-
重型柴油车的尾气排放对大气中PM2.5和NOx等污染物的贡献很大。据估算,重型柴油车的PM排放量能占机动车排放总量的78%,其中NOx排放量占机动车排放总量的57.3%[1-2]。根据《车用压燃式、气体燃料点燃式发动机与汽车排气污染物排放限值及测量方法(中国VI阶段)》(GB 17691-2018),我国将分别在2021年7月1日和2023年7月1日实施重型柴油车国VI-a阶段和VI-b阶段排放标准。为满足柴油机的国VI排放要求,后处理装置通常采用柴油机催化氧化(diesel oxidation catalyst,DOC)-颗粒物捕集器(diesel particulate filter,DPF)-选择性催化氧化(selective catalytic reduction,SCR)联用的模式。其中,DPF用于减少颗粒物排放、SCR用于减少NOx的排放。目前,我国符合国VI排放标准的发动机及后处理核心技术与国外相比仍存在较大差距[3-4]。
实际应用较多的低温SCR系统对NOx的转化效率不高。城市邮政车、公交车、环卫车运行时经常启停,在排气温度较低时尿素SCR系统无法正常运行[5-8]。为达到国IV、国V排放标准,SCR系统的平均转化效率需达到75%~85%。而由于SCR控制策略往往采用基于目标转化效率的开环控制策略[9-10],故到了国VI阶段,SCR系统平均转化效率应提升至90%~98%。为达到更高的转化效率,需要按比例过量喷射尿素,但尿素结晶的风险会随之增大。另外,国VI标准下调了NH3泄漏限值,还需在SCR下游安装氨捕集器(ammonia slip catalyst, ASC)。而在排气温度高于380 ℃时,还应考虑喷入排气气流中的尿素水溶液可能快速脱水转变成三聚氰胺沉积物的问题。这种现象会导致排气管路堵塞、发动机背压增加,由此带来功率下降、油耗上升的事件时常发生[11]。因此,降低或避免尿素结晶是亟需解决的难题[12-13]。
固态SCR技术是近年来出现的一种降低NOx排放的新技术。LACIN等[14]的研究结果表明,固态SCR在FTP72和US06测试循环有较高的NOx转化效率;FULKS等[15]对不同种类的固态氨存储材料的氨气释放特性进行了研究,发现固态SCR技术携带氨的体积密度和纯液体氨相当,在相同的容积下,可比尿素SCR系统携带更多的有效还原剂;SHOST等[16]的研究结果表明,固态SCR可将氨气直接喷射到排气管中,有较大的NOx减排潜力[16-17]。因此,固态SCR能够避免尿素SCR系统低温活性不足、排气管路结晶、低温结冰等缺陷,是一种有前景的柴油机NOx排放控制技术[18-21]。
本研究为探讨固态SCR技术对柴油机尾气的NOx减排特性,分别在发动机台架及车载道路上开展实验,并与尿素SCR技术进行了对比分析,以期为降低我国城市柴油车NOx污染物排放、轻型柴油车国VI排放标准达标提供参考。
全文HTML
-
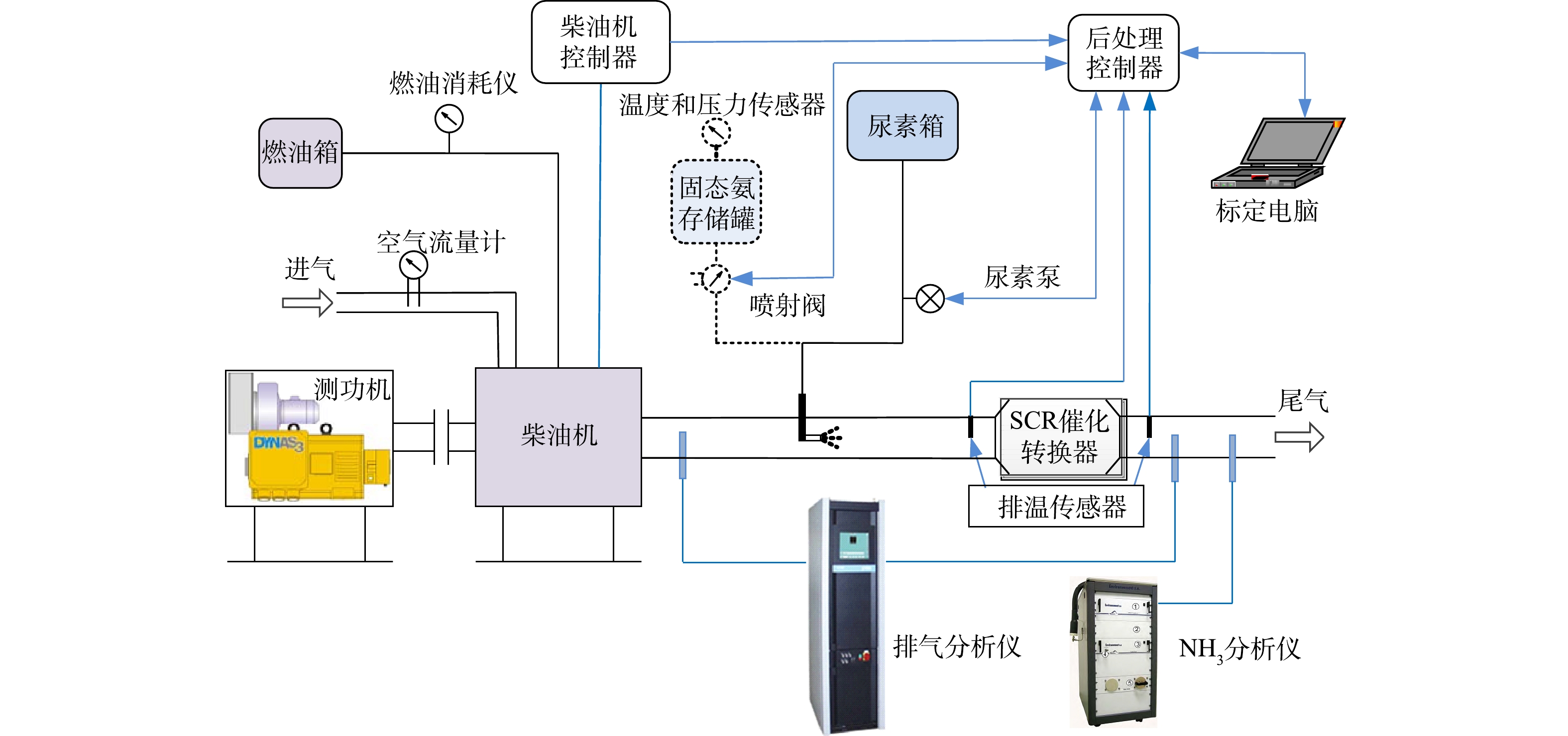
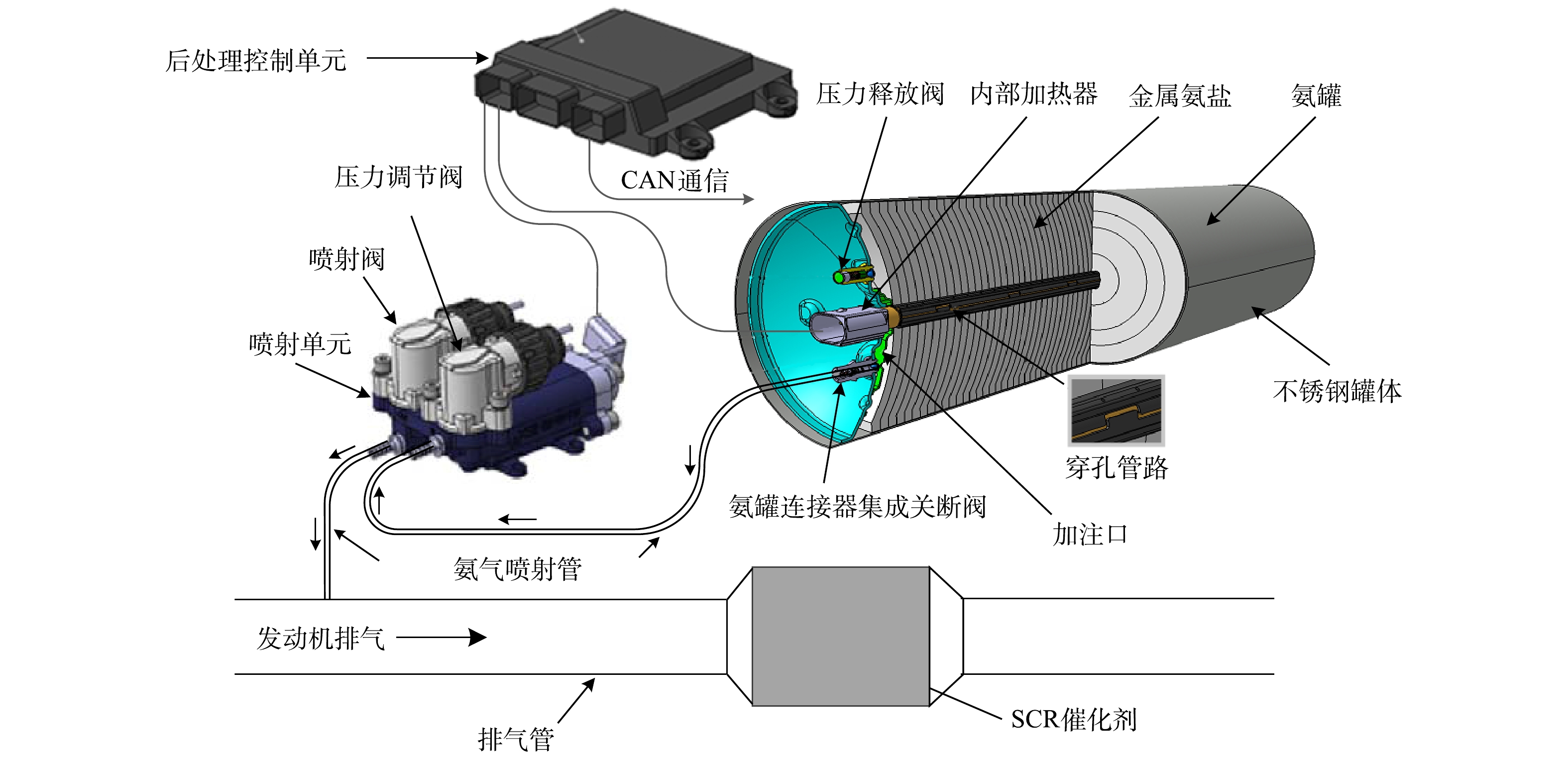
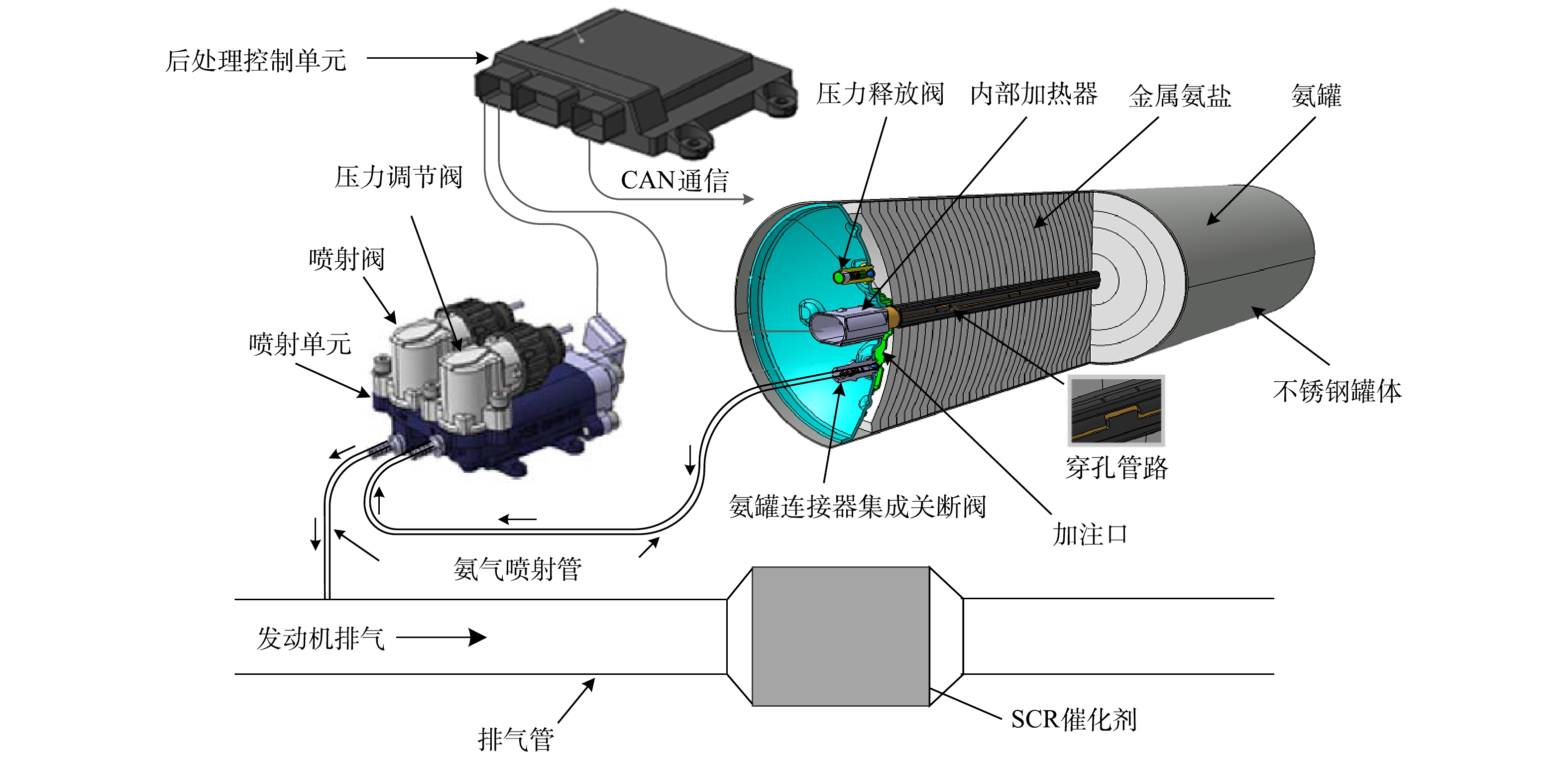
固态SCR系统分金属氨盐存储罐、内部加热器、减压阀、不锈钢罐,以及后处理控制单元(aftertreatment control unit,ACU)、喷射控制阀、喷射装置、压力调节阀和氨气输送管等组成,其结构[22-23]如图1所示。固体SCR技术原理分3步:1)将金属氨盐 (Sr(NH3)8Cl2)存于封闭不锈钢罐体内,当其受热至一定温度后,NH3以氨气形式释放出来。NH3的喷射压力通过压力调节阀闭环调节,稳定在400 kPa;2)ACU接收发动机控制器(electronic control unit,ECU)的氨氮比通信信号(controller area network,CAN)后,实时喷射NH3到发动机排气管根据柴油机不同工况实时调整NH3喷射量;3)NH3在SCR催化剂的作用下与NOx发生化学反应,从而减少柴油机尾气中NOx的排放。为使NH3剂量阀保持精确计量,对系统监测密闭容器压力值及温控单元进行闭环控制。固态SCR技术同尿素SCR技术相比,不受尿素热解水解温度的限制,不存在尿素结晶、尿素结石堵塞排气管路的风险。而金属氨盐(Sr(NH3)8Cl2)做为NH3的存储介质[24],具有氨存储效率高、低温活性高等优点。另外,催化剂氨存储量对NOx转化效率有明显的影响,所以选择催化剂氨存储性能好、低温活性好的催化剂,可以有效提升SCR系统在低温下的NOx转化效率。
-
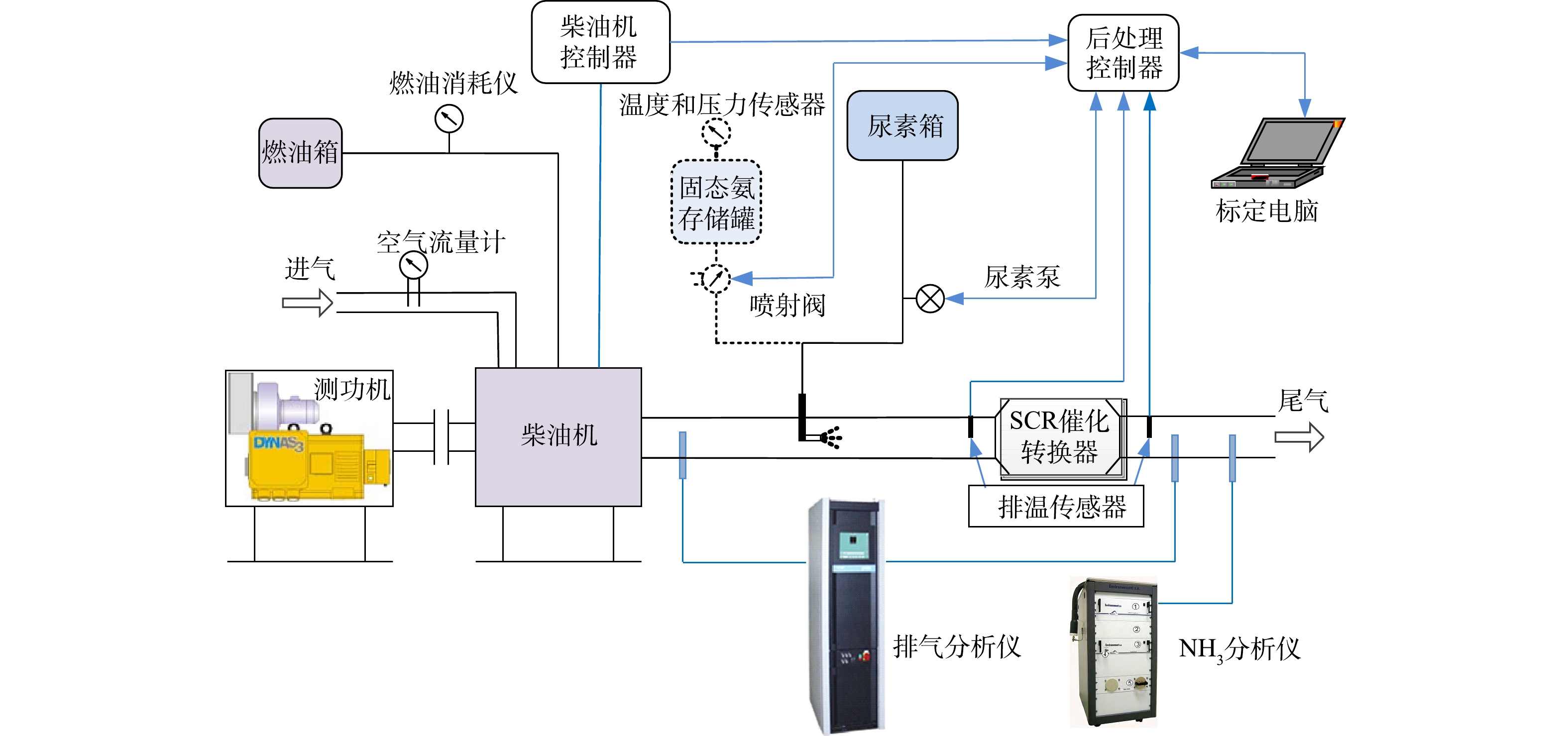
柴油发动机台架实验系统包括测功机、排放测试分析仪、固态SCR系统、尿素SCR系统和SCR催化剂系统(见图2)。催化剂采用低温转化效率高的铜基催化剂或钒基催化剂。柴油发动机及后处理的主要技术参数为:四冲程;增压中冷机型;电控高压共轨的燃油供给;缸径行程80 mm×130 mm,额定功率220 kW;最大转矩1 250 N·m;最大扭矩转速范围为1 200~1 700 r·min−1;涡轮增压器限制温度≤600 ℃;SCR催化转换器体积17 L。测试设备的主要型号及参数分别为:电力测功机(湖南湘仪实验室仪器开发有限公司,ZAC450),测量精度为±3%;油耗分析仪(AVL List GMBH,AVL735),测量精度为±1%;气体采集分析仪(HORIBA,MEXA7500),测量精度为±1%;氨气分析仪(Environment S.A,FT-UV),其测量精度为±3%;进气流量计(ABB Ltd.,Sensyflow P),测量精度为±3%。
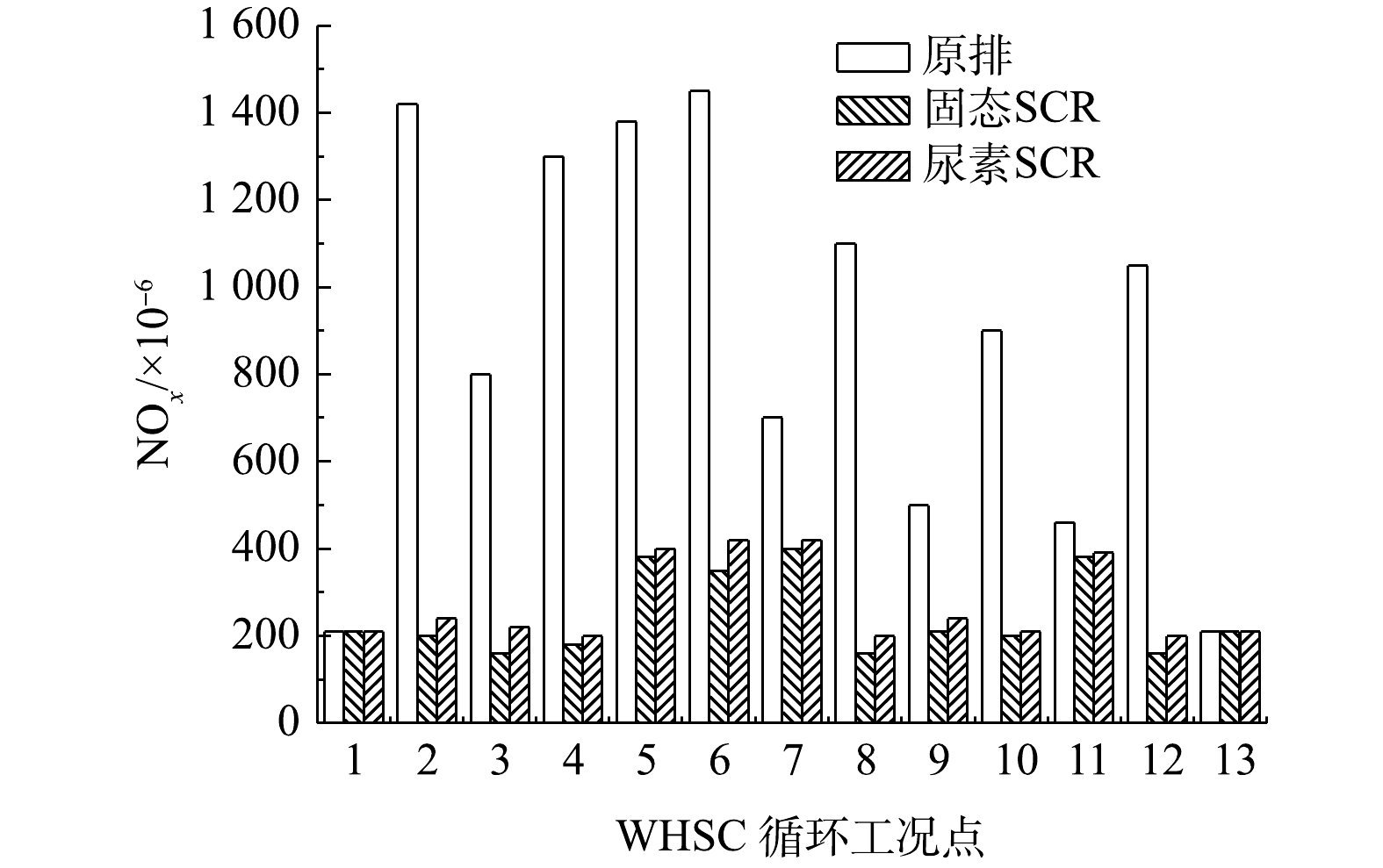
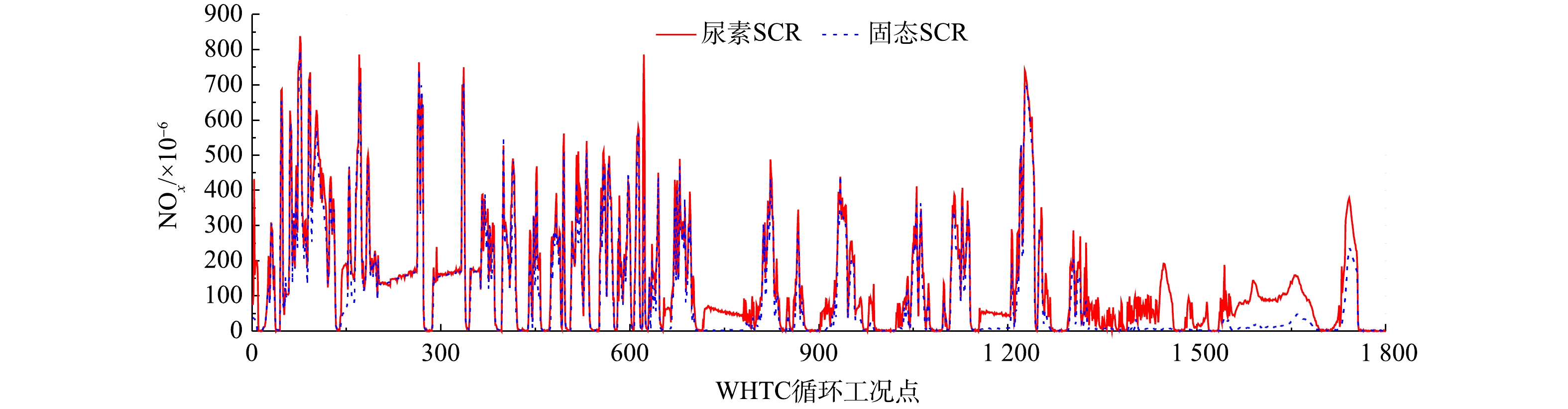
发动机台架实验系统通过同一控制器提供固态SCR系统和尿素SCR系统相同氨氮比的控制信号,测功机用来控制发动机转速和扭矩。实验基于国VI排放标准中的世界统一稳态循环(world harmonized steady cycle,WHSC)和世界统一瞬态循环(world harmonized transient cycle,WHTC)进行,分别采用固态SCR和尿素SCR喷射系统对同一台柴油发动机进行实验。气体采样装置对后处理系统排气尾管直接采样,尾气分析仪测量排放的NOx污染物,氨气分析仪测量氨气泄露量。
-
在一台增压中冷柴油机中进行固态SCR与尿素SCR系统降低NOx的对比研究:1)研究固态SCR的氨盐存储释放特性;2)基于相同的氨氮比,确保2种系统喷射的还原剂总量相同,固态SCR采用被动喷射氨气模式、尿素SCR为直接向排气尾管喷射氨气模式,进行WHSC和WHTC循环实验验证。实验条件中,柴油发动机、进气系统、排气系统、冷却系统和排气后处理系统等装备,以及润滑油、燃料油等材料均符合《车用压燃式、气体燃料点燃式发动机与汽车排气污染物排放限值及测量方法(中国Ⅲ、Ⅳ、Ⅴ阶段)》(GB17691-2005)。根据实验要求,发动机实验台实时记录发动机的工作参数,如发动机转速、扭矩、温度、催化剂的空速、氨氮比等参数。每个发动机的运行模式稳定180 s,在最后30 s记录数据,采用排气组分分析仪和NH3分析仪记录对应的排放数据。由于1 mol尿素可水解成2 mol的氨气,尿素标准水溶液的质量浓度为32.5%,所以,固态SCR氨气需求量与尿素需求量的换算关系见式(1)。SCR催化转换器的表面温度由催化转换器进出口温度的算术平均值替代。SCR催化转换器的转化效率如式(2)所示。误差分析采用标准偏差公式(式(3))。
式中:
Qu 为尿素需求量,mg·s−1;Qn 为氨气需求量,mg·s−1;Mu 为尿素的摩尔质量,g·mol−1;Mn 为氨气的摩尔质量,g·mol−1;C为标准尿素水溶液质量浓度,取值32.5%。氨气和尿素的摩尔质量分别为17 g·mol−1和60 g·mol−1。式中:
ηN 为催化转换器的NOx转化效率;CNin 为催化转换器入口处NOx浓度;CNout 为催化转换器出口处NOx浓度。式中:σ为标准偏差;N为样本总量;i为样本序列号;Xi为第i个样本的值;μ为算术平均值。
1.1. 技术原理
1.2. 台架实验系统
1.3. 道路实验方案
-
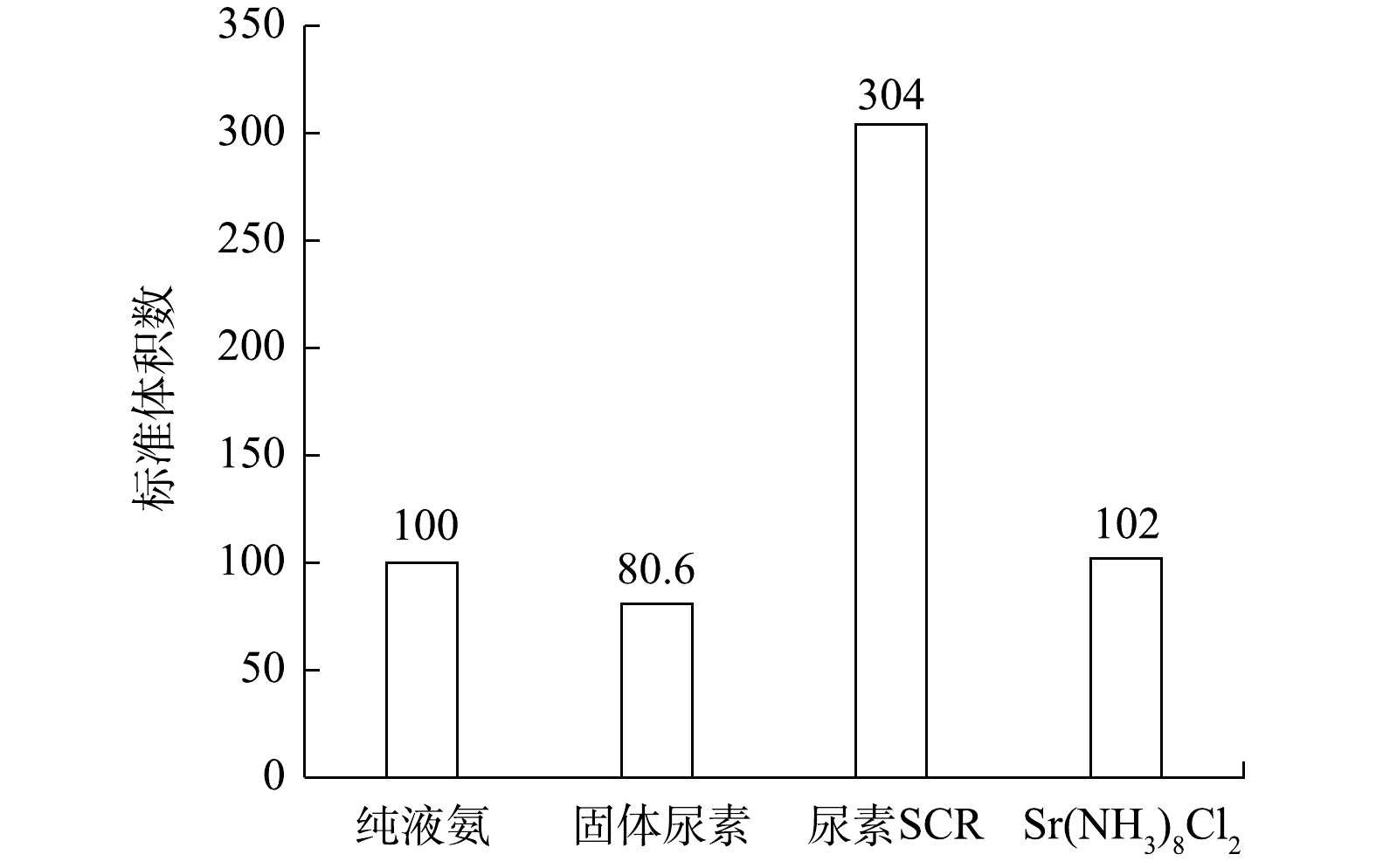
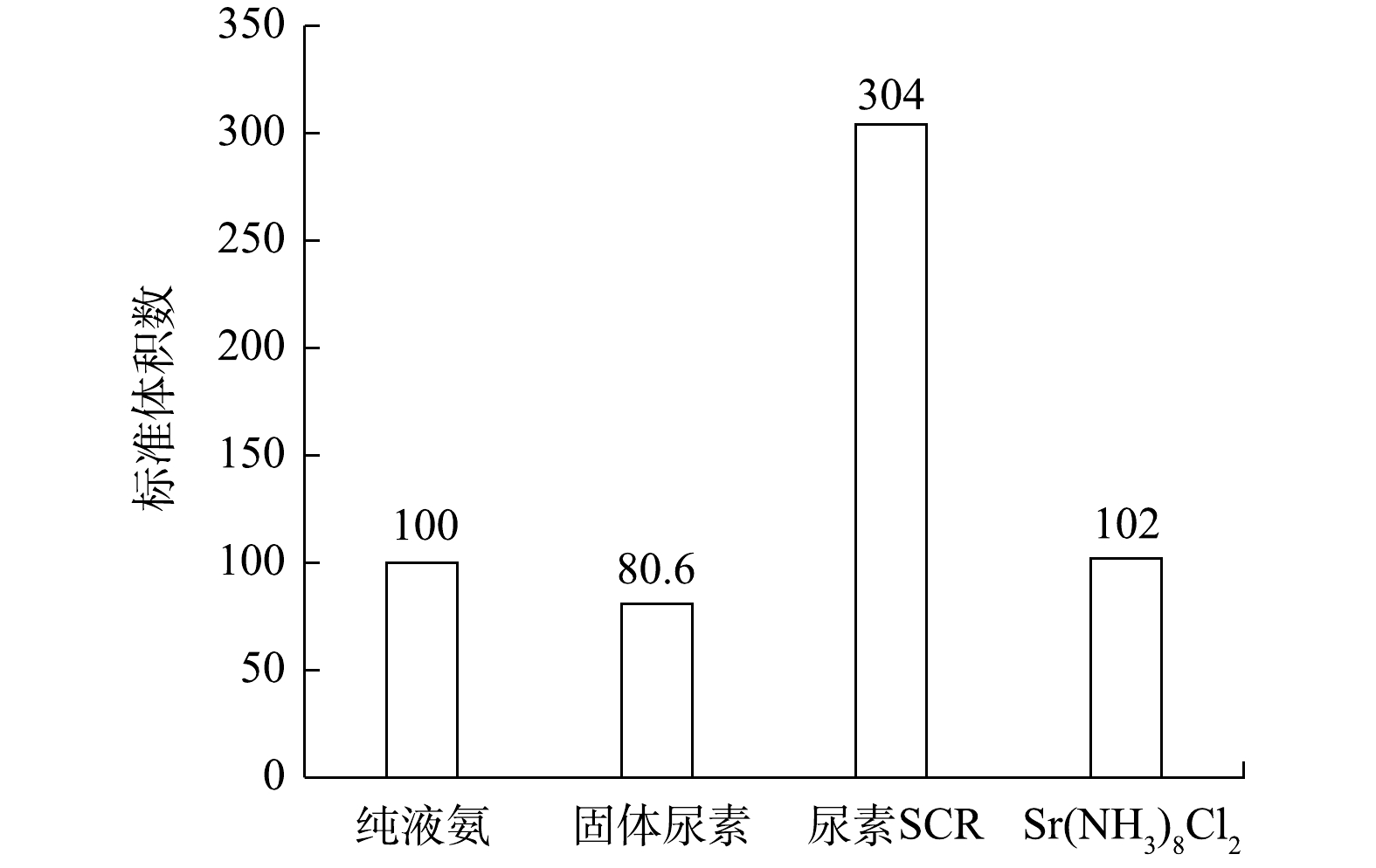
SCR系统降低柴油机NOx排放的主反应为:4NO+4NH3+O2=4N2+6H2O。实验所需各种还原剂对应的储氨特性如表1所示,由此计算出还原单位质量NO所需的纯液氨、固态SCR、尿素SCR及固态储氨物质Sr(NH3)8Cl2的体积之比为0.93∶0.75∶2.83∶0.95。以纯液氨体积为基准体积,设定为100,各还原剂体积按此标准换算出的体积数如图3所示。由图3可知,还原单位质量NO所需还原剂中,固态SCR体积数最小,尿素SCR体积数最大,Sr(NH3)8Cl2与纯液氨相当,Sr(NH3)8Cl2的体积数是尿素SCR的1/3左右。因此,在相同存储体积下,应用固态SCR系统能够比尿素SCR系统携带更多有效还原剂,即携带相同质量还原剂的固体SCR系统续航里程要高于尿素SCR系统。这也说明应用固态SCR系统能大幅减小系统体积,更有利于整车布置。
-
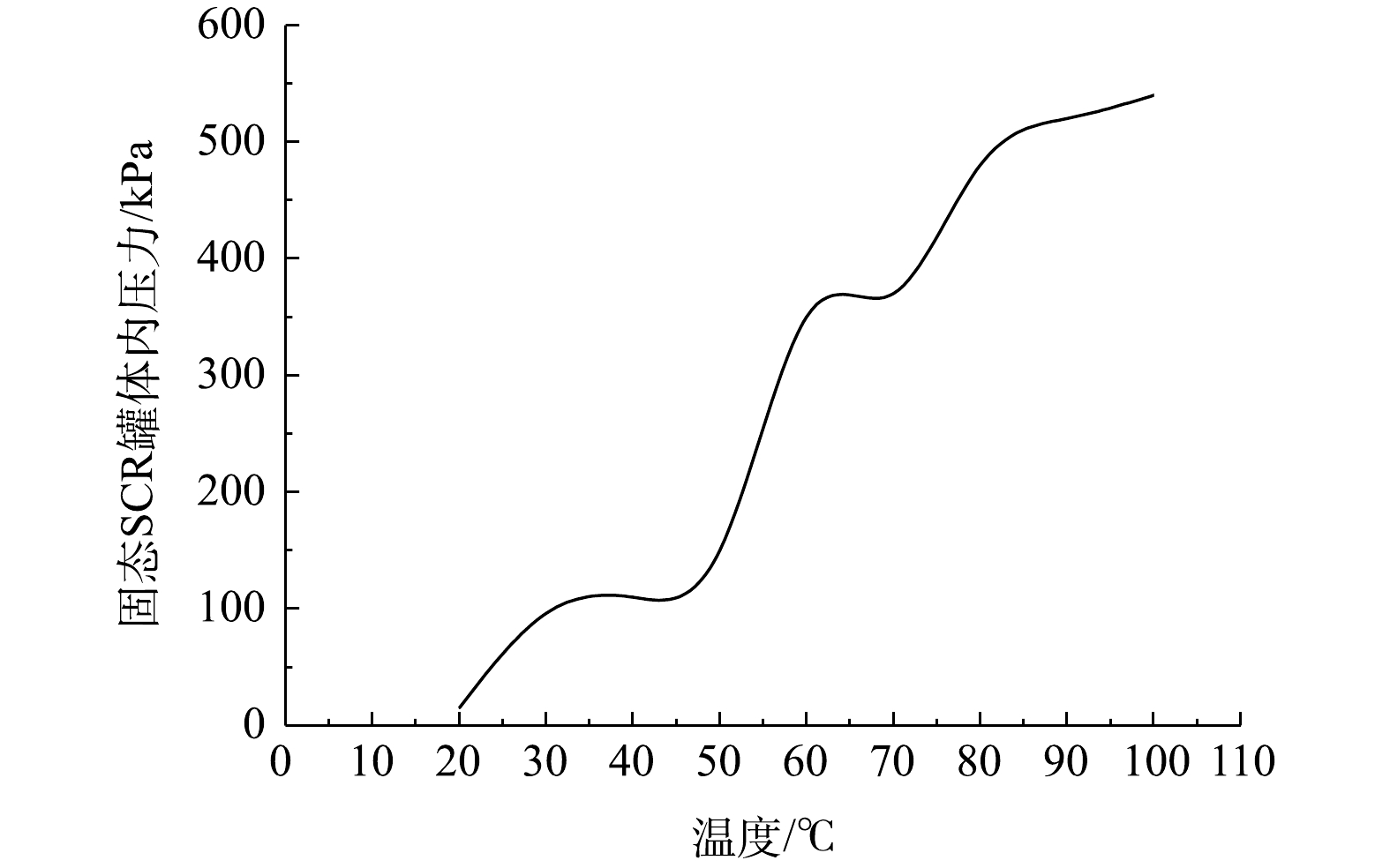
固态SCR系统中氨气随温度变化的释放情况见图4。当固态SCR不锈钢罐被加热到阈值温度,金属氨盐方可释放出氨气,相关反应的化学方程式见式(4)和式(5)。
由图4可知,当不锈钢罐体被加热到20 ℃时,氨气开始释放。安装在罐体上的压力传感器探测到压力信号为20 kPa。持续升温至70 ℃,不锈钢罐体的压力可通过稳定阀调节,并在400 kPa时满足喷射条件。通过控制电加热的PWM占空比信号,使不锈钢罐体温度稳定在60~70 ℃。这样最有利于喷射压力稳定在400 kPa。当系统预热300 s后,即可满足400 kPa喷射要求。
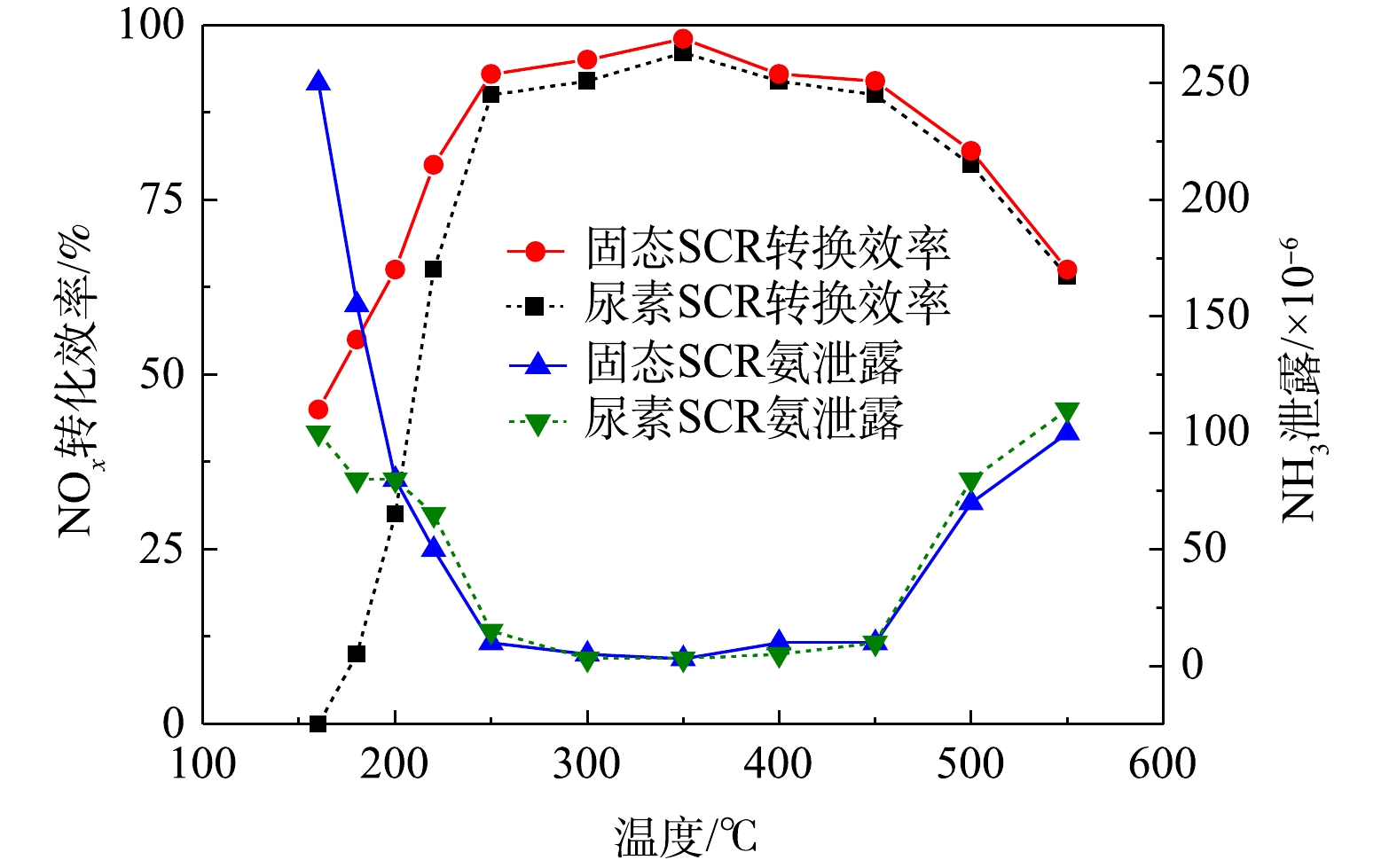
在空速为30 000 h−1、不同排气温度条件下,通过后处理电控单元(ACU)设定氨氮比为1∶1,固态SCR和尿素SCR的NOx转化效率对比如图5所示。结果表明,当排气温度低于250 ℃时,固态SCR转化效率明显高于尿素SCR。其中,在160 ℃时,固态SCR的NOx转化效率较尿素SCR提升了40%;在180 ℃时,转化效率提升了40%;在200 ℃时,转化效率提升了35%;在220 ℃时,转化效率提升了25%。而在低温时,尿素SCR的NOx转化率主要受尿素热解、水解温度的限制,故低温时的转化效率较低。在300~400 ℃时,固态SCR系统的NOx转化效率与尿素SCR相当,最高转化效率接近95%。这是由于在该温度区间催化剂活性最好,NOx转化效率最高。当排气温度低于200 ℃,固态SCR氨气泄漏量明显高于尿素SCR。这是由于该温度下,喷入排气管中的尿素水溶液不能完全水解成氨气,而在排气温度高于200 ℃时,尿素水解效率较高,故氨泄漏与固态SCR技术的量相当。
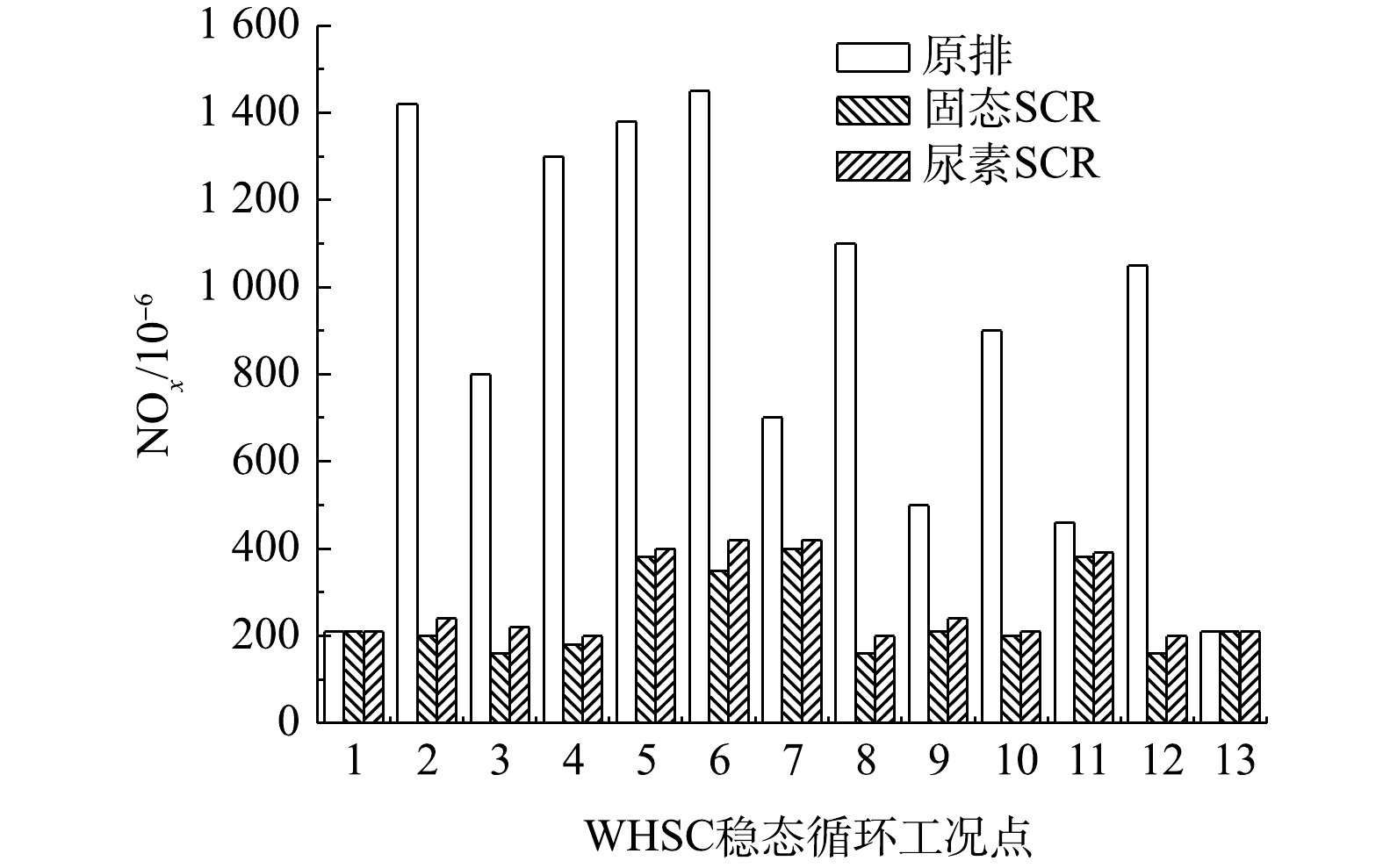
不带后处理的柴油机WHSC裸机NOx排放量为9.25 g·(kWh)−1。在WHSC循环内设置相同氨氮比,分别进行固态SCR和尿素SCR对比实验,结果如图6所示。结果表明:固态SCR和尿素SCR系统的NOx排放量分别减少至1.65 g·(kWh)−1和1.95 g·(kWh)−1;平均NOx转化效率分别为82.2%和78.9%,固态SCR的转化效率较尿素SCR提升了3.3%;平均NH3泄漏是1.2×10−6和1.7×10−6,2种技术差异较小;氨逃逸峰分别为6×10−6和8×10−6。
不带后处理的柴油机WHTC裸机NOx排放量为8.99 g·(kWh)−1。在WHTC循环内设置相同的氨氮比,分别进行固态SCR和尿素SCR对比实验,结果如图7所示。结果表明:固态SCR和尿素SCR系统的NOx排放量分别减少到1.5 g·(kWh)−1和1.9 g·(kWh)−1,平均NOx转化效率分别为83.3%和78.8%;固态SCR系统的NOx转化效率较尿素SCR提升了4.5%;氨气泄漏峰值分别出现在78×10−6和55×10−6,平均氨泄漏分别为4.3×10−6和3.0×10−6。分析其原因,WHTC循环由1 400~1 600 s切换到高速路段时,柴油机负荷突然增加,排气流量迅速变大;此时的SCR箱内氨存储量较大,温度迅速上升,氨存储量下降,从而导致氨气溢出;而铜基SCR催化剂氨存储能力较强,也容易在SCR催化器温度突然升高时产生氨泄漏。
-
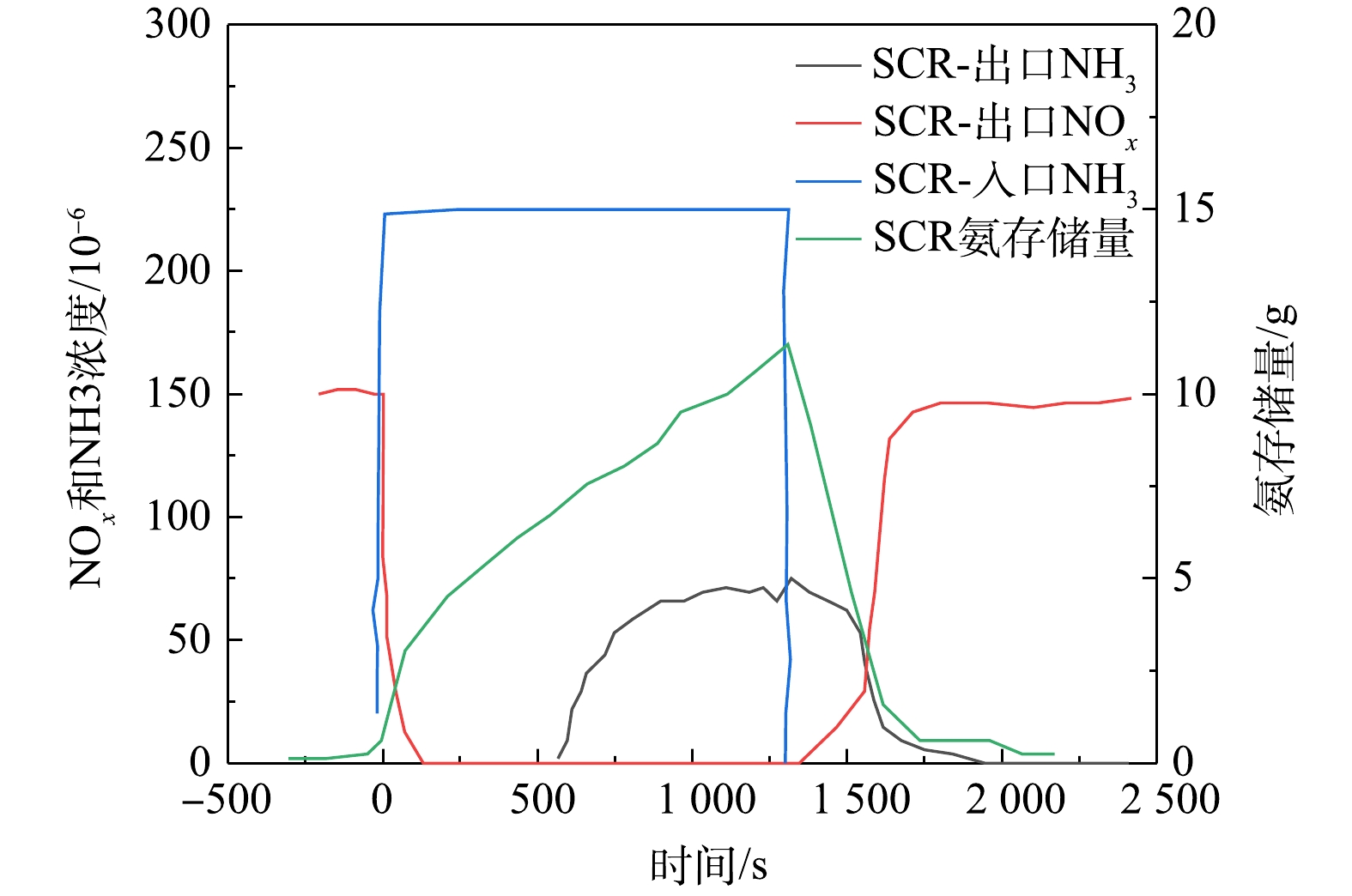
图8为SCR催化器在排温200 ℃、空速25 000 h−1下,各工况点的动态反应特性,表现了不同点的氨存储性能。在进行氨存储最大值实验时,需将ASC拆下。实验过程中,记录SCR催化器上下游温度、上下游NOx浓度、下游NH3浓度、尿素喷射量、发动机进气量、喷油量等相关参数。发动机在额定点运行10~15 min后,停止喷射尿素以清空SCR催化器内的氨存储。手动调整发动机工况,使SCR平均温度在200 ℃、空速25 000 h−1工况点。待SCR前后温度和上下游NOx浓度稳定后,开始记录数据。将氨氮比调节为1.3后开始喷射尿素,下游NOx浓度快速下降,氨存储量逐渐上升,NH3泄漏在400 s开始缓慢上升,NH3泄漏上升到70×10−6时,停止喷射尿素。下游NOx浓度快速上升到柴油机原机排放浓度时,停止记录数据。选取从尿素喷射开始,到NH3泄漏达到25×10−6这一段时间的数据。根据SCR催化器动态化学平衡,NH3in是进入催化器的NH3质量流量,NOxin进入催化器的NOx质量流量,NH3out是溢出催化器的NH3质量流量,NOxout排出催化器的NOx质量流量,积分后累计的差值即为该工况点的最大氨存储量。因满足国VI排放的NOx平均效率应达到95%以上,故一般选用低温转化效率较好的铜基催化剂。SCR催化器的氨存储随催化器温度的上升而降低。在相同温度点,随氨存储量的增加,NOx转化效率得以提升。
-
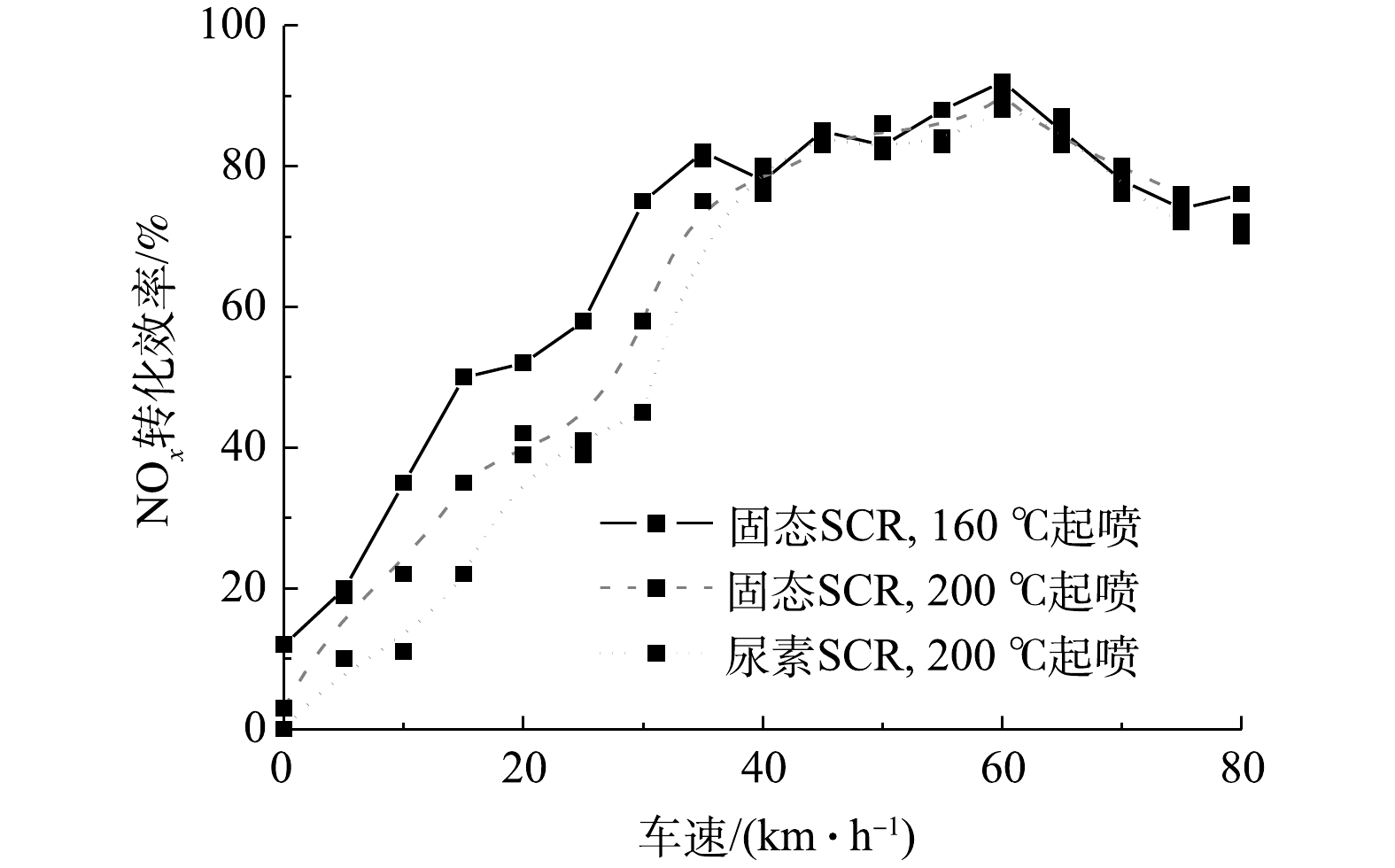
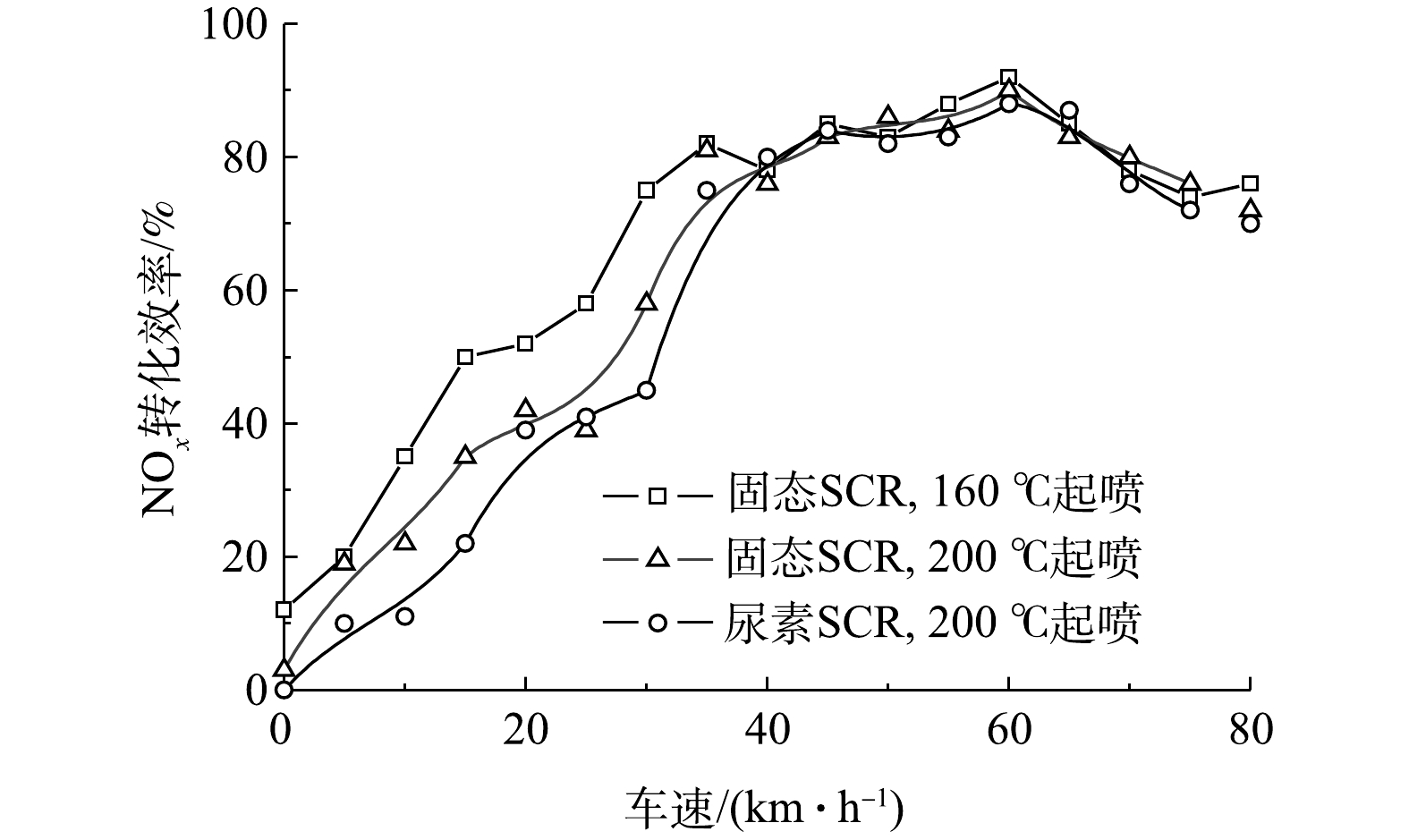
图9为固态SCR与尿素SCR随车速变化的NOx转化效率对比。由图9可知,随着车速的增加,发动机工作负荷逐渐上升,排气温度逐渐提高,相应的NOx转化效率也同步增加。由于固态SCR技术是直接向排气尾管喷射氨气,故没有尿素结晶风险。将固态SCR起喷温度调整到160 ℃进行喷射,车速控制在0~40 km·h−1,NOx的转化效率明显提升,较200 ℃起喷的固态SCR系统和尿素SCR系统分别提升了9.7%和15.5%。在车速大于40 km·h−1时,维持相同氨氮比,其转化效率变化不大。因此,对于长期在低速运行的市内柴油车辆,采用固态SCR技术并降低起喷温度,可有效提升NOx的转化效率。
功基窗口法是将实验结果划分为若干个适用于评估PEMS性能的窗口数据子集。功基窗口大小为发动机WHTC循环功,并计算功基窗口内所有采样点的平均比排放值。功基窗口移动间隔为1 s。主要包括功基窗口法和CO2基窗口法。第i个平均窗口的周期t2,i−t1,i由式(6)决定。
式中:W(tj,i)为从开始到时间tj,i内的发动机循环功,kWh;Wref为WHTC的循环功,kWh;t2,i见式(7)。
Δt为数据采样周期,≤1 s。每一个窗口和每一种污染物比排放的计算见式(8)。
式中:
m 为各污染物的排放质量,mg;W(t2,i)−W(t1,i)为第i个平均窗口的发动机循环功,同时,有效窗口平均功率大于发动机最大功率的20%,有效窗口比例至少50%。基于功基窗口法对固态SCR与尿素SCR的NOx排放效率进行了分析(见图10)。由图10可知,功基窗口数在(0,20]之间,匹配尿素SCR系统的柴油车NOx污染物排放量明显较高,分别为160 ℃起喷温度和200 ℃起喷温度下的固态SCR系统的2.38和1.73倍。这是由于尿素喷入排气尾管后需要先进行水解热解,才能生成还原剂氨气;若汽车启动时间较短,排气温度尚未达到适宜条件时还原剂的生成率较低,会导致NOx排放量较大。因此,对于频繁起停的车辆,起步阶段尿素SCR排放的污染物NOx较多。
2.1. 固态SCR与尿素SCR技术的单位储氨性能对比
2.2. 固态SCR与尿素SCR台架实验结果的对比分析
2.3. 固态SCR系统氨存储特性
2.4. 固态SCR与尿素SCR车载道路实验结果的对比分析
-
1)固态SCR系统比尿素SCR系统携带更多有效还原剂,携带同等质量还原剂,体积仅为标准尿素水溶液体积的1/3。SCR系统体积的节省可有助于整车的安装布置,实现轻量化,降低碳排放。
2)为满足国VI排放标准,SCR系统对NOx的平均去除率应达到95%以上,故一般选用低温转化效率较好的铜基SCR催化剂。台架实验表明,随反应温度的上升,SCR催化器的氨存储能力降低,故应维持适宜温度点,保证氨存储量的增加,从而提升NOx的转化效率。
3)实际道路实验表明,固态SCR直接向排气管喷射氨气,具有更低的起燃温度,可提高低温下的NOx转化效率,所以,对于长期在低速运行的市内柴油车辆,应采用固态SCR技术并降低起喷温度,以有效减少NOx的排放。



 下载:
下载: