-
自然界中铬主要以Cr(Ⅲ)和Cr(Ⅵ) 2种形式存在[1],Cr(Ⅲ)比较稳定,是人体必需的微量元素之一,而Cr(Ⅵ)以其高毒性引起了人们的广泛关注。Cr(Ⅵ)的毒性是Cr(Ⅲ)的100倍左右,因其易溶于水而可迁移到深层地下水和土壤中[2]。Cr(Ⅵ)被国际公共卫生组织列为一级致癌物,对生态系统具有严重的危害[3]。目前国内外常见的含铬废水处理方法主要有活性炭吸附、离子交换、电渗析以及生物吸附等[4],但这些方法都存在一定的局限性,如能耗大、Cr(Ⅵ)去除率低、运行维护费用高等。
近年来,纳米铁已经成为环境中Cr(Ⅵ)污染修复的研究热点。纳米铁因具有较大的比表面积和较高的反应活性而被广泛应用于环境重金属污染修复中[5]。对水中Cr(Ⅵ)的去除,相比吸附、沉淀、离子交换和膜分离等方法[6-7],纳米零价铁(nZVI)处理具有成本低、固废产生量少、易于从水体中分离等优点[8-9],但同时具有易氧化和团聚的缺点[10-11]。生物炭(BC)在土壤中具有永久的存在性、较大的比表面积和大量的含氧活性官能团等优点[12],从而进入研究人员的视野[13]。羧甲基纤维素钠(CMC-Na)因其无毒和来源广泛,是十分理想的nZVI稳定材料[14]。目前,已经有相关研究报道了BC负载纳米铁、CMC稳定纳米铁的一些优势,但BC负载的纳米铁抗氧化能力较弱,CMC稳定化纳米铁材料对纳米铁的分散性改良较弱,无法发挥纳米铁的还原优势。本研究利用液相还原法制备了生物炭负载羧甲基纤维素钠稳定化纳米铁(BC-nZVI-CMC),且探究了BC-nZVI-CMC去除水中Cr(Ⅵ)的效率及主要影响因素,为高效处理重金属废水提供参考。
全文HTML
-
1)试剂。FeSO4·7H2O购于西陇科学股份有限公司,NaHB4购于上海凌峰化学试剂有限公司,无水乙醇购于安徽安特食品股份有限公司,以上试剂纯度均为分析纯。羧甲基纤维素钠(CMC)购于国药集团化学试剂有限公司,纯度为化学纯。
2) BC。水稻秸秆用蒸馏水洗净,在105 ℃下烘至恒重,粉碎磨细,过200目筛,放于马弗炉中,在恒温缺氧的条件下,以5 ℃·min−1升温至700 ℃,恒温热解4 h,冷却后密封保存。将制备好的BC放入1.0 mol·L−1 盐酸中,搅拌处理2 h,用去离子水洗至pH中性,烘干,避光保存备用。
3) BC-nZVI-CMC。采用质量比为1∶0.5、1∶1、1∶5、1∶10生物炭/铁来制备BC-nZVI-CMC。将50 mL 0.4 mol·L−1的FeSO4溶液和上述炭/铁质量比的生物炭和50 mL乙醇置于1 L三角瓶中,不断搅拌和超声分散0.5 h,然后匀速滴入0.4 mol·L−1的NaHB4,直至反应完全,过滤,用乙醇和超纯水分别清洗2次,以去除多余的反应液,整个过程在氮气保护下进行。清洗完成后,加入CMC/BC质量比为1∶2的CMC溶液,搅拌混合。将混合体系置于−80 ℃冷冻8 h后,于冷冻干燥仪上冻干,得最终产物。
-
采用二苯碳酰二肼分光光度法(GB 7467-1987)测定溶液中Cr(Ⅵ),玻璃电极法测pH[15]。按BC-nZVI-CMC的含量为0.5、1、2、4、8 g·L−1取适量BC-nZVI-CMC于离心管中,加入30 mg·L−1 Cr(Ⅵ)溶液50 mL,在25 ℃、240 r·min−1条件下进行水浴振荡,12 h后测定Cr(Ⅵ)含量。分别取一系列50 mL浓度为50 mg·L−1的Cr(Ⅵ)溶液于离心管中,调整溶液pH分别为 2.0、4.0、6.0、8.0、10.0,各管加入BC-nZVI-CMC,使其含量为1 g·L−1,在25 ℃、240 r·min−1条件下,进行水浴振荡,12 h后,测定溶液中的Cr(Ⅵ)含量。将商品纳米铁、BC-nZVI-CMC、BC-nZVI及CMC-nZVI(CMC/nZVI=1∶10)置于空气中暴露7 d,之后各取适量,按1 g·L−1的量加入50 mg·L−1 Cr(Ⅵ)溶液中,同时以新鲜CMC-Na作对照,在25 ℃、240 r·min−1条件下进行水浴振荡,12 h后,测定溶液中Cr(Ⅵ)含量。将2、4、8 g·L−1的BC-nZVI-CMC投加入50 mL含铬电镀废水中,在25 ℃、240 r·min−1条件下进行水浴振荡,12 h后取样,测定废水中重金属的含量。
-
采用美国ThermoFisher Scientific公司Verios G4型扫描电镜对样品进行微观结构及表面形态的观察,分析纳米铁颗粒的大小。采用美国Bruker公司D8 Advance型X射线衍射仪对样品进行晶相分析,工作电压为40 kV,电流为40 mA,2
θ 为10°~90°。采用Bruker Optik GmbH德国公司的TRENSOR27型傅里叶红外光谱仪对样品进行FT-IR谱图测定,使用KBr压片,扫描范围为350~4 000 cm−1。
1.1. 实验材料
1.2. 实验方法
1.3. 材料表征
-
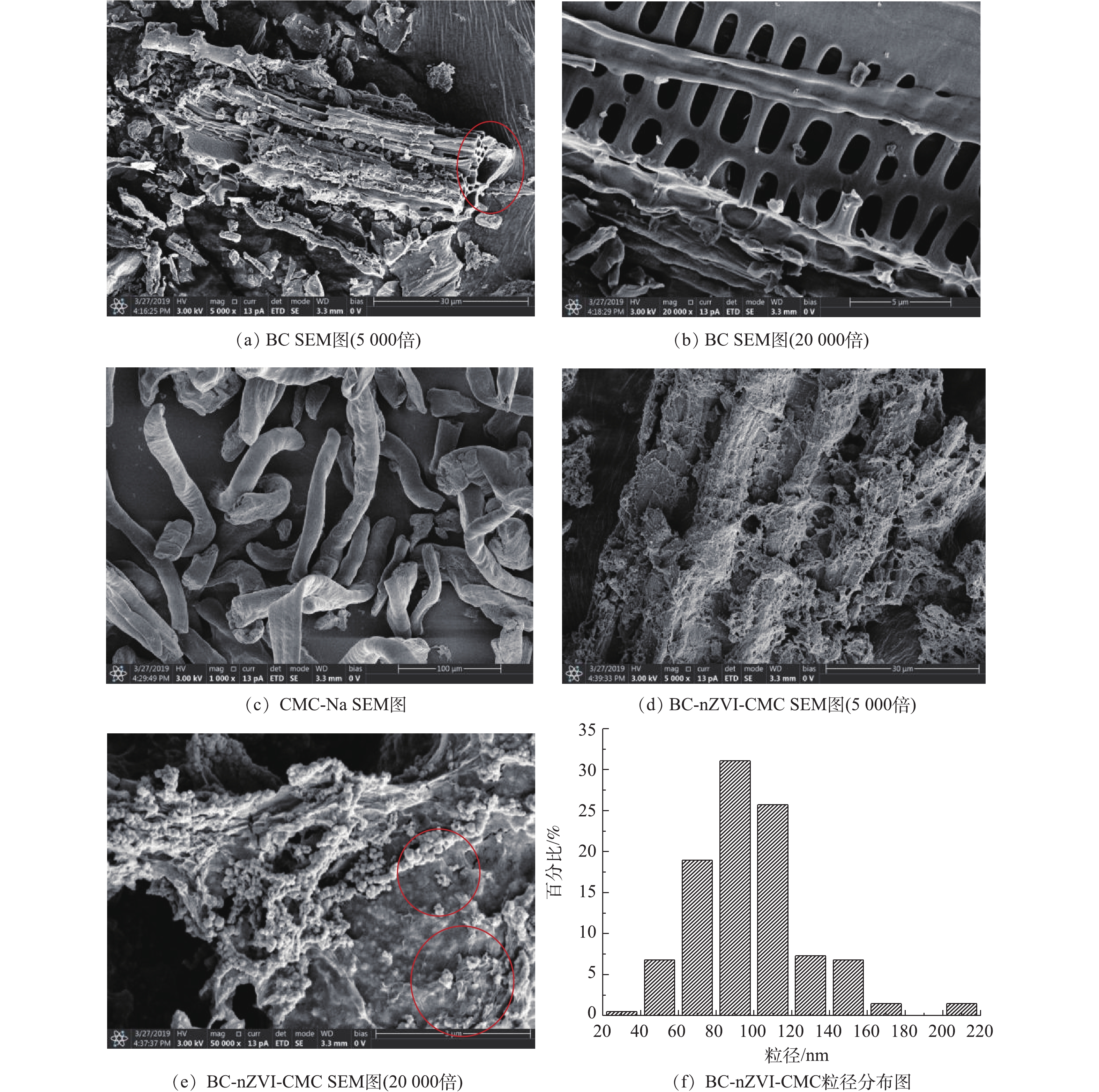
生物炭(BC)、羧甲基纤维素钠(CMC-Na)、生物炭负载羧甲基纤维素钠稳定化纳米铁(BC-nZVI-CMC)的SEM扫描结果及纳米铁颗粒粒径分布结果如图1所示。由图1(a)和图1(b)可见,700 ℃制得的生物炭具有蜂窝状孔隙及丰富的孔状结构[16]。由CMC-Na的扫描电镜结果(图1(c))可见,它是一种表面光滑的纤维状物质。由BC-nZVI-CMC的扫描电镜图(图1(d)和图1(e))可见, 纳米铁颗粒呈链状,较均匀地分散在生物炭表面,这说明作为载体的生物炭对纳米铁的团聚起到了一定的改善作用;同时,纳米铁被CMC包覆在BC的表面,说明CMC在BC-nZVI体系隔绝空气中起到了很好的保护作用。 随机选取200多个颗粒进行统计分析,由纳米铁颗粒粒径分布结果(图1(f))可知,纳米铁的粒径分布在40~160 nm,最小粒径为30 nm,平均粒径为90 nm;其中0~100 nm的颗粒占比为57.28%,这表明所制备的纳米铁颗粒符合纳米级别。
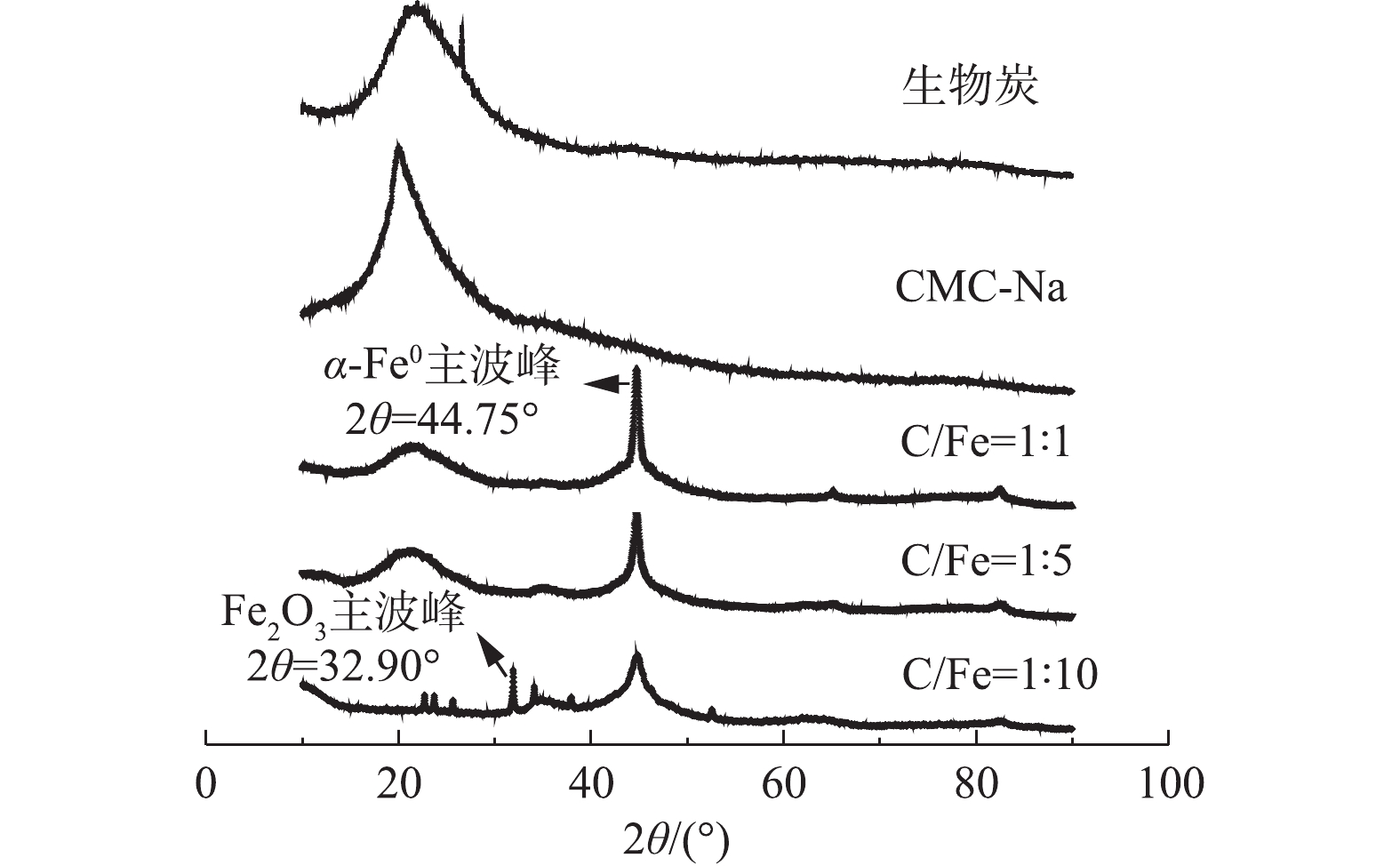
BC、CMC-Na及BC-nZVI-CMC的XRD分析结果如图2所示。由图2可知,BC在15°~30°具有明显的有机物衍射峰,在26.65°处有明显的碳峰[17]。CMC-Na在15°~30°具有和BC一样明显的有机物衍射峰。BC-nZVI-CMC在44.75°出现了一个主波峰,且在65.08°和82.52°处出现了2个伴峰,与其他研究[18-19]得出该位置为α-Fe0的特征峰结果相吻合。与PDF标准卡片对比后表明制得的为纳米单质零价铁。BC对纳米铁进行负载和CMC对纳米铁进行包裹后,有机物的衍射峰明显降低,这表明BC和CMC所携带的有机官能团会与纳米铁进行反应,使其性质稳定,并且随着纳米铁相对含量的增加,BC和CMC相对含量减少,CMC对纳米铁的包裹率降低,nZVI的衍射峰逐渐减弱,铁的氧化物衍射峰开始出现。与PDF标准卡片对比,发现主要为Fe2O3的波峰,主波峰处于31.90°,从侧面证实了CMC在纳米铁抗氧化性方面起到了决定性作用[20]。
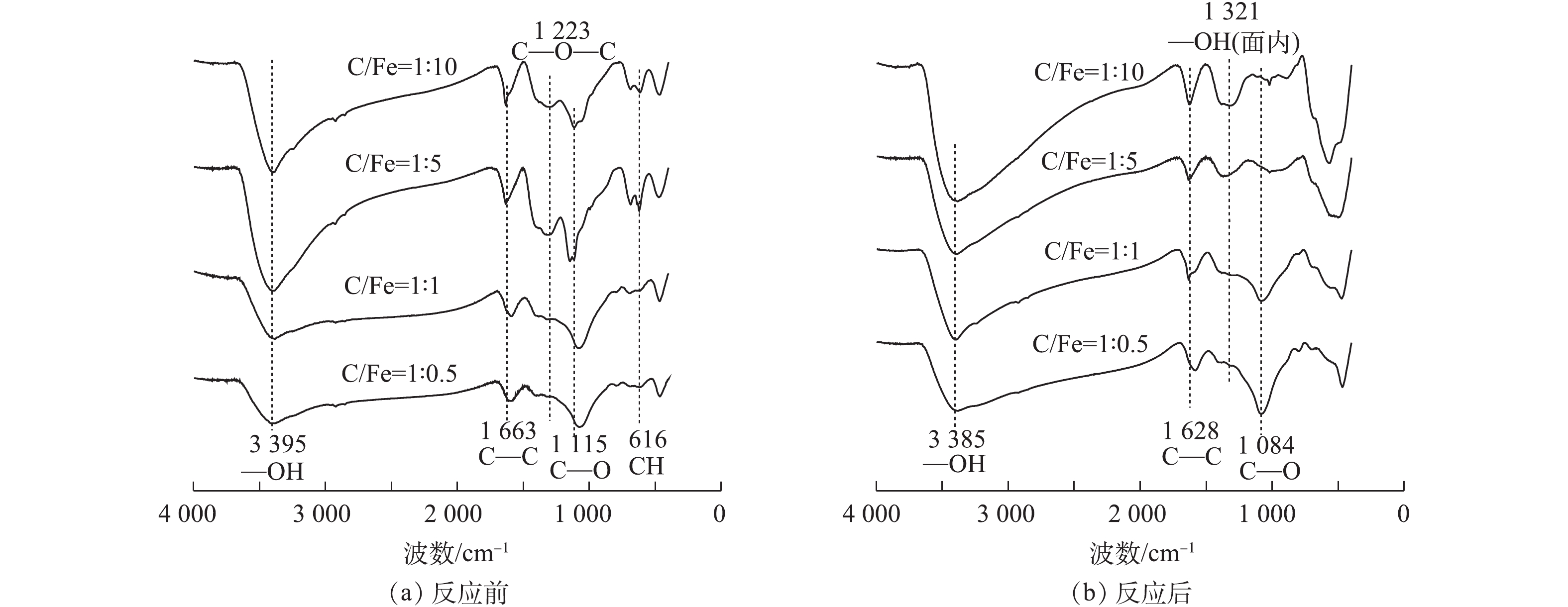
BC-nZVI-CMC与Cr(Ⅵ)反应前后的FT-IR图谱如图3所示。由图3可知,随着纳米铁含量的增加,1 223 cm−1(醚类C—O—C伸缩振动)、1 115 cm−1(醇类C—O伸缩振动)和616 cm−1(炔烃类C—H弯曲振动(面外))吸收峰由钝锋变成锐峰,且峰强度先增加后降低,这可能是nZVI与BC和CMC结合引起的。1 663 cm−1处峰强度无明显变化,表明该类官能团很稳定,有研究[21]认为是芳香C的振动峰位。3 395 cm−1处振动峰呈现峰高先升高后降低的趋势,有研究[22-23]认为很可能是nZVI与BC之间形成Fe—O—H键所致。从图3(b)可以看出,1 084 cm−1处C—O官能团特征峰随着nZVI比例的增加而明显降低,表明该类官能团是由nZVI与BC结合产生的,随着纳米铁与Cr(Ⅵ)反应后明显减少。3 385 cm−1和1 321 cm−1处特征峰随着纳米铁比例的增加,峰高逐渐增加,该类官能团属—OH振动,表明Cr(Ⅵ)与BC-nZVI-CMC反应后,被还原并以Fe(Ⅲ)/Cr(Ⅲ)络合物的形态存在于BC表面[24-26]。C—C官能团属于一类比较稳定的官能团,反应前后并无明显变化,表明该类官能团不参与纳米铁的负载及后续的还原Cr(Ⅵ)过程。
-
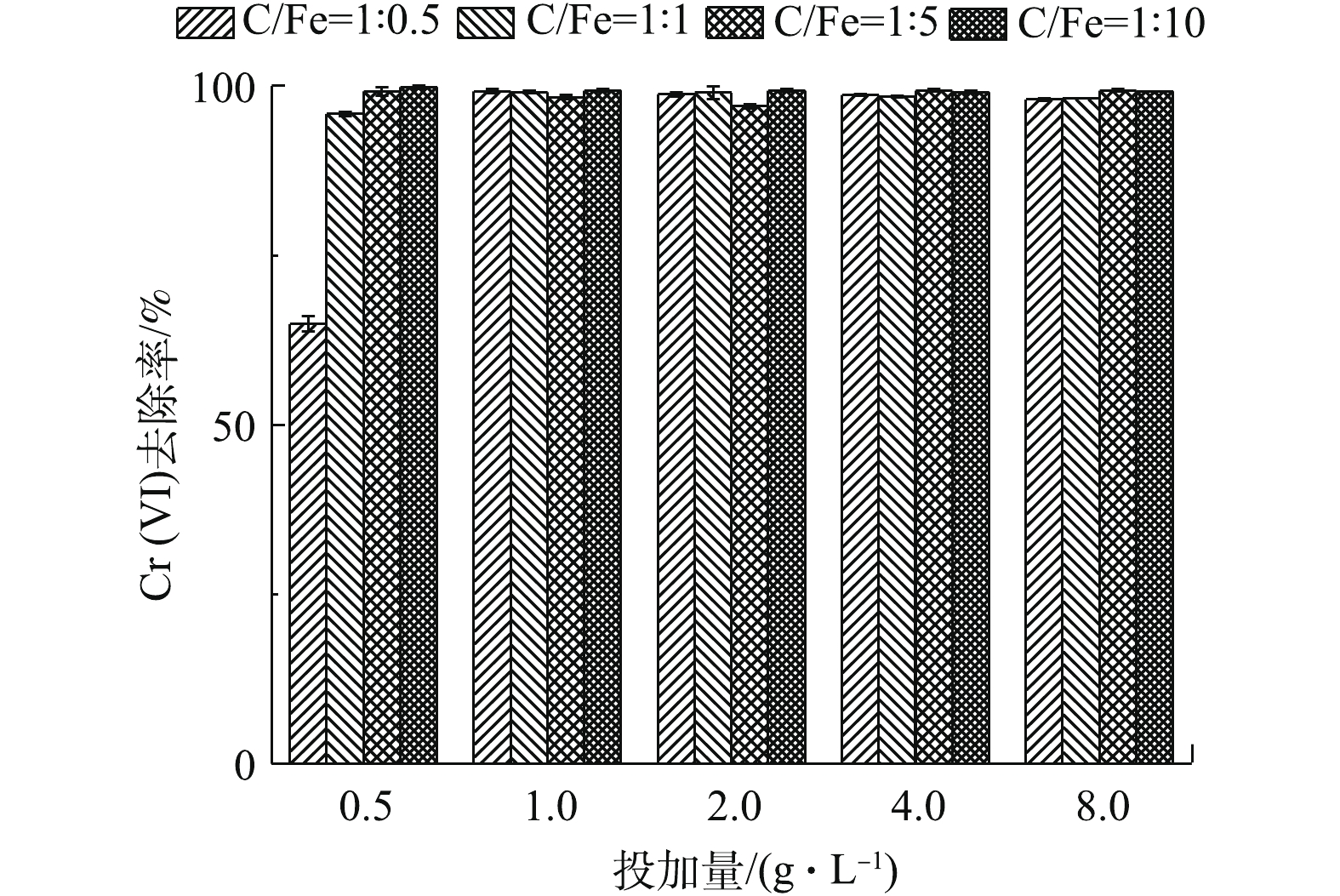
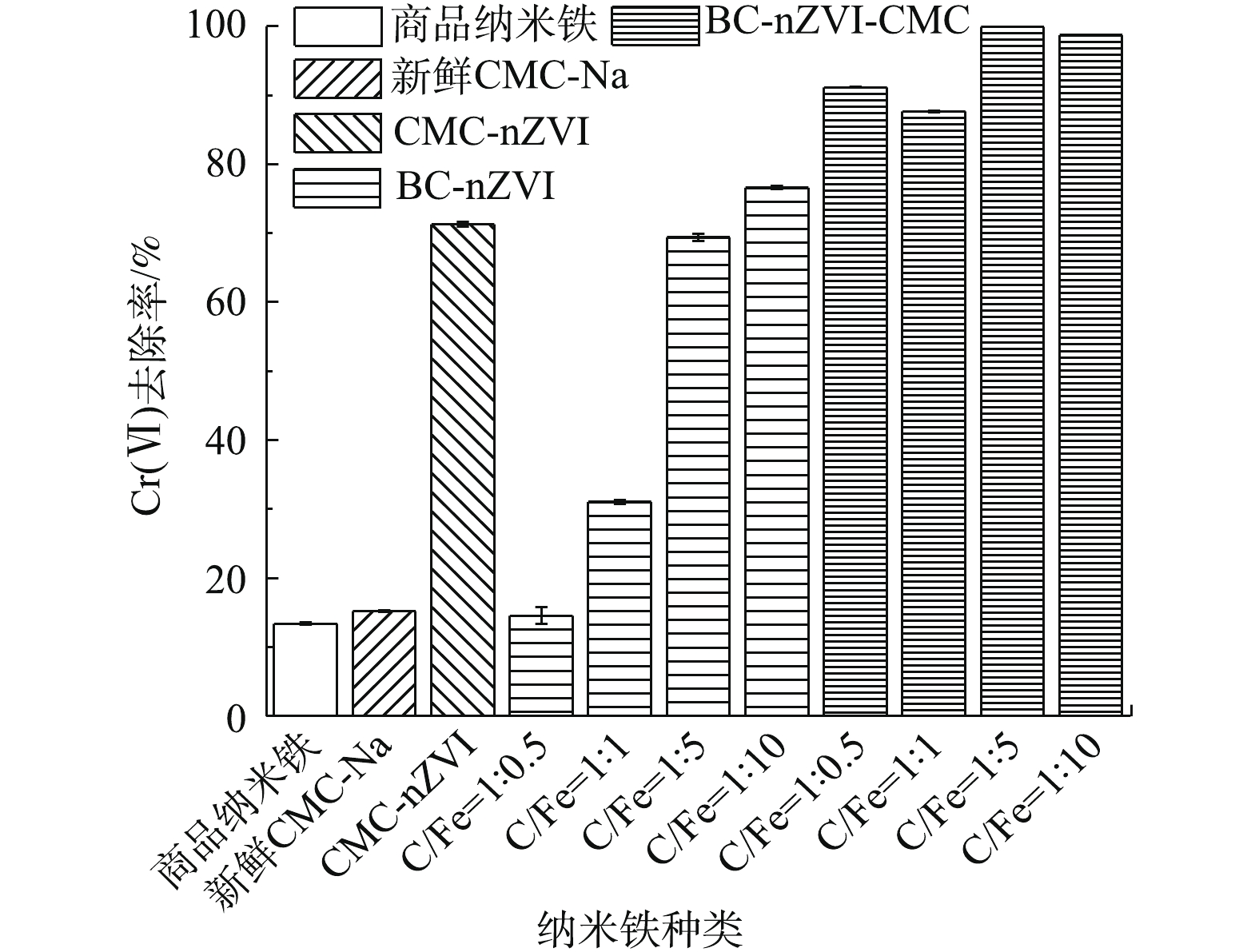
4种BC-nZVI-CMC对Cr(Ⅵ)的去除效果如图4所示。由图4可知,4种BC-nZVI-CMC对Cr(VI)均有较高的去除率,随着纳米铁投加量的增加,Cr(Ⅵ)的去除率也随之升高。在4种BC-nZVI-CMC投加量(0.5 g·L−1)较低时,随纳米铁负载量的增加,Cr(Ⅵ)的去除率逐渐上升。4种材料对Cr(Ⅵ)去除率最高达99.83%,最低为64.90%。
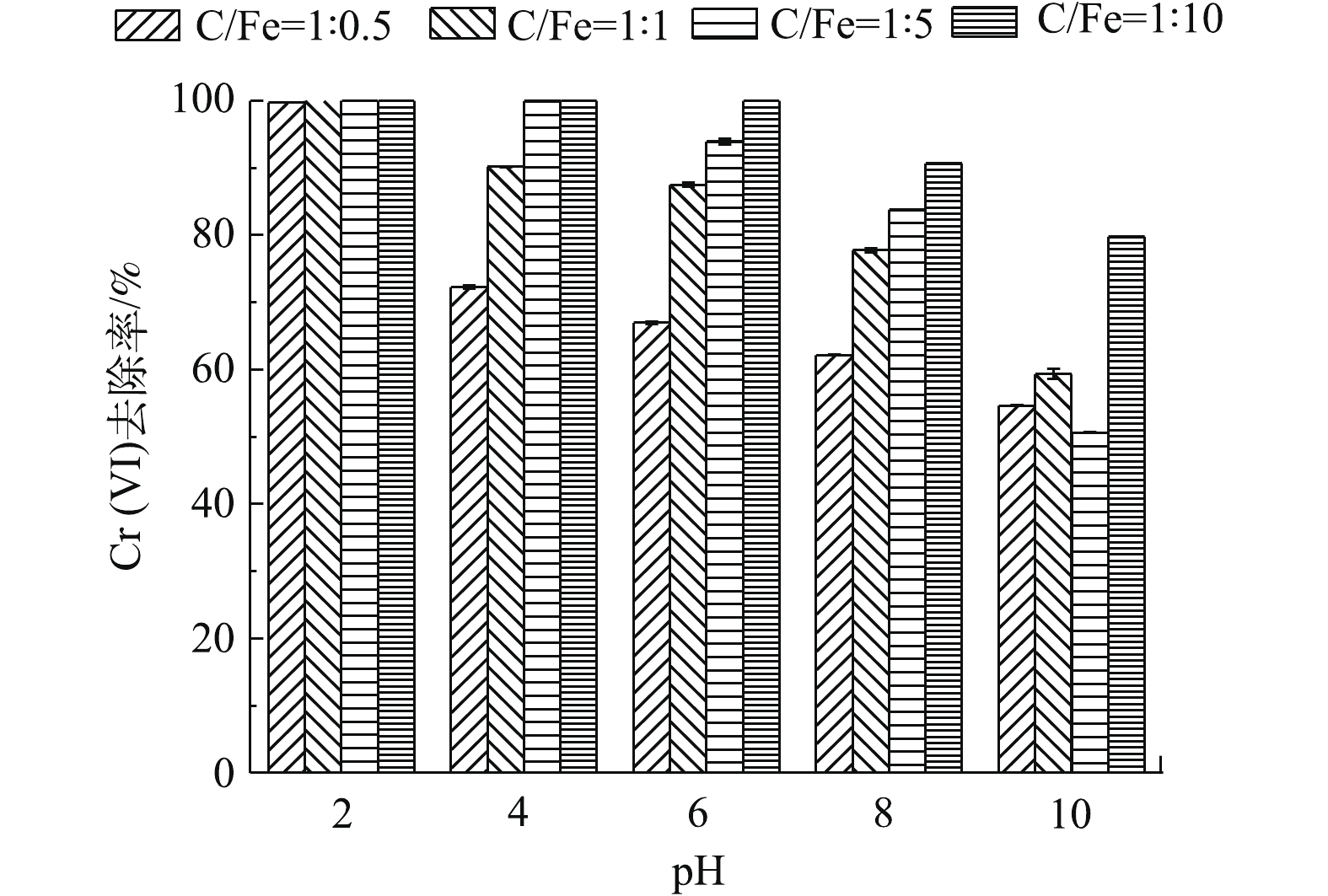
pH对4种BC-nZVI-CMC去除Cr(Ⅵ)的影响如图5所示。由图5可知,4种纳米铁颗粒对Cr(Ⅵ)的去除率随pH的升高而降低,这是由于当pH较低时,H+会促进纳米铁和Cr(Ⅵ)发生电子交换,导致溶液中Cr(Ⅵ)的去除率较高。当溶液pH较高时,OH−会与Cr(Ⅵ)发生电子争夺,存在竞争吸附。此外,在碱性条件下,溶液中OH−较多,会阻止nZVI的腐蚀,还会使纳米铁氧化物外壳加厚,进而阻止了纳米铁进一步与Cr(Ⅵ)的反应,导致溶液中Cr(Ⅵ)的去除率降低[27-28]。
4种纳米铁暴露于空气后,对Cr(Ⅵ)的去除率如图6所示。由图6可知,暴露于空气后,BC-nZVI-CMC对Cr(Ⅵ)的去除率明显高于商品纳米铁、BC-nZVI和CMC-nZVI。BC-nZVI随纳米铁比例的升高,对Cr(Ⅵ)的去除率也逐渐升高,原因是纳米铁本身具有壳核结构,纳米铁比例的升高会形成一定的团聚,从而阻止空气对纳米铁的进一步氧化[29]。氧化后的CMC-nZVI对Cr(Ⅵ)的去除率较低,原因是CMC对纳米铁分散性的改进可能不及BC。BC-nZVI-CMC对Cr(Ⅵ)的去除率较高,且随纳米铁比例的升高,呈现先降低后升高再趋于平稳的趋势,表明随着纳米铁在生物炭上负载量的增加,团聚加剧,降低了稳定化纳米铁对Cr(Ⅵ)的去除率;当纳米铁的量超出一定范围后,由于纳米铁数量较多,表现出对Cr(Ⅵ)趋于完全的去除,所以去除率上升至趋于稳定。而新鲜CMC-Na对Cr(Ⅵ)的去除率只有15.23%,表明CMC在BC-nZVI-CMC体系中所起的作用主要是包裹纳米铁,使其与空气隔绝。在此过程中,Cr(Ⅵ)被吸附于材料表面,并将Fe0氧化为Fe(Ⅱ)和Fe(Ⅲ),同时Cr(Ⅵ)被还原为Cr(Ⅲ),与Fe(Ⅲ)以共沉淀的形式((CrxFe1-x)(OH)3或CrxFe1-xOOH)存在于BC表面[30]。
-
为了验证BC-nZVI-CMC实际应用的可行性,以含铬电镀废水(采用铬酐+硫酸工艺)为对象,探讨BC-nZVI-CMC对实际废水的处理效果。该废水取自某电镀厂,pH为2.2,总Cr为380.4 mg·L−1,Cr(Ⅵ)为328.2 mg·L−1,Cu、Zn、Ni分别为159.3、356.0和99.0 mg·L−1。BC-nZVI-CMC对该废水中重金属的去除效果如图7所示。由图7可知,电镀废水重金属的去除率随BC-nZVI-CMC投加量的增加而升高,当BC-nZVI-CMC投加量大于4 g·L−1时,六价铬的去除率高于98.09%。随着纳米铁比例的提高,Cu、Zn、总铬、Cr(Ⅵ)的去除率随之升高,而Ni的去除率却下降,这是由于Cu、Zn、总铬、Cr(Ⅵ)的去除主要通过纳米铁的化学吸附作用,而Ni的去除主要通过BC的物理吸附作用。当BC-nZVI-CMC投加量为2 g·L−1时,重金属的去除率较低,原因是BC-nZVI-CMC的量少,吸附位点也较少,各重金属之间存在吸附竞争,导致各重金属的去除率均较低。
-
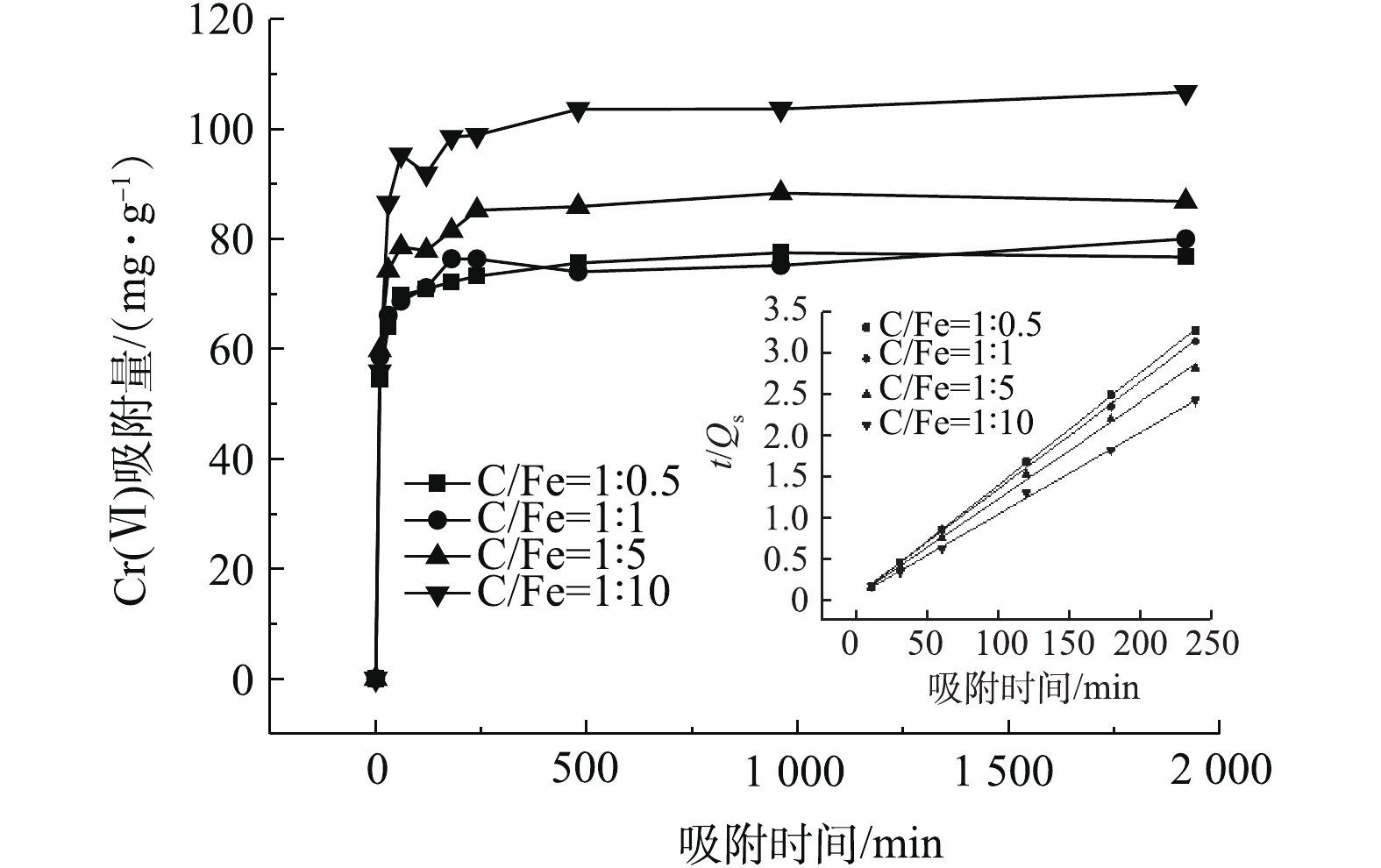
BC-nZVI-CMC对Cr(Ⅵ)的吸附结果如图8所示,采用准一级动力学和准二级动力学模型对实验数据的拟合结果如表1所示。由图8可知,BC-nZVI-CMC对Cr(Ⅵ)的吸附量先随时间的增加而逐渐升高,然后趋于稳定。在吸附开始的30 min内,BC-nZVI-CMC对Cr(Ⅵ)的吸附量快速增加,在随后的4 h内吸附达到平衡。这主要是由于吸附前期,BC-nZVI-CMC的孔隙度较高,吸附位点较多,有利于重金属离子的吸附。随着吸附的进行,材料表面的吸附位点逐渐被占据,吸附反应逐渐达到平衡[31]。由表1可知,BC-nZVI-CMC对Cr(Ⅵ)的吸附更符合二级动力学模型(R2>0.929 5),有较高的拟合度,由此可推断吸附过程为以离子交换和螯合反应为主的化学吸附[32]。
2.1. 纳米铁材料性质表征
2.2. BC-nZVI-CMC对Cr(Ⅵ)去除的影响因素
2.3. BC-nZVI-CMC对含铬电镀废水处理效果
2.4. 吸附动力学
-
1)合成的BC-nZVI-CMC颗粒为纳米单质零价铁,CMC对BC-nZVI能起到抗氧化的保护作用;与其他纳米铁相比,BC-nZVI-CMC有更强的还原和抗氧化能力。
2)在pH较低、投加量较多时,BC-nZVI-CMC对Cr(Ⅵ)的去除率较高;BC-nZVI-CMC对Cr(Ⅵ)的去除机理主要为Cr(Ⅵ)被还原,并与Fe(Ⅲ)以共沉淀的形式存在于生物炭表面。
3)吸附动力学数据表明,BC-nZVI-CMC对Cr(Ⅵ)的去除符合准二级动力学模型,吸附过程为以离子交换和螯合反应为主的化学吸附。



 下载:
下载: