-
我国作为世界印染和纺织大国,有机染料废水的排放成为一个普遍问题. 有机染料分子中大多含显色基团,还原后易形成有毒的胺,不仅会破坏生态系统,也会对人类身体健康带来健康风险[1]. 因此,有机染料废水的处理具有重要意义.
目前,许多技术被应用于有机染料废水的处理,如复合纳米纤维材料吸附[2]、光催化[3]、膜吸附[4]、电凝吸附[5]、超分子包合技术[6]等,但这些技术或多或少存在处理时间长、操作成本高、二次污染等弊端. 因此,开发高效、便捷、环境友好且价格低廉的染料废水处理技术至关重要. 传统技术对于染料的去除为物理转移,存在不能将染料彻底氧化降解,或降解效率极低等问题. 因此将废水中的染料富集后再氧化降解可有效提升染料废水的处理效率.
电容去离子(capacitive deionization, CDI)是基于双电层(electric double layer, EDL)或法拉第电容(faradic capacitor)基本原理[7],实现淡化水质的一种新型脱盐技术,具有装置操作简单、能耗低等优势,除了应用在海水淡化,也逐渐应用在去除有机污染物方面. Bayram等[8]利用CDI去除废水中的芳香醇有机酸,Senoussi等[9]采用CDI(BM CDI)首次去除并回收纺织废水中的阳离子型染料,Tang等[10]利用流动电极电容去离子同时分馏、脱盐和去除染料,都证明CDI可以有效处理废水中的有机物. 但CDI在解吸过程中的同离子效应降低了系统的电荷效率. Kim等[11]在CDI装置两电极表面加入厚度约为20 μm的阴、阳离子交换膜,形成了膜电容去离子(membrane capacitive deionization,MCDI). MCDI利用离子交换膜对离子的选择性可大大提高系统的电荷效率[12].
然而,目前并没有膜电容去离子应用于染料去除的相关研究. 其原因可能是染料属于大分子有机物,经离子交换过膜困难,处理效率低. 因此可通过只加阳离子交换膜(cation exchange membrane, CEM),组建半膜MCDI装置. 碳毡(carbon felt, CF)是一种由聚丙烯腈基(PAN)碳纤维[13]经预氧化、低温碳化、高温碳化后形成的稳定致密碳纤维材料,高纯无污染,具有优异的电化学性能[14],其表面丰富的介孔/大孔结构,突破了微孔材料的扩散限值,更有利于实现对大分子有机物的充分吸附,是较好的电极材料[15]. 研究表明碳毡电极作为电极材料时,在电场作用下,可原位产生H2O2[16],但需经活化后才能产生具有直接氧化作用的羟基自由基,而铁氧化物是活化H2O2产生活性自由基的常用催化剂. 此外,铁的掺杂不仅扩大CF内部缺陷,增加活性位点,提供更多反应空间,而且促进羟基自由基的产生[17],实现染料废水中有机物的氧化降解. 同时,优化MCDI装置结构,构建更适合去除阴离子型染料的半膜MCDI装置,可显著提高对染料的富集、降解速率.
本研究制备并详细表征了碳毡-铁氧化物复合电极,并构建了半膜MCDI装置. 考察了铁碳掺杂比、电压、染料浓度、pH等运行参数对去除染料的影响,确定了在一定范围内最佳去除条件,为MCDI去除废水中的有机染料提供参考依据,为其在有机染料盐废水中有机物和离子的同时去除建立基础.
-
CF购自内蒙古万兴碳素有限公司,刚果红染料(congo red, CR)、新胭脂红染料(new carmine, NC)、日落黄染料(sunset yellow FCF, SY FCF)购自山东德彦化工有限公司,草酸高铁铵,三水合物(Ⅲ)(ammonium iron(Ⅲ) oxalate trihydrate, 98%)购自阿法埃莎(中国)化学有限公司,所有试剂均为分析纯,且未经二次处理.
-
分别称量
0.9893 、0.3297 、0.2473 、0 g草酸高铁铵,三水合物于250 mL烧杯中,加入40 mL超纯水充分溶解. 裁剪数个3 mm×45 mm×50 mm(约0.9893 g)大小的CF,经超纯水充分洗涤至洗涤液电导率为0 μS·cm−1,置于烘箱75 ℃烘干. 最后放入真空管式炉(CHY-1200 ,河南成仪设备科技有限公司)中碳化,碳化过程采用两段升温程序,以氮气作为保护气,先以5 ℃ min−1升温至500 ℃,保温30 min,再以10 ℃·min−1继续升温到800 ℃,保温2 h. 冷却后得到1:1、1:3、1:4和无铁掺杂四种不同铁碳质量比的材料CFs,分别命名为CF-Fe(50%)、CF-Fe(25%)、CF-Fe(20%)、CF-Fe(0%). -
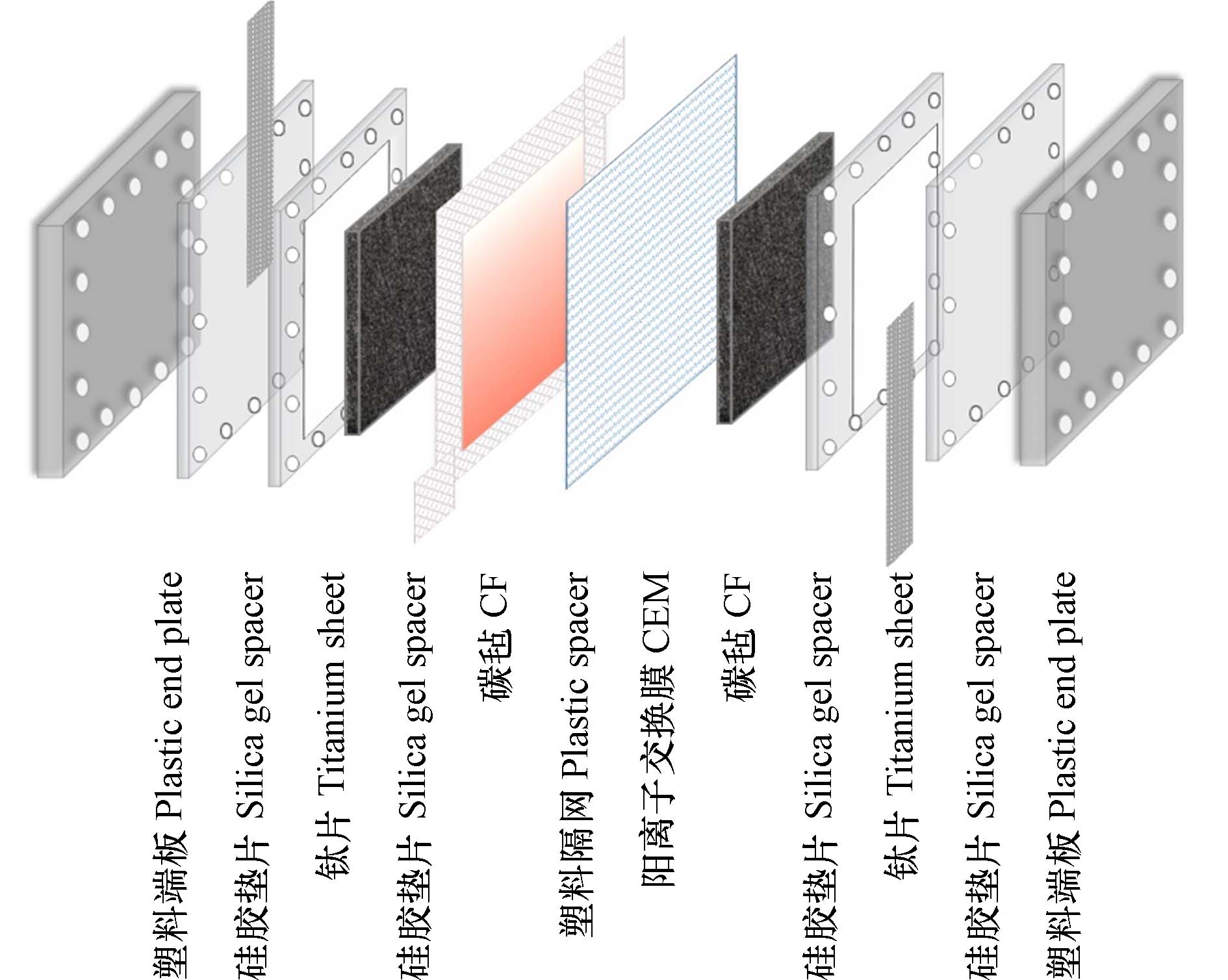
实验装置由塑料端板、硅胶垫片、钛片、硅胶垫片、CF、4.5 cm×5 cm塑料隔网(中间腔室)、CEM、CF、硅胶垫片、钛片、硅胶垫片、塑料端板依次排列,最终用螺丝进行装置固定,组成只加一侧CEM的新型半膜-MCDI装置(如图1). 采用序批模式(Batch)进水40 mL,利用蠕动泵(Longer Pump BT100-1L, 保定兰格恒流源有限公司)以3 mL·min−1流速控制阴离子型染料下进上出,流经中间腔室,正负极两端伸出钛片,电化学工作站(瑞士万通 MAC00589)连接钛片对装置进行加电,电导率仪(S475-UMIX,梅特勒Seven Excellence)实时监测染料离子浓度变化(如图2).
-
用扫描电子显微镜(SEM, Hitachi S48000, Japan)观察CFs的表面形貌,采用X射线光电子能谱仪(XPS, Thermo Scientific, France)分析改性CFs的元素组成及官能团,采用X射线衍射仪(XRD, SmartLab9KW, Japan)研究铁氧化物的有效掺杂以及CFs材料的结晶特性.
-
对电极材料CFs进行循环伏安(CV)和电化学阻抗谱(EIS)测试. 采用三电极体系,其中CFs电极为工作电极,饱和甘汞为参比电极,铂电极为对电极,在1 mol·L−1的NaCl电解液中进行. CV测试在50 mV·s−1扫描速率,扫描窗口为−0.6—0.6 V下进行. EIS测试过程在0 V偏置电压下,施加±5 mV交流电,在0.1—105 Hz的频率范围内进行检测. CV曲线的比电容根据式(1)计算:
式中:C为比电容,F·g−1;a为扫速,mV·s−1;Va和Vc分别为低电位截止值和高电位截止值,V;m为电极材料的质量,g;I为电流,A.
-
分别用超纯水配制不同浓度的CR、NC、SY FCF母液避光保存,称取某一染料溶液40 mL置于总体积为100 mL的锥形瓶中,在温度为298 K下,采用单一变量法,探究CFs材料(CF-Fe(50%)、CF-Fe(25%)、CF-Fe(20%)、CF-Fe(0%)),不同运行电压(0.6 V、1.2 V、1.8 V)、不同进水染料浓度(0.05、0.1、0.3 g·L−1)和不同pH(3、7、11)对不同阴离子型染料的去除情况. 实验加电15 min,每隔3 min,从锥形瓶中吸取1 mL染料于离心管中,稀释至30 mL,摇匀避光保存. 用紫外分光光度仪(TU-1810, 北京普析通用仪器有限责任公司)于最佳波长处定量测定染料溶液吸光度,根据绘制的染料浓度与吸光度的标准曲线计算染料去除率,并根据总有机碳分析仪(TOC-VCPN, Shimadzu, Japan)测定剩余染料中有机物浓度. 通过反向加电的方式进行5次吸附、解吸实验,验证材料的循环稳定性. 以5,5-二甲基- 1-吡咯啉-N-氧化物(DMPO)作为·OH捕获剂,采用电子顺磁共振波谱仪(EPR)(SPINSCAN X, ADANI, Belarus)测定DMPO-·OH信号及其强度. 采用LC-MS/MS检测CR降解的中间产物,色谱分析采用超高效液相色谱(Ultimate
3000 UHPLC, Dinonex, USA),使用 Eclipse Plus C18 色谱柱,流动相为A:0.1%甲酸溶液,B:乙腈,梯度洗脱. 质谱分析采用质谱仪(Q Exactive, Thermo Scientific, USA),采用离子源HESI进行正离子模式进行电离. 主扫描分辨率为70000,范围为50—600 m/z;二次扫描分辨率为17500,起始离子为50 m/z. 为保证数据科学可靠性,每组实验至少重复2次进行. 染料的吸附容量和去除率如式(2)、(3)计算:式中:qt为吸附容量(mg·g−1),C0为染料初始浓度(mg·L−1),Ct为吸附某时刻的染料浓度(mg·L−1),V代表染料溶液体积(mL),m为电极材料的质量(g),R为去除率(%).
-
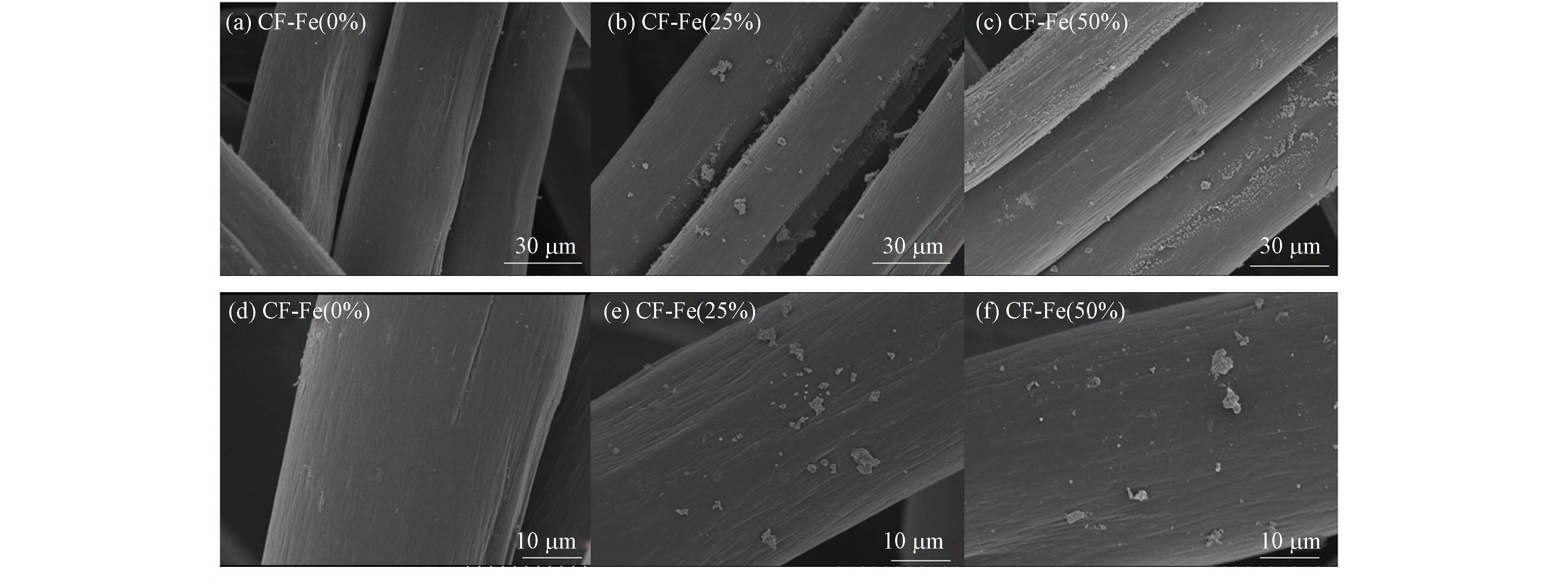
对CFs的形貌表征如图3所示,图3(a)、(d)为CF-Fe(0%),表面整体光滑,无任何杂质. 图3(b)、(e)为CF-Fe(25%),(c)、(f)为CF-Fe(50%)材料,可以看出碳毡上明显有块状物质负载,且铁掺杂比例越高,被铁氧化物覆盖的CFs面积也越大. 同时CF纤维表面存在明显沟槽,有细条纹,这可能是由于高温干燥碳化过程中氮原子和碳原子的演变,高温促进了石墨结构清晰的径向梯度分布,纤维发生横向物理收缩[18].
-
XRD谱图如图4(a)、(b)所示,电极材料在衍射角24.4°和43.4°出现了两个宽衍射峰,分别对应石墨碳纤维晶格平面(002)和(100)的晶面衍射. 随着铁元素掺杂比例的增加,在30°到45°之间,CF-Fe(50%)和CF-Fe(25%)电极材料出现四处衍射峰,分别对应铁氧化物在(104)、(110)、(311)、(220)晶格表面的衍射[19](如图4(b)),证明了铁氧化物的成功负载. 复合材料的催化性能与铁元素价态密不可分,因此采用XPS进一步测定CF和CF-Fe(25%)材料的化学组成和化学成分,从图(c)中确定了C、O、Fe的3个核心元素,表明铁氧化物成功负载在CF上.
图4(d)为Fe元素2p轨道的XPS高分辨图谱,观察到707.2、710.9、713.1、717.8、725.7、728 eV下6个峰,分别为Fe0 2p1/2、Fe3+ 2p1/2、Fe2+ 2p1/2、Fe0 2p3/2、Fe2+ 2p3/2、Fe3+ 2p3/2自旋轨道[20],表明Fe掺杂以多种价态形式存在.
-
由图5(a)可知,对CFs材料进行了电容特性表征. 观察到CF-Fe(20%)、CF-Fe(25%)和CF-Fe(50%)材料的CV曲线均存在轻微的氧化还原峰,说明复合材料在扫描电压窗口下具有接受/失去电子的能力,会发生氧化还原反应;且图像整体较为对称,说明复合材料具有良好的电化学稳定性;其中CF-Fe(25%)与其他材料相比,CV曲线的积分面积最大,一般CV曲线的积分面积代表电荷存储容量即比电容[21],说明铁氧化物掺杂为电极材料提供了更高的比表面积和更多的活性位点,因此CF-Fe(25%)材料的电容性能更优异.
CFs的EIS(如图5(b))表现出典型的电容行为,由一个半圆和一条斜线组成[22]. 一般认为,高频区半圆表示电子传递阻力,低频区的线表示离子扩散速率[23]. 与其他材料相比,CF-Fe(25%)材料半圆最小,说明其电子传递阻力最小;CF-Fe(25%)材料斜率最大,说明其离子迁移速率也最高. 而CF-Fe(0%)基本看不到圆弧,可以得出铁氧化物的负载增加了材料的电子传递阻力.
-
图6(a)为4种CFs下对CR染料的去除效果,可以看出CF-Fe(25%)材料对CR的去除率明显大于其他材料,在15 min内,1.2 V电压下,对300 mg·L−1的CR染料去除率达到45.03%. 图6(b)为不同浓度梯度下对CR的全波长扫描图,可知CR最大吸收波长为505 nm,并观察到,CR的吸光度随着CR的去除而逐渐降低,颜色也随之变浅,这证明CR染料被有效吸附去除. 通过测定反应前后的总有机碳(TOC)含量,可以得出对总有机碳的矿化率达69.40%(如图6(c)). 图6(d)为CR的吸光度与浓度的标准曲线,其中R2=0.999,接近于1 .
-
如图7(a),外加电压对染料去除性能有着显著影响,施加电压为零,即仅靠物理吸附时对CR的去除率约为24.6%,证明CF-Fe(25%)对CR具有一定的物理吸附能力,而在施加电压后,吸附速率明显增加,在1.2 V电压下CR的去除率达到了66.3%,1.8 V电压下去除率为72.5%. 因此可以得出电压与CR染料的去除率呈正相关,这可能是高电压可提供强大的静电驱动力,增强电极对染料的静电吸附作用[24],从而促进离子的转移,加快染料的吸附降解. 但是当电压超过1.2 V时,会产生水解副反应从而增加系统能耗,因此1.2 V电压作为最优电压.
由图7(b)可知,CF-Fe(25%)材料对100 mg·L−1CR的去除率最高,对50 mg·L−1CR去除率最低,可能是由于低浓度CR前期以物理吸附为主,去除率较慢;9 min后随着阳极产生羟基自由基含量增多,去除率也随之增大;300 mg·L−1 CR由于浓度过高,1.2 V电压下产生活性自由基数量有限,加之初始浓度高,因此去除率偏低. 因此,选择100 mg·L−1 CR进行后续实验.
如图7(c)可知,过碱的环境不利于对CR染料的去除,在碱性环境中,CR表面的负电荷会与羟基自由基形成静电排斥,阻止自由基与CR的相互作用,使得脱色率下降[25]. 酸性和中性环境有利于对CR的去除,且在pH在3—5范围内具有更好的去除效果,可能是因为在酸性环境中,氢离子增多,加快与氧气生成过氧化氢,从而增加了羟基自由基的生成量,从而提升对CR的去除效果[26]. 但在15 min内,pH在3—11范围内对CR的去除没有明显差别,说明MCDI去除CR等阴离子型染料对进水水质的要求并不高. 图7(d)得到在1.2 V电压,流速3 mL·min−1下,pH 7的进水环境中,对100 mg·L−1的CR、NC、SY FCF在15 min内分别达到66.3%、61.6%和60.7%的去除效果. 图7(e)、(f)为不同浓度梯度下对NC和SY FCF的全波长扫描图,可知最大吸收波长分别为506 nm和482 nm,NC和SY FCF的吸光度与浓度的标准曲线如图7(g)、(h),R2均为0.999.
-
电极材料的循环稳定性也是CDI水处理的关键指标. 经过5次吸附-解吸循环实验,结果如图8所示,CF-Fe(25%)对CR的去除率没有明显的衰减,这明CFs对染料的去除率可以稳定在65%,具有良好的循环使用性[27]. 在五次循环实验中,解吸过程中电流的变化几乎保持不变,电流的大小代表溶液离子浓度的变化,同样证明了材料的可重复利用性,为实验运行提供保障.
-
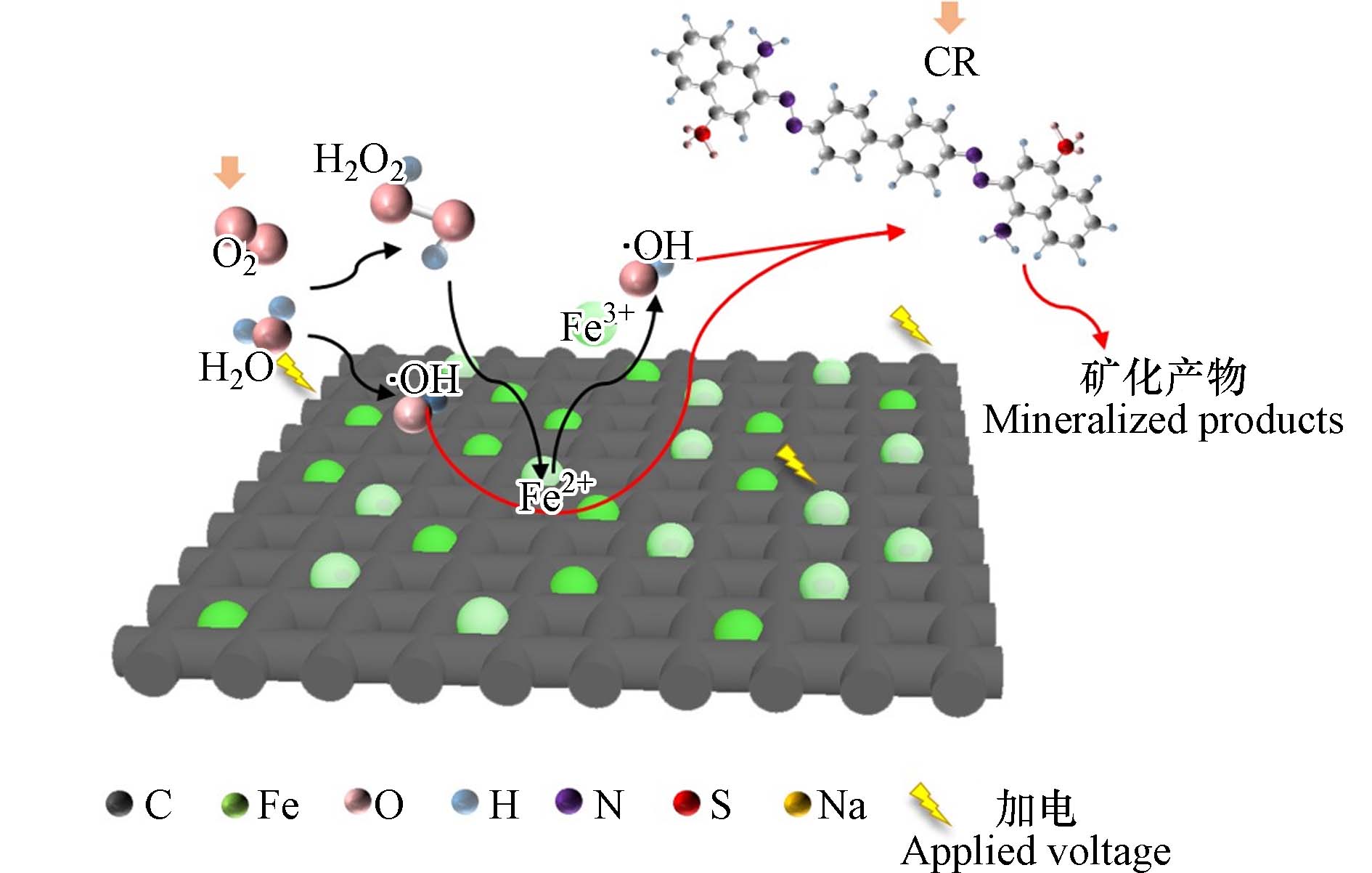
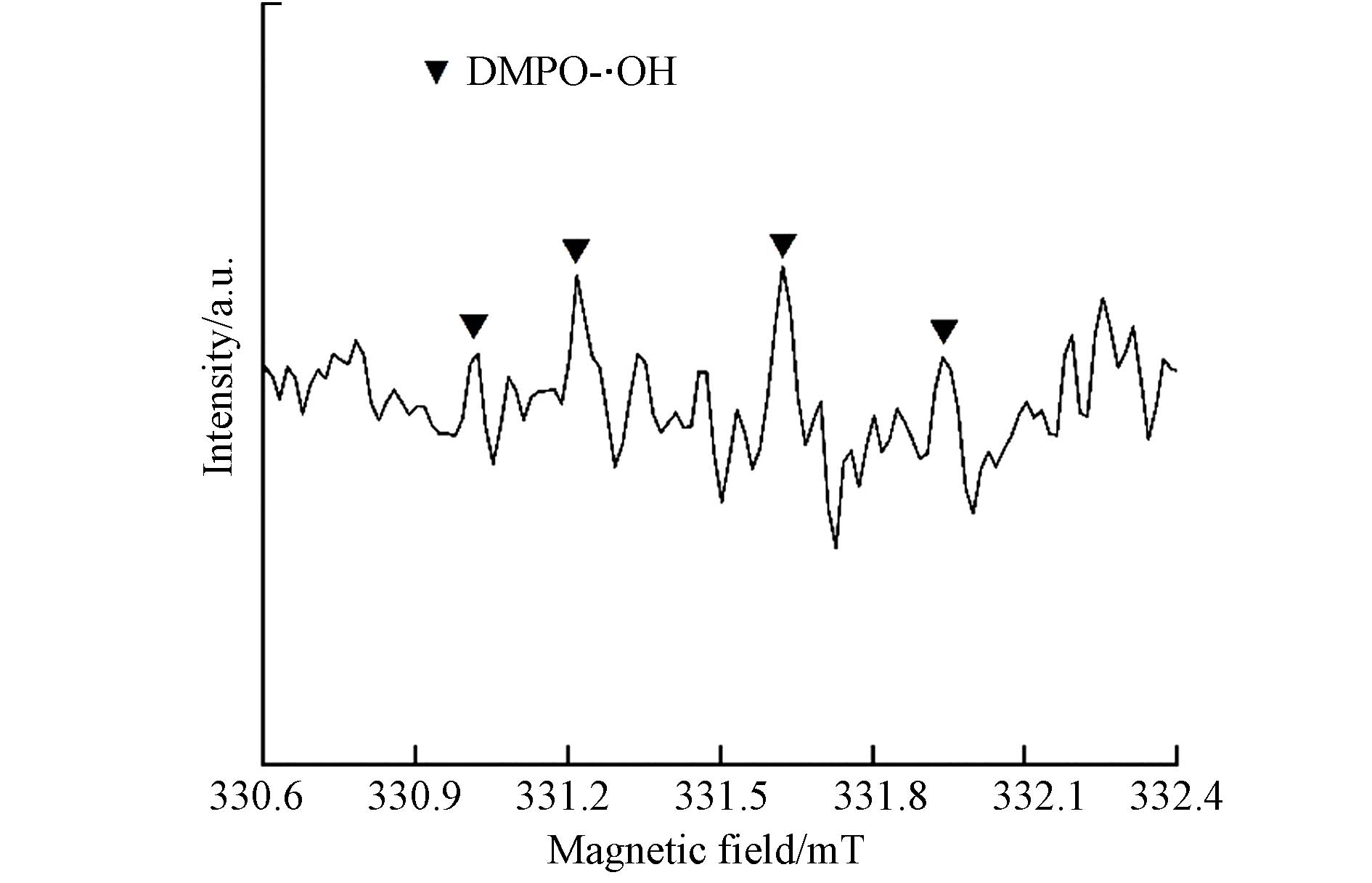
CR等阴离子染料在电场作用力的作用下进入阳极,只添加CEM的半膜MCDI装置使阴离子型染料可以直接与CF接触,CF表面大量丰富的大孔结构使得有机物被充分吸附. CR的去除主要是靠羟基自由基(·OH)的强氧化作用,·OH可能由两种方式生成:一是由于环境处于有氧状态,阳极氧气(O2)发生两电子还原反应生成H2O−,后与氢离子反应产生一些过氧化氢(H2O2)(反应式(5-6))[28],生成的H2O2会被CF表面的铁氧化物活化生成·OH(反应式(7))[29] ;二是外加电压使得水分子被电解生成氢离子、氢氧根离子和部分·OH(反应式(4)),但施加的电压(1.2 V)较低,因此这部分产生的·OH较少. 从而大量·OH与富集在CF表面活性位点上的阴离子染料接触,最终将大分子有机物降解,实现染料的去除(如图9). 因此可以总结为,阴离子染料先被碳毡大孔吸附富集,后被原位产生的·OH降解,降解后CF材料空出活性吸附位点进行再次吸附、降解,实现染料在15 min内的连续去除. 并对反应溶液进行EPR检测实验,检测出DMPO-·OH的特异信号[30] (如图10),验证反应过程中·OH的存在.
-
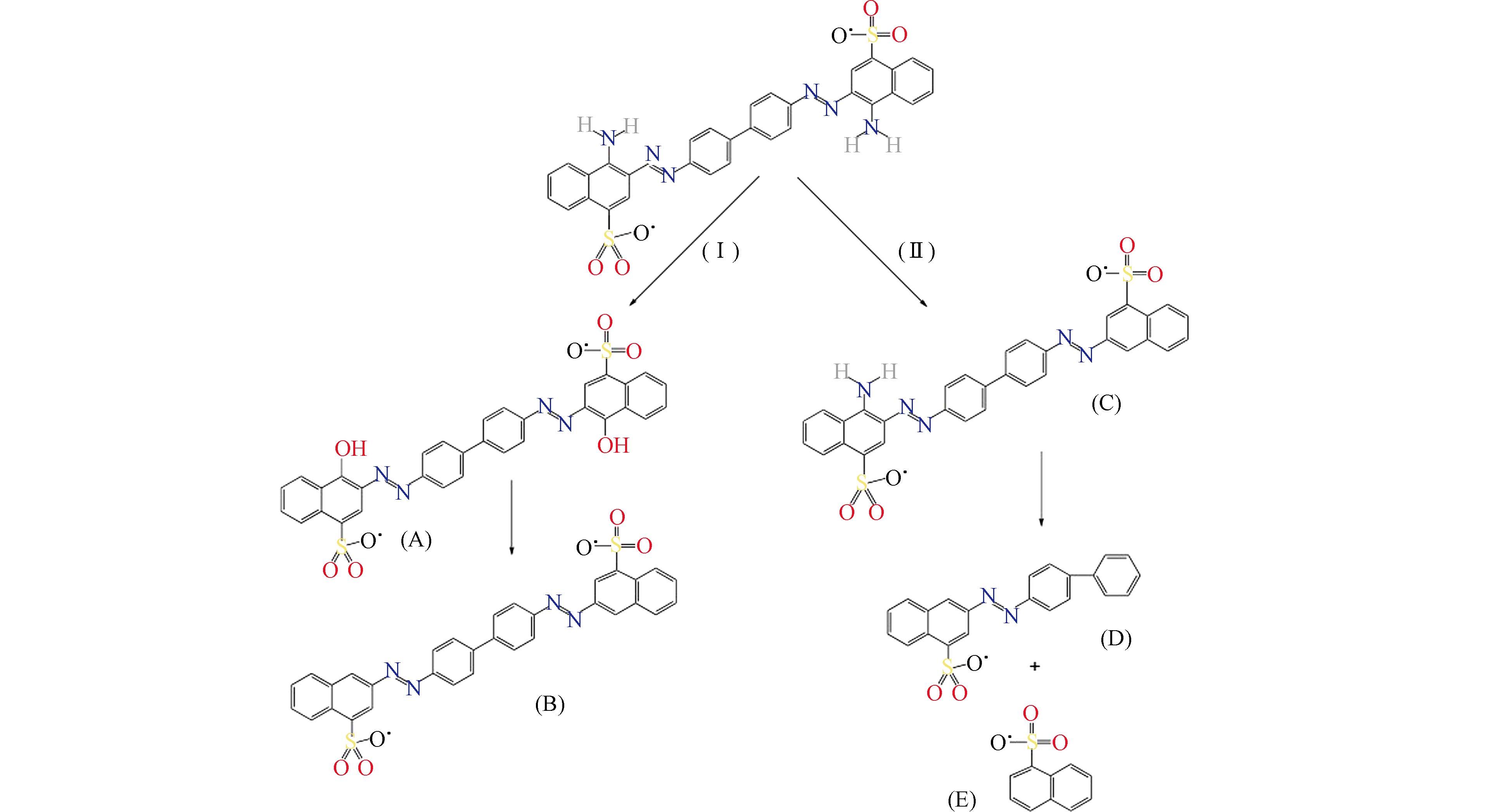
随后用LC-MS/MS研究了CR的降解途径随反应时间的变化. 推测出CR的降解可能通过以下步骤进行(图11):(Ⅰ)CR进行总脱氨和氧化,生成化合物(A),当总脱氨结束后,形成降解产物(B);(Ⅱ)CR的部分脱氨(C)和·OH在芳香环和偶氮基之间可能发生不对称断裂,得到中间体(D)和(E)[31 − 32].
-
1)本论文根据阴离子型染料特点,设计了新型杂化半膜MCDI装置,对CR、NC、SY FCF三种阴离子型染料进行去除,阐明铁氧化物掺杂CF作为电极材料的优势.
2)系统地研究了铁碳掺杂比对电极材料电化学性能和去除阴离子型染料性能的影响,得到最佳电极材料为CF-Fe(25%),并根据五次吸附-解吸实验证明材料的循环稳定性.
3)在此基础上进一步探究pH、电压和染料浓度等运行参数对染料去除的影响,结果表明在1.2 V电压,流速3 mL·min−1中性环境下,对100 mg·L−1的CR、NC、SY FCF在15 min内可达66.3%、61.6%和60.7%,并且酸性环境更有利于对染料的去除. 同时,总有机碳(TOC)去除率达69.4%,证明有机物得到彻底降解.
4)EPR试验中检测出DMPO-·OH的特异信号,证明起降解作用的主要氧化性物质为·OH. 本研究将对MCDI处理染料废水方向具有重要指导意义.
改性碳毡电极构建杂化膜电容去离子去除阴离子型染料的性能及机理
Removal of anionic dyes by modified carbon felt electrodes to construct hybrid the membrane capacitive deionization
-
摘要: 根据染料不过膜的特点,构建了新型杂化半膜膜电容去离子(MCDI)装置,首次利用MCDI对阴离子型染料进行去除. 将铁氧化物以不同铁碳质量比掺杂在碳毡(CF)中制备复合电极,通过扫描电子显微镜(SEM)、X射线衍射(XRD)和X射线光电子能谱(XPS)等多重手段表征电极材料的形貌、结构和化学组成等,表明铁氧化物的成功掺杂. 并根据循环伏安(CV)和电化学阻抗谱(EIS)证明铁氧化物掺杂比例为25%的改性材料CF-Fe(25%)具有最优的电化学性能. 对比CFs对阴离子型染料刚果红(congo red,CR)的去除效果,并探究电压、染料浓度和pH等参数对CR去除性能的影响,验证最佳条件下对其他阴离子型染料的去除效果. 结合自由基捕获实验阐述MCDI对阴离子型染料的降解机理. 得到在1.2 V电压,流速3 mL·min−1下,pH 7的进水环境中,对100 mg·L−1的CR、新胭脂红染料(new carmine,NC)和日落黄染料(sunset yellow FCF,SY FCF)在15 min内分别达到66.3%、61.6%和60.7%的去除率,且酸性环境有利于染料的去除. 新型杂化半膜MCDI装置可直接将离子型染料富集,后经阳极产生的高活性自由基(·OH)氧化降解、去除,铁氧化物的掺杂可增加电极反应活性位点,利于染料的吸附,并促进·OH的生成. ·OH是降解染料的主要作用力. 该方法去除效率高,同时避免了膜污染,为MCDI去除阴离子型染料方面提供科学依据.Abstract: A novel hybrid half-membrane membrane capacitive deionization (MCDI) device was constructed for the removal of anionic dyes using MCDI for the first time, based on the characteristic that dyes do not pass through the membrane. The composite electrodes were prepared by doping iron oxides in carbon felt (CF) with different iron-to-carbon mass ratios. The morphology, structure and chemical composition of the electrode materials were characterized by multiple means such as scanning electron microscopy (SEM), X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS), indicating the successful doping of iron oxides. It was also demonstrated that the modified material CF-Fe(25%) with 25% Fe oxide doping has the best electrochemical performance based on cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). The effect of CFs on the removal of the anionic dye congo red (CR) was compared and the effect of parameters such as voltage, dye concentration and pH on the removal performance of CR was investigated to verify the removal of other anionic dyes under optimal conditions. The mechanism of degradation of anionic dyes by MCDI was investigated in conjunction with free radical capture experiments. The removal rates of CR, new carmine (NC) and sundown yellow FCF (SY FCF) at 100 mg·L−1 were 66.3%, 61.6% and 60.7%, respectively, within 15 min at 1.2 V, a flow rate of 3 mL·min−1 and pH 7 in the feed water, and the acidic environment was favorable to the removal of dyes. The new hybrid half-membrane MCDI device can directly enrich ionic dyes, which are later degraded and removed by the oxidation of highly reactive radicals (·OH) generated by the anode. The doping of iron oxides increases the active site of the electrode reaction, which facilitates the adsorption of dyes and promotes the production of ·OH. ·OH is the main force for the degradation of dyes. The method is highly efficient in removal, while avoiding membrane contamination, and provides a scientific basis for MCDI in the removal of anionic dyes.
-
我国作为世界印染和纺织大国,有机染料废水的排放成为一个普遍问题. 有机染料分子中大多含显色基团,还原后易形成有毒的胺,不仅会破坏生态系统,也会对人类身体健康带来健康风险[1]. 因此,有机染料废水的处理具有重要意义.
目前,许多技术被应用于有机染料废水的处理,如复合纳米纤维材料吸附[2]、光催化[3]、膜吸附[4]、电凝吸附[5]、超分子包合技术[6]等,但这些技术或多或少存在处理时间长、操作成本高、二次污染等弊端. 因此,开发高效、便捷、环境友好且价格低廉的染料废水处理技术至关重要. 传统技术对于染料的去除为物理转移,存在不能将染料彻底氧化降解,或降解效率极低等问题. 因此将废水中的染料富集后再氧化降解可有效提升染料废水的处理效率.
电容去离子(capacitive deionization, CDI)是基于双电层(electric double layer, EDL)或法拉第电容(faradic capacitor)基本原理[7],实现淡化水质的一种新型脱盐技术,具有装置操作简单、能耗低等优势,除了应用在海水淡化,也逐渐应用在去除有机污染物方面. Bayram等[8]利用CDI去除废水中的芳香醇有机酸,Senoussi等[9]采用CDI(BM CDI)首次去除并回收纺织废水中的阳离子型染料,Tang等[10]利用流动电极电容去离子同时分馏、脱盐和去除染料,都证明CDI可以有效处理废水中的有机物. 但CDI在解吸过程中的同离子效应降低了系统的电荷效率. Kim等[11]在CDI装置两电极表面加入厚度约为20 μm的阴、阳离子交换膜,形成了膜电容去离子(membrane capacitive deionization,MCDI). MCDI利用离子交换膜对离子的选择性可大大提高系统的电荷效率[12].
然而,目前并没有膜电容去离子应用于染料去除的相关研究. 其原因可能是染料属于大分子有机物,经离子交换过膜困难,处理效率低. 因此可通过只加阳离子交换膜(cation exchange membrane, CEM),组建半膜MCDI装置. 碳毡(carbon felt, CF)是一种由聚丙烯腈基(PAN)碳纤维[13]经预氧化、低温碳化、高温碳化后形成的稳定致密碳纤维材料,高纯无污染,具有优异的电化学性能[14],其表面丰富的介孔/大孔结构,突破了微孔材料的扩散限值,更有利于实现对大分子有机物的充分吸附,是较好的电极材料[15]. 研究表明碳毡电极作为电极材料时,在电场作用下,可原位产生H2O2[16],但需经活化后才能产生具有直接氧化作用的羟基自由基,而铁氧化物是活化H2O2产生活性自由基的常用催化剂. 此外,铁的掺杂不仅扩大CF内部缺陷,增加活性位点,提供更多反应空间,而且促进羟基自由基的产生[17],实现染料废水中有机物的氧化降解. 同时,优化MCDI装置结构,构建更适合去除阴离子型染料的半膜MCDI装置,可显著提高对染料的富集、降解速率.
本研究制备并详细表征了碳毡-铁氧化物复合电极,并构建了半膜MCDI装置. 考察了铁碳掺杂比、电压、染料浓度、pH等运行参数对去除染料的影响,确定了在一定范围内最佳去除条件,为MCDI去除废水中的有机染料提供参考依据,为其在有机染料盐废水中有机物和离子的同时去除建立基础.
1. 材料与方法(Materials and methods)
1.1 实验材料与药品
CF购自内蒙古万兴碳素有限公司,刚果红染料(congo red, CR)、新胭脂红染料(new carmine, NC)、日落黄染料(sunset yellow FCF, SY FCF)购自山东德彦化工有限公司,草酸高铁铵,三水合物(Ⅲ)(ammonium iron(Ⅲ) oxalate trihydrate, 98%)购自阿法埃莎(中国)化学有限公司,所有试剂均为分析纯,且未经二次处理.
1.2 电极制备
分别称量
0.9893 、0.3297 、0.2473 、0 g草酸高铁铵,三水合物于250 mL烧杯中,加入40 mL超纯水充分溶解. 裁剪数个3 mm×45 mm×50 mm(约0.9893 g)大小的CF,经超纯水充分洗涤至洗涤液电导率为0 μS·cm−1,置于烘箱75 ℃烘干. 最后放入真空管式炉(CHY-1200 ,河南成仪设备科技有限公司)中碳化,碳化过程采用两段升温程序,以氮气作为保护气,先以5 ℃ min−1升温至500 ℃,保温30 min,再以10 ℃·min−1继续升温到800 ℃,保温2 h. 冷却后得到1:1、1:3、1:4和无铁掺杂四种不同铁碳质量比的材料CFs,分别命名为CF-Fe(50%)、CF-Fe(25%)、CF-Fe(20%)、CF-Fe(0%).1.3 新型半膜MCDI装置组装及实验流程
实验装置由塑料端板、硅胶垫片、钛片、硅胶垫片、CF、4.5 cm×5 cm塑料隔网(中间腔室)、CEM、CF、硅胶垫片、钛片、硅胶垫片、塑料端板依次排列,最终用螺丝进行装置固定,组成只加一侧CEM的新型半膜-MCDI装置(如图1). 采用序批模式(Batch)进水40 mL,利用蠕动泵(Longer Pump BT100-1L, 保定兰格恒流源有限公司)以3 mL·min−1流速控制阴离子型染料下进上出,流经中间腔室,正负极两端伸出钛片,电化学工作站(瑞士万通 MAC00589)连接钛片对装置进行加电,电导率仪(S475-UMIX,梅特勒Seven Excellence)实时监测染料离子浓度变化(如图2).
1.4 表征
1.4.1 物理表征
用扫描电子显微镜(SEM, Hitachi S48000, Japan)观察CFs的表面形貌,采用X射线光电子能谱仪(XPS, Thermo Scientific, France)分析改性CFs的元素组成及官能团,采用X射线衍射仪(XRD, SmartLab9KW, Japan)研究铁氧化物的有效掺杂以及CFs材料的结晶特性.
1.4.2 电化学表征
对电极材料CFs进行循环伏安(CV)和电化学阻抗谱(EIS)测试. 采用三电极体系,其中CFs电极为工作电极,饱和甘汞为参比电极,铂电极为对电极,在1 mol·L−1的NaCl电解液中进行. CV测试在50 mV·s−1扫描速率,扫描窗口为−0.6—0.6 V下进行. EIS测试过程在0 V偏置电压下,施加±5 mV交流电,在0.1—105 Hz的频率范围内进行检测. CV曲线的比电容根据式(1)计算:
C=1000∫Idv2ma(Vc−Va) (1) 式中:C为比电容,F·g−1;a为扫速,mV·s−1;Va和Vc分别为低电位截止值和高电位截止值,V;m为电极材料的质量,g;I为电流,A.
1.5 多种阴离子型染料的去除
分别用超纯水配制不同浓度的CR、NC、SY FCF母液避光保存,称取某一染料溶液40 mL置于总体积为100 mL的锥形瓶中,在温度为298 K下,采用单一变量法,探究CFs材料(CF-Fe(50%)、CF-Fe(25%)、CF-Fe(20%)、CF-Fe(0%)),不同运行电压(0.6 V、1.2 V、1.8 V)、不同进水染料浓度(0.05、0.1、0.3 g·L−1)和不同pH(3、7、11)对不同阴离子型染料的去除情况. 实验加电15 min,每隔3 min,从锥形瓶中吸取1 mL染料于离心管中,稀释至30 mL,摇匀避光保存. 用紫外分光光度仪(TU-1810, 北京普析通用仪器有限责任公司)于最佳波长处定量测定染料溶液吸光度,根据绘制的染料浓度与吸光度的标准曲线计算染料去除率,并根据总有机碳分析仪(TOC-VCPN, Shimadzu, Japan)测定剩余染料中有机物浓度. 通过反向加电的方式进行5次吸附、解吸实验,验证材料的循环稳定性. 以5,5-二甲基- 1-吡咯啉-N-氧化物(DMPO)作为·OH捕获剂,采用电子顺磁共振波谱仪(EPR)(SPINSCAN X, ADANI, Belarus)测定DMPO-·OH信号及其强度. 采用LC-MS/MS检测CR降解的中间产物,色谱分析采用超高效液相色谱(Ultimate
3000 UHPLC, Dinonex, USA),使用 Eclipse Plus C18 色谱柱,流动相为A:0.1%甲酸溶液,B:乙腈,梯度洗脱. 质谱分析采用质谱仪(Q Exactive, Thermo Scientific, USA),采用离子源HESI进行正离子模式进行电离. 主扫描分辨率为70000,范围为50—600 m/z;二次扫描分辨率为17500,起始离子为50 m/z. 为保证数据科学可靠性,每组实验至少重复2次进行. 染料的吸附容量和去除率如式(2)、(3)计算:qt=(C0−Ct)V×1000m (2) R=(C0−Ct)C0×100 (3) 式中:qt为吸附容量(mg·g−1),C0为染料初始浓度(mg·L−1),Ct为吸附某时刻的染料浓度(mg·L−1),V代表染料溶液体积(mL),m为电极材料的质量(g),R为去除率(%).
2. 结果与讨论(Results and discussion)
2.1 物理表征
2.1.1 形貌表征
对CFs的形貌表征如图3所示,图3(a)、(d)为CF-Fe(0%),表面整体光滑,无任何杂质. 图3(b)、(e)为CF-Fe(25%),(c)、(f)为CF-Fe(50%)材料,可以看出碳毡上明显有块状物质负载,且铁掺杂比例越高,被铁氧化物覆盖的CFs面积也越大. 同时CF纤维表面存在明显沟槽,有细条纹,这可能是由于高温干燥碳化过程中氮原子和碳原子的演变,高温促进了石墨结构清晰的径向梯度分布,纤维发生横向物理收缩[18].
2.1.2 结构表征
XRD谱图如图4(a)、(b)所示,电极材料在衍射角24.4°和43.4°出现了两个宽衍射峰,分别对应石墨碳纤维晶格平面(002)和(100)的晶面衍射. 随着铁元素掺杂比例的增加,在30°到45°之间,CF-Fe(50%)和CF-Fe(25%)电极材料出现四处衍射峰,分别对应铁氧化物在(104)、(110)、(311)、(220)晶格表面的衍射[19](如图4(b)),证明了铁氧化物的成功负载. 复合材料的催化性能与铁元素价态密不可分,因此采用XPS进一步测定CF和CF-Fe(25%)材料的化学组成和化学成分,从图(c)中确定了C、O、Fe的3个核心元素,表明铁氧化物成功负载在CF上.
图4(d)为Fe元素2p轨道的XPS高分辨图谱,观察到707.2、710.9、713.1、717.8、725.7、728 eV下6个峰,分别为Fe0 2p1/2、Fe3+ 2p1/2、Fe2+ 2p1/2、Fe0 2p3/2、Fe2+ 2p3/2、Fe3+ 2p3/2自旋轨道[20],表明Fe掺杂以多种价态形式存在.
2.1.3 电容特性表征
由图5(a)可知,对CFs材料进行了电容特性表征. 观察到CF-Fe(20%)、CF-Fe(25%)和CF-Fe(50%)材料的CV曲线均存在轻微的氧化还原峰,说明复合材料在扫描电压窗口下具有接受/失去电子的能力,会发生氧化还原反应;且图像整体较为对称,说明复合材料具有良好的电化学稳定性;其中CF-Fe(25%)与其他材料相比,CV曲线的积分面积最大,一般CV曲线的积分面积代表电荷存储容量即比电容[21],说明铁氧化物掺杂为电极材料提供了更高的比表面积和更多的活性位点,因此CF-Fe(25%)材料的电容性能更优异.
CFs的EIS(如图5(b))表现出典型的电容行为,由一个半圆和一条斜线组成[22]. 一般认为,高频区半圆表示电子传递阻力,低频区的线表示离子扩散速率[23]. 与其他材料相比,CF-Fe(25%)材料半圆最小,说明其电子传递阻力最小;CF-Fe(25%)材料斜率最大,说明其离子迁移速率也最高. 而CF-Fe(0%)基本看不到圆弧,可以得出铁氧化物的负载增加了材料的电子传递阻力.
2.2 对染料去除的影响
2.2.1 对CR去除性能的影响
图6(a)为4种CFs下对CR染料的去除效果,可以看出CF-Fe(25%)材料对CR的去除率明显大于其他材料,在15 min内,1.2 V电压下,对300 mg·L−1的CR染料去除率达到45.03%. 图6(b)为不同浓度梯度下对CR的全波长扫描图,可知CR最大吸收波长为505 nm,并观察到,CR的吸光度随着CR的去除而逐渐降低,颜色也随之变浅,这证明CR染料被有效吸附去除. 通过测定反应前后的总有机碳(TOC)含量,可以得出对总有机碳的矿化率达69.40%(如图6(c)). 图6(d)为CR的吸光度与浓度的标准曲线,其中R2=0.999,接近于1 .
 图 6 CFs对CR的去除效果(a), CR全波长扫描(b), 总有机碳含量变化(c)和CR的吸光度与浓度标准曲线(d)Figure 6. Removal effect of CFs on CR (a), full wavelength scan of CR (b), variation of total organic carbon content (c) and absorbance versus concentration standard curve of CR (d)(注:施加电压1.2 V,pH=7,CR染料浓度300 mg·L−1,流速3 mL·min−1)(Note: Applied voltage 1.2 V, pH=7, CR dye concentration 300 mg·L−1, flow rate 3 mL·min−1)
图 6 CFs对CR的去除效果(a), CR全波长扫描(b), 总有机碳含量变化(c)和CR的吸光度与浓度标准曲线(d)Figure 6. Removal effect of CFs on CR (a), full wavelength scan of CR (b), variation of total organic carbon content (c) and absorbance versus concentration standard curve of CR (d)(注:施加电压1.2 V,pH=7,CR染料浓度300 mg·L−1,流速3 mL·min−1)(Note: Applied voltage 1.2 V, pH=7, CR dye concentration 300 mg·L−1, flow rate 3 mL·min−1)2.2.2 运行参数对去除阴离子型染料的影响
如图7(a),外加电压对染料去除性能有着显著影响,施加电压为零,即仅靠物理吸附时对CR的去除率约为24.6%,证明CF-Fe(25%)对CR具有一定的物理吸附能力,而在施加电压后,吸附速率明显增加,在1.2 V电压下CR的去除率达到了66.3%,1.8 V电压下去除率为72.5%. 因此可以得出电压与CR染料的去除率呈正相关,这可能是高电压可提供强大的静电驱动力,增强电极对染料的静电吸附作用[24],从而促进离子的转移,加快染料的吸附降解. 但是当电压超过1.2 V时,会产生水解副反应从而增加系统能耗,因此1.2 V电压作为最优电压.
 图 7 电压(a)、CR浓度(b)、pH(c)对CR去除的影响;对不同阴离子型染料的去除(d); NC(e)和SY FCF(f)全波长扫描;NC(g)和SY FCF(h)的吸光度和浓度标准曲线Figure 7. Effect of voltage (a), CR concentration (b) and pH (c) on CR removal, removal of different anionic dyes (d), full wavelength scans of NC (e) and SY FCF (f), absorbance and concentration standard curves of NC (g) and SY FCF (h)(注:流速3 mL·min−1,CR染料浓度100 mg·L−1,pH 7 (a);电压1.2 V,CR染料pH 7(b);电压1.2 V ,CR染料浓度300 mg·L−1 (c))(Note: Flow rate 3 mL·min−1, CR dye concentration 100 mg·L−1, pH 7(Fig.(a)), applied voltage 1.2 V, CR dye with pH 7(Fig. (b)), applied voltage 1.2 V, CR dye concentration 300 mg·L−1 (Fig.(c)).
图 7 电压(a)、CR浓度(b)、pH(c)对CR去除的影响;对不同阴离子型染料的去除(d); NC(e)和SY FCF(f)全波长扫描;NC(g)和SY FCF(h)的吸光度和浓度标准曲线Figure 7. Effect of voltage (a), CR concentration (b) and pH (c) on CR removal, removal of different anionic dyes (d), full wavelength scans of NC (e) and SY FCF (f), absorbance and concentration standard curves of NC (g) and SY FCF (h)(注:流速3 mL·min−1,CR染料浓度100 mg·L−1,pH 7 (a);电压1.2 V,CR染料pH 7(b);电压1.2 V ,CR染料浓度300 mg·L−1 (c))(Note: Flow rate 3 mL·min−1, CR dye concentration 100 mg·L−1, pH 7(Fig.(a)), applied voltage 1.2 V, CR dye with pH 7(Fig. (b)), applied voltage 1.2 V, CR dye concentration 300 mg·L−1 (Fig.(c)).由图7(b)可知,CF-Fe(25%)材料对100 mg·L−1CR的去除率最高,对50 mg·L−1CR去除率最低,可能是由于低浓度CR前期以物理吸附为主,去除率较慢;9 min后随着阳极产生羟基自由基含量增多,去除率也随之增大;300 mg·L−1 CR由于浓度过高,1.2 V电压下产生活性自由基数量有限,加之初始浓度高,因此去除率偏低. 因此,选择100 mg·L−1 CR进行后续实验.
如图7(c)可知,过碱的环境不利于对CR染料的去除,在碱性环境中,CR表面的负电荷会与羟基自由基形成静电排斥,阻止自由基与CR的相互作用,使得脱色率下降[25]. 酸性和中性环境有利于对CR的去除,且在pH在3—5范围内具有更好的去除效果,可能是因为在酸性环境中,氢离子增多,加快与氧气生成过氧化氢,从而增加了羟基自由基的生成量,从而提升对CR的去除效果[26]. 但在15 min内,pH在3—11范围内对CR的去除没有明显差别,说明MCDI去除CR等阴离子型染料对进水水质的要求并不高. 图7(d)得到在1.2 V电压,流速3 mL·min−1下,pH 7的进水环境中,对100 mg·L−1的CR、NC、SY FCF在15 min内分别达到66.3%、61.6%和60.7%的去除效果. 图7(e)、(f)为不同浓度梯度下对NC和SY FCF的全波长扫描图,可知最大吸收波长分别为506 nm和482 nm,NC和SY FCF的吸光度与浓度的标准曲线如图7(g)、(h),R2均为0.999.
2.3 材料循环稳定性
电极材料的循环稳定性也是CDI水处理的关键指标. 经过5次吸附-解吸循环实验,结果如图8所示,CF-Fe(25%)对CR的去除率没有明显的衰减,这明CFs对染料的去除率可以稳定在65%,具有良好的循环使用性[27]. 在五次循环实验中,解吸过程中电流的变化几乎保持不变,电流的大小代表溶液离子浓度的变化,同样证明了材料的可重复利用性,为实验运行提供保障.
2.4 可能的吸附降解机理
CR等阴离子染料在电场作用力的作用下进入阳极,只添加CEM的半膜MCDI装置使阴离子型染料可以直接与CF接触,CF表面大量丰富的大孔结构使得有机物被充分吸附. CR的去除主要是靠羟基自由基(·OH)的强氧化作用,·OH可能由两种方式生成:一是由于环境处于有氧状态,阳极氧气(O2)发生两电子还原反应生成H2O−,后与氢离子反应产生一些过氧化氢(H2O2)(反应式(5-6))[28],生成的H2O2会被CF表面的铁氧化物活化生成·OH(反应式(7))[29] ;二是外加电压使得水分子被电解生成氢离子、氢氧根离子和部分·OH(反应式(4)),但施加的电压(1.2 V)较低,因此这部分产生的·OH较少. 从而大量·OH与富集在CF表面活性位点上的阴离子染料接触,最终将大分子有机物降解,实现染料的去除(如图9). 因此可以总结为,阴离子染料先被碳毡大孔吸附富集,后被原位产生的·OH降解,降解后CF材料空出活性吸附位点进行再次吸附、降解,实现染料在15 min内的连续去除. 并对反应溶液进行EPR检测实验,检测出DMPO-·OH的特异信号[30] (如图10),验证反应过程中·OH的存在.
H2O→⋅OH+(H++e−) (4) O2+H2O+2e−→H2O−+OH− (5) H2O−+H+→H2O2 (6) Fe2+H2O2→Fe3++OH−+⋅OH (7) 2.5 可能的降解产物分析
随后用LC-MS/MS研究了CR的降解途径随反应时间的变化. 推测出CR的降解可能通过以下步骤进行(图11):(Ⅰ)CR进行总脱氨和氧化,生成化合物(A),当总脱氨结束后,形成降解产物(B);(Ⅱ)CR的部分脱氨(C)和·OH在芳香环和偶氮基之间可能发生不对称断裂,得到中间体(D)和(E)[31 − 32].
3. 结论(Conclusion)
1)本论文根据阴离子型染料特点,设计了新型杂化半膜MCDI装置,对CR、NC、SY FCF三种阴离子型染料进行去除,阐明铁氧化物掺杂CF作为电极材料的优势.
2)系统地研究了铁碳掺杂比对电极材料电化学性能和去除阴离子型染料性能的影响,得到最佳电极材料为CF-Fe(25%),并根据五次吸附-解吸实验证明材料的循环稳定性.
3)在此基础上进一步探究pH、电压和染料浓度等运行参数对染料去除的影响,结果表明在1.2 V电压,流速3 mL·min−1中性环境下,对100 mg·L−1的CR、NC、SY FCF在15 min内可达66.3%、61.6%和60.7%,并且酸性环境更有利于对染料的去除. 同时,总有机碳(TOC)去除率达69.4%,证明有机物得到彻底降解.
4)EPR试验中检测出DMPO-·OH的特异信号,证明起降解作用的主要氧化性物质为·OH. 本研究将对MCDI处理染料废水方向具有重要指导意义.
-
图 7 电压(a)、CR浓度(b)、pH(c)对CR去除的影响;对不同阴离子型染料的去除(d); NC(e)和SY FCF(f)全波长扫描;NC(g)和SY FCF(h)的吸光度和浓度标准曲线
Figure 7. Effect of voltage (a), CR concentration (b) and pH (c) on CR removal, removal of different anionic dyes (d), full wavelength scans of NC (e) and SY FCF (f), absorbance and concentration standard curves of NC (g) and SY FCF (h)
-
[1] CAI Z Q, SUN Y M, LIU W, et al. An overview of nanomaterials applied for removing dyes from wastewater[J]. Environmental Science and Pollution Research, 2017, 24(19): 15882-15904. doi: 10.1007/s11356-017-9003-8 [2] AHMADIJOKANI F, MOLAVI H, BAHI A, et al. Electrospun nanofibers of chitosan/polyvinyl alcohol/UiO-66/nanodiamond: Versatile adsorbents for wastewater remediation and organic dye removal[J]. Chemical Engineering Journal, 2023, 457: 141176. doi: 10.1016/j.cej.2022.141176 [3] SAAD I, RALHA N, ABUKHADRA M R, et al. Recent advances in photocatalytic oxidation techniques for decontamination of water[J]. Journal of Water Process Engineering, 2023, 52: 103572. doi: 10.1016/j.jwpe.2023.103572 [4] MOKHTARI N, DINARI M, FASHANDI H. Developing polysulfone-based mixed matrix membrane containing hydrazone-linked covalent organic frameworks towards dye wastewater purification[J]. Chemical Engineering Journal, 2022, 446: 137456. doi: 10.1016/j.cej.2022.137456 [5] CASTAÑEDA-DÍAZ J, PAVÓN-SILVA T, GUTIÉRREZ-SEGURA E, et al. Electrocoagulation-adsorption to remove anionic and cationic dyes from aqueous solution by PV-energy[J]. Journal of Chemistry, 2017, 2017: 1-14. [6] DUAN Z B, LI Y L, ZHANG M, et al. Towards cleaner wastewater treatment for special removal of cationic organic dye pollutants: A case study on application of supramolecular inclusion technology with β-cyclodextrin derivatives[J]. Journal of Cleaner Production, 2020, 256: 120308. doi: 10.1016/j.jclepro.2020.120308 [7] JIA B P, ZHANG W. Preparation and application of electrodes in capacitive deionization (CDI): A state-of-art review[J]. Nanoscale Research Letters, 2016, 11(1): 64. doi: 10.1186/s11671-016-1284-1 [8] BAYRAM E, AYRANCI E. Structural effects on electrosorptive behavior of aromatic organic acids from aqueous solutions onto activated carbon cloth electrode of a flow-through electrolytic cell[J]. Journal of Electroanalytical Chemistry, 2012, 683: 14-20. doi: 10.1016/j.jelechem.2012.07.028 [9] SENOUSSI H, BOUHIDEL K E. Feasibility and optimisation of a batch mode capacitive deionization (BM CDI) process for textile cationic dyes (TCD) removal and recovery from industrial wastewaters[J]. Journal of Cleaner Production, 2018, 205: 721-727. doi: 10.1016/j.jclepro.2018.09.026 [10] TANG K X, ZHENG H, DU P H, et al. Simultaneous fractionation, desalination, and dye removal of dye/salt mixtures by carbon cloth-modified flow-electrode capacitive deionization[J]. Environmental Science & Technology, 2022, 56(12): 8885-8896. [11] KIM Y J, CHOI J H. Improvement of desalination efficiency in capacitive deionization using a carbon electrode coated with an ion-exchange polymer[J]. Water Research, 2010, 44(3): 990-996. doi: 10.1016/j.watres.2009.10.017 [12] PORADA S, ZHAO R, van der WAL A, et al. Review on the science and technology of water desalination by capacitive deionization[J]. Progress in Materials Science, 2013, 58(8): 1388-1442. doi: 10.1016/j.pmatsci.2013.03.005 [13] MINKE C, KUNZ U, TUREK T. Carbon felt and carbon fiber - A techno-economic assessment of felt electrodes for redox flow battery applications[J]. Journal of Power Sources, 2017, 342: 116-124. doi: 10.1016/j.jpowsour.2016.12.039 [14] PIGNOL G, BASSIL P, FONTMORIN J M, et al. Electrochemical properties of carbon fibers from felts[J]. Molecules, 2022, 27(19): 6584. doi: 10.3390/molecules27196584 [15] SARDINHA A F, ALMEIDA D A L, VERNASQUI L G, et al. Electrochemical response enhancement of CF and GO/CF composites using a promising CF etching[J]. Diamond and Related Materials, 2020, 108: 107997. doi: 10.1016/j.diamond.2020.107997 [16] TANG W W, HE D, ZHANG C Y, et al. Comparison of Faradaic reactions in capacitive deionization (CDI) and membrane capacitive deionization (MCDI) water treatment processes[J]. Water Research, 2017, 120: 229-237. doi: 10.1016/j.watres.2017.05.009 [17] BAI X Y, SUN H C, SUN J, et al. Efficient removal of sixteen priority polycyclic aromatic hydrocarbons from textile dyeing sludge using electrochemical Fe2+-activated peroxymonosulfate oxidation-a green pretreatment strategy for textile dyeing sludge toxicity reduction[J]. Journal of Hazardous Materials, 2022, 435: 129087. doi: 10.1016/j.jhazmat.2022.129087 [18] LU J S, LI W W, KANG H L, et al. Microstructure and properties of polyacrylonitrile based carbon fibers[J]. Polymer Testing, 2020, 81: 106267. doi: 10.1016/j.polymertesting.2019.106267 [19] WANG Y, LIN Y, YANG C P, et al. Calcination temperature regulates non-radical pathways of peroxymonosulfate activation via carbon catalysts doped by iron and nitrogen[J]. Chemical Engineering Journal, 2023, 451: 138468. doi: 10.1016/j.cej.2022.138468 [20] ZHAO C, MENG L H, CHU H Y, et al. Ultrafast degradation of emerging organic pollutants via activation of peroxymonosulfate over Fe3C/Fe@N-C-x: Singlet oxygen evolution and electron-transfer mechanisms[J]. Applied Catalysis B:Environmental, 2023, 321: 122034. doi: 10.1016/j.apcatb.2022.122034 [21] ZHANG Q L, CHENG Y L, FANG C Q, et al. Electrochemically enhanced adsorption of organic dyes from aqueous using a freestanding metal-organic frameworks/cellulose-derived porous monolithic carbon foam[J]. Bioresource Technology, 2022, 347: 126424. doi: 10.1016/j.biortech.2021.126424 [22] SHEN G, MA J J, NIU J R, et al. Mechanism of ball milled activated carbon in improving the desalination performance of flow- and fixed-electrode in capacitive deionization desalination[J]. Frontiers of Environmental Science & Engineering, 2023, 17(5): 64. [23] QIU S Y, WANG Y, WAN J Q, et al. Enhanced electro-Fenton catalytic performance with in situ grown Ce/Fe@NPC-GF as self-standing cathode: Fabrication, influence factors and mechanism[J]. Chemosphere, 2021, 273: 130269. doi: 10.1016/j.chemosphere.2021.130269 [24] XIE F S, SHI Q Y, BAI H L, et al. An anode fabricated by co electrodeposition on ZIF-8/CNTs/CF for peroxymonosulfate (PMS) activation[J]. Chemosphere, 2023, 313: 137384. doi: 10.1016/j.chemosphere.2022.137384 [25] SUN N, ZHOU H J, ZHANG H M, et al. Synchronous removal of tetracycline and water hardness ions by capacitive deionization[J]. Journal of Cleaner Production, 2021, 316: 128251. doi: 10.1016/j.jclepro.2021.128251 [26] ARAB C, EL KURDI R, PATRA D. Effect of pH on the removal of anionic and cationic dyes using zinc curcumin oxide nanoparticles as adsorbent[J]. Materials Chemistry and Physics, 2022, 277: 125504. doi: 10.1016/j.matchemphys.2021.125504 [27] ZHAO C H, SHAO B B, YAN M, et al. Activation of peroxymonosulfate by biochar-based catalysts and applications in the degradation of organic contaminants: A review[J]. Chemical Engineering Journal, 2021, 416: 128829. doi: 10.1016/j.cej.2021.128829 [28] XIA C, XIA Y, ZHU P, et al. Direct electrosynthesis of pure aqueous H2O2 solutions up to 20% by weight using a solid electrolyte[J]. Science, 2019, 366(6462): 226-231. doi: 10.1126/science.aay1844 [29] DUAN L S, LIU X H, ZHANG H D, et al. A novel way for hydroxyl radicals generation: Biochar-supported zero-valent iron composite activates oxygen to generate hydroxyl radicals[J]. Journal of Environmental Chemical Engineering, 2022, 10(4): 108132. doi: 10.1016/j.jece.2022.108132 [30] ZHANG J, LV S Y, YU Q, et al. Degradation of sulfamethoxazole in microbubble ozonation process: Performance, reaction mechanism and toxicity assessment[J]. Separation and Purification Technology, 2023, 311: 123262. doi: 10.1016/j.seppur.2023.123262 [31] JO W K, KUMAR S, ISAACS M A, et al. Cobalt promoted TiO2/GO for the photocatalytic degradation of oxytetracycline and Congo Red[J]. Applied Catalysis B:Environmental, 2017, 201: 159-168. doi: 10.1016/j.apcatb.2016.08.022 [32] ASSES N, AYED L, HKIRI N, et al. Congo red decolorization and detoxification by Aspergillus niger: Removal mechanisms and dye degradation pathway[J]. BioMed Research International, 2018, 2018: 3049686. -




 下载:
下载: