-
重金属铬及其化合物作为化工原料被广泛应用于电镀、制革、印染、冶金工业等领域。铬渣的无序堆放和含铬废水的不当排放是造成地下水铬污染的主要原因,因而对生态环境和人群健康造成了严重的威胁[1-2]。截至2018年,我国历史遗留的铬渣堆场和已关停的铬盐的生产场地约有60个(其中20个已完成修复),场区内地下水受到严重的铬污染[3]。铬在地下水中主要以三价铬(Cr(Ⅲ))和六价铬(Cr(Ⅵ))的价态存在,其中,Cr(Ⅵ)在低浓度下也具有剧毒性,毒性为Cr(Ⅲ)的100~1 000倍,常以重铬酸根离子(Cr2O72−,酸性条件)和铬酸根离子(CrO42−,中性或碱性条件)的形式存在,具有强致癌致畸性、较高的溶解度、高迁移性和环境持久性[4-7]。我国《地下水质量标准》(GB/T 14848-2017)[8]将Cr(Ⅵ)作为毒理学指标,当其质量浓度>0.10 mg·L−1时,该类水质就判定为Ⅴ类水,不适宜作为生活饮用水水源。因此,亟需研发绿色、高效的新型修复材料去除地下水中的Cr(Ⅵ)。
近年来,纳米零价铁(nanoscale zero-valent iron,nZVI)由于具有较强的还原性和反应活性、材料易回收利用等优点,被广泛应用于水体中Cr(Ⅵ)及其他重金属的去除[9]。但在实际应用中,nZVI存在易团聚和易氧化等问题,导致其迁移能力变差,从而降低了反应活性[10]。因此,通过制备纳米零价铁复合材料来优化纳米零价铁的修复性能,以提高其反应活性,从而高效地处理重金属、有机物等多种污染物[11-12]。目前,许多研究主要集中在吸附材料上,如将nZVI负载在诸如生物质炭[13]、活性炭[14]、膨润土[15]、凹凸棒石[16]等比表面积大的多孔材料上以克服其团聚的缺陷,提高在环境中的迁移性。在这些材料中,生物质炭因廉价易得、多孔结构、比表面积大、绿色环保等优点被广泛用作各类污染物的吸附剂以及nZVI的载体材料[17-19]。有研究表明,原始生物质炭材料经酸改性后可以去除灰分和硅,增大比表面积,降低表面负电荷[20-21];经碱处理可以去除生物质炭表面灰分,清除残留在孔隙中的杂质且增加生物质炭的表面积、孔体积和含氧官能团的数量,从而增加其吸附性能[21-23]。DONG等[20]发现,盐酸改性的生物质炭负载nZVI对Cr(Ⅵ)的去除率可达到35.3%,而原始生物质炭负载nZVI对Cr(Ⅵ)的去除率约为22.0%。WU等[24]研究了改性生物质炭负载铁/银双金属颗粒对四氯化碳的去除效果,结果表明,氢氧化钠改性生物质炭负载铁/银双金属颗粒对四氯化碳的去除率高于未改性生物质炭负载铁/银材料。因此,可以看出,经过酸碱改性后的生物质炭表面性能得到进一步提高,可促进nZVI的有效分散,成为nZVI理想的负载材料。迄今为止,采用HF、NaOH改性生物质炭负载nZVI复合材料去除Cr(Ⅵ)的研究尚未见报道,其去除性能及机理亦尚不清晰。
本研究选择HF和NaOH作为酸碱改性剂,对水稻秸秆生物质炭进行改性,获得氢氟酸改性生物质炭(BC-HF)和氢氧化钠改性生物质炭(BC-NaOH),并进一步负载纳米零价铁制得生物质炭负载纳米零价铁(nZVI@BC)、氢氟酸改性生物质炭负载纳米零价铁(nZVI@BC-HF)和氢氧化钠改性生物质炭负载纳米零价铁(nZVI@BC-NaOH)。利用比表面积分析、元素分析、红外光谱分析、X射线衍射分析等手段表征反应前后材料的组成与结构;通过动力学,测试复合材料对Cr(Ⅵ)的去除性能,并通过动力学过程、反应前后溶液pH分析和材料晶体结构分析等探讨其去除机制,同时探讨在空气中老化对nZVI@BC-HF和nZVI@BC-NaOH活性的影响。
-
实验所用试剂主要有硼氢化钾(KBH4)、七水合硫酸亚铁(FeSO4·7H2O)、氢氟酸(HF)、氢氧化钠(NaOH)、无水乙醇(C2H5OH)、硝酸钠(NaNO3)、重铬酸钾(K2Cr2O7)、二苯碳酰二肼(C13H14N4O)、丙酮(CH3COCH3)、硫酸(H2SO4)、磷酸(H3PO4)等。除K2Cr2O7为优级纯外,以上试剂均为分析纯,所有溶液均采用超纯水配制。
-
本研究以中国科学院南京土壤研究所常熟农业生态实验站的水稻秸秆作为生物质炭前体。在使用前,用去离子水冲洗水稻秸秆3次,自然风干,并于在60 °C烘箱中干燥24 h,然后根据文献报道的方法,在限氧条件下以5 °C·min−1的升温速率达到700 ℃后热解6 h,冷却至室温,磨碎,过100目筛备用[25]。分别将10 g生物质炭置于200 mL、1 mol·L−1的HF、NaOH溶液中,在温度为25 °C、150 r·min−1的转速下震荡24 h,用蒸馏水反复冲洗抽滤至pH稳定,BC-HF、BC-NaOH最后稳定的pH分别为3.12±0.1、10.40±0.1;原始生物质炭在相同的条件下加入超纯水清洗即可,最后将制备的3种生物质炭在80 °C下烘干,研磨过100目筛,保存备用。
采用液相还原法制备nZVI和3种生物质炭负载nZVI复合材料。预先称1 g的FeSO4·7H2O溶于50 mL的去离子水中(用预配的1 mol·L−1稀盐酸和1 mol·L−1氢氧化钾调节pH至4.0),将溶液转移至三口烧瓶中,加入50 mL乙醇,将三口烧瓶中的溶液分别用氮气除氧和搅拌15 min,使溶液混合均匀。然后,在氮气保护和机械搅拌下(150 r·min−1),将等体积的0.5 mol·L−1的KBH4溶液倒入恒压漏斗中,以5 mL·min−1的速度匀速逐滴滴入三口烧瓶中,此后继续曝气反应60 min,采用磁选法将制备得到的材料固液分离,用脱氧超纯水和脱氧无水乙醇各洗3遍,置于真空干燥箱中60 ℃干燥过夜。干燥后的样品经研磨、过筛后,置于棕色样品瓶中,放入真空干燥皿中保存备用。该过程发生的化学反应如式(1)所示。
制备nZVI@BC时,先取50 mg生物质炭加入到50 mL、20 g·L−1的FeSO4·7H2O溶液中(炭铁质量比为1∶4),放入25 ℃、150 r·min−1的恒温振荡器中震荡24 h后置于三口烧瓶中,然后按照上述合成nZVI的方法制备nZVI@BC。按照同样的步骤制得nZVI@BC-HF和nZVI@BC-NaOH。
-
配置Cr(Ⅵ)污染溶液时加入NaNO3作为背景电解质,用来平衡地下水溶液中的离子强度,其浓度为10 mmol·L−1,同时将Cr(Ⅵ)污染溶液初始pH调至3。在20 mL玻璃瓶中加入Cr(Ⅵ)污染溶液和供试材料,最终质量浓度分别为50 mg·L−1和0.3 g·L−1。将玻璃瓶置于150 r·min−1和25 ℃的恒温摇床上进行反应,然后在选定的时间间隔内(1、2、4、8、12、24、48 h)取样,样品通过0.45 μm滤膜过滤后进行分析测试,在反应过程中测量了溶液pH及总铁浓度。所有实验均重复进行3次。
-
本实验通过比表面积分析仪(Micromeritics ASAP 2460,美国麦克仪器公司)使用N2吸附-解吸法测定供试材料的比表面积和孔体积;供试材料元素组成用元素分析仪(Elementar Vario EL III,德国Elementar公司)测定;供试材料表面官能团用傅立叶变换红外光谱仪(Nicolet iS10,美国 Thermo Fisher 仪器公司)测定;反应前后的晶体结构进行用X射线衍射(XRD,RIGAKUD/MAX2550/PC,日本)表征。采用二苯碳酰二肼分光光度法测定溶液中Cr(Ⅵ)浓度;采用原子吸收法(ICP-OES)测定溶液中的总铁浓度;采用pH计(pHS-3C,Thermo)测定反应前后的溶液pH。供试材料与Cr(Ⅵ)反应的过程使用伪二阶动力学模型描述,计算公式见式(2)[26]。
式中:qt和qe分别为时间t和反应达到平衡时Cr(Ⅵ)的去除量,mg·g−1;k2是伪二阶动力学模型的速率常数,g·(mg·h)−1。
-
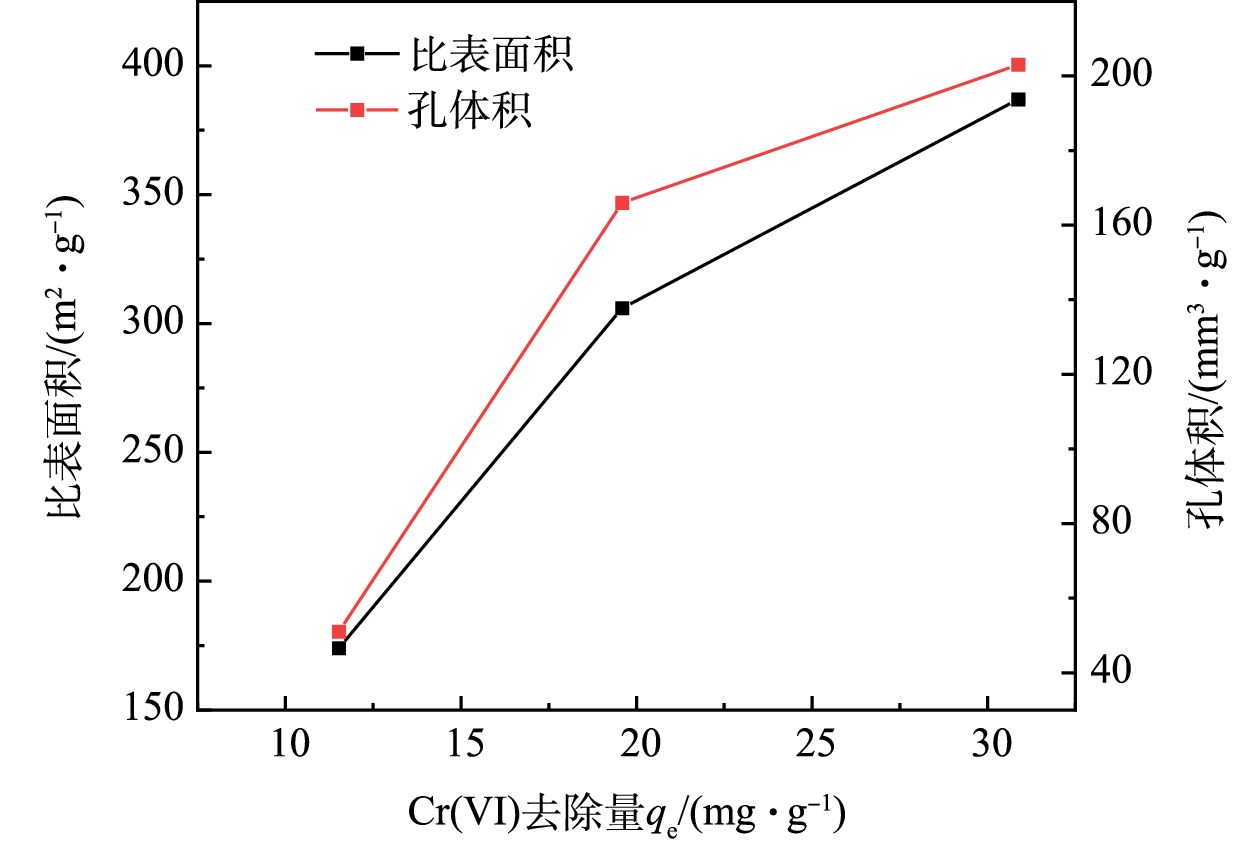
1)比表面积与孔隙结构。供试材料的比表面积与孔隙结构分析结果见表1,BC的比表面积和孔体积为173.5 m2·g−1和50.96 mm3·g−1,经酸、碱改性后的生物质炭比表面积和孔体积分别为BC的2.22、1.76倍和3.98、3.25倍,比表面积和孔体积明显增大。LIU等[27]的研究结果同样表明,生物质炭经过酸碱改性后比表面积和孔体积会明显增大。这是因为,生物质炭通过酸、碱改性去除了孔隙结构中的灰分和无机物,清除了部分可溶性杂质,使比表面积和孔隙体积增大,从而有效地改善了BC的物理性质。负载nZVI后,复合材料的比表面积和孔体积均低于酸碱改性生物质炭,这与ZHANG[4]等的研究结果一致,其主要原因是:具有较小比表面积的nZVI是复合材料中的主要组成部分(炭铁质量比为1∶4);同时,nZVI占据了生物质炭中大部分孔隙,因此,酸碱改性生物质炭负载nZVI后比表面积和孔体积明显减小。
2)元素分析。生物质炭的主要组成元素是C、H、O和N。从表2可以看出,酸碱改性前后基本元素组成不变,主要元素组成均为C和O元素,但改性前后生物质炭主要元素的质量分数有所差异。经酸碱改性后的生物质炭,由于灰分和一些可溶性杂质被清除,C的质量分数明显增大,N、H、S、O的质量分数略有增加。H/C比值用来表示生物质炭的芳香性,(O+N)/C比值用来表示生物质炭的极性[28]。相比BC,酸改性生物质炭的H/C和(O+N)/C均降低,这表明芳香性增加,疏水性增强;碱改性生物质炭的H/C比值增加,(O+N)/C比值降低,这表明芳香性降低,疏水性增加。酸碱改性都能使生物质炭疏水性增强,其主要原因是改性后的炭材料中硅氧化物气化,改善了材料表面的亲水性[29]。
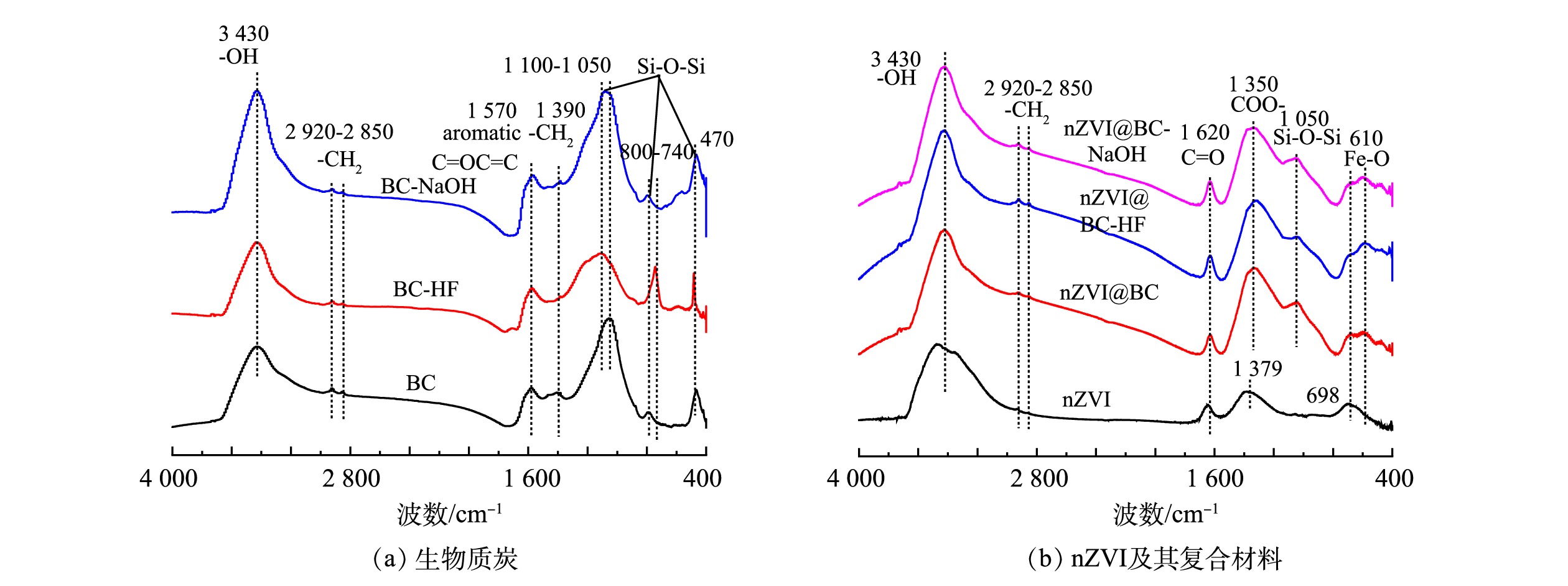
3)红外谱图分析。图1为供试材料的傅里叶变换红外光谱图。图1(a)为BC改性前后材料上的官能团变化,3 430 cm−1处的吸收峰为-OH基团伸缩振动,这表明生物质炭表面均含有大量的羟基官能团[30];2 920~2 850 cm−1和1 390 cm−1处的吸收峰为-CH2[21];1 570 cm−1的吸收峰被认为是芳香族C=C键和C=O键[31];Si-O-Si的特征带主要出现在1 100~1 050、880~740和 470 cm−1处[25]。由图1(a)可以看出,酸碱改性前后的生物质炭官能团种类没有发生变化,但经过氢氟酸改性后的BC在1 100~1 050 cm−1处的Si-O-Si特征峰减弱,这说明酸改性可洗脱BC中的硅。图1(b)为nZVI及其复合材料的红外光谱图。3 430、2 920~2 850 cm−1处分别为-OH和-CH2的吸收峰[21];在1 350 cm−1的峰值与COO-有关[32]。在1 100~1 050、880-740和 470 cm−1处的Si-O-Si特征带在3种生物质炭与 nZVI负载后减弱或者消失,这表明Si-O-Si是负责支持nZVI的主要位点之一[25, 33-34]。因此,nZVI通过与生物质炭表面Si-O-Si官能团耦合形成Si-O-Fe键,铁离子趋于稳定,降低了nZVI的氧化倾向[35]。
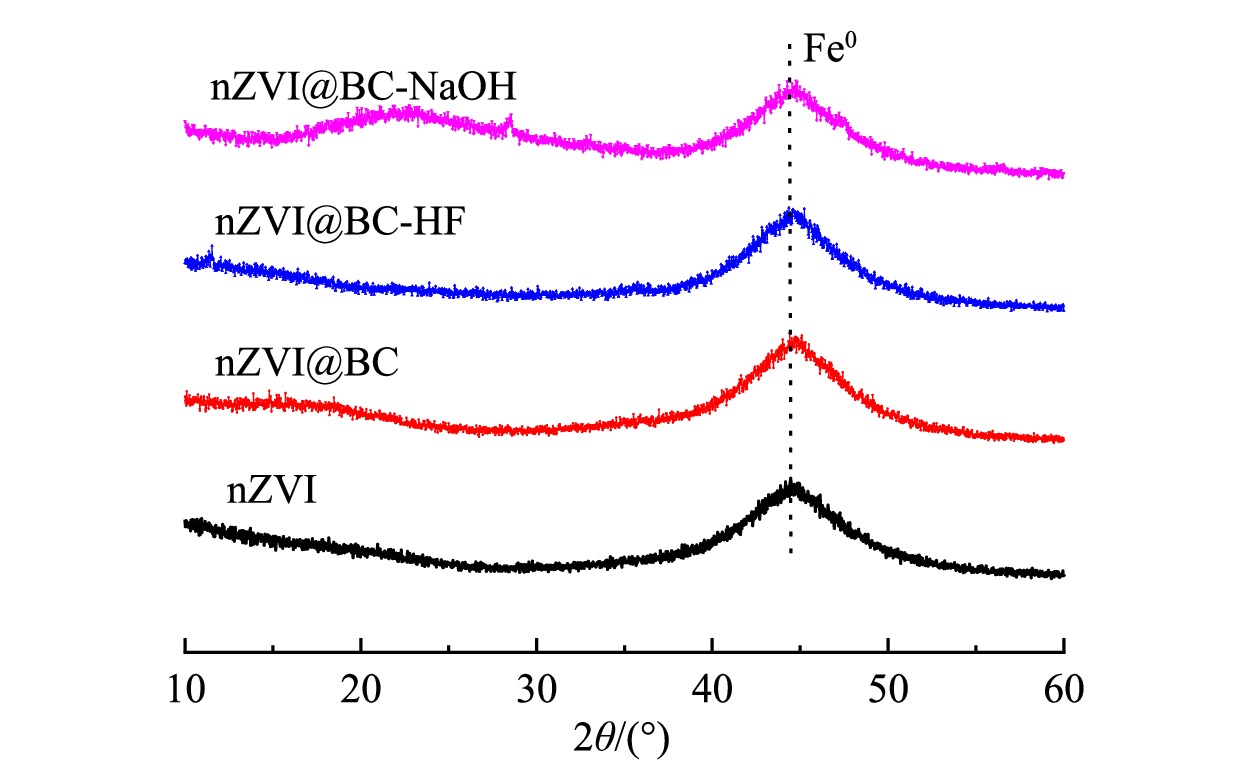
4) X射线衍射图分析。对于nZVI和改性前后的生物质炭负载nZVI复合材料,在43°~45°处都出现了1个较为明显的衍射峰(图2),该处的特征峰主要与零价铁(Fe0)相关 [16,19]。该结果表明,nZVI负载到生物质炭和酸碱改性生物质炭上不会改变nZVI颗粒的晶体类型,这与以往的研究结果一致[25,32]。
-
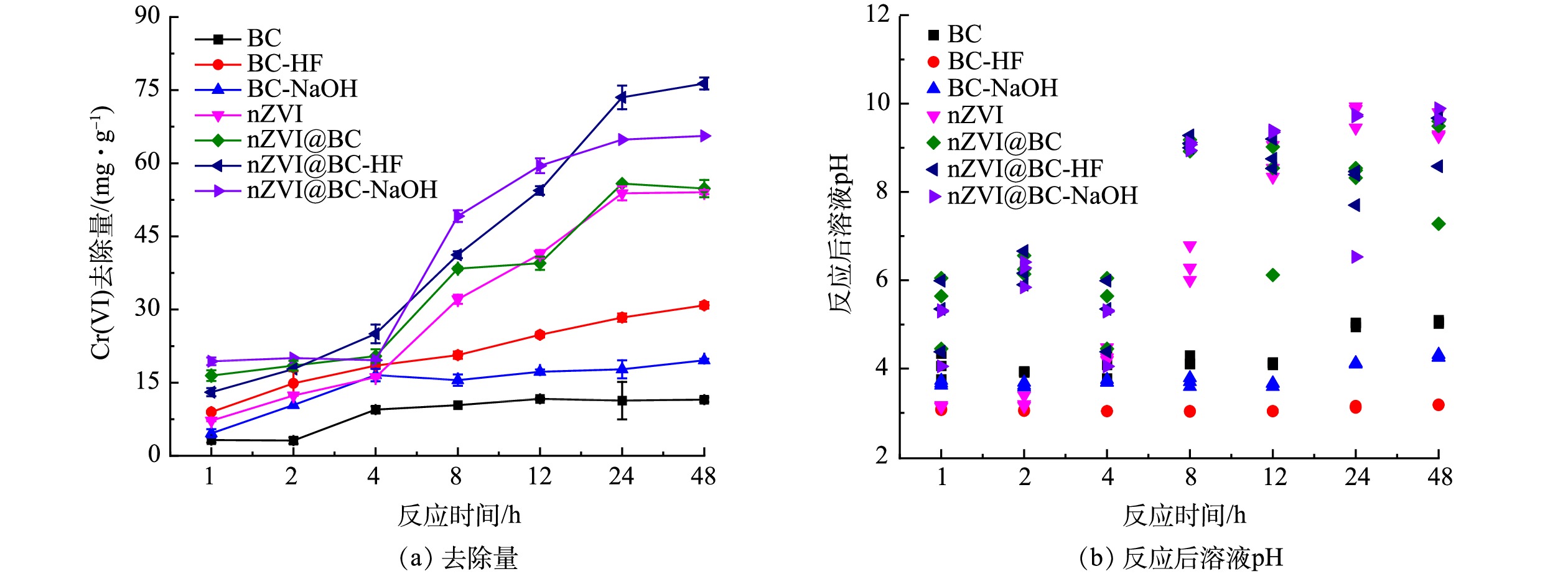
如图3所示,供试材料与Cr(Ⅵ)反应48 h后达到反应平衡。生物质炭对Cr(Ⅵ)的去除量仅为11.52 mg·g−1,经酸碱改性后的生物质炭增强了对Cr(Ⅵ)的去除效果,酸改性生物质炭的去除性能更优于碱改性生物质炭,去除量分别为30.87和19.59 mg·g−1。这是因为,经酸碱改性后的生物质炭比表面积、孔隙体积等表面性能得到了明显改善(见表1),为Cr(Ⅵ)提供了更多的吸附位点。将nZVI负载到3种生物质炭上后,对Cr(Ⅵ)表现出了更优异的去除性能。nZVI和生物质炭负载nZVI(炭铁质量比为1∶4)对Cr(Ⅵ)的去除效果无明显差异,这说明生物质炭负载nZVI中质量分数为20%的生物质炭与裸露nZVI中质量分数为20%的零价铁颗粒对Cr(Ⅵ)表现出了同等的去除效果。由于生物质炭具有较大的给电子能力,以及它的石墨碳结构有利于nZVI向Cr(Ⅵ)进行电子转移[36],故生物质炭负载nZVI后实际增强了nZVI对Cr(Ⅵ)的还原。此外,因酸碱改性后的生物质炭更大的比表面积和发达的孔隙结构,负载高反应活性的nZVI后,缓解了nZVI自身团聚和氧化导致反应活性降低的问题,因此进一步提高了Cr(Ⅵ)的去除效果,对Cr(Ⅵ)的去除量分别为76.36、65.62 mg·g−1。而相比于nZVI及其复合材料,改性前后的生物质炭对Cr(Ⅵ)的去除效果并不佳。推测其原因,一方面是由于生物质炭去除Cr(Ⅵ)主要通过吸附作用,另一方面是由于Cr(Ⅵ)在水溶液中主要以HCr2O7−,Cr2O72−及CrO42−等阴离子形式存在,生物质炭表面由于含有大量含氧官能团而带负电,因而与Cr(Ⅵ)阴离子化合物产生静电排斥作用导致吸附效果较差[19]。
改性前后的生物质炭与Cr(Ⅵ)的反应过程中溶液pH维持稳定(图3(b)),加入生物质炭后,pH略有增加,且生物质炭与Cr(Ⅵ)反应后的溶液pH略高于碱改性生物质炭与Cr(Ⅵ)反应后的溶液pH。这可能是由于BC在溶液中的缓冲pH能力弱于碱改性后的生物质炭。nZVI及其复合材料在去除Cr(Ⅵ)过程中,溶液pH由酸性最终变为强碱性,这是因为,反应过程中质子消耗或羟基离子的产生,导致溶液 pH增大;在4~8 h内溶液pH上升较为明显,这说明此过程中nZVI反应活性最高,主要发生氧化还原反应,Cr(Ⅵ)被还原成Cr(Ⅲ)。因此,nZVI对溶液中Cr(Ⅵ)的去除过程可大致分为两个阶段,在反应前期,溶液中的Cr(Ⅵ)被快速吸附到材料表面,发生吸附、还原和沉淀作用,Cr(Ⅵ)去除量快速上升,此阶段溶液pH明显增大;随着反应继续进行,材料表面的Cr(Ⅵ)结合位点逐渐减少,nZVI钝化产生的铁氧化物逐渐阻塞孔道,导致去除量逐渐趋于稳定,故该阶段溶液pH变化不明显[37]。
-
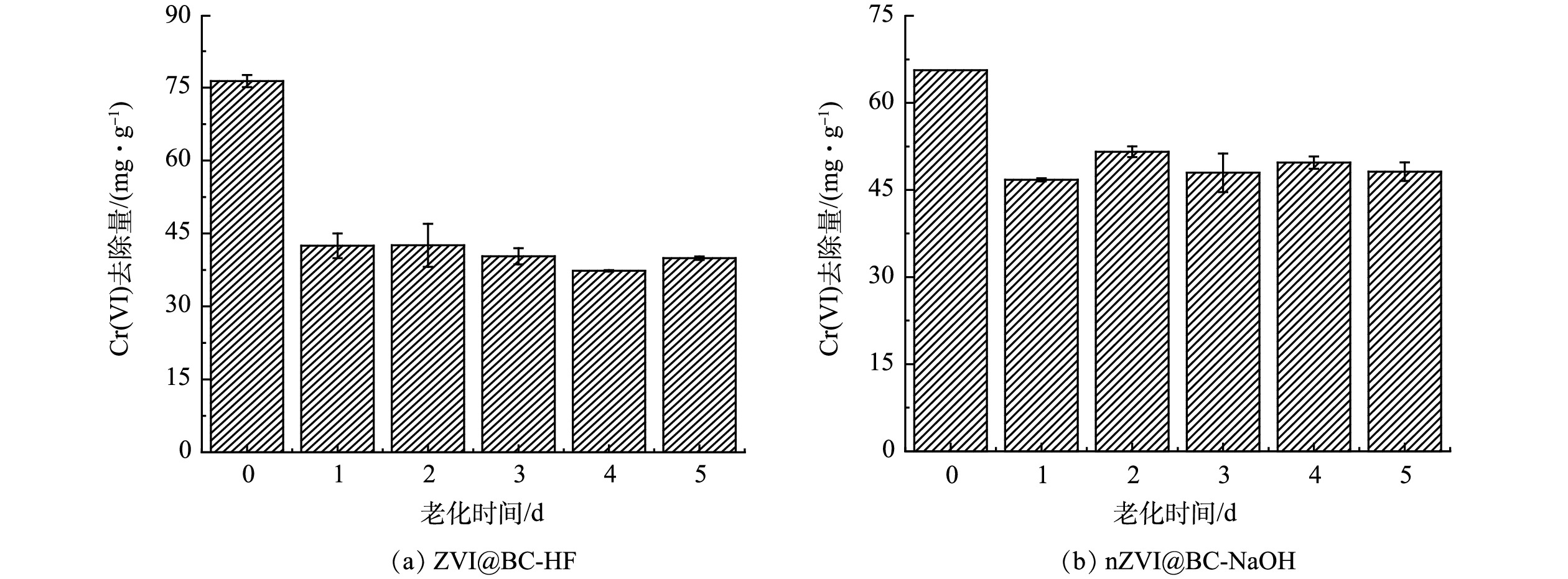
nZVI由于本身具有较高的反应活性,在空气中易被氧化而失去活性。为此,将酸碱改性生物质炭负载nZVI复合材料暴露在空气中后再进行Cr(Ⅵ)的去除实验,以探究该类复合材料的稳定性,结果如图4所示。酸碱改性生物质炭负载nZVI在空气中老化1~5 d后,对Cr(Ⅵ)的去除量分别降低44.24%~51.11%和21.41%~23.89%。这是因为,一方面nZVI活性较高,在制备过程中不可避免受到氧化;另一方面本研究中复合材料的炭铁质量比为1∶4,可能存在部分nZVI未成功负载在生物质炭上,因此,将材料置于空气中时,复合材料表面上的nZVI被快速氧化,导致对Cr(Ⅵ)的去除量降低。而在1~5 d内,随着老化时间进一步增加,材料对Cr(Ⅵ)的去除性能保持稳定。其主要原因是,成功负载在生物质炭上的nZVI颗粒处于生物质炭的孔隙中,与空气接触面积更小,抑制了氧气的侵蚀。
-
生物质炭在酸、碱改性过程中发生了比表面积、孔隙体积、平均孔径等物理性质的改变,这可能是改性生物质炭增强Cr(Ⅵ)吸附去除的主要机制之一[21,38]。通过改性前后生物质炭去除Cr(Ⅵ)的实验结果对比发现,随着生物质炭比表面积和孔体积增大,Cr(Ⅵ)的去除量也相应增加,如图5所示。因此,生物质炭经酸碱改性后,比表面积的增大以及孔隙结构的改善为Cr(Ⅵ)提供了更多的吸附位点,增强了Cr(Ⅵ)的去除。
分别用一阶、二阶、伪一阶和伪二阶动力学模型模拟了供试材料与Cr(Ⅵ)反应的过程,结果表明,伪二阶动力学模型更符合该反应过程(拟合参数见表3),其相关系数均大于0.90,这说明供试材料对Cr(Ⅵ)的吸附去除过程主要为化学吸附[17]。生物质炭、酸改性生物质炭和碱改性生物质炭与Cr(Ⅵ)的反应速率常数分别为0.04、0.03、0.03 g·(mg·h)−1。酸碱改性后的生物质炭与Cr(Ⅵ)的反应速率常数降低,这说明对生物质炭进行酸碱改性,改变了对Cr(Ⅵ)去除效率。Cr(Ⅵ)在与比表面积大的材料接触时,反应速率反而降低,这可能是由于材料比表面积越大,在同一反应条件下,污染物与材料充分接触发生反应需要更长时间,导致比表面积更大的材料与目标污染物的反应变慢,但不影响最终去除效果。
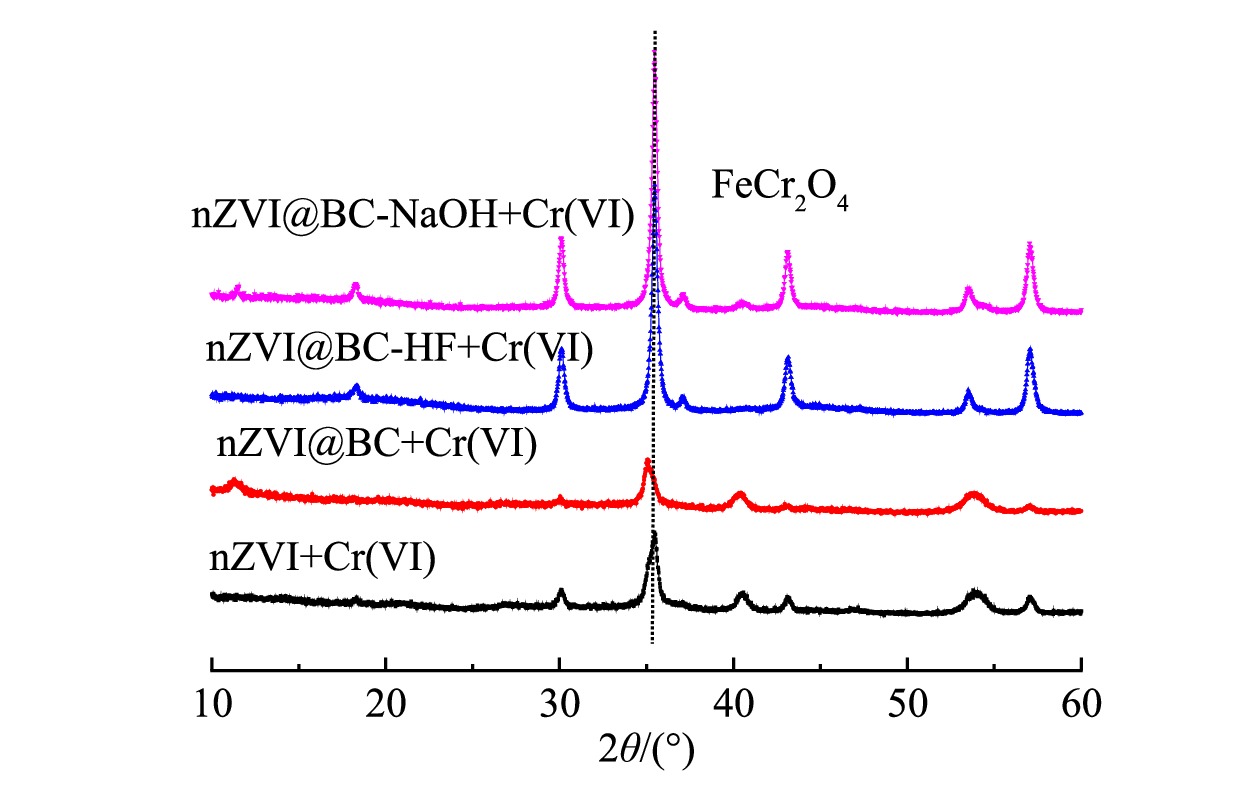
基于以上讨论,nZVI复合材料与Cr(Ⅵ)的反应速率常数明显高于裸露的nZVI。这是由于,酸碱改性生物质炭表面的Si-O-Si官能团与nZVI的成键作用使nZVI成功负载到生物质炭上,提高了nZVI的反应活性,最终提高了复合材料与Cr(Ⅵ)的反应速率。为了更好地了解酸碱改性生物质炭负载nZVI增强Cr(Ⅵ)去除的机理,对nZVI及其复合材料与Cr(Ⅵ)反应后的材料进行了X射线衍射图分析。图6表明,nZVI及其复合材料中Fe0的特征峰与Cr(Ⅵ)反应后消失,同时出现了铬铁化合物的特征峰,这说明Fe0作为主要的电子供体使得Cr(Ⅵ)被还原成Cr(Ⅲ);同时,还原产物Cr(Ⅲ)和铁离子发生了共沉淀,最终产物附着在材料表面,避免了二次污染[13,37,39]。从图6中可以看出,与nZVI和生物质炭负载nZVI相比,对生物质炭酸碱改性后再去负载nZVI增强了铬铁化合物的结晶,有利于反应的持续进行。
综上所述,酸碱改性生物质炭以及负载nZVI后增强Cr(Ⅵ)去除的途径主要有两条:一是生物质炭经过酸碱改性后更大的比表面积以及良好的孔隙结构促进了Cr(Ⅵ)的吸附;二是载体材料酸碱改性生物质炭表面的Si-O-Si官能团与nZVI耦合成Si-O-Fe键后提高了nZVI的反应活性,减少了nZVI的氧化,从而增强了对Cr(Ⅵ)的还原,同时为主要反应产物铬铁化合物提供了丰富的附着位点 (如图7所示)。
-
1)生物质炭经过酸碱改性后比表面积明显增加,孔隙结构得到改善,促进了Cr(Ⅵ)的去除,负载nZVI后进一步增强了Cr(Ⅵ)的去除。其中,氢氟酸改性生物质炭负载nZVI对Cr(Ⅵ)的去除效果最佳,去除量达到了76.36 mg·g−1。
2) 供试材料去除Cr(Ⅵ)的过程符合伪二阶动力学模型。生物质炭对Cr(Ⅵ)的主要去除机制为化学吸附;酸碱改性后的生物质炭使nZVI得到了有效分散,其表面的Si-O-Si官能团与nZVI耦合形成Si-O-Fe键,提高了nZVI的反应活性,增强了Cr(Ⅵ)的还原;同时,还原产物Cr(Ⅲ)与铁离子形成共沉淀FeCr2O4附着在材料表面,有利于反应的持续进行。
酸碱改性生物质炭-纳米零价铁增强六价铬去除的机理
Mechanism of enhanced removal of Cr(VI) by acid and alkali modified biochar-nanoscale zero-valent iron
-
摘要: 通过氢氟酸和氢氧化钠改性水稻秸秆生物质炭(BC),得到改性材料BC-HF和BC-NaOH,在此基础上负载纳米零价铁(Nanoscale zero-valent iron,nZVI)制得生物质炭负载纳米零价铁(nZVI@BC)、氢氟酸改性生物质炭负载纳米零价铁(nZVI@BC-HF)和氢氧化钠改性生物质炭负载纳米零价铁(nZVI@BC-NaOH)。通过比表面积分析、元素分析、红外光谱分析、X射线衍射分析以及动力学等方法,研究了不同材料对Cr(Ⅵ)的去除性能与机理。结果表明:酸碱改性后的生物质炭比表面积、孔体积显著增加,促进了Cr(Ⅵ)的去除,BC-HF和BC-NaOH对Cr(Ⅵ)的去除量分别为30.87、19.59 mg·g−1,为BC的2.68、1.70倍;负载nZVI后,进一步增强了Cr(Ⅵ)的去除效果,其中,nZVI@BC-HF和nZVI@BC-NaOH对Cr(Ⅵ)的去除效果显著,去除量分别为76.36、65.62 mg·g−1。酸碱改性生物质炭使nZVI得到了有效分散,其表面的Si-O-Si官能团与nZVI耦合成Si-O-Fe键,增强了nZVI对Cr(Ⅵ)的还原;同时,酸碱改性生物质炭负载nZVI促进了铁铬化合物的结晶,有利于反应的持续进行。本研究表明,酸碱改性生物质炭-纳米零价铁复合材料对于地下水中Cr(Ⅵ)的去除具有较大的应用潜力。Abstract: Following rice straw biochar (BC) was modified by hydrofluoric acid and sodium hydroxide to obtain BC-HF and BC-NaOH, respectively. And biochar loaded with nZVI (nZVI@BC), hydrofluoric acid modified biochar loaded with nZVI (nZVI@BC-HF) and sodium hydroxide modified biochar loaded with nZVI (nZVI@BC-NaOH) were prepared. The removal performance and mechanism of Cr(Ⅵ) by different materials were evaluated by the analyses of specific surface areas, elements, infrared spectroscopy, X-ray diffraction and kinetic. The results showed that specific surface area and pore volume of the biochar modified by acid and alkali were significantly increased, which promoted the removal of Cr(Ⅵ). The removal capacities of Cr(Ⅵ) by BC-HF and BC-NaOH were 30.87 and 19.59 mg·g−1, respectively, which were 2.68 and 1.70 times higher than the BC. After nZVI was loaded, the Cr(Ⅵ) removal was further enhanced, in which nZVI@BC-HF and nZVI@BC-NaOH possess significant Cr(Ⅵ) removal capacities of 76.36 and 65.62 mg·g−1, respectively. The acid and alkali modified biochar enables nZVI to be effectively dispersed and the Si-O-Si functional group on its surface with nZVI to form a Si-O-Fe bond, which enhance the Cr(Ⅵ) reduction by nZVI. At the same time, the acid and alkali modified biochar loaded with nZVI promoted the crystallization of iron chromium compounds, sustaining the reaction. The research showed that the acid and alkali modified biochar-nanoscale zero-valent iron composites had a good application potential for the removal of Cr(Ⅵ) in groundwater.
-
重金属铬及其化合物作为化工原料被广泛应用于电镀、制革、印染、冶金工业等领域。铬渣的无序堆放和含铬废水的不当排放是造成地下水铬污染的主要原因,因而对生态环境和人群健康造成了严重的威胁[1-2]。截至2018年,我国历史遗留的铬渣堆场和已关停的铬盐的生产场地约有60个(其中20个已完成修复),场区内地下水受到严重的铬污染[3]。铬在地下水中主要以三价铬(Cr(Ⅲ))和六价铬(Cr(Ⅵ))的价态存在,其中,Cr(Ⅵ)在低浓度下也具有剧毒性,毒性为Cr(Ⅲ)的100~1 000倍,常以重铬酸根离子(Cr2O72−,酸性条件)和铬酸根离子(CrO42−,中性或碱性条件)的形式存在,具有强致癌致畸性、较高的溶解度、高迁移性和环境持久性[4-7]。我国《地下水质量标准》(GB/T 14848-2017)[8]将Cr(Ⅵ)作为毒理学指标,当其质量浓度>0.10 mg·L−1时,该类水质就判定为Ⅴ类水,不适宜作为生活饮用水水源。因此,亟需研发绿色、高效的新型修复材料去除地下水中的Cr(Ⅵ)。
近年来,纳米零价铁(nanoscale zero-valent iron,nZVI)由于具有较强的还原性和反应活性、材料易回收利用等优点,被广泛应用于水体中Cr(Ⅵ)及其他重金属的去除[9]。但在实际应用中,nZVI存在易团聚和易氧化等问题,导致其迁移能力变差,从而降低了反应活性[10]。因此,通过制备纳米零价铁复合材料来优化纳米零价铁的修复性能,以提高其反应活性,从而高效地处理重金属、有机物等多种污染物[11-12]。目前,许多研究主要集中在吸附材料上,如将nZVI负载在诸如生物质炭[13]、活性炭[14]、膨润土[15]、凹凸棒石[16]等比表面积大的多孔材料上以克服其团聚的缺陷,提高在环境中的迁移性。在这些材料中,生物质炭因廉价易得、多孔结构、比表面积大、绿色环保等优点被广泛用作各类污染物的吸附剂以及nZVI的载体材料[17-19]。有研究表明,原始生物质炭材料经酸改性后可以去除灰分和硅,增大比表面积,降低表面负电荷[20-21];经碱处理可以去除生物质炭表面灰分,清除残留在孔隙中的杂质且增加生物质炭的表面积、孔体积和含氧官能团的数量,从而增加其吸附性能[21-23]。DONG等[20]发现,盐酸改性的生物质炭负载nZVI对Cr(Ⅵ)的去除率可达到35.3%,而原始生物质炭负载nZVI对Cr(Ⅵ)的去除率约为22.0%。WU等[24]研究了改性生物质炭负载铁/银双金属颗粒对四氯化碳的去除效果,结果表明,氢氧化钠改性生物质炭负载铁/银双金属颗粒对四氯化碳的去除率高于未改性生物质炭负载铁/银材料。因此,可以看出,经过酸碱改性后的生物质炭表面性能得到进一步提高,可促进nZVI的有效分散,成为nZVI理想的负载材料。迄今为止,采用HF、NaOH改性生物质炭负载nZVI复合材料去除Cr(Ⅵ)的研究尚未见报道,其去除性能及机理亦尚不清晰。
本研究选择HF和NaOH作为酸碱改性剂,对水稻秸秆生物质炭进行改性,获得氢氟酸改性生物质炭(BC-HF)和氢氧化钠改性生物质炭(BC-NaOH),并进一步负载纳米零价铁制得生物质炭负载纳米零价铁(nZVI@BC)、氢氟酸改性生物质炭负载纳米零价铁(nZVI@BC-HF)和氢氧化钠改性生物质炭负载纳米零价铁(nZVI@BC-NaOH)。利用比表面积分析、元素分析、红外光谱分析、X射线衍射分析等手段表征反应前后材料的组成与结构;通过动力学,测试复合材料对Cr(Ⅵ)的去除性能,并通过动力学过程、反应前后溶液pH分析和材料晶体结构分析等探讨其去除机制,同时探讨在空气中老化对nZVI@BC-HF和nZVI@BC-NaOH活性的影响。
1. 材料与方法
1.1 实验试剂
实验所用试剂主要有硼氢化钾(KBH4)、七水合硫酸亚铁(FeSO4·7H2O)、氢氟酸(HF)、氢氧化钠(NaOH)、无水乙醇(C2H5OH)、硝酸钠(NaNO3)、重铬酸钾(K2Cr2O7)、二苯碳酰二肼(C13H14N4O)、丙酮(CH3COCH3)、硫酸(H2SO4)、磷酸(H3PO4)等。除K2Cr2O7为优级纯外,以上试剂均为分析纯,所有溶液均采用超纯水配制。
1.2 材料制备
本研究以中国科学院南京土壤研究所常熟农业生态实验站的水稻秸秆作为生物质炭前体。在使用前,用去离子水冲洗水稻秸秆3次,自然风干,并于在60 °C烘箱中干燥24 h,然后根据文献报道的方法,在限氧条件下以5 °C·min−1的升温速率达到700 ℃后热解6 h,冷却至室温,磨碎,过100目筛备用[25]。分别将10 g生物质炭置于200 mL、1 mol·L−1的HF、NaOH溶液中,在温度为25 °C、150 r·min−1的转速下震荡24 h,用蒸馏水反复冲洗抽滤至pH稳定,BC-HF、BC-NaOH最后稳定的pH分别为3.12±0.1、10.40±0.1;原始生物质炭在相同的条件下加入超纯水清洗即可,最后将制备的3种生物质炭在80 °C下烘干,研磨过100目筛,保存备用。
采用液相还原法制备nZVI和3种生物质炭负载nZVI复合材料。预先称1 g的FeSO4·7H2O溶于50 mL的去离子水中(用预配的1 mol·L−1稀盐酸和1 mol·L−1氢氧化钾调节pH至4.0),将溶液转移至三口烧瓶中,加入50 mL乙醇,将三口烧瓶中的溶液分别用氮气除氧和搅拌15 min,使溶液混合均匀。然后,在氮气保护和机械搅拌下(150 r·min−1),将等体积的0.5 mol·L−1的KBH4溶液倒入恒压漏斗中,以5 mL·min−1的速度匀速逐滴滴入三口烧瓶中,此后继续曝气反应60 min,采用磁选法将制备得到的材料固液分离,用脱氧超纯水和脱氧无水乙醇各洗3遍,置于真空干燥箱中60 ℃干燥过夜。干燥后的样品经研磨、过筛后,置于棕色样品瓶中,放入真空干燥皿中保存备用。该过程发生的化学反应如式(1)所示。
2Fe2++BH−4+3H2O→2Fe0↓+BO−3+3H2↑+4H+ 制备nZVI@BC时,先取50 mg生物质炭加入到50 mL、20 g·L−1的FeSO4·7H2O溶液中(炭铁质量比为1∶4),放入25 ℃、150 r·min−1的恒温振荡器中震荡24 h后置于三口烧瓶中,然后按照上述合成nZVI的方法制备nZVI@BC。按照同样的步骤制得nZVI@BC-HF和nZVI@BC-NaOH。
1.3 实验方法
配置Cr(Ⅵ)污染溶液时加入NaNO3作为背景电解质,用来平衡地下水溶液中的离子强度,其浓度为10 mmol·L−1,同时将Cr(Ⅵ)污染溶液初始pH调至3。在20 mL玻璃瓶中加入Cr(Ⅵ)污染溶液和供试材料,最终质量浓度分别为50 mg·L−1和0.3 g·L−1。将玻璃瓶置于150 r·min−1和25 ℃的恒温摇床上进行反应,然后在选定的时间间隔内(1、2、4、8、12、24、48 h)取样,样品通过0.45 μm滤膜过滤后进行分析测试,在反应过程中测量了溶液pH及总铁浓度。所有实验均重复进行3次。
1.4 分析方法
本实验通过比表面积分析仪(Micromeritics ASAP 2460,美国麦克仪器公司)使用N2吸附-解吸法测定供试材料的比表面积和孔体积;供试材料元素组成用元素分析仪(Elementar Vario EL III,德国Elementar公司)测定;供试材料表面官能团用傅立叶变换红外光谱仪(Nicolet iS10,美国 Thermo Fisher 仪器公司)测定;反应前后的晶体结构进行用X射线衍射(XRD,RIGAKUD/MAX2550/PC,日本)表征。采用二苯碳酰二肼分光光度法测定溶液中Cr(Ⅵ)浓度;采用原子吸收法(ICP-OES)测定溶液中的总铁浓度;采用pH计(pHS-3C,Thermo)测定反应前后的溶液pH。供试材料与Cr(Ⅵ)反应的过程使用伪二阶动力学模型描述,计算公式见式(2)[26]。
tqt=1k2q2e+tqe (2) 式中:qt和qe分别为时间t和反应达到平衡时Cr(Ⅵ)的去除量,mg·g−1;k2是伪二阶动力学模型的速率常数,g·(mg·h)−1。
2. 结果与讨论
2.1 酸碱改性生物质炭及负载纳米零价铁材料的结构特性
1)比表面积与孔隙结构。供试材料的比表面积与孔隙结构分析结果见表1,BC的比表面积和孔体积为173.5 m2·g−1和50.96 mm3·g−1,经酸、碱改性后的生物质炭比表面积和孔体积分别为BC的2.22、1.76倍和3.98、3.25倍,比表面积和孔体积明显增大。LIU等[27]的研究结果同样表明,生物质炭经过酸碱改性后比表面积和孔体积会明显增大。这是因为,生物质炭通过酸、碱改性去除了孔隙结构中的灰分和无机物,清除了部分可溶性杂质,使比表面积和孔隙体积增大,从而有效地改善了BC的物理性质。负载nZVI后,复合材料的比表面积和孔体积均低于酸碱改性生物质炭,这与ZHANG[4]等的研究结果一致,其主要原因是:具有较小比表面积的nZVI是复合材料中的主要组成部分(炭铁质量比为1∶4);同时,nZVI占据了生物质炭中大部分孔隙,因此,酸碱改性生物质炭负载nZVI后比表面积和孔体积明显减小。
表 1 供试材料的比表面积与孔隙结构分析Table 1. Analysis of specific surface area and pore structure of selected materials供试材料 比表面积/(m2·g−1) 孔体积/(mm3·g−1) 平均孔径/nm BC 173.55 50.96 2.43 BC-HF 386.71 202.72 3.08 BC-NaOH 306.00 165.93 2.98 nZVI 13.82 57.55 5.99 nZVI@BC 43.22 79.26 4.20 nZVI@BC-HF 42.57 64.57 3.75 nZVI@BC-NaOH 53.01 80.82 3.91 2)元素分析。生物质炭的主要组成元素是C、H、O和N。从表2可以看出,酸碱改性前后基本元素组成不变,主要元素组成均为C和O元素,但改性前后生物质炭主要元素的质量分数有所差异。经酸碱改性后的生物质炭,由于灰分和一些可溶性杂质被清除,C的质量分数明显增大,N、H、S、O的质量分数略有增加。H/C比值用来表示生物质炭的芳香性,(O+N)/C比值用来表示生物质炭的极性[28]。相比BC,酸改性生物质炭的H/C和(O+N)/C均降低,这表明芳香性增加,疏水性增强;碱改性生物质炭的H/C比值增加,(O+N)/C比值降低,这表明芳香性降低,疏水性增加。酸碱改性都能使生物质炭疏水性增强,其主要原因是改性后的炭材料中硅氧化物气化,改善了材料表面的亲水性[29]。
表 2 供试材料的元素分析及比例Table 2. Elemental analysis and proportion of selected materials供试材料 N/% C/% H/% S% O/% H/C (O+N)/C BC 0.70 45.16 1.49 0.13 13.58 0.40 0.24 BC-HF 1.12 69.05 2.11 0.15 15.24 0.37 0.18 BC-NaOH 0.92 58.73 2.11 0.15 15.92 0.43 0.22 3)红外谱图分析。图1为供试材料的傅里叶变换红外光谱图。图1(a)为BC改性前后材料上的官能团变化,3 430 cm−1处的吸收峰为-OH基团伸缩振动,这表明生物质炭表面均含有大量的羟基官能团[30];2 920~2 850 cm−1和1 390 cm−1处的吸收峰为-CH2[21];1 570 cm−1的吸收峰被认为是芳香族C=C键和C=O键[31];Si-O-Si的特征带主要出现在1 100~1 050、880~740和 470 cm−1处[25]。由图1(a)可以看出,酸碱改性前后的生物质炭官能团种类没有发生变化,但经过氢氟酸改性后的BC在1 100~1 050 cm−1处的Si-O-Si特征峰减弱,这说明酸改性可洗脱BC中的硅。图1(b)为nZVI及其复合材料的红外光谱图。3 430、2 920~2 850 cm−1处分别为-OH和-CH2的吸收峰[21];在1 350 cm−1的峰值与COO-有关[32]。在1 100~1 050、880-740和 470 cm−1处的Si-O-Si特征带在3种生物质炭与 nZVI负载后减弱或者消失,这表明Si-O-Si是负责支持nZVI的主要位点之一[25, 33-34]。因此,nZVI通过与生物质炭表面Si-O-Si官能团耦合形成Si-O-Fe键,铁离子趋于稳定,降低了nZVI的氧化倾向[35]。
4) X射线衍射图分析。对于nZVI和改性前后的生物质炭负载nZVI复合材料,在43°~45°处都出现了1个较为明显的衍射峰(图2),该处的特征峰主要与零价铁(Fe0)相关 [16,19]。该结果表明,nZVI负载到生物质炭和酸碱改性生物质炭上不会改变nZVI颗粒的晶体类型,这与以往的研究结果一致[25,32]。
2.2 Cr(Ⅵ)去除动力学
如图3所示,供试材料与Cr(Ⅵ)反应48 h后达到反应平衡。生物质炭对Cr(Ⅵ)的去除量仅为11.52 mg·g−1,经酸碱改性后的生物质炭增强了对Cr(Ⅵ)的去除效果,酸改性生物质炭的去除性能更优于碱改性生物质炭,去除量分别为30.87和19.59 mg·g−1。这是因为,经酸碱改性后的生物质炭比表面积、孔隙体积等表面性能得到了明显改善(见表1),为Cr(Ⅵ)提供了更多的吸附位点。将nZVI负载到3种生物质炭上后,对Cr(Ⅵ)表现出了更优异的去除性能。nZVI和生物质炭负载nZVI(炭铁质量比为1∶4)对Cr(Ⅵ)的去除效果无明显差异,这说明生物质炭负载nZVI中质量分数为20%的生物质炭与裸露nZVI中质量分数为20%的零价铁颗粒对Cr(Ⅵ)表现出了同等的去除效果。由于生物质炭具有较大的给电子能力,以及它的石墨碳结构有利于nZVI向Cr(Ⅵ)进行电子转移[36],故生物质炭负载nZVI后实际增强了nZVI对Cr(Ⅵ)的还原。此外,因酸碱改性后的生物质炭更大的比表面积和发达的孔隙结构,负载高反应活性的nZVI后,缓解了nZVI自身团聚和氧化导致反应活性降低的问题,因此进一步提高了Cr(Ⅵ)的去除效果,对Cr(Ⅵ)的去除量分别为76.36、65.62 mg·g−1。而相比于nZVI及其复合材料,改性前后的生物质炭对Cr(Ⅵ)的去除效果并不佳。推测其原因,一方面是由于生物质炭去除Cr(Ⅵ)主要通过吸附作用,另一方面是由于Cr(Ⅵ)在水溶液中主要以HCr2O7−,Cr2O72−及CrO42−等阴离子形式存在,生物质炭表面由于含有大量含氧官能团而带负电,因而与Cr(Ⅵ)阴离子化合物产生静电排斥作用导致吸附效果较差[19]。
改性前后的生物质炭与Cr(Ⅵ)的反应过程中溶液pH维持稳定(图3(b)),加入生物质炭后,pH略有增加,且生物质炭与Cr(Ⅵ)反应后的溶液pH略高于碱改性生物质炭与Cr(Ⅵ)反应后的溶液pH。这可能是由于BC在溶液中的缓冲pH能力弱于碱改性后的生物质炭。nZVI及其复合材料在去除Cr(Ⅵ)过程中,溶液pH由酸性最终变为强碱性,这是因为,反应过程中质子消耗或羟基离子的产生,导致溶液 pH增大;在4~8 h内溶液pH上升较为明显,这说明此过程中nZVI反应活性最高,主要发生氧化还原反应,Cr(Ⅵ)被还原成Cr(Ⅲ)。因此,nZVI对溶液中Cr(Ⅵ)的去除过程可大致分为两个阶段,在反应前期,溶液中的Cr(Ⅵ)被快速吸附到材料表面,发生吸附、还原和沉淀作用,Cr(Ⅵ)去除量快速上升,此阶段溶液pH明显增大;随着反应继续进行,材料表面的Cr(Ⅵ)结合位点逐渐减少,nZVI钝化产生的铁氧化物逐渐阻塞孔道,导致去除量逐渐趋于稳定,故该阶段溶液pH变化不明显[37]。
2.3 酸碱改性生物质炭负载nZVI在空气中的老化
nZVI由于本身具有较高的反应活性,在空气中易被氧化而失去活性。为此,将酸碱改性生物质炭负载nZVI复合材料暴露在空气中后再进行Cr(Ⅵ)的去除实验,以探究该类复合材料的稳定性,结果如图4所示。酸碱改性生物质炭负载nZVI在空气中老化1~5 d后,对Cr(Ⅵ)的去除量分别降低44.24%~51.11%和21.41%~23.89%。这是因为,一方面nZVI活性较高,在制备过程中不可避免受到氧化;另一方面本研究中复合材料的炭铁质量比为1∶4,可能存在部分nZVI未成功负载在生物质炭上,因此,将材料置于空气中时,复合材料表面上的nZVI被快速氧化,导致对Cr(Ⅵ)的去除量降低。而在1~5 d内,随着老化时间进一步增加,材料对Cr(Ⅵ)的去除性能保持稳定。其主要原因是,成功负载在生物质炭上的nZVI颗粒处于生物质炭的孔隙中,与空气接触面积更小,抑制了氧气的侵蚀。
2.4 酸碱改性生物质炭负载nZVI增强Cr(Ⅵ)去除的机理
生物质炭在酸、碱改性过程中发生了比表面积、孔隙体积、平均孔径等物理性质的改变,这可能是改性生物质炭增强Cr(Ⅵ)吸附去除的主要机制之一[21,38]。通过改性前后生物质炭去除Cr(Ⅵ)的实验结果对比发现,随着生物质炭比表面积和孔体积增大,Cr(Ⅵ)的去除量也相应增加,如图5所示。因此,生物质炭经酸碱改性后,比表面积的增大以及孔隙结构的改善为Cr(Ⅵ)提供了更多的吸附位点,增强了Cr(Ⅵ)的去除。
分别用一阶、二阶、伪一阶和伪二阶动力学模型模拟了供试材料与Cr(Ⅵ)反应的过程,结果表明,伪二阶动力学模型更符合该反应过程(拟合参数见表3),其相关系数均大于0.90,这说明供试材料对Cr(Ⅵ)的吸附去除过程主要为化学吸附[17]。生物质炭、酸改性生物质炭和碱改性生物质炭与Cr(Ⅵ)的反应速率常数分别为0.04、0.03、0.03 g·(mg·h)−1。酸碱改性后的生物质炭与Cr(Ⅵ)的反应速率常数降低,这说明对生物质炭进行酸碱改性,改变了对Cr(Ⅵ)去除效率。Cr(Ⅵ)在与比表面积大的材料接触时,反应速率反而降低,这可能是由于材料比表面积越大,在同一反应条件下,污染物与材料充分接触发生反应需要更长时间,导致比表面积更大的材料与目标污染物的反应变慢,但不影响最终去除效果。
表 3 供试材料去除Cr(VI)的伪二阶动力学模型拟合参数Table 3. Pseudo second-order kinetic model fitting parameters of selected materials for removing Cr(VI)拟合参数 BC BC-HF BC-NaOH nZVI nZVI@BC nZVI@BC-HF nZVI@BC-NaOH k2/ (g·mg−1·h−1) 0.04 0.03 0.03 0.02 0.09 0.10 0.11 qe/(mg·g−1) 11.52 30.87 19.59 54.06 54.83 76.36 65.62 R2 0.97 0.99 0.99 0.96 0.99 0.99 0.99 基于以上讨论,nZVI复合材料与Cr(Ⅵ)的反应速率常数明显高于裸露的nZVI。这是由于,酸碱改性生物质炭表面的Si-O-Si官能团与nZVI的成键作用使nZVI成功负载到生物质炭上,提高了nZVI的反应活性,最终提高了复合材料与Cr(Ⅵ)的反应速率。为了更好地了解酸碱改性生物质炭负载nZVI增强Cr(Ⅵ)去除的机理,对nZVI及其复合材料与Cr(Ⅵ)反应后的材料进行了X射线衍射图分析。图6表明,nZVI及其复合材料中Fe0的特征峰与Cr(Ⅵ)反应后消失,同时出现了铬铁化合物的特征峰,这说明Fe0作为主要的电子供体使得Cr(Ⅵ)被还原成Cr(Ⅲ);同时,还原产物Cr(Ⅲ)和铁离子发生了共沉淀,最终产物附着在材料表面,避免了二次污染[13,37,39]。从图6中可以看出,与nZVI和生物质炭负载nZVI相比,对生物质炭酸碱改性后再去负载nZVI增强了铬铁化合物的结晶,有利于反应的持续进行。
综上所述,酸碱改性生物质炭以及负载nZVI后增强Cr(Ⅵ)去除的途径主要有两条:一是生物质炭经过酸碱改性后更大的比表面积以及良好的孔隙结构促进了Cr(Ⅵ)的吸附;二是载体材料酸碱改性生物质炭表面的Si-O-Si官能团与nZVI耦合成Si-O-Fe键后提高了nZVI的反应活性,减少了nZVI的氧化,从而增强了对Cr(Ⅵ)的还原,同时为主要反应产物铬铁化合物提供了丰富的附着位点 (如图7所示)。
3. 结论
1)生物质炭经过酸碱改性后比表面积明显增加,孔隙结构得到改善,促进了Cr(Ⅵ)的去除,负载nZVI后进一步增强了Cr(Ⅵ)的去除。其中,氢氟酸改性生物质炭负载nZVI对Cr(Ⅵ)的去除效果最佳,去除量达到了76.36 mg·g−1。
2) 供试材料去除Cr(Ⅵ)的过程符合伪二阶动力学模型。生物质炭对Cr(Ⅵ)的主要去除机制为化学吸附;酸碱改性后的生物质炭使nZVI得到了有效分散,其表面的Si-O-Si官能团与nZVI耦合形成Si-O-Fe键,提高了nZVI的反应活性,增强了Cr(Ⅵ)的还原;同时,还原产物Cr(Ⅲ)与铁离子形成共沉淀FeCr2O4附着在材料表面,有利于反应的持续进行。
-
表 1 供试材料的比表面积与孔隙结构分析
Table 1. Analysis of specific surface area and pore structure of selected materials
供试材料 比表面积/(m2·g−1) 孔体积/(mm3·g−1) 平均孔径/nm BC 173.55 50.96 2.43 BC-HF 386.71 202.72 3.08 BC-NaOH 306.00 165.93 2.98 nZVI 13.82 57.55 5.99 nZVI@BC 43.22 79.26 4.20 nZVI@BC-HF 42.57 64.57 3.75 nZVI@BC-NaOH 53.01 80.82 3.91 表 2 供试材料的元素分析及比例
Table 2. Elemental analysis and proportion of selected materials
供试材料 N/% C/% H/% S% O/% H/C (O+N)/C BC 0.70 45.16 1.49 0.13 13.58 0.40 0.24 BC-HF 1.12 69.05 2.11 0.15 15.24 0.37 0.18 BC-NaOH 0.92 58.73 2.11 0.15 15.92 0.43 0.22 表 3 供试材料去除Cr(VI)的伪二阶动力学模型拟合参数
Table 3. Pseudo second-order kinetic model fitting parameters of selected materials for removing Cr(VI)
拟合参数 BC BC-HF BC-NaOH nZVI nZVI@BC nZVI@BC-HF nZVI@BC-NaOH k2/ (g·mg−1·h−1) 0.04 0.03 0.03 0.02 0.09 0.10 0.11 qe/(mg·g−1) 11.52 30.87 19.59 54.06 54.83 76.36 65.62 R2 0.97 0.99 0.99 0.96 0.99 0.99 0.99 -
[1] 周艺艺, 刘存, 王玉军. 不同主导晶面赤铁矿对Cr(Ⅵ)吸附与迁移行为的影响[J]. 农业环境科学学报, 2021, 40(8): 1667-74. doi: 10.11654/jaes.2021-0386 [2] SHANG J G, ZONG M Z, YU Y, et al. Removal of chromium (VI) from water using nanoscale zerovalent iron particles supported on herb-residue biochar[J]. Journal of Environmental Management, 2017, 197: 331-337. doi: 10.1016/j.jenvman.2017.03.085 [3] 李克, 王芳, 陈瑛. 中国铬渣污染地块现状与政策建议[J]. 中国环境科学学会科学技术年会论文集(第一卷), 2018: 合肥. [4] ZHANG Y T, JIAO X Q, LIU N, et al. Enhanced removal of aqueous Cr(VI) by a green synthesized nanoscale zero-valent iron supported on oak wood biochar[J]. Chemosphere, 2020, 245: 125542. doi: 10.1016/j.chemosphere.2019.125542 [5] 李东, 贺丽洁, 盛培培. Tessier连续提取法用于土壤铬分析的Cr(Ⅵ)-Cr(Ⅲ)转化及适用性[J]. 环境工程学报, 2021, 15(7): 2368-2378. doi: 10.12030/j.cjee.202012141 [6] LV X S, XU J, JIANG G M, et al. Removal of chromium(VI) from wastewater by nanoscale zero-valent iron particles supported on multiwalled carbon nanotubes[J]. Chemosphere, 2011, 85(7): 1204-1209. doi: 10.1016/j.chemosphere.2011.09.005 [7] COSTA M. Potential hazards of hexavalent chromate in our drinking water[J]. Toxicology Applied Pharmacology, 2003, 188: 1-5. doi: 10.1016/S0041-008X(03)00011-5 [8] 中华人民共和国国家质量监督检验检疫总局, 中国国家标准化管理委员会. 地下水质量标准: GB/T 14848-2017[S]. 北京: 中国环境科学出版社, 2018. [9] ZOU Y T, WANG X X, KHAN A, et al. Environmental remediation and application of nanoscale zero-valent iron and its composites for the removal of heavy metal ions: a review[J]. Environmental Science & Technology, 2016, 50(14): 7290-7304. [10] 宋珍霞, 殷齐贺, 穆晓斐. 膨润土负载纳米零价铁去除废水中Cr(Ⅵ)的动力学特性研究[J]. 化工新型材料, 2018, 46(5): 199-203. [11] HAN Y L, YAN W L. Reductive Dechlorination of trichloroethene by zero-valent iron nanoparticles: reactivity enhancement through sulfidation treatment[J]. Environmental Science & Technology, 2016, 50(23): 12992-13001. [12] PONDER S M, DARAB J G, MALLOUK T E. Remediation of Cr(VI) and Pb(II) aqueous solutions using supported, nanoscale zero-valent iron[J]. Environmental Science & Technology, 2000, 34(12): 2564-2569. [13] QIAN L B, LIU S N, ZHANG W Y, et al. Enhanced reduction and adsorption of hexavalent chromium by palladium and silicon rich biochar supported nanoscale zero-valent iron[J]. Journal of Colloid and Interface Science, 2019, 533: 428-436. doi: 10.1016/j.jcis.2018.08.075 [14] 吴丽梅, 吕国诚, 廖立兵. 活性炭负载纳米零价铁去除污水中六价铬的研究[J]. 矿物学报, 2012, 32(S1): 181-182. [15] 黄超, 余兵, 李任超, 等. 有机膨润土负载纳米零价铁还原-类芬顿氧化降解2, 4-二氯苯酚[J]. 环境工程学报, 2015, 9(8): 3643-3649. doi: 10.12030/j.cjee.20150810 [16] ZHANG W Y, QIAN L B, OUYANG D, et al. Effective removal of Cr(VI) by attapulgite-supported nanoscale zero-valent iron from aqueous solution: Enhanced adsorption and crystallization[J]. Chemosphere, 2019, 221: 683-692. doi: 10.1016/j.chemosphere.2019.01.070 [17] QIAN L B and Chen B L. Dual role of biochars as adsorbents for aluminum: the effects of oxygen-containing organic components and the scattering of silicate particles[J]. Environmental Science & Technology, 2013, 47(15): 8759-8768. [18] 李云桂, 杨慧敏, 武彩霞, 等. 粒径和温度对玉米秸秆生物碳吸附锶的耦合影响[J]. 安全与环境学报. 2017, 17(4): 1459-1464. [19] QIAN L B, ZHANG W Y, YAN J C, et al. Nanoscale zero-valent iron supported by biochars produced at different temperatures: Synthesis mechanism and effect on Cr(VI) removal[J]. Environmental Pollution, 2017, 223: 153-160. doi: 10.1016/j.envpol.2016.12.077 [20] DONG H R, DENG J M, XIE Y K, et al. Stabilization of nanoscale zero-valent iron (nZVI) with modified biochar for Cr(VI) removal from aqueous solution[J]. Journal of Hazardous Materials, 2017, 332: 79-86. doi: 10.1016/j.jhazmat.2017.03.002 [21] SHANG X, YANG L, OUYANG D, et al. Enhanced removal of 1, 2, 4-trichlorobenzene by modified biochar supported nanoscale zero-valent iron and palladium[J]. Chemosphere, 2020, 249: 126518. doi: 10.1016/j.chemosphere.2020.126518 [22] ZHANG X J, ZHANG L, LI A M. Eucalyptus sawdust derived biochar generated by combining the hydrothermal carbonization and low concentration KOH modification for hexavalent chromium removal[J]. Journal of Environmental Management, 2018, 206: 989-998. doi: 10.1016/j.jenvman.2017.11.079 [23] SIZMUR T, FRESNO T, AKGUL G, et al. Biochar modification to enhance sorption of inorganics from water[J]. Bioresource Technology, 2017, 246: 34-47. doi: 10.1016/j.biortech.2017.07.082 [24] WU H W, FENG Q Y. Fabrication of bimetallic Ag/Fe immobilized on modified biochar for removal of carbon tetrachloride[J]. Journal of Environmental Sciences, 2017, 54: 346-357. doi: 10.1016/j.jes.2016.11.017 [25] QIAN L B, SHANG X, ZHANG B, et al. Enhanced removal of Cr(VI) by silicon rich biochar-supported nanoscale zero-valent iron[J]. Chemosphere, 2019, 215: 739-745. doi: 10.1016/j.chemosphere.2018.10.030 [26] YIN H B, KONG M, GU X H, et al. Removal of arsenic from water by porous charred granulated attapulgite-supported hydrated iron oxide in bath and column modes[J]. Journal of Cleaner Production, 2017, 166: 88-97. doi: 10.1016/j.jclepro.2017.08.026 [27] LIU P, LIU W J, JIANG H, et al. Modification of bio-char derived from fast pyrolysis of biomass and its application in removal of tetracycline from aqueous solution[J]. Bioresource Technology, 2012, 121: 235-240. doi: 10.1016/j.biortech.2012.06.085 [28] AHMAD M, LEE S S, RAJAPAKSHA A U, et al. Trichloroethylene adsorption by pine needle biochars produced at various pyrolysis temperature[J]. Bioresource Technology, 2013, 143: 615-622. doi: 10.1016/j.biortech.2013.06.033 [29] 肖正辉, 李学良, 邢高瞻. 酸处理对秸秆基活性炭电化学性能的影响[J]. 硅酸盐学报, 2011, 39(4): 596-600. [30] KHATAEE A, KAYAN B, KALDERIS D, et al. Ultrasound-assisted removal of acid red 17 using nanosized Fe3O4-loaded coffee waste hydrochar[J]. Ultrasonics Sonochemistry, 2017, 5: 72-80. [31] ZHANG K K, SUN P, FAYE M, et al. Characterization of biochar derived from rice husks and its potential in chlorobenzene degradation[J]. Carbon, 2018, 130: 730-740. doi: 10.1016/j.carbon.2018.01.036 [32] SU H J, FANG Z Q, TSANG P E, et al. Remediation of hexavalent chromium contaminated soil by biochar-supported zero-valent iron nanoparticles[J]. Journal of Hazardous Materials, 2016, 318: 533-540. doi: 10.1016/j.jhazmat.2016.07.039 [33] ZHANG W Y, QIAN L B, CHEN Y, et al. Nanoscale zero-valent iron supported by attapulgite produced at different acid modification: Synthesis mechanism and the role of silicon on Cr(VI) removal[J]. Chemosphere, 2020, 267: 129183. [34] WU H W, FENG Q Y, YANG H, et al. Modified biochar supported Ag/Fe nanoparticles used for removal of cephalexin in solution: characterization, kinetics and mechanisms[J]. Colloids and Surfaces a-Physicochemical and Engineering Aspects, 2017, 517: 63-71. [35] YUAN Y, BOLAN N, PREVOTEAU A, et al. Applications of biochar in redox-mediated reactions[J]. Bioresource Technology, 2017, 246: 271-281. doi: 10.1016/j.biortech.2017.06.154 [36] YUAN Y F, ZHOU M, SHI J, et al. The significant role of electron donating capacity and carbon structure of biochar to electron transfer of zerovalent iron[J]. Chemosphere, 2021, 287: 132381. [37] SHI L N, LIN Y M, ZHANG X, et al. Synthesis, characterization and kinetics of bentonite supported nZVI for the removal of Cr(VI) from aqueous solution[J]. Chemical Engineering Journal, 2011, 171(2): 612-617. doi: 10.1016/j.cej.2011.04.038 [38] LI R N, WANG Z W, GUO J L, et al. Enhanced adsorption of ciprofloxacin by KOH modified biochar derived from potato stems and leaves[J]. Water Science and Technology, 2018, 77(4): 1127-1136. doi: 10.2166/wst.2017.636 [39] WANG S S, ZHAO M Y, ZHOU M, et al. Biochar-supported nZVI (nZVI/BC) for contaminant removal from soil and water: a critical review[J]. Journal of Hazardous Materials, 2019, 373: 820-834. doi: 10.1016/j.jhazmat.2019.03.080 -




 DownLoad:
DownLoad: