-
城市化和工业化的发展导致废水治理和水环境的污染问题日益凸显,这对居民用水安全乃至生态环境保护都有不利影响. 近年来针对污水治理进行了大量研究,包括采用热、酸/碱、超声波、紫外(UV)、微波、高级氧化过程(AOPs)、生物发酵技术和相应的组合技术等[1]. 其中AOPs是目前工程技术人员青睐的热门技术之一. AOPs是指通过物理化学方法生成自由基等高反应活性物质以高效氧化降解甚至矿化污染物的过程[2 − 3]. 从早期的活化过氧化氢产生羟基自由基,到活化过硫酸盐产生硫酸根自由基,基于过氧化物活化的AOPs技术在迅速发展[4 − 5].
过氧乙酸(PAA)是一种高效有机过氧酸,除了具有直接杀菌的能力,还能够经活化产生羟基、乙酰氧基、乙酰过氧基等自由基实现污染物的降解[6]. 研究人员在20世纪初合成了PAA,之后PAA在食品、化工、医疗以及水处理领域中的应用不断发展. PAA最开始在水处理中的应用是作为消毒剂,研究者试图用PAA来作为氯消毒的替代品,因为PAA在水处理应用中只产生少量消毒副产物,且具有广谱的杀菌性和经济性. 进一步地,美国环保局在1999年批准了将PAA用于下水道污水消毒,又在2012年批准了PAA在废水消毒方面的应用[7]. 近年来,由于PAA具有高氧化还原电位(E0=1.96 V),有学者们将PAA用来氧化降解水中的有机污染物[8]. 而最近的研究主要集中在如何借助外界能量或者助催化剂来提高PAA的氧化能力[9]. 另一方面,相比于过硫酸盐或过氧化氢,PAA和在水处理领域中的应用存在着优势与缺点[10]. 过硫酸盐作为氧化剂容易产生硫酸根离子从而导致二次污染,相对来说PAA造成的二次污染较小. 当过氧化氢作为微生物消毒剂时,相较于PAA,过氧化氢需要投入更大剂量来达到相同的微生物灭活水平. 然而,相比于过硫酸盐和过氧化氢来说,PAA的稳定性更差,这在一定程度上限制了它的应用.
根据图1呈现的共线性分析结果可以看到,消毒、污染物去除、废水处理以及高级氧化等是近10年PAA研究的主要方向,其中又涉及臭氧、过氧化氢、次氯酸钠、超声等均相技术的联用,以及有机自由基、协同作用等机制的探讨.
与其他过氧化物活化技术类似,PAA活化的均相体系同样存在能耗大、成本高、对基质透光度要求高、过渡金属离子污染水质等问题,导致实际水环境应用受到阻碍. 因此,探索低成本、环境友好的非均相活化材料是未来基于PAA活化的AOPs研究的重要方向. 然而,目前有关非均相活化PAA的研究尚处于起步阶段,关于PAA活化功能材料的开发、材料界面PAA的活化机制,以及非均相活化PAA体系的复杂水环境中的应用等方面的研究仍然缺乏系统性与针对性. 已有综述文献阐述了基于PAA的高级氧化技术在水污染环境中的应用,分析了相关水环境基质、PAA的投加量、催化剂的投加量对污染物降解的影响以及PAA在消毒领域的应用. 然而在均相和非均相体系中对PAA的活化的机制探究,以及理论计算和模型拟合在活化PAA的研究,仍缺乏相关文献给出总结或评述. 基于此,本文综述了基于PAA活化的功能材料开发与水环境应用的新近研究进展,以期为以PAA活化为导向的功能材料开发与技术运用提供思路.
-
PAA的分子式为CH3C(O)OOH,是一种无色液体,属于有机过氧化物,其理化性质如表1. PAA是一种人造化工产品,通常由乙酸和过氧化氢在硫酸催化反应条件下制备. 实验中常用的PAA溶液,一般是由32% 的PAA、6% 的H2O2以及62%的乙酸水溶液所组成的混合物. PAA的稳定性较差,多在4 ℃下保存在增厚的聚乙烯瓶中,其在碱性环境(pH > 8.2)中会水解产生乙酸和过氧化氢,在酸性环境中则水解产生CH 3C(O)OO-和H3O+[11].
-
如表2所示,PAA单独作用于水环境中能够灭活致病微生物. 这是由于PAA能够影响细菌膜转运基因的调控,并选择性地诱导与细胞增殖和保护有关的基因,使基因的转录和表达受到抑制,进而造成微生物的失活. 此外,PAA还能够在细胞膜打孔进而直接破坏病原微生物的细胞结构[13 − 15]. 研究发现PAA对水中的病原微生物具有显著的抑制作用,当PAA浓度-时间值(CT值)为32、47、69 mg·min·L−1时,分别能够导致废水中的诺如病毒减少1—3个数量级,当PAA浓度为250 mg·min·L−1时,城市废水中80%区域的大肠杆菌含量降至100 CFU·L−1[16 − 19]. 另一方面,PAA单独作用也能够实现有机污染物的降解. Zhang等[20]发现PAA能够攻击β-内酰胺的硫醚键,从而实现对抗生素的降解. PAA还能够有效分解复杂工艺中的污泥,溶解和破坏污泥絮凝体中的细菌和胞外聚合物(EPS),并将其转化为细胞碎片和可溶性胞外聚合物,其中类蛋白物质则被降解为小分子[21].
然而,单独使用PAA用来灭活微生物和降解有机物的能力仍然有限. 有研究则指出在二级废水中过氧甲酸和次氯酸的单独作用比PAA对大肠杆菌的抑制作用更强,保持大肠杆菌浓度为50000 CFU·L−1时所需过氧甲酸的CT值仅为过氧乙酸的33.18%[22 − 23]. 另外,在单独作用的降解试验中,PAA对双酚A(BPA)的氧化降解效果仍不如过氧甲酸[24]. 除了自身的氧化性能较弱,PAA在使用过程中还可能产生羧酸类和醛类等有毒副产物,在溴化物存在下,1.5 mmol·L−1的PAA能产生1—10 μg·L−1溴仿等潜在的致癌化合物[25 − 26]. 另一方面,生产工艺要求高、转化率低、产品不稳定等问题也在一定程度上限制了单独PAA的推广应用[27]. 因此,开发高效的均相和非均相技术提升PAA的活化效能成为了当前研究的热点.
-
与其他过氧化物相比,PAA的分子内过氧键键能相对低,理论上更容易在外源条件作用下生成氧化活性强的自由基物种. 近年来,基于PAA活化的高级氧化技术引起了研究人员的关注,其中均相活化技术的研究发展较早,受关注度大[28]. 均相活化PAA的方法主要有辐射活化、金属离子活化以及非金属活化.
-
辐射活化主要通过外加能量的方式破坏O—O键,从而产生自由基[29 − 30]. 常见的活化方法包括紫外(UV)、太阳辐射、超声波、微波等. UV是光活化技术最主要的研究,在水处理方面应用广泛[31]. UV激活PAA可产生羟基自由基(·OH)、碳中心自由基(R—C·)和过氧乙酸基自由基(CH3C(O)OO·),这些自由基进一步攻击有机污染物从而使其降解. 同时UV也能与PAA产生协同作用,导致病原微生物细胞结构或组分受到破坏,使与繁殖相关的关键功能基因表达受到抑制,从而降解污染物和灭活菌群[30,32 − 34]. Kibbee等[35]发现,单独PAA处理对柯萨奇病毒B3无显著抑制作用,而在低压UV/PAA体系中3 mg·L−1 PAA处理导致毒株减少了4个数量级. 另一项研究中,中压UV能够激活PAA产生·OH和CH3C(O)OO·来降解诺氟沙星,并且两种自由基在不同pH条件下对目标污染物的降解能力不同[36]. 除UV外,太阳辐射、超声波、微波同样能够促进PAA产生活性氧物种用于水处理[37 − 39]. 作为辐射的另一种形式,热活化作用同样能够激活PAA产生自由基降解污染物. 在热活化PAA体系中,双氯芬酸(DCF)和磺胺甲恶唑(SMX)的去除率分别达到96%、86% [29,40]. 然而,在实际应用中辐射活化技术存在对水环境透光能力要求高、能源成本高昂等问题,难以大规模应用[41].
-
金属离子催化是目前较为热门的研究方向[42]. 其中包括Fe(Ⅱ)/Fe(Ⅲ)、Co(Ⅱ)/Co(Ⅲ)及其他金属离子. 金属离子通过向PAA提供电子实现价态变化,从而产生具有降解能力的活性物种(式(1)—(7))[43 − 45]. Fe(Ⅱ)/Fe(Ⅲ)能够激活PAA产生高活性自由基[45]. 在最近的研究中,Fe(Ⅱ)-Fe(Ⅲ)/PAA体系中的主要作用物质还包括高价金属物种(式(4),式(6))[46 − 50]. Co(Ⅱ)是另一种活化PAA的有效金属离子试剂,同样能够产生自由基和高价金属物种(式(5)),并对污染物产生显著降解效果[50,52 − 54]. 与Fe(Ⅲ)、Co(Ⅱ)相比,Cu(Ⅱ)、Ag(Ⅰ)等对PAA的活化作用则相对较弱[55]. 值得一提的是,贵金属Ru(Ⅲ)同样能够激活PAA产生自由基降解污染物[56]. 金属离子具有活化效果显著、投加方便等优点. 然而,金属离子的活化作用本身可能会导致二次污染,而且当pH值升高时,金属离子可能会转化为氢氧化物沉淀失去激活能力,这限制了它们的应用[57].
-
均相体系中某些非金属也能够活化PAA. 例如,氯离子能够激活PAA产生自由基和单线态氧(1O2)降解污染物[58 − 59]. 碳酸盐也能与PAA反应生成碳酸根离子自由基,用于降解难降解污染物[60]. 研究发现磷酸盐缓冲液(PBS)同样能够导致PAA的活化,并且有效去除了DCF[61]. 这一过程受到碳酸氢根离子和天然有机质的显著干扰,并且说明用于控制pH的PBS在基于PAA的AOPs中使用时应该谨慎对待. 除了非金属离子,醌也能够激活PAA并产生氧中心自由基和1O2,从而降解污染物[9]. 然而,在反应过程中产生的副产物可能会增加后处理的难度.
-
均相体系中还存在不同活化剂之间的协同作用. 当使用热辐射处理Cu(Ⅱ)/PAA体系时,热活化作用能够显著增强PAA的降解能力. 在60 ℃下,Cu(Ⅱ)/PAA体系中DCF的去除率是常温下Cu(Ⅱ)/PAA体系的5.54倍[62]. UV处理则促进了体系中Fe(Ⅱ)与Fe(Ⅲ)的转化(式(7))[63]. 在Fe(Ⅱ)/PAA中引入2,2’-联氮双(3-乙基苯并噻唑啉-6-磺酸)二铵盐(ABTS)不仅加速了体系中Fe(Ⅲ)向Fe(Ⅱ)的转化,还产生了ABTS·+作为主要反应活性物质参与反应(式(8)),导致体系中DCF去除率比对照组提高了8.2倍[64].
如表3所示,基于PAA活化的均相体系能够有效杀灭病原微生物或降解有机污染物. 然而,均相体系中存在对水环境背景基质敏感、能耗大、投量大、成本高、二次污染等诸多问题,这在很大程度上限制了其推广和应用. 非均相体系能够一定程度上规避上述限制因素,有望成为基于PAA活化的高级氧化技术向应用转化的关键.
-
当前非均相PAA活化功能材料可归纳为金属基和非金属基材料两大类别,具体包括金属及其化合物、金属有机框架(MOFs)、碳材料、陶瓷膜、非金属化合物、非金属有机框架等(表4).
-
纳米级零价金属,具有反应活性高、比表面积大和表面活性位点丰富等优点[65]. 周高峰等[66]发现零价钴能够原位生成Co(Ⅱ)激活PAA产生CH3C(O)O ·和 CH3C(O)OO·,使罗丹明B在180 s内去除率达到98.3%. 硫化零价铁则激活PAA产生了·OH、R—C·和Fe(Ⅳ),导致15 min内SMX、双氯芬酸钠、布洛芬、卡马西平、萘普生和左氧氟沙星的去除率分别达到99.64%、98.5%、99.2%、99.8%、97.5%和97.4%[67]. 向均相体系中引入零价金属可以强化污染物的去除. 例如,添加纳米零价铁(nZVI)促进了UV/PAA体系中·OH和R—C·的生成,使螺旋霉素在20 min内完全降解[68].
金属化合物也能够活化PAA,其中非金属元素可能对金属元素的活化机制产生影响. Yang等[69]发现,FeS可有效活化PAA使得SMX在3 min时的去除率达80.32%. 在该体系中S元素不仅参与了Fe(Ⅱ)的循环再生,而且消耗了体系生成的有机自由基(R—O·),使得·OH的占据主导作用. 黄铁矿(FeS2)同样能够激活PAA,使四环素在30 min内完全去除. 在该过程中CH3C(O)OO·是主要作用物种[70]. 另一项研究表明,纳米级锐钛矿型TiO2 能够加速PAA的分解,但并不产生活性自由基,其存在反而抑制了PAA对大肠杆菌的灭活作用[71]. 与均相活化方法联用能够提高金属化合物活化PAA体系的效果. Li等[72]合成了钴取代锰铁氧体,在微波辐射作用下激活PAA产生1O2,导致盐酸四环素在5 min内去除率达98.61%. 钴铁氧体也能够通过激活PAA产生CH3C(O)O·和CH3C(O)OO·,使SMX的去除率在反应30 min后达到74.7%[73]. 超声处理促进了MnO2活化PAA并产生·OH,显著提高了苯酚在第一阶段(60 min)的去除率[74]. 辐射对金属氧化物活化PAA的协同或强化效果被认为与金属氧化物表面的能量吸收以及电子转移过程有关[75].
MOFs是一种由有机体和无机金属簇通过配位作用构建而成的杂化多孔晶态材料,具有结构多样、比表面积大、孔径和拓扑结构可控、活性位点丰富等优点[76 − 77]. Fu等[78]综述了近5年MOFs在水处理中的研究进展,指出MOFs本身即具有高效的污染物降解和重金属去除能力. Yi等[79]分别以Cd和Co为配位中心构建了二维MOFs,对甲基橙的光催化去除率在30 min时分别达到85%和100%. MOFs也能够活化PAA增强对污染物的降解. Duan等[80]构建了一种以Co为配位中心的咪唑骨架,并用于活化PAA产生CH3C(O)OO·,使体系中磺胺氯哒嗪在3 min内被完全降解.
将金属与其他载体复合也能获得有效的PAA活化材料. Zhang等[81]构建了一种二维夹心状Co@MXenes材料,可以有效激活PAA产生CH3C(O)OO·,并选择性破坏富电子有机污染物. 郑婷露等[82]制备了纳米核壳Co@NC催化剂,可以激活PAA产生CH3C(O)O·和CH3C(O)OO·,导致体系中SMX在中性条件下5 min去除率达到98%. Liu等[83]合成了钴掺杂g-C3N4材料,并发现主要通过双电子转移机制激活PAA产生Co(IV),从而实现SMX在20 min内完全降解.
此外,值得注意的是,PAA中共存的H2O2可能对非均相活化PAA的过程产生影响. 例如,Zhang等[84]发现,在纳米-CuO/PAA体系中,H2O2显著促进了Cu(Ⅱ)向Cu(Ⅰ)的转化,从而加快了卡马西平的降解. 总之,在非均相金属基材料活化PAA的过程中,所产生的活性氧物种可能是CH3C(O)OO·,HO·,1O2以及高价金属氧物种等, 而加速金属离子不同价态的循环是提升PAA反应性的重要手段.
-
非金属基材料主要以碳材料、陶瓷等为主要研究对象. 碳材料具有比表面积大、热稳定性好、无金属浸出污染等优点,其降解路径包括自由基、电子转移路径和1O2[85]. Kong等[86]发现还原氧化石墨烯(rGO)能够激活PAA,并通过电子转移和1O2路径降解SMX,SMX的去除率在2 min时达95%. Dai等[87]制备了一种热改性活性炭,通过激活PAA产生自由基CH3C(O)O·和CH3C(O)OO·以及电子转移途径实现SMX的降解,150 min时去除率达到99.4%. 基于废弃有机物合成的生物炭材料同样能够被用于活化PAA降解污染物. 有学者通过混合热解初级污泥和次级污泥制备了混合污泥生物炭,并成功激活PAA产生R-O·实现对氯苯酚的降解[88].
陶瓷膜具有合理设计的孔结构和功能(如分离、催化和吸附活性),在水处理领域有良好的应用前景[89]. 通过经功能化修饰的陶瓷膜可以实现PAA的有效活化. 利用陶瓷膜与PAA活化反应生成的·OH和1O2,可以实现牛血清白蛋白的快速降解,这为缓解膜污染问题提供思路[90 − 91].
Liao等[92]基于三聚氰胺和1,3,5-三甲酰基间苯三酚合成了共价有机框架(COFs),并用于活化过氧化物. 研究发现COFs对过氧化物的活化速率比碳材料高了两个数量级. 该类无机非金属材料具有形式多样、活化能力强等优势,但目前有关PAA活化的研究报道较少.
非金属基材料还能够与金属材料协同活化PAA. 例如,铁粉与生物炭能够共同促进PAA产生R—O·、·OH和1O2,导致体系中酸性橙染料去除率达93.3%,其中R—O·是主要的反应物质[93]. 孙义才等[94]进一步构建了碳铁微电解材料,发现材料激活PAA产生·OH,导致罗丹明B体系脱色率在40 min时达到92.1%,总有机碳(TOC)的去除率高达80.6%. Li等[95]将PAA活化技术与传统反硝化生物滤池耦合,负载钴的陶粒能够有效活化PAA,并实现了生化尾水中的杂环药物和总氮的高效去除.
-
如前文所述,材料界面的PAA活化除污过程可分为自由基途径和非自由基途径. 自由基途径通常指PAA在活化材料界面断键形成自由基活性物种(例如CH3C(O)OO·,CH3C(O)O· 和HO· 等)的过程. 自由基活性物种往往具有高效降解和矿化污染物的能力,但是选择性较差和受水质影响较大. 零价金属被认为在活化过程中首先失去电子转化为金属离子,之后遵循金属离子活化PAA的机制[96]. 金属化合物则可通过其表面的化合态金属以及溶出在体系中的金属离子激活PAA,其中非金属元素在反应过程中可能促进金属的转化和再生[69,97]. MOFs中的金属中心也能够接触并活化PAA产生自由基物种[80 − 81,83]. 以碳材料为代表的非金属基材料含有丰富的缺电子和富电子官能团,能够吸附并活化PAA产生R—O·,其中CH3C(O)OO·在体系中能够进一步分解产生CH3·[87]. 陶瓷膜对PAA的激活作用主要来源于陶瓷膜上负载的金属元素,而陶瓷膜的高亲水性和低表面粗糙度则促进了金属元素活化PAA产生自由基的过程[90]. 金属元素和非金属载体之间存在协同作用,金属元素的引入可作为非金属材料的反应位点,而非金属材料稳定的结构和丰富的比表面积为PAA的活化提供了理想的反应场所[93,95]. Xiao等[98]使用石墨烯作为金属包覆外壳时进一步促进了金属与PAA的电子传递,加快了Cu0和Cu2+的转化以及自由基的产生.
随着研究的深入,PAA活化的非自由基机制被逐渐揭露. PAA活化的非自由基过程主要包括1O2氧化、高价金属物种氧化和界面电子转移氧化. 同自由基途径相比,非自由基途径往往具有更高的选择性,并且受到水基质的影响较小. 然而非自由基途径对有机污染物的矿化程度可能不如自由基途径. 醌能够通过一系列反应产生1,2-二氧杂螺[2.5]八-4,7-二烯-6-酮,并与过氧化物反应诱导产生1O2[9]. 与醌结构类似,非均相催化剂中的羰基官能团也可能与PAA反应最终生成1O2. 在涉及高价金属物种的机制中,PAA通常与金属位点发生双电子转移反应(氧转移反应),生成对应的高价金属[83]. 而在界面电子转移氧化机制中,PAA首先被吸附在碳材料的表面,形成氧化还原电位更高的活性复合体,随后与表面吸附的微污染物发生电子转移实现氧化降解[86]. 目前,关于PAA在材料界面上的活化机理研究仍然较少,影响PAA分子吸附和反应的功能基团和活性位点等均有待系统揭示.
-
体系酸碱度是影响PAA活化的主要因素之一. 对于PAA本身,酸性条件下其氧化还原电位显著高于碱性环境,而提高pH值则有利于促进PAA去质子化并分解产生自由基[39,99 − 100]. 研究指出,较低的pH能够吸引更多的零价铁以Fe(Ⅱ)的形式进入体系,并提高PAA的活化效率[101]. Li等[72]认为高pH环境下PAA-的静电斥力对反应物之间的接触和反应的抑制作用可能是导致碱性条件不利于PAA活化的原因之一,并指出中性条件最有利于钴铁氧体/PAA体系对污染物的降解. 在均相体系金属基活化PAA的研究中也有相似的现象[46,55]. 酸碱度还会影响污染物的主要存在形态从而影响体系的氧化除污效果. SMX在碱性条件下去质子化转化为SMX−,导致SMX−与热改性活性炭/PAA体系的氧化物种之间存在静电斥力,从而抑制了降解[87]. Luukkonen等[55]则发现在pH 3环境下, Co(Ⅳ)的产生使得有机聚合物负载的Co(Ⅱ)对PAA的活化作用以及对有机微污染物的降解作用强于中性环境.
无机阴离子能够影响PAA活化除污的效果. 一方面Cl-能够与PAA反应产生具有降解能力的HOCl,并与
HO+2 进一步反应产生1O2,从而促进了热改性活性炭/PAA体系和活性炭纤维/PAA体系对污染物的降解[87,102]. 另一方面,Cl−也能与PAA活化产生的自由基发生反应,轻微抑制了nZVI/PAA体系对四环素的降解.HCO−3 和HPO2−4 也能够与体系中的金属离子反应产生络合物,抑制对污染物的降解[67,101].PAA体系氧化除污过程中涉及的背景有机物主要包括天然有机物(NOM)和人工化学合成的有机物质. 尽管他们可能不直接参与PAA活化,但却能够与体系中的其他物质发生作用从而对PAA活化产生影响. 研究表明,NOM能与活性氧物种反应或与活化剂直接反应,从而抑制体系降解污染物. 例如,当体系中存在腐殖酸时,能够与R—C·反应,抑制nZVI/PAA体系对四环素的降解作用[101]. 腐殖酸还有可能吸附在活化剂MoS2表面并对体系降解污染物产生抑制作用[7]. 在另一项研究中,L-半胱氨酸(L-Cys)的存在促进了Fe(Ⅲ)向Fe(Ⅱ)的再生循环,对PAA的活化产生了积极的作用(式(9)—(10))[103].
除了以上因素,目标污染物自身的理化性质,也决定了PAA体系的降解作用效果. 例如,芳香环中的氯取代有利于CH3C(O)O·反应,使得二氯苯酚在MOFs/PAA体系中反应性要高于苯酚和氯苯酚[81].
-
近年来,基于密度泛函(DFT)理论的计算化学在解析环境功能材料微观电子结构、模拟材料界面催化活化反应进程、揭示有机物降解路径等方面做出了重要贡献[83,104 − 107]. DFT能够被用于计算分子内部能量、O—O键长、O—H键长、Bader电荷等描述符,进而分析反应活性位点[108]. Wang等[73]基于DFT计算了CoFe2O4/PAA/SMX体系PAA分子内化学键的强度指数,分析认为其倾向于与SMX的富电子部分反应,进而推测污染物的芳香环、氨基和S-N可能是反应位点. Zhang等[109]采用福井函数计算了零价钴/PAA降解SMX反应中的正值最大区域,得到了SMX的亲电自由基位点为RO+·攻击下的最有利活性位点. Liu等[83]利用Bader电荷分析发现Co掺杂改变了g-C3N4的原始分布,导致C、N电子密度较低,电荷密度差异分析表明PAA和Co-N-C之间存在显著的电子转移,推测Co—N—C是激活PAA的反应位点,并根据状态密度函数分析发现PAA的O原子在费米能级附近表现出非对称的自旋轨道,因此认为PAA吸附后O原子的pz轨道与Co原子的3d轨道相互作用形成杂化态以充分激活PAA. Miao等[110]使用反应能垒作为描述符计算了在不同反应位点发生作用时的能量势垒,结果表明PAA断键生成R—O·和·OH的临界势垒能小于吸附到碳纳米管基体时的势垒能且大于吸附到碳纳米管边缘的势垒能,说明sp2杂化碳是自由基路径的反应位点,而键长分析表明PAA的C—OH是非自由基路径的反应位点. Silva等[111]使用DFT和混合泛函计算了PAA的构象和PAA的分解机理,提出了自发分解、在pH 5.5—10.2之间自发分解和水解分解机理,并根据体系溶解氧含量确定了不同机理的适用性. Duan等[80]在得到PAA的活性氧物种和反应中间体的基础上使用了福井函数分析活性氧物种的攻击位点,发现CH3C(O)OO·作为主要反应物质通过单电子转移降解磺胺氯哒嗪,并通过一系列反应将其转化为H2O、SO2和CO2. Gu等[9]则根据量子化学计算(包括DFT、量子力学电荷密度等)提出了醌与过氧化物的反应路径.
模型拟合是探究灭活机制的手段之一. Balachandran等[112]使用基于残留消毒剂随时间的浓度变化(ICT)的Chick-Watson灭活动力学模型拟合PAA病原菌的消长变化,提出了大肠杆菌和肠球菌的耐药性和灭活机制. Lin等[47]根据实验结果改进了PAA对大肠杆菌的双指数微生物灭活模型. Maffettone等[113]使用基于ICT的动力学模型分析了PAA和过氧甲酸对诺如病毒、粪便大肠菌群和肠球菌灭活动力学,根据灭活动力学模型的ICT要求指出过氧甲酸的灭活能力远高于PAA. Dunkin等[16]则使用灭活动力学模型比较了PAA和氯化铵对诺如病毒和MS2噬菌体的灭活作用,结果表明PAA和氯化铵对诺如病毒的灭活能力不同,PAA低于氯化铵,而二者对MS2噬菌体的灭活能力相似. Foschi等[114]建立了包括基于接触罐液压、PAA衰变动力学和PAA灭活大肠杆菌动力学的连续流动体系PAA灭活模型,并通过中试反应器验证模型,模拟结果表明大肠杆菌的灭活模型遵循一维分散模型,试验结果表明模型能够很好地预测大肠杆菌的动态变化. Manoli等[115]开发了PAA对二次废水的化学物质降解和病原菌灭活模型,并用于通过保持恒定的ICT剂量来控制消毒过程的新策略,对研究的中试结果表明该模型能够有效预测消毒剂残留量和病原菌变化,该策略也能够很好控制ICT剂量.
-
近年来,基于PAA活化的高级氧化技术的发展迅速,从经典的均相系统扩展到非均相系统. 开发经济、高效、环境友好的非均相PAA活化功能材料是促进PAA活化氧化技术可持续环境应用的重要一步. PAA活化材料可大体分为两类:金属基和非金属基材料,两者之间还存在着协同和复合作用. 除传统的自由基氧化途径外,非均相活化过程还涉及材料界面的非自由基机理,包括单线态氧氧化、高价金属氧化和界面电子转移氧化,逐渐被揭示. 尽管非均相PAA活化技术在有机污染物降解、水体消毒灭菌方面表现出了极大的潜力,但复杂的水质环境因素,包括pH值、无机离子、天然有机物等,可能会影响氧化效果,甚至会影响系统的机理. 计算化学在环境催化领域中发挥着重要作用,包括材料的微观结构分析、反应位点的预测、模拟材料界面和PAA之间的接触反应过程、以及氧化体系中污染物分解途径的推导. 模型拟合则更多用于探究病原微生物的灭活效果和机制. 本文基于现有的成果,提出以下关于PAA活化材料开发和水环境应用的未来研究的参考思路:(1)进一步揭示PAA在材料界面上的特定活化机理,阐明影响PAA分子吸附和反应的功能基团和活性位点,建立材料结构-PAA活化机制-氧化效率的相关性;(2)有针对性地设计和合成PAA活化功能材料,结合实际应用要求实现氧化机制的可控性,并考虑实际应用成本问题,大力发展如生物炭等可持续的功能材料;(3) 拓宽PAA活化技术在水环境中的应用场景,检测更多有机污染物在水体中的降解效率,研究PAA活化技术对病原微生物和抗生素耐药基因的削减效果,探究PAA活化技术对复合水环境污染的处理效果,进一步探讨水环境基质对PAA活化体系去除效率的影响; (4)未来的研究中应考虑对PAA消毒应用中的精确风险评估,并对产生有害消毒副产物的机理给与足够重视; (5) 考虑PAA活化材料的多重催化功能特性,将PAA活化技术与其他高级氧化技术(如Fenton氧化、过硫酸盐氧化、光催化等)相结合,探索可能的协同作用和机理; (6) 基于非均相PAA活化水处理技术单元或设备的开发,考虑如何实现先进氧化技术与现有处理工艺的互补耦合,使用生命周期评估和经济可持续性分析评价整个处理过程,并促进工程应用.
过氧乙酸活化功能材料开发与水环境应用
Development and application of peracetic acid activation-oriented functional materials in water treatment
-
摘要: 近年来,水处理消毒剂过氧乙酸(PAA)在高级氧化过程(AOPs)中受到了越来越多的关注. 虽然基于PAA活化的均相体系已经得到了大量报道,但非均相活化材料具有成本低、易操控、应用性更强、反应路径多元化等优势,逐渐发展为研究的热门方向. 本文首先梳理了均相活化PAA技术的研究进展,随后着重综述了基于非均相材料活化的PAA技术在水处理中的新近研究成果,总结了涉及自由基和非自由基的材料界面氧化机制,讨论了影响PAA活化除污效果的主要因素,并介绍了理论计算和模型拟合在PAA技术研究中的具体运用. 最后,总结了现阶段非均相活化PAA技术研究的现状,并对未来以PAA活化功能为导向的材料开发与水环境应用进行了展望.Abstract: In recent years, peracetic acid (PAA) has gained significant attention as a disinfectant for water treatment in advanced oxidation processes (AOPs). While homogenous PAA activation systems have been extensively studied, there has been a growing interest in non-homogenous activation materials due to their advantages, including low cost, easy operability, stronger applicability, and diversified reaction pathways. In this review, we first reviewed the research progress of homogenous PAA activation technology, and then focused on summarizing the recent research results of non-homogenous material-activated PAA technology in water treatment. We elucidated the radical and non-radical oxidation mechanisms at the material interface, discussed the main factors affecting the removal of pollutants through PAA activation, and introduced the specific application of theoretical calculations in PAA technology research. Finally, we concluded the current status and shortcomings of non-homogenous PAA activation technology research and provided prospects for future material development and water environment application oriented to PAA activation functionality.
-
Key words:
- peracetic acid /
- environmental material /
- advanced oxidation process /
- free radical /
- water treatment.
-
燃煤烟气脱硫工艺分为湿法脱硫、干法脱硫和半干法脱硫3种[1-3]。其中,湿法脱硫工艺应用最广泛,SO2脱除率达95%以上。然而,该工艺存在设备腐蚀严重、易造成石膏雨,并产生大量废水等问题[4-6]。干法脱硫为气固反应,其工艺流程及装置较为简单,且具有能耗低、二次污染少等优点,但其脱硫效果较差、脱硫剂利用率较低,且系统运行可靠性不高[7]。半干法脱硫工艺为气、液、固三相反应,利用烟气显热可将湿浆料中的水分或喷入的增湿水加热蒸发以实现烟气增湿,其产物呈干燥态[8-9]。半干法脱硫工艺结合了湿法脱硫工艺和干法脱硫工艺的优势,且SO2脱除率可达90%以上[10-11],故备受关注。
半干法脱硫工艺采用Ca(OH)2为脱硫剂。在干燥条件下,Ca(OH)2几乎不与SO2反应,而在有水或水蒸气存在的条件下,Ca(OH)2与SO2具有很高的反应活性[12-15]。因此,探究水及其他因素对半干法脱硫效果的影响,对于其工况的选取及工艺的改进具有重要意义。式(1)、式(2)为有水及水蒸气存在的条件下Ca(OH)2与SO2的主要脱硫反应方程式。
Ca(OH)2+SO2+H2O→CaSO3⋅2H2O (1) CaSO3⋅2H2O+1/2O2→CaSO4⋅2H2O (2) 本研究利用固定床反应器,以Ca(OH)2作为脱硫剂,考察反应温度、脱硫剂颗粒粒径、反应空速、模拟烟气中水蒸气含量对半干法脱硫反应的影响;同时,以脉冲增湿方法改善半干法工艺的脱硫效果,以期为钙基半干法脱硫工艺的改进提供参考。
1. 实验材料和方法
1.1 实验材料
所使用的二氧化硫气体(SO2,浓度1%,氮平衡)、空气(Air,21%O2/N2)、氮气(N2,纯度99.999%)均购自北京环宇京辉京城气体科技有限公司;氢氧化钙(Ca(OH)2,AR,≥95.0%)购自天津百伦斯生物技术有限公司;无水氯化钙(CaCl2,AR,≥96.0%)购自国药集团化学试剂有限公司。
1.2 实验装置和方法
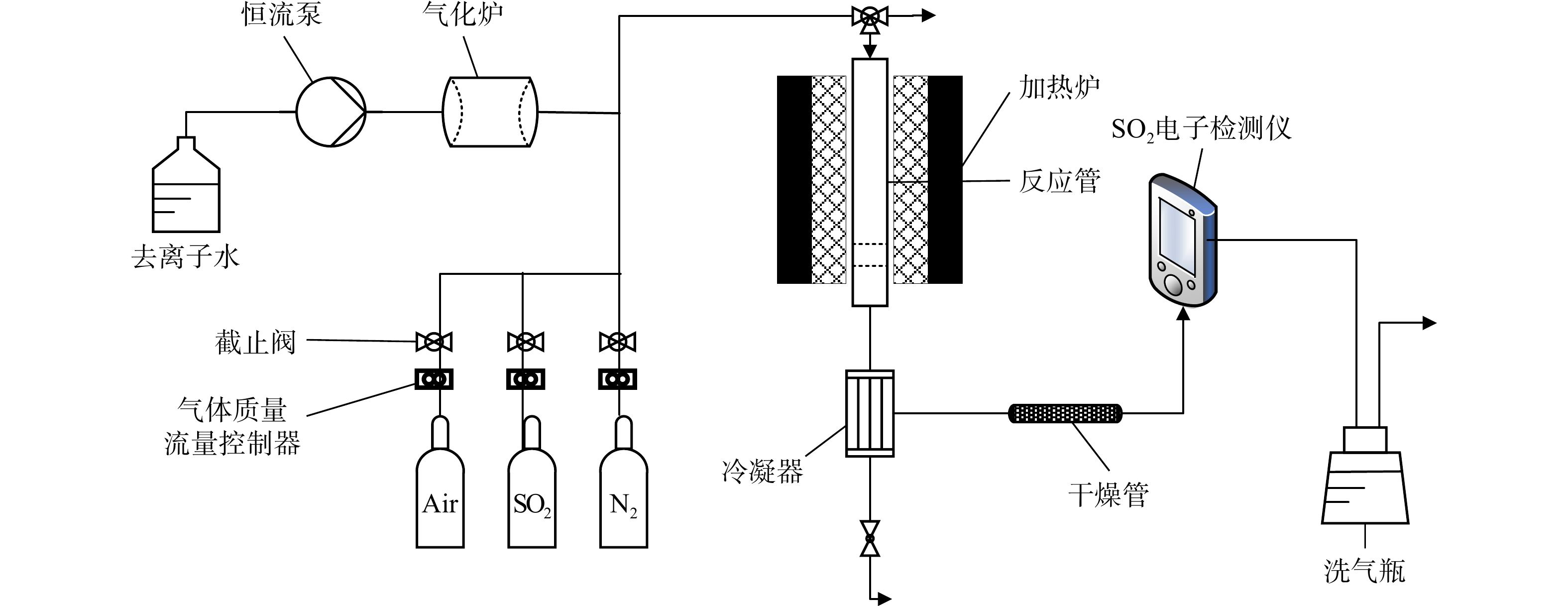
实验装置由配气系统、反应系统、出口气体检测系统、尾气处理系统4部分组成(见图1)。配气系统主要装置有恒流泵、气化炉、气瓶(SO2、N2、Air);反应系统主要有加热炉和管式固定床反应器;出口气体检测系统由冷凝器、干燥管、SO2检测仪组成;尾气处理系统为盛有NaOH水溶液的洗气瓶。
模拟烟气的SO2、N2及空气流量由质量流量控制器设定。SO2质量浓度为2 285.7 mg·Nm−3。去离子水经连接管路流入气化炉,其流量由恒流泵控制,在气化炉中产生水蒸气后再与反应气混合并通入固定床反应器中进行脱硫反应。
将200目的Ca(OH)2粉末加去离子水制浆后造粒,在120 ℃下干燥,再破碎、筛分,得到不同粒径的Ca(OH)2颗粒备用。固定床下部填充直径2 mm的刚玉球作为支撑,并在刚玉球上方装填Ca(OH)2颗粒。
反应管出口气体经过冷凝器、干燥管除水后,进入SO2检测仪进行分析,之后进入尾气处理系统经充分净化后外排至环境中。
1.3 参数定义
采用SO2检测仪分析进出口SO2质量浓度。我国火电厂燃煤烟气中SO2的质量浓度排放标准为35 mg·Nm−3[16]。本研究将该质量浓度定义为SO2穿透浓度,将SO2出口质量浓度达到穿透浓度时的反应时间定义为穿透时间。
脱硫率(η)的计算方法式为式(3)。Ca(OH)2利用率(Ue)是评价脱硫效果及脱硫反应经济性的重要指标,其计算式为式(4)。Ca/S,即Ca(OH)2的使用量与SO2穿透时的SO2脱除量之比,其计算式为式(5)。
η(%)=Cin−CoutCin×100 (3) Ue=Vt1∫0(Cin−Cout)dt2285.7Vt2 (4) Ca/S=NCa(OH)2NSO2 (5) 式中:Cin为反应气体中SO2进口质量浓度,mg·Nm−3;Cout为SO2出口质量浓度,mg·Nm−3;V为反应气体体积流量,mL·min−1;t1为脱硫反应的穿透时间,min;t2为脱硫反应的理论反应时间,min;
NCaOH2 NSO2 2. 结果和讨论
2.1 反应温度对脱硫效果的影响
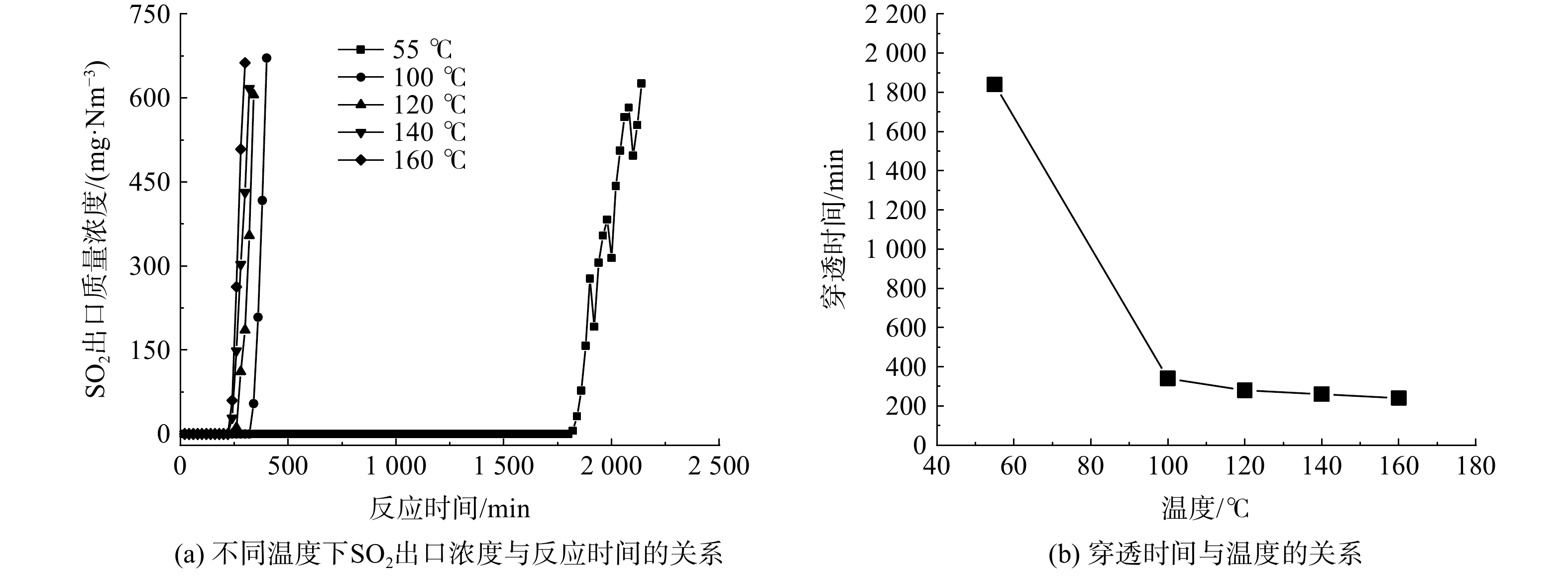
温度对Ca(OH)2与SO2的脱硫反应有一定影响。在55~160 ℃下,采用平均粒径0.58 mm的Ca(OH)2颗粒,在空速2 500 h−1、含硫气体中水蒸气体积分数5%的条件下进行脱硫实验,SO2出口质量浓度随反应时间的变化如图2(a)所示,穿透时间与反应温度的关系如图2(b)所示。穿透时间随温度的升高而降低,当温度由55 ℃逐渐升至160 ℃时,穿透时间由1 840 min降至240 min。此结果表明,在55~160 ℃下,Ca(OH)2的脱硫效果会随着反应温度的升高而下降,这与文献[17-18]的结论相吻合。
2.2 Ca(OH)2颗粒的粒径对脱硫效果的影响
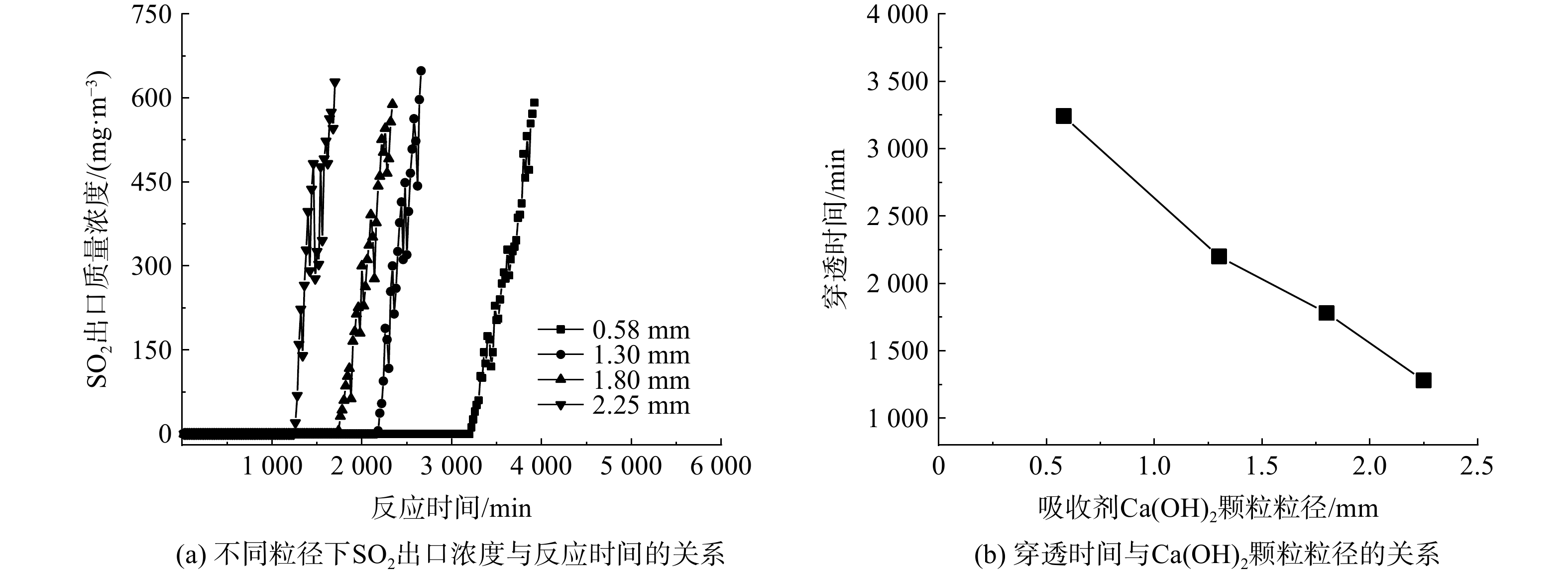
固定床反应器内填充的Ca(OH)2颗粒粒径越大,床层空隙率则越高。为避免空隙率过高影响实验结果,采用了40 mm直径的反应管。较大的管径比可有效减小壁面附近空隙增大时带来的壁面效应影响[19]。同时,Ca(OH)2颗粒粒径的增大还会造成SO2的内扩散阻力增大,不利于进行脱硫反应。图3反映了半干法脱硫在不同Ca(OH)2颗粒粒径条件下的实验结果,在55 ℃、2 500 h−1及水蒸气体积分数为12%的条件下,随着Ca(OH)2颗粒粒径的增大,脱硫反应的穿透时间不断缩短。
图4分别反映了Ca/S及Ca(OH)2利用率与Ca(OH)2颗粒粒径的变化关系。在Ca(OH)2颗粒的平均粒径为0.58 mm、反应温度为55 ℃、空速为2 500 h−1、水蒸气体积分数为12%的条件下,当出口SO2达到穿透浓度时,可获得本实验的最佳脱硫效果。根据式(4)和(5)计算出该条件下的Ca(OH)2利用率为71.3%、Ca/S为1.4。Ca/S随Ca(OH)2粒径的增加而不断增大,而Ca(OH)2利用率则不断降低。当Ca(OH)2颗粒的平均粒径由0.58 mm增至2.25 mm时,Ca/S由1.4增至3.6,Ca(OH)2的利用率则从71.3%降至27.8%。可见,Ca(OH)2颗粒的平均粒径增大不利于半干法脱硫效果的提升。这是由于:Ca(OH)2颗粒的粒径增大会造成颗粒的比表面积减小及SO2内扩散阻力的增加,导致颗粒内部大量Ca(OH)2未能与SO2发生反应,最终使得Ca(OH)2利用率降低,穿透时间随之缩短。
2.3 空速对脱硫效果的影响
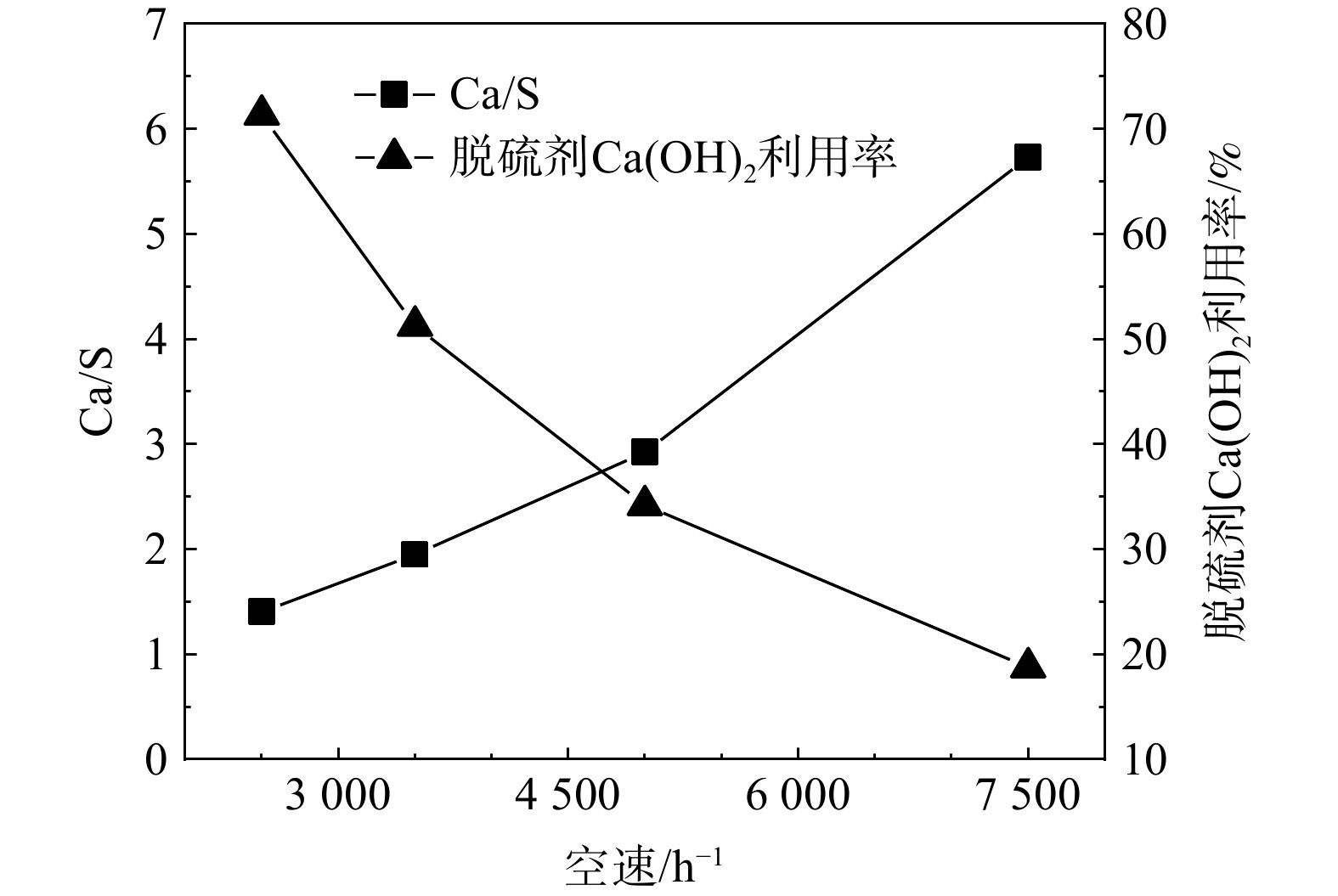
空速对脱硫效果的影响如图5所示。空速的提高使得脱硫反应的穿透时间缩短,在Ca(OH)2颗粒平均粒径为0.58 mm、水蒸气体积分数为12%、温度为55 ℃的条件下,当空速由2 500 h−1升至7 500 h−1时,穿透时间由3 240 min大幅降至260 min。
不同空速对Ca/S及脱硫剂Ca(OH)2利用率的影响如图6所示。随着反应空速的增大,Ca/S随之增大而Ca(OH)2利用率则随之降低。保持其他条件不变,当反应空速由2500 h−1增加到7500 h−1时,Ca/S由1.4提高为5.7,Ca(OH)2的利用率则由71.3%降低到18.8%。可见,空速的增大会导致脱硫效果急剧下降。在较高的空速下,烟气在床层的停留时间会缩短,脱硫反应速率则大幅提高。反应中会快速生成大量脱硫产物并覆盖至脱硫剂颗粒表面,所形成的致密产物层可阻止SO2与颗粒内部的Ca(OH)2继续反应,使得脱硫反应快速穿透。
2.4 反应气体中水蒸气含量对脱硫效果的影响
在半干法脱硫工艺中,烟气中的水蒸气含量对脱硫效果影响显著[20-21]。图7反映了水蒸气体积分数对脱硫效果的影响规律。穿透时间随着反应气体中水蒸气含量的增加而增大,在Ca(OH)2颗粒平均粒径为0.58 mm、空速为2 500 h−1、温度为55 ℃的条件下,当水蒸气的体积分数由5%升至12%时,脱硫反应的穿透时间由1 840 min大幅增至3 240 min。
水蒸气体积分数对Ca/S及脱硫剂Ca(OH)2利用率的影响如图8所示。随着水蒸气含量的增加,Ca/S相应不断降低,Ca(OH)2利用率则不断提高。水蒸气的体积分数由5%增至12%时,Ca/S由2.5降至1.4,Ca(OH)2的利用率由40.9%增至71.3%。这表明水蒸气含量的增大有利于半干法脱硫反应的进行。实际上,烟气中水蒸气的体积分数越大,反应中Ca(OH)2颗粒的表面越容易润湿从而形成液膜,这使得Ca(OH)2可游离出部分OH−,进而与烟气中的SO2进行快速、充分的离子反应[22-23]。反之,当烟气中水蒸气含量太低时,水蒸气不足以使得Ca(OH)2颗粒表面润湿,SO2与Ca(OH)2的离子反应便难以进行。当烟气为不含水蒸气的极端干燥状态时,SO2与干燥的Ca(OH)2几乎不发生反应[24]。
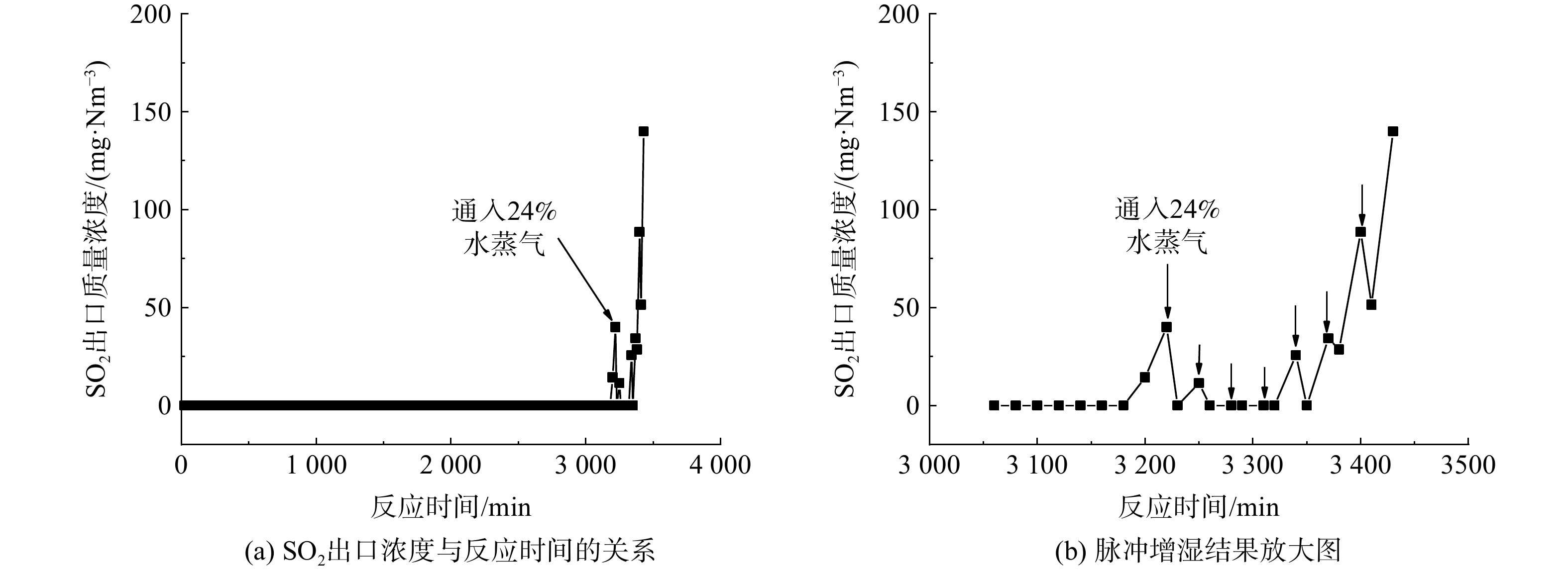
本研究提出了以脉冲式增湿方法对半干法脱硫工艺进行改进,故对水蒸气含量对半干法脱硫反应的影响进行了研究。当出口SO2质量浓度首次达到穿透浓度时,通过恒流泵调节水的流量,使反应气体中的水蒸气体积分数由12%增至24%并保持10 min。之后,将水蒸气体积分数调回原来的12%。20 min之后,再将反应气体中的水蒸气体积分数由12%增至24%并保持10 min。如此循环往复进行脉冲式的增湿,直至脱硫反应出口SO2质量浓度再次达到35 mg·Nm−3这一穿透浓度,结果如图9所示。图9(b)中的箭头为反应气体中的水蒸气体积分数由12%增至24%的时间节点。在反应3 220 min时,出口SO2质量浓度达到40 mg·Nm−3,即在水蒸气体积分数为12%条件下,脱硫反应首次穿透。而首次脉冲增湿后,出口SO2质量浓度降至0,接着水蒸气体积分数调回原先的12%并保持20 min。之后出口SO2质量浓度逐渐增至11 mg·Nm−3。这表明水蒸气含量的增加促进了脱硫,即使在反应穿透后,通过增加水蒸气含量仍可增加脱硫剂颗粒表面的润湿程度,使得SO2与颗粒内部未反应的Ca(OH)2得以继续反应。如此循环往复,直至第7次脉冲增湿时还有明显效果。在前6次脉冲增湿期间,出口SO2质量浓度一直保持低于穿透浓度。通过脉冲增湿,反应穿透时间延长了约160 min,Ca(OH)2利用率提高了4.4%,Ca/S由1.43降至1.36。
又研究了Ca(OH)2颗粒的平均粒径为2.25 mm时的脱硫反应当出口SO2浓度首次达到穿透浓度时脉冲增湿方法对脱硫效果的影响。实验结果如图10所示。结果表明,脉冲增湿使得该条件下的脱硫反应的穿透时间延长了约720 min,计算得到的Ca(OH)2利用率提高了15.6%,Ca/S由3.6降低到了2.3。2.25 mm的Ca(OH)2颗粒粒径条件下的脉冲增湿结果虽然仍与本实验最佳脱硫效果有较大差距,但脉冲增湿对脱硫效果的改善幅度较0.58 mm Ca(OH)2颗粒粒径的条件下有了较大提升。
分析脉冲式增湿方法对半干法脱硫效果的促进原因可发现,由于水蒸气体积分数为24%时,反应气体处于过饱和状态,在进入反应管后易凝结成为液态水并覆盖在Ca(OH)2颗粒表面形成液膜,使得脱硫剂表面及内部未反应的Ca(OH)2得以继续游离出部分OH−并与SO2发生反应,最终导致出口SO2质量浓度降低,穿透时间也相应延长。然而,长时间通入24%体积分数的水蒸气会发生Ca(OH)2颗粒团聚和床层板结,而以2:1的时长比循环通入水蒸气体积分数为12%和24%的脉冲增湿方法,则可保证在脱硫效果改善的同时有效避免上述不利情形发生。因此,采用脉冲增湿方法可优化半干法脱硫技术。
3. 结论
1)较低的反应温度、Ca(OH)2颗粒粒径的减小、反应空速的降低、烟气中水蒸气含量的提高均有利于半干法脱硫效果的提升,即有利于穿透时间的增加、脱硫剂利用率的提高及Ca/S的降低。当反应温度为55 ℃、Ca(OH)2颗粒平均粒径为0.58 mm、反应空速2 500 h−1、烟气中水蒸气体积分数12%时,可获得最佳脱硫效果。在SO2质量浓度为穿透浓度(35 mg·Nm−3)时,Ca(OH)2利用率达可到71.3%,Ca/S为1.4。
2)脉冲增湿方法可促进半干法脱硫工艺的脱硫效果。在上述最佳条件下,当反应器出口SO2达到穿透浓度时,通过实施脉冲增湿,即间歇提高反应气体中水蒸气体积分数可使穿透时间延长160 min,达到3 380 min,此时Ca(OH)2利用率提高了4.4%。采用脉冲增湿方法可优化半干法脱硫工艺。
-
表 1 PAA和H2O2理化性质
Table 1. Physiochemistry properties of PAA and H2O2
指标 Property PAA H2O2 摩尔质量/(g·mol−1) 76.05 34.01 密度/(g mL−1) 1.0375 1.71 沸点/℃ 105 150.2 闪点/℃ 41 107 酸度(pKa) 8.2 11.75 稳定性 不稳定 较稳定 过氧键键能/(kJ·mol−1) 159.0 213.4 氧化还原电位/V 1.96 1.78 对人体健康造成相似损害的浓度比(PAA/H2O2)[12] 0.24:1 表 2 单独PAA的作用效果及机制
Table 2. The performance and mechanism of PAA alone
目标物质 Target pollutant 处理效果 Performance 作用机制 Mechanism 大肠杆菌[13 − 15] 浓度为250 mg·min·L−1时菌群含量降至100 CFU·L-1 在细胞膜打孔并破坏膜蛋白;影响膜转运基因的调控和DNA修复、小分子转运蛋白的合成以及细胞保护过程相关基因的表达 消毒处理29 h后仍未出现再生长 诺如病毒[16 − 19] 浓度为 69 mg·min·L−1时能够导致废水中病毒减少3个数量级 能够导致病毒蛋白质和核酸的改变 30—60 mg·min·L−1处理导致GII毒株减少1.5个数量级 β-内酰胺[22] 10 mg L−1 PAA处理10 min导致内酰胺减少了62%—95% 攻击分子内的硫醚键 EPS[23] 浓度为0.36 g·g−1悬浮物时可溶性EPS和分散状态EPS分别达到186 mg·g−1和65 mg·g−1悬浮物 溶解和破环凝聚状态的EPS并将类蛋白物质降解为小分子 表 3 均相体系中PAA活化的降解效果和主要反应物质
Table 3. Degradation performance of activating PAA in homogeneous system and main active species
反应体系 System 目标污染物 Target pollutant 降解效果 Degradation performance 主要反应物质 Main active species UV/PAA[35] 柯萨奇病毒B3 3 mg·L−1 PAA处理导致毒株减少4个数量级. PAA-UV/PAA[35] 病原微生物 杜兰斯菌、表皮葡萄球菌、大肠杆菌分别降低了3.1、6.2 和5.6个数量级 中压UV /PAA[36] 诺氟沙星 pH 5、7、9条件下去除率分别达到85.8%、96.6%、97.2% ·OH, CH3C(O)OO· UV/PAA[33] 萘普生 kobs值为0.11 min−1 R—C· UV/Fe0/PAA[34] 磺胺嘧啶 60 min后去除率达到85%. R—C· UV/PAA[31] 四环素、四环素抗性细菌 四环素降至检测限以下,细菌表达受到显著抑制(3.2%—38.9%) ·OH, R—C· 太阳光/PAA[37] 病原微生物、SMX QUV = 38.03 kJ L−1处理210 min后大肠杆菌降至检测限 ·OH 超声波/PAA[38] 肠道沙门氏菌 附着在生菜上的病菌降低了3.0个数量级 微波/PAA[39] SMX 碱性条件下反应30 min后的去除率达到94.2% 1O2, R—C· 热/PAA[29,40] DCF 65 ℃下体系中去除率达到96% CH3C(O)O·, CH3C(O)OO· 热/PAA[29,40] SMX 60 ℃处理0.2 mmol·L−1 PAA去除率达到86% CH3C(O)O·, CH3C(O)OO· 热/Cu(Ⅱ) /PAA[42] DCF 60 ℃处理DCF被完全降解 CH3C(O)O·, ·OH,CH3C(O)OO· Fe(Ⅲ)/ PAA[44] 亚甲蓝染料 30 min后去除率达到98.98% CH3C(O)OO·, CH3C(O)·, ·OH Fe(Ⅱ)/ PAA[43 − 44,51] 聚丙烯酰胺 10 mg·L−1 PAA处理15 min去除率达81% ·OH 亚甲蓝、萘普生、BPA 12 min后亚甲蓝、萘普生、BPA最大去除率分别达到89.4%、98.2%和87.7% CH3C(O)O·, CH3C(O)·, CH3·, Fe(Ⅳ) 羟胺/Fe(Ⅱ)/ PAA[47] DCF 10 min内去除率接近95% Fe(IV)O2, R—O· 吡啶甲酸/Fe(Ⅲ)/ PAA[48] 微污染物 10 min内萘普生和磺酸甲恶唑完全降解 Fe(Ⅳ), Fe(V) ABTS/ Fe(Ⅱ)/PAA[49] DCF 30 min内DCF的去除率接近92% ABTS·+ Co(Ⅱ)/ PAA[50,52 − 54] 酸性橙 60 min后去除率达到92% CH3C(O)O·, CH3C(O)OO· 芳香族化合物 萘普生和2-萘酚的KPAA分别达到6.83×10−2、6.67×10−2 CH3C(O)OO· SMX 中性条件下反应15 min后去除率达到89.4% CH3C(O)OO·, CH3C(O)O· 有机微污染物 酸性条件下(pH 3.5)BPA和SMX分别在15 min和20 min完全降解,甲基苯基亚砜和卡马西平的去除率分别达到88.7%和84.5% Co(IV), CH3C(O)OO·, CH3C(O)O· 苯酚 最大去除率达到99% 产生自由基 Cu(Ⅱ)/ PAA[55] 苯酚 最大去除率达到65% 产生自由基 Ag(Ⅰ)/ PAA[55] 苯酚 最大去除率达到20% 产生自由基或激活H2O2 Ru(Ⅲ)/ PAA[56] SMX 200 μmol·L−1 PAA处理SMX 2 min内完全去除 CH3C(O)O·, CH3C(O)OO· Cl-/PAA[58] 罗丹明B 10 min后去除率达到96.2% CH3C(O)O·,1O2, CH3C(O)OO· 磷酸盐缓冲液/PAA[59] DCF pH 7.4环境中45 min后去除率达到96% CH3C(O)O·,·OH, CH3C(O)OO· Na2CO3/阳光/PAA[60] 亚甲蓝染料 第一、二阶段kobs值分别达到0.0139 min−1和0.0494 min−1 1O2 醌/PAA[9] 1O2, ·OH, CH3O· 表 4 非均相活化材料对过氧乙酸的激活作用
Table 4. Activation of PAA by different heterogeneous materials
活化材料 Material 目标污染物 Target pollutant 降解效果 Degradation performance 主要反应物质 Main active species 零价钴[66] 罗丹明B 180 s内去除率达到98.3% CH3C(O)O ·, CH3C(O)OO· nZVI/UV[68] 螺旋霉素 在20 min内完全降解 ·OH, R—C· 硫化零价铁[67] 微污染物 15 min内SMX、DCF钠、布洛芬、卡马西平、萘普生和左氧氟沙星的去除率分别达到99.64%、98.5%、99.2%、99.8%、97.5%、97.4% ·OH FeS[69] SMX 3 min内去除率达到80.32% ·OH 黄铁矿[70] 四环素 在30 min内完全去除 CH3C(O)OO· 纳米铜[65,84] 卡马西平 表观反应速率常数为0.07 min−1,达到对照组的2倍. CH3C(O)OO· 微波/钴取代锰铁氧体[72] 盐酸四环素 5 min内去除率达到98.61% 1O2 钴铁氧体[73] SMX 30 min内去除率达到74.7% CH3C(O)O·, CH3C(O)OO· 超声/MnO2[74] 苯酚 第一阶段(60 min)去除率接近70%. ·OH 分子筛咪唑骨架(ZIF)67[80] 磺胺氯达嗪 3 min内去除率达到100% CH3C(O)OO· Co@ MXenes[81] 富电子有机污染物 四环素、氟喹诺酮类、2,4-二氯苯酚和罗丹明B分别在5 min、10 min和20 min、20 min内完全降解,环丙沙星、SMX、磺胺嘧啶在30 min时的去除率分别达到95%、90%和89% CH3C(O)OO· 钴掺杂g-C3N4[83] SMX 在20 min内完全降解 Co(Ⅳ) 纳米核壳Co@NC[82] SMX 在中性条件下5 min时去除率达到98% CH3C(O)O·, CH3C(O)OO· rGO[86] SMX 去除率在2 min时达到95% 电子转移和1O2 热改性活性炭[87] SMX 150 min去除率达到99.4% CH3C(O)O·, CH3C(O)OO·电子转移 污泥生物炭[88] 对氯苯酚 去除率达到85%—100% R—O· FeOCl功能化的陶瓷膜[90] 牛血清白蛋白 20 min后牛血清白蛋白几乎被完全降解 ·OH和1O2 锐钛矿型TiO2[71] 电子转移 碳铁微电解材料[94] 罗丹明B 脱色率在40 min时达到92.1%,TOC的 去除率达到80.6% ·OH 铁-生物炭[93] 酸性橙染料 去除率达到93.3% R—O· 负载钴陶粒[95] 微污染物 SMX、磺胺嘧啶、卡马西平的去除率分别达到67.77%、70.20%、89.25% -
[1] LUO H C, GUO W Q, ZHAO Q, et al. Compared effects of “solid-based” hydrogen peroxide pretreatment on disintegration and properties of waste activated sludge[J]. Chinese Chemical Letters, 2022, 33(3): 1293-1297. doi: 10.1016/j.cclet.2021.08.002 [2] SI Q S, GUO W Q, WANG H Z, et al. Carbon quantum dots-based semiconductor preparation methods, applications and mechanisms in environmental contamination[J]. Chinese Chemical Letters, 2020, 31(10): 2556-2566. doi: 10.1016/j.cclet.2020.08.036 [3] HOANG N T, MANH T D, NGUYEN V T, et al. Kinetic study on methylene blue removal from aqueous solution using UV/chlorine process and its combination with other advanced oxidation processes[J]. Chemosphere, 2022, 308: 136457. doi: 10.1016/j.chemosphere.2022.136457 [4] SHI C J, LI C, WANG Y, et al. Review of advanced oxidation processes based on peracetic acid for organic pollutants[J]. Water, 2022, 14(15): 2309. doi: 10.3390/w14152309 [5] SHAH N S, HE X X, KHAN H M, et al. Efficient removal of endosulfan from aqueous solution by UV-C/peroxides: A comparative study[J]. Journal of Hazardous Materials, 2013, 263: 584-592. doi: 10.1016/j.jhazmat.2013.10.019 [6] 万思文, 徐志威, 易琳雅, 等. 过氧乙酸在高级氧化工艺中的应用研究[J]. 环境科学与技术, 2021, 44(8): 64-74. doi: 10.19672/j.cnki.1003-6504.0763.21.338 WAN S W, XU Z W, YI L Y, et al. Research on the application of peracetic acid in advanced oxidation process[J]. Environmental Science & Technology, 2021, 44(8): 64-74 (in Chinese). doi: 10.19672/j.cnki.1003-6504.0763.21.338
[7] AZAIZEH H, LINDEN K G, BARSTOW C, et al. Constructed wetlands combined with UV disinfection systems for removal of enteric pathogens and wastewater contaminants[J]. Water Science and Technology, 2013, 67(3): 651-657. doi: 10.2166/wst.2012.615 [8] WANG J W, WANG Z P, CHENG Y J, et al. Molybdenum disulfide (MoS2): A novel activator of peracetic acid for the degradation of sulfonamide antibiotics[J]. Water Research, 2021, 201: 117291. doi: 10.1016/j.watres.2021.117291 [9] GU J, SONG Y, YANG Y, et al. Mechanical insights into activation of peroxides by quinones: Formation of oxygen-centered radicals or singlet oxygen[J]. Environmental Science & Technology, 2022, 56(12): 8776-8783. [10] CORREA-SANCHEZ S, PEÑUELA G A. Peracetic acid-based advanced oxidation processes for the degradation of emerging pollutants: A critical review[J]. Journal of Water Process Engineering, 2022, 49: 102986. doi: 10.1016/j.jwpe.2022.102986 [11] CHEN J C, PAVLOSTATHIS S G. Peracetic acid fate and decomposition in poultry processing wastewater streams[J]. Bioresource Technology Reports, 2019, 7: 100285. doi: 10.1016/j.biteb.2019.100285 [12] KITIS M. Disinfection of wastewater with peracetic acid: A review[J]. Environment International, 2004, 30(1): 47-55. doi: 10.1016/S0160-4120(03)00147-8 [13] CHANG W, SMALL D A, TOGHROL F, et al. Microarray analysis of toxicogenomic effects of peracetic acid on Pseudomonas aeruginosa[J]. Environmental Science & Technology, 2005, 39(15): 5893-5899. [14] CHANG W, TOGHROL F, BENTLEY W E. Toxicogenomic response of Staphylococcus aureus to peracetic acid[J]. Environmental Science & Technology, 2006, 40(16): 5124-5131. [15] ZHANG C Q, BROWN P J B, MILES R J, et al. Inhibition of regrowth of planktonic and biofilm bacteria after peracetic acid disinfection[J]. Water Research, 2019, 149: 640-649. doi: 10.1016/j.watres.2018.10.062 [16] DUNKIN N, WENG S, SCHWAB K J, et al. Comparative inactivation of murine norovirus and MS2 bacteriophage by peracetic acid and monochloramine in municipal secondary wastewater effluent[J]. Environmental Science & Technology, 2017, 51(5): 2972-2981. [17] SANTORO D, CRAPULLI F, RAISEE M, et al. Nondeterministic computational fluid dynamics modeling of Escherichia coli inactivation by peracetic acid in municipal wastewater contact tanks[J]. Environmental Science & Technology, 2015, 49(12): 7265-7275. [18] DUNKIN N, WENG S, COULTER C G, et al. Reduction of human norovirus GI, GII, and surrogates by peracetic acid and monochloramine in municipal secondary wastewater effluent[J]. Environmental Science & Technology, 2017, 51(20): 11918-11927. [19] ANTONELLI M, ROSSI S, MEZZANOTTE V, et al. Secondary effluent disinfection: PAA long term efficiency[J]. Environmental Science & Technology, 2006, 40(15): 4771-4775. [20] ZHANG K J, ZHOU X Y, DU P H, et al. Oxidation of β-lactam antibiotics by peracetic acid: Reaction kinetics, product and pathway evaluation[J]. Water Research, 2017, 123: 153-161. doi: 10.1016/j.watres.2017.06.057 [21] ZHANG W J, CAO B D, WANG D S, et al. Influence of wastewater sludge treatment using combined peroxyacetic acid oxidation and inorganic coagulants re-flocculation on characteristics of extracellular polymeric substances (EPS)[J]. Water Research, 2016, 88: 728-739. doi: 10.1016/j.watres.2015.10.049 [22] CAMPO N, de FLORA C, MAFFETTONE R, et al. Inactivation kinetics of antibiotic resistant Escherichia coli in secondary wastewater effluents by peracetic and performic acids[J]. Water Research, 2020, 169: 115227. doi: 10.1016/j.watres.2019.115227 [23] RAGAZZO P, CHIUCCHINI N, PICCOLO V, et al. Wastewater disinfection: Long-term laboratory and full-scale studies on performic acid in comparison with peracetic acid and chlorine[J]. Water Research, 2020, 184: 116169. doi: 10.1016/j.watres.2020.116169 [24] LUUKKONEN T, HEYNINCK T, RÄMÖ J, et al. Comparison of organic peracids in wastewater treatment: Disinfection, oxidation and corrosion[J]. Water Research, 2015, 85: 275-285. doi: 10.1016/j.watres.2015.08.037 [25] DOMÍNGUEZ HENAO L, TUROLLA A, ANTONELLI M. Disinfection by-products formation and ecotoxicological effects of effluents treated with peracetic acid: A review[J]. Chemosphere, 2018, 213: 25-40. doi: 10.1016/j.chemosphere.2018.09.005 [26] FARINELLI G, COHA M, VIONE D, et al. Formation of halogenated byproducts upon water treatment with peracetic acid[J]. Environmental Science & Technology, 2022, 56(8): 5123-5131. [27] 曹聪, 张土乔, 张富标, 等. 饮用水中的新型消毒剂: 过氧乙酸的研究进展[J]. 中国给水排水, 2018, 34(4): 36-40. CAO C, ZHANG T Q, ZHANG F B, et al. Research progress of peracetic acid (PAA): An emerging disinfectant in drinking water[J]. China Water & Wastewater, 2018, 34(4): 36-40 (in Chinese).
[28] 戴寅豪, 杨绍贵, 祁承都, 等. 活化过氧乙酸技术去除水体有机污染物研究进展[J]. 环境化学, 2021, 40(2): 497-508. doi: 10.7524/j.issn.0254-6108.2020083001 Dai Y H, Yang S G, Qi C D, et al. Activation of peracetic acid process for aquatic organic pollutants degradation: A review[J]. Environmental Chemistry, 2021, 40(2): 497-508 (in Chinese). doi: 10.7524/j.issn.0254-6108.2020083001
[29] DENG J W, LIU S L, FU Y S, et al. Heat-activated peracetic acid for degradation of diclofenac: Kinetics, influencing factors and mechanism[J]. Environmental Technology, 2022: 1-9. [30] PING Q, YAN T T, WANG L, et al. Insight into using a novel ultraviolet/peracetic acid combination disinfection process to simultaneously remove antibiotics and antibiotic resistance genes in wastewater: Mechanism and comparison with conventional processes[J]. Water Research, 2022, 210: 118019. doi: 10.1016/j.watres.2021.118019 [31] 李文涛, 李梦凯, 强志民. 化学剂量法原理及其在水处理紫外线技术研究中的应用[J]. 环境化学, 2020, 39(2): 326-333. doi: 10.7524/j.issn.0254-6108.2019031502 LI W T, LI M K, QIANG Z M. Principles and application of chemical actinometry in the research of UV technology for water treatment[J]. Environmental Chemistry, 2020, 39(2): 326-333 (in Chinese). doi: 10.7524/j.issn.0254-6108.2019031502
[32] ZHANG T Q, WANG T, MEJIA-TICKNER B, et al. Inactivation of bacteria by peracetic acid combined with ultraviolet irradiation: Mechanism and optimization[J]. Environmental Science & Technology, 2020, 54(15): 9652-9661. [33] CHEN S A, CAI M Q, LIU Y Z, et al. Effects of water matrices on the degradation of naproxen by reactive radicals in the UV/peracetic acid process[J]. Water Research, 2019, 150: 153-161. doi: 10.1016/j.watres.2018.11.044 [34] WANG L Q, YE J Y, ZHANG J Y, et al. Removal of sulfamethazine using peracetic acid activated by Fe0 and UV: Efficiency and mechanism study[J]. Journal of Environmental Chemical Engineering, 2021, 9(6): 106358. doi: 10.1016/j.jece.2021.106358 [35] KIBBEE R, ÖRMECI B. Peracetic acid (PAA) and low-pressure ultraviolet (LP-UV) inactivation of Coxsackievirus B3 (CVB3) in municipal wastewater individually and concurrently[J]. Water Research, 2020, 183: 116048. doi: 10.1016/j.watres.2020.116048 [36] AO X W, WANG W B, SUN W J, et al. Degradation and transformation of norfloxacin in medium-pressure ultraviolet/peracetic acid process: An investigation of the role of pH[J]. Water Research, 2021, 203: 117458. doi: 10.1016/j.watres.2021.117458 [37] RIZZO L, AGOVINO T, NAHIM-GRANADOS S, et al. Tertiary treatment of urban wastewater by solar and UV-C driven advanced oxidation with peracetic acid: Effect on contaminants of emerging concern and antibiotic resistance[J]. Water Research, 2019, 149: 272-281. doi: 10.1016/j.watres.2018.11.031 [38] SILVEIRA L O, DO ROSÁRIO D K A, GIORI A C G, et al. Combination of peracetic acid and ultrasound reduces Salmonella Typhimurium on fresh lettuce (Lactuca sativa L. var. crispa)[J]. Journal of Food Science and Technology, 2018, 55(4): 1535-1540. doi: 10.1007/s13197-018-3071-8 [39] DAI Y H, QI C D, CAO H, et al. Enhanced degradation of sulfamethoxazole by microwave-activated peracetic acid under alkaline condition: Influencing factors and mechanism[J]. Separation and Purification Technology, 2022, 288: 120716. doi: 10.1016/j.seppur.2022.120716 [40] WANG J W, WAN Y, DING J Q, et al. Thermal activation of peracetic acid in aquatic solution: The mechanism and application to degrade sulfamethoxazole[J]. Environmental Science & Technology, 2020, 54(22): 14635-14645. [41] SGROI M, SNYDER S A, ROCCARO P. Comparison of AOPs at pilot scale: Energy costs for micro-pollutants oxidation, disinfection by-products formation and pathogens inactivation[J]. Chemosphere, 2021, 273: 128527. doi: 10.1016/j.chemosphere.2020.128527 [42] WANG S X, WANG H B, LIU Y Q, et al. Effective degradation of sulfamethoxazole with Fe2+-zeolite/peracetic acid[J]. Separation and Purification Technology, 2020, 233: 115973. doi: 10.1016/j.seppur.2019.115973 [43] LING X, CAI A H, CHEN M J, et al. A comparison of oxidation and re-flocculation behaviors of Fe2+/PAA and Fe2+/H2O2 treatments for enhancing sludge dewatering: A mechanism study[J]. Science of the Total Environment, 2022, 847: 157690. doi: 10.1016/j.scitotenv.2022.157690 [44] CARLOS T D, BEZERRA L B, VIEIRA M M, et al. Fenton-type process using peracetic acid: Efficiency, reaction elucidations and ecotoxicity[J]. Journal of Hazardous Materials, 2021, 403: 123949. doi: 10.1016/j.jhazmat.2020.123949 [45] 侯琳萌, 清华, 吉庆华. 类芬顿反应的催化剂、原理与机制研究进展[J]. 环境化学, 2022, 41(6): 1843-1855. doi: 10.7524/j.issn.0254-6108.2021030301 HOU L M, QINGHUA, JI Q H. Research progress on catalysts, principles and mechanisms of Fenton-like reactions[J]. Environmental Chemistry, 2022, 41(6): 1843-1855 (in Chinese). doi: 10.7524/j.issn.0254-6108.2021030301
[46] KIM J, ZHANG T Q, LIU W, et al. Advanced oxidation process with peracetic acid and Fe(II) for contaminant degradation[J]. Environmental Science & Technology, 2019, 53(22): 13312-13322. [47] LIN J B, ZOU J, CAI H Y, et al. Hydroxylamine enhanced Fe(Ⅱ)-activated peracetic acid process for diclofenac degradation: Efficiency, mechanism and effects of various parameters[J]. Water Research, 2021, 207: 117796. doi: 10.1016/j.watres.2021.117796 [48] KIM J, WANG J Y, ASHLEY D C, et al. Enhanced degradation of micropollutants in a peracetic acid–Fe(Ⅲ) system with picolinic acid[J]. Environmental Science & Technology, 2022, 56(7): 4437-4446. [49] MANOLI K, LI R B, KIM J, et al. Ferrate(Ⅵ)-peracetic acid oxidation process: Rapid degradation of pharmaceuticals in water[J]. Chemical Engineering Journal, 2022, 429: 132384. doi: 10.1016/j.cej.2021.132384 [50] LIU B H, GUO W Q, JIA W R, et al. Insights into the oxidation of organic contaminants by Co(Ⅱ) activated peracetic acid: The overlooked role of high-valent cobalt-oxo species[J]. Water Research, 2021, 201: 117313. doi: 10.1016/j.watres.2021.117313 [51] 阿尔娜·海萨尔, 贾剑平, 任定益, 等. 类Fenton氧化技术去除聚丙烯酰胺的研究[J]. 精细石油化工, 2016, 33(6): 55-58. AERNA· HAISAER, JIA J P, REN D Y, et al. The removal of HPAM with Fenton-like reagents oxidation process[J]. Speciality Petrochemicals, 2016, 33(6): 55-58(in Chinese).
[52] 田丹, 吴玮, 沈芷璇, 等. Co(Ⅱ)活化过氧乙酸降解有机染料研究[J]. 环境科学学报, 2018, 38(10): 4023-4031. TIAN D, WU W, SHEN Z X, et al. Degradation of organic dyes with peracetic acid activated by Co (Ⅱ)[J]. Acta Scientiae Circumstantiae, 2018, 38(10): 4023-4031 (in Chinese).
[53] KIM J, DU P H, LIU W, et al. Cobalt/peracetic acid: Advanced oxidation of aromatic organic compounds by acetylperoxyl radicals[J]. Environmental Science & Technology, 2020, 54(8): 5268-5278. [54] WANG Z P, WANG J W, XIONG B, et al. Application of cobalt/peracetic acid to degrade sulfamethoxazole at neutral condition: Efficiency and mechanisms[J]. Environmental Science & Technology, 2020, 54(1): 464-475. [55] LUUKKONEN T, von GUNTEN U. Oxidation of organic micropollutant surrogate functional groups with peracetic acid activated by aqueous Co(II), Cu(II), or Ag(I) and geopolymer-supported Co(II)[J]. Water Research, 2022, 223: 118984. doi: 10.1016/j.watres.2022.118984 [56] LI R B, MANOLI K, KIM J, et al. Peracetic acid–ruthenium(Ⅲ) oxidation process for the degradation of micropollutants in water[J]. Environmental Science & Technology, 2021, 55(13): 9150-9160. [57] BOKARE A D, CHOI W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes[J]. Journal of Hazardous Materials, 2014, 275: 121-135. doi: 10.1016/j.jhazmat.2014.04.054 [58] 王静晓, 朱柯安, 陈飞. 氯离子活化过氧乙酸对罗丹明B的降解性能及机理研究[J]. 环境科学研究, 2021, 34(12): 2850-2858. WANG J X, ZHU K A, CHEN F. Degradation performance and mechanism of rhodamine B by chloride activated peracetic acid[J]. Research of Environmental Sciences, 2021, 34(12): 2850-2858 (in Chinese) .
[59] WANG H Z, LIU B H, SI Q S, et al. Developing functional carbon nitride materials for efficient peroxymonosulfate activation: From interface catalysis to irradiation synergy[J]. Environmental Functional Materials, 2022, 1(1): 21-33. doi: 10.1016/j.efmat.2022.05.007 [60] DAS NEVES A P N, CARLOS T D, BEZERRA L B, et al. Carbonate anion photolyzed by solar radiation or combined with peracetic acid to form reactive species for dye degradation[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2021, 420: 113511. doi: 10.1016/j.jphotochem.2021.113511 [61] DENG J W, WANG H B, FU Y S, et al. Phosphate-induced activation of peracetic acid for diclofenac degradation: Kinetics, influence factors and mechanism[J]. Chemosphere, 2022, 287: 132396. doi: 10.1016/j.chemosphere.2021.132396 [62] 邓杰文, 张琳悦, 付永胜, 等. Cu(Ⅱ)协同热活化过氧乙酸降解水中双氯芬酸[J]. 环境化学, 2023, 42(4 ): 1222-1229. doi: 10.7524/j.issn.0254-6108.2021111602 DENG J W, ZHANG L Y, FU Y S, et al. Degradation of diclofenac in water by Cu(Ⅱ)-combined with heat activation of peracetic acid[J]. Environmental Chemistry, 2023, 42(4 ): 1222-1229 (in Chinese). doi: 10.7524/j.issn.0254-6108.2021111602
[63] GHANBARI F, GIANNAKIS S, LIN K Y A, et al. Acetaminophen degradation by a synergistic peracetic acid/UVC-LED/Fe(Ⅱ) advanced oxidation process: Kinetic assessment, process feasibility and mechanistic considerations[J]. Chemosphere, 2021, 263: 128119. doi: 10.1016/j.chemosphere.2020.128119 [64] LIN J B, HU Y Y, XIAO J Y, et al. Enhanced diclofenac elimination in Fe(Ⅱ)/peracetic acid process by promoting Fe(III)/Fe(Ⅱ) cycle with ABTS as electron shuttle[J]. Chemical Engineering Journal, 2021, 420: 129692. doi: 10.1016/j.cej.2021.129692 [65] 徐文露, 张凌燕, 邱杨率, 等. 石墨基复合材料负载零价纳米铁吸附重金属离子的研究进展[J]. 环境化学, 2022, 41(1): 376-385. doi: 10.7524/j.issn.0254-6108.2020091601 XU W L, ZHANG L Y, QIU Y S, et al. Research progress on adsorption of heavy metal ions by graphite based composites supported with nano zero valent iron[J]. Environmental Chemistry, 2022, 41(1): 376-385 (in Chinese). doi: 10.7524/j.issn.0254-6108.2020091601
[66] 周高峰, 周润宇, 刘义青, 等. 零价钴活化过氧乙酸降解水中罗丹明B的研究[J]. 环境科学学报, 2022, 42(11): 47-55. ZHOU G F, ZHOU R Y, LIU Y Q, et al. Degrdation of rhodamine B by peracetic acid activated with zero-valent cobalt[J]. Acta Scientiae Circumstantiae, 2022, 42(11): 47-55 (in Chinese).
[67] HE M F, LI W Q, XIE Z H, et al. Peracetic acid activation by mechanochemically sulfidated zero valent iron for micropollutants degradation: Enhancement mechanism and strategy for extending applicability[J]. Water Research, 2022, 222: 118887. doi: 10.1016/j.watres.2022.118887 [68] WANG L, YAN T T, TANG R J, et al. Motivation of reactive oxidation species in peracetic acid by adding nanoscale zero-valent iron to synergic removal of spiramycin under ultraviolet irradiation: Mechanism and N-nitrosodimethylamine formation potential assessment[J]. Water Research, 2021, 205: 117684. doi: 10.1016/j.watres.2021.117684 [69] YANG S R, HE C S, XIE Z H, et al. Efficient activation of PAA by FeS for fast removal of pharmaceuticals: The dual role of sulfur species in regulating the reactive oxidized species[J]. Water Research, 2022, 217: 118402. doi: 10.1016/j.watres.2022.118402 [70] XING D Y, SHAO S J, YANG Y Y, et al. Mechanistic insights into the efficient activation of peracetic acid by pyrite for the tetracycline abatement[J]. Water Research, 2022, 222: 118930. doi: 10.1016/j.watres.2022.118930 [71] ZHANG L L, CHEN J B, ZHANG Y L, et al. Interactions between peracetic acid and TiO2 nanoparticle in wastewater disinfection: Mechanisms and implications[J]. Chemical Engineering Journal, 2021, 412: 128703. doi: 10.1016/j.cej.2021.128703 [72] LI S, YANG Y L, ZHENG H S, et al. Introduction of oxygenvacancy to Manganese ferrite by Co substitution for enhanced peracetic acid activation and 1O2 dominated tetracycline hydrochloride degradation under microwave irradiation[J]. Water Research, 2022, 225: 119176. doi: 10.1016/j.watres.2022.119176 [73] WANG J W, XIONG B, MIAO L, et al. Applying a novel advanced oxidation process of activated peracetic acid by CoFe2O4 to efficiently degrade sulfamethoxazole[J]. Applied Catalysis B: Environmental, 2021, 280: 119422. doi: 10.1016/j.apcatb.2020.119422 [74] ROKHINA E V, MAKAROVA K, LAHTINEN M, et al. Ultrasound-assisted MnO2 catalyzed homolysis of peracetic acid for phenol degradation: The assessment of process chemistry and kinetics[J]. Chemical Engineering Journal, 2013, 221: 476-486. doi: 10.1016/j.cej.2013.02.018 [75] CUERDA-CORREA E M, ALEXANDRE-FRANCO M F, FERNÁNDEZ-GONZÁLEZ C. Advanced oxidation processes for the removal of antibiotics from water. an overview[J]. Water, 2019, 12(1): 102. doi: 10.3390/w12010102 [76] 王崇臣, 王恂. 金属-有机骨架在水处理中的应用研究进展[J]. 工业水处理, 2020, 40(11): 1-9 WANG C C, WANG X. The application of metal-organic frameworks in the wastewater treatment: A state-of-the-art review[J]. Industrial Water Treatment, 2020, 40(11): 1-9(in Chinese) .
[77] 楚弘宇, 王天予, 王崇臣. MOFs基材料高级氧化除菌[J]. 化学进展, 2022, 34(12): 2700-2714. doi: 10.7536/220501 CHU H Y, WANG T Y, WANG C C. Advanced oxidation processes(AOPs)for bacteria removal over MOFs-based materials[J]. Progress in Chemistry, 2022, 34(12): 2700-2714 (in Chinese). doi: 10.7536/220501
[78] FU H F, WANG C C, LIU W. MOFs for water purification[J]. Chinese Chemical Letters, 2022, 33(4): 1647-1649. doi: 10.1016/j.cclet.2021.08.065 [79] YI X H, WANG F X, DU X D, et al. Highly efficient photocatalytic Cr(Ⅵ) reduction and organic pollutants degradation of two new bifunctional 2D Cd/Co-based MOFs[J]. Polyhedron, 2018, 152: 216-224. doi: 10.1016/j.poly.2018.06.041 [80] DUAN J, CHEN L, JI H D, et al. Activation of peracetic acid by metal-organic frameworks (ZIF-67) for efficient degradation of sulfachloropyridazine[J]. Chinese Chemical Letters, 2022, 33(6): 3172-3176. doi: 10.1016/j.cclet.2021.11.072 [81] ZHANG L L, CHEN J B, ZHANG Y L, et al. Activation of peracetic acid with cobalt anchored on 2D sandwich-like MXenes (Co@MXenes) for organic contaminant degradation: High efficiency and contribution of acetylperoxyl radicals[J]. Applied Catalysis B: Environmental, 2021, 297: 120475. doi: 10.1016/j.apcatb.2021.120475 [82] 郑婷露, 张龙龙, 陈家斌, 等. 纳米核壳Co@NC催化剂活化过氧乙酸降解磺胺甲噁唑[J]. 环境科学, 2023, 44(5): 2635-2645. ZHENG T L, ZHANG L L, CHEN J B, et al. Degradation of SMX with peracetic acid activated by nano core-shell Co@NC catalyst[J]. Environmental Science, 2023, 44(5): 2635-2645 (in Chinese).
[83] LIU B H, GUO W Q, JIA W R, et al. Novel nonradical oxidation of sulfonamide antibiotics with Co(Ⅱ)-doped g-C3N4-activated peracetic acid: Role of high-valent cobalt–oxo species[J]. Environmental Science & Technology, 2021, 55(18): 12640-12651. [84] ZHANG L L, CHEN J B, ZHANG Y L, et al. Highly efficient activation of peracetic acid by nano-CuO for carbamazepine degradation in wastewater: The significant role of H2O2 and evidence of acetylperoxy radical contribution[J]. Water Research, 2022, 216: 118322. doi: 10.1016/j.watres.2022.118322 [85] HU C G, LIN Y, CONNELL J W, et al. Carbon-based metal-free catalysts for energy storage and environmental remediation[J]. Advanced Materials (Deerfield Beach, Fla. ), 2019, 31(13): e1806128. doi: 10.1002/adma.201806128 [86] KONG D Z, ZHAO Y M, FAN X R, et al. Reduced graphene oxide triggers peracetic acid activation for robust removal of micropollutants: The role of electron transfer[J]. Environmental Science & Technology, 2022, 56(16): 11707-11717. [87] DAI C M, LI S, DUAN Y P, et al. Mechanisms and product toxicity of activated carbon/peracetic acid for degradation of sulfamethoxazole: Implications for groundwater remediation[J]. Water Research, 2022, 216: 118347. doi: 10.1016/j.watres.2022.118347 [88] WU L Y, LI Z Y, CHENG P T, et al. Efficient activation of peracetic acid by mixed sludge derived biochar: Critical role of persistent free radicals[J]. Water Research, 2022, 223: 119013. doi: 10.1016/j.watres.2022.119013 [89] QIU M H, CHEN X F, FAN Y Q, et al. 1.11 ceramic membranes[M]//Comprehensive Membrane Science and Engineering. Amsterdam: Elsevier, 2017: 270-297. [90] ZHAO Y M, ZHAO Y X, YU X, et al. Peracetic acid integrated catalytic ceramic membrane filtration for enhanced membrane fouling control: Performance evaluation and mechanism analysis[J]. Water Research, 2022, 220: 118710. doi: 10.1016/j.watres.2022.118710 [91] WANG S Y, ZHOU S M, TAO Y, et al. Organic peroxides and sulfur dioxide in aerosol: Source of particulate sulfate[J]. Environmental Science & Technology, 2019, 53(18): 10695-10704. [92] LIAO Q B, WANG D N, KE C, et al. Metal-free Fenton-like photocatalysts based on covalent organic frameworks[J]. Applied Catalysis B: Environmental, 2021, 298: 120548. doi: 10.1016/j.apcatb.2021.120548 [93] SHI C J, WANG Y, ZHANG K, et al. Fe-biochar as a safe and efficient catalyst to activate peracetic acid for the removal of the acid orange dye from water[J]. Chemosphere, 2022, 307: 135686. doi: 10.1016/j.chemosphere.2022.135686 [94] 孙义才, 孙德栋, 王佳莹, 等. 铁碳微电解与过氧乙酸联用处理罗丹明B废水[J]. 工业水处理, 2018, 38(12): 93-96. doi: 10.11894/1005-829x.2018.38(12).093 SUN Y C, SUN D D, WANG J Y, et al. Combination of iron-carbon micro-electrolysis and peracetic acid for the treatment of Rhodamine B containing wastewater[J]. Industrial Water Treatment, 2018, 38(12): 93-96. (in Chinese) doi: 10.11894/1005-829x.2018.38(12).093
[95] LI T, JIN L L, ZHU S S, et al. Simultaneous removal of heterocyclic drugs and total nitrogen from biochemical tailwater by peracetic acid/cobalt-loaded ceramsite-based denitrification biofilter[J]. Environmental Pollution, 2022, 314: 120279. doi: 10.1016/j.envpol.2022.120279 [96] ZHANG L, FU Y S, WANG Z R, et al. Removal of diclofenac in water using peracetic acid activated by zero valent copper[J]. Separation and Purification Technology, 2021, 276: 119319. doi: 10.1016/j.seppur.2021.119319 [97] LI Y J, DONG H R, XIAO J Y, et al. Oxidation of sulfamethazine by a novel CuS/calcium peroxide/tetraacetylethylenediamine process: High efficiency and contribution of oxygen-centered radicals[J]. Chemical Engineering Journal, 2022, 446: 136882. doi: 10.1016/j.cej.2022.136882 [98] XIAO J Y, DONG H R, LI Y J, et al. Graphene shell-encapsulated copper-based nanoparticles (G@Cu-NPs) effectively activate peracetic acid for elimination of sulfamethazine in water under neutral condition[J]. Journal of Hazardous Materials, 2023, 441: 129895. doi: 10.1016/j.jhazmat.2022.129895 [99] KATARIA J, VADDU S, RAMA E N, et al. Evaluating the efficacy of peracetic acid on Salmonella and Campylobacter on chicken wings at various pH levels[J]. Poultry Science, 2020, 99(10): 5137-5142. doi: 10.1016/j.psj.2020.06.070 [100] ZHANG C Q, BROWN P J B, HU Z Q. Thermodynamic properties of an emerging chemical disinfectant, peracetic acid[J]. Science of the Total Environment, 2018, 621: 948-959. doi: 10.1016/j.scitotenv.2017.10.195 [101] ZHANG P Y, ZHANG X F, ZHAO X D, et al. Activation of peracetic acid with zero-valent iron for tetracycline abatement: The role of Fe(II) complexation with tetracycline[J]. Journal of Hazardous Materials, 2022, 424: 127653. doi: 10.1016/j.jhazmat.2021.127653 [102] ZHOU F Y, LU C, YAO Y Y, et al. Activated carbon fibers as an effective metal-free catalyst for peracetic acid activation: Implications for the removal of organic pollutants[J]. Chemical Engineering Journal, 2015, 281: 953-960. doi: 10.1016/j.cej.2015.07.034 [103] DAI Y H, CAO H, QI C D, et al. L-cysteine boosted Fe(III)-activated peracetic acid system for sulfamethoxazole degradation: Role of L-cysteine and mechanism[J]. Chemical Engineering Journal, 2023, 451: 138588. doi: 10.1016/j.cej.2022.138588 [104] XIONG Z K, JIANG Y N, WU Z L, et al. Synthesis strategies and emerging mechanisms of metal-organic frameworks for sulfate radical-based advanced oxidation process: A review[J]. Chemical Engineering Journal, 2021, 421: 127863. doi: 10.1016/j.cej.2020.127863 [105] KHARISSOVA O V, KHARISOV B I, GONZÁLEZ L T. Recent trends on density functional theory–assisted calculations of structures and properties of metal–organic frameworks and metal-organic frameworks-derived nanocarbons[J]. Journal of Materials Research, 2020, 35(11): 1424-1438. doi: 10.1557/jmr.2020.109 [106] CERRÓN-CALLE G A, SENFTLE T P, GARCIA-SEGURA S. Strategic tailored design of electrocatalysts for environmental remediation based on density functional theory (DFT) and microkinetic modeling[J]. Current Opinion in Electrochemistry, 2022, 35: 101062. doi: 10.1016/j.coelec.2022.101062 [107] ZOU Y D, WANG X X, KHAN A, et al. Environmental remediation and application of nanoscale zero-valent iron and its composites for the removal of heavy metal ions: A review[J]. Environmental Science & Technology, 2016, 50(14): 7290-7304. [108] ZHANG P P, YANG Y Y, DUAN X G, et al. Density functional theory calculations for insight into the heterocatalyst reactivity and mechanism in persulfate-based advanced oxidation reactions[J]. ACS Catalysis, 2021, 11(17): 11129-11159. doi: 10.1021/acscatal.1c03099 [109] ZHOU G F, ZHOU R Y, LIU Y Q, et al. Efficient degradation of sulfamethoxazole using peracetic acid activated by zero-valent cobalt[J]. Journal of Environmental Chemical Engineering, 2022, 10(3): 107783. doi: 10.1016/j.jece.2022.107783 [110] MIAO F, YUE X T, CHENG C, et al. Insights into the mechanism of carbocatalysis for peracetic acid activation: Kinetic discernment and active site identification[J]. Water Research, 2022, 227: 119346. doi: 10.1016/j.watres.2022.119346 [111] Da SILVA W P, CARLOS T D, CAVALLINI G S, et al. Peracetic acid: Structural elucidation for applications in wastewater treatment[J]. Water Research, 2020, 168: 115143. doi: 10.1016/j.watres.2019.115143 [112] BALACHANDRAN S, CHARAMBA L V C, MANOLI K, et al. Simultaneous inactivation of multidrug-resistant Escherichia coli and enterococci by peracetic acid in urban wastewater: Exposure-based kinetics and comparison with chlorine[J]. Water Research, 2021, 202: 117403. doi: 10.1016/j.watres.2021.117403 [113] MAFFETTONE R, MANOLI K, SANTORO D, et al. Performic acid disinfection of municipal secondary effluent wastewater: Inactivation of murine norovirus, fecal coliforms, and enterococci[J]. Environmental Science & Technology, 2020, 54(19): 12761-12770. [114] FOSCHI J, BIANCHI G F, TUROLLA A, et al. Disinfection efficiency prediction under dynamic conditions: Application to peracetic acid disinfection of wastewater[J]. Water Research, 2022, 222: 118879. doi: 10.1016/j.watres.2022.118879 [115] MANOLI K, SARATHY S, MAFFETTONE R, et al. Detailed modeling and advanced control for chemical disinfection of secondary effluent wastewater by peracetic acid[J]. Water Research, 2019, 153: 251-262. doi: 10.1016/j.watres.2019.01.022 -






 下载:
下载: