛的吸附作用失效。4Å分子筛除了主要的离子交换吸附作用外,还存在部分表面化学沉淀作用[41]。本研究结果表明,4Å分子筛处理后废液均偏碱性,很好地证实了这一点,这也可能是4Å分子筛相比于化学沉淀剂处理更为高效的原因之一。4Å分子筛作为优良的吸附剂,还可以通过NaCl、Na2EDTA等化学洗脱方法再生[16, 42],更具有经济价值,同时也避免了化学沉淀过程产生大量污泥的二次处理的复杂问题。
2.1. 不同提取剂对龙葵中Cd的萃取效果
2.2. 不同提取剂对伴矿景天中Cd的萃取效果
2.3. 不同处理下龙葵萃取废液中Cd的去除效果
-
1) 10种提取剂对植物体中Cd的萃取效果差异较大,其中,以0.25 mol∙L−1浓度的盐酸、硝酸、硫酸和0.10 mol∙L−1 EDTA的效果最好且相近,龙葵茎部Cd萃取率最高达88.2%,龙葵叶部Cd萃取率最高达89.8%。但提取剂对伴矿景天中Cd的萃取效果不佳,Cd萃取率均在50%以下,最高Cd萃取率仅46.6%。综合提取剂的成本与危险性、萃取废液中Cd的净化问题以及萃取后生物质中Cd的活性状况,0.25 mol∙L−1盐酸作为液相萃取龙葵生物质中Cd的提取剂最为合适。
2)比较了碳酸钾、氢氧化钾和4Å分子筛吸附去除萃取废液中Cd的潜力,4Å分子筛最为高效,0.25 mol∙L−1 HCl萃取废液中Cd的去除率达91.9%,处理后溶液中Cd质量浓度达污水排放标准(GB 8978-1996,Cd≤0.10 mg∙L−1),溶液pH达排放标准(GB 8978-1996,6≤pH≤9),3种材料对EDTA萃取废液中Cd均没有清除能力,因而,EDTA完全不适合作为修复植物生物质中重金属的提取剂。




 下载:
下载: