-
铬(chromium, Cr)是水中典型的重金属污染物,主要来源于造纸、制革、冶金和染料等行业的废水排放[1]。铬在水体中以三价、四价和六价存在,Cr(Ⅲ)和Cr(Ⅵ)最稳定[2],Cr(Ⅵ)对生物的毒性是Cr(Ⅲ)的100~1 000倍[3],可诱发多种致癌性、诱变性疾病,如慢性溃疡、肝脏炎症、肾损伤、肺癌等[4]。因此,高效地去除水中的Cr(Ⅵ)是至关重要的。由于Cr(Ⅵ)具有极高的氧化性,通常将高毒性的Cr(Ⅵ)还原为低毒性的Cr(Ⅲ),作为Cr(Ⅵ)的有效去除方法[5]。
纳米零价铁(nano-zero-valent iron, nZVI)具有大的比表面积、丰富的活性位点、高的反应性和环境友好性[6],在去除水溶液中污染物方面具有很好的应用前景。但是,其易团聚、易钝化和迁移性差等缺陷使得单一的nZVI系统已经不能满足当今废水处理的实际需求[7]。复合型nZVI成为了近几年研究的热点。负载型、表面修饰型、硫化型和双金属型是常用的复合型nZVI技术。QIAN等[8]使用红麻棒、铁盐和绿茶提取物制备多孔生物炭,并制备了多孔生物炭负载的纳米零价铁(biochar loaded nano-zero-valent iron, BC-nZVI)。HE等[9]采用羧甲基纤维素(carboxymethyl cellulose, CMC)作为稳定剂,对nZVI进行了分散研究。LI等[10]采用球磨、真空化学气相沉积(vacuum chemical vapor deposition, CVD)和液相还原法制备出硫化纳米零价铁(sulfurized nano-zero-valent iron, S-nZVI)。HE,ZHAO[11]采用水溶性淀粉作为稳定剂,制备了淀粉稳定的nZVI粒子和Fe-Pd双金属纳米粒子。复合型nZVI对重金属、有机物和放射性核素等方面的有着良好的去除效果。
黄铁矿是自然界中常见的金属矿物,主要成分是FeS2,在尾矿中含量很大,尾矿中的黄铁矿会对周围环境形成污染[12]。但黄铁矿具有价格低和良好的电化学性质等特点。因此,黄铁矿的有效利用在环境治理方面具有广泛的研究前景。天然的黄铁矿S和Fe之间以共价键结合,具有良好的稳定性,反应活性较低[13]。使用机械活化可以将黄铁矿制备成微纳米级粉末,降低黄铁矿粒径,增加其晶格畸变率[14],使黄铁矿粒子表面的悬空未配位键和点缺陷等增多[15-16]。有研究[17]表明,使用天然黄铁矿和铁粉进行球磨,通过黄铁矿和铁粉的协同作用对Cr(Ⅵ)产生了良好的去除效果,但由于黄铁矿和铁粉活性较差、反应较慢,对于Cr(Ⅵ)含量高废水,需要消耗大量材料[18]。而化学合成的nZVI具有极强的反应活性,更容易和机械活化的黄铁矿产生协同作用。并且通过黄铁矿的负载可以减轻nZVI的团聚,从而提升nZVI对Cr(Ⅵ)的去除效果。除此之外,还可以对黄铁矿进行有效利用,同时减少硼氢化钠(NaBH4)的使用量,节约成本。基于此,本文利用乙醇作为介质,通过优化球料比、磨球数量和磨球大小比例,采用机械活化的方式,制备了微纳米级天然黄铁矿,以此作为载体材料,通过液相还原法制备了微纳米级天然黄铁矿负载nZVI(pyrite loaded nano-zero-valent iron, nZVI/黄铁矿),用于去除水体中的Cr(Ⅵ)。并通过动力学、等温线模型和热力学分析对Cr(Ⅵ)去除机理进行了探讨。
-
天然黄铁矿(北京水远山长矿物标本有限公司,采自云南石林西纳村);无水乙醇(C2H6O,AR,天津市德恩化学试剂有限公司);七水合硫酸亚铁(FeSO4·7H2O,AR,西陇化工股份有限公司);硼氢化钠(NaBH4,AR,天津市进丰化工有限公司);盐酸(HCl,AR);氢氧化钠(NaOH,AR);重铬酸钾(K2Cr2O7,AR,北京化工厂),高纯氮气和混合气(太原宜虹气体工业有限公司);实验所用水均为无氧超纯水。
-
Optima7300V型电感耦合等离子体发射光谱仪(inductively coupled plasma-optical emission spectrometer, ICP-OES,美国PE公司);COY低氧手套箱(天美科学仪器有限公司);QM-3SP04型PM系列行星式球磨机(卓的仪器设备上海有限公司);KQ-500DB型透射电子显微镜(transmission electron microscope, TEM,昆山舒美超声仪器有限公司);S-4800型扫描电子显微镜(scanning electron microscope, SEM,日本日立公司);纯水仪(MILLIPORE公司);XRD-6000型X射线衍射仪(X-ray diffraction, XRD,日本岛津公司);Noran system 7型X射线能谱仪(X-ray energy spectrum, EDS,美国Thermo Fisher科技有限公司)。
-
将天然黄铁矿人工粉碎成小块状,放入1.0 mol·L−1的稀盐酸中浸泡1.0 h,以去除杂质和氧化物,洗涤和干燥后研磨至粉末状;按照球料比为10:1的比例,向50.0 mL的氧化锆球磨罐内加入相同数量直径为3 mm和8 mm的氧化锆磨球,再加入矿浆体积比为1:1的黄铁矿和无水乙醇,设置球磨机转速为450 r·min−1,机械活化24.0 h,再于无水中乙醇超声分散30.0 min,干燥后获得微纳米级天然黄铁矿,备用。
-
采用液相还原法制备nZVI。本研究使用500.0 mL三口烧瓶作为反应容器,并使用N2进行曝气。称取1.390 g FeSO4·7H2O溶于100.0 mL无氧水中,搅拌30 min,再以每秒1~2滴的速度,滴加过量的NaBH4溶液,至溶液变黑,继续搅拌20.0 min,以保证FeSO4·7H2O和NaBH4完全反应。反应完成后,转移至无氧手套箱内,通过磁选的方式,用无氧水和无水乙醇各洗3次,然后转移至培养皿中,在真空40 ℃的条件下,干燥8.0 h。得到黑色粉末,备用。
-
分别称取0.280、0.140和0.070 g黄铁矿加入三口烧瓶内,加入50.0 mL无氧水,搅拌30.0 min。再称取1.390 g FeSO4·7H2O溶于50.0 mL无氧水中,加入三口烧瓶内,继续搅拌30.0 min,之后步骤同nZVI的制备方法,制备出质量比(黄铁矿:nZVI)为1:1、1:2和1:4 nZVI/黄铁矿复合材料。
-
称取2.829 g的K2Cr2O7用无氧超纯水溶于1 000.0 mL的容量瓶中,配置成1.0 g·L−1的Cr(Ⅵ)储备液。实验所用的Cr(Ⅵ)污染物溶液均经1.0 g·L−1的Cr(Ⅵ)储备液稀释而来,稀释得70.0、80.0、90.0、100.0和110.0 mg·L−1的Cr(Ⅵ)污染物原液,备用。以50.0 mL的离心管作为反应容器,取设定质量浓度的Cr(Ⅵ)污染物原液50.0 mL于离心管中,并用1.0 mol·L−1、0.1 mol·L−1 HCl和1.0 mol·L−1、0.1 mol·L−1 NaOH溶液调节初始pH,称取0.020 g的nZVI/黄铁矿和nZVI于离心管中。将离心管置于旋转仪上,调整转速为40.0 r·min−1,在预设的时间点从离心管中取1.0 mL溶液,用0.22 μm滤膜过滤后,使用ICP-OES测量溶液中剩余Cr(Ⅵ)的质量浓度。除温度影响实验外,其余实验反应温度均为25 ℃。除初始pH影响实验外,其余实验反应初始pH均为3.00。
-
nZVI的制备所使用的NaBH4和FeSO4·7H2O分别是天然黄铁矿价格的5 800倍和160倍。以天然黄铁矿和nZVI质量比为1:2计算,可减少约1/3的NaBH4和FeSO4·7H2O的使用量,相当于减少了约1/3的成本。这表明该材料具有良好的经济效益。
-
通过去除率判断nZVI/黄铁矿对Cr(Ⅵ)的去除能力。根据准一级和准二级动力学方程分析反应动力学过程,使用Langmuir和Freundlich吸附等温式和热力学参数评价吸附过程。
-
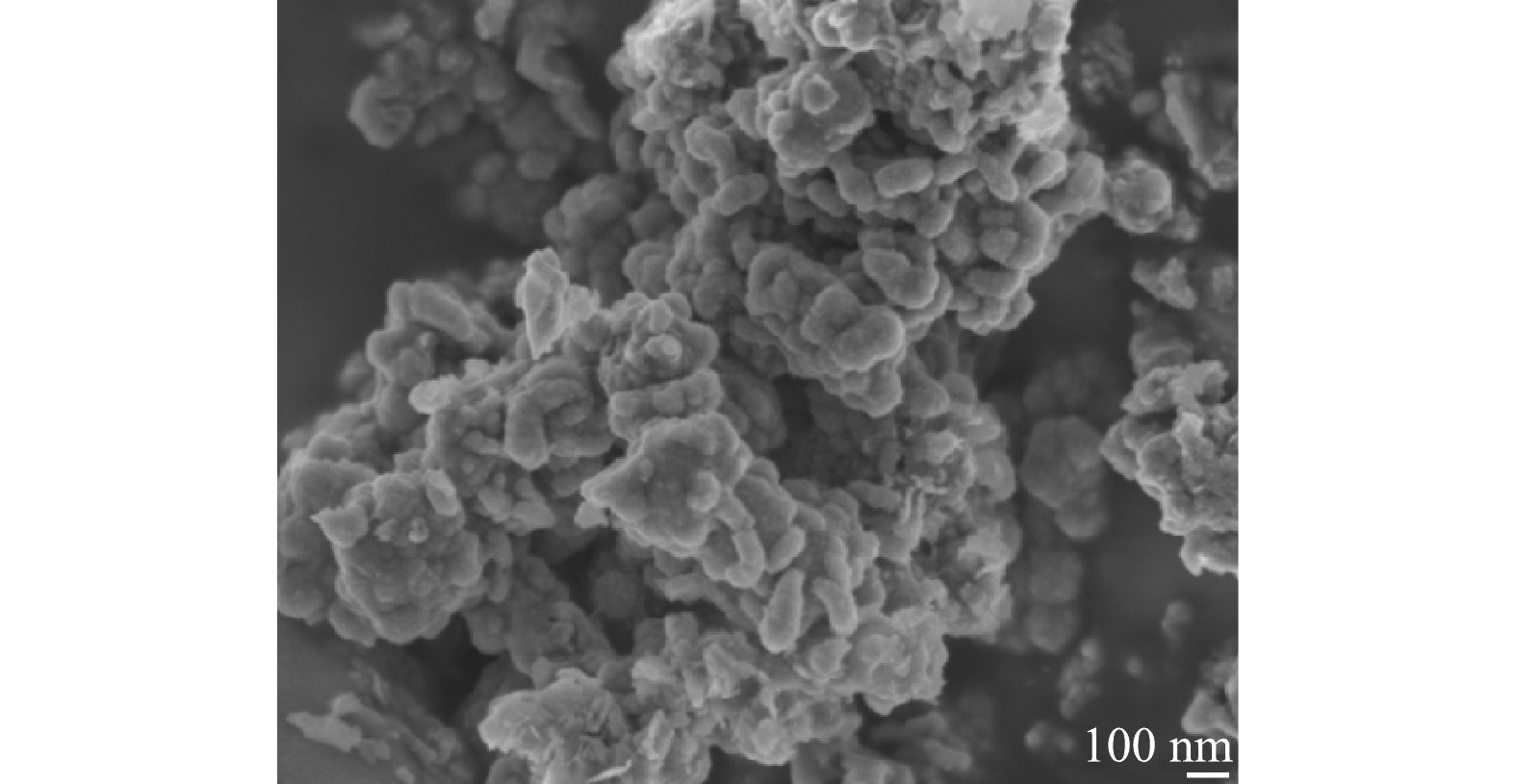
1) SEM分析。在真空手套箱内,将导电胶带粘在样品台上,再使用棉签粘取适量上述制备的nZVI/黄铁矿粉末轻弹于导电胶带上,用洗耳球吹去未黏住的样品,并对样品进行1 min的喷金处理,进行SEM表征,表征结果如图1所示。将nZVI/黄铁矿放大50 000倍可看出,机械活化后的黄铁矿呈块状和片层状,粒径在50~200 nm。nZVI呈球形和椭球形,原本光滑的表面变得粗糙。表明黄铁矿的负载使得nZVI的表面结构发生了改变,产生了铁硫化物。铁硫化物可与nZVI建立良好的电子传递路径[17],在去除污染物的过程中,加快nZVI的腐蚀可提升污染物的去除效率。
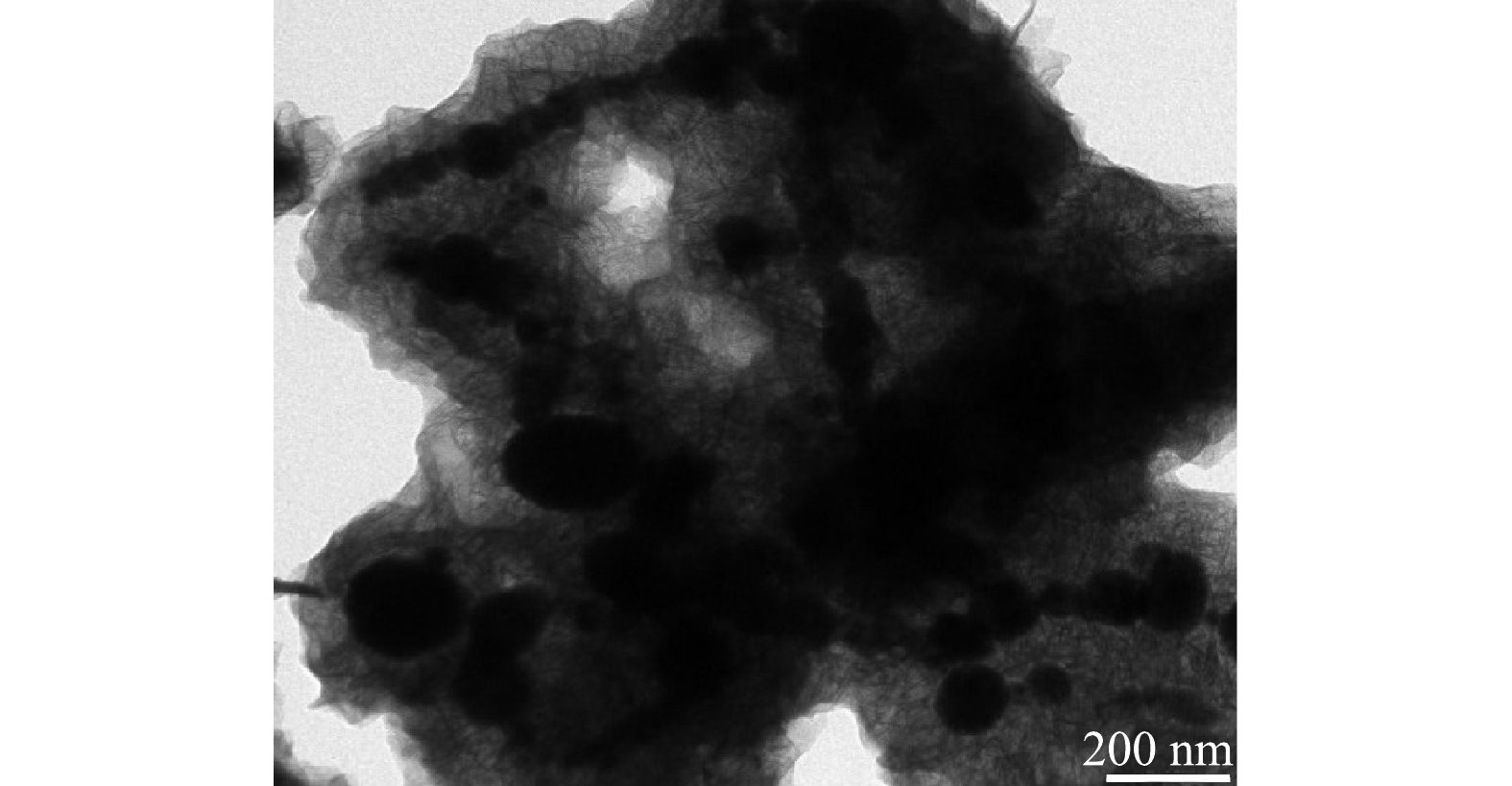
2) TEM分析。取适量上述制备的nZVI/黄铁矿粉末放入无水乙醇中,超声10 min后,用针管吸取少量混合液,滴在双联铜网上,在真空手套箱中干燥,进行TEM表征,表征结果见图2。将nZVI/黄铁矿放大25 000倍可明显观察到,黑色的nZVI颗粒的粒径在50~120 nm,并附着于黄铁矿上,表明nZVI成功的负载到了机械活化黄铁矿的表面,团聚现象也明显减少。
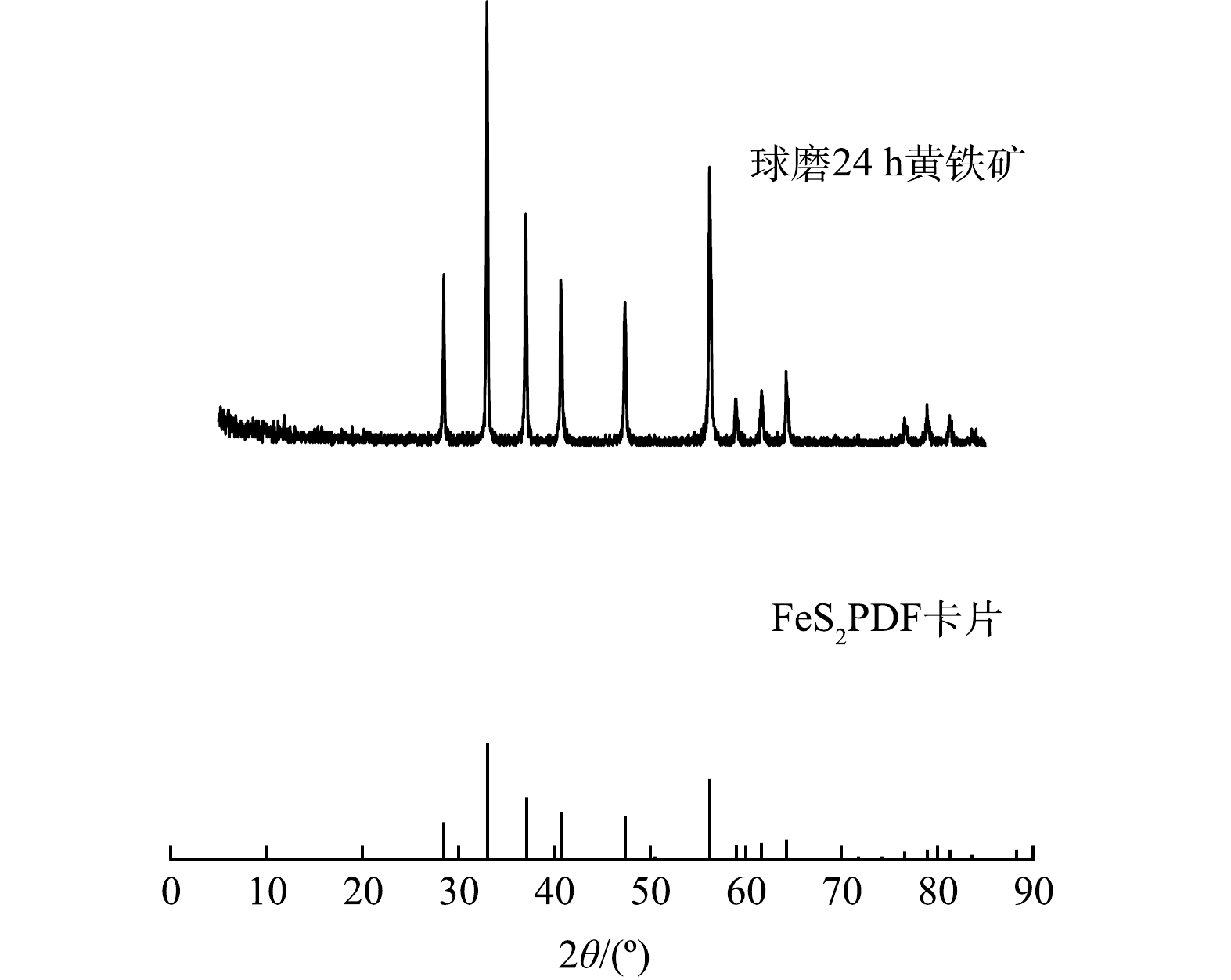
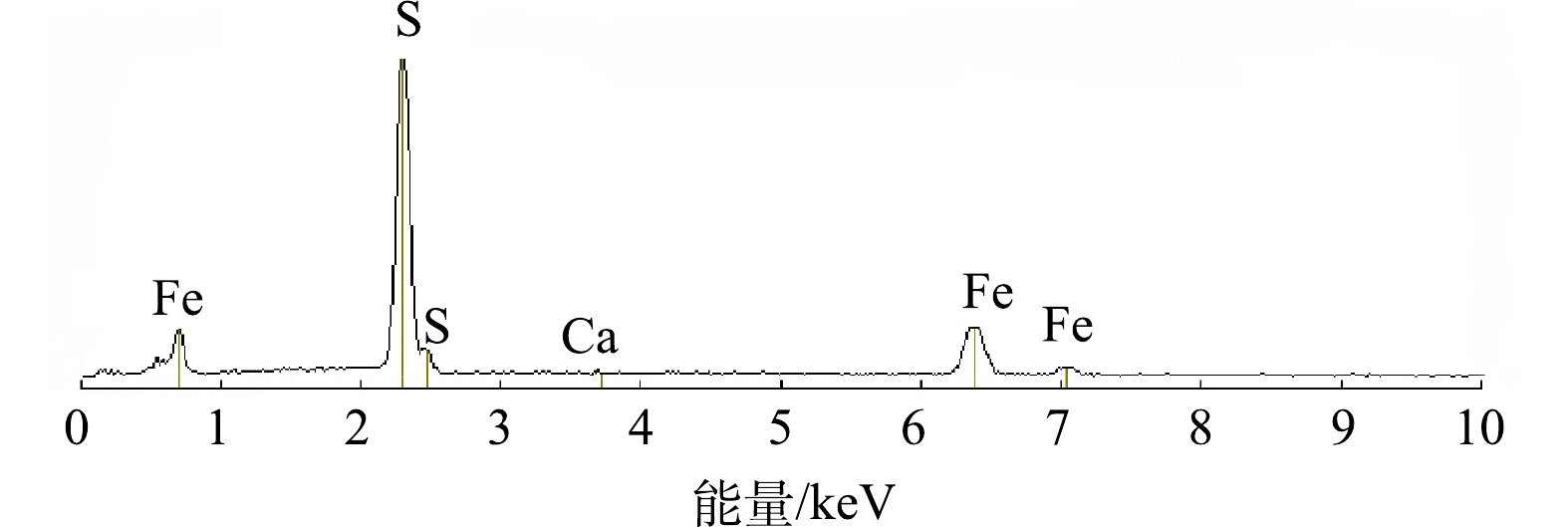
3) XRD和EDS分析。使用Cu Kα辐射(40 kV,30 mA),设置扫描速度为8o·min-1,衍射角(2θ)为5o~85o,进行XRD表征,表征结果如图3所示。通过与黄铁矿标准卡片的对比可看出,该天然黄铁矿杂质少,结晶度好,且机械活化后未产生其他物质。通过EDS对机械活化的天然黄铁矿进行定性分析,图4为微纳米级黄铁矿的EDS图谱,表1为元素含量分析结果。由图4中可看出,该黄铁矿含有Fe、S和Ca 3种元素,结合表1的元素分析结果可知,该黄铁矿的主要元素组成为Fe和S,还有微量的Ca,表明该黄铁矿纯度很高。
-
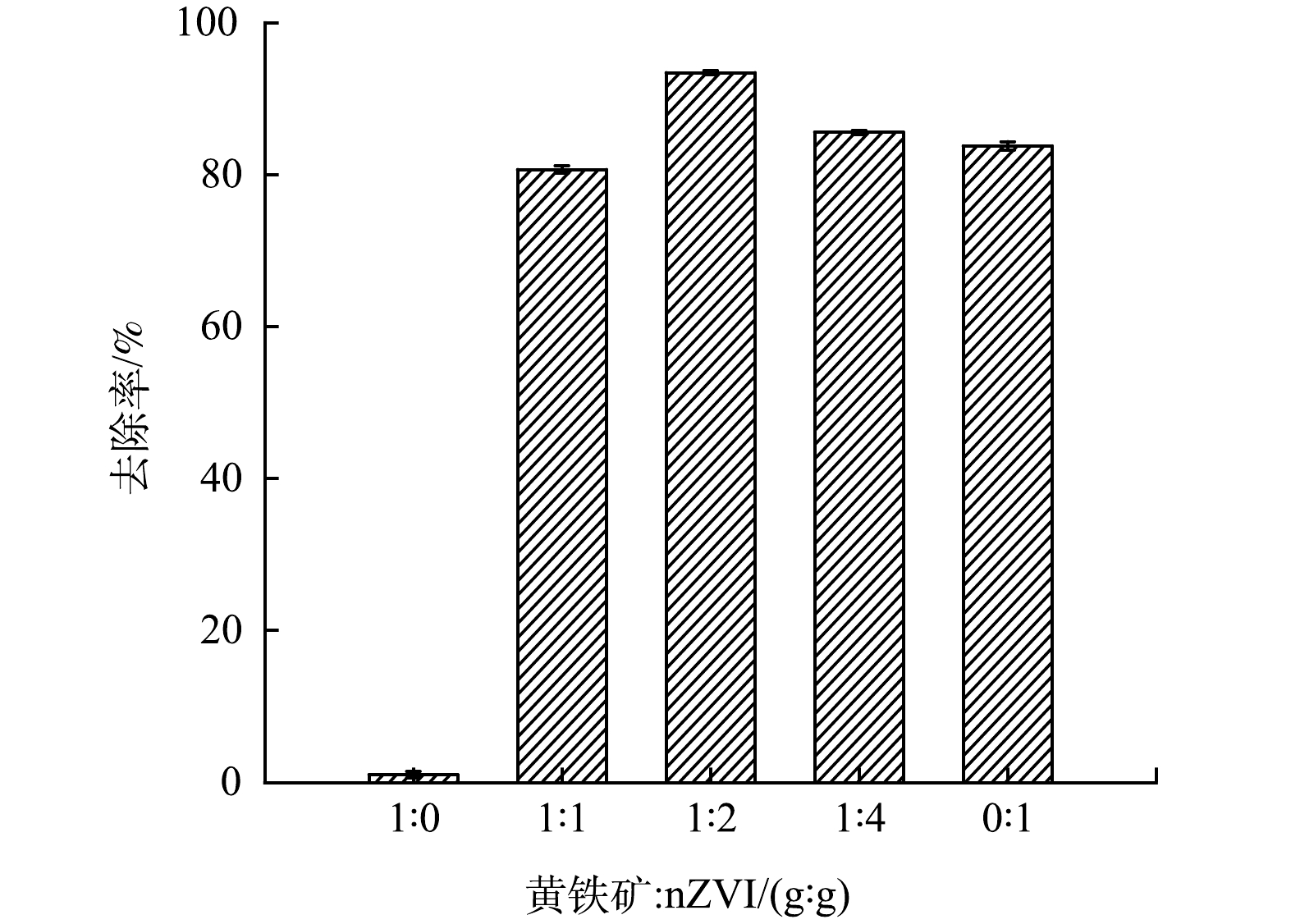
图5为黄铁矿:nZVI质量比分别为1:0、1:1、1:2、1:4和0:1的nZVI/黄铁矿对80.0 mg·L−1 Cr(Ⅵ)(初始pH=3.00,投加量为0.020 g)的去除效果。由图5可知,反应8.0 h后,质量比为1:1的nZVI/黄铁矿复合材料对Cr(Ⅵ)的去除率为80.6%,比nZVI的去除率(83.7%)低。这是因为复合材料中黄铁矿含量较多,0.020 g的黄铁矿对Cr(Ⅵ)去除率为1%,影响了nZVI/黄铁矿对Cr(Ⅵ)的去除效果;当质量比达到1:2时,复合材料对Cr(Ⅵ)的去除率为93.4%。这是因为nZVI在黄铁矿表面得到了很好的分散效果,比表面积增大。且黄铁矿表面产生的S22-发生S22-→S2-的还原过程,该过程产生FeS的附着于黄铁矿和nZVI表面,FeS的存在会加速nZVI的腐蚀,使得更多的nZVI与Cr(Ⅵ)进行接触反应,因此,在黄铁矿和nZVI协同的作用下,达到了较高的Cr(Ⅵ)去除率;当质量比为1:4时,去除率为85.5%,与nZVI的去除率相差不大,主要是因为过多的nZVI无法在黄铁矿表面均匀分布,且发生了团聚现象,被包覆的黄铁矿难以与nZVI发生协同作用,导致去除率降低。因此,后续实验复合材料的质量比均采用1:2,nZVI和nZVI/黄铁矿投加量均为0.020 g。
-
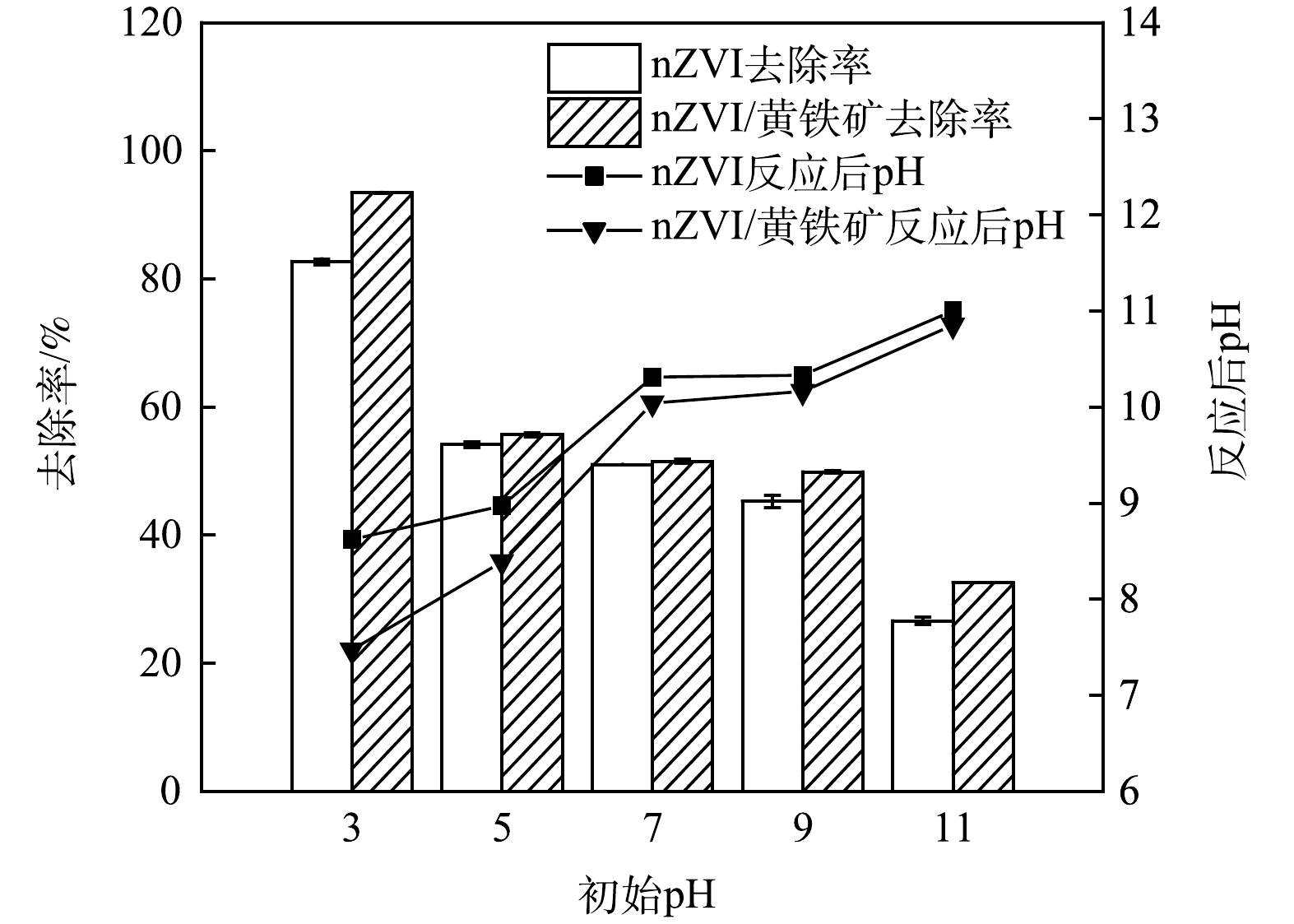
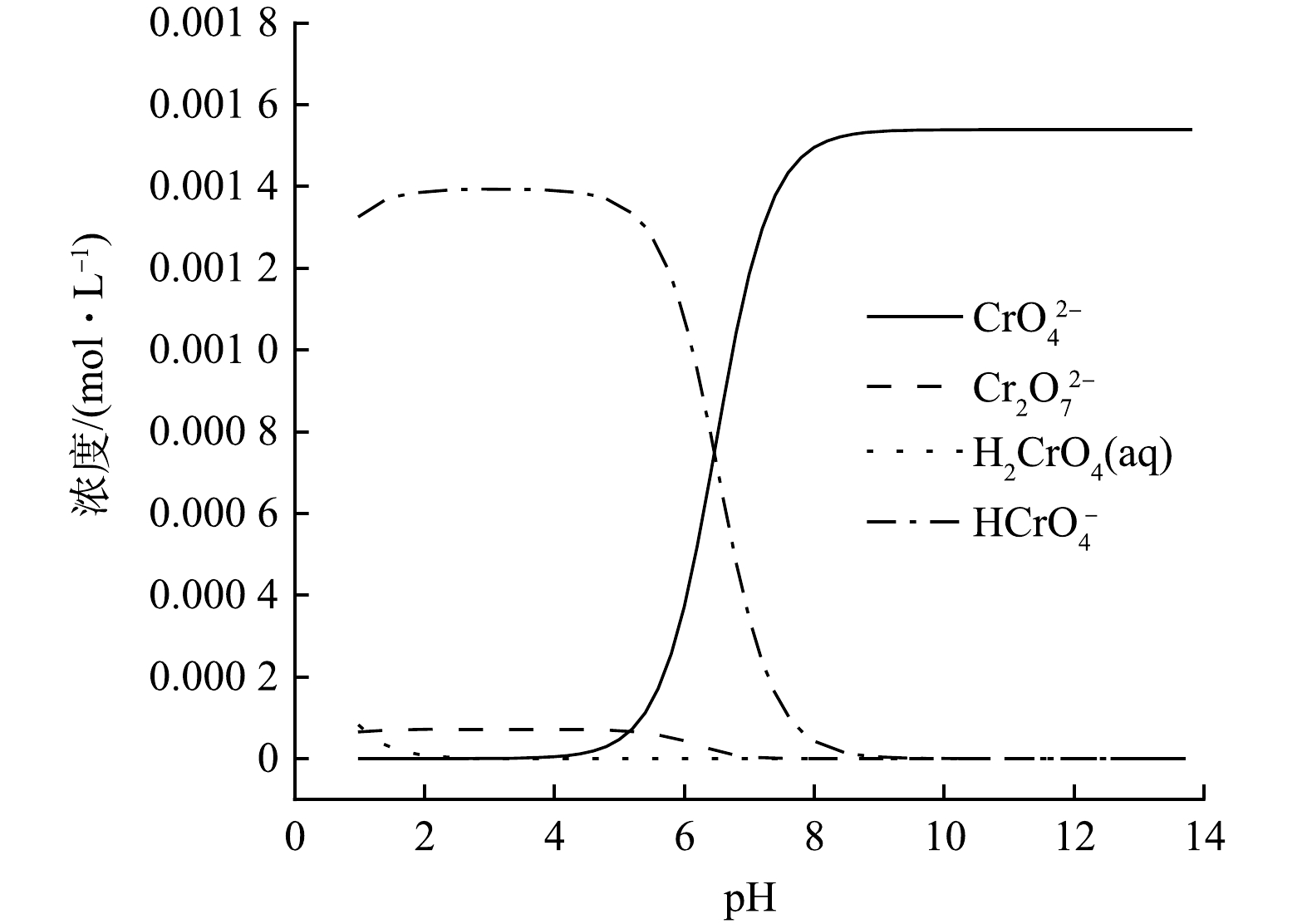
图6为反应8.0 h后不同初始pH(pH=3.00、5.00、7.00、9.00、11.00)对Cr(Ⅵ)(80.0 mg·L−1)去除效率的影响。由图6可明显观察到,随着pH的升高,nZVI和nZVI/黄铁矿的去除率不断下降。因为酸性条件下,Cr(Ⅵ)主要以HCrO4−的形式存在(图7),Cr(Ⅵ)的标准氧化还原电位(Eθ)为+1.33 V, 随着H+浓度升高,溶液pH降低,由氧化还原电位公式(式(1))可得,在一定温度下,给定
abB /arR 值,Cr(Ⅵ)的氧化还原电位(E)增加,Cr(Ⅵ)的氧化性增强,从而化学吸附显著增强。此外,在酸性条件下,Fe3+主要以离子态存在,不易生成Fe(OH)3沉淀,减少了nZVI表面的钝化,更易于接触到污染物,加强了物理吸附,物理吸附的加强又促进了化学吸附,从而导致去除率较高。而在碱性条件下,Cr(Ⅵ)主要以CrO42-的形式存在(图7),其标准氧化还原电位为-0.13 V,根据式(1)可知,随着pH的增加,氧化还原电位不断下降,Cr(Ⅵ)的氧化性减弱,从而化学吸附减弱;且当pH较高时,Fe3+会沉淀为Fe(OH)3,这些沉淀会附着在nZVI表面,形成钝化层,阻碍nZVI与污染物的接触,减弱了物理吸附,从而大幅影响对Cr(Ⅵ)的去除率。当初始pH=3.00时,nZVI/黄铁矿可去除93.4%的Cr(Ⅵ),nZVI对Cr(Ⅵ)去除率为82.6%。这是因为黄铁矿对nZVI具有良好的分散作用,使得nZVI可与污染物更好地接触,进一步增强了物理吸附能力,从而使得nZVI对Cr(Ⅵ)的去除效率进一步提升。而当初始pH为9.00和11.00时,nZVI/黄铁矿对Cr(Ⅵ)去除效率仍高于nZVI,主要是因为在碱性条件下,经过机械活化后的黄铁矿产生的Fe3+会与水体中的OH−离子反应,生成Fe(OH)3,Fe3+的水解使得水中H+的浓度上升,使得反应过程中的pH略微下降(图6),从而减轻nZVI表面的钝化,提升了nZVI对Cr(Ⅵ)的去除效率。结果表明,黄铁矿提升了nZVI在酸性和碱性条件下对Cr(Ⅵ)的去除能力。
-
在初始pH=3.00,投加量为0.020 g,Cr(Ⅵ)的初始质量浓度为70.0、80.0、90.0、100.0和110.0 mg·L−1,温度为25、35和45 ℃的条件下,测得反应12.0 h内指定时间剩余Cr(Ⅵ)的质量浓度,并通过式(2)计算qt。
式中:qt为t时的Cr(Ⅵ)吸附量,mg·g−1;c0为Cr(Ⅵ)的初始质量浓度,mg·L−1;ct为t时刻剩余Cr(Ⅵ)的质量浓度;mg·L−1V为Cr(Ⅵ)的体积,L;m为材料投加量,g。
在不同Cr(Ⅵ)初始质量浓度条件下,采用准一级(式(3))和准二级动力学(式(4))模型对反应过程进行拟合。
式中:qe为平衡时的Cr(Ⅵ)吸附量,mg·g−1;k1为准一级动力学方程吸附速率常数,min−1;k2为准二级动力学方程吸附速率常数,g·(mg∙min)−1。
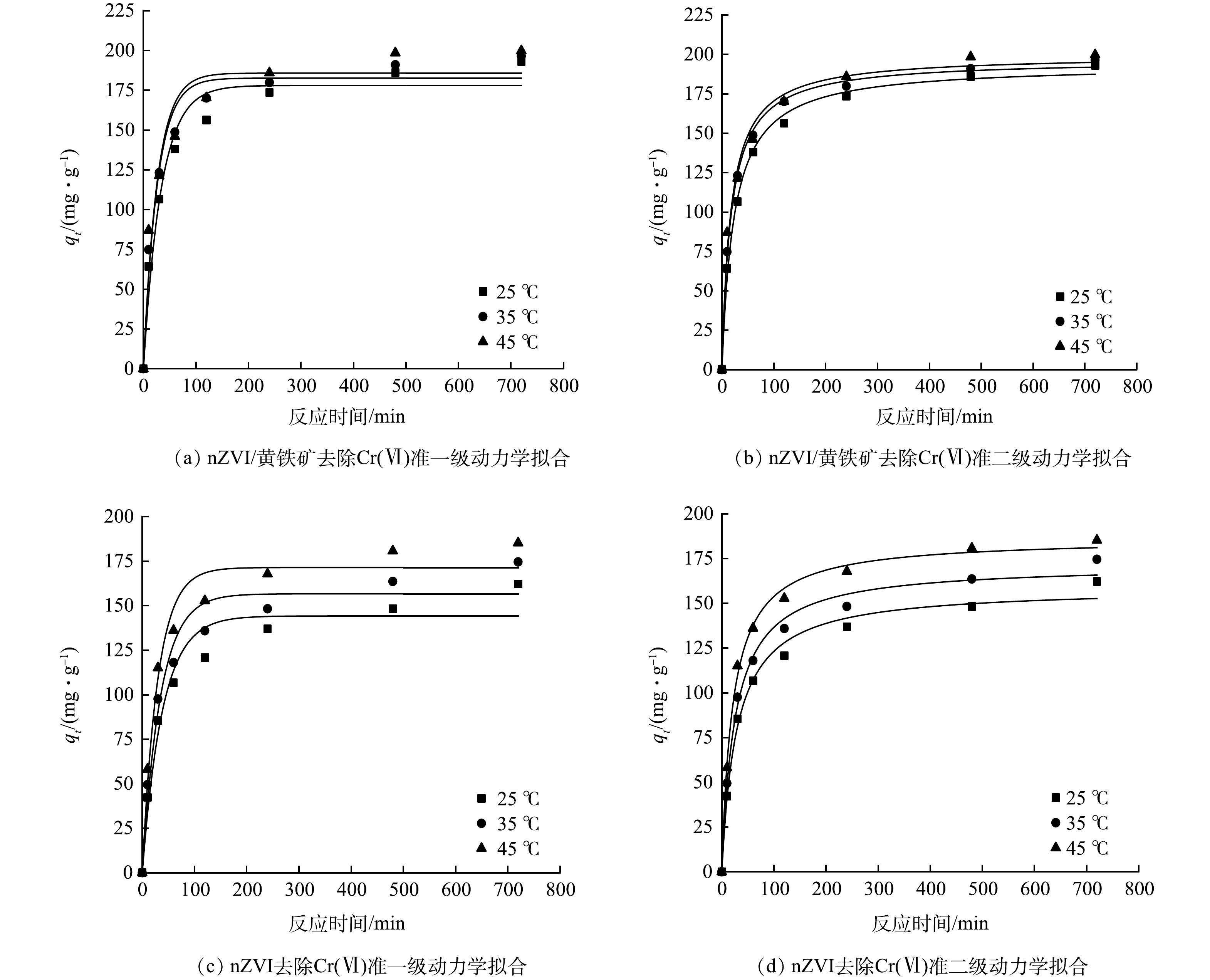
nZVI/黄铁矿和nZVI对Cr(Ⅵ)去除的准一级和准二级动力学拟合结果如图8和表2所示。根据表2可知,随着Cr(Ⅵ)初始质量浓度的上升,nZVI/黄铁矿和nZVI的准二级动力学速率常数(k2)逐渐下降,平衡吸附量逐渐上升。当Cr(Ⅵ)初始质量浓度大于100.0 mg·L−1时,nZVI去除Cr(Ⅵ)的k2明显低于nZVI/黄铁矿去除Cr(Ⅵ)的k2。这表明黄铁矿的负载提升了nZVI对较高质量浓度含Cr(Ⅵ)的去除性能。在不同初始质量浓度条件下,nZVI/黄铁矿和nZVI准二级动力学拟合的相关系数均大于准一级动力学拟合。且平衡吸附量的拟合结果与实际计算相差不大。因此,nZVI/黄铁矿和nZVI对Cr(Ⅵ)的去除过程可以用准二级动力学模型进行描述。表明去除过程是由物理吸附和化学吸附共同作用的,且化学吸附为主要限速步骤[18]。
在不同温度条件下,采用准一级动力和准二级动力学模型对反应过程进行拟合。从图9中可观察到,随着反应温度的上升,nZVI/黄铁矿和nZVI对Cr(Ⅵ)的去除效果逐渐上升。且当反应温度为25 ℃时,nZVI/黄铁矿对Cr(Ⅵ)的吸附量明显大于nZVI。根据表3可知,三种温度下的nZVI/黄铁矿和nZVI对Cr(Ⅵ)的去除更符合准二级动力学,R2 > 0.98,反应速率常数(k2)和平衡吸附量(qe)也随着温度的增加而增加。表明温度的上升有利于推动nZVI/黄铁矿对Cr(Ⅵ)氧化还原反应的进行。
-
采用Langmuir和Freundlich吸附等温线用来分析nZVI/黄铁矿对Cr(Ⅵ)的吸附平衡特征。Langmuir吸附等温线的方程如式(5)所示。Freundlich吸附等温线是一个基于非均相表面的经验方程,如式(6)所示。
式中:qe为平衡时的Cr(Ⅵ)吸附量,mg·g−1;qmax为完全单层覆盖的最大吸附量,mg·g−1;Ce为吸附平衡时溶液中离子质量浓度,mg·L−1;KL为Langmuir常数,L·mg−1。
式中:KF为Freundlich常数,mg·g−1(mg·L−1)−1/n;1/n为吸附指数。
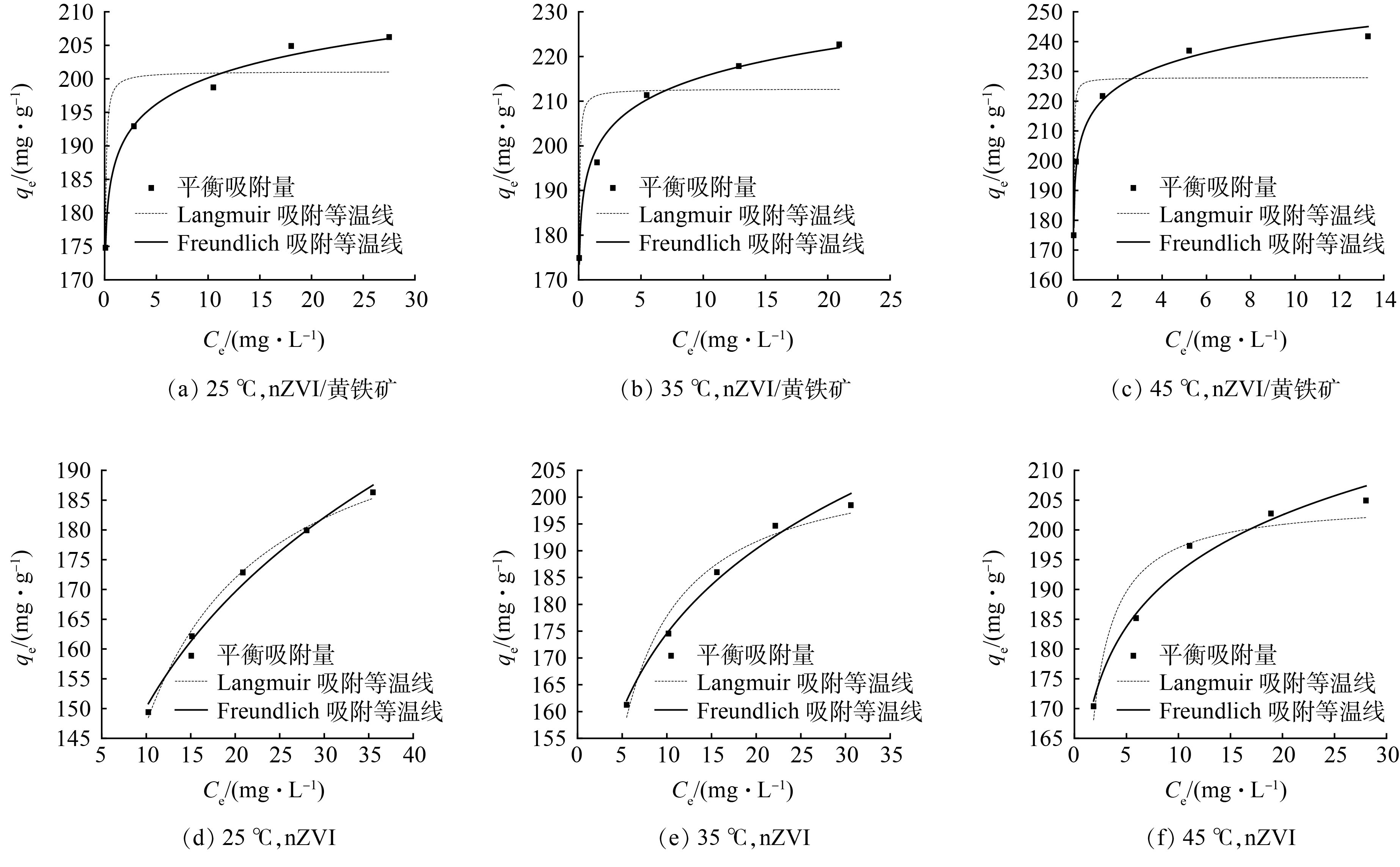
以qe为纵坐标,Ce为横坐标作图进行拟合。根据吸附等温线拟合结果(图10和表4)可知,在3种温度下,Freundlich吸附等温线的相关系数均大于Langmuir吸附等温线。因此,Freundlich吸附等温线更符合nZVI/黄铁矿和nZVI对Cr(Ⅵ)的吸附过程。表明nZVI/黄铁矿和nZVI对Cr(Ⅵ)的吸附可归因于多分子层吸附。从表4中可观察到, nZVI/黄铁矿的1/n 明显小于nZVI,1/n越小说明吸附过程越容易发生[19],因此,nZVI/黄铁矿比nZVI更容易吸附Cr(Ⅵ)。
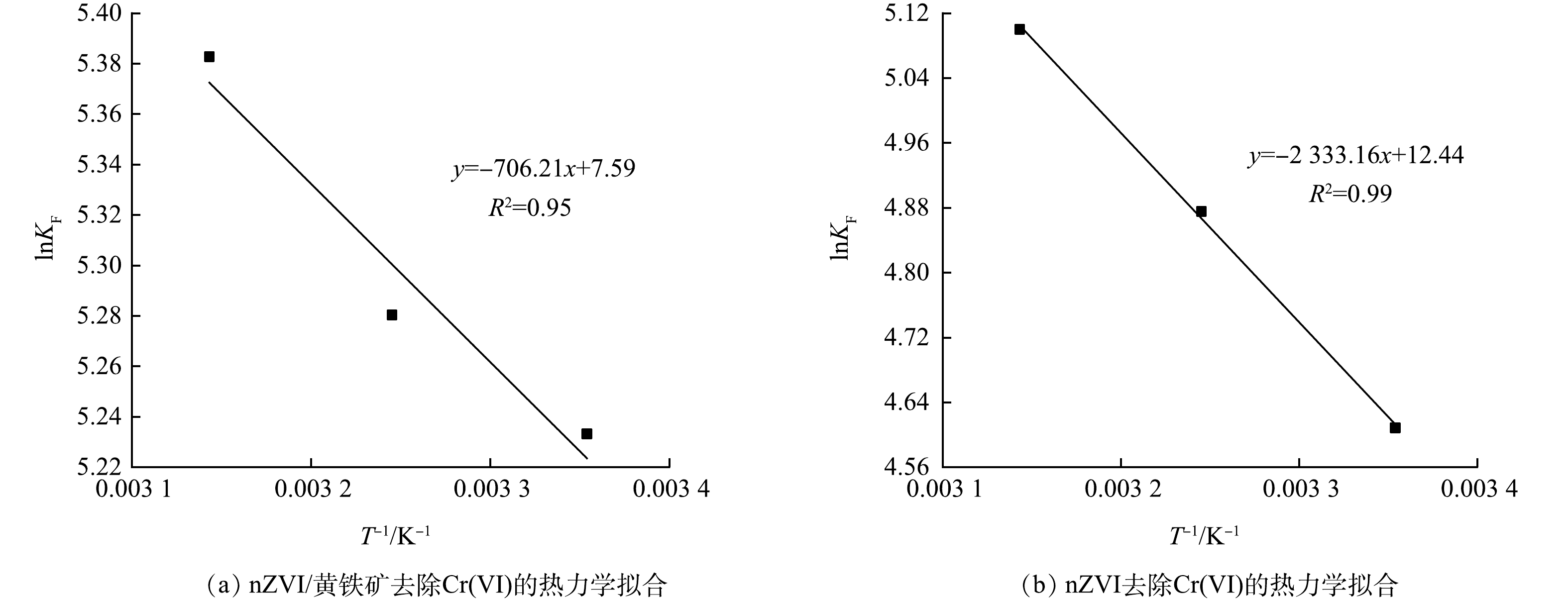
由于反应符合Freundlich吸附等温线,因此,使用KF作为范特霍夫方程的平衡常数[20-21]。利用式(8)计算吉布斯自由能,利用式(9)计算焓变和熵变。由式(7)和式(8)得范特霍夫方程(Van 't Hoff equation)(式(9))。
式中:ΔG为吉布斯自由能,kJ·mol−1;ΔH为焓变,kJ·mol−1;ΔS为熵变,kJ·(mol·K)−1;KF为Freundlich常数;R为理想气体常数,J·(mol·K)−1;T为绝对温度,K。
根据式(9),以lnKF为纵坐标,T−1为横坐标进行线性拟合。由图11(a)可知,nZVI/黄铁矿去除Cr(Ⅵ)的热力学拟合直线的斜率为−706.21,截距为7.59,因此,可计算ΔH为5.87 kJ·mol−1,ΔS为0.06 kJ·(mol·K)−1。由图11(b)可知,nZVI去除Cr(Ⅵ)的热力学拟合直线的斜率为−2 333.16,截距为12.44,因此,可计算ΔH为19.40 kJ·mol−1,ΔS为0.01 kJ·(mol·K)−1。由表5可知,nZVI/黄铁矿和nZVI对Cr(Ⅵ)的吸附的ΔS > 0,表明随着反应的进行,反应体系变的无序性和混乱性增加,固-液界面自由度增加[22]。ΔH > 0,表明反应过程是吸热反应。在3种温度下ΔG < 0,表明反应可以自发进行。且同一温度下,nZVI/黄铁矿ΔG小于nZVI,表明nZVI/黄铁矿去除Cr(Ⅵ)的反应更容易进行。
-
1)机械活化后的黄铁矿粒径在50~200 nm,微纳米级天然黄铁矿成功制备。该黄铁矿主要由Fe和S元素组成,且纯度很高。纳米零价铁(nZVI)附着于黄铁矿上,粒径在50~120 nm,表明微纳米级天然黄铁矿负载nZVI(nZVI/黄铁矿)成功制备,且黄铁矿对nZVI产生了良好的分散效果。
2) nZVI/黄铁矿中黄铁矿和nZVI最佳质量比为1:2。当初始pH=3.00、pH=9.00和pH=11.00时,nZVI/黄铁矿对80.0 mg·L−1 50.0 mL的Cr(Ⅵ)去除率明显高于nZVI,表明黄铁矿的存在加强了nZVI在酸性和碱性条件下对Cr(Ⅵ)去除能力。
3) nZVI/黄铁矿和nZVI对Cr(Ⅵ)去除过程均符合准二级动力学模型(R2 > 0.97),反应过程中既有物理吸附也有化学吸附。nZVI/黄铁矿和nZVI对Cr(Ⅵ)吸附更符合Freundlich等温吸附曲线(R2>0.97,1/n<1),表明吸附过程为极易发生的多层吸附过程。热力学分析表明反应体系中ΔG<0、ΔH>0、ΔS>0,反应为熵增吸热反应,可以自发进行。通过对比nZVI/黄铁矿和nZVI去除Cr(Ⅵ)的动力学、吸附等温线和热力学分析结果可得,nZVI/黄铁矿对Cr(Ⅵ)具有更好的吸附性能和还原固定效果,且对Cr(Ⅵ)的去除反应更容易进行。研究结果为天然黄铁矿和零价铁协同处理重金属提供了理论依据和反应机理支撑。
微纳米级天然黄铁矿负载nZVI去除水中Cr(Ⅵ)
Removal of Cr (VI) from water by micro-nano-scale natural pyrite loaded nZVI
-
摘要: 采用机械活化(球磨)的方式,制备了微纳米级天然黄铁矿。同时以此作为载体材料,通过液相还原法制备了微纳米级天然黄铁矿负载纳米零价铁(nZVI/黄铁矿)去除水中的Cr(VI)。分析了nZVI/黄铁矿中黄铁矿和nZVI的质量比、反应初始pH对Cr(Ⅵ)去除效果的影响,并结合动力学、吸附等温线和热力学的拟合结果,对反应机理进行了探讨。结果表明,在nZVI/黄铁矿去除Cr(Ⅵ)的实验中,黄铁矿和nZVI的最佳质量比为1:2,且黄铁矿的负载加强了nZVI在酸性和碱性条件下对Cr(Ⅵ)去除能力。除此之外,nZVI/黄铁矿和nZVI对Cr(Ⅵ)去除过程均符合准二级动力学模型和Freundlich等温吸附曲线,吸附过程由物理吸附和化学吸附共同控制,为极易发生的多层吸附。反应体系中ΔG<0、ΔH>0、ΔS>0,为熵增吸热反应,可以自发进行。nZVI/黄铁矿对Cr(Ⅵ)具有更好的吸附性能和还原固定效果,反应更容易进行。以上研究结果可为天然黄铁矿和零价铁协同处理重金属的实际应用提供技术支持。Abstract: In order to remove Cr(VI) from water, micro-nano-scale natural pyrite was prepared by mechanical activation (ball milling). At the same time, as a carrier material, micro-nano-scale natural pyrite loaded nano-zero-valent iron (nZVI/pyrite) was prepared by a liquid phase reduction method. The effects of the mass ratio of pyrite to nZVI in nZVI/pyrite and the initial pH of the reaction on the removal of Cr(VI) were analyzed. The reaction mechanism was discussed based on the fitting results of kinetics, adsorption isotherms and thermodynamics. The results show that in the experiment of Cr(VI) removal by nZVI/pyrite, the optimal mass ratio of pyrite to nZVI was 1:2, and the loading of pyrite enhanced the Cr(VI) removal ability of nZVI under acidic and alkaline conditions. In addition, the removal process of Cr(VI) by nZVI/pyrite and nZVI conformed to the pseudo-second-order kinetic model and Freundlich isothermal adsorption curve. The adsorption process was controlled by physical and chemical adsorption, and was a very easily occurred multi-layer adsorption. In the reaction system, ΔG < 0, ΔH > 0, ΔS > 0, which was an entropy increase endothermic reaction and could happened spontaneously. Moreover, nZVI/pyrite had a better adsorption performance and reduction fixation effect on Cr(VI), and the reaction was easier to happen than nZVI alone. The research results can provide a theoretical basis and technical support for the practical application of natural pyrite and nano-zero-valent iron in the synergistic treatment of heavy metals.
-
Key words:
- nano-zero-valent iron /
- natural pyrite /
- chromium /
- mechanical activation
-
铬(chromium, Cr)是水中典型的重金属污染物,主要来源于造纸、制革、冶金和染料等行业的废水排放[1]。铬在水体中以三价、四价和六价存在,Cr(Ⅲ)和Cr(Ⅵ)最稳定[2],Cr(Ⅵ)对生物的毒性是Cr(Ⅲ)的100~1 000倍[3],可诱发多种致癌性、诱变性疾病,如慢性溃疡、肝脏炎症、肾损伤、肺癌等[4]。因此,高效地去除水中的Cr(Ⅵ)是至关重要的。由于Cr(Ⅵ)具有极高的氧化性,通常将高毒性的Cr(Ⅵ)还原为低毒性的Cr(Ⅲ),作为Cr(Ⅵ)的有效去除方法[5]。
纳米零价铁(nano-zero-valent iron, nZVI)具有大的比表面积、丰富的活性位点、高的反应性和环境友好性[6],在去除水溶液中污染物方面具有很好的应用前景。但是,其易团聚、易钝化和迁移性差等缺陷使得单一的nZVI系统已经不能满足当今废水处理的实际需求[7]。复合型nZVI成为了近几年研究的热点。负载型、表面修饰型、硫化型和双金属型是常用的复合型nZVI技术。QIAN等[8]使用红麻棒、铁盐和绿茶提取物制备多孔生物炭,并制备了多孔生物炭负载的纳米零价铁(biochar loaded nano-zero-valent iron, BC-nZVI)。HE等[9]采用羧甲基纤维素(carboxymethyl cellulose, CMC)作为稳定剂,对nZVI进行了分散研究。LI等[10]采用球磨、真空化学气相沉积(vacuum chemical vapor deposition, CVD)和液相还原法制备出硫化纳米零价铁(sulfurized nano-zero-valent iron, S-nZVI)。HE,ZHAO[11]采用水溶性淀粉作为稳定剂,制备了淀粉稳定的nZVI粒子和Fe-Pd双金属纳米粒子。复合型nZVI对重金属、有机物和放射性核素等方面的有着良好的去除效果。
黄铁矿是自然界中常见的金属矿物,主要成分是FeS2,在尾矿中含量很大,尾矿中的黄铁矿会对周围环境形成污染[12]。但黄铁矿具有价格低和良好的电化学性质等特点。因此,黄铁矿的有效利用在环境治理方面具有广泛的研究前景。天然的黄铁矿S和Fe之间以共价键结合,具有良好的稳定性,反应活性较低[13]。使用机械活化可以将黄铁矿制备成微纳米级粉末,降低黄铁矿粒径,增加其晶格畸变率[14],使黄铁矿粒子表面的悬空未配位键和点缺陷等增多[15-16]。有研究[17]表明,使用天然黄铁矿和铁粉进行球磨,通过黄铁矿和铁粉的协同作用对Cr(Ⅵ)产生了良好的去除效果,但由于黄铁矿和铁粉活性较差、反应较慢,对于Cr(Ⅵ)含量高废水,需要消耗大量材料[18]。而化学合成的nZVI具有极强的反应活性,更容易和机械活化的黄铁矿产生协同作用。并且通过黄铁矿的负载可以减轻nZVI的团聚,从而提升nZVI对Cr(Ⅵ)的去除效果。除此之外,还可以对黄铁矿进行有效利用,同时减少硼氢化钠(NaBH4)的使用量,节约成本。基于此,本文利用乙醇作为介质,通过优化球料比、磨球数量和磨球大小比例,采用机械活化的方式,制备了微纳米级天然黄铁矿,以此作为载体材料,通过液相还原法制备了微纳米级天然黄铁矿负载nZVI(pyrite loaded nano-zero-valent iron, nZVI/黄铁矿),用于去除水体中的Cr(Ⅵ)。并通过动力学、等温线模型和热力学分析对Cr(Ⅵ)去除机理进行了探讨。
1. 材料与制备方法
1.1 实验材料
天然黄铁矿(北京水远山长矿物标本有限公司,采自云南石林西纳村);无水乙醇(C2H6O,AR,天津市德恩化学试剂有限公司);七水合硫酸亚铁(FeSO4·7H2O,AR,西陇化工股份有限公司);硼氢化钠(NaBH4,AR,天津市进丰化工有限公司);盐酸(HCl,AR);氢氧化钠(NaOH,AR);重铬酸钾(K2Cr2O7,AR,北京化工厂),高纯氮气和混合气(太原宜虹气体工业有限公司);实验所用水均为无氧超纯水。
1.2 实验仪器
Optima7300V型电感耦合等离子体发射光谱仪(inductively coupled plasma-optical emission spectrometer, ICP-OES,美国PE公司);COY低氧手套箱(天美科学仪器有限公司);QM-3SP04型PM系列行星式球磨机(卓的仪器设备上海有限公司);KQ-500DB型透射电子显微镜(transmission electron microscope, TEM,昆山舒美超声仪器有限公司);S-4800型扫描电子显微镜(scanning electron microscope, SEM,日本日立公司);纯水仪(MILLIPORE公司);XRD-6000型X射线衍射仪(X-ray diffraction, XRD,日本岛津公司);Noran system 7型X射线能谱仪(X-ray energy spectrum, EDS,美国Thermo Fisher科技有限公司)。
1.3 微纳米级天然黄铁矿的制备
将天然黄铁矿人工粉碎成小块状,放入1.0 mol·L−1的稀盐酸中浸泡1.0 h,以去除杂质和氧化物,洗涤和干燥后研磨至粉末状;按照球料比为10:1的比例,向50.0 mL的氧化锆球磨罐内加入相同数量直径为3 mm和8 mm的氧化锆磨球,再加入矿浆体积比为1:1的黄铁矿和无水乙醇,设置球磨机转速为450 r·min−1,机械活化24.0 h,再于无水中乙醇超声分散30.0 min,干燥后获得微纳米级天然黄铁矿,备用。
1.4 nZVI的制备
采用液相还原法制备nZVI。本研究使用500.0 mL三口烧瓶作为反应容器,并使用N2进行曝气。称取1.390 g FeSO4·7H2O溶于100.0 mL无氧水中,搅拌30 min,再以每秒1~2滴的速度,滴加过量的NaBH4溶液,至溶液变黑,继续搅拌20.0 min,以保证FeSO4·7H2O和NaBH4完全反应。反应完成后,转移至无氧手套箱内,通过磁选的方式,用无氧水和无水乙醇各洗3次,然后转移至培养皿中,在真空40 ℃的条件下,干燥8.0 h。得到黑色粉末,备用。
1.5 nZVI/黄铁矿的制备
分别称取0.280、0.140和0.070 g黄铁矿加入三口烧瓶内,加入50.0 mL无氧水,搅拌30.0 min。再称取1.390 g FeSO4·7H2O溶于50.0 mL无氧水中,加入三口烧瓶内,继续搅拌30.0 min,之后步骤同nZVI的制备方法,制备出质量比(黄铁矿:nZVI)为1:1、1:2和1:4 nZVI/黄铁矿复合材料。
1.6 nZVI/黄铁矿去除Cr(Ⅵ)实验
称取2.829 g的K2Cr2O7用无氧超纯水溶于1 000.0 mL的容量瓶中,配置成1.0 g·L−1的Cr(Ⅵ)储备液。实验所用的Cr(Ⅵ)污染物溶液均经1.0 g·L−1的Cr(Ⅵ)储备液稀释而来,稀释得70.0、80.0、90.0、100.0和110.0 mg·L−1的Cr(Ⅵ)污染物原液,备用。以50.0 mL的离心管作为反应容器,取设定质量浓度的Cr(Ⅵ)污染物原液50.0 mL于离心管中,并用1.0 mol·L−1、0.1 mol·L−1 HCl和1.0 mol·L−1、0.1 mol·L−1 NaOH溶液调节初始pH,称取0.020 g的nZVI/黄铁矿和nZVI于离心管中。将离心管置于旋转仪上,调整转速为40.0 r·min−1,在预设的时间点从离心管中取1.0 mL溶液,用0.22 μm滤膜过滤后,使用ICP-OES测量溶液中剩余Cr(Ⅵ)的质量浓度。除温度影响实验外,其余实验反应温度均为25 ℃。除初始pH影响实验外,其余实验反应初始pH均为3.00。
1.7 材料成本评估
nZVI的制备所使用的NaBH4和FeSO4·7H2O分别是天然黄铁矿价格的5 800倍和160倍。以天然黄铁矿和nZVI质量比为1:2计算,可减少约1/3的NaBH4和FeSO4·7H2O的使用量,相当于减少了约1/3的成本。这表明该材料具有良好的经济效益。
1.8 数据处理
通过去除率判断nZVI/黄铁矿对Cr(Ⅵ)的去除能力。根据准一级和准二级动力学方程分析反应动力学过程,使用Langmuir和Freundlich吸附等温式和热力学参数评价吸附过程。
2. 结果与分析
2.1 材料表征与分析
1) SEM分析。在真空手套箱内,将导电胶带粘在样品台上,再使用棉签粘取适量上述制备的nZVI/黄铁矿粉末轻弹于导电胶带上,用洗耳球吹去未黏住的样品,并对样品进行1 min的喷金处理,进行SEM表征,表征结果如图1所示。将nZVI/黄铁矿放大50 000倍可看出,机械活化后的黄铁矿呈块状和片层状,粒径在50~200 nm。nZVI呈球形和椭球形,原本光滑的表面变得粗糙。表明黄铁矿的负载使得nZVI的表面结构发生了改变,产生了铁硫化物。铁硫化物可与nZVI建立良好的电子传递路径[17],在去除污染物的过程中,加快nZVI的腐蚀可提升污染物的去除效率。
2) TEM分析。取适量上述制备的nZVI/黄铁矿粉末放入无水乙醇中,超声10 min后,用针管吸取少量混合液,滴在双联铜网上,在真空手套箱中干燥,进行TEM表征,表征结果见图2。将nZVI/黄铁矿放大25 000倍可明显观察到,黑色的nZVI颗粒的粒径在50~120 nm,并附着于黄铁矿上,表明nZVI成功的负载到了机械活化黄铁矿的表面,团聚现象也明显减少。
3) XRD和EDS分析。使用Cu Kα辐射(40 kV,30 mA),设置扫描速度为8o·min-1,衍射角(2θ)为5o~85o,进行XRD表征,表征结果如图3所示。通过与黄铁矿标准卡片的对比可看出,该天然黄铁矿杂质少,结晶度好,且机械活化后未产生其他物质。通过EDS对机械活化的天然黄铁矿进行定性分析,图4为微纳米级黄铁矿的EDS图谱,表1为元素含量分析结果。由图4中可看出,该黄铁矿含有Fe、S和Ca 3种元素,结合表1的元素分析结果可知,该黄铁矿的主要元素组成为Fe和S,还有微量的Ca,表明该黄铁矿纯度很高。
表 1 天然黄铁矿的元素分析Table 1. Elements analysis of natural pyrite元素 质量占比/% 质量占比误差/% 原子占比/% 原子占比误差/% S 53.36 ±0.75 66.53 ±0.89 Ca 0.27 ±0.16 0.29 ±0.15 Fe 46.37 ±0.89 33.18 ±0.60 2.2 不同质量比nZVI/黄铁矿对Cr(Ⅵ)去除率的影响
图5为黄铁矿:nZVI质量比分别为1:0、1:1、1:2、1:4和0:1的nZVI/黄铁矿对80.0 mg·L−1 Cr(Ⅵ)(初始pH=3.00,投加量为0.020 g)的去除效果。由图5可知,反应8.0 h后,质量比为1:1的nZVI/黄铁矿复合材料对Cr(Ⅵ)的去除率为80.6%,比nZVI的去除率(83.7%)低。这是因为复合材料中黄铁矿含量较多,0.020 g的黄铁矿对Cr(Ⅵ)去除率为1%,影响了nZVI/黄铁矿对Cr(Ⅵ)的去除效果;当质量比达到1:2时,复合材料对Cr(Ⅵ)的去除率为93.4%。这是因为nZVI在黄铁矿表面得到了很好的分散效果,比表面积增大。且黄铁矿表面产生的S22-发生S22-→S2-的还原过程,该过程产生FeS的附着于黄铁矿和nZVI表面,FeS的存在会加速nZVI的腐蚀,使得更多的nZVI与Cr(Ⅵ)进行接触反应,因此,在黄铁矿和nZVI协同的作用下,达到了较高的Cr(Ⅵ)去除率;当质量比为1:4时,去除率为85.5%,与nZVI的去除率相差不大,主要是因为过多的nZVI无法在黄铁矿表面均匀分布,且发生了团聚现象,被包覆的黄铁矿难以与nZVI发生协同作用,导致去除率降低。因此,后续实验复合材料的质量比均采用1:2,nZVI和nZVI/黄铁矿投加量均为0.020 g。
2.3 初始pH对Cr(Ⅵ)去除率的影响
图6为反应8.0 h后不同初始pH(pH=3.00、5.00、7.00、9.00、11.00)对Cr(Ⅵ)(80.0 mg·L−1)去除效率的影响。由图6可明显观察到,随着pH的升高,nZVI和nZVI/黄铁矿的去除率不断下降。因为酸性条件下,Cr(Ⅵ)主要以HCrO4−的形式存在(图7),Cr(Ⅵ)的标准氧化还原电位(Eθ)为+1.33 V, 随着H+浓度升高,溶液pH降低,由氧化还原电位公式(式(1))可得,在一定温度下,给定
abB arR 当初始pH=3.00时,nZVI/黄铁矿可去除93.4%的Cr(Ⅵ),nZVI对Cr(Ⅵ)去除率为82.6%。这是因为黄铁矿对nZVI具有良好的分散作用,使得nZVI可与污染物更好地接触,进一步增强了物理吸附能力,从而使得nZVI对Cr(Ⅵ)的去除效率进一步提升。而当初始pH为9.00和11.00时,nZVI/黄铁矿对Cr(Ⅵ)去除效率仍高于nZVI,主要是因为在碱性条件下,经过机械活化后的黄铁矿产生的Fe3+会与水体中的OH−离子反应,生成Fe(OH)3,Fe3+的水解使得水中H+的浓度上升,使得反应过程中的pH略微下降(图6),从而减轻nZVI表面的钝化,提升了nZVI对Cr(Ⅵ)的去除效率。结果表明,黄铁矿提升了nZVI在酸性和碱性条件下对Cr(Ⅵ)的去除能力。
stringUtils.convertMath(!{formula.content}) (1) 2.4 动力学分析
在初始pH=3.00,投加量为0.020 g,Cr(Ⅵ)的初始质量浓度为70.0、80.0、90.0、100.0和110.0 mg·L−1,温度为25、35和45 ℃的条件下,测得反应12.0 h内指定时间剩余Cr(Ⅵ)的质量浓度,并通过式(2)计算qt。
stringUtils.convertMath(!{formula.content}) (2) 式中:qt为t时的Cr(Ⅵ)吸附量,mg·g−1;c0为Cr(Ⅵ)的初始质量浓度,mg·L−1;ct为t时刻剩余Cr(Ⅵ)的质量浓度;mg·L−1V为Cr(Ⅵ)的体积,L;m为材料投加量,g。
在不同Cr(Ⅵ)初始质量浓度条件下,采用准一级(式(3))和准二级动力学(式(4))模型对反应过程进行拟合。
stringUtils.convertMath(!{formula.content}) (3) stringUtils.convertMath(!{formula.content}) (4) 式中:qe为平衡时的Cr(Ⅵ)吸附量,mg·g−1;k1为准一级动力学方程吸附速率常数,min−1;k2为准二级动力学方程吸附速率常数,g·(mg∙min)−1。
nZVI/黄铁矿和nZVI对Cr(Ⅵ)去除的准一级和准二级动力学拟合结果如图8和表2所示。根据表2可知,随着Cr(Ⅵ)初始质量浓度的上升,nZVI/黄铁矿和nZVI的准二级动力学速率常数(k2)逐渐下降,平衡吸附量逐渐上升。当Cr(Ⅵ)初始质量浓度大于100.0 mg·L−1时,nZVI去除Cr(Ⅵ)的k2明显低于nZVI/黄铁矿去除Cr(Ⅵ)的k2。这表明黄铁矿的负载提升了nZVI对较高质量浓度含Cr(Ⅵ)的去除性能。在不同初始质量浓度条件下,nZVI/黄铁矿和nZVI准二级动力学拟合的相关系数均大于准一级动力学拟合。且平衡吸附量的拟合结果与实际计算相差不大。因此,nZVI/黄铁矿和nZVI对Cr(Ⅵ)的去除过程可以用准二级动力学模型进行描述。表明去除过程是由物理吸附和化学吸附共同作用的,且化学吸附为主要限速步骤[18]。
表 2 不同初始质量浓度下nZVI/黄铁矿和nZVI对Cr(VI)的吸附动力学参数Table 2. Adsorption kinetic parameters of nZVI/pyrite and nZVI to Cr(VI) at different initial mass concentrations材料 c0/(mg·L−1) 准一级动力学 准二级动力学 k1/(min−1) qe/(mg·g−1) R2 k2/(g·(mg∙min)−1) qe/(mg·g−1) R2 nZVI/黄铁矿 70 0.042 87 170.114 0.96 0.000 39 180.673 0.99 80 0.029 69 177.949 0.96 0.000 22 193.522 0.99 90 0.023 54 182.501 0.96 0.000 16 200.588 0.99 100 0.022 89 182.593 0.95 0.000 15 201.090 0.98 110 0.022 82 185.211 0.94 0.000 14 205.297 0.98 nZVI 70 0.044 46 139.088 0.95 0.000 48 148.170 0.99 80 0.025 74 144.170 0.95 0.000 22 158.760 0.99 90 0.022 48 160.690 0.97 0.000 18 176.352 0.99 100 0.012 48 159.316 0.93 0.000 08 182.419 0.97 110 0.010 57 167.112 0.95 0.000 06 194.791 0.98 在不同温度条件下,采用准一级动力和准二级动力学模型对反应过程进行拟合。从图9中可观察到,随着反应温度的上升,nZVI/黄铁矿和nZVI对Cr(Ⅵ)的去除效果逐渐上升。且当反应温度为25 ℃时,nZVI/黄铁矿对Cr(Ⅵ)的吸附量明显大于nZVI。根据表3可知,三种温度下的nZVI/黄铁矿和nZVI对Cr(Ⅵ)的去除更符合准二级动力学,R2 > 0.98,反应速率常数(k2)和平衡吸附量(qe)也随着温度的增加而增加。表明温度的上升有利于推动nZVI/黄铁矿对Cr(Ⅵ)氧化还原反应的进行。
表 3 不同温度下nZVI/黄铁矿和nZVI对Cr(VI)的吸附动力学参数Table 3. Adsorption kinetics parameters of nZVI/pyrite and nZVI for Cr(VI) at different temperatures材料 温度/ ℃ 准一级动力学 准二级动力学 k1/(min−1) qe/(mg·g−1) R2 k2/( g·(mg∙min)−1) qe/(mg·g−1) R2 nZVI/黄铁矿 25 0.029 69 177.949 0.96 0.000 22 193.522 0.99 35 0.037 98 182.560 0.96 0.000 28 196.636 0.99 45 0.038 07 185.723 0.93 0.000 29 199.543 0.98 nZVI 25 0.025 74 144.170 0.95 0.000 22 158.760 0.99 35 0.028 53 156.597 0.96 0.000 23 171.621 0.99 45 0.033 65 171.351 0.97 0.000 25 186.333 0.99 2.5 吸附等温线和热力学分析
采用Langmuir和Freundlich吸附等温线用来分析nZVI/黄铁矿对Cr(Ⅵ)的吸附平衡特征。Langmuir吸附等温线的方程如式(5)所示。Freundlich吸附等温线是一个基于非均相表面的经验方程,如式(6)所示。
stringUtils.convertMath(!{formula.content}) (5) 式中:qe为平衡时的Cr(Ⅵ)吸附量,mg·g−1;qmax为完全单层覆盖的最大吸附量,mg·g−1;Ce为吸附平衡时溶液中离子质量浓度,mg·L−1;KL为Langmuir常数,L·mg−1。
stringUtils.convertMath(!{formula.content}) (6) 式中:KF为Freundlich常数,mg·g−1(mg·L−1)−1/n;1/n为吸附指数。
以qe为纵坐标,Ce为横坐标作图进行拟合。根据吸附等温线拟合结果(图10和表4)可知,在3种温度下,Freundlich吸附等温线的相关系数均大于Langmuir吸附等温线。因此,Freundlich吸附等温线更符合nZVI/黄铁矿和nZVI对Cr(Ⅵ)的吸附过程。表明nZVI/黄铁矿和nZVI对Cr(Ⅵ)的吸附可归因于多分子层吸附。从表4中可观察到, nZVI/黄铁矿的1/n 明显小于nZVI,1/n越小说明吸附过程越容易发生[19],因此,nZVI/黄铁矿比nZVI更容易吸附Cr(Ⅵ)。
表 4 Langmuir和Freundlich吸附等温线拟合参数Table 4. Langmuir and Freundlich adsorption isotherm fitting parameters材料 温度/ ℃ Langmuir Freundlich KL/(L·mg−1) qmax/(mg·g−1) R2 KF/(mg·g−1(mg·L−1)−1/n) 1/n R2 nZVI/黄铁矿 25 79.160 0 201.099 0.84 187.394 0.028 58 0.99 35 98.416 3 212.737 0.76 196.441 0.040 19 0.99 45 252.956 227.934 0.75 217.623 0.045 87 0.99 nZVI 25 0.252 15 206.006 0.99 100.342 0.175 19 0.99 35 0.589 78 207.936 0.96 131.043 0.124 58 0.98 45 2.472 81 204.977 0.90 164.061 0.070 30 0.97 由于反应符合Freundlich吸附等温线,因此,使用KF作为范特霍夫方程的平衡常数[20-21]。利用式(8)计算吉布斯自由能,利用式(9)计算焓变和熵变。由式(7)和式(8)得范特霍夫方程(Van 't Hoff equation)(式(9))。
stringUtils.convertMath(!{formula.content}) (7) stringUtils.convertMath(!{formula.content}) (8) 式中:ΔG为吉布斯自由能,kJ·mol−1;ΔH为焓变,kJ·mol−1;ΔS为熵变,kJ·(mol·K)−1;KF为Freundlich常数;R为理想气体常数,J·(mol·K)−1;T为绝对温度,K。
stringUtils.convertMath(!{formula.content}) (9) 根据式(9),以lnKF为纵坐标,T−1为横坐标进行线性拟合。由图11(a)可知,nZVI/黄铁矿去除Cr(Ⅵ)的热力学拟合直线的斜率为−706.21,截距为7.59,因此,可计算ΔH为5.87 kJ·mol−1,ΔS为0.06 kJ·(mol·K)−1。由图11(b)可知,nZVI去除Cr(Ⅵ)的热力学拟合直线的斜率为−2 333.16,截距为12.44,因此,可计算ΔH为19.40 kJ·mol−1,ΔS为0.01 kJ·(mol·K)−1。由表5可知,nZVI/黄铁矿和nZVI对Cr(Ⅵ)的吸附的ΔS > 0,表明随着反应的进行,反应体系变的无序性和混乱性增加,固-液界面自由度增加[22]。ΔH > 0,表明反应过程是吸热反应。在3种温度下ΔG < 0,表明反应可以自发进行。且同一温度下,nZVI/黄铁矿ΔG小于nZVI,表明nZVI/黄铁矿去除Cr(Ⅵ)的反应更容易进行。
表 5 nZVI/黄铁矿和nZVI去除Cr(Ⅵ)的热力学拟合参数Table 5. Thermodynamic fitting parameters for the removal of Cr(Ⅵ) by nZVI/pyrite and nZVI材料 温度/K ΔH/(kJ·mol−1) ΔS/(kJ·(mol·K)−1) ΔG/(kJ·mol−1) nZVI/黄铁矿 298.15 5.87 0.06 −12.95 308.15 −13.58 318.15 −14.21 nZVI 298.15 19.40 0.10 −10.42 308.15 −11.42 318.15 −12.42 3. 结论
1)机械活化后的黄铁矿粒径在50~200 nm,微纳米级天然黄铁矿成功制备。该黄铁矿主要由Fe和S元素组成,且纯度很高。纳米零价铁(nZVI)附着于黄铁矿上,粒径在50~120 nm,表明微纳米级天然黄铁矿负载nZVI(nZVI/黄铁矿)成功制备,且黄铁矿对nZVI产生了良好的分散效果。
2) nZVI/黄铁矿中黄铁矿和nZVI最佳质量比为1:2。当初始pH=3.00、pH=9.00和pH=11.00时,nZVI/黄铁矿对80.0 mg·L−1 50.0 mL的Cr(Ⅵ)去除率明显高于nZVI,表明黄铁矿的存在加强了nZVI在酸性和碱性条件下对Cr(Ⅵ)去除能力。
3) nZVI/黄铁矿和nZVI对Cr(Ⅵ)去除过程均符合准二级动力学模型(R2 > 0.97),反应过程中既有物理吸附也有化学吸附。nZVI/黄铁矿和nZVI对Cr(Ⅵ)吸附更符合Freundlich等温吸附曲线(R2>0.97,1/n<1),表明吸附过程为极易发生的多层吸附过程。热力学分析表明反应体系中ΔG<0、ΔH>0、ΔS>0,反应为熵增吸热反应,可以自发进行。通过对比nZVI/黄铁矿和nZVI去除Cr(Ⅵ)的动力学、吸附等温线和热力学分析结果可得,nZVI/黄铁矿对Cr(Ⅵ)具有更好的吸附性能和还原固定效果,且对Cr(Ⅵ)的去除反应更容易进行。研究结果为天然黄铁矿和零价铁协同处理重金属提供了理论依据和反应机理支撑。
-
表 1 天然黄铁矿的元素分析
Table 1. Elements analysis of natural pyrite
元素 质量占比/% 质量占比误差/% 原子占比/% 原子占比误差/% S 53.36 ±0.75 66.53 ±0.89 Ca 0.27 ±0.16 0.29 ±0.15 Fe 46.37 ±0.89 33.18 ±0.60 表 2 不同初始质量浓度下nZVI/黄铁矿和nZVI对Cr(VI)的吸附动力学参数
Table 2. Adsorption kinetic parameters of nZVI/pyrite and nZVI to Cr(VI) at different initial mass concentrations
材料 c0/(mg·L−1) 准一级动力学 准二级动力学 k1/(min−1) qe/(mg·g−1) R2 k2/(g·(mg∙min)−1) qe/(mg·g−1) R2 nZVI/黄铁矿 70 0.042 87 170.114 0.96 0.000 39 180.673 0.99 80 0.029 69 177.949 0.96 0.000 22 193.522 0.99 90 0.023 54 182.501 0.96 0.000 16 200.588 0.99 100 0.022 89 182.593 0.95 0.000 15 201.090 0.98 110 0.022 82 185.211 0.94 0.000 14 205.297 0.98 nZVI 70 0.044 46 139.088 0.95 0.000 48 148.170 0.99 80 0.025 74 144.170 0.95 0.000 22 158.760 0.99 90 0.022 48 160.690 0.97 0.000 18 176.352 0.99 100 0.012 48 159.316 0.93 0.000 08 182.419 0.97 110 0.010 57 167.112 0.95 0.000 06 194.791 0.98 表 3 不同温度下nZVI/黄铁矿和nZVI对Cr(VI)的吸附动力学参数
Table 3. Adsorption kinetics parameters of nZVI/pyrite and nZVI for Cr(VI) at different temperatures
材料 温度/ ℃ 准一级动力学 准二级动力学 k1/(min−1) qe/(mg·g−1) R2 k2/( g·(mg∙min)−1) qe/(mg·g−1) R2 nZVI/黄铁矿 25 0.029 69 177.949 0.96 0.000 22 193.522 0.99 35 0.037 98 182.560 0.96 0.000 28 196.636 0.99 45 0.038 07 185.723 0.93 0.000 29 199.543 0.98 nZVI 25 0.025 74 144.170 0.95 0.000 22 158.760 0.99 35 0.028 53 156.597 0.96 0.000 23 171.621 0.99 45 0.033 65 171.351 0.97 0.000 25 186.333 0.99 表 4 Langmuir和Freundlich吸附等温线拟合参数
Table 4. Langmuir and Freundlich adsorption isotherm fitting parameters
材料 温度/ ℃ Langmuir Freundlich KL/(L·mg−1) qmax/(mg·g−1) R2 KF/(mg·g−1(mg·L−1)−1/n) 1/n R2 nZVI/黄铁矿 25 79.160 0 201.099 0.84 187.394 0.028 58 0.99 35 98.416 3 212.737 0.76 196.441 0.040 19 0.99 45 252.956 227.934 0.75 217.623 0.045 87 0.99 nZVI 25 0.252 15 206.006 0.99 100.342 0.175 19 0.99 35 0.589 78 207.936 0.96 131.043 0.124 58 0.98 45 2.472 81 204.977 0.90 164.061 0.070 30 0.97 表 5 nZVI/黄铁矿和nZVI去除Cr(Ⅵ)的热力学拟合参数
Table 5. Thermodynamic fitting parameters for the removal of Cr(Ⅵ) by nZVI/pyrite and nZVI
材料 温度/K ΔH/(kJ·mol−1) ΔS/(kJ·(mol·K)−1) ΔG/(kJ·mol−1) nZVI/黄铁矿 298.15 5.87 0.06 −12.95 308.15 −13.58 318.15 −14.21 nZVI 298.15 19.40 0.10 −10.42 308.15 −11.42 318.15 −12.42 -
[1] LIU B, XIN Y N, ZOU J, et al. Removal of chromium species by adsorption: fundamental principles, newly developed adsorbents and future perspectives[J]. Molecules, 2023, 28(2): 639. doi: 10.3390/molecules28020639 [2] EHSANPOUR S, SAMANI M R, TOGHRAIE D. Removal of chromium (VI) from aqueous solution using eggshell/poly pyrrole composite[J]. Alexandria Engineering Journal, 2023, 64: 581-589. doi: 10.1016/j.aej.2022.09.018 [3] YANG X, LIU L H, ZHANG M Z, et al. Improved removal capacity of magnetite for Cr(VI) by electrochemical reduction[J]. Journal of Hazardous Materials, 2019, 374: 26-34. doi: 10.1016/j.jhazmat.2019.04.008 [4] BULIN C, LI B, ZHANG Y, et al. Removal performance and mechanism of nano α-Fe2O3/graphene oxide on aqueous Cr(Ⅵ)[J]. Journal of Physics and Chemistry of Solids, 2020, 147: 109659. doi: 10.1016/j.jpcs.2020.109659 [5] QIU Y, ZHANG Q, GAO B, et al. Removal mechanisms of Cr(VI) and Cr(III) by biochar supported nanosized zero-valent iron: synergy of adsorption, reduction and transformation [J]. Environmental Pollution, 2020, 265(Pt B): 115018. [6] TANG H, WANG J, ZHANG S, et al. Recent advances in nanoscale zero-valent iron-based materials: characteristics, environmental remediation and challenges[J]. Journal of Cleaner Production, 2021, 319: 128641. doi: 10.1016/j.jclepro.2021.128641 [7] WANG P, FU F, LIU T. A review of the new multifunctional nano zero-valent iron composites for wastewater treatment: emergence, preparation, optimization and mechanism[J]. Chemosphere, 2021, 285: 131435. doi: 10.1016/j.chemosphere.2021.131435 [8] QIAN W, LIANG J Y, ZHANG W X, et al. A porous biochar supported nanoscale zero-valent iron material highly efficient for the simultaneous remediation of cadmium and lead contaminated soil[J]. Journal of environmental sciences, 2021, 113: 231-241. [9] HE F, ZHAO D Y. Manipulating the size and dispersibility of zerovalent iron nanoparticles by use of carboxymethyl cellulose stabilizers[J]. Environmental Science & Technology, 2007, 41(17): 6216-6221. [10] LI L, JIN HW, LUO N, et al. Sulfurized nano zero-valent iron prepared via different methods: effect of stability and types of surface corrosion products on removal of 2, 4, 6-trichlorophenol[J]. Ecotoxicology and Environmental Safety, 2023, 256: 114864. doi: 10.1016/j.ecoenv.2023.114864 [11] HE F, ZHAO D Y. Preparation and characterization of a new class of starch-stabilized bimetallic nanoparticles for degradation of chlorinated hydrocarbons in water[J]. Environmental Science & Technology, 2005, 39(9): 3314-3320. [12] JEFFERSON M, YENIAL-ARSLAN U, EVANS C, et al. Effect of pyrite textures and composition on flotation performance: A review[J]. Minerals Engineering, 2023, 201: 108234. doi: 10.1016/j.mineng.2023.108234 [13] 丁峰. 微纳米级天然黄铁矿还原固定水中铼的研究[D]. 太原: 太原科技大学, 2015. [14] 丁庆伟, 秦莹莹, 罗学强. 纳米天然黄铁矿对土壤和地下水中铬的原位固定技术[J]. 环境工程学报, 2020, 14(9): 2568-2575. [15] 丁峰, 钱天伟, 丁庆伟, 等. 不同pH下纳米级天然黄铁矿对水中ReO4−的去除规律[J]. 环境工程学报, 2016, 10(1): 55-59. doi: 10.12030/j.cjee.20160109 [16] 崔晋艳, 钱天伟, 丁庆伟, 等. 纳米级天然黄铁矿去除水中Cr6+, Cd2+和Pb2+[J]. 环境工程学报, 2016, 10(12): 7103-7108. doi: 10.12030/j.cjee.201507166 [17] HE X Y, MIN X B, PENG T Y, et al. Mechanochemically activated microsized zero-valent iron/pyrite composite for effective hexavalent chromium sequestration in aqueous solution[J]. Journal of chemical and engineering data, 2020, 65(4): 1936-1945. doi: 10.1021/acs.jced.9b01110 [18] ZHANG R M, NAPOLANO R, XI B D, et al. Mechanistic insights into Cr(VI) removal by a combination of zero-valent iron and pyrite[J]. Chemosphere, 2023, 330: 138693. doi: 10.1016/j.chemosphere.2023.138693 [19] KHAN M, ALI F, RAMZAN S, et al. N-phenyl acrylamide-incorporated porous silica-bound graphene oxide sheets with excellent removal capacity for Cr(iii) and Cr(vi) from wastewater[J]. RSC Advances, 2023, 13(24): 16047-16066. doi: 10.1039/D3RA02568C [20] YANG J, YU M, QIU T. Adsorption thermodynamics and kinetics of Cr(VI) on KIP210 resin[J]. Journal of Industrial & Engineering Chemistry, 2014, 20(2): 480-486. [21] 姜传东, 黄玮, 丛玉凤, 等. 载铜活性炭吸附二甲基硫醚的热力学和动力学[J]. 石油炼制与化工, 2021, 52(8): 64-70. doi: 10.3969/j.issn.1005-2399.2021.08.015 [22] 马芳, 白妙妍, 毛仁俊, 等. 茶树枝叶制备生物炭负载纳米零价铁净化水中Cr(Ⅵ)[J]. 农业工程学报, 2023, 39(11): 212-219. -




 下载:
下载: