-
砷(As)是一种广泛存在于自然环境中的有毒类金属,砷污染问题已成为全球关注的问题。世界卫生组织(WHO)和美国环境保护局(USEPA)已将饮用水中砷的最大污染物水平(MCL)从50 μg·L−1降低到10 μg·L−1[1-3]。水中的砷一般以三价砷As(Ⅲ)和五价砷As(Ⅴ)两种价态存在。As(Ⅲ)的毒性是As(Ⅴ)的60倍左右,移动性强且具有亲水性[4]。而常规给水处理工艺对砷的去除能力有限,故采取有效的方法强化去除饮用水中的砷非常必要[5]。
相较于膜法、生物法、离子交换法和混凝法等,吸附法因其操作简单、经济高效且再生能力强等优点,因而被小型社区和农村分散式砷去除工艺普遍采用[3-5]。近年来的研究表明,单质铁及其氧化物与水体中的砷存在很强的亲和力,且来源丰富、绿色高效,更适合大规模应用于水体除砷工艺[6-7]。但一方面铁氧化物与中性分子形式存在的As(Ⅲ)结合力相对较弱,对As(Ⅲ)去除效果并不理想[8];另一方面铁氧化物尤其是吸附性能良好的水合铁氧化物粒径极细甚至为纳米级粒径,致使其在实际应用时水头损失过大,固液分离困难[9-10],易造成构筑物管道或设备堵塞,因而限制了其工业化应用。
活性炭具有较大的比表面积,对污染物吸附能力强,是常用的吸附剂。然而,单纯使用活性炭无法有效去除As(Ⅲ),为了获得理想的除砷效果,有必要对活性炭加以改性处理。因此,绿色安全地应用活性炭和水合氧化铁的吸附性能,同时又解决水合氧化铁固液分离的难题,成为强化吸附除砷的研究热点。有研究表明,采用FeSO4浸渍和H2O2氧化的组合[8]、FeSO4浸渍和NaClO氧化的组合[11]以及FeCl3浸渍和NaClO氧化的组合[12]所制备出的载铁活性炭,对水中的As均能达到良好的去除效果。这些研究为铁盐浸渍和氧化组合方式进行活性炭改性负载铁氧化物研究提供了理论基础和技术借鉴,但利用氧化性和吸附性良好的高铁酸钾(K2FeO4),经过适当的改性处理,将原位生成的多种吸附性能优良的铁氧化物负载在多孔材料活性炭上,制备出吸附和固液分离性能良好的载铁活性炭复合材料还鲜有研究。基于上述背景,本研究利用FeSO4和少量K2FeO4通过恒温浸润的方式,制备获得原位铁氧化物载铁活性炭(AFPAC)。研究AFPAC对水中As(Ⅲ)的吸附特性,并结合系列表征分析以阐明其除As(Ⅲ)机理,以期为地下水除砷治理技术的探索提供参考。
-
所用活性炭购自国药的煤质活性炭粉(粒径200目左右,其碘值为1000 mg·g−1,亚甲基蓝吸附量120 mg·L−1,水分为10%,灰分为9%);亚砷酸钠标准溶液(上海安谱实验科技有限公司,浓度为1000 µg·mL−1);七水合硫酸亚铁(FeSO4·7H2O)、硝酸(HNO3)、氯化铁(FeCl3)等化学试剂均购自国药集团化学试剂有限公司,纯度均为分析纯;高铁酸钾通过湿式氧化法制得,其纯度大于96%。
-
电子天平(AUW120D,上海力辰西仪器有限公司)、真空干燥箱(DZF-6020A,上海力辰西仪器有限公司)、电热恒温鼓风干燥箱(DHG-9023A,上海一恒科学仪器有限公司)、PH计(PHS-3E,上海力辰西仪器有限公司)、数显恒温磁力搅拌器(85-2,常州市鑫鑫仪器有限公司)、超声波清洗机(DS-031S,深圳市品凰仪器有限公司)、数显恒温水浴锅(HH-1,上海力辰西仪器有限公司)、水浴恒温振荡器(SHA-C,上海力辰西仪器有限公司)、悬臂式电动搅拌器(LC-OES-60SH,上海力辰西仪器有限公司)、循环水式多用真空泵(SHZ-D(Ⅲ),上海力辰西仪器有限公司)、电感耦合等离子体发射光谱仪(ICP-OES,Optima 8300,美国铂金埃尔默公司)、傅立叶红外光谱仪(FTIR,Nicolet 6700,美国尼力高仪器公司)、场发射电子扫描显微镜(SEM,Sigma500,德国卡尔蔡司公司)、X射线衍射仪(XRD,XRD-6100,日本岛津公司)、X射线光电子能谱仪(XPS,PHI 5700 ESCA,美国物理电子公司)。
-
1) K2FeO4的制备。采用湿式法制备K2FeO4,具体方法见文献[13]。制备原理如式(1)和式(2)所示。采用ABTS法检测K2FeO4的纯度,经过检测,其纯度大于96%。
2)载铁活性炭的制备。在均匀的磁力搅拌条件下,将清洗后的活性炭粉PAC在0.01 mol·L−1的稀硝酸中进行浸渍24 h预处理,随后用超纯水清洗活性炭粉至上清液pH不变,在60 ℃条件下真空干燥后研磨制得活性炭粉末APAC。取适量的APAC和一定量的FeSO4·7H2O置于盛有100 mL超纯水锥形瓶中,调节pH为4,在25 ℃下以150 r·min−1转速振荡12 h,随后向锥形瓶内缓慢滴加少量浓度为0.1 mol·L−1的K2FeO4溶液,继续在上述条件下振荡12 h,反复清洗后经一定温度熟化即制得原位铁氧化物载铁活性炭粉AFPAC。
3)静态吸附除As(Ⅲ)实验及机理研究。静态吸附实验:准确称取适量的AFPAC,投加到一定浓度的As(Ⅲ)溶液锥形瓶中,置于一定温度下以150 r·min−1的恒温气浴振荡器中振荡24 h,取适量上清液经0.45 μm滤膜过滤后测滤液中砷浓度,其中As(Ⅲ)的去除率以及单位质量活性炭的As(Ⅲ)吸附容量由式(3)和式(4)计算所得。
式中:R为去除率,%;qc为吸附容量,μg·g−1;C0和Ct分别为溶液中As(Ⅲ)的初始质量浓度和t时刻的质量浓度,μg·L−1;V是溶液体积,L;m是AFPAC的投加质量,g。
AFPAC除As(Ⅲ)吸附性能研究:探究AFPAC投加量、溶液初始pH、反应温度以及As(Ⅲ)初始质量浓度等操作条件下AFPAC吸附除As(Ⅲ)效能,同时对其吸附动力学和等温吸附过程进行研究。采用拟一级和拟二级动力学模型对水中As(Ⅲ)的吸附去除过程进行动力学拟合,其线性模拟公式如式(5)和式(6)所示。采用弗雷德利希(Freundlich)吸附等温式和朗格缪尔(Langmuir)吸附等温式来描述吸附平衡时吸附质分子液相和固相中的分配。Freundlich和Langmuir的线性模拟公式如式(7)和式(8)所示。
式中:q、qt分别表示平衡和t时刻吸附量,μg·g−1,K1和K2分别为拟一级和拟二级的反应速率常数。
式中:KF与1/n为Freundlich常数;b为Langmuir常数;qe为吸附平衡时吸附质吸附在AFPAC上的质量浓度,mg·L−1;Ce为吸附平衡时吸附质残留于液相的质量浓度,mg·L−1;qm为单分子层饱和吸附量,mg·L−1。
AFPAC除As(Ⅲ)机理研究:对吸附除As(Ⅲ)前后的AFPAC进行相关系列表征,分析AFPAC除As(Ⅲ)前后表面形貌、官能团、元素组成、物质形态及结合状态等变化,阐明AFPAC吸附除As(Ⅲ)的作用机制。
4)分析方法。采用电感耦合等离子体(ICP-OES)测定溶液中砷和铁的浓度;采用扫描电镜(SEM)观察样品的表面形貌和界面结构;利用傅里叶变换红外光谱(FTIR)分析样品表面官能团的变化;采用X射线衍射(XRD)和Rietveld全谱拟合的方法测定物质元素组成、相丰度和原子占位等参数;利用X射线光电子能谱(XPS)分析物质结合状态。
-
对载铁活性炭的改性方法进行优选,确定合理的组合改性方式。不同改性方法制备的载铁活性炭除As(Ⅲ)效果如表1所示。结果表明,将活性炭采用酸预处理后进行FeSO4恒温浸渍结合K2FeO4氧化组合改性,制备获得的载铁活性炭(AFPAC)对水中As(Ⅲ)具有显著的去除效果,As(Ⅲ)去除率达到99.5%。而原始新鲜活性炭在相同条件下对As(Ⅲ)的去除率只有33.0%,FeCl3/K2FeO4组合改性的载铁活性炭对As(Ⅲ)的去除率为85.5%。因此,推测在FeSO4/K2FeO4组合改性过程中,FeSO4不仅浸渍在活性炭上,还可能与K2FeO4发生了复杂的氧化还原反应,影响了原位载铁物种的生成,同时K2FeO4也起到优化活性炭内部结构的作用,协同强化了载铁活性炭对As(Ⅲ)的去除效果。
另外,对比相关研究载铁活性炭除砷改性方法(表2),本研究中的FeSO4/K2FeO4组合方式显著提高了载铁活性炭吸附除砷效果,对As(Ⅲ)的最大吸附容量达到6.17 mg·g−1。因此,确定FeSO4/K2FeO4组合改性方式,并在此基础上优化载铁活性炭的制备条件。
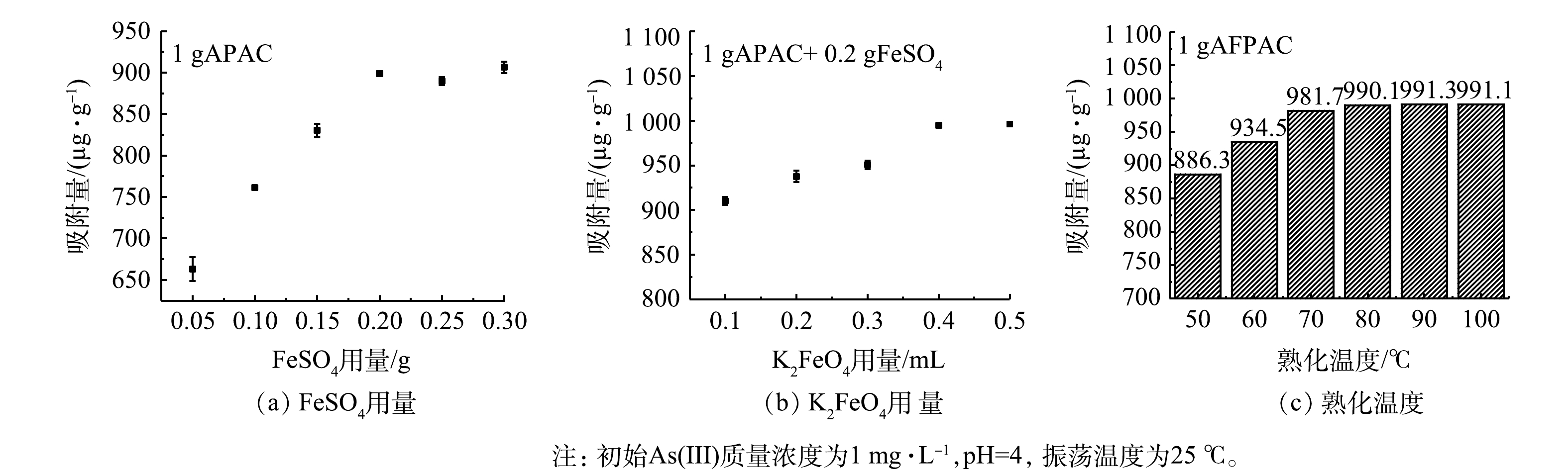
考察FeSO4和K2FeO4的用量比及改性活性炭的熟化温度对改性活性炭吸附除As(Ⅲ)效果的影响,结果如图1所示。由图1(a)~(b)可见,在一定铁盐投量范围内,随着FeSO4投加量的增加,所制备的AFPAC对As(Ⅲ)的吸附量不断增加,K2FeO4用量的增加同样可以提高对As(Ⅲ)的吸附量。此结果与肖静和陈苹等的研究结果一致[8,12]。AFPAC上负载的铁含量越高,与砷结合位点碰撞概率越大,有利于对As(Ⅲ)的吸附去除。但当活性炭为1 g时,FeSO4的用量超过0.2 g,0.1 mol·L−1的 K2FeO4的用量超过0.4 mL后,所制备的AFPAC对As(Ⅲ)的去除率基本稳定甚至有所下降。这是由于过高的铁盐投加量会导致活性炭孔结构堵塞,原来已经负载的铁氧化物容易从活性炭表面脱落,无法充分发挥其吸附能力[14]。因此,制备1 g的AFPAC时FeSO4与K2FeO4的用量比为0.200∶0.008。由图1(c)可见,当熟化温度达到80 ℃以上,所制备的活性炭去除As(Ⅲ)效果基本稳定,继续提高熟化温度对活性炭的除As(Ⅲ)效果基本没有影响。从经济角度考虑,本研究中最佳熟化温度设定为80 ℃。
-
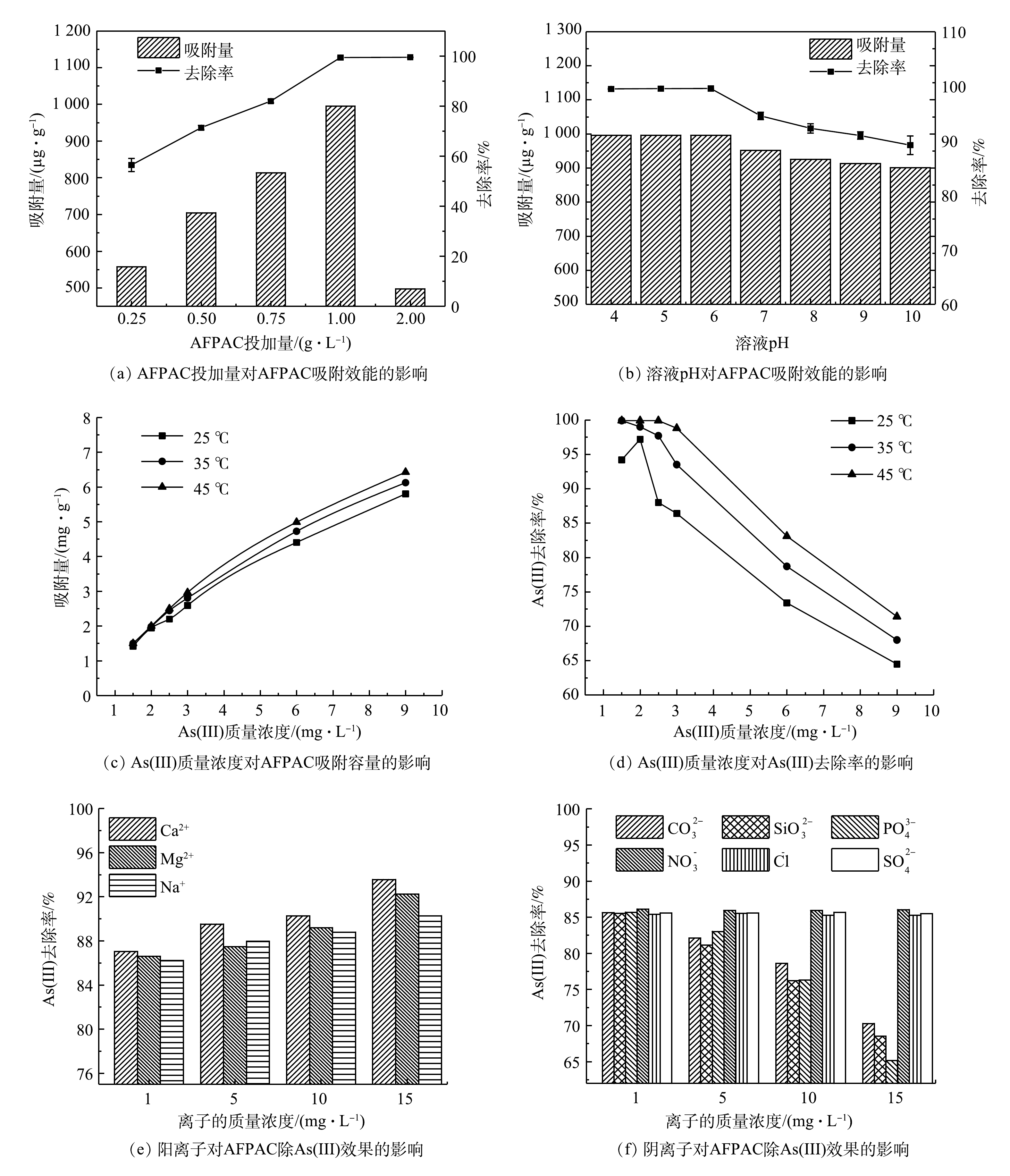
考察AFPAC投加量、溶液pH及初始As(Ⅲ)质量浓度对AFPAC除As(Ⅲ)效果的影响,结果如图2所示。图2(a)表明,当AFPAC投加量由0.25 g·L−1增加至1.0 g·L−1时,As(Ⅲ)的去除率由56.4%增加至99.4%。这是由于AFPAC投加量的增加可提供更多不饱和吸附位点。而继续增大AFPAC的投加量,As(Ⅲ)的去除率基本保持不变。这是由于吸附位点不能被有效利用,且随着传质阻力增大,单位质量的AFPAC对As(Ⅲ)的吸附容量减小[14]。由图2(b)可以看出,在pH为4~10内,AFPAC对As(Ⅲ)均有良好的去除效果,对As(Ⅲ)的吸附容量大于900 µg·g−1。这说明AFPAC除As(Ⅲ)具有很宽的pH适应性。在pH为4~6时,As(Ⅲ)的去除率达到99.4%以上,随着溶液pH继续增大,As(Ⅲ)的去除率呈一定的下降趋势;pH增大至10时,As(Ⅲ)的去除率降至90.1%。这说明偏酸性的溶液环境更适宜AFPAC对As(Ⅲ)的去除。据此可推测:当pH<6时,AFPAC表面羟基质子化形成带正电的OH2+,促进了配位体与As(Ⅲ)阴离子的交换;随着pH增大至碱性时,AFPAC表面开始去质子化反应而带负电荷,与溶液中As(OH)4−存在静电斥力,不利于其吸附去除[11]。
由图2(c)和图2(d)可见,随着温度的升高,AFPAC对As(Ⅲ)吸附去除率有所提高。这是由于温度的升高促进了AFPAC与溶液的界面反应并提高了AFPAC与As(Ⅲ)的接触效率。同时,在一定温度下,随着As(Ⅲ)初始质量浓度的增加,AFPAC对As(Ⅲ)的去除率逐渐下降,吸附量逐渐增加。这是由于随着As(Ⅲ)质量浓度的增加,AFPAC表面的活性位点被逐渐占据,继续增加As(Ⅲ)质量浓度至6 mg·L−1后,吸附除As(Ⅲ)的速率开始减缓,吸附趋于饱和,此时As(Ⅲ)的去除率降至70%左右。
图2(e)和图2(f)为共存离子对AFPAC去除As(Ⅲ)的效果的影响。Ca2+、Mg2+、Na+等阳离子对AFPAC去除As(Ⅲ)具有一定的促进作用,呈现出Ca2+>Mg2+>Na+的促进趋势,随着离子质量浓度的增大其促进作用降低。共存阴离子Cl−、SO42-和NO3−等对AFPAC吸附除As(Ⅲ)效果基本没有影响,而PO43-、SiO32-以及CO32-等对AFPAC吸附除As(Ⅲ)存在明显的抑制作用,呈现出PO43->SiO32->CO32-的抑制趋势。已有研究表明,离子强度是决定吸附剂对重金属的特异性和非特异性吸附去除率的重要参数[15]。重金属的特异性吸附过程基本不受溶液离子强度的影响,而金属离子的非特异性吸附一般受溶液离子强度变化的影响[16],因此,本研究中AFPAC对As(Ⅲ)的吸附属于非特异性吸附行为。
-
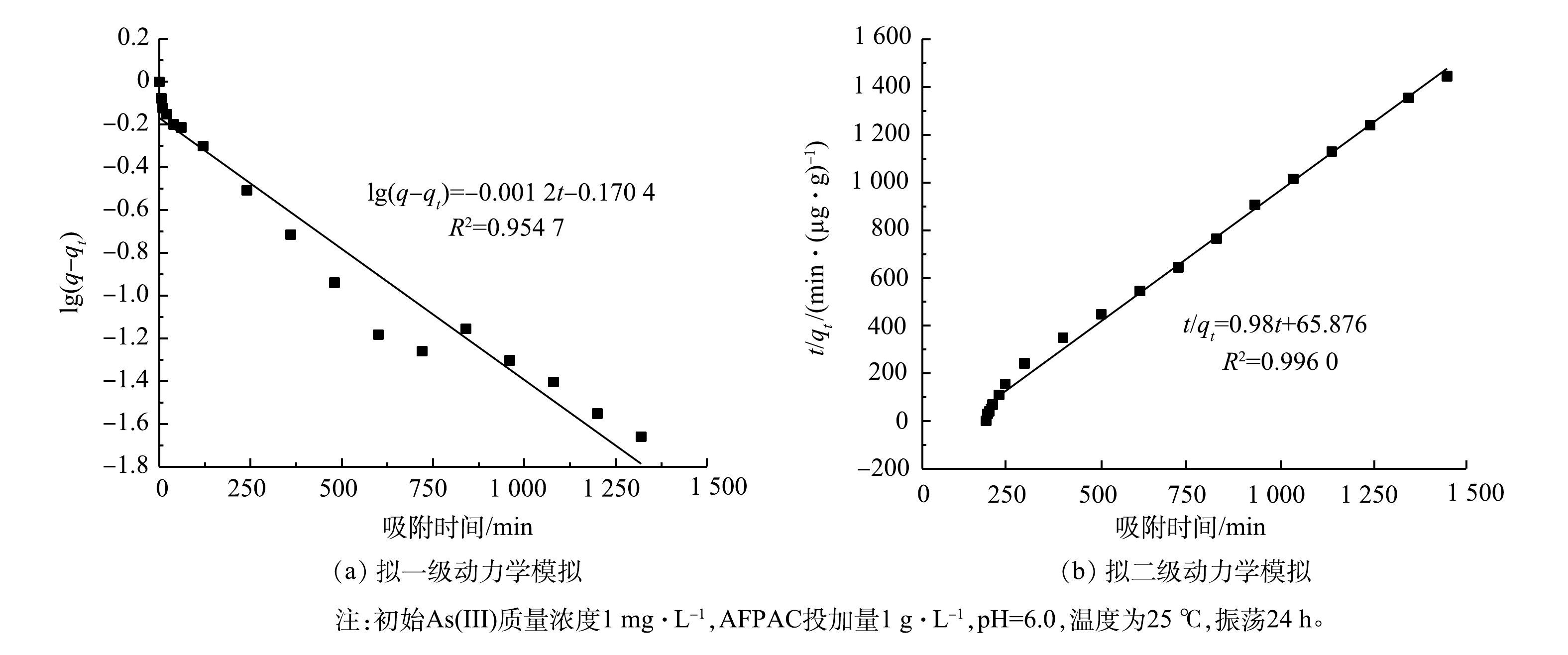
AFPAC对As(Ⅲ)的吸附动力学拟合结果及拟合参数如图3和表3所示。由图3和表3可见,AFPAC对As(Ⅲ)的吸附过程更好地遵循拟二级反应动力学。这与目前关于活性炭吸附除重金属的研究报道一致[8,12]。计算得出的AFPAC对As(Ⅲ)的平衡吸附量(1.020 4 mg·g−1)接近实际平衡吸附量(0.995 mg·g−1),表明AFPAC吸附As(Ⅲ)的决定性步骤是化学吸附。
-
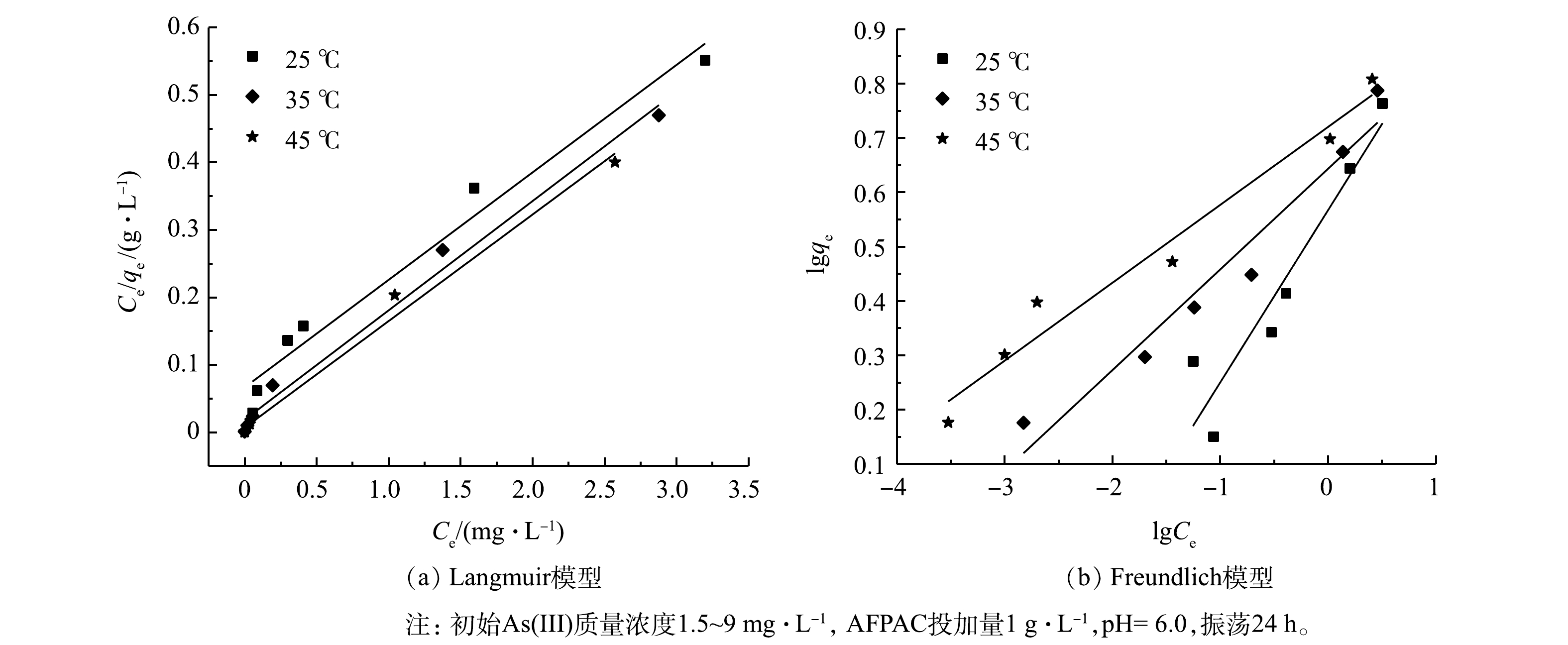
Langmuir和Freundlich吸附等温模型对AFPAC去除As(Ⅲ)的吸附过程拟合结果及拟合参数如图4和表4所示。可见,AFPAC对As(Ⅲ)的去除特征更适于使用Langmuir等温吸附模型进行描述。这与公绪金[5]、陈苹[8]和罗成[11]等有关载铁活性炭除砷的研究结果一致。据此可推测AFPAC对As(Ⅲ)的吸附方式为单层吸附。AFPAC对As(Ⅲ)的最大吸附容量在25、35和45 ℃时分别为6.17、6.27和6.33 mg·g−1。由Freundlich等温吸附模型拟合可知0<1/n<1,说明AFPAC与As(Ⅲ)之间有较强的亲和力。当lnCe较低时,lnqe随lnCe的增大呈明显线性增加,但当lnCe较高时,lnqe的增速逐渐变缓。这可能是较高的As(Ⅲ)质量浓度影响了AFPAC与As(Ⅲ)之间的相互作用力,导致吸附过程偏离理想状态。
-
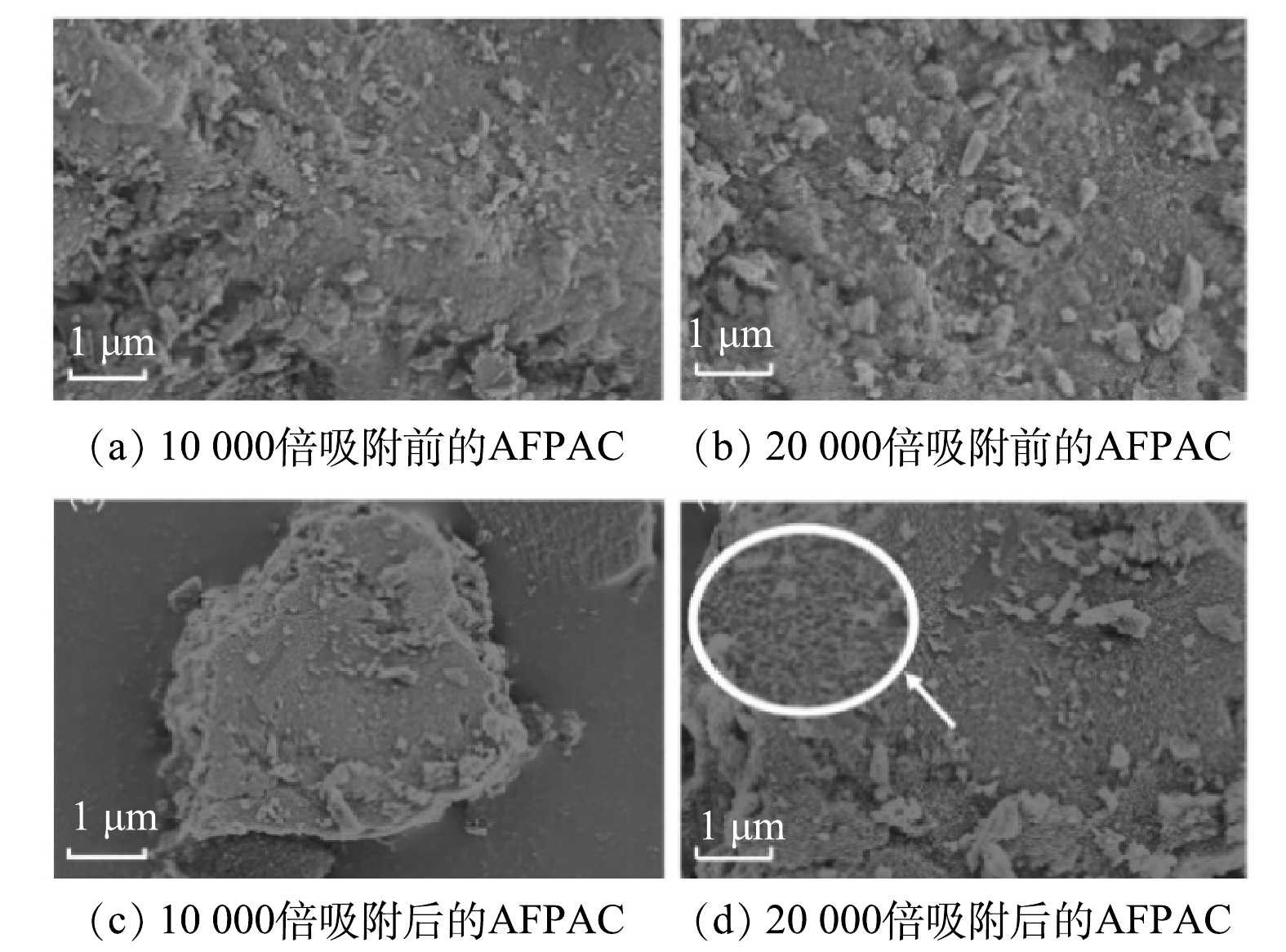
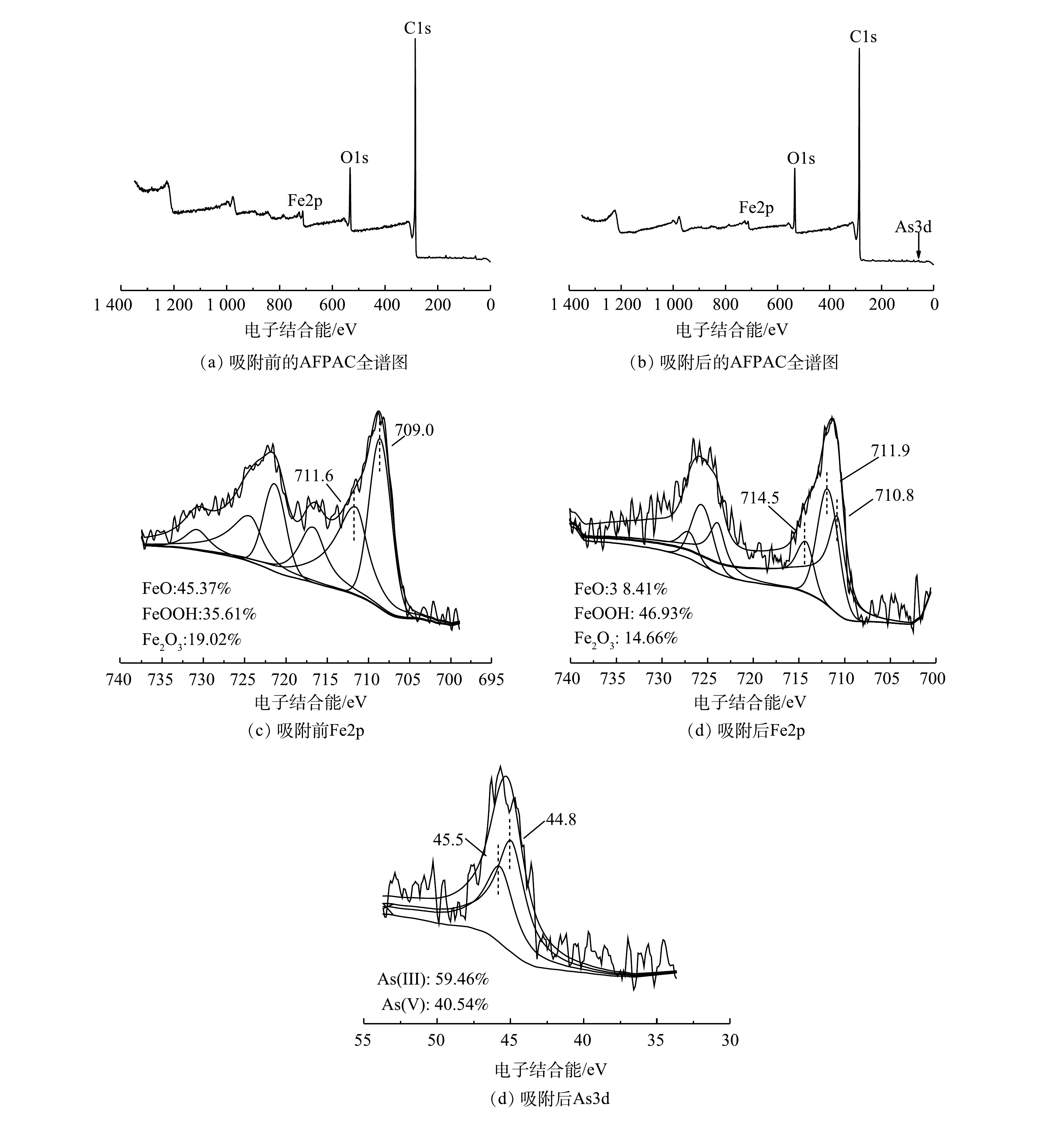
1)表面形貌变化。利用SEM对吸附除As(Ⅲ)前后的AFPAC进行了表征。如图5所示,AFPAC表面的铁氧化物与As(Ⅲ)发生明显的吸附作用,致使其表面形貌发生变化。由图5(a)~(b)可见,吸附除As(Ⅲ)前,AFPAC表面较为粗糙,凹凸不平有明显的棒状、块状的铁氧化物颗粒物。这不仅提高了AFPAC的比表面积,强化了与水溶液的界面反应,更为重要的是提供了丰富的吸附位点[17]。吸附As(Ⅲ)后,AFPAC表面呈现较多的精细颗粒,以膜网状态堆叠在一起,将吸附后的As(Ⅲ)紧紧的固定在材料上,不易断裂浸出。
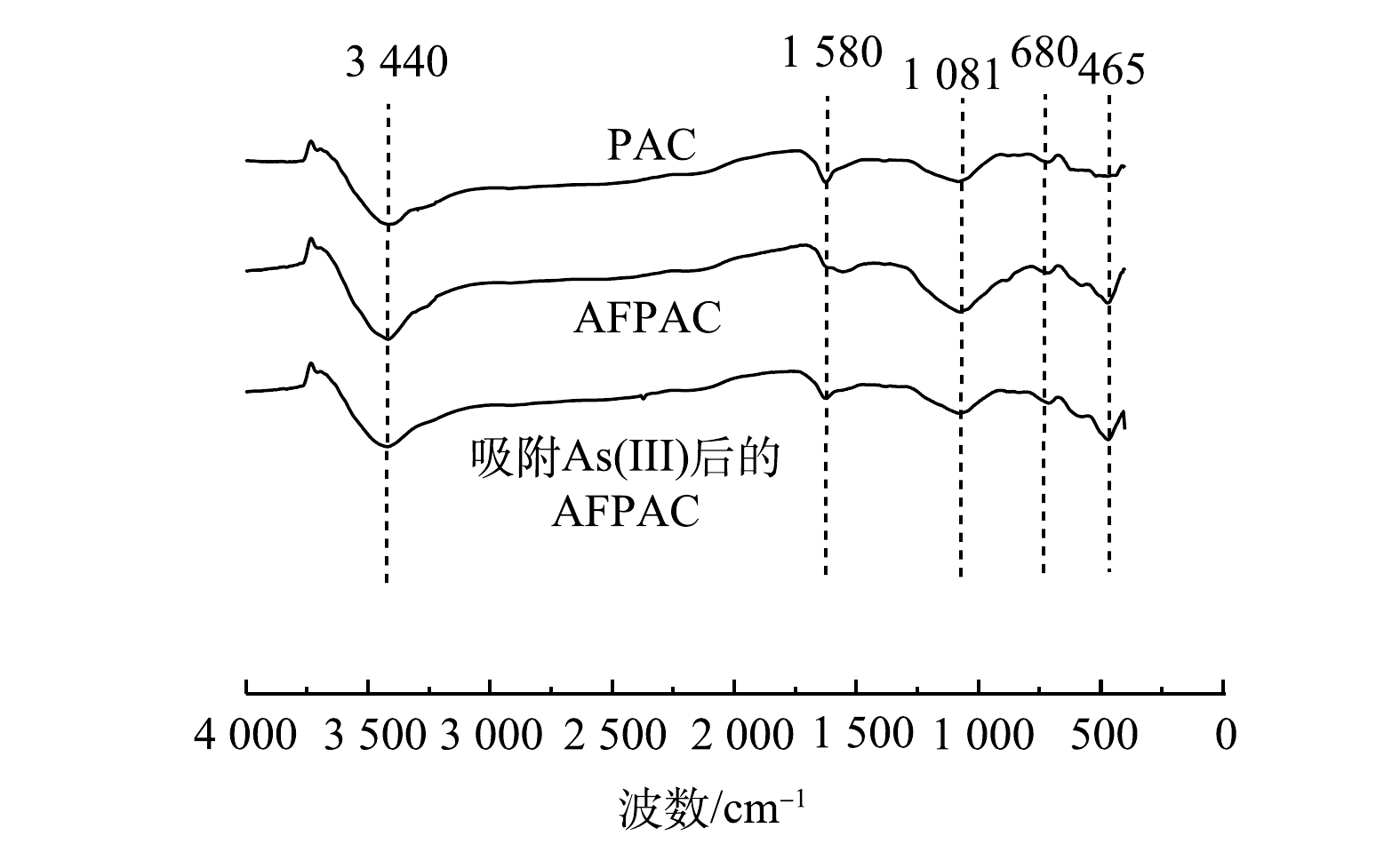
2)红外光谱的变化。对吸附As(Ⅲ)前后的AFPAC进行了红外光谱表征,结果如图6所示。其中1 081 cm−1为C—O的伸缩振动峰,3 440 cm−1附近为O—H的伸缩振动峰,1 570~1 580 cm−1为C=C的伸缩振动峰,1 400 cm−1为C—H的弯曲振动峰,676~680 cm−1为S—O的弯曲振动峰,900~1 300 cm−1的宽波峰为Si—O的振动峰[18-19]。由上述结果可知,吸附前后的AFPAC红外光谱发生了一定的变化,其中改性后的AFPAC在464.8 cm−1处出现了一个Fe—O 特征峰[8],据此可推测预处理后的活性炭在浸渍过程中有效负载了铁氧化物。改性后的AFPAC在3 440 cm−1的O—H峰增强,这是由于改性过程中FeSO4与K2FeO4发生氧还反应促进了铁氢氧化物的生成并原位负载在活性炭上。吸附As(Ⅲ)后的AFPAC主要吸收峰的位置基本未发生变化,说明吸附除As(Ⅲ)并未明显改变其自身的结构。此外,吸附As(Ⅲ)后O—H 振动峰的强度减弱,推测是由于AFPAC上的FeOOH与As(Ⅲ)形成了Fe—O—As络合物所致[11]。
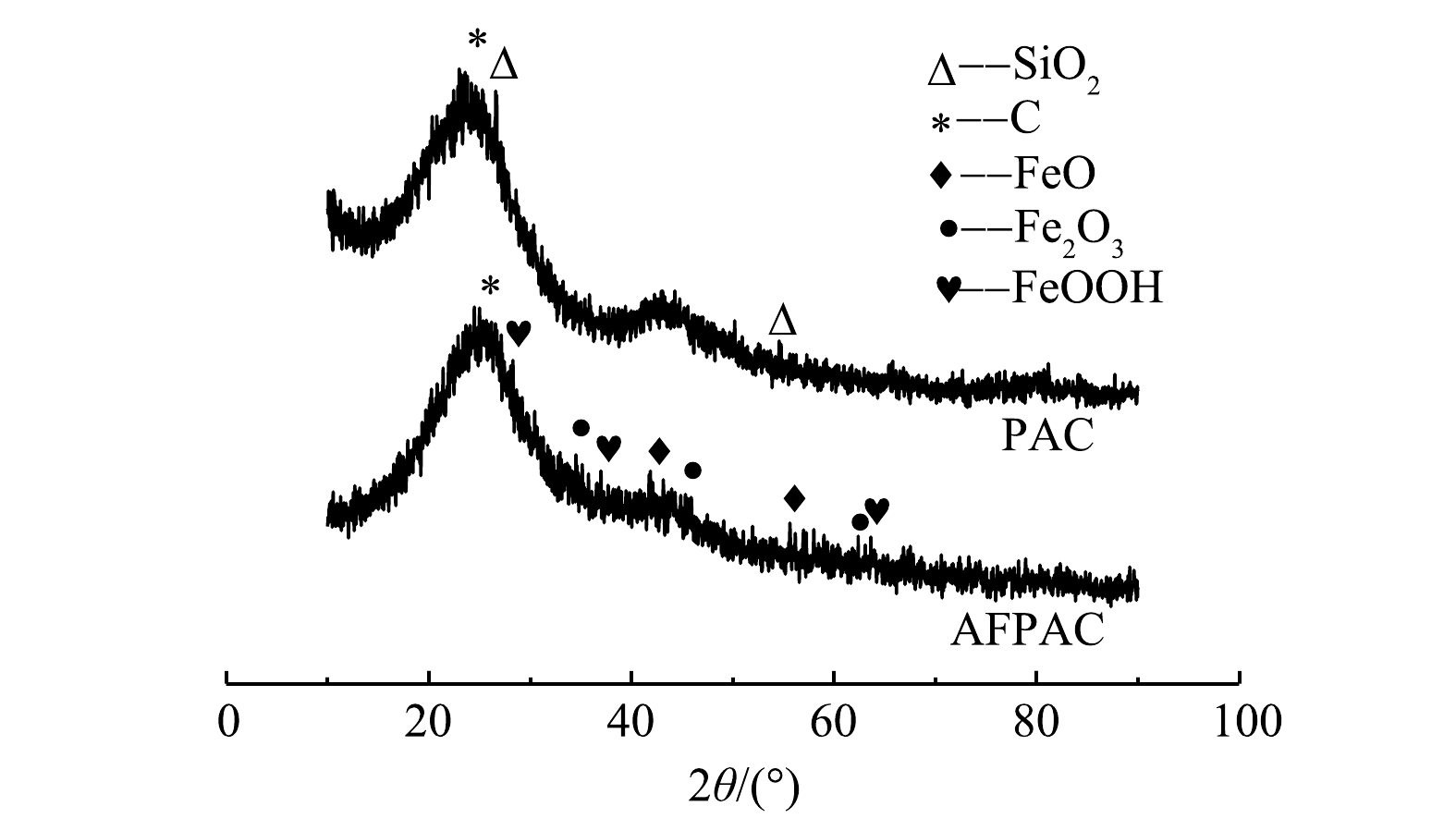
3) XRD分析。利用XRD分析样品的晶体结构并确定了改性后的活性炭上铁氧化物的种类。由图7可见,PAC和AFPAC在26.6º附近有较宽的C的特征峰。这说明两者主要组成结构是非晶体结构。PAC中26.2º和58.1º为SiO2特征峰,而改性后AFPAC的SiO2特征峰消失不见。这说明酸预处理及载铁改性有效清除了活性炭表面杂质,优化了活性炭结构。需要注意的是,制备获得的AFPAC中出现了3种铁氧化物的特征峰:位于33.9º、41.8º、57.7º的FeO特征峰;位于35.9º、43.2º、62.4º的Fe2O3的特征峰;位于28.4º、35.6º、63.8º的FeOOH特征峰[20]。不过,峰的强度相对较弱,推测是由于Fe主要以无定形态负载在活性炭上所致[21]。
4) XPS分析。对吸附除As(Ⅲ)前后的AFPAC进行了XPS表征分析。由图8(a)~(b)可见,AFPAC吸附除As(Ⅲ)前后C1s的结合能均未曾改变,为炭纤维中炭骨架峰,无谱峰分裂现象。结合图8(c)中Fe2p的精细谱可以看出,AFPAC上在709.0 eV和711.6 eV处出现了Fe的特征峰,Fe的价态分别对应Fe(Ⅱ)和Fe(Ⅲ),主要以Fe(Ⅱ)形式存在。结合有关研究报道和XPS标准谱库可知,载铁改性过程中原位生成了物种丰富的铁氧化物,包括FeO、Fe2O3和FeOOH等[22],与本研究中XRD研究结论一致。而吸附除As(Ⅲ)后AFPAC的Fe2p精细谱有一定的变化,Fe主要以Fe(Ⅲ)形式存在,原位产生的FeOOH在吸附除As(Ⅲ)中发挥了重要的作用。不同铁氧化物形态的来源主要由于溶液中少量游离态的HFeO4−与FeSO4形成的Fe(OH)2发生氧化还原反应,原位产生了Fe(OH)3,并最终以FeO、Fe2O3和FeOOH等形式负载于AFPAC上。具体反应机制如式(9)~式(13)所示。
同时,由As3d的精细谱(图8(e))可以看出,吸附As(Ⅲ)后AFPAC的As3d结合能的特征峰位于44.8 eV和45.5 eV,分别对应As(Ⅲ)和As(Ⅴ)[23],其中As(Ⅴ)占约40%,砷的价态在吸附过程中发生了变化。其原因可能是:一方面由于AFPAC呈现一定的核壳结构现象,铁元素由表到里呈现出不同的价态分布,存在少量的高价态铁与As(Ⅲ)发生氧化还原反应;另一方面可能是由于部分As(Ⅲ)与活性炭上的O原子在传递电子过程中失去电子所致。由此可见,AFPAC去除As(Ⅲ)的过程不仅存在化学吸附,还存在一定的氧化还原作用。
-
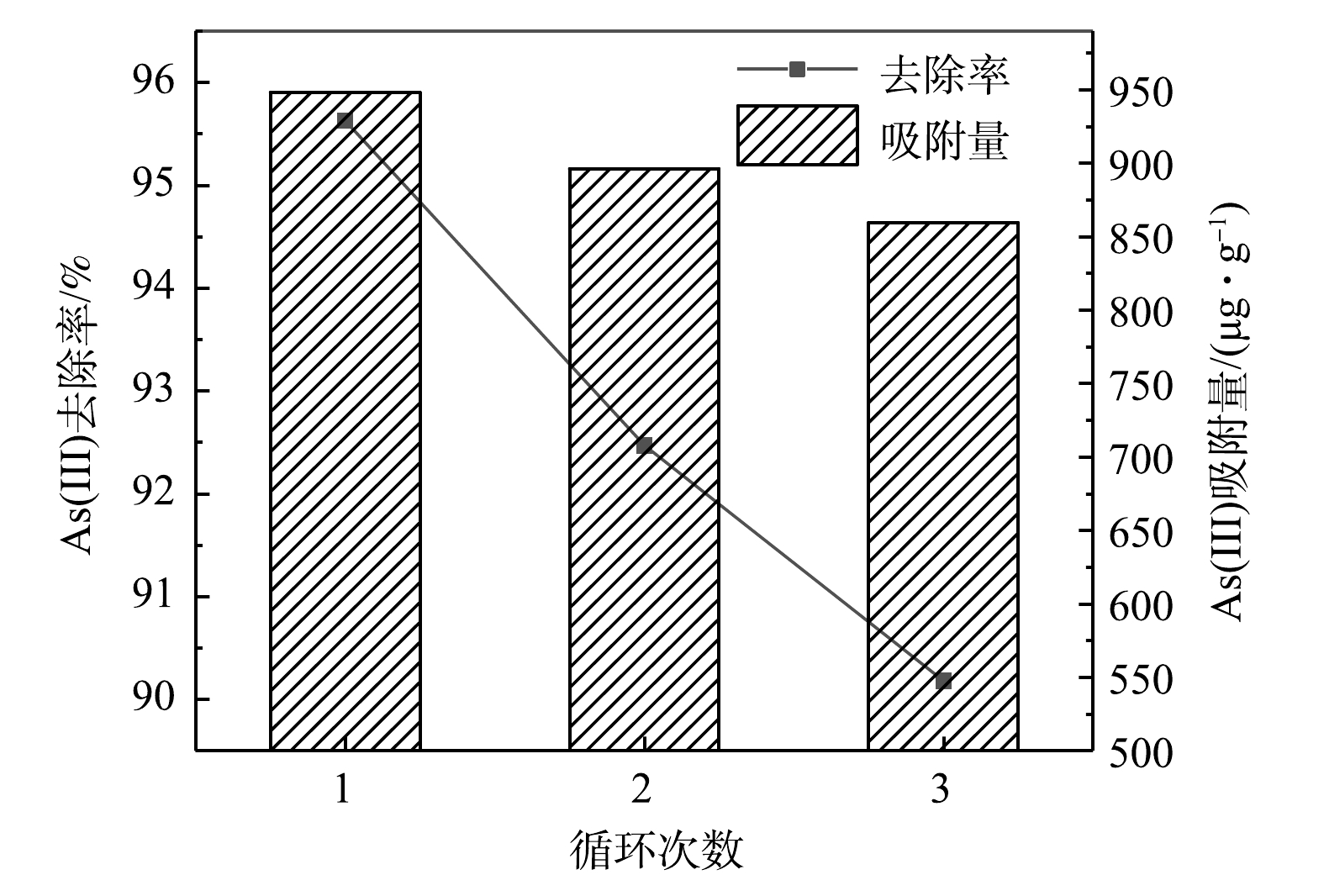
由图9可见,经过3次解吸再生后,AFPAC对As(Ⅲ)的去除率依次为95.63%、92.47%和90.18%,再生后的AFPAC对As(Ⅲ)依然保持较高的去除效果。这说明AFPAC的再生性能良好,多次使用后仍具有稳定的除As(Ⅲ)性能。
-
1)采用FeSO4/K2FeO4组合改性制备获得的原位铁氧化物载铁活性炭(AFPAC)对水中As(Ⅲ)具有显著的去除效果,As(Ⅲ)的最高去除率可达到99.5%。
2)拟二级动力学模型和Langmuir等温吸附模型能较好地拟合AFPAC对As(Ⅲ)的吸附过程,表明其吸附以化学吸附为主,属于单层吸附,最大吸附容量为6.17 mg·g−1。
3) FeSO4/K2FeO4组合改性可在活性炭表面原位生成丰富的铁(氢)氧化物形态,为吸附As(Ⅲ)提供大量的吸附位点,具有较强的吸附结合能力。吸附过程存在明显的氧化还原反应。AFPAC对As(Ⅲ)的去除存在配位络合、静电吸附及氧化还原等多重协同作用机制。
原位铁氧化物载铁活性炭的制备及其对水中As(III)的去除性能
Preparation of in situ iron oxides-loaded activated carbon and its performance on arsenite removal from water
-
摘要: 针对水中三价砷As(III)毒害大、难去除的问题,本研究采用FeSO4/K2FeO4组合处理方式以恒温浸润法对活性炭进行改性,制备获得原位铁氧化物载铁活性炭(AFPAC),利用负载的铁氧化物的吸附性能,耦合活性炭的吸附和有效的固液分离功能,实现了As(Ⅲ)的高效去除。结果表明,当AFPAC投加量1 g·L−1时,对初始质量浓度1 mg·L−1的As(Ⅲ)的去除率达到99.5%以上,吸附容量达到6.17 mg·g−1;其吸附行为属于非均质表面吸附,以化学吸附为主。AFPAC表面原位生成的铁氧化物形态和物种丰富,具有较强的络合和吸附能力,对As(Ⅲ)的去除呈现出配位络合、静电吸附及氧化还原等多重协同作用机制。Abstract: In view of the problem that arsenic trivalent As(Ⅲ) in water is highly toxic and difficult to remove, in this study, the in-situ ferric oxide ferric-loaded activated carbon (AFPAC) was prepared by the dipping method at constant temperature with FeSO4/K2FeO4 combined treatment mode, and As (Ⅲ) could be efficiently removed by coupling the adsorption ability of loaded iron oxides with the adsorption and effective solid-liquid separation abilities of activated carbon. The results showed that at AFPAC dosage of 1 g·L−1, the removal rate of As(Ⅲ) at the initial concentration of 1 mg·L−1 was above 99.5%, and the corresponding adsorption capacity reached 6.17 mg·g−1. The adsorption behavior of As(Ⅲ) was heterogeneous surface adsorption, mainly belonged to chemical adsorption. The iron oxides generated in situ on the surface of AFPAC were rich in form and species with strong complexation and adsorption capacity for As(Ⅲ) removal. Multiple synergistic mechanisms for As(Ⅲ) removal were ligand complexation, electrostatic adsorption and adsorption and oxidation reduction.
-
Key words:
- iron-loaded activated carbon /
- in-situ iron oxide /
- ferrate /
- arsenite /
- ligand complexation
-
伊朗位于亚洲西南部,南临波斯湾和阿曼湾、北濒里海,地理位置优越,有“欧亚路桥”之称。中伊同为文明古国,古丝绸之路将两国紧密相连。2016年1月,习近平主席访问伊朗,双方发表了建立全面战略伙伴关系的联合声明,内容涉及经济、航天、粮食、环保等诸多领域。2017年4月,在刘延东副总理和伊朗科技副总统萨塔里共同见证下,中国科学院生态环境研究中心与伊朗德黑兰大学农业与自然资源学院签订合作备忘录。该备忘录强调双方将共建“中-伊水与环境联合研究中心”,并以该研究中心为平台,开展人才培养、技术培训、合作研究及示范工程建设等互利合作,为相关技术及产业开拓伊朗市场提供协助。伊朗长期面临水资源匮乏与水环境污染,对其实现可持续发展造成了严峻挑战。因此,中伊两国深化在水与环境领域合作,将有助于缓解伊朗面临的紧迫性环境、民生问题。
1. 伊朗在水与环境领域的问题
1.1 伊朗面临的主要环境问题
联合国针对伊朗发布的国别发展援助框架(United Nations Development Assistance Framework, UNDAF, 2017-2021)明确指出了伊朗发展的4个优先事项,其中环境问题居首位。该框架指出,由于气候干燥、城市化加速和经济发展导致对石油和天然气生产的高度依赖,伊朗正面临着极其严峻的环境挑战。这些挑战包括:能源利用强度过高导致的城市空气污染和温室气体排放;不可持续的农业和土地管理模式导致的植被覆盖减少、土壤侵蚀、荒漠化、旱灾和湿地萎缩;地下水过度开发利用导致的河流和湖泊的干涸、部分湿地的消失;波斯湾地区石油产业造成的海洋污染和生态系统破环;里海地区不可持续性的生物资源开发、区域性污染对生态系统造成威胁等。此外,工业污染、废弃物处理、过度放牧、森林砍伐、土地退化和土壤侵蚀也都对伊朗的生物多样性产生负面影响。因此,伊朗应采取坚决措施,着力保护关键的环境资产,才可实现国家的可持续发展[1]。
1.2 伊朗的水资源状况及问题
世界资源研究所(World Resources Institute, WRI)最新公布的一份报告指出,截至2019年,共有17个国家面临“极高”的水资源压力,其中伊朗排名第4位[2]。伊朗国土总面积1.75×106 km2,52%国土面积被高山和沙漠覆盖,约16%面积的地区海拔在2 000 m以上,属于典型的高原国家[3]。伊朗北部、西部、东部均为山脉或高地,中部为荒漠地带,仅西南部波斯湾沿岸与北部里海沿岸有小面积的冲积平原。国土总面积的64.7%为干旱和半干旱气候。近50年来,伊朗年均降水量为248 mm,近10年的年均降水量仅有220 mm,减少幅度为11%,可知伊朗国内的水资源呈持续减少趋势。此外,伊朗国内的降水量还存在严重的时空分布不均。北部、西部和西南部的占国土总面积的30%,降水量却占全国总量的56%。而中部和东部地区面积占70%,降水量却仅为全国总量的43%[4]。从时间分布来看,降水呈现显著的季节特征:全国平均降水量的70%集中在冬春两季(每年11月—翌年3月);而夏季(每年6—8月)几乎无雨[5]。综上所述,水资源的持续减少、严重短缺和时空分布不均,导致伊朗在生态环境保护、社会经济和农业发展等方面都承受着巨大的压力。
伊朗近50年间的年均水资源量约为1.16×1011 m3,年均水资源消费量约为1×1011 m3,即每年水资源总量的86%为人类生产、生活所消耗,远高于世界平均9.3%的水平。从伊朗水资源消费构成来看,约0.4×1011 m3用水源于地表水,约0.6×1011 m3源于地下水,说明对地下水资源的依赖度高。从水资源的用途来看,农业用水占92%、生活用水占6%、工业用水占2%,即90%以上用于农业生产、约10%用于生活和工业生产,说明农业用水量占比极高。此外,根据水井数量的变化,可推断伊朗国内地下水的利用情况。公开数据显示,截至2017年末,伊朗国内的水井总数为8.07×105口。伊朗法律(The Fair Water Distribution Law, 1983)规定,开凿水井必须获得行政管理部门的许可。然而,事实上约40%的水井属于无许可的违规水井,且违规水井数量仍以每年约1×104口的速度增加。以上水井数据说明,伊朗水资源开发主要来自地下水,同时也说明相关行政部门对地下水的管理力度需要加强[4]。
伊朗水资源短缺问题不仅归因于地理和气候等自然条件导致的水资源先天不足,人口的持续增长致使水资源消耗量大幅增加也是造成短缺的原因。20世纪60年代,伊朗人口为3×107人,到2018年,已增至约8.2×107人[6]。另外,伊朗是以小麦为主食的农业大国。自20世纪80年代起,政府推动小麦自给自足政策,并于2004年实现了年产1.4×107 t小麦,达到政策制定目标[7]。然而,这项政策亦造成了严重后果,即耕地面积增加导致农业用水量剧增,过度开采和利用地表水和地下水,导致生态用水严重不足;同时,因管理不到位和灌溉技术原始粗放,农业灌溉浪费水资源现象极其严重[8]。近年来,伊朗国内已出现大量湖泊干涸、湿地萎缩、河流断流等状况。2014年4月,位于伊朗东北部的乌尔米耶湖有90%的湖面出现干涸。该湖是伊朗最大的湖泊,其周边湿地也是服务1.3×107人口的重要水源地。据报道[9],伊朗乌尔米耶大学校长HOBBENAGHI Rahim认为:“人类对自然环境的破坏,多年来过度的农业开发和水资源的无序使用,最终导致乌尔米耶湖今天岌岌可危的局面。”2018年,伊朗国内遭遇旱灾,冬春雨季的降水量仅为往年的30%左右。同年6月,在伊朗西南部的港口城市和石油基地阿巴丹市、以及与伊拉克毗邻的边境港口城市霍拉姆沙赫尔,先后发生了因自来水水质和频繁断水引发的民众抗议游行[10-11]。
综上所述,伊朗的水资源存在持续减少、时空分布不均、用水量不断增加、过度依赖地下水、农业用水占比过高、管理不到位等一系列问题,导致伊朗面临极大的水资源短缺压力,从而对整个社会的稳定和经济的发展产生不可逆的负面影响。
1.3 伊朗供水现状
据报道[12],2018年,伊朗能源部长ARDAKANIYAN Reza在公开报告中指出:“2017年是过去50年来最干旱的一年。降水量的减少是主要原因之一,如果不能采取有效措施和管理,伊朗大部分地区的水资源短缺问题会日益严重。”他同时还表示:“2017年整个伊朗的饮用水供应已经出现问题,今后几年饮用水供应不足的情况会成为常态。”
根据伊朗能源部发布的最新数据,伊朗国内517座城市面临严重的供水不足,2013年3月以来持续出现城市用水量超过供水能力的情况。为解决这一问题,主管水务的伊朗能源部在原先的《第五次5年供水规划》基础上,追加了投资总额达3.75×108美元的供水系统优化计划,涉及130项供水项目,包括12个大型供水设施。这项供水系统优化计划主要包括:多源头供水保障(含海水淡化)、老旧供水基础设施的修缮和改进、不合理农业用水量的削减、再生水利用的强化、在各领域提倡并开展节水措施等。这项计划原定2016年底完成,但因资金保障不足问题而滞后[13]。
以首都德黑兰市为例。该市水源主要来自周边山区的4座水库。4座水库中库容最大的Laar Dam水库的储水量约为9.6×108 m3,干旱年份的可利用水量仅有1.8×107 m3[13]。由于德黑兰市的地势北高南低,海拔高度为1 100~1 800 m,所以政府在市区建设了118个泵站和187个蓄水池。其中,北部海拔较高地区主要通过泵进行提升供水,而南部地区则依靠重力流进行供水。德黑兰市现有自来水厂6座,日供水能力可达2×106 m3,而德黑兰市每天的水消费量为3.3×106 m3。为解决供水不足问题,德黑兰市还开凿了441口平均深度达250 m的深水井,单口井的最大产能达600 m3·h−1,导致地下水常年过度开采[14]。德黑兰市居住人口约为1.2×106人。该市的供水人口占伊朗全国的12%,却消耗了伊朗全国供水量约25%。然而,尽管德黑兰市面临严重的水资源短缺危机,市民节水意识却普遍不强,人均日生活用水量高达400 L·d−1,远高于全国人均250 L·d−1的水平[15]。
1.4 伊朗国内水务市场及合作可能性
分析伊朗的水与环境领域面临的问题可知,在农业用水循环利用(节水灌溉)、生活污水(分散型污水)处理、雨水回收利用、工业废水处理、再生水技术和应用等水务领域蕴含巨大商机。自2016年联合国解除对伊朗的经济制裁后,世界各国均对伊朗国内的水务市场表现出浓厚兴趣。
2016年,韩国的斗山重工业中标了伊朗的大型海水淡化厂项目,投资总额约2.2×1011韩元(约1.9×108美元)。该项目得到了韩伊两国总统的共同推动,成为联合国解除对伊经济制裁后,首个外国企业进军伊朗海水淡化市场的案例。2017年10月,法国水务企业联合体与伊朗供排水专家协会(Iranian Association of Water and Wastewater Experts, IAWWE)签署全面合作协议。该协会将全面引进法国水务企业联合体的专业技术和经验,以填补伊朗国内民营企业与外国企业之间的技术差距。同年10月,德国水、污水和废弃物处理协会(German Association for Water, Wastewater and Waste, DWA)也与伊朗国营供排水工程公社签署了合作备忘录。双方确认共同出资在伊朗国内面临严重缺水的大城市之一——伊斯法罕市建设针对供排水领域技术人员的培训中心,该中心已于2018年3月正式启用[16]。
伊朗政府将水务领域作为2017年度政府重点支持建设的3个领域之一,并下达了总额约6.5×108美元的中央政府预算。此外,根据伊朗能源部公布的资料,今后将在水务领域开展总额约5.5×109美元的投资计划,并希望采取不同的方式(BOT、BOO、ROT、Buy-Back等)吸引外国企业进行投资,投资计划[16]见表1。
表 1 伊朗能源部发布的水务领域投资计划Table 1. Investment plan for water sector released by Ministry of Energy of Iran类别 项目内容 规模 投资方式 投资金额/(104美元) 海水淡化 海水淡化及供水基本规划 2项 BOO 360 000 海水淡化设施 4座 BOO 8 300 供水设施 自来水供水设施 2座 融资/BOT 4 000 未收益水量管理项目 35处 BOT/ROT 20 000 智慧水表安装 3×106台 ROT 28 100 污水处理 城市大型污水厂 4座 BOT 20 400 污水处理厂 17座 Buy-Back 77 000 能源部办公楼小型污水处理设施 100处 采购 18 000 农村污水 农村污水处理设施 70个村落 BOT 14 000 2. 水与环境领域开展的对伊合作回顾
过去20多年中,我国在水与环境领域取得了显著的治理成效,积累了丰富的技术、经验和产品。为响应并推动《联合国千年发展目标(2015)》中目标7C“改善饮用水和卫生设施”的实现[17],2013年3月,中国科学院生态环境研究中心(Research Center for Eco-Environmental Sciences, RCEES)申请成立了中科院-发展中国家科学院水与环境卓越中心(CAS-TWAS Center of Excellence for Water and Environment, CEWE)。CEWE的愿景是通过培训、教育和联合研究方案,协助发展中国家在水和环境领域进行人力资源能力建设,并为水与环境领域的具体问题提供切实可行的解决方案。
2016年起,CEWE积极推动中伊双方在水与环境领域的合作。合作初期,双方以共同申请科研项目、加强人员交流为主,开展了多次互访活动。通过实地考察和技术交流等,加强了相互了解,并在分析伊朗水环境领域具体问题的同时,掌握了伊方的具体合作需求。为更好地推进后续合作,CEWE分别与伊朗德黑兰大学、桂兰大学签署了合作备忘录,确立了双方的合作机制和框架,即通过搭建合作平台,推动双方在水与环境领域深化合作。合作备忘录还明确了今后开展合作的方向:科研项目合作、共同发表研究成果、技术合作与交流、人才培养培训、合作举办学术会议、推动中国环保企业在伊朗的投资等。2017年4月,中伊双方在德黑兰大学农业与自然资源学院(University of Tehran, College of Agriculture & Natural Resources, UTCAN)正式成立了“中-伊水与环境联合研究中心(International Research Center for Water and Environment, IRCWE)”。该中心将在中伊双方水与环境领域的科技合作中起到平台作用,通过该平台与伊朗水与环境领域中央和地方政府部门、研究机构、大学等建立合作关系。UTCAN为该平台提供了专用的实验和办公用房,并配备了专职的科研、技术和管理人员。同年10月,中方组织研究机构、大学和环保企业等访问伊朗,期间向IRCWE捐赠了水质和废水分析相关实验室仪器。迄今为止,以IRCWE为平台,中方已与伊朗政府部门、大学、企业等举行了30多次学术或技术交流会议,并组织伊朗环境部研究和技术人员参加了中方举办的“水与卫生”和“二恶英检测技术”培训班;伊方组织了针对中方研究生的夏令营活动,同时推动了在UTCAN校区和附属农场等开展雨水回收利用、学生宿舍生活污水处理、农场高浓度畜禽废水处理、水质监测站建设等4项示范工程的建设工作。截至2019年末,组织中国企业向该平台捐赠的实验室仪器和示范工程设备总额约5×1010伊朗里亚尔(按官方汇率,约合1.2×106美元),为中伊两国进一步拓展在水与环境领域的合作打下了良好的基础。
3. 示范工程进展及问题
经过多次技术交流和现场考察,中伊双方逐步对在水与环境领域如何开展合作制订了明确的目标和计划。除了继续开展科研项目合作、技术交流及人才培养之外,中伊双方有计划地组织和推动了4项示范工程建设,相关示范工程在2019年取得积极进展。
3.1 大楼雨水回收利用示范工程
伊朗国内干旱少雨,冬春季节降雨比较集中。为开发宝贵的雨水资源,CEWE联合北京泰宁科创雨水利用技术股份有限公司在UTCAN的环境科学与工程系大楼开展了雨水回收利用示范工程建设(见图1)。该工程包括“PP模块式地下雨水储存池”设施,雨水储存在其中经净化处理后输送到大楼内部,用于楼内喷水池景观用水。示范工程现已完成建设,双方正针对后期运行维护等开展技术合作,以保障设施稳定运行。
3.2 学生宿舍生活污水处理示范工程
为解决UTCAN校区学生宿舍生活污水的处理问题,开展了相关示范工程建设。该工程采用集成到标准集装箱中的污水处理一体化设备,采用缺氧/好氧(anoxic/oxic,A/O)法与膜生物反应器(membrane bioreactor,MBR)的组合工艺处理生活污水。一体化设备由江苏金梓环保科技有限公司负责加工,CEWE提供技术支持。德黑兰大学负责用电、调节池施工、排放水体等。目前,一体化污水处理设备已运抵伊朗示范工程现场(见图2),由于新型冠状病毒疫情影响,原定由中方开展的安装调试等工作延后开展。该示范工程可为伊朗城市小型污水处理设施和农村地区分散型污水处理提供参考和借鉴,有利于城市地区卫生环境的改善和湿地、湖泊、河流等地区村落污水的处理。
3.3 农场高浓度畜禽废水处理示范工程
按照2017年CEWE、北京京润环保科技有限公司(北京京润)以及UTCAN签署的三方协议,农场畜禽高浓度污水处理示范工程由北京京润承担建设,CEWE提供技术支持。为推动该示范工程的建设,2018年CEWE及企业代表团考察了UTCAN附属农场,包括农场养殖设施、水源、现有污水处理设施及污水排放源等,掌握了一手的现场数据。例如,汇总了养鸡设施、奶牛降温喷淋设备、挤奶清洗设施、小牛养护设施等(为了维护卫生环境,保护新生小牛的养护设施用水量最大)不同设施的排水量,测算出日废水量约为17 m3。最终确定生物处理技术为主要处理工艺,处理设备的设计污水处理量为30 m3。农场具有独立的井水水源,设有水塔和储水池,安装有5台抽水泵抽取地下水,水质较清澈。另外,在拟建污水处理设施旁的隔离栅栏外,农场方面新建了养鱼池(见图3)以接收处理设施的出水。该养鱼池安装有曝气泵,多余水量用于周边农地(油菜和小麦)的灌溉,从而实现从井水-畜禽养殖用水-养鱼用水-农田灌溉用水的水资源循环利用,促进节水型养殖业和农业的结合。
3.4 水质监测站示范工程
根据中伊合作协议,计划在UTCAN校园内人工引水渠(兼具校园景观河道作用)附近建设水质监测站,对流经卡拉季市的卡拉季河进行常年的水质监测。相关监测数据除供德黑兰大学中-伊水与环境联合研究中心科研项目使用之外,还将提供给当地环保部门,为水资源保护、河流水质检测、农业灌溉用水水质变化等提供依据。
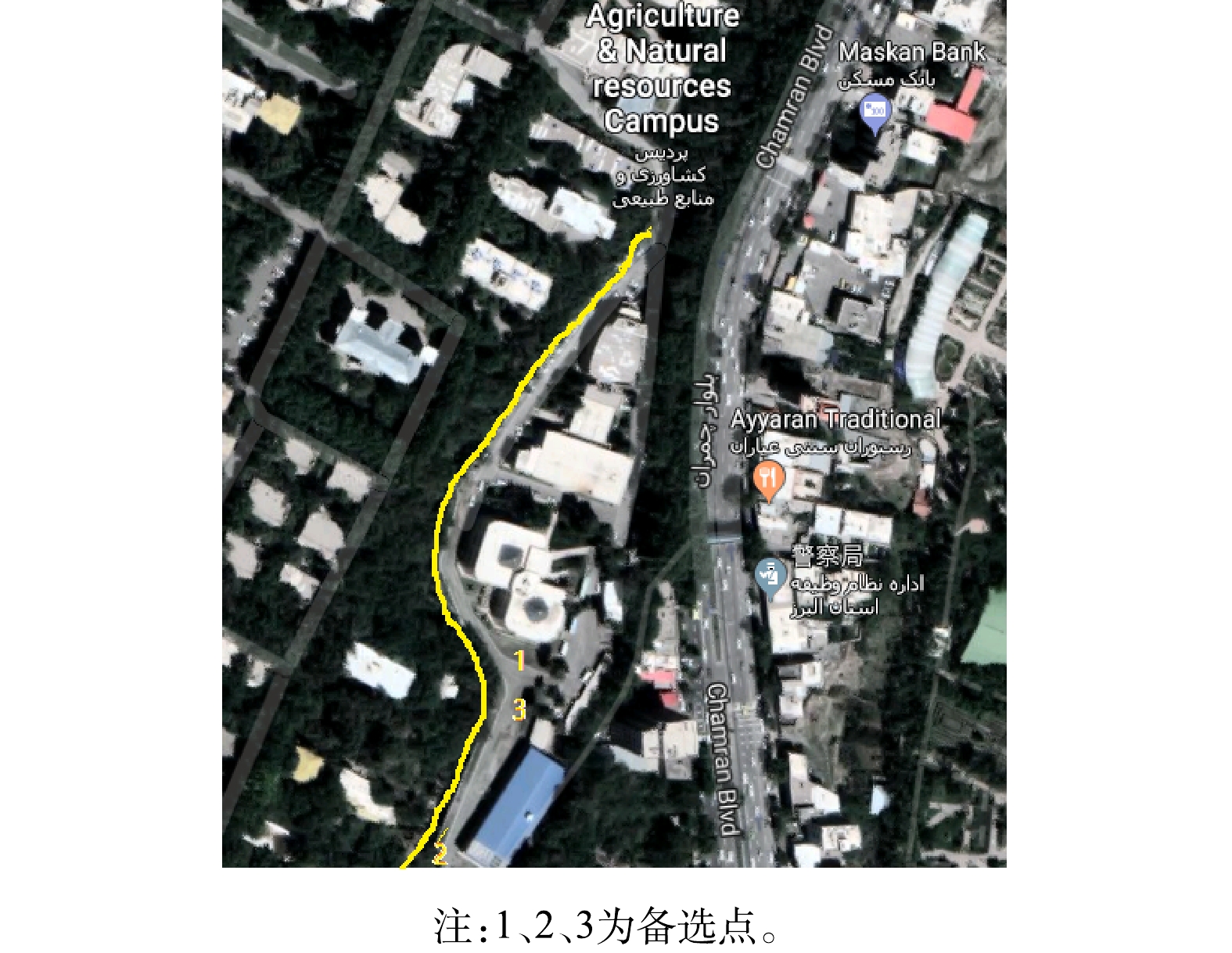
示范工程承建单位力合科技(湖南)股份有限公司(湖南力合)技术人员对预建水质监测站的情况进行了确认。水质监测站位于UTCAN卡拉季校区,以流经校园的人工引水渠(卡拉季河的分支)为水质监测对象。该渠宽3~5 m,渠岸与水面落差约2 m。现场考察了3个建站备选点(图4中1、2、3点),最终选定距引水渠较近、有空置实验室可供水质监测站使用的位置作为选定的建站点(图4中3点)。该位置取水直线距离在10 m以内,取水高度8 m以内,需设置潜水泵从河道取水采样。
4. 结语
1)伊朗水资源严重不足,水质问题又加重了水资源的短缺。2016年以来,中国科学院生态环境研究中心依托中科院-发展中国家科学院水与环境卓越中心,与伊朗大学、研究机构及环保行政管理部门开展了一系列学术和人员交流,对伊朗国内水与环境领域的问题进行了系统分析,为在该领域对伊开展持续、有效的国际合作打下了良好基础。
2)以德黑兰大学“中-伊水与环境联合研究中心”为平台,推动了4项示范工程建设,其中环境科学与工程系大楼雨水回收利用示范工程、学生宿舍生活污水处理示范工程进展良好。农场高浓度畜禽废水处理示范工程和水质监测站示范工程正在积极推进。示范工程将为中伊两国在水与环境领域合作提供成功案例,也成为中国环保企业及成熟环保技术、设备展示的平台。
3)伊朗面临严重的环境问题,水资源短缺及水污染问题尤为突出。中国环保企业在湿地保护、湖泊生态修复、再生水利用、雨水回收利用、水污染防治、工业废水治理、饮用水安全保障等领域积累了丰富的技术和经验。可通过开展国际合作、技术输出、示范工程,助力中国环保企业特别是民营环保企业开拓伊朗市场,实现企业发展国际贸易与当地提升环境质量的双赢。
-
表 1 不同组合改性后的活性炭除砷效果
Table 1. Arsenic removal effect of activated carbon modified by different combinations
不同铁盐和氧化剂组合 As(Ⅲ)的去除率/% 铁含量/% 原始新鲜活性炭 33.00 — 酸预处理活性炭 35.80 — 碱预处理活性炭 32.60 — FeCl3+K2FeO4 85.50 3.20 FeSO4·7H2O+ K2FeO4 99.50 4.50 注:吸附剂投量为1 g·L−1;As(Ⅲ)初始质量浓度为1 mg·L−1。 表 2 不同研究中载铁活性炭除砷效果
Table 2. Arsenic removal effect of iron-loaded activated carbon in different studies
表 3 吸附动力学模型拟合结果与参数值
Table 3. Adsorption kinetics model fitting results and parameter values
拟一级吸附动力学模型 拟二级吸附动力学模型 R2 q/(mg·g-1) K1 R2 q/(mg·g-1) K2 0.954 7 0.675 5 0.002 8 0.996 0 1.020 4 0.014 6 表 4 吸附等温线拟合结果与参数值
Table 4. Adsorption isotherm fitting results and parameter values
T/℃ Freundlich吸附等温线模型 Langmuir吸附等温线模型 n KF R2 qm/(mg·g−1) KL R2 25 3.155 3.687 0.899 7 6.169 1.416 0.970 3 35 5.397 4.395 0.953 7 6.274 8.810 0.989 6 45 6.974 5.248 0.968 7 6.329 23.939 0.990 7 -
[1] 刘兆民, 李耸耸, 黄贝贝, 等. 零价铁去除饮水中砷As(III)性能的研究[J]. 化工中间体, 2015,17(1): 34-36. DOI: CNKI:SUN:ZJTY.0.2015-01-017. [2] 彭映林, 肖斌 . 两级中和-铁盐沉淀法处理高砷废水[J]. 工业水处理, 2016, 36(6): 64-68. DOI: CNKI:SUN:GYSC.0.2016-06-016. [3] LUO Q, CHENG L, ZHANG M, et al. Comparison and characterization of polyacrylonitrile, polyvinyliden fluoride, and polyvinyl chloride composites functionalized with ferric hydroxide for removing arsenic from water[J]. Environmental Technology & Innovation, 2021, 24: 101927. DOI: 10.1016/j.eti.2021.101927. [4] 肖静, 田凯勋, 高怡. 载铁活性炭吸附剂的制备及除砷(Ⅲ)性能研究[J]. 工业水处理, 2012, 32(11): 28-32. doi: 10.3969/j.issn.1005-829X.2012.11.008 [5] 公绪金, 董玉奇, 李伟光. 原位载铁中孔活性炭吸附As和天然有机物效能[J]. 中国环境科学, 2019, 39(9): 3857-3865. doi: 10.3969/j.issn.1000-6923.2019.09.031 [6] 宋志莲. 铁锰氧化物/煅烧牡蛎壳复合吸附剂对水中As(Ⅲ)的吸附性能及机理[D]. 大连: 大连理工大学, 2021. [7] ZHANG Q L, LIN Y C, CHEN X, et al. A method for preparing ferric activated carbon composites adsorbents to remove arsenic from drinking water[J]. Journal of Hazardous Materials, 2007, 148(3): 671-678. doi: 10.1016/j.jhazmat.2007.03.026 [8] 陈苹. 载铁活性炭吸附材料的制备及对水中砷(Ⅴ)的吸附研究[D]. 南昌: 江西理工大学, 2015. [9] 朱慧杰, 贾永锋, 吴星, 等. 负载型纳米铁吸附剂去除饮用水中As(Ⅲ)的研究[J]. 环境科学, 2009, 30(6): 1644-1648. doi: 10.3321/j.issn:0250-3301.2009.06.014 [10] NIETO-DELGADO C, RANGEL-MENDEZ J R. Anchorage of iron hydro(oxide) nanoparticles onto activated carbon to remove As(V) from water[J]. Water Research, 2012, 46(9): 2973-2982. doi: 10.1016/j.watres.2012.03.026 [11] 罗成. 载铁活性炭的制备及对水中三价砷的吸附研究[D]. 哈尔滨: 哈尔滨工业大学, 2019. [12] 肖静. 载铁活性炭吸附剂的制备及除砷机理研究[D]. 湘潭: 湘潭大学, 2013. [13] 刘前进. 高铁酸钾的制备及其联合MIEX树脂去除水源中富里酸的特性研究[D]. 马鞍山: 安徽工业大学, 2016. [14] 周娟娟, 李战军. 活性炭/纳米零价铁复合吸附剂的制备及对砷的去除应用[J]. 环境科学与管理, 2012,037(010):106-108. DOI: 10. 3969/j.issn.1673-1212.2012.10.027 [15] EGBOSIUBA T C, EGWUNYENGA M C, TIJANI J O, et al. Activated multi-walled carbon nanotubes decorated with zero valent nickel nanoparticles for arsenic, cadmium and lead adsorption from wastewater in a batch and continuous flow modes[J]. Journal of Hazardous Materials, 2022,423:126993. DOI: 10.1016/j.jhazmat.2021.126993 [16] ZHANG Y H, WANG Y C, ZHANG H H, et al. Recycling spent lithium-ion battery as adsorbents to remove aqueous heavy metals: Adsorption kinetics, isotherms, and regeneration assessment[J]. Resources, Conservation & Recycling, 2020,156:104688. DOI: 10.1016/j.resconrec.2020.104688 [17] 夏新星, 马腾, 王志强, 等. 载铁活性炭烧结滤芯的制备及其除砷性能[J]. 环境工程学报, 2019, 13(07): 1534-1540. DOI: 10.12030/j.cjee.201809199. [18] 王正芳. 载铁活性炭的制备及对P(V)的吸附性能研究[D]. 南京: 南京大学, 2011. [19] NASSEH N, KHOSRAVI R, RUMMAN G A, et al. Adsorption of Cr(VI) ions onto powdered activated carbon synthesized from Peganum harmala seeds by ultrasonic waves activation[J]. Environmental Technology &Innovation, 2021, 21: 101277. DOI: 10.1016/j.eti.2020.101277. [20] KAUR J, KAUR M, UBHI M K, et al. Composition optimization of activated carbon-iron oxide nanocomposite for effective removal of Cr(VI)ions[J]. Materials Chemistry and Physics, 2021, 258: 124002. doi: 10.1016/j.matchemphys.2020.124002 [21] FIERRO V, MUÑIZ G, GONZALEZ-SÁNCHEZ G, et al. Arsenic removal by iron-doped activated carbons prepared by ferric chloride forced hydrolysis[J]. Journal of Hazardous Materials, 2009, 168(1): 430-437. doi: 10.1016/j.jhazmat.2009,168(1):430-437. [22] SHI-QI TIAN, L W. Degradation of organic pollutants by ferratebiochar Enhanced formation of strong intermediate oxidative iron species[J]. Water Research, 2020 , 183 : 116054. DOI: 10.1016/j.watres.2020.116054. [23] LIU A, WANG W, LIU J, et al. Nanoencapsulation of arsenate with nanoscale zero-valent iron (nZVI): A 3D perspective[J]. Science Bulletin, 2018, 63(24): 1641-1648. doi: 10.1016/j.scib.2018.12.002 -




 DownLoad:
DownLoad: