-
随着氯消毒工艺在饮用水处理中得到广泛应用[1],消毒副产物也随之产生。其中,卤代消毒副产物(DBPs)特别是卤乙酸(HAAs)等虽然在一些水体中的化学浓度相对较低,但因其具有高毒性、易发生致癌等特征,受到了人们越来越多的重视和关注[2]。我国《生活饮用水卫生标准》将卤乙酸的最高可允污染物排放量限制在150 μg·L−1[3]。美国环境保护署(USEPA)还提出饮用水中痕量卤代乙酸(HAA5)的污染物排放限值为60 μg·L−1[4]。
碘乙酸(MIAA)具有卤乙酸大多数的化学性质,在氯消毒过程中出现的浓度高达1.7 μg·L−1,但较其他卤代乙酸(如氯乙酸,溴乙酸)具有更强的细胞毒性和遗传毒性[5-9]。目前,常用的消毒副产物去除方法包括吸附法、生物法、氧化法、膜分离法等[10-14],但大多针对氯乙酸和溴乙酸,由于碘乙酸是近年来发现的新型消毒副产物[9],目前去除碘乙酸的方法鲜少报道,仅有的一些方法如采用UV-光解法降解碘乙酸的去除率只有3.35%[15],因此迫切地需要探索能够有效降解碘乙酸的方法。
催化加氢脱碘(hydrodeiodination,HDI)是一种简单、高效的去除碘代污染物的方法,该方法使用 H2 作为还原剂,能够在常温常压下将碘原子取代进而完成脱碘[16]。催化加氢脱碘已被证实可以去除多种碘代污染物,是一种新兴且相对绿色的方案。
本文采用硼氢化钠还原法制备了一系列不同载体(氧化铈、氧化铝、氧化硅和商用碳)负载的Pt基催化剂,并将其应用于水体中的MIAA的催化加氢脱碘,进一步探究了不同载体对催化剂活性的影响以及碘乙酸的脱碘机制。
-
载体γ- Al2O3 和SiO2分别购于上海五四化学试剂有限公司、上海市奉贤奉城试剂厂;CeO2载体采用沉积法合成,步骤如下:在剧烈搅拌条件下,将0.5 mol·L−1的氨水逐滴滴入250 mL 0.5 mol·L−1 Ce(NO3)3溶液中至pH=10,继续在室温下搅拌1 h后过滤、清洗、烘干(105 ℃,6 h)、焙烧(500 ℃,4 h)。得到的CeO2研磨过筛(400目)备用[17]。负载量为1.0 %wt的商用催化剂Pt/commercial C购于阿法埃莎(中国)化学有限公司。
催化剂采用硼氢化钠还原法制备,具体制备方法如下:首先,在含有氯铂酸溶液的30 mL去离子水中,加入一定量的载体,充分搅拌1h后,在0℃条件下向其中缓慢滴加2.5 mL 1.0 mol·L−1的NaBH4溶液,继续搅拌平衡2 h后,过滤、清洗,置于60 ℃真空烘箱干燥后,即得所需催化剂Pt(X)/B,其中X为负载量(以质量分数计),B为载体。
透射电镜分析(TEM) :透射电子显微镜,日本电子株式会社,JEM-200CX。X射线衍射分析(XRD):X射线衍射仪,日本理学株式会社,D/max-RA。电感耦合等离子体发射光谱( ICP-OES) :电感耦合等离子体发射光谱仪,美国Jarrel-Ash公司,J-A1100。X射线光电子能谱( XPS):X射线光电子能谱仪,美国赛默飞世尔科技公司,ESCALAB250。Zeta电位:Zeta电位仪,Zeta PALS Brookhaven公司。
-
在配备有pH计的 250 mL 四口烧瓶反应器中进行MIAA 的催化加氢脱碘反应,反应过程中用恒温浴锅中将反应温度控制在25 oC。简言之,在剧烈搅拌 (1500 r·min−1) 下,向200 mL 含一定浓度碘乙酸的反应液中,加入5—20 mg催化剂,用质量流量计控制的 N2 以50 mL·min−1的速率吹扫0.5 h以去除溶解氧。当 N2 流切换到 H2 流 (150 mL·min−1) 时,还原反应开始。在预设的时间点收集样品,样品通过 0.45 μm 滤膜过滤,并采用离子色谱(ICS1100,Dionex,美国)使用 10 mmol L−1 KOH 作为流动相进行MIAA的浓度分析。
-
采用初活性(r0)和翻转频率(TOF)对催化剂活性进行评价。其中,初活性是指MIAA转化率小于25%时,单位催化剂单位时间内MIAA浓度降低的速率,单位为mmol·L−1·g−1·h−1Cat。翻转频率指单位时间单位活性位点转化的反应物的量,单位为h−1。
-
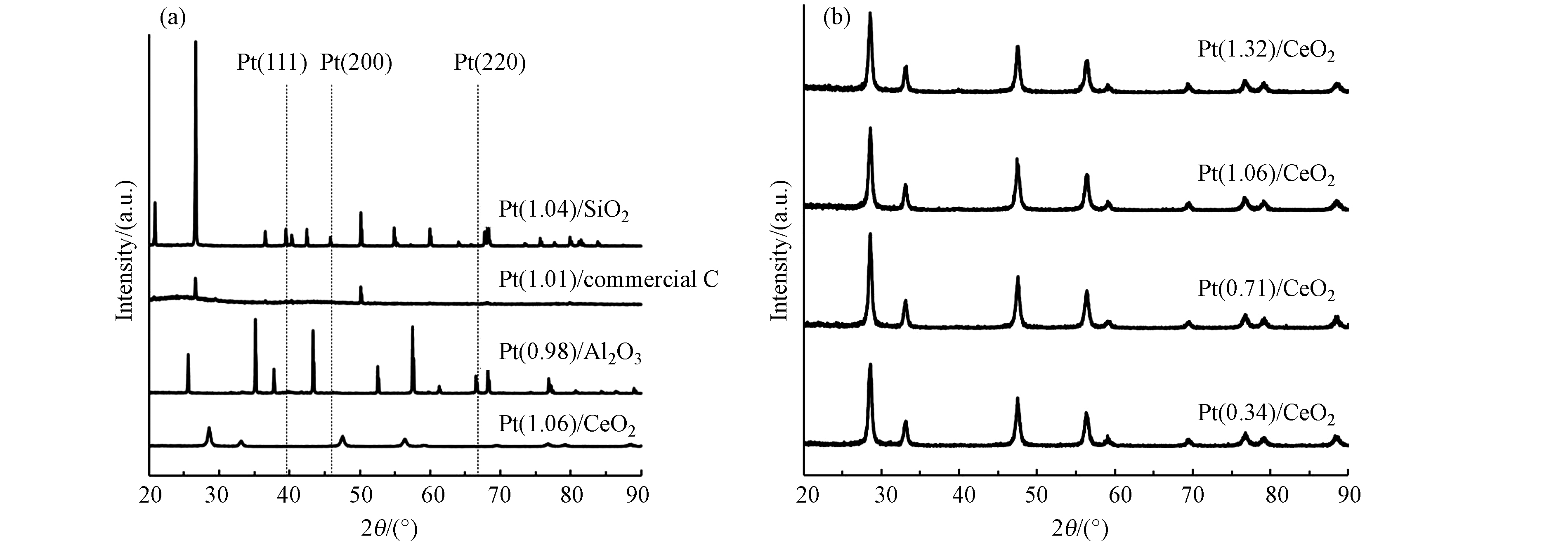
催化剂Pt(1.04)/SiO2、Pt(0.98)/Al2O3、Pt(1.01)/commercial C、Pt(1.06)/CeO2的XRD图如图1(a) 所示,各载体均有明显的特征峰,说明在合成过程中载体的结构是稳定的。对于 Pt(1.04)/SiO2、Pt(0.98)/Al2O3和Pt(1.01)/commercial C,在 2θ 为 39.8o、46.2o和67.5o 处观察到的衍射峰,对应于金属Pt的 (002)、(100) 和 (101) 晶面。而在催化剂Pt(0.34)/CeO2、Pt(0.71)/CeO2、Pt(1.06)/CeO2、Pt(1.32)/CeO2的XRD图(图1(b))上,均未观察到 Pt的特征峰,可能是Pt的负载量较低,颗粒粒径较小,分散度较高导致的。
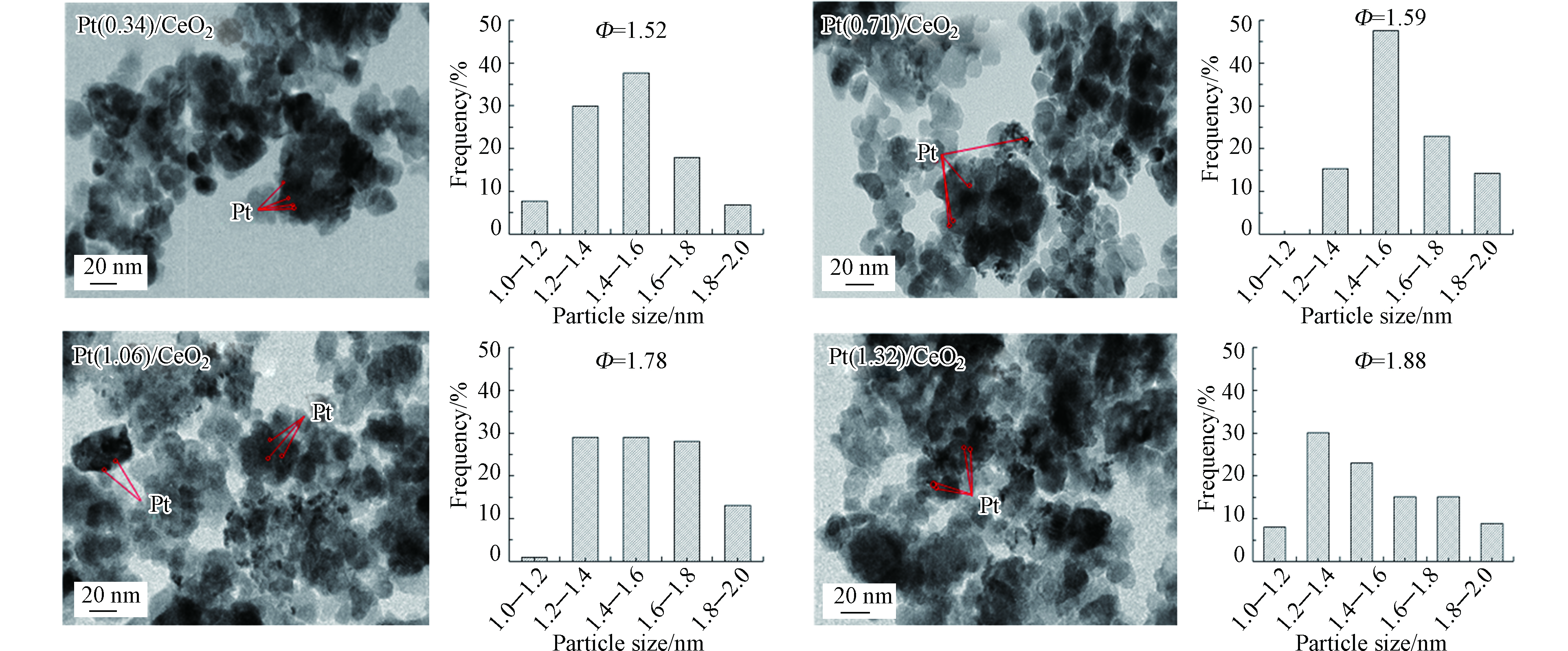
图2为不同负载量的Pt/CeO2催化剂的TEM和对应的粒径分布直方图,根据表面积加权直径估计催化剂中 Pt 颗粒的平均粒径:
式中,
−ds :催化剂表面Pt粒子的平均粒径,nm;ni:直径为di且总数超过100的颗粒的数量。从图2可以看出,Pt颗粒均匀的分散在载体CeO2上。随着Pt负载量的提高,Pt颗粒在催化剂表面发生团聚,表现为Pt纳米粒子的粒径不断增大。
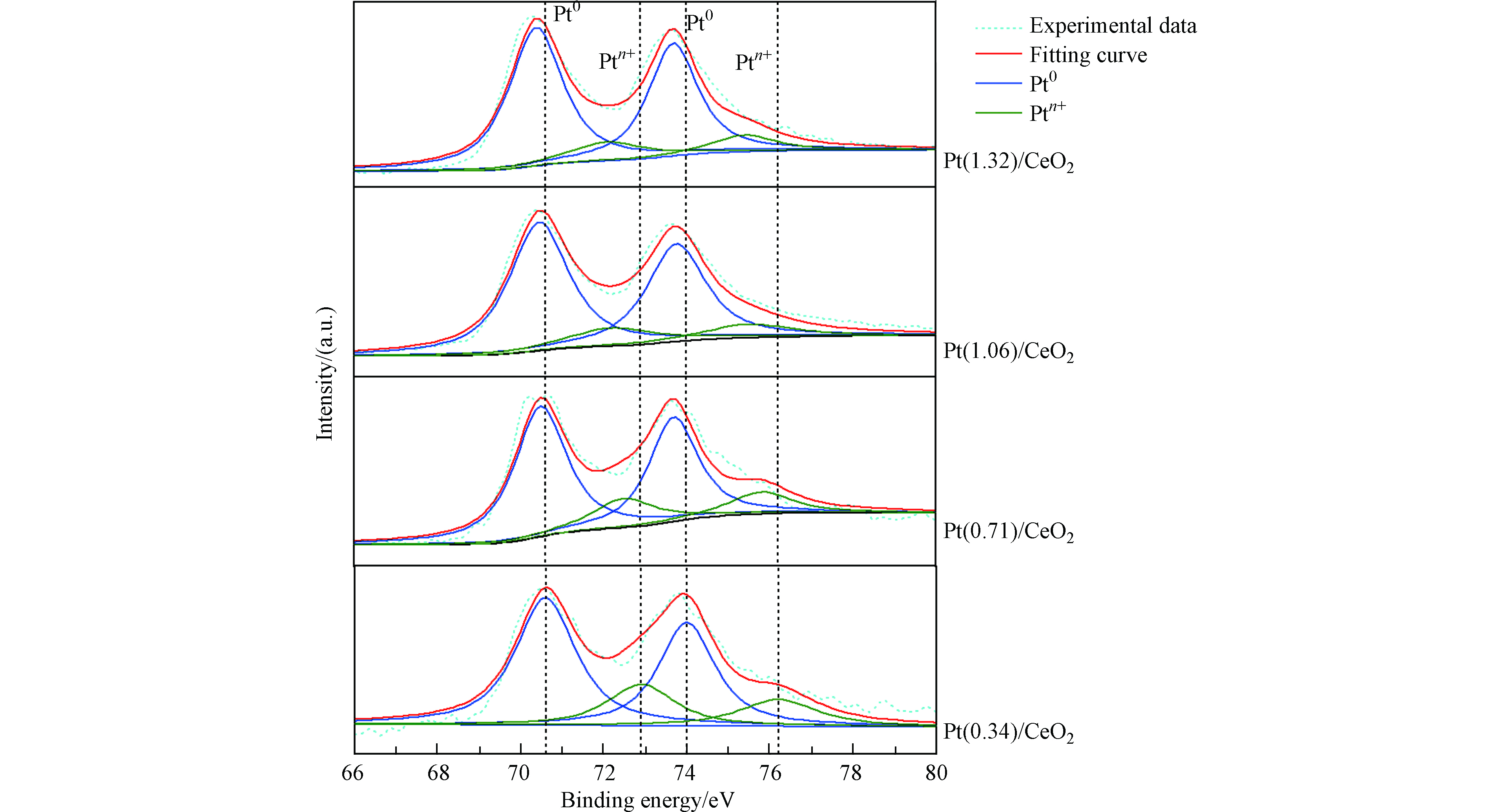
为了进一步了解具有不同负载量的催化剂Pt/CeO2中Pt的化学状态,进行了XPS测量,图3为金属Pt 4f区域的XPS图谱。从图3可以看出,所有催化剂的 Pt 4f7/2 峰均含有两个亚峰,位于70.4 eV和71.5 eV,分别归属于金属态Pt和阳离子态Pt。表1中总结了各催化剂的Ptn+与Pt0的比值,该比值越大,表明金属颗粒Pt与载体CeO2表面官能团之间的相互作用越强。可以发现,随着Pt的负载量从0.34%wt增加到1.32%wt,Ptn+/Pt0比值逐渐减小。表明高负载量的催化剂载体表面与Pt颗粒金属-载体相互作用较弱而低负载量的更强[18]。
-
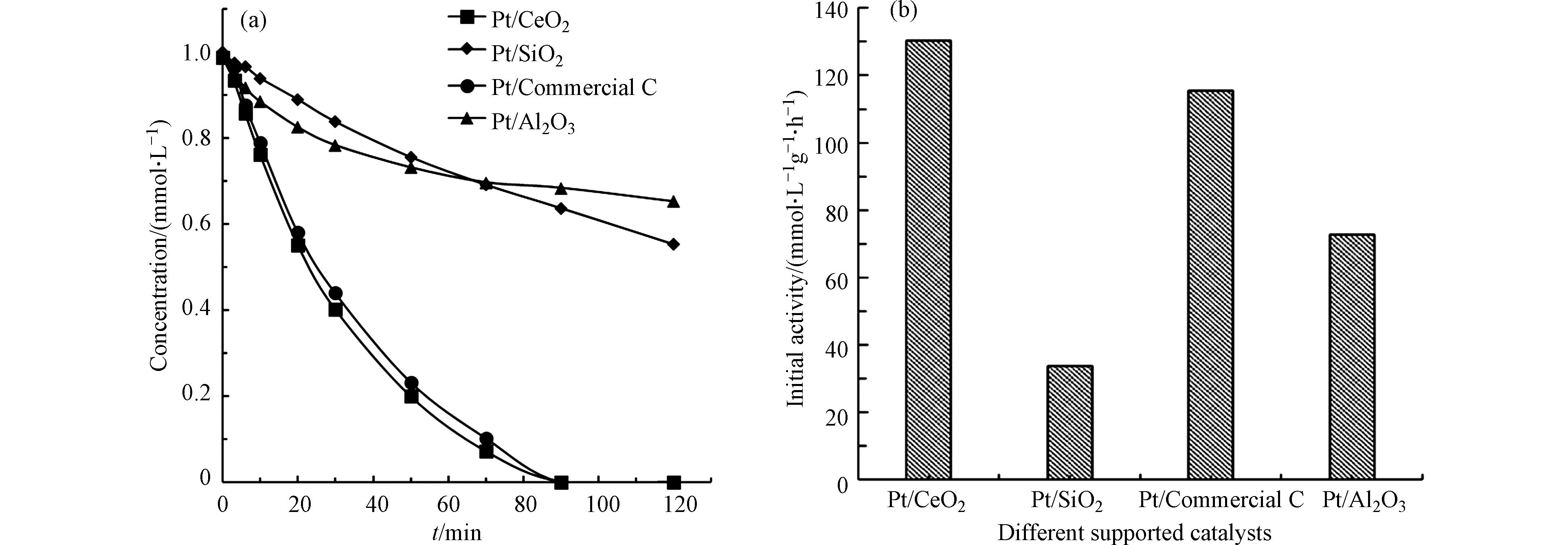
在初始pH值为5.6,MIAA的初始浓度为1.0 mmol·L−1, H2的流速为200 mL·min−1的反应条件下,分别以Pt(1.06)/CeO2、Pt(0.98)/Al2O3、Pt(1.04)/SiO2和Pt(1.01)/commercial C为催化剂进行液相加氢脱碘反应。结果如图4所示,可以看出Pt(1.04)/SiO2对MIAA催化加氢还原的初活性为33.66 mmol·L−1·g−1·h−1Cat,明显低于其他催化剂。经Zeta电位测定确定了CeO2、Al2O3、commercial C的等电点分别为6.4、9.0、2.0,SiO2的等电点小于2.0。在pH=5.6条件下,MIAA多以阴离子的形式存在(pKa=3.12)[19],因此阴离子化的MIAA与SiO2表面存在较强的静电斥力抑制了催化反应的进行。相反,Pt/CeO2表现出最高的催化活性(130.22 mmol·L−1·g−1·h−1Cat)这是CeO2载体较高的等电点和Pt在CeO2上高分散共同作用的结果。
-
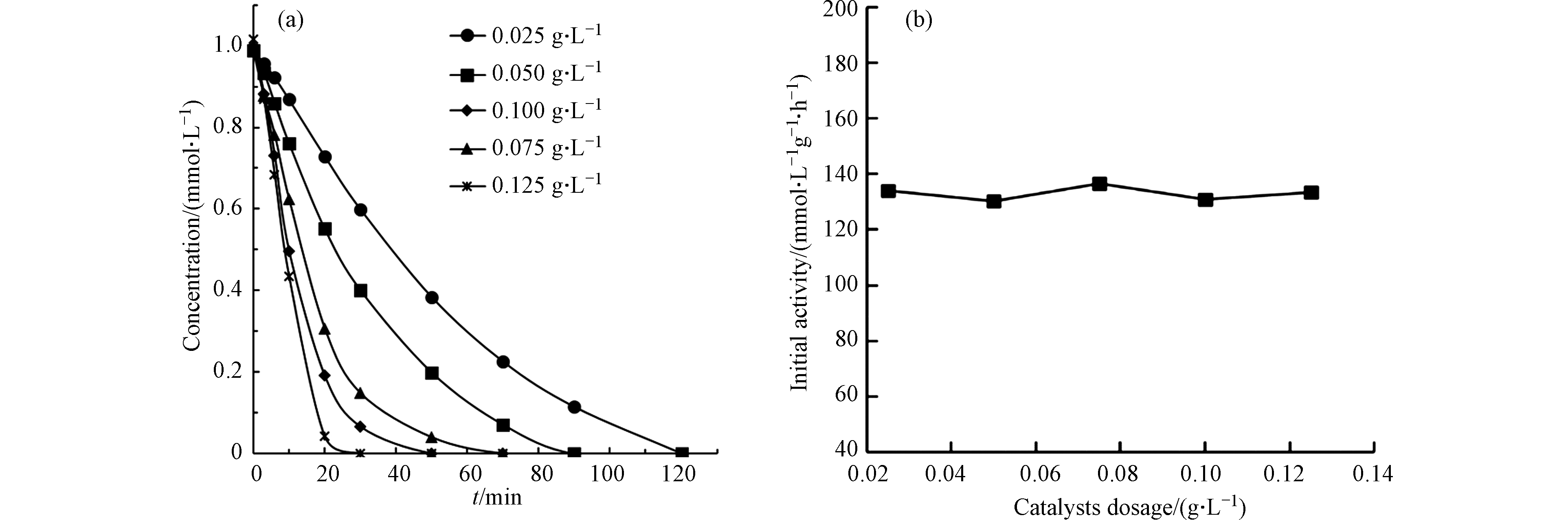
根据上述实验结果,选用Pt(1.06)/CeO2为催化剂,进一步探究反应条件对催化过程的影响。首先,调节反应初始pH值为5.6,MIAA的初始浓度为1.0 mmol·L−1,H2的流速为200 mL·min−1,改变催化剂投加量进行反应,结果如图5(a)所示。可以看出,随着催化剂投加量的增大,MIAA 的去除效率不断提高。而对催化剂投加量进行标化后,初活性基本保持不变(图5(b)),表明在本实验条件下,MIAA的催化加氢脱碘反应不受传质阻力的影响[20]。
-
为了进一步探究污染物在催化剂表面的吸附对催化过程产的影响,在初始反应pH为5.6,催化剂投加量为0.050 g·L−1条件下,以Pt(1.06)/CeO2为催化剂,对不同初始浓度的MIAA进行的催化还原脱碘反应。从图6可以看出,随着反应物的浓度的升高,反应初活性也逐渐提高表明反应速率与吸附的MIAA浓度之间呈正相关。结果进一步拟合了 Langmuir-Hinshelwood 模型 [21]:
式中,r 为初始MIAA的反应速率,C0 为初始MIAA浓度,k为反应速率常数,K为催化剂表面MIAA的吸附平衡常数,θs为催化剂表面MIAA的覆盖率。
实验所得的1/r与1/C0的关系如图6(c)所示,可以看出1/r与1/C0之间有良好的线性关系(R2>0.99),说明污染物MIAA在催化剂表面的吸附是反应的速率控制步骤。
-
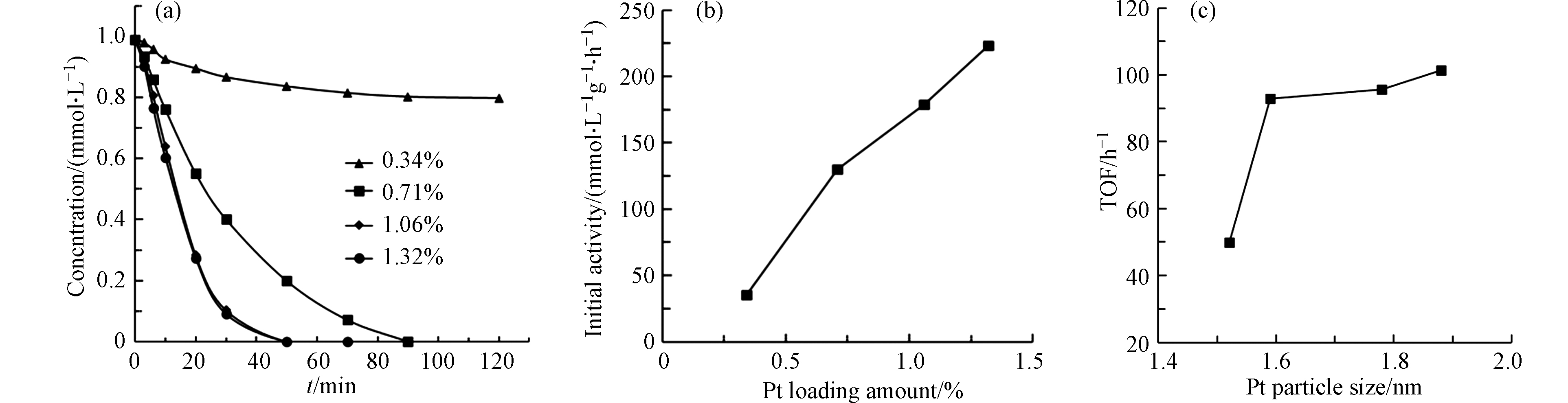
在pH为5.6,MIAA的初始浓度为1.0 mmol·L−1 ,H2的流速为200 mL·L−1,催化剂投加量为10 mg条件下,考察了Pt/CeO2催化剂的Pt负载量对加氢脱碘反应的影响。从图7可以看出,随着催化剂的负载量从0.34%wt增加到1.32%wt,催化剂的初始活性从35.05 mmol·L−1·g−1·h−1Cat提高到223.43 mmol·L−1·g−1·h−1Cat,这可能是由于高负载量产生更多的活性位点造成的。为进一步探究Pt活性位的性质对活性的影响,本研究比较了对应的TOF值。根据先前的报道,在液相催化加氢脱卤反应中,阳离子态Ptn+活化C—X键和金属态Pt0活化H—H键是反应的关键步骤[22]。图7(c)显示,催化剂的TOF值随着Pt纳米颗粒粒径的增加而提高,主要由于大颗粒Pt催化剂具有更高的Pt0比例,有助于活化H2,从而催化剂活性得到提高,进一步说明了在本反应中与C—I键活化相比,H—H键的活化起更重要的作用。
-
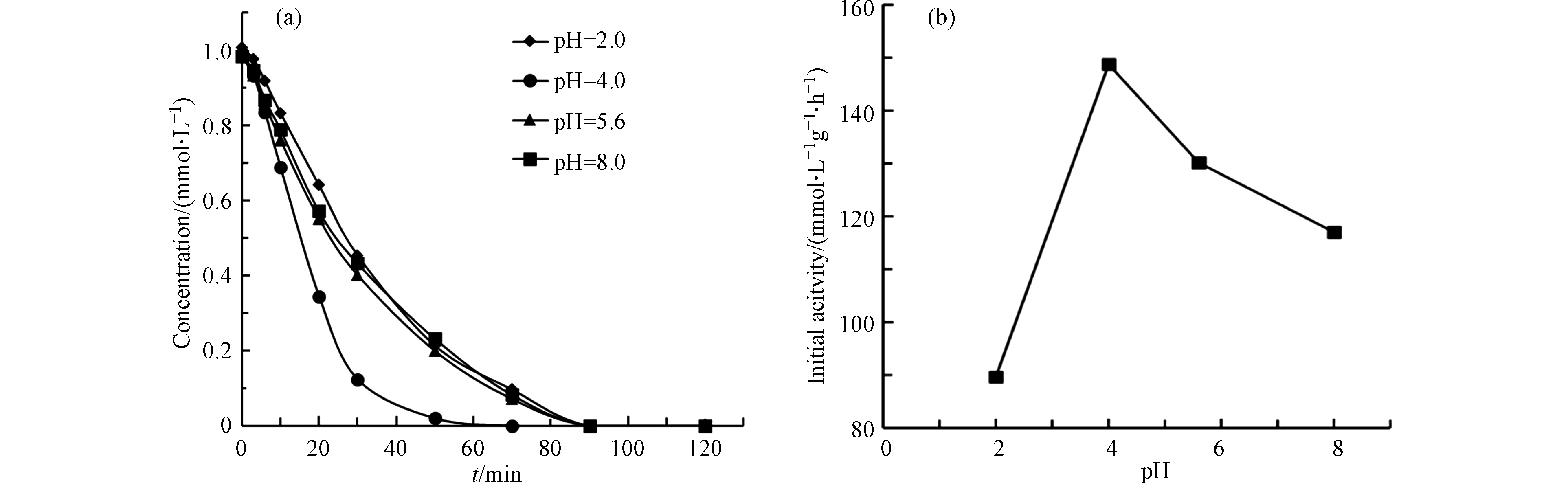
由于pH会大大影响污染物形态和催化剂表面电性,因此,在催化剂Pt(1.06)/CeO2用量为0.050 g·L−1,MIAA的初始浓度为1.0 mmol·L−1的条件下,调节反应的初始pH,探索不同pH对MIAA加氢脱碘反应的影响。结果如图8所示,可以发现,催化剂的初活性随pH的增大先升后降,呈火山型变化。
当反应初始 pH值为2.0时,MIAA(pKa =3.12)在溶液中以分子态存在,不利于其在催化剂表面的吸附; 当pH值升高到4.0时,MIAA开始以阴离子态存在,同时由于催化剂Pt(1.06)/CeO2的Zeta电位为6.1,因此去质子化的MIAA和质子化的催化剂表面产生了较强的静电引力,进而使初活性由89.69 mmol·L−1·g−1·h−1Cat上升到148.73 mmol·L−1·g−1·h−1Cat。当反应溶液的pH进一步提高至8.0,去质子化的催化剂表面与阴离子态的MIAA之间产生了越来越强的静电斥力,故活性也随之降低。此结果进一步说明了污染物在催化剂表面的吸附对反应有重要作用。
-
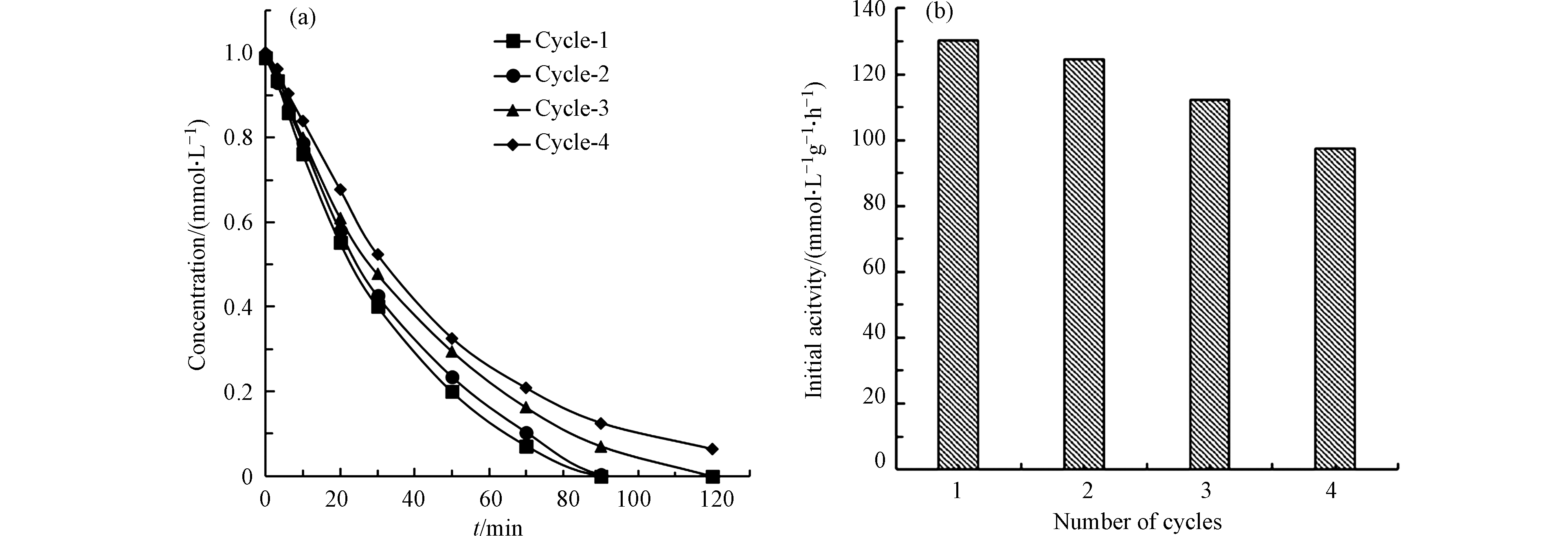
以5.6的初始pH,1.0 mmol·L−1的MIAA初始浓度, 0.050 g·L−1的催化剂Pt(1.06)/CeO2用量,200 mL·min−1的H2流速为反应条件,进行了MIAA的加氢脱碘反应,在每次反应结束后,向反应器中直接添加等量的MIAA,进行了4次循环实验。从图9可以看出,循环结束后初活性虽降低了约25%,仍然能将93.4%反应物MIAA去除,说明材料经过4次循环仍然能具有比较好的活性。活性的下降可能是Pt颗粒在反应过程中由于剧烈搅拌脱落造成的,该现象在先前的研究中也有报道[23]。
-
(1)分别以SiO2、Al2O3、商用C、CeO2为载体,通过硼氢化钠还原法制备了不同的金属Pt基负载型催化剂,其中Pt/CeO2的催化加氢脱碘活性最高,Pt/SiO2活性最低。
(2)Pt/CeO2催化剂中Pt高度分散,对碘乙酸的催化加氢脱碘符合Langmuir-Hinshelwood模型,其催化活性随Pt负载量的增大而增强,受pH影响较大。
(3)多次循环使用后,Pt/CeO2催化剂仍具有较高的MIAA转化率,保持了良好的稳定性。
负载型Pt/CeO2催化剂对碘乙酸的催化加氢脱碘研究
Study on catalytic hydrodeiodination of iodoacetic acid on CeO2 supported Pt catalyst
-
摘要: 采用硼氢化钠还原法制备了一系列不同载体(氧化铈、氧化铝、氧化硅和商用碳)的负载型Pt基催化剂。通过透射电子显微镜(TEM)、X射线衍射(XRD)、X射线光电子能谱(XPS)、电感耦合等离子体发射光谱(ICP-OES)等手段对催化剂的结构进行了详细表征,并对碘乙酸(MIAA)的液相催化加氢脱碘反应进行了研究。结果表明,Pt/CeO2具有比其他催化剂高得多的MIAA催化加氢脱碘活性;Pt/CeO2催化活性与溶液pH呈现先升后降的火山型变化,且随Pt负载量的增加而提高;MIAA的催化加氢脱碘反应符合朗格缪尔-欣谢尔伍德(Langmuir-Hinshelwood)模型,表明MIAA脱碘反应的速率控制步骤是污染物MIAA在催化剂表面的吸附过程;经4次循环使用后,Pt/CeO2催化剂仍能去除93.4%的MIAA,具有较高的活性。Abstract: The Pt catalysts supported on varied material (CeO2, Al2O3, SiO2 and commercial C) were synthesized by the sodium borohydride reduction method. The structural properties of the catalysts were characterized in detail by TEM, XRD,XPS and ICP-OES, and their catalytic activities of liquid phase hydrodeiodination of iodoacetic acid (MIAA) were examined. The results showed that Pt/CeO2 had much higher MIAA catalytic hydrodeiodination activity than other catalysts. The initial activity of Pt/CeO2 showed an up-side down volcano-type dependency on pH, and enhanced with Pt loading amount. The catalytic hydrodeiodination reaction of MIAA conformed to the Langmuir-Hinshelwood model, which indicated that the adsorption of MIAA on the catalyst surface is the rate-controlling step of this reaction. In addition, the Pt/CeO2 catalyst can still remove 93.4% of MIAA after 4 cycles of use, and exhibits high catalytic stability during catalyst reuse.
-
随着氯消毒工艺在饮用水处理中得到广泛应用[1],消毒副产物也随之产生。其中,卤代消毒副产物(DBPs)特别是卤乙酸(HAAs)等虽然在一些水体中的化学浓度相对较低,但因其具有高毒性、易发生致癌等特征,受到了人们越来越多的重视和关注[2]。我国《生活饮用水卫生标准》将卤乙酸的最高可允污染物排放量限制在150 μg·L−1[3]。美国环境保护署(USEPA)还提出饮用水中痕量卤代乙酸(HAA5)的污染物排放限值为60 μg·L−1[4]。
碘乙酸(MIAA)具有卤乙酸大多数的化学性质,在氯消毒过程中出现的浓度高达1.7 μg·L−1,但较其他卤代乙酸(如氯乙酸,溴乙酸)具有更强的细胞毒性和遗传毒性[5-9]。目前,常用的消毒副产物去除方法包括吸附法、生物法、氧化法、膜分离法等[10-14],但大多针对氯乙酸和溴乙酸,由于碘乙酸是近年来发现的新型消毒副产物[9],目前去除碘乙酸的方法鲜少报道,仅有的一些方法如采用UV-光解法降解碘乙酸的去除率只有3.35%[15],因此迫切地需要探索能够有效降解碘乙酸的方法。
催化加氢脱碘(hydrodeiodination,HDI)是一种简单、高效的去除碘代污染物的方法,该方法使用 H2 作为还原剂,能够在常温常压下将碘原子取代进而完成脱碘[16]。催化加氢脱碘已被证实可以去除多种碘代污染物,是一种新兴且相对绿色的方案。
本文采用硼氢化钠还原法制备了一系列不同载体(氧化铈、氧化铝、氧化硅和商用碳)负载的Pt基催化剂,并将其应用于水体中的MIAA的催化加氢脱碘,进一步探究了不同载体对催化剂活性的影响以及碘乙酸的脱碘机制。
1. 材料与方法 (Materials and methods)
1.1 催化剂的制备与表征方法
载体γ- Al2O3 和SiO2分别购于上海五四化学试剂有限公司、上海市奉贤奉城试剂厂;CeO2载体采用沉积法合成,步骤如下:在剧烈搅拌条件下,将0.5 mol·L−1的氨水逐滴滴入250 mL 0.5 mol·L−1 Ce(NO3)3溶液中至pH=10,继续在室温下搅拌1 h后过滤、清洗、烘干(105 ℃,6 h)、焙烧(500 ℃,4 h)。得到的CeO2研磨过筛(400目)备用[17]。负载量为1.0 %wt的商用催化剂Pt/commercial C购于阿法埃莎(中国)化学有限公司。
催化剂采用硼氢化钠还原法制备,具体制备方法如下:首先,在含有氯铂酸溶液的30 mL去离子水中,加入一定量的载体,充分搅拌1h后,在0℃条件下向其中缓慢滴加2.5 mL 1.0 mol·L−1的NaBH4溶液,继续搅拌平衡2 h后,过滤、清洗,置于60 ℃真空烘箱干燥后,即得所需催化剂Pt(X)/B,其中X为负载量(以质量分数计),B为载体。
透射电镜分析(TEM) :透射电子显微镜,日本电子株式会社,JEM-200CX。X射线衍射分析(XRD):X射线衍射仪,日本理学株式会社,D/max-RA。电感耦合等离子体发射光谱( ICP-OES) :电感耦合等离子体发射光谱仪,美国Jarrel-Ash公司,J-A1100。X射线光电子能谱( XPS):X射线光电子能谱仪,美国赛默飞世尔科技公司,ESCALAB250。Zeta电位:Zeta电位仪,Zeta PALS Brookhaven公司。
1.2 实验方法
在配备有pH计的 250 mL 四口烧瓶反应器中进行MIAA 的催化加氢脱碘反应,反应过程中用恒温浴锅中将反应温度控制在25 oC。简言之,在剧烈搅拌 (1500 r·min−1) 下,向200 mL 含一定浓度碘乙酸的反应液中,加入5—20 mg催化剂,用质量流量计控制的 N2 以50 mL·min−1的速率吹扫0.5 h以去除溶解氧。当 N2 流切换到 H2 流 (150 mL·min−1) 时,还原反应开始。在预设的时间点收集样品,样品通过 0.45 μm 滤膜过滤,并采用离子色谱(ICS1100,Dionex,美国)使用 10 mmol L−1 KOH 作为流动相进行MIAA的浓度分析。
1.3 催化剂活性评估
采用初活性(r0)和翻转频率(TOF)对催化剂活性进行评价。其中,初活性是指MIAA转化率小于25%时,单位催化剂单位时间内MIAA浓度降低的速率,单位为mmol·L−1·g−1·h−1Cat。翻转频率指单位时间单位活性位点转化的反应物的量,单位为h−1。
2. 结果讨论 (Results and discussion)
2.1 催化剂的表征结果
催化剂Pt(1.04)/SiO2、Pt(0.98)/Al2O3、Pt(1.01)/commercial C、Pt(1.06)/CeO2的XRD图如图1(a) 所示,各载体均有明显的特征峰,说明在合成过程中载体的结构是稳定的。对于 Pt(1.04)/SiO2、Pt(0.98)/Al2O3和Pt(1.01)/commercial C,在 2θ 为 39.8o、46.2o和67.5o 处观察到的衍射峰,对应于金属Pt的 (002)、(100) 和 (101) 晶面。而在催化剂Pt(0.34)/CeO2、Pt(0.71)/CeO2、Pt(1.06)/CeO2、Pt(1.32)/CeO2的XRD图(图1(b))上,均未观察到 Pt的特征峰,可能是Pt的负载量较低,颗粒粒径较小,分散度较高导致的。
图2为不同负载量的Pt/CeO2催化剂的TEM和对应的粒径分布直方图,根据表面积加权直径估计催化剂中 Pt 颗粒的平均粒径:
−ds=∑nid3i/∑nid2i 式中,
−ds 从图2可以看出,Pt颗粒均匀的分散在载体CeO2上。随着Pt负载量的提高,Pt颗粒在催化剂表面发生团聚,表现为Pt纳米粒子的粒径不断增大。
为了进一步了解具有不同负载量的催化剂Pt/CeO2中Pt的化学状态,进行了XPS测量,图3为金属Pt 4f区域的XPS图谱。从图3可以看出,所有催化剂的 Pt 4f7/2 峰均含有两个亚峰,位于70.4 eV和71.5 eV,分别归属于金属态Pt和阳离子态Pt。表1中总结了各催化剂的Ptn+与Pt0的比值,该比值越大,表明金属颗粒Pt与载体CeO2表面官能团之间的相互作用越强。可以发现,随着Pt的负载量从0.34%wt增加到1.32%wt,Ptn+/Pt0比值逐渐减小。表明高负载量的催化剂载体表面与Pt颗粒金属-载体相互作用较弱而低负载量的更强[18]。
表 1 催化剂的基本性质Table 1. Basic properties of the catalysts催化剂Catalyst Pt负载量/%wt aPt loading amount Pt平均粒径/nm bPt particle sizes 催化剂Zeta电位 cCatalyst Zeta potential Ptn+/Pt0 d/% Pt/SiO2 1.04 N.D. < 2.0 N.D. Pt/Al2O3 0.98 N.D. 9.0 N.D. Pt/commercial C 1.01 N.D. 2.0 N.D. Pt(0.34)/CeO2 0.34 1.52 N.D. 36 Pt(0.71)/CeO2 0.71 1.59 N.D. 27 Pt(1.06)/CeO2 1.06 1.78 6.4 21 Pt(1.32)/CeO2 1.32 1.88 N.D. 19 注:a 电感耦合等离子发射光谱仪测定;b 根据透射电镜测定;c Zeta电位仪测定;d X射线光电子能谱仪测定;N.D. 未测 Notes:a by ICP; b by TEM; c by Zeta potentiometer; dby X-ray photoelectron spectrometer; N.D. no determined. 2.2 负载型Pt基催化剂对MIAA的催化加氢脱碘
2.2.1 Pt基催化剂载体对MIAA加氢脱碘反应的影响
在初始pH值为5.6,MIAA的初始浓度为1.0 mmol·L−1, H2的流速为200 mL·min−1的反应条件下,分别以Pt(1.06)/CeO2、Pt(0.98)/Al2O3、Pt(1.04)/SiO2和Pt(1.01)/commercial C为催化剂进行液相加氢脱碘反应。结果如图4所示,可以看出Pt(1.04)/SiO2对MIAA催化加氢还原的初活性为33.66 mmol·L−1·g−1·h−1Cat,明显低于其他催化剂。经Zeta电位测定确定了CeO2、Al2O3、commercial C的等电点分别为6.4、9.0、2.0,SiO2的等电点小于2.0。在pH=5.6条件下,MIAA多以阴离子的形式存在(pKa=3.12)[19],因此阴离子化的MIAA与SiO2表面存在较强的静电斥力抑制了催化反应的进行。相反,Pt/CeO2表现出最高的催化活性(130.22 mmol·L−1·g−1·h−1Cat)这是CeO2载体较高的等电点和Pt在CeO2上高分散共同作用的结果。
2.2.2 催化剂投加量对MIAA催化加氢脱碘活性的影响
根据上述实验结果,选用Pt(1.06)/CeO2为催化剂,进一步探究反应条件对催化过程的影响。首先,调节反应初始pH值为5.6,MIAA的初始浓度为1.0 mmol·L−1,H2的流速为200 mL·min−1,改变催化剂投加量进行反应,结果如图5(a)所示。可以看出,随着催化剂投加量的增大,MIAA 的去除效率不断提高。而对催化剂投加量进行标化后,初活性基本保持不变(图5(b)),表明在本实验条件下,MIAA的催化加氢脱碘反应不受传质阻力的影响[20]。
2.2.3 MIAA初始浓度对加氢脱碘反应的影响
为了进一步探究污染物在催化剂表面的吸附对催化过程产的影响,在初始反应pH为5.6,催化剂投加量为0.050 g·L−1条件下,以Pt(1.06)/CeO2为催化剂,对不同初始浓度的MIAA进行的催化还原脱碘反应。从图6可以看出,随着反应物的浓度的升高,反应初活性也逐渐提高表明反应速率与吸附的MIAA浓度之间呈正相关。结果进一步拟合了 Langmuir-Hinshelwood 模型 [21]:
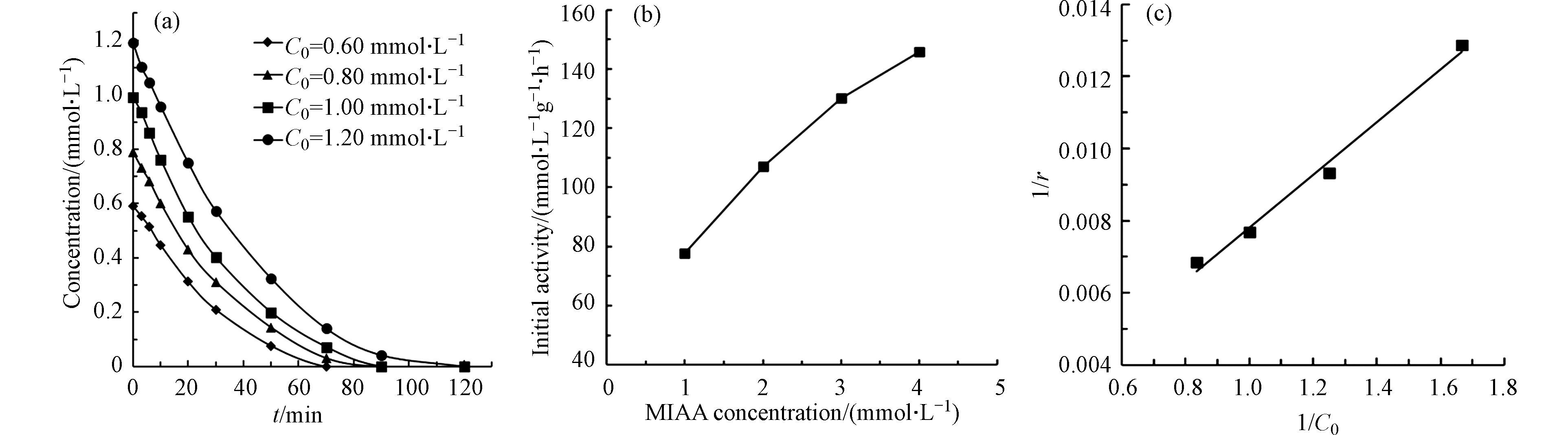 图 6 MIAA初始浓度对催化加氢脱碘反应的影响Figure 6. Effect of initial MIAA concentration on catalytic hydrodeiodination of MIAA(a. 不同初始浓度下MIAA的催化加氢脱碘反应; b. 初活性的变化; c. 1/r与1/C0的关系)(a. Catalytic hydrodeiodination of MIAA on Pt/CeO2 at different initial concentration; b. Initial activity; c.Initial activities (r0) as a function of C0)
图 6 MIAA初始浓度对催化加氢脱碘反应的影响Figure 6. Effect of initial MIAA concentration on catalytic hydrodeiodination of MIAA(a. 不同初始浓度下MIAA的催化加氢脱碘反应; b. 初活性的变化; c. 1/r与1/C0的关系)(a. Catalytic hydrodeiodination of MIAA on Pt/CeO2 at different initial concentration; b. Initial activity; c.Initial activities (r0) as a function of C0)r=−dC0dt=kθs=kKC01+KC0 1r=1kKC0+1k 式中,r 为初始MIAA的反应速率,C0 为初始MIAA浓度,k为反应速率常数,K为催化剂表面MIAA的吸附平衡常数,θs为催化剂表面MIAA的覆盖率。
实验所得的1/r与1/C0的关系如图6(c)所示,可以看出1/r与1/C0之间有良好的线性关系(R2>0.99),说明污染物MIAA在催化剂表面的吸附是反应的速率控制步骤。
2.2.4 Pt负载量对MIAA加氢脱卤反应的影响
在pH为5.6,MIAA的初始浓度为1.0 mmol·L−1 ,H2的流速为200 mL·L−1,催化剂投加量为10 mg条件下,考察了Pt/CeO2催化剂的Pt负载量对加氢脱碘反应的影响。从图7可以看出,随着催化剂的负载量从0.34%wt增加到1.32%wt,催化剂的初始活性从35.05 mmol·L−1·g−1·h−1Cat提高到223.43 mmol·L−1·g−1·h−1Cat,这可能是由于高负载量产生更多的活性位点造成的。为进一步探究Pt活性位的性质对活性的影响,本研究比较了对应的TOF值。根据先前的报道,在液相催化加氢脱卤反应中,阳离子态Ptn+活化C—X键和金属态Pt0活化H—H键是反应的关键步骤[22]。图7(c)显示,催化剂的TOF值随着Pt纳米颗粒粒径的增加而提高,主要由于大颗粒Pt催化剂具有更高的Pt0比例,有助于活化H2,从而催化剂活性得到提高,进一步说明了在本反应中与C—I键活化相比,H—H键的活化起更重要的作用。
2.2.5 pH对MIAA催化加氢脱碘反应的影响
由于pH会大大影响污染物形态和催化剂表面电性,因此,在催化剂Pt(1.06)/CeO2用量为0.050 g·L−1,MIAA的初始浓度为1.0 mmol·L−1的条件下,调节反应的初始pH,探索不同pH对MIAA加氢脱碘反应的影响。结果如图8所示,可以发现,催化剂的初活性随pH的增大先升后降,呈火山型变化。
当反应初始 pH值为2.0时,MIAA(pKa =3.12)在溶液中以分子态存在,不利于其在催化剂表面的吸附; 当pH值升高到4.0时,MIAA开始以阴离子态存在,同时由于催化剂Pt(1.06)/CeO2的Zeta电位为6.1,因此去质子化的MIAA和质子化的催化剂表面产生了较强的静电引力,进而使初活性由89.69 mmol·L−1·g−1·h−1Cat上升到148.73 mmol·L−1·g−1·h−1Cat。当反应溶液的pH进一步提高至8.0,去质子化的催化剂表面与阴离子态的MIAA之间产生了越来越强的静电斥力,故活性也随之降低。此结果进一步说明了污染物在催化剂表面的吸附对反应有重要作用。
2.3 催化剂稳定性研究
以5.6的初始pH,1.0 mmol·L−1的MIAA初始浓度, 0.050 g·L−1的催化剂Pt(1.06)/CeO2用量,200 mL·min−1的H2流速为反应条件,进行了MIAA的加氢脱碘反应,在每次反应结束后,向反应器中直接添加等量的MIAA,进行了4次循环实验。从图9可以看出,循环结束后初活性虽降低了约25%,仍然能将93.4%反应物MIAA去除,说明材料经过4次循环仍然能具有比较好的活性。活性的下降可能是Pt颗粒在反应过程中由于剧烈搅拌脱落造成的,该现象在先前的研究中也有报道[23]。
3. 结论 (Conclusion)
(1)分别以SiO2、Al2O3、商用C、CeO2为载体,通过硼氢化钠还原法制备了不同的金属Pt基负载型催化剂,其中Pt/CeO2的催化加氢脱碘活性最高,Pt/SiO2活性最低。
(2)Pt/CeO2催化剂中Pt高度分散,对碘乙酸的催化加氢脱碘符合Langmuir-Hinshelwood模型,其催化活性随Pt负载量的增大而增强,受pH影响较大。
(3)多次循环使用后,Pt/CeO2催化剂仍具有较高的MIAA转化率,保持了良好的稳定性。
-
表 1 催化剂的基本性质
Table 1. Basic properties of the catalysts
催化剂Catalyst Pt负载量/%wt aPt loading amount Pt平均粒径/nm bPt particle sizes 催化剂Zeta电位 cCatalyst Zeta potential Ptn+/Pt0 d/% Pt/SiO2 1.04 N.D. < 2.0 N.D. Pt/Al2O3 0.98 N.D. 9.0 N.D. Pt/commercial C 1.01 N.D. 2.0 N.D. Pt(0.34)/CeO2 0.34 1.52 N.D. 36 Pt(0.71)/CeO2 0.71 1.59 N.D. 27 Pt(1.06)/CeO2 1.06 1.78 6.4 21 Pt(1.32)/CeO2 1.32 1.88 N.D. 19 注:a 电感耦合等离子发射光谱仪测定;b 根据透射电镜测定;c Zeta电位仪测定;d X射线光电子能谱仪测定;N.D. 未测 Notes:a by ICP; b by TEM; c by Zeta potentiometer; dby X-ray photoelectron spectrometer; N.D. no determined. -
[1] PALACIOS M, F -PAMPILLÓN J, RODRı́GUEZ M E. Organohalogenated compounds levels in chlorinated drinking waters and current compliance with quality standards throughout the European Union [J]. Water Research, 2000, 34(3): 1002-1016. doi: 10.1016/S0043-1354(99)00191-8 [2] RICHARDSON S D, PLEWA M J, WAGNER E D, et al. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research [J]. Mutation Research/Reviews in Mutation Research, 2007, 636(1/2/3): 178-242. [3] 中华人民共和国卫生部, 中国国家标准化管理委员会. 中华人民共和国国家标准: 生活饮用水卫生标准 GB 5749—2006[S]. 北京: 中国标准出版社, 2007. Ministry of Health of the People's Republic of China, Standardization Administration of the People's Republic of China. National Standard (Mandatory) of the People's Republic of China: Standards for drinking water quality. GB 5749—2006[S]. Beijing: Standards Press of China, 2007(in Chinese).
[4] US EPA. Microbial and disinfection by-product rules-simultaneous compliance guidance manual [S]. United States Environmental Protection Agency, EPA 815-R-99-015, 1999. [5] RICHARDSON S D, FASANO F, ELLINGTON J J, et al. Occurrence and mammalian cell toxicity of iodinated disinfection byproducts in drinking water [J]. Environmental Science & Technology, 2008, 42(22): 8330-8338. [6] CEMELI E, WAGNER E D, ANDERSON D, et al. Modulation of the cytotoxicity and genotoxicity of the drinking water disinfection byproduct lodoacetic acid by suppressors of oxidative stress [J]. Environmental Science & Technology, 2006, 40(6): 1878-1883. [7] ESCOBAR-HOYOS L F, HOYOS-GIRALDO L S, LONDOÑO-VELASCO E, et al. Genotoxic and clastogenic effects of monohaloacetic acid drinking water disinfection by-products in primary human lymphocytes [J]. Water Research, 2013, 47(10): 3282-3290. doi: 10.1016/j.watres.2013.02.052 [8] PALS J A, ANG J K, WAGNER E D, et al. Biological mechanism for the toxicity of haloacetic acid drinking water disinfection byproducts [J]. Environmental Science & Technology, 2011, 45(13): 5791-5797. [9] PLEWA M J, WAGNER E D, RICHARDSON S D, et al. Chemical and biological characterization of newly discovered iodoacid drinking water disinfection byproducts [J]. Environmental Science & Technology, 2004, 38(18): 4713-4722. [10] RODRIGUEZ M J, SERODES J, ROY D. Formation and fate of haloacetic acids (HAAs) within the water treatment plant [J]. Water Research, 2007, 41(18): 4222-4232. doi: 10.1016/j.watres.2007.05.048 [11] CHALATIP R, CHAWALIT R, NOPAWAN R. Removal of haloacetic acids by nanofiltration [J]. Journal of Environmental Sciences, 2009, 21(1): 96-100. doi: 10.1016/S1001-0742(09)60017-6 [12] LI A Z, ZHAO X, HOU Y N, et al. The electrocatalytic dechlorination of chloroacetic acids at electrodeposited Pd/Fe-modified carbon paper electrode [J]. Applied Catalysis B:Environmental, 2012, 111/112: 628-635. doi: 10.1016/j.apcatb.2011.11.016 [13] BAYLESS W, ANDREWS R C. Biodegradation of six haloacetic acids in drinking water [J]. Journal of Water and Health, 2008, 6(1): 15-22. doi: 10.2166/wh.2007.002 [14] WANG K P, GUO J S, YANG M, et al. Decomposition of two haloacetic acids in water using UV radiation, ozone and advanced oxidation processes [J]. Journal of Hazardous Materials, 2009, 162(2/3): 1243-1248. [15] XIAO Y J, ZHANG L F, ZHANG W, et al. Comparative evaluation of iodoacids removal by UV/persulfate and UV/H2O2 processes [J]. Water Research, 2016, 102: 629-639. doi: 10.1016/j.watres.2016.07.004 [16] LIU H, YU Q, FU H Y, et al. Pt supported on ordered microporous carbon as highly active catalyst for catalytic hydrodeiodination of iodinated X-ray contrast media [J]. Applied Catalysis B:Environmental, 2018, 222: 167-175. doi: 10.1016/j.apcatb.2017.10.006 [17] YUAN G, KEANE M A. Liquid phase catalytic hydrodechlorination of 2, 4-dichlorophenol over carbon supported palladium: An evaluation of transport limitations [J]. Chemical Engineering Science, 2003, 58(2): 257-267. doi: 10.1016/S0009-2509(02)00476-1 [18] NING X M, LI Y H, DONG B Q, et al. Electron transfer dependent catalysis of Pt on N-doped carbon nanotubes: Effects of synthesis method on metal-support interaction [J]. Journal of Catalysis, 2017, 348: 100-109. doi: 10.1016/j.jcat.2017.02.011 [19] XIA Y, MO Y, YANG Q Y, et al. Iodoacetic acid disrupting the thyroid endocrine system in vitro and in vivo [J]. Environmental Science and Technology, 2018, 52(13): 7545-7552. doi: 10.1021/acs.est.8b01802 [20] de PEDRO Z M, CASAS J A, GOMEZ-SAINERO L M, et al. Hydrodechlorination of dichloromethane with a Pd/AC catalyst: Reaction pathway and kinetics [J]. Applied Catalysis B:Environmental, 2010, 98(1/2): 79-85. [21] ILINITCH O M, CUPERUS F P, NOSOVA L V, et al. Catalytic membrane in reduction of aqueous nitrates: Operational principles and catalytic performance [J]. Catalysis Today, 2000, 56(1/2/3): 137-145. [22] BAEZA J A, CALVO L, GILARRANZ M A, et al. Catalytic behavior of size-controlled palladium nanoparticles in the hydrodechlorination of 4-chlorophenol in aqueous phase [J]. Journal of Catalysis, 2012, 293: 85-93. doi: 10.1016/j.jcat.2012.06.009 [23] LI M H, SUN Y H, TANG Y Q, et al. Efficient removal and recovery of copper by liquid phase catalytic hydrogenation using highly active and stable carbon-coated Pt catalyst supported on carbon nanotube [J]. Journal of Hazardous Materials, 2020, 388: 121745. doi: 10.1016/j.jhazmat.2019.121745 -




 下载:
下载: