-
铁是一种生物必须的营养元素,直接影响浮游植物的光合作用和碳水化合物形成,由于高含氧量和无机态铁的低溶解性,铁通常是制约HNLC海区(High nutrition low chlorophyll)初级生产力的关键微量元素[1-2]。大规模海洋施铁实验表明,水生态系统中生物可利用铁的增加可以显著提高浮游植物的生物量和光合作用率,从而提高初级生产力,并促使浮游植物的群落结构发生变化[3-7]。以往研究表明,光自养微生物对碳循环和全球气候起关键作用[8-9]。初级生产力的提高,深刻地影响着全球尺度的二氧化碳固定,对温室气体的控制具有重要意义。
水生态系统中,99%溶解性铁(dissolved iron,DFe)与有机配体结合。尽管大部分有机络合态铁不能直接被藻类利用,但通过一些地球化学转化过程,可转变为生物可利用铁[10-11]。Blazevic等[12]研究发现,海洋中腐殖酸结合态铁可以发生光还原反应,进而提高铁的生物可利用性。沼泽性河流是海洋DFe的重要来源[13]。沼泽性河流中大量存在的溶解性有机碳(DOC, dissolved organic carbon)与铁离子形成有机络合物,使水中保持较高浓度DFe。有机质中羧基和酚羟基是与铁络合的主要官能团。泥炭源中的酚酸类物质,含有稳定的芳香环结构。部分酚酸与铁有较高的配合能力,这类物质的存在保护了长距离迁移的DFe,保证了陆源DFe向水生态系统的有效输出[14]。
泥炭沼泽中存在多种类型酚酸,前人在金川泥炭中检测出了9种酚酸,包括对-羟基苯甲酸、丁香酸、香草酸、阿魏酸、对-香豆酸、没食子酸、原儿茶酸和咖啡酸,许多泥炭沼泽中都有这些酚酸的存在[14]。研究证实,酚酸等有机质可以和铁形成较为稳定的配合物,使其可以在淡水运输过程中迁移更长的距离[15]。其中,具有儿茶酚或者没食子酰基结构的原儿茶酸、没食子酸以及咖啡酸可以和Fe(Ⅱ)形成较为稳定的络合物,使得Fe(Ⅱ)在极易被氧化的碱性条件下也可以保存较长时间。而咖啡酸、没食子酸、原儿茶酸以及龙胆酸还对Fe(Ⅲ)有着明显的还原作用,同样有助于这两种铁形态之间的平衡[16]。
植物或微生物分泌代谢物质对环境中其他植物或微生物体产生不利或有利的影响,这种作用称为化感作用。在化感作用过程中分泌的物质即被称为化感物质,自然界的化感物质种类非常丰富,主要包括酚酸类、苯醌类、倍半萜类、黄酮类等几大类物质[17]。迄今发现的化感物质几乎都是植物的次生代谢物质,分子量较小,结构简单,其中酚酸类物质是一类重要的次生代谢产物,也是研究较多,被证实是化感活性较强的一类物质[18]。酚酸具有一定生物毒性。目前对于酚酸抑藻的机制还不十分清楚,其抑制作用可能通过多种方式实现。研究表明,酚酸与蛋白质分子易遵循疏水键-氢键多点键合理论结合。在酚酸存在的情况下,藻细胞的胞外磷酸酶活性受到抑制,碱性磷酸酶活性的抑制使藻利用磷的能力下降。酚酸与细胞膜蛋白的结合,会破坏生物体细胞膜结构,使植物多酚物质进一步穿过细胞膜,进入细胞体内,从而改变微生物细胞酶活性,减少藻类对外源性蛋白质的利用,并通过对细胞外酶的抑制达到抑藻的目的[19]。另外,如果酚酸进入细胞体后,通过与金属离子发生络合反应,形成沉淀而破坏微生物的正常新陈代谢也是植物多酚抑藻的原因所在[20]。尽管酚酸存在生物毒性,但适量前提下,对藻类生长有积极作用[21]。泥炭源典型酚酸与铁的络合物是否对藻类利用铁有显著影响尚待进一步研究。因此,探究酚-铁配合物络合稳定性及其生物可利用性有助于进一步了解生物对铁的吸收,更好地理解全球铁碳耦合循环。
铜绿微囊藻(microcystic aeruginosa) 是中国湖泊、水库及其他水域生态系统水体富营养化蓝藻水华的代表性藻类。本文铜绿微囊藻为培养对象,利用泥炭源典型酚酸及泥炭溶解有机质(DOM)开展了一系列培养试验,以期了解泥炭沼泽源酚酸以及酚-铁络合物对铜绿微囊藻生长的影响。
-
试验所用铜绿微囊藻藻源,由中国科学院水生生物研究所提供,采用BG-11培养基培养。
4种酚酸的配制:以1.7 g·L−1的浓度配制BG-11培养基,然后将对羟基苯甲酸、对香豆酸、水杨酸、咖啡酸加入,分别配制4份浓度为0.24 g·L−1的酚酸溶液。用0.22 μm滤膜在超净台中过滤,并用紫外光照射30 min,消除微生物的影响,现配现用。
藻种培养条件:实验前5天,将铜绿微囊藻进行扩大培养。光照强度4000 lx;光暗比24 h∶0 h;温度(25±1)°C;每天摇动培养瓶5次,使藻类生长进入对数生长期。进入对数增长期后,取铜绿微囊藻各300 mL加入到1 L锥形瓶,再加入BG-11培养基100 mL进行驯化培养。
-
由于对羟基苯甲酸和对香豆酸在泥炭中含量相对较高,水杨酸和咖啡酸和铁离子可以络合,实验选择这4种酚酸进行实验。
使用细胞计数仪确定当前藻液浓度,并根据藻液浓度取一定量的处于对数生长期的藻液加入250 mL锥形瓶内,其中分别添加稀释了不同倍数的酚酸溶液,最后用培养基补足,使得锥形瓶内的液体总体积达到150 mL。每个锥形瓶内藻的初始密度为105 cell·mL−1,酚酸的最终浓度梯度分别为0、10、20、40、60、80 mg·L−1,每组3个平行,置于光照培养箱内。培养温度为(20±1)℃,光照强度为4000 lx,24 h光照,每天震荡3—5次。
-
选用水杨酸和咖啡酸与铁形成络合物,探究酚铁对藻类生长的影响。实验选择的酚酸浓度为5×10−5 mol·L−1,铁浓度为1×10−6 mol·L−1,在此条件下酚酸浓度为铁浓度的50倍,可以有效保护体系中的二价铁。此外,由于泥炭沼泽中也普遍存在草酸、柠檬酸、酒石酸、乙酸等无苯环的小分子有机酸,所以实验选择草酸、柠檬酸、乙酸作为干扰物质加入到酚铁体系中进行藻类的培养实验。
藻类的培养实验分为10组,每组添加的物质如下:A.水杨酸+硫酸亚铁;B.咖啡酸+硫酸亚铁;C.水杨酸+草酸+硫酸亚铁;D.水杨酸+乙酸+硫酸亚铁;E.咖啡酸+草酸+硫酸亚铁;F.咖啡酸+乙酸+硫酸亚铁;G.水杨酸+柠檬酸+硫酸亚铁;H.咖啡酸+柠檬酸+硫酸亚铁;I.不添加酸和铁的对照组;J.只添加铁的对照组。
以上试验均为期15 d,每隔48 h取样1次,记录藻细胞数量的变化,以及藻存活情况的变化和pH值的变化。培养周期结束后分别取10 mL和5 mL样品,测量样品中叶绿素a的含量和叶绿素荧光参数Fv/Fm(最大光能转化效率)。其中Fv/Fm常用来表征叶绿素PsⅡ(低铁环境藻类光系统Ⅱ)反应中心内禀光能转换效率,反映当时所有的PsⅡ反应中心均处于开放态时最大光量子产量。
-
为测藻细胞存活率及藻细胞数量,使用5-CFDA染色,具体操作方法如下:
(1)用DMSO(二甲基亚砜)5-CFDA稀释至10 mmol·L−1。将99 μL已经配制好的BG-11培养基与1 μL的5-CFDA混合作为A液,摇晃10 s混匀;
(2)将50 μL样品与50 μL A液混匀,用移液枪至少吹打10次;
(3)在25℃条件下将上一步准备好的样品避光放置30 min;
(4)用移液枪将待测样品吹打10次或摇晃,使藻细胞分散,然后用移液枪取20 μL加入计数板内。在计数板插入仪器之前,稳定1 min,使样品在其中稳定下来。
将细胞染色后,使用细胞计数仪计数,并观察细胞的存活状态。
测量样品叶绿素a的含量:
(1)取藻液10 mL,4500 r·min-1离心15 min,去掉上层清液,将样品在4℃冰箱中放置1 d;
(2)取出后迅速加入5 mL的90%热乙醇(80℃)于80℃的热水浴萃取2 min,再用超声处理10 min,放在暗处萃取4 h后,用0.22 μm的滤头过滤。用酶标仪于波长665 nm和750 nm处测吸光值,然后滴加1滴1 mol·L−1的盐酸酸化,于波长665 nm和750 nm处再测吸光值。计算公式为:
其中,Chla乙醇为热乙醇法测定的叶绿素a含量(μg·L−1);E665是乙醇萃取液于波长665 nm的吸光值;E750是乙醇萃取液于波长750 nm的吸光值;A665为乙醇萃取液酸化后在665 nm处的吸光值;A750为乙醇萃取液在750 nm处的吸光值;V乙醇为乙醇萃取液的体积(mL),V样品为所取样品的体积(mL)。
抑制率计算公式:IR=(1-N/N0×100%)抑制率为负值则有促进效果,抑制率为正则抑制。
-
培养前准备:
(1)提取泥炭中的DOM;
(2)将样品通过H型阳离子柱交换柱,去除样品中存在的金属离子;然后将DOM样品按照<1 KDa,1—3 KDa,>3 KDa分成3份;
(3)将3份样品中加入FeSO4,待稳定一段时间后,取10 mL加入培养基中,加入后Fe的浓度为5×10−6 mol·L−1。
铜绿微囊藻培养实验分为5组,每组3份平行,每组添加的物质如下:A.无添加;B.Fe,浓度为5×10−6 mol·L−1;C.DOM-Fe(<1 KDa),Fe浓度为5×10−6 mol·L−1;D.DOM-Fe(1—3 KDa),Fe浓度为5×10−6 mol·L−1;E.DOM-Fe(>3 KDa),Fe浓度为5×10−6 mol·L−1。
实验均为期15 d,每隔48 h取样1次,记录藻细胞数量的变化,以及藻存活情况的变化。
-
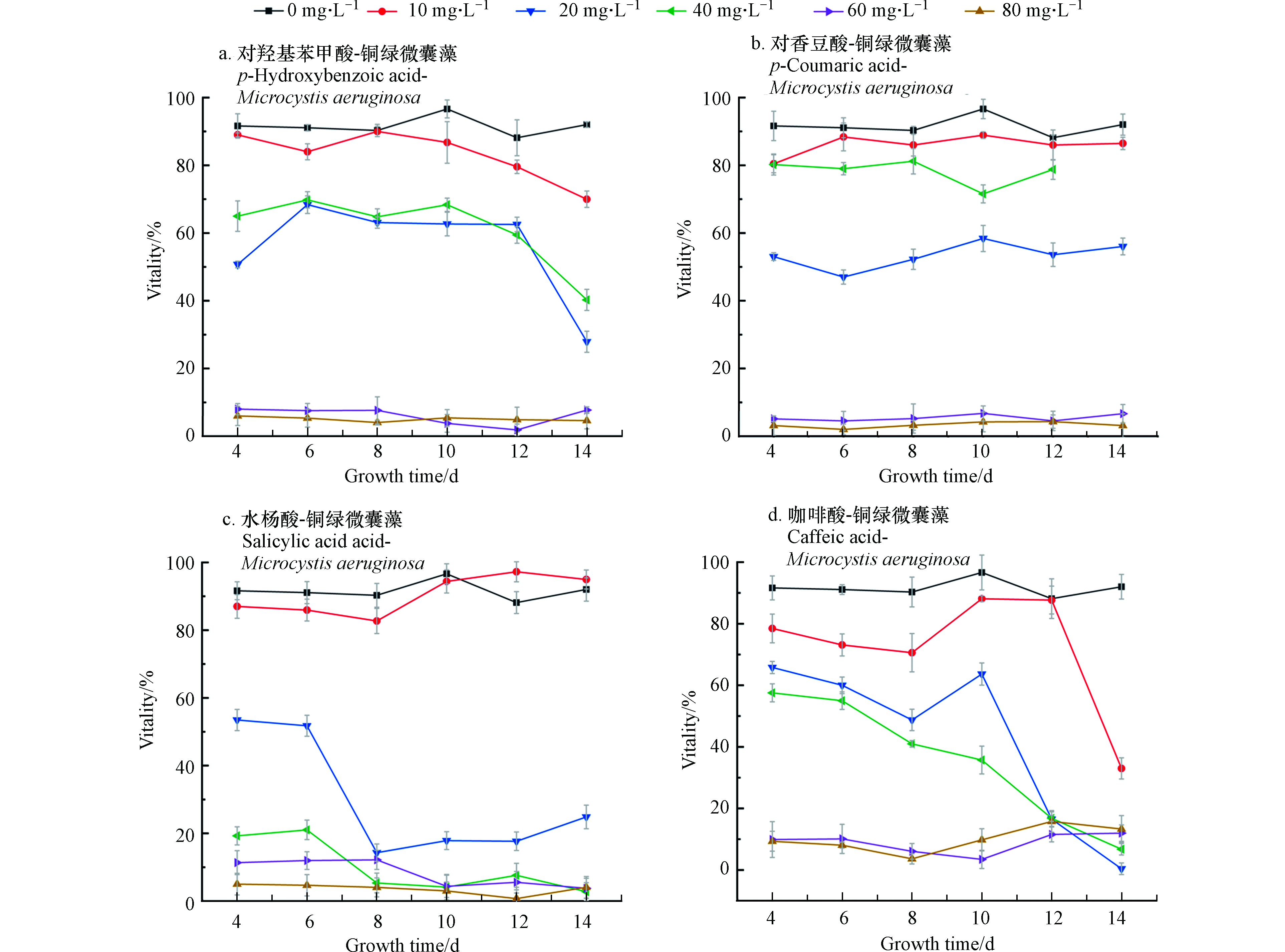
在微囊藻培养体系中分别加入不同浓度四种酚酸溶液,经过14 d培养和检测,得到微囊藻-酚酸的生长曲线如图1所示。图1中A、B为不同浓度的对羟基苯甲酸和对香豆酸对微囊藻生长情况的影响。可以看出,当酚酸浓度为10 mg·L−1和20 mg·L−1时,微囊藻的生长速率和最终达到的终点浓度明显高于控制组(0)抑制率为-23.5%—18%。从微囊藻浓度上来看,当酚酸浓度为10 mg·L−1和20 mg·L−1时,生长情况比较接近,说明在此浓度下,这两种酚酸对微囊藻的生长有一定的刺激作用。在A、B组中当酚酸浓度超过20 mg·L−1时,藻液浓度和藻的生长速率明显低于控制组;当浓度增加到40 mg·L−1时,这两种酚酸对微囊藻的生长起到了明显的抑制作用,而对羟基苯甲酸的对微囊藻生长的抑制作用更加明显;浓度继续增加到60—80 mg·L−1时,微囊藻前4—5天略有增长,然后基本停止了增长,保持在3×106 cell·mL−1左右。对香豆酸60 mg·L−1组的藻数量略高于80 mg·L−1组。
在C组水杨酸-微囊藻的实验中,水杨酸浓度为10 mg·L−1时,增长的速度与控制组接近,在8—10 d快速增长后,藻数量和控制组趋于一致。而当浓度大于10 mg·L−1时,都出现了明显的抑藻效果,抑制率约为80%。
结合图2中存活率来看(从培养的第4天开始测微囊藻的存活率)当浓度为10 mg·L−1时,微囊藻的存活率都略低于控制组,差值在10%左右浮动,当浓度为20—40 mg·L−1,在开始计数时,藻类的存活率就已不同程度地低于控制组,并且A、C、D存活率在4—14 d整体处于下降的趋势,可以看出,水杨酸对微囊藻的抑制作用最强。
表1是各组样品的Fv/Fm,最大光能转化效率Fv/Fm常用来表征叶绿素PsII反应中心内禀光能转换效率,反映当时所有的PsII反应中心均处于开放态时最大光量子产量。
在非胁迫环境下,植物叶片叶绿素荧光参数Fv/Fm变化极小,表现出稳定的特点,但在胁迫条件下,该参数明显下降[22]。Fv/Fm可作为植物受环境胁迫的响应指标[23]。控制组的Fv/Fm为0.308。一般情况下,微囊藻的Fv/Fm在0.3左右,当Fv/Fm过低表明藻类受到环境胁迫,PSII中心受到损伤进而降低光合作用效率。由表1可以看出,当水杨酸浓度大于20 mg·L−1时会对微囊藻的光合作用产生明显抑制,当各组酚酸浓度超过60 mg·L−1时,藻类基本停止了光合作用,这和前文中藻类的生物量变化和存活率相吻合。表2是各个组叶绿素含量的均值,数据表明:各组中相对低浓度的酚酸,不仅对藻类数量的增长有促进作用,也促进了叶绿素含量的增加。
-
根据上述试验结果可以得出,当这4种典型泥炭源酚酸的浓度达10 mg·L−1时,从生物量、存活率以及光合作用强度来说对微囊藻的生长没有明显抑制作用。普遍认为,藻类吸收Fe主要是离子形态,而不是有机络合态Fe[24]。目前,仅观察到在产生铁载体的微生物中存在铁载体复合物的吸收及其在细胞中的还原[25]。已有研究发现,有机络合态铁中铁的释放途径不同。其中包括简单的配体-金属平衡(Ligand-Metal balance),平衡在初级生产者消耗铁后发生变化,促进铁从络合物中分离。另一个途径是基于配体的降解,这也导致了Fe和配体的分离。第三种可能是通过有机态铁的还原,降低配体对铁的亲和力,并导致Fe的释放。这个过程可能是由于络合物的自还原/氧化而发生的,这意味着配体氧化同时也释放产生Fe[26]。尤其是光还原分解对铁的生物利用度有很大影响,这一释放铁途径被称为AHS(aquatic humic substances)机制[12]。
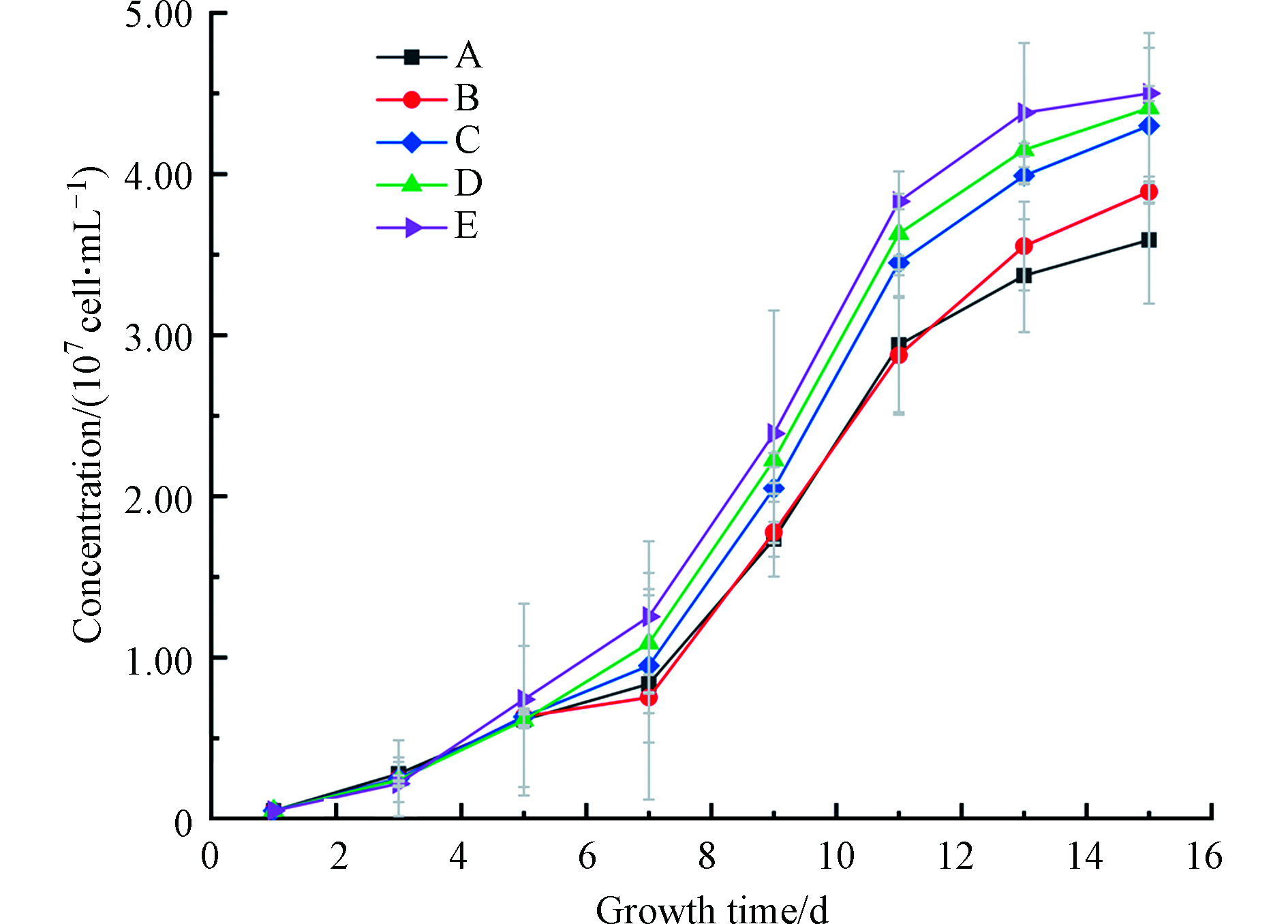
图3是加入不同酸和Fe2+的微囊藻生长曲线。施加和不加Fe2+的对照试验表明,在培养1—9 d,两组生长速度以及生物量大致相同。在第9天后,施加Fe2+组的生长速度放缓,最终的藻密度低于对照组。图3表明在没有配体存在的情况下,加入一定量的铁对微囊藻生长促进作用不明显。同时,观察发现,在藻类指数生长阶段出现了较高的pH值(高达10.5),这与二氧化碳生物需求增加有关。在指数生长结束和平台期开始后,pH值略下降。在藻类生长平缓或生长不良的样品中,pH值无显著变化,pH值大多保持在6—8。
能促进藻类生长的酚酸应当与三价铁有较高的亲和力,与二价铁有较低的亲和力[27]。试验表明,相对其他3种酚酸铁配合物体系,水杨酸铁不能有效地为微囊藻提供生物可利用性铁,这可能与水杨酸的稳定性较强有关[27]。对照表明,用咖啡酸处理的微囊藻生长良好,最高浓度达到2.19×107 cell·mL−1。这可能是咖啡酸中的儿茶酚基结构所引起的,并且有更高的氧化还原潜力,更容易将Fe从配合物中释放。Santana等 [28]的研究也证实了在生物条件下还原络合物的可能性。总体上,酚酸的加入提高了藻类对Fe的生物可利用率。
图2显示藻类存活率从第10天开始明显下降,藻类计数可能包括了死藻,因此选择第11天数据进行显著性分析。结果表明(表3),在0.05的置信水平下,咖啡酸的加入对微囊藻生长有显著促进作用,而水杨酸在0.05的置信水平下,对微囊藻生长无显著促进作用。
-
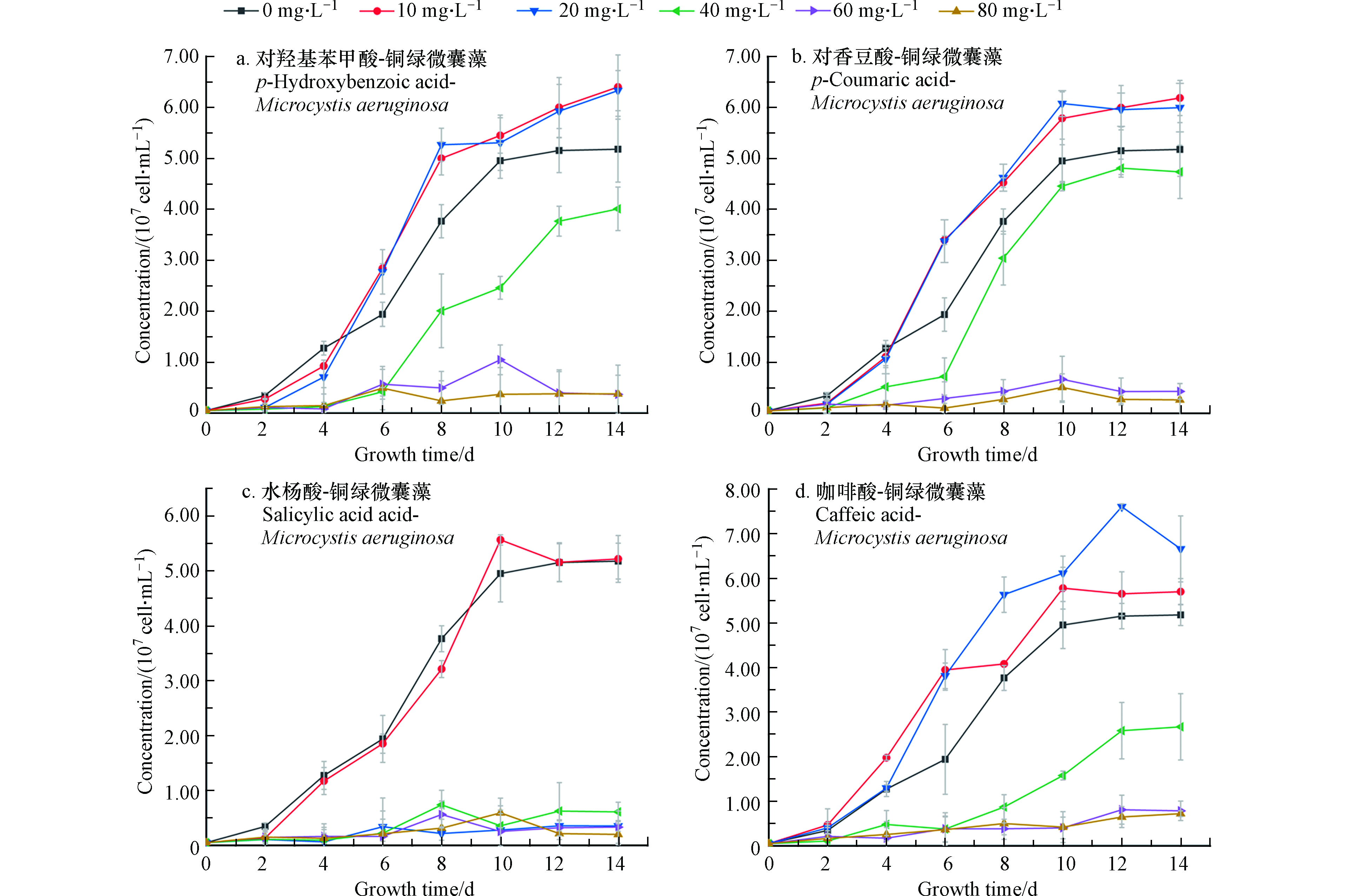
许多泥炭源有机质同时含有酚羟基和羧基,具有酚酸性质。但由于泥炭有机质组成复杂多样,现有技术尚不能有效分离不同性质有机化合物。因此,利用不同分子量段有机质与铁的络合物,开展藻类培养试验有助于客观评估泥炭源DOM-Fe的藻类可利用性。利用不同分子量段DOM-Fe,进行培养试验,铜绿微囊藻的生长情况如图4。表4 结果表明,在0.05 的置信水平下,不同组之间差异不具统计显著性,但图4 还是反映出有机态铁对藻类生长的促进趋势.
结果显示(图3和图4),不同分子量结合态Fe均促进了铜绿微囊藻的生长,但影响程度不同。添加Fe后,铜绿微囊藻的生物量和控制组相比均有增加,微囊藻生长得到促进,最终达到107 cell·mL−1。其中,微囊藻在7—11 d增长最快;其次,对比生长终点可以发现不同DOM-Fe促进效果存在差异:E组>D组>C组>B组>A组>无Fe组;第三,添加DOM显著促进了藻的生长。研究表明,相对于Fe3+,藻类更倾向于利用Fe2+[29]。这是由于具有一定还原能力的DOM可以减缓二价铁的氧化[30],从而提高了藻类对铁的利用率。此外,不同分子量段DOM与Fe的络合稳定常数略有不同,较高分子量的DOM(>3 kD)与Fe的络合稳定常数较小[30],在光照或者其他条件下容易发生解离,产生易被藻类利用的Fe。而泥炭源低分子量DOM(<1 KD)络合态铁,由于其络合稳定的常数相对较高,在培养体系中更加稳定,相对不易被藻类利用。
-
(1)4种酚酸对藻类生长的影响均呈现“低促高抑”的规律。从藻类生物量和叶绿素含量来看,抑藻效果从高到低:水杨酸>对羟基苯甲酸>对香豆酸>咖啡酸;结合藻类的存活率,虽然低浓度酚酸刺激了藻类生物量的增长,但是也对藻类的生存产生了一定的负面影响:在添加10 mg·L−1酚酸的几组样品中,微囊藻的存活率都略低于控制组。
(2)添加酚酸的藻类样品中,当水杨酸浓度达到20 mg·L−1时,Fv/Fm明显降低(0.3降低到0.02左右),而其它3种酚酸浓度达到60 mg·L−1才出现抑制,说明水杨酸抑制作用最强。
(3)不同酚铁络合物的生物可利用性存在差异:相对咖啡酸和水杨酸,水杨酸络合态铁更难被藻类利用,除酚毒性效应外,还与其较高的络合稳定性有关。
(4)泥炭源不同分子段DOM-Fe对藻类生长的促进作用从高到低依次为:>3 KD,1—3 KD,<1 KD。高分子段DOM(>3 kD)与Fe的络合稳定常数最小,在光照或者其他条件下容易发生解离,更易释放Fe而被藻类利用;泥炭源低分子量DOM(<1 KD)络合态铁,因其络合稳定常数相对较高,相对不易被藻类利用。
致谢:感谢中国科学院水生生物研究所的大力支持。
典型泥炭源酚铁络合物的藻类可利用性——以铜绿微囊藻为例
Algae availability of typical peat-derived iron phenol complexes: A study based on Microcystis aeruginosa
-
摘要: 铁是影响水生态系统初级生产力的关键微量元素。泥炭沼泽普遍含有丰富的溶解有机质,其中酚酸类物质具有稳定的芳香环结构,与铁有较强的络合能力,提高了陆源溶解性铁向水生态系统的有效输出。泥炭沼泽源酚铁配合物的生物可利用性对藻类生长及铁的生物地球化学循环有重要影响。本文通过一系列培养试验,研究了泥炭源典型酚铁络合物的藻类可利用性及其影响因素。结果表明,4种酚酸对藻类生长的影响均呈现“低促高抑”的规律,从藻类生物量和叶绿素含量来看,抑藻效果从高到低:水杨酸>对羟基苯甲酸>对香豆酸>咖啡酸。当水杨酸浓度达到20 mg·L−1时,对藻类的光合作用抑制最强。对照试验表明,水杨酸络合态铁更难被藻类利用,这与其络合物稳定性较高有关。利用不同分子量段泥炭源DOM-Fe的培养试验显示,对藻类生长的促进作用从高到低依次为:>3 KD,1—3 KD,<1 KD。低分子量DOM(<1KD )络合态铁,由于其在培养体系中更加稳定,相对不易被藻类利用。Abstract: Iron is a key trace element that affects the primary productivity of aquatic ecosystems. Pealands are generally rich in dissolved organic matter. Among them, phenolic acids have a stable aromatic ring structure and have a high complexing ability with iron, which improves the effective output of terrestrial dissolved iron to the water ecosystem. The bioavailability of the peat-derived phenol-iron complex has an important impact on the growth of algae and the biogeochemical cycle of iron. In this paper, through a series of culture experiments, the algae availability and influencing factors of typical phenolic iron complexes from peat sources were studied. The results show that the effects of the four phenolic acids on the growth of algae present the law of “low concentration promotes, and high concentration inhibits”. From the perspective of algal biomass and chlorophyll content, the algae inhibitory effect is from high to low: salicylic acid>p-hydroxybenzoic acid> To coumaric acid>caffeic acid. When the concentration of salicylic acid reaches 20 mg·L−1, the photosynthesis inhibition of algae is the strongest. Control experiments show that the iron in the complexed form of salicylic acid is more difficult to be used by algae, which is related to the higher stability of the complex. Cultivation experiments using peat DOM-Fe of different molecular weights showed that the promotion of algae growth from high to low was: >3 KD, 1—3 KD, <1 KD. Low molecular weight DOM (<1 KD) complexed iron, because it is more stable in the culture system, is relatively difficult to be used by algae.
-
Key words:
- peat /
- phenolic acid /
- iron /
- microcystic aeruginosa /
- bioavailability
-
铁是一种生物必须的营养元素,直接影响浮游植物的光合作用和碳水化合物形成,由于高含氧量和无机态铁的低溶解性,铁通常是制约HNLC海区(High nutrition low chlorophyll)初级生产力的关键微量元素[1-2]。大规模海洋施铁实验表明,水生态系统中生物可利用铁的增加可以显著提高浮游植物的生物量和光合作用率,从而提高初级生产力,并促使浮游植物的群落结构发生变化[3-7]。以往研究表明,光自养微生物对碳循环和全球气候起关键作用[8-9]。初级生产力的提高,深刻地影响着全球尺度的二氧化碳固定,对温室气体的控制具有重要意义。
水生态系统中,99%溶解性铁(dissolved iron,DFe)与有机配体结合。尽管大部分有机络合态铁不能直接被藻类利用,但通过一些地球化学转化过程,可转变为生物可利用铁[10-11]。Blazevic等[12]研究发现,海洋中腐殖酸结合态铁可以发生光还原反应,进而提高铁的生物可利用性。沼泽性河流是海洋DFe的重要来源[13]。沼泽性河流中大量存在的溶解性有机碳(DOC, dissolved organic carbon)与铁离子形成有机络合物,使水中保持较高浓度DFe。有机质中羧基和酚羟基是与铁络合的主要官能团。泥炭源中的酚酸类物质,含有稳定的芳香环结构。部分酚酸与铁有较高的配合能力,这类物质的存在保护了长距离迁移的DFe,保证了陆源DFe向水生态系统的有效输出[14]。
泥炭沼泽中存在多种类型酚酸,前人在金川泥炭中检测出了9种酚酸,包括对-羟基苯甲酸、丁香酸、香草酸、阿魏酸、对-香豆酸、没食子酸、原儿茶酸和咖啡酸,许多泥炭沼泽中都有这些酚酸的存在[14]。研究证实,酚酸等有机质可以和铁形成较为稳定的配合物,使其可以在淡水运输过程中迁移更长的距离[15]。其中,具有儿茶酚或者没食子酰基结构的原儿茶酸、没食子酸以及咖啡酸可以和Fe(Ⅱ)形成较为稳定的络合物,使得Fe(Ⅱ)在极易被氧化的碱性条件下也可以保存较长时间。而咖啡酸、没食子酸、原儿茶酸以及龙胆酸还对Fe(Ⅲ)有着明显的还原作用,同样有助于这两种铁形态之间的平衡[16]。
植物或微生物分泌代谢物质对环境中其他植物或微生物体产生不利或有利的影响,这种作用称为化感作用。在化感作用过程中分泌的物质即被称为化感物质,自然界的化感物质种类非常丰富,主要包括酚酸类、苯醌类、倍半萜类、黄酮类等几大类物质[17]。迄今发现的化感物质几乎都是植物的次生代谢物质,分子量较小,结构简单,其中酚酸类物质是一类重要的次生代谢产物,也是研究较多,被证实是化感活性较强的一类物质[18]。酚酸具有一定生物毒性。目前对于酚酸抑藻的机制还不十分清楚,其抑制作用可能通过多种方式实现。研究表明,酚酸与蛋白质分子易遵循疏水键-氢键多点键合理论结合。在酚酸存在的情况下,藻细胞的胞外磷酸酶活性受到抑制,碱性磷酸酶活性的抑制使藻利用磷的能力下降。酚酸与细胞膜蛋白的结合,会破坏生物体细胞膜结构,使植物多酚物质进一步穿过细胞膜,进入细胞体内,从而改变微生物细胞酶活性,减少藻类对外源性蛋白质的利用,并通过对细胞外酶的抑制达到抑藻的目的[19]。另外,如果酚酸进入细胞体后,通过与金属离子发生络合反应,形成沉淀而破坏微生物的正常新陈代谢也是植物多酚抑藻的原因所在[20]。尽管酚酸存在生物毒性,但适量前提下,对藻类生长有积极作用[21]。泥炭源典型酚酸与铁的络合物是否对藻类利用铁有显著影响尚待进一步研究。因此,探究酚-铁配合物络合稳定性及其生物可利用性有助于进一步了解生物对铁的吸收,更好地理解全球铁碳耦合循环。
铜绿微囊藻(microcystic aeruginosa) 是中国湖泊、水库及其他水域生态系统水体富营养化蓝藻水华的代表性藻类。本文铜绿微囊藻为培养对象,利用泥炭源典型酚酸及泥炭溶解有机质(DOM)开展了一系列培养试验,以期了解泥炭沼泽源酚酸以及酚-铁络合物对铜绿微囊藻生长的影响。
1. 材料与方法(Materials and methods)
1.1 实验材料
试验所用铜绿微囊藻藻源,由中国科学院水生生物研究所提供,采用BG-11培养基培养。
4种酚酸的配制:以1.7 g·L−1的浓度配制BG-11培养基,然后将对羟基苯甲酸、对香豆酸、水杨酸、咖啡酸加入,分别配制4份浓度为0.24 g·L−1的酚酸溶液。用0.22 μm滤膜在超净台中过滤,并用紫外光照射30 min,消除微生物的影响,现配现用。
藻种培养条件:实验前5天,将铜绿微囊藻进行扩大培养。光照强度4000 lx;光暗比24 h∶0 h;温度(25±1)°C;每天摇动培养瓶5次,使藻类生长进入对数生长期。进入对数增长期后,取铜绿微囊藻各300 mL加入到1 L锥形瓶,再加入BG-11培养基100 mL进行驯化培养。
1.2 实验设计
1.2.1 单一酚酸对铜绿微囊藻的抑制作用
由于对羟基苯甲酸和对香豆酸在泥炭中含量相对较高,水杨酸和咖啡酸和铁离子可以络合,实验选择这4种酚酸进行实验。
使用细胞计数仪确定当前藻液浓度,并根据藻液浓度取一定量的处于对数生长期的藻液加入250 mL锥形瓶内,其中分别添加稀释了不同倍数的酚酸溶液,最后用培养基补足,使得锥形瓶内的液体总体积达到150 mL。每个锥形瓶内藻的初始密度为105 cell·mL−1,酚酸的最终浓度梯度分别为0、10、20、40、60、80 mg·L−1,每组3个平行,置于光照培养箱内。培养温度为(20±1)℃,光照强度为4000 lx,24 h光照,每天震荡3—5次。
1.2.2 酚络合态铁对铜绿微囊藻生长的影响
选用水杨酸和咖啡酸与铁形成络合物,探究酚铁对藻类生长的影响。实验选择的酚酸浓度为5×10−5 mol·L−1,铁浓度为1×10−6 mol·L−1,在此条件下酚酸浓度为铁浓度的50倍,可以有效保护体系中的二价铁。此外,由于泥炭沼泽中也普遍存在草酸、柠檬酸、酒石酸、乙酸等无苯环的小分子有机酸,所以实验选择草酸、柠檬酸、乙酸作为干扰物质加入到酚铁体系中进行藻类的培养实验。
藻类的培养实验分为10组,每组添加的物质如下:A.水杨酸+硫酸亚铁;B.咖啡酸+硫酸亚铁;C.水杨酸+草酸+硫酸亚铁;D.水杨酸+乙酸+硫酸亚铁;E.咖啡酸+草酸+硫酸亚铁;F.咖啡酸+乙酸+硫酸亚铁;G.水杨酸+柠檬酸+硫酸亚铁;H.咖啡酸+柠檬酸+硫酸亚铁;I.不添加酸和铁的对照组;J.只添加铁的对照组。
以上试验均为期15 d,每隔48 h取样1次,记录藻细胞数量的变化,以及藻存活情况的变化和pH值的变化。培养周期结束后分别取10 mL和5 mL样品,测量样品中叶绿素a的含量和叶绿素荧光参数Fv/Fm(最大光能转化效率)。其中Fv/Fm常用来表征叶绿素PsⅡ(低铁环境藻类光系统Ⅱ)反应中心内禀光能转换效率,反映当时所有的PsⅡ反应中心均处于开放态时最大光量子产量。
1.2.3 生理指标的测定方法:
为测藻细胞存活率及藻细胞数量,使用5-CFDA染色,具体操作方法如下:
(1)用DMSO(二甲基亚砜)5-CFDA稀释至10 mmol·L−1。将99 μL已经配制好的BG-11培养基与1 μL的5-CFDA混合作为A液,摇晃10 s混匀;
(2)将50 μL样品与50 μL A液混匀,用移液枪至少吹打10次;
(3)在25℃条件下将上一步准备好的样品避光放置30 min;
(4)用移液枪将待测样品吹打10次或摇晃,使藻细胞分散,然后用移液枪取20 μL加入计数板内。在计数板插入仪器之前,稳定1 min,使样品在其中稳定下来。
将细胞染色后,使用细胞计数仪计数,并观察细胞的存活状态。
测量样品叶绿素a的含量:
(1)取藻液10 mL,4500 r·min-1离心15 min,去掉上层清液,将样品在4℃冰箱中放置1 d;
(2)取出后迅速加入5 mL的90%热乙醇(80℃)于80℃的热水浴萃取2 min,再用超声处理10 min,放在暗处萃取4 h后,用0.22 μm的滤头过滤。用酶标仪于波长665 nm和750 nm处测吸光值,然后滴加1滴1 mol·L−1的盐酸酸化,于波长665 nm和750 nm处再测吸光值。计算公式为:
Chla乙醇=27.9×[(E665−E750)−A665+A750)]×V乙醇/V样品 其中,Chla乙醇为热乙醇法测定的叶绿素a含量(μg·L−1);E665是乙醇萃取液于波长665 nm的吸光值;E750是乙醇萃取液于波长750 nm的吸光值;A665为乙醇萃取液酸化后在665 nm处的吸光值;A750为乙醇萃取液在750 nm处的吸光值;V乙醇为乙醇萃取液的体积(mL),V样品为所取样品的体积(mL)。
抑制率计算公式:IR=(1-N/N0×100%)抑制率为负值则有促进效果,抑制率为正则抑制。
1.2.4 泥炭沼泽源酚络合态铁对铜绿微囊藻生长的影响
培养前准备:
(1)提取泥炭中的DOM;
(2)将样品通过H型阳离子柱交换柱,去除样品中存在的金属离子;然后将DOM样品按照<1 KDa,1—3 KDa,>3 KDa分成3份;
(3)将3份样品中加入FeSO4,待稳定一段时间后,取10 mL加入培养基中,加入后Fe的浓度为5×10−6 mol·L−1。
铜绿微囊藻培养实验分为5组,每组3份平行,每组添加的物质如下:A.无添加;B.Fe,浓度为5×10−6 mol·L−1;C.DOM-Fe(<1 KDa),Fe浓度为5×10−6 mol·L−1;D.DOM-Fe(1—3 KDa),Fe浓度为5×10−6 mol·L−1;E.DOM-Fe(>3 KDa),Fe浓度为5×10−6 mol·L−1。
实验均为期15 d,每隔48 h取样1次,记录藻细胞数量的变化,以及藻存活情况的变化。
2. 结果与讨论(Results and discussion)
2.1 不同酚酸影响微囊藻生长的浓度效应
在微囊藻培养体系中分别加入不同浓度四种酚酸溶液,经过14 d培养和检测,得到微囊藻-酚酸的生长曲线如图1所示。图1中A、B为不同浓度的对羟基苯甲酸和对香豆酸对微囊藻生长情况的影响。可以看出,当酚酸浓度为10 mg·L−1和20 mg·L−1时,微囊藻的生长速率和最终达到的终点浓度明显高于控制组(0)抑制率为-23.5%—18%。从微囊藻浓度上来看,当酚酸浓度为10 mg·L−1和20 mg·L−1时,生长情况比较接近,说明在此浓度下,这两种酚酸对微囊藻的生长有一定的刺激作用。在A、B组中当酚酸浓度超过20 mg·L−1时,藻液浓度和藻的生长速率明显低于控制组;当浓度增加到40 mg·L−1时,这两种酚酸对微囊藻的生长起到了明显的抑制作用,而对羟基苯甲酸的对微囊藻生长的抑制作用更加明显;浓度继续增加到60—80 mg·L−1时,微囊藻前4—5天略有增长,然后基本停止了增长,保持在3×106 cell·mL−1左右。对香豆酸60 mg·L−1组的藻数量略高于80 mg·L−1组。
在C组水杨酸-微囊藻的实验中,水杨酸浓度为10 mg·L−1时,增长的速度与控制组接近,在8—10 d快速增长后,藻数量和控制组趋于一致。而当浓度大于10 mg·L−1时,都出现了明显的抑藻效果,抑制率约为80%。
结合图2中存活率来看(从培养的第4天开始测微囊藻的存活率)当浓度为10 mg·L−1时,微囊藻的存活率都略低于控制组,差值在10%左右浮动,当浓度为20—40 mg·L−1,在开始计数时,藻类的存活率就已不同程度地低于控制组,并且A、C、D存活率在4—14 d整体处于下降的趋势,可以看出,水杨酸对微囊藻的抑制作用最强。
表1是各组样品的Fv/Fm,最大光能转化效率Fv/Fm常用来表征叶绿素PsII反应中心内禀光能转换效率,反映当时所有的PsII反应中心均处于开放态时最大光量子产量。
在非胁迫环境下,植物叶片叶绿素荧光参数Fv/Fm变化极小,表现出稳定的特点,但在胁迫条件下,该参数明显下降[22]。Fv/Fm可作为植物受环境胁迫的响应指标[23]。控制组的Fv/Fm为0.308。一般情况下,微囊藻的Fv/Fm在0.3左右,当Fv/Fm过低表明藻类受到环境胁迫,PSII中心受到损伤进而降低光合作用效率。由表1可以看出,当水杨酸浓度大于20 mg·L−1时会对微囊藻的光合作用产生明显抑制,当各组酚酸浓度超过60 mg·L−1时,藻类基本停止了光合作用,这和前文中藻类的生物量变化和存活率相吻合。表2是各个组叶绿素含量的均值,数据表明:各组中相对低浓度的酚酸,不仅对藻类数量的增长有促进作用,也促进了叶绿素含量的增加。
表 1 铜绿微囊藻的Fv/FmTable 1. Fv/Fm of Microcystis aeruginosa对羟基苯甲酸P-hydroxybenzoic acid 对香豆酸P-coumaric acid 水杨酸Salicylic acid 咖啡酸Caffeic acid 010×10−620×10−640×10−660×10−680×10−6 0.3080.3100.2920.3020.0390.000 0.3080.3100.3140.3240.0490.000 0.3080.3090.0230.0140.0000.000 0.3080.3310.2760.3600.0380.000 注:0—80×10−6分别对应添加的4种酚酸浓度,表中数据是在微囊藻培养期结束时测得的Fv/Fm。 Note: 0—80×10−6 respectively correspond to the four added phenolic acid concentrations. The data in the table are the Fv/Fm measured at the end of the Microcystis culture period. 表 2 铜绿微囊藻叶绿素含量(g·L-1)Table 2. Chlorophyll content of Microcystis aeruginosa对羟基苯甲酸P-hydroxybenzoic acid 对香豆酸P-coumaric acid 水杨酸Salicylic acid 咖啡酸Caffeic acid 010×10−620×10−640×10−660×10−680×10−6 0.5681.0141.1380.7750.0000.000 0.5680.7370.8620.5680.0000.000 0.5680.7960.0090.0000.0000.000 0.5682.0932.2011.3590.0000.000 注:0—80×10−6分别对应添加的4种酚酸浓度,表中数据是微囊藻培养期结束时测得的叶绿素含量。 Note: 0—80×10−6 respectively correspond to the four phenolic acid concentrations added. The data in the table is the chlorophyll content measured at the end of the Microcystis culture period. 2.2 酚络合态铁的生物可利用性
根据上述试验结果可以得出,当这4种典型泥炭源酚酸的浓度达10 mg·L−1时,从生物量、存活率以及光合作用强度来说对微囊藻的生长没有明显抑制作用。普遍认为,藻类吸收Fe主要是离子形态,而不是有机络合态Fe[24]。目前,仅观察到在产生铁载体的微生物中存在铁载体复合物的吸收及其在细胞中的还原[25]。已有研究发现,有机络合态铁中铁的释放途径不同。其中包括简单的配体-金属平衡(Ligand-Metal balance),平衡在初级生产者消耗铁后发生变化,促进铁从络合物中分离。另一个途径是基于配体的降解,这也导致了Fe和配体的分离。第三种可能是通过有机态铁的还原,降低配体对铁的亲和力,并导致Fe的释放。这个过程可能是由于络合物的自还原/氧化而发生的,这意味着配体氧化同时也释放产生Fe[26]。尤其是光还原分解对铁的生物利用度有很大影响,这一释放铁途径被称为AHS(aquatic humic substances)机制[12]。
图3是加入不同酸和Fe2+的微囊藻生长曲线。施加和不加Fe2+的对照试验表明,在培养1—9 d,两组生长速度以及生物量大致相同。在第9天后,施加Fe2+组的生长速度放缓,最终的藻密度低于对照组。图3表明在没有配体存在的情况下,加入一定量的铁对微囊藻生长促进作用不明显。同时,观察发现,在藻类指数生长阶段出现了较高的pH值(高达10.5),这与二氧化碳生物需求增加有关。在指数生长结束和平台期开始后,pH值略下降。在藻类生长平缓或生长不良的样品中,pH值无显著变化,pH值大多保持在6—8。
能促进藻类生长的酚酸应当与三价铁有较高的亲和力,与二价铁有较低的亲和力[27]。试验表明,相对其他3种酚酸铁配合物体系,水杨酸铁不能有效地为微囊藻提供生物可利用性铁,这可能与水杨酸的稳定性较强有关[27]。对照表明,用咖啡酸处理的微囊藻生长良好,最高浓度达到2.19×107 cell·mL−1。这可能是咖啡酸中的儿茶酚基结构所引起的,并且有更高的氧化还原潜力,更容易将Fe从配合物中释放。Santana等 [28]的研究也证实了在生物条件下还原络合物的可能性。总体上,酚酸的加入提高了藻类对Fe的生物可利用率。
图2显示藻类存活率从第10天开始明显下降,藻类计数可能包括了死藻,因此选择第11天数据进行显著性分析。结果表明(表3),在0.05的置信水平下,咖啡酸的加入对微囊藻生长有显著促进作用,而水杨酸在0.05的置信水平下,对微囊藻生长无显著促进作用。
 图 3 微囊藻生长曲线Figure 3. Microcystis growth curveA.水杨酸+硫酸亚铁 B.咖啡酸+硫酸亚铁 C.水杨酸+草酸+硫酸亚铁 D.水杨酸+乙酸+硫酸亚铁 E.咖啡酸+草酸+硫酸亚铁 F.咖啡酸+乙酸+硫酸亚铁 G.水杨酸+柠檬酸+硫酸亚铁 H.咖啡酸+柠檬酸+硫酸亚铁 1_3.不添加酸和铁的对照组 Fe1_3硫酸亚铁A. Salicylic acid+Ferrous sulfate B. Caffeic acid+Ferrous sulfate C. Salicylic acid+Oxalic acid+Ferrous sulfate D. Salicylic acid+Acetic acid+Ferrous sulfate E. Caffeic acid+Oxalic acid+Ferrous sulfate F. Caffeic acid+Acetic acid+Ferrous sulfate G. Salicylic acid+Citric acid+Ferrous sulfate H. Caffeic acid+Citric acid+Ferrous sulfate 1_3. Control group without acid and iron Fe1_3. Ferrous sulfate表 3 第11天不同试验组微囊藻浓度变化的相关性矩阵Table 3. Correlation matrix of changes in the concentration of Microcystis in different test groups on the 11th day
图 3 微囊藻生长曲线Figure 3. Microcystis growth curveA.水杨酸+硫酸亚铁 B.咖啡酸+硫酸亚铁 C.水杨酸+草酸+硫酸亚铁 D.水杨酸+乙酸+硫酸亚铁 E.咖啡酸+草酸+硫酸亚铁 F.咖啡酸+乙酸+硫酸亚铁 G.水杨酸+柠檬酸+硫酸亚铁 H.咖啡酸+柠檬酸+硫酸亚铁 1_3.不添加酸和铁的对照组 Fe1_3硫酸亚铁A. Salicylic acid+Ferrous sulfate B. Caffeic acid+Ferrous sulfate C. Salicylic acid+Oxalic acid+Ferrous sulfate D. Salicylic acid+Acetic acid+Ferrous sulfate E. Caffeic acid+Oxalic acid+Ferrous sulfate F. Caffeic acid+Acetic acid+Ferrous sulfate G. Salicylic acid+Citric acid+Ferrous sulfate H. Caffeic acid+Citric acid+Ferrous sulfate 1_3. Control group without acid and iron Fe1_3. Ferrous sulfate表 3 第11天不同试验组微囊藻浓度变化的相关性矩阵Table 3. Correlation matrix of changes in the concentration of Microcystis in different test groups on the 11th day1_3 Fe1_3 A B C D E F G H 1_3 1 0.963 0.971 −0.358 0.474 0.657 0.246 0.461 0.983 0.491 Fe1_3 0.963 1.00 1.000* −0.095 0.221 0.835 −0.022 0.682 0.996 0.707 A 0.971 1.000* 1.00 −0.126 0.251 0.818 0.008 0.659 0.999* 0.685 B −0.358 −0.095 −0.126 1.00 −0.992 0.468 −0.993 0.663 −0.179 0.637 C 0.474 0.221 0.251 −0.992 1.00 −0.352 0.970 −0.563 0.302 −0.534 D 0.657 0.835 0.818 0.468 −0.352 1.00 −0.569 0.972 0.785 0.979 E 0.246 −0.022 0.008 −0.993 0.970 −0.569 1.00 −0.746 0.062 −0.723 F 0.461 0.682 0.659 0.663 0.663 0.972 −0.746 1.00 0.618 0.999* G 0.983 0.996 0.999* −0.179 0.302 0.785 0.062 0.618 1.00 0.645 H 0.491 0.707 0.685 0.637 −0.534 0.979 −0.723 0.999* 0.645 1.00 注:*. 在 0.05 水平(双侧)上显著相关。Notes:*. Significant correlation at 0.05(bilateral) level. 2.3 泥炭沼泽源DOM-Fe的藻类可利用性
许多泥炭源有机质同时含有酚羟基和羧基,具有酚酸性质。但由于泥炭有机质组成复杂多样,现有技术尚不能有效分离不同性质有机化合物。因此,利用不同分子量段有机质与铁的络合物,开展藻类培养试验有助于客观评估泥炭源DOM-Fe的藻类可利用性。利用不同分子量段DOM-Fe,进行培养试验,铜绿微囊藻的生长情况如图4。表4 结果表明,在0.05 的置信水平下,不同组之间差异不具统计显著性,但图4 还是反映出有机态铁对藻类生长的促进趋势.
结果显示(图3和图4),不同分子量结合态Fe均促进了铜绿微囊藻的生长,但影响程度不同。添加Fe后,铜绿微囊藻的生物量和控制组相比均有增加,微囊藻生长得到促进,最终达到107 cell·mL−1。其中,微囊藻在7—11 d增长最快;其次,对比生长终点可以发现不同DOM-Fe促进效果存在差异:E组>D组>C组>B组>A组>无Fe组;第三,添加DOM显著促进了藻的生长。研究表明,相对于Fe3+,藻类更倾向于利用Fe2+[29]。这是由于具有一定还原能力的DOM可以减缓二价铁的氧化[30],从而提高了藻类对铁的利用率。此外,不同分子量段DOM与Fe的络合稳定常数略有不同,较高分子量的DOM(>3 kD)与Fe的络合稳定常数较小[30],在光照或者其他条件下容易发生解离,产生易被藻类利用的Fe。而泥炭源低分子量DOM(<1 KD)络合态铁,由于其络合稳定的常数相对较高,在培养体系中更加稳定,相对不易被藻类利用。
表 4 第11天不同试验组铜绿微囊藻生长浓度变化的相关性矩阵Table 4. Correlation matrix of growth concentration changes of Microcystis aeruginosa in different test groups on the 11th dayA B C D E A 1 −.751 .350 −.167 .770 B −.751 1 −.881 .776 −.158 C .350 −.881 1 −.982 −.327 D −.167 .776 −.982 1 .500 E .770 −.158 −.327 .500 1 注:*. 在 0.05 水平(双侧)上显著相关. Notes:*. Significant correlation at 0.05(bilateral) level. 3. 结论(Conclusion)
(1)4种酚酸对藻类生长的影响均呈现“低促高抑”的规律。从藻类生物量和叶绿素含量来看,抑藻效果从高到低:水杨酸>对羟基苯甲酸>对香豆酸>咖啡酸;结合藻类的存活率,虽然低浓度酚酸刺激了藻类生物量的增长,但是也对藻类的生存产生了一定的负面影响:在添加10 mg·L−1酚酸的几组样品中,微囊藻的存活率都略低于控制组。
(2)添加酚酸的藻类样品中,当水杨酸浓度达到20 mg·L−1时,Fv/Fm明显降低(0.3降低到0.02左右),而其它3种酚酸浓度达到60 mg·L−1才出现抑制,说明水杨酸抑制作用最强。
(3)不同酚铁络合物的生物可利用性存在差异:相对咖啡酸和水杨酸,水杨酸络合态铁更难被藻类利用,除酚毒性效应外,还与其较高的络合稳定性有关。
(4)泥炭源不同分子段DOM-Fe对藻类生长的促进作用从高到低依次为:>3 KD,1—3 KD,<1 KD。高分子段DOM(>3 kD)与Fe的络合稳定常数最小,在光照或者其他条件下容易发生解离,更易释放Fe而被藻类利用;泥炭源低分子量DOM(<1 KD)络合态铁,因其络合稳定常数相对较高,相对不易被藻类利用。
致谢:感谢中国科学院水生生物研究所的大力支持。
-
表 1 铜绿微囊藻的Fv/Fm
Table 1. Fv/Fm of Microcystis aeruginosa
对羟基苯甲酸P-hydroxybenzoic acid 对香豆酸P-coumaric acid 水杨酸Salicylic acid 咖啡酸Caffeic acid 010×10−620×10−640×10−660×10−680×10−6 0.3080.3100.2920.3020.0390.000 0.3080.3100.3140.3240.0490.000 0.3080.3090.0230.0140.0000.000 0.3080.3310.2760.3600.0380.000 注:0—80×10−6分别对应添加的4种酚酸浓度,表中数据是在微囊藻培养期结束时测得的Fv/Fm。 Note: 0—80×10−6 respectively correspond to the four added phenolic acid concentrations. The data in the table are the Fv/Fm measured at the end of the Microcystis culture period. 表 2 铜绿微囊藻叶绿素含量(g·L-1)
Table 2. Chlorophyll content of Microcystis aeruginosa
对羟基苯甲酸P-hydroxybenzoic acid 对香豆酸P-coumaric acid 水杨酸Salicylic acid 咖啡酸Caffeic acid 010×10−620×10−640×10−660×10−680×10−6 0.5681.0141.1380.7750.0000.000 0.5680.7370.8620.5680.0000.000 0.5680.7960.0090.0000.0000.000 0.5682.0932.2011.3590.0000.000 注:0—80×10−6分别对应添加的4种酚酸浓度,表中数据是微囊藻培养期结束时测得的叶绿素含量。 Note: 0—80×10−6 respectively correspond to the four phenolic acid concentrations added. The data in the table is the chlorophyll content measured at the end of the Microcystis culture period. 表 3 第11天不同试验组微囊藻浓度变化的相关性矩阵
Table 3. Correlation matrix of changes in the concentration of Microcystis in different test groups on the 11th day
1_3 Fe1_3 A B C D E F G H 1_3 1 0.963 0.971 −0.358 0.474 0.657 0.246 0.461 0.983 0.491 Fe1_3 0.963 1.00 1.000* −0.095 0.221 0.835 −0.022 0.682 0.996 0.707 A 0.971 1.000* 1.00 −0.126 0.251 0.818 0.008 0.659 0.999* 0.685 B −0.358 −0.095 −0.126 1.00 −0.992 0.468 −0.993 0.663 −0.179 0.637 C 0.474 0.221 0.251 −0.992 1.00 −0.352 0.970 −0.563 0.302 −0.534 D 0.657 0.835 0.818 0.468 −0.352 1.00 −0.569 0.972 0.785 0.979 E 0.246 −0.022 0.008 −0.993 0.970 −0.569 1.00 −0.746 0.062 −0.723 F 0.461 0.682 0.659 0.663 0.663 0.972 −0.746 1.00 0.618 0.999* G 0.983 0.996 0.999* −0.179 0.302 0.785 0.062 0.618 1.00 0.645 H 0.491 0.707 0.685 0.637 −0.534 0.979 −0.723 0.999* 0.645 1.00 注:*. 在 0.05 水平(双侧)上显著相关。Notes:*. Significant correlation at 0.05(bilateral) level. 表 4 第11天不同试验组铜绿微囊藻生长浓度变化的相关性矩阵
Table 4. Correlation matrix of growth concentration changes of Microcystis aeruginosa in different test groups on the 11th day
A B C D E A 1 −.751 .350 −.167 .770 B −.751 1 −.881 .776 −.158 C .350 −.881 1 −.982 −.327 D −.167 .776 −.982 1 .500 E .770 −.158 −.327 .500 1 注:*. 在 0.05 水平(双侧)上显著相关. Notes:*. Significant correlation at 0.05(bilateral) level. -
[1] GEIDER R J. Biological oceanography: Complex lessons of iron uptake [J]. Nature, 1999, 400(6747): 815. doi: 10.1038/23582 [2] BILLER D V, BRULAND K W. The central california current transition zone: A broad region exhibiting evidence for iron limitation [J]. Progress in Oceanography, 2014, 120: 370-382. doi: 10.1016/j.pocean.2013.11.002 [3] WATSON A, LISS P, DUCE R. Design of a small‐scale in situ iron fertilization experiment [J]. Limnology and Oceanography, 1991, 36(8): 1960-1965. doi: 10.4319/lo.1991.36.8.1960 [4] DAI M H, MARTIN J M. First data on trace metal level and behaviour in two major Arctic river-estuarine systems (Ob and Yenisey) and in the adjacent Kara Sea, Russia [J]. Earth and Planetary Science Letters, 1995, 131(3-4): 127-141. doi: 10.1016/0012-821X(95)00021-4 [5] COALE K H, FITZWATER S E, GORDON R M, et al. Control of community growth and export production by upwelled iron in the equatorial Pacific Ocean [J]. Nature, 1996, 379(6566): 621. doi: 10.1038/379621a0 [6] MARTIN J H, FITZWATER S E. Iron deficiency limits phytoplankton growth in the north-east Pacific subarctic [J]. Nature, 1988, 331(6154): 341. doi: 10.1038/331341a0 [7] 穆景利, 韩建波, 霍传林, 等. 海洋铁施肥研究进展 [J]. 海洋环境科学, 2011, 30(2): 282-286. doi: 10.3969/j.issn.1007-6336.2011.02.031 MU J L, HAN J B, HUO C L, et al. Study progress on ocean iron fertilization [J]. Marine Environmental Science, 2011, 30(2): 282-286(in Chinese). doi: 10.3969/j.issn.1007-6336.2011.02.031
[8] RAVEN J A, GEIDER R J. Temperature and algal growth [J]. New Phytologist, 1988, 110(4): 441-461. doi: 10.1111/j.1469-8137.1988.tb00282.x [9] GREGG W W, CONKRIGHT M E. Decadal changes in global ocean chlorophyll[J]. Geophysical Research Letters, 2002, 29(15): 20-1–20-4. [10] KONDO Y, TAKEDA S, FURUYA K. Distinct trends in dissolved Fe speciation between shallow and deep waters in the Pacific Ocean [J]. Marine Chemistry, 2012, 134: 18-28. [11] KUHN K M, MAURICE P A, NEUBAUER E, et al. Accessibility of humic-associated Fe to a microbial siderophore: Implications for bioavailability [J]. Environmental Science & Technology, 2014, 48(2): 1015-1022. [12] BLAZEVIC A, ORLOWSKA E, KANDIOLLER W, et al. Photoreduction of terrigenous Fe‐Humic substances leads to bioavailable iron in oceans [J]. Angewandte Chemie International Edition, 2016, 55(22): 6417-6422. doi: 10.1002/anie.201600852 [13] KRACHLER R, KRACHLER R F, von der KAMMER F, et al. Relevance of peat-draining rivers for the riverine input of dissolved iron into the ocean [J]. The Science of the Total Environment, 2010, 408(11): 2402-2408. doi: 10.1016/j.scitotenv.2010.02.018 [14] WANG Y, XIANG W, YANG W, et al. Photo-stability of iron-phenolic complexes derived from peatland upon irradiation in waters under simulated sunlight [J]. Chemical Geology, 2018, 485: 14-23. doi: 10.1016/j.chemgeo.2018.03.016 [15] HARWOOD C S, PARALES R E. The beta-ketoadipate pathway and the biology of self-identity [J]. Annual Review of Microbiology, 1996, 50: 553-590. doi: 10.1146/annurev.micro.50.1.553 [16] WU Y, XIANG W, FU X F, et al. Geochemical interactions between iron and phenolics originated from peatland in Hani, China: Implications for effective transport of iron from terrestrial systems to marine [J]. Environmental Earth Sciences, 2016, 75(4): 1-12. [17] 倪利晓, 任高翔, 陈世金, 等. 酚酸和不饱和脂肪酸对铜绿微囊藻的联合作用 [J]. 环境化学, 2011, 30(8): 1428-1432. NI L X, REN G X, CHEN S J, et al. Study on joint action of phenolic acids and unsaturated fatty acids to Microcystis aeruginosa [J]. Environmental Chemistry, 2011, 30(8): 1428-1432(in Chinese).
[18] 吴安平, 张庭廷, 何梅, 等. 水杨酸对水华鱼腥藻的化感抑制作用及相关毒理学的初步研究 [J]. 生物学杂志, 2008, 25(5): 44-47. doi: 10.3969/j.issn.2095-1736.2008.05.013 WU A P, ZHANG T T, HE M, et al. A preliminary study on Anabena Flos-aquae minitigation of salicylic acid and its related toxicity [J]. Journal of Biology, 2008, 25(5): 44-47(in Chinese). doi: 10.3969/j.issn.2095-1736.2008.05.013
[19] 李慧. 植物多酚用于饮用水消毒的试验研究[D]. 北京: 北京建筑工程学院, 2005. LI H. The Study on Plant Polyphenols Used in Disinfection of Drinking Water. Beijing Institute of Architectural Engineering[D]. Beijing, Beijing Institute of Architectural Engineering, , 2005(in Chinese).
[20] KORNER S. Allelopathic growth inhibition of selected phytoplankton species by submerged macrophyes [J]. Journal of Phycology, 2002, 38: 862-871. doi: 10.1046/j.1529-8817.2002.t01-1-02001.x [21] ORLOWSKA E, ROLLER A, PIGNITTER M, et al. Synthetic iron complexes as models for natural iron-humic compounds: Synthesis, characterization and algal growth experiments [J]. The Science of the Total Environment, 2017, 577: 94-104. doi: 10.1016/j.scitotenv.2016.10.109 [22] 陈辰, 何小定, 秦金舟, 等. 4种含笑叶片叶绿素荧光参数Fv/Fm特性的比较 [J]. 安徽农业大学学报, 2013(1): 32-37. CHEN C, HE X D, QIN J Z, et al. Comparison of chlorophyll fluorescence Fv/Fm characteristics of four Michelia trees [J]. Journal of Anhui Agricultural University, 2013(1): 32-37(in Chinese).
[23] ANNIKA J, GAKU K. Short-Term responses in maximum quantum yield of PSII (Fv/Fm) to ex situ temperature treatment of populations of bryophytes originating from different sites in Hokkaido, Northern Japan [J]. Plants, 2016, 5(2): 22. doi: 10.3390/plants5020022 [24] JOE M, CHRIS B. Iron utilization in marine cyanobacteria and eukaryotic algae [J]. Frontiers in Microbiology, 2012, 3(43): 1-13. [25] HOPKINSON B M, MOREL F M M. The role of siderophores in iron acquisition by photosynthetic marine microorganisms [J]. BioMetals, 2009, 22(4): 659-669. doi: 10.1007/s10534-009-9235-2 [26] ROSE A L, WAITE T D. Predicting iron speciation in coastal waters from the kinetics of sunlight-mediated iron redox cycling [J]. Aquatic Sciences, 2003, 65(4): 375-383. doi: 10.1007/s00027-003-0676-3 [27] ORLOWSKA E, ROLLER A, WIESINGER H, et al. Benzoic hydroxamate-based iron complexes as model compounds for humic substances: Synthesis, characterization and algal growth [J]. Royal Society of Chemistry Experiments, 2016, 6(46): 40238-40249. doi: 10.1039/C5RA25256C [28] SANTANA-CASIANO J M, GONZALEZ-DAVLIA M , MILLERO F J. Comment on “oxygenation of Fe(II) in natural waters revisited: Kinetic modelling approaches, rate constant estimation and the importance of various reaction pathways”by pham and waite (2008) [J]. Geochimica et Cosmochimica Acta, 2010, 74(17): 5150-5153. doi: 10.1016/j.gca.2009.12.032 [29] WELLS M L, ZORKIN N G, LEWIS A G. The role of colloid chemistry in providing a source of iron to phytoplankton [J]. Journal of Marine Research, 1983, 41: 731-746. doi: 10.1357/002224083788520478 [30] 黄玉冰, 赵甜甜, 向武, 等. 大九湖泥炭沼泽源铁有机配合物的络合稳定性及其生态环境意义[J]. 地球科学, 2021, 46(5): 1862-1870. ZHUANG Y B, HAO T T , XIANG W, et al. Stability of organic iron complexes in Dajiuhu Peats and its ecological significance[J]. Earth Science, 2021, 46(5): 1862-1870 (in Chinese).
-




 下载:
下载: