-
催化氧化技术是目前去除挥发性有机物(volatile organic compounds, VOCs)最有效的方法之一,其核心在于催化剂的研发。根据催化剂中活性组分的不同,一般将其分为贵金属催化剂(如Pd、Pt、Au、Ru等)[1-4]和非贵金属催化剂(Cu、Mn、Co、Ce、Ni等)[5-11]。贵金属催化剂由于具有良好的低温催化活性和选择性,使得部分贵金属催化剂(Pd和Pt)已实现了工业化应用。但在实际工业应用中,贵金属催化剂存在价格昂贵、活性组分易挥发和易中毒失活等问题,因此,在一定程度上限制了其推广应用[12]。非贵金属催化剂主要指过渡金属氧化物及其混合物,由于价格低廉、资源丰富,同时具有良好的氧化还原性能等优点,近年来受到越来越多的关注,被认为是贵金属催化剂的良好替代品[12]。
单一锰氧化物催化剂(如Mn3O4、Mn2O3和MnO2)在VOCs的催化氧化中表现出良好的活性,但其催化氧化性能受到催化剂的比表面积、Mn4+含量、表面氧物种及催化剂的氧化还原性等诸多因素的影响[13-16]。与贵金属催化剂的催化氧化性能相比,单一金属氧化物的性能难以满足要求,因此,近年来,许多科研工作者都在探索提高其催化氧化性能的方法。其中,过渡金属元素掺杂改性形成混合金属氧化物催化剂的研究最为广泛,如Mn-Ce、Mn-Co、Mn-Cu等的催化氧化性能相对于单一氧化物催化剂均能得到提高[12]。然而,这些二元复合氧化物催化剂通常是通过物理混合、共沉淀、浸渍等方法制备的,这些方法可能受到元素扩散速率和金属前驱体沉淀速率的影响,从而易形成非均相催化剂[17-19]。为了避免形成非均相催化剂,ARENA等[20-21]首次报道了采用氧化还原共沉淀法制备“分子”级别高分散态Mn-Ce复合氧化物催化剂,其对于邻二甲苯的催化氧化性能优异,表明金属元素混合均匀的高分散态催化剂有助于提高其催化氧化性能。
本研究采用氧化还原共沉淀法制备Mn-Ce二元复合氧化物催化剂,考察其对苯的催化氧化性能,并与共沉淀法制备的Mn-Ce催化剂及单一金属氧化物(Mn2O3和CeO2)进行对比,结合催化剂的多种表征结果,建立了催化剂的结构-活性关系(构效关系);在此基础上,研究了氧化还原共沉淀法制备的其他二元复合锰氧化物催化剂(Mn-Co、Mn-Cu和Mn-Sn)对苯的催化氧化性能,发现Ce和Sn掺杂锰氧化物催化剂性能最好。
全文HTML
-
硝酸锰溶液、氢氧化钠、七水合硝酸钴、六水合硝酸铈、硝酸铜(均购于阿拉丁试剂(上海)有限公司,分析纯);30%过氧化氢、高锰酸钾(均购于北京化工厂,分析纯);无水四氯化锡(购于山东西亚化学工业有限公司,分析纯)。
-
采用氧化还原共沉淀法和共沉淀法,通过掺杂不同元素(Cu、Ce、Co和Sn),在相同的焙烧温度下(500 °C),合成了二元锰氧化物催化剂。氧化还原共沉淀法的制备过程如下:将KMnO4(6 g,38 mmol)和Ce(NO3)3·6H2O(5.5 g,12.7 mmol)溶解于400 mL去离子水中,并用400 mL去离子水稀释30%的过氧化氢溶液(19.45 g)。将稀释后的过氧化氢溶液逐滴滴加到KMnO4和Ce(NO3)3的混合溶液中,并剧烈搅拌,此过程中伴随着沉淀产生。过氧化氢溶液滴加完成后,继续搅拌0.5 h,之后静置老化4 h,再用去离子水过滤和洗涤,并于100 °C下过夜干燥,得到深棕色粉末,之后放入马弗炉中,于500 °C焙烧4 h(升温速率5 °C·min−1)。得到的锰铈氧化物催化剂记为OP-Mn3Ce1,其中Mn/Ce的化学计量摩尔比为3∶1。同理,采用Co(NO3)2·6H2O、Cu(NO3)2·3H2O和SnCl4制备锰钴、锰铜和锰锡复合氧化物,分别记为OP-Mn2Co1、OP-Mn2Cu1和OP-Mn4Sn1,其中Mn/Co、Mn/Cu和Mn/Sn的化学计量摩尔比分别为2∶1、2∶1和4∶1。在OP-Mn4Sn1制备的过程中,须用稀盐酸将KMnO4和SnCl4的混合溶液的pH调至1.0。共沉淀法的制备过程如下:将Ce(NO3)3·6H2O(17.4 g,40 mmol)和Mn(NO3)2(42.9 g,120 mmol)溶解于400 mL去离子水中,并将NaOH(15 g,376 mmol)溶解于400 mL去离子水中。将NaOH溶液逐滴滴加到Mn(NO3)2和Ce(NO3)3的混合溶液中,并剧烈搅拌,此过程中伴随着沉淀的产生。后续制备过程与OP-Mn3Ce1的一致。焙烧后得到的锰铈氧化物催化剂记为CP-Mn3Ce1,其中Mn/Ce的化学计量摩尔比为3∶1。同理,Mn2O3制备时Mn(NO3)2质量为28.632 g(80 mmol),NaOH质量为7.04 g(176 mmol);CeO2制备时Ce(NO3)3·6H2O质量为17.4 g(40 mmol),NaOH质量为5.44 g(136 mmol)。
-
利用X射线衍射仪(Rigaku D/Max-RA),以步长为0.02°、步时为8 s的条件,测量得到样品的X射线衍射图谱。采用物理吸附仪(Autosorb-IQ-MP, Quantachrome),在−198 °C(77 K)条件下,进行N2吸附/脱附实验,来表征样品的多孔结构。利用FEI公司的Tecnai G2 F20场发射电子显微镜,进行透射电镜(TEM)和高分辨透射电镜(HR-TEM)图像测试,程序升温还原(TPR)实验在ChemBET Pulsar TPR型化学吸附仪上进行。
-
催化剂评价装置主要由气体管路、固定床反应器、流量控制系统和气体检测系统等4部分组成,在实验过程中,使用质量流量计实现对各路气体的精确控制。采用外径为6 mm,内径为4 mm的石英管作为催化反应器,反应器由电阻炉进行加热,并通过K型热电偶监测反应器内温度。实验气路中各气路流量通过质量流量计进行控制,所有气体均为标准气体。混合气体的总流量为100 mL·min−1,其中O2的体积分数为20%,苯的浓度为3 482.1 mg·m−3。苯为由N2稀释且低于其饱和蒸气压的标准钢瓶气体,其浓度很低,通过调节流量计的阀门开度,设置不同浓度的苯。实验条件下反应气流中苯的分压远小于苯的饱和蒸气压,苯并不会在气路中发生冷凝,为了减少气路对苯的吸附,实验中对管路进行了加热,总体温度保证在80 ℃以上,苯的吸附量很少。在实验过程中,催化剂的装填量为200 mg,粒径为250~425 μm(40~60目),所对应的质量空速(WHSV)为30 000 mL·(g·h)−1。因实验所用高纯气纯度很高,可以基本排除CO2对催化反应的干扰。此外,实验室也采用一定浓度的CO2,测试了其对催化过程的影响,从测试结果来看,CO2对于催化过程基本无影响。
首先,将催化剂置于石英筛板上,使催化床的温度升高至100 °C,在此条件下,使苯的浓度达到稳定状态;之后,以5 °C·min−1的升温速率,25 °C的温度梯度,每个温度点稳定在55 min的条件下,从100 °C升温至300 °C进行催化活性评价。在催化剂的条件下进行空白运行,实验结果显示:苯的浓度基本无变化,表明既没有发生催化反应,也没有发生气相均相反应;催化实验过程中没有观察到副产物的生成,且催化剂床层进出口可以实现碳平衡。反应物和产物(CO2和CO)通过气相色谱仪(GC 2010 Plus,Shimadzu)进行在线监测和分析。反应物的转化率通过式(1)计算。
式中:x为转化率;Cin和Cout为苯的入口和出口浓度。
CO2的选择性通过式(2)计算。
式中:S(CO2)为CO2的选择性;C(CO2)和C(CO)为出口CO2和CO的浓度。
1.1. 实验材料
1.2. 催化剂制备方法
1.3. 催化剂表征
1.4. 催化剂评价
-
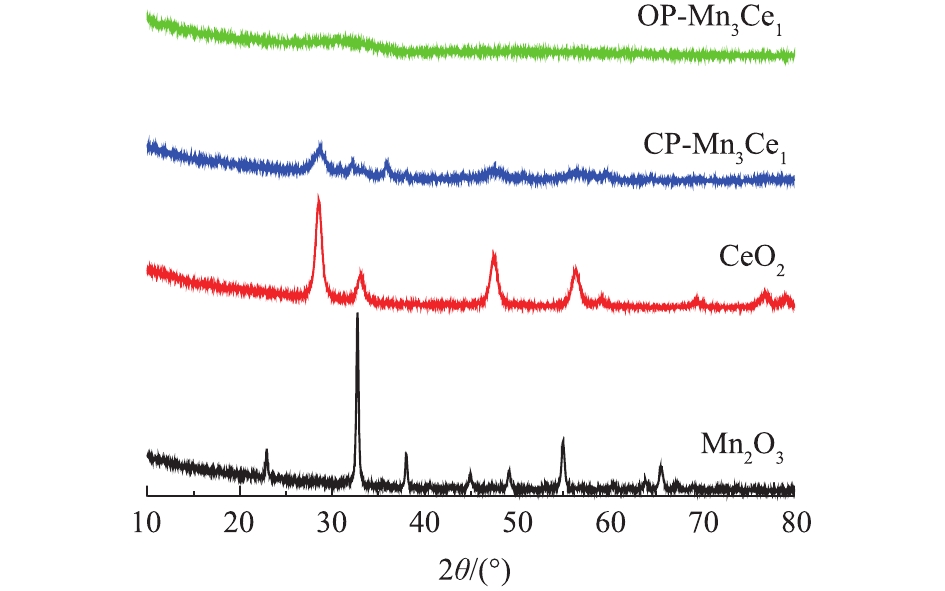
氧化还原共沉淀法和共沉淀法制备得到的OP-Mn3Ce1、CP-Mn3Ce1、Mn2O3和CeO2催化剂的XRD谱图如图1所示。对于纯铈氧化物,其在2θ=28.5°、33.1°、47.5°、56.3°、59.1°、69.4°、76.7°、79.1°和88.4°出现的强峰全部归属于CeO2晶型(PDF 03-065-2975),而纯锰氧化物在2θ=23.1°、32.9°、38.1°、49.2°、55.0°和65.6°出现的衍射峰则归属于Mn2O3(PDF 01-078-0390)。从CP-Mn3Ce1的XRD谱图上可以观察到CeO2和Mn2O3的特征峰,但出峰位置略有偏移,表明MnOx与CeOy之间存在着强烈的相互作用。但采用氧化还原共沉淀法制备的OP-Mn3Ce1的XRD谱图中并未观察到明显的衍射峰,说明催化剂的晶粒较小或具有非定型结构。
样品的N2吸附/脱附实验结果如表1所示。可以看出,单一的锰氧化物和铈氧化物比表面积分别为31.6 m2·g−1和61.8 m2·g−1,而CP-Mn3Ce1和OP-Mn3Ce1的比表面积分别为63.6 m2·g−1和105.6 m2·g−1,明显高于单一金属氧化物。同时,采用氧化还原共沉淀法制备的OP-Mn3Ce1的平均孔径(12.4 nm)大于共沉淀法制备的CP-Mn3Ce1(9.6 nm),这主要是由于氧化还原反应过程中O2的大量释放抑制了小颗粒的团聚。
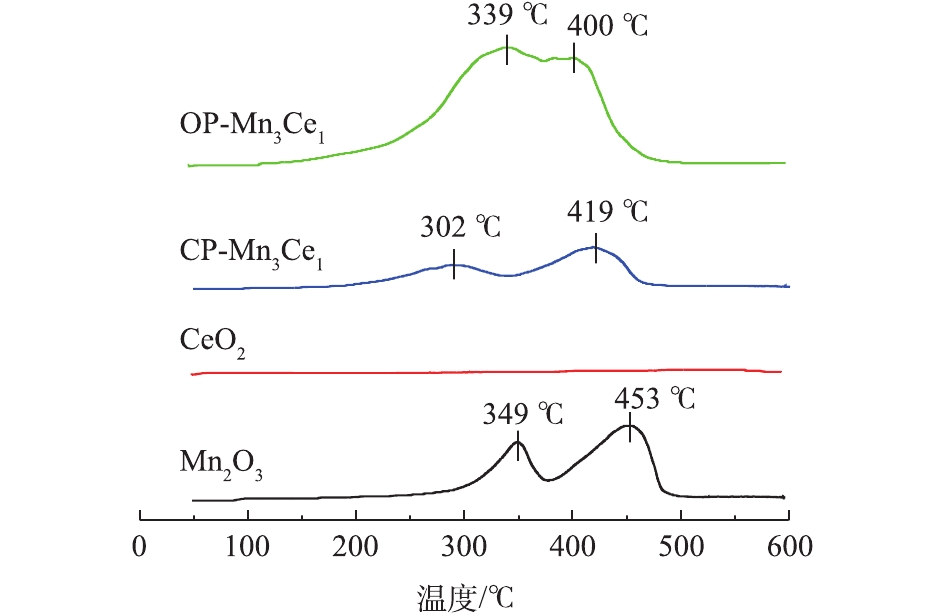
为了研究OP-Mn3Ce1、CP-Mn3Ce1、CeO2和Mn2O3的氧化还原性,对催化剂进行了H2-TPR实验,结果如图2所示。在349 °C和453 °C时,Mn2O3谱图有2个明显的还原峰,对应于Mn2O3还原为Mn3O4及Mn3O4还原为MnO的过程。然而,在CeO2谱图中,并未发现明显的还原峰,可能是由于采用NaOH作为沉淀剂得到的CeO2晶格缺陷较少所致,类似的现象已有相关报道[22-23]。相对于单一氧化物催化剂,OP-Mn3Ce1和CP-Mn3Ce1催化剂的谱图中均出现2个明显的还原峰,且对应的温度均低于Mn2O3的还原峰,表明Ce掺杂可以提高锰氧化物催化剂的还原性。而OP-Mn3Ce1谱图在100~500 °C时对应的峰面积相对于CP-Mn3Ce1要大很多,表明OP-Mn3Ce1的活性氧较多。这说明采用氧化还原共沉淀制备的二元锰氧化物催化剂,不同过渡金属之间掺杂更为均匀,相互作用更强,显著提高了催化剂的氧化还原能力。
为进一步研究各元素在OP-Mn3Ce1催化剂表面的分布特性,对其进行了HAADF-STEM和STEM-EDS mapping表征。图3为OP-Mn3Ce1的高角度环形暗场-扫描透射电子显微镜图及Mn、Ce元素的分布,可以看出,Mn元素和Ce元素均匀分布,证实了采用氧化还原共沉淀法可以有效形成高分散态Mn-Ce复合氧化物催化剂。
OP-Mn3Ce1、CP-Mn3Ce1、Mn2O3和CeO2催化剂对苯的催化氧化性能评价结果如图4所示。由图4可知,所有复合锰氧化物催化剂(OP-Mn3Ce1和CP-Mn3Ce1)的活性均高于单一金属氧化物(Mn2O3和CeO2),其中OP-Mn3Ce1的催化氧化活性最高,而CeO2的活性最差。随着温度的升高,苯在CeO2上的转化率甚至出现了下降,这可能与CeO2表面活性氧物种逐渐耗尽却无法有效补充有关,与H2-TPR表征结果一致。产物(CO2和CO)通过气相色谱仪进行在线监测和分析,结果表明,CO2的选择性均为100%,且无其他副产物。总之,采用氧化还原共沉淀法制备的OP-Mn3Ce1催化剂具有较大的孔径和比表面积,晶体缺陷多,低温还原性好,对苯具有良好的催化氧化性能。由图4可知,OP-Mn3Ce1和CP-Mn3Ce1在250 °C下实现了苯的完全转化。
-
图5为氧化还原共沉淀法制备的OP-Mn2Co1、OP-Mn2Cu1、OP-Mn3Ce1和OP-Mn4Sn1的XRD谱图。由此可知,对于OP-Mn2Co1催化剂,其在2θ=36.7°出现的强峰归属于Co3O4晶型(PDF 01-080-1534),而OP-Mn2Cu1催化剂在2θ=30.5°、35.9°、43.7°、54.2°、55.4°、57.8°和63.5°出现的衍射峰全部归属于Cu1.5Mn1.5O4晶型(PDF 01-070-0260),表明采用氧化还原共沉淀法掺杂Co、Cu 2种元素,焙烧500 °C的条件下会有其他晶相析出。OP-Mn3Ce1和OP-Mn4Sn1的XRD谱图上并未观察到明显的衍射峰,说明催化剂中2种过渡金属元素混合较为均匀。
图6为氧化还原共沉淀法制备的OP-Mn2Co1、OP-Mn2Cu1、OP-Mn3Ce1和OP-Mn4Sn1的H2-TPR结果。由此可知,所有二元复合锰氧化物催化剂的谱图中均出现2个明显的还原峰。Cu、Co掺杂时,H2-TPR中2个还原峰的出峰位置分别为243、365 °C和273、399 °C;而Ce、Sn掺杂时,2个还原峰的出峰位置分别为339、400 °C和331、425 °C,相比单一金属氧化物Mn2O3(349、453 °C),4种元素掺杂时均能使还原峰向低温区移动,其中Cu、Co的效果最佳。
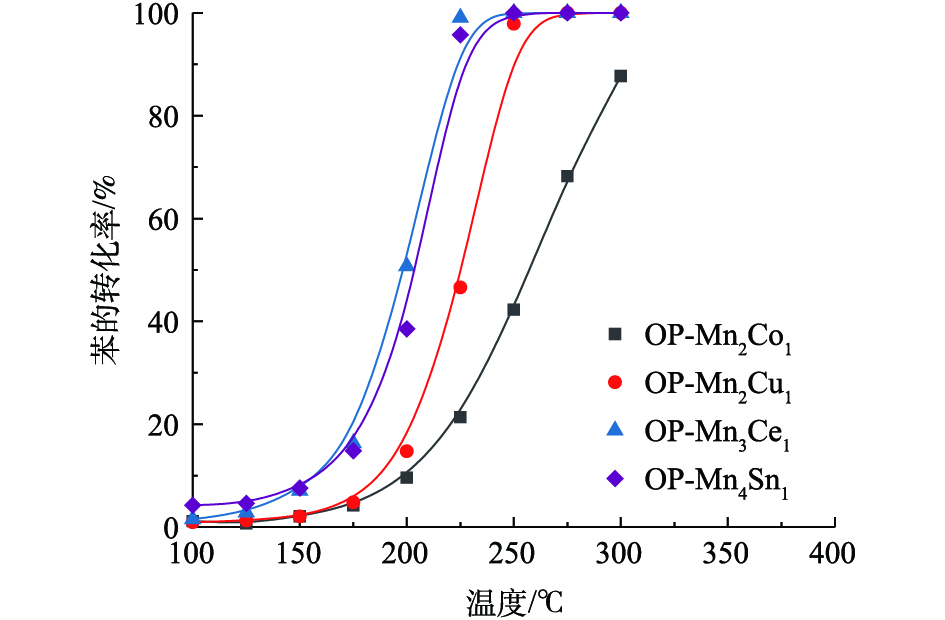
氧化还原共沉淀制备的二元复合锰氧化物催化剂对苯的催化氧化性能评价结果如图7所示。由此可知,二元复合锰氧化物催化剂的活性顺序为OP-Mn4Sn1 ≈ OP-Mn3Ce1 > OP-Mn2Cu1 > OP-Mn2Co1。产物(CO2和CO)通过气相色谱仪进行在线监测和分析,发现CO2的选择性均为100%,且无其他副产物。与单一金属氧化物Mn2O3相比,不同金属元素(Co、Cu、Ce、Sn)掺杂均能增强锰基催化剂的活性,其中Sn、Ce掺杂的效果最佳。可见,对于不同催化体系,催化剂的氧化还原性与催化性能之间没有必然联系。OP-Mn3Ce1和OP-Mn4Sn1催化剂的性能最好,可能与Ce和Sn可以与Mn形成更好的固溶体结构有关,不同过渡金属元素之间的相互作用更强。
2.1. 氧化还原共沉淀法和共沉淀法制备的Mn3Ce1的催化活性的比较
2.2. 氧化还原共沉淀法制备的不同锰氧化物催化剂的催化活性比较
-
1) 经Ce掺杂后,2种方法制备的锰铈氧化物催化剂均可以提高单一锰催化剂的还原性与苯的催化氧化活性,但相对于共沉淀法而言,通过氧化还原共沉淀法制备的锰铈催化剂有较好的还原性与苯的催化氧化性能,并且具有较大的孔径和比表面积。
2) 通过氧化还原共沉淀法制备了不同元素(Co、Cu、Ce、Sn)掺杂的锰氧化物催化剂,并进行苯的催化氧化评价;XRD数据显示,Co和Cu 2种元素掺杂的锰催化剂,在焙烧500 °C的条件下,会有其他晶相析出,而Ce和Sn 2种元素掺杂的锰催化剂的XRD谱图上并未观察到明显的衍射峰,说明催化剂中2种过渡金属元素混合较为均匀。
3) 苯的催化研究结果表明,相比于单一锰氧化物(Mn2O3),4种元素掺杂时的还原峰均向低温区移动,并且均能提高Mn2O3的催化氧化活性,其中Ce、Sn掺杂的锰氧化物催化剂的催化氧化性能最佳;而对于不同催化体系,催化剂的氧化还原性与催化性能之间没有必然联系。





 下载:
下载:




 百度学术
百度学术


