-
农村生活污水的可持续治理是建设宜居宜业和美乡村的重要内容之一。传统的农村生活污水治理方式存在建设及运维成本高、污染物收集率低、运行不稳定、运维复杂等问题,是一种消耗能源处理资源的非可持续治理路径。农村生活污水的资源化利用已成为共识,在地形条件复杂或农房较为分散的农村地区,基于黑灰分离的分散式污水资源化利用系统是更为有效且可持续的治理模式[1]。
生活污水根据其来源可以分为来自厕所的黑水和来自厨房、洗涤、洗澡等的灰水,其中黑水主要包括粪便、尿液和冲厕水等[2]。人粪尿的日均排放量仅占生活污水的1%~2%(体积比),但生活污水中约60% 的化学需氧量(Chemical Oxygen Demand, COD)、97%的总氮(Total Nitrogen, TN)和90%的总磷(Total Phosphorus, TP)源自于黑水[3]。因此,源分离是一种能充分利用黑水中营养物质并大幅降低污水处理成本的有效方案[4]。通过“黑灰分离”,对黑水进行处理,获得液态或固态肥,实现黑水资源化利用。化粪池是农村地区常见的无动力黑水简易处理设施[5],通过厌氧消化实现对黑水中有机物的降解,然而,化粪池的简易厌氧消化不能有效去除黑水中所含有的大量病原微生物。王玉华等[6]研究发现在三格式化粪池中,经30 d以上的厌氧消化,粪大肠菌值无法满足无害化卫生要求,增加了利用过程中的安全风险。通过提高发酵温度或与餐厨垃圾、畜禽粪便、厌氧污泥、农业废弃物共消化等方式,可有效灭活病原微生物,同时产生清洁能源甲烷,实现黑水的资源化,但需要额外的设施收集农户黑水和产生的甲烷,增加了建设成本,在广大农村地区推广应用难度较大。因此,强化黑水在化粪池内厌氧发酵,促进大分子有机物的水解和病原微生物的灭活,是进行黑水安全资源化利用的关键[7]。
本研究应用厌氧发酵菌剂,强化黑水厌氧发酵产酸过程,促进黑水中有机物的快速水解并积累有机酸,抑制后续的产甲烷过程,保留黑水中的小分子有机物和营养物质,获得液态肥,同时有效控制黑水中的臭味物质和粪大肠菌群等病原微生物,保障发酵液回田利用的安全性。通过批次实验,考察了厌氧发酵菌剂投加量、发酵温度对黑水中有机物转化、有机酸积累、臭味控制和病原微生物灭活的影响,分析了发酵体系中微生物群落结构的变化,提出了适宜的黑水强化处理方法,为实现农村黑水资源化安全利用提供依据。
-
本文使用的厌氧发酵菌剂由课题组与登高环保科技有限公司联合研发,外观为黑色液体,有少许沉淀。厌氧发酵菌剂的性质为:pH为7.6,总化学需氧量(total chemical oxygen demand, TCOD)为150 000 mg·L−1,溶解性化学需氧量(soluble chemical oxygen demand, SCOD)为270 mg·L−1,总固体浓度(total solids, TS)为1 500 mg·L−1,挥发性固体浓度(volatile solids, VS)为1 480 mg·L−1。
高通量测序结果显示厌氧发酵菌剂含有16种细菌门,优势菌门为厚壁菌门和变形菌门,相对丰度分别为88.3%和11.2%;含有69种菌属,优势菌属为乳杆菌属、罗尔斯通菌属、假单胞菌属和寡养单胞菌属,相对丰度分别为87.4%、6.5%、2.1%和1.1%,表1列出了相对丰度排名前10的细菌门/属。
实验用黑水取自东南大学校园厕所,厕所冲水量为每次5 L。取样后,将黑水混匀并分装至厌氧反应器内进行厌氧发酵实验。黑水pH为9.2±0.3,TCOD为(2 900±100) mg·L−1,SCOD为(300±20) mg·L−1,TS为(1 950±150) mg·L−1,VS为(1 200±100) mg·L−1,TN为(545±25) mg·L−1, TP为(125±15) mg·L−1,总钾(Total Potassium, TK)为(3.5±1.7) mg·L−1,粪大肠菌群数为 (1.6±0.8)×1010 MPN·L−1,蛔虫卵数为(2 650±350) MPN·L−1。
-
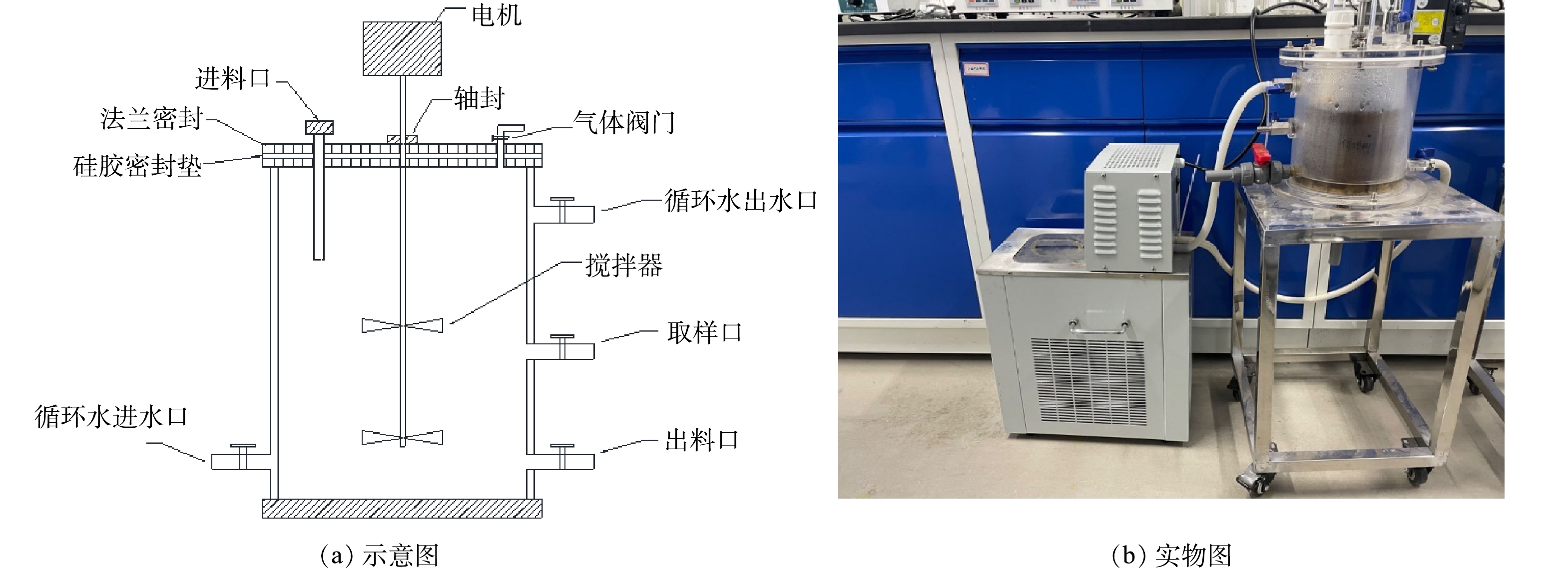
本研究实验装置为如图1所示的双层厌氧反应釜,有效容积为2 L,设有转速为30 r·min−1的搅拌机。使用带有循环泵的恒温水浴锅,在反应釜夹层中注入恒温水控制反应温度。
-
1)厌氧发酵菌剂适宜投加量的确定。在正式实验前,分别在0、0.2、0.4、0.6、0.8、1 mL和0、2、4、6、8、10 mL两个菌剂投加量梯度下进行了黑水发酵预实验,黑水量为2 L,发酵时间为20 d。预实验结果表明,1 mL以下的菌剂投加量下,厌氧发酵效果较差,而在2~10 mL投加量下,厌氧发酵菌剂的效果差异并不显著,因此将菌剂投加量梯度设置为0.5、1.0、1.5、2.0和2.5 mL。发酵10 d后,SCOD、VFA等指标均趋于平稳,故确定发酵时间为10 d。
在5个分别装有2 L黑水的反应釜内,分别投加0.5、1.0、1.5、2.0和2.5 mL的厌氧发酵菌剂,编号分别为F1~F5,并设置不投加厌氧发酵菌剂的对照组F0。在室温(20±1) ℃下进行为期10 d的厌氧发酵实验。搅拌速度30 r·min−1,反应釜内投加聚氨酯海绵填料,填充体积比70%。每天取上清液,测定TCOD、SCOD、VFA、氨氮、TN、TP等指标。经0.45 μm膜过滤后的黑水原水和发酵后水样均在4 ℃下保存,并在3 d内完成测定。每天取样后,反应器内通氮气5 min以保证厌氧环境。
2)温度对厌氧发酵菌剂强化黑水厌氧发酵的影响。在3个装有2 L黑水的反应釜内各投加2.0 mL厌氧发酵菌剂(投加量由前述实验确定),搅拌速度30 r·min−1,聚氨酯填料填充体积比为70%,分别在10、20、30 ℃下进行为期10 d的厌氧发酵实验,实验编号为T1、T2和T3,并设置20 ℃下不添加厌氧发酵菌剂的对照组T0。经0.45 μm膜过滤后的黑水原水和发酵后水样均在4 ℃下保存,并在3 d内完成测定。每天取样后,反应器内通氮气5 min以保证厌氧环境。
3)黑水厌氧发酵过程中微生物群落结构分析。常温下,在有效容积为2 L的厌氧反应釜内进行厌氧发酵实验,厌氧发酵菌剂投加量为2.0 mL(由实验1确定),搅拌速率为30 r·min−1,聚氨酯填料填充率70%,记为M1。设置不添加厌氧发酵菌剂的对照组为M0,其他条件一致。取黑水原水及发酵时间为1、5、10 d的M0、M1发酵液,经12 000 r·min−1离心后获得沉淀,分析其微生物群落结构。
-
1)微生物群落分析方法。经离心处理后,沉淀物的高通量测序委托广东美格基因科技有限公司进行。使用引物341F(CCTACGGGNGGCWGCAG)和805R(GACTACHVGGGTATCTAATCC)对细菌16srDNA的V3V4区域进行扩增,使用Illumina Novaseq
6000 / Miseq高通量测序平台的PE250 / PE150 / PE300模式进行测序。使用QIIME2(Version QIIME2-202202 )软件进行降噪,并获得最终的ASV(Amplicon Sequence Variants)以及特征表。使用QIIME2软件的SILVA138.1数据库对进行物种注释分析[8-9]。2)其他指标分析方法。pH、TS、VS、TCOD、SCOD、氨氮、TN、TP按照国家标准方法测定[10]。使用pH100A型pH计(HACH, Loveland, Colorado, USA)测定pH。使用配有HPFFAP毛细管柱(30 m×0.25 mm×0.25 mm)和氢火焰离子化检测器的气相色谱仪(7890B,美国Agilent公司)测定VFA[11]。UV254、E4/E6使用分光光度计(UV8100A,中国Labtech公司)进行测定。蛋白质质量浓度采用比色法测定[12-13]。纤维素采用中性洗涤剂洗涤、蒽酮比色定糖法测定[14]。粪大肠菌值采用《粪便无害化卫生标准》(GB7959)中的推荐方法测定[15]。粪大肠菌群数采用多管发酵法测定[16]。三维荧光光谱采用荧光光谱仪(Cary Eclipse,美国Agilent公司)测定。粪臭素采用高效液相色谱仪(6890A,美国Agilent公司)测定,色谱条件如下:色谱柱为Hypersill BDSC18(250 mm×4.6 mm×5 μm),柱温30 ℃,紫外检测波长254 nm,流动相为甲醇:水(75:25,体积比),流速为0.6 mL·min−1,进样量为20 μL。
-
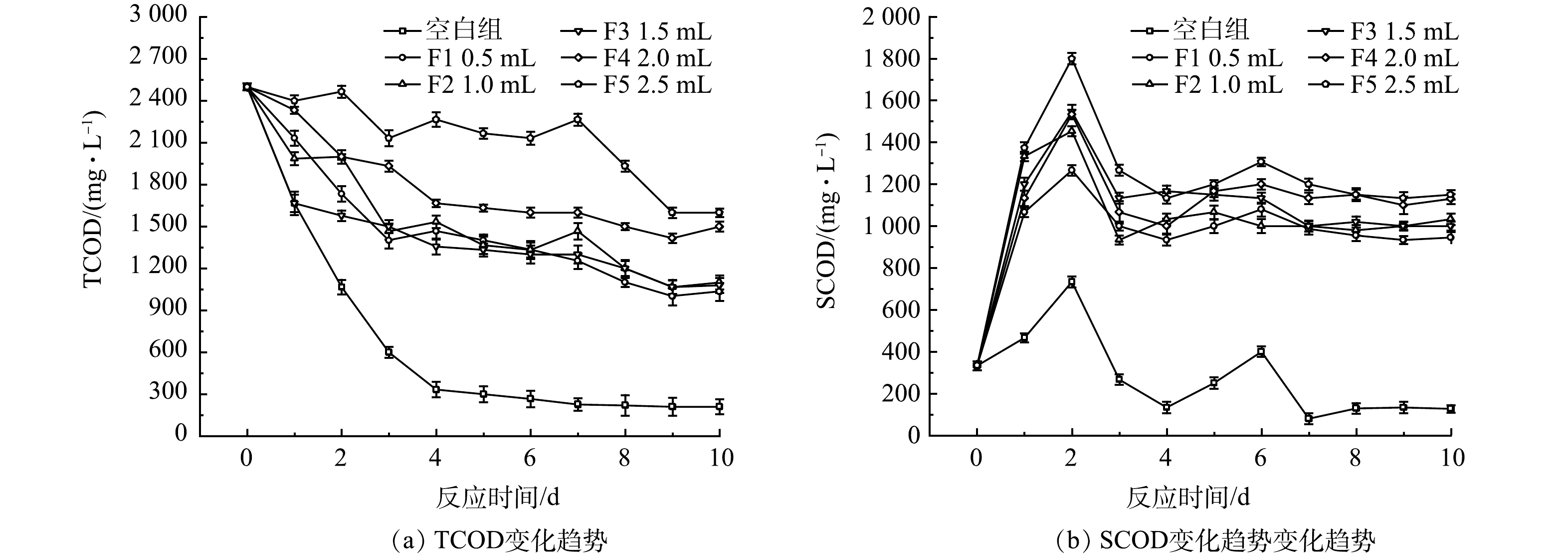
1)厌氧发酵菌剂投加量对TCOD与SCOD的影响。不同厌氧发酵菌剂投加量下的黑水TCOD与SCOD值在不同时间的变化如图2所示。在常温且没有投加菌剂的情况下,TCOD整体呈波动降低的趋势。与对照组相比,添加了厌氧发酵菌剂的反应器内,TCOD值降低较慢。其中F5号反应器添加了最多剂量的厌氧发酵菌剂,其TCOD的去除率为36%,低于F4号反应器的43.3%。F1、F2和F3号反应器有着相似的TCOD去除率,均在67%左右。这种TCOD变化的差异可能是由于菌剂的添加导致黑水中的更易通过物理沉降作用沉淀的大颗粒物质被分解为粒度更小、更不易沉降的小颗粒物质。这点也可以从黑水的表观形态看出,添加了菌剂的反应器内絮状物更多,而对照组内大部分固体物质均沉降在底部。
与TCOD不同,SCOD表现出不同的趋势。随着反应时间的增加,SCOD整体呈现出先上升后略微下降,随后保持稳定的状态。在反应器内部同时存在2种反应,一种是粒径较大的不溶性的颗粒在微生物的作用下逐步转化为粒径较小的不溶性颗粒,并且进一步转化为可溶性物质,这会导致SCOD的升高。同时,溶解在水中的有机物会通过微生物的同化作用和异化分解,导致SCOD的减少。出现上述趋势的原因可能是,在反应前3 d,反应器内的主要反应过程为非溶解性物质转化为溶解性有机物,因此SCOD值在前3 d迅速积累,呈上升趋势,随后2 d,黑水中溶解性有机物含量的增加导致微生物大量生长,同化作用和异化分解开始占据主要地位,SCOD呈下降趋势,之后这两类反应过程逐渐达到平衡。6个反应器内,SCOD值均在第3天达到峰值,随后开始下降。其中F5反应器内SCOD峰值最高,为1 800 mg·L−1,而不添加菌剂的对照组峰值最低。上述结果说明添加厌氧发酵菌剂可以有效促进黑水中不溶性物质转化为溶解性有机物,进而有利于黑水中微生物的增殖,这一点在后续进一步讨论。厌氧发酵菌剂的投加量影响了黑水SCOD值,较多的厌氧发酵菌剂促进了黑水中SCOD的积累。F5反应器添加了2.5 mL厌氧发酵菌剂,获得最高的SCOD值,但反应10 d后SCOD的积累量仅比添加2.0 mL的F4反应器提高3%,而F4反应器比F3中的SCOD值提高了10%。该结果表明厌氧发酵菌剂添加的越多,越有利于黑水中不溶性有机质的水解,但菌剂的添加量增加至一定值后,其效果不再明显提升。
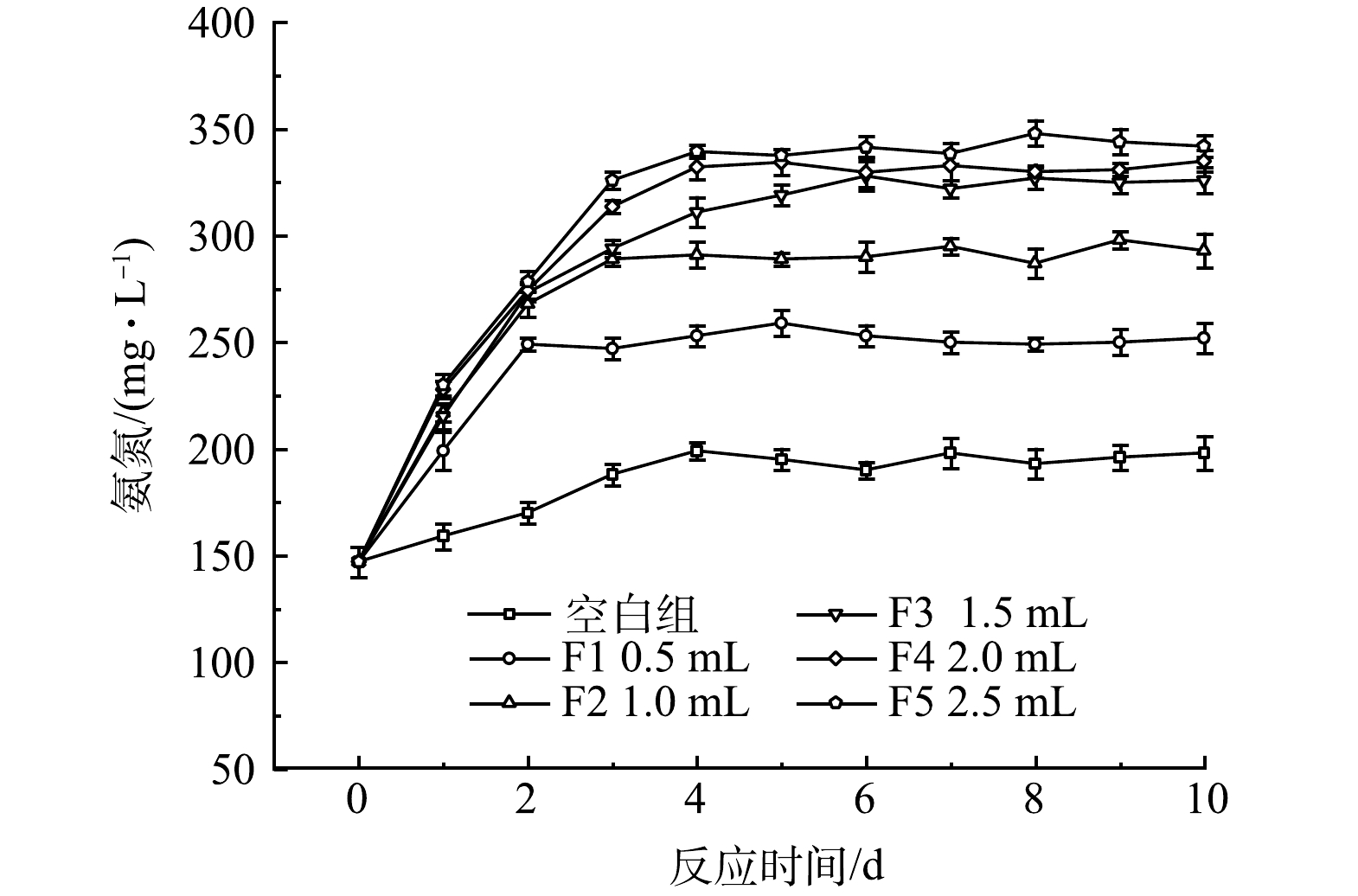
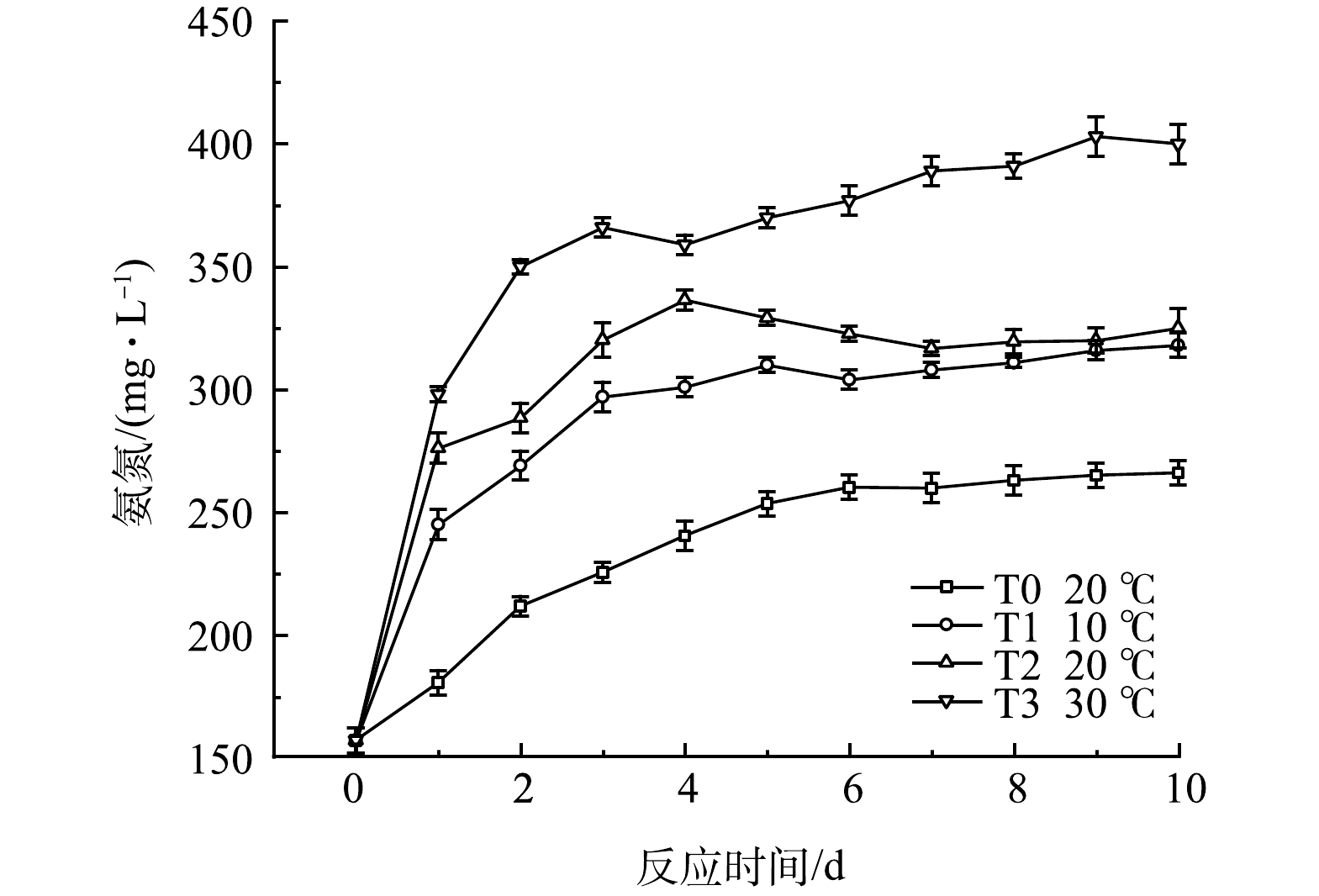
2)厌氧发酵菌剂投加量对氨氮的影响。不同厌氧发酵菌剂投加量对黑水氨氮值的影响如图3所示。由于黑水中含有大量蛋白质,在微生物的分解作用下可形成高质量浓度的氨氮[17]。由图3可见,氨氮由最开始的147 mg·L−1开始快速积累,最高达到342 mg·L−1。有研究[18]表明,厌氧发酵过程氨氮质量浓度应保持在200 mg·L−1以下,以避免高质量浓度氨氮抑制产甲烷菌的活性。但本文使用的厌氧发酵菌剂旨在促进厌氧产酸过程,即厌氧发酵的第一阶段,通过这一阶段产生富含有机物和氮磷营养物质的发酵液,进而实现黑水的资源化。因此,尽管在很多文献中氨氮积累被极力避免,但在本文中氨氮积累可以提高发酵液中营养盐的含量,有利于黑水资源化效率。添加厌氧发酵菌剂的反应器中氨积累量明显高于对照组,反应10 d后F5反应器的氨氮质量浓度达到了342 mg·L−1,明显高于对照组的198 mg·L−1。YAN等[19]研究表明,黑水在厌氧发酵过程中产生的高质量浓度氨氮主要来源于尿素和蛋白质的分解。因此,厌氧发酵菌剂的添加能够明显促进蛋白质和尿素等的水解过程,对于提高黑水中的营养物质量浓度有贡献较大,该结果在后文的蛋白质的分析中也能进一步证明。与SCOD类似,仅添加0.5 mL的厌氧发酵菌剂就可以使黑水中的氨氮质量浓度提升27%,而F5与F4相比氨氮质量浓度积累仅提高2%,因此,在提高厌氧发酵菌剂量至2.0 mL后,再增加菌剂使用量并不能明显提高氨氮的积累效率。
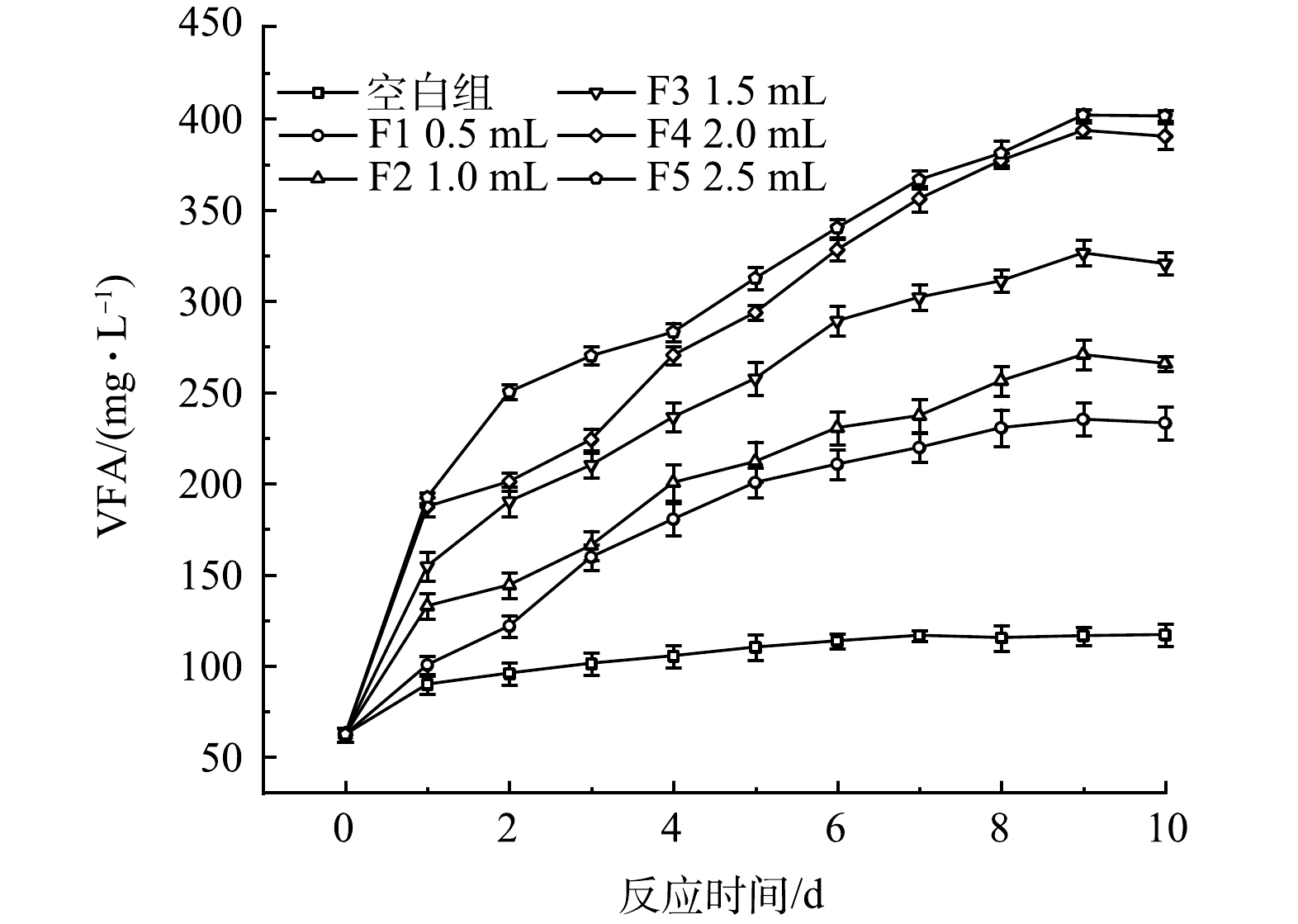
3)厌氧发酵菌剂投加量对VFA生成的影响。厌氧发酵菌剂投加量对黑水VFA值的影响如图4所示。黑水中的大分子有机物通过厌氧发酵转化可为微生物、植物利用的小分子有机物,本研究使用的厌氧发酵菌剂强化了厌氧产酸的过程,促进黑水中VFA的积累。对照组中的VFA积累量最低,反应10 d后从最开始的62.16 mg·L−1仅增加至116.84 mg·L−1。而在添加了厌氧发酵菌剂的反应器内,VFA的积累量明显高于对照组,表明厌氧发酵菌剂对黑水厌氧发酵的产酸阶段具有积极的促进作用,增加发酵菌剂的投加量可以提高VFA的产量,但投加量从F4的2 mL提高至F5的2.5 mL时,VFA产量和积累速率相近。
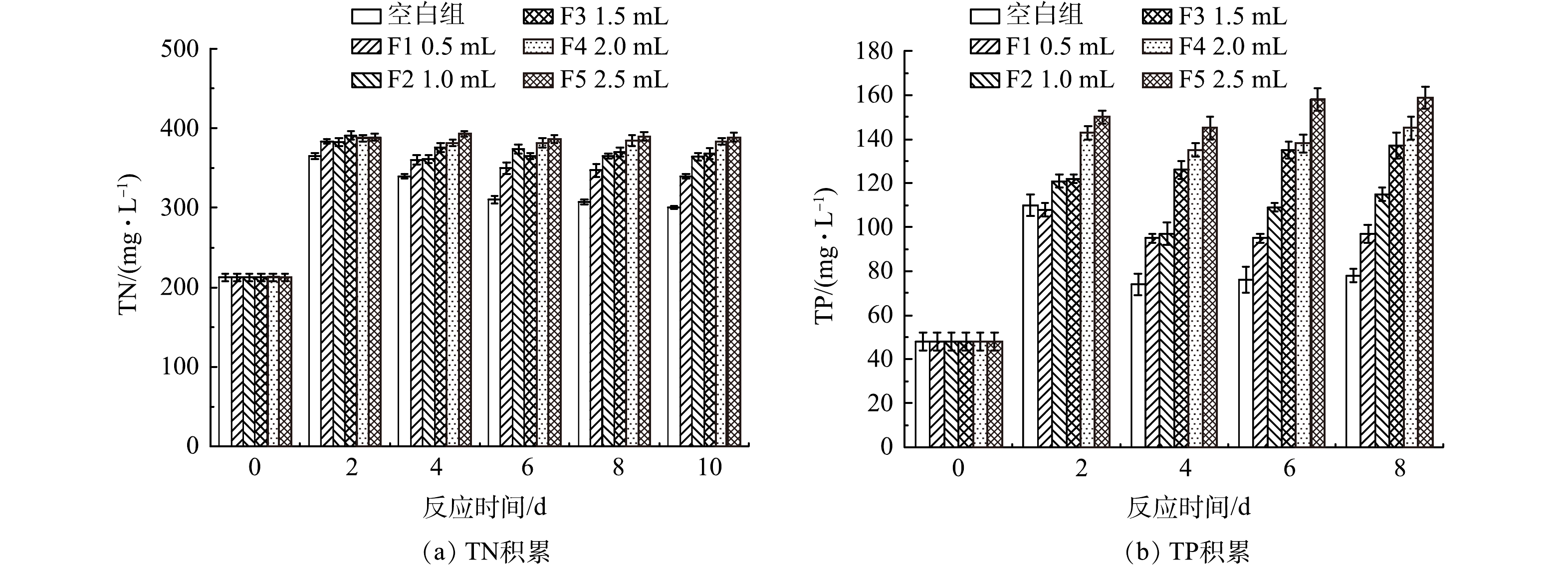
4)厌氧发酵菌剂投加量对TN与TP的影响。厌氧菌剂投加量对黑水中TP与TN值的影响如图5所示。6个反应器的TN质量浓度在第2天均有明显积累,且积累量相当,但在随后的反应中均有所衰减。发酵过程中各反应器pH由9.2下降至8.1~8.4,始终处于碱性条件,因此部分游离氨的挥发导致TN下降,但由于氨氮的产生量大于氨的挥发量,氨氮质量浓度没有下降(图3)。添加了厌氧发酵菌剂的反应器内,由于发酵液中氨氮质量浓度的增加,TN的衰减速度略慢于对照组,但菌剂投加量对于TN质量浓度的变化没有显著影响。TP的积累与TN有所差异,反应第2天由于颗粒态含磷有机物的水解,各组TP质量浓度均有增加,F0~F2组在反应2 d后,TP质量浓度开始有所降低,而F3~F5组的TP质量浓度缓慢增加,其中F5组在反应10 d后TP质量浓度最高,达到159 mg·L−1。这可能与固磷微生物的作用有关,即添加1.5 mL及以上的厌氧发酵菌剂的反应体系中固磷微生物菌群在厌氧发酵菌剂的作用下得到更好生长[19]。
5)厌氧发酵菌剂投加量对粪大肠菌值的影响。在《粪便无害化卫生要求》(GB7959-2012)ak/>中,粪大肠菌值是评价黑水无害化的一个重要指标[15]。本研究监测了黑水原水和发酵10 d后黑水的粪大肠菌值,结果如表2所示。由表可知,经过10 d的厌氧发酵后,黑水中粪大肠菌值均升高。粪大肠菌值越高,表明检测出粪大肠菌存在所需要的稀释倍数越高,也表明该体系内可能含有的粪大肠菌的个数越少。表2表明,添加了厌氧发酵菌剂的实验组,其发酵液粪大肠菌值均显著高于对照组F0,证实了本研究使用的厌氧发酵菌剂可以高效促进黑水的无害化。
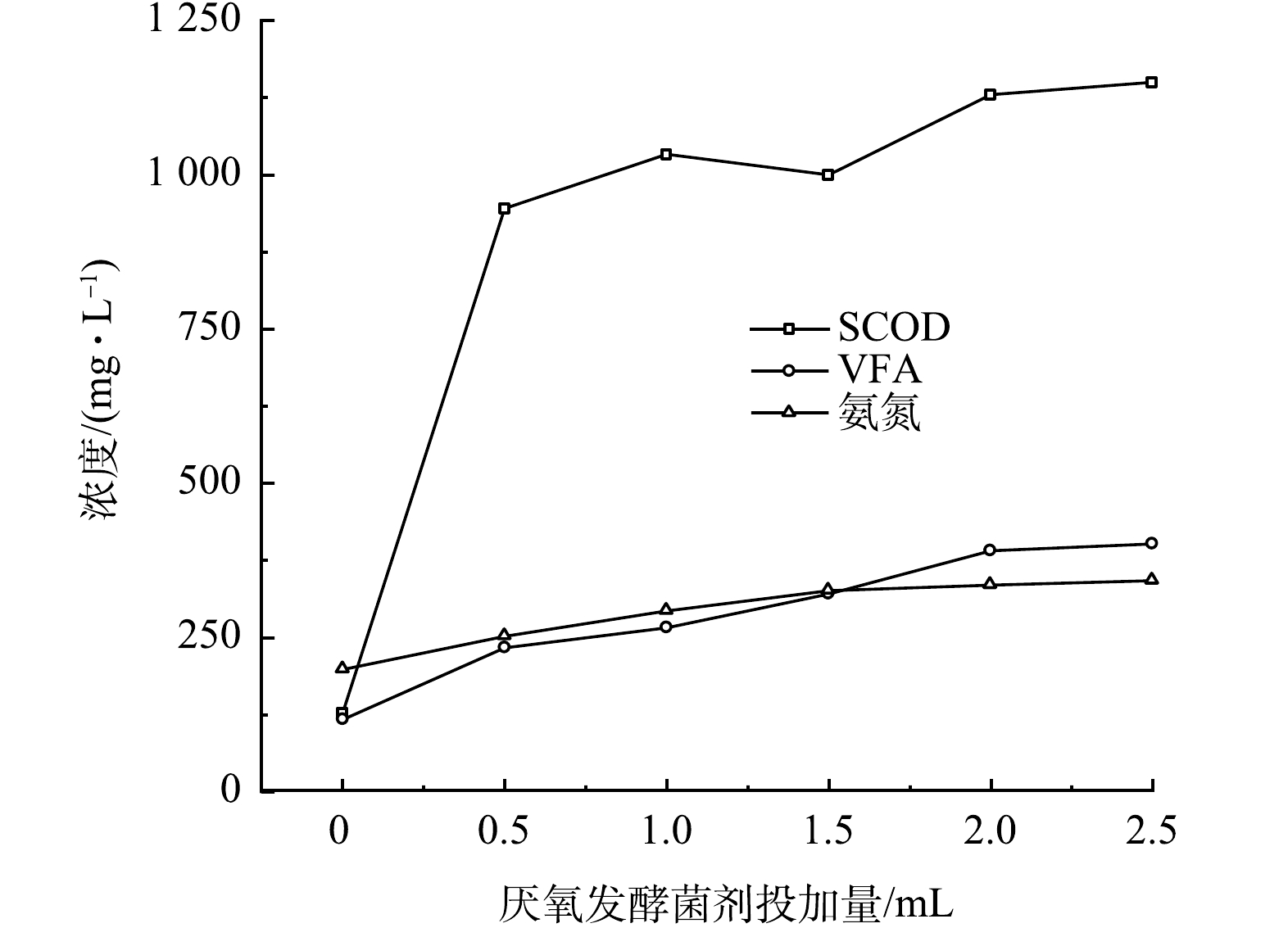
黑水在不同厌氧发酵菌剂投加量时经10 d厌氧发酵后,SCOD、氨氮和VFA的积累如图6所示。由图可知,各指标的最终积累的质量浓度与厌氧发酵菌剂投加量呈正相关,表明厌氧发酵菌剂可以促进黑水在厌氧发酵过程中固态及大分子有机物的水解、VFA的产生和致病菌的去除,菌剂量从0增加至2 mL时促进作用显著,而当菌剂投加量从2.0 mL增加至2.5 mL时, SCOD值、VFA值和氨氮质量浓度变化不显著,因此厌氧发酵菌剂的适宜投加量为1.0 mL·L−1黑水,即黑水量的1‰。
-
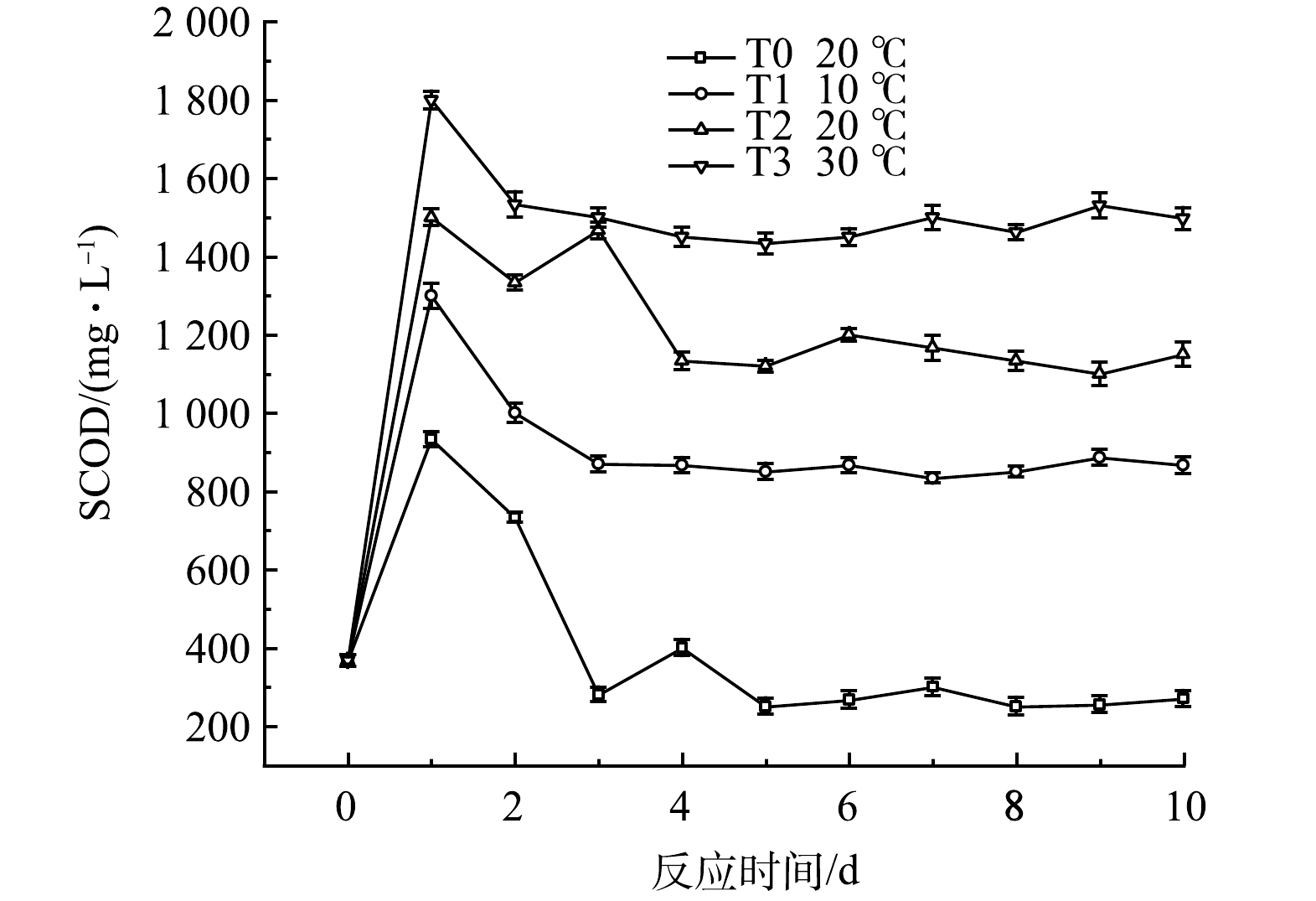
1)对水质指标的影响。SCOD可以直观表现出黑水中溶解性有机物的积累,不同温度时黑水的SCOD值变化如图7所示。由于黑水中颗粒态有机物在发酵初期的有效水解,各组SCOD值快速上升,随后由于微生物的同化作用及异化分解,SCOD值逐渐下降并维持稳定。温度对水解过程有显著影响,温度越高,发酵液SCOD的峰值越高,T3组的SCOD值在反应第1天就达到1 800 mg·L−1的峰值,而T1组的峰值仅为1 300 mg·L−1,但均高于对照组T0的933 mg·L−1,各组在实验4 d后SCOD值趋于稳定,该结果表明在10 ℃至30 ℃的范围内,提高温度可以促进黑水厌氧发酵过程的水解阶段。而在10 ℃时,添加厌氧发酵菌剂的黑水与常温下不添加菌剂的黑水相比,其SCOD值的积累也较高,说明在较低温度下,厌氧发酵菌剂依然可以有效促进黑水中颗粒态有机物的水解。
温度对黑水氨氮质量浓度的影响如图8所示。各组氨氮质量浓度呈现先升高后稳定的趋势,其中T1和T2两组在反应4 d后,氨氮值的增长达到相对稳定的状态,T2的氨氮积累量在反应10 d后达到325 mg·L−1,略高于T1的318 mg·L−1,而T3中氨氮值在反应7 d后趋于稳定,积累量为403 mg·L−1,高于其余实验组。该结果表明较低温度仍然可以实现氨氮的积累,但适当升高温度可促进黑水中含氮有机物的生物分解,使氨氮的积累更多。
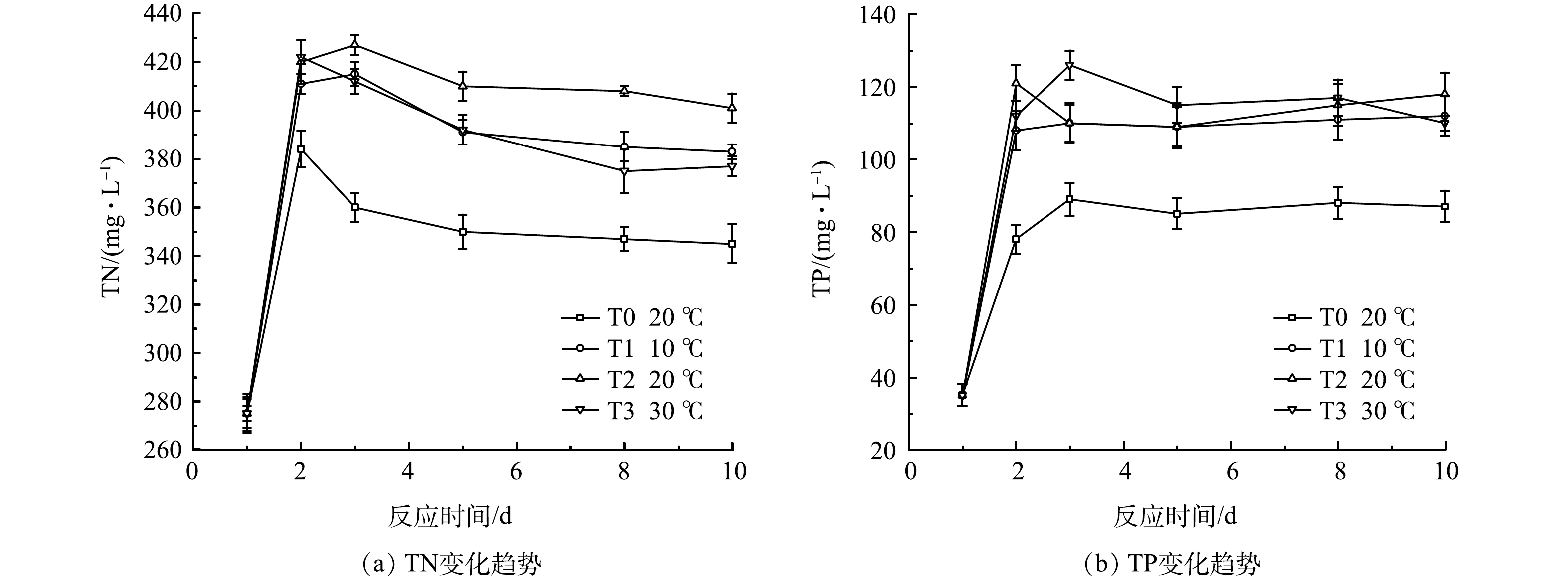
图9为各组黑水发酵中TN变化。在经历1~2 d的快速积累后,由于氨的挥发,总氮值均有所下降,其中T3组TN积累最多,第2天达到峰值422 mg·L−1,但其随后的下降量也最多, 第10 d时质量浓度降至377 mg·L−1,低于T1和T2组,可能原因是较高的温度促进了氨的挥发,导致TN下降。低温下的T1组TN值在各反应阶段均低于T2常温组,其原因为低温条件下微生物活性下降,颗粒态含氮有机物的分解较慢,达到平衡时的TN值也较低。不同温度时各组TP质量浓度均高于对照组T0,但T1~T3组间差异不大,说明投加发酵菌剂时温度对于总磷的积累影响不大。
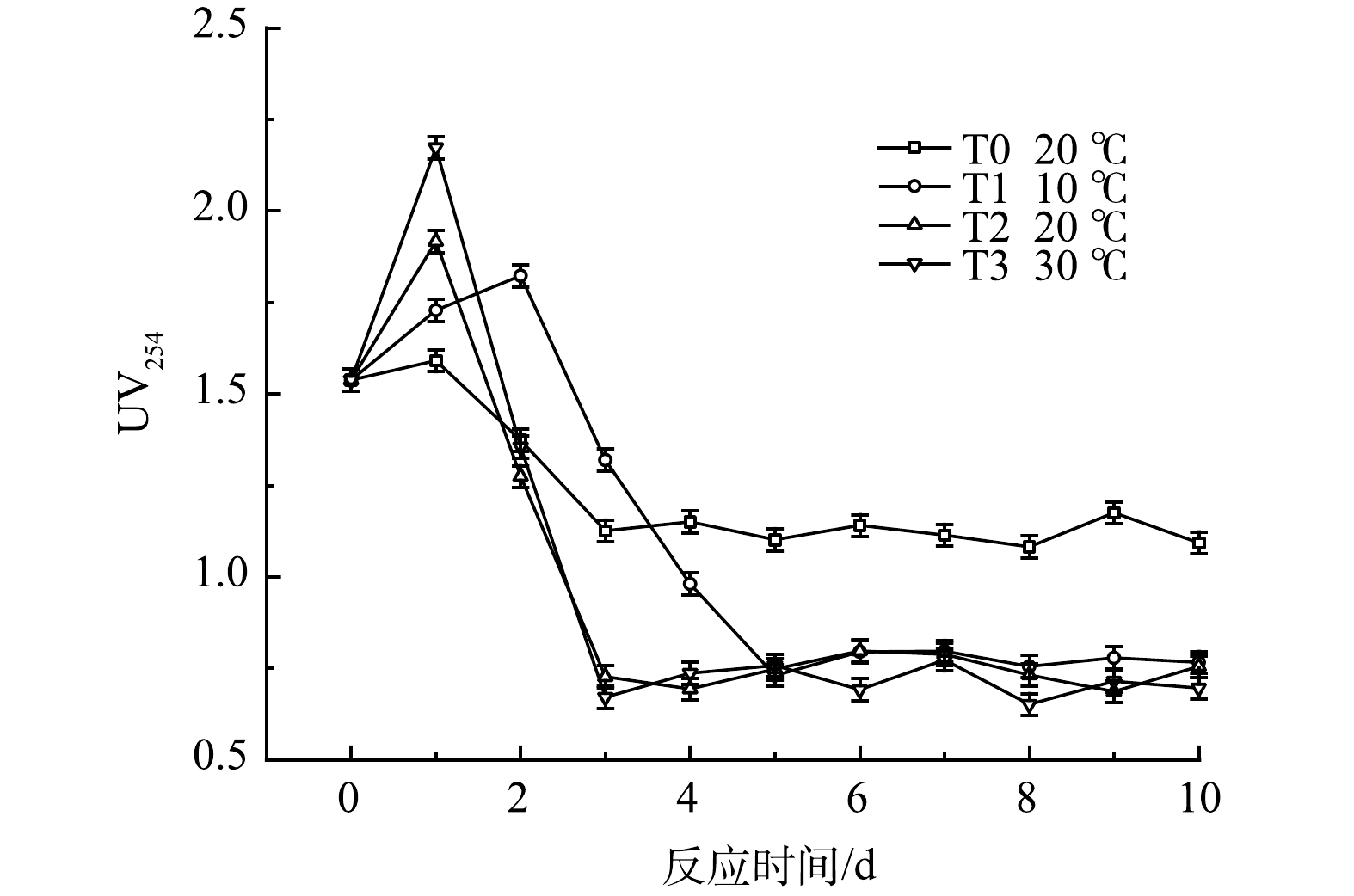
UV254是衡量水中有机物的一个重要指标,其与COD、DOC等均具有良好的关联性[20-21]。由图10可知,厌氧发酵1~2 d内 UV254值即达到峰值,随后快速下降,在第3~5天开始稳定,这种变化是黑水中颗粒态有机物的水解和溶解性有机物生物降解共同作用的结果。有研究表明,木质素、丹宁等有机污染物在254 nm处均有较强的吸收[22],UV254的下降表明在厌氧发酵菌剂的作用下,这类物质很好地被降解[23],而未投加发酵菌剂的对照组T0中黑水UV254值变化量较小。温度对UV254有显著影响,温度越高,其值变化速率越高,其原因为温度影响黑水中微生物活性。T1低温组黑水的UV254在第5天后才达到稳定状态,但稳定后UV254的值与T2和T3组无显著区别,表明温度对黑水中有机物的水解程度并无显著影响。
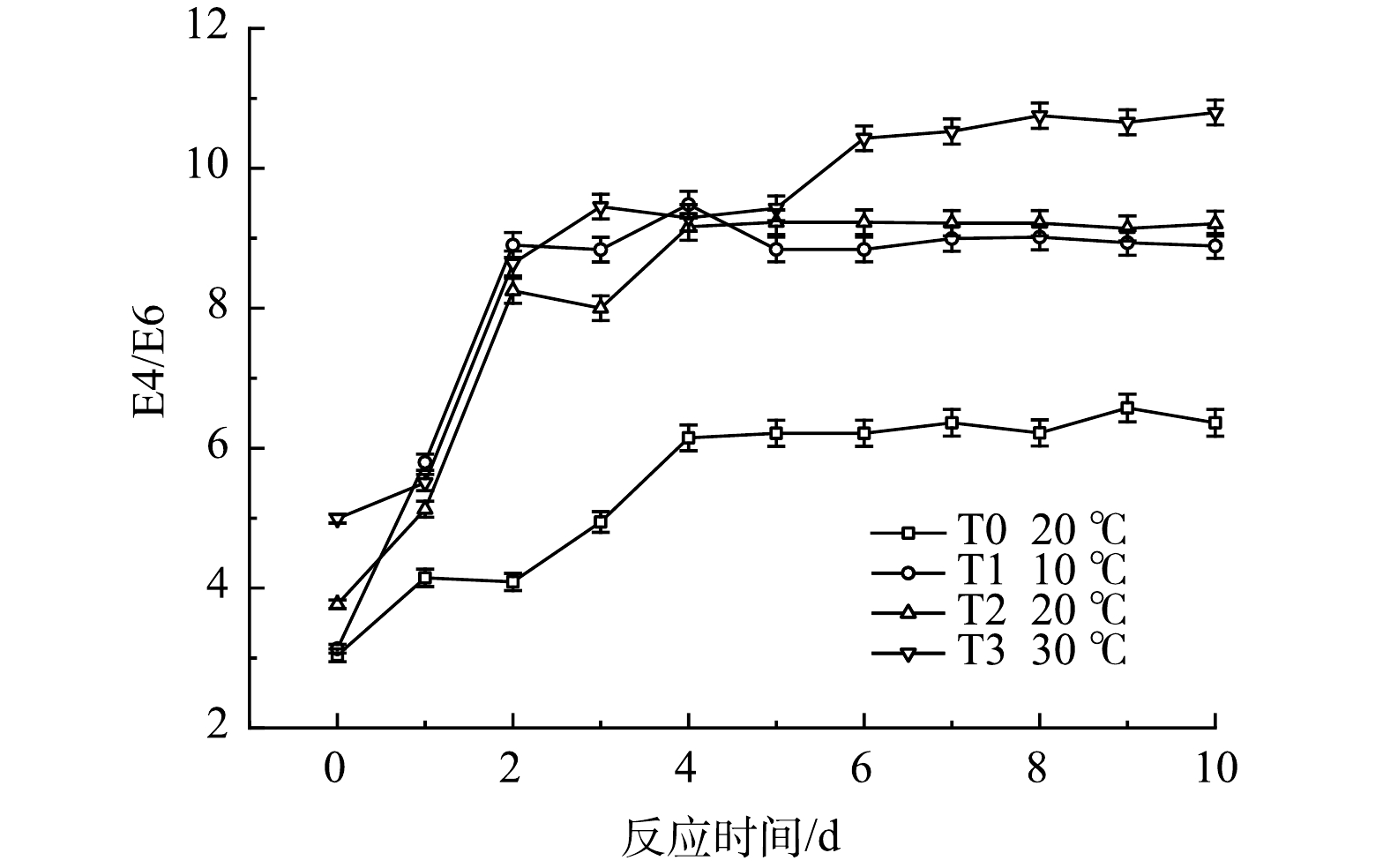
E4/E6值可用于表征苯环 C 骨架的聚合程度及羰基共轭度,其值越小表示有机物的聚合程度越高[24]。温度对黑水E4/E6值的影响如图11所示。在厌氧发酵的前期,E4/E6快速升高,表明在反应起始阶段大分子物质占总溶解性有机物的比例较高,随着反应的进行,小分子物质占总溶解性有机物的比例快速增加,其原因为发酵菌剂在反应前期即可将大分子有机物有效分解为小分子物质。研究表明,分子质量小于1kDa的有机物含量是水体是否具有生物稳定性的关键[25],因此在厌氧发酵菌剂作用下发酵的黑水具有更强的生物稳定性。T1与T2组相比没有明显差异,T3组由于温度升高,黑水的E4/E6值在反应后期略高于另外两个实验组,该现象表明温度升高可以使黑水中小分子有机物占比提高。
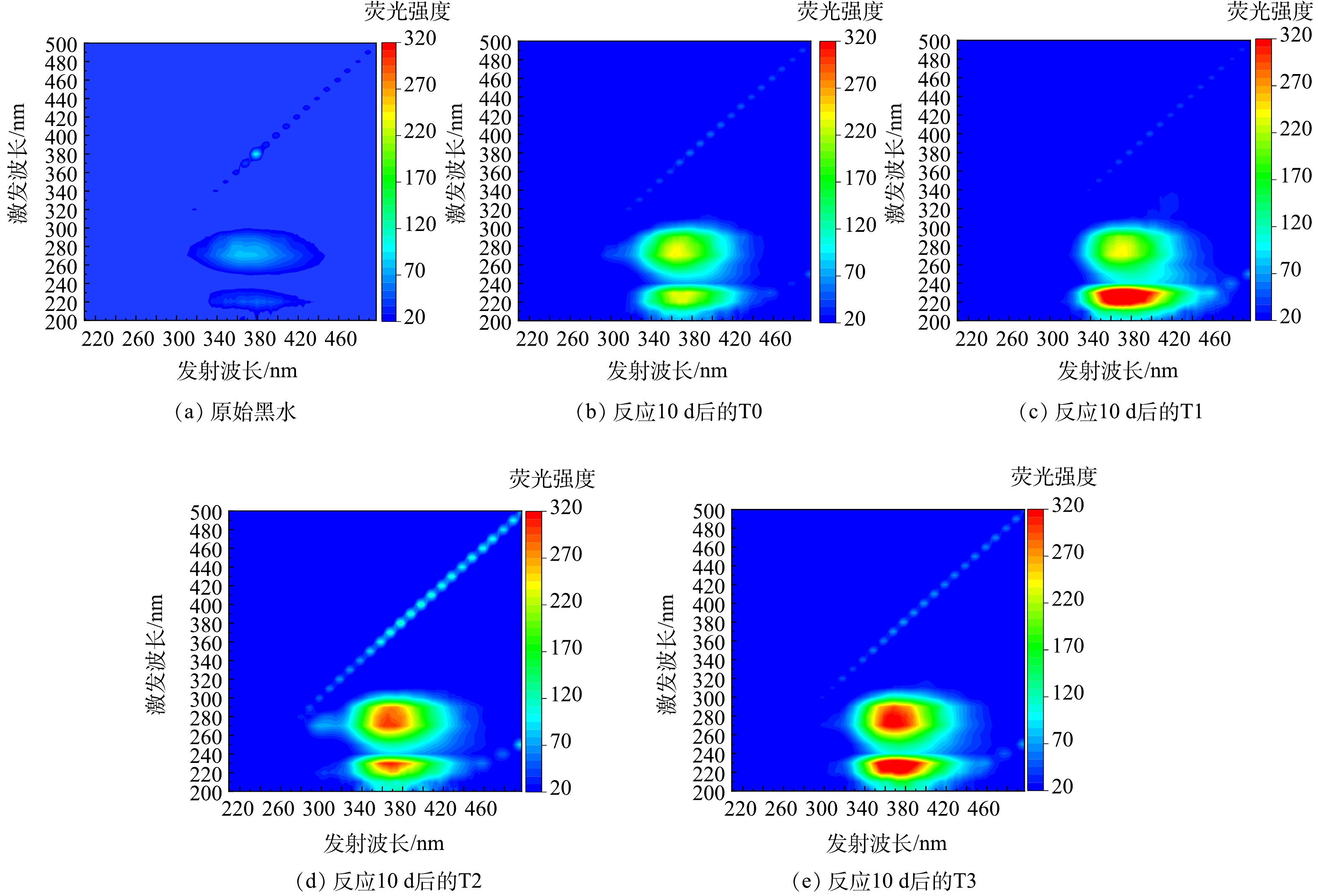
2)对有机物转化的影响。黑水中溶解性有机物(dissolved organic matter, DOM),如腐殖酸和蛋白质等,受特定激发光线照射的影响,会产生各异的荧光信号[26]。对黑水的三维荧光光谱图进行分析,可以揭示黑水在不同温度时发酵前后DOM化学组分的变化,结果如图12所示。由图12可知,黑水的三维荧光光谱图主要由两个明显的荧光峰组成,分别为类腐殖酸峰(Ex/Em=260~300 nm/350~390 nm)与类蛋白质峰(Ex/Em=215~235 nm/350~410 nm)[27-28],这与王明月等[22]对黑水进行三维荧光光谱强度测定,所检测到的峰一致。图12表明,经10 d发酵后,黑水中类腐殖酸与类蛋白质物质含量明显升高,且与对照组相比,T1~T3实验组黑水中类腐殖酸与类蛋白质物质的积累明显高于对照组,表明添加厌氧发酵菌剂可以极大促进黑水在厌氧发酵的过程中类腐殖酸与类蛋白质物质的积累。T1组中,黑水经发酵后,类腐殖酸物质的积累低于T2与T3,表明低温条件不利于将有机物分解为类腐殖酸物质。而不同温度条件下,对于类蛋白物质的积累并无明显影响。
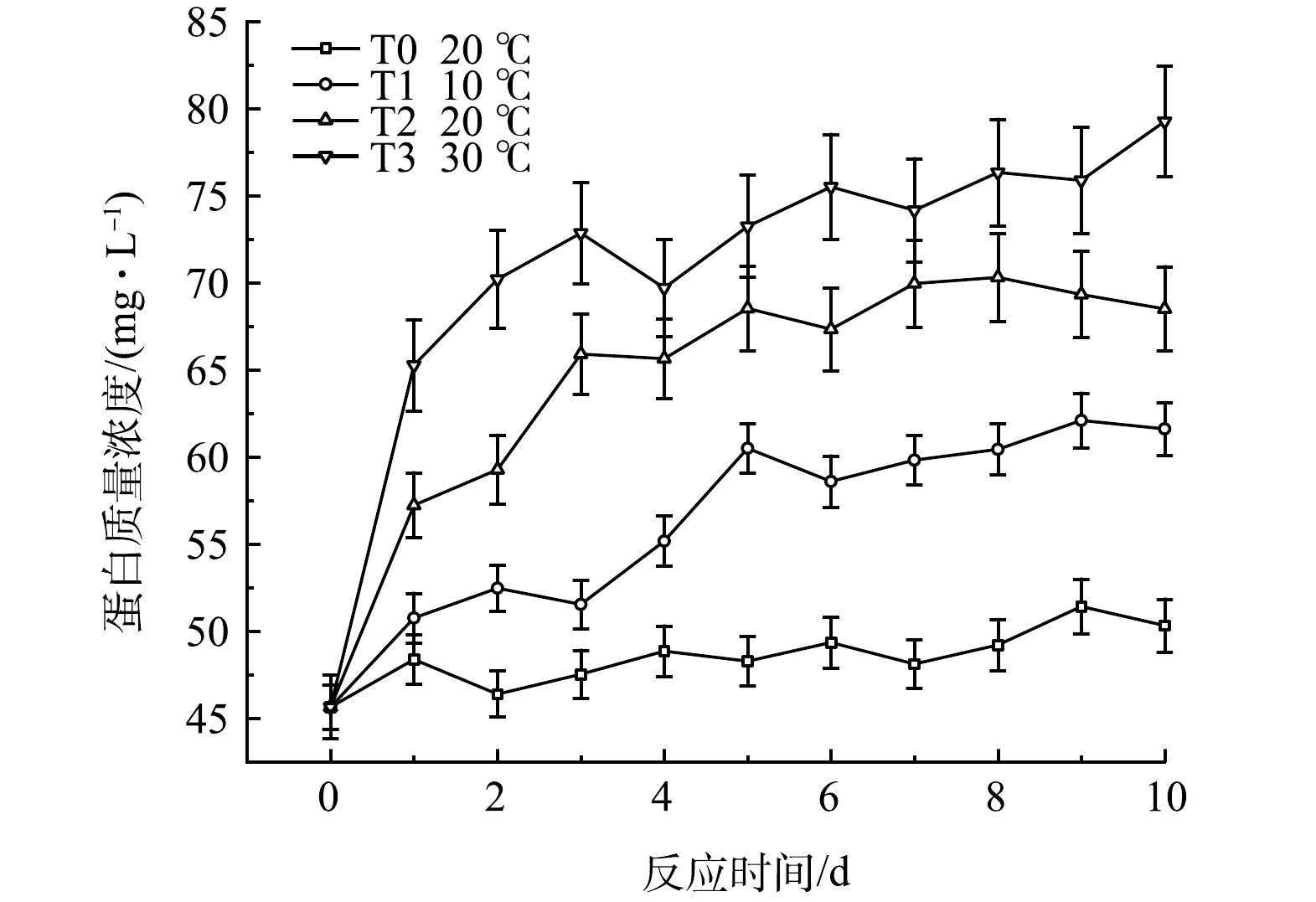
黑水发酵过程中的蛋白质量浓度的变化如图13所示,呈现先升高、后逐渐趋于稳定的趋势。由图图13可知,温度越高,黑水中蛋白质量浓度升高越快,积累量也越多,T3中黑水的蛋白质量浓度在发酵10 d后达到79.8 mg·L−1,而T2与T1组中的蛋白质量浓度分别为61.6 mg·L−1和68.5 mg·L−1。与对照组T0相比,T1中的蛋白质量浓度积累速率与最终积累量都较高,表明低温条件下添加厌氧发酵菌剂也可以促进黑水中蛋白质量浓度的提高。
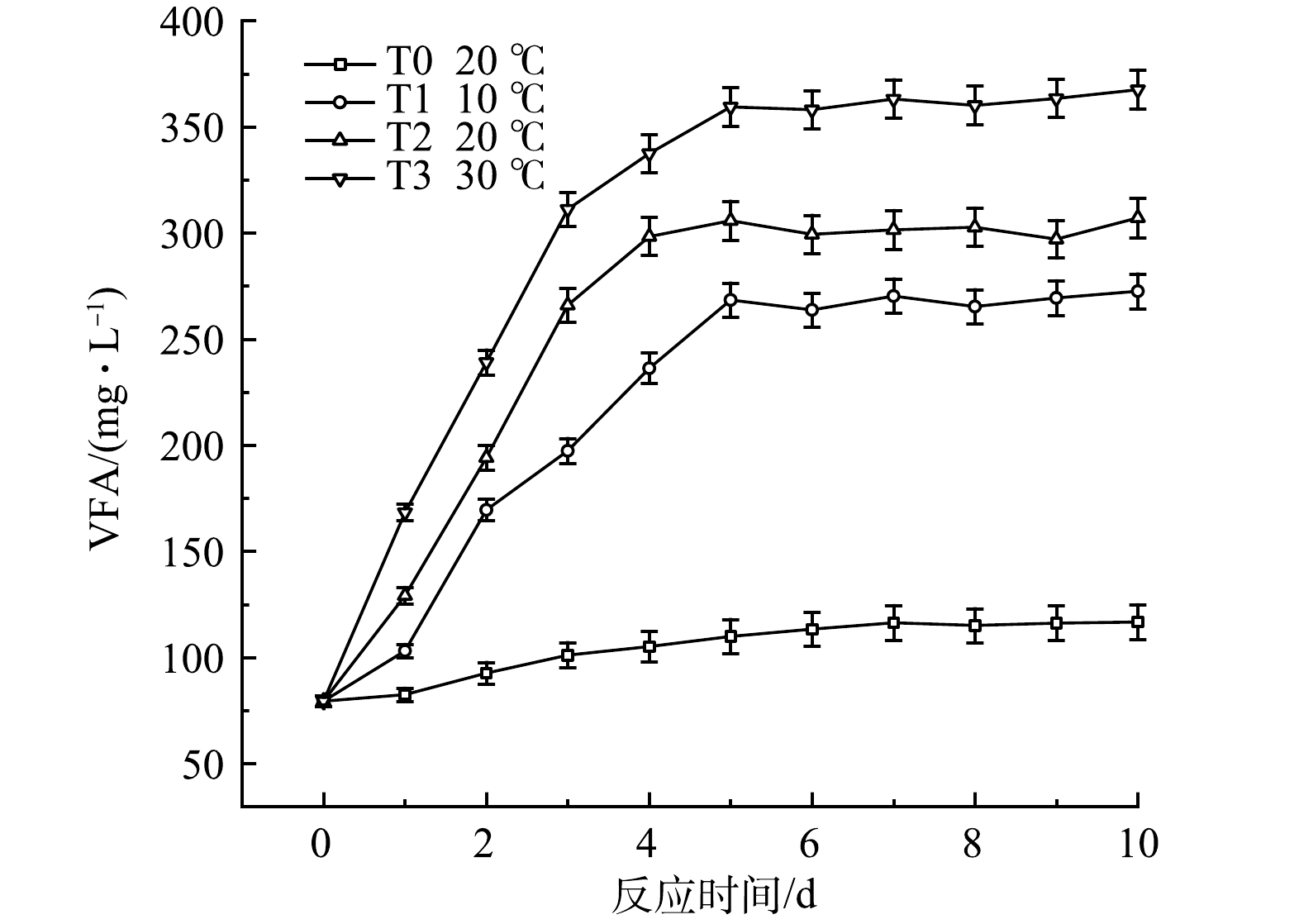
温度对黑水厌氧发酵过程中VFA的积累速率有显著明显影响,结果如图14所示。T3组VFA积累最快,在反应第5天达到359 mg·L−1,T2与T1组也在第5d达到最高值,分别为305 mg·L−1与268 mg·L−1,T1~T3组均在第5天后维持稳定,表明温度的升高可以促进黑水厌氧发酵中的产酸过程,使VFA积累量提高。T1组与T0组相比,VFA的积累速率与积累量也有明显升高,这一现象表明在低温条件下,可以通过投加厌氧发酵菌剂的方式来促进黑水的厌氧发酵产酸过程。
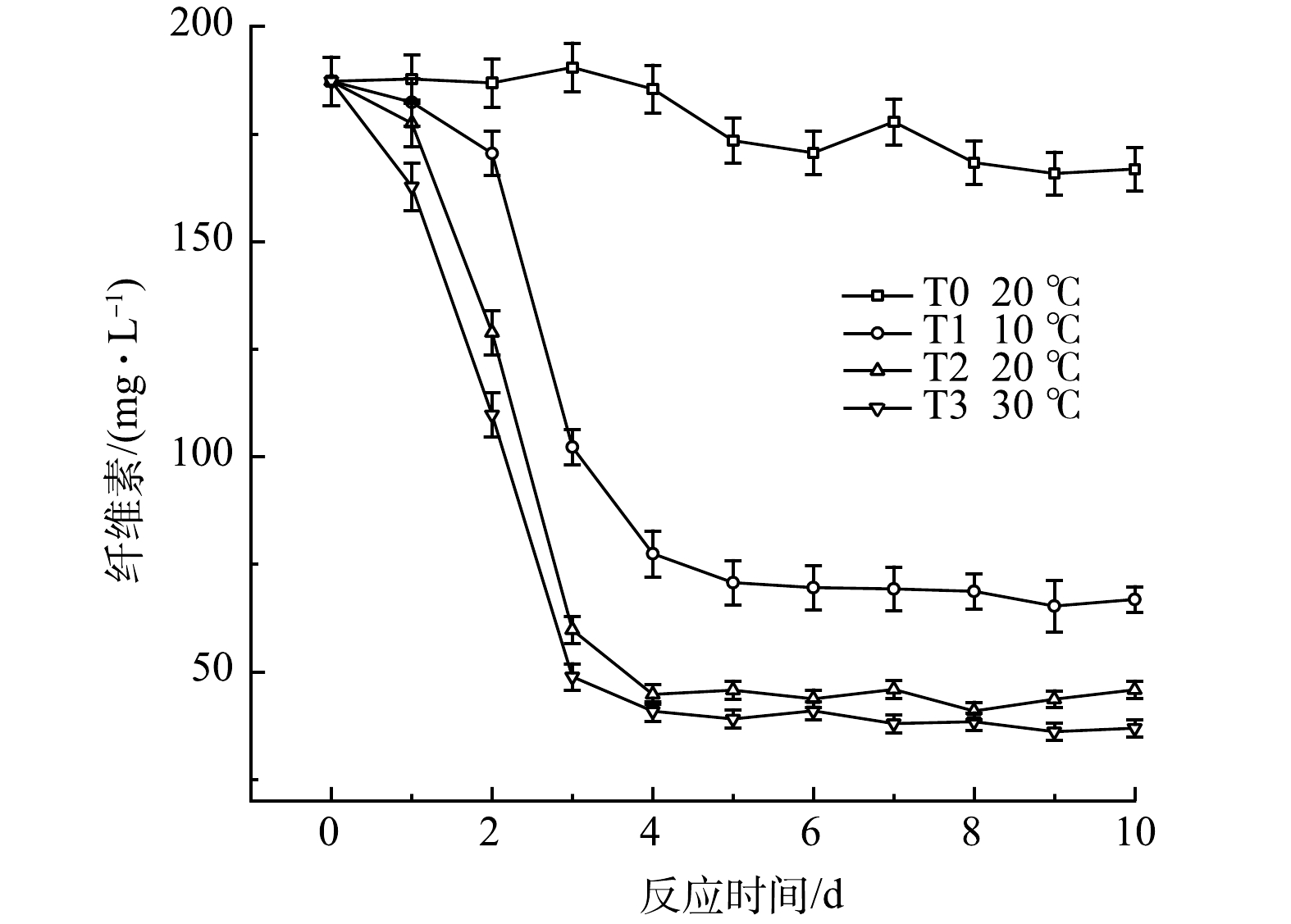
纤维素是一类分子量大且难以被微生物降解利用的有机物,这也是导致厌氧发酵启动困难的一个很重要的因素[29]。图15反映了黑水厌氧发酵过程中的纤维素含量的变化。由图可知,在常温且不添加厌氧发酵菌剂的情况下,T0组经10 d的厌氧发酵后,纤维素仅被降解10.9%左右,而添加了厌氧发酵菌剂的实验组,纤维素降解速率明显高于T0组,表明厌氧发酵菌剂对纤维素的降解具有明显促进作用。T1组在发酵初期纤维素质量浓度下降较慢,这可能是由于在低温条件下,纤维素降解菌群较不活跃,导致降解速度较低,并且在发酵10 d后,其纤维素降解率为64.3%,低于T2组的75.5%和T3组的80.3%。该结果表明较高温度促进了纤维素的生物降解,而低温对纤维素的生物降解有一定抑制作用,但仍显著优于空白组中的纤维素降解。
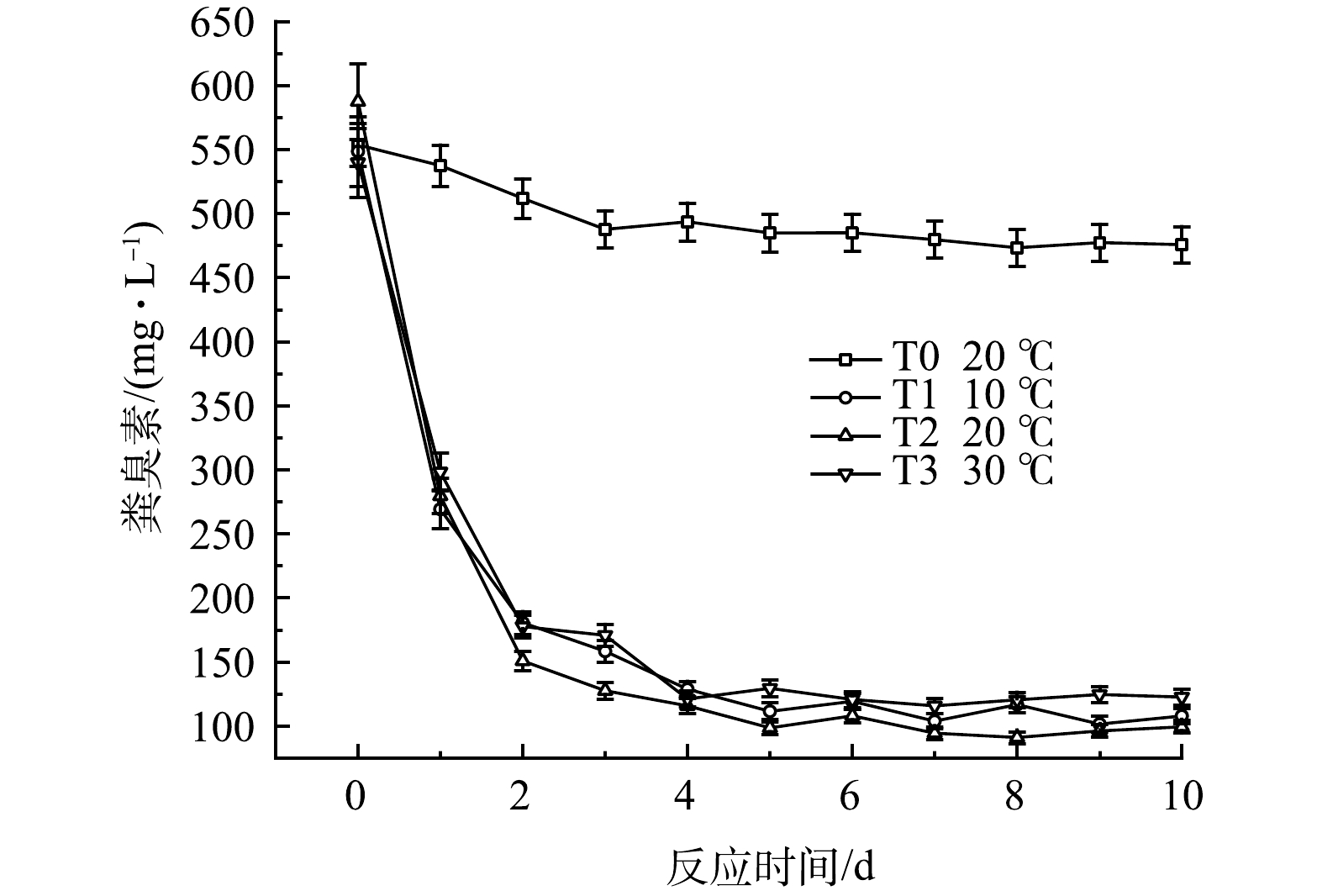
3)对无害化指标的影响。粪臭素(3-甲基吲哚)是一类有难闻气味的有机物,是黑水中臭味物质的主要来源[30]。通过液相色谱法对粪臭素含量进行监测,结果如图16所示。常温下,不添加发酵菌剂的黑水厌氧发酵10 d后,对粪臭素的降解能力有限,粪臭素质量浓度由553.5 mg·L−1降低至475.7 mg·L−1,去除率为14%,因此,单独黑水在厌氧发酵过程中会有难闻的气味。T1~T3组粪臭素降解效果显著,降解率在77.3%~83.1%之间。有文献报道了单独培养粪臭素降解菌Burkholderia sp. IDO3,在pH 4.0~9.0,30~35 °C 时降解粪臭素性能最佳,降解率为82%[31],MA等[32]的研究中,两种粪臭素降解菌Rhodococcus sp. DMU1 和 Rhodococcus sp. DMU2 在接种量为1%、pH为7.0、温度30 °C、转速150 r·min−1时可降解250 mg·L−1的粪臭素,MENG等[33]培养了Lactobacillus brevis 1.12菌株,在1 mL的粪臭素质量浓度为1.0 μg·mL−1系统中,粪臭素降解率为65%。本研究通过添加厌氧发酵菌剂,可以实现最高83.1%的粪臭素降解率。
粪大肠菌是一种常见的致病菌,多见于人与动物的排泄物中。在2.1节中检测了黑水在发酵过程中粪大肠菌值的变化,在本小节进一步采用多管发酵法确定具体的粪大肠菌群数,从而可以更直观反映黑水中粪大肠菌群的数量变化。各反应组不同时间的粪大肠菌群数的最大可能数(most probable number, MPN)如表3所示。在对照组T0中,黑水的粪大肠菌群数在厌氧发酵过程中均处于较高的数值,远超我国农业推荐标准中规定的10 000个·L−1[34]。添加厌氧发酵菌剂后,在反应初期对粪大肠菌并没有高效灭活,T2与T3组甚至出现了粪大肠菌群数升高的现象,这可能是由于在反应初期,反应器内还未处于完全厌氧状态,发酵系统的还未稳定,导致粪大肠菌群数出现波动。从反应第3天开始,T1~T3组黑水中的粪大肠菌群数均开始迅速下降,直到反应第7天时,黑水中的粪大肠菌开始保持在较低的水平。在发酵后期,添加了厌氧发酵菌剂的黑水中,粪大肠菌群数远低于我国农业推荐标准。可能的原因是厌氧发酵菌剂的添加,显著减少了Bacteroides菌属的相对丰度,文献研究表明这是一类与肠道疾病有关的菌属,对粪大肠菌群的贡献较高[35]。该结果表明,在低温、常温和较高温度条件下,厌氧发酵菌剂可以促进高效灭活黑水中的粪大肠菌群,有效降低利用黑水发酵液作为液态肥施用于农田时的安全风险。在实际应用中,应保证黑水的厌氧发酵时间不少于7 d,以确保黑水的无害化效果,控制资源化利用的安全风险。
对黑水中的蛔虫卵个数在发酵前后的数量变化也进行了检测和分析,结果如表4所示。根据我国粪便无害化卫生标准,蛔虫卵死亡率应≥95%,由表4可知,T1~T3均达到标准要求限值,蛔虫卵死亡率均在97%以上,而T0组未添加厌氧发酵菌剂,经过10 d的发酵,蛔虫卵指标难以满足无害化要求。表明厌氧发酵菌剂的投加可以快速有效灭活黑水中蛔虫卵等寄生虫卵,使其满足我国农业推荐标准和无害化卫生要求。T0组的蛔虫卵数量最低,T3组最高,表明温度越低,可能越有利于蛔虫卵的灭活,这与宋伟民等[36]的研究结果一致。
-
空白组(M0)与实验组(M1)同时进行为期10 d的厌氧发酵实验,取黑水原水及发酵时间为1、5、10 d的发酵液,编号分别为BW(黑水未发酵时的原水)、D1M0(发酵1 d的M0组)、D1M1(发酵1 d的M1组)、D5M0(发酵5 d的M0组)、D5M1(发酵5 d的M1组)、D10M0(发酵10 d的M0组)、D10M1(发酵10 d的M1组)。经12 000 r·min−1离心后获得沉淀,对沉淀物进行微生物群落组成分析。
1)微生物群落多样性及丰富度。由表5可知,各检测样本的Coverage指数均为1,表明该检测的结果覆盖了样本中的全部微生物,检测结果可以反映出样本中微生物的真实情况。Richness指数整合了OTUs、ACE、Chao1 3种指数,其值越高表明样本中微生物群落的丰富度越高;Simpson指数和Shannon指数可以用来表征微生物群落的多样性,Simpson指数越小,Shannon指数越大,则表明样本微生物群落多样性越高[37]。由表可知,在未添加菌剂的空白组(F0)内,随着反应时间的增加,Richness指数并没有出现显著升高或降低,表明在空白组中微生物的丰富度并没有显著变化;添加菌剂的样本(F1)中Richness指数较空白组有明显升高,表明添加厌氧发酵菌剂可以提高样本的微生物丰富度。随着菌剂的投加,样本中的Simpson指数均大于空白组,而Shannon指数均小于空白组,表明添加菌剂可以提高样本中的微生物多样性。
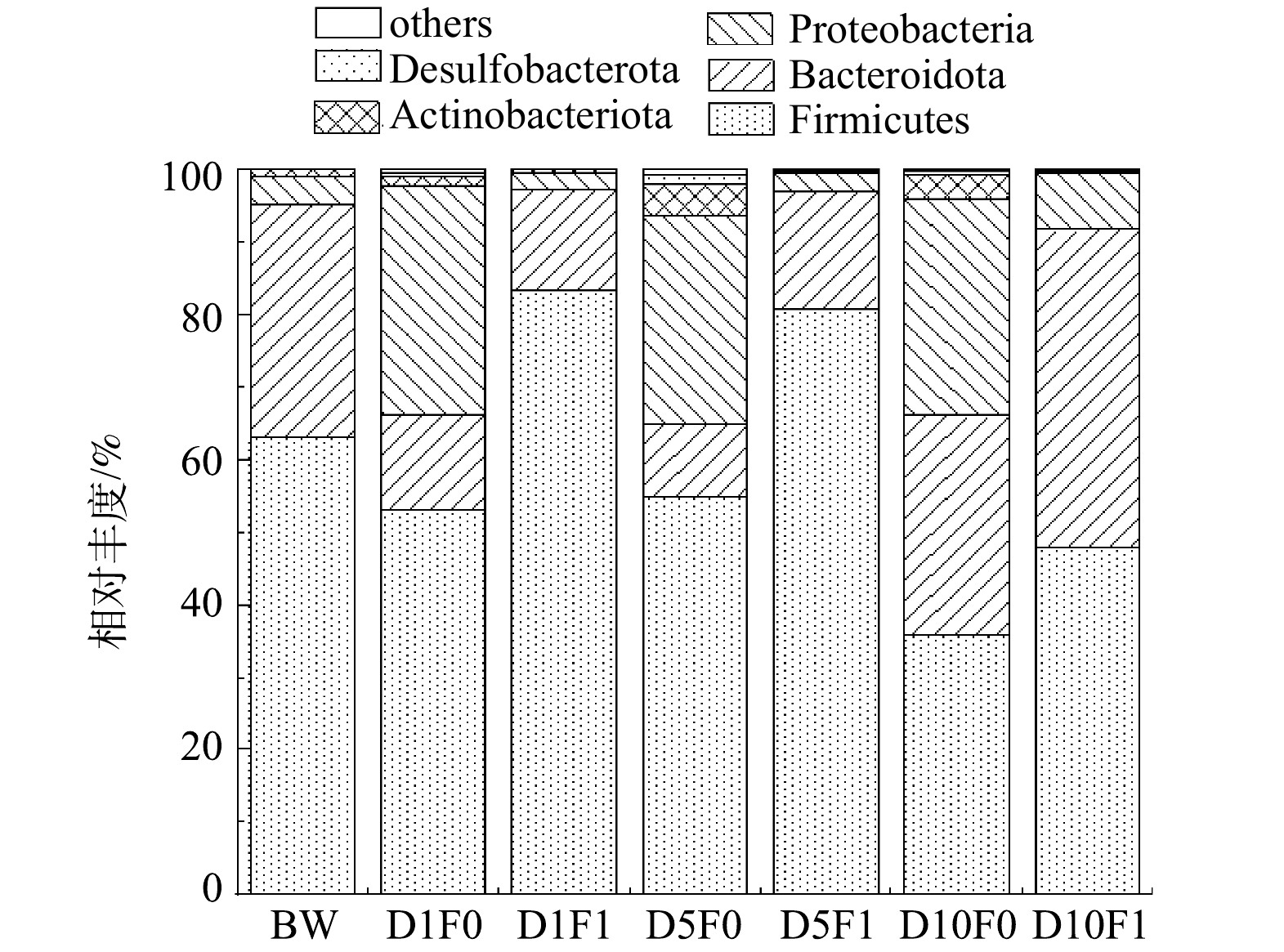
2)微生物门水平组成分析。微生物门水平群落相对丰度如图17所示。在门水平上,M0与M1在不同时间下的优势菌门相似,主要的优势菌门为厚壁菌门、拟杆菌门、变形菌门,这与韦采妮等[38]在粪污厌氧发酵系统中检测到的优势菌门类似。在黑水厌氧发酵过程中,大分子有机物的水解、产乙酸为主要的过程。厚壁菌门主要参与水解酸化的过程,且其广泛存在于富含纤维素的底物中,是将木质纤维素转化为有机酸的主要细菌群[39]。与原始黑水相比,添加菌剂1 d后的样本中厚壁菌门的丰度由63.0%提高至83.3%,而空白组的厚壁菌门丰度则下降至53.0%,表明厌氧发酵菌剂的添加促进了黑水中厚壁菌门的富集。在反应后期(10 d),厚壁菌门的丰度有所降低,空白组与实验组分别降低至35.8%和47.8%,这可能是由于在反应后期,黑水中的木质纤维素大部分已被分解成有机酸,系统中有机酸含量升高,对厚壁菌门的有所抑制。拟杆菌门在富含木质素和多糖的环境中比较丰富,其参与将有机物转化为乙酸的过程。拟杆菌门丰度在空白组与实验组中相对稳定,菌剂的投加对于拟杆菌门并没有显著的促进或抑制作用,且在反应前中期,拟杆菌门丰度低于反应后期(10 d),表明在反应后期,更多的微生物参与到合成乙酸的反应过程中来,底物中有机酸的含量增加更有利于拟杆菌门的生长。变形菌门可以促进粗蛋白的降解,使其转化为小分子物质,为产甲烷阶段的微生物转化过程提供底物,同时变形菌门还能够促进粪便中碳氮的转化[40]。与空白组相比,投加厌氧发酵菌剂使样本中变形菌门的丰都明显低于空白组,表明添加菌剂对变现菌门有抑制作用,这可能是黑水厌氧发酵过程中产生的高质量浓度氨导致的[18]。这一现象不利于甲烷的产生,但可能更有利于体系中有机酸的积累,使黑水发酵液更适合作为液态肥回用于农田。
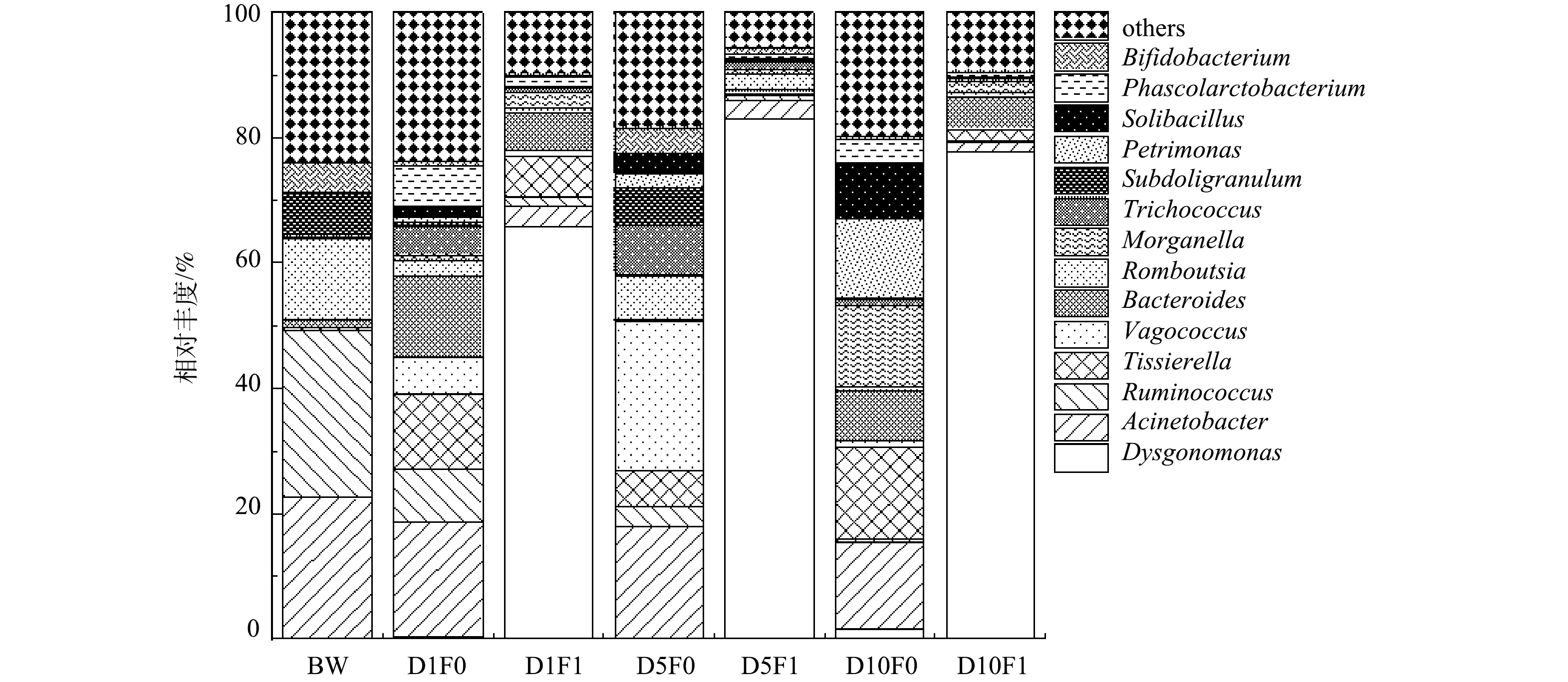
2)微生物属水平组成分析。微生物属水平群落相对丰度如图18所示。在原始黑水及不添加厌氧发酵菌剂的空白组中,各反应时间的优势菌属有所不同,但Acinetobacter、Tissierella、Bacteroides是其共有的3种优势菌属。Acinetobacter是一种与肺部感染、皮肤感染、泌尿生殖系统感染相关的重要致病菌, Bacteroides 被报道与肠道疾病等有关[35],而Tissierella 常见于发酵乳制品中,可产生酯、酸、酮等风味物质,对厌氧发酵过程有一定的促进作用。另外,随着反应进行,存在于黑水原水中的一些菌属丰度逐渐降低,如Ruminococcus 和 Romboutsia。有研究表明,这2种菌属均是常见致病菌,Ruminococcus与消化道疾病有关而Romboutsia 与尿路感染有关[41]。随着反应进行,空白组内 Ruminococcus 的相对丰度由34%下降至0.5%,Romboutsia 的相对丰度则由16%下降至0.7%,表明在不添加厌氧发酵菌剂的情况下,黑水自身微生物菌群在厌氧情况下也可以自发灭活一些致病菌,但仍有许多可能致病的菌属没有被灭活,如Morganella菌属,这是一种与呼吸道疾病有关的致病菌[42],其相对丰度在发酵10 d后仍在14%。
添加厌氧发酵菌剂的反应体系内的优势菌属与空白组有较大差异。添加厌氧发酵菌剂后,优势菌属主要为Dysgonomonas,这是一种可以有效降解木质素、纤维素等有机物的菌属[43-44]。表明厌氧发酵菌剂对该菌属具有显著促进作用,有利于降解黑水中的有机物。Acinetobacter的相对丰度在添加厌氧发酵菌剂后迅速降低,相比于空白组的18%,实验组的数值仅在3.2%,并且经过10 d发酵后,空白组降低至14%,实验组降低至1.5%。这表明厌氧发酵菌剂的添加可以很好地抑制Acinetobacter这类致病菌的增长,并且该现象在Ruminococcus和Romboutsia 两种菌属中同样有所体现。
-
1)增加厌氧发酵菌剂的投加量可以提高黑水厌氧发酵过程中的水解和产酸效果,但投加量增加到一定水平后,继续增加投加量对水解产酸效果提升不显著,厌氧发酵菌剂的适宜投加量为1‰黑水量。
2)不同温度下,厌氧发酵菌剂对黑水厌氧发酵的影响有所不同,但在10、20、30 ℃下,都可以促进黑水厌氧水解产酸的效果。温度越高,对黑水中有机物的水解及VFA、氨氮积累的促进效果越明显。在较低的温度下,厌氧发酵菌剂对于大分子有机物的水解和厌氧产酸过程也有明显的促进作用,并能有效灭活致病菌、去除臭味物质。
3)添加发酵菌剂的实验组与空白组相比,在细菌门水平上,二者在不同时间下的优势菌门相似,主要的优势菌门为厚壁菌门、拟杆菌门、变形菌门,相对丰度分别为35.8%~63.0%、9.9%~43.8%、2.6%~31.5%。发酵菌剂的投加使黑水中厚壁菌门的丰度增加至47.8%~83.3%,降低变形菌门的丰度至2.3%~8.0%,对于拟杆菌门的丰度并没有显著影响,厚壁菌门是将木质纤维素转化为有机酸的主要细菌群,其丰度增加表明水解酸化过程被促进。在细菌属水平上,二者在不同的反应时间内共有的优势菌属为Acinetobacter、Tissierella、 Bacteroides菌属,相对丰度分别为14.0%~22.5%、6.4%~14.5%和1.3%~7.9%。添加发酵菌剂显著提高黑水中Dysgonomonas丰度至65.8%~82.9%,并使Acinetobacter、Ruminococcus和 Romboutsia丰度显著降低,Dysgonomonas可以有效降解木质素、纤维素等有机物,其丰度增加有利于有机物的水解。
厌氧发酵菌剂对黑水厌氧发酵和无害化效果的影响
Influence of anaerobic fermentation bacterial agents on the effectiveness of anaerobic fermentation and harmlessness of blackwater
-
摘要: 为实现农村黑水的安全资源化利用,本研究通过添加厌氧发酵菌剂,分别考察了菌剂投加量、发酵温度对黑水中有机物的转化和病原微生物灭活效果的影响,分析了发酵体系中微生物群落结构的变化。结果表明厌氧发酵菌剂对黑水厌氧发酵有明显促进作用,适宜投加量为黑水量的1‰。较高温度有利于黑水发酵,SCOD积累量最高达
1497 mg·L−1,VFA积累量为359 mg·L−1,80.3%的纤维素被降解,粪大肠菌群数、蛔虫卵数均达到无害化要求。低温时微生物活性降低但仍有明显促进效果。发酵体系中,厚壁菌门、拟杆菌门和变形菌门是主要优势菌门,相对丰度分别为35.8%~63.0%、9.9%~43.8%和2.6%~31.5%;优势菌属为Acinetobacter, Tissierella, Bacteroides菌属,相对丰度分别为14.0%~22.5%、6.4%~14.5%和1.3%~7.9%。厌氧发酵菌剂的投加提高了具有水解酸化功能的厚壁菌门的丰度,降低了变形菌门的丰度;同时提高了具有降解木质素、纤维素的Dysgonomonas丰度,降低了Acinetobacter, Ruminococcus和 Romboutsia的丰度。本研究结果可为应用厌氧发酵菌剂促进黑水厌氧发酵、臭味控制、实现黑水的高效无害化和资源化提供依据。Abstract: In order to safely and resourceful utilization of rural blackwater, the anaerobic fermentation bacterial agents were added in the blackwater, the effects of bacterial dosage and fermentation temperature on the conversion of organic matter and the inactivation effect of pathogenic microorganisms in black water were investigated, and the changes in the microbial community structure of the fermentation system were analysed, respectively. The results showed that anaerobic fermentation bacterial agents significantly promoted the anaerobic fermentation of black water, and the optimal dosage was 1‰ of black water. The high temperature was conducive to the fermentation process, the highest SCOD and VFA accumulation were1497 mg·L−1 and 359 mg·L−1, respectively; 80.3% of cellulose was degraded, and the counts of Fecal coliform and Ascaris lumbricoides egg met the requirements of harmlessness. At lower temperatures, the microbial activity decreased, but still exhibited a considerable promotional effect. In the fermentation system, Firmicutes, Bacteroidota and Proteobacteria were the main dominant phyla, with relative abundances of 35.8%~63.0%, 9.9%~43.8%, and 2.6%~31.5%, respectively; and the dominant genera were Acinetobacter, Tissierella, and Bacteroides, with relative abundances of 14.0%~22.5%, 6.4%~14.5%, and 1.3%~7.9%, respectively. 22.5%, 6.4%~14.5% and 1.3%~7.9%, respectively. The addition of anaerobic fermentation agents increased the abundance of the Firmicutes with hydrolytic acidification function and decreased the abundance of the Bacteroidota. Meanwhile, it increased the abundance of the Dysgonomonas with the function of degrading lignin and cellulose, and decreased the abundance of the Acinetobacter, Ruminococcus, and Romboutsia. This study provides a reference for the application of anaerobic fermentation bacterial agents to promote the anaerobic fermentation of blackwater, control odour production and effectively achieve the harmlessness and resourcefulness of blackwater. -
大气环境颗粒物中的铬(Cr)主要以三价铬(Cr(Ⅲ))和六价铬(Cr(Ⅵ))2种价态存在。Cr(Ⅲ)是人体进行糖类和脂类等代谢活动必不可少的微量物质;Cr(Ⅵ)则是剧毒物质,具有致癌性。1990年,美国的《清洁空气法》将Cr(Ⅵ)化合物列入188种有害空气污染物,美国环境保护局也将其列入18种核心污染物[1]。我国的《环境空气质量标准》规定其年平均浓度限值为25 pg·m−3[2]。近年来,相关学者围绕Cr这种环境颗粒物中重要的有害过渡金属[3-5],展开了Cr的污染特征和来源解析、潜在生物危害和健康风险评估以及在环境中可能存在的化学作用等[6-9]方面的研究。
大气颗粒物产生化学过程往往只持续较短时间(大约几个小时)。切实模拟和研究其产生、传输、消耗的动态过程,需要在该时间精度内进行分析测定[10-11]。在高时空分辨率背景下,研究大气化学过程中过渡金属的污染特征,可为探索此类气溶胶的理化特性、并控制其潜在毒性提供重要见解。建立灵敏而可靠的测量方法需要解决3个问题:1)如何提高测量的灵敏度(达到更低的检测限);2)如何降低测量过程中的潜在干扰;3)如何在处理和测量过程中降低样品中待测物质的损失。目前,多数研究者倾向于使用原子吸收光谱技术测定样品中的总Cr,包括火焰原子吸收光谱法(FAAS)、石墨炉原子吸收光谱法、电热原子吸收光谱法(ET-AAS)等[12-13]。但是,此类方法的样品前处理较为繁琐,所需仪器设备造价昂贵。紫外/可见光分光光度法(UV/Vis)灵敏度很高,通过适当的显色试剂进行络合反应可在高吸收率条件下测量水溶性金属,常被用于溶液中金属浓度的高精度测量[14-15]。以此为基础,可通过配备长光液体波导毛细管来实现UV/Vis定量测定环境样品中的水溶性金属[16-17],结合测量系统的开发,还能有序测量环境颗粒物样品中的金属浓度[18-19]。
结合采样和前处理操作,设计并评估了一种基于分光光度法连续测量大气环境细颗粒物(PM2.5)中铬浓度的系统,以便于精确了解大气颗粒物中铬的污染水平及其来源,提供一种测量环境大气中水溶性痕量金属的思路,以期为开发实际监测技术奠定基础。
1. 材料与方法
1.1 试剂标准
实验所需化学药品纯度至少为光谱纯,配制的试剂和溶液使用由纯水机(Smart-DUV(F) SAIDE)生产(电阻率不低于18.2 MΩ)的超纯水和去离子水。用于试剂配制的实验器材和试剂保存的聚丙烯瓶在使用前均先用浓度为4 mol·L−1的HCl进行清洗,然后用超纯水反复冲洗干净后烘干置于干净环境中备用。
用于标定和校准的Cr(Ⅵ)标准溶液通过用重铬酸钾(K2Cr2O7,纯度为99.9%)和去离子水制备的100 μg·m−3母液稀释获得,在使用前取母液用去离子水稀释到需要的浓度梯度(0.5~8 ng·mL−1)。制备4 mol·L−1的HNO3和3 mol·L−1的NaOH用于酸化和调整溶液pH。用于前处理过程的0.1%的H2O2试剂通过用0.1 mol·L−1的NaOH溶液将0.044 mL 30%的H2O2水溶液定容稀释至1 L制备。将167 mg的二苯碳酰二肼(diphenylcarbazide,DPC)溶解于100 mL的丙酮(C3H6O,纯度为99.9%)中,再与1.67%的H2SO4溶液按照1∶1的体积比混合,制备用于光度法检测前络合反应的DPC试剂[16]。除Cr(Ⅵ)标准溶液,所有的试剂均不含铬。
1.2 样品采集与预处理
用中流量颗粒物采样器(XY-2200,青岛旭宇)通过石英膜(QMA-Whatman,20.3 cm
× 25.4 cm,Φ90 mm)采集环境大气细颗粒物(PM2.5),采样流速100 L·min−1。为降低膜上Cr的本底浓度并减少其在采样中的损失,在采样前将待采样的空白石英膜通过450 ℃高温烘烤6 h,再用2 g·L−1的NaHCO3溶液浸泡后置于清洁恒温箱内晾干密封。采样口距离地面150 cm,用除湿装置保证过程中采样器的环境干燥。样品膜用密封的聚丙烯袋封装并且在-10 ℃下低温保存。所有的样品在采样完成1 d内取回,在3 d内完成前处理和分析测定,以此减少采样过程中Cr的损失,减少测量误差。1.3 分光光度法检测系统及误差避免方法
分光光度检测系统由容量为112 μL、光程为50 cm的长光程流通池(liquid waveguide capillary cell,LWCC,LWCC-3050, World Precision Instruments, Inc., Sarasota, FL),钨灯光源(HL-2000-FHSA-LL,Ocean Optics, Inc., Dunedin, FL),光缆(QP450-1-XSR, Ocean Optics, Inc., Dunedin, FL)和光谱范围为200~900 nm的小型光谱仪(USB4000-UV-VIS,Ocean Optics, Inc., Dunedin, FL)组成。
分析过程中,Cr浓度由Cr(Ⅵ)与DPC反应产生的络合物在540 nm的吸光度进行测算。尽管DPC与大气环境中部分金属离子(Fe3+、Hg2+、Mo6+、Cu2+和V5+)会发生类似络合反应,但这些金属络合物的最大光吸收波长范围均不含540 nm,且相差较大[6]。因此,检测前加入DPC可保证结果基本不受其他金属离子干扰。设定光谱的积分时间为8 ms,每个样品平均次数为20次,光吸收谱图上800 nm处吸光度信号用于设定基线(即设定系统背景信号)。为去除检测仪器自身产生的背景吸光度,通过断开光源测得仪器的暗光谱,并在光谱分析软件中设定扣除暗光谱得到实测光谱数据,以便对系统进行自吸收校正。
1.4 系统运行流程
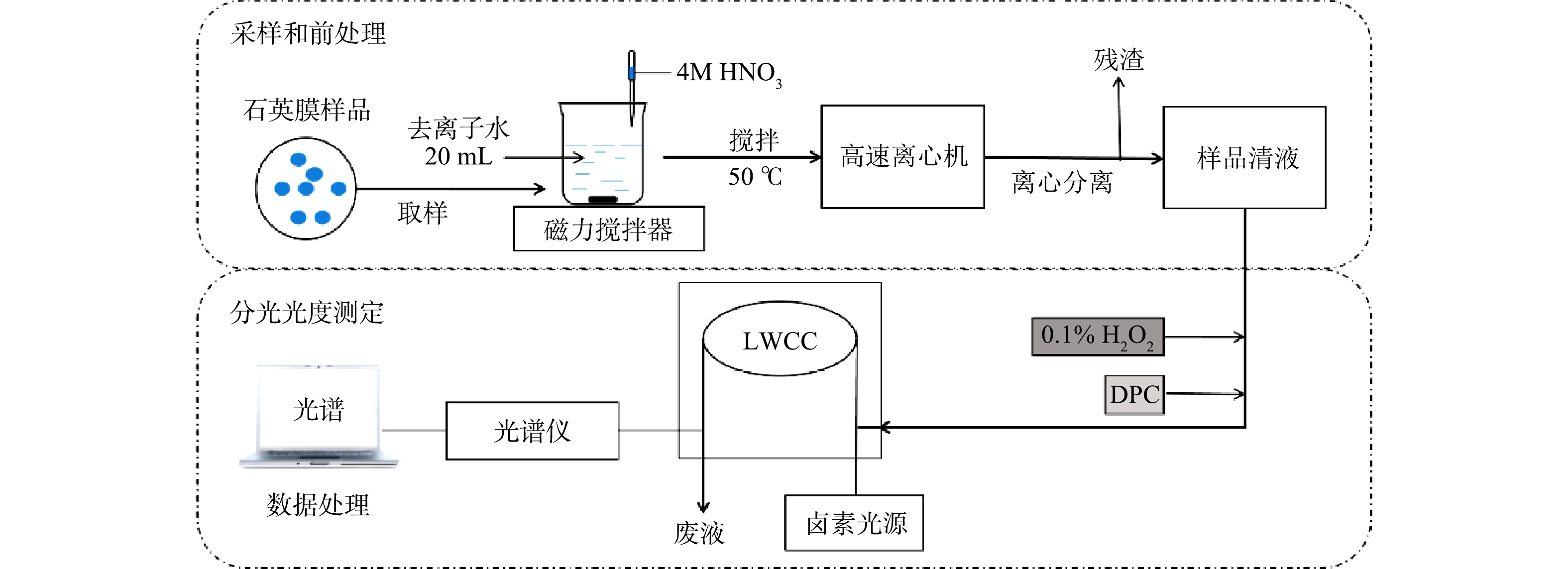
系统分为2个部分(见图1):石英膜采样和前处理操作的样品采集模块;以分光光度检测为基础的浓度分析模块。将采样后的石英膜用清洁陶瓷剪刀取样、剪碎,放入烧杯后加入20 mL的去离子水和1 mL浓度为4 mol·L−1的HNO3溶液。为尽可能溶解石英膜上采集的环境颗粒物,用磁力搅拌机将石英膜制成的酸化浆液加热(温度为50 ℃)搅拌40 min,分离出10 mL样品清液,装入棕色聚丙烯瓶中避光储存。每次测量后,用4 mol·L−1的HCl溶液清洗所有样品管路,再用超纯水洗净。
分光光度方法测定样品中的Cr浓度:1)络合反应。向样品溶液中加入0.1 mL 0.1%的H2O2试剂将样品溶液中的Cr(Ⅲ)转化为Cr(Ⅵ),同时加入0.1 mL的DPC试剂,静置10 min,待Cr络合物形成并稳定;2)样品注入。用带0.22 μm微孔滤膜(聚四氟乙烯,PTFE)的聚丙烯注射器将1 mL样品注入LWCC中;3)测定浓度。通入样品2~3 min后,待光谱稳定,得到光吸收谱图上540 nm处Cr络合物的最大吸收峰值,将该值扣减设定的800 nm基线值得出净峰高,并通过与标定结果的对比计算得出总Cr浓度。
1.5 采样点
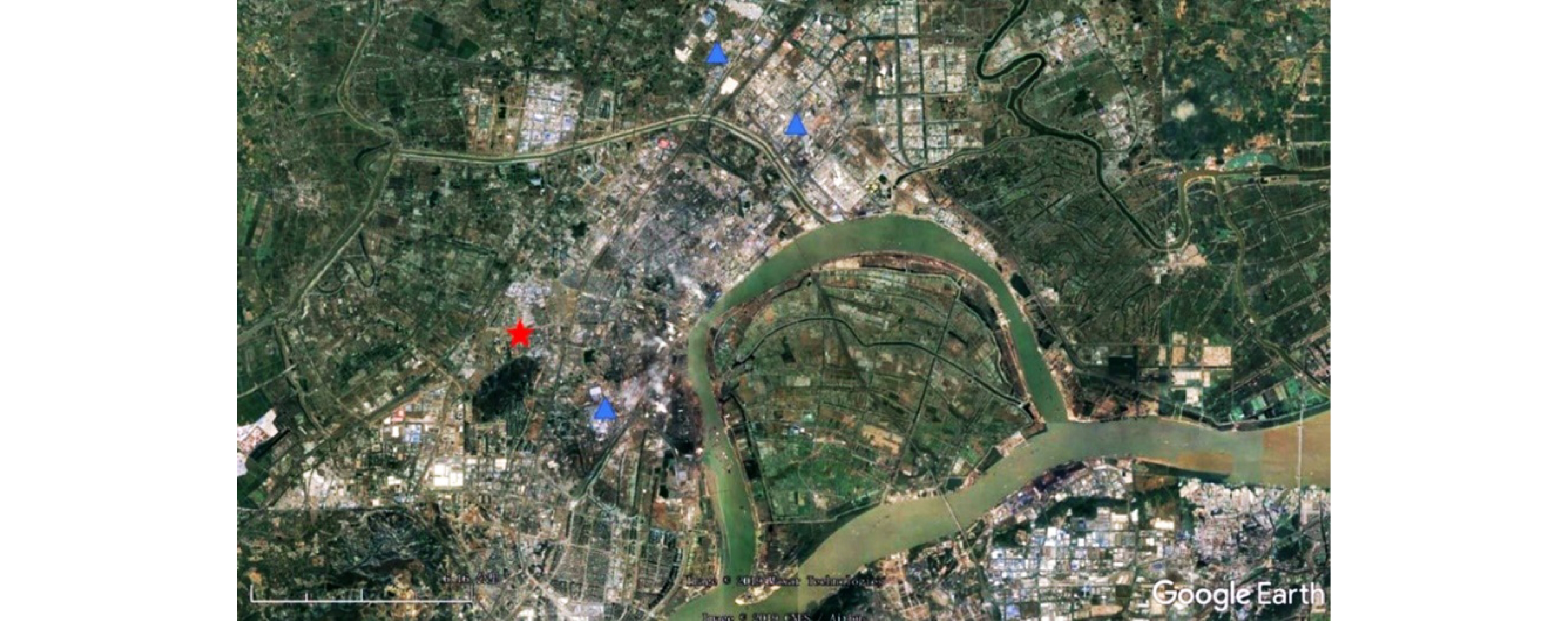
采样点设在南京信息工程大学气象观测场内,按12 h的时间间隔采集南京北郊大气颗粒物样品。设定样品采集的起始时间为每日6:30和18:30。采样点(红色星标)位于南京市中心以北大约15 km处,大型工业区(蓝色三角标)以西,靠近城区日常交通干道(见图2)。该地的PM2.5质量浓度数据由Met One Instruments公司的PM2.5在线监测仪进行实时在线监测,时间分辨率为1 h。
2. 结果与讨论
2.1 最佳样品测定条件的设定
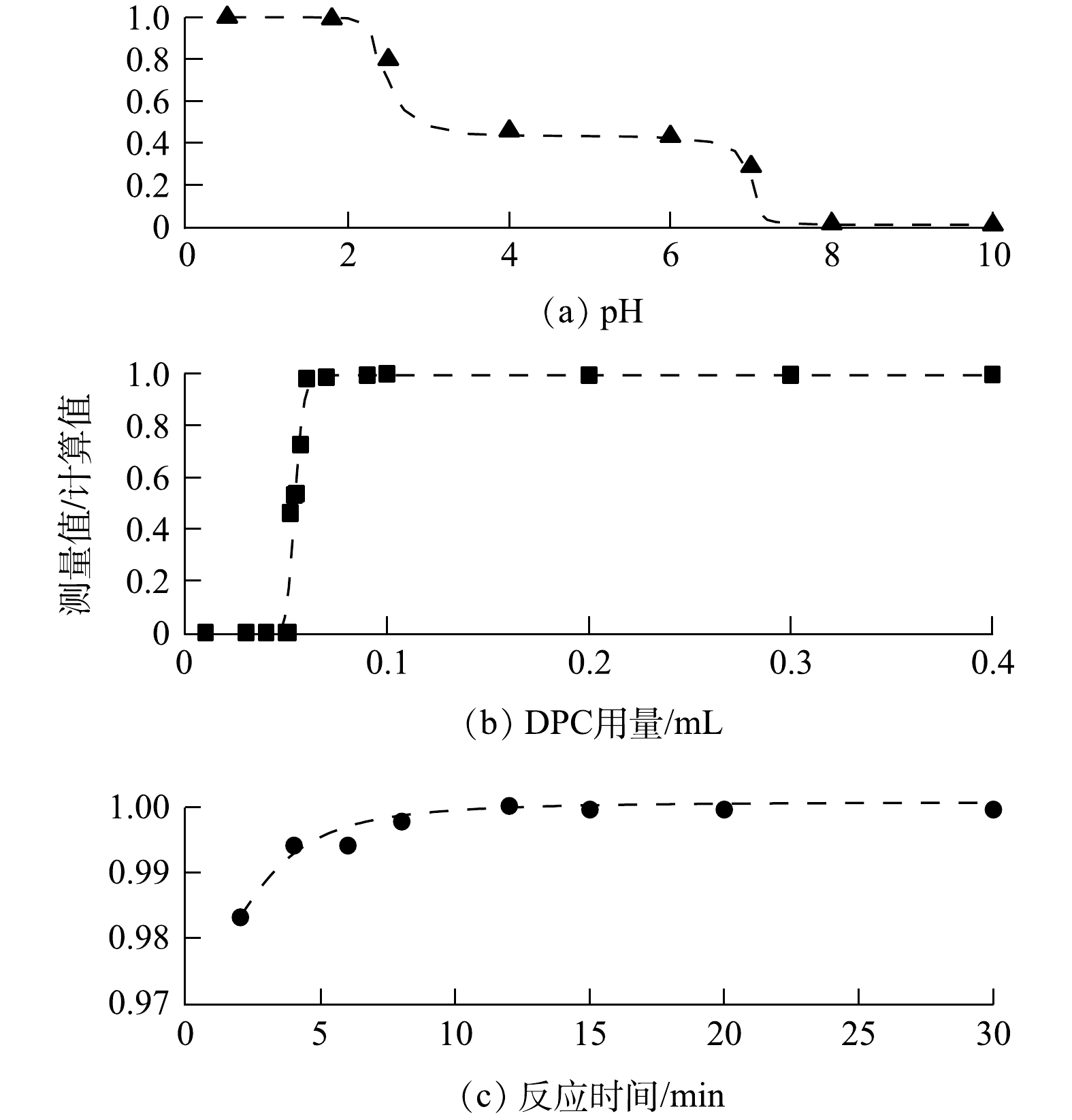
由于大气本底Cr浓度较低,每次取用10 mL浓度为5 ng·mL−1的Cr(Ⅵ)标准溶液进行条件实验。通过改变溶液的pH、DPC试剂的用量及预留的络合反应时间,根据条件实验结果来确定前述各项参数的最佳值或阈值。将仪器的测量值与标准样品浓度的计算值之比作为判别标准,比值越接近1,则结果对应的条件和参数更优。
在酸性环境下,Cr(Ⅵ)与DPC络合的效率随溶液pH的升高而降低,导致测定结果偏低;在碱性环境下,Cr(Ⅵ)无法发生络合反应。因此,需要对溶液体系进行酸化。按体积比加入去离子水和HNO3溶液,得到混合浆液的pH约为0.5。用3 mol·L−1 NaOH溶液调节混合浆液pH,结果如图3(a)所示。加入DPC的Cr络合物反应后溶液的最佳pH为0.5~1,故在前处理中无需调节溶液pH。溶液中过量的氧化剂H2O2将Cr(Ⅲ)氧化成Cr(Ⅵ)后,会优先和DPC发生反应,干扰Cr络合物的生成。DPC试剂的用量为0.1 mL时,可去除过量H2O2的干扰(见图3(b))。同时,所有试剂加入后静置10 min以保证溶液中的Cr络合物完全产生(见图3(c))。
2.2 标定曲线和检测限
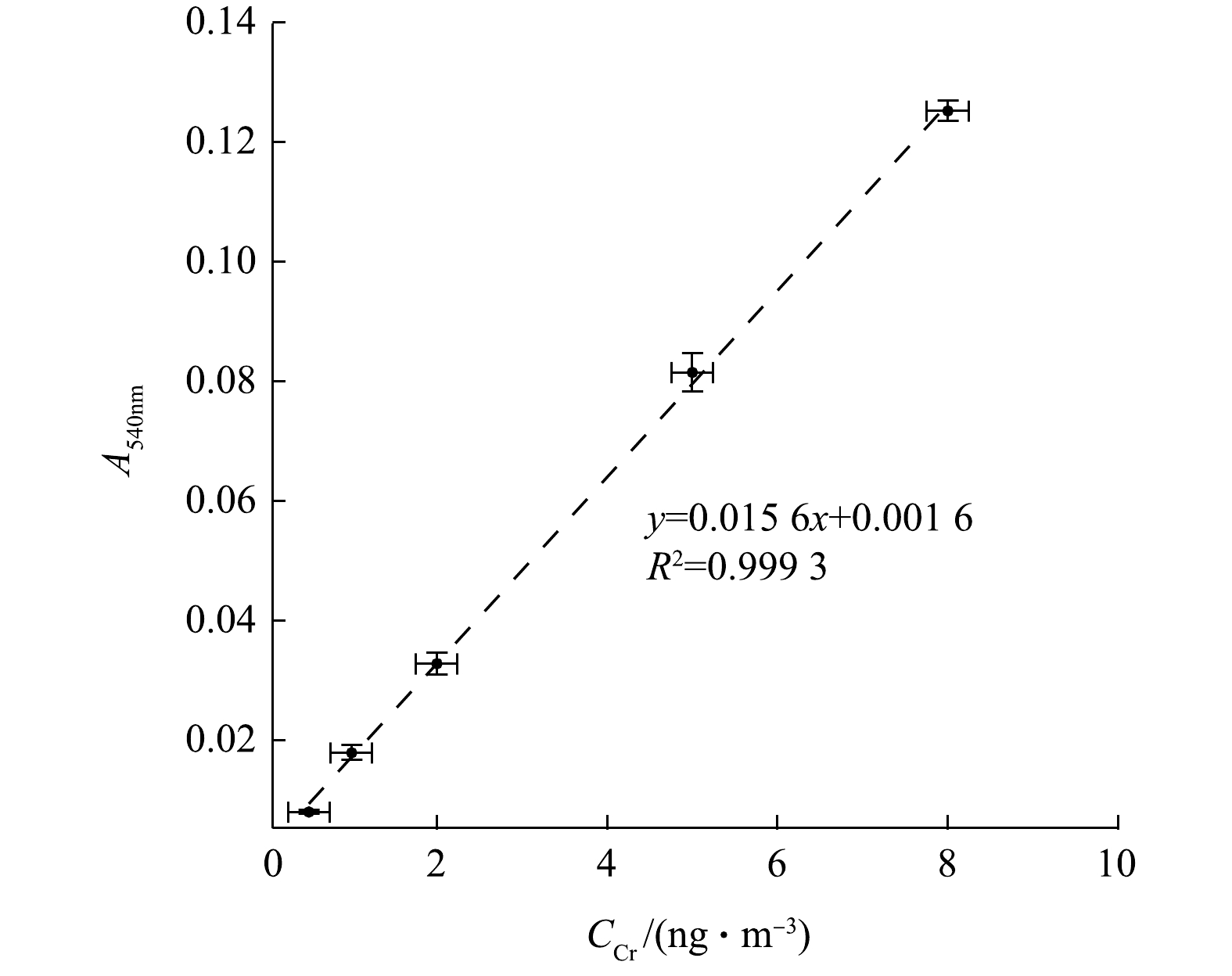
根据实际环境大气颗粒物中总Cr浓度,选择合适浓度梯度(0.5、1、2、5、8 ng·mL−1)的Cr(Ⅵ)标准溶液确定标准曲线。每次标定使用相同Cr(Ⅵ)母液,用去离子水稀释至所需浓度。图4为14 d内不同时间进行的5次标定结果的平均值,相同浓度的多次测定值范围以error bar形式在图中标出。几次标定得到的线性方程斜率在(0.015 6±0.000 2)mL·ng−1范围变化,并且所有标定曲线的R2均能达到0.99。系统分光光度测量的标定范围涵盖了一般环境大气颗粒物中Cr浓度范围。Cr浓度与其络合物的吸光度峰值呈稳定线性关系,多次标定反映出该结果重现性好。
根据空白样品吸光度峰值标准偏差的3倍来测算系统的检测限(limit of detection,LOD)。空白样品由空白石英膜按照样品的前处理过程制得。将加入DPC的空白样品通入LWCC测量吸光度峰值,LOD约为0.158 ng·mL−1,对应采样空气体积约为12 m−3。经过计算对应的环境颗粒物中Cr浓度为0.133 ng·m−3,大气颗粒物中Cr浓度至少比该LOD高一个数量级,故检测系统对Cr浓度的测定灵敏度较高,可满足大气监测需求。从上述空白样品的测定结果还表明,石英膜上Cr本底浓度极低,为(0.971±0.087)ng·g−1,不会干扰实际测量。
2.3 回收率实验
检测系统的回收率实验包括空白加标回收和样品加标回收两部分。空白样品的加标回收通过在空白石英膜上加入10 ng Cr(Ⅵ),再按照样品前处理步骤进行处理和分析测定,测定结果与理论值之比即空白加标回收率。用微升注射器(HAMILTON-7000)取用1 μL由相同Cr(Ⅵ)母液稀释所得浓度为10 μg·mL−1的Cr(Ⅵ)标液,直接加注在石英膜表面进行样品加标,以相同浓度的Cr(Ⅲ)标液(由纯度为99.99%的CrCI3·6H2O和去离子水制得)进行加标实验,通过加入0.1%的H2O2氧化Cr(Ⅲ)后,检测Cr(Ⅳ)浓度并与配置的Cr(Ⅲ)标液比较得到转化率。样品的加标回收则选用NIST商品化的城市颗粒物标准物质(NIST 1648 PM)作为样品。用清洁研钵将NIST样品磨碎,取2份(各5 mg)分别涂抹在空白石英膜上,其中1份加入10 ng Cr(Ⅵ)。将2份样品通过检测系统进行分析,2份样品测量的差值与加入标准Cr(Ⅵ)物质的理论值之比即加标回收率。上述实验均重复进行多次以减少偶然性误差。
由于使用高纯度的药品且系统测量受到干扰误差很小,Cr(Ⅵ) 空白加标回收率均值可达98%,标准偏差为2.4%(样品数为10);Cr(Ⅲ)的转化率为(95±2.6)%(样品数为10)。样品加标回收实验结果显示:在15个加标的标准环境样品中Cr(Ⅵ)回收率为(90.3±8.1)%;而对不加标的NIST标准样品中Cr浓度测定的误差范围为(9.6±3.3)%。ERG使用相同样品加标,通过碳酸盐缓冲液提取与离子色谱(IC)测得的回收率为((89.8±10)%, N=10)[20]。2组样品加标回收率经中位数检验并不存在统计上的显著差异,故该系统具有较好的采集和测量精度。
2.4 与ICP-MS方法的对比测试
ICP-MS是目前颗粒物中痕量金属的热门测定方法之一[21-22]。2014年,我国环境保护部将其确定为测量环境水样中多种痕量元素的标准方法(HJ 700-2014)。近年来,ICP-MS与各种分离技术联用已常见于Cr的相关研究中[23-25]。
将2016-12-17—2016-12-27在福州三中地区(PM2.5>80 μg·m−3)采集的高浓度大气颗粒物石英膜样品(膜规格:QMA-Whatman, 1851-050, Φ50 mm;采样时长3 h;采样流速16.7 L·min−1),分别通过本系统和ICP-MS测量总Cr浓度。
在进行ICP-MS测定前,将膜样品做密闭微波消解前处理。取用与本系统分析等量样品置于Teflon-TFM消解罐中,再加入10 mL HNO3和HCl的混合液(体积比为1:1),摇匀浸湿样品膜后放入微波消解仪(XT-9900A,上海新拓)中消解8 h。待消解罐冷却至室温,将其中的混合物用0.45 μm的醋酸纤维素滤膜进行过滤。将得到滤液用去离子水稀释至50 mL,用ICP-MS(X-Series2, US Thermo fisher)进行分析。
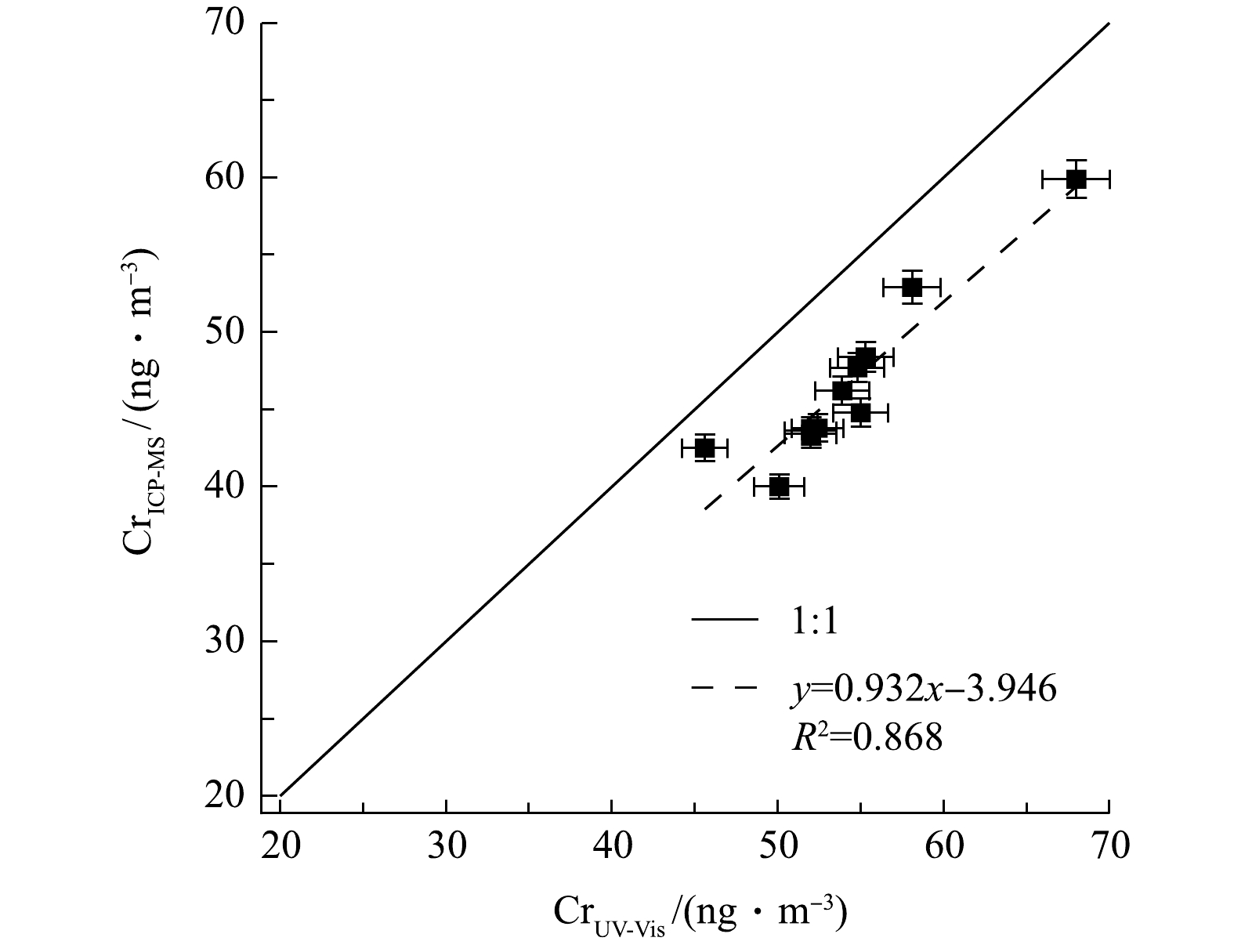
2种方法测量结果的相关性如图5所示,表明结果呈线性关系(斜率为0.932,R2为0.868)。该结果表明本系统对环境颗粒物中总Cr浓度测定的准确性与稳定性较好,也进一步证明本系统的前处理操作(包括酸化、加热搅拌等)能基本确保样品中所有含铬组分充分溶解,以得到可靠的测量结果。同时也表明若颗粒物样品含Cr组分中耐酸且难溶物质较多,会影响系统测量结果。
2.5 大气PM2.5中Cr浓度的外场实测
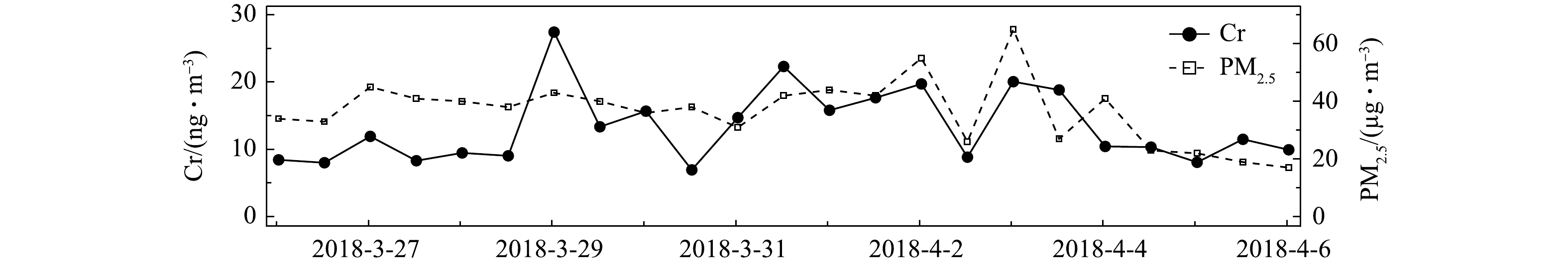
在2018-03-26—2018-04-06对环境大气PM2.5进行样品采集,每12 h采集1次,得到1周多的连续数据如图6所示。测得Cr浓度在采样期间出现了明显的变化,浓度为6.9~27.4 ng·m−3。该浓度水平与其他观测结果相似[27-28],Cr浓度的变化趋势与PM2.5的变化趋势基本一致。整个采样期间,Cr的日平均浓度为13.4 ng·m−3。而这段时间Cr浓度变化较大,这是由于春季空气污染过程频发,且受风向风速变化的影响较大。
分析Cr浓度的变化可发现,2018-03-28夜间到2018-03-29凌晨、2018-03-31白天、2018-04-01夜间到2018-04-02凌晨,以及2018-04-02夜间到2018-04-03凌晨这几个时段达到观测期间的峰值,平均值比日平均浓度高50%~67%;2018-03-30全天、2018-04-02白天Cr浓度降至观测期间日平均浓度的52%~60%;2018-04-04下午到2018-04-06凌晨,Cr浓度基本与采样期间的Cr日平均浓度持平。由于Cr被广泛用于工业生产,如冶金、铬镁耐火材料制造和电镀工艺等[29-31],交通工具的尾气排放和机械磨损[32]也会使环境大气颗粒物中含有这类过渡金属,Cr是地壳中的金属物质之一,故土壤粉尘和地面扬尘也为大气颗粒物中贡献了Cr[33-34]。结合采样点的地理位置分析可推断,大气颗粒物中的Cr主要来自早晚期间强烈的交通活动导致的直接排放和扬尘排放,以及偏东风带来的工业园区排放的污染气团。上述结果表明,本系统可对PM2.5中Cr浓度进行准确连续的测量。
3. 结语
1)设计并评估了一种基于分光光度法测量环境PM2.5中微量Cr的系统。系统通过膜采样经过前处理制成待测样品,再基于分光光度法测量样品中总Cr浓度。系统具有很好的灵敏度和重现性,采集效率较高,测量精度较好。用相同样品与ICP-MS测量结果的对比表明,系统可靠性和准确性均较好。外场采样观测的结果显示,系统具有足够的可靠性,在较少人工监管的情况下能够对环境细颗粒物中Cr进行较快速的连续测量。
2)设计中用到的通过替换金属络合试剂及对前处理步骤进行的优化方法,不仅能测定Cr的价态,还能帮助测定大气环境中部分金属浓度,为实现连续快捷地分析大气环境中水溶性金属离子的污染特征提供了参考。同时,根据大气颗粒物中Cr的来源可以判断,还应进一步研究不同季节大气中Cr的来源和日变化特征。
-
表 1 厌氧发酵菌剂微生物群落丰度
Table 1. Microbial community abundance of the anaerobic fermentation bacterial agent
门水平分类 相对丰度/% 属水平分类 相对丰度/% 厚壁菌门(Firmicutes) 88.28 乳杆菌属(Lactobacillus) 87.40 变形菌门(Proteobacteria) 11.21 罗尔斯通菌属(Ralstonia) 6.55 拟杆菌门(Bacteroidota) 0.25 假单胞菌属(Pseudomonas) 2.06 放线菌门(Actinobacteriota) 0.08 寡养单胞菌属(Stenotrophomonas) 1.07 蓝藻菌门(Cyanobacteria) 0.05 李斯特菌属(Listeria) 0.48 脱硫杆菌门(Desulfobacterota) 0.02 根瘤菌属(Allorhizobium-Rhizobium) 0.37 疣微菌门(Verrucomicrobiota) 0.02 气单胞菌属(Aeromonas) 0.19 脱铁杆菌门(Deferribacterota) 0.02 弧菌属(Vibrio) 0.13 奇异球菌门(Deinococcota) 0.02 固氮螺菌属(Azospirillum) 0.12 梭杆菌门(Fusobacteriota) 0.02 普氏菌属(Prevotella_9) 0.08 表 2 不同菌剂投加量下,黑水的粪大肠菌群值
Table 2. Fecal coliform values of blackwater at different dosages of bacterial agents
发酵时间/d F0 F1 F2 F3 F4 F5 0 4.0×10−6 4.0×10−6 4.0×10−6 4.0×10−6 4.0×10−6 4.0×10−6 10 4.0×10−5 0.4 0.6 3.6 4.3 4.3 表 3 不同温度下,黑水的粪大肠菌群的最大可能数
Table 3. Most probable number of Fecal coliform in black water at different temperatures
发酵时间/d T0/(MPN·L−1) T1/(MPN·L−1) T2/(MPN·L−1) T3/(MPN·L−1) 0 9.2×109 9.2×109 9.2×109 9.2×109 1 1.7×1010 9.2×109 1.1×1010 1.3×1010 2 1.9×1010 5.3×109 7.4×109 9.1×109 3 2.1×1010 3.7×106 4.3×106 8.3×108 4 2.3×109 4.7×105 2.9×105 3.5×106 5 1.9×109 7.3×104 1.3×105 7.2×105 6 2.3×1010 5 900 8.3×104 5.5×105 7 2.5×1010 3 700 9 300 1.3×104 8 2.3×1010 2 800 4 900 8 600 9 2.3×1010 3 300 3 100 7 900 10 2.5×1010 3 400 5 400 6 400 表 4 不同温度下,黑水的蛔虫卵的最大可能数
Table 4. Most probable number of Ascaris lumbricoides eggs in black water at different temperatures
MPN·L−1 发酵时间/d T0 T1 T2 T3 0 2.6×103 2.6×103 2.6×103 2.6×103 10 1.2×103 4 11 60 表 5 微生物多样性及丰富度指数统计
Table 5. Microbial diversity and the statistics of abundance index
样本 Reads Richness Simpson Shannon Coverage BW 76 843 624 0.123 4.68 1 D1M0 84 338 649 0.125 4.64 1 D1M1 79 960 1 276 0.021 7 6.91 1 D5M0 85 827 577 0.066 5 4.55 1 D5M1 76 468 1 194 0.032 2 6.7 1 D10M0 71 067 682 0.076 6 4.71 1 D10M1 83 976 1 038 0.039 6 6.59 1 -
[1] DISSANAYAKE P, TENNAKOON M. Guide to on-site wastewater management for industrial and commercial establishments and other institutions: guide for hotel and restaurant owners and managers in Kurunegala, Sri Lanka[J]. Iwmi Books, 2008, 68(3): 20-21. [2] SABHARWAL S, SARMA K S S. Appliance of electron beam technology for disinfection of sewage water to minimize public health risks[J]. European Journal of Sustainable Development, 2013, 2(2): 31-31. doi: 10.14207/ejsd.2013.v2n2p31 [3] WALKER R, BEADSWORTH A. The potential of source-separated human urine to be used as a partial replacement for synthetic fertilisers[J]. Aspects of Applied Biology, 2011: 171-176. [4] 王国田, 郭芳, 温禾, 等. 基于源分离的农村污水资源化技术现状与展望[J]. 资源节约与环保, 2024, 1(02): 41-46. [5] SANTIAGO-DIAZ A L, GARCIA-ALBORTANTE J, SALAZAR-PELAEZ M L. UASB-septic tank as an alternative for decentralized wastewater treatment in Mexico[J]. Environmental Technology, 2019, 40(13): 1780-1792. [6] 王玉华, 方颖, 焦隽. 江苏农村“三格式”化粪池污水处理效果评价[J]. 生态与农村环境学报, 2008, 24(2): 80-83. [7] MAHMOUD A, ZAGHLOUL M, HAMZA R, et al. Comparing VFA composition, biomethane potential, and methane production kinetics of different substrates for anaerobic fermentation and digestion[J]. Fermentation, 2023, 9: 138-138. doi: 10.3390/fermentation9020138 [8] PETER J A C, CHRISTOPHER J F, NAOHISA Goto, et al. The Sanger FASTQ file format for sequences with quality scores, and the Solexa/Illumina FASTQ variants[J]. Nucleic Acids Research, 2009, 38(6): 1767-1771. [9] KASPER D H, STEVEN E B, et al. Biases in Illumina transcriptome sequencing caused by random hexamer priming[J]. Nucleic Acids Research, 2010, 38(12): 131-131. doi: 10.1093/nar/gkq224 [10] 国家环境保护总局. 水和废水监测分析方法[M]. 4版. 北京: 中国环境科学出版社, 2002: 211-213. [11] WEN L, HUANG X W, LI X Y. Enhanced production of short-chain fatty acids from sludge by thermal hydrolysis and acidogenic fermentation for organic resource recovery[J]. Science of the Total Environment, 2022, 8(28): 154-389. [12] DIAZ-ELSAYED N, REZAEI N, GUO T, et al. Wastewater-based resource recovery technologies across scale: A review[J]. Resources Conservation and Recycling, 2019, 14(5): 94-112. [13] TAYLOR K A C C. A modification of the phenol/sulfuric acid assay for total carbohydrates giving more comparable absorbances[J]. Applied Biochemistry and Biotechnology, 1995, 53(3): 207-214. doi: 10.1007/BF02783496 [14] 王玉万, 徐文玉. 木质纤维素固体基质发酵物中半纤维素、纤维素和木素的定量分析程序[J]. 微生物学通报, 1987, 1(2): 81-84. [15] 卫生部, 国家标准管理委员会. GB7959-2012 粪便无害化卫生标准[S]. 北京: 中国标准出版社, 2012. [16] 生态环境部. HJ347.2-2018 水质 粪大肠菌群的测定 多管发酵法[S]. 北京: 中国环境出版集团, 2019. [17] YAN, Han, NING. Pathways in bacterial and archaeal communities dictated by ammonium stress in a high solid anaerobic digester with dewatered sludge[J]. Bioresource Technology, 2017, 2(41): 95-102. [18] CHEN Y, CHENG J J, CREAMER K S. Inhibition of anaerobic digestion process: A review[J]. Bioresource Technology, 2008, 99(10): 4044-4064. doi: 10.1016/j.biortech.2007.01.057 [19] HARDER R, WIELEMAKER R, LARSEN A T, et al. Recycling nutrients contained in human excreta to agriculture: Pathways, processes, and products[J]. Critical Reviews in Environmental Science and Technology, 2019, 49(8): 695-743. doi: 10.1080/10643389.2018.1558889 [20] 林星杰, 杨慧芬, 宋存义. UV254在水质监测中应用的研究[J]. 能源与环境, 2006, 1(22): 22-24. [21] 刘莹, 盛飞, 陈文婷, 等. UV254在煤制气废水处理中的指示作用[J]. 环境工程学报, 2015, 9(4): 1809-1814. [22] 王明月, 程丽华, 刘健, 等. 污水处理对黑水/灰水中溶解性有机物紫外光谱及荧光光谱特性的影响[J]. 环境工程学报, 2019, 13(4): 910-917. [23] YAMAMOTO H, LILJESTRAND H M, SHIMIZU Y, et al. Effects of physical -chemical characteristics on the sorption of selected endocrine disrnptors by dissolved organic matter surrogates[J]. Environmental Science & Technology, 2003, 37(12): 2646-2657. [24] 谷洁, 李生秀, 秦清军, 等. 微生物及胡敏酸E4/E6值在农业废弃物静态高温腐解中的变化[J]. 西北农林科技大学学报 (自然科学版), 2005, 33(12): 98-102. [25] 程丽华, 王奇, 韩婷, 等. 混凝对二级出水中有机物分布特性的影响[J]. 环境工程学报, 2013, 7(8): 2919-2924. [26] 安莹, 李云辉, 李震, 等. 改良型 AO 法组合工艺中有机物的三维荧光分析[J]. 环境工程学报, 2013, 7(1): 159-163. [27] DU Y X, LU Y H, ROEBUCK J A, et al. Direct versus indirect effects of human activities on dissolved organic matter in highly impacted lakes[J]. Science of the Total Environment, 2020, 75(2): 141-839. [28] YU H , LIANG H , QU F , et al. Impact of dataset diversity on accuracy and sensitivity of parallel factor analysis model of dissolved organic matter fluorescence excitation-emission matrix[J]. Scientific Reports, 2015, 5(1): 2-7. [29] 汤昀, 朱教宁, 庞震鹏, 等. 菌剂预处理秸秆与牛粪混合对厌氧发酵产气的影响[J]. 天津农业科学, 2022, 28(6): 56-60. [30] MA Q, MENG N, LI Y, et al. Occurrence, impacts, and microbial transformation of 3-methylindole (skatole): A critical review[J]. Journal of Hazardous Materials, 2021, 41(6): 126-181. [31] MA Q, QU H, MENG N, et al. Biodegradation of skatole by Burkholderia sp. IDO3 and its successful bioaugmentation in activated sludge systems[J]. Environmental Research, 2020, 18(2): 109-123. [32] MA Q, LIU SW, LI SZ, HU JB, et al. Removal of malodorant skatole by two enriched microbial consortia: performance, dynamic, function prediction and bacteria isolation[J]. Science of the Total Environment, 2020, 72(5): 138-416. [33] MENG X, HE ZF, LI HJ, ZHAO X. Removal of 3-methylindole by lactic acid bacteria in vitro[J]. Experimental and Therapeutic Medicine, 2013, 6(4): 983-988. doi: 10.3892/etm.2013.1251 [34] 农业部. NY/T1168-2006 畜禽粪便无害化处理技术规范[S] . 北京: 中国标准出版社, 2007. [35] HASSAN Z, MILTON H, SAIER J. Gut Bacteroides species in health and disease, Gut Microbes, 2021, 13(1). [36] 宋伟民, 卢纯惠, 李锦梅. 土壤渗滤处理三格化粪池粪液的可行性论证[J]. 上海环境科学, 1997, 16(3): 36-37. [37] 唐山青, 谢彦培, 刘智峰, 等. 温度转换对厌氧消化系统连续运行及微生物群落的影响[J/OL]. 中国环境科学. [38] 韦采妮, 赵晨菊, 黄河笑, 等. 微量元素Fe、Co、Ni对养殖粪污厌氧发酵产气性能及微生物群落结构影响[J]. 当代化工研究, 2022, 20(6): 23-25. [39] FLINT H J, BAYER E A, RINCON M T, et al. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis[J]. Nature reviews Microbiology, 2008, 6(2): 121-131. doi: 10.1038/nrmicro1817 [40] XIAO Z Z, SHI C M, SHI P W, et al. A comparative study of composting the solid fraction of dairy manure with or without bulking material: performance and microbial community dynamics[J]. Bioresource Technology, 2018, 247: 443-452. doi: 10.1016/j.biortech.2017.09.116 [41] CHUA HH, CHOU HC, et al. Intestinal dysbiosis featuring abundance of ruminococcus gnavus associates with allergic diseases in Infants[J]. Gastroenterology, 2018, 154(1): 154-167. doi: 10.1053/j.gastro.2017.09.006 [42] 李晓萍, 张龙, 刘华. 摩根菌致肺内感染合并菌血症1例[J]. 宁夏医科大学学报, 2013, 35(3): 357-358. [43] DUAN J, LIANG J D, WANG Y P, et al. Kraft lignin biodegradation by Dysgonomonas sp. WJDL-Y1, a new anaerobic bacterial strain isolated from sludge of a pulp and paper mill[J]. Journal of Microbiology and Biotechnology, 2016, 26(10): 1765-1773. doi: 10.4014/jmb.1602.02014 [44] ZHANG M L, LIU N, QIAN C L, et al. Phylogenetic and functional analysis of gut Microbiota of a fungus-growing higher termite: Bacteroidetes from higher termites are a rich source of β-glucosidase genes[J]. Microbial Ecology, 2014, 68(2): 416-425. doi: 10.1007/s00248-014-0388-3 -






 下载:
下载: