-
多杀菌素是一种发酵产生的无公害农药[1],属于农用抗生素,因其杀虫效率高,在农业上的应用前景广阔[2]。多杀菌素菌渣是抗生素发酵提取后残留菌丝体和培养基的混合物,若直接进入环境可能造成潜在环境危害。因此,多杀菌素菌渣在2008年被列入中国的危险废物管理清单。考虑到菌渣有机物含量丰富[3],有效地处理多杀菌素菌渣以实现无害化和资源化具有巨大潜力。
抗生素发酵菌渣无害化方法很多,包括微波分解[4-5]、热水解[6]、高级氧化工艺[7]、厌氧堆肥[8]和好氧堆肥[9],其中好氧堆肥处理以其低成本、技术成熟和可推广性受到企业的青睐。LIU et al[10]将庆大霉素残留物和洛伐他汀发酵残留物混合堆肥,实现了庆大霉素最大降解率96.7%。YANG et al[11]将肉鸡粪便堆肥42 d,去除粪便中75.4%的诺氟沙星。因此,抗生素残留物的肥料化是一个很有前景的资源利用途径。目前,暂无关于多杀菌素菌渣无害化和资源化的相关研究。
本文通过好氧发酵对多杀菌素菌渣进行无害化与稳定化处理,系统研究其堆肥化效能。通过土壤模拟试验,从土壤中多杀菌素残留降、土壤理化性质及微生物活性与多样性等多层面分析多杀菌素菌渣的肥料化应用效果,以期为多杀菌素菌渣的无害化与资源化提供理论与技术支持。
-
实验用多杀菌素菌渣取自山东省某生物制药公司,经脱水处理,含水率(5.61±0.71)%。菌渣样品采用已消毒的塑料桶收集,并在运回实验室后立刻放置于4 ℃冰箱内冷藏储存。实验用土壤取自江苏省某农场纯天然田园土。在进行土壤模拟实验前,已将土壤阴干14 d,并过2 mm筛网以去除石块和植物根系。实验用菌渣和土壤的理化性质,见表1和表2。
-
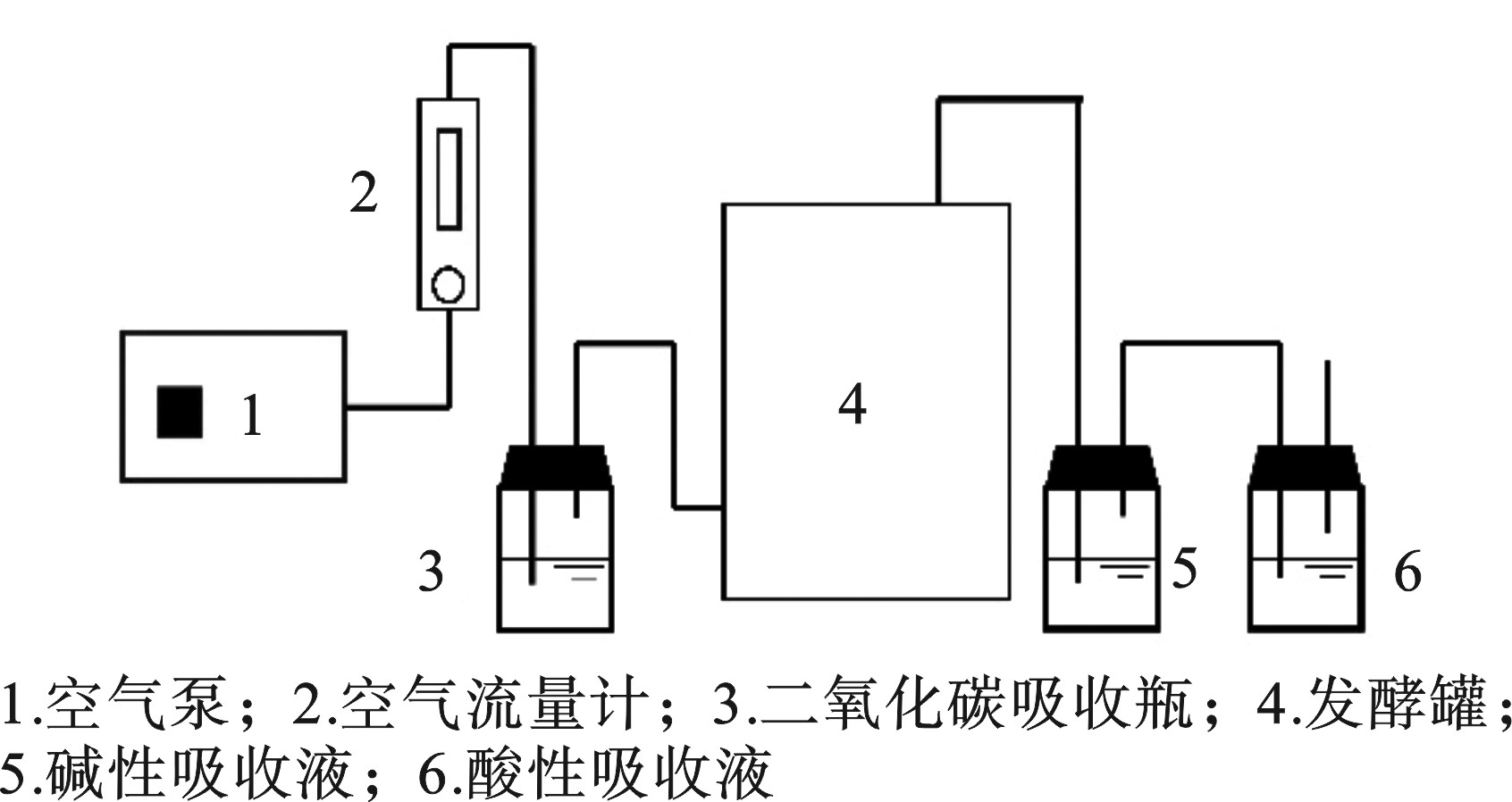
实验取稻草秸秆作为碳源,控制堆体C/N分别约为15、20、25进行堆肥,控制堆体含水率控制在60%。考虑到多杀菌素菌渣含有的微生物种类较为单一,单独堆肥难达理想效果,故投加约2%的高效菌(即EM菌,属混合菌,含光合菌、乳酸菌、酵母菌等)。同时堆体内投加约5%的腐殖酸,一方面能为堆体提供碳源,另一方面也能减少堆肥过程中的氮素损失。堆体体积约为15 L。机械曝气量为0.4 L/(min·kg),采用间歇式曝气法,曝气2 h,暂停1 h。与此同时,每天进行人工翻堆保证有机质能够被微生物充分利用。堆肥一共42 d,取样日期分别为0,1,2,3,4,6,8,10,14,18,22,32,42 d,每次取样均从堆体内上、中、下以及发酵罐相应截面的中心、四周均匀取样50 g,取出样品装袋标记后立刻放进−20 ℃的冰箱内进行冷冻保存,以备后续指标检测。发酵设备见图1。
-
本研究通过实验室土壤模拟试验法,研究了菌渣有机肥对土壤性能的影响。参考文献[12],本文设置1%、6%、12%的质量比,并另设空白组和1%鲜菌渣投加组进行对比。每组土壤模拟实验使用土壤量约为1 kg,设置3组平行对照,放置于直径17.5 cm、高16 cm的花盆中培养,定期浇水保证土壤湿度约为10%。在0、3、7、12、20、30、42 d取样(约50 g)。每组样品分为2部分,一部分储存于−20 ℃用于检测残留多杀菌素残留量,另一部分储存于4 ℃以检测相关理化性质。
-
pH和EC的检测分别参考《土壤检测 第2部分:土壤pH的测定:NY/T 1121.2—2006》和《土壤 电导率的测点 电极法:HJ 802—2016》。堆体三维荧光光谱检测:使用质量比1∶10的超纯水提取土壤样品5 g,在水平振动器中震荡24 h,10 000 r/min离心20 min后过0.45 μm滤膜,用于三维激发发射矩阵(3D-EEMs)荧光光谱分析,检测数据进行拉曼归一化[13]。土壤酶活采用比色法进行测定[14]。利用生物工业微生物测序仪对细菌群落进行16S基因测序。GI的检测参考了《有机肥料:NY/T 525—2021》。
抗生素残留检验检测方法:(1)流动相为甲醇:1%乙酸铵=7∶3(V/V),流速0.3 mL/min;(2)使用C18柱色谱柱,柱温30 ℃,进样量10 μL;(3)质谱选择采集多级反应监测模式,电喷雾离子源电压4.5 kV,雾化气流流速700 L/h,锥孔气流流速35 L/h;(4)多杀菌素A母离子质荷比(m/z)732.5,子离子质荷比(m/z)142.2;多杀菌素D母离子质荷比(m/z)746.5,子离子质荷比(m/z)142.2。
预处理方法:称取样品2.0 g(精确至0.01 g)于50 mL离心管中,加入饱和氯化钙溶液10 mL,同时再加入2.0 mL乙酸乙酯,将混合液摇匀后置于多管涡旋仪上以2 000 r/min的转速充分振荡20 min,4 000 r/min离心10 min之后,取1.0 mL乙酸乙酯相液体在氮吹仪上吹干,之后使用1 mL流动相复溶,过0.22 µm滤膜待测。
-
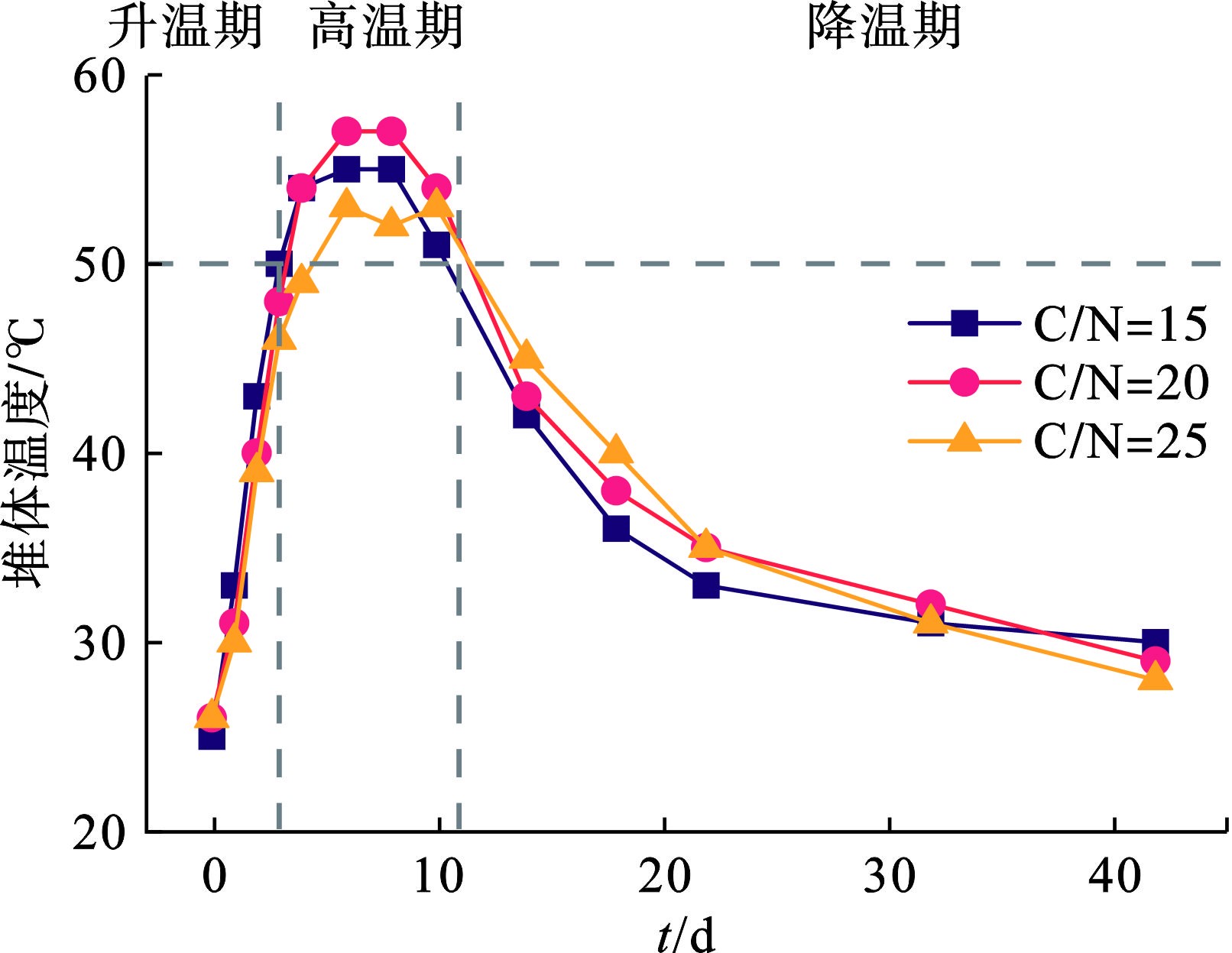
温度反映出好氧堆肥中微生物的新陈代谢水平,是判定堆肥成品达到无害化的重要指标。图2可知,3组实验的初始温度基本一致,在0—3 d内从室温快速升至50 ℃左右。在此阶段,水溶性糖类、淀粉类等易降解的可溶性有机物及大分子有机质被微生物利用,并产生热量上的累积。在4—10 d,堆体温度维持在50 ℃以上,嗜热微生物大量繁殖,有效地分解大分子蛋白质、纤维素、木质素等在升温过程难分解的有机物。到了降温期(11—42 d),嗜温性微生物开始进一步分解残余难降解有机物,温度进一步降低至室温。
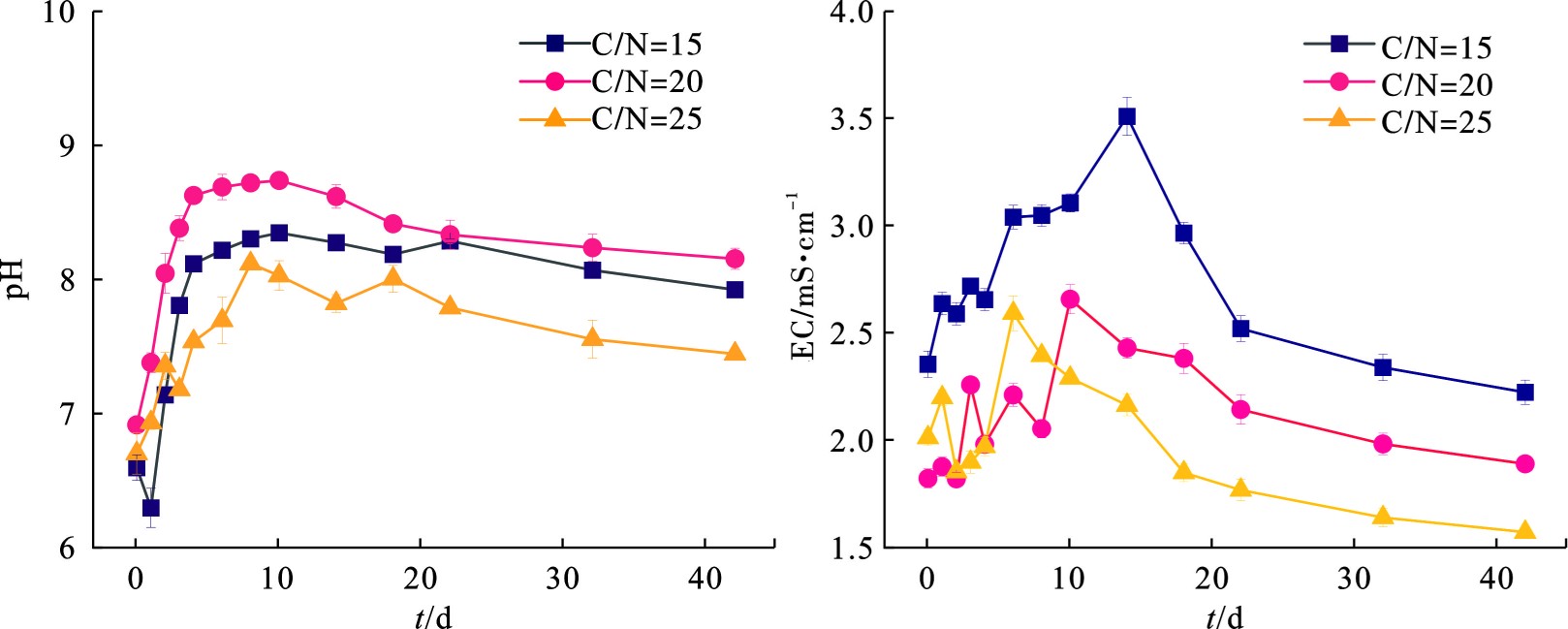
pH是影响体系中微生物活性和堆肥性能的重要参数,大多数微生物最适宜生长代谢的pH环境为中性或弱碱性[15],pH过高或过低均会影响到堆肥腐熟的进程。图3可知,在堆肥过程中A、B、C 3组的初始pH均为6.8左右,这主要是因为添加物料中含有一定量的腐殖酸,中和了菌渣等物料本身的碱度。在0—3 d内堆体pH迅速上升,大量含氮有机物被微生物利用产生氨气[16]以及小分子有机酸的降解[17]。在10—22 d进入降温期,功能微生物群落发生转变,堆体内有机物被进一步分解,小分子有机酸和部分盐基离子被合成大分子腐殖质(胡敏酸)[18],3组的pH缓慢下降并趋于稳定,这一点与图3中电导率后期呈现下降趋势相吻合。堆肥结束后,3组的pH稳定在堆肥适宜的7.5~8.5[19],EC稳定在<3 000 μS/cm范围内[20]。
-
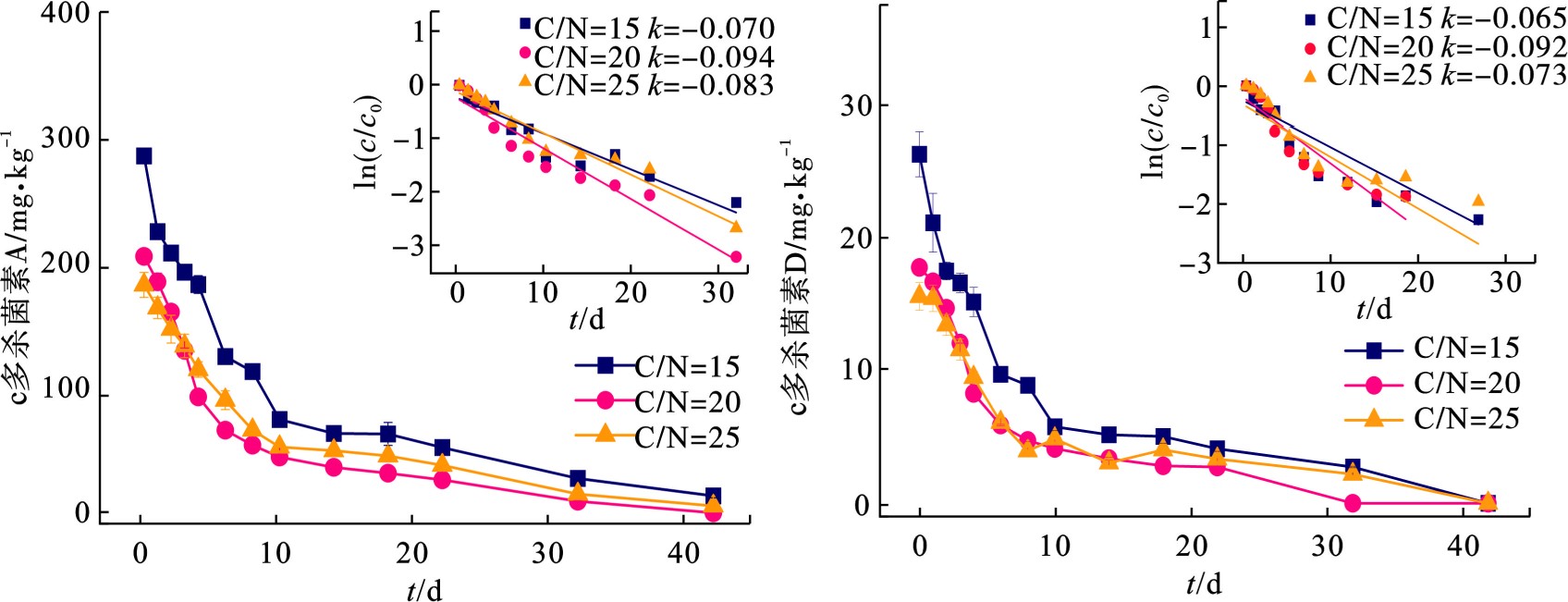
在多杀菌素菌渣好氧堆肥过程中,多杀菌素残留量是评价菌渣无害化水平的重要指标。多杀菌素包含2种成分,即多杀菌素A和多杀菌素D。在C/N为15、20、25条件下,多杀菌素A和D残留量的变化曲线,见图4。反应至22 d时,C/N为15、20、25实验组的多杀菌素A去除率分别为81.71%、87.16%、79.13%,多杀菌素D的降解率分别为84.40%、84.64%、78.59%。其中,B组其内抗生素残留量最快,多杀菌素A 26.30 mg/kg,多杀菌素D 2.73 mg/kg。到42 d时,3组的多杀菌素降解率均达90%以上。另外,图4可知,在0—10 d内,3组多杀菌素去除率均可达70%以上,这表明多杀菌素的降解主要发生在升温期和高温期。以上结果表明,通过堆肥进行多杀菌素的降解是可行的。
-
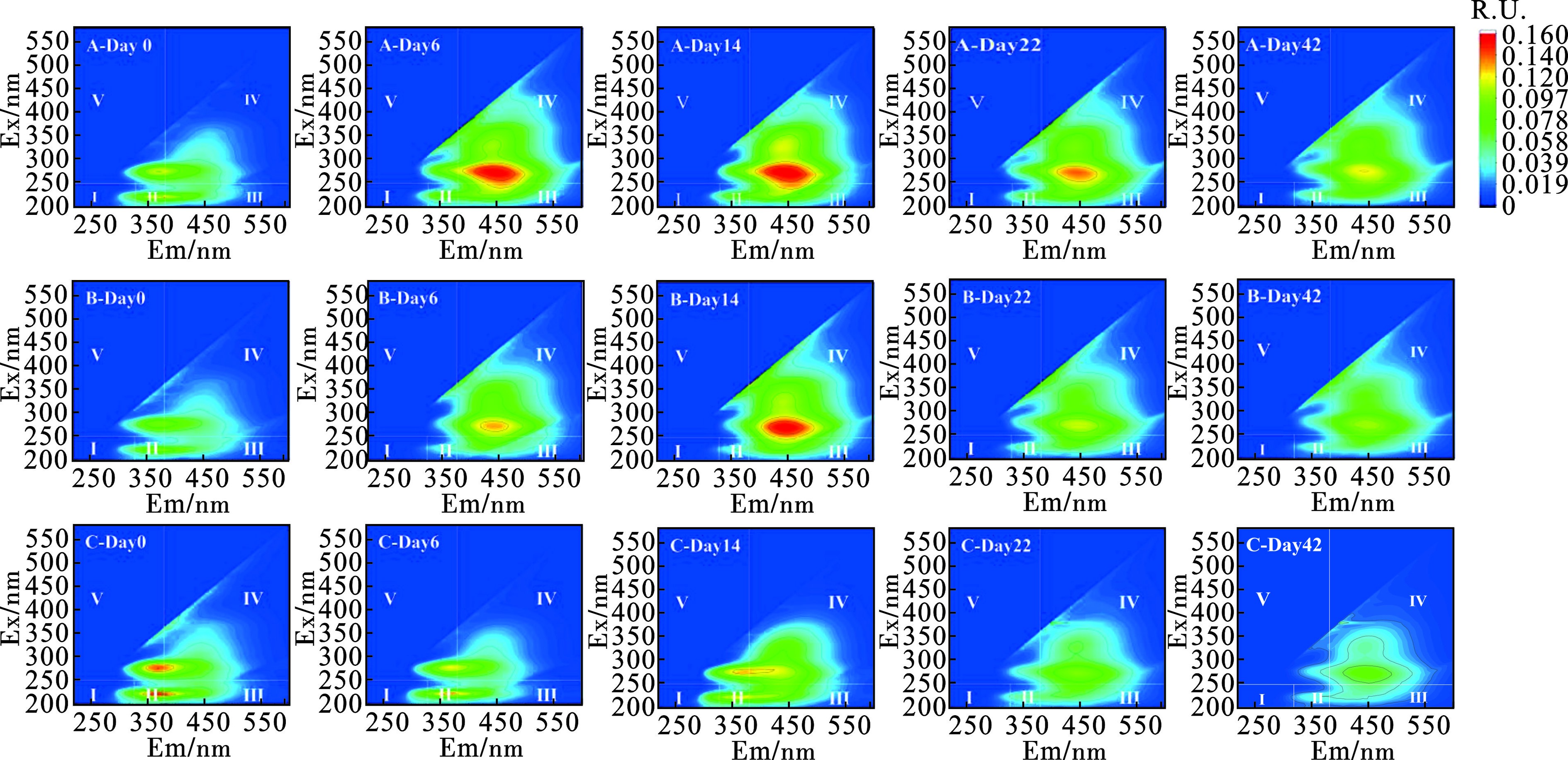
三维荧光光谱(3D-EEMs)可以直观地展示与微生物活动相关的蛋白质类物质的光谱信息及堆肥过程中形成的腐殖质类物质的结构信息。在数据处理过程中,将空白样品数据扣除并进行拉曼归一化处理,见图5。在堆肥过程中Peak Ⅰ(225/370 nm Ex/Em)代表的色氨酸类物质峰荧光强度下降,说明好氧堆肥过程中类蛋白物质逐渐发生降解,到堆肥后期基本降解完全。Peak Ⅱ(275/370 nm Ex/Em)表示堆肥初期就存在溶解性微生物代谢产物,这是因为菌渣是由微生物经发酵作用而产生的,菌渣内会残留一定的微生物代谢产物。Peak Ⅲ和Peak Ⅳ在高温期同时出现,Ex/Em波长分别为275/449和325/424 nm。Peak Ⅲ和Ⅳ均和腐殖酸类物质相关。LV et al[21]在牛粪蚯蚓堆肥过程中的第60 d检测到与腐殖酸相关的320/416 nm Ex/Em波长对的峰值Peak Ⅲ和Peak Ⅳ的出现表明在堆肥过程中产生了腐殖质的积累,标志着堆体不断腐熟,趋于稳定,见表3。
-
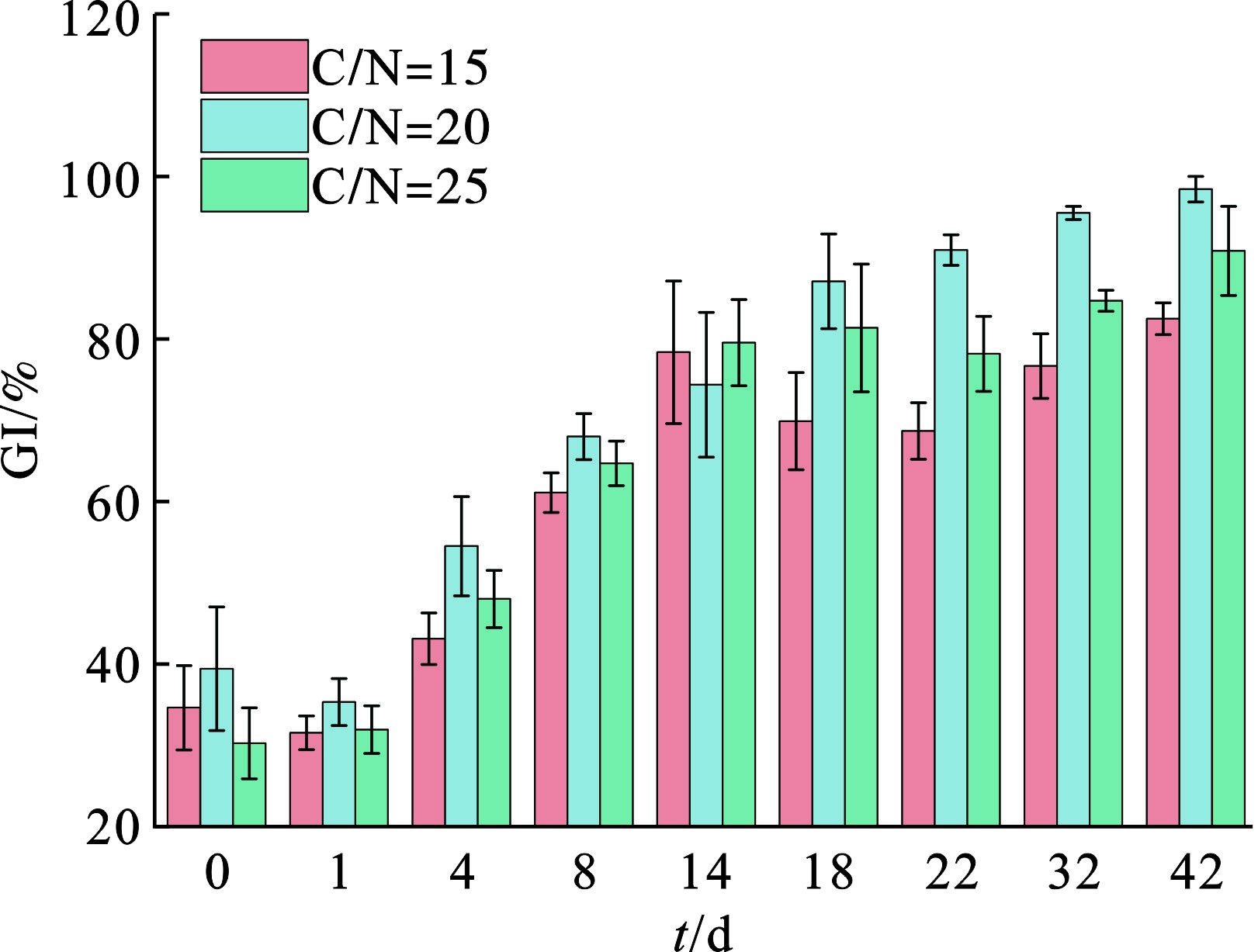
种子发芽指数(GI)综合反映生物性安全,广泛用于堆肥中物料的植物毒性评价,GI受到多种因素的影响,包括残留物浓度、重金属离子浓度等。图6可知,在堆肥初期,3个堆体的GI相对较低,表明植物毒性相对较高。随着时间推进,GI均有所提升,表明植物毒性大幅降低。第42 d C/N=20、25的GI分别为98.29%和90.70%,而C/N=15的GI为82.34%。这一现象的原因可能与C/N=15组含盐量较大有关[22]。总体上,GI在高温期实现较大提升,原因在于在高温阶段,氨气固定和挥发,有机质分解,毒性化合物降解,堆体开始稳定。通常认为好氧堆肥处理GI>80%说明产品无植物毒性且达到腐熟状态,结果表明3组均达到了GI层面的腐熟状态,较为理想。
-
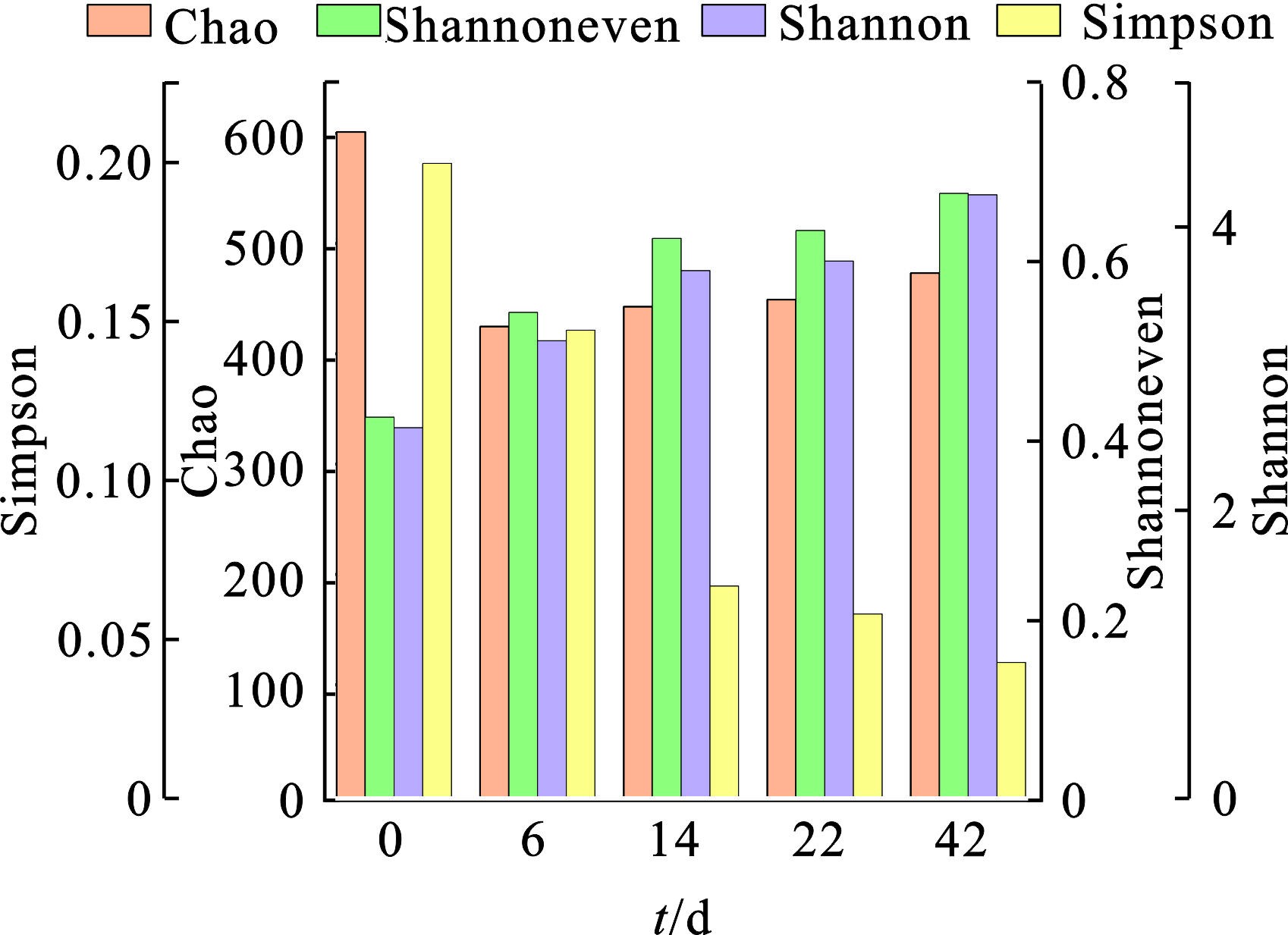
作为一个生物反应过程,微生物群落结构的变化会直接影响堆肥过程中的物质转化以及堆体的稳定性。结合2.1.1至2.1.4节数据分析,我们初步判断B组(C/N=20)堆肥较为理想,并对B组0、6、14、22 d的样品进行16S rRNA高通量测序。Alpha多样性可以反映微生物群落的丰度和多样性。C/N=15、20、25 3组的Alpha指数变化见图7。在整个堆肥过程中,Shannon指数和Shannoneven指数呈上升趋势,Simpson指数呈下降趋势,表明细菌多样性有所提高,同时群落分布均匀度有所提升。而Chao指数先下降后上升,说明群落丰富度先下降后上升。原因可能在于堆肥使用的EM菌种较为复杂,其中存在一些不适于堆肥条件下生存的微生物种类。综合C/N=20组的微生物群落分析,可初步判定其达到理想的堆肥效果。
-
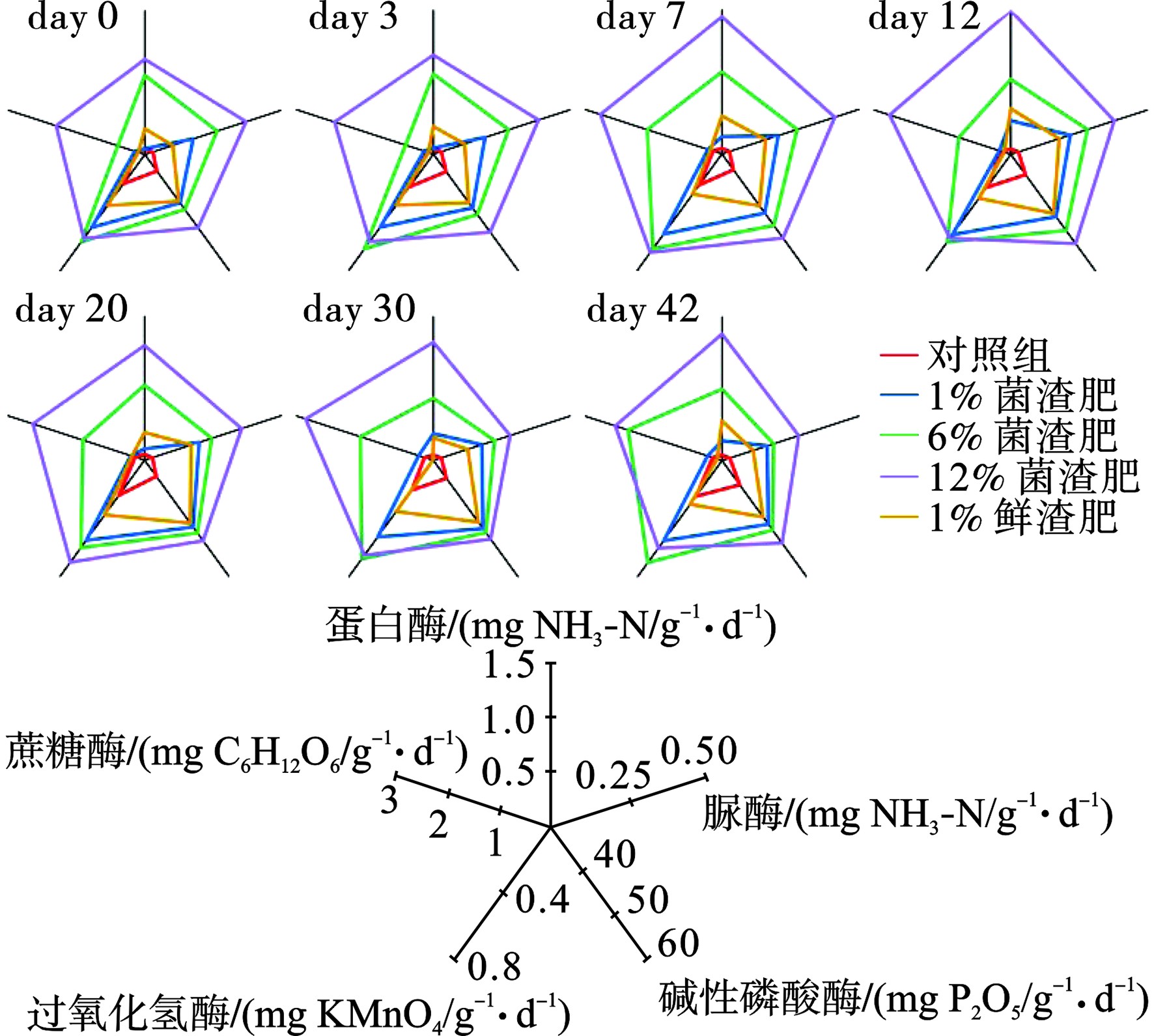
土壤酶作为一类在土壤中广泛存在的酶类物质,能够催化包括有机质分解、养分循环等过程在内的土壤中的化学反应。土壤酶活性可以用来衡量土壤生态系统功能,具有重要的意义和作用[23]。通过分析土壤酶活性的变化可以反映出菌渣肥对土壤生物活动、土壤物质循环以及土壤生物区系的影响,进而明确对土壤肥力的作用效果。将各检测结果以雷达图的形式展示,各组分所占据面积即可表示土壤酶活性的整体水平,见图8。菌渣肥的投加促进了土壤酶活性,反观鲜菌渣,其投加对酶活促进效果较弱。
磷酸酶在有机磷矿化中起着重要作用,其通过水解有机分子中磷酸基团的磷酸酯键来催化磷酸盐的释放,可表征土壤的供磷能力。图8可知,在模拟前期,磷酸酶的活性随着施肥量的增加而增强,这可能是因为前期高浓度菌渣肥对土壤微生物活性有一定的促进作用,导致土壤酶活性升高;在土壤模拟实验中,不同施肥浓度下的磷酸酶活性均高于空白值且,随着施肥浓度的增加而增加。这与菌渣肥中大量有机质和营养物质的供应有关。
土壤脲酶能促进土壤中有机化合物尿素分子酰胺碳氮键的水解,其产物是植物最重要的土壤速效氮,在氮肥利用和土壤氮素代谢方面有着重要的意义[24]。不同施肥比条件下菌渣肥均显著提高了土壤脲酶的活性,且随着施肥比的提升有进一步的增强。在第12 d时,脲酶活性达到峰值,而后随着含氮有机物的消耗,脲酶活性逐渐降低,并在第30 d后趋于稳定。培养结束后,施加1%、6%、12%菌渣肥土壤的脲酶活性则显著高于菌渣施肥土壤的脲酶活性,表明多杀菌素菌渣肥增强土壤脲酶活性效果优于菌渣效果。
-
通过考察多杀菌素在土壤中的降解规律,分析了菌渣肥土壤施用过程中多杀菌素在土壤中的稳定性及累积的可能性。土壤模拟施肥实验过程中各土壤样品中多杀菌素的含量变化,见表4。在鲜菌渣施入土壤后,显著提高了土壤中多杀菌素的含量。随着培养时间的延长,多杀菌素的含量逐渐降低,分别经过12、20、30 d后,鲜菌渣施加比例为1%、6%的土壤中的多杀菌素已低于检测值,表明多杀菌素在土壤中难以稳定存在,可以被有效降解,不存在累积的风险。而在多杀菌素菌渣肥施入土壤后,在各时期的土壤中多杀菌素残留量均远高于菌渣肥组,表明经过无害化处理后,施肥过程中多杀菌素剩余量大大减少。另外,在《食品安全国家标准 食品中农药最大残留限量:GB 2763—2021》中,多杀菌素在坚果和马铃薯中的最大残留量分别为10和70 μg/kg,这也说明了该结果的安全性。
-
土壤细菌群落结构对土壤理化性质以及物质循环的意义重大:(1)土壤细菌群落结构与土壤养分的循环和分布密切相关。一些细菌可以将氮、磷等元素固定,促进土壤养分循环,为植物的生长提供了必要的物质条件。同时,细菌还可以促进有机质的分解,释放出养分为植物利用。(2)土壤细菌群落结构对土壤结构的稳定作用明显。某些细胞产生的胞外多糖物质能够促进土壤结构的形成和稳定,提高土壤的水分保持能力和抗侵蚀能力。(3)土壤细菌群落结构对植物生长影响重大,除了提供营养物质徐进植物生长之外,细菌还可以产生包括植物生长素在内的生长因子,促进植物生长发育,提升免疫力和抗逆性。
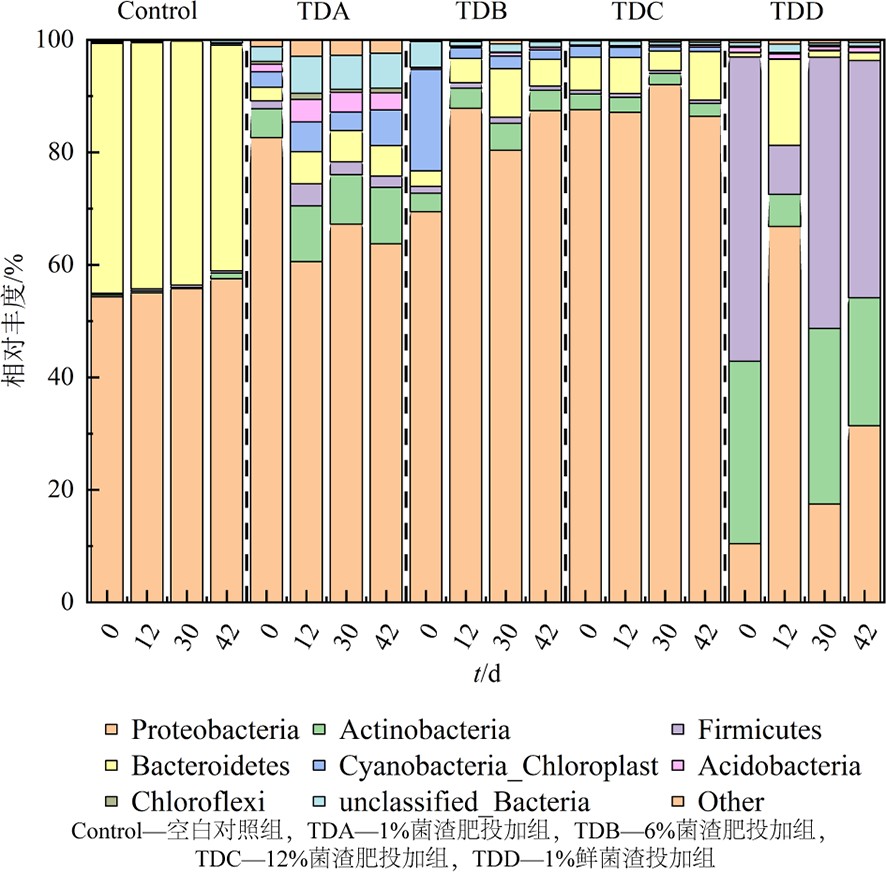
空白对照组、1%、6%、12%菌渣肥投加组和1%菌渣投加组土壤模拟过程中门水平上相对丰度变化,见图9。在对照组中,变形菌(Proteobacteria)和拟杆菌(Bacteroidetes)占比极大,在42 d占比分别为57.52%和40.09%,而菌渣投加组则相对更加均匀,土壤质量得到明显提升。放线菌(Actinobacteria)、厚壁菌门(Firmicutes)、酸杆菌门(Acidobacteria)的相对丰度有明显的提。另外,1%鲜菌渣投加组相对丰度变化更大,放线菌(Actinobacteria)、厚壁菌门(Firmicutes)相对丰度提升的原因并非因为菌种数量有所上升,而是因为变形菌门(Proteobacteria)等原本在土壤中的占比较大的细菌数量大幅下降所导致的。因此,结合以上分析可以认为下鲜菌渣投加组的土壤细菌群落结构在不同程度上得到了优化,而鲜菌渣的影响结果相反,不利于细菌的生长。
-
本文通过好氧堆肥工艺探究了多杀菌素菌渣的无害化与稳定化的可行性,同时进一步分析了多杀菌素菌渣肥料对土壤性能的影响效果。(1)好氧堆肥结果表明,C/N=20的条件下对多杀菌素进行好氧堆肥处理,堆体pH、EC等理化指标达适宜范围,三维荧光数据表明堆体已达腐熟状态,多杀菌素降解率达90%以上;(2)土壤模拟实验结果表明,土壤酶活水平明显提升,多杀菌素残留低于检测水平,而微生物多样性总体也呈上升趋势。总体而言,好氧堆肥工艺可以有效去除菌渣中的残留多杀菌素,并且制备而成的多杀菌素菌渣肥能够改善土壤肥力。后续仍应进行相关田间种植试验,以进一步探究多杀菌素菌渣无害化处理的可行性。
多杀菌素菌渣无害化处理与肥料化利用研究
Experimental study on composting for fertilization utilization and harmless treatment of spinosad residues
-
摘要: 针对抗生素发酵生产残余菌渣所引发的环境危害问题,以多杀菌素菌渣为研究对象,通过好氧堆肥工艺探究其资源化的可行性,设定不同的初始C/N,分析了反应过程中堆体pH、电导率(EC)、三维荧光图谱(3D-EEMs)等指标变化规律,并用初始C/N=20组的堆肥样品进行土壤模拟试验,以评估多杀菌素菌渣肥在使用过程中对土壤环境和微生物的影响。堆肥实验结果表明:多杀菌素去除率达到90%以上,堆体理化指标均处于适宜范围;3D-EEMs表明:堆肥过程中色氨酸类物质被微生物充分利用,腐殖酸类物质累积,堆体达到较好的腐熟效果;土壤模拟实验结果表明:1%和6%的抗生素残留低于检测限(多杀菌素A 2.0 µg/kg,多杀菌素D 1.5 µg/kg)。此外,投加了菌渣肥使土壤的微生物多样性提升。以上结果表明,多杀菌素菌渣经过堆肥化处理有助于改善土壤性能。Abstract: To address the environmental hazards caused by fermentation residues from antibiotic fermentation production, the feasibility of resource utilization of spinosad fermentation residue (SFR) by aerobic composting process with different initial C/N was investigated with the different pH, conductivity, three-dimensional fluorescence and other indicators. Soil simulation tests were conducted subsequently with the product of the initial C/N=20 group to evaluate the impact of composted SFR on the soil environment and soil microorganisms. The results of composting experiments showed that the removal rate of spinosad reached more than 90%, and the physical and chemical indexes of the pile were in the suitable range. The three-dimensional fluorescence pattern showed that tryptophan substances were fully utilized by microorganisms during the composting process, humic acid substances were accumulated, and the pile achieved a good maturation effect. The results of soil simulation experiments showed that composted SFR at dosages of 1% and 6% were below the detection limits (2.0 µg/kg for spinosad A and 1.5 µg/kg for spinosad D). In addition, the overall microbial diversity of the soils fertilized with composted SFR was increased. These results indicated that composite treatment of SFR could improve various soil properties.
-
Key words:
- spinosad /
- fermentation residue /
- aerobic composting /
- safety assessment /
- soil utilization
-
多杀菌素是一种发酵产生的无公害农药[1],属于农用抗生素,因其杀虫效率高,在农业上的应用前景广阔[2]。多杀菌素菌渣是抗生素发酵提取后残留菌丝体和培养基的混合物,若直接进入环境可能造成潜在环境危害。因此,多杀菌素菌渣在2008年被列入中国的危险废物管理清单。考虑到菌渣有机物含量丰富[3],有效地处理多杀菌素菌渣以实现无害化和资源化具有巨大潜力。
抗生素发酵菌渣无害化方法很多,包括微波分解[4-5]、热水解[6]、高级氧化工艺[7]、厌氧堆肥[8]和好氧堆肥[9],其中好氧堆肥处理以其低成本、技术成熟和可推广性受到企业的青睐。LIU et al[10]将庆大霉素残留物和洛伐他汀发酵残留物混合堆肥,实现了庆大霉素最大降解率96.7%。YANG et al[11]将肉鸡粪便堆肥42 d,去除粪便中75.4%的诺氟沙星。因此,抗生素残留物的肥料化是一个很有前景的资源利用途径。目前,暂无关于多杀菌素菌渣无害化和资源化的相关研究。
本文通过好氧发酵对多杀菌素菌渣进行无害化与稳定化处理,系统研究其堆肥化效能。通过土壤模拟试验,从土壤中多杀菌素残留降、土壤理化性质及微生物活性与多样性等多层面分析多杀菌素菌渣的肥料化应用效果,以期为多杀菌素菌渣的无害化与资源化提供理论与技术支持。
1. 材料与方法
1.1 实验材料
实验用多杀菌素菌渣取自山东省某生物制药公司,经脱水处理,含水率(5.61±0.71)%。菌渣样品采用已消毒的塑料桶收集,并在运回实验室后立刻放置于4 ℃冰箱内冷藏储存。实验用土壤取自江苏省某农场纯天然田园土。在进行土壤模拟实验前,已将土壤阴干14 d,并过2 mm筛网以去除石块和植物根系。实验用菌渣和土壤的理化性质,见表1和表2。
表 1 多杀菌素菌渣的理化性质Table 1. Physicochemical properties of SFR参数 数值 pH 7.93±0.02 含水率/% 5.61±0.71 有机质/% 41.52±3.15 多杀菌素A/ mg·kg−1 2.19×103±238 多杀菌素D/ mg·kg−1 2.89×102±196 P/%(by P2O5) 1.02±0.09 K/%(by K2O) 0.25±0.05 As/mg·kg−1 0.41±0.04 Cd/mg·kg−1 0.048±0.001 Cr/mg·kg−1 48.61±0.12 Hg/mg·kg−1 0.39±0.17 Pb/mg·kg−1 15.47±3.84 C/N 6.45±0.66 1.2 好氧堆肥试验设计
实验取稻草秸秆作为碳源,控制堆体C/N分别约为15、20、25进行堆肥,控制堆体含水率控制在60%。考虑到多杀菌素菌渣含有的微生物种类较为单一,单独堆肥难达理想效果,故投加约2%的高效菌(即EM菌,属混合菌,含光合菌、乳酸菌、酵母菌等)。同时堆体内投加约5%的腐殖酸,一方面能为堆体提供碳源,另一方面也能减少堆肥过程中的氮素损失。堆体体积约为15 L。机械曝气量为0.4 L/(min·kg),采用间歇式曝气法,曝气2 h,暂停1 h。与此同时,每天进行人工翻堆保证有机质能够被微生物充分利用。堆肥一共42 d,取样日期分别为0,1,2,3,4,6,8,10,14,18,22,32,42 d,每次取样均从堆体内上、中、下以及发酵罐相应截面的中心、四周均匀取样50 g,取出样品装袋标记后立刻放进−20 ℃的冰箱内进行冷冻保存,以备后续指标检测。发酵设备见图1。
表 2 实验土壤的理化性质Table 2. Physicochemical properties of experimental soil指标 数值 pH 7.2±0.1 电导率/μS·cm−1 155.2±2.1 含水率/% 12.05±0.14 土壤有机质含量/% 1.6±0.1 总磷含量/mg·kg−1 15.5±0.1 总钾含量/mg·kg−1 100.9±0.7 1.3 土壤模拟试验设计
本研究通过实验室土壤模拟试验法,研究了菌渣有机肥对土壤性能的影响。参考文献[12],本文设置1%、6%、12%的质量比,并另设空白组和1%鲜菌渣投加组进行对比。每组土壤模拟实验使用土壤量约为1 kg,设置3组平行对照,放置于直径17.5 cm、高16 cm的花盆中培养,定期浇水保证土壤湿度约为10%。在0、3、7、12、20、30、42 d取样(约50 g)。每组样品分为2部分,一部分储存于−20 ℃用于检测残留多杀菌素残留量,另一部分储存于4 ℃以检测相关理化性质。
1.4 分析方法
pH和EC的检测分别参考《土壤检测 第2部分:土壤pH的测定:NY/T 1121.2—2006》和《土壤 电导率的测点 电极法:HJ 802—2016》。堆体三维荧光光谱检测:使用质量比1∶10的超纯水提取土壤样品5 g,在水平振动器中震荡24 h,10 000 r/min离心20 min后过0.45 μm滤膜,用于三维激发发射矩阵(3D-EEMs)荧光光谱分析,检测数据进行拉曼归一化[13]。土壤酶活采用比色法进行测定[14]。利用生物工业微生物测序仪对细菌群落进行16S基因测序。GI的检测参考了《有机肥料:NY/T 525—2021》。
抗生素残留检验检测方法:(1)流动相为甲醇:1%乙酸铵=7∶3(V/V),流速0.3 mL/min;(2)使用C18柱色谱柱,柱温30 ℃,进样量10 μL;(3)质谱选择采集多级反应监测模式,电喷雾离子源电压4.5 kV,雾化气流流速700 L/h,锥孔气流流速35 L/h;(4)多杀菌素A母离子质荷比(m/z)732.5,子离子质荷比(m/z)142.2;多杀菌素D母离子质荷比(m/z)746.5,子离子质荷比(m/z)142.2。
预处理方法:称取样品2.0 g(精确至0.01 g)于50 mL离心管中,加入饱和氯化钙溶液10 mL,同时再加入2.0 mL乙酸乙酯,将混合液摇匀后置于多管涡旋仪上以2 000 r/min的转速充分振荡20 min,4 000 r/min离心10 min之后,取1.0 mL乙酸乙酯相液体在氮吹仪上吹干,之后使用1 mL流动相复溶,过0.22 µm滤膜待测。
2. 结果与分析
2.1 堆肥参数分析
2.1.1 理化参数变化
温度反映出好氧堆肥中微生物的新陈代谢水平,是判定堆肥成品达到无害化的重要指标。图2可知,3组实验的初始温度基本一致,在0—3 d内从室温快速升至50 ℃左右。在此阶段,水溶性糖类、淀粉类等易降解的可溶性有机物及大分子有机质被微生物利用,并产生热量上的累积。在4—10 d,堆体温度维持在50 ℃以上,嗜热微生物大量繁殖,有效地分解大分子蛋白质、纤维素、木质素等在升温过程难分解的有机物。到了降温期(11—42 d),嗜温性微生物开始进一步分解残余难降解有机物,温度进一步降低至室温。
pH是影响体系中微生物活性和堆肥性能的重要参数,大多数微生物最适宜生长代谢的pH环境为中性或弱碱性[15],pH过高或过低均会影响到堆肥腐熟的进程。图3可知,在堆肥过程中A、B、C 3组的初始pH均为6.8左右,这主要是因为添加物料中含有一定量的腐殖酸,中和了菌渣等物料本身的碱度。在0—3 d内堆体pH迅速上升,大量含氮有机物被微生物利用产生氨气[16]以及小分子有机酸的降解[17]。在10—22 d进入降温期,功能微生物群落发生转变,堆体内有机物被进一步分解,小分子有机酸和部分盐基离子被合成大分子腐殖质(胡敏酸)[18],3组的pH缓慢下降并趋于稳定,这一点与图3中电导率后期呈现下降趋势相吻合。堆肥结束后,3组的pH稳定在堆肥适宜的7.5~8.5[19],EC稳定在<3 000 μS/cm范围内[20]。
2.1.2 多杀菌素残留量分析
在多杀菌素菌渣好氧堆肥过程中,多杀菌素残留量是评价菌渣无害化水平的重要指标。多杀菌素包含2种成分,即多杀菌素A和多杀菌素D。在C/N为15、20、25条件下,多杀菌素A和D残留量的变化曲线,见图4。反应至22 d时,C/N为15、20、25实验组的多杀菌素A去除率分别为81.71%、87.16%、79.13%,多杀菌素D的降解率分别为84.40%、84.64%、78.59%。其中,B组其内抗生素残留量最快,多杀菌素A 26.30 mg/kg,多杀菌素D 2.73 mg/kg。到42 d时,3组的多杀菌素降解率均达90%以上。另外,图4可知,在0—10 d内,3组多杀菌素去除率均可达70%以上,这表明多杀菌素的降解主要发生在升温期和高温期。以上结果表明,通过堆肥进行多杀菌素的降解是可行的。
2.1.3 堆肥过程的三维荧光光谱分析
三维荧光光谱(3D-EEMs)可以直观地展示与微生物活动相关的蛋白质类物质的光谱信息及堆肥过程中形成的腐殖质类物质的结构信息。在数据处理过程中,将空白样品数据扣除并进行拉曼归一化处理,见图5。在堆肥过程中Peak Ⅰ(225/370 nm Ex/Em)代表的色氨酸类物质峰荧光强度下降,说明好氧堆肥过程中类蛋白物质逐渐发生降解,到堆肥后期基本降解完全。Peak Ⅱ(275/370 nm Ex/Em)表示堆肥初期就存在溶解性微生物代谢产物,这是因为菌渣是由微生物经发酵作用而产生的,菌渣内会残留一定的微生物代谢产物。Peak Ⅲ和Peak Ⅳ在高温期同时出现,Ex/Em波长分别为275/449和325/424 nm。Peak Ⅲ和Ⅳ均和腐殖酸类物质相关。LV et al[21]在牛粪蚯蚓堆肥过程中的第60 d检测到与腐殖酸相关的320/416 nm Ex/Em波长对的峰值Peak Ⅲ和Peak Ⅳ的出现表明在堆肥过程中产生了腐殖质的积累,标志着堆体不断腐熟,趋于稳定,见表3。
表 3 荧光区域与对应物质类别Table 3. Fluorescence regions and corresponding substance classes荧光区域 对应物质类别 Ex/nm Em/nm Ⅰ 酪氨酸类 200~250 250~330 Ⅱ 色氨酸类 200~250 330~380 Ⅲ 富里酸类 200~250 380~500 Ⅳ 腐殖酸类 250~500 380~500 Ⅴ 溶解性微生物代谢产物 250~500 250~380 2.1.4 种子发芽指数变化
种子发芽指数(GI)综合反映生物性安全,广泛用于堆肥中物料的植物毒性评价,GI受到多种因素的影响,包括残留物浓度、重金属离子浓度等。图6可知,在堆肥初期,3个堆体的GI相对较低,表明植物毒性相对较高。随着时间推进,GI均有所提升,表明植物毒性大幅降低。第42 d C/N=20、25的GI分别为98.29%和90.70%,而C/N=15的GI为82.34%。这一现象的原因可能与C/N=15组含盐量较大有关[22]。总体上,GI在高温期实现较大提升,原因在于在高温阶段,氨气固定和挥发,有机质分解,毒性化合物降解,堆体开始稳定。通常认为好氧堆肥处理GI>80%说明产品无植物毒性且达到腐熟状态,结果表明3组均达到了GI层面的腐熟状态,较为理想。
2.1.5 Alpha指数变化
作为一个生物反应过程,微生物群落结构的变化会直接影响堆肥过程中的物质转化以及堆体的稳定性。结合2.1.1至2.1.4节数据分析,我们初步判断B组(C/N=20)堆肥较为理想,并对B组0、6、14、22 d的样品进行16S rRNA高通量测序。Alpha多样性可以反映微生物群落的丰度和多样性。C/N=15、20、25 3组的Alpha指数变化见图7。在整个堆肥过程中,Shannon指数和Shannoneven指数呈上升趋势,Simpson指数呈下降趋势,表明细菌多样性有所提高,同时群落分布均匀度有所提升。而Chao指数先下降后上升,说明群落丰富度先下降后上升。原因可能在于堆肥使用的EM菌种较为复杂,其中存在一些不适于堆肥条件下生存的微生物种类。综合C/N=20组的微生物群落分析,可初步判定其达到理想的堆肥效果。
2.2 菌渣肥对土壤性能的影响
2.2.1 土壤酶活性变化
土壤酶作为一类在土壤中广泛存在的酶类物质,能够催化包括有机质分解、养分循环等过程在内的土壤中的化学反应。土壤酶活性可以用来衡量土壤生态系统功能,具有重要的意义和作用[23]。通过分析土壤酶活性的变化可以反映出菌渣肥对土壤生物活动、土壤物质循环以及土壤生物区系的影响,进而明确对土壤肥力的作用效果。将各检测结果以雷达图的形式展示,各组分所占据面积即可表示土壤酶活性的整体水平,见图8。菌渣肥的投加促进了土壤酶活性,反观鲜菌渣,其投加对酶活促进效果较弱。
磷酸酶在有机磷矿化中起着重要作用,其通过水解有机分子中磷酸基团的磷酸酯键来催化磷酸盐的释放,可表征土壤的供磷能力。图8可知,在模拟前期,磷酸酶的活性随着施肥量的增加而增强,这可能是因为前期高浓度菌渣肥对土壤微生物活性有一定的促进作用,导致土壤酶活性升高;在土壤模拟实验中,不同施肥浓度下的磷酸酶活性均高于空白值且,随着施肥浓度的增加而增加。这与菌渣肥中大量有机质和营养物质的供应有关。
土壤脲酶能促进土壤中有机化合物尿素分子酰胺碳氮键的水解,其产物是植物最重要的土壤速效氮,在氮肥利用和土壤氮素代谢方面有着重要的意义[24]。不同施肥比条件下菌渣肥均显著提高了土壤脲酶的活性,且随着施肥比的提升有进一步的增强。在第12 d时,脲酶活性达到峰值,而后随着含氮有机物的消耗,脲酶活性逐渐降低,并在第30 d后趋于稳定。培养结束后,施加1%、6%、12%菌渣肥土壤的脲酶活性则显著高于菌渣施肥土壤的脲酶活性,表明多杀菌素菌渣肥增强土壤脲酶活性效果优于菌渣效果。
2.2.2 多杀菌素在土壤残留变化
通过考察多杀菌素在土壤中的降解规律,分析了菌渣肥土壤施用过程中多杀菌素在土壤中的稳定性及累积的可能性。土壤模拟施肥实验过程中各土壤样品中多杀菌素的含量变化,见表4。在鲜菌渣施入土壤后,显著提高了土壤中多杀菌素的含量。随着培养时间的延长,多杀菌素的含量逐渐降低,分别经过12、20、30 d后,鲜菌渣施加比例为1%、6%的土壤中的多杀菌素已低于检测值,表明多杀菌素在土壤中难以稳定存在,可以被有效降解,不存在累积的风险。而在多杀菌素菌渣肥施入土壤后,在各时期的土壤中多杀菌素残留量均远高于菌渣肥组,表明经过无害化处理后,施肥过程中多杀菌素剩余量大大减少。另外,在《食品安全国家标准 食品中农药最大残留限量:GB 2763—2021》中,多杀菌素在坚果和马铃薯中的最大残留量分别为10和70 μg/kg,这也说明了该结果的安全性。
表 4 多杀菌素A和D在土壤残留变化Table 4. Changes in spinosad residues in soilt/d CK 1%菌渣肥 6%菌渣肥 12%菌渣肥 1%鲜菌渣 A D A D A D A D A D 0 — — 2.24 — 6.74 — 12.53 — 1 863.93 402.65 3 — — — — 4.00 — 7.32 — 1 443.80 347.38 7 — — — — 2.35 — 5.84 — 741.23 171.73 12 — — — — 2.68 — 3.69 — 725.64 156.42 20 — — — — 2.17 — 3.16 — 705.47 141.42 30 — — — — 2.72 — 3.73 — 593.68 137.01 42 — — — — — — 3.42 — 499.07 96.44 注:多杀菌素A检测限:2.0 μg·kg−1;多杀菌素D检测限:1.5 μg·kg−1;“—”表示低于检测限。 2.2.3 对土壤细菌群落结构的影响
土壤细菌群落结构对土壤理化性质以及物质循环的意义重大:(1)土壤细菌群落结构与土壤养分的循环和分布密切相关。一些细菌可以将氮、磷等元素固定,促进土壤养分循环,为植物的生长提供了必要的物质条件。同时,细菌还可以促进有机质的分解,释放出养分为植物利用。(2)土壤细菌群落结构对土壤结构的稳定作用明显。某些细胞产生的胞外多糖物质能够促进土壤结构的形成和稳定,提高土壤的水分保持能力和抗侵蚀能力。(3)土壤细菌群落结构对植物生长影响重大,除了提供营养物质徐进植物生长之外,细菌还可以产生包括植物生长素在内的生长因子,促进植物生长发育,提升免疫力和抗逆性。
空白对照组、1%、6%、12%菌渣肥投加组和1%菌渣投加组土壤模拟过程中门水平上相对丰度变化,见图9。在对照组中,变形菌(Proteobacteria)和拟杆菌(Bacteroidetes)占比极大,在42 d占比分别为57.52%和40.09%,而菌渣投加组则相对更加均匀,土壤质量得到明显提升。放线菌(Actinobacteria)、厚壁菌门(Firmicutes)、酸杆菌门(Acidobacteria)的相对丰度有明显的提。另外,1%鲜菌渣投加组相对丰度变化更大,放线菌(Actinobacteria)、厚壁菌门(Firmicutes)相对丰度提升的原因并非因为菌种数量有所上升,而是因为变形菌门(Proteobacteria)等原本在土壤中的占比较大的细菌数量大幅下降所导致的。因此,结合以上分析可以认为下鲜菌渣投加组的土壤细菌群落结构在不同程度上得到了优化,而鲜菌渣的影响结果相反,不利于细菌的生长。
3. 结论
本文通过好氧堆肥工艺探究了多杀菌素菌渣的无害化与稳定化的可行性,同时进一步分析了多杀菌素菌渣肥料对土壤性能的影响效果。(1)好氧堆肥结果表明,C/N=20的条件下对多杀菌素进行好氧堆肥处理,堆体pH、EC等理化指标达适宜范围,三维荧光数据表明堆体已达腐熟状态,多杀菌素降解率达90%以上;(2)土壤模拟实验结果表明,土壤酶活水平明显提升,多杀菌素残留低于检测水平,而微生物多样性总体也呈上升趋势。总体而言,好氧堆肥工艺可以有效去除菌渣中的残留多杀菌素,并且制备而成的多杀菌素菌渣肥能够改善土壤肥力。后续仍应进行相关田间种植试验,以进一步探究多杀菌素菌渣无害化处理的可行性。
-
表 1 多杀菌素菌渣的理化性质
Table 1. Physicochemical properties of SFR
参数 数值 pH 7.93±0.02 含水率/% 5.61±0.71 有机质/% 41.52±3.15 多杀菌素A/ mg·kg−1 2.19×103±238 多杀菌素D/ mg·kg−1 2.89×102±196 P/%(by P2O5) 1.02±0.09 K/%(by K2O) 0.25±0.05 As/mg·kg−1 0.41±0.04 Cd/mg·kg−1 0.048±0.001 Cr/mg·kg−1 48.61±0.12 Hg/mg·kg−1 0.39±0.17 Pb/mg·kg−1 15.47±3.84 C/N 6.45±0.66 表 2 实验土壤的理化性质
Table 2. Physicochemical properties of experimental soil
指标 数值 pH 7.2±0.1 电导率/μS·cm−1 155.2±2.1 含水率/% 12.05±0.14 土壤有机质含量/% 1.6±0.1 总磷含量/mg·kg−1 15.5±0.1 总钾含量/mg·kg−1 100.9±0.7 表 3 荧光区域与对应物质类别
Table 3. Fluorescence regions and corresponding substance classes
荧光区域 对应物质类别 Ex/nm Em/nm Ⅰ 酪氨酸类 200~250 250~330 Ⅱ 色氨酸类 200~250 330~380 Ⅲ 富里酸类 200~250 380~500 Ⅳ 腐殖酸类 250~500 380~500 Ⅴ 溶解性微生物代谢产物 250~500 250~380 表 4 多杀菌素A和D在土壤残留变化
Table 4. Changes in spinosad residues in soil
t/d CK 1%菌渣肥 6%菌渣肥 12%菌渣肥 1%鲜菌渣 A D A D A D A D A D 0 — — 2.24 — 6.74 — 12.53 — 1 863.93 402.65 3 — — — — 4.00 — 7.32 — 1 443.80 347.38 7 — — — — 2.35 — 5.84 — 741.23 171.73 12 — — — — 2.68 — 3.69 — 725.64 156.42 20 — — — — 2.17 — 3.16 — 705.47 141.42 30 — — — — 2.72 — 3.73 — 593.68 137.01 42 — — — — — — 3.42 — 499.07 96.44 注:多杀菌素A检测限:2.0 μg·kg−1;多杀菌素D检测限:1.5 μg·kg−1;“—”表示低于检测限。 -
[1] MORETTI E A, TAYLOR A G, WICKINGS K, et al. Insights into How Spinosad Seed Treatment Protects Onion From Onion Maggot (Diptera: Anthomyiidae)[J]. Journal of Economic Entomology, 2021, 114(2): 694 − 701. doi: 10.1093/jee/toaa332 [2] HERRON G A, GUNNING R V, COTTAGE E L, et al. Spinosad resistance, esterase isoenzymes and temporal synergism in Frankliniella occidentalis (Pergande) in Australia[J]. Pesticide Biochemistry and Physiology, 2014, 114: 32 − 37. doi: 10.1016/j.pestbp.2014.07.006 [3] ZHAO F, ZHANG C, YIN J, et al. Coupling of Spinosad Fermentation and Separation Process via Two-Step Macroporous Resin Adsorption Method[J]. Applied Biochemistry and Biotechnology, 2015, 176(8): 2144 − 2156. doi: 10.1007/s12010-015-1704-1 [4] CAI C, LIU H. Performance of microwave treatment for disintegration of cephalosporin mycelial dreg (CMD) and degradation of residual cephalosporin antibiotics[J]. Journal of Hazardous Materials, 2017, 331: 265 − 272. doi: 10.1016/j.jhazmat.2017.02.034 [5] JIANG M, SONG S, LIU H, et al. Effect of gentamicin mycelial residues disintegration by microwave-alkaline pretreatment on methane production and gentamicin degradation during anaerobic digestion[J]. Chemical Engineering Journal, 2021, 414(8): 128790. [6] ZHANG Y, LIU H, XIN Y, et al. Erythromycin degradation and ERY-resistant gene inactivation in erythromycin mycelial dreg by heat-activated persulfate oxidation[J]. Chemical Engineering Journal, 2019, 358: 1446 − 1453. doi: 10.1016/j.cej.2018.10.157 [7] GONG P, LIU H, CAI C, et al. Alkaline-thermally treated penicillin V mycelial residue amendment improved the soil properties without triggering antibiotic resistance[J]. Waste Management, 2020, 105: 248 − 255. doi: 10.1016/j.wasman.2020.02.008 [8] LI Y, ZHONG W, NING Z, et al. Effect of biochar on antibiotic resistance genes in the anaerobic digestion system of antibiotic mycelial dreg[J]. Bioresource Technology, 2022, 364: 128052. doi: 10.1016/j.biortech.2022.128052 [9] SHA G, ZHANG L, WU X, et al. Integrated meta-omics study on rapid tylosin removal mechanism and dynamics of antibiotic resistance genes during aerobic thermophilic fermentation of tylosin mycelial dregs[J]. Bioresource Technology, 2022, 351: 127010. doi: 10.1016/j.biortech.2022.127010 [10] LIU Y, FENG Y, CHENG D, et al. Gentamicin degradation and changes in fungal diversity and physicochemical properties during composting of gentamicin production residue [J]. Bioresource Technology, 2017: 905-912. [11] YANG B, MENG L, XUE N. Removal of five fluoroquinolone antibiotics during broiler manure composting[J]. Environmental Technology, 2018, 39(3): 373 − 381. doi: 10.1080/09593330.2017.1301568 [12] ZHANG Y, LIU H, DAI X, et al. Impact of application of heat-activated persulfate oxidation treated erythromycin fermentation residue as a soil amendment: Soil chemical properties and antibiotic resistance[J]. Science of The Total Environment, 2020, 736: 139668. doi: 10.1016/j.scitotenv.2020.139668 [13] STEDMON C A, BRO R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: a tutorial[J]. Limnology and Oceanography-Methods, 2008, 6: 572 − 579. doi: 10.4319/lom.2008.6.572 [14] 肖进红. 利普司他汀菌渣的无害化及菌渣有机肥对土壤性能的影响研究 [D].上海: 上海电力大学, 2022. [15] AWASTHI M K, PANDEY A K, KHAN J, et al. Evaluation of thermophilic fungal consortium for organic municipal solid waste composting[J]. Bioresource Technology, 2014, 168: 214 − 221. doi: 10.1016/j.biortech.2014.01.048 [16] WEI L, SHUTAO W, JIN Z, et al. Biochar influences the microbial community structure during tomato stalk composting with chicken manure[J]. Bioresource Technology, 2014, 154: 148 − 154. doi: 10.1016/j.biortech.2013.12.022 [17] EZZARIAI A, BARRET M, MERLINA G, et al. Evaluation of the antibiotics effects on the physical and chemical parameters during the co-composting of sewage sludge with palm wastes in a bioreactor[J]. Waste Management, 2017, 68: 388 − 397. doi: 10.1016/j.wasman.2017.06.036 [18] HUANG G F, WONG J W, WU Q T, et al. Effect of C/N on composting of pig manure with sawdust[J]. Waste Management, 2004, 24(8): 805 − 813. doi: 10.1016/j.wasman.2004.03.011 [19] AULINAS MASO M, BONMATI BLASI A. Evaluation of composting as a strategy for managing organic wastes from a municipal market in Nicaragua[J]. Bioresource Technology, 2008, 99(11): 5120 − 5124. doi: 10.1016/j.biortech.2007.09.083 [20] SOUMARE M, DEMEYER A, TACK F M G, et al. Chemical characteristics of Malian and Belgian solid waste composts[J]. Bioresource Technology, 2002, 81(2): 97 − 101. doi: 10.1016/S0960-8524(01)00125-0 [21] LV B, XING M, YANG J, et al. Chemical and spectroscopic characterization of water extractable organic matter during vermicomposting of cattle dung[J]. Bioresource Technology, 2013, 132: 320 − 326. doi: 10.1016/j.biortech.2013.01.006 [22] TIQUIA S M. Reduction of compost phytotoxicity during the process of decomposition[J]. Chemosphere, 2010, 79(5): 506 − 512. doi: 10.1016/j.chemosphere.2010.02.040 [23] XIAO J, WANG G, LIU H, et al. Application of composted lipstatin fermentation residue as organic fertilizer: Temporal changes in soil characteristics and bacterial community[J]. Chemosphere, 2022, 306: 135637. doi: 10.1016/j.chemosphere.2022.135637 [24] BURKE D J, WEINTRAUB M N, HEWINS C R, et al. Relationship between soil enzyme activities, nutrient cycling and soil fungal communities in a northern hardwood forest[J]. Soil Biology and Biochemistry, 2011, 43(4): 795 − 803. doi: 10.1016/j.soilbio.2010.12.014 -




 DownLoad:
DownLoad: