-
高级氧化工艺(advanced oxidation processes, AOPs)是一种先进的污水处理技术,相比于传统的生物技术,AOPs可将难降解、毒性有机污染物进行有效去除,在环境保护、水处理、废弃物处理等领域收到了广泛的关注[1]. AOPs是基于通过各种化学、光化学、声化学或电化学反应生成以羟基自由基(·OH)为主的活性氧物种来攻击有机污染物[2 − 4]使其氧化分解,并进一步完全矿化生成水、二氧化碳和无机盐,从而达到净化水体的目的[3, 5]. 近年来,AOPs领域的研究方向主要集中在以下几个方面[6]:1) AOPs新技术的研发;2) AOPs机理的探索;3) AOPs与生物、吸附和膜技术等的联用;4) AOPs的实际应用;5) AOPs效率和能耗等方面综合调控.
由于AOPs技术复杂度较高,存在能源消耗、副产物产生等问题,因此降低能耗提升效率成为了AOPs研究的重点,而AOPs水体净化效率受到多种因素包括反应条件、废水性质、处理时间和工艺本身等的限制[7 − 8]. 研究人员通过改进反应器设计、使用新型催化剂和调节反应条件等手段,降低AOPs的能耗,提高处理效率和工程经济性[9]. 改进AOPs的反应条件对提高其处理效率有着重要的帮助. 前人主要研究了反应温度、反应时间、酸碱值 (pH)、催化剂用量和氧化剂用量等对体系的影响[10]. 随着AOPs机理研究的不断深入,人们注意到初始溶解氧条件与AOPs处理效率的关联机制,并推动领域朝着高选择性、可持续性和高效率性的方向发展[11 − 12]. 溶解氧在体系中的影响机制是通过利用电子顺磁共振技术和化学竞争手段定性定量研究体系主要活性物种,并根据曝气实验、溶解氧梯度浓度实验掌握溶解氧对体系的宏观影响,再通过反应方程式、产物分析、氧化机制分析,以及对活性自由基浓度的监测综合得出. 近年来在不同种类的AOPs中有关溶解氧的研究主要集中在以下3个方面:1) 不同溶解氧浓度条件下污染物的降解及矿化效果;2) 溶解氧在氧化过程中导致的自由基浓度变化;3) 氧化过程中涉及溶解氧的反应机制.
由此可见,溶解氧是AOPs体系中的一个重要参数,本文通过综述了溶解氧在光催化氧化、芬顿氧化、过硫酸盐氧化、臭氧氧化、声化学氧化和电化学氧化体系中对有机污染物降解速率、反应产物,以及自由基反应路径的动力学分析以及体系反应能耗的热力学影响(图1),相关研究有助于深刻认知AOPs应用过程溶解氧的影响机制,进一步推进AOPs应用于高效处理水中有机污染物.
-
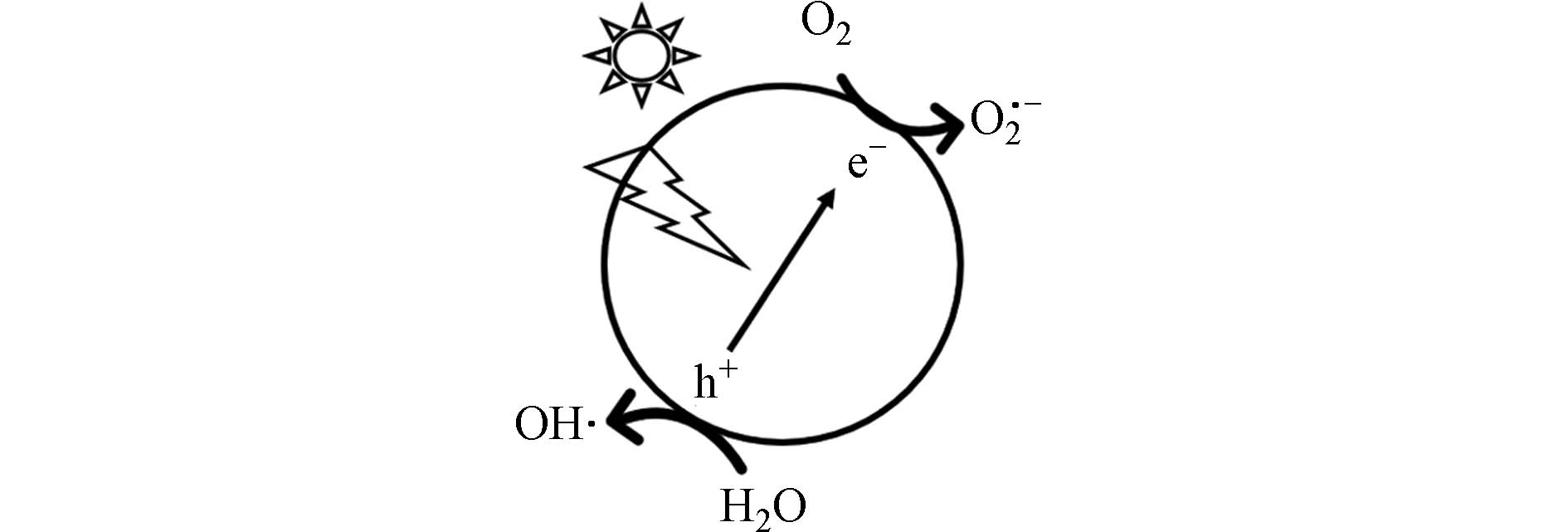
光催化降解是一种在AOPs水处理应用中被广泛使用的技术[19]. 光催化技术的机理如图2所示,是一种以光化学反应为基础,利用光催化剂的活性位点在紫外/可见光(UV/visible light)激发下产生活性物质(Reactive species,RSs),如羟基自由基(·OH)、超氧自由基(O2·−)和光生空穴(h+)等[20],对污染物进行攻击降解,使之转化为环境无害物质的过程.
当前研究中溶解氧对光催化氧化体系的作用主要体现在影响RSs生成、光催化剂表面电荷分离以及降解产物迁移转化过程上. 由机理可知,光催化反应需要光源和溶解氧等作为反应的驱动力. 作为光催化反应的氧化剂,溶解氧浓度的增加可以提高反应速率;在无氧或低氧条件时,光催化反应的速率将大大减缓. 利用对苯醌(p-benzoquinone, p-BQ)作为O2·−的清除剂[21]进行淬灭实验并结合曝气实验可发现,在以O2·−为主要贡献的污染物去除体系中[22],光催化剂的导带电位均高于O2/ O2·−的基本电位(-0.046 V vs. NHE),说明来自光催化剂导带的光激发电子(eCB−)可以被O2捕获,产生O2·−(式 1)[23 − 24]. 由于光催化技术常因光催化剂内部(或表面)电子-空穴的重组而消耗不必要的能量导致催化降解效率降低[25]. 热力学上,氧分子的电子亲和力大,提高溶解氧浓度意味着为体系提供了更多的电子接受体,促进光生电子的再生、电子-空穴对分离,增强反应的热力学驱动力并提高体系的光催化活性,减少能量损失. 研究表明,溶解氧被光生电子还原成O2·−后可进一步分解或还原生成·OH [26]. Kondrakov等[27]通过同位素追踪18O,发现光生电子还原溶解氧途径最终能生成·OH,但占体系中·OH总量较少,约为5%. 另有研究表明[28],溶解氧还可以参与生成H2O2的反应(式2—3),生成的H2O2在光催化剂的作用下产生·OH,从而参与污染物降解. 另外,Ilisz等[29]发现溶解的O2可以与形成的有机自由基发生反应(式 4),所产生的有机氧自由基能加快与主物质反应致使其转化为中间产物.
Youn等[29 − 32]在利用TiO2异质光催化剂去除污染物时发现,溶解氧在异质光催化剂表面能够有效清除电子[33 − 34]、避免电子空穴对重新结合[32],并提高两者的分离效率从而促进光催化进程[35]. 为了能够更好地捕获电子,催化剂需要具备吸附水中溶解氧的功能,如典型光催化剂TiO2的Ti3+位点能够吸附溶解氧且该位点上的溶解氧有很高的电子消耗率[36 − 37],换而言之,该类光催化剂对溶解氧有依赖性. 水溶液中溶解氧的增加能够提高·OH的形成速度[38],但超越一定浓度时,过多的气泡吸附在光催化剂的周围,在一定程度上会阻碍光催化剂表面对污染物的吸附[39],导致污染物降解效率下降. 另一方面,初始溶解氧浓度也可以通过影响光催化剂的表面电荷状态,实现对反应内在速率的调节[40];一些具有缺陷设计的半导体材料会改善光催化剂的电子结构、光吸收性能和表面吸附性能等[41],缺陷可以作为电子捕获中心[42],与溶解氧协同促进RSs的生成,同时反应速率也随之增加[38, 43]. 此外,溶液中的氧气流作为搅拌介质还可以放大光照辐射系统中的传质作用[44].
一般来说,光催化反应通过光源激发催化剂表面上的电子跃迁从而致使污染物降解[45]. 然而,对于一些能够直接光解的光敏性污染物,其吸收光后能够受激发形成电子,形成具有反应活性的自由基或激发态,从而发生光化学反应. 溶解氧在污染物直接光解的过程中可通过奇电子或三重态反应形成光生自由基参与降解,加快动力学反应速率. 然而,溶解氧对一些污染物的激发态有淬灭吸收作用,如萘普生的激发态NP*,氧氟沙星的激发态3OFL*以及双氯芬酸的激发态DCF*,溶解氧会抑制其直接光解[46 − 48].
综上,初始溶解氧浓度在光催化降解反应中能够影响光催化反应中自由基的产生和传递、催化剂表面的电荷转移以及污染物降解转化产物的种类. 提高初始溶解氧浓度可加速光催化降解过程. 然而,过多的鼓气气泡也会影响光催化剂的稳定性,降低反应效率. 因此,在光催化降解的工程应用中,可通过增加氧气供应量或进行曝气等措施来提高溶解氧浓度,以增强光催化反应的效果. 同时,也需要结合具体反应条件如应温度、污染物种类及浓度、光源强度和波长等因素的影响,进行合理的溶解氧浓度控制提高反应速率和稳定性,达到理想的处理效果.
-
芬顿(Fenton)反应是经典的AOPs之一[49],它是基于过氧化氢(H2O2)和亚铁离子(Fe2+)的化学氧化反应[50],并进一步产生·OH来降解污染物(式5—7)[51]. 芬顿氧化的效率受到温度、H2O2浓度、pH和Fe2+浓度等参数的影响,需要一系列特定的条件,以最低能耗来最大限度实现对有机物的去除. 由反应机理可知,H2O2、Fe2+和Fe3+转换以及产生RSs攻击污染物是芬顿反应的关键步骤,溶解氧浓度对这些步骤中的含氧活性物种生成速率都有不同程度的影响.
随着技术的更新,零价铁(Fe0)作为一种廉价和环保的强还原剂,常被用作芬顿体系中Fe2+的来源[52 − 53],一些研究表明:在酸性的水溶液中,Fe0还能够与溶解氧生成H2O2(式8),大大降低了反应成本[54 − 55]. 随着芬顿技术的发展,光-芬顿、电-芬顿等类芬顿反应以及耦合反应因更优异的污染物去除效率而引起人们关注[56 − 57],溶解氧在这些复合催化体系中作用也有所不同. 光芬顿反应对抗生素污染处理的活性严重依赖H2O2的用量,Du等[58]充分利用了溶解氧所带来的效益,构建磷酸盐改性的TiO2-Fe双位S型异质结,使得H2O2分子在磷酸盐位点上分解产生电子[59],并促使电子在内部电场的驱动下流向金属位点,将吸附的溶解氧还原成O2·−,巧妙地通过双位点配制和定向电子转移来提高溶解氧的利用率,从而减少H2O2消耗. 不仅如此,自Pan等[60]报道了具有高选择性和宽pH值稳定性的单线态氧1O2介导的类芬顿反应降解有机污染物后,人们尝试解决类芬顿体系中1O2产率低的问题[61],并且发现溶解氧浓度与其产率息息相关[62]. 从氧转移机制分析来看,体系中存在溶解氧接收单电子转移生成O2·−以及接收双电子转移生成H2O2的反应(式9—11);生成的H2O2经过Fe(Ⅱ)/Fe(Ⅲ)的氧化还原循环(E0 = 0.77 V)诱导传统芬顿反应生成·OH,而O2·−则可通过异质催化剂掺杂金属—如Mn(Ⅳ)/Mn(Ⅲ)的氧化还原循环(E0 = 1.06 V)生成1O2(E0 = −0.65 V)[63]. 此外,异质电芬顿体系中使用过渡金属化合物做阴极[64],能够直接吸附水中的溶解氧发生双电子的氧还原反应(oxygen reduction reaction, ORR)[65 − 66],进一步生成 H2O2,随后在活性位点上原位催化生成·OH [67 − 68],该过程被广泛应用于水中痕量有机污染物的降解[69]. 值得注意的是,当类芬顿体系中存在草酸等容易受光激发产生有机自由基物质时,溶解氧会起到淬灭有机自由基的作用,从而抑制降解反应(式12—13)[70]. 除此之外,在利用芬顿反应还原重金属六价铬Cr (Ⅵ)时,由于Cr(Ⅵ)和O2都是电子受体,彼此存在竞争性,溶解氧的存在会降低Cr(Ⅵ)的还原率.
由此可见,溶解氧在芬顿、类芬顿以及芬顿耦合工艺体系中起着至关重要的作用,是与电子反应生成O2·−和H2O2,进而转变成羟基自由基的关键物质. 在实际工程中可以通过搅拌增加液体的氧气弥散、提高反应温度、改进气体分配系统等方式调控溶解氧浓度,同时设计改进反应器形式、催化剂来提高溶解氧的利用率有助于减少能耗,实现更好的工程应用效果.
-
基于过硫酸盐(PS)的AOPs在基础研究和实际应用中都受到越来越多的关注[71 − 72],其主要通过热、光或过渡金属等激活PS(包括过一硫酸盐PMS和过二硫酸盐PDS)生成各种RSs(·OH、SO4·−和1O2等)来降解有机污染物(式 14—18)[73].
溶解氧在PS体系中对有机污染物降解表现出两面性[74]:在还原PS体系降解六氯乙烷过程中,溶解氧具有消极作用[75]. 然而在氧化PS体系中,溶解氧有利于苯[76]、环烷酸[77]和一些微污染物的降解[78]. Zhang等[78]根据量子化学计算研究了溶解氧的影响,表明氧分子可以增加有机物的吉布斯自由能,从而促进SO4·−引发对污染物的氧化反应. Xu等[74]的动力学实验揭示了在热活化PS氧化体系中,溶解氧作为有效的氧化剂可以促进降解环烷酸. 在超过80 ℃的条件下,能实现完全矿化,其中四到六成的总有机碳(TOC)和溶解氧的存在有关[77]. 在腐殖酸类有机物激活PS处理复杂有机污染物时,Fang等利用GC-MS、LC-QTOF-MS检测比较有氧和无氧条件下的降解副产物,推测过程中可能形成有机自由基(R·),这些有机自由基倾向于与溶解氧反应形成O2·−/HO2·(式 19—22),并在PS体系中起到积极的作用[79]. 因此,适量的溶解氧可以促进PS的热力学过程和氧化过程,并提高PS与污染物相互作用的频率和强度[80].
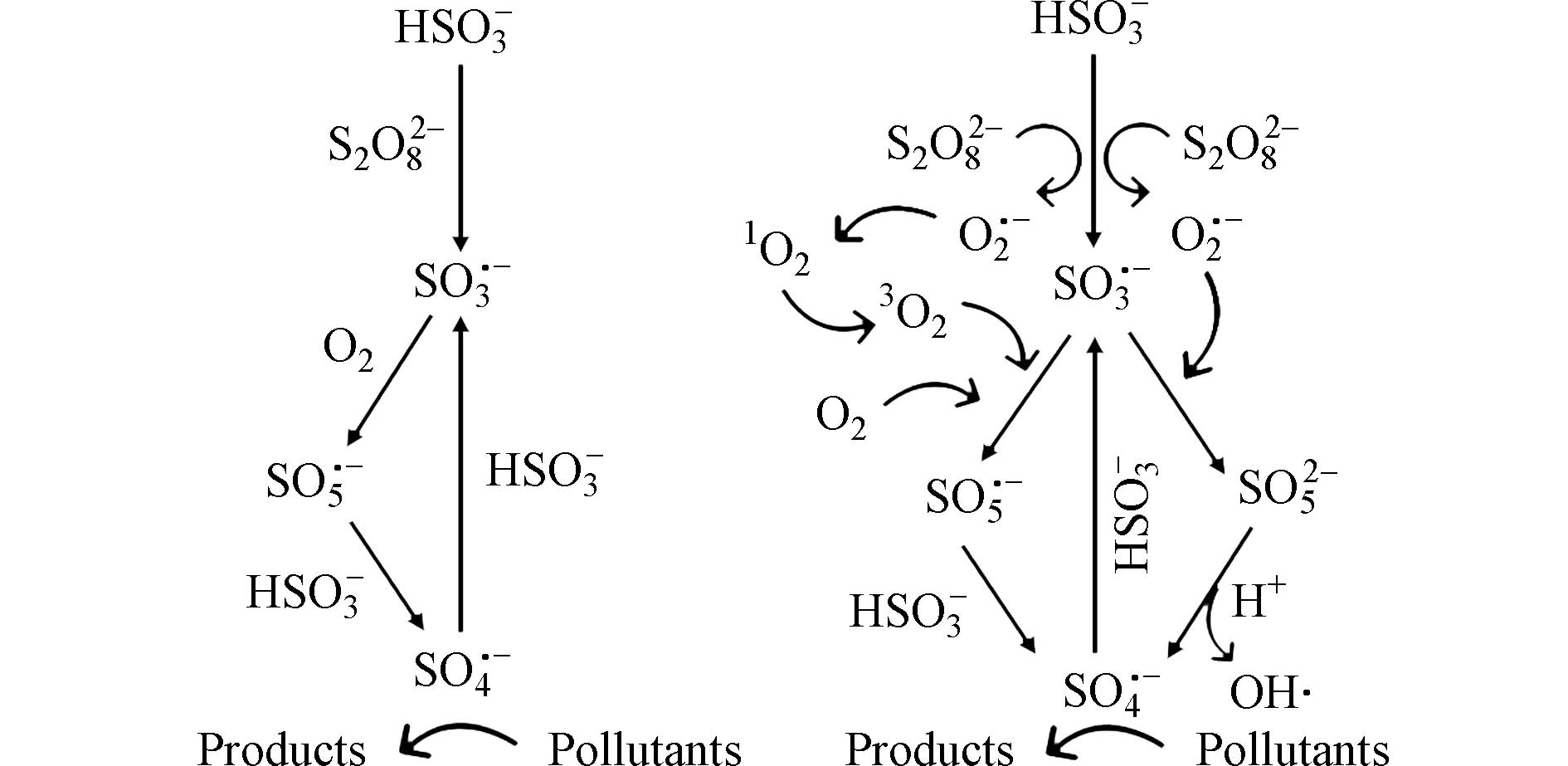
溶解氧浓度还可以影响PS氧化技术中RSs的产生路径和循环反应. Wang等[81]研究溶解氧和O2·−在PS/bisulfite体系下可能的反应机制(图3),表明溶解氧能够与SO3·−反应生成SO5·−,最终转化为SO4·−,换而言之,除了上述对有机污染物降解效率的直接影响,初始溶解氧浓度还可以影响PS技术中复杂反应的速率和机理并促进PS体系中氧、硫化合物的转化循环[82]. 由于氧、硫转化路径中溶解氧不可或缺的作用,在基于过硫酸盐氧化的实际应用中,可以根据具体反应条件和要求选择不同的方法来进行溶解氧浓度的调节和优化,如通过空气通气、微气泡法等来增加溶解氧浓度,提高过硫酸盐氧化处理的效率和效果.
-
臭氧(ozone)是一种强效氧化剂(氧化电位2.07 V),可用于直接氧化有机污染物(直接作用在具有碳碳双键的化合物上,使其断裂)或作为其他活性物种(如·OH)的前体物参与有机污染物降解. 当臭氧与水接触时,它变得极不稳定,通过一系列复杂的反应进行分解(式23—31)生成多种RSs. 紫外线与臭氧的耦合高级氧化过程UV/O3是一种常见的AOPs,可用于水和空气的净化[83]. 类似于UV/H2O2和UV/Cl等这些过程,其技术原理是利用UV的能量激发氧分子和氧化剂,产生高活性的RSs(如·OH、O2·−和活性氯物种等)氧化和降解污染物,并达到去除水中有机污染物的目的[84]. 一般来说,UV/O3也从臭氧的光解开始—在一定波长的UV照射下臭氧衍生·OH从而分解和矿化污染物[85]. 其中,溶解氧能够抑制已经形成的自由基阳离子和电子的重组,增加O2·−和过氧羟基自由基(HO2·)等由溶解氧质子化形成的含氧活性物种的浓度[86]. 研究证明了在UV作用下的氧转移机制:氧分子会发生光解反应生成两个氧原子,氧原子和另一氧分子相结合形成臭氧[87],同时臭氧也因吸收UV而分解. 由于两者是可逆反应,溶解氧浓度会影响其反应速率,当达到平衡浓度时,升高溶解氧浓度对体系没有进一步的促进作用[88]. 以UV/O3降解水中的苯酚为例,相较于无氧条件,水中溶解氧的存在能够明显提高苯酚的去除率,但提高溶解氧浓度对苯酚去除率的提升不明显[89]. 实验结果表明,溶解氧在臭氧氧化体系中具有强电子竞争性,能够争夺电子来提高含氧活性物种的浓度,并促进有机污染物的降解,但当溶解氧浓度达到可逆反应平衡浓度时,对体系的促进作用甚微,明确地指示了溶解氧浓度-效应关系. 因此,利用好溶解氧与臭氧的转换特性及其电子受体的特点,提高溶解氧利用率,实际应用中需确定并保持最佳溶解氧浓度范围,对优化臭氧氧化体系具有重要意义.
-
声化学氧化是一种利用超声波“气蚀现象”产生的能量将水分子热解(式32)成RSs(OH·、HO2·和H2O2等)的技术[90],能够有效去除废水中的污染物并且不产生二次污染. 由于超声波氧化的主要动力来源于高能量的超声波在液体中产生微小气泡并快速爆破塌陷,因此溶液中的气体浓度水平对超声氧化体系的影响不容忽视.
研究发现,溶解氧可以通过促进声波空化现象(超声波在液体中传播时引起液体中的气泡形成和破裂)的发生,从而影响声波空化过程中产生的氧化剂的种类和数量,提高超声氧化的效果[90 − 91]. 空化过程中会产生大量的自由基和其他反应物质(式 33—36),较高的溶解氧浓度可以增加产生的·OH和O2·−的数量对水中的有机污染物进行氧化降解,从而增强超声氧化的效果[92]. 此外,影响声波空化的因素有超声波频率、液体的表面张力与黏滞系数以及液体的温度等,声波空化的频率会随着条件变化而降低,导致超声氧化的速率减缓[93 − 94]. 因此在实际应用中应采用溶解氧分压计检测并通过控制气泡鼓入速度调节溶液中溶解氧浓度.
除了自由基影响,Moriwaki等[95]进一步解释了不同种类气体喷射条件下,超声氧化效率随着气体比热比的增加而增加,声空化的热量不易从气蚀气泡流失到溶液中;并且维持较高的热梯度,能保证能量释放,因此比热比高的气体曝气条件下超声氧化降解效率较好. 然而,超声氧化技术的主要缺点是能耗和较长的反应时间[96],因此超声氧化技术常与其他高级氧化过程耦合[97 − 99],其中与溶解氧耦合被多次证明能转化成一定数量的·OH,并成为主要的RSs,进一步增强有机污染物的降解和矿化[100]. 超声处理中加入高效可回收的压电催化剂(如ZnO、MoS2、BiFeO3和BaTiO3 等)[101 − 105],在超声波振动下利用自然机械能来实现水体净化,溶解氧参与的反应可解释为(式37—41). 结果表明,在设计压电催化材料时通过修饰可控氧空位来增强材料对水中溶解氧的吸附和活化[106],提高后续的RSs产出.
-
电化学氧化法因其高效率、环境适应性和安全性成为目前较有前景的有机污染物降解方法之一. 在电化学体系中,降解途径分为直接氧化(污染物被吸附在电极表面后被电子破坏)和间接氧化(阳极和阴极反应生成强氧化性的自由基攻击污染物)[107],前者受限于有机化合物在体系中传输速度的差异[108],而后者受电极材料性质、电解液和实验条件的影响[109].
溶解氧作为唯一的氧源,对电极和活性基团均有影响[110]. 研究发现,提高溶解氧浓度能够有效抑制TiO2负载的光阳极表面电子-空穴对复合[111],增大了催化效率. 光催化燃料电池在有氧和无氧条件下对氧氟沙星的最终降解效率相差无几[28],但却存在截然不同的降解途径:溶解氧存在条件下,与电子反应形成O2·−,O2·−是参与氧氟沙星降解的主要RSs;而无氧条件下,水合电子eaq−会替代O2·−成为主要的RSs,对有机污染物发起直接电子攻击,进一步发现氧氟沙星的降解速率常数随着溶解氧浓度的提高而加快,且电池电压也随之增大,原因是溶解氧在水中形成的电位差能够增强两个电极之间的电势[112]. 另外,溶解氧在阴极[113]能够通过双电子还原形成H2O2,随即H2O2被激活[114],产生·OH [115];或通过单电子转移形成O2·−的方式来促进酚类化合物和染料等有机污染物的降解(图4). 值得注意的是,由于溶解氧可以和金属电极发生氧化反应(式 42),因此溶解氧浓度过高(还原剂不足)会导致电极材料的腐蚀[116]. 更有研究表明,高浓度的溶解氧通过水的自离子化反应生成的氢离子和氢氧根离子会影响电解液的导电性,降低电解质的导电率[117],从而影响电化学反应的进行. 因此,在利用电化学氧化法去除污染物的实际工程应用中,可加强氧气通气、改善温度和pH增加溶解氧的浓度,同时可适当添加氧化剂来增加氧气的利用效率,提高反应速率.
-
初始溶解氧浓度作为影响AOPs反应过程的重要因素,其作用机制主要包括动力学(反应速率、反应机制)和热力学(能量变化和反应平衡)两个方面. 本文系统地综述了溶解氧在6种代表性AOPs体系(光催化氧化、芬顿氧化、过硫酸盐氧化、臭氧氧化、声化学氧化和电化学氧化)中的影响机制,为AOPs反应条件改进以及溶解氧参与的反应路径的探索提供参考.
总体来看,初始溶解氧浓度通过直接影响反应的速率和有机污染物去除效率作用于高级氧化反应. 提高初始溶解氧浓度可以提高AOPs的反应活性,同时还可以增加反应产物的选择性. 然而,初始溶解氧浓度对反应的影响并不总是线性的,不同的AOPs对初始溶解氧的依赖程度不同. 对于光催化反应,热力学分析表明溶解氧在光催化体系中能够快速结合电子,降低电子-空穴对的复合率,大大提升催化剂的使用效率和有机污染物的降解速率;在芬顿和过硫酸盐等含氧化剂体系中,溶解氧机制较为复杂——在参与氧物种循环的同时协同促进耦合氧化技术的反应,因此从动力学角度来看,溶解氧浓度的增加可以加快反应速率;臭氧氧化体系中,溶解氧能够促进含氧活性物种的生成;声化学氧化中,溶解氧可以促进声波空化现象的发生来加强超声氧化降解;同时,溶解氧作为电化学阴极反应物的同时,其浓度变化也会影响电化学氧化体系的稳定性、电极材料的腐蚀以及电解液的导电性. 在实际应用中,需要根据具体技术和反应条件,综合考虑溶解氧的浓度、流速、温度等因素,以最优条件促进反应过程的高效进行.
通过分析评价溶解氧对AOPs的影响机制可知,调控溶解氧浓度以提升AOPs效率的研究仍需深入科学层面和应用层面的探索,需明确氧原子的转移机制;耦合工艺中溶解氧在不同界面、不同物质循环中复杂的作用机制还需深入探讨和完善. 目前对于通过调节溶解氧浓度的手段来降低AOPs的能耗、提高处理效率和工程经济性的研究大多处于实验阶段,因效能或人为控制问题未能广泛应用于实际,未来研究需加强实验与应用相结合,将实验研究成果转化为实际工程中的技术与设备,进一步推动AOPs的发展和应用.
溶解氧对高级氧化工艺影响机制研究进展
Review: Mechanism of dissolved oxygen influence on advanced oxidation processes
-
摘要: 水溶液中的有机污染物引起了一系列生态环境问题. 现阶段,高级氧化工艺(AOPs)广泛应用于水环境中有机污染物的高效处理,其处理过程的初始溶解氧浓度是影响高级氧化反应的重要参数条件. 通过调节溶解氧浓度,以及协同控制pH和其他技术条件可以有效降低AOPs的能耗,提高处理效率和经济效益. 本文针对6种代表性AOPs,开展初始溶解氧浓度对氧化体系的影响机制分析,并分别对溶解氧参与的活性物种生成、污染物分解、催化体系影响过程进行了梳理. 最后总结了溶解氧在AOPs中的重要性及工程应用中的调控思路,为今后利用参数调控对AOPs降低能耗提升效率的优化研究提供参考.Abstract: Organic pollutants in aqueous solutions cause a series of ecological and environmental issues. At present, advanced oxidation processes (AOPs) are widely used for efficient wastewater treatment of organic pollutants. Initial dissolved oxygen concentration of the treatment is an important parameter that affects advanced oxidation reactions. By regulating the concentration of dissolved oxygen as well as synergistically controlling pH and other reaction conditions, the energy consumption of AOPs can be reduced, the treatment efficiency and economic feasibility can be further improved. This paper analyzes the impact of initial dissolved oxygen concentration on oxidation systems for six representative AOPs, and summarizes the involvement of dissolved oxygen in the generation of active species, pollutant decomposition, and catalytic processes. The importance of dissolved oxygen in AOPs and its regulatory ideas in engineering applications are discussed, providing references for future optimization research on parameter regulation to reduce AOPs energy consumption and improve efficiency.
-
Key words:
- dissolved oxygen /
- AOPs /
- mechanisms.
-
-
[1] 向伟铭, 杨绍贵, 孙敦宇, 等. 高级氧化技术去除水中碘代X射线造影剂研究进展[J]. 环境化学, 2022, 41(1): 260-275. doi: 10.7524/j.issn.0254-6108.2020101602 XIANG W M, YANG S G, SUN D Y, et al. Research progress of advanced oxidation technology to remove iodine X-ray contrast media in water[J]. Environmental Chemistry, 2022, 41(1): 260-275(in Chinese). doi: 10.7524/j.issn.0254-6108.2020101602
[2] USHANI U, LU X Q, WANG J H, et al. Sulfate radicals-based advanced oxidation technology in various environmental remediation: A state-of-the–art review[J]. Chemical Engineering Journal, 2020, 402: 126232. doi: 10.1016/j.cej.2020.126232 [3] VIDAL-DORSCH D E, BAY S M, MARUYA K, et al. Contaminants of emerging concern in municipal wastewater effluents and marine receiving water[J]. Environmental Toxicology and Chemistry, 2012, 31(12): 2674-2682. doi: 10.1002/etc.2004 [4] GARRIDO-CARDENAS J A, ESTEBAN-GARCÍA B, AGÜERA A, et al. Wastewater treatment by advanced oxidation process and their worldwide research trends[J]. International Journal of Environmental Research and Public Health, 2019, 17(1): 170. doi: 10.3390/ijerph17010170 [5] TRAN N H, REINHARD M, GIN K Y H. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review[J]. Water Research, 2018, 133: 182-207. doi: 10.1016/j.watres.2017.12.029 [6] CRINI G, LICHTFOUSE E. Advantages and disadvantages of techniques used for wastewater treatment[J]. Environmental Chemistry Letters, 2019, 17(1): 145-155. doi: 10.1007/s10311-018-0785-9 [7] National Research Council. Drinking water distribution systems: Assessing and reducing risks[M]. Washington, DC: The National Academies Press, 2006. doi. org/10.17226/11728 [8] McDONALD R I, GREEN P, BALK D, et al. Urban growth, climate change, and freshwater availability[J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(15): 6312-6317. [9] HODGES B C, CATES E L, KIM J H. Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials[J]. Nature Nanotechnology, 2018, 13(8): 642-650. doi: 10.1038/s41565-018-0216-x [10] CHEN P, BLANEY L, CAGNETTA G, et al. Degradation of ofloxacin by perylene diimide supramolecular nanofiber sunlight-driven photocatalysis[J]. Environmental Science & Technology, 2019, 53(3): 1564-1575. [11] CHEN N, SHANG H, TAO S Y, et al. Visible light driven organic pollutants degradation with hydrothermally carbonized sewage sludge and oxalate via molecular oxygen activation[J]. Environmental Science & Technology, 2018, 52(21): 12656-12666. [12] GUO F, ZHANG H, LI H, et al. Modulating the oxidative active species by regulating the valence of palladium cocatalyst in photocatalytic degradation of ciprofloxacin[J]. Applied Catalysis B: Environmental, 2022, 306: 121092. doi: 10.1016/j.apcatb.2022.121092 [13] SUSLICK K S. Mechanochemistry and sonochemistry: Concluding remarks[J]. Faraday Discussions, 2014, 170((10): ): 411-422. [14] MO Y M, XU W, ZHANG X P, et al. Enhanced degradation of rhodamine B through peroxymonosulfate activated by a metal oxide/carbon nitride composite[J]. Water, 2022, 14(13): 2054. doi: 10.3390/w14132054 [15] BRILLAS E. Recent development of electrochemical advanced oxidation of herbicides. A review on its application to wastewater treatment and soil remediation[J]. Journal of Cleaner Production, 2021, 290: 125841. doi: 10.1016/j.jclepro.2021.125841 [16] TAN X J, DING W H, JIANG Z Y, et al. Reinventing MoS2 Co-catalytic Fenton reaction: Oxygen-incorporation mediating surface superoxide radical generation[J]. Nano Research, 2022, 15(3): 1973-1982. doi: 10.1007/s12274-021-3848-3 [17] ZHANG C Y, YU Z S, WANG X Y. A review of electrochemical oxidation technology for advanced treatment of medical wastewater[J]. Frontiers in Chemistry, 2022, 10: 1002038. doi: 10.3389/fchem.2022.1002038 [18] KOE W S, LEE J W, CHONG W C, et al. An overview of photocatalytic degradation: Photocatalysts, mechanisms, and development of photocatalytic membrane[J]. Environmental Science and Pollution Research, 2020, 27(3): 2522-2565. doi: 10.1007/s11356-019-07193-5 [19] BENITEZ F J, ACERO J L, REAL F J, et al. Comparison of different chemical oxidation treatments for the removal of selected pharmaceuticals in water matrices[J]. Chemical Engineering Journal, 2011, 168(3): 1149-1156. doi: 10.1016/j.cej.2011.02.001 [20] ZU M, ZHOU X S, ZHANG S S, et al. Sustainable engineering of TiO2-based advanced oxidation technologies: From photocatalyst to application devices[J]. Journal of Materials Science & Technology, 2021, 78: 202-222. [21] WANG H, WU Y, FENG M B, et al. Visible-light-driven removal of tetracycline antibiotics and reclamation of hydrogen energy from natural water matrices and wastewater by polymeric carbon nitride foam[J]. Water Research, 2018, 144: 215-225. doi: 10.1016/j.watres.2018.07.025 [22] SI Q S, GUO W Q, WANG H Z, et al. Difunctional carbon quantum dots/g-C3N4 with in-plane electron buffer for intense tetracycline degradation under visible light: Tight adsorption and smooth electron transfer[J]. Applied Catalysis B: Environmental, 2021, 299: 120694. doi: 10.1016/j.apcatb.2021.120694 [23] LU Z, ZENG L, SONG W L, et al. in situ synthesis of C-TiO2/g-C3N4 heterojunction nanocomposite as highly visible light active photocatalyst originated from effective interfacial charge transfer[J]. Applied Catalysis B: Environmental, 2017, 202: 489-499. doi: 10.1016/j.apcatb.2016.09.052 [24] LI L P, DAI K, LI J Y, et al. A Boron-10 nitride nanosheet for combinational boron neutron capture therapy and chemotherapy of tumor[J]. Biomaterials, 2021, 268: 120587. doi: 10.1016/j.biomaterials.2020.120587 [25] WANG Q, ZHU F, CHENG H, et al. Efficient activation of persulfate by Ti3C2 MXene QDs modified ZnFe2O4 for the rapid degradation of tetracycline[J]. Chemosphere, 2023, 328: 138546. doi: 10.1016/j.chemosphere.2023.138546 [26] DIESEN V, JONSSON M. Formation of H2O2 in TiO2 photocatalysis of oxygenated and deoxygenated aqueous systems: A probe for photocatalytically produced hydroxyl radicals[J]. The Journal of Physical Chemistry C, 2014, 118(19): 10083-10087. doi: 10.1021/jp500315u [27] KONDRAKOV A O, IGNATEV A N, LUNIN V V, et al. Roles of water and dissolved oxygen in photocatalytic generation of free OH radicals in aqueous TiO2 suspensions: An isotope labeling study[J]. Applied Catalysis B: Environmental, 2016, 182: 424-430. doi: 10.1016/j.apcatb.2015.09.038 [28] CHEN X Y, YAO J J, XIA B, et al. Influence of pH and DO on the ofloxacin degradation in water by UVA-LED/TiO2 nanotube arrays photocatalytic fuel cell: Mechanism, ROSs contribution and power generation[J]. Journal of Hazardous Materials, 2020, 383: 121220. doi: 10.1016/j.jhazmat.2019.121220 [29] ILISZ I, LÁSZLÓ Z, DOMBI A. Investigation of the photodecomposition of phenol in near-UV-irradiated aqueous TiO2 suspensions. I: Effect of charge-trapping species on the degradation kinetics[J]. Applied Catalysis A: General, 1999, 180(1/2): 25-33. [30] XEKOUKOULOTAKIS N P, DROSOU C, BREBOU C, et al. Kinetics of UV-A/TiO2 photocatalytic degradation and mineralization of the antibiotic sulfamethoxazole in aqueous matrices[J]. Catalysis Today, 2011, 161(1): 163-168. doi: 10.1016/j.cattod.2010.09.027 [31] KU Y, JUNG I L. Decomposition of monocrotophos in aqueous solution by UV irradiation in the presence of titanium dioxide[J]. Chemosphere, 1998, 37(13): 2589-2597. doi: 10.1016/S0045-6535(98)00158-1 [32] YOUN N K, HEO J E, JOO O S, et al. The effect of dissolved oxygen on the 1, 4-dioxane degradation with TiO2 and Au–TiO2 photocatalysts[J]. Journal of Hazardous Materials, 2010, 177(1/2/3): 216-221. [33] FAN W, LI Y H, WANG C L, et al. Enhanced photocatalytic water decontamination by micro-nano bubbles: Measurements and mechanisms[J]. Environmental Science & Technology, 2021, 55(10): 7025-7033. [34] YU C L, WU Z, LIU R Y, et al. Novel fluorinated Bi2MoO6 nanocrystals for efficient photocatalytic removal of water organic pollutants under different light source illumination[J]. Applied Catalysis B: Environmental, 2017, 209: 1-11. doi: 10.1016/j.apcatb.2017.02.057 [35] KIM J, DOHNÁLEK Z, KAY B D. Cryogenic CO2 formation on oxidized gold clusters synthesized via reactive layer assisted deposition[J]. Journal of the American Chemical Society, 2005, 127(42): 14592-14593. doi: 10.1021/ja055764z [36] LEE H, PARK S H, CHEONG C J, et al. Contribution of dissolved oxygen to methyl orange decomposition by liquid phase plasma processes system[J]. Ozone: Science & Engineering, 2014, 36(3): 244-248. [37] ZHONG J B, MA D, ZHAO H, et al. RETRACTED: Kinetic study on photocatalytic degradation of reactive orange 5 solution with phosphotungstic acid[J]. Journal of Molecular Catalysis A: Chemical, 2008, 283(1/2): 93-98. [38] WANG X N, BRIGANTE M, MAILHOT G, et al. Bismuth catalyst mediated degradation of p-hydroxyphenylacetic acid: Photoactivation, interfacial mechanism, and influence of some critical parameters[J]. Chemical Engineering Journal, 2018, 349: 822-828. doi: 10.1016/j.cej.2018.05.097 [39] PARK Y K, HA H H, YU Y H, et al. The photocatalytic destruction of cimetidine using microwave-assisted TiO2 photocatalysts hybrid system[J]. Journal of Hazardous Materials, 2020, 391: 122568. doi: 10.1016/j.jhazmat.2020.122568 [40] CHEN F, LIU L L, ZHANG Y J, et al. Enhanced full solar spectrum photocatalysis by nitrogen-doped graphene quantum dots decorated BiO2- x nanosheets: Ultrafast charge transfer and molecular oxygen activation[J]. Applied Catalysis B: Environmental, 2020, 277: 119218. doi: 10.1016/j.apcatb.2020.119218 [41] NAKAMURA I, NEGISHI N, KUTSUNA S, et al. Role of oxygen vacancy in the plasma-treated TiO2 photocatalyst with visible light activity for NO removal[J]. Journal of Molecular Catalysis A: Chemical, 2000, 161(1/2): 205-212. [42] SUN X S, LUO X, ZHANG X D, et al. Enhanced superoxide generation on defective surfaces for selective photooxidation[J]. Journal of the American Chemical Society, 2019, 141(9): 3797-3801. doi: 10.1021/jacs.8b13051 [43] TRUONG H B, HUY B T, RAY S K, et al. Visible light-activated NGQD/nsC3N4/Bi2WO6 microsphere composite for effluent organic matter treatment[J]. Chemical Engineering Journal, 2021, 415: 129024. doi: 10.1016/j.cej.2021.129024 [44] MOONSIRI M, RANGSUNVIGIT P, CHAVADEJ S, et al. Effects of Pt and Ag on the photocatalytic degradation of 4-chlorophenol and its by-products[J]. Chemical Engineering Journal, 2004, 97(2/3): 241-248. [45] WEI D D, WU J Q, WANG Y F, et al. Dual defect sites of nitrogen vacancy and cyano group synergistically boost the activation of oxygen molecules for efficient photocatalytic decontamination[J]. Chemical Engineering Journal, 2023, 462: 142291. doi: 10.1016/j.cej.2023.142291 [46] ZHANG N, LIU G G, LIU H J, et al. Diclofenac photodegradation under simulated sunlight: Effect of different forms of nitrogen and Kinetics[J]. Journal of Hazardous Materials, 2011, 192(1): 411-418. [47] MA D J, LIU G G, LV W Y, et al. Photodegradation of naproxen in water under simulated solar radiation: Mechanism, kinetics, and toxicity variation[J]. Environmental Science and Pollution Research, 2014, 21(13): 7797-7804. doi: 10.1007/s11356-014-2721-2 [48] ALBINI A, MONTI S. Photophysics and photochemistry of fluoroquinolones[J]. Chemical Society Reviews, 2003, 32(4): 238-250. doi: 10.1039/b209220b [49] CHEN Q, LÜ F, ZHANG H, et al. Where should Fenton go for the degradation of refractory organic contaminants in wastewater?[J]. Water Research, 2023, 229: 119479. doi: 10.1016/j.watres.2022.119479 [50] SUN H W, XIE G H, HE D, et al. Ascorbic acid promoted magnetite Fenton degradation of alachlor: Mechanistic insights and kinetic modeling[J]. Applied Catalysis B: Environmental, 2020, 267: 118383. doi: 10.1016/j.apcatb.2019.118383 [51] HE P J, LIU W Y, QIU J J, et al. Improvement criteria for different advanced technologies towards bio-stabilized leachate based on molecular subcategories of DOM[J]. Journal of Hazardous Materials, 2021, 414: 125463. doi: 10.1016/j.jhazmat.2021.125463 [52] MYLON S E, SUN Q, WAITE T D. Process optimization in use of zero valent iron nanoparticles for oxidative transformations[J]. Chemosphere, 2010, 81(1): 127-131. doi: 10.1016/j.chemosphere.2010.06.045 [53] LI X Q, ELLIOTT D W, ZHANG W X. Zero-valent iron nanoparticles for abatement of environmental pollutants: Materials and engineering aspects[J]. Critical Reviews in Solid State and Materials Sciences, 2006, 31(4): 111-122. doi: 10.1080/10408430601057611 [54] SHIMIZU A, TOKUMURA M, NAKAJIMA K, et al. Phenol removal using zero-valent iron powder in the presence of dissolved oxygen: Roles of decomposition by the Fenton reaction and adsorption/precipitation[J]. Journal of Hazardous Materials, 2012, 201/202: 60-67. doi: 10.1016/j.jhazmat.2011.11.009 [55] WANG K S, LIN C L, WEI M C, et al. Effects of dissolved oxygen on dye removal by zero-valent iron[J]. Journal of Hazardous Materials, 2010, 182(1/2/3): 886-895. [56] BRILLAS E. A review on the photoelectro-Fenton process as efficient electrochemical advanced oxidation for wastewater remediation. Treatment with UV light, sunlight, and coupling with conventional and other photo-assisted advanced technologies[J]. Chemosphere, 2020, 250: 126198. doi: 10.1016/j.chemosphere.2020.126198 [57] MÁRQUEZ A A, SIRÉS I, BRILLAS E, et al. Mineralization of Methyl Orange azo dye by processes based on H2O2 electrogeneration at a 3D-like air-diffusion cathode[J]. Chemosphere, 2020, 259: 127466. doi: 10.1016/j.chemosphere.2020.127466 [58] DU Z L, ZHU C Q, JING S C, et al. Boosting dissolved oxygen utilization by oriented electron transfer on dual-site S-scheme heterojunction for low-H2O2-consumption photo-Fenton reaction[J]. Chemical Engineering Journal, 2023, 462: 142146. doi: 10.1016/j.cej.2023.142146 [59] WANG Y F, ZHANG M T. Proton-coupled electron-transfer reduction of dioxygen: The importance of precursor complex formation between electron donor and proton donor[J]. Journal of the American Chemical Society, 2022, 144(27): 12459-12468. doi: 10.1021/jacs.2c04467 [60] YIN Y, LV R L, ZHANG W M, et al. Exploring mechanisms of different active species formation in heterogeneous Fenton systems by regulating iron chemical environment[J]. Applied Catalysis B: Environmental, 2021, 295: 120282. doi: 10.1016/j.apcatb.2021.120282 [61] HONG P D, WU Z J, YANG D D, et al. Efficient generation of singlet oxygen (1O2) by hollow amorphous Co/C composites for selective degradation of oxytetracycline via Fenton-like process[J]. Chemical Engineering Journal, 2021, 421: 129594. doi: 10.1016/j.cej.2021.129594 [62] WANG Z Y, HE J G, YU D H, et al. N-doped coralline Co9S8− xN x for inducing Amitriptyline decontamination in Electro-Fenton Process: Degradation scheme Elucidation, nitrogen activating catalyst delocalized electron and enhancing 2-Electron oxygen reduction reaction mechanism investigation[J]. Chemical Engineering Journal, 2023, 457: 141171. doi: 10.1016/j.cej.2022.141171 [63] YANG Y J, SHEN H Y, XU L J. Three-dimensional graphene anchored nZVI hybrid MnO2 as a dissolved oxygen activated Fenton-like catalyst for efficient mineralization of oxytetracycline[J]. Chemical Engineering Journal, 2023, 464: 142781. doi: 10.1016/j.cej.2023.142781 [64] YAO B, LUO Z R, ZHI D, et al. Current progress in degradation and removal methods of polybrominated diphenyl ethers from water and soil: A review[J]. Journal of Hazardous Materials, 2021, 403: 123674. doi: 10.1016/j.jhazmat.2020.123674 [65] LIU Y M, CHEN S, QUAN X, et al. Efficient mineralization of perfluorooctanoate by electro-Fenton with H2O2 electro-generated on hierarchically porous carbon[J]. Environmental Science & Technology, 2015, 49(22): 13528-13533. [66] YU F K, ZHANG Y F, ZHANG Y, et al. Promotion of the degradation perfluorooctanoic acid by electro-Fenton under the bifunctional electrodes: Focusing active reaction region by Fe/N co-doped graphene modified cathode[J]. Chemical Engineering Journal, 2023, 457: 141320. doi: 10.1016/j.cej.2023.141320 [67] ZHU Y S, DENG F X, QIU S, et al. A self-sufficient electro-Fenton system with enhanced oxygen transfer for decontamination of pharmaceutical wastewater[J]. Chemical Engineering Journal, 2022, 429: 132176. doi: 10.1016/j.cej.2021.132176 [68] YU D H, HE J G, WANG Z Y, et al. Mineralization of norfloxacin in a CoFe–LDH/CF cathode-based heterogeneous electro-Fenton system: Preparation parameter optimization of the cathode and conversion mechanisms of H2O2 to ·OH[J]. Chemical Engineering Journal, 2021, 417: 129240. doi: 10.1016/j.cej.2021.129240 [69] GUO D L, JIANG S T, JIN L M, et al. CNT encapsulated MnOx for an enhanced flow-through electro-Fenton process: The involvement of Mn(iv)[J]. Journal of Materials Chemistry A, 2022, 10(30): 15981-15989. doi: 10.1039/D2TA03445J [70] ARIS A, SHARRATT P N. Influence of initial dissolved oxygen concentration on Fenton’s reagent degradation[J]. Environmental Technology, 2006, 27(10): 1153-1161. doi: 10.1080/09593332708618729 [71] KOHANTORABI M, MOUSSAVI G, GIANNAKIS S. A review of the innovations in metal- and carbon-based catalysts explored for heterogeneous peroxymonosulfate (PMS) activation, with focus on radical vs. non-radical degradation pathways of organic contaminants[J]. Chemical Engineering Journal, 2021, 411: 127957. doi: 10.1016/j.cej.2020.127957 [72] GAO Y, WANG Q, JI G Z, et al. Degradation of antibiotic pollutants by persulfate activated with various carbon materials[J]. Chemical Engineering Journal, 2022, 429: 132387. doi: 10.1016/j.cej.2021.132387 [73] LEE J, von GUNTEN U, KIM J H. Persulfate-based advanced oxidation: Critical assessment of opportunities and roadblocks[J]. Environmental Science & Technology, 2020, 54(6): 3064-3081. [74] XU X Y, PLIEGO G, ALONSO C, et al. Reaction pathways of heat-activated persulfate oxidation of naphthenic acids in the presence and absence of dissolved oxygen in water[J]. Chemical Engineering Journal, 2019, 370: 695-705. doi: 10.1016/j.cej.2019.03.213 [75] ZHU C Y, ZHU F X, LIU C, et al. Reductive hexachloroethane degradation by S2O8•– with thermal activation of persulfate under anaerobic conditions[J]. Environmental Science & Technology, 2018, 52(15): 8548-8557. [76] LIU H Z, BRUTON T A, LI W, et al. Oxidation of benzene by persulfate in the presence of Fe(Ⅲ)- and Mn(Ⅳ)-containing oxides: Stoichiometric efficiency and transformation products[J]. Environmental Science & Technology, 2016, 50(2): 890-898. [77] XU X Y, PLIEGO G, ZAZO J A, et al. Mineralization of naphtenic acids with thermally-activated persulfate: The important role of oxygen[J]. Journal of Hazardous Materials, 2016, 318: 355-362. doi: 10.1016/j.jhazmat.2016.07.009 [78] ZHANG R, WANG X X, ZHOU L, et al. The impact of dissolved oxygen on sulfate radical-induced oxidation of organic micro-pollutants: A theoretical study[J]. Water Research, 2018, 135: 144-154. doi: 10.1016/j.watres.2018.02.028 [79] FANG G D, GAO J, DIONYSIOU D D, et al. Activation of persulfate by quinones: Free radical reactions and implication for the degradation of PCBs[J]. Environmental Science & Technology, 2013, 47(9): 4605-4611. [80] YANG X R, DING X, ZHOU L, et al. New insights into clopyralid degradation by sulfate radical: Pyridine ring cleavage pathways[J]. Water Research, 2020, 171: 115378. doi: 10.1016/j.watres.2019.115378 [81] WANG J Q, WANG C J, GUO H G, et al. Crucial roles of oxygen and superoxide radical in bisulfite-activated persulfate oxidation of bisphenol AF: Mechanisms, kinetics and DFT studies[J]. Journal of Hazardous Materials, 2020, 391: 122228. doi: 10.1016/j.jhazmat.2020.122228 [82] WEI Y, ZOU Q C, YE P, et al. Photocatalytic degradation of organic pollutants in wastewater with g-C3N4/sulfite system under visible light irradiation[J]. Chemosphere, 2018, 208: 358-365. doi: 10.1016/j.chemosphere.2018.06.006 [83] GUO W Q, YANG Z Z, DU J S, et al. Degradation of sulfadiazine in water by a UV/O3 process: Performance and degradation pathway[J]. RSC Advances, 2016, 6(62): 57138-57143. doi: 10.1039/C6RA09078H [84] MANSOURI L, TIZAOUI C, GEISSEN S U, et al. A comparative study on ozone, hydrogen peroxide and UV based advanced oxidation processes for efficient removal of diethyl phthalate in water[J]. Journal of Hazardous Materials, 2019, 363: 401-411. doi: 10.1016/j.jhazmat.2018.10.003 [85] WANG J, LIU H B, GAO Y, et al. Pilot-scale advanced treatment of actual high-salt textile wastewater by a UV/O3 pressurization process: Evaluation of removal kinetics and reverse osmosis desalination process[J]. Science of the Total Environment, 2023, 857: 159725. doi: 10.1016/j.scitotenv.2022.159725 [86] ALAPI T, BERECZ L, ARANY E, et al. Comparison of the UV-induced photolysis, ozonation, and their combination at the same energy input using a self-devised experimental apparatus[J]. Ozone: Science & Engineering, 2013, 35(5): 350-358. [87] WU Q Y, YANG Z W, DU Y, et al. The promotions on radical formation and micropollutant degradation by the synergies between ozone and chemical reagents (synergistic ozonation): A review[J]. Journal of Hazardous Materials, 2021, 418: 126327. doi: 10.1016/j.jhazmat.2021.126327 [88] HUA Z C, GUO K H, KONG X J, et al. PPCP degradation and DBP formation in the solar/free chlorine system: Effects of pH and dissolved oxygen[J]. Water Research, 2019, 150: 77-85. doi: 10.1016/j.watres.2018.11.041 [89] ILAN Y, RABANI J. On some fundamental reactions in radiation chemistry: Nanosecond pulse radiolysis[J]. International Journal for Radiation Physics and Chemistry, 1976, 8(5): 609-611. doi: 10.1016/0020-7055(76)90030-9 [90] GASMI I, HAMDAOUI O, FERKOUS H, et al. Sonochemical advanced oxidation process for the degradation of furosemide in water: Effects of sonication’s conditions and scavengers[J]. Ultrasonics Sonochemistry, 2023, 95: 106361. doi: 10.1016/j.ultsonch.2023.106361 [91] KERBOUA K, MEROUANI S, HAMDAOUI O, et al. How do dissolved gases affect the sonochemical process of hydrogen production? An overview of thermodynamic and mechanistic effects–On the “hot spot theory”[J]. Ultrasonics Sonochemistry, 2021, 72: 105422. doi: 10.1016/j.ultsonch.2020.105422 [92] GAO Y Q, GAO N Y, DENG Y, et al. Factors affecting sonolytic degradation of sulfamethazine in water[J]. Ultrasonics Sonochemistry, 2013, 20(6): 1401-1407. doi: 10.1016/j.ultsonch.2013.04.007 [93] MARGULIS M A. Fundamental aspects of sonochemistry[J]. Ultrasonics, 1992, 30(3): 152-155. doi: 10.1016/0041-624X(92)90065-T [94] MAKINO K, MOSSOBA M M, RIESZ P. Chemical effects of ultrasound on aqueous solutions. Formation of hydroxyl radicals and hydrogen atoms[J]. The Journal of Physical Chemistry, 1983, 87(8): 1369-1377. doi: 10.1021/j100231a020 [95] MORIWAKI H, TAKAGI Y, TANAKA M, et al. Sonochemical decomposition of perfluorooctane sulfonate and perfluorooctanoic acid[J]. Environmental Science & Technology, 2005, 39(9): 3388-3392. [96] RAYAROTH M P, ARAVIND U K, ARAVINDAKUMAR C T. Degradation of pharmaceuticals by ultrasound-based advanced oxidation process[J]. Environmental Chemistry Letters, 2016, 14(3): 259-290. doi: 10.1007/s10311-016-0568-0 [97] HU Y B, LO S L, LI Y F, et al. Autocatalytic degradation of perfluorooctanoic acid in a permanganate-ultrasonic system[J]. Water Research, 2018, 140: 148-157. doi: 10.1016/j.watres.2018.04.044 [98] XU D, MA H L. Degradation of rhodamine B in water by ultrasound-assisted TiO2 photocatalysis[J]. Journal of Cleaner Production, 2021, 313: 127758. doi: 10.1016/j.jclepro.2021.127758 [99] LU X H, QIU W, PENG J L, et al. A review on additives-assisted ultrasound for organic pollutants degradation[J]. Journal of Hazardous Materials, 2021, 403: 123915. doi: 10.1016/j.jhazmat.2020.123915 [100] LU X H, ZHAO J N, WANG Q, et al. Sonolytic degradation of bisphenol S: Effect of dissolved oxygen and peroxydisulfate, oxidation products and acute toxicity[J]. Water Research, 2019, 165: 114969. doi: 10.1016/j.watres.2019.114969 [101] ZHAO Z, WANG D D, GAO R, et al. Magnetic-field-stimulated efficient photocatalytic N2 fixation over defective BaTiO3 perovskites[J]. Angewandte Chemie International Edition, 2021, 60(21): 11910-11918. doi: 10.1002/anie.202100726 [102] MA W, YAO B H, ZHANG W, et al. Fabrication of PVDF-based piezocatalytic active membrane with enhanced oxytetracycline degradation efficiency through embedding few-layer E-MoS2 nanosheets[J]. Chemical Engineering Journal, 2021, 415: 129000. doi: 10.1016/j.cej.2021.129000 [103] HUANG J, WANG Y, LIU X Q, et al. Synergistically enhanced charge separation in BiFeO3/Sn: TiO2 nanorod photoanode via bulk and surface dual modifications[J]. Nano Energy, 2019, 59: 33-40. doi: 10.1016/j.nanoen.2019.02.025 [104] WU J M, CHANG W E, CHANG Y T, et al. Piezo-catalytic effect on the enhancement of the ultra-high degradation activity in the dark by single- and few-layers MoS2 nanoflowers[J]. Advanced Materials, 2016, 28(19): 3718-3725. doi: 10.1002/adma.201505785 [105] CHEN X Y, LIU L F, FENG Y W, et al. Fluid eddy induced piezo-promoted photodegradation of organic dye pollutants in wastewater on ZnO nanorod arrays/3D Ni foam[J]. Materials Today, 2017, 20(9): 501-506. doi: 10.1016/j.mattod.2017.08.027 [106] WAN L C, TIAN W R, LI N J, et al. Hydrophilic porous PVDF membrane embedded with BaTiO3 featuring controlled oxygen vacancies for piezocatalytic water cleaning[J]. Nano Energy, 2022, 94: 106930. doi: 10.1016/j.nanoen.2022.106930 [107] COMNINELLIS C. Electrocatalysis in the electrochemical conversion/combustion of organic pollutants for waste water treatment[J]. Electrochimica Acta, 1994, 39(11/12): 1857-1862. [108] BONFATTI F, FERRO S, LAVEZZO F, et al. Electrochemical incineration of glucose as a model organic substrate. I. role of the electrode material[J]. Journal of the Electrochemical Society, 1999, 146(6): 2175-2179. doi: 10.1149/1.1391909 [109] PANIZZA M, CERISOLA G. Direct and mediated anodic oxidation of organic pollutants[J]. Chemical Reviews, 2009, 109(12): 6541-6569. doi: 10.1021/cr9001319 [110] YU T T, LIU L F, LI L, et al. A self-biased fuel cell with TiO2/g-C3N4 anode catalyzed alkaline pollutant degradation with light and without light—What is the degradation mechanism?[J]. Electrochimica Acta, 2016, 210: 122-129. doi: 10.1016/j.electacta.2016.05.162 [111] XIA B, YAO J J, HAN C X, et al. Degradation of ofloxacin by UVA-LED/TiO2 nanotube arrays photocatalytic fuel cells[J]. Chemical Papers, 2018, 72(2): 359-368. doi: 10.1007/s11696-017-0285-6 [112] LIU X W, SUN X F, LI D B, et al. Anodic Fenton process assisted by a microbial fuel cell for enhanced degradation of organic pollutants[J]. Water Research, 2012, 46(14): 4371-4378. doi: 10.1016/j.watres.2012.05.044 [113] PENG Y, HE X, ZHENG N C, et al. Transferring waste of biomass and heavy metal into photocatalysts for hydrogen peroxide activation[J]. Chemical Engineering Journal, 2021, 420: 129867. doi: 10.1016/j.cej.2021.129867 [114] HE X, ZHENG N C, HU R T, et al. Hydrothermal and pyrolytic conversion of biomasses into catalysts for advanced oxidation treatments[J]. Advanced Functional Materials, 2021, 31(7): 2006505. doi: 10.1002/adfm.202006505 [115] KUANG C Z, ZENG G S, ZHOU Y J, et al. Integrating anodic sulfate activation with cathodic H2O2 production/activation to generate the sulfate and hydroxyl radicals for the degradation of emerging organic contaminants[J]. Water Research, 2023, 229: 119464. doi: 10.1016/j.watres.2022.119464 [116] XU C M, TIAN Y, SUN J R, et al. Novel preoxidation-assisted mechanism to preciously form and disperse Bi2O3 nanodots in carbon nanofibers for ultralong-life and high-rate sodium storage[J]. ACS Applied Materials & Interfaces, 2023, 15(1): 1891-1902. [117] AGMON N, BAKKER H J, CAMPEN R K, et al. Protons and hydroxide ions in aqueous systems[J]. Chemical Reviews, 2016, 116(13): 7642-7672. doi: 10.1021/acs.chemrev.5b00736 -






 下载:
下载: