-
随着我国工业的快速发展,广泛应用于电镀、金属加工、制革、染料、钢铁和化工等行业的铬(Cr)已成为一种主要的工业场地土壤污染物[1-2]。Cr主要是以六价铬Cr(Ⅵ)和三价铬Cr(Ⅲ)两种价态形式存在,而且Cr(Ⅵ)的毒性是Cr(Ⅲ)的500倍[3]。Cr(Ⅵ)活性较高,不易被土壤吸附,容易对环境造成影响。因此,土壤中重金属Cr(Ⅵ)的去除已成为污染土壤修复的一个重要课题[4-6]。
Cr污染场地的治理途径主要有两种:一是将Cr从被污染的土壤及地下水中清除;二是改变Cr在土壤中的赋存形态,将Cr(Ⅵ)还原为Cr(Ⅲ),降低其毒性。但是,第二种修复技术并未将Cr(Ⅵ)从土壤中彻底去除,存在修复后受扰动被再次氧化,释放到环境的风险。同时,常用还原剂(如Na2S2O8等)存在二次污染的风险。土壤电动力学修复技术能有效去除土壤中的Cr(Ⅵ),而且具有二次污染风险小的优点,是目前Cr(Ⅵ)污染土壤修复领域研究的热点。
传统电动修复工艺一般从控制土壤pH[7-9]、增加土壤中污染物的溶解能力[10-12]、电动修复与其他修复技术联用[13-14]、优化电极空间构型[15]、在电极室与土壤室之间添加可渗透性反应墙(PRB)[16-17]等几个方面提高效率。Cr(Ⅵ)常以
CrO2−4 、Cr2O2−7 、HCrO−4 等的可溶形态在土壤中迁移、扩散[18]。这些带负电的离子在电动力的驱动下向阳极迁移[19-20]。但是,在实际Cr(Ⅵ)污染场地中,常常同时存在Cl−、NO−3 等负电的离子[21]。这些带负电离子的存在,会增加修复过程中电能的消耗,影响Cr(Ⅵ)的去除效率[22]。目前,多数研究都着重于调整运行参数来提高修复效率。了解土壤中常见的阴离子对Cr(Ⅵ)污染土壤电动修复效率的影响有重要意义,有必要对其进行探究,但由于真实的土壤体系中涉及的干扰离子过多,同时土壤中的还原性物质可能会将Cr (Ⅵ)还原,不便于计算单位能量损耗,因此,本文借鉴国内外学者利用黏土矿物纯体系开展研究[17]的思路,也选择在黏土矿物的纯体系中开展研究。为了明晰这些离子对电动力学修复过程中Cr(Ⅵ)迁移的影响,本研究选用高纯蒙脱石模拟Cr(Ⅵ)污染土壤。这是因为,含水率是影响电动修复效率的重要因素之一[23],相比于高岭土和伊利石,蒙脱石在配置成饱水且适用于电动修复的土壤时,含水率最接近实际污染土壤。参考已有的研究成果[24-27],选择以纯水作为电解液,在电压梯度为2 V·cm−1的条件下,选用石墨电极进行电动修复实验。同时,分别向人工配制的Cr(Ⅵ)污染蒙脱石中添加一定浓度的NaNO3、NaCl、NaOH、Na2SO4、Na2CO3、Na3PO4,探究单一阴离子在电动修复中的行为特征,分析其对Cr(Ⅵ)迁移的影响。
全文HTML
-
pH计(pH-3C型,上海越平科学仪器有限公司)、电导率仪(DDS-307型,上海越平科学仪器有限公司)、恒温鼓风干燥箱(DHG-9030A,上海一恒科学仪器有限公司)、电子天平(FA2204,上海力辰仪器科技有限公司)、火焰原子吸收分光光度计(TAS-990F型,北京普析通用仪器有限责任公司)、直流恒压电源/电流/电压表(MS-305D型,深圳迈盛科技有限公司)、水平恒温振荡器(SHA-BA型,绍兴苏珀仪器有限公司)、离心机(LC-LX-H185C型,上海力辰仪器科技有限公司)、紫外分光光度计(754N型,上海精科仪器有限公司)。
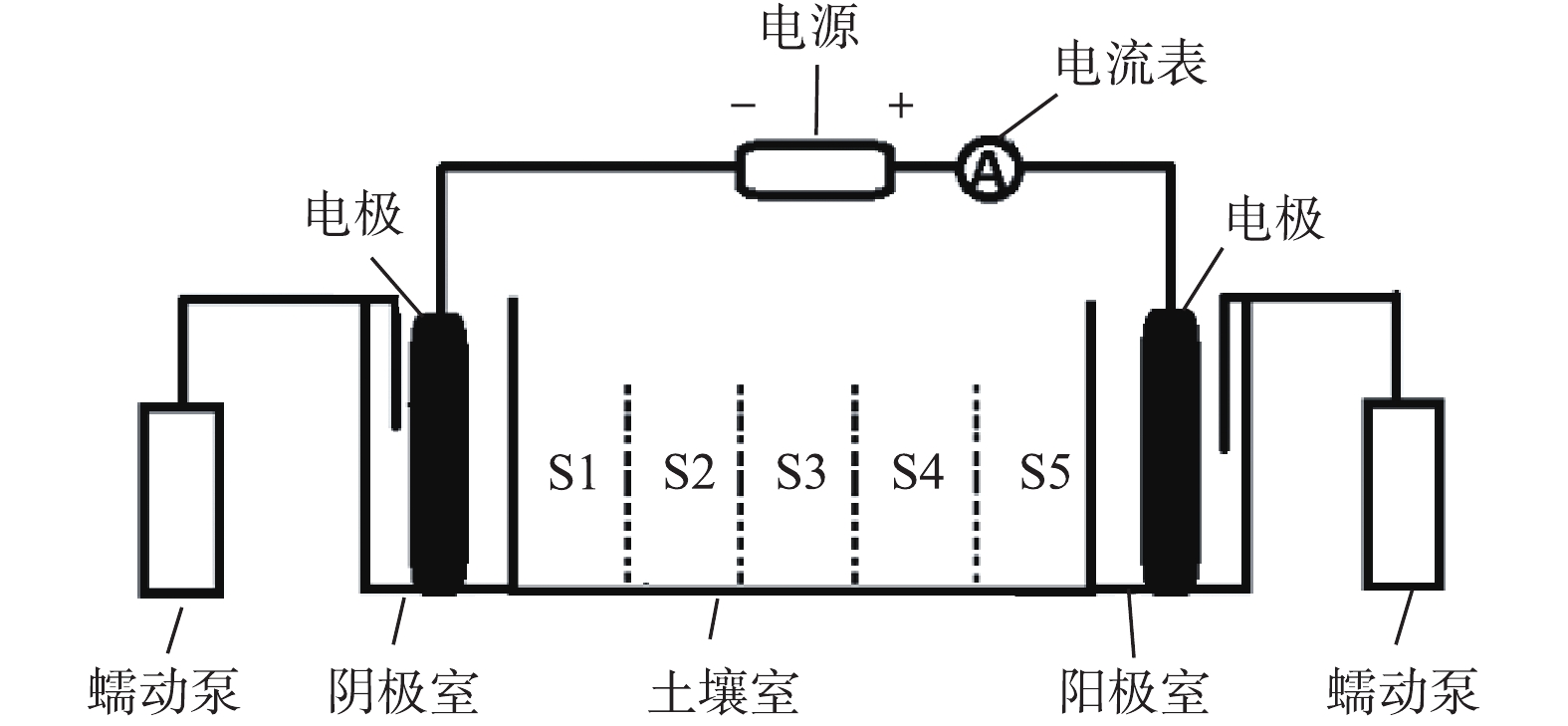
电动修复实验装置是长×宽×高为45 cm×15 cm×15 cm的自制有机玻璃槽,电极采用柱状石墨电极(L×φ=15 cm×3 cm),阴阳极室各放置3个电极,见图1。其中,土壤室尺寸为25 cm×15 cm×15 cm,阴阳极室尺寸均为10 cm×15 cm×15 cm;阴阳极室与土壤室之间均使用隔板隔开,隔板为均匀多孔的有机玻璃隔板;阴阳极隔板上附有多孔滤膜,用于防止土壤进入电极室;与阴阳极室相连的是蠕动泵,用于保证电解液的体积恒定。
-
实验试剂:重铬酸钾(K2Cr2O7)、盐酸(HCl)、硝酸(HNO3)、硫酸(H2SO4)均为优级纯;氯化钾(KCl)、磷酸(H3PO4)、二苯碳酰二肼(C13H14N4O)、氢氧化钠(NaOH)、氯化镁(MgCl2)、磷酸三钠(Na3PO4)、硝酸钠(NaNO3)、氯化钠(NaCl)、碳酸钠(Na2CO3)、硫酸钠(Na2SO4)均为分析纯。
实验供试土壤由高纯蒙脱石模拟,电导率为61 μS·cm−1,有机物质含量为0.02%,pH为8.67,矿物组成为74.8%SiO2和19.0%Al2O3,其他成分为6.2%。借鉴GUEMIZA等[28]的观点,Cr(Ⅵ)含量为2 400 mg·kg−1可用于模拟高浓度Cr(Ⅵ)污染土壤进行实验。为保证蒙脱石中Cr(Ⅵ)物质的量与目标阴离子相同,向400 mL水中分别加入0.025 mol K2Cr2O4和0.05 mol NaNO3(或0.05 mol NaCl或0.05 mol NaOH或0.05 mol Na2SO4或0.05 mol Na2CO3或0.05 mol Na3PO4),充分搅拌溶解,然后将所得的溶液加入1 kg蒙脱石中,充分搅拌混匀,放置48 h,作为供试土壤。
-
将1 kg高纯蒙脱石置于土壤室之中,铺平、压实;在阴阳2个电极室中倒入300 mL去离子水,使水平面略低于土壤界面,将柱状石墨电极连接到直流电源正负极,接通直流电源,土壤间的电压梯度为2 V·cm−1,连续工作168 h;使用蠕动泵保持阴阳极电解液的体积不变,阴阳极电极室内的电解液每24 h更换1次,每天测试阴阳极电解液中的Cr(Ⅵ)浓度和目标阴离子的浓度、电解液pH、电导率,并且记录工作电流。实验结束后,将土壤从阴极到阳极0~5、5~10、10~15、15~20、20~25 cm划分为5部分,分别记作S1、S2、S3、S4、S5;并测定不同区域的含水率、pH、电导率、Cr(Ⅵ)浓度以及目标阴离子浓度。实验分为7组,分别为Cr组(未添加其他阴离子)、CN组(添加
NO−3 )、CCl组(添加Cl−)、CO组(添加OH−)、CS组(添加SO2−4 )、CC组(添加CO2−3 )、CP组(添加PO3−4 )。 -
阴阳极电解液以及土样pH和电导率的测定参照《土壤pH值的测定 电位法》(HJ 962-2018)和《土壤 电导率的测定 电极法》(HJ 802-2016)[29]。土壤中Cr(Ⅵ)含量的测试参照《固体废物 Cr(Ⅵ)的测定 碱消解/火焰原子吸收分光光度法》(HJ 687-2014)[25]。电极室中液体的Cr(Ⅵ)浓度测试参照《水质 六价铬的测定 二苯碳酰二肼分光光度法》(GB 7467-1987)[25]。土样含水率的测试参照《土壤水分测定法》(GB 7172-1987)[30]。土样中
NO−3 、Cl−、SO2−4 、CO2−3 和PO3−4 的提取参照《固体废物浸出毒性浸出方法 水平振荡法》(HJ 557-2010)[30]。土样以及电解液中的PO3−4 的测定参照《水质 磷酸根离子的测定 离子色谱法》(HJ 669-2013)[31]、NO−3 的测定参照《水质 硝酸盐氮的测定 紫外分光光度法(试行)》(HJ/T 346-2007)[32]、SO2−4 的测定参照《水质 硫酸盐的测定 铬酸钡分光光度法》(HJ/T 342-2007)[32]、CO2−3 的测定参照《地下水质检验方法 滴定法测定碳酸根、重碳酸根和氢氧根》(DZ/T 0064.49-1993)[33]、Cl−的测定参照《水质 氯化物的测定 硝酸银滴定法》(GB 11896-1989)[32]。 -
本研究中的单位能量损耗(energy consumption,Qec)均为电能的消耗[34],计算方法见式(1)。
式中:Qec为单位能量损耗,kWh·kg−1;mCr(Ⅵ)为去除Cr(Ⅵ)的质量,mg;V为工作电压,V;I为工作电流,mA;t为修复时间,h。
1.1. 实验仪器和装置
1.2. 实验材料
1.3. 实验方法
1.4. 分析方法
1.5. 单位能量损耗
-
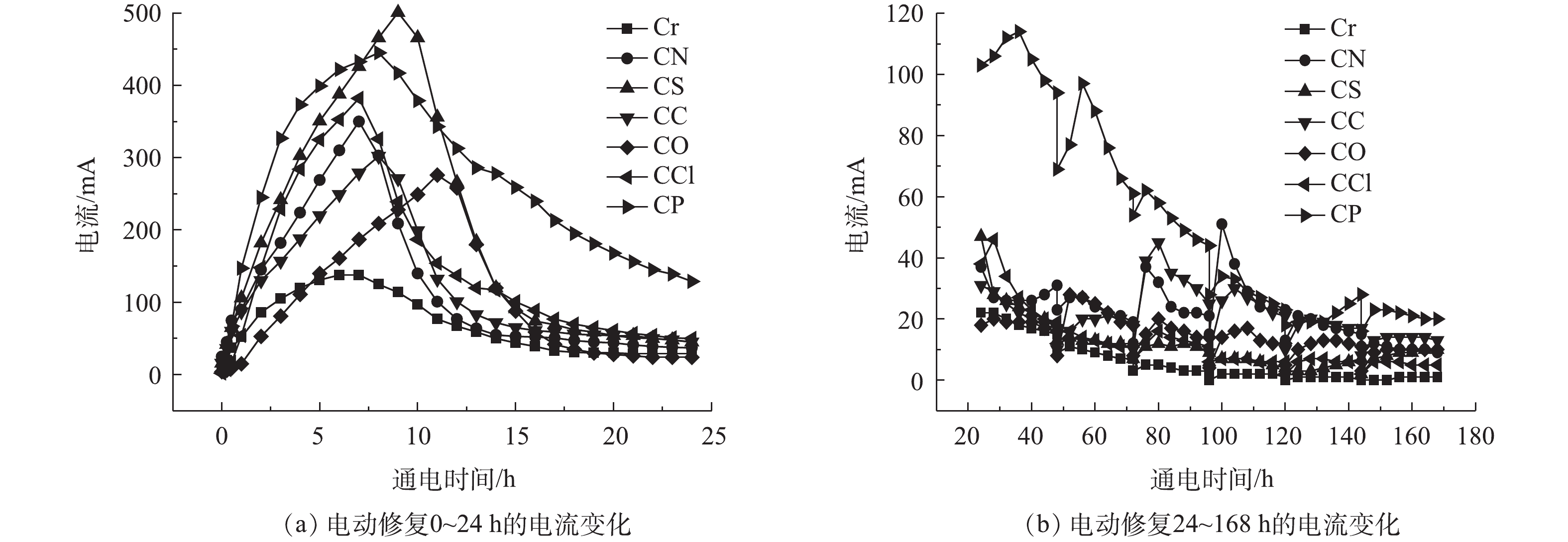
各组实验在电动修复0~24 h时间内的电流变化情况如图2所示。从图2(a)中可以看出,在第1天的修复过程中,每组实验的电流都是在短时间内快速上升,大约在5~10 h达到电流峰值,然后下降,逐渐稳定。这与电流与土壤介质溶液中存在的溶解离子浓度呈正相关的规律[35]一致。从土壤添加的成分可知,Cr组中土壤离子强度最小,因此电流峰值最低。CN和CCl组离子强度大致相同,所以电流曲线也大致相同,而CO组的电流峰值介于CCl和Cr组之间。这是因为,
Cr2O2−7 在溶液中可逐渐水解为CrO2−4 [36-37](见式(1)),导致K2Cr2O7溶液与蒙脱石混合后土壤的初始pH约为4,CO组在添加NaOH后OH−与H+反应生成H2O,降低了离子强度,导致电流峰值低于CCl组。相比Cr组,CO组中存在Na+,离子强度较高,因此电流峰值高于Cr组。CC组的电流峰值略低于CN组。这是因为,添加到土壤中的CO2−3 与土壤中存在的H+反应生成HCO−3 (见式(2)),降低了离子强度;而且,随着时间的积累,阳极电解水[38]产生的H+会进入土壤使HCO−3 转化为CO2和H2O(见式(3)),进一步减小电流。CS组的电流峰值最高,这与CS组离子强度较大,而且SO2−4 也会以溶解离子形态迁移有关。CP组离子总量大,所以电流较高,但是CP组峰值小于CS组。这可能是因为CP组中离子迁移快,当电流达到峰值时,CP组较CS组有更多的离子从土壤室迁移出来;同时,CP组中PO3−4 的酸缓冲能力能降低系统中H+浓度。因此,CP组电流峰值小于CS组。实验时间进行到24~168 h的电流变化如图2(b)所示。由于每天更换电解液移除了电极室中积累的离子,导致两极间的离子浓度降低,因此更换电解液前后产生了电流的巨变。随着阴阳两极电解水过程的进行,产生了H+和OH−,电流逐渐增大而达到峰值,当大部分可移动离子迁移出土壤后,电流出现相应的降低[32]。Cr、CS、CCl、CO组总体呈逐渐下降的趋势,CN组和CC组在72 h后电流的突然增大。这是因为,在实验前期发生了离子的聚焦现象[39-40],阻碍了离子的迁移;而随着实验的进行,土壤逐渐酸化,电渗流[41]对离子迁移的作用增强,本应该向阳极移动的阴离子随着电渗流向阴极回流,聚焦现象逐渐缓解,土壤中的离子分布趋向平均,导致电流不减反增。CP组电流一直高于其他组。这是因为,CP组土壤中的离子分布均匀,土壤不同位置离子强度相近,更利于离子的迁移,未出现明显的聚焦现象。
-
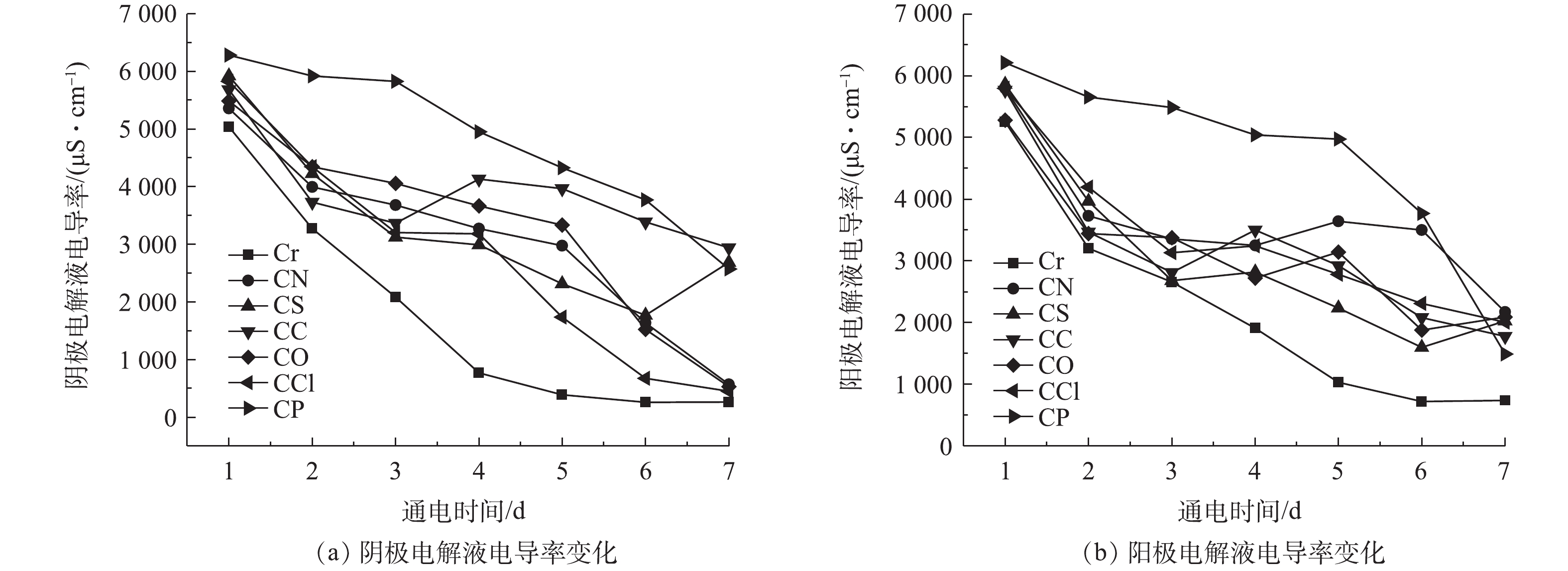
实验过程中阴、阳两极电解液的电导率变化情况如图3所示。综合图3(a)和图3(b)可知,电导率随着每天更换电解液整体呈下降趋势,部分实验组在实验进行时有电导率回升的现象。这是因为,聚焦现象逐渐的缓解,电流增大,阴阳两极电解水更强烈,产生了更多离子[4],使电导率上升。实验的前6 d,CP组阴阳极电解液的电导率大于其他组,说明CP组中离子的迁移速度快,更多的离子迁移到电极室;同时,CP组电流大,电极室中产生的H+或者OH−更多,因此电解液的电导率高于其他组。
土壤电导率直接反映了该区域的离子强度。图4为实验前后土壤电导率的变化。土壤电导率高,表明此区域发生了离子聚焦现象。Cr组、CN组、CCl组、CS组有很明显的聚焦现象发生。这4组实验中都存在某一区域土壤电导率远高于初始电导率的现象,离子聚集的区域电阻相对较小,这片区域的分压也会随之减小,导致电迁移力下降;当离子经过此区域时移动减缓,于是积累的离子越来越多,导致Cr(Ⅵ)的去除效率下降。CO组、CC组和CP组的聚焦现象不明显。这是因为,OH−、
CO2−3 和PO3−4 的酸缓冲作用,中和了土壤体系中的H+,降低了离子浓度。 -
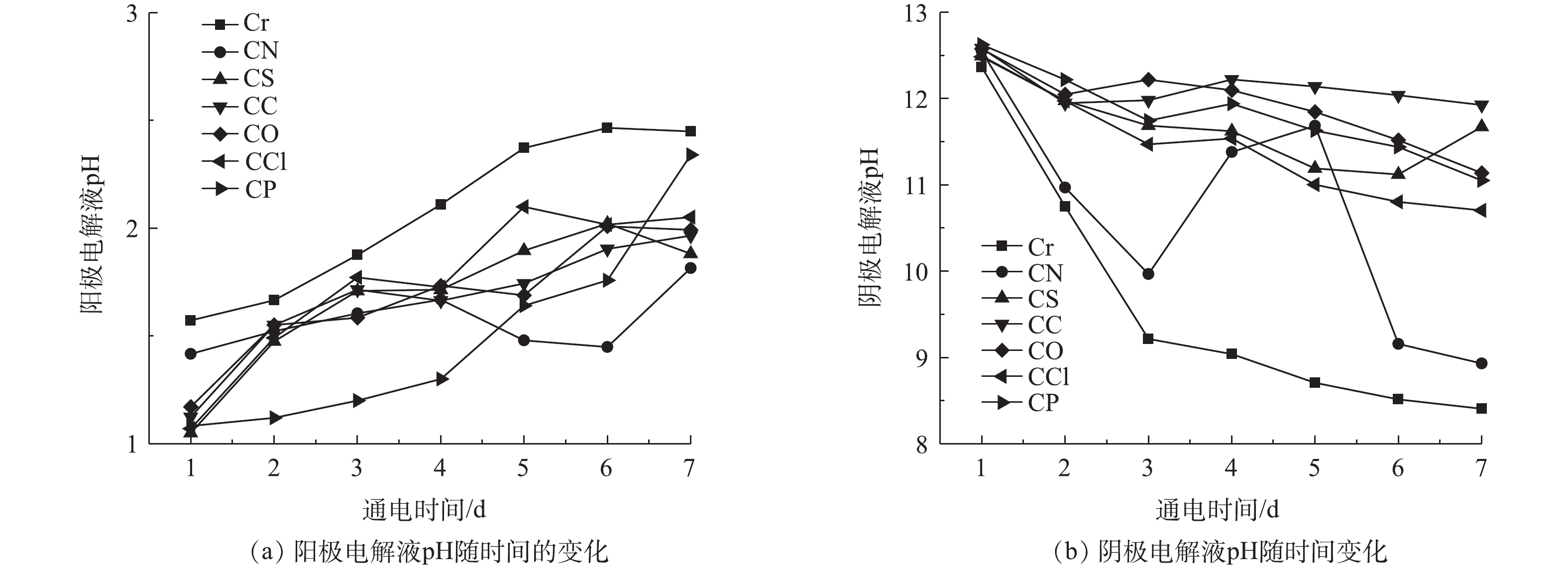
实验过程中阴阳极电解液的pH变化如图5所示。综合图5(a)和图5(b)可知,电解液pH与电流大小变化密切相关,电流大,阴极产生更多的OH−,阳极产生更多H+[42],从而影响pH。
土壤pH变化过程见图6,由于阴极产生了OH−,所以靠近阴极的S1区域pH普遍高于其他部分。理论上,阳极产生的H+进入土壤会使得靠近阳极的S5区域pH很低。但是,S5区域pH略高于土壤中间部分。这是因为,多数实验组都发生了聚焦现象,导致H+迁移到聚焦处时移动速度减缓,也聚集在此区域,使得聚焦区域pH降低,低于S5区域。从图6中还能发现,S2、S3、S4、S5区域的pH均低于初始值。这是因为,H+电迁移速率大于OH−[43],导致H+和OH−汇合的区域不是土壤的中间区域,而是更靠近阴极的区域。
-
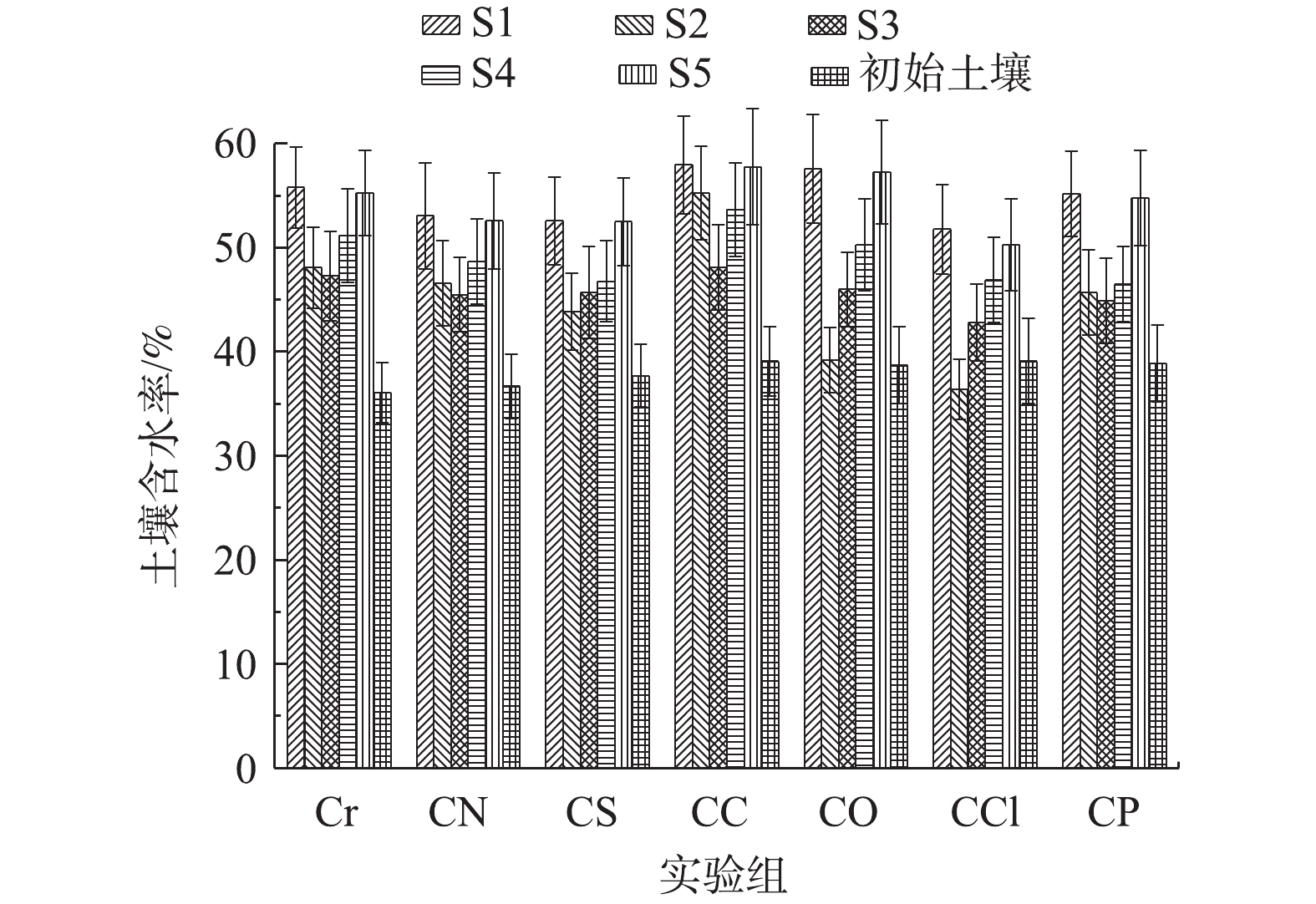
实验前后土壤含水率变化如图7所示。电动修复后,各部分土壤含水率较初始值均有增加,靠近阴阳两极的区域含水率高于其它区域。这主要是由于液体从电极室直接扩散至土壤中。含水率最低的区域出现在远离电极室的S3区域或S2区域。低含水率出现在S2区域而非S4区域是因为,随着实验的进行,土壤pH逐渐降低,电渗流向阴极流动,S2区域的水分向S1转移,S3区域距离电极室远,含水率低,从S3向S2通过电渗流输送的水分较少,而S5区域的含水率很高,因此通过电渗流向S4输送的水分较多,导致S4的含水率大于S2。
-
在本实验过程中,几乎所有的Cr(Ⅵ)都在阳极室收集并去除,迁移到阴极室的Cr(Ⅵ)几乎为0。这是因为,Cr(Ⅵ)以含氧酸根的阴离子形式存在,在电迁移的作用下向阳极移动,尽管电渗流向阴极,但在实验初期Cr(Ⅵ)浓度很高,电迁移对Cr(Ⅵ)的作用远远大于电渗流[38]。
由表1中可以看出,随着时间的增加,每天移除的Cr(Ⅵ)大体呈下降趋势。CP组第1天的去除量领先于其他实验组;CO组、CC组和CS组紧随其后;CCl组、CN组和Cr组分别为最后3位。这是因为,土壤对Cr(Ⅵ)的吸附能力随pH增加而降低[13],CP组、CO组和CC组的土壤初始pH都接近7,而其他组pH约为4。所以CP组、CO组和CC组中的Cr(Ⅵ)更容易解吸。同时,CP组离子强度最高,且电动修复过程中未出现明显的聚焦现象,电流持续处于高水平状态。因此,CP组去除量最大。CS组离子强度也比CCl组、CN组和Cr组高,Cr(Ⅵ)的迁移相对较快。在实验后期,部分实验组出现了Cr(Ⅵ)的去除量突然增大的现象。这说明实验过程中的聚焦现象正在逐渐解除,不同区域土壤中可移动离子的浓度趋向于平均,加快了离子的迁移。
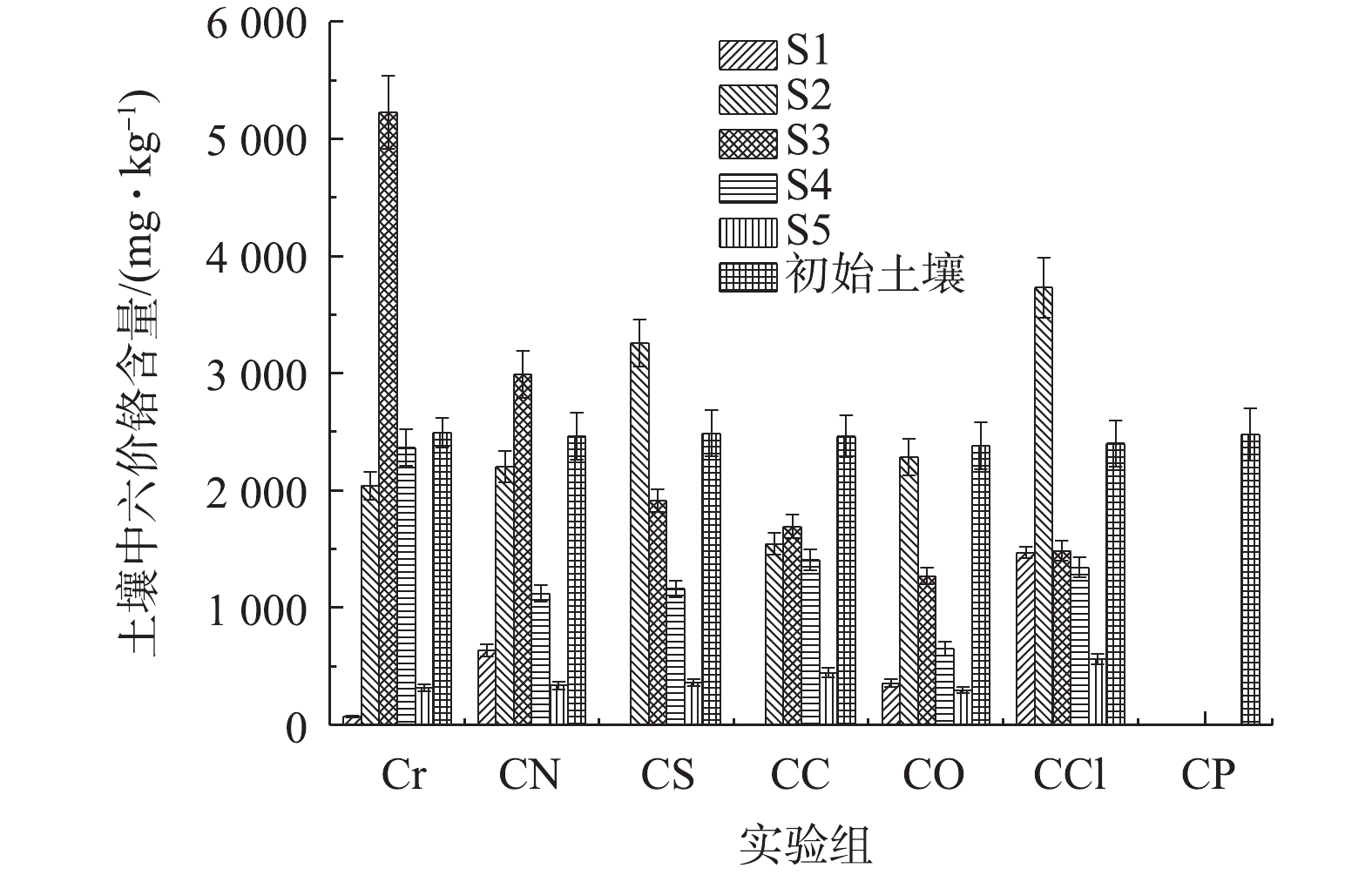
由图8可以看出,除CP组外,其他组均出现了Cr(Ⅵ)的聚焦现象。其中,Cr组、CN组、CS组和CCl组的聚焦现象较为严重,Cr(Ⅵ)分别聚集在S2或者S3区域且土壤含量均高于初始值。CO组中Cr(Ⅵ)虽然也聚集在S2区域,但含量也小于初始值,CC组中S2、S3、S4区域的Cr(Ⅵ)含量相当,均低于初始值,CC组的Cr(Ⅵ)分布较CO组均匀。这是因为,相同物质的量的
CO2−3 较OH−能结合更多的H+,有利于Cr(Ⅵ)从土壤解吸,缓解了聚焦现象。CP组的Cr(Ⅵ)去除效果很好,每部分土壤的去除率均达到99.9%以上。 -
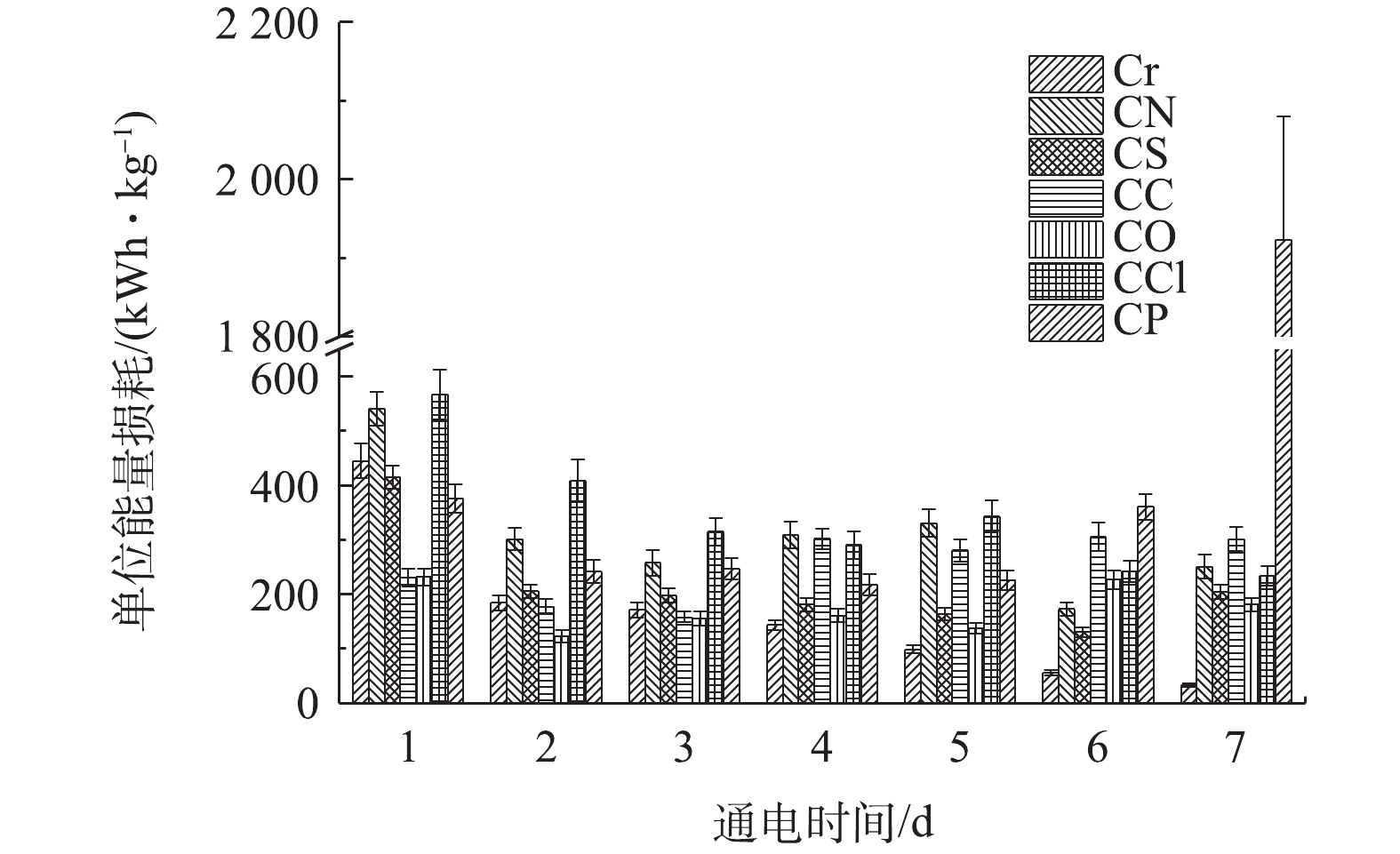
根据能量消耗模型所计算的每去除1 kg Cr(Ⅵ)所消耗的能量如图9所示。电动修复的前3 d,CC组和CO组的Qec较低。这是因为,这两组的初始pH最接近中性,一方面有利于Cr(Ⅵ)的解吸;另一方面避免了OH−或者H+对能量的消耗。而随着阳极产生H+的量增多,土壤逐渐酸化,聚焦现象发生,Qec较之前略有增加。Cr组初始离子强度低,而且初始pH很低,Cr(Ⅵ)从土壤解吸相对困难,易形成聚焦现象,所以刚开始的Qec较高。CN组和CCl组前几天Qec很高,可能是因为
NO−3 和Cl−在实验土壤中的迁移优先于Cr(Ⅵ);而且,实验土壤中H+含量也很高,导致前几天消耗的电能主要用于迁移其他离子。因此,前几天Cr(Ⅵ)的移除量较少,后两天由于其他离子减少,大部分能量用于Cr(Ⅵ)迁移,导致Qec降低。CS组的Qec较CN组和CCl组低,这可能是因为SO2−4 与Cr(Ⅵ)的迁移能力相当,或者略低于Cr(Ⅵ),使相对更多的电能用于Cr(Ⅵ)的迁移。CP组前期Qec一直处于中等水平,最后2 d的Qec明显增大。这是因为,实验前期CP组土壤离子强度高,电能用于各种离子的迁移,而且CP组的电流一直较高,阴阳极也会产生更多的OH−和H+,增加能耗;而在实验的最后2 d,土壤中Cr(Ⅵ)已经有很大一部分迁移出土壤,能量全部都消耗在其他离子的迁移上,故Qec远高于其他实验组。这一现象也证明PO3−4 (或HPO2−4 、H2PO−4 )[44]在实验土壤中的迁移显著慢于Cr(Ⅵ),有助于Cr(Ⅵ)的去除。综上所述,5种阴离子在土壤电动修复过程中的迁移顺序为NO−3 >Cr(Ⅵ)≈Cl−>SO2−4 >>PO3−4 。 -
表2显示了4组实验土壤中Cr(Ⅵ)和其他主要阴离子的去除率,由于阴阳两极产生OH−和H+,因此CC组和CO组中的
CO2−3 和OH−无法计算去除率,所以不参与讨论。从表中可以看出,CN组中Cr(Ⅵ)在S3区域聚集,而NO−3 在S2区域聚集。根据所观察到的实验现象以及之前所分析的迁移规律可知,随着实验进行到后期,电渗流对离子迁移的作用逐渐超过电迁移,阴离子会随着电渗流向阴极移动,原本聚集在S3的阴离子向S2方向迁移。由此推测,NO−3 的迁移超前于Cr(Ⅵ)。从总体上来看,NO−3 的去除率也是高于Cr(Ⅵ)的,符合推测。CCl组中Cl−和Cr(Ⅵ)均在S2区域聚集,而且其他区域的去除率也相近,证明Cl−在实验土壤中与Cr(Ⅵ)的迁移速率相仿。CS组中,SO2−4 与Cr(Ⅵ)都在S2区域聚集,但是SO2−4 在S2区域的去除率远小于Cr(Ⅵ)。这是因为,随着实验的进行,聚焦现象会逐渐缓解,聚焦区域的离子浓度会降低;同时,Cr(Ⅵ)在其他区域的去除率也高于SO2−4 。所以推测,SO2−4 在实验土壤中的迁移落后于Cr(Ⅵ)。CP组中PO3−4 和Cr(Ⅵ)的去除率都很高,但是Cr(Ⅵ)在每个区域的去除率都达到99.9%以上。而且,由图9也可以观察到,前5 d的实验中CP组的Qec较低。这说明,能量大部分用于Cr(Ⅵ)的移除,后2 d的Qec急剧升高。这是因为,大部分Cr(Ⅵ)在前5 d已经迁移出了土壤,后2 d消耗的电能大部分用于PO3−4 的移除,所以Cr(Ⅵ)的迁移是远远领先于PO3−4 的。此外,PO3−4 具有强大的的酸缓冲能力,中和了H+,缓和了聚焦现象,降低了能耗,PO3−4 同时也具备与金属离子配合的能力[45],使得Cr(Ⅵ)的去除率大大提高。
2.1. 电动修复过程中的电流变化
2.2. 电动修复过程中阴阳极电解液和土壤电导率的变化
2.3. 电动修复过程中土壤和阴阳极电解液pH的变化
2.4. 电动修复前后土壤含水率的变化
2.5. 电动修复过程中Cr(Ⅵ)的去除情况
2.6. 电动修复过程中去除Cr(Ⅵ)的能量消耗情况
2.7. 电动修复前后Cr(Ⅵ)以及其他阴离子去除率比较
-
1)受试的土壤典型阴离子在2 V·cm−1的电压梯度下,以去离子水为电解液进行土壤电动修复过程中的迁移顺序为:
NO−3 >Cr(Ⅵ)≈Cl−>SO2−4 >>PO3−4 。2)相比于酸性土壤,偏中性的土壤环境更利于Cr(Ⅵ)的迁移;土壤呈酸性会增加电动修复的能耗,而且更容易导致聚焦现象不利于Cr(Ⅵ)的迁移。
3)土壤中
CO2−3 、OH−和PO3−4 的存在能有效的缓解聚焦现象,加速Cr(Ⅵ)的去除。4)
PO3−4 在实验土壤中的迁移远远落后于Cr(Ⅵ),缓慢的迁移速度使得实验土壤电导率平均,不会出现离子强度过小,电阻过大的区域,保证了Cr(Ⅵ)迁移所需的电动力,降低了能耗,可使 Cr(Ⅵ)的去除率达到99.9%以上,可用于制备电动修复的电解液。

















 下载:
下载: