-
磷是生命体不可或缺的营养元素,在生物的生长、发育和繁殖过程中起着至关重要的作用[1]1。它是一种不可再生资源,主要源自磷矿石的开采。有研究表明,按目前的开采速率,现存的磷矿储备最多仅够维持372a[2]。另一方面,水体中过量的磷容易引发水体富营养化,进而破坏生态环境[3]。为了保护资源和环境,磷回收技术应运而生,并逐渐受到人们的关注。其中,鸟粪石(MgNH4PO4·6H2O)结晶法由于具备氮磷去除效果好、反应速率快、产品为优质缓释肥料等特点而备受青睐[4-5]。
鸟粪石产品的商品化是鸟粪石法能否实际应用的关键,而鸟粪石的商品化价值取决于其产品质量。鸟粪石的产品质量与所使用的结晶反应器密切相关,目前主流的鸟粪石结晶反应器为搅拌釜和流化床[6]。有研究表明,完全混合式的搅拌釜无法将杂质与鸟粪石产品进行有效分离,所得的鸟粪石污染物含量较高;流化床采用上升水流作为物料混合和颗粒流化的推动力,可实现轴向上的水力分级,大幅降低产品中杂质含量,所得产品纯度高,安全性好[7-8]。
截至目前,鸟粪石结晶流化床尚无设计规范。研究人员多根据经验或半经验公式计算流化速度,并设计不同的管径以实现鸟粪石在反应器中的分级[9-11]。管径的变化除了具备分级效果,还可创造一定的湍流以促进物料的混合及晶体的聚并[12]。目前较为常见的流化床构型主要有多段式和锥体式。FATTAH等[11]使用多段式流化床对污水处理厂的污泥压滤浓缩液开展磷回收,磷酸盐去除率超过90%,磷回收率高于85%,所得产品中鸟粪石纯度高达96%。李咏梅等[7]采用锥体式流化床反应器对污泥脱水上清液进行处理,
PO3−4 -P的去除率最高可达90.5%,产生颗粒的最大粒径在2.0~3.2 mm之间,纯度在80%以上。2种流化床反应器构型均具备理想的磷去除效果及产品特性,但结构较复杂,加工难度大。鸟粪石微晶的流失会导致总磷(TP)去除率下降,是鸟粪石流化床面临的首要问题,目前主要通过设置沉淀池、安装筛网或投加混凝剂进行截留[13-15]。其中,外置沉淀池的目的是为了保证沉淀效果,须设计较大容积的沉淀池,从而增加了基建成本;安装筛网虽经济有效,但须频繁清理,人工维护成本较高;投加混凝剂则需额外的药剂费用及污泥处置费用。综上所述,无论是复杂的反应器外部构型或是额外的微晶截留措施,均不可避免地增加了加工难度及运行维护成本。鉴于结晶反应器内流体运动的复杂性,完全从实验角度开展反应器优化研究将会非常费时、费力。随着计算机性能的不断提高,计算流体力学(computational fluid dynamics,CFD)已被广泛应用于反应器结构的优化,其避免了传统经验方法中繁复的实验过程,对结晶反应器的设计、优化及放大提供更加可靠的依据和详尽的信息。针对鸟粪石结晶流化床构型设计的不确定性及复杂性,本研究首先采用数值模拟的方法,探明多粒径体系下不同构型流化床的湍流强度、分级特性和微晶截留效率;然后通过实验研究新构型鸟粪石结晶流化床的磷去除效果与产品特性,以验证数值模拟优化方法的可靠性和合理性。
全文HTML
-
采用冷态数值模拟的方法研究流化床外部构型对鸟粪石产品分级情况与湍流强度的影响以及内部构件对鸟粪石微晶截留的影响,确定适宜鸟粪石流化床的外部构型及内部构件。
-
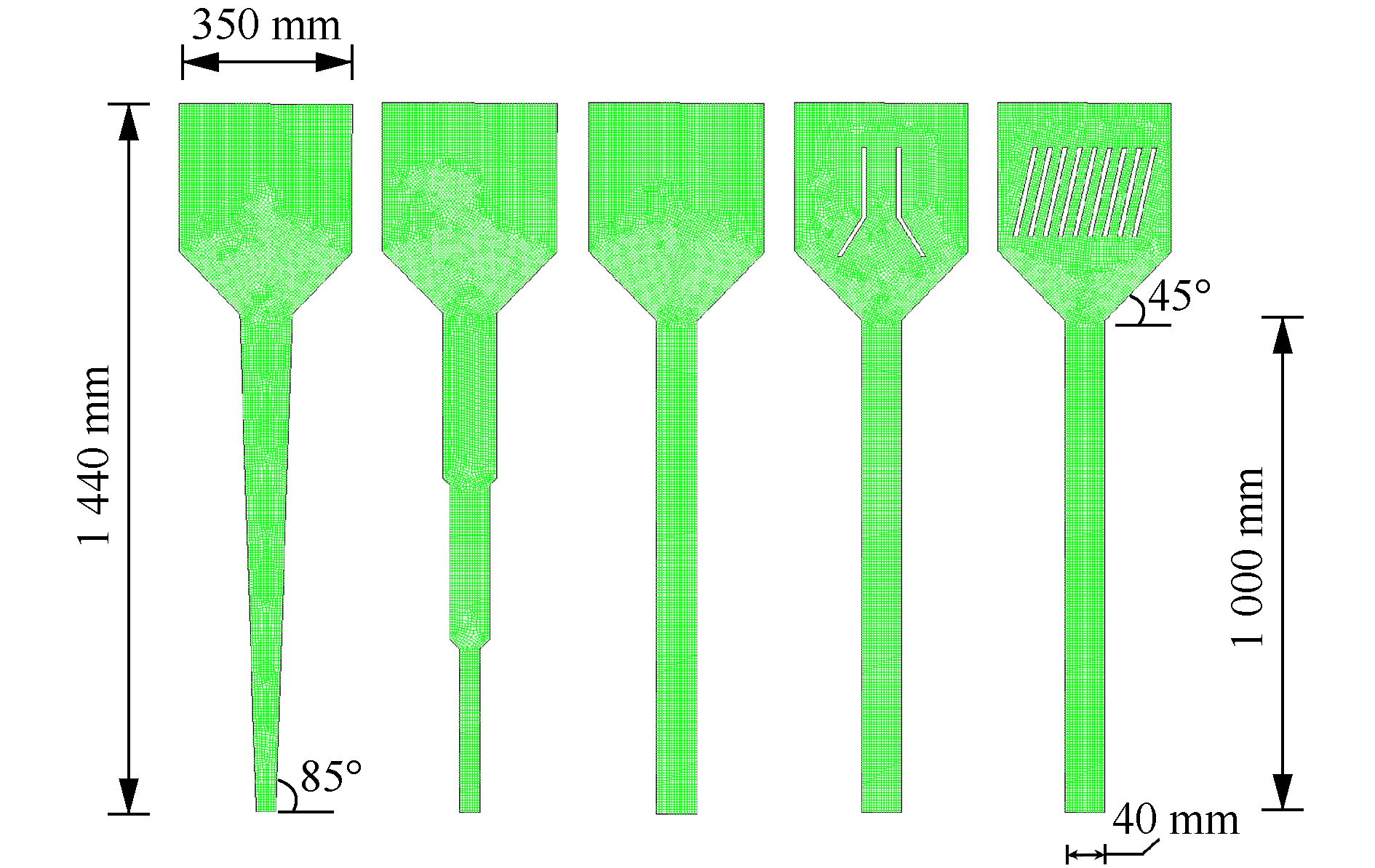
考察的流化床外部构型包括目前常见的多段圆柱式、一段锥体式和一段圆柱式。3种构型的流化床均由反应区和沉淀区组成。所对比的流化床内部构件为三相分离器及斜板,安装于沉淀区中,用以考察其对鸟粪石微晶的截留效果。鉴于流化床结构的规则性,采用Gambit 2.4软件建立流化床二维模型(X-Z平面),并采用四边形结构性网格进行划分。流化床外部构型及内部构件的具体构型与尺寸如图1所示。
-
根据文献中报道的鸟粪石粒径设置模拟粒径[10],考察流化床外部构型对3类混合粒径的分级情况及湍流特性,同时明确内部构件对小粒径鸟粪石的截留情况。具体条件设定如表1所示。
-
研究表明,曳力是固液相间运动的主要作用力。前期研究结果[16]已证实了曳力模型对鸟粪石流化体系模拟精度的重要性。由于Syamlal-O′Brien曳力模型在较宽的流速及粒径范围内对鸟粪石床层膨胀的模拟精度优于其他曳力模型,因此,本研究选用Syamlal-O′Brien曳力模型开展模拟研究。其余控制方程的表达式见鸟粪石冷态流化模拟的研究[16],模拟参数设定如下。基本设置:分离式求解器,欧拉双流体模型,Dispersed湍流模型,一阶迎风格式,残差为1×10−3,最大迭代次数为100次;边界条件:速度进口v=0.05 m·s−1,压力出口,无滑移壁面,标准壁面函数;液相参数:密度为998.2 kg·m−3,黏度为1.003×10−3;固相参数:密度为1 580.23 kg·m−3,粒径分别为0.2、0.5、1.0、2.0、3.0和4.0 mm,初始固相体积分数为60%,颗粒床层体积为380 cm3。
-
流化床反应器数值模拟计算采用Fluent 14.5,后处理采用Ensight 10.0。程序运行平台的主要参数:Intel Xeon十二核处理器(2颗),主频3.1 GHz,64 GB DDR3双通道内存。
-
为验证数值模拟结果的可靠性,明确结构优化后流化床的运行效果,本研究采用人工配水的方式考察不同进水磷浓度条件下,流化床的磷去除及产品颗粒粒径分布情况。配制的进水磷浓度为240、480和1 000 mg·L−1,采用磷酸二氢铵(纯度≥98%,武汉无机盐化肥有限公司)为磷源和氮源,采用六水合氯化镁(纯度≥99%,REDOX公司)为镁源,控制反应过程Mg/N/P摩尔比为1∶1∶1。采用氢氧化钠(纯度≥96%,沪试)调节反应液pH。
-
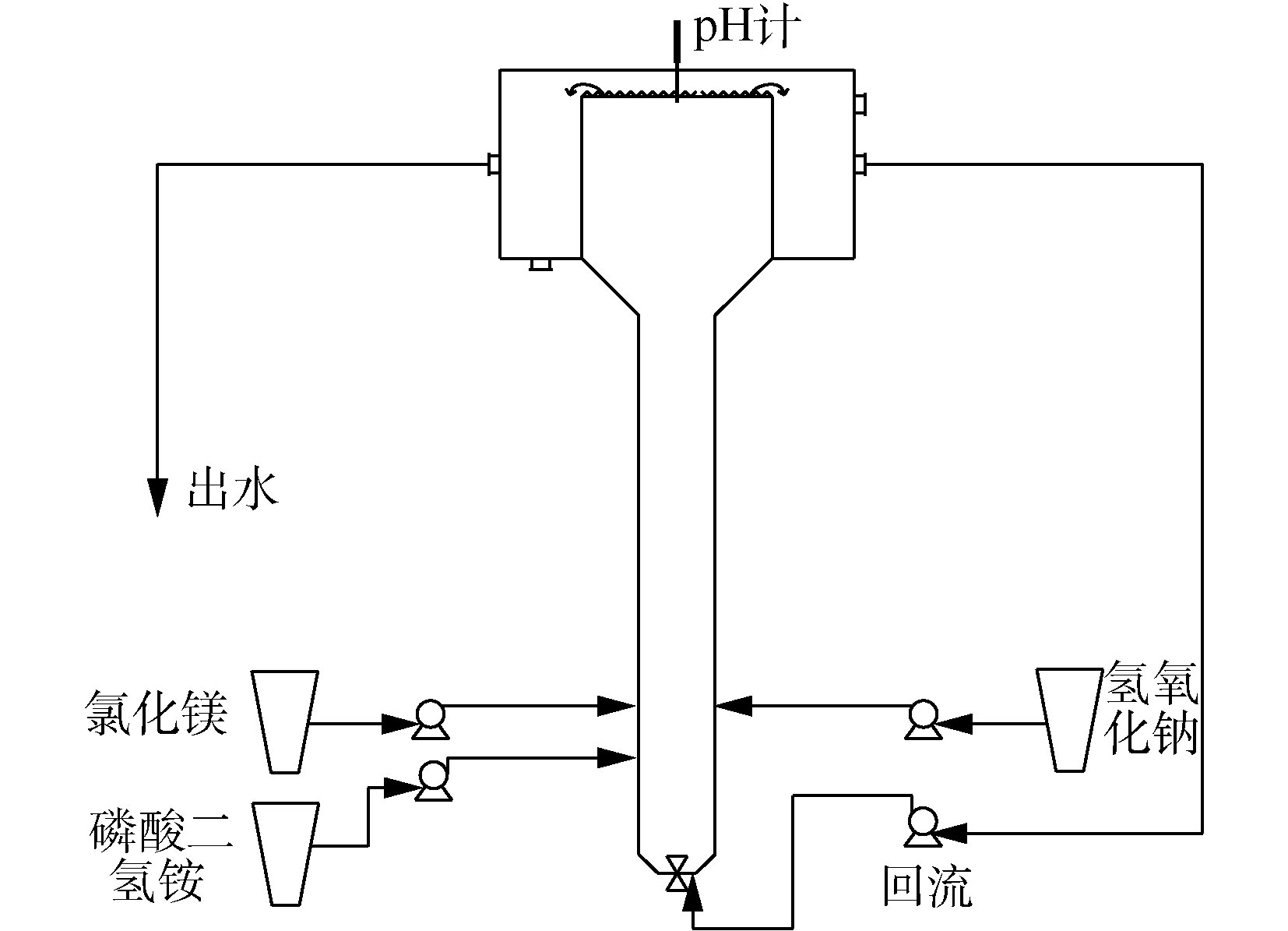
实验装置为一段式流化床,材质为有机玻璃,有效容积为50 L,由流化区和沉淀区组成(图2)。实验过程pH设置为8.5,进水流量为33 L·h−1。采用流化床出水回流作为物料混合及流化的推动力,设置流化区的上升流速为50 mm·s−1。
-
pH采用PC-3100(Suntex)在线pH计进行测定;
PO3−4 -P和TP采用钼锑抗分光光度法测定(HACH DR5000,USA);鸟粪石产品于38 ℃烘干24 h,采用标准筛(0.3 mm/1.25 mm/2.5 mm/3.2 mm)测定粒径;使用扫描电子显微镜(S-4800,Hitachi,Japan)观察鸟粪石产品的微观形貌。
1.1. 模型建立及计算
1.1.1. 几何建模及网格划分
1.1.2. 模拟条件设定
1.1.3. 模拟参数设定
1.1.4. 模型计算及后处理
1.2. 实验材料及方法
1.2.1. 实验水质
1.2.2. 实验装置及运行条件
1.2.3. 分析方法
-
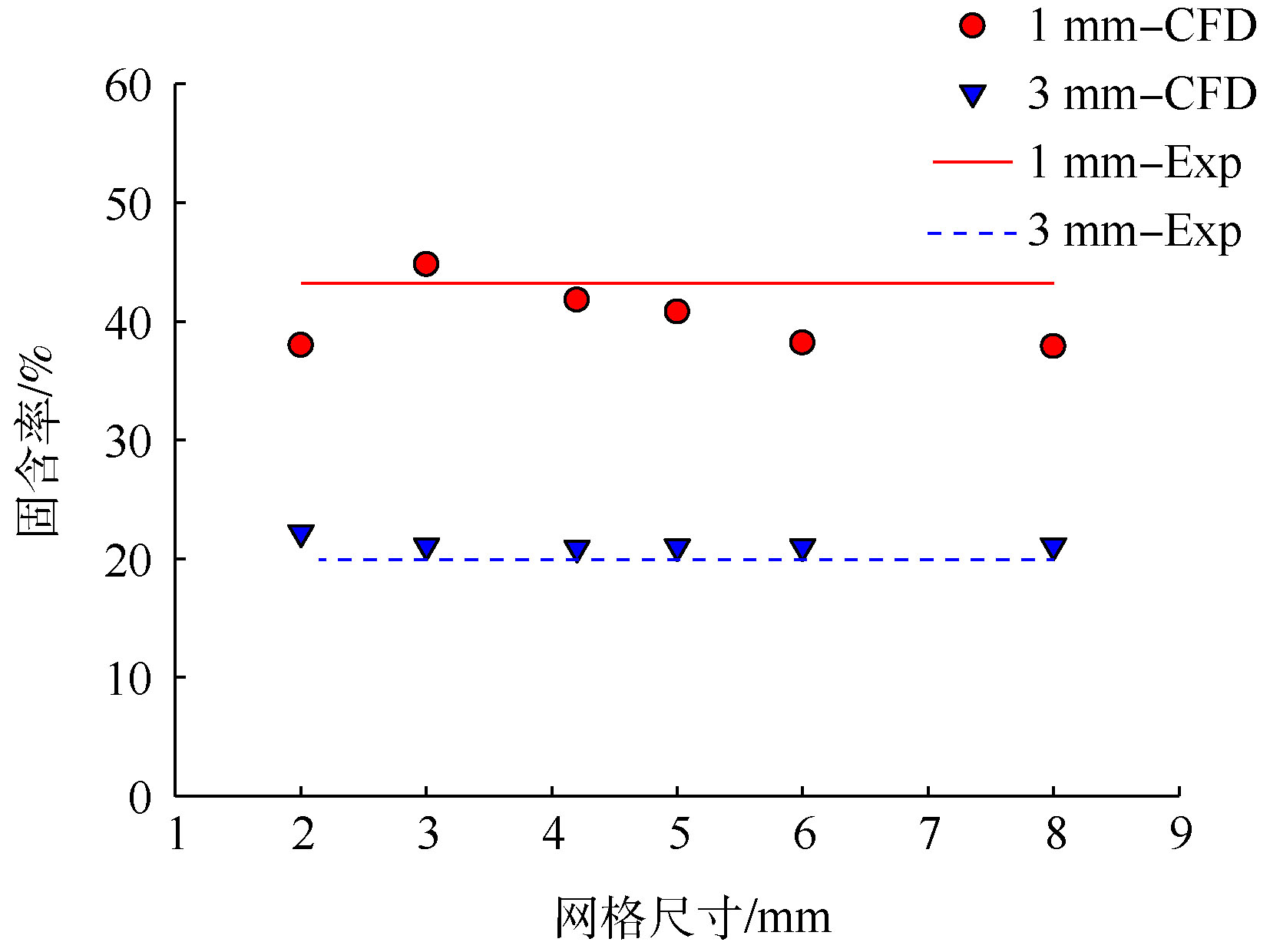
数值计算的基础是网格划分。当前的主流偏微分方程数值离散方法都是先计算节点上的物理量,而后通过插值方式求得节点间的值。因此,理论上网格点布置得越密集,所得到的计算结果也越精确。但网格加密带来了较大的计算量及舍入误差,所以从计算的效率及求解结果的精度来说,网格并非越多越好。网格过疏或过密均可能产生误差过大的计算结果。只有当网格数的增加对计算结果影响不大时,此时的数值模拟计算结果才具有意义,因此,首先必须进行网格无关性检验,可采用一段式流化床进行网格无关性研究(图3)。设置2.0、3.0、4.2、5.0、6.0和8.0 mm这6种网格尺寸,对应的网格数量分别为53 433、23 863、12 099、8 624、5 844和3 335个。通过对比体积平均粒径为1 mm和3 mm的鸟粪石颗粒在特定上升流速和初始堆积高度条件下的床层膨胀情况来判断其网格无关性。
图3为不同网格尺寸下,模拟床层与实验床层的膨胀情况对比。由图3可知,所建立的冷态流化模型对3 mm颗粒的模拟精度较好,不同网格尺寸差异较小,与实验结果偏差均在6%以内;但对1 mm颗粒的模拟结果波动较大,与实验偏差为3.3%~12.4%,其中,网格尺寸为3 mm和4.2 mm的精度最佳。综合考虑模拟精度与计算成本,选择尺寸为4.2 mm、数量为12 099个网格以供后续模拟。
-
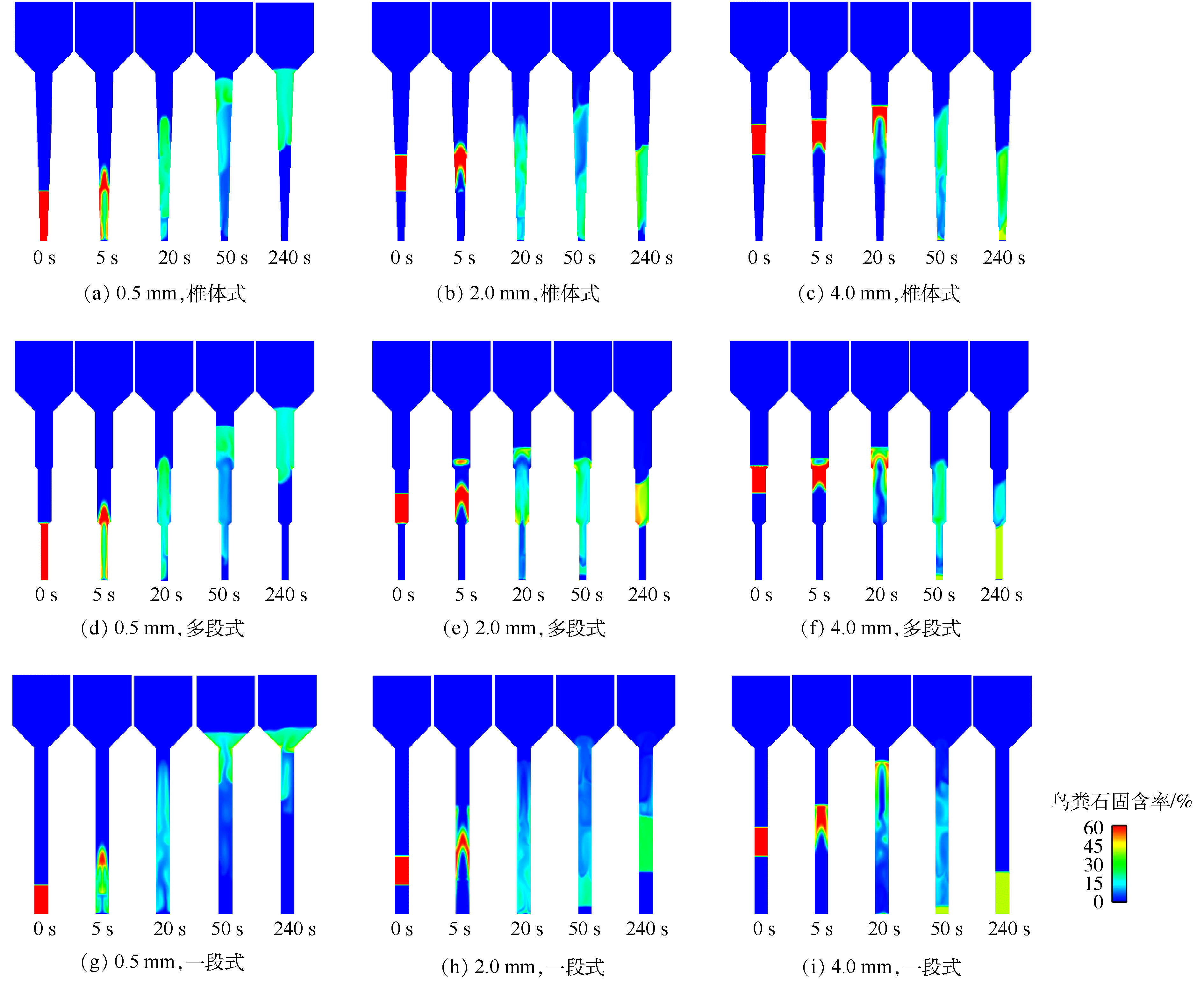
图4模拟了3种不同构型流化床在流化区进口上升流速为50 mm·s−1时,鸟粪石固含率随时间的分布云图。为了突出流化床构型对不同粒径鸟粪石产品的空间分级特性,选择颗粒粒径分别为0.5、2.0和4.0 mm。由图4可知,水流从流化床底部沿轴线穿过颗粒床层向上运动,此时空隙率的增大造成床层抬升,床层平均密度下降。在密度差的作用下,颗粒在反应器内循环运动。由于粒径的自由沉降速度随颗粒粒径的增大而增加,在相同的上升流速下,不同粒径颗粒的膨胀高度不同。反应器构型上的差异也导致了不同轴向高度上流速的不同。除了一段式流化床在流化区内上升流速不变外,锥体式流化床与多段式流化床的上升流速均随轴向高度的升高而减小,其中,锥体式流化床为逐步减小,而多段式流化床为阶梯性减小(图1)。粒径的不同与上升流速的变化综合导致了颗粒分级效果的差异。由图4可知,在相同操作条件下,多段式和一段式流化床均能对3种粒径的鸟粪石颗粒实现空间分级(图4(d)~(f),(g)~(i)),锥体式流化床对大粒径颗粒的分级效果较差(图4(b)和图4(c))。
-
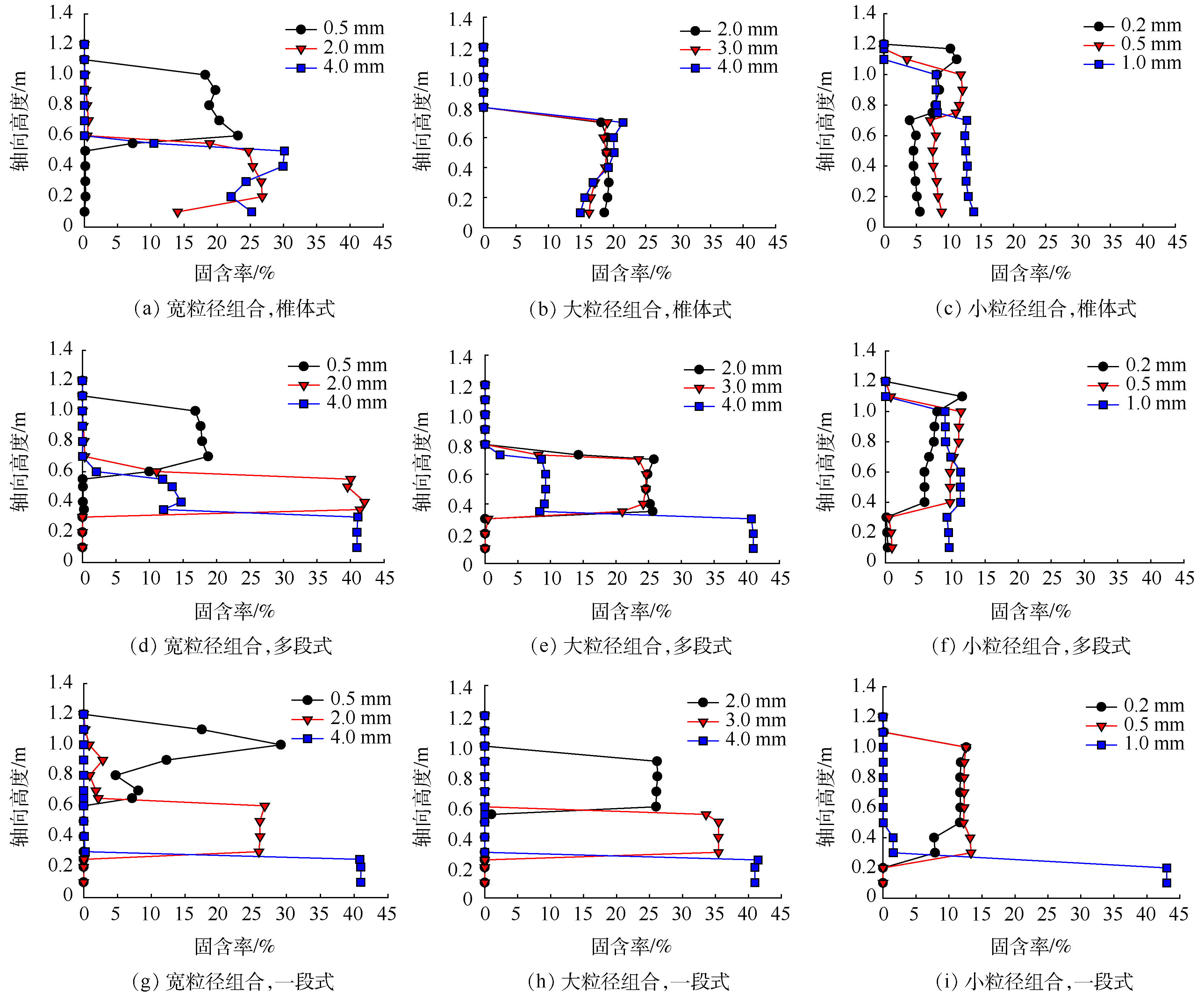
流化床结构是影响颗粒分级特性的关键因素。图5系统对比了不同鸟粪石粒径组合在3种不同流化床构型下的分级情况。
当鸟粪石粒径较大时(0.5、2.0和4.0 mm),锥体式流化床仅能将0.5 mm的颗粒与2.0 mm和4.0 mm的颗粒分离开,但不能将2.0 mm颗粒与4.0 mm颗粒分开(图5(a));多段式与一段式流化床均展示了良好的分级效果,3种粒径颗粒分布在不同的轴向位置(图5(d)和图5(g))。
当鸟粪石粒径较大时(2.0、3.0和4.0 mm),3种粒径的颗粒在锥体式流化床内混合在一起,分布在同一轴向高度上(图5(b))。多段式流化床仅能将部分4.0 mm颗粒与2.0 mm和3.0 mm颗粒分开,而对2.0 mm和3.0 mm颗粒无分级效果(图5(e)),这与多段式流化床的流化区管径设置有关[17]。在此案例中,为了确保3种流化床的流化段体积相同,多段式流化床的底部第1流化段管径较小,体积有限,4.0 mm颗粒部分被挤至中部第2流化段。另一方面,第2流化段的上升流速由于管径的增大而下降,仅略高于2.0 mm和3.0 mm颗粒的初始流化速度[17],因此,无法分离这2种粒径的颗粒。一段式流化床由于整个流化段管径无变化,水流的上升流速维持恒定,不同粒径颗粒所承受的上升推动力差异较大,因此,能较好地实现大粒径鸟粪石颗粒的分级(图5(h))。
当鸟粪石粒径较小时(0.2、0.5和1.0 mm),锥体式和多段式流化床均无法实现颗粒的分级(图5(c)和图5(f)),一段式流化床也仅能将1.0 mm颗粒与0.2 mm和0.5 mm颗粒分开,而对0.2 mm和0.5 mm颗粒无分级效果(图5(i))。
根据以上数值模拟结果,当流化段体积相同时,一段式流化床对3种不同粒径组合的分级效果最优,多段式流化床次之,锥体式流化床无分级效果。当采用多段式流化床时,为确保分离效果,流化段的管径与高度选择至关重要。
-
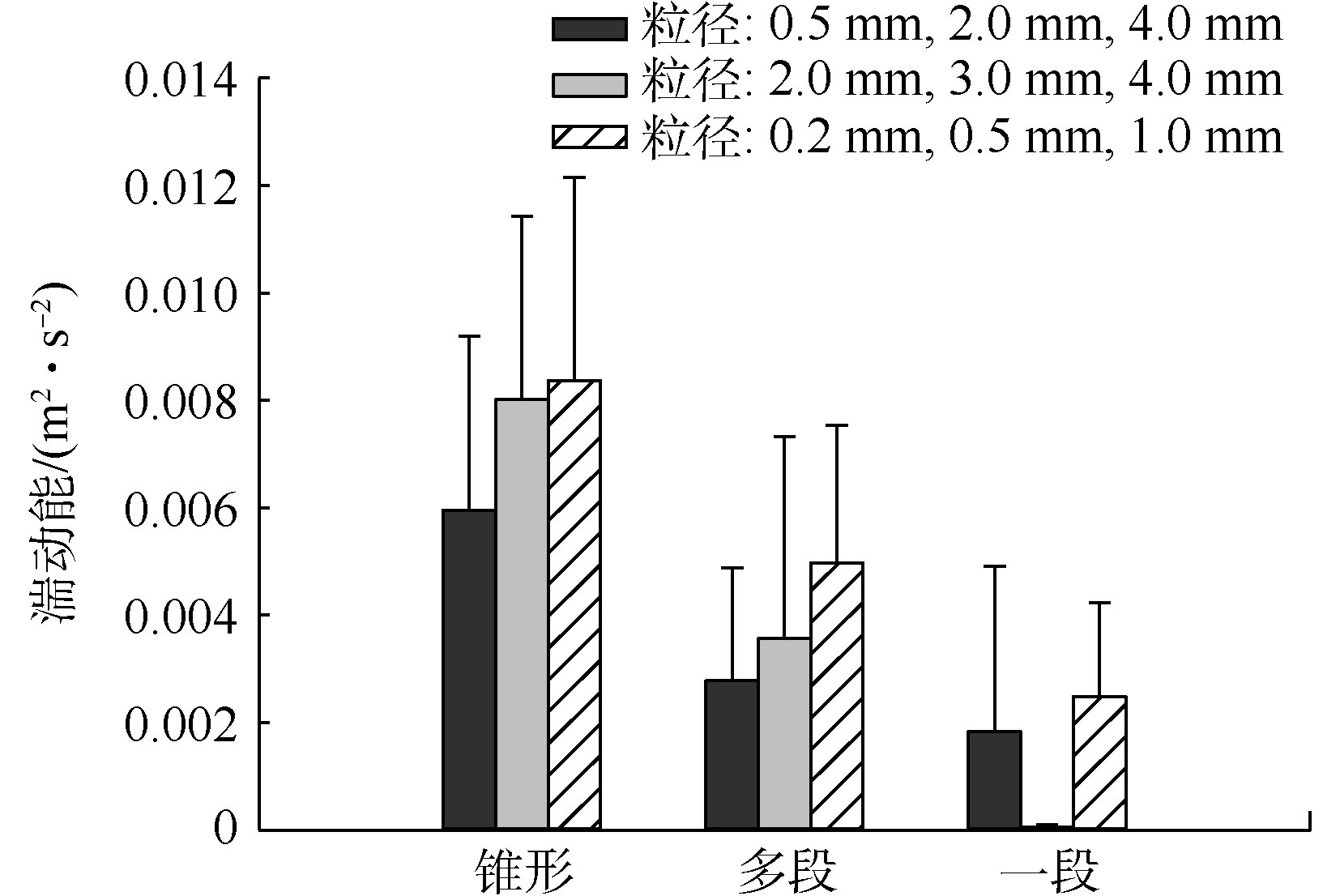
截至目前,湍流对鸟粪石颗粒化的影响并不明确。FATTAH等[18]通过调节上升流速,间接得出当上升流速高于500 cm·min−1时,上升水流产生的湍流会导致颗粒破碎的结论。YE等[10]明确了上升流速与鸟粪石颗粒粒径的正相关性。尽管上升水流形成的湍流是物料混合和颗粒流化及碰撞的推动力,但以上研究均没有直接分析湍流大小。由于实验测定湍流难度较大,本节采用数值模拟的方式对比3种构型流化床流化段的湍动能,来表征流化床结构对湍流的影响程度。
由图6可知,3种构型流化床的湍动能大小为锥体式最大,多段式次之,一段式最小,且随着流化粒径的减小而增大。由于存在变径,锥体式和多段式流化床内大粒径颗粒(如2.0、3.0和4.0 mm)的湍动能与小粒径(如0.2、0.5和1.0 mm)相差较小,导致鸟粪石粒径变大后速度波动仍然剧烈,对流明显,碰撞强度较大,这可能是前人报道的大粒径破碎的主要原因[18]。一段式流化床不存在变径,颗粒的运动速度随粒径的增大而减小,因此,颗粒粒径增大后碰撞强度降低。综上所述,一段式流化床的湍流特征可能更有助于鸟粪石造粒,此推测在2.2节的实验中也得到证实。
-
流化床采用上升水流作为物料混合和流化的推动力,细小的鸟粪石微晶易受上升水流夹带而流失,进而影响流化床的磷回收率。已有研究表明,提高回流比或降低上升流速等方式能有效减少微晶的流失[10]。但在相同的处理负荷条件下,提高回流比将增大流化床容积,同时须使用更大的回流水泵,从而不可避免地增加了基建与运行成本。降低上升流速从力学角度上虽能减少部分微晶流失,但同时也降低了物料混合效果,易造成构晶离子的局部过饱和,进而产生更多的微晶。
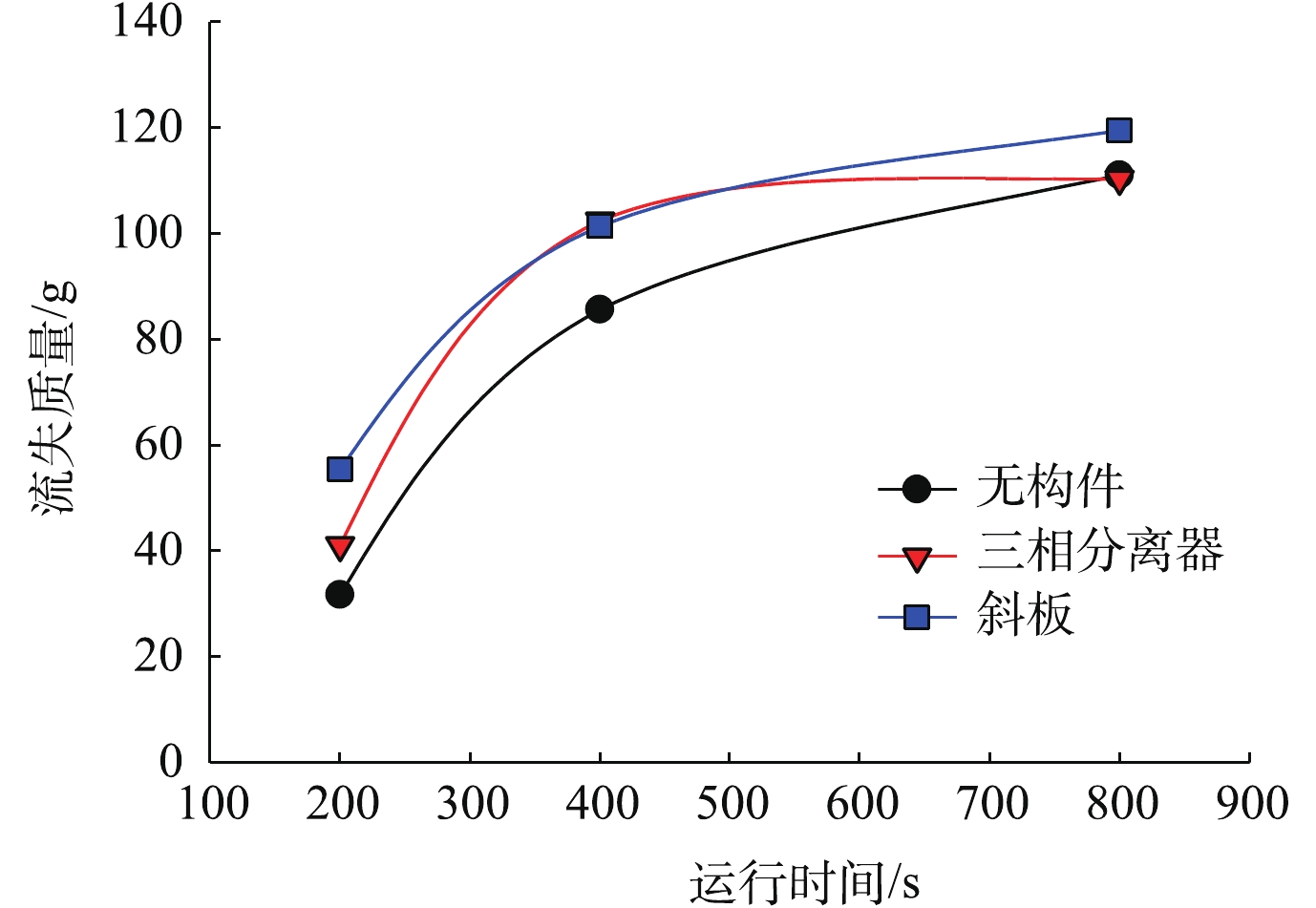
增加内部固液分离构件是增强颗粒沉淀效果的一种方式,本节通过数值模拟,在一段式流化床内流化粒径为0.2 mm的鸟粪石微晶,比较流化床沉淀区内安装构件前后鸟粪石微晶的截留效果。
如图7所示,增加内部构件后,流化前期(200 s和400 s)的微晶流失量略高于不加内部构件的工况,这是由于内部构件的设置减小了上升水流的过流面积,致使流速增大,加快了微晶的流失。在流化后期(800 s),3个工况的微晶总流失量差别不大,因此,通过增加内部固液分离构件来增强鸟粪石微晶截留的效果并不显著。
-
采用不带内部固液分离构件的一段式流化床反应器开展鸟粪石结晶连续实验。由于操作条件对除磷效果影响的研究已较为成熟,主要考察不同进水浓度下,一段式流化床反应器对磷的去除效果及鸟粪石的产品特性。
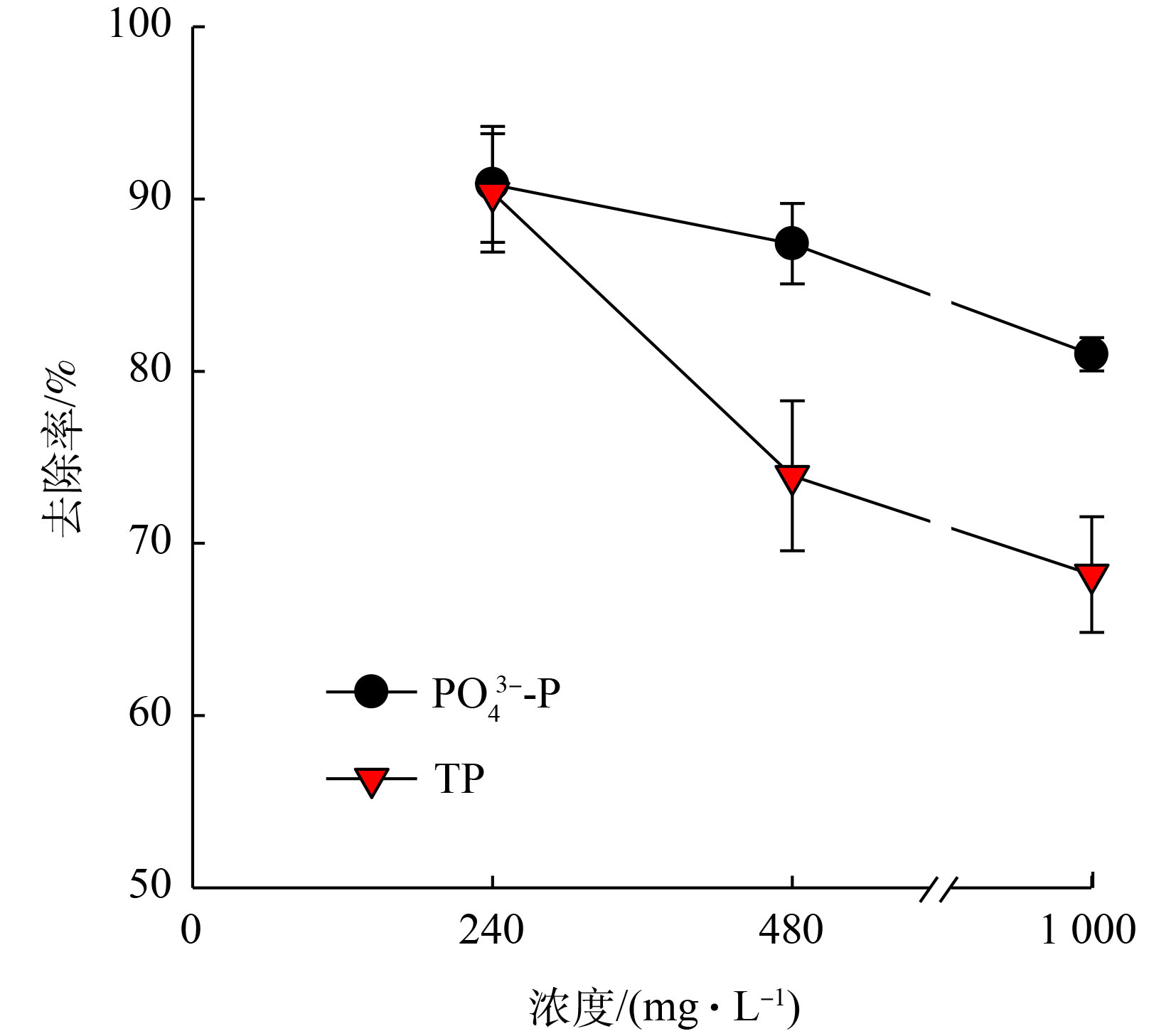
如图8所示,
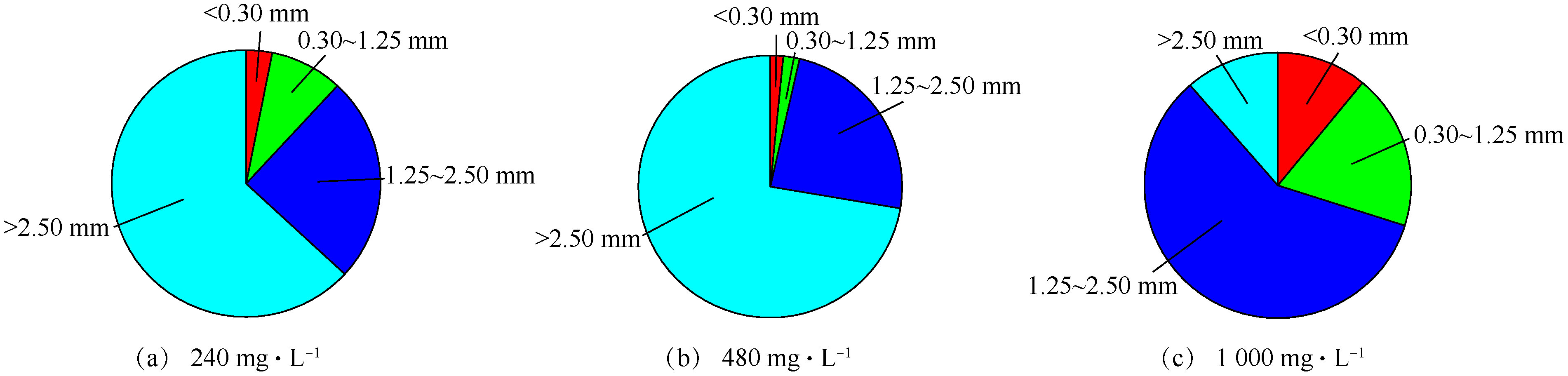
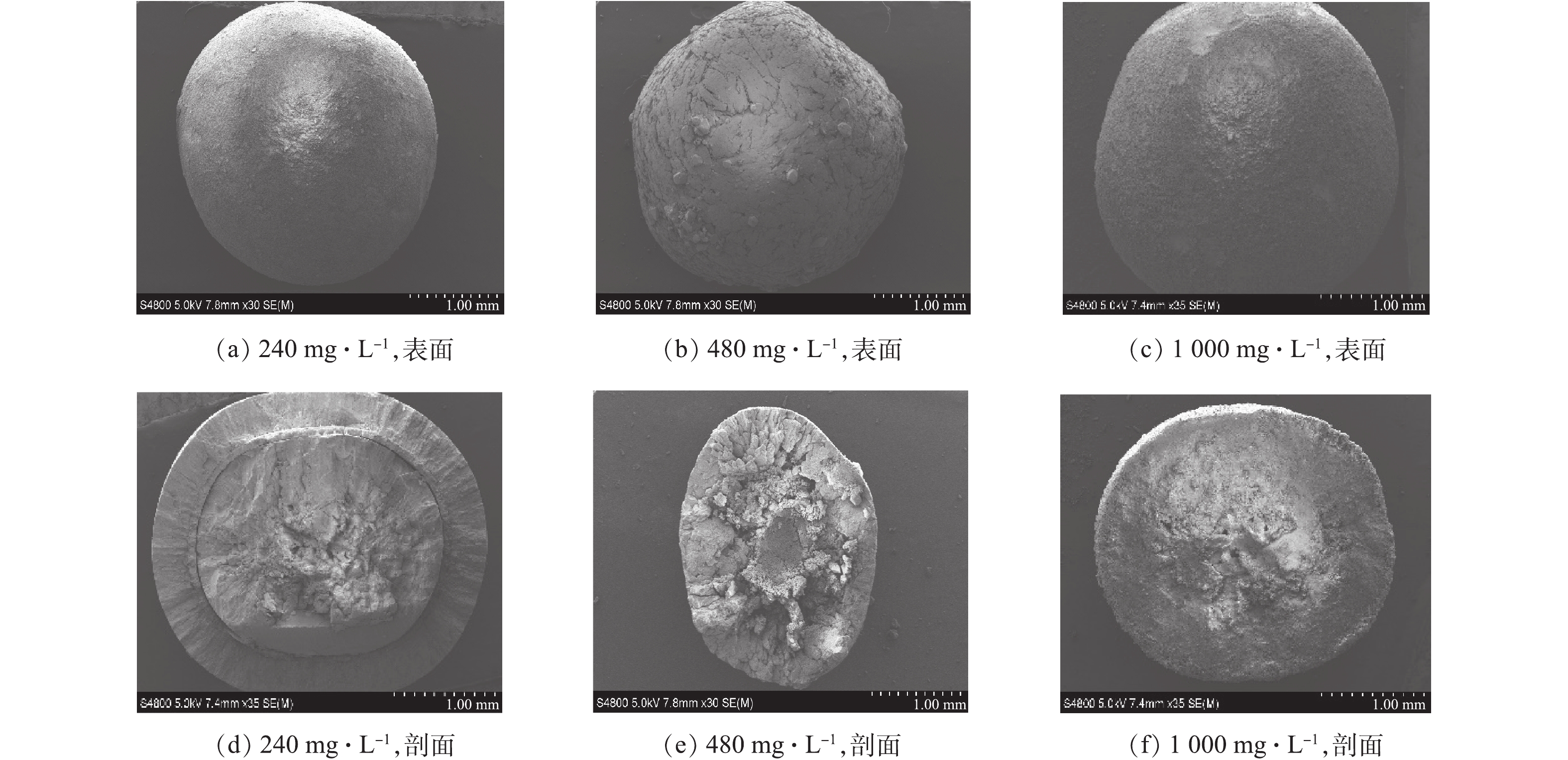
PO3−4 -P去除率及TP去除率均随着进水磷浓度的升高而降低。当进水磷浓度为240 mg·L−1时,PO3−4 -P去除率与TP去除率相当,分别为90.9%和90.4%,说明在该浓度下,生成的鸟粪石均能截留在流化床内,几乎没有鸟粪石微晶流失。当进水磷浓度增至480 mg·L−1时,PO3−4 -P和TP的去除率分别降至87.4%和73.9%;继续增高至1 000 mg·L−1时,二者的去除率分别为81.0%和68.2%。在相同的pH、Mg/N/P及水力条件下,PO3−4 -P去除率的略微降低主要缘于水力停留时间不足,而TP去除率的降低说明存在鸟粪石微晶的流失。在处理高浓度含磷废水时,反应段构晶离子局部的过饱和现象是导致微晶生成及流失的主要原因,可通过提高回流比和分散进料等方式[10]进行改善。有研究[10]表明,多段式流化床同样存在类似的问题。因此,从磷去除的角度来看,一段式流化床与多段式流化床并无显著差别。在3种进水磷浓度下,一段式流化床所得的鸟粪石产品粒径较大。其中,大于1.25 mm的产品占比分别为88.1%、96.4%和70.1%(图9),且呈规则椭球状(图10)。从2.1节的数值模拟结果得知,一段式流化床具有良好的颗粒分级特性及适合造粒的湍流强度,实验所得的颗粒特征也较好地验证了这一结论。
2.1. 流化床数值模拟分析
2.1.1. 网格无关性检验
2.1.2. 流动特性分析
2.1.3. 颗粒分级特性分析
2.1.4. 湍流特性分析
2.1.5. 微晶截留分析
2.2. 流化床实验验证研究
-
1)一段式流化床的颗粒分级效果最佳,多段式次之,锥体式较差。针对不同粒径混合体系,一段式流化床均能表现出良好的分级效果。
2)锥体式流化床的湍流强度最大,多段式次之,一段式最小,且随着流化粒径的减小而增大;一段式流化床的湍流特征可能更有助于鸟粪石造粒。
3)增设内部固液分离构件对增强鸟粪石微晶截留效果不显著。
4)验证实验结果表明,在不同的进水磷浓度条件下,一段式流化床的磷去除率与多段式流化床相当,所得的鸟粪石产品粒径多大于1.25 mm,呈规则椭球状,确证了一段式流化床是理想的鸟粪石结晶反应器。




 下载:
下载: