-
细胞毒性药物作为抗肿瘤治疗的重要手段之一,临床应用广泛. 长期从事细胞毒性药物准备工作的医护人员可能受到职业暴露危害主要有基因突变、生殖影响和致癌[1-3]. 国际癌症研究机构将10余种细胞毒性药物归类为1类致癌物与 2A 类疑似致癌物[4]. 目前有研究报道,细胞毒性药物职业暴露生殖毒性可能导致流产、暂时性或永久性不孕、早产、先天畸形和引起接触者子女学习障碍[5];长期职业暴露将增加患脱发、感染、器官毒性、骨髓毒性、粘膜溃疡、疲劳、出血和头痛的风险[6];此外,职业暴露个体的DNA损伤、染色体异常和癌症的发生率更高[7-8]. 根据国家卫生健康委发布的《静脉用药调配中心建设与管理指南》规定,细胞毒性药物必须在静脉药物调配中心 (Pharmacy Intravenous Admixture Services)中实行集中配置和供应. 工作人员每天参与的排药、混合调配和成品复核等工作,不可避免地接触各类细胞毒性药物,面临着很大的职业暴露风险. PIVAS工作环境物体表面上残留的细胞毒性药物主要是一些低分子量药物,皮肤接触和经皮吸收是其职业暴露的主要途径[9-11]. 因此对PIVAS工作环境,尤其是工作区域物体表面细胞毒性药物残留情况的评估和监测至关重要[12-13].
目前并没有统一的表面污染阈值水平,环境污染的代表药物一般是根据当地所在医院细胞毒性药物使用特性来决定的[14]. 我国目前评估细胞毒性药物残留情况所选取的目标药大多仅研究了环磷酰胺与阿糖胞苷两种药物,而对用量大、毒性大的药物,如吉西他滨、紫杉醇类药物等少有研究[15-17]. 近期有研究利用UPLC-MS/MS对我国10家医院PIVAS进行了环境污染评估和监测,发现其检测的两种药物(环磷酰胺和阿糖胞苷)污染最严重的区域是生物安全柜[15]. 擦拭取样已经广泛应用与环境监测[18-19],本研究采用UPLC-MS/MS,选取了5种使用频率高,性质差异大的细胞毒性药物作为研究对象,通过对生物安全柜及PIVAS内常用材质玻璃、不锈钢和PVC表面进行模拟污染,通过擦拭取样,建立了PIVAS生物安全柜、地面及工作区域其他物体表面上多种细胞毒性药物残留的测定方法,对评估PIVAS工作人员职业暴露风险具有重要意义.
-
AB SCIEX Triple Quad TM 4500MD液相色谱串联质谱检测系统、高效液相色谱仪(Jasper TM)和软件Analyst ® MD(板本号:1.6)组成(AB SCIEX公司);H1-16KR型医用离心机(湖南可成仪器设备有限公司);SB-5200D型超声波清洗机(宁波新芝生物科技股份有限公司);Milli-Q纯水系统(美国Millipore公司);Waters ACQUITY UPLC BEH C18色谱柱(2.1 mm×50 mm,1.7 µm);Quintix125D-1CN万分之一分析天平(SQP型,Sartorius公司).
标准品:吉西他滨(Gemcitabine, GEM,批号:T26S6T3870,纯度≥99%)、依托泊苷(Etoposide, VP-16,批号:C09S11S123704,纯度≥98%)、紫杉醇(Paclitaxel,TAX,批号:J04S11H123510,纯度≥98%)、表柔比星(Epirubicin,EPI,批号:AQW905,纯度≥98%)、环磷酰胺(Cyclophosphamide,CTX,批号:CMA739,纯度≥98%)、安替比林(Antipyrine, APR,批号:Y22A10C86317,纯度≥98%),均来自源叶生物科技有限公司. 主要试剂:乙腈、甲醇(色谱纯,美国TEDIA公司);甲酸、二甲基亚砜(色谱纯,Macklin公司);水为Milli-Q高纯水;滤纸(100张/盒,英国Whatman公司).
内标溶液的配制:准确称取安替比林20.0 mg,置于20 mL容量瓶中,用甲醇溶解并定容,得到1 mg·mL−1储备液. 精密移取内标储备液0.4 mL至20 mL容量瓶中,用甲醇稀释为 20 μg·mL−1的标准溶液,内标储备液和标准溶液均保存在4 ℃冰箱中. 进一步用20%乙腈(含0.1%甲酸)溶液稀释为10 ng·mL−1内标工作液,测试当日新鲜配制.
标准溶液的配制:准确称取吉西他滨、依托泊苷、紫杉醇、表柔比星和环磷酰胺标准品20.0 mg,分别用二甲基亚砜溶解并定容于20 mL 容量瓶中,得到1 mg·mL−1单标储备液. 分别精密移取单标储备液0.4 mL至20 mL容量瓶中,用甲醇稀释,配成浓度各为 20 μg·mL−1的混合标准溶液. 准确移取混合标准溶液至容量瓶中,配制成200、1000、4000 ng·mL−1的混合标准中间液. 用10 ng·mL−1内标工作液逐级稀释成0.1、0.5、1、10、50、200、800 ng·mL−1系列标准工作溶液,上机测定. 所有储备液和标准溶液均在4 ℃避光保存.
-
用2 cm×2 cm的滤纸擦拭10 cm×10 cm面积区域,0.1 mL乙腈-0.1%甲酸溶液(80:20,V/V)为预湿溶液,重复擦拭2遍。将擦拭后的2张滤纸置于2 mL EP管中,加入1 mL 内标工作液,超声提取20 min. 上清液移至另一个4 mL离心管中,残渣用1 mL 内标工作液重复提取1次,合并上清液,14000 r·min−1 离心5 min,取上清液200 μL供UPLC-MS/MS测试.
-
色谱柱:Waters ACQUITY UPLC BEH C18柱(2.1 mm×50 mm,1.7 µm);柱温:35 ℃;进样量:3 µL;流动相:A相为水, B 相为0.1%的甲酸乙腈溶液;流速:0.5 mL·min−1;梯度洗脱程序为:0—1.0 min,10%B;1.01—2.50 min,20%→30%B;2.51—3.00 min,30%→80%B;3.01—3.50 min,80%B;3.51—4.00 min,80%→10%B;4.01—5.00 min,10%B.
-
电喷雾电离源ESI(+),采用多反应监测(MRM)模式. 气帘气压力:35 psi,碰撞气压力:8 psi,离子源喷雾电压:5500 V,温度:500 ℃, 离子源气1压力50 psi,离子源气2压力50 psi,5种细胞毒性药物的串联质谱参数见表1.
-
根据世界卫生组织国际癌症研究机构发布的2020年全球最新癌症负担数据显示,我国是癌症发病率最高的国家,而细胞毒性药物目前仍是抗肿瘤治疗非常重要的手段,在临床使用广泛[20]. 对我院2019—2020年细胞毒性药物使用情况进行统计分析,筛选出排名靠前10位的药物. 根据细胞毒性药物的理化性质和结构类型,从10种药物中筛选出5种具有代表性的药物,包含了水溶性和脂溶性药物,其中水溶性药物为吉西他滨、环磷酰胺、表柔比星,脂溶性药物为依托泊苷和紫杉醇.
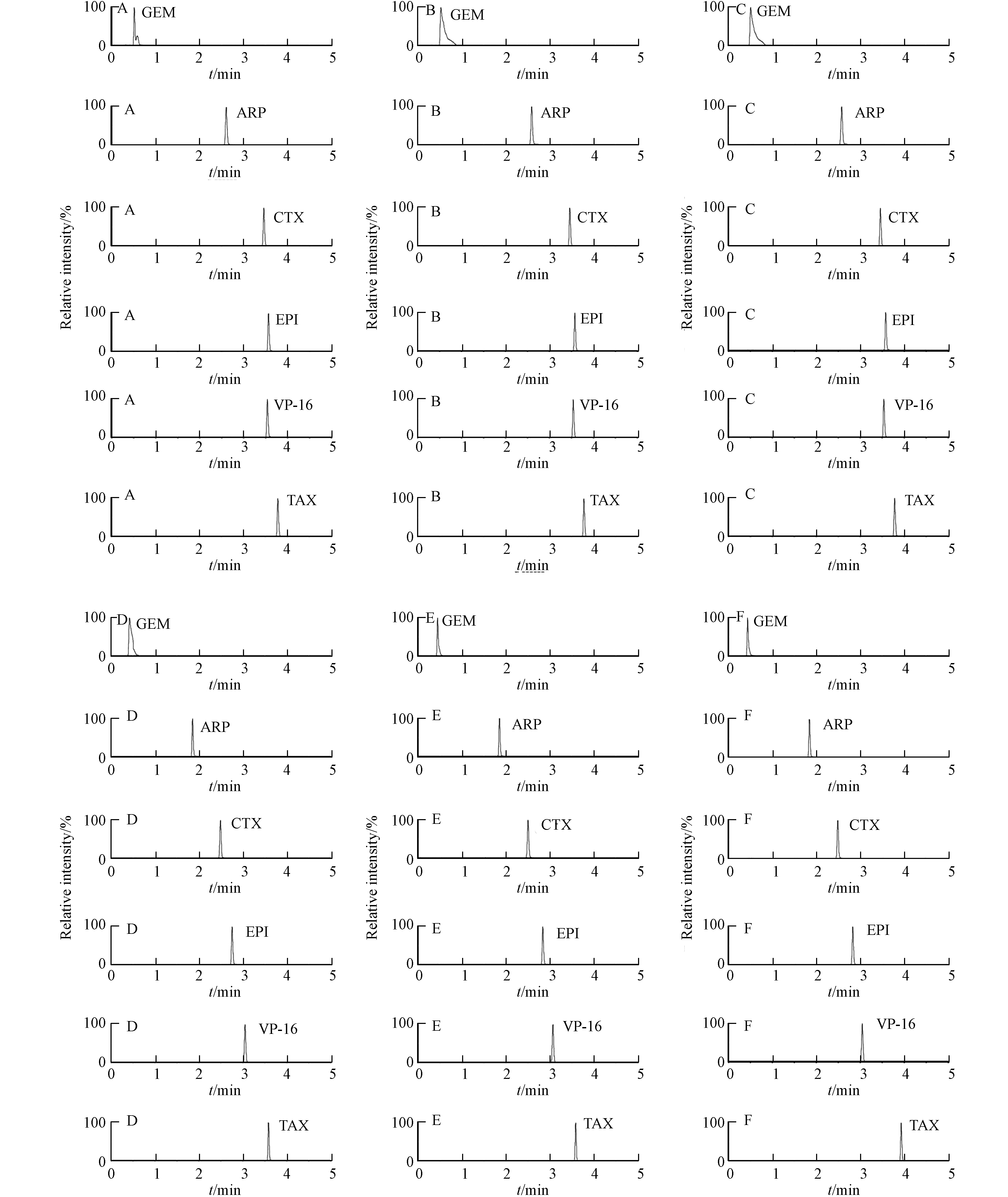
内标物的选择首选稳定同位素标记内标,同时测定多种药物时需要多种药物同位素内标,但同位素内标不易得到,实际操作比较困难. 有文献报道安替比林作为异环磷酰胺内标进行危害药物表面污染检测方法可行[21]. 本研究选择安替比林为内标,其色谱峰的位置在5种细胞毒性药物色谱峰中间且完全分离,能够满足分析需要.
-
用 100 ng·mL−1的 5 种细胞毒性药物的混合标准品溶液,以 ESI+ 扫描方式进行一级质谱扫描,考察目标化合物的准分子离子峰[M+H]+,确定母离子后,进行二级质谱扫描,取丰度较高且相对稳定的碎片离子作为定量、定性离子,并采用MRM模式进一步优化碰撞能量、解簇电压等质谱参数. 表柔比星对定性离子进行优化时,调整碰撞能量后m/z 544.3/397.1与m/z 544.3/379.0离子对响应较好,但实际测定中发现相对响应较高的m/z 544.3/397.1离子对存在较多干扰,故采用m/z 544.3/379.0作为定量离子对.
-
本研究比较了0.1%的甲酸水溶液-0.1%的甲酸乙腈溶液、0.1%的甲酸水溶液-乙腈溶液、水-0.1%的甲酸乙腈溶液、0.1%的甲酸水溶液-0.1%的甲酸甲醇溶液、0.1%的甲酸水溶液-甲醇溶液、水-0.1%的甲酸甲醇溶液作为流动相时5种细胞毒性药物的分离效果. 结果表明,用乙腈为流动相吉西他滨色谱峰拖尾现象得到改善,对称性较好. 水-0.1%的甲酸乙腈溶液为流动相时,采用梯度洗脱,分析时间5 min,各待测化合物响应好,离子化效率最佳,5种细胞毒性药物和内标物的色谱图见图1.
-
取6份2 cm×2 cm空白滤纸用1 mL 20%乙腈-0.1%甲酸溶液重复提取2次,合并提取液,14000 r·min−1离心5 min,上清液即为经过前处理的空白基质. 分别用溶剂和经过前处理的空白基质配制5、25、100 ng·mL−1质控样品,5种细胞毒性药物和IS的基质效应=100%×经过前处理的空白基质配制样品的峰面积/溶剂配制样品的峰面积,内标归一化的基质效应=100%×细胞毒性药物的基质效应/IS的基质效应.
在2 cm×2 cm空白滤纸添加50 μL,200、1000、4000 ng·mL−1 的3个水平的混合标准物质,用1 mL 20%乙腈-0.1%甲酸溶液重复提取2次,合并提取液,14000 r·min−1离心5 min,去上清液进样;经过前处理的空白基质配制相应浓度的质控样品. 5种细胞毒性药物和IS的回收率=100%×加标样品测得的峰面积/空白基质配制样品测得的峰面积,内标归一化的回收率=100%×细胞毒性药物的回收率/IS的回收率. 对于基质效应与回收率RSD不大于15%,结果见表2.
-
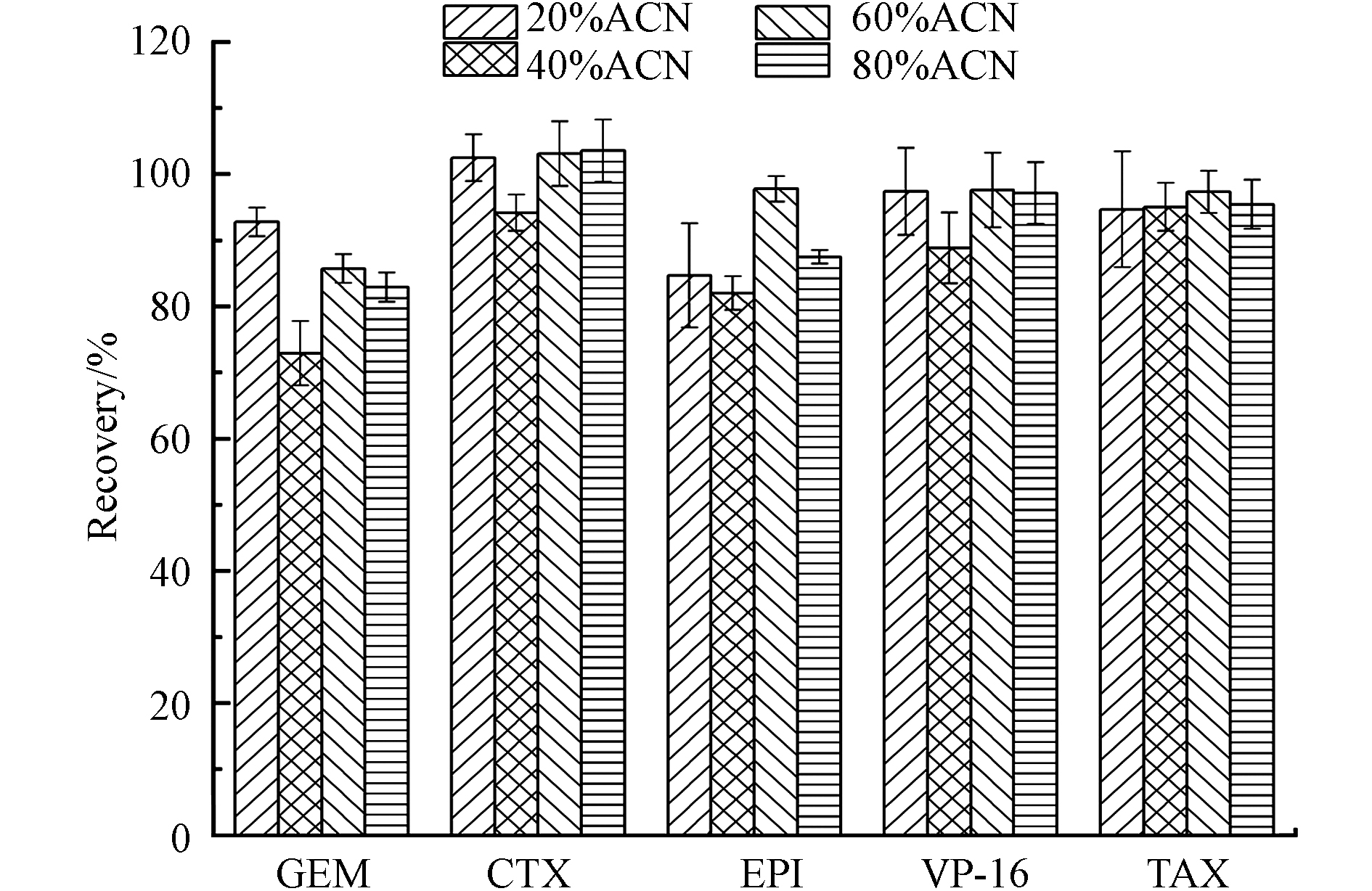
提取溶液的选择以提取更多的目标化合物和更少的杂质为目的. 水、甲醇和乙腈是最常用的提取溶剂[22]. 本研究比较了不同比例(20%、40%、60%和80%)的乙腈水溶液(含0.1%甲酸)对5种细胞毒性药物的提取效率. 将浓度4000 ng·mL−1工作液50 μL喷洒到2 cm×2 cm滤纸上作为模拟样品进行考察,结果显示如图2,20%乙腈-0.1%甲酸溶液为提取溶剂时,5种药物的平均提取回收率即可达到84.7%—102%,能够满足OSHA表面采样评估指南建议分析物的采集回收率需高于75%[23]. 乙腈浓度的增加并未显著提高提取回收率,而当乙腈浓度增加到80%时,表柔比星的峰型变得很差,且考虑到有机溶剂的有毒性,因此选择20%乙腈-0.1%甲酸溶液为提取溶剂.
-
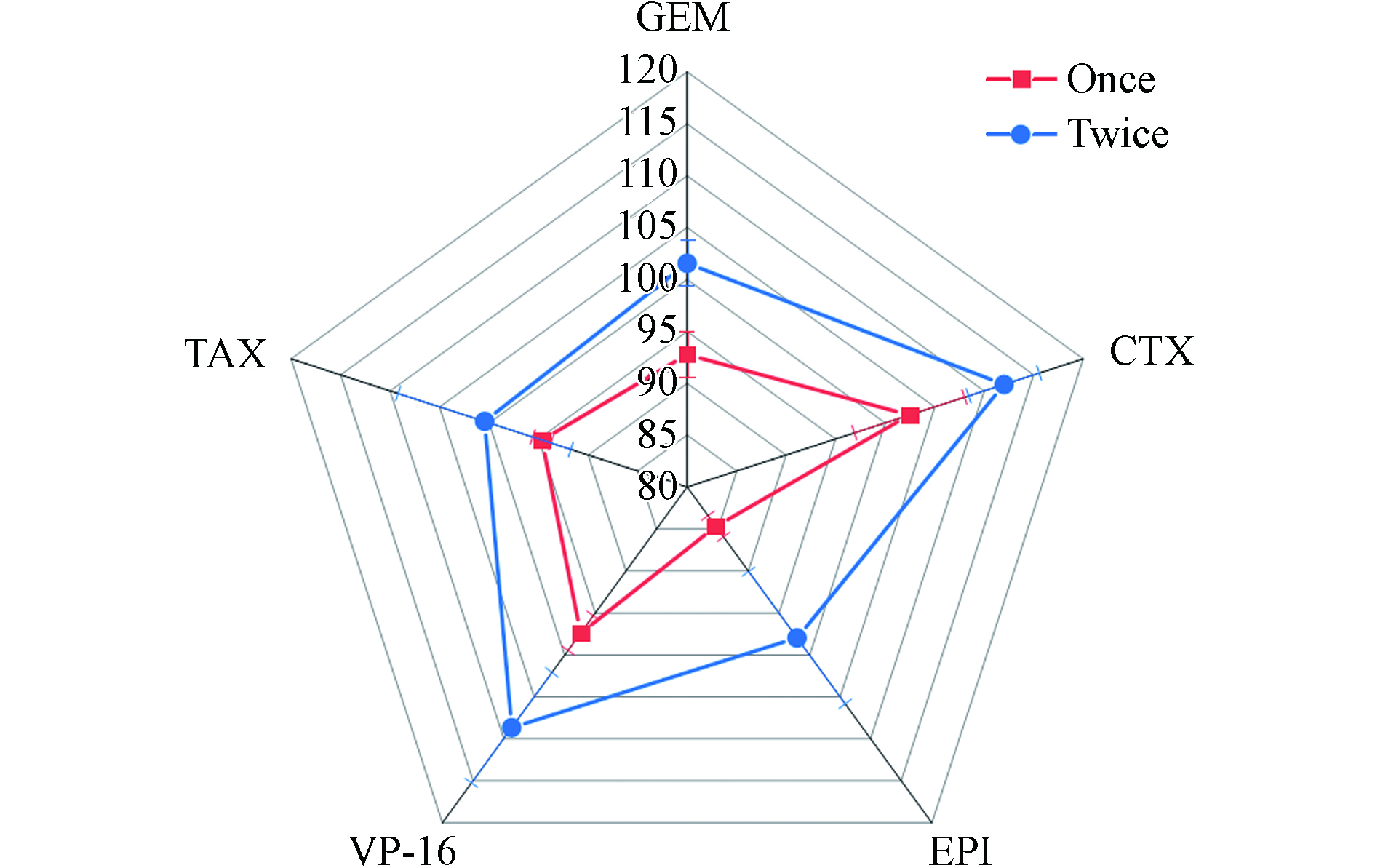
本实验分别考察了用1 mL 20%乙腈−0.1%甲酸溶液对4000 ng·mL−1工作液50 μL喷洒到2 cm×2 cm滤纸上作的模拟样品提取1次和提取2次的提取效率. 结果如图3所示,提取1次和提取2次的平均回收率分别为84.7%—102%和98.0%—112%,尤其是表柔比星的提取效率从84.7%提高至98.0%,故本实验选择1 mL 20%乙腈−0.1%甲酸溶液提取2次为提取方法.
-
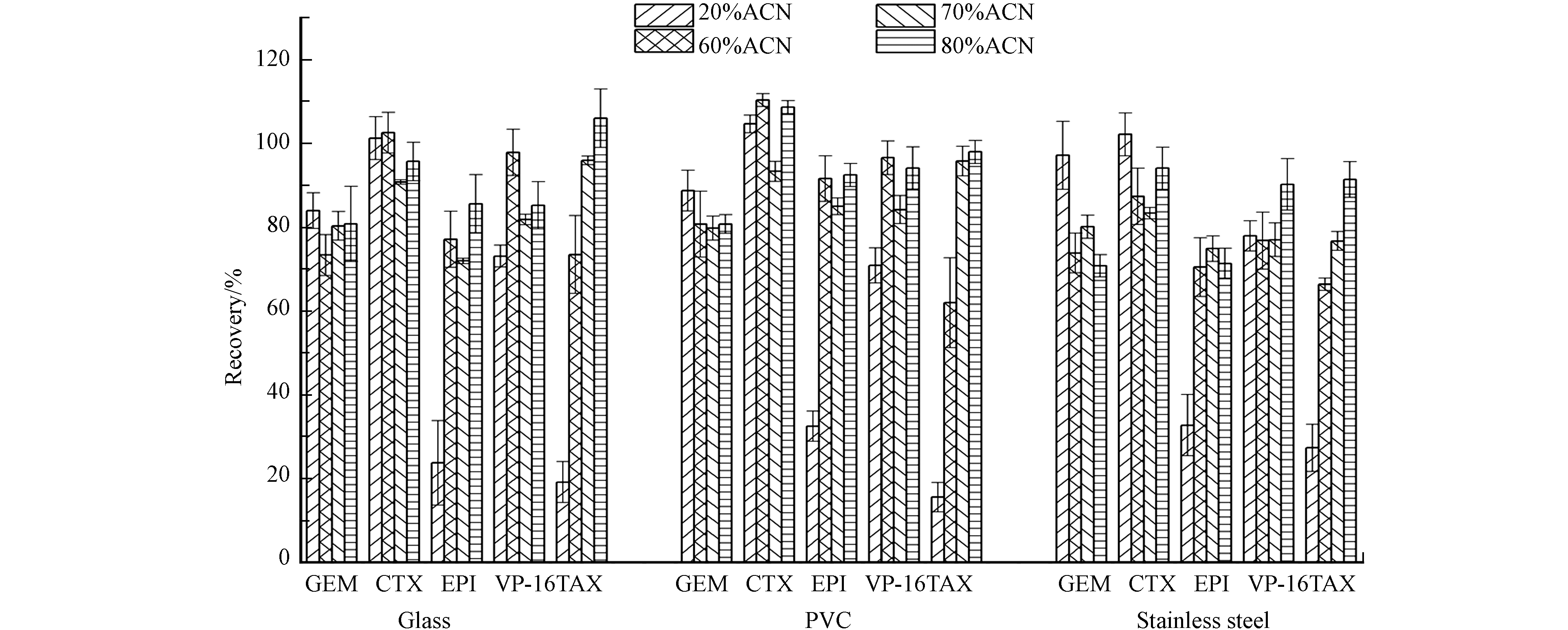
评估了几种擦拭溶剂分别在10 cm×10 cm的不锈钢、玻璃和PVC板上进行擦拭取样的回收率. 用4000 ng·mL−1的标准溶液50 μL对10 cm×10 cm的不锈钢、玻璃和PVC板进行模拟污染,静置待干过夜,按照1.2.1节进行样品采集和处理,并计算平均回收率. 结果显示如图4,在20%乙腈-0.1%甲酸溶液擦拭在3种材质物表对于表柔比星、紫杉醇的回收率均较低,表柔比星擦拭回收率18.2%—37.6%,紫杉醇13.1%—32.2%. 而增加乙腈比例,表柔比星、紫杉醇的回收率能够得到改善. 当80%乙腈−0.1%甲酸溶液为擦拭溶液时,表柔比星和紫杉醇的回收率分别达到71.3%—92.5%和88.1%—114%. 从总体上看,不锈钢材质上的擦拭回收率较玻璃和PVC上低,5在种药物在玻璃板、PVC板和不锈钢板上的平均回收率分别为80.8%—106%、80.7%—108%、70.8%—94.1%,RSD分别为4.8%—11.1%、1.4%—5.3%、2.0%—6.8%,这可能与表面本身粗糙度相关,有研究表明粗糙度较大的表面回收率较低[24-25]. 尽管如此,5种药物在玻璃板、PVC板和不锈钢板上的平均回收率均能满足本研究的分析要求. 因此,选择80%乙腈−0.1%甲酸溶液用于不锈钢、玻璃和PVC板表面细胞毒性药物残留擦拭取样.
-
在最佳的串联质谱条件下, 测定不同浓度的细胞毒性药物混合标准工作液,以质量浓度为横坐标(X),待测物与内标峰面积的比值(Y)为纵坐标绘制标准曲线,结果如表3所示,5种药物在线性范围内的线性关系良好,R2均大于0.999,检出限(LODs)为0.2—1 pg·cm−2,吉西他滨和环磷酰胺定量限0.6 pg·cm−2,表柔比星和依托泊苷定量限1.2 pg·cm−2,紫杉醇定量限3.2 pg·cm−2,均低于文献报道的定量限,表明该方法灵敏度高[11,14].
-
分别在10 cm×10 cm不同空白物表(不锈钢、玻璃和PVC板)上添加50 μL 200、1000、4000 ng·mL−1 的3个水平的混合标准物质,相当于每种药物添加10、50、200 ng,每个浓度分别制备 6个平行样,按照“1.2.1”进行样品采集和处理,计算其平均回收率和相对标准偏差(RSD),结果见表4.
实验结果表明,在 3个加标水平下,5种药物在玻璃板上的平均回收率为84.9%—108%,RSD为1.9%—8.3%;PVC板上的平均回收率为85.1%—107%,RSD为0.8%—13.3%;不锈钢板上的平均回收率为67.1%—101%,RSD为1.3%—11.0%,本方法的准确度和精密度较好.
-
每日细胞毒性药物配置结束后,工作人员会对生物安全柜、地面、传递窗等进行清洁消毒. 对清洁后的生物安全柜工作台面、侧壁、玻璃挡板、推车、地面和传递窗等表面按照“1.2.1”进行样品采集处理,并用本文建立的UPLC-MS/MS方法进行细胞毒性药物残留测定. 结果见表5,在生物安全柜内的细胞毒性药物的残留量最大,其中以环磷酰胺残留最严重,残留量为34.3—10898.8 pg·cm−2,显著高于MEWIP研究中的目标污染水平100 pg·cm−2 [26];其次,在门把手和配置间推车上发现大量细胞毒性药物残留,同时在PIVAS工作区域其他物体表面、地板等区域亦检测出不同程度的药物残留.
-
本研究建立了在PIVAS环境物体表面中同时检测分析吉西他滨、环磷酰胺、表柔比星、依托泊苷及紫杉醇5种细胞毒性药物残留的UPLC-MS/MS测定方法. 该方法前处理简单,对不同材质表面细胞毒性药物残留检测均具有较好的准确度和精密度,通过内标法检测提高了灵敏度与准确性,为PIVAS工作环境细胞毒性药物残留检测提供了新的技术手段,对于进一步促进PIVAS工作流程优化和完善工作人员的保护措施,降低职业暴露风险意义重大.
超高效液相色谱-串联质谱法同时测定PIVAS工作环境中5种细胞毒性药物残留
Simultaneous determination of 5 cytotoxic drugs residues in PIVAS workplace ultra-performance liquid chromatography-tandem mass spectrometry
-
摘要: 建立超高效液相色谱-串联质谱技术同时测定医院静脉用药调配中心(PIVAS)工作环境中5种细胞毒性药物吉西他滨、环磷酰胺、表柔比星、依托泊苷及紫杉醇在玻璃、不锈钢和PVC材质表面残留含量的检测方法. 将2 cm×2 cm滤纸用100 μL 80%乙腈-0.1%甲酸溶液润湿后对10 cm×10 cm物表进行擦拭取样,擦拭样品用1 mL 20%乙腈含0.1%甲酸溶液重复提取2次,合并提取液离心后取上清液,以水-乙腈(0.1%甲酸)为流动相梯度洗脱,采用电喷雾电离源正离子模式和MRM模式检测. 结果表明,吉西他滨和环磷酰胺在0.1—800 ng·mL−1、表柔比星和依托泊苷在0.1—200 ng·mL−1、紫杉醇在0.2—200 ng·mL−1范围内线性关系良好,相关系数均大于0.999,方法的检出限为 0.2—1 pg·cm−2,定量限(LOQ)为0.6—3.2 pg·cm−2. 在玻璃、PVC和不锈钢3种材质上3个加标水平(200、1000、4000 ng·mL−1)的回收率为67.1%—108%,相对标准偏差(RSD)为0.8%—13.3%. 用本文建立的方法检测本中心PIVAS环境物体表面,发现5种细胞毒性药物可被不同程度检出,浓度范围为1.8—10898.8 pg·cm−2,PIVAS医务人员存在一定的细胞毒性药物职业暴露风险.
-
关键词:
- 静脉用药调配中心(PIVAS) /
- 细胞毒性药物 /
- 残留 /
- 超高效液相色谱-串联质谱(UPLC-MS/MS).
Abstract: An ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) analytical method was established and validated for the simultaneous determination of 5 cytotoxic drugs on the surface of glass, stainless steel, and PVC in the working environment of the pharmacy intravenous admixture service (PIVAS). The 10 cm×10 cm surface was wiped and sampled by a 2 cm×2 cm filter paper moistened with 100 μL of 80% ACN (0.1% FA) solution. Then the sample of filter paper was extracted with 1 mL 20% acetonitrile containing 0.1% formic acid solution two times. The supernatant was taken after centrifugation of the combined extraction solution, then the supernatant was used directly quantitated by UPLC-MS/MS in MRM mode after gradient elution with water and ACN with -0.1% FA as mobile phase. The results showed that good linearity was observed for GEM and CTX from 0.1 ng·mL−1 to 800 ng·mL−1, EPI and VP-16 from 0.1 ng·mL−1 to 200 ng·mL−1, TAX from 0.2 ng·mL−1 to 200 ng·mL−1, with correlation coefficients larger than 0.999. The limits of detection (LODs) were 0.2—1 pg·cm−2 and limits of quantitation (LOQs) were 0.6—3.2 pg·cm−2. The average recoveries at three different spiked levels (200, 1000, 4000 ng·mL−1) ranged from 67.1% to 108%, with relative standard deviations (RSD) of 0.8%—13.3%. This method was used to detect the surface of the PIVAS environment, and it was found that five cytotoxic drugs could be detected with concentrations range from 1.8 pg·cm−2 to 10898.8 pg·cm−2, which indicated that unintended occupational exposure to antineoplastic drugs may occur in PIVAS. -
高氯酸盐(
ClO−4 )作为一种新型环境污染物,主要来源于军工企业和火箭推进剂制造厂等[1]。ClO−4 会抑制人体甲状腺对碘离子的吸收,干扰甲状腺正常功能、代谢和发育,严重时会对骨髓和肌肉组织产生病变影响,诱发甲状腺癌,危害人类的健康[1-2]。常见的ClO−4 的去除方法主要包括物化法[3-4]和生物法[5-6]。物化法的主要缺点是操作成本高,选择性低,处理后产生二次卤水废物。相反,微生物法可以使ClO−4 完全转化为Cl−,具有对ClO−4 降解速率快、反应条件温和等优点,因而被认为是有前景的ClO−4 处理技术。在微生物处理技术中,厌氧颗粒污泥已逐步应用到
ClO−4 废水处理中[7-8]。然而,由于ClO−4 具有强氧化性会抑制厌氧颗粒污泥中微生物的生长[9],延长了颗粒污泥的培养周期。因此,如何快速培养具有去除ClO−4 能力的高氯酸盐还原颗粒污泥,成为国内外研究的热点。在颗粒污泥驯化过程中,微生物作为颗粒污泥的主要组成部分,所选用的接种污泥对高氯酸盐还原颗粒污泥的形成起着至关重要的作用。有研究[10]表明,絮状活性污泥中含有少量高氯酸盐还原菌,具有降解ClO−4 的潜力。WAN等[11]在硫填充床反应器中接种活性污泥同时去除硝酸盐(NO−3 )和ClO−4 的过程中发现,部分反硝化细菌可以以ClO−4 为电子受体。同时,WANG等[12]的研究表明,以颗粒污泥为接种污泥会有利于加速颗粒污泥的形成。YIN等[8]使用反硝化颗粒污泥为接种污泥,通过直接逐步增加高氯酸盐负荷的方法,经过99 d培养出高氯酸盐还原颗粒污泥,并发现高氯酸盐还原颗粒污泥中高氯酸盐还原菌和反硝化细菌为优势菌群。然而,在逐步增加高氯酸盐负荷的过程中,同时逐步降低硝酸盐进水浓度,能否加快高氯酸盐还原颗粒污泥的培养,目前并未有相关报道。本研究以反硝化颗粒污泥为接种污泥,通过逐步降低进水
NO−3 同时逐步升高进水ClO−4 浓度的方法,开展了高氯酸盐还原颗粒污泥快速培养的研究,考察了高氯酸盐还原颗粒污泥的培养过程中ClO−4 去除性能和颗粒污泥特性(混合液悬浮固体浓度,混合液挥发性固体浓度,粒径分布和胞外聚合物组分),并对反硝化颗粒污泥和高氯酸盐还原颗粒污泥的菌群结构进行了分析,为进一步研究高氯酸盐还原颗粒污泥在ClO−4 废水处理中的实际应用提供理论基础。1. 材料与方法
1.1 实验装置
UASB反应器材料为有机玻璃(图1),内径为80 mm,高为750 mm,有效容积为3.50 L。顶部内设三相分离器,沿反应器不同高度设4个取样口。反应器运行温度保持在(30±2) ℃,水力停留时间(HRT)为3.50 h。
1.2 接种污泥
接种污泥取自实验室UASB反应器成功培养出的反硝化颗粒污泥。其颗粒污泥成熟时间为50 d,硝酸盐去除率稳定在85%以上,混合液悬浮固体浓度(MLSS)为42.33 g·L−1,混合液挥发性固体浓度(MLVSS)为25.84 g·L−1,MLVSS/MLSS为0.50,胞外聚合物(EPS)为24.62 mg·g−1,蛋白质(PN)为8.47 mg·g−1,多糖(PS)为11.29 mg·g−1。
1.3 废水成分
实验废水为人工配置的硝酸盐和高氯酸盐混合进水,其中硝酸盐主要进水成分为硝酸钠和无水乙酸钠,碳氮比(C∶N)为4∶1。高氯酸盐主要进水成分为高氯酸钠和无水乙酸钠,CH3COO−∶
ClO−4 (摩尔比)为1.5∶1.0。其他主要成分为0.13 g·L−1的磷酸二氢钾和0.52 g·L−1的硫酸铵,微量元素为2.00 mL·L−1。微量元素溶液组成为0.50 mg·L−1 MnSO4·H2O、0.10 mg·L−1 FeSO4·7H2O、0.01 mg·L−1 CuSO4·5H2O、0.01 mg·L−1 Na2MoO4·2H2O、0.01 mg·L−1 Na2WO4·2H2O、0.02 mg·L−1 NiCl2·6H2O、0.50 mg·L−1 EDTA。1.4 运行条件
为实现高氯酸盐还原颗粒污泥的快速培养,本实验采用逐步降低
NO−3 同时逐步增加ClO−4 浓度的方法,其间NO−3 浓度从200 mg·L−1降低至0,ClO−4 浓度从50 mg·L−1增加至250 mg·L−1,具体的运行参数调控见表1。水样每天取自UASB反应器出水口,每隔10 d,由UASB反应器的取样口取颗粒污泥样品。表 1 运行参数Table 1. Operation parameters阶段 时间/d HRT/h 进水流速/(L·h−1)  /(mg·L−1)
/(mg·L−1) /(mg·L−1)
/(mg·L−1)Ⅰ 0~10 3.50 1.00 50 200 Ⅱ 11~20 3.50 1.00 100 150 Ⅲ 21~30 3.50 1.00 150 100 Ⅳ 31~40 3.50 1.00 200 50 Ⅴ 41~50 3.50 1.00 250 0 1.5 分析方法
ClO−4 采用离子色谱仪(美国戴安ICS-1100)进行测定,保护柱为AG20(4 mm×50 mm),分析柱为AS20(4 mm×250 mm)。样品分析前均经8 000 r·min−1离心10 min,然后经过0.22 μm微孔滤膜过滤。采用梯度淋洗的方式进行,淋洗液为KOH溶液,流速为1.00 mL·min−1,柱温为30 ℃。NO−3 采用紫外分光光度法[13]测定,COD采用重铬酸钾消解法[13]测定,粒径分布采用湿式筛法[13]测定,MLSS和MLVSS采用重量法[13]测定。EPS采用加热法[14-15]提取。具体实验方法为:将颗粒污泥加入磷酸盐缓冲液(pH=7)中,进行超声处理,然后在80 ℃水浴中加热;加入0.30 mL甲醛,放入摇床,以300 r·min−1振荡0.50 h;再加入1.00 mL 2.00 mol·L−1的NaOH,以300 r·min−1振荡1 h;最后经6 000 g·min−1的转速离心10 min,再用0.22 μm的滤膜过滤。EPS用总有机碳测定仪测定提取液中的总有机碳含量,来表示EPS含量,其中PN采用考马斯亮蓝法进行测定,以牛血清白蛋白作为标准物质;PS采用蒽酮比色法[16]进行测定。1.6 高通量测序
为探究高氯酸盐还原颗粒污泥形成过程中微生物菌群结构的变化,分别对反硝化颗粒污泥和高氯酸盐还原颗粒污泥进行高通量测序分析。建库和测序工作委托上海美吉医药生物科技有限公司(上海,中国)完成,高通量测序是在Miseq(Illumina,美国)测序平台上完成的。
2. 结果与讨论
2.1 高氯酸盐还原颗粒污泥形成过程中反应器运行性能
通过不断降低进水
NO−3 同时逐步升高进水ClO−4 浓度的方法,培养高氯酸盐还原颗粒污泥,高氯酸盐还原颗粒污泥形成过程中ClO−4 和NO−3 浓度及去除率的变化如图2所示。由图2可知,第0~10天(第Ⅰ阶段),进水ClO−4 浓度为50 mg·L−1,进水NO−3 浓度为200 mg·L−1,ClO−4 和NO−3 的去除率都维持在90%以上。先前研究表明,反硝化细菌也具有代谢ClO−4 的能力[17],因此,反应器中反硝化颗粒污泥可以代谢ClO−4 。第10~30天(第Ⅱ~Ⅲ阶段),当进水ClO−4 和NO−3 浓度为100 mg·L−1和150 mg·L−1时,ClO−4 的去除率在90%以上,而NO−3 的去除率虽有小幅波动,但基本维持在80%以上;当进水ClO−4 和NO−3 浓度为150 mg·L−1和100 mg·L−1时,ClO−4 的去除率在90%以上,而NO−3 的去除率发生明显波动,经几天的运行恢复到90%以上。这可能是因为ClO−4 对微生物具有毒性,随着进水ClO−4 浓度的增加,导致大量反硝化细菌被杀死或抑制。另外,在ClO−4 还原过程中会产生氧气,氧气的产生会抑制反应体系中厌氧菌的生长。因此,NO−3 的去除率明显降低,但随着高氯酸盐还原菌的富集,NO−3 的去除率又逐渐恢复,这与先前的研究结果[18]一致,高氯酸盐还原菌可以去除NO−3 。第30~40天(第Ⅳ阶段),进水ClO−4 浓度为200 mg·L−1,进水NO−3 浓度为50 mg·L−1,ClO−4 和NO−3 的去除率均可达到90%以上。第40~50天(第Ⅴ阶段),进水ClO−4 浓度为250 mg·L−1,进水中无NO−3 浓度的加入,ClO−4 和NO−3 的去除率可以维持在90%以上,这表明经过了50 d的培养,反应器中成功培养出了高氯酸盐还原颗粒污泥。与先前99 d培养出高氯酸盐还原颗粒污泥[8]相比,本研究通过逐步降低NO−3 浓度的同时逐步增加ClO−4 浓度的培养方法,较为显著地减少了高氯酸盐还原颗粒污泥的培养时间。这可能是由于NO−3 与ClO−4 共同代谢过程中,反硝化细菌的NO−3 代谢过程中会产生一定量的胞外分泌物,可以在一定程度上减弱ClO−4 对颗粒污泥中各类菌体细胞的毒性,从而减少在高氯酸盐还原细菌不断富集的过程中其他微生物的大量死亡和颗粒污泥的瓦解,进而加快了反硝化颗粒污泥向高氯酸盐还原颗粒污泥的转化。谢宇轩等[19]的研究中也发现,在ClO−4 和NO−3 的混合体系中,NO−3 的存在会促进所有参与ClO−4 降解的细菌(高氯酸盐还原菌和部分反硝化细菌)的生长和发育。2.2 高氯酸盐还原颗粒污泥形成过程中颗粒污泥特性
颗粒污泥特性(MLSS、MLVSS、粒径分布和EPS组分)直接影响着反应器运行以及污染物的去除效果。因此,本研究对高氯酸盐还原颗粒污泥形成过程中的颗粒污泥特性进行了研究。
MLSS和MLVSS可以代表反应器中颗粒污泥浓度,在高氯酸盐还原颗粒污泥培养的过程中,MLSS和MLVSS的变化如图3所示。由图3可知,第0~20天(第Ⅰ~Ⅱ阶段),MLSS的含量由42.33 g·L−1降低到30.64 g·L−1,MLVSS的含量由25.84 g·L−1降低到21.28 g·L−1,反应器内的生物量呈现下降的趋势。这可能是因为
ClO−4 的加入对微生物产生毒害作用,并且随着ClO−4 浓度的增加,其毒害作用有所加强,从而抑制了反硝化细菌的生长,且与高氯酸盐还原代谢相关的菌种还未形成优势菌种。第20~50天(第Ⅲ~Ⅴ阶段),MLSS的含量由30.64 g·L−1增加到50.68 g·L−1,MLVSS的含量由21.28 g·L−1增加到40.58 g·L−1。反应器内生物量呈现上升的趋势,这可能是因为随着反应器的运行,反应器中逐渐富集了大量的高氯酸盐还原菌,因此,MLSS和MLVSS开始逐步升高。另外,在整个实验过程中,MLVSS/MLSS的变化趋势呈逐步上升的趋势,MLVSS/MLSS由0.61增加到0.80,本研究结果与先前的研究结果[20-21]相似。在颗粒污泥的驯化初期,反应体系内MLSS、MLVSS和MLVSS/MLSS均呈现下降的趋势,但随着运行时间的延长,污泥对污染物去除能力不断增强,MLSS、MLVSS和MLVSS/MLSS逐渐增加。TANG等[22]的研究发现,UASB反应器经过450 d培养出颗粒化较好的厌氧氨氧化颗粒污泥,其具有较高的脱氮能力,反应器内MLVSS为42.00~57.70 g·L−1。本研究经过50 d所得到的高氯酸盐还原颗粒污泥,其MLSS和MLVSS分别为50.68 g·L−1和40.58 g·L−1,MLVSS/MLSS为0.80,考虑到去除不同污染物的颗粒污泥性能上会稍有差异,且ClO−4 去除率维持在90%以上,因此,确认高氯酸盐颗粒污泥培养成功。在高氯酸盐还原颗粒污泥培养过程中,污泥颗粒粒径分布的变化如图4所示。由图4可知,在整个反应器运行期间(第Ⅰ~Ⅴ阶段),颗粒污泥粒径呈先减小后增大的趋势。第0~20天(第Ⅰ~Ⅱ阶段),粒径范围在<0.60 mm和0.60~1.00 mm的颗粒有所增加,分别由40.00%和22.00%增加到45.00%和25.00%;粒径为1.00~2.00、2.00~4.75和>4.75 mm的颗粒有所减少,分别由26.00%、10.00%和2.00%降低到23.00%、6.50%和0.50%。这可能是因为随着
ClO−4 浓度的增加,对微生物的毒害作用增强,容易造成颗粒解体,进而形成较小的颗粒。第21~50天(第Ⅲ~Ⅴ阶段),粒径范围在<0.60 mm和0.60~1.00 mm的颗粒逐步减少,所占百分比分别降低到36.49%和20.27%;粒径范围为1.00~2.00、2.00~4.75和>4.75 mm的颗粒增加,其对应的占比分别增加到28.46%、12.30%和2.48%。这可能是因为随培养时间的延长,微生物群落中能代谢ClO−4 的细菌大量富集,从而促进了反应器内颗粒污泥的颗粒化。有研究[23-24]表明,颗粒粒径也是表征颗粒污泥是否培养成功的重要指标。唐鹏等[23]的研究发现,UASB反应器连续运行127 d便可成功培养出厌氧氨氧化颗粒污泥,其脱氮能力高且颗粒粒径为1.00~3.00 mm。丛岩等[24]经过80 d培养出脱氮能力较高的厌氧氨氧化颗粒污泥,其平均直径为0.556 mm。本研究经过50 d的培养得到的高氯酸盐还原颗粒污泥,其主要粒径范围在<0.60 mm和1.00~2.00 mm,且ClO−4 去除率维持在90%以上,高氯酸盐颗粒污泥培养成功。EPS是由PN、PS和其他物质构成的高分子聚合物,其在颗粒污泥团聚和保护细菌在不利条件下生成中起着重要作用[25-26]。高氯酸盐还原颗粒污泥培养过程中EPS及其组分变化如图5所示。由图5可知,在整个反应器运行期间(第Ⅰ~Ⅴ阶段),EPS、PN和PS呈先下降后上升的趋势。第0~20天(第Ⅰ~Ⅱ阶段),EPS从开始时的24.62 mg·g−1下降到20.66 mg·g−1,PN从开始时的8.47 mg·g−1下降到6.85 mg·g−1,PS从开始时的11.29 mg·g−1下降到9.48 mg·g−1。这可能是因为
ClO−4 的加入对细菌具有毒性,导致部分微生物死亡脱落,因此,EPS及其组分的含量有所下降。第20~50天(第Ⅲ~Ⅴ阶段),EPS从20.66 mg·g−1上升到26.40 mg·g−1,PN从6.85 mg·g−1上升到10.02 mg·g−1,PS从9.48 mg·g−1上升到13.48 mg·g−1。这可能是因为随着高氯酸盐还原菌的富集,促进了EPS及其组分的分泌。然而,在高氯酸盐还原颗粒污泥培养的过程中,虽然EPS,PN和PS均呈先下降后上升的趋势,但其数值变化幅度较小,且PN/PS基本维持在0.75左右。以上结果表明,在还原颗粒污泥培养过程中,当有NO−3 存在时,ClO−4 浓度的增加并不会导致EPS及其组分的明显改变,推测该现象是由于反硝化细菌代谢NO−3 过程中,其产生的EPS及其组分可减少在高氯酸盐还原细菌富集过程中其他细菌的大量死亡和颗粒污泥的瓦解,从而加快了反硝化颗粒污泥向高氯酸盐还原颗粒污泥的转化。2.3 菌群结构分析
微生物群落对颗粒污泥的形成及特性具有重要影响,不同微生物有不同的功能。有些细菌具有促进颗粒污泥形成的作用,有些细菌可以代谢污染物。因此,为了进一步解读高氯酸盐还原颗粒污泥的形成过程,对反硝化颗粒污泥和高氯酸盐还原颗粒污泥的菌群结构进行了研究。
α多样性可以反映微生物群落中物种的数目,其中用Chao1和ACE指数评估群落的物种丰度,用Shannon和Simpson指数评估群落的物种多样性。反硝化颗粒污泥和高氯酸盐还原颗粒污泥的α多样性参数如表2所示。由表2可知,反硝化颗粒污泥和高氯酸盐还原颗粒污泥的覆盖率(coverage)均大于95%,表明样品中未被检测出来的序列的概率较低。在高氯酸盐还原颗粒污泥培养的过程中,Chao1和ACE指数从1 446.26和1 507.87降低到831.47和868.04,这表明颗粒污泥中的物种丰度降低。另外,在高氯酸盐还原颗粒污泥培养的过程中,Shannon指数从4.71降低到3.18,Simpson指数从0.04上升到0.12,这表明颗粒污泥中的物种多样性降低。以上结果表明,随着
ClO−4 浓度的增加,ClO−4 对菌的抑制作用增强,导致菌群的丰度和多样性均有所降低。表 2 α多样性参数Table 2. Alpha diversity parameters样品 OTU Shannon Simpson ACE Chao1 覆盖率 反硝化颗粒污泥 1 306 4.71 0.04 1 507.87 1 446.26 0.99 高氯酸盐还原颗粒污泥 656 3.18 0.12 868.04 831.47 0.99 将微生物检测相对丰度>1%的菌门作为主要菌门,反硝化颗粒污泥和高氯酸盐还原颗粒污泥在门水平的群落结构分析如图6所示。由图6可知,反硝化颗粒污泥和高氯酸盐还原颗粒污泥中共发现4个主要菌门,分别为Proteobacteria、Bacteroidetes、Chloroflexi和Firmicutes。LIAN等[27]的研究表明,大部分高氯酸盐还原菌和反硝化细菌属于Proteobacteria和Chloroflexi。在本实验中,Proteobacteria和Chloroflexi在反硝化颗粒污泥中为主要的优势菌,所占的比例分别为82.66%和3.82%;Proteobacteria和Chloroflexi在高氯酸盐还原颗粒污泥中也为主要的优势菌,所占的比例分别为45.47%和26.15%。
为了进一步分析群落结构的变化,反硝化颗粒污泥和高氯酸盐还原颗粒污泥在属水平的群落结构分析结果如图7所示。有研究表明:Methyloversatilis[17]、Thauera[8]和Hydrogenophaga[8]为反硝化细菌;Azospira[8]、Longilinea[17]和Ancalomicrobium[8]为高氯酸盐还原菌。在高氯酸盐还原颗粒污泥培养过程中,Methyloversatilis、Thauera和Hydrogenophaga在颗粒污泥中所占的比例由46.07%、2.67%和1.33%下降到0.96%、1.11%和0.42%,这表明在培养过程中反硝化细菌数量有所减少;而Azospira、Longilinea和Ancalomicrobium在颗粒污泥中所占的比例由11.50%、1.62%和0.01%升高到15.15%、9.95%和3.95%,这表明在培养过程中高氯酸盐还原颗粒污泥数量有所增加。此外,Ornatilinea在高氯酸盐还原颗粒污泥培养过程中所占的比例由0.01%升高到1.43%,这表明Ornatilinea可能为高氯酸盐还原菌。
3. 结论
1)以反硝化颗粒污泥为接种污泥,通过逐步降低
NO−3 浓度的同时逐步增加ClO−4 浓度的方法,可在50 d快速培养出高氯酸盐还原颗粒污泥。NO−3 浓度的逐步降低,可缓解ClO−4 对颗粒污泥中各类微生物的毒性,是本研究利用反硝化颗粒污泥快速培养高氯酸盐还原颗粒污泥的技术关键之一。2)高氯酸盐还原颗粒污泥培养成功后,颗粒污泥特性良好,MLSS和MLVSS分别为50.68 g·L−1和40.58 g·L−1,主要粒径分布在<0.60 mm和1.00~2.00 mm。培养过程中EPS及其组分变化幅度较小,这说明逐步降低进水
NO−3 浓度,对促进高氯酸盐颗粒污泥快速培养具有重要作用。 -
表 1 5种细胞毒性药物和内标的串联质谱参数
Table 1. Detection parameters of 5 cytotoxic drugs and internal standard with tandem mass spectrometry
化合物Compounds 保留时间/min Retention time 母离子 Precursor ion (m/z) 子离子 Product ions (m/z) 锥孔电压/V Cone voltage 碰撞能量/V Collision energy GEM 0.42 264.3 112.0 72 23 APR 1.84 189.3 56.0 78 49 CTX 2.6 261.1 140.1 92 29 EPI 2.86 544.3 379.0 105 27 VP-16 3.19 589.3 229.1 116 20 TAX 3.61 876.4 308.2 60 28 表 2 5种细胞毒性药物和内标物的基质效应及回收率
Table 2. The matrix effect and recovery of 5 cytotoxic drugs and IS
化合物Compounds 浓度/(ng·mL−1)Concentration 基质效应/%Matrix effect 内标归一化的基质效应/%IS-normalized matrix effect 回收率/%Recovery 内标归一化的回收率/%IS-normalized recovery GEM 5 98.2±6.2 94.8±5.5 94.3±9.8 92.2±5.0 25 96.2±1.1 94.5±0.7 92.8±8.4 105.9±11.9 100 96.5±1.3 94.0±2.4 97.8±5.2 108.7±3.0 CTX 5 100.2±2.4 96.7±1.2 91.6±10.8 89.5±7.2 25 98.9±7.5 97.1±7.0 92.5±3.7 105.4±7.3 100 103.0±3.7 100.3±2.5 90.8±3.4 101.3±2.3 EPI 5 101.9±6.4 98.4±8.7 95.6±4.7 93.7±4.3 25 100.5±4.2 98.7±5.7 93.3±3.0 106.2±2.9 100 101.9±1.4 99.3±2.6 91.3±1.6 102.8±1.8 VP-16 5 98.6±11.2 95.0±8.5 101.3±5.6 99.3±8.0 25 96.0±7.0 94.2±6.1 95.2±9.2 108.3±8.0 100 101.4±2.1 98.8±2.7 95.9±2.9 109.3±3.5 TAX 5 101.0±10.0 97.7±12.9 87.6±7.6 85.6±2.1 25 91.5±8.3 89.9±9.8 87.0±10.7 98.9±10.1 100 99.6±6.4 97.1±6.8 89.2±6.2 99.2±7.1 APR 10 102.7±1.9 92.5±9.1 表 3 5种细胞毒性药物的线性方程、线性范围、相关系数、检出限与定量限
Table 3. Linear regression equation, R2, linear range, detection limit and quantitative limit of 5 cytotoxic drugs
化合物Compounds 线性回归方程Regression equation 线性范围/( ng·mL−1) Linear range R2 LOD/ ( pg·cm−2) LOQ/ ( pg·cm−2) GEM y=0.186x+0.00628 0.1—800 0.9990 0.2 0.6 CTX y=0.0475x-0.00165 0.1—800 0.9996 0.2 0.6 EPI y=0.0099x+0.000765 0.1—200 0.9994 0.4 1.2 VP-16 y=0.0209x+0.00026 0.1—200 0.9996 0.4 1.2 TAX y=0.00189x+0.0000717 0.2—200 0.9994 1 3.2 表 4 不同材质物表上5种细胞毒性药物的回收率及相对标准偏差(n=6)
Table 4. Recovery and RSD of 5 cytotoxic drugs on different materials surfaces (n=6)
化合物Compounds 添加10 ng Added 添加50 ng Added 添加200ng Added Recovery/% RSD/% Recovery /% RSD/% Recovery /% RSD/% 玻璃表面Glass surface GEM 86.4 4.9 104 4.5 84.9 4.3 CTX 108 1.9 106 2.7 98.0 2.4 EPI 98.5 7.1 93.0 4.8 97.0 6.6 VP-16 102 4.3 97.9 8.3 99.5 6.9 TAX 105 2.9 99.3 4.2 101 6.6 PVC 表面PVC surface GEM 87.7 5.5 89.1 6.4 85.1 1.4 CTX 107 4.7 98.6 4.0 98.7 3.8 EPI 97.5 2.2 99.2 0.8 94.0 6.1 VP-16 96.7 3.1 95.1 0.8 92.1 4.8 TAX 93.1 13.3 101 6.8 97.6 5.1 不锈钢表面Stainless steel surface GEM 89.0 4.7 88.8 6.3 87.2 9.7 CTX 85.7 4.5 101 1.5 97.9 3.6 EPI 68.3 3.8 67.1 1.3 69.4 7.7 VP-16 83.5 2.5 90.3 9.0 89.3 8.4 TAX 76.0 8.8 87.4 4.8 85.9 11.0 表 5 PIVAS工作区域5种细胞毒性药物残留情况
Table 5. Residues of 5 cytotoxic drugs in PIVAS working area
残留量/ (pg·cm−2 ) Residues GEM CTX EPI VP-16 TAX 准备区桌面Preparation area -desktop 13.6 34.3 — 1.8 24.8 准备区推车Preparation area-trolley worktop 69.0 83.0 — 37.8 69.0 准备区地面Preparation area-floor 9.1 64.7 — — 66.6 成品复核区桌面Check area-desktop 48.5 184.5 — 27.4 47.6 配置间推车Clean room- trolley worktop 339.3 594.3 — 14.9 47.8 传递窗Delivery window 95.1 246.4 — 12.8 23.4 传递窗门把手Delivery window handles 443.5 888.6 2.6 87.5 75.6 配置间门把手Clean room- door handles 443.0 689.9 — 127.5 60.2 配置间地面Clean room-floor 54.3 1463.6 — 24.1 32.7 生物安全柜台面BSC workbench 792.9 6391.9 1.9 292.3 349.0 生物安全柜侧壁BSC side wall 1552.7 3545.7 — 375.8 314.2 生物安全柜内侧玻璃Inside the windscreen of BSC 1013.4 10898.8 10.8 364.8 404.2 生物安全柜外侧玻璃Outside the windscreen of BSC 604.9 2253.2 — 85.1 102.7 生物安全柜外沿Outer edge of BSC 819.9 2650.8 1.6 1109.4 503.6 注: “ —” 表示无对应值. Note:“ —” Represented no corresponded value -
[1] SKOV T, MAARUP B, OLSEN J, et al. Leukaemia and reproductive outcome among nurses handling antineoplastic drugs [J]. British Journal of Industrial Medicine, 1992, 49(12): 855-861. [2] FRANSMAN W, ROELEVELD N, PEELEN S, et al. Nurses with dermal exposure to antineoplastic drugs: reproductive outcomes [J]. Epidemiology, 2007, 18(1): 112-119. doi: 10.1097/01.ede.0000246827.44093.c1 [3] SUSPIRO A, PRISTA J. Biomarkers of occupational exposure do anticancer agents: A minireview [J]. Toxicology Letters, 2011, 207(1): 42-52. doi: 10.1016/j.toxlet.2011.08.022 [4] National Toxicology Program. NTP 12th Report on Carcinogens[R]. Report on Carcinogens: Carcinogen Profiles, 2011, 12: 499. [5] CONNOR TH, LAWSON CC, POLOVICH M, et al. Reproductive health risks associated with occupational exposures to antineoplastic drugs in health care settings: a review of the evidence [J]. Journal of Occupational and Environmental Medicine, 2014, 56(9): 901-910. doi: 10.1097/JOM.0000000000000249 [6] FRANSMAN W, PEELEN S, HILHORST S, et al. A pooled analysis to study trends in exposure to antineoplastic drugs among nurses [J]. The Annals of Occupational Hygiene, 2007, 51(3): 231-239. [7] VIEGAS S, OLIVEIRA AC, CAROLINO E, et al. Occupational exposure to cytotoxic drugs: the importance of surface cleaning to prevent or minimise exposure [J]. Archives of Industrial Hygiene and Toxicology, 2018, 69(3): 238-249. doi: 10.2478/aiht-2018-69-3137 [8] HON C Y, TESCHKE K, CHU W, et al. Antineoplastic drug contamination of surfaces throughout the hospital medication system in Canadian hospitals [J]. Journal of Occupational and Environmental Hygiene, 2013, 10(7): 374-383. doi: 10.1080/15459624.2013.789743 [9] CONNOR TH, SESSINK PJ, HARRISON BR, et al. Surface contamination of chemotherapy drug vials and evaluation of new vial-cleaning techniques: results of three studies [J]. American Journal of Health-System Pharmacy, 2005, 62(5): 475-484. doi: 10.1093/ajhp/62.5.475 [10] MARIE P, CHRISTOPHE C, MANON R, et al. Environmental monitoring by surface sampling for cytotoxics: a review [J]. Environmental Monitoring and Assessment, 2017, 189(2): 52. doi: 10.1007/s10661-016-5762-9 [11] ACRAMEL A, CHOUQUET T, PLÉ A, et al. Development and validation of a liquid chromatography tandem mass spectrometry quantification method for 14 cytotoxic drugs in environmental samples [J]. Rapid Communications in Mass Spectrometry, 2020, 34(4): e8594. [12] 包健安, 沈国荣, 王人英, 等. 多中心PIVAS集中调配人员抗肿瘤药物职业暴露评估 [J]. 中国医院药学杂志, 2016, 36(9): 701-706. doi: 10.13286/j.cnki.chinhosppharmacyj.2016.09.01 BAO J A, SHEN G R, WANG R Y, et al. A multicenter investigation of occupational exposure risks in workers to antineoplastic drugs in pharmacy intravenous admixture services [J]. Chinese Journal of Hospital Pharmacy, 2016, 36(9): 701-706(in Chinese). doi: 10.13286/j.cnki.chinhosppharmacyj.2016.09.01
[13] 胡小刚, 张小华, 陈开杰, 等. 基于生物学指标评估我院PIVAS医务人员职业性接触抗肿瘤药物的风险 [J]. 中国药房, 2020, 31(18): 2283-2288. doi: 10.6039/j.issn.1001-0408.2020.18.19 HU X G, ZHANG X H, CHEN K J, et al. Evaluation of the risk of medical staff occupationally exposed to antineoplastic drugs in PIVAS of our hospital based on biological indicators [J]. China Pharmacy, 2020, 31(18): 2283-2288(in Chinese). doi: 10.6039/j.issn.1001-0408.2020.18.19
[14] ROSSIGNOL E, AMIAND M B, SORRIEUL J, et al. A fully validated simple new method for environmental monitoring by surface sampling for cytotoxics [J]. Journal of Pharmacological and Toxicological Methods, 2020, 101: 106652. doi: 10.1016/j.vascn.2019.106652 [15] 李芸, 沈国荣, 张晶晶, 等. 我国10家医院静脉药物调配中心抗肿瘤药物环境污染评估及工作人员防护措施效果比较 [J]. 中国医院药学杂志, 2021, 41(24): 2591-2596. doi: 10.13286/j.1001-5213.2021.24.17 LI Y, SHEN GR, ZHANG JJ, et al. Antineoplastic drug environmental pollution assessment and outcome comparisons of different staff protective measures in pharmacy intravenous admixture services at ten domestic hospitals [J]. Chinese Journal of Hospital Pharmacy, 2021, 41(24): 2591-2596(in Chinese). doi: 10.13286/j.1001-5213.2021.24.17
[16] 邹东岑, 徐帆. 4种细胞毒性药物对不同材质手套的穿透性研究 [J]. 华西药学杂志, 2019, 34(5): 475-480. doi: 10.13375/j.cnki.wcjps.2019.05.009 ZOU D C, XU F. Investigation on 4 cytotoxic drugs penetrating gloves made from different materials [J]. West China Journal of Pharmaceutical Sciences, 2019, 34(5): 475-480(in Chinese). doi: 10.13375/j.cnki.wcjps.2019.05.009
[17] 尹红梅. 某医院静脉用药调配中心工作人员抗肿瘤药物职业暴露评估研究 [J]. 中国当代医药, 2020, 27(18): 179-182. doi: 10.3969/j.issn.1674-4721.2020.18.052 YIN H M. Study of occupational exposure risk in workers to anticancer drugs in pharmacy intravenous admixture service of a hospital [J]. China Modern Medicine, 2020, 27(18): 179-182(in Chinese). doi: 10.3969/j.issn.1674-4721.2020.18.052
[18] NUSSBAUMER S, GEISER L, SADEGHIPOUR F, et al. Wipe sampling procedure coupled to LC-MS/MS analysis for the simultaneous determination of 10 cytotoxic drugs on different surfaces [J]. Analytical and Bioanalytical Chemistry, 2012, 402(8): 2499-2509. doi: 10.1007/s00216-011-5157-2 [19] TURCI R, SOTTANI C, SPAGNOLI G, et al. Biological and environmental monitoring of hospital personnel exposed to antineoplastic agents: a review of analytical methods [J]. Journal of Chromatography B, 2003, 789(2): 169-209. doi: 10.1016/S1570-0232(03)00100-4 [20] SUNG H, FERLAY J, SIEGEL RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries [J]. CA:a Cancer Journal for Clinicians, 2021, 71(3): 209-249. doi: 10.3322/caac.21660 [21] COX J, SPEED V, O'NEAL S, et al. Development and evaluation of a novel product to remove surface contamination of hazardous drugs [J]. Journal of Oncology Pharmacy Practice, 2017, 23(2): 103-115. doi: 10.1177/1078155215621151 [22] PORTILHA-CUNHA MF, ALVES A, SANTOS MSF. Cytostatics in indoor environment: an update of analytical methods [J]. Pharmaceuticals, 2021, 14(6): 574. doi: 10.3390/ph14060574 [23] Occupational Safety and Health Administration (OSHA). Evaluation Guidelines for Surface Sampling Methods; OSHA Salt Lake Technical Center: Salt Lake City, UT, USA, 2001. [24] COLOMBO M, JERONIMO M, ASTRAKIANAKIS G, et al. Wipe sampling method and evaluation of environmental variables for assessing surface contamination of 10 antineoplastic drugs by liquid chromatography/tandem mass spectrometry [J]. Annals of Work Exposures and Health, 2017, 61(8): 1003-1014. doi: 10.1093/annweh/wxx070 [25] JERONIMO M, COLOMBO M, ASTRAKIANAKIS G, et al. A surface wipe sampling and LC-MS/MS method for the simultaneous detection of six antineoplastic drugs commonly handled by healthcare workers [J]. Analytical and Bioanalytical Chemistry, 2015, 407(23): 7083-7092. doi: 10.1007/s00216-015-8868-y [26] KIFFMEYER T K, TUERK J, HAHN M, et al. Application and assessment of a regular environmental monitoring of the antineoplastic drug contamination level in pharmacies - the MEWIP project [J]. The Annals of Occupational Hygiene, 2013, 57(4): 444-455. -







 下载:
下载: