-
近年来,随着十溴联苯醚(decabromodiphenyl ether,Deca-BDE)的逐步禁用,十溴二苯乙烷(decabromodiphenyl ethane,DBDPE;化学结构见图1)作为其主要替代产品,生产量和使用量呈逐年升高的趋势,现已成为使用量最大的新型溴代阻燃剂(novel brominated flame retardants,NBFRs)[1-2]. DBDPE作为一种添加型NBFRs,不与产品产生化学键结合,因而在生产、加工和使用等过程中极易释放到周围环境中[3]. 此外,DBDPE还具有潜在的内分泌干扰效应[4]、生殖发育毒性[5]以及肝脏毒性[6]等,进而对生物和人体造成健康危害. 我国作为DBDPE生产和使用大国,其环境污染问题以及由此带来的潜在健康风险不容忽视[7-8]. 研究表明,在广州市污水处理厂中DBDPE是主要的卤代阻燃剂类(HFRs)污染物之一,且呈现逐年递增的趋势[9]. 在我国东江下游水体中DBDPE也是检出的主要污染物,占HFRs总量的64%[10]. 因此,DBDPE潜在的环境健康风险问题需引起高度重视.
DBDPE亲脂性较高(辛醇-分配系数,lg Kow = 11.1)[11],具有在水生生物体内富集和放大的潜力. 目前关于DBDPE生物富集特征的相关研究普遍集中于野外食物链调查,且研究结果并不一致. 针对东江流域鲮鱼、罗非鱼和鲢鱼等3种鱼类的研究结果表明,DBDPE可以在鱼体内显著富集[12]. 关于我国南海海域水生食物网的研究发现,DBDPE具有明显的生物放大作用[13]. 然而在太湖水域水生食物链中DBDPE的研究结果显示,DBDPE出现营养级稀释现象[14]. 除污染物自身的理化性质外,污染物在生物体内的吸收和代谢转化等也是影响其生物富集特征的重要因素. 然而,目前仅有少量研究报道了DBDPE的生物转化过程. 将DBDPE暴露斑马鱼胚胎后,在胚胎内共检出7种可能的脱溴代谢产物[15]. 以大鼠为模式生物,经口暴露DBDPE后也检出7种代谢产物[16]. 通过开展沉积物-水-泥螺暴露实验结果发现,DBDPE在泥螺体内的生物转化主要为脱溴过程[17]. 以上研究结果表明,DBDPE生物富集特征仍然存在争议,在生物体内的转化过程具有潜在的物种差异性. 目前对DBDPE在鱼体内的生物富集以及生物转化尚缺乏深入认识,且有关生物转化过程对DBDPE生物富集的影响仍有待进一步研究.
综上所述,本研究以斑马鱼作为受试生物,开展室内DBDPE饮食暴露实验,探讨DBDPE在肌肉、肝脏、性腺和脑等不同组织内的生物富集特征;并对暴露鱼体血清中DBDPE潜在的生物转化产物进行筛查和鉴定,推导其可能的生物转化路径,进而评估生物转化对DBDPE生物富集特征的影响. 主要目的是探讨DBDPE在鱼类中的组织特异性分布特征,以及DBDPE在斑马鱼体内的生物转化特征. 相关研究结果有望为深入认识DBDPE的环境行为和归趋、准确评估其生态环境健康风险提供科学依据,具有重要的意义.
-
仪器:气相色谱-质谱联用仪(GC-MS,7890N-5977A;Agilent,美国),色谱柱DB-5HT(15 m×0.25 mm×0.1 µm;Agilent,美国),超高效液相色谱-四极杆-飞行时间质谱联用仪(UPLC-QTOF-MS,1290-G6545;Agilent,美国),RRHD Eclipse Plus95-C18色谱柱(3 mm×150 mm,1.8 µm;Agilent,美国),离心机(Sigma,德国),氮吹仪(Organomation,美国),冷冻干燥机(北京博医康实验仪器有限公司,中国),数控超声波清洗器(上海安谱实验科技公司,中国),涡旋振荡器(Troemner,美国),电子天平(梅特勒托利多公司,瑞士).
耗材:CNWBOND HC-C18 SPE 填料(40—63 μm),CNWBOND PSA QuEChERS专用超洁净填料(40—63 μm),10 mL和15 mL玻璃旋盖离心管,1 mL 无菌连针注射器,亲水PTFE针式滤器(0.22 μm)(上海安谱实验科技有限公司,中国),鱼饲料(茂名市粤诚饲料有限公司,中国),无水硫酸钠(阿拉丁生化科技公司,中国),Supelco Florisil® ENVI固相萃取(SPE)柱(500 mg, 3 mL;Merck,德国),巴斯德吸管(Witeg Labortechnik Gmb,德国).
实验所用试剂均为色谱纯,包括正己烷(HEX),丙酮(ACE),二氯甲烷(DCM),甲醇(MeOH),乙酸乙酯(EtAC)和异辛烷(上海安谱实验科技有限公司,中国);优级纯甲酸(阿拉丁生化科技公司,中国);标准品包括DBDPE,OH-BDE85,BDE128和BDE181(> 99.0%,AccuStandard,美国).
受试生物:性成熟雌性斑马鱼(Brachydanio rerio,AB种野生型;购自中国科学院水生生物研究所).
-
将性成熟雌性斑马鱼(体长(4.01±0.16) cm,体重(0.65±0.21) g)养殖于15 L水族玻璃缸内(n = 40);养殖水为经过爆气和活性炭过滤的自来水(pH = 7.4±0.2;水温(28±0.5) ℃;光暗比为14 h:10 h),每天食物投放量以全部鱼体重2%计;待斑马鱼适应14 d后开始暴露实验,并于实验前5 d停止喂食.
-
斑马鱼暴露实验参考经合组织(Organization for Economic Co-operation and Development; OECD)推荐的化学物质鱼体生物富集测试指南[18-20],简述如下:将斑马鱼(n = 200)随机分配于5个15 L玻璃水族箱中(每缸40条)作为暴露组;另取50条作为实验对照组. 暴露剂量参考我国华南地区河流污泥样品中DBDPE平均浓度(1995 ng·g−1 dw)[8],将染毒鱼食设计剂量为2000 ng·g−1,以模拟DBDPE在实际环境中的生物富集潜力;对照组用未染毒的相同鱼食进行喂养.
暴露期间,以前述饲养环境,每天用加标鱼食喂养斑马鱼. 连续暴露28 d后,将其转至干净的水族箱继续开展为期77 d净化实验,净化期采用未染毒的鱼食喂养. 实验过程中,每2 天更换1次实验水体. 分别于暴露期第7、14、21、28 d,以及清除期7、21、35、49、63、77 d进行取样;每次取样,从每个暴露组水族箱中随机选取4尾斑马鱼,共计20尾. 测量每条鱼的体长和体重;断尾取血,解剖分离鱼体性腺、肝脏、脑和肌肉等组织,并记录其重量. 每次取样的血液合并为一个样品,离心(4000 r·min−1,10 min)后获得血清;肌肉、肝脏和脑等则分别将5尾鱼对应组织合并为一个样品(n = 4). 除血清外,其他生物样品在合并、称重后冷冻干燥. 每次取样时,随机从对照组中取5尾斑马鱼,各组织处理方式与暴露组相同.
-
鱼食和斑马鱼组织样品前处理参考文献报道的方法[15, 17, 21],并做适当优化,简述如下:准确称取鱼食或鱼体组织样品置于干净的玻璃离心管中,冷冻干燥后加入适量无水硫酸钠、3 mL HEX:ACE混合溶剂(1:1,V/V)、内标溶液20 μL(BDE128,1000 ng·mL−1),静置过夜. 样品经超声萃取(100 kHz,15 min,35 ℃)、离心(4000 r·min−1,10 min)后,取上清液转移至干净的10 mL玻璃离心管;重复上述超声萃取步骤2次,合并萃取液. 取1 mL萃取液用于测定脂肪含量,剩余萃取液(8 mL)氮吹浓缩至近干,HEX复溶至1 mL. 浓缩液采用Florisil 固相萃取(SPE)柱(500 mg, 3 mL)分离纯化;SPE柱依次用6 mL EtAC和6 mL HEX预淋洗;上样后,用10 mL HEX:DCM混合溶剂(1:1,V/V)进行洗脱. 洗脱液氮吹浓缩至近干,加入180 μL异辛烷和20 μL回收率指示物(BDE181,1000 ng·mL−1),涡旋混合均匀后转至进样瓶中,置于-20 ℃冷冻保存,待上机测试.
斑马鱼血清样品分析参考Chen等[22]的处理方法,并对其进行优化:准确称重合并的血清(—100 μL)样品于2 mL离心管,加入100 μL MeOH和20 μL甲酸,以及20 μL内标溶液(OH-BDE85,1000 ng·mL−1),混匀后静置15 min;加入200 μL MeOH:ACE (1:1,V/V),涡旋混匀(1800 r·min−1,2 min);离心(8000 r·min−1,10 min)后取上清转移至干净的离心管中;重复萃取步骤3次,合并上清液;萃取液加入5 mg PSA和5 mg C18除脂;涡旋(1800 r·min−1,2 min)、离心(8000 r·min−1,10 min)后取上清;过亲水PTFE针式滤器(0.22 μm),并氮吹至近干,加入100 μL MeOH复溶,涡旋混合均匀后转移至进样2 mL瓶中,置于−20 ℃冷冻保存,用于DBDPE脱溴产物筛查分析.
-
DBDPE定量参考Tang等[21]报道的仪器方法,分析仪器为Agilent GC-MS(7890N-5977A);离子源为负化学电离源(ECNI),采用选择离子检测模式(SIM). 载气为高纯氦气,甲烷为反应气体;色谱柱采用DB-5 HT毛细管柱(15 m×0.25 mm,0.10 µm;Agilent). DBDPE扫描离子为m/z 79和81. 升温程序:初始温度为110 ℃,保持5 min;以20 ℃·min−1升至200 ℃,保持4.5 min;以10 ℃·min−1升至310 ℃,保持5 min. 采用不分流进样,进样体积为1 μL. 传输线、进样口和离子源温度分别为280 ℃、290 ℃、250 ℃. 此外,采用全扫描(SCAN)模式下分析斑马鱼组织及血清中DBDPE的脱溴代谢产物.
参考Wang[20]等报道的方法,使用安捷伦超高效液相色谱-四极杆-飞行时间质谱(UPLC-QTOF-MS;Agilent 1290-6465)对斑马鱼血清中DBDPE的代谢产物进行筛查分析. 采用RRHD Eclipse Plus95-C18色谱柱(3 mm×150 mm,1.8 µm)分离,柱温40 ℃;进样体积4 µL;流动相A为乙腈,B为含0.05%(V/V)甲酸的超纯水,洗脱梯度见表1,流速为0.3 L·min−1. 采用电喷雾离子源正负(ESI+和ESI-)两种模式采集数据;扫描模式的参数设置:采集模式为自动(Auto MS/MS)和靶向(Targeted MS/MS)两种相结合;扫描质荷比(m/z)50—1200之间;毛细管电压:3500V(ESI-)/4000V(ESI+);碰撞能设置:5、10以及20 eV;采集速率:MS为2.5 spectra·s−1,MS/MS为4 spectra·s−1;气体流速:9 L·min−1,气体温度:325 °C;鞘气流速:11 L·min−1,鞘气温度:350 °C;锥孔电压:65 V.
-
使用未染毒生长发育正常的雌性斑马鱼肌肉作为基质,采用与样品相同流程,分析基质(n = 3)、基质加标(n = 3)、空白(n = 3)以及空白加标(n = 3)等4组平行样品. DBDPE的基质加标、空白加标回收率分别为109%±14.8%和84.2%±5.60%,相对标准偏差(RSD)均小于20%. 实验过程中,每批16个生物样品,设置2个程序空白样品同时分析,以检测实验过程中可能产生的背景污染. 程序空白样品中有少量DBDPE检出,并在报道浓度时做相应的空白校正. 定量限(LOQ)定义为程序空白样品中DBDPE浓度均值加上3倍标准偏差,为2.90 ng·g−1 lw(脂重). 所有样品中,内标(BDE 128)的回收率为87.5%±8.92%. 血清样品实验分析的过程中,设置两个程序空白样品,以检测实验过程中是否产生背景污染,程序空白样品中未检测出DBDPE,对照组样品基质对照,以检测是否为斑马鱼自身基质产生的影响.
-
参考(OECD)推荐的化学物质鱼体生物富集测试指南[18-19, 23-24],计算DBDPE在斑马鱼体内的生物富集常数.
净化阶段鱼体组织中DBDPE的浓度变化采用准一级动力学模型拟合:
式中,C0和Ct分别为清除开始(t = 0)及清除时间t时,鱼体组织中DBDPE的浓度;kd为清除速率常数(depuration rate constant). 为获得多个净化时间点的kd,将DBDPE浓度的对数值与净化时间进行线性拟合,kd为回归趋线的斜率值,即:
DBDPE在鱼体组织中的生物半衰期(half-life,t1/2)通过以下公式计算:
暴露阶段DBDPE的同化效率(assimilation efficiency,α)采用以下方程进行拟合:
式中,F为喂食比例(食物/g 斑马鱼/d,脂肪校正);Cfood为斑马鱼组织中DBDPE的浓度.
生物放大因子(biological magnification factor,BMF)计算公式为:
采用SPSS21.0对暴露组与对照组的体长、体重、不同组织浓度的差异是否具有显著性差异进行单因素方差分析(one-way ANOVA),显著性标准设置为P < 0.05,绘图采用Origin 2017.
-
DBDPE代谢产物鉴定如下:(1)根据相关的文献报道[16-17, 25-26]以及Biotransformer 3.0[27]软件预测的代谢产物,并运用ChemDraw Ultra 20.0软件绘制化合物结构,构建PCDL数据库构建;(2)将MS数据导入Profinder进行批量处理,快速确定可能的产物精确分子量及出峰时间;(3)通过Auto MS/MS及Targeted MS/MS模式获取所有可能的产物的二级质谱图,将MS/MS数据导入Qualitative Analysis运用“Find by Auto-MS/MS”或者“Find by Targeted-MS/MS”查找化合物,并将结果以cef格式导出;(4)最后,将cef文件导入MSC分析,调用前期构建的PCDL数据库或者选择一些在线的化合物数据库(PubChem或ChemSpider)进行匹配,根据子离子的碎片信息推测产物的二级结构.
-
在整个实验期间,未发生斑马鱼死亡现象;且暴露组和对照组斑马鱼的体长和体重较实验开始前的初始值相比均无显著差异(one-way ANOVA,P > 0.05). 因此,生长稀释作用对鱼体中DBDPE浓度的影响较小. 对照组斑马鱼组织样品和鱼食中DBDPE的浓度均小于LOQ,表明DBDPE的背景干扰可以忽略. 斑马鱼暴露加标鱼食中DBDPE的实际检测浓度1995 ng·g−1,略低于设计浓度(2000 ng·g−1),可能与配制时鱼油的稀释作用、配置过程中DBDPE的挥发,以及装染毒鱼食的玻璃瓶表面吸附损失等有关. 计算生物富集常数时,以实测的鱼食中DBDPE浓度为准.
-
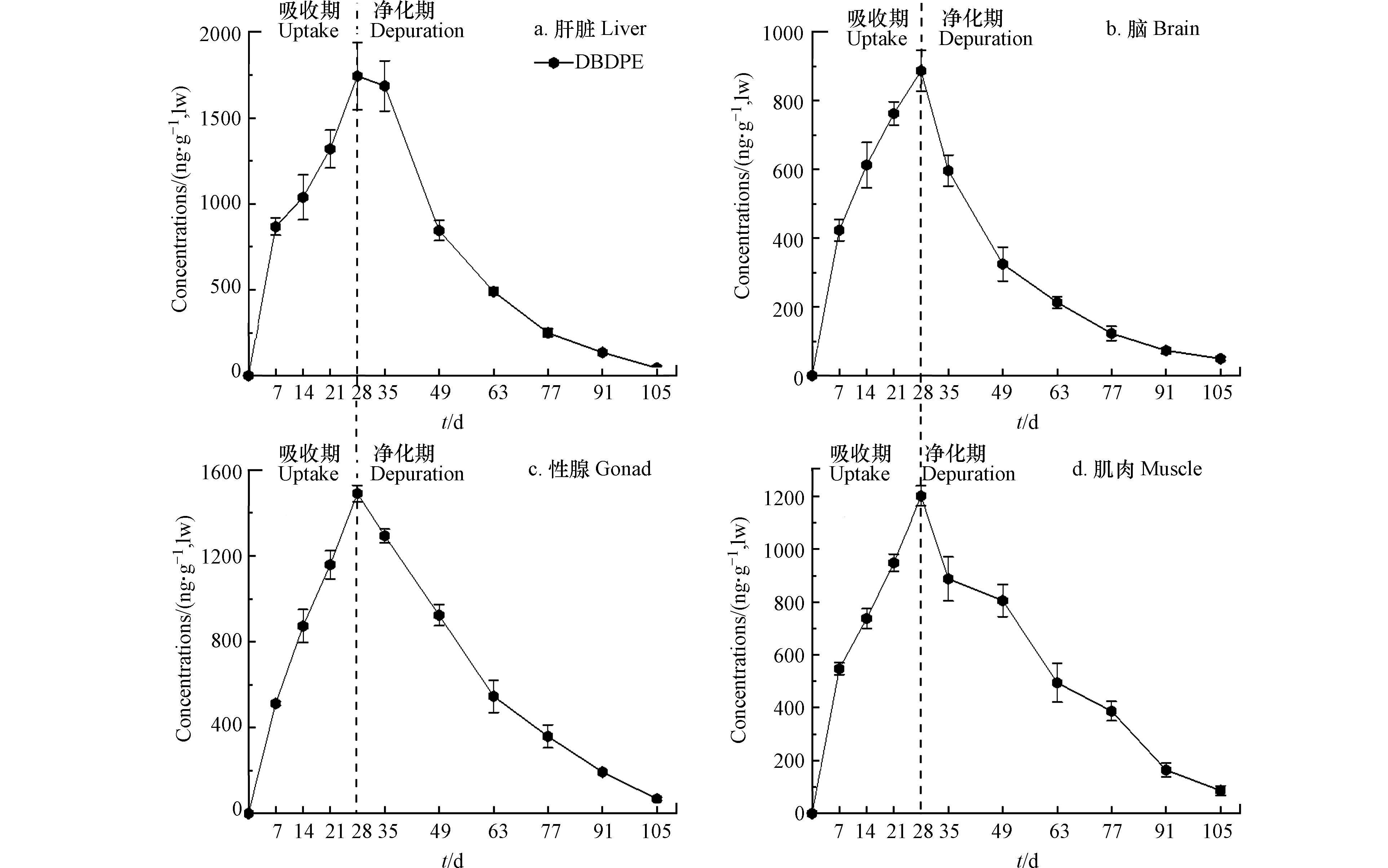
肝脏是生物体内主要的代谢器官,肌肉是最大的生物组织,脑组织以及性腺中含有较高浓度的脂质;同时,由于斑马鱼个体较小,心脏、脾脏等其他组织不易分离或分离后量太少不足以开展实验分析. 因此,本研究主要分析了DBDPE在斑马鱼肌肉、肝脏、性腺和脑4种组织中的吸收和清除过程. 暴露7 d后,斑马鱼各组织中均有DBDPE检出,且吸收曲线相似,均呈现随暴露时间延长而递增的趋势. 暴露结束(28 d)时,各组织中DBDPE的浓度均达到峰值,浓度分别为肝脏(1740 ng·g−1 lw)> 性腺(1490 ng·g−1 lw)> 肌肉(1200 ng·g−1 lw)> 脑(885 ng·g−1 lw);暴露期间,DBDPE在斑马鱼组织内的吸收可能尚未达到平衡状态(图2).
进入净化期,斑马鱼各组织内DBDPE的浓度均呈现快速下降的趋势;在净化期结束时,各组织中DBDPE清除率均超过90%. DBDPE在斑马鱼各组织中的清除符合准一级动力学方程,不同组织中的清除速率常数(kd)、生物半衰期(t1/2)、同化效率(α)和生物放大因子(BMF)等生物富集常数计算结果如表2所示.
DBDPE在斑马鱼肝脏中的同化效率最高18.3%±3.78%,可能与肝脏是污染物经胃肠道吸收后首先沉积的器官有关[28];其次是性腺14.6%±0.53%和肌肉12.8%±1.94%,脑组织中最低10.1%±1.34%. 有关同化效率的研究结果显示,多氯联苯(PCBs)同系物lg Kow> 7时,化合物的同化效率随着lg Kow值升高而降低[29]. 本研究中DBDPE的lg Kow = 11.1,故而导致其在斑马鱼体内的同化效率较低. 斑马鱼各组织中DBDPE的清除速率常数为(3.66±0.16)×10−2·d−1至(4.69±0.06)×10−2·d−1,清除半衰期介于(14.8±0.19)d和(19.0±0.83)d(表2). 肝脏中DBDPE的清除速率最高、生物半衰期最短;可能与肝脏的代谢功能有关[6];因此相比其他组织,更不利于污染物的富集. 而肌肉的代谢活性相对较低,因此肌肉中DBDPE清除速率常数最低、生物半衰期最长(表2).
开展水体微宇宙暴露的实验结果与本研究结果相似,斑马鱼肝脏中DBDPE的生物半衰期最短,仅为1.72—2.14 d;在肌肉中的生物半衰期最长,为1.46—8.33 d[23]. 此外,通过建立沉积物-水-泥螺暴露系统,得到DBDPE在泥螺体内的清除速率常数为0.181×10−2—0.232×10−2·d−1,生物半衰期仅为3.0—3.8 d[17]. 通过建立沉积物-水-蛤蜊水生生物系统的研究发现,蛤蜊中DBDPE的清除速率常数为0.06—0.76(10−2·d−1),生物半衰期为0.91—11.6 d[25]. 而Zheng等开展鲫鱼、鲶鱼和黄头鲶鱼等3种鱼的肝微粒体体外代谢实验研究结果表明,DBDPE的生物半衰期为15.8—17.3 d[30] . 不同研究中DBDPE的清除速率不尽相同,可能与生物受试体种类和暴露途径有关.
DBDPE在斑马鱼肝脏和性腺中的BMF值均大于1,分别为1.12±0.23和1.09±0.04(表2);其次是肌肉,BMF值为0.92±0.15;脑组织中DBDPE的BMF值较低(0.72±0.04),可能与DBDPE的分子量较大(MW = 971.22 g·mol−1),以及血脑屏障的影响有关. 以上结果表明,DBDPE在斑马鱼组织内具有潜在的生物富集性. 而通过室内喂养虹鳟鱼的实验结果显示,DBDPE在虹鳟鱼的膳食吸收效率接近于零,无明显的生物富集作用[31]. 针对我国太湖水生食物链的研究发现,DBDPE的脂重浓度随着生物营养级的升高而下降,呈现营养级稀释现象(TMF = 0.37)[14]. 而在关于南海海域水生食物链的研究则发现,各水生生物体内DBDPE的lg BAF值均大于3.7,是生物富集潜力最高的NBFRs化合物,且呈现营养级放大作用(TMF = 4.22)[13].
不同水域、不同食物链中DBDPE生物富集潜力结果的差异,可能与不同研究中食物链的长短、构成食物链的物种不同有关,生物体对DBDPE的生物转化率差异可能是影响其在生物体内富集与否的关键因素之一. HOU等[13]研究显示,淡水鱼中DBDPE的生物转化率高于海鱼,DBDPE在海洋食物网中具有更高的生物富集和放大潜力.
-
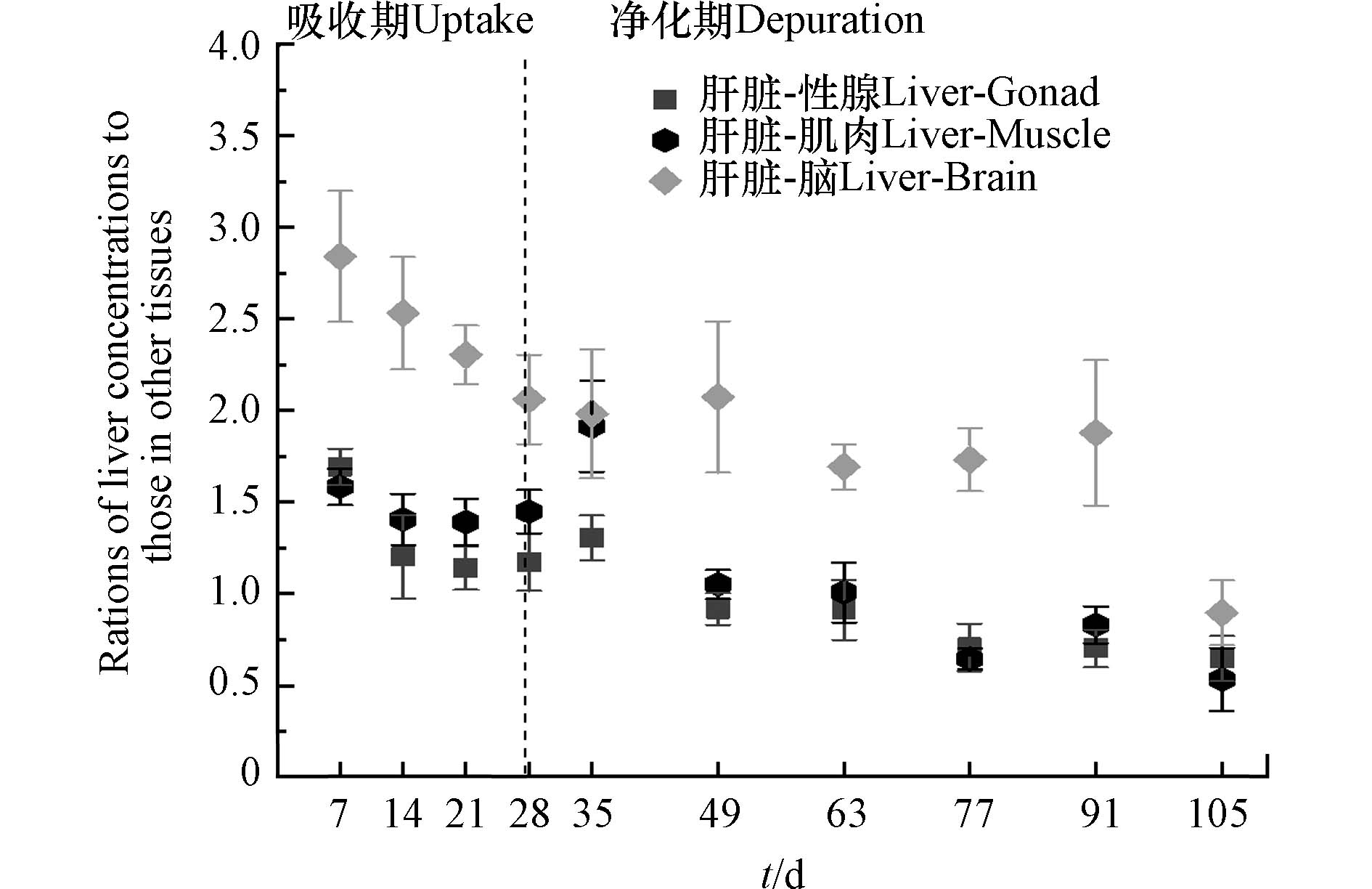
生物体内异源化学物质或药物的主要转运方式是被动扩散,其在组织中的分布与血液灌注率和膜通透性有关. 房室模型(compartment model)是药代动力学和药效学研究中的重要数学模型:异源化学物质经胃肠道吸收后,通过血液灌流进入中央腔室(如心脏和肝脏),然后从中央腔室转移到血液灌流不良的外周腔室(如肌肉和脂肪)[32-33]. 为探讨DBDPE在斑马鱼不同组织间的分布特征,计算了整个实验期间肝脏与其他组织中DBDPE浓度的动态比值. 如图3所示,在暴露开始时(7 d),肝脏与性腺、肌肉和脑中DBDPE的比值最高,分别为1.69±0.10、1.58±0.10和2.84±0.35;随后,随着暴露和清除时间的延长总体呈现下降趋势,并逐渐趋于平衡. 本研究中,DBDPE通过食物暴露喂养斑马鱼,胃肠道吸收是化合物进入鱼体的主要途径. 而肝脏是生物体中血液充盈的组织,也是生物体内重要的代谢器官;污染物从胃肠道吸收后首先传输进入肝脏,随后再经血液传输在不同组织间分配. 因此,进一步计算了DBDPE在斑马鱼不同组织中的负荷占比,如图4所示.
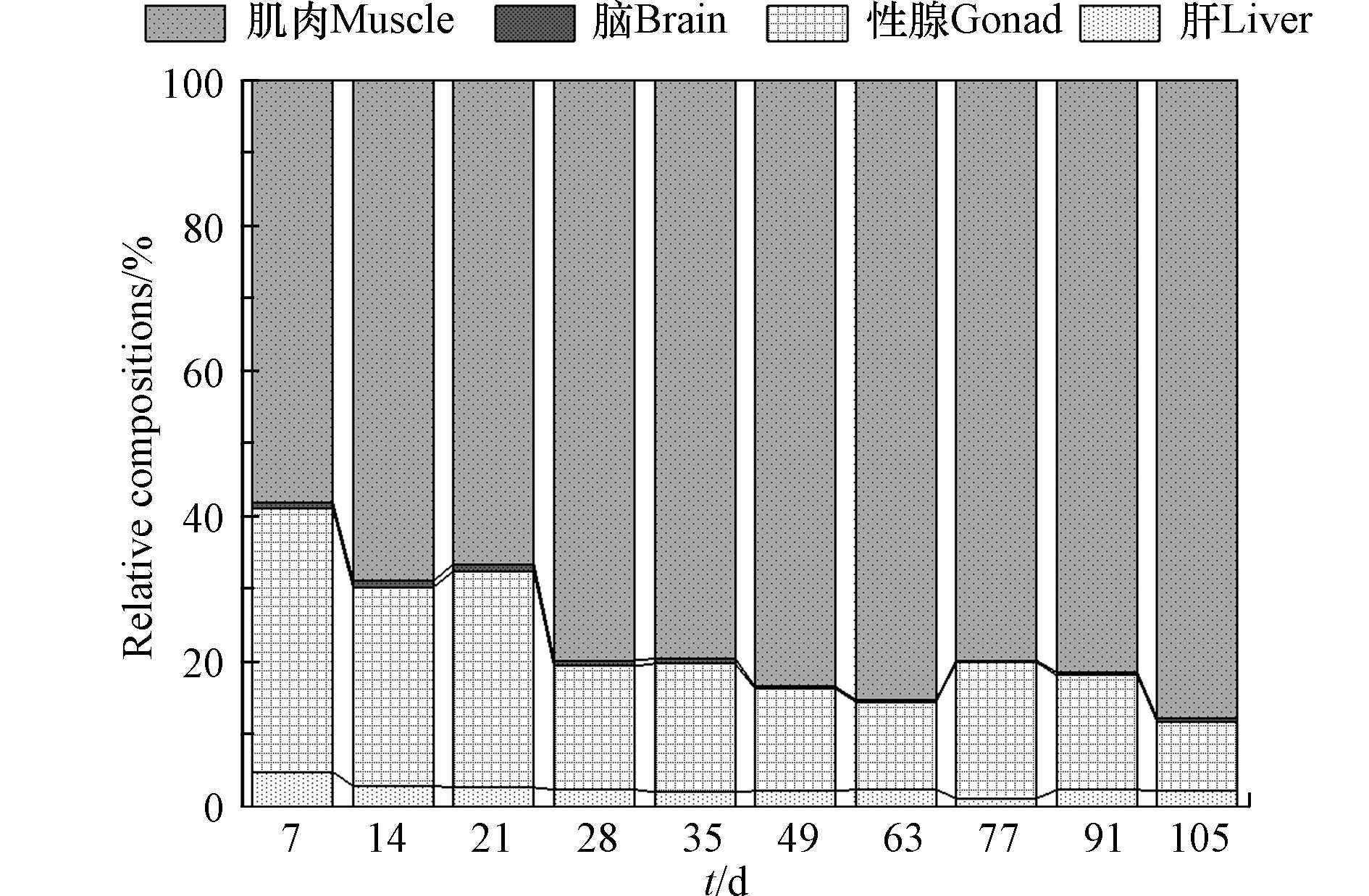
与浓度动态比值结果类似,暴露7 d时肝脏中DBDPE负荷占比较高(5%),随后逐渐呈现降低趋势并趋于稳定,维持在2%左右(图4). 整个实验期间,DBDPE在肌肉中的占比远高于其他组织;且随着时间的延长,其负荷占比总体呈现递增的趋势,从58%增加到88%(图4). 该结果与构建微宇宙暴露实验中,DBDPE在斑马鱼肌肉中富集的结果一致[23]. 在性腺中,DBDPE的负荷占比呈现为随时间延长而略有下降的趋势,在清除后期逐渐呈稳定状态(图4). 其主要原因可能是,本研究采用性成熟的雌性斑马鱼作为实验对象,卵巢是仅次于肌肉的第二大组织,且其中DBDPE的浓度高于肌肉(图2). 此外,本研究结果表明DBDPE在卵巢中具有富集的潜力,可能造成代际传递,对子代产生不利影响. 而对于脑组织,DBDPE的负荷占比无明显变化(图4);且其负荷占比为4种组织中最小的,在清除实验后期,基本趋于0. 可能是由于DBDPE具有较大的分子量,不易穿过血浆与脑脊液之间的血脑屏障;该结果也与前述DBDPE进入脑组织的浓度最低相一致. 综上所述,组织功能是影响DBDPE在斑马鱼体内分布的重要因素.
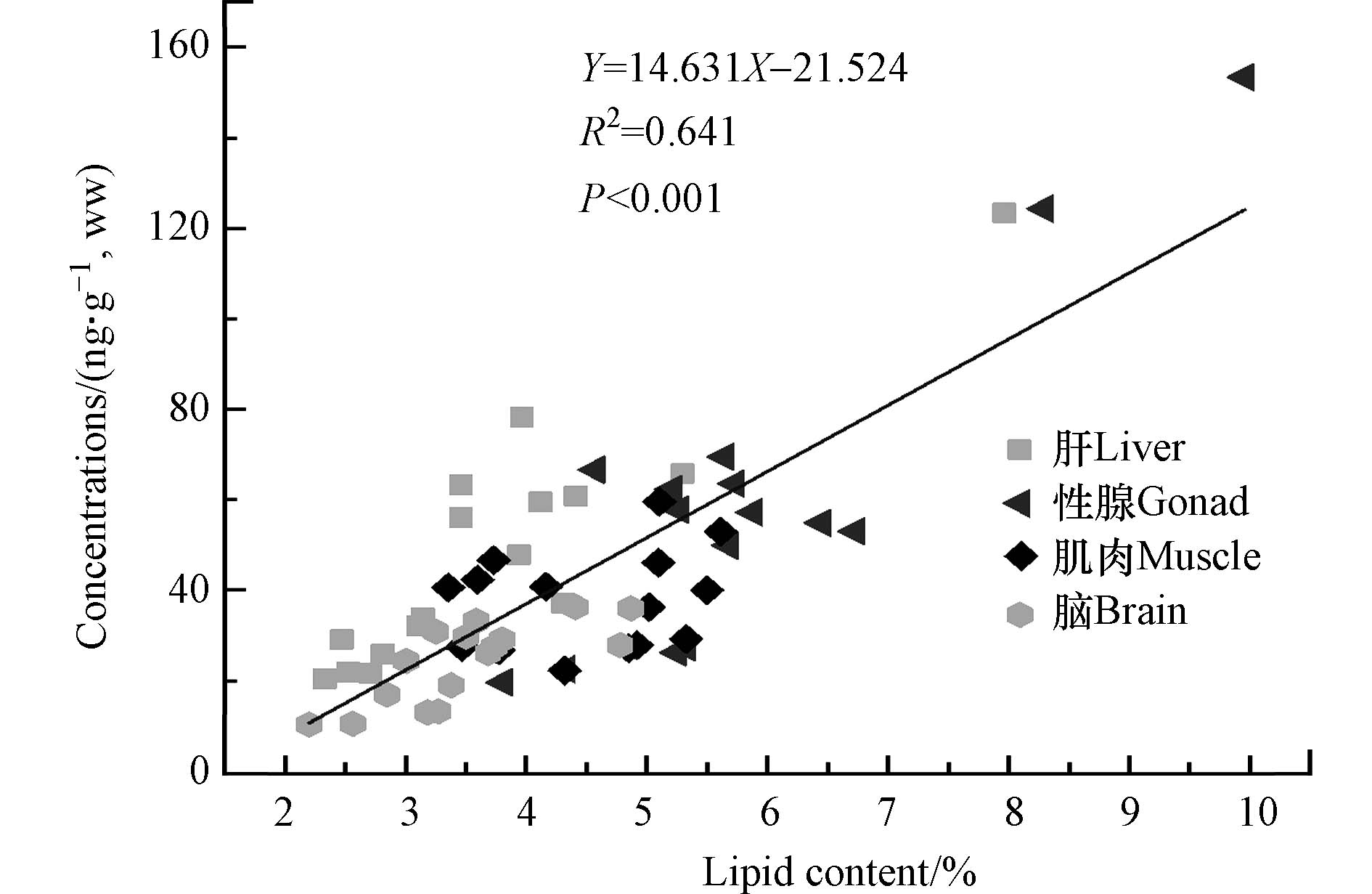
本研究中,斑马鱼不同组织的脂肪百分含量依次为:脑组织3.51%±0.73%< 肝脏3.75%±1.39%< 肌肉4.66%±0.94%< 性腺5.92%±1.51%. 如图5所示,斑马鱼不同组织中DBDPE的湿重浓度与其脂肪含量呈显著正相关关系(R2 = 0.641,P < 0.01).
结果表明,DBDPE在鱼体组织间的富集主要是通过被动运输将其转移到含脂介质实现. 针对格陵兰岛海豹、北极熊等生物体的研究也发现类似结果,DBDPE在脂肪含量高的组织中富集浓度显著高于脂肪含量低的组织[34]. 大鼠经口暴露DBDPE的研究结果也显示,DBDPE更容易在脂肪含量较高的组织中富集[16]. 综上所述,脂肪含量也是影响DBDPE在斑马鱼组织间分布的重要因素之一.
-
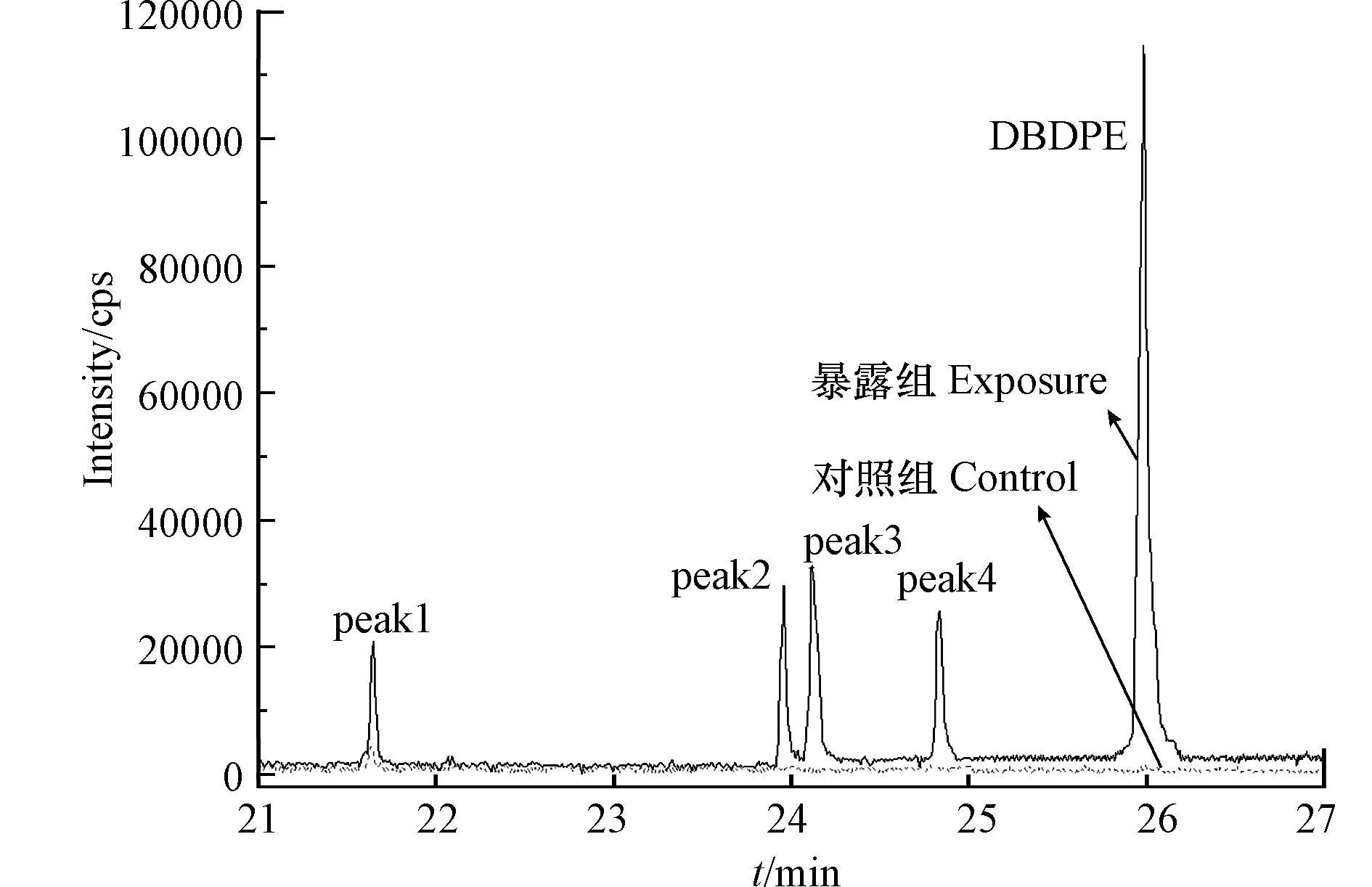
基于GC-MS全扫描分析,通过提取含溴特征离子(m/z 79和81)与对照组斑马鱼组织样品的色谱图对比,在暴露组组织样品共检测到4个未知的含溴化合物峰(图6),其可能是DBDPE的脱溴转化产物,提示DBDPE在斑马鱼体内发生了生物转化. 已有研究发现,DBDPE能够在斑马鱼体内发生生物转化,且通过GC-MS全扫描分析共检测到7个未知峰,但未对转化产物开展进一步鉴定[15].
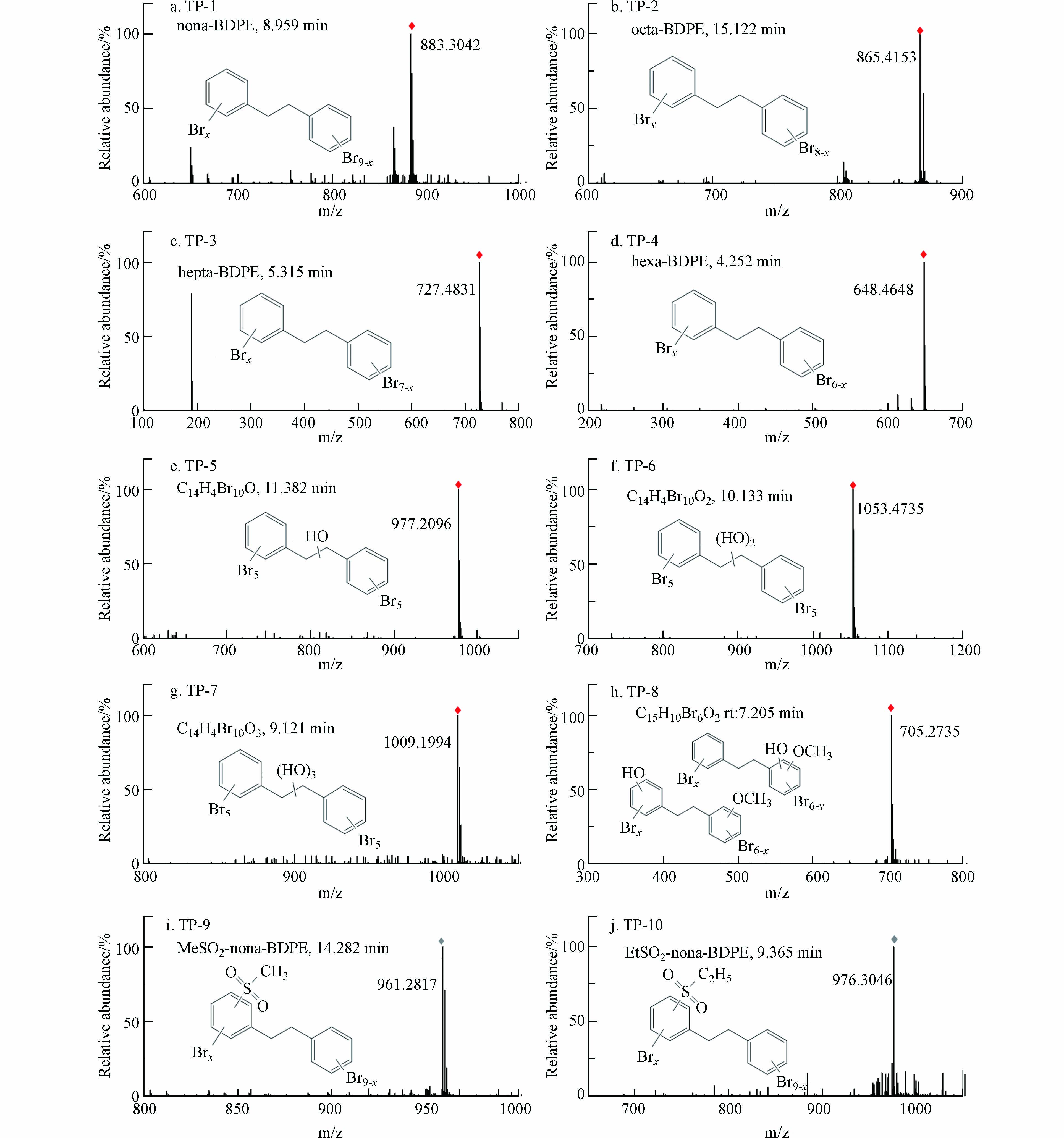
为进一步探究DBDPE的生物转化特征,本研究采用UPLC-QTOF-MS对斑马鱼血清样品中DBDPE的产物进行筛查和鉴定;共鉴定出10种转化产物(transform products,TP),包括4种脱溴代谢产物、1种羟基化-甲基化代谢产物,以及2种酚类代谢产物.
斑马鱼血清样品中的4种脱溴产物包括,九溴二苯乙烷(TP-1,nona-BDPE,m/z 883.3024)、八溴二苯乙烷(TP-2,octa-BDPE,m/z 865.4153)、七溴二苯乙烷(TP-3,hepta-BDPE,m/z 727.4831)和六溴二苯乙烷(TP-4,hexa-BDPE,m/z 648.4648),进一步证实DBDPE在斑马鱼体内发生了脱溴转化反应. 在DBDPE暴露的泥螺体内共检出5种脱溴产物,包括nona-BDPE、octa-BDPE、hepta-BDPE、hexa-BDPE等脱溴产物[17]. 通过蚯蚓暴露实验,在蚯蚓体内检出11个可能的DBDPE代谢产物峰,并鉴定了hepta-BDPE、octa-BDPE和nona-BDPE等脱溴产物[26]. 以上结果表明,脱溴代谢是DBDPE在水生生物体内较为普遍的一种转化路径. 此外,本研究在斑马鱼血清中还检测出3种羟基以及1种羟基化甲氧基化的代谢产物(TP-5,m/z 977.2096;TP-6,m/z 1053.4735;TP-7,m/z 1009.1994;TP-8,m/z 705.2735),其可能的结构如图7所示. 在沉积物-水-蛤蜊暴露系统中,检测到DBDPE的羟基化和甲基化的代谢产物,与本研究结果一致[25]. 同时,本实验还在血清中筛查到2种DBDPE甲磺基类代谢物(TP-9,MeSO2-nona-BDPE,m/z 961.2871;TP-10,EtSO2-nona-BDPE,m/z 976.3046). DBDPE暴露的大鼠样品中检测到2种类似的甲磺基代谢产物,该研究显示还原脱溴并不是DBDPE在大鼠体内的主要代谢途径[16].
以上结果表明,斑马鱼体内可能发生了渐进性脱溴以及羟基化代谢过程:苯环上脱去1—4个溴,产生了TP-1—TP-4等4种脱溴代谢产物,或直接转化生成TP-5和TP-6等羟基化产物. BDPE及其脱溴产物还可在能斑马鱼体内发生进一步转化:如,六溴二苯乙烷(hexa-BDPE)经羟基化以及甲氧基化等Ⅰ相反应产生TP-8;而九溴二苯乙烷(nona-BDPE)则进一步发生甲磺基(MeSO2、EtSO2)等Ⅱ相反应,生成TP-9和TP-10. 综合本研究筛查鉴定的血清中DBDPE转化产物,推导出斑马鱼体内DBDPE的可能代谢路径如图8所示. DBDPE在斑马鱼体内存在脱溴和羟基化过程等Ⅰ相反应的过程,并进一步发生甲磺基化等Ⅱ相反应.
-
本研究结果表明DBDPE在斑马鱼体内具有潜在的生物富集性;且组织功能及其脂肪含量对DBDPE的富集潜力具有重要作用. 此外,在斑马鱼体组织中筛查到DBDPE的脱溴代谢产物;而在血清中,除脱溴产物外,还筛查鉴定出DBDPE的羟基化代谢产物以及其他产物;表明DBDPE在斑马鱼体内除脱溴反应外,还存在氧化羟基化和甲氧基化等代谢反应,代谢转化也是影响DBDPE生物富集潜力的重要因素之一;同时,DBDPE代谢产物对环境的影响也值得引起关注. 本文系统探讨了DBDPE在斑马鱼体内的生物富集和转化过程,为准确评估其生态环境风险提供科学依据.




 下载:
下载: