-
哌拉西林(piperacillin,PIP)属于青霉素类抗生素. 受现有污水处理设施去除率低[1]、污水直接排放等因素影响,PIP在环境中已有检出[2-3]. PIP的预测无效应浓度(Predicted No-effect Concentration, PNEC)较低(0.5 μg·L−1),表明其在很低的浓度水平就具有环境风险[4]. 因此,有必要了解PIP在水环境中的迁移转化规律.
水解是PIP在水环境中的主要代谢途径[5]. PIP的主要特征结构是分子内的β-内酰胺环与哌嗪结构,除此以外还含有—NH2、—COOH和—OH等. β-内酰胺环的水解是青霉素类抗生素在环境中水解的重要途径,即β-内酰胺环结构中的α-氨基和羧酸根基团之间的分子内酰胺化反应,其反应速率受温度、pH、金属离子等影响[5-10].
本研究测定PIP在不同温度、pH条件下浓度随时间的变化,计算了PIP的水解速率常数、半衰期与活化能. 结合液相色谱质谱分析,推导PIP在不同pH条件下的水解机理.
-
仪器:液相色谱(1260 infinity Ⅱ,安捷伦,美国),pH计(PHS-3C,上海越平,中国),恒温培养箱(SPX-250B,上海琅玕实验设备有限公司,中国),液相色谱质谱(Xevo-TQD,Waters,美国).
材料:邻苯二甲酸氢钾(GR,北京化工厂,中国),2-环己氨基乙磺酸(CHES)(≥99.5%,阿拉丁,中国),4-吗啉乙磺酸(MES)(≥99%,阿拉丁,中国),3-吗啉丙磺酸(MOPS)(≥99.5%,阿拉丁,中国),PIP(99.0%,坛墨质检,中国),盐酸(CMOS,国药集团,中国),氢氧化钠(AR,西陇化工股份有限公司,中国),甲醇(HPLC,阿拉丁,中国).
-
正交反应体系:温度为15 ℃、25 ℃、35 ℃,pH为3、5、5.6(无缓冲盐)、7、9,每一种实验条件的反应体系平行配置3组. 反应体系均在避光条件下培养,实验所用器具均预先使用高压灭菌处理.在反应开始后的4周内,按一定时间间隔取样测定。
-
液相色谱:色谱柱(Poroshell EC-C18,4 μm ×4.6 mm × 150 mm),柱温30 ℃,于220 nm处使用VWD检测器进行测定. 进样量为10 μL,流动相A和流动相B分别为0.025 mol·L−1 NaH2PO4(45%)和甲醇(55%),流速1.0 mL·min−1 .
液相色谱质谱:色谱柱Waters ACQUITY UPLC BEH C18(2.1 mm × 50 mm × 7 μm);流动相A和流动相B分别为0.1%甲酸-水溶液和0.1%甲酸-甲醇溶液,流速为0.20 mL·min−1;色谱柱温度为40 ℃;样品进样量为10 μL. 采用全扫描模式检测对质荷比(m/z)为50—600的范围检测,离子源温度为150 ℃;毛细管电压为3.2 V;碰撞气和脱溶剂气流量分别为50 L·h−1和550 L·h−1,去溶剂温度为550 ℃. 流动相为在0—2 min(A 95%,B 5%),2—7 min(A由95%下降至5%, B由5%上升至95%), 7—8 min(A 5%,B 95%), 8—10 min (A由5%上升至95%, B由95%下降至5%).
-
PIP在不同温度下的一级动力学水解速率、半衰期及活化能见表1. 在pH一定的条件下,PIP水解速率均随温度的上升而加快. 这与前人的总结的规律相同,即随着温度升高,抗生素更容易水解[6, 11]. 在不同pH条件下,PIP半衰期受温度影响变化趋势相近. 计算PIP的平均水解速率变化因子[6],在pH = 3、5、5.6、7、9时,每增加10 ℃,PIP的平均水解速率因子分别增加0.498、0.499、0.499、0.496、0.494 h−1. PIP的平均水解速率变化因子不受pH影响,在不同pH条件下,每增加10 ℃,PIP的平均水解速率变化因子为0.497 h−1.
-
pH对PIP的水解速率影响见表1. PIP的水解速率受pH影响较大,当pH = 9时的水解速率最快,pH = 3时次之,pH = 7较慢,在pH = 5和无缓冲盐的体系中更慢. 当pH不同时,PIP水解反应的活化能差异较大,表明PIP的水解应存在不同的反应路径. 当反应超过500 h后,PIP在非碱性条件下的吸收峰面积不再继续降低,因此本文中关于PIP的动力学计算仅使用了500 h前的数据.
研究表明,碱性条件有利于青霉素类抗生素的水解[12],青霉素类抗生素在碱性条件下,多以阴离子状态存在,以阿莫西林(Amoxicillin,AMX)为例,当pH大于9.63时,AMX以AMX2-的形式存在[13]. 青霉素类抗生素处于离子状态时,更容易发生水解反应[6]. 除此以外,Zhang等研究发现,羰基对青霉素类抗生素的水解过程有催化作用[8]. 头孢拉定属于青霉素类抗生素,分子结构内具有β-内酰胺环. 头孢拉定分子内β-内酰胺环C8-N5的开环反应存在两种方式. 一是H2O中的H与—OH同时进攻C8与N5;二是—OH先进攻C8,随后H转移至羰基的O上,再转移至N5上. 经计算,第二种开环反应的吉布斯自由能低于第一种开环反应,第二种开环反应更容易发生[8]. 随溶液pH的上升,溶液中游离的—OH增加,有利于第二种开环反应的正向移动,进而加速β-内酰胺环结构的水解.
-
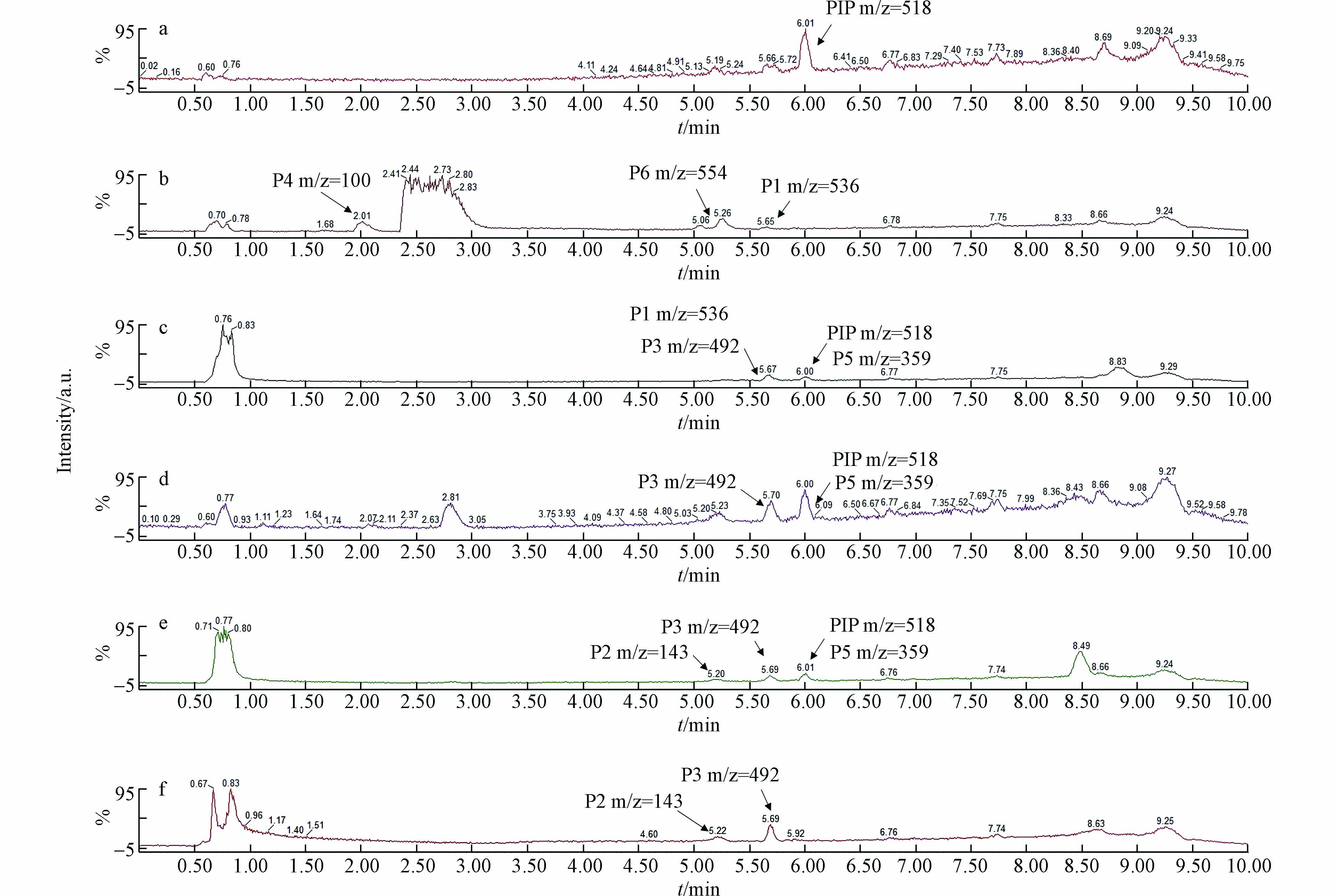
利用液相色谱质谱对15 ℃不同pH条件下反应40 d后的PIP的水解产物进行检测. 如图1 a所示,PIP (m/z = 518)的保留时间(retention time,RT)为6.0 min(图1 a). pH = 9时(图1 b),PIP完全水解(在6.0 min处无色谱峰). PIP的主要水解产物为P1(m/z = 536)(RT = 5.7 min)、P4(m/z = 100)(RT = 2.0 min)、P6(m/z = 554)(RT = 5.2 min). P1为PIP的β-内酰胺环开环反应的直接水解产物[14].
pH=3时,PIP完全水解,主要水解产物为P2(m/z = 143)(RT = 5.2 min)、P3(m/z = 492)(RT = 5.7 min)(图1 f). P3是P1失去一个羧基后的水解产物,该反应在pH = 3时更容易发生[14]. P2是PIP的常见杂质与水解产物[14-15],也是商业合成PIP的原料之一[16]. 对比PIP在pH = 3和pH = 9时的水解产物. 在pH = 9时,未检出P2,因此推测P4为P2在碱性条件下的水解产物. 由于哌嗪结构在碱性条件下不稳定,推测P6为P1中哌嗪结构在碱性条件下水解的产物.
pH = 7、5.6、5时(图1 c,d,e),PIP不完全水解,PIP的剩余浓度分别为5.98、3.63、5.69 μg·L−1. PIP的主要水解产物为P3、P5(m/z = 359)(RT = 6.0 min). 在pH = 7时,P1检出,在pH = 5时,P2(m/z = 143)(RT = 5.2 min)检出. P5存在于PIP的杂质谱中,是PIP在中性条件下常见的水解产物[15]. 与pH=3和pH=9时不同,在pH = 7、5.6、5时PIP未完全水解. 原因可能是P1在中性和弱酸性条件下较为稳定,容易与PIP形成稳定的二聚体[15],二聚体的存在会抑制PIP的水解. 当pH = 9时,PIP快速水解,不易与P1形成P7(m/z = 1035)(图2). 研究表明,弱酸条件下更利于P7的生成[15],这与我们测定的PIP剩余浓度的差异具有相同的规律. P7的存在使得在中性和弱酸条件下,PIP在500 h后达到PIP、P1与P7之间的化学平衡,进而抑制了PIP的水解. PIP可能的水解机理见图2.
-
(1)温度每增加10 ℃,PIP的平均水解速率因子增加0.497 h−1.
(2)pH通过影响PIP在反应体系中的存在形式,进而影响其水解的反应途径. 碱性条件下PIP水解更快.
(3)碱性条件下,PIP分子内的哌嗪结构不稳定,会进一步水解,m/z = 143的产物进一步水解为m/z = 100的水解产物,m/z = 536的产物进一步水解为m/z = 554的水解产物.
(4)PIP与其水解产物(m/z = 536)形成的二聚体在弱酸性和中性条件下会抑制PIP的水解.
哌拉西林的水解机理研究
Mechanism study on hydrolysis of piperacillin
-
摘要: 水解是哌拉西林(piperacillin,PIP)在环境中迁移转化的主要途径之一. 本文测定了PIP在不同温度、pH条件下的水解速率常数、半衰期及活化能. 在pH为3、5、5.6(无缓冲盐)、7的条件下,每增加10 ℃,PIP的平均水解速率因子增加0.497 h-1. pH 为9时的PIP水解速率大于pH为3、5、5.6(无缓冲盐)、7时的水解速率. PIP的水解反应途径受pH影响,在酸性条件下,PIP的主要水解产物含有哌嗪结构,质荷比为143与492;在弱酸性和中性条件下,PIP会与其水解产物形成较为稳定的二聚体,抑制PIP的进一步水解;在碱性条件下,PIP的水解产物中哌嗪结构不稳定,会进一步水解,质荷比为143的产物进一步水解为质荷比为100的水解产物,质荷比为536的产物进一步水解为质荷比为554的产物.Abstract: Hydrolysis is a significant pathway for the migration and transformation of piperacillin (PIP) in the environment. This study aimed to determine the hydrolysis rate constant, half-life, and activation energy of PIP under different temperature and pH . The results showed that the average hydrolysis rate factor of PIP increased by 0.497 h-1 for every 10 ℃ increase in temperature under the conditions of pH 3, 5, 5.6 (no buffer salt), and 7. Moreover, when pH = 9, the hydrolysis rate of PIP was greater than that at pH 3, 5, 5.6 (buffer free), and 7. The hydrolysis pathway of PIP is influenced by pH. Under acidic conditions, the main hydrolysis product of PIP contains a pyrazine structure with a m/z = 143 and m/z = 492; under weakly acidic and neutral conditions, PIP will form a relatively stable dimer with its hydrolysis product, inhibiting further hydrolysis of PIP; under alkaline conditions, the pyrazine structure in PIP’s hydrolysis product is unstable and will undergo further hydrolysis. Additionally, the product with a m/z = 143 further hydrolyzes into a product with a m/z = 100, the product with a m/z = 536 further hydrolyzes into a product with a m/z = 554.
-
Key words:
- piperacillin /
- hydrolysis /
- hydrolysis product
-
表 1 温度对PIP在不同pH条件下水解速率、半衰期及活化能的影响
Table 1. Effect of temperature on the degradation rate, half-life, and activation energy of PIP under different pH conditions
pH 水解速率常数/h−1|半衰期/h 活化能/(kJ·mol−1) 15 ℃ 25 ℃ 35 ℃ 3 0.0029|240.8 0.0029|236.4 0.0095|72.6 45.3 5 0.0014|503.8 0.0016|439.2 0.0019|364.8 12.0 7 0.0018|384.8 0.0108|64.3 0.0044|155.9 31.2 9 0.0269|25.8 0.1159|6.0 0.1515|4.6 62.9 5.6(无缓冲盐) 0.0013|536.6 0.0013|544.5 0.0032|217.6 34.1 -
[1] YAO S J, YE J F, YANG Q, et al. Occurrence and removal of antibiotics, antibiotic resistance genes, and bacterial communities in hospital wastewater [J]. Environmental Science and Pollution Research, 2021, 28(40): 57321-57333. doi: 10.1007/s11356-021-14735-3 [2] 蒋宝, 隋珊珊, 孙成一, 等. 北京市北运河水体中抗生素污染特征及风险评估 [J]. 环境科学, 2023, 44(6): 3198-3205. [3] 王若男, 曹阳, 高超, 等. 沱江干流抗生素污染的时空变化和生态风险评估 [J]. 环境化学, 2021, 40(8): 2505-2514. doi: 10.7524/j.issn.0254-6108.2021022003 [4] BENGTSSON-PALME J, JOAKIM LARSSON D G. Concentrations of antibiotics predicted to select for resistant bacteria: Proposed limits for environmental regulation [J]. Environment International, 2016, 86: 140-149. doi: 10.1016/j.envint.2015.10.015 [5] MITCHELL S M, ULLMAN J L, TEEL A L, et al. pH and temperature effects on the hydrolysis of three β-lactam antibiotics: Ampicillin, cefalotin and cefoxitin [J]. Science of the Total Environment, 2014, 466/467: 547-555. doi: 10.1016/j.scitotenv.2013.06.027 [6] ZHANG H Q, BAI J C, XUE W F, et al. Quantum chemical prediction of effects of temperature on hydrolysis rate of penicillin under weakly acidic condition [J]. Science of the Total Environment, 2022, 806: 150509. doi: 10.1016/j.scitotenv.2021.150509 [7] BLOTEVOGEL J, MAYENO A N, SALE T C, et al. Prediction of contaminant persistence in aqueous phase: A quantum chemical approach [J]. Environmental Science & Technology, 2011, 45(6): 2236-2242. [8] ZHANG H Q, XIE H B, CHEN J W, et al. Prediction of hydrolysis pathways and kinetics for antibiotics under environmental pH conditions: A quantum chemical study on cephradine [J]. Environmental Science & Technology, 2015, 49(3): 1552-1558. [9] CHEN J B, SUN P Z, ZHANG Y L, et al. Multiple roles of Cu(II) in catalyzing hydrolysis and oxidation of β-lactam antibiotics [J]. Environmental Science & Technology, 2016, 50(22): 12156-12165. [10] SHENG F, LING J Y, WANG C, et al. Rapid hydrolysis of penicillin antibiotics mediated by adsorbed zinc on goethite surfaces [J]. Environmental Science & Technology, 2019, 53(18): 10705-10713. [11] SHIN J, LEE S, PARK H, et al. Effects of thermal hydrolysis on anaerobic digestion and abundance of antibiotic resistance genes during recuperative thickening digestate treatment of sewage sludge [J]. Chemical Engineering Journal, 2022, 450: 138128. doi: 10.1016/j.cej.2022.138128 [12] 李国傲, 孙成一, 雒梅, 等. 哌拉西林的解离常数测定及其在水溶液中的存在形态 [J]. 分析试验室, 2022, 41(10): 1121-1126. [13] JAVAD I, AMIRHOSSIEN G, MOHSEN M, et al. Fast and effective adsorption of amoxicillin from aqueous solutions by L-methionine modified montmorillonite K10 [J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2020(prepublish): 125792. [14] LÄNGIN A, ALEXY R, KÖNIG A, et al. Deactivation and transformation products in biodegradability testing of ß-lactams amoxicillin and piperacillin [J]. Chemosphere, 2009, 75(3): 347-354. doi: 10.1016/j.chemosphere.2008.12.032 [15] 常祎卓. 基于分析目标(Analytic Target Profile)的理念探讨青霉素杂质谱分析方法的普适性[D]. 北京: 中国食品药品检定研究院, 2018. [16] XIE Y, FENG M J, ZHANG M, et al. Kinetics model of piperacillin synthesis in a microreactor [J]. Chemical Engineering Science, 2022, 259: 117821. doi: 10.1016/j.ces.2022.117821 -




 下载:
下载:


