-
磷酸铁锂电池的使用寿命一般为3~8 a[1],报废后会产生一系列的环境问题。电池内电解液以及黏结剂的挥发,对人、动物、植物都会有危害;潜在的短路会在短时间内释放出巨大的热量而引起火灾或爆炸等事故;重金属泄露进入土壤后会在植物、动物体内富集,经过食物链传输,最终会被人体摄入,对人的健康造成不可逆的危害[2-4]。对于废旧锂电池的回收处理刻不容缓。
针对废旧磷酸铁锂电池的处理,主要是针对正极材料(LiFePO4,LFP)中Li资源的回收。Li的回收一方面可保证资源的再利用;另一方面对于将P、Fe等元素制备成环境修复、重金属吸附、有机染料催化降解等方面的环境功能材料具有积极的促进作用,也可以实现废弃物资源再利用。因此,Li的回收不仅实现了废旧磷酸铁锂电池的高附加值资源利用,而且可以降低其对环境的影响,保证这类电池的回收效益。目前,Li的回收一般采用湿法和火法2种工艺。传统的“浸出-分步沉淀-锂回收”湿法冶金方式需要依赖强酸强碱[5-6],且Li的回收处于最后阶段,分步沉淀时会损失部分Li[7],降低了Li的回收率;新兴的选择性浸出工艺虽然有效地提升了Li的回收率,但是需要大量的试剂[8-10],工艺成本增加。火法工艺中,Li在高温煅烧时容易进入炉渣相中[7, 11],损失严重。传统的回收工艺容易产生酸雾、Cl2等有毒有害气体[12-14],对环境以及人体健康易造成危害。
机械化学活化(MCA)可产生摩擦、剪切等机械力,由此引起的物理和化学变化可有效降低物质活化能、增强反应活性[10, 15-16],改善物质的浸出特性[17],有助于缓解上述废旧锂电池回收过程中出现的问题。YANG等[18]将EDTA-2Na作为共磨剂与LFP进行机械化学活化,稀H3PO4溶液作为浸出剂,Fe和Li的浸出率分别为97.7%和94.3%;WANG等[19]选用EDTA作为共磨剂与LiCoO2进行MCA,水作为浸出剂,Co和Li的浸出率分别为98.0%和99.0%。研究人员选用EDTA等共磨试剂,与锂电池正极材料通过固-固反应形成稳定的可溶性螯合物,经过水洗[20]或者酸浸[18, 21]后以离子态存在于溶液中,虽然提高了浸出率,但是并没有达到选择性浸出Li的目的,依旧需要分步沉淀回收Li,降低了Li的整体回收率。由此可见,同时实现Li的选择性提取和高效率回收是当前面临的主要挑战。
传统的湿法以及火法冶金虽然有效地回收了锂电池中的贵重金属,机械活化法对重金属的回收具有较大的改善效果,但是对于选择性地高效回收单个金属的研究相对较少;此外,机械化学活化过程中,研究人员选用的共磨剂多为螯合型或酸性试剂,对共磨剂的研究还不是很全面,还要对共磨剂展开研究。本研究选用非强酸碱试剂与LFP共磨,以分解产物为H2O和O2的H2O2作为浸出剂,建立了机械化学活化协同湿法冶金的Li回收工艺,并对Fe在整个浸出过程中的浸出表现,以及Fe对Li选择性浸出的影响与作用进行了探究,为机械化学活化选择性回收废旧锂电池中的贵重金属提供参考。
全文HTML
-
废弃磷酸铁锂电池由广东某动力公司提供。实验所用试剂盐酸、硫酸铵、过氧化氢、磷酸三钠均为分析纯(国药集团化学试剂公司)。
-
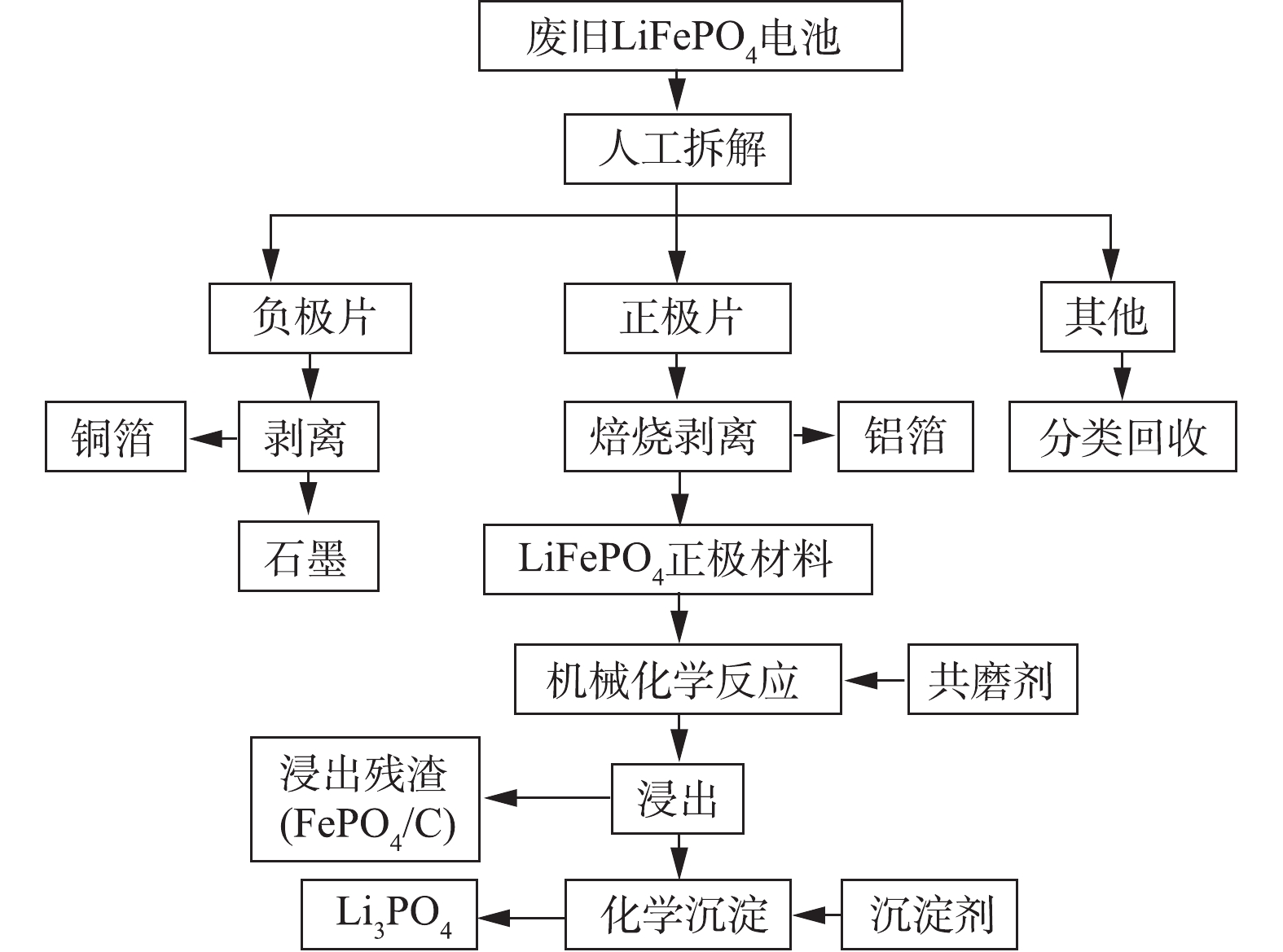
首先,将废弃磷酸铁锂电池在饱和NaCl溶液中浸泡48 h放电;然后,进行拆卸将电池单体分离成正、负极极片。负极片放入水中约2 min分离负极材料与铜箔;正极片切成2 cm×4 cm的小长条,放入管式炉中,在N2氛围下360 ℃焙烧2 h分离正极材料与铝箔,所得粉末作为本研究的目标回收原料。取一定量的供试粉末用HCl溶解,用ICP-OES测得正极材料中Fe含量为27.71%、P为13.84%、Li为2.90%、Al为0.07%、Cu为0.01%。通过行星式球磨机(长沙德科仪器有限公司)将正极材料与共磨剂进行MCA,MCA后的样品用去离子水清洗转入圆底锥形瓶,随后加入H2O2进行浸出实验(浸出零点以加入H2O2为准);浸出完成后过滤分离浸出液及浸出渣,向浸出液中加入Na3PO4进行沉淀回收Li。本研究的实验流程如图1所示。
-
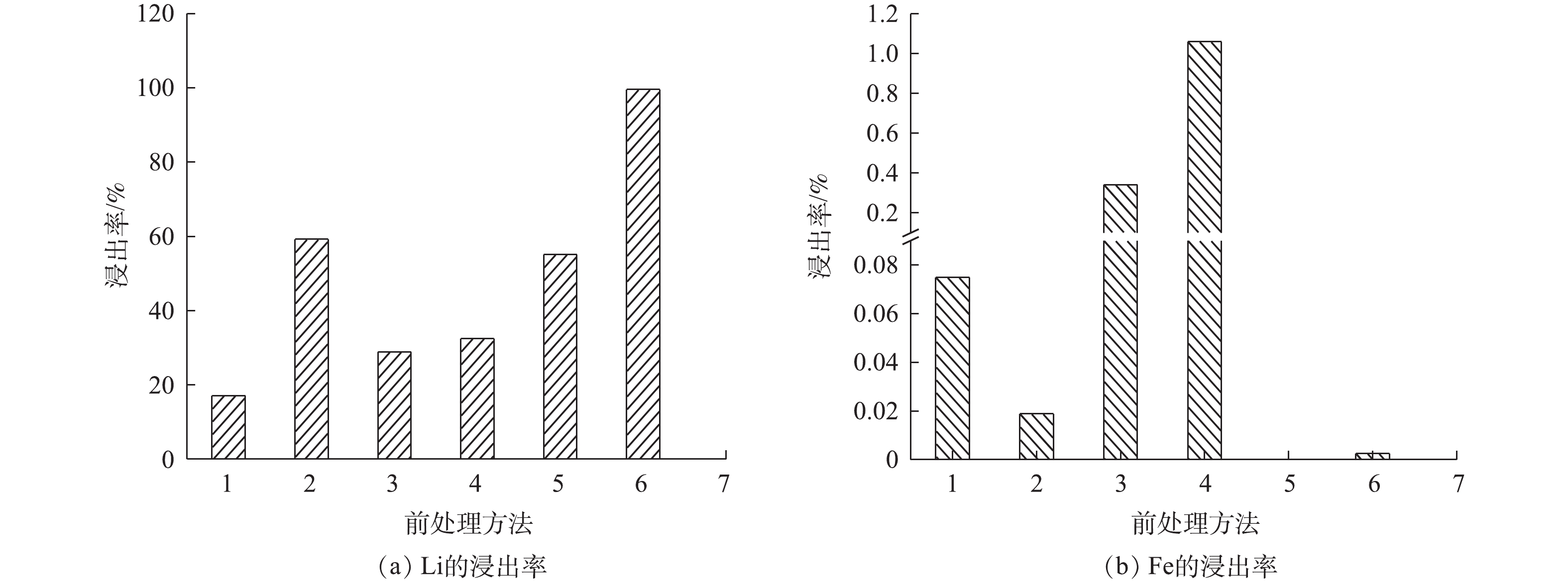
1)无机械化学活化前处理浸出实验。称取1 g正极材料进行浸出实验,该组编号为1;称取1 g正极材料、0.837 g (NH4)2SO4进行浸出实验,该组编号为2。2组实验浸出剂的体积分数为4%、浸出时间为60 min、浸出温度为80 ℃。
2)机械化学活化前处理浸出实验。取1 g正极材料进行干磨后进行浸出,该组编号为3;取1 g正极材料、0.6 g水进行湿磨后进行浸出,该组编号为4。2组实验的球磨时间为30 min、转速为600 r·min−1、球料比为10:1(g:g)、浸出剂的体积分数为4%、浸出时间为60 min、浸出温度为80 ℃。
3)添加共磨剂进行机械化学活化前处理浸出实验。取1 g正极材料、0.837 g (NH4)2SO4混合干磨后进行浸出,该组编号为5;取1 g正极材料、0.837 g (NH4)2SO4、0.6 g水,混合湿磨后进行浸出,该组编号为6。2组实验的球磨时间为30 min、转速为600 r·min−1、球料比为10:1(g:g)、浸出剂的体积分数为4%、浸出时间为60 min、浸出温度为80 ℃。
-
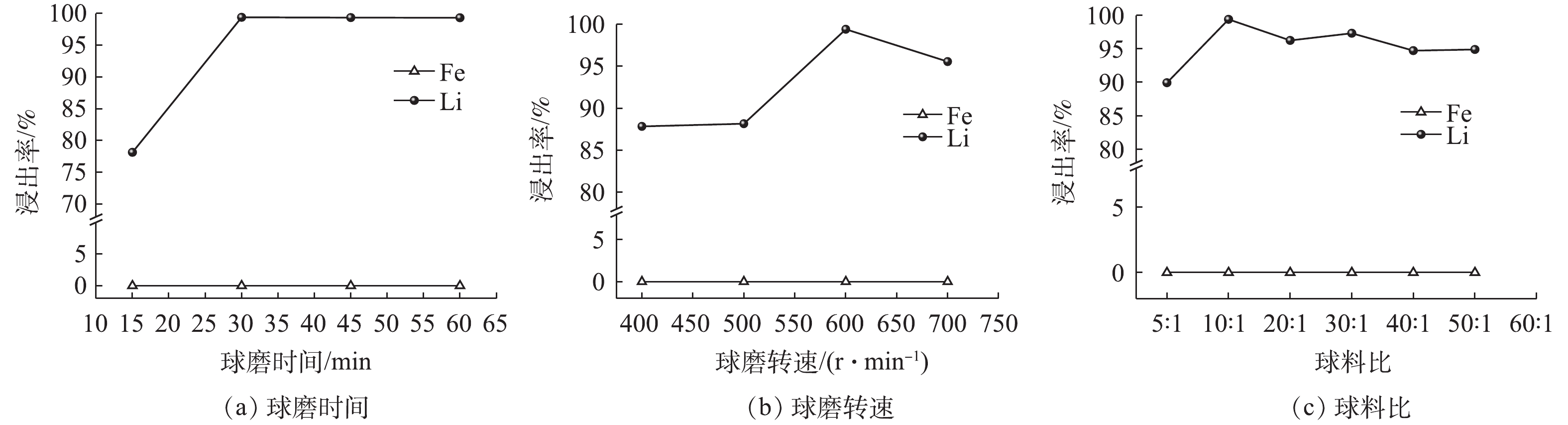
称取1 g正极材料、0.837 g (NH4)2SO4、0.6 g水混合湿磨后进行浸出,浸出剂的体积分数为4%、浸出时间为60 min、浸出温度为80 ℃。实验设定的变化参数包括:时间(15、30、45、60 min)、转速(400、500、600、700 r·min−1)、球料比(5∶1、10∶1、20∶1、30∶1、40∶1、50∶1 (g∶g))。
-
该实验称取5 g正极材料、4.188 g (NH4)2SO4、3 g水混合湿磨后进行浸出,球磨时间、球料比、转速的参数为1.4节实验选出的最佳条件。实验设定的变化参数包括:浸出剂的体积分数(1%、2%、3%、4%、5%、6%)、温度(25、40、50、60、70、80 ℃)、固液比(30∶1、40∶1、50∶1、60∶1、70∶1(g∶L))、时间(10、20、30、40、50、60 min)。
-
采用电感耦合等离子体发射光谱仪(ICP-OES,American Perkin-Elmer Company Optima 8300)测定溶液中金属元素的含量;采用激光粒度仪(MS,Masterizer 2000)表征球磨前、后正极材料的粒径分布;通过X射线衍射仪(XRD,X'pert PRO,PANalytical)表征固体样品的晶体结构,其扫描速度和扫描角度(2θ)分别为6 (°)·min−1和5°~90°;用场发射扫描电子显微镜(SEM,Merlin Compact,Zeiss)观察固体颗粒的形貌;采用X射线光电子能谱仪(XPS,ESCALAB 250Xi,Thermo Scientific)分析浸出后固体样品中元素价态的变化。
1.1. 实验原料及试剂
1.2. 废旧磷酸铁锂电池回收处理流程
1.3. 不同活化方式对浸出率的影响
1.4. 球磨参数对浸出率的影响
1.5. 浸出参数对浸出率的影响
1.6. 分析方法
-
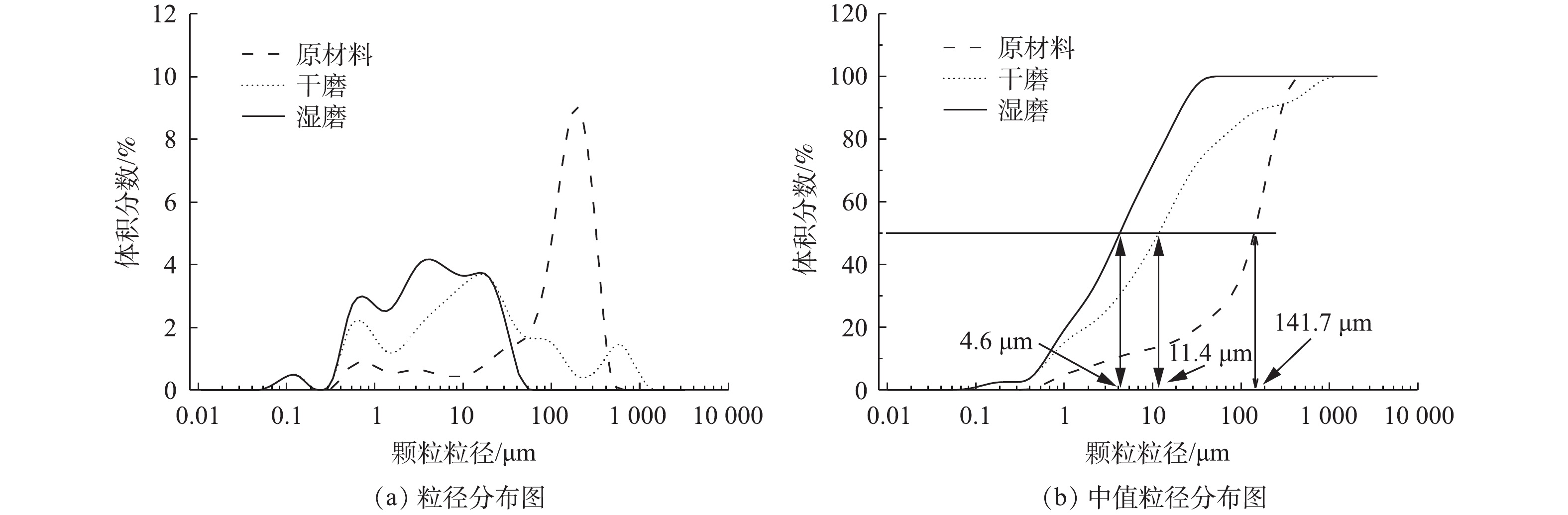
样品的粒径分布见图2。从图2(a)中可以看出,原始LFP颗粒的粒径分布区域主要集中在100~1 000 μm;经过机械化学活化30 min后,湿法MCA样品的粒径分布区域则集中在100 μm以下,干法MCA样品的粒径分布区域多数集中在100 μm以下,有少量分布于100~1 000 μm之间。从图2(b)中可以看出,原始LFP颗粒的中值粒径(中值粒径:D50)为141.7 μm,湿法MCA样品的D50为4.6 μm,干法MCA样品的D50为11.4 μm。由此可知,MCA可显著降低样品的粒径。
样品的形貌见图3。从图3中可以看出,湿磨样品分散均匀,而干磨样品则呈现出团聚现象。团聚现象不利于颗粒分散,会造成颗粒粒径较大;本研究结果表明,添加共磨剂进行湿磨时可有效抑制颗粒的团聚,有研究表明,湿磨比干磨更能有效地将各种材料的粒度降低到亚微米级[22]。其主要原因是,干磨过程中会发生团聚,导致D50增加,而湿法球磨由于具备一定的流动性,可有效减少团聚现象的产生[23-24]。
MCA对物质晶体结构的影响见图4。从图4(a)可以看出,正极材料中只存在LiFePO4的晶相,由图4(b)(图4(a)的区域放大图)可以看出,MCA 30 min后,正极材料的部分晶格面发生错位。其中,(220)、(020)、(011)晶面的衍射角减小,说明其晶面间距增大;(120)、(111)、(131)、(211)晶面的衍射角增大,说明其晶面间距减小。XRD结果表明,MCA产生的剪切、撞击和挤压等机械应力[16]可以造成正极材料的晶格扭曲,从而改变LFP的晶体结构,而湿法MCA中由于物料流动性较强,与共磨剂接触充分,使得其错位率高于干法MCA。
MCA处理及共磨剂对Li、Fe浸出率的影响见图5。从图5(a)可以看出,没有经过MCA的样品,用(NH4)2SO4+H2O2作为复合浸出剂比只用H2O2作为浸出剂时,Li的浸出率提高了42.08%;以(NH4)2SO4作为共磨剂与LFP进行干磨后用H2O2溶液浸出比不添加(NH4)2SO4进行MCA后用H2O2溶液浸出,Li的浸出率至少提高23.64%。由此可知,(NH4)2SO4的存在可以有效地促进Li的浸出率。以(NH4)2SO4作为共磨剂进行湿磨后用H2O2溶液浸出,湿法共磨时Li的浸出率比没有MCA时提高40.34%,比干法共磨提高44.40%。由此可知,湿法共磨可以有效的提升Li的浸出率。其原因在于,干法MCA时,样品粘附在球磨罐璧上发生团聚现象,而湿法MCA没有研磨死角,物料流动性较强,可有效避免颗粒的团聚,且颗粒粒径相对较小[24],与共磨剂接触充分促进了反应的进行。从图5(b)可以看出,在所有处理方法中,Fe的最高浸出率为1.2%,由此可知,Li浸出到溶液中以可溶态存在时,Fe是以不可溶的固态形式存在的。
-
球磨参数对Li、Fe浸出率的影响见图6。从图6(a)可以看出,Fe在整个过程中没有浸出到溶液中;而Li的浸出率则随着球磨时间的增加而增长,当球磨时间为15 min时,Li的浸出率为78.14%;球磨时间为30 min时,Li的浸出率99.40%。由此可以认为,延长球磨时间会为反应系统提供更多的机械能,促使晶格扭曲[25]。继续延长球磨时间,Li的浸出率没有发生改变,而球磨时间更长意味着能耗更高,因此,选择球磨时间为30 min。
球磨转速对Li、Fe浸出率的影响见图6(b)。从图6(b)可以看出,Fe在整个过程中没有浸出到溶液中;而Li的浸出率则随着转速的增加呈现先浸出率增加后降低的趋势,当转速从400 r·min−1 增加至600 r·min−1时,Li的浸出率从87.83%增至99.40%;当转速为700 r·min−1时,浸出率下降为95.55%。这是因为,增加转速可降低浸出体系表观活化能和反应级数,增强活化效果[26-27]。因此,增加转速可强化球磨浸出过程,促进Li浸出;但转速过大时,球的离心力大于重力,使得机械活化的效果减弱[28],Li浸出率降低。综合比较Li浸出率及能耗成本,选择最佳转速为600 r·min−1。
球磨球料比对Li、Fe浸出率的影响见图6(c)。从图6(c)可以看出,Fe在整个过程中没有浸出到溶液中;而Li的浸出率随着球料比的增加先达到最高点后呈现波动下降,当球料比为5∶1(g∶g)时,Li的浸出率为89.93%;球料比10∶1(g∶g)时,Li的浸出率达到最佳;继续增加球料比,Li的浸出率呈现下降趋势。这是因为,球料比较小时,产生的有效碰撞次数不够,而较大的球料比则会则会占据系统中物料的空间[25],有效碰撞次数降低,使得物料在预定时间内不能充分共磨。综合比较Li浸出率及及球磨体系的损耗,选择最佳球料比为10∶1。
-
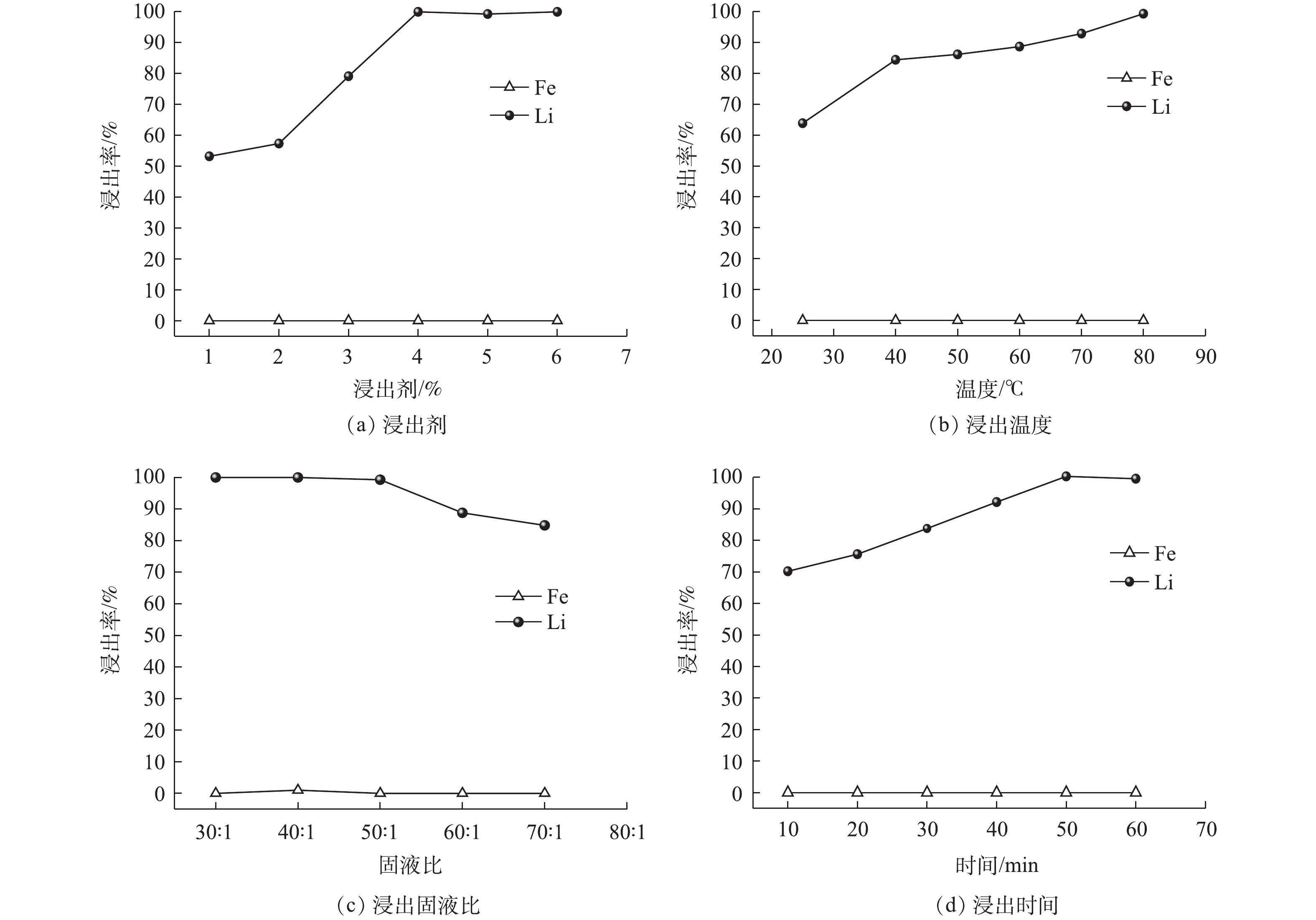
浸出参数对Li、Fe浸出率的影响见图7。从图7(a)可以看出,整个浸出过程中Fe的浸出率为0,Li的浸出率随着浸出剂用量增加而提高,当H2O2<4%(体积分数)时,Li的浸出率低于80%;当H2O2≥4%时,Li的浸出率达到99.80%。这是由于H2O2的用量会影响反应时·OH的产生量[29],H2O2<4%时,产生的·OH较少,氧化能力不足;增加H2O2后,·OH数量增加,氧化能力增强,促进Fe2+向Fe3+的转化,同时也促进了LFP晶格中Li的脱嵌。考虑到过量的H2O2容易产生大量泡沫使得反应物溢出反应器,且过量的H2O2会与·OH作用生成·
O−2 ,·O−2 与H2O2再进一步发生反应使得H2O2发生自耗[30],从而降低其氧化能力影响Li的浸出率。因此,选择H2O2的用量为4%体积分数。浸出温度对Li浸出率的影响见图7(b)。从图7(b)可以看出,整个浸出过程中Fe的浸出率为0,而Li的浸出率随浸出温度升高而增大,在40 ℃时Li的浸出率达到84.39%;随着温度提高,浸出率缓慢增加,在80 ℃时达到99.31%。温度较低时,离子的扩散速率较慢,反应速度缓慢,从而影响了Li的浸出速率;此外,H2O2需在较高的温度下才能产生·OH[31],且高温在降低液体黏度的同时可以加速离子的扩散并增加活化分子的数量,加速氧化剂的传质速度,提高Li的浸出率。考虑到能耗及高温带来的溶液蒸发,选择浸出温度为80 ℃。
浸出固液比对Li浸出率的影响见图7(c)。从图7(c)可以看出,整个浸出过程中Fe的浸出率为0,而Li的浸出率随浸出固液比增加而下降,当固液比<50∶1(g∶L)时,Li的浸出率都维持在99.30%以上;当固液比>50∶1(g∶L)时,浸出率低于90%。这是由于,低的固液比有利于物料的分散,增加粉末与浸出液的接触面积,促进反应的进行;而固液比较高时,溶液中颗粒的浓度过高,使得物料的流动受到物料之间黏度的影响抑制扩散[32],从而导致了浸出率的下降。选择最优固液比为50∶1(g∶L)。
浸出时间对Li浸出率的影响见图7(d)。从图7(d)可以看出,整个浸出过程中Fe的浸出率为0,而Li的浸出率随浸出时间增加而增加,10 min可以浸出70.23%的Li;50 min时Li的浸出率可达到99.55%。这说明Li的高效浸出需要一定的反应时间,而浸出时间的延长,会使得反应能耗升高。为了最大限度地提高Li的浸出率,50 min为最佳浸出时间。
-
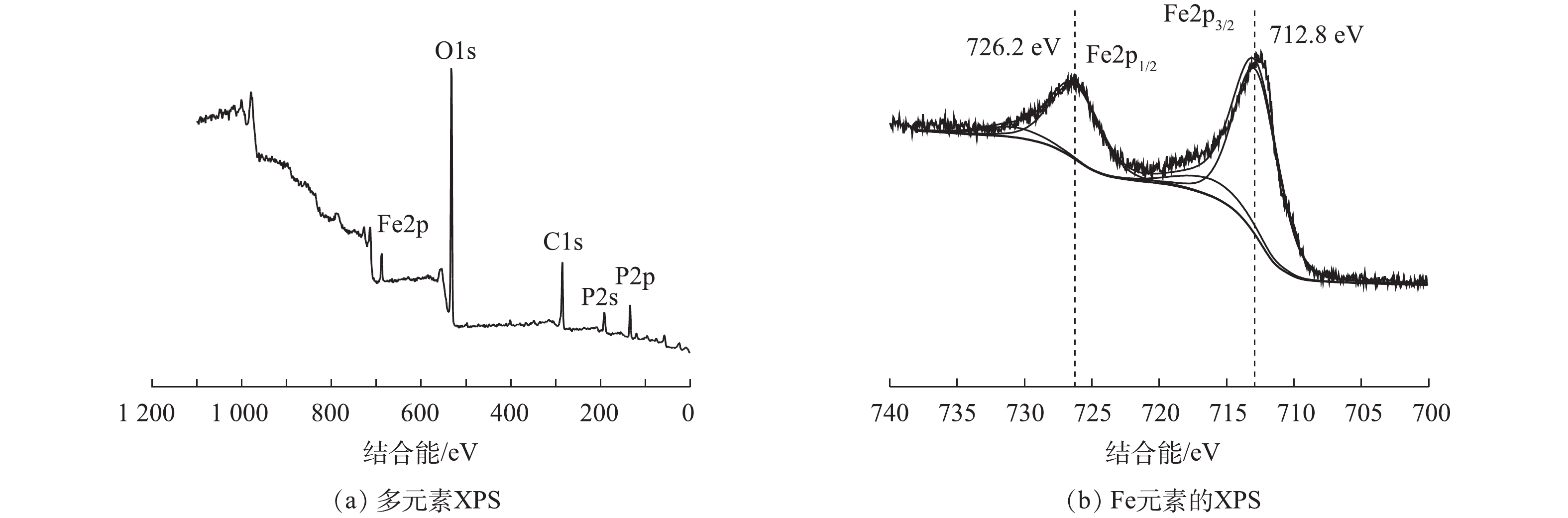
为了阐明反应原理,对浸出完成后的浸出残渣及将浸出液烘干后进行XRD分析表征,对浸出残渣进行了XPS分析表征,结果如图8和图9所示。从图8(a)可以看出,残渣的X射线衍射峰与FePO4(PDF#34-0134)的峰完全吻合,没有检测到其他物质的衍射峰。残渣的XPS图谱如图9所示,132.7、531.2和711.5 eV位置处的特征峰分别对应于P2p、O1s和Fe2p[33],XPS谱图中Fe2p3/2和Fe2p1/2峰由主峰和卫星峰组成,726.2 eV为Fe3+在Fe2p1/2的特征峰,712.8 eV 为Fe2+在Fe2p3/2的特征峰[34],没有观察到Fe2+的特征峰,只观察到Fe3+的特征峰,说明浸出残渣中Fe是以Fe3+化合物形式存在。有研究认为,Fe2+转变为Fe3+在锂的选择性浸出中起重要作用[35]。从图8(b)中可以看出,浸出液烘干后的化合物XRD图谱中只检索到Li(NH4)SO4和(NH4)2SO4。这说明硫酸铵过量,在反应过程中(NH4)2SO4与Li+络合形成Li(NH4)SO4。由此,浸出反应方程式如式(1)所示。
综上所述,LFP中Li的选择性浸出原理为:在机械活化过程中的剪切、撞击和挤压等机械力对晶体结构的冲击作用下[18],颗粒尺寸减小,不断出现新的活性表面,暴露的化学反应活性的活性位点越多,在浸出过程中H2O2的作用下氧化LFP中的Fe2+成为Fe3+;FePO4的溶度积Ksp=1.3×10−27,远小于Li3PO4的Ksp=2.37×10−4,因此,Fe3+优先与
PO3−4 结合生成FePO4沉淀,FePO4在酸性条件下是热力学稳定的[35],不会再发生溶解,这也解释了浸出液中没有Fe;而在酸性条件下,Li+不会与PO3−4 结合生成Li3PO4沉淀,这说明了Fe元素化学价态的变化使得Li在LFP的晶格中处于一种游离的状态,在热力学的作用下,游离态的Li从晶格中脱嵌以可溶态的Li+进入溶液中,实现Li的选择性浸出。正极材料中微量的Al、Cu等元素对Li的选择性浸出没有影响。 -
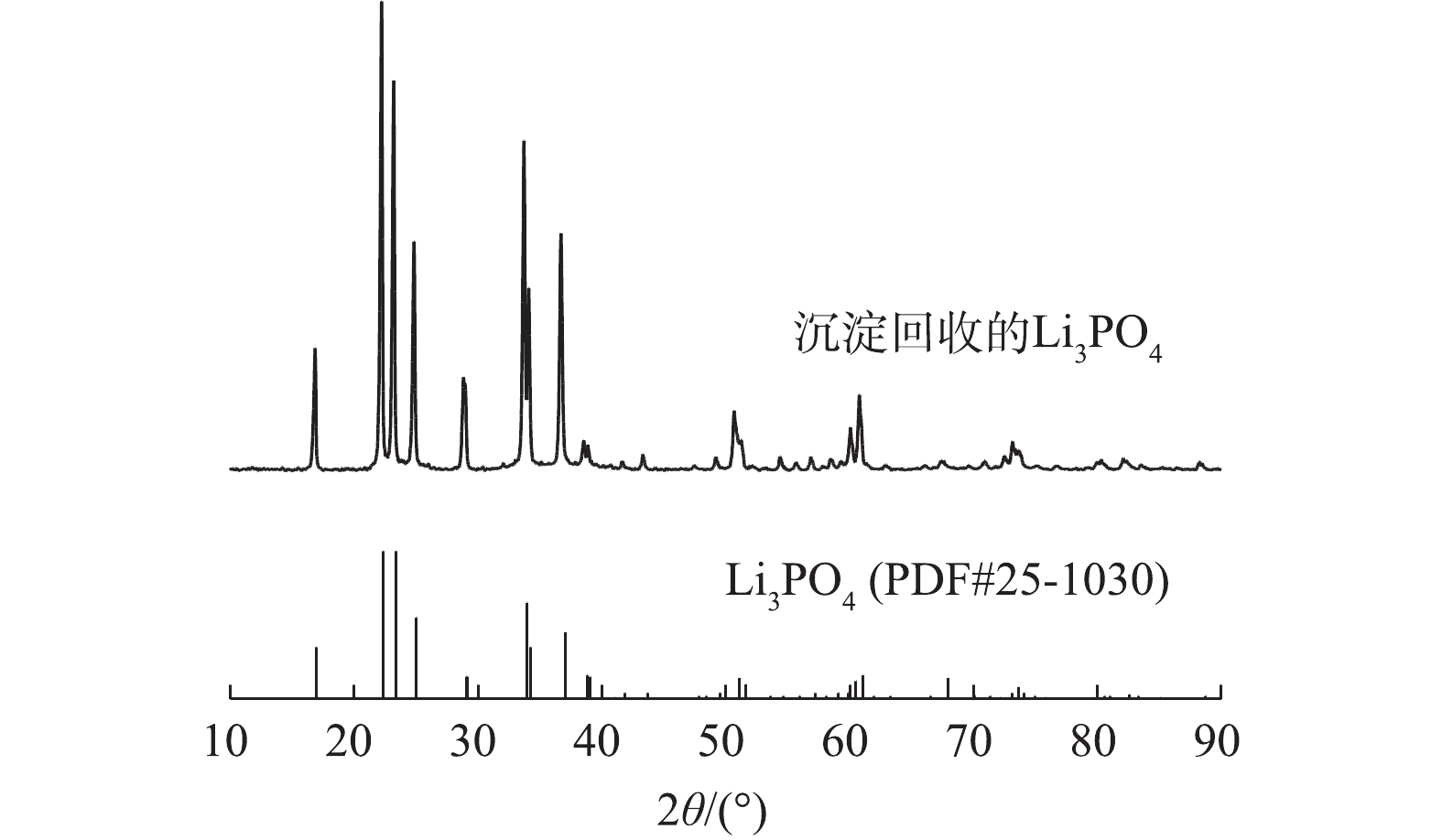
向含Li溶液中加入氢氧化钠,将溶液的pH调节为11~13,再加入化学计量比微过量的Na3PO4,在90 ℃下搅拌约1 h后过滤收集沉淀物进行Li的回收。沉淀物的XRD图谱如图10所示,回收产物的所有衍射峰与Li3PO4标准图谱吻合,未观察到其他物质的衍射峰,结晶度很高。由表1的对比结果可知,本研究在同时实现Li的选择性以及高效率浸出方面具有一定优势。对Li3PO4进行纯度分析,ICP-OES测得纯度为95.23%,具备较高的纯度。Li3PO4的晶度以及纯度分析结果表明该产品具备作为合成磷酸铁锂正极材料锂源的潜力。
2.1. 不同活化方式对原料性质及浸出率的影响
2.2. 球磨参数对元素浸出率的影响
2.3. 浸出参数对元素浸出率的影响
2.4. 浸出反应机理分析
2.5. 锂的回收效率
-
1)利用机械化学法,在室温下添加摩尔比(NH4)2SO4∶LiFePO4为1∶1、球料比为10∶1、质量比LiFePO4∶H2O为5∶3的混合材料进行湿磨30 min,可以有效地破坏LiFePO4的晶体结构,造成晶面错位。
2) LiFePO4中元素的最佳的浸出条件是:H2O2体积分数为4%、浸出温度为80 ℃、浸出固液比为50∶1(g∶L)、浸出时间为50 min。浸出后Li的浸出率为99.55%,Fe的浸出率为0;回收的Li3PO4结晶度较高,晶体结构良好,具备作为再制备LiFePO4电池正极材料的潜力。
3)结合机械活化前后及浸出前后固体样品的晶体、形貌、价态等表征结果,阐述了MCA对晶体结构的影响以及浸出机理:MCA使得LiFePO4晶体尺寸减小,晶体缺陷与化学活性增强,改善了Li的浸出特性,促进Li的浸出。



 下载:
下载: