-
噻虫胺(Clothianidin,CLO)是第2代新烟碱类杀虫剂,与吡虫啉、噻虫嗪并列为3大最广谱的新烟碱类农药,其占新烟碱类农药使用量的14.3%,具有广谱、触杀、胃杀和內吸性等特点,可用于控制半翅目、鞘翅目和某些鳞翅目等害虫[1-3]。从20世纪90年代进入市场以来,新烟碱类杀虫剂的生产量、销售量和使用量持续增长,并在环境水体中被广泛检出且具有较高存在水平[4]。2016年珠江流域的3条主干河流水样检测结果显示噻虫嗪、噻虫胺和啶虫脒等3种新烟碱类杀虫剂检出率为100%[5]。2019年我国地下水中检出噻虫胺的最大质量浓度达到0.137 μg·L−1[6]。残留的新烟碱类杀虫剂会对环境生物产生毒害作用,研究表明当花粉中噻虫胺等新烟碱类杀虫剂达到亚致死剂量时会对蜜蜂等授粉类昆虫生存造成多方面困难,包括掌握花卉特性、导航以及觅食等[7]。因此,如何有效去除环境中残留的新烟碱类杀虫剂成为环境污染与修复领域亟待解决的问题之一。
生物炭是生物质(包括农业残留物、富含生物质的动植物、粪肥、污泥等)在厌氧或缺氧的条件下热解和碳化后产生的高度芳香化物质,具有比表面积大、孔隙发达及含氧官能团丰富等优点[8-9]。生物炭能够通过π-π电子供体-受体相互作用、静电吸附作用、氢键作用、疏水分配和孔填充效应等方式吸附去除包括抗生素[10-11]、农药[12-13]、苯系物[14-15]等污染物。目前,越来越多的研究开始通过物理、化学和生物等方法对生物炭进行改性,以进一步提高其对污染物的去除能力[16-20]。
化学改性是目前最常用的改性方法,通常包括酸碱改性法、氧化还原改性法和高分子材料改性法[21]。高锰酸钾具有强氧化性,且能够分解生成价态丰富的锰氧化物,能够对生物炭起到良好的改性效果。一方面能够通过氧化作用对生物炭的比表面积、孔隙结构、官能团等理化性质产生影响,另一方面其分解生成的锰氧化物具有较高的比表面积、较强的吸附和氧化能力以及在酸性和中性条件下稳定存在的优点,负载在生物炭上可提高污染物去除效率[22-23]。杉木是一种栽培广、生长快的树种,具有良好的生态和经济价值,本研究以杉木原木为原料,在700 ℃条件下烧制得到杉木生物炭,通过高锰酸钾在高温条件下改性生物炭,考察了改性后生物炭对水中噻虫胺的吸附性能及机理,为新烟碱类杀虫剂环境污染修复提供参考。
-
噻虫胺(C6H8ClN5O2S,纯度大于97.8%)购自上海阿拉丁生化科技股份有限公司,解离常数pKa=11.1,土壤半衰期24.3~26.4 d[24]。NaNO3为分析纯,购自无锡市晶科化工有限公司,Ca(NO3)·4H2O为分析纯,购自上海阿拉丁生化科技股份有限公司,HNO3为分析纯,购自无锡市展望化工试剂有限公司,NaOH为分析纯,购自上海泰坦科技股份有限公司,KMnO4为分析纯,购自无锡市展望化工试剂有限公司,实验用水均为超纯水,电导率为18.2 MΩ·cm。
-
高效液相色谱仪(e2695,美国Waters),用于CLO浓度定量分析;X射线衍射仪(XRD-6100,日本岛津),用于改性后生物炭表面锰氧化物晶型分析;扫描电子显微镜(SU8100,日本Hitachi),用于观察生物炭的表面形貌;傅里叶红外光谱仪(Cary660,美国Agilent),用于分析样品表面官能团;全自动比表面和孔隙度测定仪(Mini Ⅱ,日本Belsorp),用于测定生物炭的比表面积及孔隙结构;X射线光电子能谱仪(K-Alpha+,美国Thermo)用于分析MnOx-BC中Mn价态;X射线衍射(X´Pert PRO MPD,荷兰PANalytical B.V.),用于分析MnOx-BC中MnOx晶型。
-
1)杉木生物炭制备。将杉木切割成方形薄块(长×宽×高为3 cm×2 cm×0.5 cm),每个小块用锡箔纸包裹,放入150 mL石英坩埚内,置于马弗炉中,于700 ℃条件下高温热解6 h,炉内自然冷却后取出,研磨,过100目筛,封装备用。此生物炭标记为BC。
2)改性生物炭制备。采用LI等[25]的方法,在50 mL的刚玉坩埚中分别加入5 g BC,0.5 g高锰酸钾和40 mL超纯水,用玻璃棒搅拌均匀后,置于超声波清洗仪中超声2 h,80 ℃下烘干,将烘干的样品置于马弗炉中以20 ℃·min−1的升温速率加热到700 ℃,保持0.5 h后冷却至室温,取出样品,经反复真空抽滤后,真空冷冻干燥60 h,研磨过100目筛,装入自封袋中备用,将上述样品分别标记为MnOx-BC。
-
生物炭的零电荷点(pHpzc)采用WU等[26]的方法测定。具体方法为:实验在250 mL的具塞锥形瓶中进行,反应总体积为100 mL,首先,向具塞锥形瓶中加入1 mol·L−1的NaNO3溶液1 mL用于控制离子强度,然后,通过1 、0.1和0.01 mol·L−1的NaOH和HCl,依次将溶液的pH调节为3~11,加入30 mg生物炭,向具塞锥形瓶中通入0.8 L·min−1的N2,最后,将配好的溶液置于组合式恒温摇床中,摇床转速为200 r·min−1,经48 h后测定最终pH,ΔpH为最终pH与初始pH的差值,以初始pH与ΔpH作图,ΔpH=0时,即为生物炭的pHpzc。
-
实验在250 mL的具塞锥形瓶中进行,反应总体积为100 mL,噻虫胺母液质量浓度为100 mg·L−1,使用0.01 mol·L−1 HCl和NaOH溶液调节溶液pH,使用1.0 mol·L−1 NaNO3溶液调节离子强度。吸附反应在组合式恒温摇床中进行,摇床转速为200 r·min−1。采用5 mL的针筒注射器取样,经0.45 μm的混合纤维素滤膜过滤后,使用高效液相色谱检测溶液中剩余污染物浓度。
在进行吸附动力学实验时,溶液初始pH为5.0,温度为25 ℃,噻虫胺质量浓度初值为10 mg·L−1,生物炭添加量为0.3 g·L−1,取样时间分别为0.25、0.5、1、2、4、6、8、12、24 h。测定各时间点时溶液中剩余污染物浓度,用准一级动力学模型和准二级动力学模型对实验数据进行拟合,取3个平行组的实验结果平均值。
在进行等温吸附实验时,噻虫胺的质量浓度设为3.0、5.0、8.0、10、15、18、20 mg·L−1,生物炭添加浓度为0.3 g·L−1,温度分别为15、25和35 ℃。吸附24 h后取样,测定溶液中噻虫胺浓度,用Langmuir和Freundlich模型拟合实验数据。
在考察初始pH和离子强度对吸附的影响时,本实验使用1、0.1和0.01 mol·L−1 HCl和NaOH分别将噻虫胺溶液初始pH调节为3.0、5.0、7.0、9.0、11.0,以考察初始pH对吸附效果的影响。用1.0 mol·L−1的NaNO3和Ca(NO3)2调节溶液中金属离子质量浓度分别为0、0.01、0.1和0.5 mol·L−1,以考察离子强度对吸附效果的影响,其余条件与吸附动力学实验一致。
-
噻虫胺采用高效液相色谱检测,吸收波长为265 nm,色谱柱为XBridge@C18(250 mm × 4.6 mm,5 μm),柱温为30 ℃,流动相甲醇与水的体积比为45∶55。生物炭对噻虫胺的去除率(R)和吸附量(qe)分别根据式(1)和式(2)计算;采用准一级动力学方程(式(3))、准二级动力学方程(式(4))和内部扩散方程(式(5))对吸附动力学过程进行拟合;采用Langmuir(式(6))和Freundlich(式(7))吸附模型对吸附过程进行拟合。
式中:R为生物炭对噻虫胺的去除率;qe为生物炭平衡吸附量,mg·g−1;C0和Ce分别为噻虫胺的初始质量浓度和平衡质量浓度,mg·L−1; V为反应溶液体积,L;m为生物炭添加量,g。
式中:qt和qe分别为t时刻和吸附平衡时噻虫胺吸附量,mg·g−1;k1为准一级吸附动力学速率常数,min−1;k2为准二级吸附动力学速率常数,g·(mg·min)−1;k3为颗粒内扩散速率常数,mg·(g·min1/2)−1。
式中:qe和qm分别为平衡吸附量和理论最大吸附量,mg·g−1;Ce为吸附平衡质量浓度,mg·L−1;KF和KL分别是Langmuir和Freundlich方程的相关系数,1/n为Freundlich的经验系数。
-
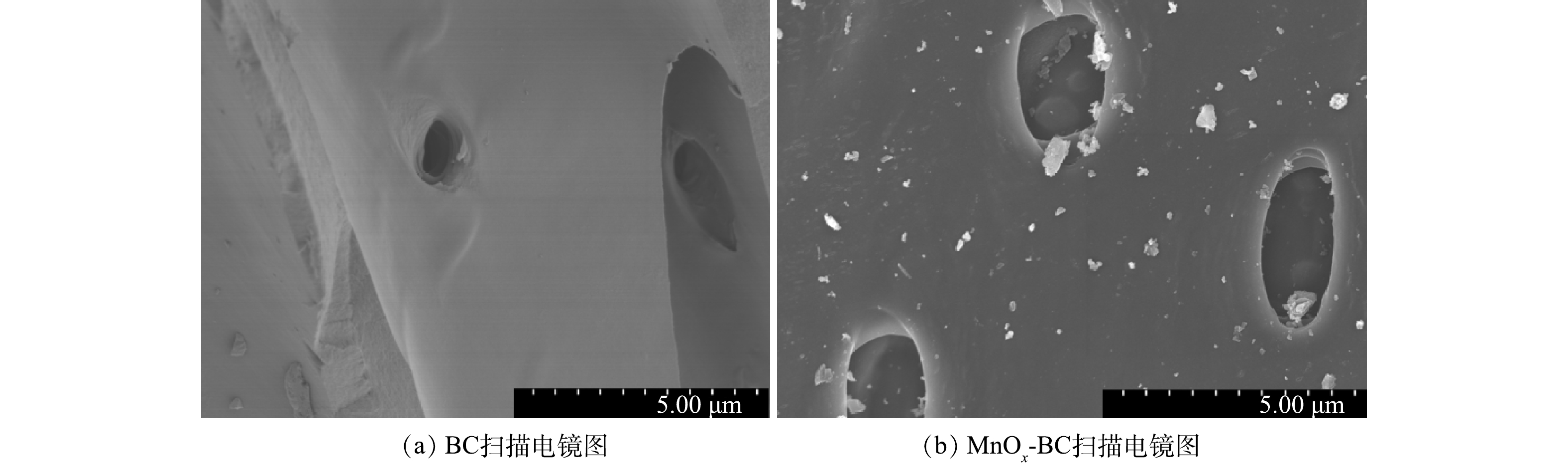
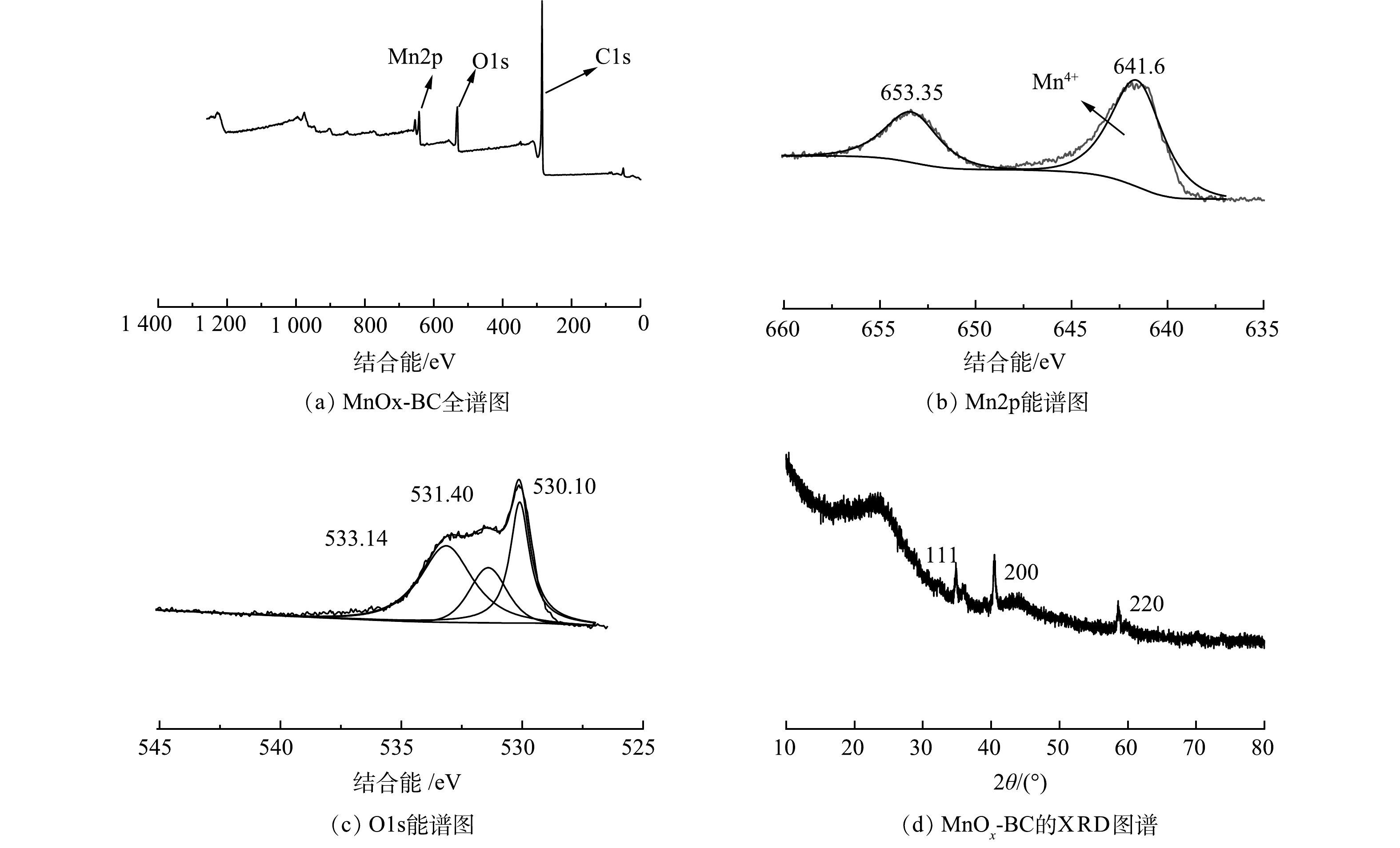
图1为BC和MnOx-BC的扫描电镜图。可见,BC和MnOx-BC表面形貌并无明显差异,生物炭表面均有一些圆孔结构。这可能是因杉木组分中的纤维素等在缺氧条件下分解为有机气体在高温条件下挥发溢出所致[27]。MnOx-BC表面出现了许多颗粒物,尤其在圆孔结构内颗粒更多,可能是高锰酸钾改性后生物炭表面生成的含锰化合物。通过抗坏血酸溶解和微波消解处理高锰酸钾改性生物炭,测得改性后生物炭中Mn的质量分数达到4.09%。
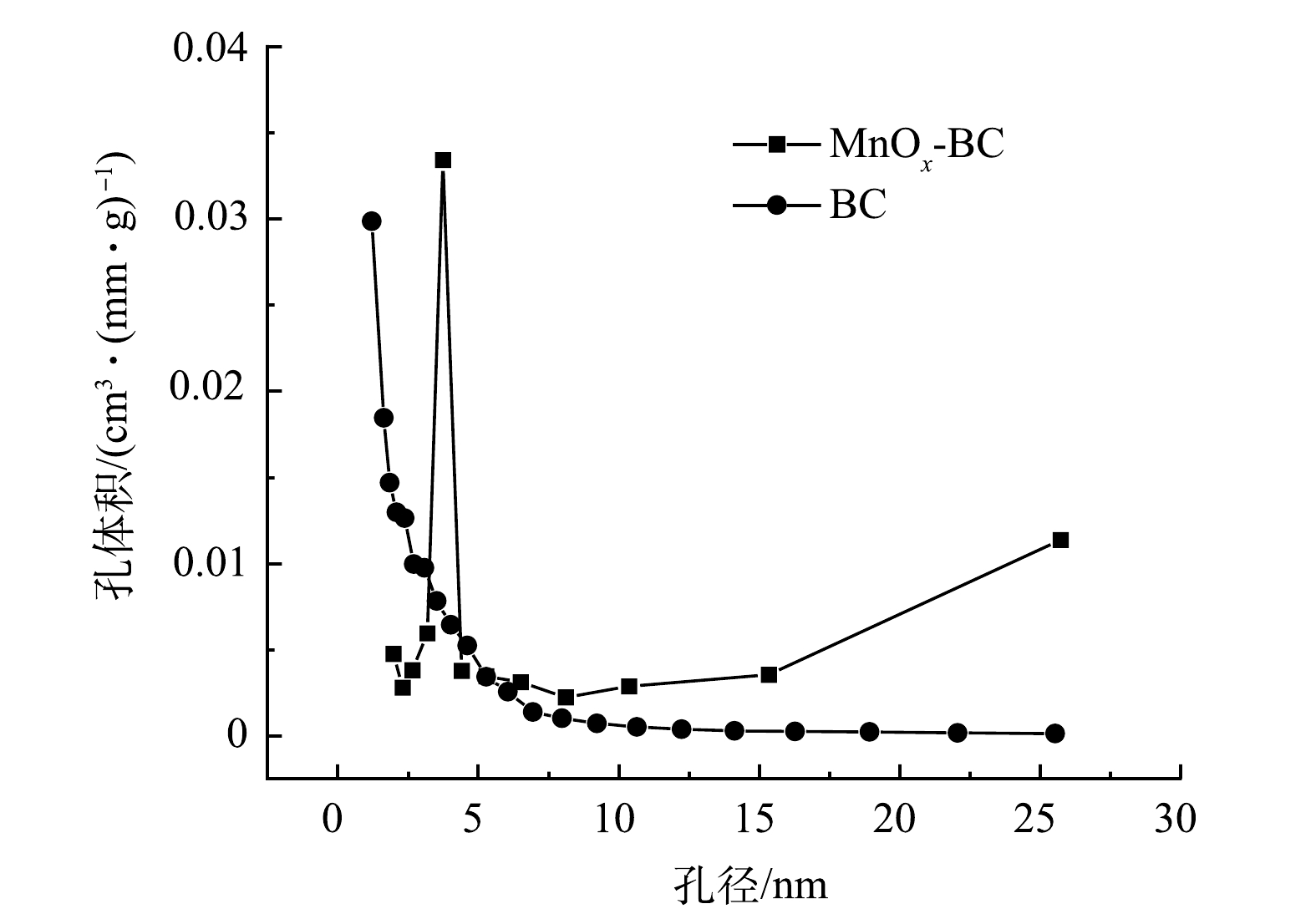
对改性前后生物炭的比表面积和孔隙结构进行表征分析,结果见图2和表1。经过高锰酸钾改性后生物炭比表面积、孔体积和平均孔径均得到一定程度的提升,比表面积和孔体积分别较原始生物炭提高了12.08%和12.33%。改性生物炭比表面积提高可能是因为高锰酸钾活化了杉木生物炭的表面以及负载于生物炭表面的MnOx本身具有一定的比表面积所致[28]。改性后生物炭的零电荷点有所升高,表明改性后生物炭表面可带有更多的负电荷,这种变化则更有利于其对噻虫胺的吸附。
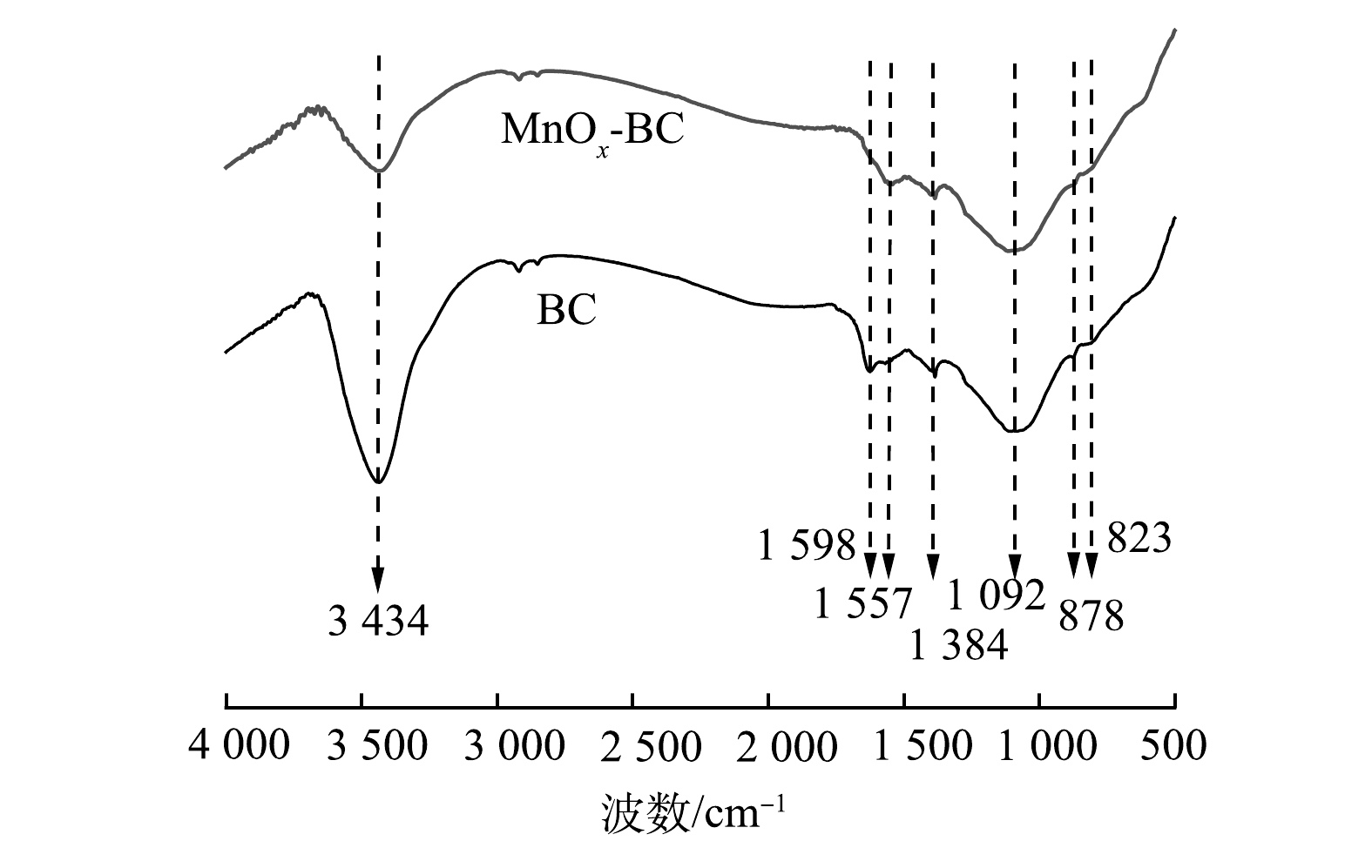
图3为BC和MnOx-BC的傅里叶红外光谱图。可以看出,BC和MnOx-BC具有许多相似的特征峰。3 434 cm−1处的宽峰是酚羟基(—OH)的伸缩振动峰,1 598 cm−1处的吸收峰是芳香环中的C=O伸缩振动峰,1 384和1 092 cm−1处的吸收峰可以归因于羧基(—OH)的弯曲模式和C—O拉伸振动,878 cm−1和823 cm−1附近的吸收峰主要是由于C—H伸缩振动引起的[29-32]。BC和MnOx-BC差异较大的是改性后生物炭在1 520 cm−1处出现了不饱和C=C伸缩振动特征峰。此外,在500~800 cm−1内没有发现明显的Mn—O振动峰,这可能与负载量相关。
采用X-射线光电子能谱(XPS)对MnOx-BC表面的Mn元素进行价态分析,采用X射线衍射(XRD)对MnOx-BC的晶体结构进行分析。由图4(a)可知,MnOx-BC主要含有的元素为C、O和Mn。分别对Mn和O的谱图进行分峰拟合,结果表明,对MnOx-BC的Mn2p能谱图拟合出现了2个峰,其中结合能在653.35 eV和641.60 eV处的谱峰属于Mn2p1/2和Mn2p3/2,Mn2p1/2与Mn2p3/2之间的间距为11.75 eV,表明在MnOx中有Mn4+的存在[33]。O1s能谱图中在530.10、531.40和533.14 eV处出现了3个特征峰,表明MnOx-BC存在着MnO2、金属锰氧化物(Mn-O)以及羟基(-OH)[34]。由图4(d)可见,整体上X射线衍射图谱衍射峰不尖锐,表面改性后生成的锰氧化物的结晶度差,属于无定型锰氧化物。MnOx-BC在34.9°、40.5°和58.7°处出现衍射峰,与γ-MnO2的衍射峰位置最为接近[35]。
-
BC和MnOx-BC对噻虫胺的吸附动力学如图5所示。由图5可见,BC和MnOx-BC对溶液中的噻虫胺呈现出相近的吸附动力学过程。BC和MnOx-BC对噻虫胺的吸附量在短时间内(30 min)随着时间的推移而迅速增大,之后伴随吸附增速减缓过程,8 h后,吸附量趋于稳定。此现象与生物炭表面的活性位点有限性有关,吸附初始阶段,生物炭表面的活性位点充分,目标污染物能够迅速占据活性位点而呈现出吸附量的急速上升。随着吸附的继续进行,可供吸附的活性位点逐渐减少,吸附速率开始下降,最终,吸附趋于平衡。此现象与已有研究结果相类似[36]。
为了更好的评价2种生物炭的吸附特性及其速率控制步骤和机理,采用准一级和准二级动力学方程对BC和MnOx-BC吸附噻虫胺的动力学过程进行拟合,结果见表2。BC和MnOx-BC对噻虫胺的准二级动力学拟合R2(0.944和0.964),高于准一级动力学拟合的R2(0.877和0.900),且准二级动力学拟合的平衡吸附量较准一级更加接近于实验值。这说明MnOx-BC和BC对CLO的吸附过程受到2种以上因素共同影响[37]。
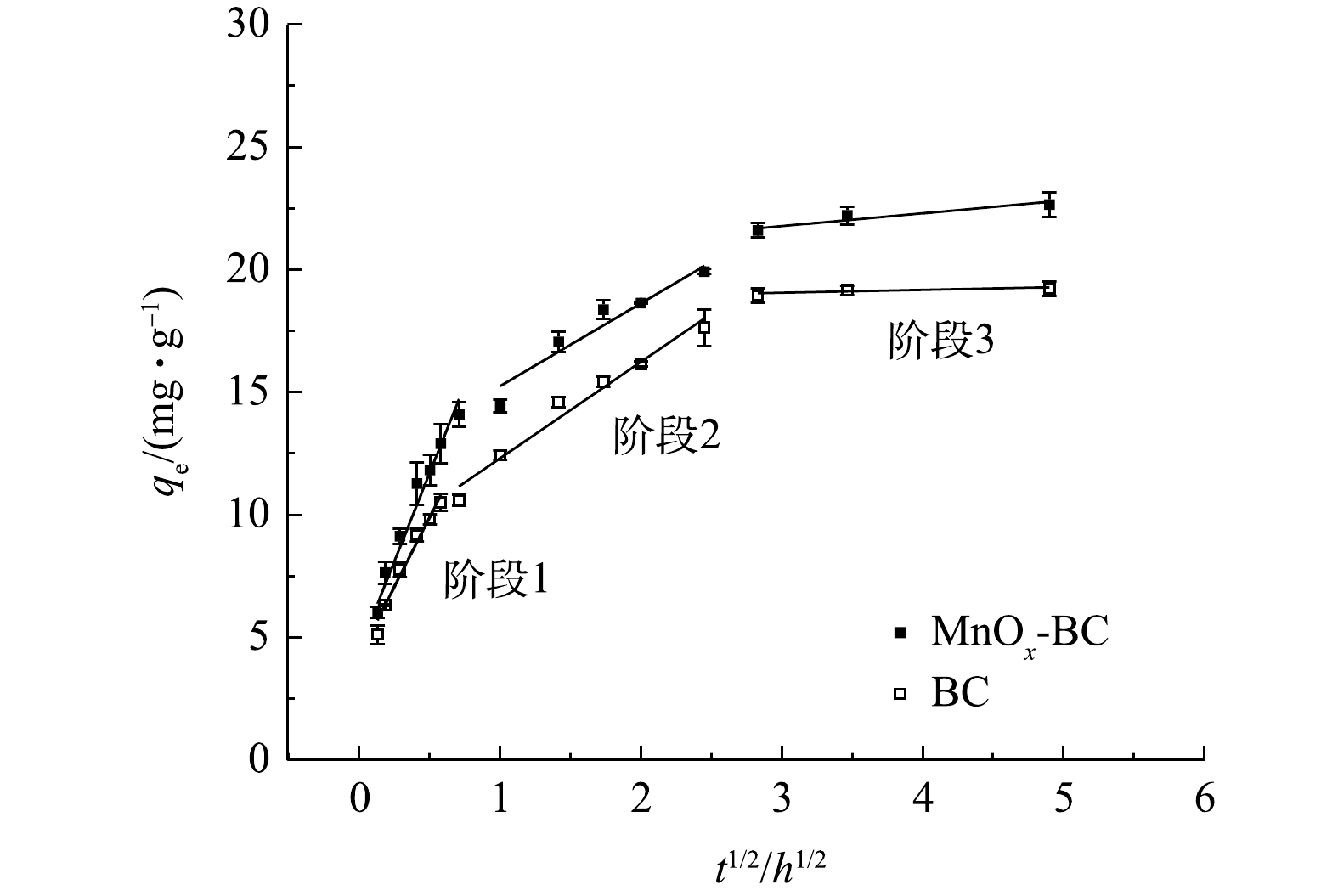
颗粒内扩散模型反映了吸附过程中的实际控速步骤和吸附机理[38]。由于实验条件及吸附材料和目标污染物的理化性质不同,吸附过程通常可分为阶段2和阶段3[39-40]。阶段2吸附机理为污染物通过液膜扩散到吸附剂表面和污染物在吸附剂表面发生吸附的2个过程。阶段3吸附机理为污染物跨水膜扩散到吸附剂表面、污染物在吸附剂孔隙内扩散和吸附趋平衡的3个过程。本研究中颗粒内扩散拟合结果见图6和表3。如图6所示,BC和MnOx-BC对噻虫胺的吸附过程存在明显的3个阶段,分别为污染物跨水膜扩散到吸附剂表面,污染物在吸附剂孔隙内扩散和吸附趋平衡3个过程。对比表3中的k值,每种生物炭吸附拟合斜率大小均为k1 > k2 > k3,说明污染物向吸附剂的扩散是速率逐渐减小直至达到吸附平衡的过程。BC和MnOx-BC阶段3的模型拟合程度均较低,此外,各阶段的拟合曲线均未经过原点,说明颗粒内扩散不是唯一的速率控制步骤。这与前述准二级动力学拟合结果一致,其他研究中也有类似结果[41]。本研究结果表明,BC和MnOx-BC对噻虫胺的吸附过程可能存在着表面吸附和孔隙填充作用。
-
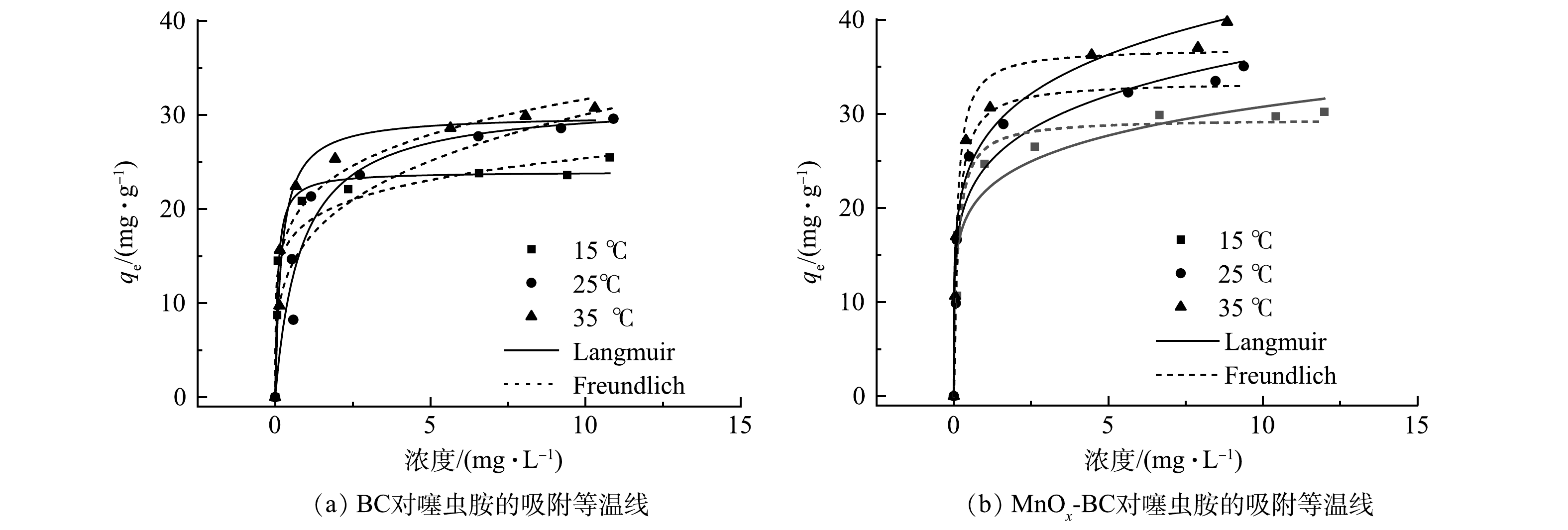
为了进一步了解BC和MnOx-BC对噻虫胺吸附容量和吸附机理,分别采用Langmuir和Freundlich吸附模型对数据进行拟合,结果见图7和表4。总体上,随着噻虫胺初始质量浓度的增加,BC和MnOx-BC的吸附量均呈现出先增加后稳定的现象。此外,随着温度的上升,BC和MnOx-BC的吸附量也同样有所增加,说明该吸附过程为吸热过程。Langmuir模型拟合的相关系数明显高于Freundlich,说明2种生物炭对噻虫胺的吸附属于单分子层吸附,温度对杀虫剂在BC和MnOx-BC上的吸附形式没有影响。使用Langmuir模型拟合计算出BC和MnOx-BC对噻虫胺的理论最大吸附容量分别为31.56 mg·g−1和37.00 mg·g−1。
-
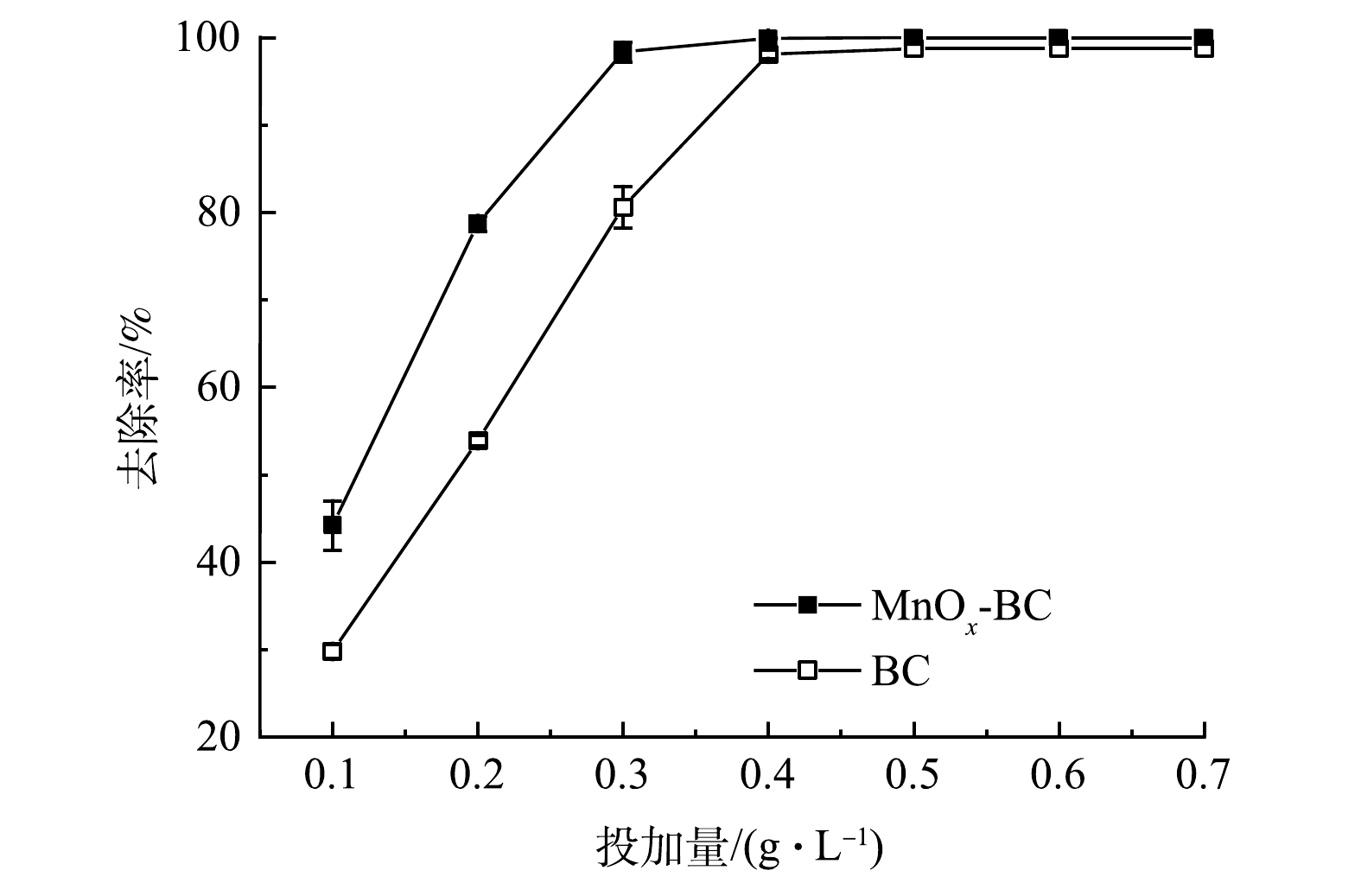
由图8可见,随着吸附剂投加量的增加,BC和MnOx-BC对噻虫胺的去除率均呈现出先增加后趋于平缓的趋势,在相同投加量下,MnOx-BC比BC有更好污染物去除效果。当MnOx-BC的投加量达到0.3 g·L−1时,噻虫胺的去除率达到98.36%,而达到相近的噻虫胺去除率,BC的投加量需要0.4 g·L−1。
-
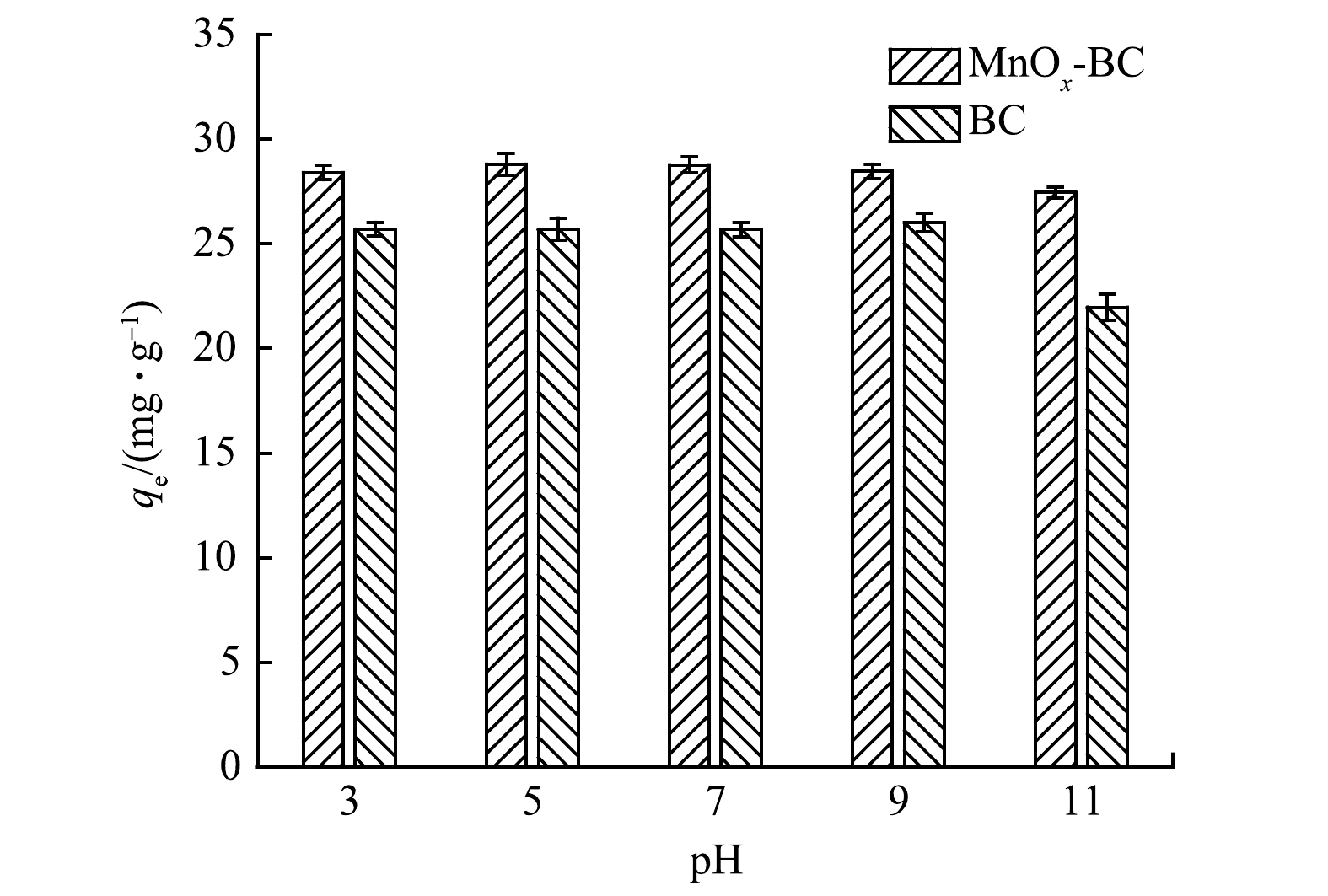
溶液pH是影响吸附效果的重要因素之一,影响目标污染物的解离程度和生物炭的表面电荷[42]。初始pH对BC和MnOx-BC吸附噻虫胺的影响如图9所示。由图9可以看出,当pH为3.0~9.0时,2种生物炭的吸附量未发生明显变化。这说明2种生物炭均能在较宽的pH范围内对新烟碱类杀虫剂具有良好的吸附效果。当初始pH为11时,BC和MnOx-BC对噻虫胺的吸附量均有所下降。这可能是由于碱性条件钝化了生物炭表面的活性位点和减弱了质子化的BC和MnOx-BC噻虫胺分子间的氢键作用[43-44]。此外,BC吸附量的下降幅度大于MnOx-BC,分别为15.4%和4.5%,说明改性后生物炭比原始生物炭有更好的吸附稳定性。
-
溶液离子强度对BC和MnOx-BC吸附噻虫胺的影响如图10所示。随着Na+和Ca2+浓度的增加,BC和MnOx-BC的吸附容量均有所下降。当Na+质量浓度由0增加到0.5 mol·L−1时,BC和MnOx-BC对噻虫胺的吸附量分别下降了和8.18%和5.49%。Ca2+质量浓度由0增加到0.5 mol·L−1时,BC和MnOx-BC对噻虫胺的吸附量分别下降了18.11%和13.65%。由此可知,Na+和Ca2+的添加对噻虫胺的吸附均有一定的抑制作用,且Ca2+的抑制作用大于Na+,这可能与2种离子的电荷量和电负性有关。一方面,相同浓度的Ca2+呈现的离子强度比Na+更高;另一方面,Ca2+的电负性(1.00)大于Na+(0.93),具有更强的水合能力,能更有效地占据生物炭表面的活性位点[45-46]。
-
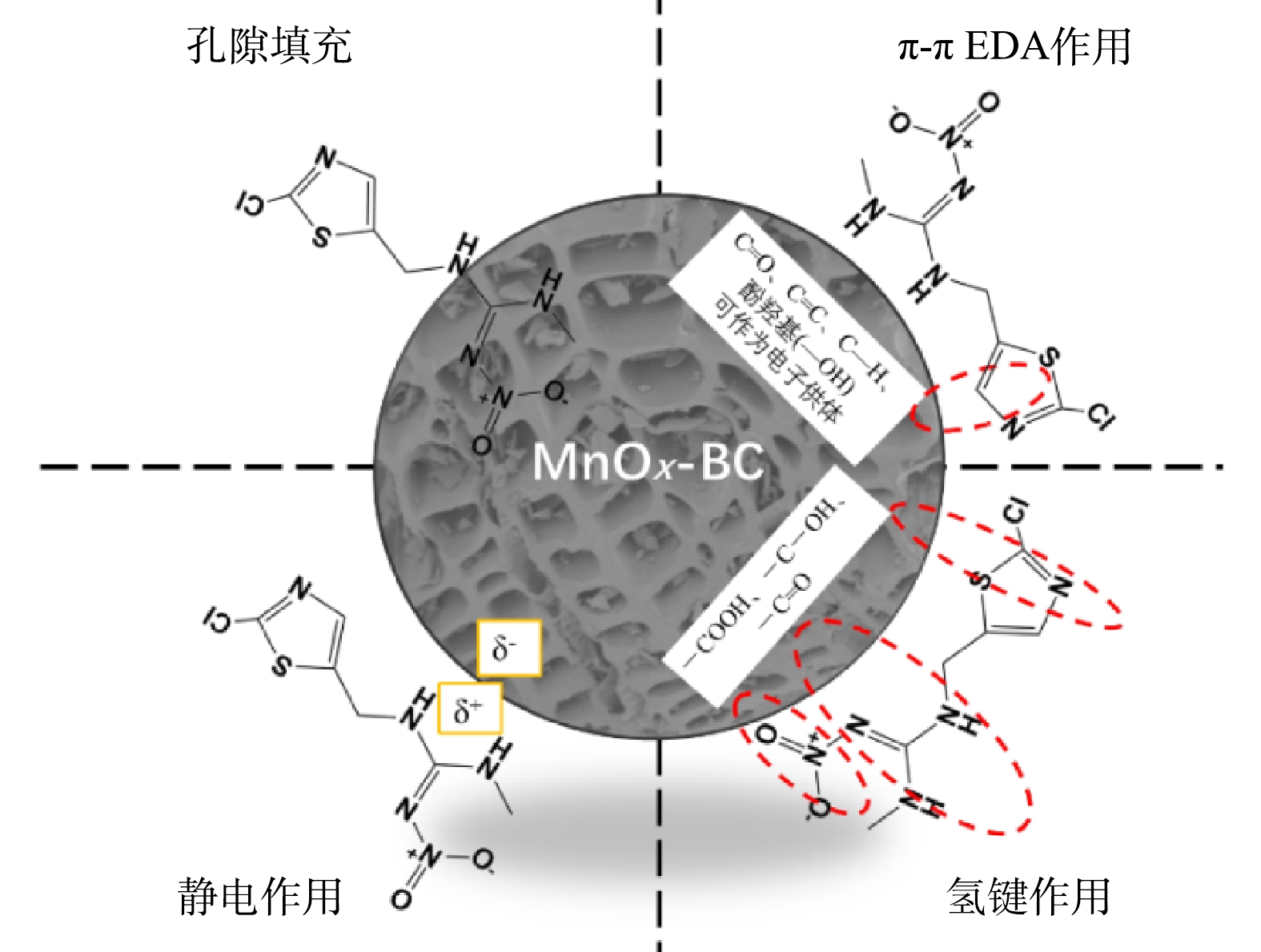
1)孔隙填充作用。孔隙填充作用主要由炭质材料的孔隙结构特性所决定[47]。由图2和表1可知,BC和MnOx-BC孔隙结构主要为中孔结构。通过颗粒内扩散方程拟合图7中颗粒内扩散的阶段2可知,噻虫胺在MnOx-BC孔隙内都得到一定量的富集。ZHU等[48]发现了比表面积和孔隙结构是影响生物炭吸附有机物的重要因素,并提出了孔填充机制。ZHAO等[49]也报道了孔隙填充作用在花生壳生物炭吸附吡虫啉中起到重要作用。基于此,孔隙填充作用是MnOx-BC能够吸附噻虫胺的重要机制之一。
2) π-π电子供体-受体(EDA)相互作用。噻虫胺的分子结构中含有芳香环π系统,使噻虫胺的π电子密度较低;苯杂环中的N、S原子的强吸电子作用会使得芳香环π系统的π电子密度进一步降低,使得噻虫胺可作为很强的π-电子受体;此外,由于噻虫胺分子中的氨基官能团可以将其孤立电子对给予芳香环,使得氨基官能团也可做为π-电子受体[44]。由图3可知,BC和MnOx-BC均具有C=O、C=C、C—H、羧基、酚羟基等官能团,其中C=O、C=C、C―H、酚羟基等芳香性官能团均可作为电子供体与噻虫胺形成π-π电子供体-受体相互作用[50-51]。
3)氢键作用。通过FT-IR分析可知,MnOx-BC表面均具有羧基(―COOH)、酚羟基(―OH)和羰基(C=O)等含氧官能团,这些官能团均能与噻虫胺分子中O和N原子容易形成氢键作用,促进MnOx-BC对噻虫胺的吸附。
4)静电作用。在本研究的初始pH范围内,噻虫胺以阳离子的形式存在于水溶液中。由表1可知,MnOx-BC的pHPZC=7.22,当平衡pH<pHPZC,MnOx-BC表面带正电,此时,MnOx-BC与噻虫胺并非表现为静电排斥,而是以阳离子-π相互作用发生吸附;当平衡pH>pHPZC时,MnOx-BC表面带负电,此时,MnOx-BC与噻虫胺之间可发生静电吸附作用。由图10可见,Na+和Ca2+对吸附过程呈现出的抑制作用也可以说明MnOx-BC对噻虫胺的吸附过程中存在静电吸附作用[52]。
综上所述,MnOx-BC对水溶液中噻虫胺的吸附机制主要包括孔隙填充、π-π电子供体-受体相互作用、氢键作用和静电作用,如图11所示。
-
1)高锰酸钾改性杉木生物炭,在生物炭表面负载晶型结构差的锰氧化物,改性后生物炭的孔隙更加丰富、比表面积更大,pHpzc更高,吸附性能得到进一步提高,使用Langmuir模型拟合得到MnOx-BC对噻虫胺的理论最大吸附量达到37 mg·g−1。
2) BC和MnOx-BC对噻虫胺的吸附动力学符合准二级动力学方程,且吸附过程存在3个阶段,依次为污染物跨水膜扩散到吸附剂表面阶段、污染物在吸附剂孔隙内扩散阶段和吸附平衡阶段。Langmuir模型能更好地拟合等温吸附数据,说明吸附过程属于单分子层吸附。MnOx-BC对pH和离子强度的变化表现出更好的吸附稳定性。
3) MnOx-BC对水溶液中噻虫胺的吸附机理主要包括孔隙填充、π-π电子供体-受体相互作用、氢键作用和静电作用。
高锰酸钾改性生物炭对水中噻虫胺吸附性能及机理
Adsorption of clothianidin by potassium permanganate modified biochar in aqueous solution
-
摘要: 以700 ℃热解制备的杉木生物炭(BC)为原料,采用高锰酸钾高温氧化法制备了改性生物炭(MnOx-BC),考察了其对广泛使用的新烟碱类杀虫剂噻虫胺的强化去除及作用机理。结果表明,高锰酸钾改性生物炭对噻虫胺的去除能力较原始生物炭有所提高,Langmuir模型拟合得到MnOx-BC对噻虫胺的最大吸附容量达到37 mg·g−1。MnOx-BC对噻虫胺的吸附动力学符合准二级动力学方程,颗粒内扩散模型拟合显示吸附过程分为3个阶段。MnOx-BC对噻虫胺的吸附过程属于单分子层吸附,不同pH条件下MnOx-BC比BC具有更好的吸附稳定性,共存离子Ca2+对MnOx-BC吸附抑制作用大于Na+。MnOx-BC对噻虫胺的吸附机理主要包括孔隙填充、π-π电子供体-受体相互作用、氢键作用和静电作用。Abstract: In this study, a type of biochar (BC) prepared from Chinese fir pyrolysis at 700℃ was taken as raw material, then the modified biochar (MnOx-BC) was prepared by potassium permanganate oxidation method. The enhanced removal effect and mechanism of clothianidin (CLO) by MnOx-BC were investigated. The results showed that CLO removal ability by potassium permanganate modified biochar was improved compared with the original biochar, and the maximum adsorption capacity obtained from Langmuir model fitting was up to 37 mg·g−1. The kinetics of CLO adsorption by MnOx-BC was in accordance with the quasi-second-order kinetic equation, and the fitting of the diffusion model showed that the adsorption process was divided into three stages. The adsorption process of CLO on MnOx-BC belonged to monolayer adsorption. MnOx-BC had better adsorption stability than BC at different pHs, and the inhibition effect of Ca2+ on MnOx-BC adsorption capacity was greater than that of Na+. The adsorption mechanisms mainly included pore filling, π-π electron donor-acceptor interaction and hydrogen bonding and electrostatic interaction.
-
Key words:
- biochar /
- potassium permanganate /
- modification /
- clothianidin /
- adsorption
-
随着现代工业化的蓬勃发展,大量水资源被用于各行各业,其中大部分作为废水排放,这些废水通常含有大量有机物和复杂的基质[1]。目前生化处理工艺是最主要的处理技术,可以有效去除废水中有机物,然而工业废水中含有许多难降解有机污染物,因此,这些处理工艺的出水通常难以满足环境法规及回用需求[2-4]。因此,亟需开发更先进的方法来高效去除工业废水中的难降解有机污染物。
最近,基于多孔阳极开发的新型反应性电化学膜(reactive electrochemical membrane, REM)氧化技术在去除废水中的难降解有机物方面显示出潜力[5-6]。REM氧化系统不仅保留了传统电化学氧化系统(即采用平行板电极设置,溶液仅在电极之间流动)的优点(如无需添加化学试剂、反应条件温和、易于扩大规模等),还通过依靠其微孔结构压缩边界扩散层,强制废水在多孔阳极微孔中的对流传输从而解决了传统系统受传质限制的问题[7]。同时,废水的对流传输也激活了传统系统中难以接触的内部活性位点[8]。因此,与传统系统相比,REM氧化系统能将污染物的氧化动力学提高几倍到几十倍[9]。而且,除了•OH介导的间接氧化过程外,阳极氧化还存在另一种氧化途径,即直接电子转移(direct electron transfer,DET)过程[7]。因此,与基于•OH的高级氧化技术(如光催化氧化、芬顿氧化等)相比,REM氧化工艺的效率受废水基质的影响较小[10]。同时,通过调节电位降低DET过程的活化能垒,阳极可以有效降解各种难降解污染物,甚至是极稳定的全氟辛基磺酸[11]。
当前的研究已经充分证实REM氧化技术能够高效地处理各种实际废水(如垃圾填埋场渗滤液、反渗透浓缩液等)[6,12]。然而,废水中普遍存在的Cl‒虽然可通过形成活性氯提高处理效率,但Cl‒的过度氧化存在生成氯化副产物(主要指ClO3‒和ClO4‒)的风险[13-14]。这些氯化副产物会危害人体健康,如ClO4‒会损害甲状腺功能[14]。因此,对REM氧化过程中形成的氯化副产物有必要进行严格审查和控制。最近,阳极氧化系统中通常被忽略的阴极反应过程越来越受到重视,许多将阳极氧化(anodic oxidation,AO)和阴极还原(cathodic reduction,CR)耦合的系统已被频繁报道[15]。有研究[16-17]表明,氯化副产物能够在阴极被还原为无毒的Cl‒,从而有效降低处理后废水的生物毒性。因此,可以推测使用合适的电极材料构建将AO和CR耦合的REM系统(AO-CR-REM),处理含氯工业废水可能在保证有机物去除的同时抑制含氯副产物的生成。
基于此,本研究旨在系统地评估AO-CR-REM系统对生化处理过的工业废水的处理效果。首先,选择了2种价格低廉、性能稳定、具备工程化应用的REM阳极材料,即Ti4O7和SnO2-Sb,从有机物去除对其处理效率进行比较,并评估配备筛选过的阳极的REM氧化系统中含氯副产物的生成情况。随后,研究了REM氧化系统对工业废水中有机物的氧化机理。最后,结合筛选的多孔阳极和商业化的多孔RuO2阴极,设计了一个双功能AO-CR-REM系统,评估了其在去除有机物的同时抑制氯化副产物的生成的情况,以期为反应性电化学膜技术在高效、安全地处理高含氯、难处理的工业废水方面的应用提供参考。
1. 材料与方法
1.1 实验材料
本研究中工业废水样品采集自山东某工业园区污水处理站,该工业园区主要包括基础化工原料、农药及其制剂、医药原料、食品添加剂等生产企业。采集的废水为经厌氧工艺和膜生物反应器(membrane bioreactor, MBR)处理后出水,废水中的悬浮颗粒及胶体已被MBR有效拦截。采集的废水水质特征如下:COD值为230 mg·L−1、Cl‒含量和电导率分别高达2 300 mg·L−1和13 000 μS·cm−1、pH为8.2、NH4+-N和NO3‒-N分别为8.5 mg·L−1和9.2 mg·L−1。其中,废水出水的COD值远超过处理规定限值(100 mg·L−1) 。
1.2 REM电极的制备和表征
本研究中使用的REM电极(即Ti4O7和SnO2-Sb)由直径50 mm、厚度2 mm的多孔钛基底制备。SnO2-Sb电极采用溶胶-凝胶法制备,同时制备出SnO2-Sb电极也被用作制备Ti4O7电极的中间体,通过真空等离子喷涂技术进一步负载Ti4O7催化层[9,18-19]。
使用德国Zeiss公司的Sigma 300型扫描电子显微镜(scanning electron microscope,SEM)、日本RigakuD公司的max-3C型X射线衍射仪(X-ray diffractometer,XRD)和Micromeritics公司的AutoPoreIV 9500型的汞孔度计分别对两种电极的表面形态、结晶相和孔隙结构进行表征。采用三电极系统在0.1 mmol·L−1 KH2PO4溶液中以20 mV·s−1的扫描速率进行线性扫描伏安法(linear sweep voltammetry, LSV)和循环伏安法(cyclic voltammetry, CV)测试,获得电极的析氧电位(oxygen evolution potential, OEP)及与电活性位点相关的电流面积。
1.3 实验装置
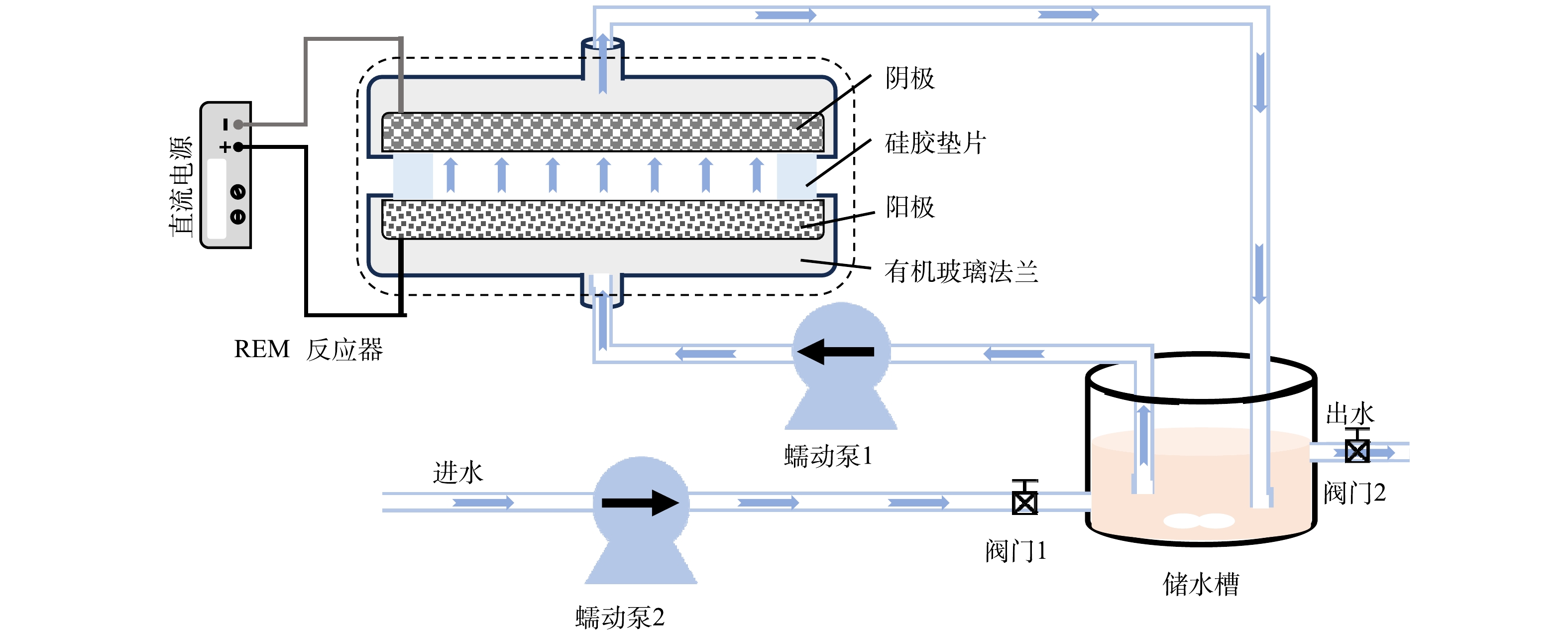
REM反应系统原理图如图1所示,反应系统包括1个REM反应器、1个直流电源、1个储水槽以及2个蠕动泵。其中,REM反应器由阴极、阳极、2个有机玻璃法兰和相应的硅胶垫圈组成。硅胶垫片的厚度、外径和内径分别为6、50和40 mm。制作的多孔Ti4O7和SnO2-Sb REM电极被用作阳极,每个电极的有效表观面积约为12.56 cm2。REM氧化系统运行时,阴极为不锈钢穿孔板,其直径为50 mm,厚度为1 mm。在进行耦合的AO-CR-REM工艺时,阴极为商用的多孔RuO2电极(直径=50 mm,厚度=2 mm)。该反应系统可在循环或连续模式下运行,循环模式下,蠕动泵1驱动废水进入REM反应器中,流经多孔电极后返回储水槽,待测样品从储水槽中获取;连续模式下,保持循环模式继续运行,打开阀门1和2,同时蠕动泵2将原始废水不断送入储水槽,然后在溢流的作用下从出水口流出储水槽,待测样品从出水口获取。
1.4 废水处理实验
收集的工业废水样品首先通过REM氧化工艺在循环模式下进行处理,以评估两种阳极材料(即Ti4O7和SnO2-Sb)去除有机物的效率以及选定的多孔阳极生成氯化副产物的情况。实验使用150 mL工业废水,水循环通量为 150 mL·min−1,电流密度为10~50 mA·cm−2。随后,进一步评估循环模式下AO-CR-REM工艺处理废水的效率以及对氯化副产物抑制情况。实验使用150 mL工业废水,水循环通量为 75~300 mL·min−1。电流密度为20 mA·cm−2和30 mA·cm−2。最后,对AO-CR-REM工艺在连续模式下的应用进行了评估。储水槽中的废水以150 mL·min−1的水循环通量进行循环,原废水以4 mL·min−1的水通量持续注入储水槽,电流密度设定为30 mA·cm−2。
在含有4 mmol·L−1 K4Fe(CN)6、250 μmol·L−1 TPA或2 300 mg·L−1 Cl‒的150 mL溶液中进行模型探针氧化实验,以评估AO-CR-REM工艺在循环模式下去除废水中有机物的机理。溶液的电导率用KH2PO4调节到与收集的工艺废水相同的水平(13 000 μS·cm−1)。实验在水循环通量为150 mL·min−1和电流密度为10~30 mA·cm−2的条件下进行。
1.5 样品分析与数据处理
工业废水中的COD值使用美国Hach公司的DR1900型COD仪测定;ClO3‒和ClO4‒浓度采用美国Thermo Fisher Scientific公司的离子色谱仪测定;活性氯通过美国Hach公司的DPD试剂粉包测定(1407099-CN); NH4+-N和NO3−-N采用国标法测定;K4Fe(CN)6浓度采用紫外可见分光光度计(Hach,DR6000)在420 nm波长下测定;对苯二甲酸(terephthalic acid,TPA)使用配备2489 UV-Vis检测器和XBridge C18柱的高效液相色谱仪进行分析,检测波长为254 nm,柱温保持在35 ℃。流动相为0.1%甲酸溶液和甲醇(40:60),流速设置为1 mL·min−1。
去除有机物的电流效率根据式(1)计算得出,TPA或K4Fe(CN)6氧化的电流效率根据式(2)计算。
stringUtils.convertMath(!{formula.content}) (1) 式中:η为电流效率,%;C0和Ct分别为0和t时的COD值,g·L−1;F为法拉第常数,96 485.3 C·mol−1;V为待处理溶液体积,0.15 L;I为外加电流,A;t为处理时间,h;n=8为氧气当量。
stringUtils.convertMath(!{formula.content}) (2) 式中:C0和Ct分别为0和t时的TPA或K4Fe(CN)6的浓度,mol·L−1;x代表反应中的电子转移次数。参与TPA氧化的•OH是水分子通过单电子转移反应在阳极表面生成的;类似地,K4Fe(CN)6氧化也是单电子转移反应。因此,在计算TPA或K4Fe(CN)6氧化的电流效率时,x为1。根据式(3)计算出TPA或K4Fe(CN)6的氧化动力学速率常数,连续运行模式下处理工业废水的电能消耗根据式(4)或(5)计算。
stringUtils.convertMath(!{formula.content}) (3) 式中:Kobs 为氧化动力学速率常数,m·s−1;A为REM阳极的反应面积,0.125 6×10−2 m2;Cb为K4Fe(CN)6或TPA的质量浓度,mg·L−1。
stringUtils.convertMath(!{formula.content}) (4) stringUtils.convertMath(!{formula.content}) (5) 式中:E为电能消耗,kWh·m−3 或 kWh·g−1(以COD计);U为反应池电压,V;Q为连续废水流量,m3·h−1;Cef为出水中的COD值,mg·L−1。
2. 结果与讨论
2.1 电极材料表征
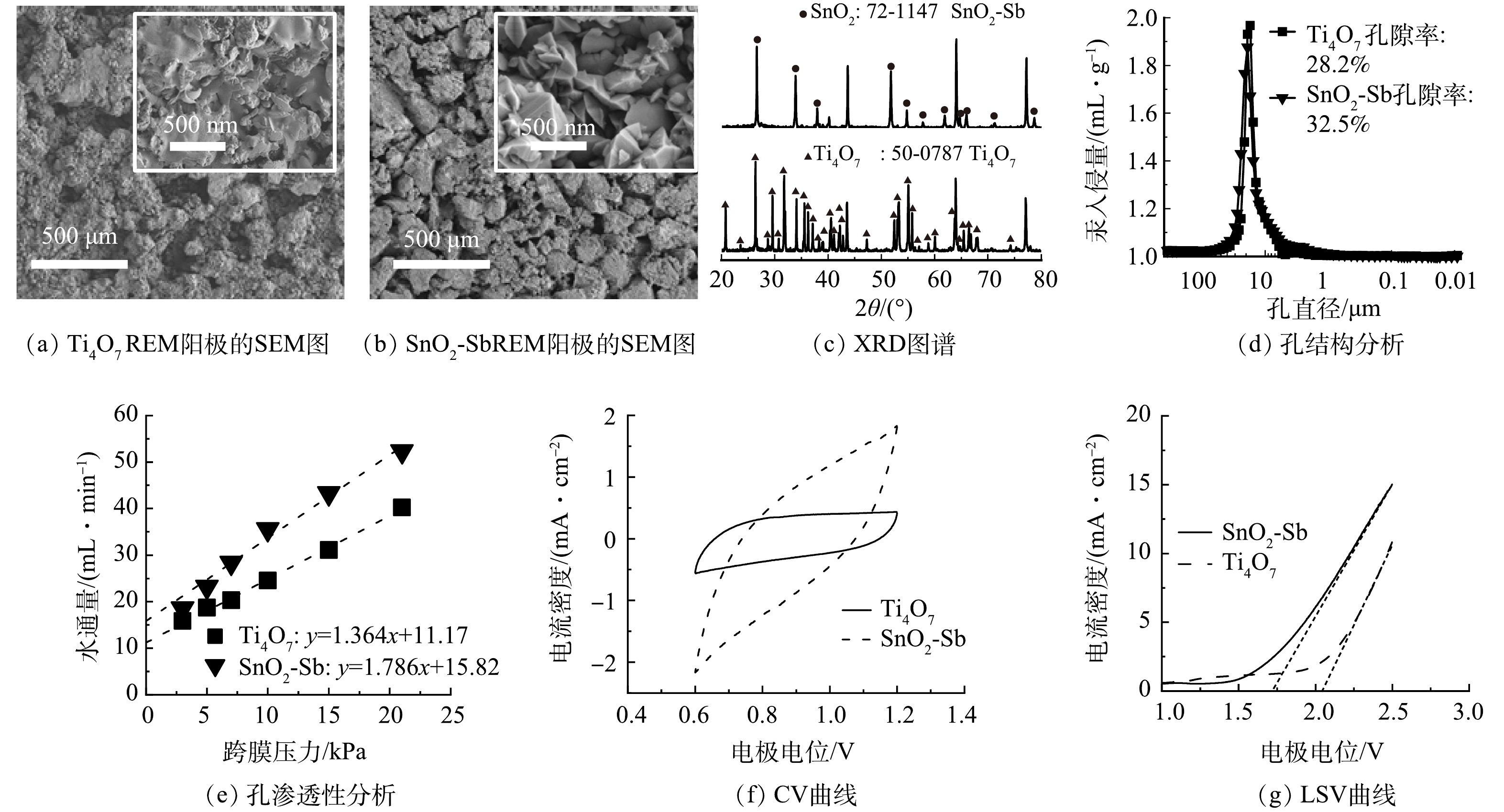
由图2(a)和图2(b)可见, SnO2-Sb和Ti4O7电极均具有发达的孔隙结构,表面形态粗糙。由XRD图谱(图2(c))可见,2个电极上分别出现SnO2和Ti4O7特征峰,证明电极成功负载了SnO2-Sb和Ti4O7催化层。由图2(d)可知,2种电极显示出类似的孔结构,2种电极显示出类似的孔结构,孔径分布、中值孔径分别为5~50 μm和20.0 μm,Ti4O7和SnO2-Sb电极孔隙率分别为28.2和32.5%。。发达的孔隙结构促使两种电极在极低的压力下也能获得极佳的渗透性(图2(e))。同时,SnO2-Sb和Ti4O7电极的孔径远大于传统意义上膜材料(如微滤膜(0.1~1 μm)和纳滤膜(< 2 nm))的孔径促使制备的电极材料几乎不具备拦截水中胶体(1~100 nm)的能力,为后续连续运行实验提供了良好的基础条件[6]。由图2(f)可见,SnO2-Sb电极对应的循环伏安曲线(CV)的电流面积大于Ti4O7电极,表明SnO2-Sb电极可能比Ti4O7电极具有更多的电化学反应活性位点。由图2(g)可知,以Ag/AgCl为参比电极,SnO2-Sb和Ti4O7的析氧电位分别约为1.73 V和2.05 V,符合已报道的SnO2-Sb和Ti4O7电极材料的特征[9,20]。上述表征结果证实目标REM电极被成功制备。
2.2 REM氧化工艺处理工业废水
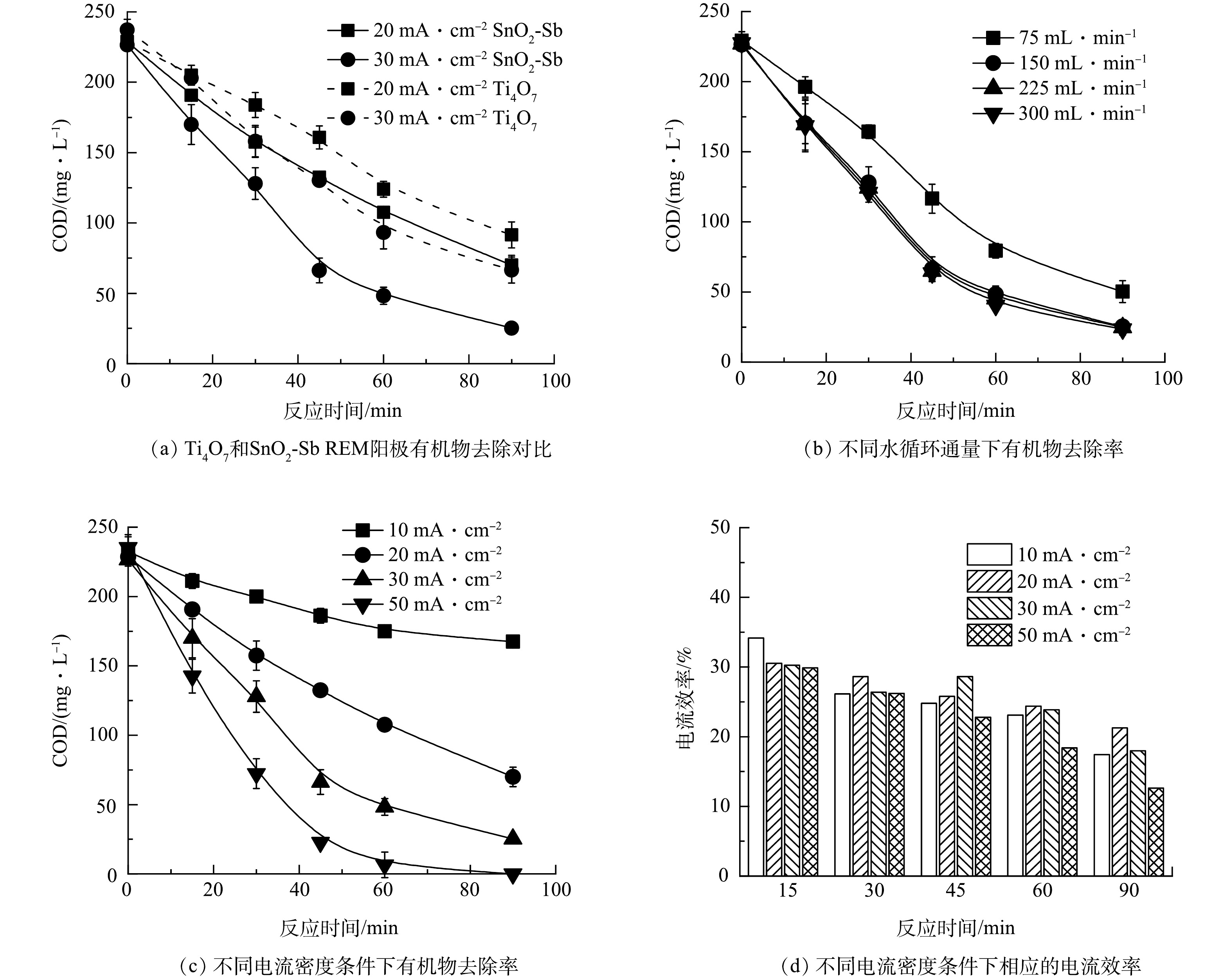
1)有机物的去除。图3(a)对比了2个电极材料处理废水的效率。在相同电流密度下,SnO2-Sb REM阳极对有机物(以COD计)的去除率明显高于Ti4O7,表明SnO2-Sb REM阳极在去除难降解有机物方面更具优势。因此,后续的研究选择SnO2-Sb REM阳极作为研究对象。水通量和电流密度是REM氧化系统运行的2个关键参数,前者影响污染物的传质过程,后者制约着电极的反应动力学.因此,为了进一步优化反应系统的处理效率,研究了水通量和电流密度对反应系统处理工业废水效率的影响。如图3(b)所示,当水循环通量从75 mL·min−1增加到150 mL·min−1时,由于传质增强,90 min处理结束时,有机物去除率从78.1%提升到88.8%;当水循环通量进一步增加到225 mL·min−1和300 mL·min−1时, 有机物去除率几乎没有变化,这表明REM氧化系统达到传质的极限。图3(c)显示了不同电流密度下废水COD的去除情况。随着电流密度的增加,由于电极电子转移能力的提升,有机物去除率显著提升。而由图3(d)可知,当电流密度在10~30 mA·cm−2内,有机物去除的电流效率保持在较高水平,而随着电流密度进一步增加到50 mA·cm−2,由于析氧副反应显著增加,电流效率大幅降低。例如,当电流密度从 30 mA·cm−2增加到50 mA·cm−2时,电流效率由18.0%降低到12.6%。因此,在SnO2-Sb REM阳极采用150 mL·min−1的水循环通量及30 mA·cm−2的电流密度来处理收集的工业废水更为合理。值得注意的是,当有机物去除率在45 min内达到国内的处理规定(100 mg·L−1)的情况下,SnO2-Sb REM阳极的电流效率接近30%,该值远高于达到类似处理效果的传统系统(< 20%)[21-23]。由此可见,新型REM氧化技术能够高效处理工业废水。
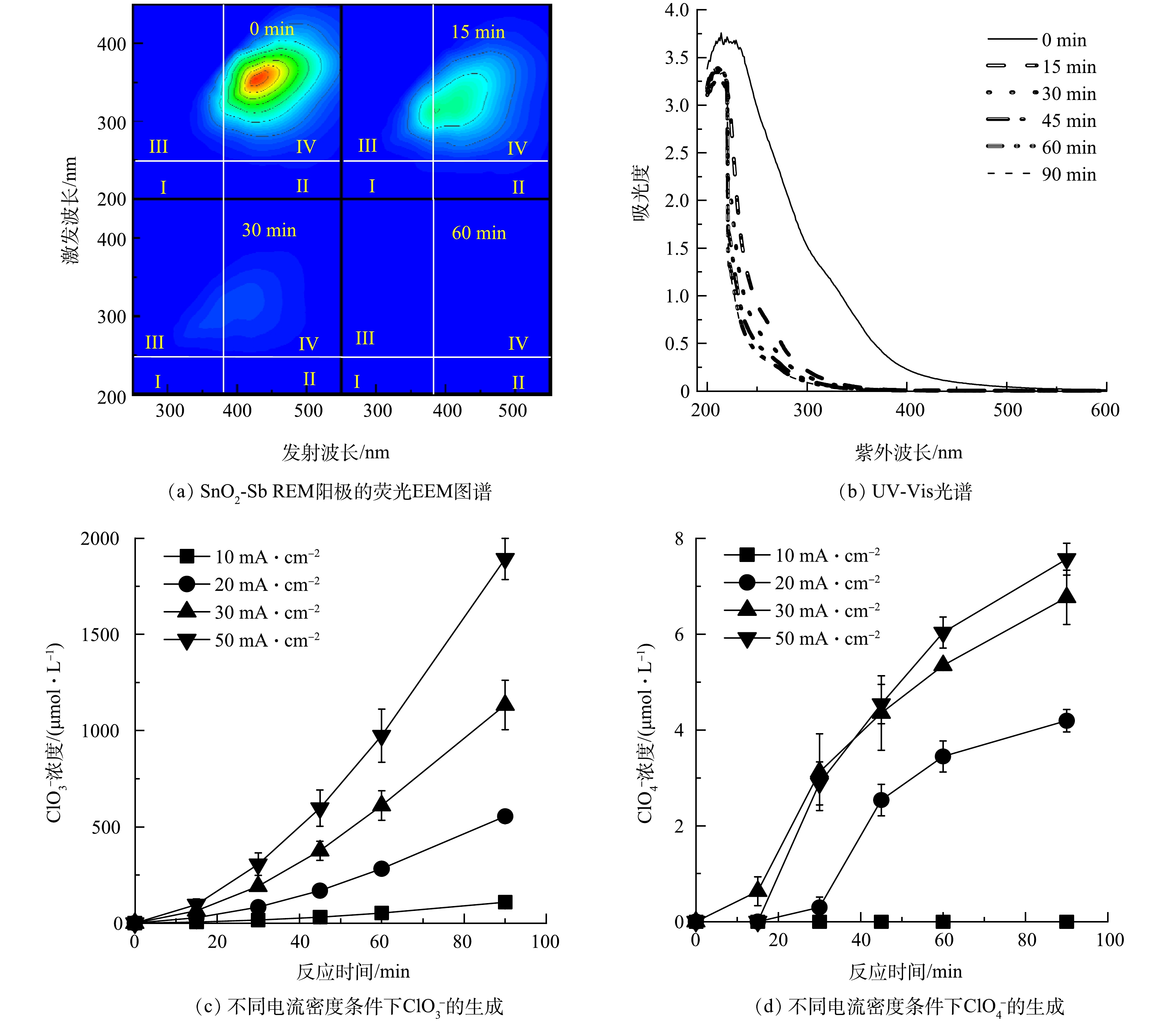
2)有机质转化及氯化副产物生成。为了分析工业废水处理前后有机物特征,进行了三维荧光光谱和UV-Vis吸光光谱分析(图4)。根据不同的激发波长/发射波长(Ex/Em)区域,三维荧光光谱主要反映了不同类型的溶解有机质(dissolved organic matter, DOM):芳香族蛋白区(I),Ex/Em=220~250 nm/280~380 nm;富勒烯酸区(II),Ex/Em=220~250 nm/380~500 nm;可溶性微生物副产物区(III),Ex/Em=250~280 nm/280~380 nm;腐殖酸区(IV), Ex/Em=250~400 nm/380~500 nm[23]。此外,紫外可见光光谱根据特定波长对应的吸光度值或比率同样可以反映有机物的类型。通常,UV250/UV365比值代表腐殖酸类物质,UV254和UV410代表芳香族化合物和共轭大分子[6,24]。如图4(a)和图4(b)所示,在电流密度为30 mA cm−2时,三维荧光强度和UV-Vis吸光度随时间变化与COD有机物去除趋势一致。未经处理的废水荧光区域位于Ex/Em=275~425 nm/350~550 nm的区域内,表明该原始废水中的DOM主要为腐殖酸类物质。随着处理过程,IV区域的荧光强度逐渐消失,III区域荧光强先略微增强随后消失。这些结果表明,工业废水中腐殖酸类物质被氧化分解,并可能产生少量可溶性微生物副产物类物质,这些物质最终被进一步分解。同时,UV250/UV365比值从5.64显著增加到42.90,也表明腐植酸类物质向低分子质量有机物的转化;UV254和UV410经过处理后均大幅降低,说明其中的芳香族化合物和共轭大分子发生了分解。上述结果表明REM氧化工艺能够有效地去除工业废水中的DOM。
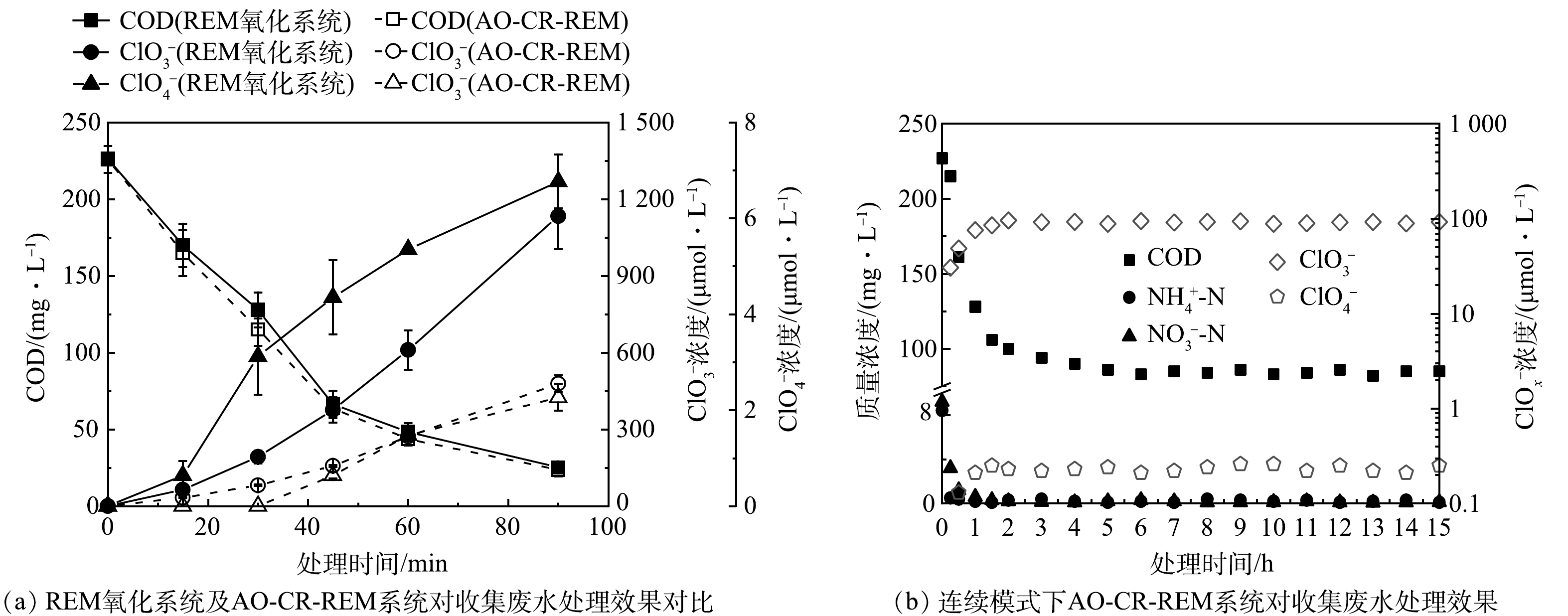
图4(c)和图4(d)为处理过程中检测到氯化副产物的生成情况。废水经过处理后同时检测到ClO3‒和ClO4‒的生成,其生成量均随处理时间和电流密度的增加而增加。值得注意的是,本研究中的REM氧化系统中检测到的ClO3‒和ClO4‒的产量远远低于传统系统[25-27]。而且,REM氧化系统中的ClO4‒浓度比低毒的ClO3‒浓度低约2个数量级,而在传统系统中,ClO4‒通常是主要产物[25-26,28]。本研究团队最近的研究还证明,在REM氧化系统中不仅产生的ClO3‒远远少于传统系统,在很短的处理时间内也检测不到ClO4‒的生成[6]。这些结果表明,REM氧化系统与传统系统相比在处理含氯废水时在氯化副产物生成方面具有很大的优势。
2.3 有机物的去除机制
阳极氧化工艺去除有机物的机理主要涉及DET过程及•OH介导的间接氧化(式(6)~(8))[29]。当未处理废水中存在的Cl−可在阳极表面转化为活性氯,从而参加有机物的氧化(式(9)~(12))[28]。
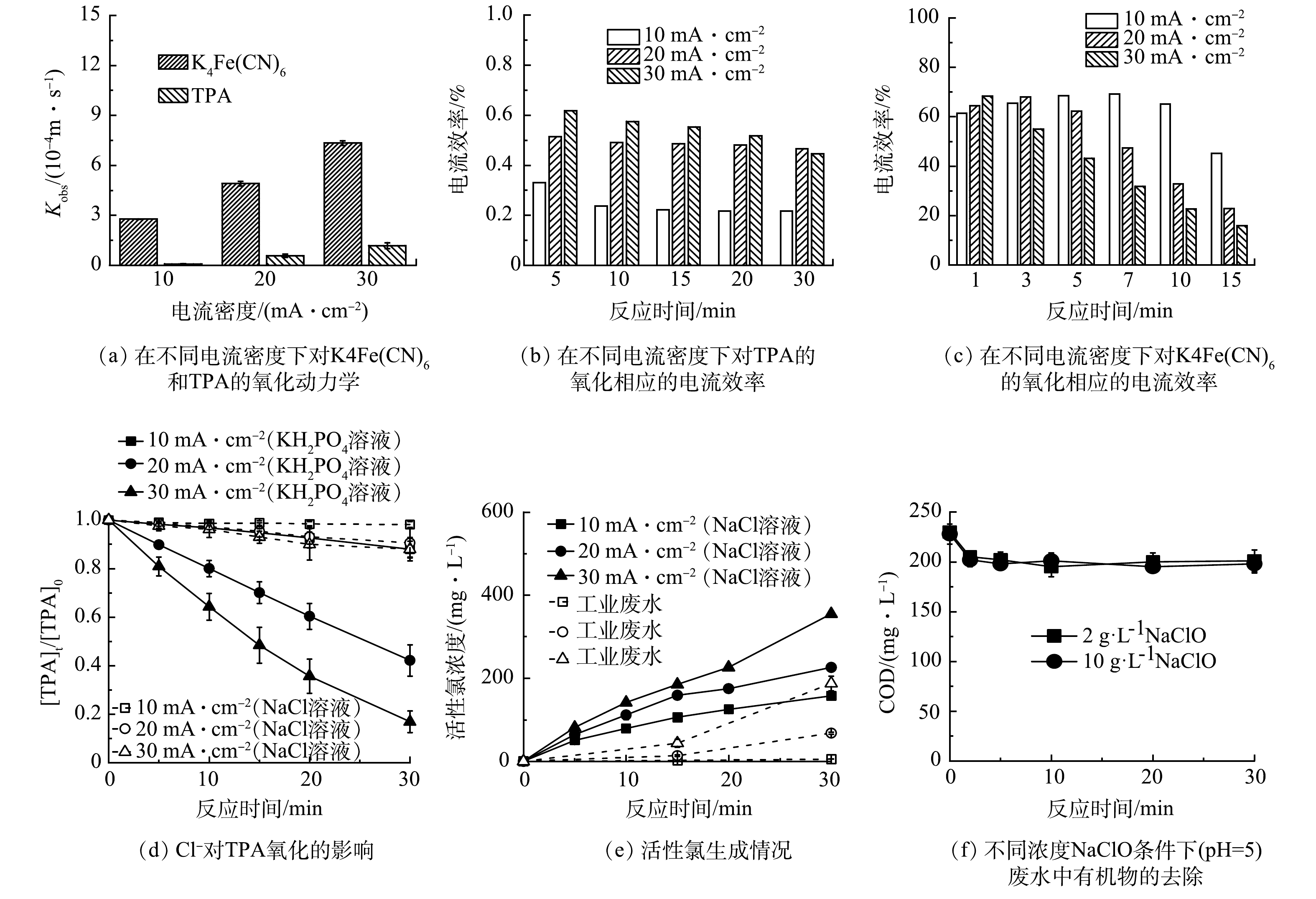
stringUtils.convertMath(!{formula.content}) (6) stringUtils.convertMath(!{formula.content}) (7) stringUtils.convertMath(!{formula.content}) (8) stringUtils.convertMath(!{formula.content}) (9) stringUtils.convertMath(!{formula.content}) (10) stringUtils.convertMath(!{formula.content}) (11) stringUtils.convertMath(!{formula.content}) (12) 首先,选择K4Fe(CN)6作为探针分子评估了SnO2-Sb REM氧化系统的电化学反应性能,这是因为K4Fe(CN)6不仅极易发生单电子转移反应(Fe(CN)64‒ → Fe(CN)63‒+e‒, E0 = 0.358 V),而且与•OH的反应活性极高(1.2×1010 L·(mol·s)−1)[20]。接着,由于对苯二甲酸(TPA)对•OH具有高选择性(4×109 L·(mol·s)−1),选择TPA作为•OH探针来评估•OH的生成情况[30]。如图5(a)所示,随着电流密度增加,K4Fe(CN)6的Kobs显著增加,这与有机物去除结果一致。在REM氧化系统中,氧化K4Fe(CN)6的Kobs高达2.8~7.3×10−4 m·s−1,比TPA高数倍至数十倍(0.1~1.2×10−4 m·s−1),表明REM氧化具有强大的DET能力,这一结论已在最近的研究报道[6,20,31]。同时, 由图5(b)可知,氧化TPA的电流效率不足1%,与工业废水处理的电流效率(图3(d))相差甚远,表明生成的•OH远无法实现观察到的高有机物去除率。然而,氧化K4Fe(CN)6的电流效率大大提高(图5(c)),与处理工业废水时的电流效率匹配。同时,由于•OH与Cl‒的高反应活性(4.3×109 mol·(L·s)−1),生成的•OH将会被废水中的Cl‒淬灭,如图5(d)中所示,NaCl溶液中TPA氧化动力学与相同电导条件下KH2PO4溶液中相比大幅降低也证明了这一结论。因此,根据上述结果,DET过程在REM氧化工艺处理废水过程中发挥重要作用。为了进一步评价生成的活性氯在废水处理过程的作用,我们测试了处理与工业废水电导率和Cl‒浓度相同的KH2PO4替代溶液时活性氯的生成情况。由图5(e)可见,替代溶液中产生的活性氯浓度远高于处理工业废水时生成的活性氯,尤其是在初始阶段,这表明活性氯参与了有机物的去除。更进一步,我们在工业废水中添加2~10 g·L−1 NaClO,同时用2%稀H2SO4溶液调节pH至5,以确保NaClO转化为活性氯。如图5(f)所示,仅在初始阶段观察到约10%的有机物去除率,而且增加NaClO浓度对有机物的去除几乎没有影响。这一结果表明,氧化能力较低的活性氯难以有效氧化收集的工业废水中大部分原始有机化合物。
根据上述分析,REM氧化工艺去除废水中的有机质机理可能遵循以下过程:首先,工业废水中原始有机物主要依靠DET过程分解;随后,分别形成的二次产物在DET过程和生成的活性氯共同作用下被进一步去除。由于废水中含有大量的Cl‒,生成的•OH会被废水中存在的Cl‒淬灭掉,导致•OH对处理收集的工业废水有机物去除的贡献较小。
2.4 采用AO-CR-REM工艺同时去除有机物和减少氯化副产物
上述结果表明,REM氧化工艺能够高效去除难处理工业废水中的有机物,但伴随着也产生了一定量氯化副产品ClO3‒和ClO4‒[14]。因此,为了去除有机物的同时抑制或者减少氯化副产物的产生,我们进一步采用图1描述的兼具电氧化-还原于一体的双功能AO-CR-REM系统来处理收集的工业废水。如图6(a)所示,在相同的实验条件下,AO-CR-REM系统对于有机物的去除效果与REM氧化系统几乎保持一致,而ClO3‒和ClO4‒的产量则大大减少。例如,在AO-CR-REM系统中,处理30 min后仅测定到约81.5 μmol·L−1的ClO3‒,仅为REM氧化系统的42.3%(约192.3 μmol·L−1),且未检测到ClO4‒的生成。这些结果证明AO-CR-REM系统可以同时去除有机物并显著抑制氯化副产物的生成。需要指出的是,处理过的废水中的ClO3‒仍处于较高水平,因此,今后仍需设计和开发更先进的阴极材料,以进一步抑制氯化副产品的生成。
在实际的废水处理中,通常希望采用连续运行反应系统。目前,主要通过减少水通量或串联多级REM系统来设计连续运行的REM氧化系统。然而,降低水通量会大幅降低污染物的传质效率,从而削减反应系统的处理效率。串联多级REM系统虽然可以保证处理效率,但设备投资成本将急剧上升。据估计,REM氧化技术应用过程中设备的投资成本约占总成本的90%[6]。在此,我们尝试采用一种新的连续运行模式来处理废水,即图1中蠕动泵1以150 mL·min−1水循环通量运行,以确保使AO-CR-REM系统反应不受传质限制,蠕动泵2将原废水不断输送到系统中。由于循环模式下,AO-CR-REM去除有机物遵循伪一级动力学(R2=0.99),对应的动力学常数计算为0.026 min−1。由此可以估算当处理时间为31.4 min时,AO-CR-REM系统能够将150 mL废水的COD削减至接近排放标准(100 mg·L−1),即在连续运行模式下,废水的连续进水速率约为4.8 mL·min−1。为了确保出水水质满足排放标准,设置蠕动泵2以4 mL·min−1的流速不断将原始工业废水输送到反应系统,实验结果如图6(b)所示。处理4 h之后,出水的COD稳定在80 mg·L−1,ClO3‒和ClO4‒分别稳定在90 mg·L−1和0.25 mg·L−1。在连续运行模式下,对废水的处理效果基本维持稳定,反应系统未出现多孔电极堵塞污染问题。出水符合中国的处理规定的情况下,所需电能为7.91 kWh·m−3。同时,还观察到NH4+-N和NO3‒-N几乎被完全去除,这分别归因于活性氯的氧化作用和REM阴极的电还原作用[32-34]。此外,值得注意的是, MBR工艺能够有效去除水中的悬浮物和胶体,避免多孔阳极堵塞和污染,但该工艺无法有效去除废水中普遍存在的硬度离子(Ca2+、Mg2+等),而阴极区域由于析氢反应聚集了大量OH‒,废水中的硬度离子将可能会以氧化物或盐的形式在阴极附近沉降,从而造成阴极结垢。尽管本研究在15 h的运行过程中并未观察到阴极结垢堵塞的情况,但在未来的研究中有必要进一步聚焦于阴极反应过程,研究阴极结垢情况以及应对措施。
3. 结论
本研究从电极材料、处理效能、副产物生成与抑制等全面评估了REM工艺处理含氯量高的难降解工业废水的可行性,所得主要结论如下。
1)在Ti4O7和SnO2-Sb 2种常见的电极材料中,SnO2-Sb REM阳极对有机物的去除率比Ti4O7 REM阳极高约9%~17%。
2)废水中的腐殖酸类物质、芳香族化合物和共轭大分子均能够被有效氧化分解。由于Cl‒对生成的•OH有较强的淬灭作用,有机物主要由DET过程分解,接着再由DET过程和生成的活性氯结合进一步去除。
3)进一步开发的AO-CR-REM系统可有效去除有机物,出水符合中国的处理规定的情况下,可有效抑制ClO3‒ 和ClO4‒的生成,同时废水中的NH4+-N和NO3‒-N也被有效去除。
-
表 1 BC和MnOx-BC的结构特征
Table 1. Structural characteristics of BC and MnOx-BC
吸附剂 比表面积/(cm3·g-1) 孔体积/(cm3·g-1) 平均孔径/nm 零电荷点 BC 440.27 0.227 2.06 6.82 MnOx-BC 493.45 0.255 2.58 7.22 表 2 BC和MnOx-BC吸附噻虫胺动力学参数
Table 2. Kinetic parameters of CLO adsorption by BC and MnOx-BC
吸附剂 准一级动力学方程 准二级动力学方程 q1/(mg·g−1) k1/h−1 R2 q2/(mg·g−1) k2/(g·(mg·min)−1) R2 BC 17.56 1.87 0.900 18.81 0.15 0.964 MnOx−BC 19.48 2.23 0.877 21.13 0.15 0.944 表 3 BC和MnOx-BC吸附噻虫胺的颗粒内扩散方程拟合参数
Table 3. Fitting parameters of the intraparticle diffusion equation for CLO adsorption by BC and MnOx-BC
吸附剂 阶段1 阶段2 阶段3 k1 C1 R2 k2 C2 R2 k3 C3 R2 BC 11.32 4.26 0.990 3.99 0.972 0.972 0.11 18.72 0.595 MnOx-BC 14.41 4.52 0.974 3.38 11.88 0.957 0.52 20.22 0.891 表 4 BC和MnOx-BC吸附噻虫胺的吸附等温线拟合参数
Table 4. Fitting parameters of adsorption isotherms for BC and MnOx-BC toward CLO
吸附剂 温度/℃ Langmuir Freundlich qm/(mg g−1) KL/(L·mg−1) R2 1/n KF R2 BC 15 23.96 2.70 0.939 0.14 18.49 0.869 25 30.00 1.20 0.902 0.16 16.51 0.835 35 31.56 4.88 0.949 0.18 20.10 0.900 MnOx-BC 15 29.48 8.08 0.968 0.15 21.72 0.952 25 33.43 7.79 0.983 0.17 24.23 0.952 35 37.00 10.09 0.946 0.19 25.67 0.981 -
[1] 张敏恒, 赵平, 严秋旭, 等. 新烟碱类杀虫剂市场与环境影响[J]. 农药, 2012, 51(12): 859-862. [2] BASS C, DENHOLM I, WILLIAMSON M S, et al. The global status of insect resistance to neonicotinoid insecticides[J]. Pesticide Biochemistry and Physiology, 2015, 121: 78-87. doi: 10.1016/j.pestbp.2015.04.004 [3] 胡倩, 阳海, 石妮, 等. 光催化体系中噻虫胺降解动力学及机制[J]. 环境科学, 2016, 37(9): 3524-3531. [4] SIMON-DELSO N, AMARAL-ROGERS V, BELZUNCES L P, et al. Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites[J]. Environmental Science and Pollution Research, 2015, 22(1): 5-34. doi: 10.1007/s11356-014-3470-y [5] ZHANG C, TIAN D, YI X H, et al. Occurrence, distribution and seasonal variation of five neonicotinoid insecticides in surface water and sediment of the Pearl Rivers, South China[J]. Chemosphere, 2019, 217: 437-446. doi: 10.1016/j.chemosphere.2018.11.024 [6] MAHAI G, WAN Y J, XIA W, et al. A nationwide study of occurrence and exposure assessment of neonicotinoid insecticides and their metabolites in drinking water of China[J]. Water Research, 2021, 189: 116630. doi: 10.1016/j.watres.2020.116630 [7] WHITEHORN P R, O'CONNOR S, WACKERS F L, et al. Neonicotinoid pesticide reduces bumble bee colony growth and queen production[J]. Science, 2012, 336(6079): 351-352. doi: 10.1126/science.1215025 [8] MANDAL A, SINGH N, PURAKAYASTHA T J. Characterization of pesticide sorption behaviour of slow pyrolysis biochars as low cost adsorbent for atrazine and imidacloprid removal[J]. Science of the Total Environment, 2017, 577: 376-385. doi: 10.1016/j.scitotenv.2016.10.204 [9] KIM K H, KIM J Y, CHO T S, et al. Influence of pyrolysis temperature on physicochemical properties of biochar obtained from the fast pyrolysis of pitch pine (Pinus rigida)[J]. Bioresource Technology, 2012, 118: 158-162. doi: 10.1016/j.biortech.2012.04.094 [10] NIE T, HAO P, ZHAO Z, et al. Effect of oxidation-induced aging on the adsorption and co-adsorption of tetracycline and Cu2+ onto biochar[J]. Science of the Total Environment, 2019, 673: 522-532. doi: 10.1016/j.scitotenv.2019.04.089 [11] YIN Y Y, GUO X T, PENG D. Iron and manganese oxides modified maize straw to remove tylosin from aqueous solutions[J]. Chemosphere, 2018, 205: 156-165. doi: 10.1016/j.chemosphere.2018.04.108 [12] YU X Y, MU C L, GU C, et al. Impact of woodchip biochar amendment on the sorption and dissipation of pesticide acetamiprid in agricultural soils[J]. Chemosphere, 2011, 85(8): 1284-1289. doi: 10.1016/j.chemosphere.2011.07.031 [13] TAHA S M, AMER M E, ELMARSAFY A E, et al. Adsorption of 15 different pesticides on untreated and phosphoric acid treated biochar and charcoal from water[J]. Journal of Environmental Chemical Engineering, 2014, 2(4): 2013-2025. doi: 10.1016/j.jece.2014.09.001 [14] 马锋锋, 赵保卫. 不同热解温度制备的玉米芯生物炭对对硝基苯酚的吸附作用[J]. 环境科学, 2017, 38(2): 837-844. [15] 李政剑, 石宝友, 苏宇, 等. 粉末活性炭粒径对水中菲吸附动力学的影响效应研究[J]. 环境科学学报, 2013, 33(1): 67-72. [16] LYU H H, XIA S Y, TANG J C, et al. Thiol-modified biochar synthesized by a facile ball-milling method for enhanced sorption of inorganic Hg2+ and organic CH3Hg+[J]. Journal of Hazardous Materials, 2020, 384: 121357. doi: 10.1016/j.jhazmat.2019.121357 [17] LIATSOU I, PASHALIDIS I, DOSCHE C. Cu(II) adsorption on 2-thiouracil-modified Luffa cylindrica biochar fibres from artificial and real samples, and competition reactions with U(VI)[J]. Journal of Hazardous Materials, 2020, 383: 120950. doi: 10.1016/j.jhazmat.2019.120950 [18] ZHU S H, ZHAO J J, ZHAO N, et al. Goethite modified biochar as a multifunctional amendment for cationic Cd(II), anionic As(III), roxarsone, and phosphorus in soil and water[J]. Journal of Cleaner Production, 2020, 247: 119579. doi: 10.1016/j.jclepro.2019.119579 [19] 赵志伟, 陈晨, 梁志杰, 等. 锰氧化物改性生物炭对水中四环素的强化吸附[J]. 农业环境科学学报, 2021, 40(1): 194-201. doi: 10.11654/jaes.2020-0803 [20] LIU Y, ZHANG L X, ZHANG Z F, et al. Citrate-modified biochar for simultaneous and efficient plant-available silicon release and copper adsorption: Performance and mechanisms[J]. Journal of Environmental Management, 2022, 301: 113819. doi: 10.1016/j.jenvman.2021.113819 [21] 杨广西. 生物炭的化学改性及其对铜的吸附研究[D]. 合肥: 中国科学技术大学, 2014. [22] 蒋子旸, 徐敏, 伍钧. 高铁酸钾/高锰酸钾改性生物炭对Cd2+的吸附研究[J]. 农业环境科学学报, 2021, 40(4): 876-883. doi: 10.11654/jaes.2020-1123 [23] ZHANG J Z, MA X F, YUAN L, et al. Comparison of adsorption behavior studies of Cd2+ by vermicompost biochar and KMnO4-modified vermicompost biochar[J]. Journal of Environmental Management, 2020, 256: 109959. doi: 10.1016/j.jenvman.2019.109959 [24] 管欢, 黄慧俐, 行艳景, 等. 噻虫胺在甘蔗和土壤中的残留分析及消解动态[J]. 现代农药, 2015, 14(2): 42-45. doi: 10.3969/j.issn.1671-5284.2015.02.014 [25] LI R N, WANG Z W, ZHAO X T, et al. Magnetic biochar-based manganese oxide composite for enhanced fluoroquinolone antibiotic removal from water[J]. Environmental science and pollution research international, 2018, 25(31): 31136-31148. doi: 10.1007/s11356-018-3064-1 [26] LIU W J, ZENG F X, JIANG H, et al. Preparation of high adsorption capacity bio-chars from waste biomass[J]. Bioresource Technology, 2011, 102(17): 8247-8252. doi: 10.1016/j.biortech.2011.06.014 [27] 张涵瑜, 王兆炜, 高俊红, 等. 芦苇基和污泥基生物炭对水体中诺氟沙星的吸附性能[J]. 环境科学, 2016, 37(2): 689-696. [28] GAO J, HEDMAN C, LIU C, et al. Transformation of sulfamethazine by manganese oxide in aqueous solution[J]. Environmental Science & Technology, 2012, 46(5): 2642-2651. [29] LIN L, ZHOU S W, HUANG Q, et al. Capacity and mechanism of arsenic adsorption on red soil supplemented with ferromanganese oxide-biochar composites[J]. Environmental science and pollution research international, 2018, 25(20): 20116-20124. doi: 10.1007/s11356-018-2188-7 [30] TAN X L, FANG M, CHEN C L, et al. Counterion effects of nickel and sodium dodecylbenzene sulfonate adsorption to multiwalled carbon nanotubes in aqueous solution[J]. Carbon, 2008, 46(13): 1741-1750. doi: 10.1016/j.carbon.2008.07.023 [31] OUYANG D, CHEN Y, YAN J C, et al. Activation mechanism of peroxymonosulfate by biochar for catalytic degradation of 1, 4-dioxane: Important role of biochar defect structures[J]. Chemical Engineering Journal, 2019, 370: 614-624. doi: 10.1016/j.cej.2019.03.235 [32] LIU S, XU W H, LIU Y G, et al. Facile synthesis of Cu(II) impregnated biochar with enhanced adsorption activity for the removal of doxycycline hydrochloride from water[J]. Science of the Total Environment, 2017, 592: 546-553. doi: 10.1016/j.scitotenv.2017.03.087 [33] JOSHI T P, ZHANG G, CHENG H Y, et al. Transformation of para arsanilic acid by manganese oxide: Adsorption, oxidation, and influencing factors[J]. Water Research, 2017, 116: 126-134. doi: 10.1016/j.watres.2017.03.028 [34] WANG X, HUANG K, CHEN Y, et al. Preparation of dumbbell manganese dioxide/gelatin composites and their application in the removal of lead and cadmium ions[J]. Journal of Hazardous Materials, 2018, 350: 46-54. doi: 10.1016/j.jhazmat.2018.02.020 [35] HUANG J Z, ZHONG S F, DAI Y F, et al. Effect of MnO2 Phase Structure on the Oxidative Reactivity toward Bisphenol A Degradation[J]. Environmental Science & Technology, 2018, 52(19): 11309-11318. [36] 孙航, 蒋煜峰, 石磊平, 等. 不同热解及来源生物炭对西北黄土吸附敌草隆的影响[J]. 环境科学, 2016, 37(12): 4857-4866. [37] 谭珍珍, 张学杨, 骆俊鹏, 等. 小麦秸秆生物炭对四环素的吸附特性研究[J]. 水处理技术, 2019, 45(2): 32-38. [38] ZHOU Y Y, LIU X C, XIANG Y J, et al. Modification of biochar derived from sawdust and its application in removal of tetracycline and copper from aqueous solution: Adsorption mechanism and modelling[J]. Bioresource Technology, 2017, 245(Pt A): 266-273. [39] 张连科, 王洋, 王维大, 等. 生物炭负载纳米羟基磷灰石复合材料的制备及对铅离子的吸附特性[J]. 化工进展, 2018, 37(9): 3492-3501. [40] 孙绪兵, 吴雪梅, 朱建发, 等. 羧基甲壳素对Pb(Ⅱ)的吸附性能及机理研究[J]. 中国环境科学, 2018, 38(8): 3018-3028. doi: 10.3969/j.issn.1000-6923.2018.08.030 [41] 徐大勇, 张苗, 杨伟伟, 等. 氧化铝改性污泥生物炭粒制备及其对Pb(Ⅱ)的吸附特性[J]. 化工进展, 2020, 39(3): 1153-1166. [42] LIU P, LIU W J, JIANG H, et al. Modification of bio-char derived from fast pyrolysis of biomass and its application in removal of tetracycline from aqueous solution[J]. Bioresource Technology, 2012, 121: 235-240. doi: 10.1016/j.biortech.2012.06.085 [43] LI H Q, HU J T, MENG Y, et al. An investigation into the rapid removal of tetracycline using multilayered graphene-phase biochar derived from waste chicken feather[J]. Science of the Total Environment, 2017, 603-604: 39-48. doi: 10.1016/j.scitotenv.2017.06.006 [44] 杨奇亮, 吴平霄. 改性多孔生物炭的制备及其对水中四环素的吸附性能研究[J]. 环境科学学报, 2019, 39(12): 3973-3984. [45] PEIRIS C, GUNATILAKE S R, MLSNA T E, et al. Biochar based removal of antibiotic sulfonamides and tetracyclines in aquatic environments: A critical review[J]. Bioresource Technology, 2017, 246: 150-159. doi: 10.1016/j.biortech.2017.07.150 [46] 李蕊宁. 改性马铃薯秸秆生物炭对水体中典型抗生素的吸附性能研究[D]. 兰州: 兰州大学, 2018. [47] 赵华轩, 郎印海. 磁性生物炭对水中CIP和OFL的吸附行为和机制[J]. 环境科学, 2018, 39(8): 3729-3735. [48] ZHU X D, LIU Y C, ZHOU C, et al. A novel porous carbon derived from hydrothermal carbon for efficient adsorption of tetracycline[J]. Carbon, 2014, 77: 627-636. doi: 10.1016/j.carbon.2014.05.067 [49] ZHAO R L, MA X X, XU J Q, et al. Removal of the Pesticide Imidacloprid from Aqueous Solution by Biochar Derived from Peanut Shell[J]. BioResources, 2018, 13(3): 5656-5669. [50] AHMED M B, ZHOU J L, NGO H H, et al. Single and competitive sorption properties and mechanism of functionalized biochar for removing sulfonamide antibiotics from water[J]. Chemical Engineering Journal, 2017, 311: 348-358. doi: 10.1016/j.cej.2016.11.106 [51] 王宇宙, 吴安心. 芳环超分子体系中的π-π作用[J]. 有机化学, 2008, 28(6): 997-1011. [52] KANG J, LIU H J, ZHENG Y M, et al. Application of nuclear magnetic resonance spectroscopy, Fourier transform infrared spectroscopy, UV-Visible spectroscopy and kinetic modeling for elucidation of adsorption chemistry in uptake of tetracycline by zeolite beta[J]. Journal of Colloid and Interface Science, 2011, 354(1): 261-267. doi: 10.1016/j.jcis.2010.10.065 -




 下载:
下载: