-
溶解空气气浮(DAF)已经被广泛应用于给水处理、废水处理和中水回用过程[1-2]。虽然DAF对COD、BOD5和TSS有一定程度的去除,但其限制了溶解性有机物的去除[3-4]。由于臭氧具有较强的氧化性,故其作为消毒剂和氧化剂也被广泛的应用于给水和废水处理中[5-6],臭氧氧化的主要目的是脱色和去除天然难降解有机物[7-8]。相比传统的深度处理工艺(混凝+沉淀+过滤),DOF工艺有着较高的脱色、脱臭和有机物去除率[9]。同时,由于DOF工艺将混凝、分离、脱色、除臭和消毒等多个过程集中于同1个操作单元[3],比常规深度处理工艺具有低于4倍的水力停留时间[9],节省了建设费用和土地成本。但臭氧气浮工艺对溶解性有机物的去除效果仍有一定的局限性,未能高效去除二级出水中的残余有机物。
目前,有关气浮工艺的研究大多仍处于基础操作条件的优化[10-11]和气泡大小的改变对气浮工艺去除性能的影响[12-14]。为了进一步提高气浮工艺的去除特性,之前的研究[15-18]着重于溶气水中气泡电荷性质及表面性质的改性对污染物去除特性的影响。ARABLOO等[19]和PASDAR等[20]分别研究了不同浓度表面活性剂和高分子聚合物对气泡理化性质及大小分布的影响。RAO等[21-22]从气泡Zeta电位和PAM剩余浓度等角度对比了聚合物甲基丙烯酸二甲胺基乙酯(N,N-dimethylaminoethyl methacrylate)和N,N-二烯丙基-N,N-二甲基氯化铵(N,N-diallyl-N,N-dimethylammonium chloride)对气泡表面电荷性质改善的效果,并揭示了PAM和微气泡的作用机理。
先前的研究对絮体表面性质的改性,依然停留在不同价态电解质对表面的改性阶段[23-24]。高分子聚合物PAM应用于气浮工艺中大多是改性气泡表面的性质[17-25],很少涉及对絮体表面特性改善的研究。而且,在气浮工艺中微气泡和絮凝体的结合特性通常用去除性能、接触角及絮体形态特征等表征[26-27],尚未从气载絮体的尺寸大小、形态特征等角度阐明气浮效果。本研究探究了阴阳离子型PAM对以腐殖酸为代表的天然有机物在DOF工艺中去除特性的影响,从去除性能、气载絮体大小及分形维数和接触角等多角度阐明污染物和微气泡的结合特性,揭示絮体表面性质对溶解性有机物去除性能的影响。
-
原水用商品腐殖酸配制,称取10 g商品腐殖酸在pH=12的强碱条件溶解于5 L水中,充分搅拌24 h后,用0.45 μm的滤膜过滤保证水样中的有机物为溶解态。实验时水样的腐殖酸浓度为5 mg·L−1 TOC左右。
实验所用的主要试剂包括聚合氯化铝(PAC)和阴、阳离子型聚丙烯酰胺(ployacrylamide,PAM),电荷密度用电离度表示。阳离子型聚合物FO 4125 SSH分子质量为7×106 Da,电荷密度为2%;阴离子型聚合物AN 905 SH分子质量为11×106 Da,电荷密度为9%。
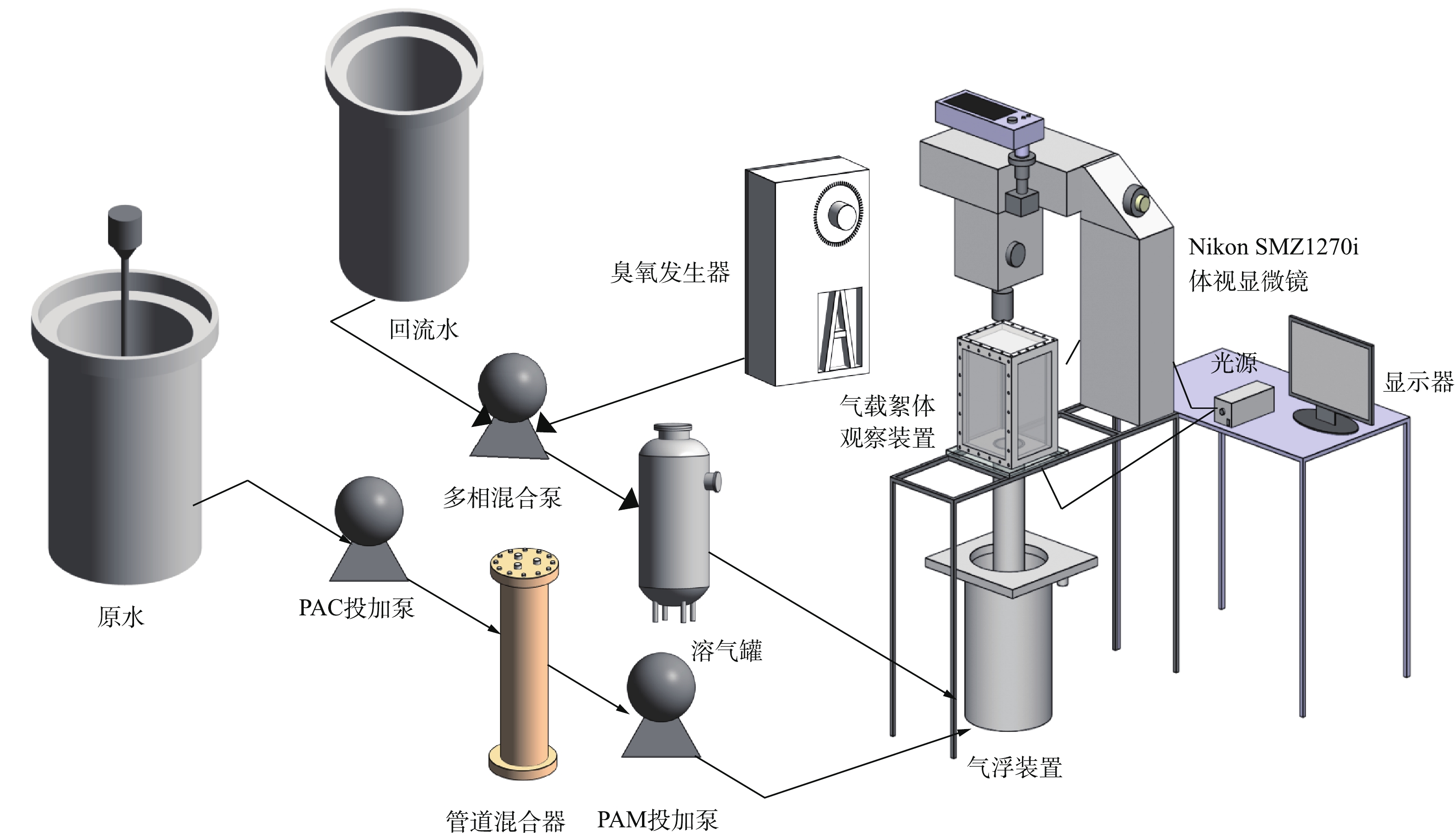
臭氧气浮工艺的实验装置见图1,主要由进水系统和溶气水系统2个部分组成。原水加入PAC后在管道混合器中充分混合,经快速混凝后进入接触区底部。回流水在溶气泵、溶气罐的作用下充分溶解臭氧气体,在接触区压力的释放产生大量的微气泡,与原水中的微小絮体结合形成气载絮体,到达分离区后继续上升至顶部形成浮渣。其中,DOF工艺中最重要的一部分是气载絮体的表征技术,利用抽吸装置将气载絮体溶液通过长45 cm和内径5 cm的有机玻璃圆柱体上升管缓慢抽吸到上部观察容器(10 cm×10 cm×15 cm),Nikon SMZ1270i体视显微镜放置在其上表面的正上方。单孔双光纤卤素灯冷光源(21 V,150 W)分别放置在CCD相机的对面和侧面,为图像捕捉提供合适的对比度。实验过程中DOF工艺的运行参数见表1。
-
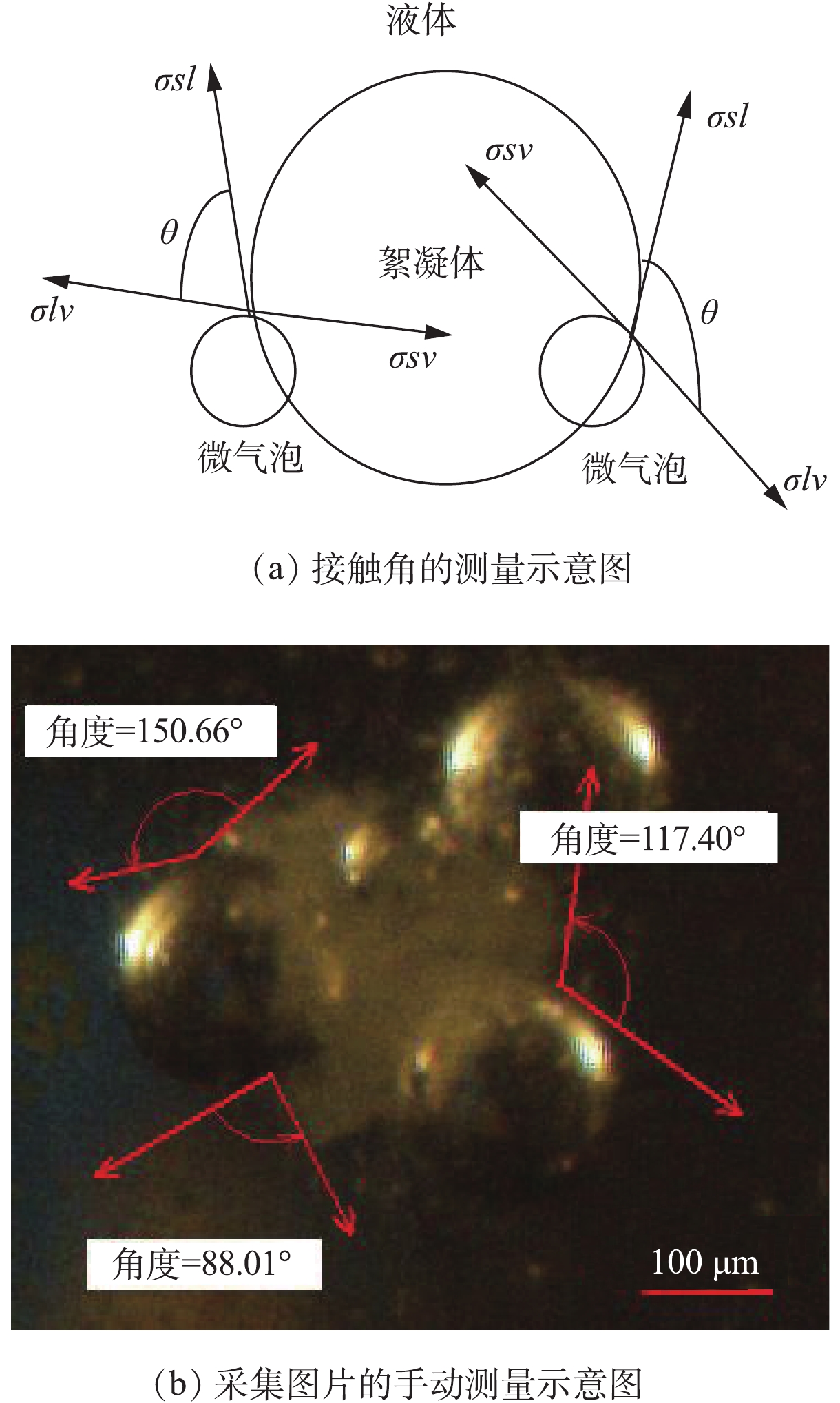
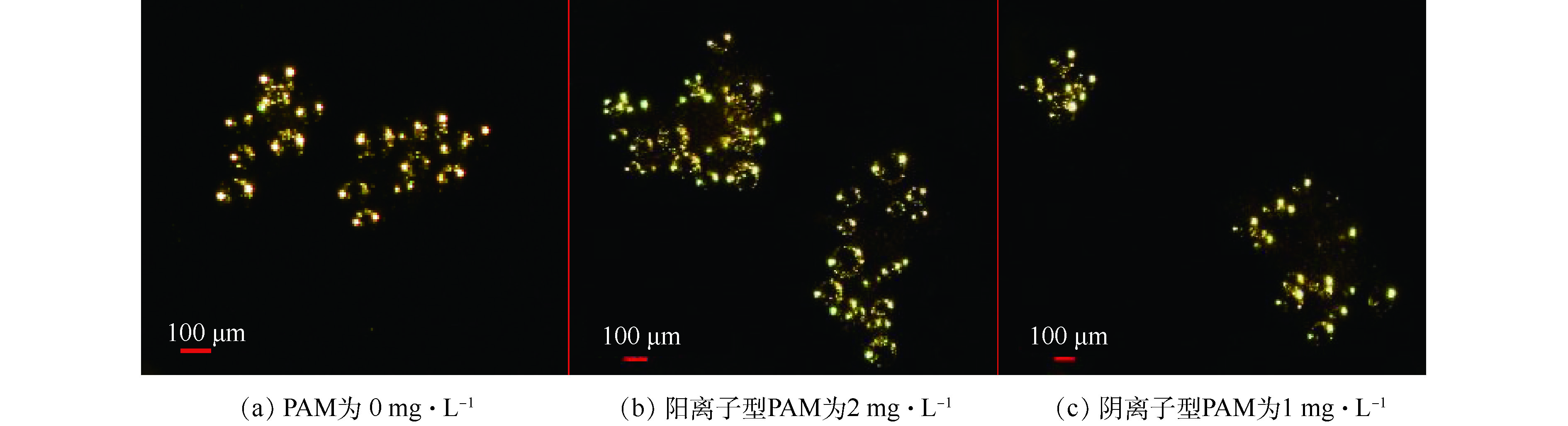
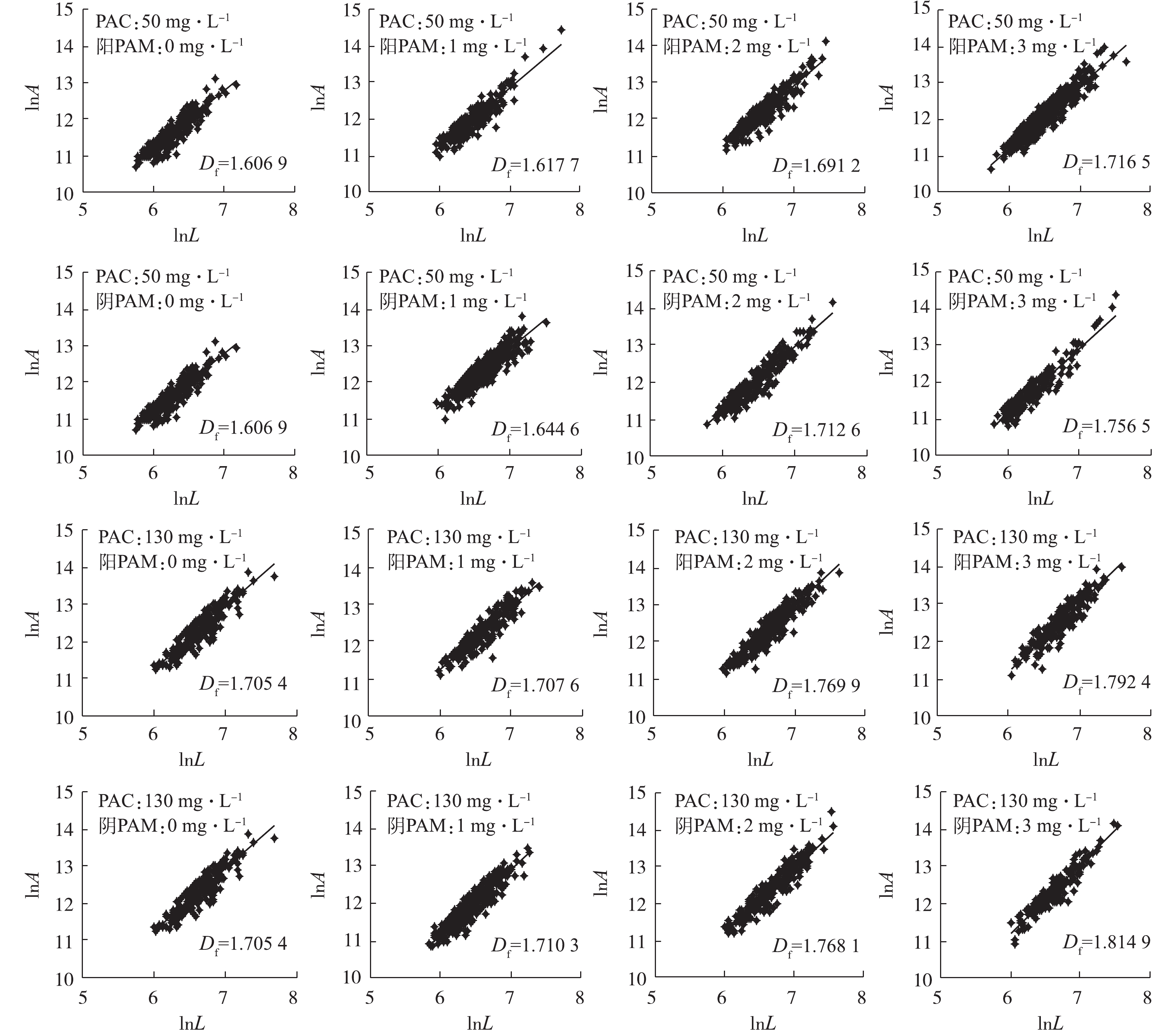
本实验涉及的测定项目主要包括Zeta电位、色度、UV254、TOC、气载絮体的尺寸大小、二维分形维数和接触角。其中,Zeta电位采用马尔文Zeta电位分析仪(Zetasizer Nano ZS90型,英国马尔文仪器有限公司)测定;色度用分光光度铂钴比色法测定(UV-4802型,UNIC);UV254采用紫外分光光度计(UV-4802型,UNIC)在254 nm下的吸光度值;TOC采用日本岛津公司生产的TOC-VCPH分析仪直接分析总有机碳浓度;采集的图像借助图像分析测量软件(NIS-Elements D 3.2)测量气载絮体粒径、特征长度、接触角等参数,气载絮体平均粒径(d50)按等面积圆直径求得;根据气载絮体的投影面积A与其最大长度L的函数关系lnA=D2 lnL+lnα,在对数坐标上作图,所得直线的斜率即为气载絮体的二维分形维数;接触角描述多相体系中絮凝体和微气泡间结合特性,采集的图片根据杨氏方程手动测量,如图2所示。在实验过程中,400多个气载絮体被用于尺寸大小、二维分形维数和接触角等分析。
-
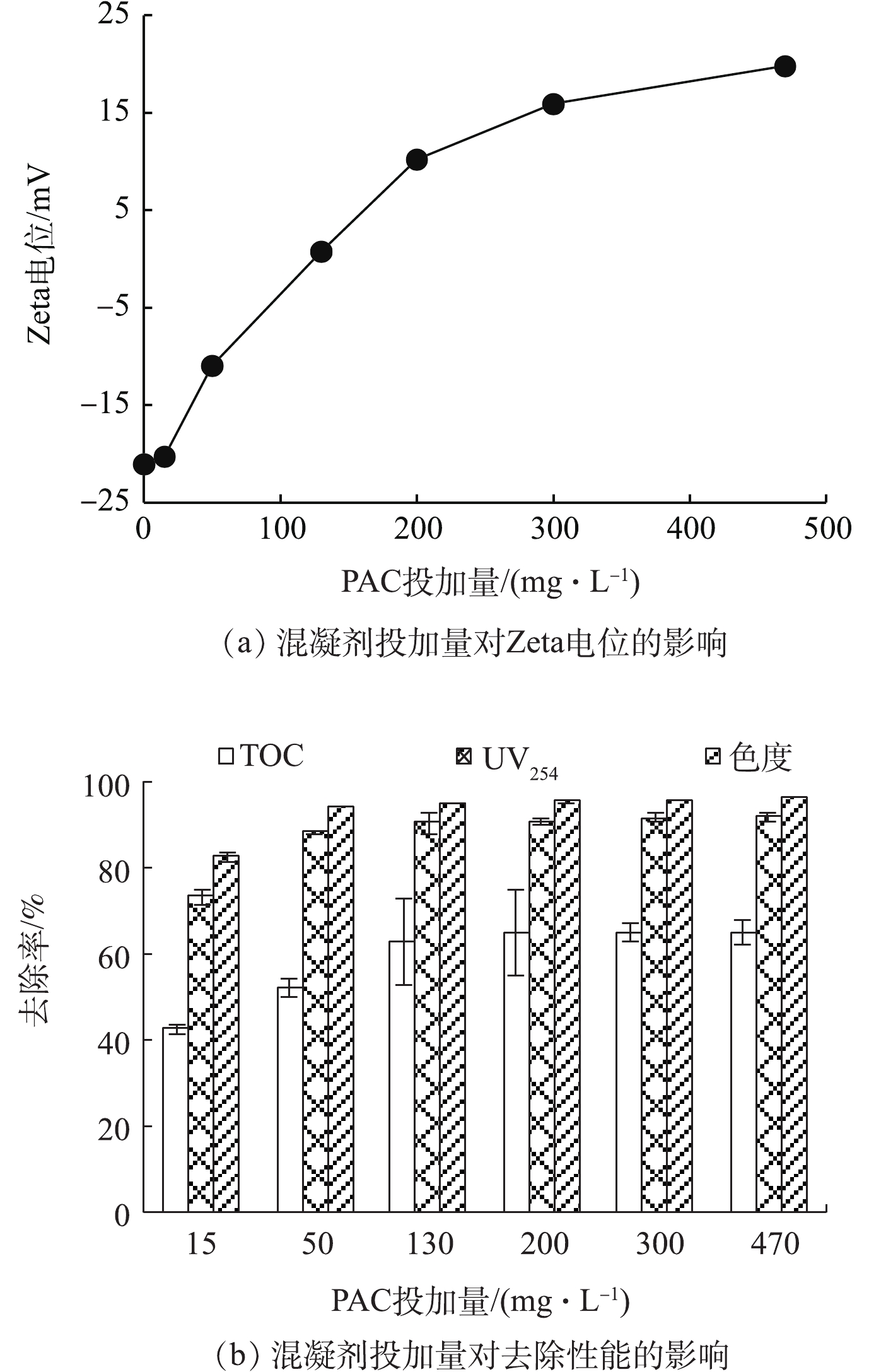
为了考察混凝剂投加量对DOF工艺中絮体Zeta电位及去除性能的影响,调节混凝剂投加量为0~470 mg·L−1,结果如图3所示。随着PAC投加量的增加,Zeta电位呈升高趋势,TOC、UV254和色度去除率在低PAC投加量下显著升高,在高PAC投加量下趋于稳定趋势。UV254反映的是能够吸收紫外光的有机物,如含有不饱和键和芳香结构的有机物,一般认为色度是由C=C、苯环物质及金属离子等引起的真实色度。在低PAC投加量下,处于亚稳定状态的絮体与微气泡碰撞、结合,但由于带负电的絮体与带负电的微气泡间存在静电排斥力,使得气浮效果并未达到最优。随着PAC投加量的增加,同一混合体系中混凝剂和臭氧之间的互促增效作用产生了具有强氧化性的羟基自由基[28],其可氧化降解大分子有机化合物,从而形成了更多的小分子有机化合物[29],通过微气泡的上升带动污染物的去除,从而实现TOC、UV254和色度的去除。当PAC投加量高于130 mg·L−1时,絮凝体以网扫卷捕的混凝机理脱稳,形成较大的絮体,需要大量微气泡的粘附,故存在微气泡的结合,致使其破灭,不利于气浮,从而导致TOC、UV254和色度的变化呈稳定趋势。这与腐殖酸在混凝过程中最佳混凝剂投加量[30]相一致,即在等电点附近,这表明混凝预处理对DOF工艺去除性能起着重要作用。
-
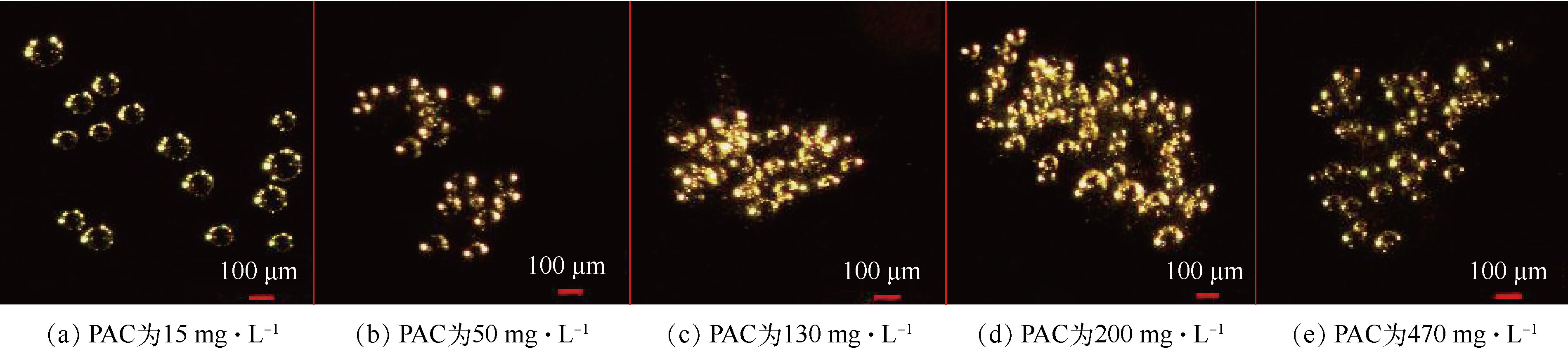
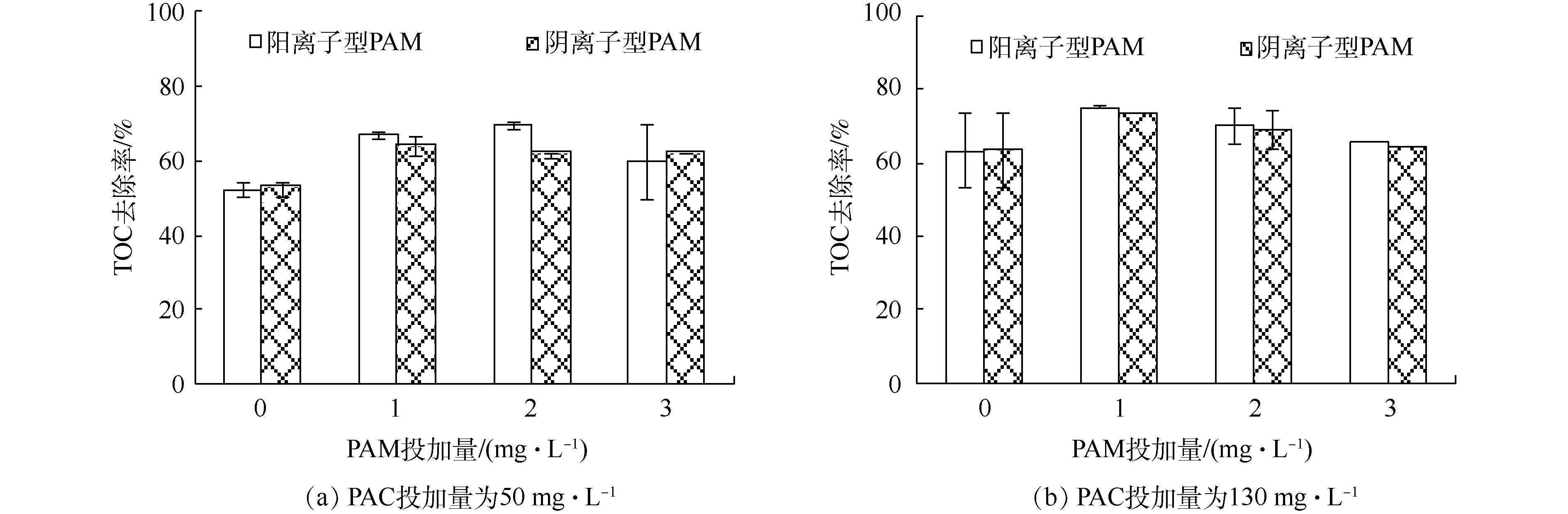
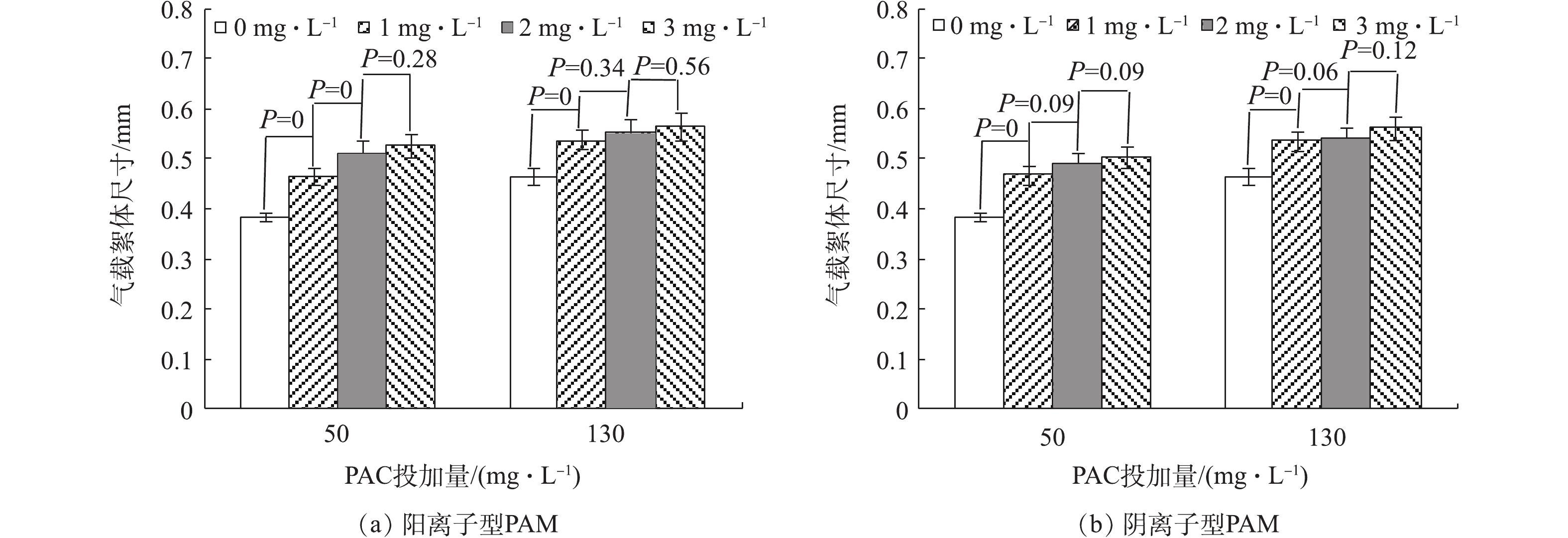
为了探究不同种类的PAM投加量对气浮效果的去除性能,对比了不同PAC投加量下对腐殖酸的去除效果及气载絮体的微观拍摄图(图4)。结果表明,PAC投加量小于50 mg·L−1未形成气载絮体,PAC投加量大于130 mg·L−1形成较大的气载絮体,不利于气浮工艺。因此,选择使絮体处于亚稳定状态的PAC投加量50 mg·L−1和最佳脱稳状态的PAC投加量130 mg·L−1,研究了不同种类的PAM投加量对DOF工艺中溶解性有机物去除性能的影响,结果如图5所示。对于2种不同类型的PAM来说,PAM的投加均有利于TOC的去除,在达到最佳投加量后,变化趋势并不明显。在相同的PAC投加量下,阳离子PAM在最佳剂量下的去除效果高于阴离子PAM最佳剂量下的去除效果。针对阳离子型PAM,在PAC投加量为50 mg·L−1、PAM投加量为2 mg·L−1时,腐殖酸去除效果最佳;而对于PAC剂量为130 mg·L−1、PAM投加量为1 mg·L−1时,腐殖酸去除效果总体达到最优。对于阴离子型PAM,不论哪种PAC投加量,PAM投加量均为1 mg·L−1时腐殖酸去除效果均达到最优。这与絮凝体、PAM和微气泡间静电引力有着密切的关系。阳离子PAM的投加,通过电荷中和作用,利于污染物的气浮分离。而阴离子PAM的投加,覆盖在微气泡表面形成的气泡群利于絮体的气浮分离[31],同时絮凝体、PAM和微气泡间强的静电斥力不利于微气泡和絮体间的结合,因此,低PAM投加量下微气泡群占主导优势,腐殖酸去除性能呈增加趋势,而高投加量下强负电荷间的静电排斥力也占重要作用,使腐殖酸去除效果变化不明显。
-
比较不同种类PAM剂量下气载絮体的粒径变化,结果如图6所示。对于不同种类的PAM,与PAC投加量为50 mg·L−1时形成气载絮体的尺度相比,PAC投加量为130 mg·L−1时形成的气载絮体粒径较大,这与混凝过程中絮体的脱稳状态相关。在PAC投加量为130 mg·L−1时,颗粒处于最佳的脱稳状态,颗粒间和微气泡的碰撞几率较大,利于气载絮体的形成,则粒径较大。此外,不论哪种PAC投加量,在最佳PAM投加量之前,随着PAM剂量的增加,气载絮体粒径呈先增大后稳定的趋势,这表明在絮体表面PAM适量的吸附利于气载絮体的长大。阳离子型PAM最佳剂量下对应形成的气载絮体粒径大于阴离子型PAM最佳剂量下的粒径。即:在PAC投加量50 mg·L−1时,d阳离子(0.51 mm)>d阴离子(0.46 mm),如图7所示;在PAC投加量为130 mg·L−1时,阴、阳离子型PAM最佳剂量下形成的气载絮体粒径差异较小,如图8所示。这与PAM在颗粒表面的吸附方式有关。PAM剂量对气载絮体粒径的影响可从絮体、PAM高分子和微气泡间的相互作用关系解释。在PAC投加量为50 mg·L−1时,带负电的絮体间存在较强的静电斥力。加入阳离子型PAM后,一方面,带负电的腐殖酸颗粒被电中和进一步脱稳,粒间距离缩短;另一方面,表面吸附的PAM可电中和带负电的微气泡,利于气浮分离。在PAC投加量为130 mg·L−1时,等电点絮体间的静电力可忽略不计,通过氢键作用吸附在颗粒表面的阳离子型PAM可进一步中和微气泡的电荷,以此增加了颗粒和微气泡的碰撞和结合效率。有研究[32]表明,絮体和微气泡结合形成气载絮体的Zeta电位越接近等电点,越有利于气浮分离。这也解释了达到最佳气浮效果时,PAC投加量50 mg·L−1所需的阳离子型PAM剂量高于PAC投加量为130 mg·L−1时的PAM剂量。当PAM浓度较高时,高分子PAM在颗粒表面的覆盖率接近100%,颗粒表面已无吸附空位,架桥作用无法实现,颗粒因位阻效应较大而分散,絮体粒径反而减小,但微气泡的吸附使得气载絮体的粒径并未减小(P>0.05)。同样,对于阴离子型 PAM来说,吸附PAM的微气泡群利于在颗粒表面黏附,利于气浮分离,但颗粒、PAM 和微气泡间存在强的静电斥力,不利于气浮分离,是否有利于气浮分离主要取决于PAM的剂量。
-
图9反映了二维分形维数随不同种类PAM投加量变化的关系。王晓昌等[33-34]认为絮凝体分形维数与密度呈正相关,即分形维数越大,絮凝体越密实。由此可以推断,在DOF工艺中,气载絮体的分形维数越小,越利于气浮分离。对于同一类型PAM,2种PAC投加量对应的分形维数的大小与气载絮体尺度的变化相一致。即:在PAC投加量为130 mg·L−1时,絮体完全脱稳,形成致密的絮体,分形维数较大;而对于PAC剂量为50 mg·L−1时,絮体处于亚稳定状态,形成疏松的絮体,相应的分形维数较小。对于不同类型PAM,最优剂量选取变化曲线与二维分形维数变化曲线趋势相同。但对于阳离子型PAM、PAC投加量为50 mg·L−1的分形维数,在最佳PAM剂量时,气载絮体的分形维数并不是最小的,而随着PAM投加量的增加呈增加趋势,这与在颗粒表面通过强的静电引力吸附了大量的PAM有关,使得分形维数较大。
上述实验结果仍归因于絮体、PAM高分子与气泡间的相互作用。PAM剂量为0 mg·L−1时,絮体的生长主要靠电中和机理,易形成较致密的絮体,分形维数较大。对于阳离子型PAM,随着PAM投加量的增加,通常由于高分子伸展的链状吸附及远距离胶体间的架桥作用形成疏松的絮体结构,但由于絮体和微气泡在强静电引力的作用力下彼此靠近、结合,形成致密的气载絮体,分形维数增大;对于阴离子型PAM,颗粒、PAM高分子与微气泡间强的静电斥力使的少量微气泡的黏附,因此,较阳离子型PAM形成的气载絮体分形维数较大。
-
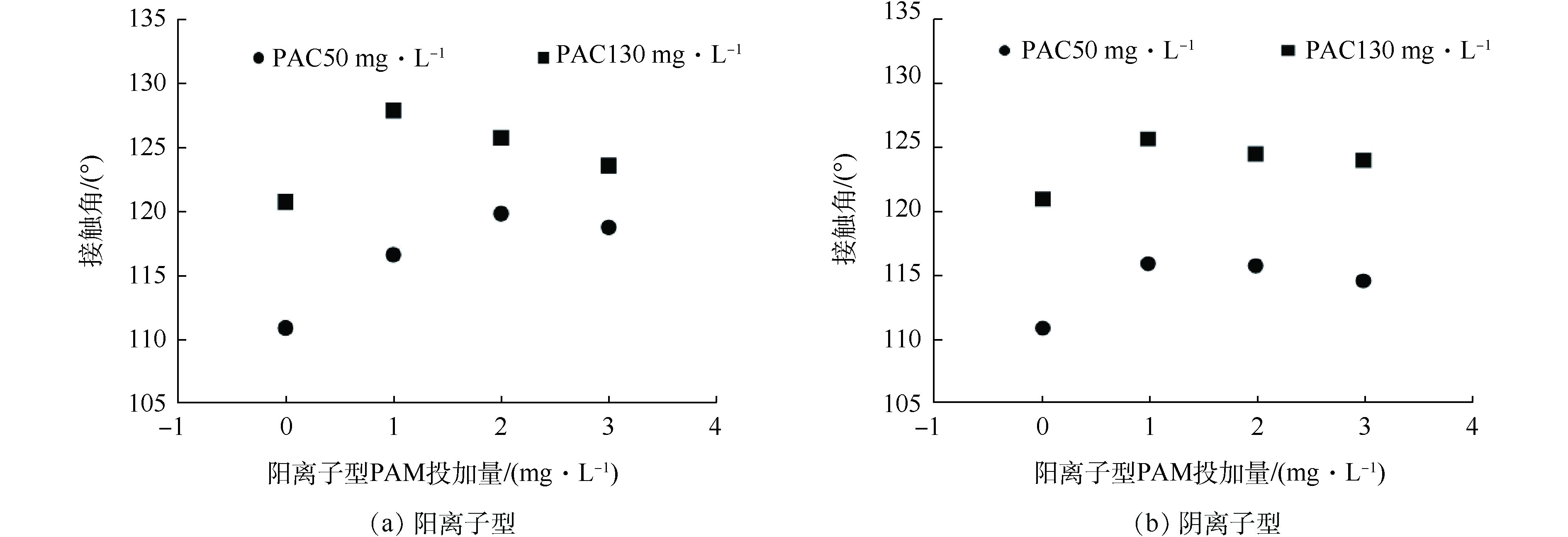
多相混合体系中微气泡和絮凝体间接触角随PAM剂量变化的关系如图10所示。图中反映的是颗粒表面的亲疏水性质。通常,接触角越大,表面的疏水性越强,气泡附着在表面的效果越好,则气浮效果越好[35]。对比去除性能曲线与接触角变化曲线可发现,接触角的变化趋势与去除效果呈正相关关系,即在PAC投加量不变时,随着PAM投加量的增加,存在最佳接触角。在低PAM投加量时,PAM的亲水基团以相互作用力吸附在絮体表面,疏水端远离水溶液,利用微气泡的上升力带动絮凝体远离水溶液,利于气载絮体的形成,从而利于污染物分离;随着PAM投加量增加,由于空间位阻的原因,根据热力学定律,絮体表面的PAM处于压缩状态,不利于微气泡和PAM的相互作用,则絮凝体和微气泡间的接触角降低。同样,固定PAC投加量时,阳离子PAM的接触角大于相同投加量阴离子PAM剂量下的接触角,这与颗粒、阳离子型PAM和微气泡间强静电吸附密切相关。
-
1)与PAC投加量50 mg·L−1相比,在PAC投加量为130 mg·L−1时,溶解性有机物的去除率较高,气载絮体的粒径、分形维数和接触角均较大。
2)PAM的投加提高了DOF工艺中的去除性能,且气载絮体尺寸、分形维数和接触角均较大。在PAC投加量为50 mg·L−1时,阴离子型PAM和阳离子型PAM最佳投加量分别为1 mg·L−1和2 mg·L−1;在PAC投加量130 mg·L−1时,阴离子型PAM和阳离子型PAM最佳投加量均为1 mg·L−1。
3)在最佳PAM投加量下,与阴离子PAM相比,阳离子型PAM形成气载絮体的大小和接触角较大,分形维数较小,且去除效果较好。
臭氧气浮工艺絮体尺度与界面理化特性调控
Regulation of the size and interfacial physicochemical properties of flocs in dissolved ozone flotation
-
摘要: 针对臭氧气浮工艺对有机物脱色和去除性能的限制,通过阴、阳离子型聚丙烯酰胺(ployacrylamide,PAM)对以腐殖酸为代表的天然有机物絮体的尺度调控,采用气载絮体尺度、二维分形维数及絮体与微气泡间的接触角对PAM浓度梯度下形成的气载絮体进行了表征;研究了阴阳离子型PAM浓度梯度对腐殖酸的降解效果和气载絮体尺度;并探讨了阴阳离子型PAM、絮体与微气泡间的结合机理。结果表明:PAM的投加提高了臭氧气浮工艺中腐殖酸的去除性能,且气载絮体尺寸、分形维数和接触角均有所增大;在不同类型PAM中均存在最佳PAM剂量,在此剂量下,体系内去除效果、气载絮体大小和接触角最大、分形维数最小;在最佳PAM剂量下,与阴离子型PAM相比,阳离子型PAM形成气载絮体的大小和接触角较大,分形维数较小,且去除效果较好。在臭氧气浮体系中,通过改善PAM、絮体与微气泡结合静电作用力的强弱形成了利于气浮的气载絮体尺度,提高了对腐殖酸的去除效果。
-
关键词:
- 臭氧气浮工艺(DOF) /
- 腐殖酸 /
- 气载絮体 /
- 形态特征
Abstract: In response to the performance limitations on the decolorization and removal of organic matter by dissolved ozone flotation process(DOF), anionic and cationic polyacrylamide (PAM) were used to regulate floc size of the natural organic matter represented by humic acid, and aerated flocs formed at different PAM concentration gradients were characterized by aerated floc size, two-dimensional fractal dimension and contact angle between flocs and microbubbles. In addition, the effects of anionic and cationic PAM concentration gradients on humic acid degradation and aerated floc size were studied, and binding mechanism among anionic and cationic PAM, flocs and microbubbles was discussed. The results indicated that PAM addition improved humic acid removal in DOF process, and aerated floc size, fractal dimension and contact angle increased. There was an optimal dosage for different kinds of PAM, at which the removal efficiency, aerated flocs size and contact angle were the largest, and fractal dimension was the smallest. Compared with the anionic PAM at its optimum dosage, the formed aerated flocs using cationic PAM were characterized as larger size, higher contact angle, smaller fractal dimension and better humic acid removal effect. In DOF system, favorable aerated floc size could be formed to improve the removal efficiency of humic acid by ameliorate the electrostatic interaction strength among PAM, flocs and microbubbles. -
表 1 臭氧气浮装置的操作条件
Table 1. Standard operational condition for DOF reactor
运行参数 数值 运行参数 数值 原水进水流速/(L·h−1) 120 分离区高度/m 1.5 管道混合时间/s 30 直径/m 0.3 臭氧投加量/(mL·min−1) 60 表面流速/(m·h−1) 2.21 回流水的流速/(L·h−1) 36 水力停留时间/min 40 压力/MPa 0.4 总体积/m3 0.10 -
[1] RUBIO J, SOUZA M L, SMITH R W. Overview of flotation as a wastewater treatment technique[J]. Minerals Engineering, 2002, 15(3): 139-155. doi: 10.1016/S0892-6875(01)00216-3 [2] RODRIGUES R T, RUBIO J. DAF-dissolved air flotation: Potential applications in the mining and mineral processing industry[J]. International Journal of Mineral Processing, 2007, 82(1): 1-13. doi: 10.1016/j.minpro.2006.07.019 [3] LEE B H, SONG W C, MANNA B, et al. Dissolved ozone flotation (DOF): A promising technology in municipal wastewater treatment[J]. Desalination, 2008, 225(1): 260-273. [4] WILINSKI P, NAUMCZKY J. Dissolved ozone flotation as an innovative and prospect method for treatment of micropollutants and wastewater treatment costs reduction[EB/OL]. [2018-12-10]. https://hal-enpc.archives-ouvertes.fr/file/index/docid/709736/filename/06-WWW-YES-2012-Wilinski-Paper-DT-2012-06-12.pdf. [5] GRAHAM J L, STRIEBICH R, PATTERSON C L, et al. MTBE oxidation byproducts from the treatment of surface waters by ozonation and UV-ozonation[J]. Chemosphere, 2004, 54(7): 1011-1016. doi: 10.1016/j.chemosphere.2003.09.017 [6] JOHN D E, HASS C N, NWACHUKU N, et al. Chlorine and ozone disinfection of Encephalitozoon intestinalis spores[J]. Water Research, 2005, 39(11): 2369-2375. doi: 10.1016/j.watres.2005.04.013 [7] SELCUK H. Decolorization and detoxification of textile wastewater by ozonation and coagulation processes[J]. Dyes & Pigments, 2005, 64(3): 217-222. [8] SHU H Y, CHANG M C. Decolorization effects of six azo dyes by O3, UV/O3 and UV/H2O2 processes[J]. Dyes & Pigments, 2005, 65(1): 25-31. [9] JIN P K, WANG X C, HU G. A dispersed-ozone flotation (DOF) separator for tertiary wastewater treatment[J]. Water Science & Technology, 2006, 53(9): 151-157. [10] OLIVEIRA C, RODRIGUES R T, RUBIO J. Operating parameters affecting the formation of kaolin aerated flocs in water and wastewater treatment[J]. Clean-Soil Air Water, 2014, 42(7): 909-916. doi: 10.1002/clen.v42.7 [11] OLIVEIRA C, RODRIGUES R T, RUBIO J. A new technique for characterizing aerated flocs in a flocculation-microbubble flotation system[J]. International Journal of Mineral Processing, 2010, 96(1): 36-44. [12] REAY D, RATCLIFF G A. Removal of fine particles from water by dispersed air flotation: Effects of bubble size and particle size on collection efficiency[J]. Canadian Journal of Chemical Engineering, 1973, 51(2): 178-185. doi: 10.1002/cjce.v51:2 [13] CALGAROTO S, AZEVEDO A, RUBIO J. Separation of amine-insoluble species by flotation with nano and microbubbles[J]. Minerals Engineering, 2016, 89: 24-29. doi: 10.1016/j.mineng.2016.01.006 [14] AZEVEDO A, ETCHEPARE R, RUBIO J. Raw water clarification by flotation with microbubbles and nanobubbles generated with a multiphase pump[J]. Water Science & Technology, 2017, 75(10): 2342-2349. [15] OLIVEIRA C, RUBIO J. Zeta potential of single and polymer-coated microbubbles using an adapted microelectrophoresis technique[J]. International Journal of Mineral Processing, 2011, 98(1/2): 118-123. [16] HENDERSON R K, PARSONS S A, JEFFERSON B. Surfactants as bubble surface modifiers in the flotation of algae: Dissolved air flotation that utilizes a chemically modified bubble surface[J]. Environmental Science & Technology, 2008, 42(13): 4883-4888. [17] YAP R K L, WHITTAKER M, DIAO M, et al. Hydrophobically-associating cationic polymers as micro-bubble surface modifiers in dissolved air flotation for cyanobacteria cell separation[J]. Water Research, 2014, 61(18): 253-262. [18] HENDERSON R, PARSON S, JEFFERSON B. The potential for using bubble modification chemicals in dissolved air flotation for algae removal[J]. Separation Science & Technology, 2009, 44(9): 1923-1940. [19] ARABLOO M, SHAHRI M P. Effect of surfactant and polymer on the characteristics of aphron-containing fluids[J]. Canadian Journal of Chemical Engineering, 2016, 94(6): 1197-1201. doi: 10.1002/cjce.v94.6 [20] PASDAR M, KAZEMZADEH E, KAMARI E, et al. Monitoring the role of polymer and surfactant concentrations on bubble size distribution in colloidal gas aphron based fluids[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2018, 556: 93-98. [21] RAO N R H, GRANVILLE A M, BROWNE C I, et al. Determining how polymer-bubble interactions impact algal separation using the novel " posi”-dissolved air flotation process[J]. Separation & Purification Technology, 2018, 201: 139-147. [22] RAO N R H, YAP R, WHITTAKER M, et al. The role of algal organic matter in the separation of algae and cyanobacteria using the novel " posi”-dissolved air flotation process[J]. Water Research, 2017, 130: 20-30. [23] GHANNAM M T. Wetting behavior of aqueous solutions of polyacrylamide over polyethylene substrat[J]. Journal of Chemical & Engineering Data, 2002, 47(2): 274-277. [24] GRAY S R, HARBOUR P J, DIXON D R. Effect of polyelectrolyte charge density and molecular weight on the flotation of oil in water emulsions[J]. Colloids & Surfaces A: Physicochemical & Engineering Aspects, 1997, 126(2/3): 85-95. [25] SHI Y, YANG J, MA J, et al. Feasibility of bubble surface modification for natural organic matter removal from river water using dissolved air flotation[J]. Frontiers of Environmental Science & Engineering, 2017, 11(6): 1-10. [26] JAMESON G J. Hydrophobicity and floc density in induced-air flotation for water treatment[J]. Colloids & Surfaces A: Physicochemical & Engineering Aspects, 1999, 151(1/2): 269-281. [27] WANG Y H, ZHUO S G, LI N, et al. Influences of various aluminum coagulants on algae floc structure, strength and flotation effect[J]. Procedia Environmental Sciences, 2011, 8(1): 75-80. [28] JIN X, JIN P K, HOU R, et al. Enhanced WWTP effluent organic matter removal in hybrid ozonation-coagulation (HOC) process catalyzed by Al-based coagulant[J]. Journal of Hazardous Materials, 2017, 327: 216-224. doi: 10.1016/j.jhazmat.2016.12.043 [29] JIN X, JIN P K, WANG X C. A study on the effects of ozone dosage on dissolved-ozone flotation (DOF) process performance[J]. Water Science & Technology, 2015, 71(9): 1423-1428. [30] 王晓昌, 金鹏康. 腐殖酸铝盐絮凝体的动态特性[J]. 环境科学, 2002, 23(4): 71-75. doi: 10.3321/j.issn:0250-3301.2002.04.015 [31] OLIVEIRA C, RUBIO J. Kaolin aerated flocs formation assisted by polymer-coated microbubbles[J]. International Journal of Mineral Processing, 2011, 93(2): 31-36. [32] LI C, XING Y, GUI X, et al. Enhancement of oxidized coal flotation by pre-conditioning with positive charged microbubbles[J]. International Journal of Coal Preparation & Utilization, 2017, 11(2): 1-11. [33] 王晓昌, 丹保宪仁. 絮凝体形态学和密度的探讨(Ⅰ):从絮凝体分形构造谈起[J]. 环境科学学报, 2000, 20(3): 257-262. doi: 10.3321/j.issn:0253-2468.2000.03.001 [34] 王晓昌, 丹保宪仁. 絮凝体形态学和密度的探讨(Ⅱ):致密型絮凝体形成操作模式[J]. 环境科学学报, 2000, 20(4): 385-390. doi: 10.3321/j.issn:0253-2468.2000.04.001 [35] MULLENEERS H A E, KOOPAL L K, BRUNING H, et al. Selective separation of fine particles by a new flotation approach[J]. Separation Science and Technology, 2002, 37(9): 2097-2112. doi: 10.1081/SS-120003503 -




 下载:
下载: