-
在众多的污水处理方法中,活性污泥法受到人们的广泛关注,活性污泥法作为重要的处理污水方法之一,具有很多优势. 但是随着国内外对污水治理的日益重视和城市污水处理厂的不断建设,大量的剩余污泥作为活性污泥法处理污水的副产物排出[1]. 污泥因其含水率高、含有大量病原体和微生物等有害生物、重金属及有机物含量高等特点,容易对环境造成二次污染[2],污泥的有效处理处置是亟待解决的重要问题. 污泥脱水是常规的污泥处理方法,在污泥脱水之前需要经过一定的调理使其满足后续脱水要求,所以,选择合适的污泥调理方法对改善污泥脱水性能尤为重要.
过氧化钙(CaO2)作为一种热稳定性好的环境友好型材料,被广泛应用于农业种植、水产养殖、食品保存、医疗以及环境领域[3]. CaO2具有高能的过氧化物共价键,当CaO2与水接触时,能够缓慢释放过氧化氢(H2O2),同时还会生成羟基自由基、过氧化氢自由基等具有强氧化性的自由基(反应式见式(1—5))[4]. 近年来,因其具有稳定的氧化性,CaO2在污泥处理方面的应用成为一个新的研究热点. Wang 等研究发现,通过CaO2预处理污泥后,难降解有机物可以转化为可生物降解,促进污泥中可生物降解基质的水解和分解代谢,进而增强污泥厌氧消化效果[5]. 有研究表明,CaO2可以破解污泥EPS结构,释放污泥中的束缚水[6]. Wang等的研究表明,通过联合CaO2和微波预处理污泥,预处理后污泥的CST值相较于原泥下降52% [7]. 通过热处理与CaO2联合调理,可以提升污泥脱水性能[8].
除了直接使用CaO2对目标物进行氧化,对CaO2进行活化也是一种常用的技术[8]. 有研究认为,通过微波活化CaO2,能促进CaO2产生更多的HO·和·O2-[7]. 通过过渡金属(Fe2+/Fe3+和Ag+)活化CaO2分解是常用的活化方法[9]. 利用Fe2+活化CaO2可以形成类芬顿反应,但如果不进行pH调节, Fe2+易于被氧化成Fe3+,限制了芬顿反应的效率. 有研究指出,利用含铁矿物对H2O2进行活化可以克服这一缺陷[10]. 黄铁矿(FeS2)是一种常见的脉石矿物,与矿床中的有价矿物伴生,可通过常规浮选方法轻松处理[11]. 最近有研究发现,利用黄铁矿活化CaO2降解磺胺,相比常规的芬顿反应,磺胺的氧化效率从30%提升至80%,(主要反应见式(6—9))[12]. Zhou等研究表明利用黄铁矿活化CaO2处理邻苯二甲酸二乙酯(DEP),78%的DEP在24 h内被降解[13]. 这些结果说明,通过黄铁矿活化CaO2能有效促进HO·产生,但目前尚未发现关于利用黄铁矿活化过氧化钙调理污泥的研究,其对污泥脱水性能的影响及机理尚未清晰,因此本研究利用黄铁矿-CaO2作为一种新型的芬顿法对污泥进行调理,以期达到破解EPS从而释放结合水的效果,并通过EPS性质及污泥絮体性质变化探究其对污泥脱水性能的影响机理.
本研究对不同污泥样品进行EPS的提取,并对提取出来的EPS样品进行含量测定、三维荧光光谱检测,以表征调理前后污泥EPS性质变化. 同时对不同污泥样品的粒径分布进行检测,探究调理方法对污泥絮体团聚性能变化的影响.
-
本研究中污泥取自于广州市某污水处理厂二沉池,污泥取至实验室后,先过20目筛,去除大颗粒杂质和毛发,之后置于冰箱在4 ℃下保存. CaO2采购于上海麦克林生化科技有限公司. 黄铁矿采购于佛山市大昌顺材料科技有限公司,黄铁矿在使用之前对其进行研磨,并过100目筛,利用0.1 mol·L−1HNO3 洗去表面杂质及氧化层,干燥后备用[14].
-
为了探究不同调理条件对污泥脱水性能的影响,本研究对黄铁矿单独调理、CaO2单独调理以及两者复合调理污泥进行实验室规模的污泥脱水性能实验,250 mL的烧杯作为污泥调理容器,在调理容器中加入100 mL污泥样品进行实验. 利用重量法对污泥总固体(TS)进行测定[15]. 在黄铁矿单独调理实验中,设置6组不同黄铁矿调理剂量实验组,各组黄铁矿投加量分别为0、1、2、4、6 g·L−1. CaO2单独调理实验中,设置6组不同CaO2调理剂量实验组,各组CaO2投加量分别为10、30、50、80、100 mg·g−1 TS. 为了研究单独调理与复合调理以及不同复合调理方法之间的污泥脱水性能变化,设置了两组复合调理实验,第一组:CaO2投加量30 mg·g−1 TS,黄铁矿投加剂量1 g·L−1,第二组:CaO2投加量100 mg·g−1 TS,黄铁矿投加剂量1 g·L−1. 将单独调理和复合调理的实验组分别设置为A30、A100和B30、B100. 其中,A30为30 mg·g−1 TS CaO2单独调理,B30为30 mg·g−1 TS CaO2 +1 g·L−1黄铁矿复合调理,A100为100 mg·g−1 TS CaO2单独调理,B100为100 mg·g−1 TS CaO2 +1 g·L−1黄铁矿复合调理.
-
本研究中利用毛细吸水时间(CST)作为评价污泥脱水性能的指标. CST利用CST测定仪进行测定(HDFC-10A),利用测定后CST数据进行标准化CST(SCST)计算[16],计算公式如下:
其中,CSTa为调理后污泥样品的CST值,CST0为原泥的CST值.
-
在本研究中,EPS根据其存在形态分类为溶解性EPS(S-EPS)、松散束缚EPS(LB-EPS)和紧密束缚EPS(TB-EPS)[17],本研究采用一种改进的热提取方式对EPS进行提取,具体方法参照文献[18]. EPS中的多糖含量利用硫酸-蒽酮法测定,蛋白质含量利用福林酚法进行测定[19].
-
本研究中利用荧光光谱仪(Hitachi F-4600)对提取出的EPS进行3D-EEM的测定,光谱数据的发射波长(Em)以及激发波长(Ex)范围从220 nm到450 nm,采集间隔为10 nm. 光谱数据的利用5 nm的发射和激发狭缝带宽以及1500 nm·min−1的扫描速度进行收集.
-
本研究利用激光粒度仪(Mastersize 3000)对污泥絮体粒径分布及絮体粒径D50和D90值的测定. 其中,D50与D90分别定义为颗粒直径的第50和第90百分位数[20].
-
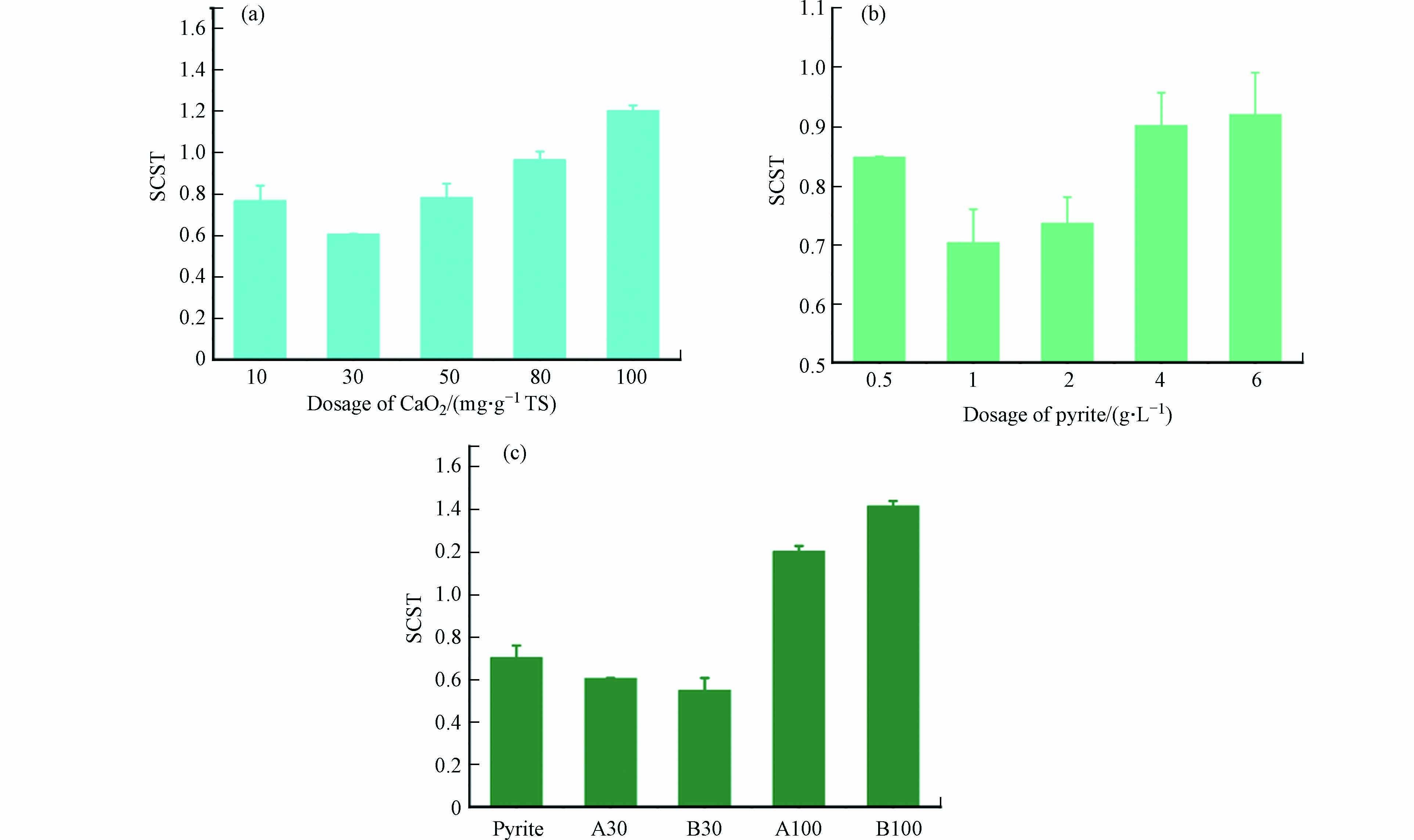
由图1可见,单独投加CaO2之后,污泥SCST值随着CaO2的投加量的增加呈现先下降再上升的趋势,单独投加CaO2,投加量为30 mg·g−1 TS的实验组SCST值最低为0.61. 在投加剂量不高于80 mg·g−1 TS时,CaO2单独调理有利于提升脱水性能,但当CaO2投加量增加至100 mg·g−1 TS时,SCST值增加至1.39,说明过量的CaO2不仅不会提升污泥脱水性能,反而会使得原污泥脱水性能下降. 随着黄铁矿投加量增加,黄铁矿单独调理的SCST值也表现出先下降再上升的,最优黄铁矿单独调理剂量为1 g·L−1,SCST值为0.70. 但当投加量继续增加时,黄铁矿单独调理对污泥脱水性能的提升效果变弱,在投加量为6 g·L−1的单独调理下,SCST值为0.92,污泥脱水性能提升不明显. 这说明过量的过氧化钙投加,带来过强的氧化性能,会使得污泥的脱水性能下降,这一趋势与Chen等的研究结果相似,过强的氧化性可能会导致过量的EPS释放,降低污泥脱水性能[6]. 但在CaO2投加量为30 mg·g−1 TS复合调理时,虽然氧化性能更强,但污泥有更佳的脱水性能,SCST值下降至0.55,这说明利用黄铁矿活化过氧化钙对污泥进行复合调理能有效提升污泥的脱水性能.
-
不同结构的EPS对剩余污泥的脱水性能影响程度可能不同,Dai等认为S-EPS中有机物含量较高或LB-EPS中有机物含量较低,具有较好的脱水性能[21]. He等指出污泥脱水性与S-EPS中有机物浓度呈正相关,而与LB-EPS中生物聚合物含量呈负相关[22]. 剩余污泥脱水性能除了和EPS的组成结构有关,还与EPS的组成成分相关,Wei等研究发现,污泥脱水性能与EPS中蛋白质含量呈负相关性[23],而且蛋白质含量是决定污泥脱水性能的关键因素[24],为了进一步探究污泥调理过程中污泥性质的变化,本研究对提取出的EPS样品进行蛋白质和多糖含量的测定. CaO2调理后污泥EPS结构发生明显的变化(图2a),在30 mg·g−1 TS的CaO2投加量下,S-EPS蛋白质含量略有下降,而内层EPS(LB-EPS、TB-EPS)蛋白质含量增加,相较于单独调理,CaO2/黄铁矿复合调理由于其更强的氧化性能,在CaO2投加量为30 mg·g−1 TS时的复合调理污泥样品中,内层EPS蛋白质含量增加幅度更大. 当CaO2投加量增加至100 mg·g−1 TS后,所有层EPS中蛋白质含量均增加,与低CaO2投加量相似,复合调理因其更强的氧化性,内部EPS含量较单独调理增加更多. 调理后污泥的总EPS(T-EPS)蛋白质含量均增加,高剂量CaO2导致更多的蛋白质释放,而复合调理对蛋白质含量的提升高于单独调理.
调理前后EPS多糖含量的变化见图2b,随着CaO2投加量增加,内外层EPS多糖含量均增加. 值得注意的是,高CaO2投加剂量的复合调理样品中,S-EPS和LB-EPS的多糖含量较单独调理均下降. T-EPS中多糖的变化趋势与蛋白质不同,T-EPS中多糖含量随着氧化性能的增强表现出先增加后下降的趋势,这可能是低CaO2剂量调理下,EPS结构被破解,内层EPS释放至外层. 但在高剂量CaO2的复合调理下,多糖类物质可能被分解为更小的有机分子或直接被矿化,导致T-EPS中多糖含量下降.
有研究认为,LB-EPS中蛋白质/多糖比率(PN/PS)与脱水性有负相关性[25]. 本实验中,B30样品LB-EPS的PN/PS最小(图2c),且无论高剂量或低剂量,在同一剂量下复合调理得到的LB-EPS样品,其PN/PS值均小于单独调理. 但当用高剂量过氧化钙对污泥进行调理后,LB-EPS中的PN/PS上升,污泥脱水性能下降. 但本实验发现,高剂量的过氧化钙调理后虽然PN/PS上升,但仍然低于原泥,这与脱水性能变化不一致,这是因为污泥脱水性能的变化影响十分复杂,并不能只靠EPS中的PN/PS进行指示.
从EPS含量变化可以看出,使用CaO2单独调理以及CaO2/黄铁矿复合调理都可以改变EPS原有结构,破解EPS结构. 在同一CaO2投加量下,复合调理得到的EPS破解效果更加明显. 结合污泥脱水结果分析,污泥调理方法在一定范围内对EPS结构进行破解,可能有利于污泥脱水性能的提升,但对EPS结构的过度破解可能会使得大量有机质的释放,进而使得污泥脱水性能下降.
-
为了更深入地了解调理前后以及各调理方法对各层EPS的性质以及其含量的影响,本研究利用三维荧光光谱对各层EPS的有机成分进行表征,各样品EPS的三维荧光光谱见图3. 本研究中EPS的荧光光谱峰主要有两个,分别为A峰(Em/Ex:340 nm/225 nm)和B峰(Em/Ex:350 nm/280 nm). 根据Wen等提出的三维荧光光谱分区方法,A峰位于区域Ⅱ,归类为芳香类蛋白物质,B峰位于区域Ⅳ,归类为色氨酸和类蛋白物质[26].
A峰在原泥S-EPS中强度较低,但经过调理后,A峰强度上升,芳香类蛋白含量增加. 在A100中,S-EPS中的A峰出现最强的荧光强度,说明在此调理方法下内层EPS和胞内的芳香类蛋白向外释放,聚集在外层EPS中. 但经过氧化性更强的B100调理后,A峰强度下降,这可能是由于芳香类蛋白的分解导致含量下降. S-EPS中B峰的荧光强度在A30和B30调理下均下降,当CaO2投加量增加后,S-EPS的B峰强度增加,S-EPS中B峰最强峰强度出现在B100调理下. 原泥中LB-EPS中A峰和B峰强度稍强于S-EPS,经过预处理后污泥LB-EPS中A、B峰强度增加,且两峰强度的增加幅度明显大于S-EPS. 不同调理方法对LB-EPS的荧光光谱图影响与S-EPS相似,A、B峰在B100调理下均出现最强荧光强度. 原泥TB-EPS中的芳香类蛋白和色氨酸含量明显高于S-EPS和LB-EPS,这一结果与EPS含量一致. 不同调理手段下B峰强度在TB-EPS中的变化与在S-EPS、LB-EPS中的变化相似,B峰在A100调理下出现最大荧光强度,随后下降. 但与 S-EPS、LB-EPS 变化趋势不一致的是,TB-EPS 中 A 峰的最大荧光强度出现在 B30 调理下, 这一结果说明,芳香类蛋白比色氨酸更易于从胞内和内层 EPS 释放至胞外和外层 EPS.
荧光峰强度变化趋势可以说明,在一定条件下,随着调理方法的氧化性的增强,EPS中物质被分解,EPS结构破解程度增加,胞内物质向TB-EPS转移,同时TB-EPS中的物质向外层的LB-EPS和S-EPS转移. 当调理方法氧化性能过强,各层EPS中物质被分解甚至矿化,导致各层EPS中荧光峰强度下降,同时还发现,各层EPS中不同物质对于不同调理方法的变化趋势并不完全相同.
-
由图4a可以看出,经过调理后的污泥絮体粒径分布曲线均向左移动,同时图4b中看到原泥有最大的D90以及D50值,调理后污泥的D50以及D90均有明显的下降,说明调理后污泥的絮体粒径下降. 这是由于强氧化性的调理方法将EPS结构破解后,会使得污泥絮体分解,形成尺寸更小的絮体[27]. 随着调理方法的氧化性能增强,污泥的粒径分布曲线左移程度越大,且有更小的D50和D90值,可以认为氧化性能越强的调理方法能够更高效、更彻底地破坏原有污泥絮体结构,使得原有稳定的大颗粒絮体失稳进而形成众多小尺寸的絮体. 这一现象与Ling等研究结果一致,通过对污泥絮体的破解,可以有效地释放束缚水,提升污泥脱水性能[28]. 在本研究中,在同一CaO2投加量下,复合调理后的污泥样品相较于单独调理后的污泥样品有更小的粒径,这也再次说明本研究中复合调理有更高效的EPS破解性能,但高剂量的过氧化钙投加量可能会过度破解絮体结构,过度破解絮体使得絮体粒径下降可能会增加小颗粒污泥对过滤介质的堵塞作用,降低污泥的脱水性能[29].
-
本研究提出一种利用黄铁矿活化CaO2的污泥调理技术,结果表明,单独利用CaO2或者黄铁矿对污泥进行调理,随着CaO2或黄铁矿投加量的增加,污泥脱水性能呈现先上升后下降的趋势,在30 mg·g−1 TS CaO2和1 g·L−1黄铁矿的投加量下分别得到过氧化钙和黄铁矿的最优单独调理效果,同时发现,当CaO2和黄铁矿投加量为30 mg·g−1 TS和1g L−1时,复合调理后的污泥样品脱水性能优于单独调理. 但实现污泥脱水性能的提升需要对调理药剂投加量进行控制,过多的药剂投加可能会带来污泥脱水性能的下降.
黄铁矿活化过氧化钙(CaO2)调理提升污泥脱水性能
Improving sludge dewatering performance by conditioning of activated calcium peroxide (CaO2) with pyrite
-
摘要: 本研究提出了一种利用黄铁矿活化过氧化钙(CaO2)的污泥调理技术,考察了黄铁矿和CaO2单独/复合调理污泥对污泥脱水性能的影响. 本研究中结合胞外聚合物(EPS)性质变化(各层EPS含量、三维荧光光谱)以及污泥絮体结构变化(污泥絮体粒径)分析不同调理方法对污泥性质的影响. 结果表明,在黄铁矿和CaO2投加量分别为1 g·L−1和30 mg·g−1 TS调理条件下,污泥样品有最低的SCST值(0.55). 在研究中发现,通过破解EPS结构虽然能提升污泥脱水性能,但过度的EPS结构破解,会导致大量污泥内部有机物释放,絮体粒径大幅下降,反而降低污泥的脱水性能.Abstract: In this study, a sludge conditioning technology using pyrite activated calcium peroxide (CaO2) was proposed to improve sludge dewaterability, and the effect of pyrite and CaO2 alone/combined conditioning sludge on sludge dewatering performance was investigated. The effects of different conditioning methods on the properties of sludge were analyzed by combining the changes of extracellular polymeric substance (EPS) properties (EPS content and Three-Dimention fluorescence spectrum of each layer) and the structural changes of sludge floc (particle size of sludge floc). The results showed that the sludge samples had a lowest SCST value (0.55) when the dosage of pyrite and CaO2 was 1 g·L−1 and 30 mg·g−1TS, respectively. It was found that cracking EPS structure can improve the sludge dewatering performance, however, excessive decomposing EPS structure will lead to the release of a large number of organic compounds into the sludge and the particle size of flocs will decrease significantly, resulting in the poor sludge dewatering performance.
-
Key words:
- pyrite /
- calcium peroxide /
- sludge dewatering.
-
滨海土壤盐渍化严重,仅靠降雨淋洗和植物演替进行土壤改良需数十年、甚至更长时间,必须通过技术措施进行改良才能用于绿林建设[1]。近年来,烟气脱硫石膏对盐渍土壤的改良和修复效应得到较好地验证,其被认为是一项成本低、修复速率快的滨海盐渍土壤改良剂[2-4]。烟气脱硫石膏可以有效降低盐渍土壤pH和碱化度[5-7],但也存在一些不足,程镜润等[7]研究发现烟气脱硫石膏在显著降低pH和碱化度的同时,也会增加土壤含盐量,并降低了土壤有效磷含量;毛玉梅等[8]研究发现烟气脱硫石膏会导致土壤全盐量增加,并降低了土壤有机质和速效磷,影响了黑麦草的发芽率;贺坤等[9]研究发现,烟气脱硫石膏改良滨海盐渍土会使土壤EC有明显增加,土壤速效磷、速效钾含量降低。城市园林废弃物通过堆肥处理和微生物分解会转化形成腐殖质,可增加土壤营养物质含量、促进养分的转化,提高营养物质的有效性[10-11]。张强[12]、顾兵等[13]研究表明园林废弃物堆肥可以改善植物生长状况和基质通气性、保水性和养分供应能力,提高土壤有机物质含量和土壤肥力,并对土壤有害阴、阳离子能起到缓冲作用,明显改善土壤物理性状和盐分组成。
为克服烟气脱硫石膏改良滨海盐渍土壤中产生的土壤含盐量增大、营养物质减少等不足的缺点,本研究通过盆栽实验开展了不同重量配比的园林废弃物堆肥与适量烟气脱硫石膏混合施用对滨海盐渍土的改良效果研究,分析了混合改良剂对盐渍土壤理化性质和植物生长发育的影响,以期为滨海盐渍土改良提供技术参考,并为城市固废提供适合的处置方式和综合利用途径。
1. 实验材料与方法
1.1 实验材料
实验土样取自上海南汇东滩的表层盐渍土,自然风干后磨碎过2 mm筛作为原土备用;园林废弃物堆肥取自上海植物园,主要原料是植物树枝及落叶等;烟气脱硫石膏取自上海外高桥电厂,主要成分为CaSO4.2H2O (CaSO4占含量的90.0%),营养物质含量较少,主要实验材料的理化性质见表1。
表 1 实验材料主要理化性质Table 1. Physical and chemical properties of test materials实验材料 pH EC/(Ms·cm−1) 有机质/% 全氮/(g·kg−1) 全磷/(g·kg−1) 全钾/(g·kg−1) 土壤 8.7 1.30 2.25 0.4 1.05 12.7 堆肥 7.3 0.37 112.0 1.36 2.31 10.44 烟气脱硫石膏 7.2 — — <0.001 <0.001 <0.1 注:实验植物为黑麦草(Lolium perenne L.),种植前于实验室恒温箱内进行发芽率实验,发芽率约为90.0%。 1.2 实验设计
根据程镜润等[7]和MAO等[8]的实验结果,25 g·kg−1是该区域盐渍土改良剂的适宜施用量,因此,本研究中烟气脱硫石膏施加量为固定的25 g·kg−1。实验于上海应用技术大学玻璃温室内进行,设6个水平的处理,分别为:T1处理(原土,空白对照)、T2处理(原土+烟气脱硫石膏)、T3处理(原土+烟气脱硫石膏+5%堆肥)、T4处理(原土+烟气脱硫石膏+10%堆肥)、T5处理(原土+烟气脱硫石膏+20%堆肥)、T6处理(原土+烟气脱硫石膏+40%堆肥),其中堆肥百分比均为重量配比,每个处理的土壤重量均为2.0 kg,各5次重复。
首先将50 g烟气脱硫石膏与原土混合(T1处理除外),然后将堆肥按不同重量配比分别加入制成样品装盆。实验过程中保持良好的温度和湿度条件,不断浇水以保持土壤湿度,保证改良剂与土壤充分反应,并通过浇灌排出土壤盐分。60 d后种植黑麦草,每个样盆放入20颗黑麦草种子,种植期间浇水量以盆底刚刚渗出水为宜,各样盆统一浇水和管理措施,种植20 d后统计黑麦草发芽情况,50 d左右盆栽实验结束。
1.3 指标测定
盆栽实验结束后,选取10株长势相近的黑麦草植株冲洗干净,分别测定地上、地下部分长度以及总重;然后将植株放置于烘箱内以85 ℃进行12 h烘干,取出后分别测定地上、地下部分干重。最后将植株样品研磨成粉末状,过1 mm筛后,采用H2SO4-H2O2消煮,测株全氮、全磷和全钾含量。实验结束后,收集土壤样品,风干后过1 mm筛以备测试理化性质。土壤pH采用酸度计实测,土壤电导率值采用电导仪测定[14];土壤有机质、有效磷、有效氮、速效钾的含量测定依照《森林土壤分析方法》[15]。
1.4 数据处理
实验结果统计与分析采用Excel 2015和SPSS 17.0软件处理。土壤理化性质、养分含量、植物生长指标等均以实验重复平均值显示,不同处理间指标的差异采用Duncan法检验。
2. 结果与讨论
2.1 改良剂对盐渍土壤理化性质的影响
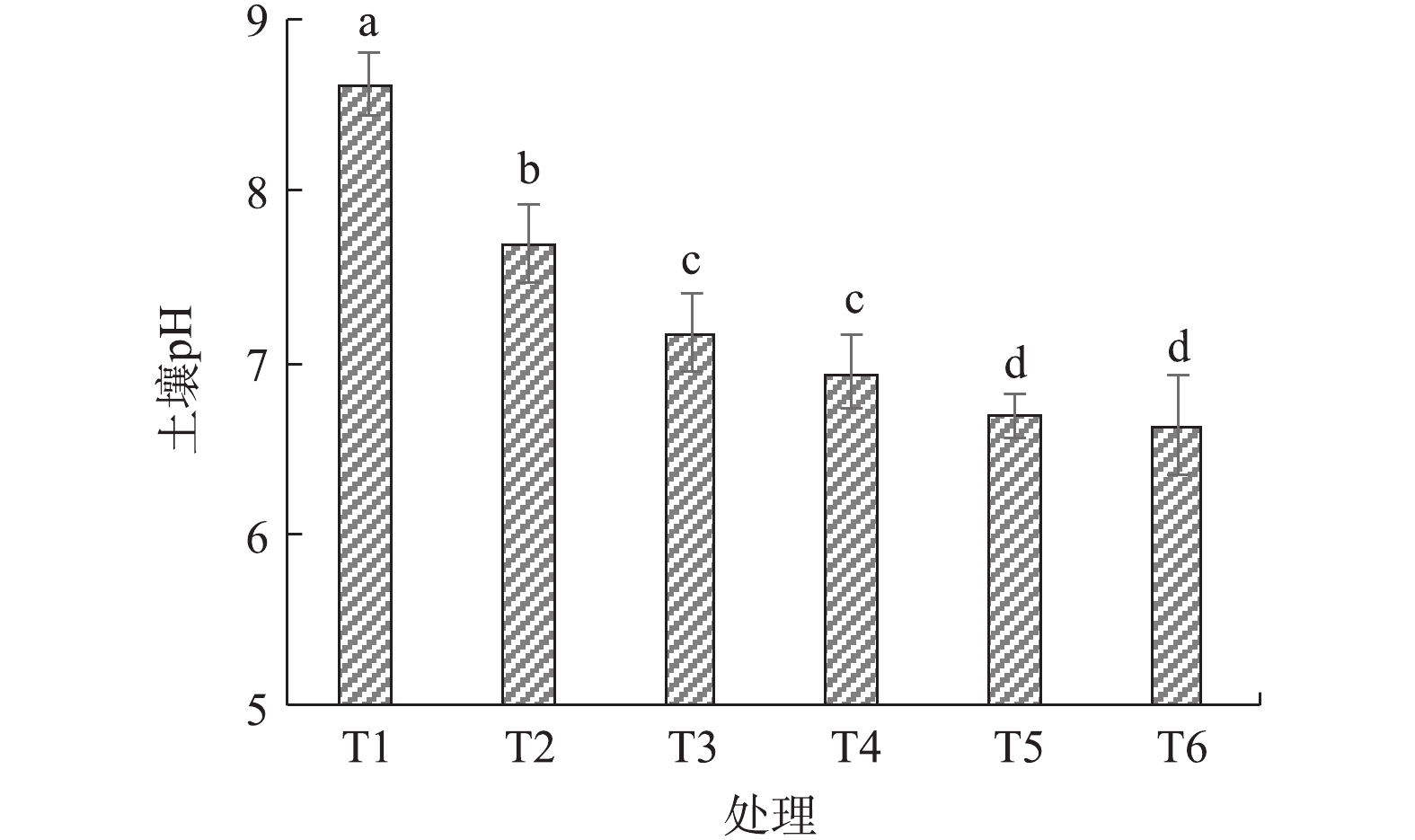
图1为改良剂对土壤pH的影响。施加烟气脱硫石膏后土壤pH明显低于对照处理,由8.61降至7.67,降幅为10.9%,结果与已有研究[2, 6-7]的结果一致。烟气脱硫石膏施用量固定的情况下,混合施用园林废弃物堆肥,随着堆肥施用量的增加,pH呈现逐步降低的趋势,降幅为6.8%~13.8%;当堆肥重量配比在15%~20%时,盐渍土壤的pH下降最为明显。各处理下土壤pH均降到8.0以下,符合多数植物的生长要求。
园林废弃物堆肥混合施用后,堆肥中的腐解酸能够与土壤碱性物质发生中和反应,致使土壤pH进一步降低。堆肥腐解酸还可以保持土壤水分和提高微生物活性[13],也会降低土壤pH。腐殖酸类物质也可以结合烟气脱硫石膏中的钙离子,减少钙离子对钠离子的置换,因此,在大量使用堆肥的情况下土壤pH能够较快地趋于稳定。
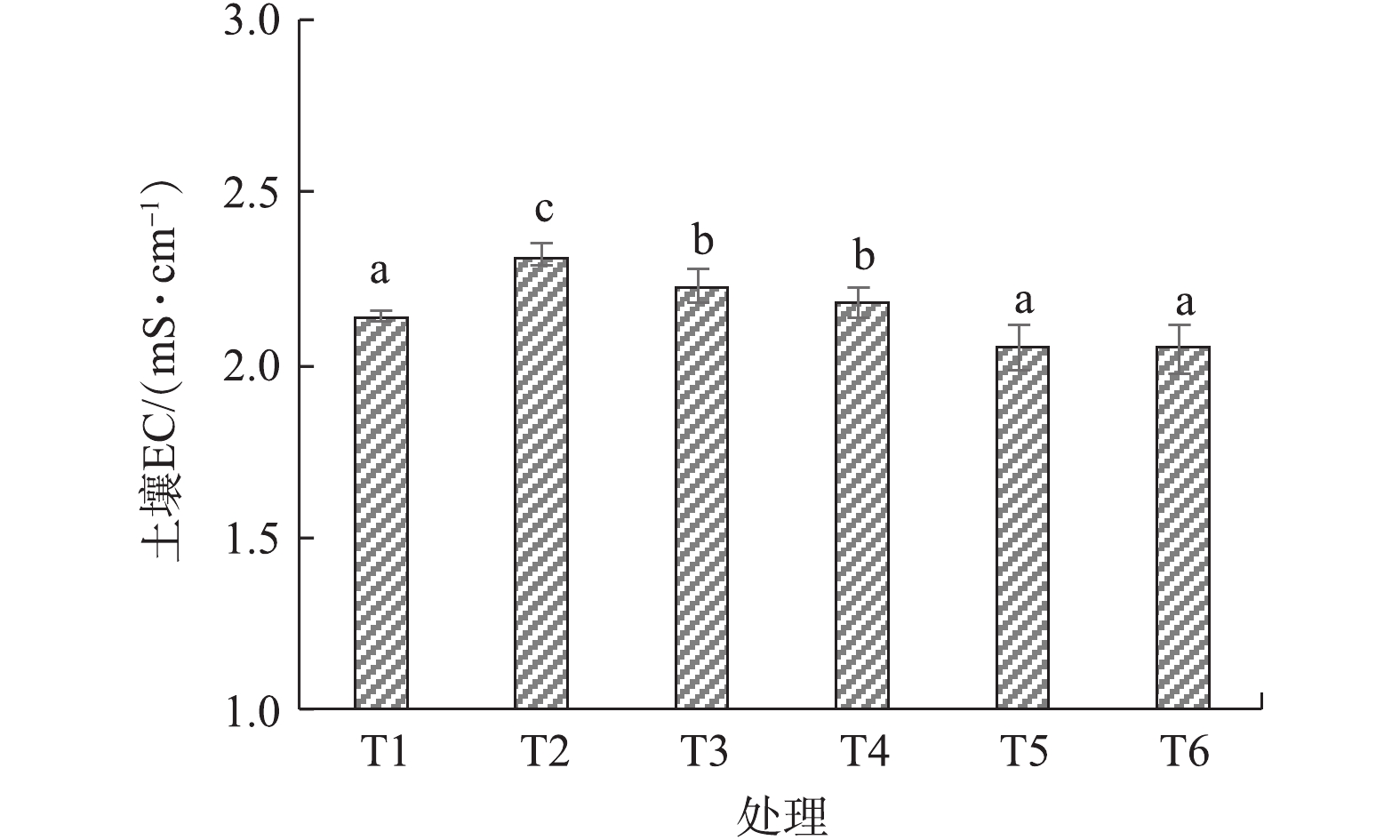
图2为改良剂对土壤EC的影响。在盐渍土壤中加入烟气脱硫石膏后EC显著升高,增幅可高达8.4%,其原因是烟气脱硫石膏是一种中等溶解度盐,可以连续释放硫酸根离子和钙离子[16]。在烟气脱硫石膏施用量固定的情况下,进一步增施堆肥可以降低土壤EC,堆肥重量配比20%~40%时,对比T2处理,EC降低了11.6%,对比T1处理,EC也降低了4.2%左右。这说明混合改良剂可以降低土壤全盐含量,堆肥增加了土壤孔隙度,提高了土壤渗透性,随着时间的推移,土壤中随水运移速度较快的盐离子会被进一步淋洗掉。
2.2 改良剂对土壤营养元素的影响
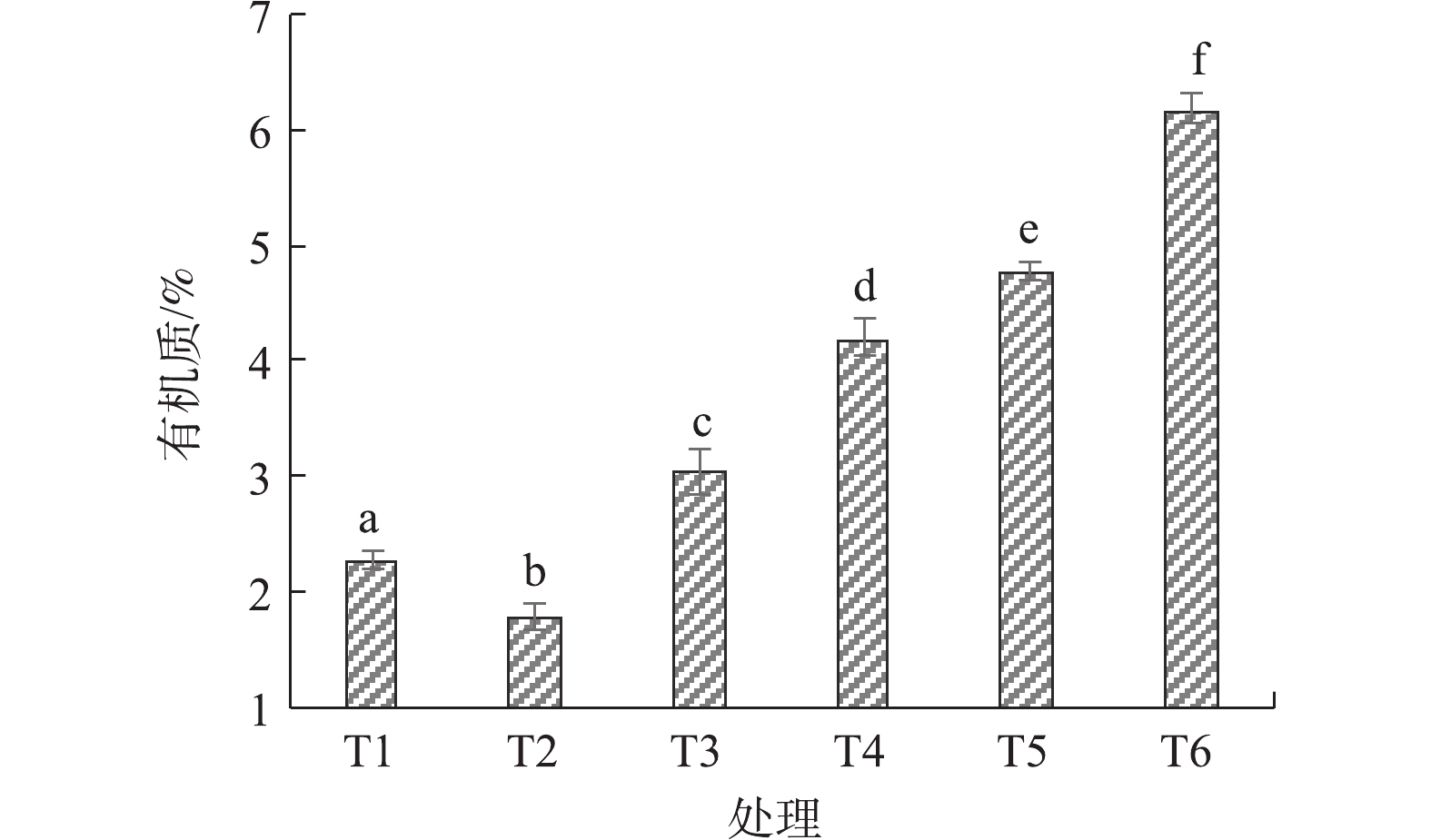
图3是改良剂对土壤有机质含量的影响。施加烟气脱硫石膏后盐渍土壤有机质含量下降21.6%左右。施加不同重量配比的堆肥后,土壤有机质含量随堆肥施用量增加逐步升高,T6处理时,盐渍土壤有机质含量比对照处理高出171.6%。在施加烟气脱硫石膏后,土壤有机质含量下降的主要原因是土壤pH的降低减少了有机质在水中的溶解,降低了水溶性有机质的含量[17]。园林废弃物堆肥中的有机质含量很高,增施到盐渍土壤后可使土壤有机质含量有较大程度的增加。
图4为改良剂对土壤营养物质含量的影响。施加烟气脱硫石膏后盐渍土壤有效磷、有效氮和速效钾含量均有所降低,降幅分别为30.1%、40.5%和36.1%。相对于T2处理,施加堆肥后盐渍土壤中有效磷、有效氮和速效钾含量均有明显增加,增幅分别为96.0%~182.7%,40.0%~186.7%和71.7%~157.5%。
烟气脱硫石膏中的钙离子在交换盐渍土壤胶体上的钠离子后,仍会以交换态形式留在土壤中吸附土壤中富集的磷酸根离子[18],或置换土壤中的铵离子和交换性钾离子,并随水流出而降低了土壤营养物质含量[8, 19]。由于堆肥的主要原料来源于植物枝条和落叶等,可促进土壤中小团聚体向大团聚体转化,提高土壤中毛管孔隙度和饱和导水率,可以显著提高氮、磷、钾含量[20]。此外,随着土壤pH的降低,土壤微生物活性增大,这可能将部分磷转化为易于被植物吸收的形态,从而导致土壤有效磷的明显增加[21]。而有效氮的增加还可能与烟气脱硫石膏促进了堆肥中的有机氮释放有关[22],吕子文等[10]的研究结果则表明堆肥可以促进土壤中钾的活性,增加了土壤速效钾的含量。
2.3 改良剂对植物发芽率和叶片数量的影响
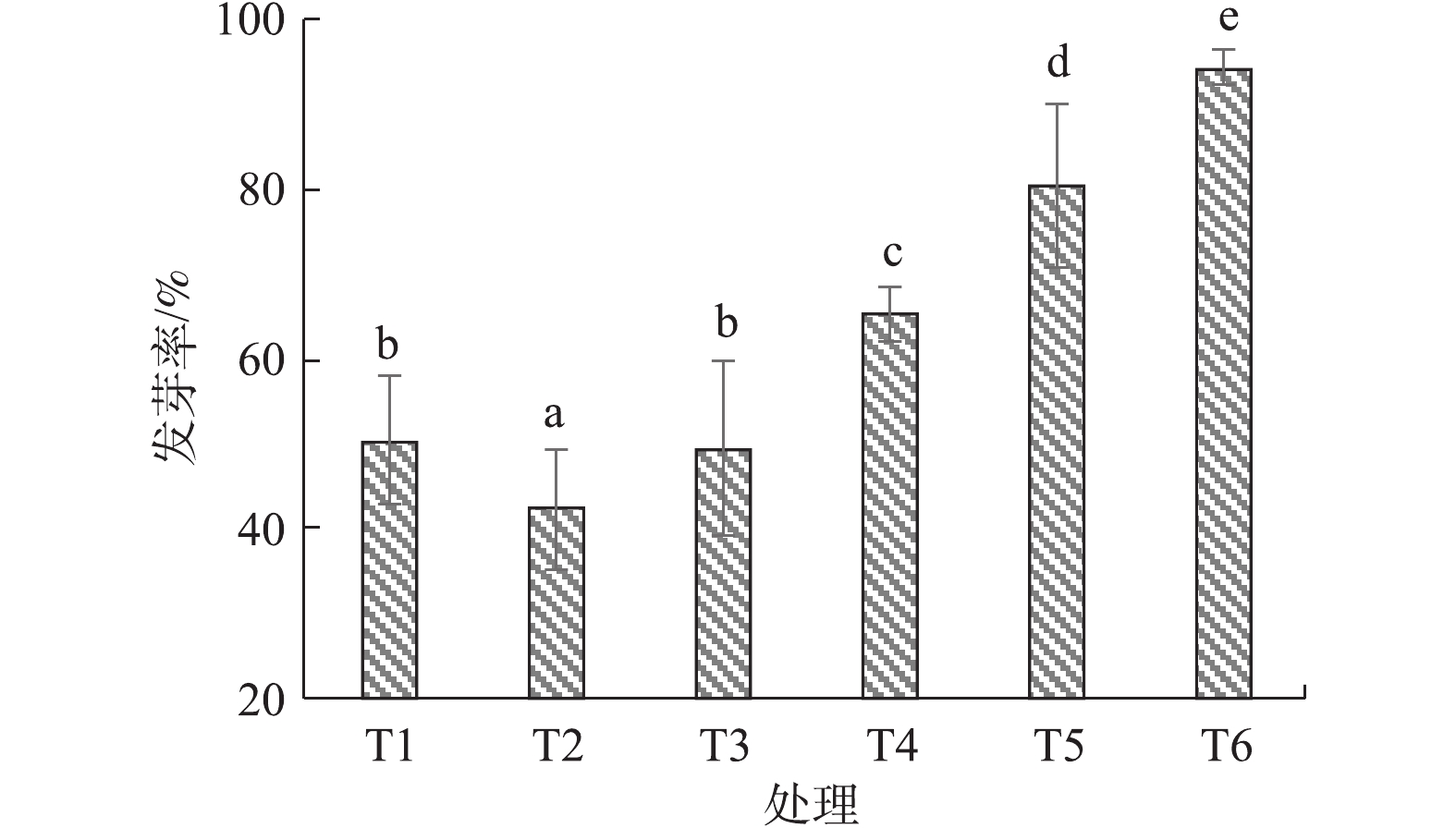
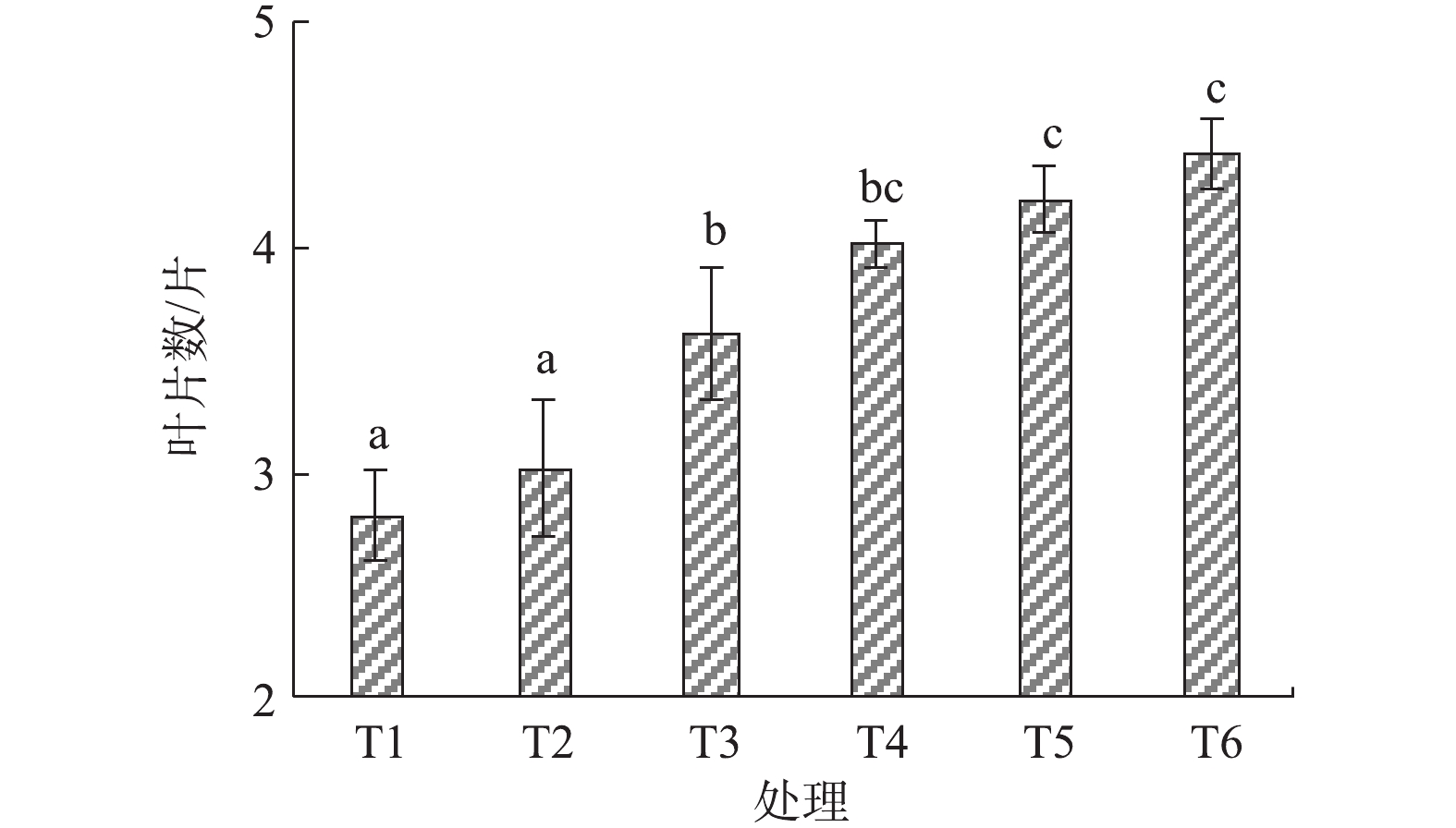
由图5可知,黑麦草在盐渍土壤条件下的发芽率仅有50.0%,施加烟气脱硫石膏后发芽率降至42.0%左右,这说明烟气脱硫石膏虽能降低土壤碱化度,但短时间内过量施用会导致盐分过量积累,以致土壤含盐浓度超过植物正常的耐受力,从而影响了黑麦草发芽率,该结果与CLARK等[23]和毛玉梅等[24]的研究结果相一致。施加园林废弃物堆肥后,土壤孔隙度增大,土壤pH降低,均可促进植物种子萌发和发芽率的提升。结果表明,随着堆肥施用量的增加,黑麦草发芽率也逐渐增加,当堆肥重量配比为40%时,发芽率达到90.0%左右。
由图6可知,施加烟气脱硫石膏后的黑麦草叶片分蘖数大于对照处理,但差异不显著。随着堆肥施用量的增加,黑麦草叶片分蘖数也逐步增加,但堆肥重量配比为20%~40%时,差异并不显著。
2.4 改良剂对植物生物量的影响
表2为改良剂对黑麦草生长特征变化的影响结果。施加烟气脱硫石膏后黑麦草的重量和高度均比对照处理有所增加,但差异并不显著。混合园林废弃物堆肥后,黑麦草重量和高度等指标均随堆肥施用量增加而呈现逐渐增加的趋势。T6处理时,黑麦草总湿重、地上干重、株高和根长等均达到最大值,分别较对照处理增加了154.1%、100.0%、89.2%和103.6%,比单一施加烟气脱硫石膏的处理增加了94.2%、75.0%、66.2%和71.3%。
表 2 不同处理中黑麦草生长特征的变化Table 2. Changes of growth characteristics of ryegrass under different soil amendments处理 重量/g 高度/cm 总湿重 地上干重 地下干重 株高 根长 T1 0.146±0.012a 0.021±0.001a 0.009±0.001a 13.0±1.20a 13.8±2.25a T2 0.191±0.052b 0.024±0.004a 0.010±0.002a 14.8±0.52a 16.4±2.30a T3 0.201±0.004b 0.029±0.001b 0.011±0.001a 16.8±0.36b 19.6±1.52b T4 0.281±0.009c 0.030±0.003b 0.013±0.001b 19.6±0.52c 22.0±3.01b T5 0.303±0.041c 0.036±0.002c 0.014±0.002b 21.8±0.07c 24.4±2.10c T6 0.371±0.020c 0.042±0.002d 0.013±0.001b 24.6±0.21d 28.1±2.10d 烟气脱硫石膏中所含高价离子可降低土壤胶体表面由负电荷相互排斥而产生的电位势,促进土壤胶体的凝聚,从而利于土壤团粒结构形成,改善作物根系的生长环境,促进作物的生长和发育[1, 7-8, 25]。烟气脱硫石膏中大量的钙和硫等营养物质也会促进植物的生长[7],因此部分土壤营养物质的减少并没有影响到植物生物量的增加。混合改良剂施用后,由于土壤pH、全盐的降低以及土壤有机质、营养物质的增加,进一步促进了黑麦草的生长。园林废弃物堆肥不仅进改善了盐渍土壤的容重与孔隙度[26],提高了土壤养分含量,而且烟气脱硫石膏经过一段时间的灌溉溶解后脱盐程度也会逐步增大[9],两者混合施用对盐碱地植物的根系生长更加有利[27]。
2.5 改良剂对植物体内营养物质的影响
表3是改良剂对黑麦草植株内营养物质变化的影响。施加烟气脱硫石膏后植株全氮、全钾含量均有所升高。混合施用园林废弃物堆肥后,植株全氮、全钾含量再度升高,且随堆肥施加量增加而呈现上升的趋势,与T1和T2处理相比差异显著。T6处理时,植株全氮、全钾含量增加效果最为明显,分别较对照处理增加了139.9%和40.8%,比单一施加烟气脱硫石膏的处理增加了83.3%和29.8%。植株全磷含量在施加烟气脱硫石膏后有所下降,降幅为25.4%。混合施加园林废弃物堆肥后,随着堆肥施加量的增加,植株全磷含量也逐渐增加,T6处理时略高于对照处理。结果表明烟气脱硫石膏抑制了植物对土壤有效磷的吸收,施用肥后土壤有效磷增加,植物吸收磷的数量也增加,与龚小强的研究结果一致[28]。
表 3 不同处理中黑麦草植株内营养物质的变化Table 3. Changes of nutrients in ryegrass under different soil amendments处理 全氮/(mg·kg−1) 全磷/(mg·kg−1) 全钾/(mg·kg−1) T1 4.268±0.232a 0.206±0.015a 5.200±0.192a T2 5.584±0.158b 0.154±0.015b 5.641±0.067b T3 6.957±0.176c 0.159±0.020b 6.000±0.307c T4 8.662±0.337d 0.166±0.013b 6.041±0.232c T5 9.333±0.197d 0.191±0.004a 6.803±0.081d T6 10.238±0.348e 0.207±0.017a 7.324±1.263d 施加烟气脱硫石膏或者2种改良剂混合施用,植物体内的全氮、全钾含量均有所增加,说明烟气脱硫石膏的施用虽然降低了土壤营养物质含量,但并未影响到植物对氮、钾两种营养物质的吸收,其原因应该是由于土壤孔隙度的增大和pH的降低促进了植物的根系生长(植物的根系长度和地下重量均增加),而植物根系对养分的生物有效性有重要作用。
烟气脱硫石膏施用后植株的全磷含量有明显下降,这说明烟气脱硫石膏抑制了植物对磷的吸收。相关研究表明土壤中的磷大部分都是迟效性的,植物生长对磷的利用率本来就比较低,一般为5%~15%,因此,土壤有效磷的含量直接影响植物体内的磷含量[29-30],烟气脱硫石膏减少了土壤有效磷含量,也就降低了植株中的全磷含量。混合园林废弃物堆肥后,植物体内全磷含量逐步增加,这说明堆肥能将自身磷转换成易被植物吸收的有效磷[13],植株全磷含量相应增加。
2.6 烟气脱硫石膏与园林废弃物堆肥施用量及经济效益
研究结果表明烟气脱硫石膏施用量固定的情况下,园林废弃物堆肥占比越高,植物生长越旺盛。但张强等等[12]的研究结果表明,过高的比例会影响植物生长,对花卉生长和品质的影响效果出现降低趋势,园林废弃物堆肥的添加比例以30%~50%为宜[11, 13]。根据本研究的结果,推荐使用25 g·kg−1作为烟气脱硫石膏最佳施用量,以及20%~40%作为园林废弃物堆肥的最佳重量比,在此条件下即可取得较好的盐渍土改良效果。目前,上海地区烟气脱硫石膏出厂费用大约60元·t−1,而园林废弃物堆肥的生产成本约为100元·t−1,因此,按照改良10 000平方米(翻深30 cm,土壤容重1.40 g·cm−3)盐渍土壤计算,需要105 kg的烟气脱硫石膏和840 kg的园林废弃物堆肥,合计成本费用大约仅需要90.3元。目前,滨海盐渍土改良普遍采用灌溉压盐、埋管排盐等方法,轻、中度碱化盐土的改良多在一定灌排条件下结合农业生物措施改良,重碱化盐土的改良则主要是配合化学改良剂[31],以上方法无论是时间和成本都相对较高。本研究中2种改良剂的施用成本相对于传统的工程措施和材料而言都相对较低,还能够降低城市固体废弃物的处理成本。
3. 结论
1)烟气脱硫石膏能显著降低滨海盐渍土壤pH,但增加了土壤全盐含量。混合园林废弃物堆肥后,土壤pH进一步降低,同时增加了土壤盐离子的流失,进而降低了土壤全盐含量。
2)烟气脱硫石膏和园林废弃物堆肥2种改良剂的混合施用,能够显著增加盐渍土壤营养物质的含量,从而改善了单独施用烟气脱硫石膏改良盐碱土所造成的土壤营养物质降低的不足。
3)烟气脱硫石膏混合园林废弃物堆肥一起施用可以提高盐渍土壤植物发芽率,有效改善土壤的理化性质,增加土壤和植物体内的营养物质含量,最终增加植物的生物量。
4)相对于传统的工程措施和改良材料,烟气脱硫石膏混合园林废弃物堆肥施用成本较低,还可以作为城市固体废弃物处理的有效手段,降低固废处理成本,一举两得。
-
-
[1] CHRISTENSEN M L, KEIDING K, NIELSEN P H, et al. Dewatering in biological wastewater treatment: A review[J]. Water Research, 2015, 82: 14-24. doi: 10.1016/j.watres.2015.04.019 [2] 汤连生, 罗珍贵, 张龙舰, 等. 污泥脱水研究现状与新认识[J]. 水处理技术, 2016, 42(6): 12-17. doi: 10.16796/j.cnki.1000-3770.2016.06.003 TANG L S, LUO Z G, ZHANG L J, et al. Research status and new views of sludge dewatering[J]. Technology of Water Treatment, 2016, 42(6): 12-17 (in Chinese). doi: 10.16796/j.cnki.1000-3770.2016.06.003
[3] MA Y, ZHANG B T, ZHAO L X, et al. Study on the generation mechanism of reactive oxygen species on calcium peroxide by chemiluminescence and UV-visible spectra[J]. Luminescence, 2007, 22(6): 575-580. doi: 10.1002/bio.1003 [4] XU Q X, HUANG Q S, WEI W, et al. Improving the treatment of waste activated sludge using calcium peroxide[J]. Water Research, 2020, 187: 116440. doi: 10.1016/j.watres.2020.116440 [5] WANG C, WEI W, DAI X H, et al. Calcium peroxide significantly enhances volatile solids destruction in aerobic sludge digestion through improving sludge biodegradability[J]. Bioresource Technology, 2022, 346: 126655. doi: 10.1016/j.biortech.2021.126655 [6] CHEN Z, ZHANG W J, WANG D S, et al. Enhancement of waste activated sludge dewaterability using calcium peroxide pre-oxidation and chemical re-flocculation[J]. Water Research, 2016, 103: 170-181. doi: 10.1016/j.watres.2016.07.018 [7] WANG J, SHANG K K, DA L J, et al. Synergetic effect of combined CaO2 and microwave treatment on waste active sludge dewaterability and organic contaminants’ removal[J]. Frontiers in Environmental Science, 2021, 9: 734277. doi: 10.3389/fenvs.2021.734277 [8] ZHOU Y B, FANG X B, WANG T H, et al. Chelating agents enhanced CaO2 oxidation of bisphenol A catalyzed by Fe3+ and reuse of ferric sludge as a source of catalyst[J]. Chemical Engineering Journal, 2017, 313: 638-645. doi: 10.1016/j.cej.2016.09.111 [9] ZHANG X, GU X G, LU S G, et al. Degradation of trichloroethylene in aqueous solution by calcium peroxide activated with ferrous ion[J]. Journal of Hazardous Materials, 2015, 284: 253-260. doi: 10.1016/j.jhazmat.2014.11.030 [10] CHEN H, ZHANG Z L, YANG Z L, et al. Heterogeneous Fenton-like catalytic degradation of 2, 4-dichlorophenoxyacetic acid in water with FeS[J]. Chemical Engineering Journal, 2015, 273: 481-489. doi: 10.1016/j.cej.2015.03.079 [11] WANG L P, CHANG Y Z, LI A M. Hydrothermal carbonization for energy-efficient processing of sewage sludge: A review[J]. Renewable and Sustainable Energy Reviews, 2019, 108: 423-440. doi: 10.1016/j.rser.2019.04.011 [12] KIM J G, KIM H B, JEONG W G, et al. Enhanced-oxidation of sulfanilamide in groundwater using combination of calcium peroxide and pyrite[J]. Journal of Hazardous Materials, 2021, 419: 126514. doi: 10.1016/j.jhazmat.2021.126514 [13] ZHOU Y, HUANG M, WANG X L, et al. Efficient transformation of diethyl phthalate using calcium peroxide activated by pyrite[J]. Chemosphere, 2020, 253: 126662. doi: 10.1016/j.chemosphere.2020.126662 [14] LI Y, CHEN J M, ZHONG J, et al. Acceleration of traces of Fe3+-activated peroxymonosulfate by natural pyrite: A novel cocatalyst for improving Fenton-like processes[J]. Chemical Engineering Journal, 2022, 435: 134893. doi: 10.1016/j.cej.2022.134893 [15] DAI Z H, LIU L, DUAN H R, et al. Improving sludge dewaterability by free nitrous acid and lysozyme pretreatment: Performances and mechanisms[J]. Science of the Total Environment, 2023, 855: 158648. doi: 10.1016/j.scitotenv.2022.158648 [16] GUO J Y, JIA X J, GAO Q F. Insight into the improvement of dewatering performance of waste activated sludge and the corresponding mechanism by biochar-activated persulfate oxidation[J]. Science of the Total Environment, 2020, 744: 140912. doi: 10.1016/j.scitotenv.2020.140912 [17] NIU M Q, ZHANG W J, WANG D S, et al. Correlation of physicochemical properties and sludge dewaterability under chemical conditioning using inorganic coagulants[J]. Bioresource Technology, 2013, 144: 337-343. doi: 10.1016/j.biortech.2013.06.126 [18] LI X Y, YANG S F. Influence of loosely bound extracellular polymeric substances (EPS) on the flocculation, sedimentation and dewaterability of activated sludge[J]. Water Research, 2007, 41(5): 1022-1030. doi: 10.1016/j.watres.2006.06.037 [19] McDONALD C E, CHEN L L. The Lowry modification of the Folin reagent for determination of proteinase activity[J]. Analytical Biochemistry, 1965, 10(1): 175-177. doi: 10.1016/0003-2697(65)90255-1 [20] CHEN D Y, YANG J. Effects of explosive explosion shockwave pretreatment on sludge dewaterability[J]. Bioresource Technology, 2012, 119: 35-40. doi: 10.1016/j.biortech.2012.05.129 [21] DAI Q X, MA L P, REN N Q, et al. Investigation on extracellular polymeric substances, sludge flocs morphology, bound water release and dewatering performance of sewage sludge under pretreatment with modified phosphogypsum[J]. Water Research, 2018, 142: 337-346. doi: 10.1016/j.watres.2018.06.009 [22] HE D Q, WANG L F, JIANG H, et al. A Fenton-like process for the enhanced activated sludge dewatering[J]. Chemical Engineering Journal, 2015, 272: 128-134. doi: 10.1016/j.cej.2015.03.034 [23] WEI H, GAO B Q, REN J, et al. Coagulation/flocculation in dewatering of sludge: A review[J]. Water Research, 2018, 143: 608-631. doi: 10.1016/j.watres.2018.07.029 [24] LIU J, YANG Q, WANG D B, et al. Enhanced dewaterability of waste activated sludge by Fe(Ⅱ)-activated peroxymonosulfate oxidation[J]. Bioresource Technology, 2016, 206: 134-140. doi: 10.1016/j.biortech.2016.01.088 [25] GUO Z Y, MA L P, DAI Q X, et al. Role of extracellular polymeric substances in sludge dewatering under modified corn-core powder and sludge-based biochar pretreatments[J]. Ecotoxicology and Environmental Safety, 2020, 202: 110882. doi: 10.1016/j.ecoenv.2020.110882 [26] CHEN W, WESTERHOFF P, LEENHEER J A, et al. Fluorescence excitation–emission matrix regional integration to quantify spectra for dissolved organic matter[J]. Environmental Science & Technology, 2003, 37(24): 5701-5710. [27] XIAO K K, PEI K Y, WANG H, et al. Citric acid assisted Fenton-like process for enhanced dewaterability of waste activated sludge with in-situ generation of hydrogen peroxide[J]. Water Research, 2018, 140: 232-242. doi: 10.1016/j.watres.2018.04.051 [28] LING X, DENG J, YE C, et al. Fe(Ⅱ)-activated sodium percarbonate for improving sludge dewaterability: Experimental and theoretical investigation combined with the evaluation of subsequent utilization[J]. Science of the Total Environment, 2021, 799: 149382. doi: 10.1016/j.scitotenv.2021.149382 [29] LI Y F, ZHU Y Q, WANG D B, et al. Fe(Ⅱ) catalyzing sodium percarbonate facilitates the dewaterability of waste activated sludge: Performance, mechanism, and implication[J]. Water Research, 2020, 174: 115626. doi: 10.1016/j.watres.2020.115626 -




 DownLoad:
DownLoad: