-
金属有机框架(MOF)材料MIL-125,由法国拉瓦锡材料研究所首先合成并报道[1],而后由法国巴黎东部化学与材料研究所首先合成并报道了氨基功能化的MIL-125-NH2[2]. MIL-125和MIL-125-NH2具有比表面积大、孔径尺寸较小及结构具有柔性特点[3 − 4]. 由于MIL-125和MIL-125-NH2独特的结构和性能,使其在气体存储[5]、吸附与分离[6 − 10]、(光)催化[11 − 12]等领域获得广泛应用. 目前,越来越多的研究人员对MIL-125和MIL-125-NH2的光催化性能产生兴趣. MIL-125因具有较宽带隙(Eg = 3.6 eV)而仅能被紫外光激发,而MIL-125-NH2具有较窄带隙(Eg = 2.6 eV)可被可见光激发[13],这也就使得MIL-125-NH2更加受到科研人员的关注. 但是,MIL-125-NH2自身仍然存在一些问题,使得MIL-125-NH2仍需要与一些功能材料复合以进一步增强其光催化性能. 总体来看,相关研究者将MIL-125或MIL-125-NH2与半导体类型的光催化剂进行复合,使其在可见光照射下表现出较为优异的光催化性能.
本文选择了部分典型MIL-125/ MIL-125-NH2复合光催化剂,介绍其制备的方法,并详细地探讨其光催化还原Cr(Ⅵ)和光催化降解染料、药物及个人护理品(PPCPs)的性能与机理,总结出它们的优势与不足.
-
MIL-125系列复合材料在光催化还原Cr(Ⅵ)、降解有机染料和新污染物等方面取得了一些进展. 本文将结合近年来利用MIL-125系列复合材料光催化去除水体污染物的案例(表1),来详细阐述一些有代表性的研究,并着重论述一些典型的研究工作.
-
MIL-125和MIL-125-NH2的合成方法以溶剂热法[3,14]为主,溶剂一般选用甲醇或DMF(N,N-二甲基甲酰胺),反应物为含有Ti(Ⅳ)的盐类和对苯二甲酸(H2BDC)或氨基-对苯二甲酸(NH2-H2BDC). 反应温度一般为150 ℃,反应时间一般为24 h. MIL-125样品一般呈白色粉末状晶体,而MIL-125-NH2样品一般呈亮黄色粉末状晶体.
-
MIL-125系列复合材料的合成一般是采用(光)沉积法、原位法或两步法,其中原位法更常用.
-
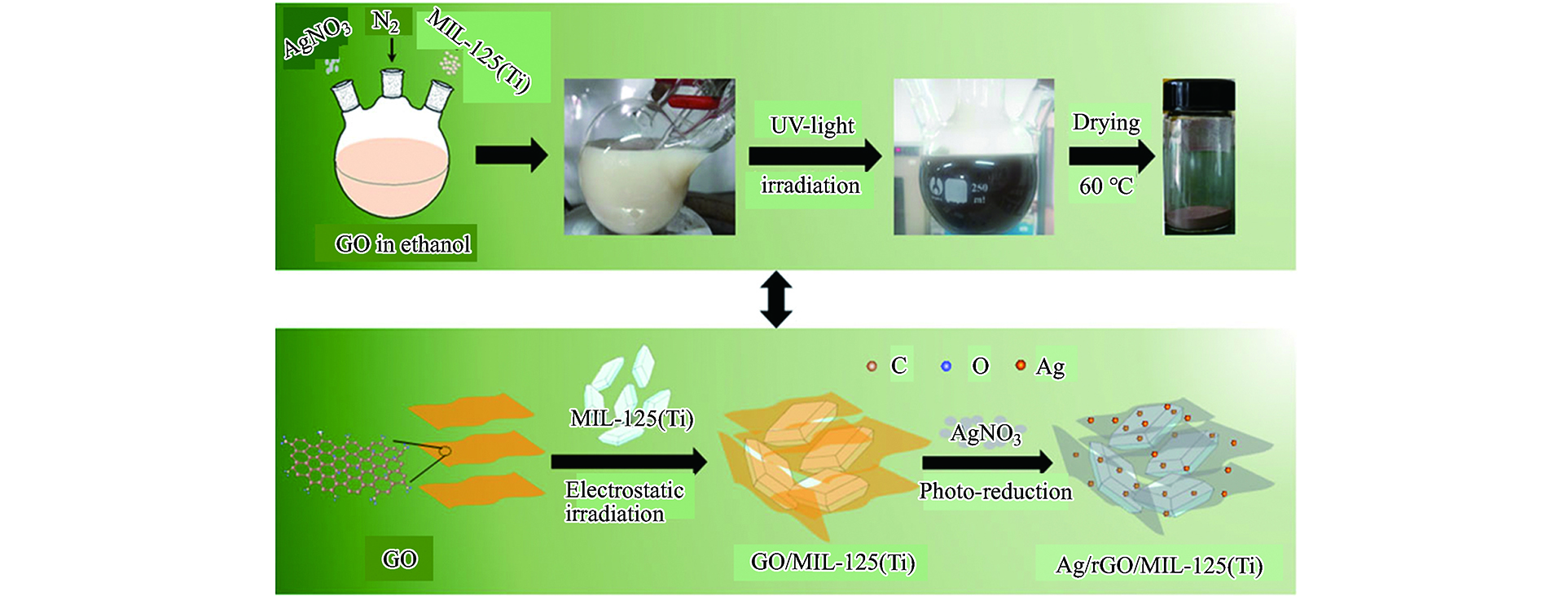
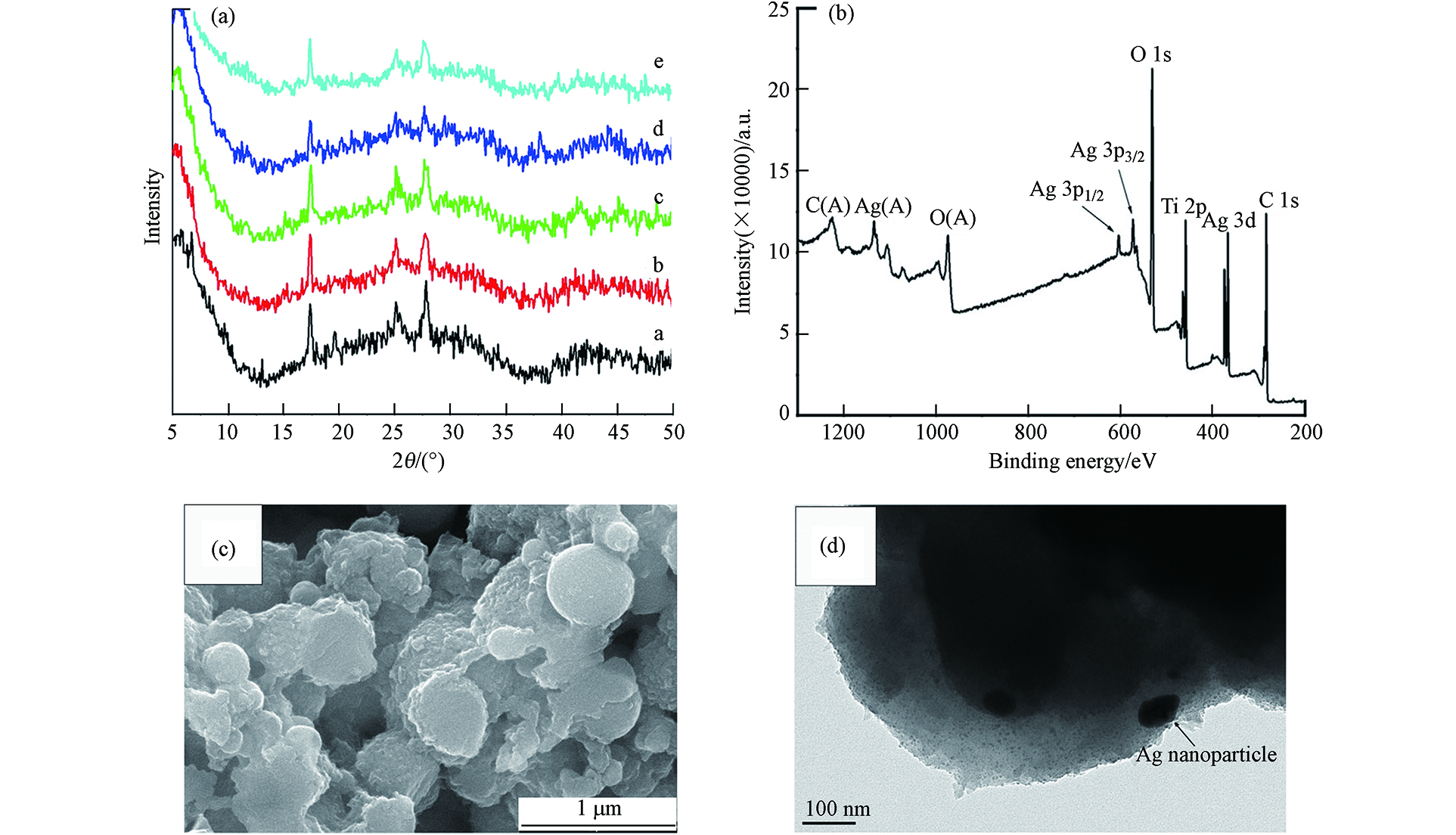
Yuan等[23]用光沉积法将MIL-125与Ag纳米粒子以及还原型氧化石墨烯(rGO)复合制备了Ag/rGO/MIL-125复合材料,制备过程如图1所示. 先通过超声处理60 min,得到GO(15.5 mg)和无水乙醇(200 mL)的均匀分散溶液. 然后,在前述的混合物中加入0.5 g粉末状MIL-125,再超声处理15 min. 再通过用力搅拌,将AgNO3(24.3 mg)分散到前述的溶液中. 之后在黑暗条件下,向悬浮液中注入氮气30 min,并将悬浮液在紫外光(紫外光源功率密度为100 mW cm−2)下照射2 h. 最后,取用无水乙醇共计3次过滤洗涤悬浮液中的物质,在60°C下将所得物质通过真空干燥运行12 h.
-
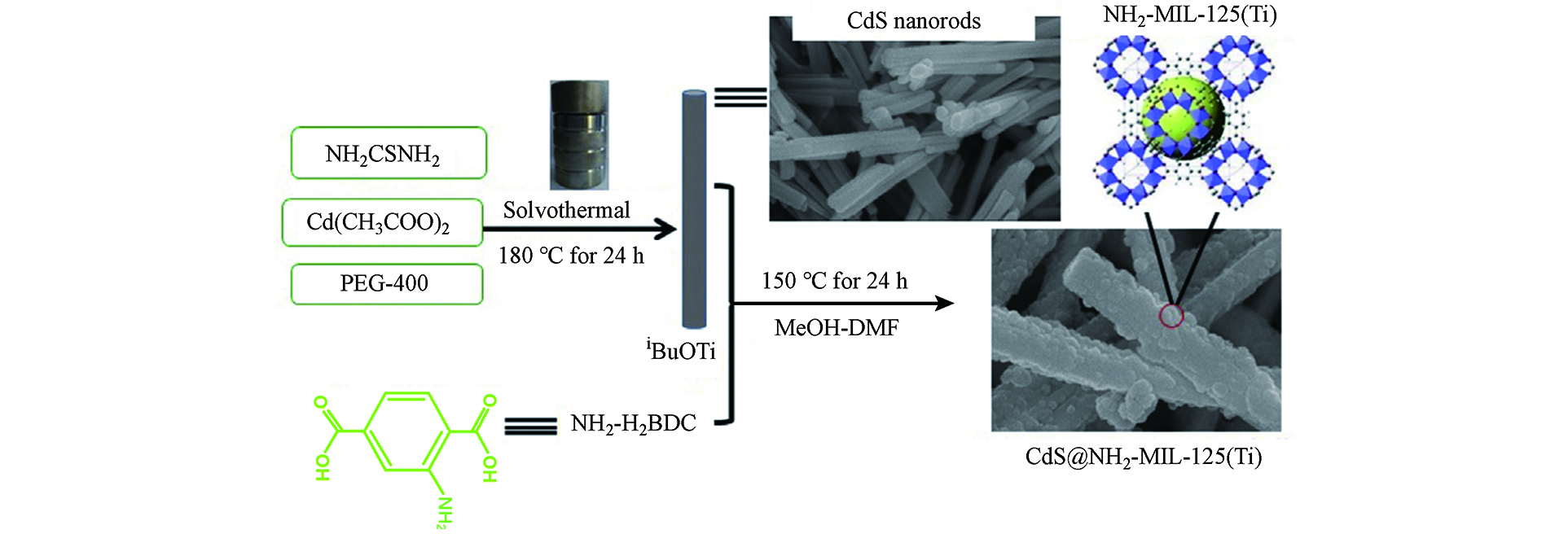
Wang等[30]通过原位法合成S@T-n复合材料(根据CdS纳米棒的质量占比,n=6、10和20)的过程如图2所示. 通常情况下,将预先合成的CdS纳米棒(0.15 mmol)连续搅拌以分散到甲醇(2.0 mL)中,然后加入含有NH2-H2BDC(0.56 g,3.1 mmol)的DMF(8.0 mL)溶液. 搅拌1 h后,在超声条件下将iBuOTi(0.6 mL,2.0 mmol)缓慢注入上述溶液中,生成前驱体溶液. 而后,将前驱体溶液转移到内衬钢高压釜(23 mL)中,在烘箱中以150 °C对其加热24 h. 随后,高压釜冷却至室温,离心分离其中黄色产物,再用DMF和甲醇(10 mL,5次)洗涤,最后在80 ℃下干燥.
Xu等[59]通过两步法合成NH2-MIL-125-P@TiO2复合材料的过程,如图3所示. 将合成的NH2-MIL-125放入150 ℃的真空干燥器中处理12 h以去除孔隙中的有机溶剂. 称取400 mg NH2-MIL-125,在40 mL甲醇中分散30 min. 将钛酸四丁酯以400 μL缓慢加入上述溶液中,室温搅拌1 h,然后快速加热至85 ℃,回流12 h,使钛酸四丁酯完全水解. 将回流装置转化为蒸馏装置,将上述溶液蒸制成膏体,蒸发甲醇. 浆料中加入0.1 mol·L−1磷酸溶液1 mL,磁力搅拌6 h,80 ℃烘箱加热至干燥,100 ℃洗涤干燥12 h,合成磷酸化NH2-MIL-125.
-
铬是水体中一种十分常见污染物,水体中少量存在的铬就足以威胁生命健康. 使用光催化剂就能高效率且低成本地实现对Cr(Ⅵ)的去除,还可以不产生任何有害物质[60 − 65]. 对于这方面应用,因为MIL-125-NH2本体的带隙较窄,一般有关MIL-125-NH2复合材料[15 − 18]的报道较多,但MIL-125复合材料中,也有性能优异的[14].
(1) MIL-125复合材料光催化还原Cr(Ⅵ)
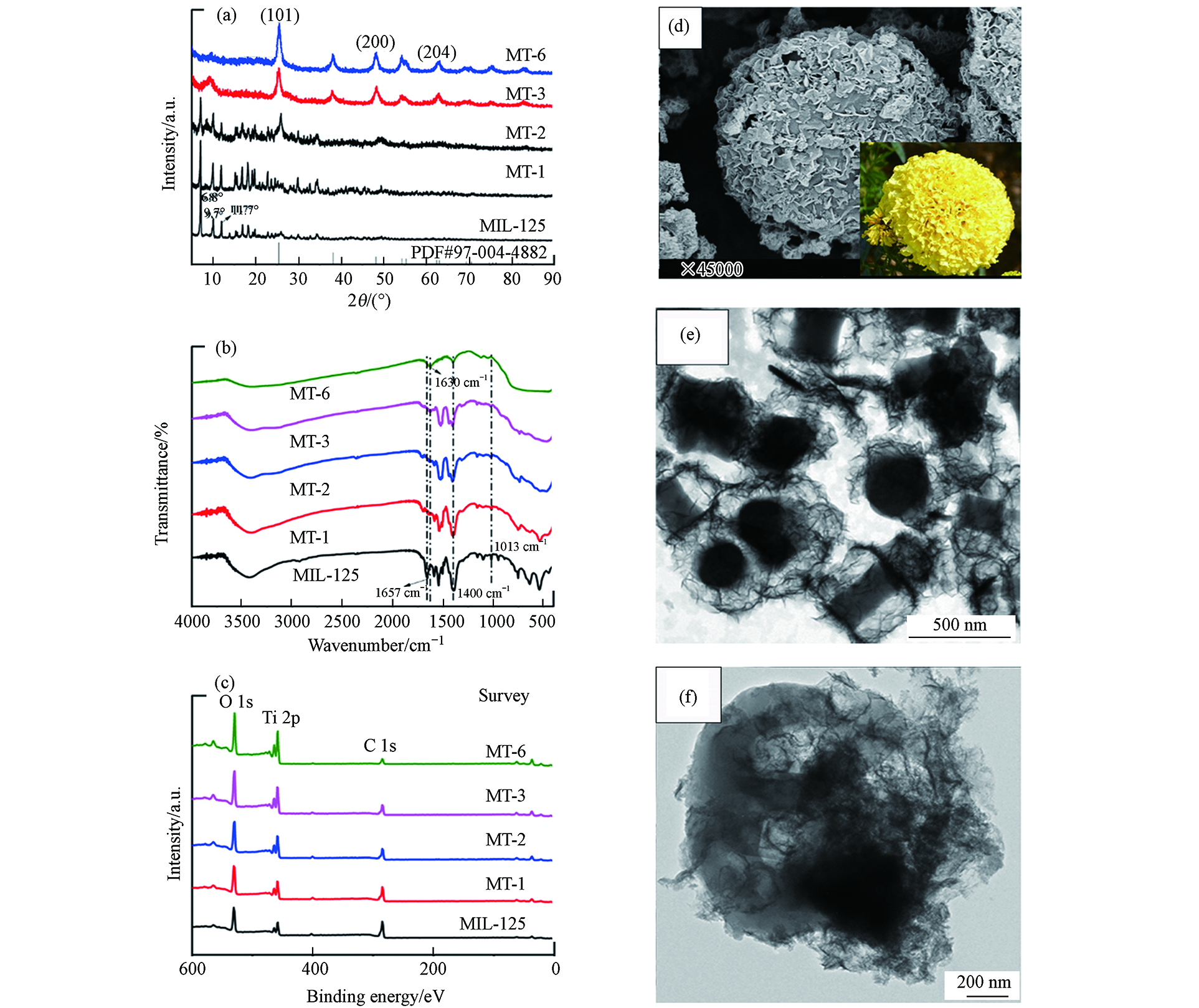
Li等[14]制备了万寿菊状TiO2@MIL-125(MT-n, n= 1, 2, 3, 6)核-壳型复合光催化剂,并将其用于还原Cr(Ⅵ). 复合材料MT-n的X射线衍射(XRD)图谱中的衍射峰与原材料基本一致(图4a),类似的情况也体现在傅立叶变换红外吸收光谱(FT-IR)图谱中(如图4b所示). 所制备样品的化学状态通过X-射线光电子能谱(XPS)测定之后(如图4c所示)可知,在MIL-125和MT-2中均可观察到Ti 2p峰,这表明TiO2@MIL-125核-壳复合材料的成功制备. MT-2的扫描电子显微镜(SEM)和透射电子显微镜(TEM)图像分别如图4d、4e和4f所示,直观地证明了TiO2已成功沉积在MIL-125上,形成了TiO2@MIL-125的核-壳型复合材料.
紫外-可见漫反射光谱(图5a)表明所制备MT-n样品的光吸收特性随硫代乙酰胺(TAA)处理时间的逐渐延长,表明引入TAA可将MIL-125的吸光能力提高,这也有望提高MIL-125的光催化活性. 图5b表明所有制备的MT-n均具有光催化还原Cr(Ⅵ)的能力,而MT-2在60 min内对初始浓度为 5 mg·L−1的Cr(Ⅵ)还原效率达100%. 5 次循环实验表明,MT-2的光催化活性在反应过程中几乎保持不变(图5c),仍具有稳定的催化剂组分和晶体结构. 基于上述实验结果,作者提出了增强MT-2光催化活性的机理与机制(如图5d所示). 所制备的MT-2中MIL-125与TiO2形成的异质结,可有效提高电荷分离效率. 更多的来自于MIL-125导带(LUMO)的光生电子可以转移到TiO2上,加速了Cr(Ⅵ)向Cr(Ⅲ)的还原过程. 在没有MIL-125的情况下,e−和h+容易复合. MIL-125引入后,TiO2与MIL-125之间的协同效应将增强复合材料的电子利用能力和光催化还原活性.
(2)MIL-125-NH2复合材料光催化还原Cr(Ⅵ)
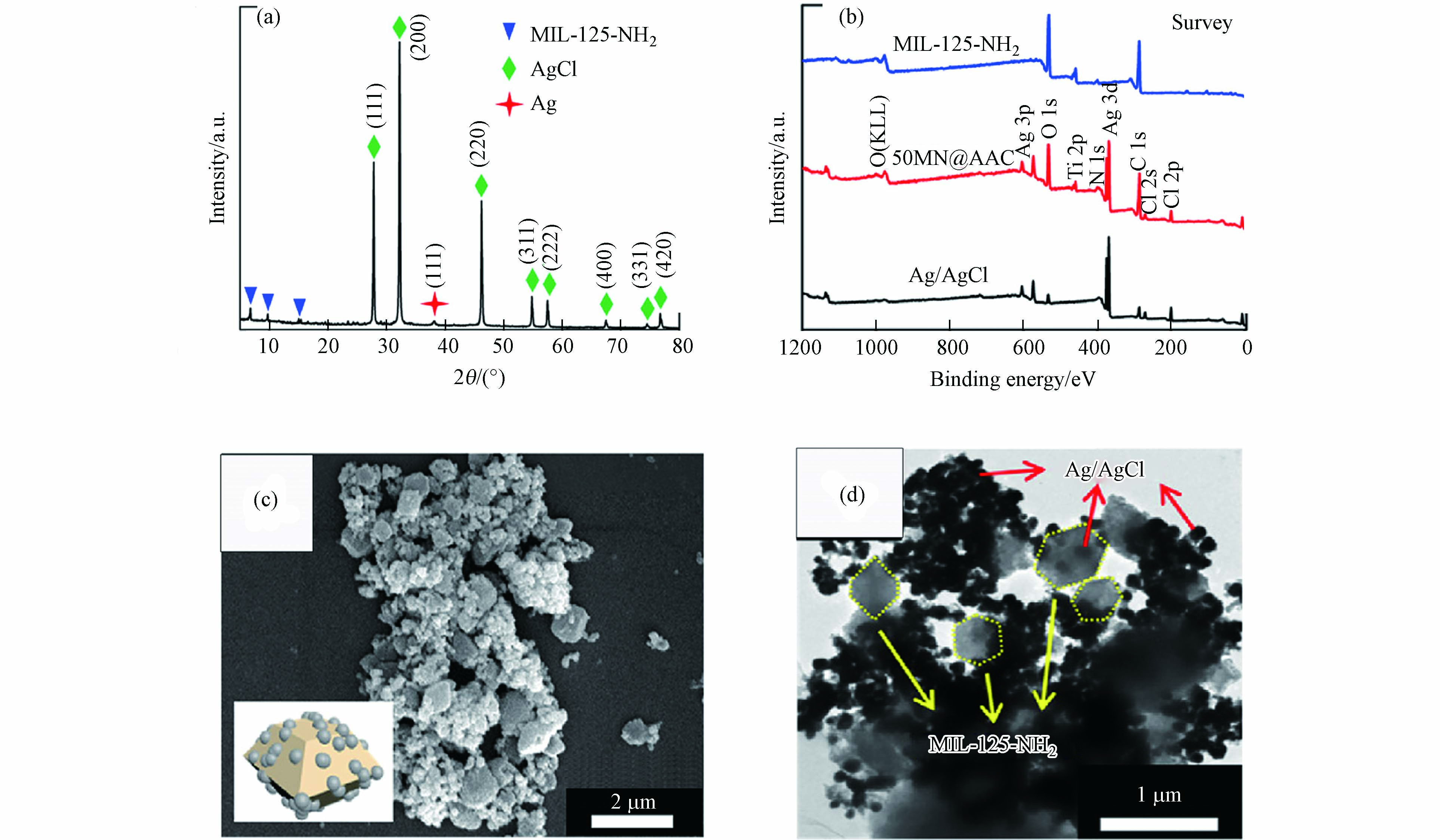
Qiu等[16]制备了MIL-125-NH2@Ag/AgCl(nMN@AAC, n= 30, 40, 50, 60)复合催化剂,并将其应用于光催化还原Cr(Ⅵ). 复合材料nMN@AAC的XRD图谱中的衍射峰是与原材料基本一致(如图6a所示),MIL-125-NH2、Ag和AgCl的特征峰均被发现. 通过XPS测定所制备样品的化学状态(如图6b所示)可知,在MIL-125-NH2和50MN@AAC中均可观察到Ti 2p峰,而在Ag/AgCl和50MN@AAC中均可观察到Ag 3d峰和Cl 2s和2p峰. 图6c和6d分别显示了50MN@AAC的SEM和TEM 图像,直观地证明了Ag/AgCl已成功沉积在MIL-125-NH2上,形成了MIL-125-NH2@Ag/AgCl复合材料.
图7a表明所有制备的nMN@AAC均具有光催化还原Cr(Ⅵ)的能力,而50MN@AAC在120 min内对初始浓度为5 mg·L−1的Cr(Ⅵ)还原效率达100%,图7b中的光电流图谱从侧面表明了50MN@AAC具备相关潜能. 经过5 次循环后,50MN@AAC的光催化活性几乎保持不变(图7c),表明在反应过程中,催化剂组分和晶体结构稳定. 基于上述的实验结果,作者提出了增强50MN@AAC光催化活性的机理与机制(如图7d所示). 所制备的50MN@AAC中MIL-125-NH2与Ag/AgCl形成的异质结,可有效提高电荷分离效率. 更多来自于MIL-125-NH2导带(LUMO)的e−可以转移到Ag/AgCl上,加速了Cr(Ⅵ)向Cr(Ⅲ)的还原过程. 在没有MIL-125-NH2的情况下,e−和h+容易复合. MIL-125-NH2引入后,Ag/AgCl与MIL-125-NH2之间的协同效应将会增强复合材料的电子利用能力和光催化还原活性.
-
有机染料类污染物会导致环境和健康问题[66 − 67],而研究表明,MIL-125系列复合材料可通过光催化降解过程有效处理含有机染料的有色废水. 例如,MIL-125-NH2分别与Ag3PO4和Ag3VO4复合降解MB[20 − 21];MIL-125分别与Ag、BiVO4等复合降解RhB[22 − 27];MIL-125-NH2分别与BiOBr、CdS、g-C3N4等复合降解RhB[28 − 38];MIL-125-NH2分别与Cu、Ag/AgBr和Ca/TiO2复合降解MO[40 − 42].
(1) MIL-125复合材料光催化降解有机染料
Guo等[22]制备了异质结构Ag/MIL‐125,Ag/MIL‐125异质结在室温条件下可通过光催化实现对罗丹明B(RhB)的高效降解. 如图8a所示,Ag/MIL‐125复合材料的XRD 谱图和Ag标准谱图及MIL‐125拟合谱图吻合较好. 通过XPS测定所制备样品的化学状态(如图8b所示)可知,在MIL-125和Ag/MIL‐125中均可观察到Ti 2p峰. 从SEM(如图8c所示)和TEM(如图8d所示)表征结果可见,Ag/MIL‐125复合材料的微观形貌显示出了圆盘状的形态.
Ag/MIL‐125复合材料在可见光照射下降解有机染料RhB的光催化活性如图9a所示,其中Ag/MIL‐125(3% Ag)复合材料的光催化降解效率最好,在40 min内能将初始浓度为10 mg·L−1的RhB降解92%(同样时间内,无催化剂的空白实验自降解效率极低). 作者以Ag/MIL‐125(3% Ag)为例研究了催化剂的稳定性和可重复使用性(如图9b所示),经过5次循环实验,Ag/MIL‐125(3% Ag)的光催化降解RhB的效率仅降低了7%,表明复合材料Ag/MIL‐125(3% Ag)具有良好的水稳定性和可循环利用性. 为了解Ag/MIL‐125(3% Ag)的光催化机理,作者进行了活性物质捕捉实验(如图9c所示). 添加甲醇(空穴捕捉剂)后,RhB降解速率发生的变化很小,表明h+并不是该体系光催化反应中的关键活性物质. 通过添加电子捕捉剂三乙醇胺(TEOA),确定e−是该光催化反应体系中重要的活性物质. 而添加羟基自由基捕捉剂异丙醇(IPA)和超氧自由基捕捉剂对苯醌(BQ)后,确定·O2−和·OH也是该光催化反应体系中重要的活性物质. 因此,作者提出了Ag/MIL‐125(3% Ag)光催化剂最有可能的光催化机理(图9d).
(2) MIL-125-NH2复合材料光催化降解有机染料
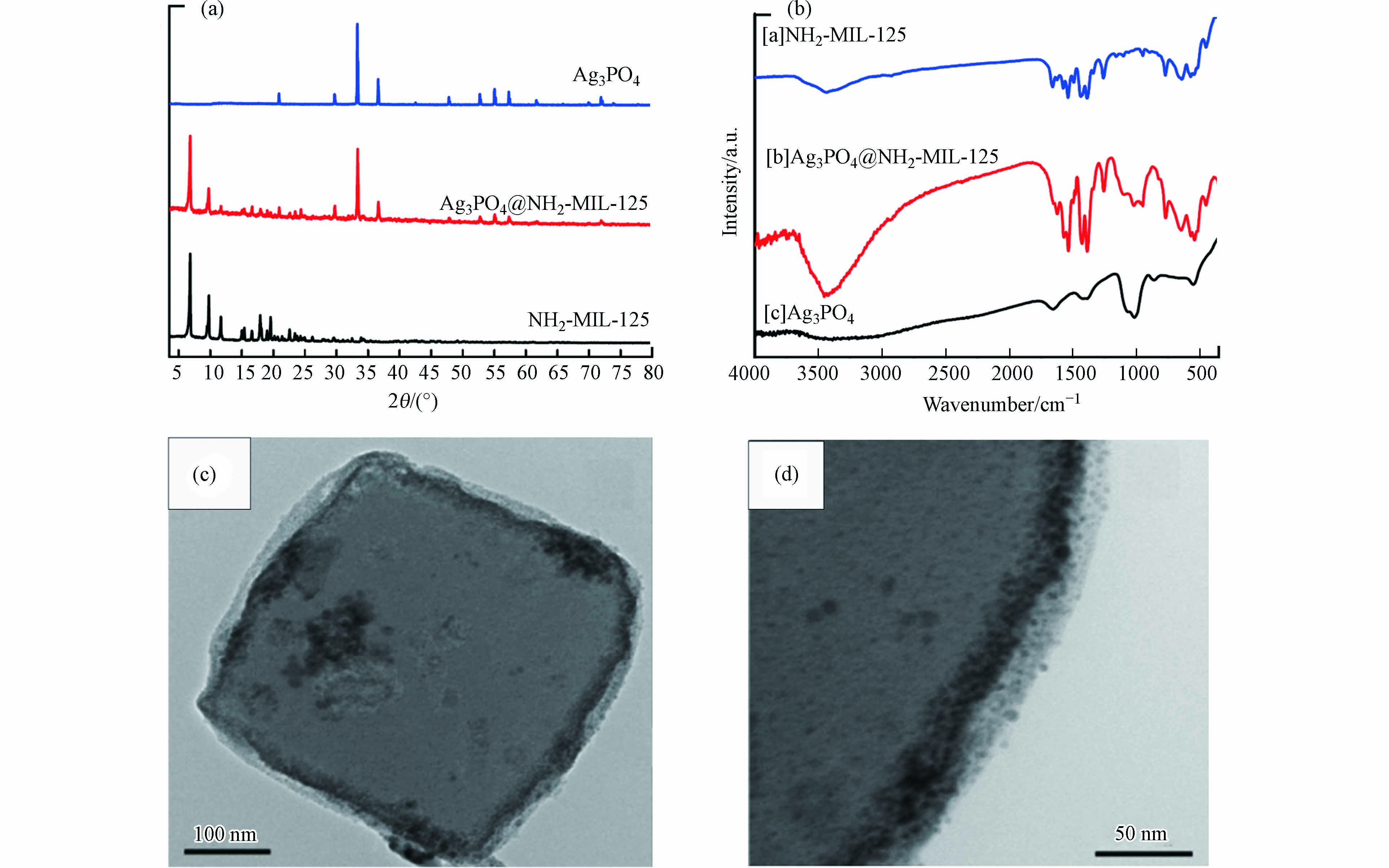
Abdelhameed等[20]成功制备了在室温下可以对亚甲基蓝(MB)进行高效光催化降解的异质结构Ag3PO4@NH2-MIL-125. 如图10a所示,Ag3PO4@NH2-MIL-125复合材料的XRD谱图和Ag3PO4谱图及MIL‐125-NH2谱图吻合较好. 通过FT-IR测定所制备样品的吸收峰(如图10b所示)也能知道,Ag3PO4@NH2-MIL-125复合材料被成功制备. 从TEM(如图10c和10d所示)的表征结果来看,Ag3PO4@NH2-MIL-125复合材料的微观形貌显示出了方块状的形态,而且异质结的界面获得了清晰的显示,也从侧面证明了Ag3PO4@NH2-MIL-125复合材料被成功制备.
Ag3PO4@NH2-MIL-125复合材料在可见光照射下降解有机染料MB的光催化活性如图11a所示,在50 min内能将初始浓度为10 mg·L−1的MB降解100%(同样的时间内,无催化剂的空白实验自降解效率极低),这表明Ag3PO4和MIL‐125-NH2复合具有较大的潜在优势. 经过5次循环实验(如图11b所示),Ag3PO4@NH2-MIL-125的光催化降解MB的效率基本没有明显降低,表明了复合材料Ag3PO4@NH2-MIL-125水稳定性和可循环利用性均十分良好. 为了解Ag3PO4@NH2-MIL-125的光催化机理,作者进行了活性物质捕捉实验(如图11c所示).
添加异丙醇(IPA,羟基自由基捕捉剂)后,MB降解速率常数下降很小,表明·OH并不是该体系光催化反应中的关键活性物质. 而添加超氧自由基捕捉剂对苯醌(BQ)和电子捕捉剂乙二胺四乙酸(EDTA)后,MB降解速率常数下降很大,表明·O2−和e−是该光催化系统当中最主要的活性物质. 因此,作者提出了Ag/MIL‐125(3% Ag)光催化剂最有可能的光催化机理(图11d):光照将Ag3PO4和MIL-125-NH2的价带(VB)中的电子e−激发到导带(CB),使得h+位于Ag3PO4或MIL-125-NH2的VB. 然后,e−快速从MIL-125-NH2的VB向Ag3PO4转移,h+从MIL-125-NH2的VB向Ag3PO4转移,光激发载流子的有效分离得到促进,这就使得Ag3PO4@NH2-MIL-125的光催化活性能够更高. 而Ag3PO4的CB上的e−可与溶解在水中的氧反应生成过氧化氢,而MIL-125-NH2的VB上的h+与羟基反应生成·OH,这就是完全降解MB的强氧化剂.
-
近年来,由于药物和PPCPs的广泛使用而导致其在水生环境中持续存在,已成为影响生态环境和人类健康不容忽视的新污染物. 例如,抗生素在我国长期滥用,被不断排入水体并以残留形式在水中持续存在[68 − 70]. 对于光催化降解新污染物,MIL-125分别与In2S3和Bi2WO6等形成的复合材料[43 − 45],MIL-125-NH2分别与BiOCl和BiOBr等形成的复合材料[46 − 50]被用于降解四环素这一非常典型的新污染物,而MIL-125与BiOBr形成的复合材料用于降解环丙沙星[51],MIL-125与g-C3N4形成的复合材料用于降解头孢克肟[52],等等.
(1) MIL-125复合材料光催化降解新污染物
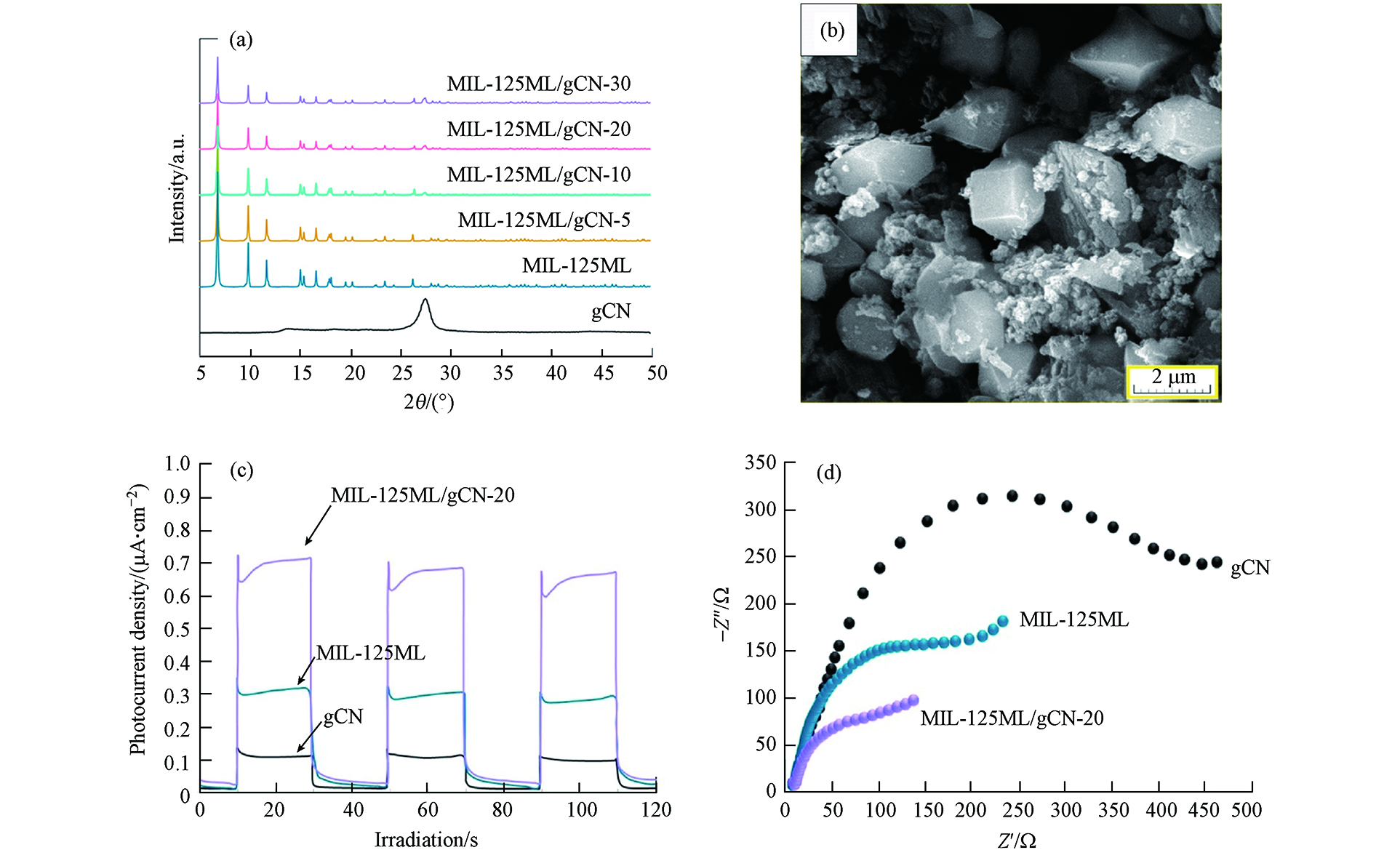
Salimi等[52]通过将g-C3N4与MIL-125复合开发了双功能复合材料(MIL-125ML/gCN),具体应用是在溶液中对头孢克肟进行高效光催化降解. 从样品的XRD图谱可以看出,样品MIL-125ML/gCN呈现出代表g-C3N4和MIL-125成分的峰(如图12a所示). 利用SEM(图12b)对制备样品的显微结构特征进行了分析,观察到典型的片层状形貌,MIL-125为高度结晶的正八面体晶体,尺寸约为1 μm,揭示了在g-C3N4基体中MIL-125晶体的原位形成. 其中,MIL-125晶体被g-C3N4纳米片所包裹. 此外,样品的光催化潜能还通过光电流(图12c)和电化学阻抗谱(EIS)分析(图12d)进行了探究.
纯g-C3N4、MIL-125和各MIL-125ML/gCN复合材料对于头孢克肟的降解效率如图13a所示. 单独的g-C3N4对头孢克肟的降解效率并不高, 在120 min内,仅有40%的头孢克肟被降解,这主要是g-C3N4纳米片较小的比表面积和较高的e−和h+复合率所致. MIL-125对头孢克肟在120 min内降解效率为45%,而复合材料MIL-125ML/gCN-20对头孢克肟在120 min内的降解效率为80%. 这说明, g-C3N4和MIL-125的有效复合,可通过协同作用增强光催化性能. 复合材料MIL-125ML/gCN-20能被循环使用4次,其间,对头孢克肟的光催化降解率仅下降了7%.
这就表明了其水稳定性和可循环利用性均足够良好(图13b). 由图13c可知,30 mg·L−1的催化剂投加量可以获得最佳的降解效果,可以100%降解溶液中的头孢克肟. 再由图13d,添加羟基自由基捕捉剂异丙醇(IPA)后,确定·OH并不是该光催化反应体系中关键的活性物质. 而添加超氧自由基捕捉剂对苯醌(BQ)和电子捕捉剂三乙醇胺(TEOA或TEA)后,表明·O2−和e−是该光催化系统当中最主要的活性物质. 因此,作者提出了MIL-125ML/gCN-20光催化剂最有可能的光催化机理(图13e):复合材料MIL-125ML/gCN-20对头孢克肟具有良好的吸附效果,能将头孢克肟吸附在催化剂的活性位点上. 在可见光照射下,溶液中未被吸附的头孢克肟与吸附在催化剂表面的头孢克肟将进一步被光催化降解. 复合材料MIL-125ML/gCN-20当中稳定的微介孔结构为增强吸附和随后的光催化活性提供了大量的活性位点,从而进一步促进了吸附过程. 此外,MIL-125ML/gCN-20复合材料对可见光吸收能力的增加也提高了其光催化性能和效率. h+、·O2−和·OH在该反应体系中,是主导光催化过程的活性物质. 在可见光照射下,复合材料MIL-125ML/gCN-20表面产生e−和h+,e−由g-C3N4的CB被转移到MIL-125的相对较低的CB,并与O2反应并形成·O2−,这些·O2−和h+使头孢克肟分子降解. 因此,由于这些成分之间的协同作用,致使e−和h+复合机会减少,光催化活性增强.
(2) MIL-125-NH2复合材料光催化降解新污染物
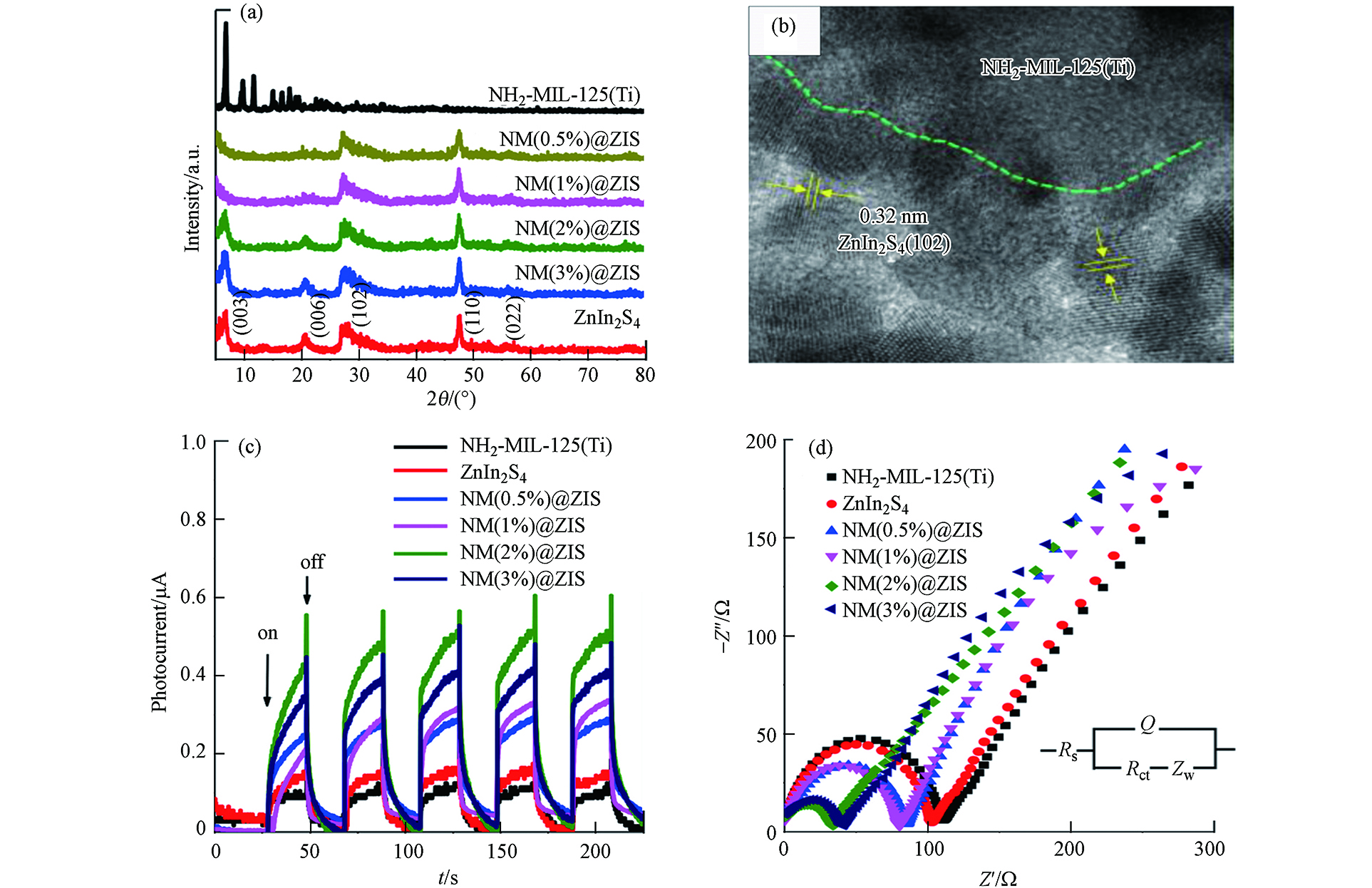
Zhu等[50]通过将ZnIn2S4与MIL-125-NH2复合开发了NH2-MIL-125@ ZnIn2S4复合材料,用于从溶液中高效光催化降解四环素. 从样品的XRD图谱可以看出,NH2-MIL-125@ ZnIn2S4复合材料从NM(0.5%)@ZIS到NM(3%)@ZIS均呈现出代表ZnIn2S4和MIL-125-NH2成分的峰(如图14a所示),高倍透射电子显微镜(HRTEM,图14b)能够观察到异质结的界面. 对样品进行了光电流(图14c)和电化学阻抗谱(EIS)分析(图14d),探究了其光催化的潜能.
纯ZnIn2S4、MIL-125-NH2和从NM(0.5%)@ZIS到NM(3%)@ZIS的复合材料对于四环素的降解效率如图15a所示. 单独的ZnIn2S4对四环素的降解效率并不高, 在80 min内,仅有不到40%的四环素被降解,这主要归因于ZnIn2S4的比表面积较小,并且e−和h+复合率较高. MIL-125-NH2对四环素在80 min内降解效率约为10%,而复合材料NM(2%)@ZIS对四环素在80 min内的降解效率为92.8%. 这说明,ZnIn2S4与MIL-125-NH2的有效复合可实现协同作用而有助于增强光催化性能. 复合材料NM(2%)@ZIS能被循环使用5次,其间,对头孢克肟的光催化降解率仅下降了3%,这就表明其水稳定性和可循环利用性均非常良好(图15b). 再由图15c,添加羟基自由基捕捉剂异丙醇(IPA)后,确定·OH并不是该光催化反应体系中关键的活性物质. 而添加超氧自由基捕捉剂对苯醌(BQ)和空穴捕捉剂草酸铵(AO)后,确定·O2−和h+是该光催化反应体系中最主要的活性物质. 因此,作者提出了NM(2%)@ZIS光催化剂最有可能的光催化机理(图15d).
复合材料NM(2%)@ZIS对四环素具有良好的吸附效果,能将四环素吸附在催化剂的活性位点上. 复合材料NM(2%)@ZIS当中稳定的微介孔结构为增强吸附和随后的光催化活性提供了大量的活性位点,从而进一步促进了吸附过程. 此外,NM(2%)@ZIS对可见光吸收能力的增加,提高了对应的光催化性能,h+、·O2−和·OH是该反应体系主导光催化过程的活性物质. 在可见光照射下,复合材料NM(2%)@ZIS表面产生e−和h+,ZnIn2S4的CB中的e−被转移到MIL-125的相对较低的CB中,并与O2反应后形成·O2−,这些·O2−和h+使四环素分子降解. 因此,由于这些成分之间的协同作用,致使e−和h+复合机会减少,光催化活性增强.
-
MIL-125和MIL-125-NH2本体具有较大的带隙,只能被紫外光激发,电子转移效率偏低. 当前的研究进展表明,将稳定性很好的MIL-125或MIL-125-NH2与半导体类功能材料复合,可以提升MIL-125或是MIL-125-NH2对光的吸收效率以及电子转移效率,这有利于光催化处理水中的污染物. 然而,当前的相关研究还处于实验室阶段,如何将相关催化剂用于实际水处理中实现高效光催化去除水体污染物尚未获得足够关注. 目前的研究做集中在研究相应的机理与机制,后续研究应加强相关光催化水处理装备及工艺. 此外,MIL-125(MIL-125-NH2)及其复合材料在环境中的迁移、转化、对应的环境效应和生物毒性、已使用材料的处置和再利用问题,也将成为今后的相关研究值得关注的问题.
MIL-125系列复合材料光催化去除水体污染物
Photocatalytic removal to water pollutants of MIL-125 series composites
-
摘要: 近年来,金属有机框架中MIL-125、MIL-125-NH2及其复合光催化剂去除水体污染物方面获得了广泛关注. 本综述具体说明了MIL-125、MIL-125-NH2及其复合光催化剂的合成方法,着重探讨了相关材料光催化去除各类水体污染物的研究进展. 通过对相应催化剂研究进展的总结,本文对其在光催化去除水体污染物方面的趋势进行了展望.Abstract: In recent years, it has been widely concerned that the water pollutants were photocatalytically removed by MIL-125, MIL-125-NH2 and their composite photocatalysts. This review described the synthesis methods and the photocatalysis performances of MIL-125, MIL-125-NH2 and their composite photocatalysts in detail, in which the research progress of photocatalytic removal toward various water pollutants was highlighted. Based on the summary of the catalysts research progress, the trend of their photocatalytic removal to water pollutants was prospected.
-
Key words:
- MIL-125 /
- composite material /
- photocatalytic /
- reduction of Cr(Ⅵ) /
- degradation of organic pollutants.
-
金属有机框架(MOF)材料MIL-125,由法国拉瓦锡材料研究所首先合成并报道[1],而后由法国巴黎东部化学与材料研究所首先合成并报道了氨基功能化的MIL-125-NH2[2]. MIL-125和MIL-125-NH2具有比表面积大、孔径尺寸较小及结构具有柔性特点[3 − 4]. 由于MIL-125和MIL-125-NH2独特的结构和性能,使其在气体存储[5]、吸附与分离[6 − 10]、(光)催化[11 − 12]等领域获得广泛应用. 目前,越来越多的研究人员对MIL-125和MIL-125-NH2的光催化性能产生兴趣. MIL-125因具有较宽带隙(Eg = 3.6 eV)而仅能被紫外光激发,而MIL-125-NH2具有较窄带隙(Eg = 2.6 eV)可被可见光激发[13],这也就使得MIL-125-NH2更加受到科研人员的关注. 但是,MIL-125-NH2自身仍然存在一些问题,使得MIL-125-NH2仍需要与一些功能材料复合以进一步增强其光催化性能. 总体来看,相关研究者将MIL-125或MIL-125-NH2与半导体类型的光催化剂进行复合,使其在可见光照射下表现出较为优异的光催化性能.
本文选择了部分典型MIL-125/ MIL-125-NH2复合光催化剂,介绍其制备的方法,并详细地探讨其光催化还原Cr(Ⅵ)和光催化降解染料、药物及个人护理品(PPCPs)的性能与机理,总结出它们的优势与不足.
1. MIL-125系列复合材料在光催化去除水体污染物领域的应用(Application of MIL-125 series composites in the field of photocatalytic removal toward water pollutants)
MIL-125系列复合材料在光催化还原Cr(Ⅵ)、降解有机染料和新污染物等方面取得了一些进展. 本文将结合近年来利用MIL-125系列复合材料光催化去除水体污染物的案例(表1),来详细阐述一些有代表性的研究,并着重论述一些典型的研究工作.
表 1 MIL-125系列复合材料光催化去除水体污染物的典型实例Table 1. Typical examples of photocatalytic removal to water pollutants by MIL-125 series composites金属有机框架 Metal-organic frameworks 复合组分 Composite component 污染物 Pollutants 初始浓度/(mg·L−1) Initial concentration 最优pH Optimal pH 反应时间/min Reaction time 去除率/% Removal efficiency 参考文献 References MIL-125 TiO2 六价铬 5 2 80 100 [14] MIL-125-NH2 Bi2S3 六价铬 10 3 90 100 [15] MIL-125-NH2 Ag/AgCl 六价铬 40 6 120 98.4 [16] MIL-125-NH2 Ag/Ag3PO4 六价铬 10 2 70 100 [17] MIL-125-NH2 PPynt 六价铬 10 2 60 99.02 [18] MIL-125 Cr-III 亚甲基蓝 20 — 120 99 [19] MIL-125-NH2 Ag3PO4 亚甲基蓝 10 — 50 100 [20] MIL-125-NH2 Ag3VO4 亚甲基蓝 5 — 60 96.4 [21] MIL-125 Ag 罗丹明B 10 — 40 95 [22] MIL-125 Ag/rGO 罗丹明B 50 — 50 95.7 [23] MIL-125 BiVO4 罗丹明B 10 — 180 92 [24] MIL-125 MnO2 罗丹明B 10 — 80 100 [25] MIL-125 ZnIn2S4 罗丹明B 15 — 50 97.7 [26] MIL-125 NiS 罗丹明B 15 — 60 98.5 [27] MIL-125-NH2 BiOBr 罗丹明B 20 — 100 96 [28] MIL-125-NH2 PHIK 罗丹明B 100 — 120 94 [29] MIL-125-NH2 CdS 罗丹明B 180 — 120 97 [30] MIL-125-NH2 CQDs 罗丹明B 10 — 120 100 [31] MIL-125-NH2 CoSx 罗丹明B 20 — 45 95.4 [32] MIL-125-NH2 Bi2WO6 罗丹明B 10 — 50 98 [33] MIL-125-NH2 BiOI 罗丹明B 40 — 240 99 [34] MIL-125-NH2 SnS2 罗丹明B 40 — 80 90.5 [35] MIL-125-NH2 CdS/石墨烯 罗丹明B 10 — 30 95 [36] MIL-125-NH2 Fe3+ 罗丹明B 14.4 4 120 82.4 [37] MIL-125-NH2 g-C3N4 罗丹明B 10 — 120 96.4 [38] MIL-125 Zn2GeO4 罗丹明6G 10 — 90 100 [39] MIL-125-NH2 Cu 甲基橙 10 — 90 99 [40] MIL-125-NH2 Ag/AgBr 甲基橙 20 — 120 70 [41] MIL-125-NH2 Ca/TiO2 甲基橙 20 5 120 87.29 [42] MIL-125 In2S3 四环素 46 3.3 60 63.3 [43] MIL-125 CQDs 四环素 20 5 240 90 [44] MIL-125 Bi2WO6 四环素 20 — 80 73 [45] MIL-125-NH2 BiOCl 四环素 20 — 120 78 [46] MIL-125-NH2 Bi2WO6 四环素 20 — 120 77.8 [47] MIL-125-NH2 TPE-2NH2 四环素 30 7 120 90 [48] MIL-125-NH2 BiOBr 四环素 25 7 90 88 [49] MIL-125-NH2 Znln2S4 四环素 20 — 80 92.8 [50] MIL-125 BiOBr 环丙沙星 10 — 80 91 [51] MIL-125 g-C3N4 头孢克肟 20 4 120 98 [52] MIL-125-NH2 ZnO 左氧氟沙星 10 7 240 90 [53] MIL-125-NH2 Pt 对乙酰氨基酚 5 — 180 100 [54] MIL-125-NH2 Ag,CdS 酮洛芬 10 4 180 94.2 [55] MIL-125-NH2 Bi2MoO6 二氯酚 10 — 180 93.28 [56] MIL-125-NH2 g-C3N4 双氯芬酸 10 — 120 100 [57] MIL-125-NH2 ZIF-67 硝基苯酚 10 — 60 100 [58] 注:MIL-125-NH2与NH2-MIL-125两种写法都正确. PHIK是聚庚氨酰亚胺钾的缩写. TPE-2NH2是一种双氨基多苯环有机配体的缩写. CQDs代表碳量子点. PPynt代表聚吡咯碳纳米管. “—”表示原文未提及. Note: Both MIL-125-NH2 and NH2-MIL-125 are correct. PHIK stands for potassium poly (heptazine imide). TPE-2NH2 is short for an organic ligand with two aminos and some phenyl rings. CQDs stands for carbon quantum dots. PPynt stands for polypyrrole carbon nanotubes. "—" indicates that it is not mentioned in the original article. 2. 合成方法与典型研究工作(Synthesis methods and typical researches)
2.1 MIL-125和MIL-125-NH2的合成方法
MIL-125和MIL-125-NH2的合成方法以溶剂热法[3,14]为主,溶剂一般选用甲醇或DMF(N,N-二甲基甲酰胺),反应物为含有Ti(Ⅳ)的盐类和对苯二甲酸(H2BDC)或氨基-对苯二甲酸(NH2-H2BDC). 反应温度一般为150 ℃,反应时间一般为24 h. MIL-125样品一般呈白色粉末状晶体,而MIL-125-NH2样品一般呈亮黄色粉末状晶体.
2.2 MIL-125和MIL-125-NH2典型复合材料的合成方法
MIL-125系列复合材料的合成一般是采用(光)沉积法、原位法或两步法,其中原位法更常用.
2.2.1 MIL-125复合材料的合成方法
Yuan等[23]用光沉积法将MIL-125与Ag纳米粒子以及还原型氧化石墨烯(rGO)复合制备了Ag/rGO/MIL-125复合材料,制备过程如图1所示. 先通过超声处理60 min,得到GO(15.5 mg)和无水乙醇(200 mL)的均匀分散溶液. 然后,在前述的混合物中加入0.5 g粉末状MIL-125,再超声处理15 min. 再通过用力搅拌,将AgNO3(24.3 mg)分散到前述的溶液中. 之后在黑暗条件下,向悬浮液中注入氮气30 min,并将悬浮液在紫外光(紫外光源功率密度为100 mW cm−2)下照射2 h. 最后,取用无水乙醇共计3次过滤洗涤悬浮液中的物质,在60°C下将所得物质通过真空干燥运行12 h.
2.2.2 MIL-125-NH2复合材料的合成方法
Wang等[30]通过原位法合成S@T-n复合材料(根据CdS纳米棒的质量占比,n=6、10和20)的过程如图2所示. 通常情况下,将预先合成的CdS纳米棒(0.15 mmol)连续搅拌以分散到甲醇(2.0 mL)中,然后加入含有NH2-H2BDC(0.56 g,3.1 mmol)的DMF(8.0 mL)溶液. 搅拌1 h后,在超声条件下将iBuOTi(0.6 mL,2.0 mmol)缓慢注入上述溶液中,生成前驱体溶液. 而后,将前驱体溶液转移到内衬钢高压釜(23 mL)中,在烘箱中以150 °C对其加热24 h. 随后,高压釜冷却至室温,离心分离其中黄色产物,再用DMF和甲醇(10 mL,5次)洗涤,最后在80 ℃下干燥.
Xu等[59]通过两步法合成NH2-MIL-125-P@TiO2复合材料的过程,如图3所示. 将合成的NH2-MIL-125放入150 ℃的真空干燥器中处理12 h以去除孔隙中的有机溶剂. 称取400 mg NH2-MIL-125,在40 mL甲醇中分散30 min. 将钛酸四丁酯以400 μL缓慢加入上述溶液中,室温搅拌1 h,然后快速加热至85 ℃,回流12 h,使钛酸四丁酯完全水解. 将回流装置转化为蒸馏装置,将上述溶液蒸制成膏体,蒸发甲醇. 浆料中加入0.1 mol·L−1磷酸溶液1 mL,磁力搅拌6 h,80 ℃烘箱加热至干燥,100 ℃洗涤干燥12 h,合成磷酸化NH2-MIL-125.
2.3 MIL-125和MIL-125-NH2典型复合材料光催化去除水体污染物
2.3.1 光催化还原Cr(Ⅵ)
铬是水体中一种十分常见污染物,水体中少量存在的铬就足以威胁生命健康. 使用光催化剂就能高效率且低成本地实现对Cr(Ⅵ)的去除,还可以不产生任何有害物质[60 − 65]. 对于这方面应用,因为MIL-125-NH2本体的带隙较窄,一般有关MIL-125-NH2复合材料[15 − 18]的报道较多,但MIL-125复合材料中,也有性能优异的[14].
(1) MIL-125复合材料光催化还原Cr(Ⅵ)
Li等[14]制备了万寿菊状TiO2@MIL-125(MT-n, n= 1, 2, 3, 6)核-壳型复合光催化剂,并将其用于还原Cr(Ⅵ). 复合材料MT-n的X射线衍射(XRD)图谱中的衍射峰与原材料基本一致(图4a),类似的情况也体现在傅立叶变换红外吸收光谱(FT-IR)图谱中(如图4b所示). 所制备样品的化学状态通过X-射线光电子能谱(XPS)测定之后(如图4c所示)可知,在MIL-125和MT-2中均可观察到Ti 2p峰,这表明TiO2@MIL-125核-壳复合材料的成功制备. MT-2的扫描电子显微镜(SEM)和透射电子显微镜(TEM)图像分别如图4d、4e和4f所示,直观地证明了TiO2已成功沉积在MIL-125上,形成了TiO2@MIL-125的核-壳型复合材料.
紫外-可见漫反射光谱(图5a)表明所制备MT-n样品的光吸收特性随硫代乙酰胺(TAA)处理时间的逐渐延长,表明引入TAA可将MIL-125的吸光能力提高,这也有望提高MIL-125的光催化活性. 图5b表明所有制备的MT-n均具有光催化还原Cr(Ⅵ)的能力,而MT-2在60 min内对初始浓度为 5 mg·L−1的Cr(Ⅵ)还原效率达100%. 5 次循环实验表明,MT-2的光催化活性在反应过程中几乎保持不变(图5c),仍具有稳定的催化剂组分和晶体结构. 基于上述实验结果,作者提出了增强MT-2光催化活性的机理与机制(如图5d所示). 所制备的MT-2中MIL-125与TiO2形成的异质结,可有效提高电荷分离效率. 更多的来自于MIL-125导带(LUMO)的光生电子可以转移到TiO2上,加速了Cr(Ⅵ)向Cr(Ⅲ)的还原过程. 在没有MIL-125的情况下,e−和h+容易复合. MIL-125引入后,TiO2与MIL-125之间的协同效应将增强复合材料的电子利用能力和光催化还原活性.
 图 5 (a)TiO2@MIL-125复合材料样品的紫外-可见漫反射谱图,通过嵌入图可得到样品的Eg值;(b)TiO2@MIL-125复合材料样品在可见光照射下的光催化性能;(c)MT-2(15 mg催化剂,50 mL Cr(Ⅵ),5 mg·L−1)的循环实验;(d)Cr(Ⅵ)的还原机理图[14]Figure 5. (a) Ultraviolet-visible diffuse reflection spectra of TiO2@MIL-125 composite samples, and the Eg value of the sample can be obtained by the embedded diagram; (b) The photocatalytic properties of TiO2@MIL-125 composite samples under visible light irradiation; (c) Cycle experiment of MT-2 (15 mg catalyst, 50 mL Cr (Ⅵ), 5 mg·L−1); (d) Reduction mechanism diagram of Cr (Ⅵ) [14]
图 5 (a)TiO2@MIL-125复合材料样品的紫外-可见漫反射谱图,通过嵌入图可得到样品的Eg值;(b)TiO2@MIL-125复合材料样品在可见光照射下的光催化性能;(c)MT-2(15 mg催化剂,50 mL Cr(Ⅵ),5 mg·L−1)的循环实验;(d)Cr(Ⅵ)的还原机理图[14]Figure 5. (a) Ultraviolet-visible diffuse reflection spectra of TiO2@MIL-125 composite samples, and the Eg value of the sample can be obtained by the embedded diagram; (b) The photocatalytic properties of TiO2@MIL-125 composite samples under visible light irradiation; (c) Cycle experiment of MT-2 (15 mg catalyst, 50 mL Cr (Ⅵ), 5 mg·L−1); (d) Reduction mechanism diagram of Cr (Ⅵ) [14](2)MIL-125-NH2复合材料光催化还原Cr(Ⅵ)
Qiu等[16]制备了MIL-125-NH2@Ag/AgCl(nMN@AAC, n= 30, 40, 50, 60)复合催化剂,并将其应用于光催化还原Cr(Ⅵ). 复合材料nMN@AAC的XRD图谱中的衍射峰是与原材料基本一致(如图6a所示),MIL-125-NH2、Ag和AgCl的特征峰均被发现. 通过XPS测定所制备样品的化学状态(如图6b所示)可知,在MIL-125-NH2和50MN@AAC中均可观察到Ti 2p峰,而在Ag/AgCl和50MN@AAC中均可观察到Ag 3d峰和Cl 2s和2p峰. 图6c和6d分别显示了50MN@AAC的SEM和TEM 图像,直观地证明了Ag/AgCl已成功沉积在MIL-125-NH2上,形成了MIL-125-NH2@Ag/AgCl复合材料.
图7a表明所有制备的nMN@AAC均具有光催化还原Cr(Ⅵ)的能力,而50MN@AAC在120 min内对初始浓度为5 mg·L−1的Cr(Ⅵ)还原效率达100%,图7b中的光电流图谱从侧面表明了50MN@AAC具备相关潜能. 经过5 次循环后,50MN@AAC的光催化活性几乎保持不变(图7c),表明在反应过程中,催化剂组分和晶体结构稳定. 基于上述的实验结果,作者提出了增强50MN@AAC光催化活性的机理与机制(如图7d所示). 所制备的50MN@AAC中MIL-125-NH2与Ag/AgCl形成的异质结,可有效提高电荷分离效率. 更多来自于MIL-125-NH2导带(LUMO)的e−可以转移到Ag/AgCl上,加速了Cr(Ⅵ)向Cr(Ⅲ)的还原过程. 在没有MIL-125-NH2的情况下,e−和h+容易复合. MIL-125-NH2引入后,Ag/AgCl与MIL-125-NH2之间的协同效应将会增强复合材料的电子利用能力和光催化还原活性.
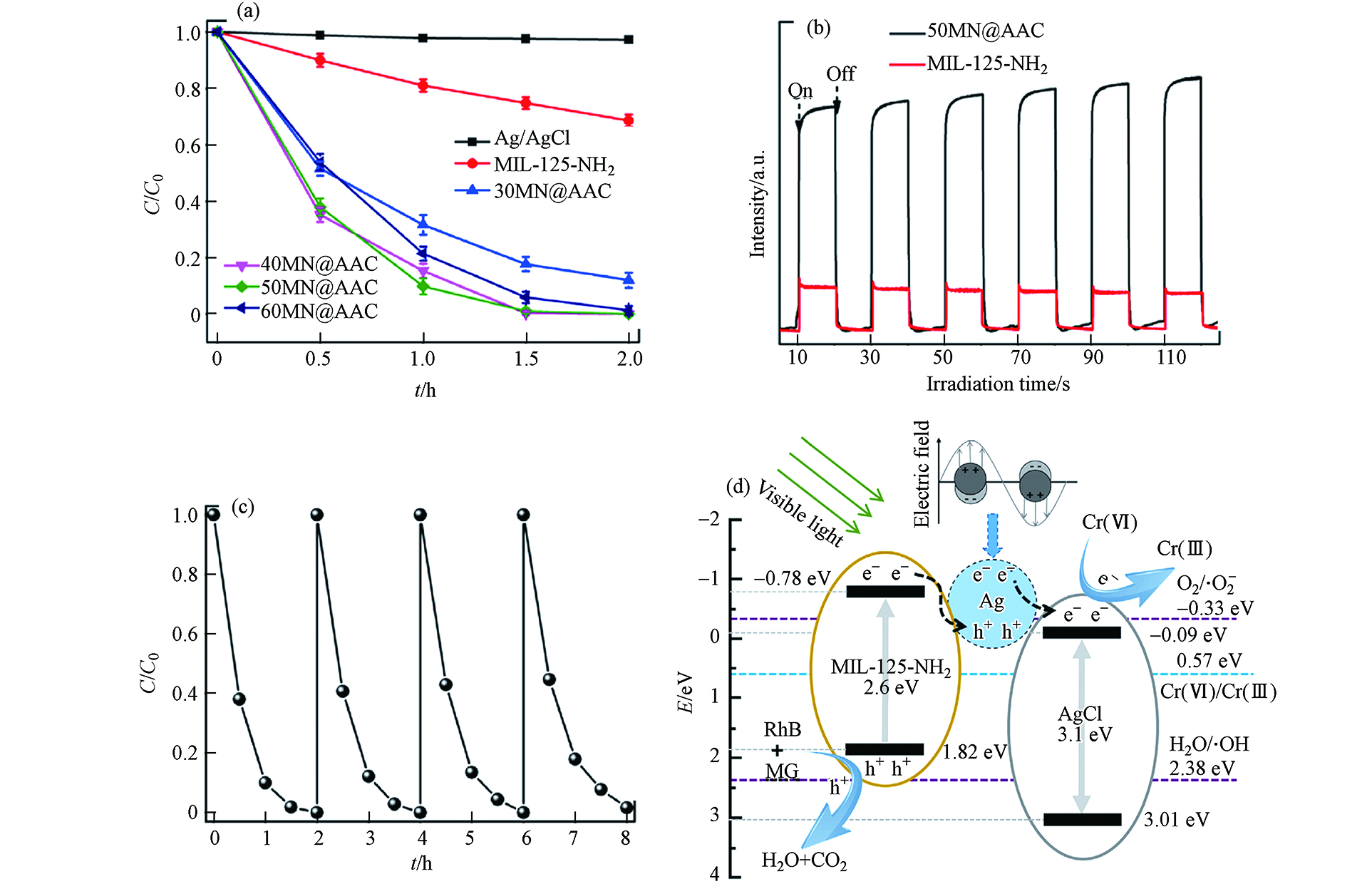 图 7 (a)MIL-125-NH2@Ag/AgCl复合材料样品在可见光照射下的光催化性能;(b)50MN@AAC复合材料样品的光电流谱图;(c)50MN@AAC(40 mg催化剂,40 mL Cr(Ⅵ),10 mg·L−1)的循环实验;(d)Cr(Ⅵ)的还原机理图[16]Figure 7. (a) Photocatalytic performance of MIL-125-NH2@Ag/AgCl composite samples under visible light irradiation;(b) Photocurrent spectra of 50MN@AAC composite samples;(c) Cycle experiment of 50MN@AAC (40 mg catalyst, 40 mL Cr (Ⅵ)),(d) Reduction mechanism diagram of Cr (Ⅵ)[16]
图 7 (a)MIL-125-NH2@Ag/AgCl复合材料样品在可见光照射下的光催化性能;(b)50MN@AAC复合材料样品的光电流谱图;(c)50MN@AAC(40 mg催化剂,40 mL Cr(Ⅵ),10 mg·L−1)的循环实验;(d)Cr(Ⅵ)的还原机理图[16]Figure 7. (a) Photocatalytic performance of MIL-125-NH2@Ag/AgCl composite samples under visible light irradiation;(b) Photocurrent spectra of 50MN@AAC composite samples;(c) Cycle experiment of 50MN@AAC (40 mg catalyst, 40 mL Cr (Ⅵ)),(d) Reduction mechanism diagram of Cr (Ⅵ)[16]2.3.2 光催化降解有机染料
有机染料类污染物会导致环境和健康问题[66 − 67],而研究表明,MIL-125系列复合材料可通过光催化降解过程有效处理含有机染料的有色废水. 例如,MIL-125-NH2分别与Ag3PO4和Ag3VO4复合降解MB[20 − 21];MIL-125分别与Ag、BiVO4等复合降解RhB[22 − 27];MIL-125-NH2分别与BiOBr、CdS、g-C3N4等复合降解RhB[28 − 38];MIL-125-NH2分别与Cu、Ag/AgBr和Ca/TiO2复合降解MO[40 − 42].
(1) MIL-125复合材料光催化降解有机染料
Guo等[22]制备了异质结构Ag/MIL‐125,Ag/MIL‐125异质结在室温条件下可通过光催化实现对罗丹明B(RhB)的高效降解. 如图8a所示,Ag/MIL‐125复合材料的XRD 谱图和Ag标准谱图及MIL‐125拟合谱图吻合较好. 通过XPS测定所制备样品的化学状态(如图8b所示)可知,在MIL-125和Ag/MIL‐125中均可观察到Ti 2p峰. 从SEM(如图8c所示)和TEM(如图8d所示)表征结果可见,Ag/MIL‐125复合材料的微观形貌显示出了圆盘状的形态.
Ag/MIL‐125复合材料在可见光照射下降解有机染料RhB的光催化活性如图9a所示,其中Ag/MIL‐125(3% Ag)复合材料的光催化降解效率最好,在40 min内能将初始浓度为10 mg·L−1的RhB降解92%(同样时间内,无催化剂的空白实验自降解效率极低). 作者以Ag/MIL‐125(3% Ag)为例研究了催化剂的稳定性和可重复使用性(如图9b所示),经过5次循环实验,Ag/MIL‐125(3% Ag)的光催化降解RhB的效率仅降低了7%,表明复合材料Ag/MIL‐125(3% Ag)具有良好的水稳定性和可循环利用性. 为了解Ag/MIL‐125(3% Ag)的光催化机理,作者进行了活性物质捕捉实验(如图9c所示). 添加甲醇(空穴捕捉剂)后,RhB降解速率发生的变化很小,表明h+并不是该体系光催化反应中的关键活性物质. 通过添加电子捕捉剂三乙醇胺(TEOA),确定e−是该光催化反应体系中重要的活性物质. 而添加羟基自由基捕捉剂异丙醇(IPA)和超氧自由基捕捉剂对苯醌(BQ)后,确定·O2−和·OH也是该光催化反应体系中重要的活性物质. 因此,作者提出了Ag/MIL‐125(3% Ag)光催化剂最有可能的光催化机理(图9d).
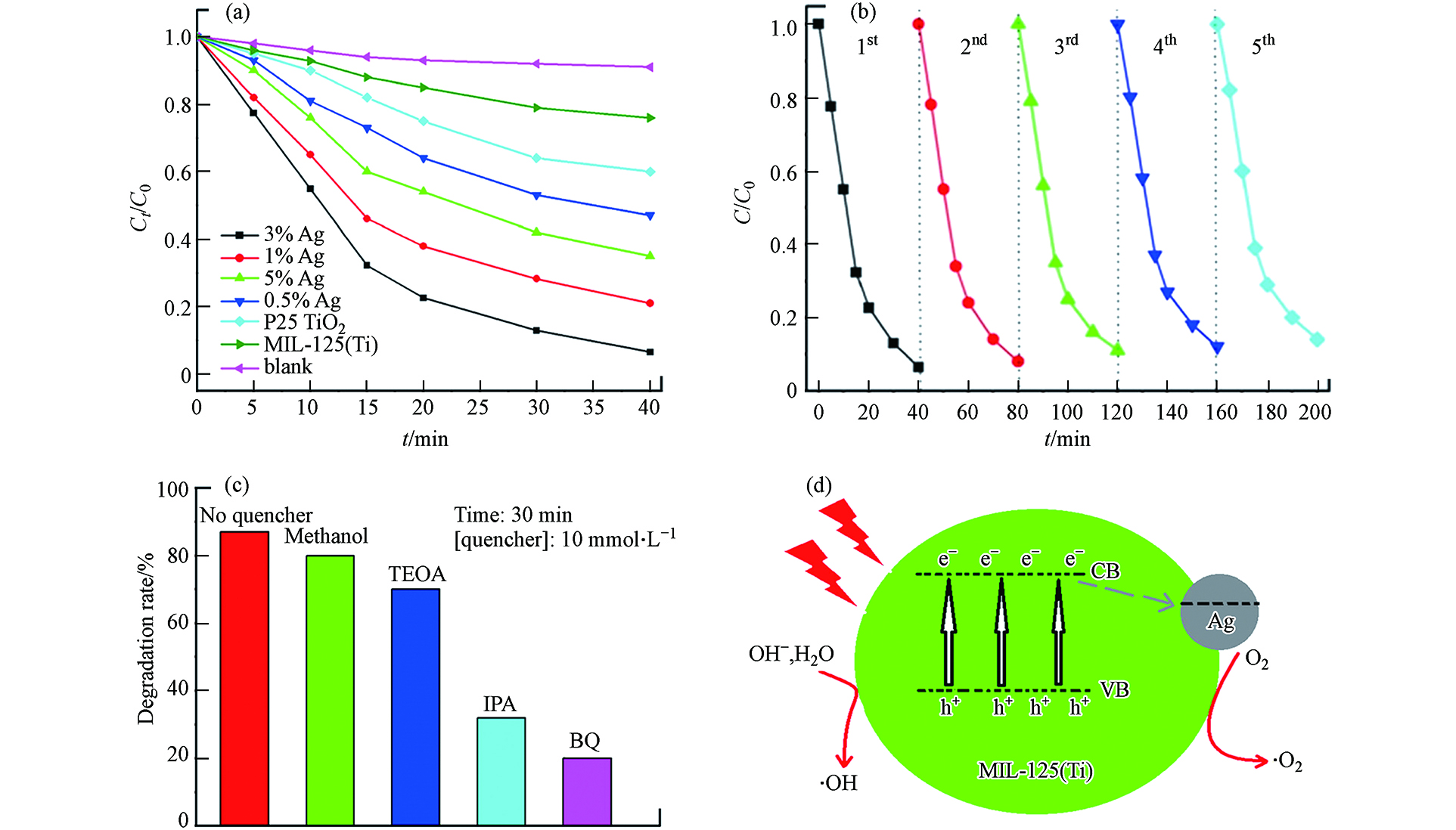 图 9 (a)Ag/MIL‐125(3% Ag)复合材料样品在可见光照射下的光催化性能;(b)Ag/MIL‐125(3% Ag)复合材料样品(20 mg催化剂,20 mL RhB,10 mg·L−1)的循环实验;(c)Ag/MIL‐125(3% Ag)复合材料样品光催化降解RhB的活性物质捕捉实验对RhB降解比率的影响;(d)Ag/MIL‐125(3% Ag)催化降解RhB的机理图[22]Figure 9. (a) Photocatalytic properties of Ag/MIL‐125 (3% Ag) composites under visible light irradiation;(b) Cycle experiment of Ag/MIL‐125 (3% Ag) composite sample (20 mg catalyst, 20 mL RhB, 10 mg·L−1); (c) The effect of the degradation ratio of RhB by capture experiments for active substance on the photocatalytic degradation of RhB by Ag/MIL‐125 (3% Ag) composite samples; (d) Catalytic degradation mechanism of RhB by Ag/MIL‐125 (3% Ag) composites[22]
图 9 (a)Ag/MIL‐125(3% Ag)复合材料样品在可见光照射下的光催化性能;(b)Ag/MIL‐125(3% Ag)复合材料样品(20 mg催化剂,20 mL RhB,10 mg·L−1)的循环实验;(c)Ag/MIL‐125(3% Ag)复合材料样品光催化降解RhB的活性物质捕捉实验对RhB降解比率的影响;(d)Ag/MIL‐125(3% Ag)催化降解RhB的机理图[22]Figure 9. (a) Photocatalytic properties of Ag/MIL‐125 (3% Ag) composites under visible light irradiation;(b) Cycle experiment of Ag/MIL‐125 (3% Ag) composite sample (20 mg catalyst, 20 mL RhB, 10 mg·L−1); (c) The effect of the degradation ratio of RhB by capture experiments for active substance on the photocatalytic degradation of RhB by Ag/MIL‐125 (3% Ag) composite samples; (d) Catalytic degradation mechanism of RhB by Ag/MIL‐125 (3% Ag) composites[22](2) MIL-125-NH2复合材料光催化降解有机染料
Abdelhameed等[20]成功制备了在室温下可以对亚甲基蓝(MB)进行高效光催化降解的异质结构Ag3PO4@NH2-MIL-125. 如图10a所示,Ag3PO4@NH2-MIL-125复合材料的XRD谱图和Ag3PO4谱图及MIL‐125-NH2谱图吻合较好. 通过FT-IR测定所制备样品的吸收峰(如图10b所示)也能知道,Ag3PO4@NH2-MIL-125复合材料被成功制备. 从TEM(如图10c和10d所示)的表征结果来看,Ag3PO4@NH2-MIL-125复合材料的微观形貌显示出了方块状的形态,而且异质结的界面获得了清晰的显示,也从侧面证明了Ag3PO4@NH2-MIL-125复合材料被成功制备.
Ag3PO4@NH2-MIL-125复合材料在可见光照射下降解有机染料MB的光催化活性如图11a所示,在50 min内能将初始浓度为10 mg·L−1的MB降解100%(同样的时间内,无催化剂的空白实验自降解效率极低),这表明Ag3PO4和MIL‐125-NH2复合具有较大的潜在优势. 经过5次循环实验(如图11b所示),Ag3PO4@NH2-MIL-125的光催化降解MB的效率基本没有明显降低,表明了复合材料Ag3PO4@NH2-MIL-125水稳定性和可循环利用性均十分良好. 为了解Ag3PO4@NH2-MIL-125的光催化机理,作者进行了活性物质捕捉实验(如图11c所示).
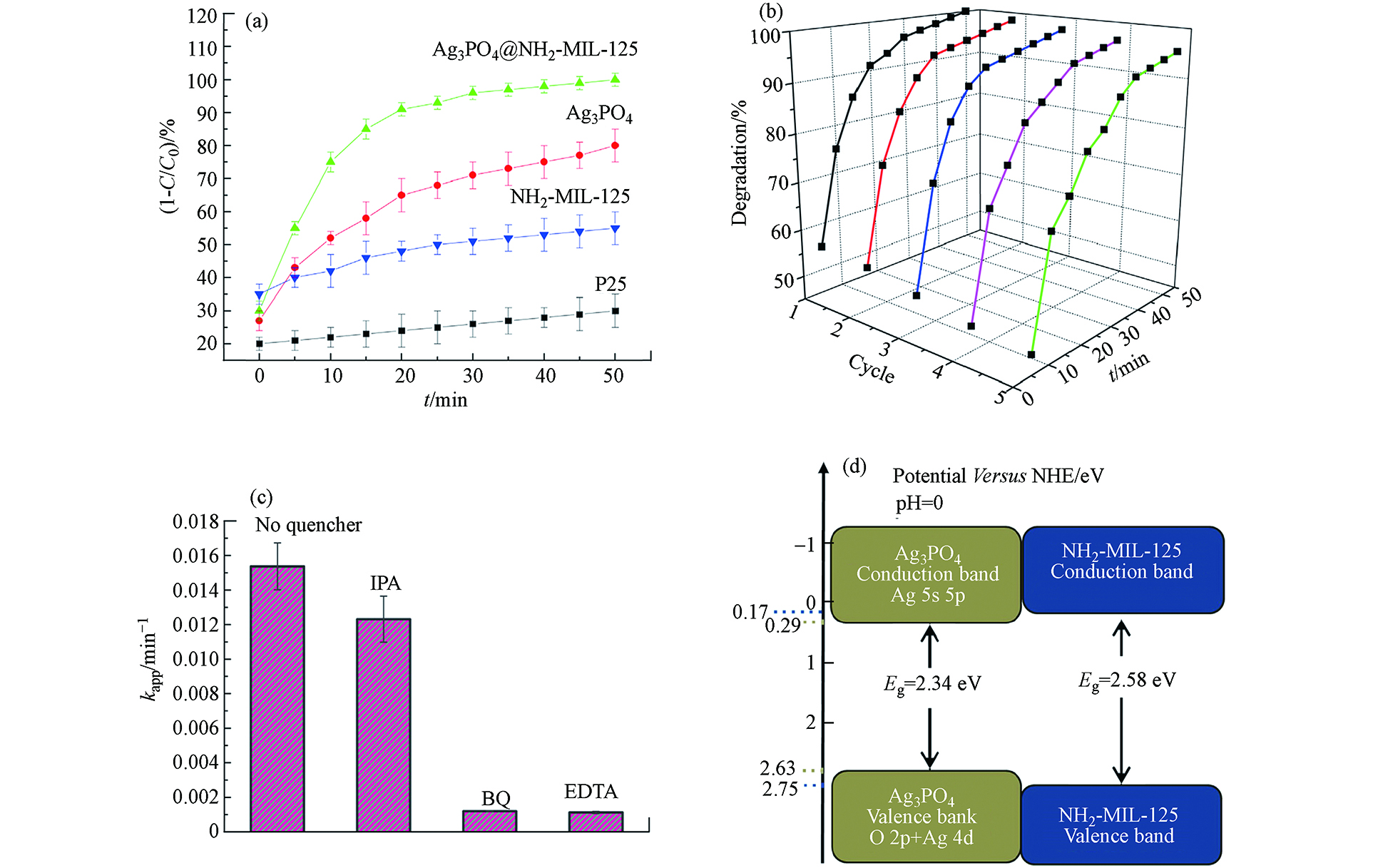 图 11 (a)Ag3PO4@NH2-MIL-125复合材料样品在可见光照射下的光催化性能;(b)Ag3PO4@ NH2-MIL-125(50 mg催化剂,100 mL MB,10 mg·L−1)的循环实验;(c)Ag3PO4@NH2-MIL-125复合材料样品光催化降解MB的活性物质捕捉实验对MB降解速率常数的影响;(d)pH = 0时Ag3PO4和NH2-MIL-125相对于标准氢电极(NHE)的能带结构[20]Figure 11. (a) The photocatalytic properties of Ag3PO4@NH2-MIL-125 composite samples under visible light irradiation; (b) Cyclic experiment of Ag3PO4@NH2-MIL-125 (50 mg catalyst, 100 mL MB, 10 mg·L−1); (c) The effect of capture experiments for active substance on the degradation rate constant of MB for the photocatalytic degradation of MB by Ag3PO4@NH2-MIL-125 composite samples; (d) Band structure of Ag3PO4 and NH2-MIL-125 related to standard hydrogen electrode (NHE) at pH = 0[20]
图 11 (a)Ag3PO4@NH2-MIL-125复合材料样品在可见光照射下的光催化性能;(b)Ag3PO4@ NH2-MIL-125(50 mg催化剂,100 mL MB,10 mg·L−1)的循环实验;(c)Ag3PO4@NH2-MIL-125复合材料样品光催化降解MB的活性物质捕捉实验对MB降解速率常数的影响;(d)pH = 0时Ag3PO4和NH2-MIL-125相对于标准氢电极(NHE)的能带结构[20]Figure 11. (a) The photocatalytic properties of Ag3PO4@NH2-MIL-125 composite samples under visible light irradiation; (b) Cyclic experiment of Ag3PO4@NH2-MIL-125 (50 mg catalyst, 100 mL MB, 10 mg·L−1); (c) The effect of capture experiments for active substance on the degradation rate constant of MB for the photocatalytic degradation of MB by Ag3PO4@NH2-MIL-125 composite samples; (d) Band structure of Ag3PO4 and NH2-MIL-125 related to standard hydrogen electrode (NHE) at pH = 0[20]添加异丙醇(IPA,羟基自由基捕捉剂)后,MB降解速率常数下降很小,表明·OH并不是该体系光催化反应中的关键活性物质. 而添加超氧自由基捕捉剂对苯醌(BQ)和电子捕捉剂乙二胺四乙酸(EDTA)后,MB降解速率常数下降很大,表明·O2−和e−是该光催化系统当中最主要的活性物质. 因此,作者提出了Ag/MIL‐125(3% Ag)光催化剂最有可能的光催化机理(图11d):光照将Ag3PO4和MIL-125-NH2的价带(VB)中的电子e−激发到导带(CB),使得h+位于Ag3PO4或MIL-125-NH2的VB. 然后,e−快速从MIL-125-NH2的VB向Ag3PO4转移,h+从MIL-125-NH2的VB向Ag3PO4转移,光激发载流子的有效分离得到促进,这就使得Ag3PO4@NH2-MIL-125的光催化活性能够更高. 而Ag3PO4的CB上的e−可与溶解在水中的氧反应生成过氧化氢,而MIL-125-NH2的VB上的h+与羟基反应生成·OH,这就是完全降解MB的强氧化剂.
2.3.3 光催化降解新污染物
近年来,由于药物和PPCPs的广泛使用而导致其在水生环境中持续存在,已成为影响生态环境和人类健康不容忽视的新污染物. 例如,抗生素在我国长期滥用,被不断排入水体并以残留形式在水中持续存在[68 − 70]. 对于光催化降解新污染物,MIL-125分别与In2S3和Bi2WO6等形成的复合材料[43 − 45],MIL-125-NH2分别与BiOCl和BiOBr等形成的复合材料[46 − 50]被用于降解四环素这一非常典型的新污染物,而MIL-125与BiOBr形成的复合材料用于降解环丙沙星[51],MIL-125与g-C3N4形成的复合材料用于降解头孢克肟[52],等等.
(1) MIL-125复合材料光催化降解新污染物
Salimi等[52]通过将g-C3N4与MIL-125复合开发了双功能复合材料(MIL-125ML/gCN),具体应用是在溶液中对头孢克肟进行高效光催化降解. 从样品的XRD图谱可以看出,样品MIL-125ML/gCN呈现出代表g-C3N4和MIL-125成分的峰(如图12a所示). 利用SEM(图12b)对制备样品的显微结构特征进行了分析,观察到典型的片层状形貌,MIL-125为高度结晶的正八面体晶体,尺寸约为1 μm,揭示了在g-C3N4基体中MIL-125晶体的原位形成. 其中,MIL-125晶体被g-C3N4纳米片所包裹. 此外,样品的光催化潜能还通过光电流(图12c)和电化学阻抗谱(EIS)分析(图12d)进行了探究.
纯g-C3N4、MIL-125和各MIL-125ML/gCN复合材料对于头孢克肟的降解效率如图13a所示. 单独的g-C3N4对头孢克肟的降解效率并不高, 在120 min内,仅有40%的头孢克肟被降解,这主要是g-C3N4纳米片较小的比表面积和较高的e−和h+复合率所致. MIL-125对头孢克肟在120 min内降解效率为45%,而复合材料MIL-125ML/gCN-20对头孢克肟在120 min内的降解效率为80%. 这说明, g-C3N4和MIL-125的有效复合,可通过协同作用增强光催化性能. 复合材料MIL-125ML/gCN-20能被循环使用4次,其间,对头孢克肟的光催化降解率仅下降了7%.
 图 13 (a)MIL-125ML/gCN复合材料样品在可见光照射下的光催化性能;(b)MIL-125ML/gCN-20(30 mg催化剂,100 mL 头孢克肟,20 mg·L−1)的循环实验;(c)MIL-125ML/gCN-20的投加量对其在可见光照射下的光催化性能的影响;(d)活性物质捕捉实验对MIL-125ML/gCN-20光催化降解头孢克肟的的影响;(e)MIL-125ML/gCN-20复合材料光催化降解头孢克肟的机理[52]Figure 13. (a) Photocatalytic performance of MIL-125ML/gCN composites under visible light irradiation; (b) Cycle experiment of MIL-125ML/gCN-20 (30 mg catalyst, 100 mL cefixime, 20 mg·L−1); (c) The effect of dosage of MIL-125ML/gCN-20 on its photocatalytic performance under visible light irradiation; (d) The effect of capture experiment for active substance on the photocatalytic degradation of cefixime by MIL-125ML/gCN-20; (e) The photocatalytic degradation mechanism of cefixime by MIL-125ML/gCN-20 composite[52]
图 13 (a)MIL-125ML/gCN复合材料样品在可见光照射下的光催化性能;(b)MIL-125ML/gCN-20(30 mg催化剂,100 mL 头孢克肟,20 mg·L−1)的循环实验;(c)MIL-125ML/gCN-20的投加量对其在可见光照射下的光催化性能的影响;(d)活性物质捕捉实验对MIL-125ML/gCN-20光催化降解头孢克肟的的影响;(e)MIL-125ML/gCN-20复合材料光催化降解头孢克肟的机理[52]Figure 13. (a) Photocatalytic performance of MIL-125ML/gCN composites under visible light irradiation; (b) Cycle experiment of MIL-125ML/gCN-20 (30 mg catalyst, 100 mL cefixime, 20 mg·L−1); (c) The effect of dosage of MIL-125ML/gCN-20 on its photocatalytic performance under visible light irradiation; (d) The effect of capture experiment for active substance on the photocatalytic degradation of cefixime by MIL-125ML/gCN-20; (e) The photocatalytic degradation mechanism of cefixime by MIL-125ML/gCN-20 composite[52]这就表明了其水稳定性和可循环利用性均足够良好(图13b). 由图13c可知,30 mg·L−1的催化剂投加量可以获得最佳的降解效果,可以100%降解溶液中的头孢克肟. 再由图13d,添加羟基自由基捕捉剂异丙醇(IPA)后,确定·OH并不是该光催化反应体系中关键的活性物质. 而添加超氧自由基捕捉剂对苯醌(BQ)和电子捕捉剂三乙醇胺(TEOA或TEA)后,表明·O2−和e−是该光催化系统当中最主要的活性物质. 因此,作者提出了MIL-125ML/gCN-20光催化剂最有可能的光催化机理(图13e):复合材料MIL-125ML/gCN-20对头孢克肟具有良好的吸附效果,能将头孢克肟吸附在催化剂的活性位点上. 在可见光照射下,溶液中未被吸附的头孢克肟与吸附在催化剂表面的头孢克肟将进一步被光催化降解. 复合材料MIL-125ML/gCN-20当中稳定的微介孔结构为增强吸附和随后的光催化活性提供了大量的活性位点,从而进一步促进了吸附过程. 此外,MIL-125ML/gCN-20复合材料对可见光吸收能力的增加也提高了其光催化性能和效率. h+、·O2−和·OH在该反应体系中,是主导光催化过程的活性物质. 在可见光照射下,复合材料MIL-125ML/gCN-20表面产生e−和h+,e−由g-C3N4的CB被转移到MIL-125的相对较低的CB,并与O2反应并形成·O2−,这些·O2−和h+使头孢克肟分子降解. 因此,由于这些成分之间的协同作用,致使e−和h+复合机会减少,光催化活性增强.
(2) MIL-125-NH2复合材料光催化降解新污染物
Zhu等[50]通过将ZnIn2S4与MIL-125-NH2复合开发了NH2-MIL-125@ ZnIn2S4复合材料,用于从溶液中高效光催化降解四环素. 从样品的XRD图谱可以看出,NH2-MIL-125@ ZnIn2S4复合材料从NM(0.5%)@ZIS到NM(3%)@ZIS均呈现出代表ZnIn2S4和MIL-125-NH2成分的峰(如图14a所示),高倍透射电子显微镜(HRTEM,图14b)能够观察到异质结的界面. 对样品进行了光电流(图14c)和电化学阻抗谱(EIS)分析(图14d),探究了其光催化的潜能.
纯ZnIn2S4、MIL-125-NH2和从NM(0.5%)@ZIS到NM(3%)@ZIS的复合材料对于四环素的降解效率如图15a所示. 单独的ZnIn2S4对四环素的降解效率并不高, 在80 min内,仅有不到40%的四环素被降解,这主要归因于ZnIn2S4的比表面积较小,并且e−和h+复合率较高. MIL-125-NH2对四环素在80 min内降解效率约为10%,而复合材料NM(2%)@ZIS对四环素在80 min内的降解效率为92.8%. 这说明,ZnIn2S4与MIL-125-NH2的有效复合可实现协同作用而有助于增强光催化性能. 复合材料NM(2%)@ZIS能被循环使用5次,其间,对头孢克肟的光催化降解率仅下降了3%,这就表明其水稳定性和可循环利用性均非常良好(图15b). 再由图15c,添加羟基自由基捕捉剂异丙醇(IPA)后,确定·OH并不是该光催化反应体系中关键的活性物质. 而添加超氧自由基捕捉剂对苯醌(BQ)和空穴捕捉剂草酸铵(AO)后,确定·O2−和h+是该光催化反应体系中最主要的活性物质. 因此,作者提出了NM(2%)@ZIS光催化剂最有可能的光催化机理(图15d).
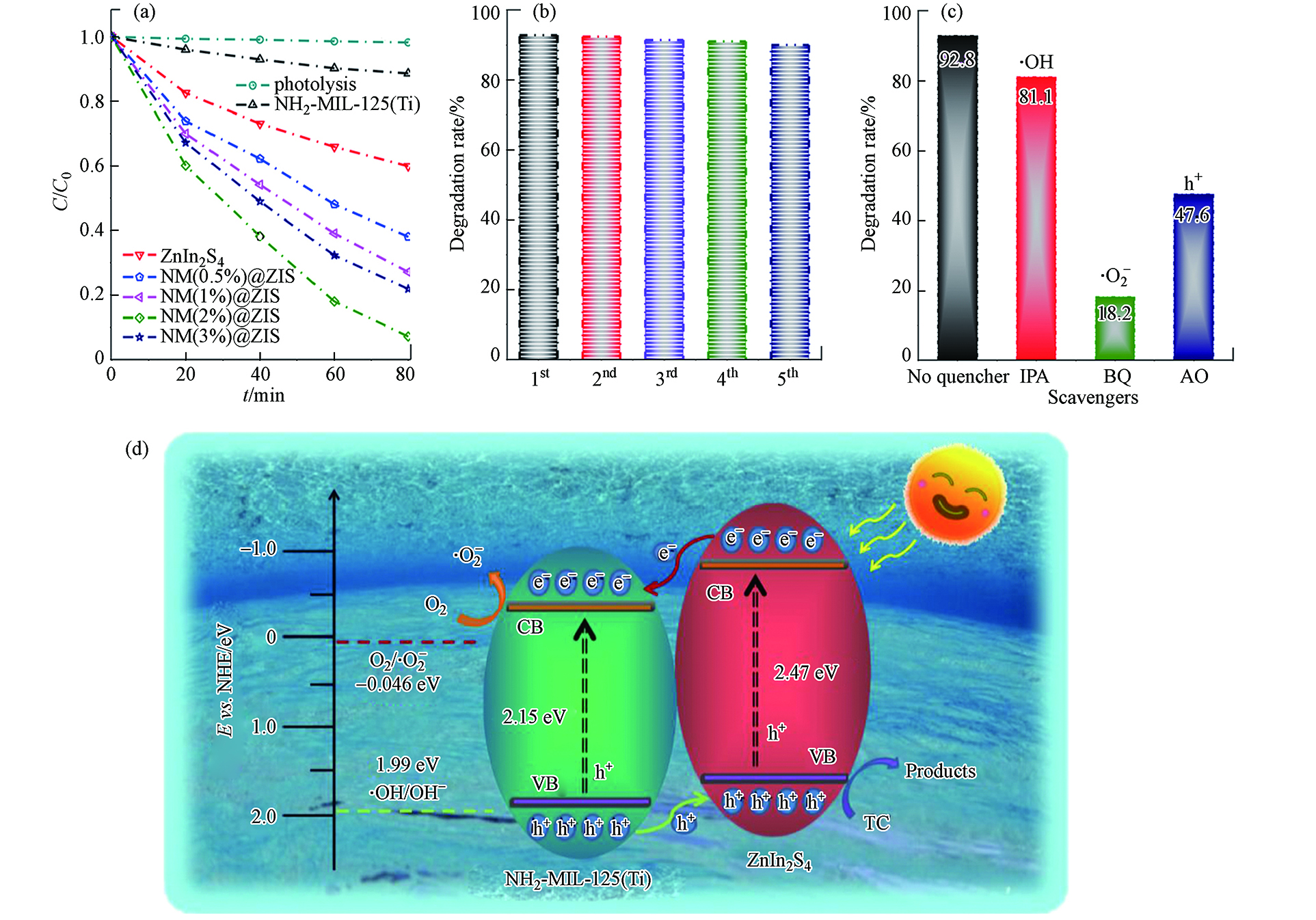 图 15 (a)NH2-MIL-125@ ZnIn2S4复合材料样品在可见光照射下的光催化性能;(b)NM(2%)@ZIS(50 mg催化剂,50 mL 四环素,20 mg·L−1)的循环实验;(c)NM(2%)@ZIS光催化降解四环素的活性物质捕捉实验对四环素降解的影响;(d)NM(2%)@ZIS复合材料光催化降解四环素的机理[50]Figure 15. (a) The photocatalytic performance of NH2-MIL-125@ZnIn2S4 composite under visible light irradiation; (b) The cyclic experiment of NM (2%) @ZIS (50 mg catalyst, 50 mL tetracycline, 20 mg·L−1); (c) The effect of capture experiment for active substance on the photocatalytic tetracycline degradation by NM (2%) @ZIS; (d) Photocatalytic degradation mechanism of tetracycline by NM (2%) @ZIS composites[50]
图 15 (a)NH2-MIL-125@ ZnIn2S4复合材料样品在可见光照射下的光催化性能;(b)NM(2%)@ZIS(50 mg催化剂,50 mL 四环素,20 mg·L−1)的循环实验;(c)NM(2%)@ZIS光催化降解四环素的活性物质捕捉实验对四环素降解的影响;(d)NM(2%)@ZIS复合材料光催化降解四环素的机理[50]Figure 15. (a) The photocatalytic performance of NH2-MIL-125@ZnIn2S4 composite under visible light irradiation; (b) The cyclic experiment of NM (2%) @ZIS (50 mg catalyst, 50 mL tetracycline, 20 mg·L−1); (c) The effect of capture experiment for active substance on the photocatalytic tetracycline degradation by NM (2%) @ZIS; (d) Photocatalytic degradation mechanism of tetracycline by NM (2%) @ZIS composites[50]复合材料NM(2%)@ZIS对四环素具有良好的吸附效果,能将四环素吸附在催化剂的活性位点上. 复合材料NM(2%)@ZIS当中稳定的微介孔结构为增强吸附和随后的光催化活性提供了大量的活性位点,从而进一步促进了吸附过程. 此外,NM(2%)@ZIS对可见光吸收能力的增加,提高了对应的光催化性能,h+、·O2−和·OH是该反应体系主导光催化过程的活性物质. 在可见光照射下,复合材料NM(2%)@ZIS表面产生e−和h+,ZnIn2S4的CB中的e−被转移到MIL-125的相对较低的CB中,并与O2反应后形成·O2−,这些·O2−和h+使四环素分子降解. 因此,由于这些成分之间的协同作用,致使e−和h+复合机会减少,光催化活性增强.
3. 结论(Conclusion)
MIL-125和MIL-125-NH2本体具有较大的带隙,只能被紫外光激发,电子转移效率偏低. 当前的研究进展表明,将稳定性很好的MIL-125或MIL-125-NH2与半导体类功能材料复合,可以提升MIL-125或是MIL-125-NH2对光的吸收效率以及电子转移效率,这有利于光催化处理水中的污染物. 然而,当前的相关研究还处于实验室阶段,如何将相关催化剂用于实际水处理中实现高效光催化去除水体污染物尚未获得足够关注. 目前的研究做集中在研究相应的机理与机制,后续研究应加强相关光催化水处理装备及工艺. 此外,MIL-125(MIL-125-NH2)及其复合材料在环境中的迁移、转化、对应的环境效应和生物毒性、已使用材料的处置和再利用问题,也将成为今后的相关研究值得关注的问题.
-
图 5 (a)TiO2@MIL-125复合材料样品的紫外-可见漫反射谱图,通过嵌入图可得到样品的Eg值;(b)TiO2@MIL-125复合材料样品在可见光照射下的光催化性能;(c)MT-2(15 mg催化剂,50 mL Cr(Ⅵ),5 mg·L−1)的循环实验;(d)Cr(Ⅵ)的还原机理图[14]
Figure 5. (a) Ultraviolet-visible diffuse reflection spectra of TiO2@MIL-125 composite samples, and the Eg value of the sample can be obtained by the embedded diagram; (b) The photocatalytic properties of TiO2@MIL-125 composite samples under visible light irradiation; (c) Cycle experiment of MT-2 (15 mg catalyst, 50 mL Cr (Ⅵ), 5 mg·L−1); (d) Reduction mechanism diagram of Cr (Ⅵ) [14]
图 7 (a)MIL-125-NH2@Ag/AgCl复合材料样品在可见光照射下的光催化性能;(b)50MN@AAC复合材料样品的光电流谱图;(c)50MN@AAC(40 mg催化剂,40 mL Cr(Ⅵ),10 mg·L−1)的循环实验;(d)Cr(Ⅵ)的还原机理图[16]
Figure 7. (a) Photocatalytic performance of MIL-125-NH2@Ag/AgCl composite samples under visible light irradiation;(b) Photocurrent spectra of 50MN@AAC composite samples;(c) Cycle experiment of 50MN@AAC (40 mg catalyst, 40 mL Cr (Ⅵ)),(d) Reduction mechanism diagram of Cr (Ⅵ)[16]
图 9 (a)Ag/MIL‐125(3% Ag)复合材料样品在可见光照射下的光催化性能;(b)Ag/MIL‐125(3% Ag)复合材料样品(20 mg催化剂,20 mL RhB,10 mg·L−1)的循环实验;(c)Ag/MIL‐125(3% Ag)复合材料样品光催化降解RhB的活性物质捕捉实验对RhB降解比率的影响;(d)Ag/MIL‐125(3% Ag)催化降解RhB的机理图[22]
Figure 9. (a) Photocatalytic properties of Ag/MIL‐125 (3% Ag) composites under visible light irradiation;(b) Cycle experiment of Ag/MIL‐125 (3% Ag) composite sample (20 mg catalyst, 20 mL RhB, 10 mg·L−1); (c) The effect of the degradation ratio of RhB by capture experiments for active substance on the photocatalytic degradation of RhB by Ag/MIL‐125 (3% Ag) composite samples; (d) Catalytic degradation mechanism of RhB by Ag/MIL‐125 (3% Ag) composites[22]
图 11 (a)Ag3PO4@NH2-MIL-125复合材料样品在可见光照射下的光催化性能;(b)Ag3PO4@ NH2-MIL-125(50 mg催化剂,100 mL MB,10 mg·L−1)的循环实验;(c)Ag3PO4@NH2-MIL-125复合材料样品光催化降解MB的活性物质捕捉实验对MB降解速率常数的影响;(d)pH = 0时Ag3PO4和NH2-MIL-125相对于标准氢电极(NHE)的能带结构[20]
Figure 11. (a) The photocatalytic properties of Ag3PO4@NH2-MIL-125 composite samples under visible light irradiation; (b) Cyclic experiment of Ag3PO4@NH2-MIL-125 (50 mg catalyst, 100 mL MB, 10 mg·L−1); (c) The effect of capture experiments for active substance on the degradation rate constant of MB for the photocatalytic degradation of MB by Ag3PO4@NH2-MIL-125 composite samples; (d) Band structure of Ag3PO4 and NH2-MIL-125 related to standard hydrogen electrode (NHE) at pH = 0[20]
图 13 (a)MIL-125ML/gCN复合材料样品在可见光照射下的光催化性能;(b)MIL-125ML/gCN-20(30 mg催化剂,100 mL 头孢克肟,20 mg·L−1)的循环实验;(c)MIL-125ML/gCN-20的投加量对其在可见光照射下的光催化性能的影响;(d)活性物质捕捉实验对MIL-125ML/gCN-20光催化降解头孢克肟的的影响;(e)MIL-125ML/gCN-20复合材料光催化降解头孢克肟的机理[52]
Figure 13. (a) Photocatalytic performance of MIL-125ML/gCN composites under visible light irradiation; (b) Cycle experiment of MIL-125ML/gCN-20 (30 mg catalyst, 100 mL cefixime, 20 mg·L−1); (c) The effect of dosage of MIL-125ML/gCN-20 on its photocatalytic performance under visible light irradiation; (d) The effect of capture experiment for active substance on the photocatalytic degradation of cefixime by MIL-125ML/gCN-20; (e) The photocatalytic degradation mechanism of cefixime by MIL-125ML/gCN-20 composite[52]
图 15 (a)NH2-MIL-125@ ZnIn2S4复合材料样品在可见光照射下的光催化性能;(b)NM(2%)@ZIS(50 mg催化剂,50 mL 四环素,20 mg·L−1)的循环实验;(c)NM(2%)@ZIS光催化降解四环素的活性物质捕捉实验对四环素降解的影响;(d)NM(2%)@ZIS复合材料光催化降解四环素的机理[50]
Figure 15. (a) The photocatalytic performance of NH2-MIL-125@ZnIn2S4 composite under visible light irradiation; (b) The cyclic experiment of NM (2%) @ZIS (50 mg catalyst, 50 mL tetracycline, 20 mg·L−1); (c) The effect of capture experiment for active substance on the photocatalytic tetracycline degradation by NM (2%) @ZIS; (d) Photocatalytic degradation mechanism of tetracycline by NM (2%) @ZIS composites[50]
表 1 MIL-125系列复合材料光催化去除水体污染物的典型实例
Table 1. Typical examples of photocatalytic removal to water pollutants by MIL-125 series composites
金属有机框架 Metal-organic frameworks 复合组分 Composite component 污染物 Pollutants 初始浓度/(mg·L−1) Initial concentration 最优pH Optimal pH 反应时间/min Reaction time 去除率/% Removal efficiency 参考文献 References MIL-125 TiO2 六价铬 5 2 80 100 [14] MIL-125-NH2 Bi2S3 六价铬 10 3 90 100 [15] MIL-125-NH2 Ag/AgCl 六价铬 40 6 120 98.4 [16] MIL-125-NH2 Ag/Ag3PO4 六价铬 10 2 70 100 [17] MIL-125-NH2 PPynt 六价铬 10 2 60 99.02 [18] MIL-125 Cr-III 亚甲基蓝 20 — 120 99 [19] MIL-125-NH2 Ag3PO4 亚甲基蓝 10 — 50 100 [20] MIL-125-NH2 Ag3VO4 亚甲基蓝 5 — 60 96.4 [21] MIL-125 Ag 罗丹明B 10 — 40 95 [22] MIL-125 Ag/rGO 罗丹明B 50 — 50 95.7 [23] MIL-125 BiVO4 罗丹明B 10 — 180 92 [24] MIL-125 MnO2 罗丹明B 10 — 80 100 [25] MIL-125 ZnIn2S4 罗丹明B 15 — 50 97.7 [26] MIL-125 NiS 罗丹明B 15 — 60 98.5 [27] MIL-125-NH2 BiOBr 罗丹明B 20 — 100 96 [28] MIL-125-NH2 PHIK 罗丹明B 100 — 120 94 [29] MIL-125-NH2 CdS 罗丹明B 180 — 120 97 [30] MIL-125-NH2 CQDs 罗丹明B 10 — 120 100 [31] MIL-125-NH2 CoSx 罗丹明B 20 — 45 95.4 [32] MIL-125-NH2 Bi2WO6 罗丹明B 10 — 50 98 [33] MIL-125-NH2 BiOI 罗丹明B 40 — 240 99 [34] MIL-125-NH2 SnS2 罗丹明B 40 — 80 90.5 [35] MIL-125-NH2 CdS/石墨烯 罗丹明B 10 — 30 95 [36] MIL-125-NH2 Fe3+ 罗丹明B 14.4 4 120 82.4 [37] MIL-125-NH2 g-C3N4 罗丹明B 10 — 120 96.4 [38] MIL-125 Zn2GeO4 罗丹明6G 10 — 90 100 [39] MIL-125-NH2 Cu 甲基橙 10 — 90 99 [40] MIL-125-NH2 Ag/AgBr 甲基橙 20 — 120 70 [41] MIL-125-NH2 Ca/TiO2 甲基橙 20 5 120 87.29 [42] MIL-125 In2S3 四环素 46 3.3 60 63.3 [43] MIL-125 CQDs 四环素 20 5 240 90 [44] MIL-125 Bi2WO6 四环素 20 — 80 73 [45] MIL-125-NH2 BiOCl 四环素 20 — 120 78 [46] MIL-125-NH2 Bi2WO6 四环素 20 — 120 77.8 [47] MIL-125-NH2 TPE-2NH2 四环素 30 7 120 90 [48] MIL-125-NH2 BiOBr 四环素 25 7 90 88 [49] MIL-125-NH2 Znln2S4 四环素 20 — 80 92.8 [50] MIL-125 BiOBr 环丙沙星 10 — 80 91 [51] MIL-125 g-C3N4 头孢克肟 20 4 120 98 [52] MIL-125-NH2 ZnO 左氧氟沙星 10 7 240 90 [53] MIL-125-NH2 Pt 对乙酰氨基酚 5 — 180 100 [54] MIL-125-NH2 Ag,CdS 酮洛芬 10 4 180 94.2 [55] MIL-125-NH2 Bi2MoO6 二氯酚 10 — 180 93.28 [56] MIL-125-NH2 g-C3N4 双氯芬酸 10 — 120 100 [57] MIL-125-NH2 ZIF-67 硝基苯酚 10 — 60 100 [58] 注:MIL-125-NH2与NH2-MIL-125两种写法都正确. PHIK是聚庚氨酰亚胺钾的缩写. TPE-2NH2是一种双氨基多苯环有机配体的缩写. CQDs代表碳量子点. PPynt代表聚吡咯碳纳米管. “—”表示原文未提及. Note: Both MIL-125-NH2 and NH2-MIL-125 are correct. PHIK stands for potassium poly (heptazine imide). TPE-2NH2 is short for an organic ligand with two aminos and some phenyl rings. CQDs stands for carbon quantum dots. PPynt stands for polypyrrole carbon nanotubes. "—" indicates that it is not mentioned in the original article. -
[1] DAN-HARDI M, SERRE C, FROT T, et al. A new photoactive crystalline highly porous titanium(IV) dicarboxylate[J]. Journal of the American Chemical Society, 2009, 131(31): 10857-10859. doi: 10.1021/ja903726m [2] ZLOTEA C, PHANON D, MAZAJ M, et al. Effect of NH2 and CF3 functionalization on the hydrogen sorption properties of MOFs[J]. Dalton Transactions, 2011, 40(18): 4879-4881. doi: 10.1039/c1dt10115c [3] REN X Y, WANG C C, LI Y, et al. Ag(I) removal and recovery from wastewater adopting NH2-MIL-125 as efficient adsorbent: A 3Rs (reduce, recycle and reuse) approach and practice[J]. Chemical Engineering Journal, 2022, 442: 136306. doi: 10.1016/j.cej.2022.136306 [4] KIM S N, KIM J, KIM H Y, et al. Adsorption/catalytic properties of MIL-125 and NH2-MIL-125[J]. Catalysis Today, 2013, 204: 85-93. doi: 10.1016/j.cattod.2012.08.014 [5] ZHANG X, LIU Y F, PANG Y P, et al. Significantly improved kinetics, reversibility and cycling stability for hydrogen storage in NaAlH4 with the Ti-incorporated metal organic framework MIL-125(Ti)[J]. Journal of Materials Chemistry A, 2014, 2(6): 1847-1854. doi: 10.1039/C3TA14202G [6] MOREIRA M A, SANTOS J C, FERREIRA A F P, et al. Toward understanding the influence of ethylbenzene in p-xylene selectivity of the porous titanium amino terephthalate MIL-125(Ti): Adsorption equilibrium and separation of xylene isomers[J]. Langmuir: the ACS Journal of Surfaces and Colloids, 2012, 28(7): 3494-3502. doi: 10.1021/la204969t [7] KIM B, LEE Y R, KIM H Y, et al. Adsorption of volatile organic compounds over MIL-125-NH2[J]. Polyhedron, 2018, 154: 343-349. doi: 10.1016/j.poly.2018.08.010 [8] LIANG X X, WANG N, QU Y L, et al. Facile preparation of metal-organic framework (MIL-125)/chitosan beads for adsorption of Pb(II) from aqueous solutions[J]. Molecules, 2018, 23(7): 1524. doi: 10.3390/molecules23071524 [9] JIANG Q S, HAN Z L, YU X B, et al. NH2-MIL-125 (Ti)/biochar fibers for enhanced direct dyes adsorption[J]. Journal of Environmental Chemical Engineering, 2021, 9(6): 106636. doi: 10.1016/j.jece.2021.106636 [10] LIU Z M, WANG C, WU Y C, et al. Synthesis of uniform-sized and microporous MIL-125(Ti) to boost arsenic removal by chemical adsorption[J]. Polyhedron, 2021, 196: 114980. doi: 10.1016/j.poly.2020.114980 [11] HE Y Z, LI H F, WU J, et al. In-situ formation of Au nanoparticles with surface plasmon resonance confined in the framework of Cu ions doped NH2-MIL-125(Ti) to enhance photocatalytic hydrogen production and NO removal[J]. Applied Surface Science, 2022, 604: 154641. doi: 10.1016/j.apsusc.2022.154641 [12] ZHANG X D, YUE K, RAO R Z, et al. Synthesis of acidic MIL-125 from plastic waste: Significant contribution of N orbital for efficient photocatalytic degradation of chlorobenzene and toluene[J]. Applied Catalysis B: Environmental, 2022, 310: 121300. doi: 10.1016/j.apcatb.2022.121300 [13] HENDON C H, TIANA D, FONTECAVE M, et al. Engineering the optical response of the titanium-MIL-125 metal–organic framework through ligand functionalization[J]. Journal of the American Chemical Society, 2013, 135(30): 10942-10945. doi: 10.1021/ja405350u [14] LI Y X, WANG C C, FU H F, et al. Marigold-flower-like TiO2/MIL-125 core−shell composite for enhanced photocatalytic Cr(VI) reduction[J]. Journal of Environmental Chemical Engineering, 2021, 9(4): 105451. doi: 10.1016/j.jece.2021.105451 [15] WANG M H, YANG L Y, YUAN J Y, et al. Heterostructured Bi2S3@NH2-MIL-125(Ti) nanocomposite as a bifunctional photocatalyst for Cr(vi) reduction and rhodamine B degradation under visible light[J]. RSC Advances, 2018, 8(22): 12459-12470. doi: 10.1039/C8RA00882E [16] QIU J H, LI M, WANG H T, et al. Integration of plasmonic effect into MIL-125-NH2: An ultra-efficient photocatalyst for simultaneous removal of ternary system pollutants[J]. Chemosphere, 2020, 242: 125197. doi: 10.1016/j.chemosphere.2019.125197 [17] ZHOU Y C, WANG P, FU H F, et al. Ternary Ag/Ag3PO4/MIL-125-NH2 Z-scheme heterojunction for boosted photocatalytic Cr(Ⅵ) cleanup under visible light[J]. Chinese Chemical Letters, 2020, 31(10): 2645-2650. doi: 10.1016/j.cclet.2020.02.048 [18] CHOE J, YANG X A, YU J, et al. Visible- light responsive PPynt@NH2-MIL-125 nanocomposite for efficient reduction of Cr(Ⅵ)[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2022, 636: 128147. doi: 10.1016/j.colsurfa.2021.128147 [19] ABDELHAMEED R M, SIMÕES M M Q, SILVA A M S, et al. Enhanced photocatalytic activity of MIL-125 by post-synthetic modification with CrIII and Ag nanoparticles[J]. Chemistry - A European Journal, 2015, 21(31): 11072-11081. doi: 10.1002/chem.201500808 [20] ABDELHAMEED R M, TOBALDI D M, KARMAOUI M. Engineering highly effective and stable nanocomposite photocatalyst based on NH2-MIL-125 encirclement with Ag3PO4 nanoparticles[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2018, 351: 50-58. doi: 10.1016/j.jphotochem.2017.10.011 [21] EMAM H E, AHMED H B, GOMAA E, et al. Doping of silver vanadate and silver tungstate nanoparticles for enhancement the photocatalytic activity of MIL-125-NH2 in dye degradation[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2019, 383: 111986. doi: 10.1016/j.jphotochem.2019.111986 [22] GUO H X, GUO D, ZHENG Z S, et al. Visible-light photocatalytic activity of Ag@MIL-125(Ti) microspheres[J]. Applied Organometallic Chemistry, 2015, 29(9): 618-623. doi: 10.1002/aoc.3341 [23] YUAN X Z, WANG H, WU Y, et al. One-pot self-assembly and photoreduction synthesis of silver nanoparticle-decorated reduced graphene oxide/MIL-125(Ti) photocatalyst with improved visible light photocatalytic activity[J]. Applied Organometallic Chemistry, 2016, 30(5): 289-296. doi: 10.1002/aoc.3430 [24] YANG Z Q, DING J E, FENG J N, et al. Preparation of BiVO4/MIL-125(Ti) composite with enhanced visible-light photocatalytic activity for dye degradation[J]. Applied Organometallic Chemistry, 2018, 32(4): e4285. doi: 10.1002/aoc.4285 [25] HAROON H, MAJID K. MnO2 nanosheets supported metal–organic framework MIL-125(Ti) towards efficient visible light photocatalysis: Kinetic and mechanistic study[J]. Chemical Physics Letters, 2020, 745: 137283. doi: 10.1016/j.cplett.2020.137283 [26] HUANG F, LI Q, LUO S, et al. Preparation and photocatalytic activity of ZnIn2S4/MIL-125 nanocomposites [J]. Journal of the Chinese Ceramic Society, 2021, 49 (6): 1167-1175. [27] LI Q, HUANG F, LI D K, et al. Synthesis of NiS/MIL-125 hybrids with expanded light absorption, fast carrier transfer and enhanced carrier separation[J]. Materials Research Bulletin, 2021, 133: 111058. doi: 10.1016/j.materresbull.2020.111058 [28] ZHU S R, LIU P F, WU M K, et al. Enhanced photocatalytic performance of BiOBr/NH2-MIL-125(Ti) composite for dye degradation under visible light[J]. Dalton Transactions, 2016, 45(43): 17521-17529. doi: 10.1039/C6DT02912D [29] RODRÍGUEZ N A, SAVATEEV A, GRELA M A, et al. Facile synthesis of potassium poly(heptazine imide) (PHIK)/Ti-based metal–organic framework (MIL-125-NH2) composites for photocatalytic applications[J]. ACS Applied Materials & Interfaces, 2017, 9(27): 22941-22949. [30] WANG H, CUI P H, SHI J X, et al. Controllable self-assembly of[email protected](Ti) heterostructure with enhanced photodegradation efficiency for organic pollutants through synergistic effect[J]. Materials Science in Semiconductor Processing, 2019, 97: 91-100. doi: 10.1016/j.mssp.2019.03.016 [31] WANG Q J, WANG G L, LIANG X F, et al. Supporting carbon quantum dots on NH2-MIL-125 for enhanced photocatalytic degradation of organic pollutants under a broad spectrum irradiation[J]. Applied Surface Science, 2019, 467/468: 320-327. doi: 10.1016/j.apsusc.2018.10.165 [32] LIU S J, ZOU Q C, MA Y, et al. A novel amorphous CoS x/NH2-MIL-125 composite for photocatalytic degradation of rhodamine B under visible light[J]. Journal of Materials Science, 2020, 55(34): 16171-16183. doi: 10.1007/s10853-020-05210-4 [33] XU Y F, ZHOU Y, DENG Y H, et al. Synthesis of Bi2WO6@NH2-MIL-125(Ti): A S-scheme photocatalyst with enhanced visible light catalytic activity[J]. Catalysis Letters, 2020, 150(12): 3470-3480. doi: 10.1007/s10562-020-03258-0 [34] DU J A, ZHANG J X, YANG T Y, et al. The research on the construction and the photocatalytic performance of BiOI/NH2-MIL-125(Ti) composite[J]. Catalysts, 2020, 11(1): 24. doi: 10.3390/catal11010024 [35] LING L Q, TU Y, LONG X Y, et al. The one-step synthesis of multiphase SnS2 modified by NH2-MIL-125(Ti) with effective photocatalytic performance for Rhodamine B under visible light[J]. Optical Materials, 2021, 111: 110564. doi: 10.1016/j.optmat.2020.110564 [36] NIVETHA R, GOTHANDAPANI K, RAGHAVAN V, et al. NH2-MIL-125(Ti) doped CdS/Graphene composite as electro and photo catalyst in basic medium under light irradiation[J]. Environmental Research, 2021, 200: 111719. doi: 10.1016/j.envres.2021.111719 [37] NGUYEN THI H T, TRAN THI K N, HOANG N B, et al. Enhanced degradation of rhodamine B by metallic organic frameworks based on NH2-MIL-125(Ti) under visible light[J]. Materials, 2021, 14(24): 7741. doi: 10.3390/ma14247741 [38] WANG J, CHEN C C, ZHAO Z H, et al. Construction of N-doped g-C3N4/NH2-MIL-125(Ti) S-scheme heterojunction for enhanced photocatalytic degradation of organic pollutants: DFT calculation and mechanism study[J]. Journal of Alloys and Compounds, 2022, 922: 166288. doi: 10.1016/j.jallcom.2022.166288 [39] KANG Q M, REN T J, ZHAO W Q, et al. Preparation of Zn2GeO4 nanosheets with MIL-125(Ti) hybrid photocatalyst for improved photodegradation of organic pollutants[J]. Materials Research Bulletin, 2021, 133: 111013. doi: 10.1016/j.materresbull.2020.111013 [40] AO D, ZHANG J, LIU H. Visible-light-driven photocatalytic degradation of pollutants over Cu-doped NH2-MIL-125(Ti)[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2018, 364: 524-533. doi: 10.1016/j.jphotochem.2018.06.044 [41] WU D Y, HAN L. Fabrication of novel Ag/AgBr/NH2-MIL-125(Ti) nanocomposites with enhanced visible-light photocatalytic activity[J]. Materials Research Express, 2019, 6(12): 125501. doi: 10.1088/2053-1591/ab540a [42] AHMADPOUR N, SAYADI M H, HOMAEIGOHAR S. Correction: A hierarchical Ca/TiO2/NH2-MIL-125 nanocomposite photocatalyst for solar visible light induced photodegradation of organic dye pollutants in water[J]. RSC Advances, 2023, 13(29): 19661. doi: 10.1039/D3RA90057F [43] WANG H, YUAN X Z, WU Y, et al. in situ synthesis of In2S3@MIL-125(Ti) core-shell microparticle for the removal of tetracycline from wastewater by integrated adsorption and visible-light-driven photocatalysis[J]. Applied Catalysis B: Environmental, 2016, 186: 19-29. doi: 10.1016/j.apcatb.2015.12.041 [44] JIANG E H, LIU X T, CHE H N, et al. Visible-light-driven Ag/Bi3O4Cl nanocomposite photocatalyst with enhanced photocatalytic activity for degradation of tetracycline[J]. RSC Advances, 2018, 8(65): 37200-37207. doi: 10.1039/C8RA07482H [45] YIN S, CHEN Y, GAO C, et al. In-situ preparation of MIL-125(Ti)/Bi2WO6 photocatalyst with accelerating charge carriers for the photodegradation of tetracycline hydrochloride[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2020, 387: 112149. doi: 10.1016/j.jphotochem.2019.112149 [46] HU Q S, DI J, WANG B, et al. In-situ preparation of NH2-MIL-125(Ti)/BiOCl composite with accelerating charge carriers for boosting visible light photocatalytic activity[J]. Applied Surface Science, 2019, 466: 525-534. doi: 10.1016/j.apsusc.2018.10.020 [47] YIN S, CHEN Y, HU Q S, et al. Construction of NH2-MIL-125(Ti) nanoplates modified Bi2WO6 microspheres with boosted visible-light photocatalytic activity[J]. Research on Chemical Intermediates, 2020, 46(7): 3311-3326. doi: 10.1007/s11164-020-04132-9 [48] SONG X L, WANG Y, ZHU T, et al. Facile synthesis a novel core-shell amino functionalized MIL-125(Ti) micro-photocatalyst for enhanced degradation of tetracycline hydrochloride under visible light[J]. Chemical Engineering Journal, 2021, 416: 129126. doi: 10.1016/j.cej.2021.129126 [49] WANG Y, FENG S, WU W, et al. Ionic liquid-assisted solvothermal construction of NH2-MIL-125(Ti)/BiOBr heterojunction for removing tetracycline under visible light[J]. Optical Materials, 2022, 123: 111817. doi: 10.1016/j.optmat.2021.111817 [50] ZHU C Z, HE Q Y, YAO H Q, et al. Amino-functionalized NH2-MIL-125(Ti)-decorated hierarchical flowerlike Znln2S4 for boosted visible-light photocatalytic degradation[J]. Environmental Research, 2022, 204: 112368. doi: 10.1016/j.envres.2021.112368 [51] YANG J, LIU T Y, ZHOU H F, et al. In situ conversion of typical type-I MIL-125(Ti)/BiOBr into type-Ⅱ heterostructure photocatalyst via MOF self-sacrifice: Photocatalytic mechanism and theoretical study[J]. Journal of Alloys and Compounds, 2022, 900: 163440. doi: 10.1016/j.jallcom.2021.163440 [52] SALIMI M, ESRAFILI A, JONIDI JAFARI A, et al. Photocatalytic degradation of cefixime with MIL-125(Ti)-mixed linker decorated by g-C3N4 under solar driven light irradiation[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2019, 582: 123874. doi: 10.1016/j.colsurfa.2019.123874 [53] KAUR M, MEHTA S K, DEVI P, et al. NH2-MIL-125(Ti) nanoparticles decorated over ZnO microrods: An efficient bifunctional material for degradation of levofloxacin and detection of Cu(II)[J]. Journal of Alloys and Compounds, 2022, 928: 166909. doi: 10.1016/j.jallcom.2022.166909 [54] MUELAS-RAMOS V, BELVER C, RODRIGUEZ J J, et al. Synthesis of noble metal-decorated NH2-MIL-125 titanium MOF for the photocatalytic degradation of acetaminophen under solar irradiation[J]. Separation and Purification Technology, 2021, 272: 118896. doi: 10.1016/j.seppur.2021.118896 [55] ZHENG X N, LI Y, YANG J, et al. Z-Scheme heterojunction Ag/NH2-MIL-125(Ti)/CdS with enhanced photocatalytic activity for ketoprofen degradation: Mechanism and intermediates[J]. Chemical Engineering Journal, 2021, 422: 130105. doi: 10.1016/j.cej.2021.130105 [56] ZHANG S Y, DU M, KUANG J Y, et al. Surface-defect-rich mesoporous NH2-MIL-125 (Ti)@Bi2MoO6 core-shell heterojunction with improved charge separation and enhanced visible-light-driven photocatalytic performance[J]. Journal of Colloid and Interface Science, 2019, 554: 324-334. doi: 10.1016/j.jcis.2019.07.021 [57] MUELAS-RAMOS V, SAMPAIO M J, SILVA C G, et al. Degradation of diclofenac in water under LED irradiation using combined g-C3N4/NH2-MIL-125 photocatalysts[J]. Journal of Hazardous Materials, 2021, 416: 126199. doi: 10.1016/j.jhazmat.2021.126199 [58] ABDELHAMEED R M, EL-SHAHAT M. Fabrication of Au@CeO2nanocomposite with enhanced visible light photoreduction activity[J]. Journal of Environmental Chemical Engineering, 2019, 7(3): 103194. doi: 10.1016/j.jece.2019.103194 [59] XU C, ZHANG W, CHEN Y T, et al. Synthesis of NH2-MIL-125/NH2-MIL-125-P@TiO2 and its adsorption to uranyl ions[J]. ChemistrySelect, 2019, 4(43): 12801-12806. doi: 10.1002/slct.201902745 [60] WANG C C, DU X D, LI J, et al. Photocatalytic Cr(Ⅵ) reduction in metal-organic frameworks: A mini-review[J]. Applied Catalysis B: Environmental, 2016, 193: 198-216. doi: 10.1016/j.apcatb.2016.04.030 [61] BESHARAT F, AHMADPOOR F, NASROLLAHZADEH M. Graphene-based (nano)catalysts for the reduction of Cr(Ⅵ): A review[J]. Journal of Molecular Liquids, 2021, 334: 116123. doi: 10.1016/j.molliq.2021.116123 [62] STERN C M, JEGEDE T O, HULSE V A, et al. Electrochemical reduction of Cr(Ⅵ) in water: Lessons learned from fundamental studies and applications[J]. Chemical Society Reviews, 2021, 50(3): 1642-1667. doi: 10.1039/D0CS01165G [63] LI X, HE J F, ZHANG W L, et al. Ag nanoparticles interlayered Fe3O4/Ag/m(TiO2-ZrO2) magnetic photocatalysts with enhanced stability and photocatalytic performance for Cr(Ⅵ) reduction[J]. Applied Surface Science, 2023, 607: 155076. doi: 10.1016/j.apsusc.2022.155076 [64] LIU C R, XIAO H, LIU Y, et al. Internal electric field induced photocarriers separation of nickel-doped pyrite/pyrite homojunction with rich sulfur vacancies for superior Cr(Ⅵ) reduction[J]. Journal of Colloid and Interface Science, 2023, 629: 847-858. doi: 10.1016/j.jcis.2022.09.129 [65] SUN H M, WANG L, ZHANG Y, et al. Photocatalytic reduction performance and mechanisms of Cr(VI) by illite-g-C3N4 under visible light[J]. Applied Surface Science, 2023, 608: 155226. doi: 10.1016/j.apsusc.2022.155226 [66] KONSTANTINOU I K, ALBANIS T A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations[J]. Applied Catalysis B: Environmental, 2004, 49(1): 1-14. doi: 10.1016/j.apcatb.2003.11.010 [67] WAGHCHAURE R H, ADOLE V A, JAGDALE B S. Photocatalytic degradation of methylene blue, rhodamine B, methyl orange and Eriochrome black T dyes by modified ZnO nanocatalysts: A concise review[J]. Inorganic Chemistry Communications, 2022, 143: 109764. doi: 10.1016/j.inoche.2022.109764 [68] KUMAR A, KHAN M, HE J H, et al. Recent developments and challenges in practical application of visible-light-driven TiO2-based heterojunctions for PPCP degradation: A critical review[J]. Water Research, 2020, 170: 115356. doi: 10.1016/j.watres.2019.115356 [69] McKAY S, TSCHARKE B, HAWKER D, et al. Calibration and validation of a microporous polyethylene passive sampler for quantitative estimation of illicit drug and pharmaceutical and personal care product (PPCP) concentrations in wastewater influent[J]. Science of the Total Environment, 2020, 704: 135891. doi: 10.1016/j.scitotenv.2019.135891 [70] ZHANG S C, TAN M Y, DU S W, et al. Base-metal oxide semiconductor electrodes for PPCP degradation: Ti-doped α-Fe2O3 for sulfosalicylic acid oxidation as an example[J]. Chemosphere, 2023, 313: 137354. doi: 10.1016/j.chemosphere.2022.137354 -






 DownLoad:
DownLoad: