-
如果大量含重金属的废水、固废等未经妥善处理直接排入环境,会造成地表水重金属污染[1]。同时,多种重金属复合污染的问题也越来越普遍[2]。重金属进入环境后,会随食物链不断富集,最后通过直接或间接摄入的方式进入人体,引起多种疾病,因而一直受到环保学者的广泛关注[3-4]。重金属不能被降解,只能通过改变其形态或价态的方式来降低其迁移能力和生物有效性。通常,重金属污染水体的治理方法主要有化学沉淀法、氧化还原、生物处理和吸附法[5-7]。随着环保标准越来越严,急切需要更有效的方法来处理重金属复合污染。锌(Zn)、镍(Ni)和铬(Cr)是水体中很常见的重金属污染物。其中,Zn和Ni主要以阳离子的形式存在于水体中,而Cr主要以Cr2O72−、CrO42−等阴离子的形式存在于水体中。对于这些以不同离子形态共存的重金属复合污染水体,其处理难度更大。
零价铁(Fe0或ZVI)具有来源广泛、价格低廉、生态风险小和中等还原性(E0= -0.44V)的特点,已成为环境污染控制和修复的重要材料之一[8-10]。通过还原反应,Fe0可以处理标准电位比其高的重金属,例如Cr(Ⅵ)、Cu(Ⅱ)、Hg(Ⅱ)、Ag(Ⅰ)、As(Ⅴ)和Se(Ⅵ)等[11-14],但对于标准电位与其非常接近的重金属元素(Ni(Ⅱ)或Zn(Ⅱ)),则主要依靠铁腐蚀产物的吸附和共沉淀作用,处理效果都不够理想[9,15]。不管是通过Fe0的直接还原,还是通过铁腐蚀产物的吸附和共沉淀,都必须保证Fe0腐蚀反应的持续进行,从而不断释放电子并且产生新的腐蚀产物。常规的Fe0体系,由于产生的三价铁腐蚀产物覆盖在Fe0表面,抑制了Fe0腐蚀反应的持续进行,从而容易导致Fe0表面钝化,这是限制Fe0技术被广泛应用的关键瓶颈[16]。为了延缓Fe0表面钝化,很多学者提出了不同的方法,如酸洗[17]、超声处理[18]、弱磁场[19]、双金属体系[20]等。TANG等[21-22]通过直接加入或反应生成Fe3O4,形成复合体系Fe0/Fe3O4,可显著提高Fe0对Se(Ⅵ)的还原速率。由于Fe3O4是半导体,不会阻碍电子传递,从而可以持续保持Fe0的活性。同时,利用Fe3O4的磁性,能实现固-液的快速分离。目前,大多数的研究是直接加入Fe3O4或利用化学反应生成Fe3O4。这样做操作比较麻烦,且难以保证只生成Fe3O4,往往是不同铁氧化物/氢氧化物的混合物。如果能通过简单的方法,在Fe0表面只生成Fe3O4,则在实际应用中将更方便。基于上述研究,本研究通过简便方法,原位制备了Fe0/Fe3O4复合体系,并且考察了该体系同时去除Zn2+、Ni2+和Cr(Ⅵ) 3种重金属的效果和机理;通过批处理实验和连续进出水的流化床反应器考察了不同反应条件对复合体系去除这3种重金属的影响;并借助XRD、SEM和XPS,探讨了对不同金属的去除机理。
-
还原铁粉(98%)(100目和400目)、锌标样、镍标样、铬标样、乙酸铵、乙酸、氯化锌(ZnCl2)、氯化镍、重铬酸钾、氯化亚铁、盐酸、硝酸、邻菲罗啉等试剂均为分析纯;溶液配制及材料制备均采用去离子水。
-
1)批处理实验。称取0.15 g和0.25 g的铁粉置于20 mL血清瓶中,再加入14 mL去离子水和1 mL FeCl2储备液,此时反应瓶内顶部还有一定体积的空气,再密封后将反应瓶置于360°旋转的木箱中,以30 r·min−1的转速开始反应一定时间。预处理完成后,再用微量注射器加入300 μL的Zn2+、Ni2+和Cr(Ⅵ)的混合储备液,得到10 mg·L−1的Ni2+和Zn2+以及不同浓度的Cr(Ⅵ),再将反应瓶放入旋转箱中反应并开始计时,在预定时间每次取出2个反应瓶作为重复,打开塞子后先测定溶液pH,再经0.45 μm滤膜过滤,收集滤液测定Fe2+和重金属离子的浓度。在Fe2+的样品中加入2滴3 mol·L−1的HCl酸化保存待测。其余滤液加入1滴3 mol·L−1HNO3保存,待测金属离子。采用准一级(式(1))、准二级(式(2))动力学方程拟合各重金属去除情况。
式中:
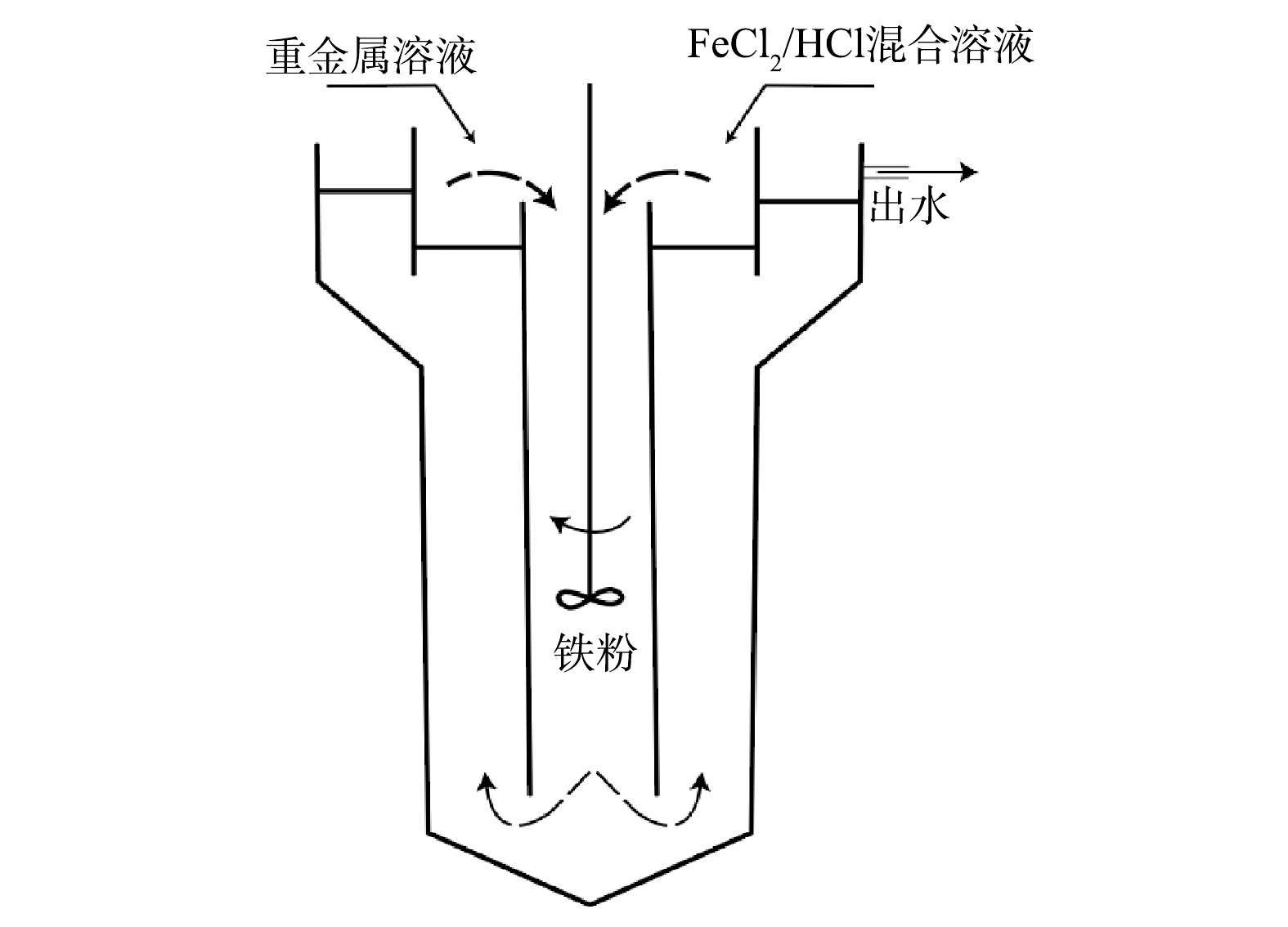
qe 为平衡吸附量,mg·g−1;qt 为时间t的吸附量,mg·g−1;t 为吸附时间,min;k1 为准一级动力学方程常数,g·(mg·min)−1;k2 为准二级动力学方程常数,g·(mg·min)−1。2)流化床反应器实验。使用纯水配制50 mmol·L−1FeCl2和30 mmol·L−1HCl混合液,进水中Zn、Ni和Cr的质量浓度均为10 mg·L−1。4个流化床反应器的操作条件见表1,流化床反应器的示意图见图1。通过可调式电子机械搅拌器搅动溶液,使铁粉悬浮于溶液中,防止铁粉沉积到反应器底部。反应器的有效体积为11.5 L。与批处理实验类似,流化床反应器也需经过预处理,先制备Fe0/Fe3O4复合体系。预处理过程中,只添加不同的氧化剂,反应2~3 d完成预处理,后再通入重金属模拟废水。模拟废水用自来水配置,为防止重金属沉淀,先用HCl将溶液pH调为7.0,再加入重金属试剂,采用连续进出水的方式运行。在反应区反应后,通过溢流口排出,固体会自动下沉流入反应区,从而保证出水所含悬浮物少,减少铁粉的流失。
-
采用原子吸收光谱(PerkinElmer 900T)测定滤液中的Zn2+、Ni2+和总Cr;采用二苯碳酰二肼分光光度法测定Cr(Ⅵ);采用邻菲啰啉分光光度法(紫外可见光度计,TU-1810,北京普析)在波长510 nm测定Fe2+浓度。文中的结果均为2个平行样。
在制备SEM样品时,按照批处理实验的条件,反应后分离固液,将固体在高纯氮气保护下干燥,再用SEM(日本电子,JSM-7500F)分析。在制备XPS样品时,为了增大待测元素的含量,所加入的各重金属浓度比实验高1倍,其它反应物均相应提高1倍。反应一定时间后,通过超声处理将铁腐蚀物从铁粉表面剥落下来,再过滤分离腐蚀物,通过连续吹入高纯氮气风干样品用于XPS(日本岛津,AXIS Supra)分析。XPS采用单色铝Kα X射线(hv=1 486.6 eV),管电压15 kV,管电流12 mA,功率180 W,步长为0.05 eV,分析Ni和Cr反应前后的价态变化,以C1s 284.5 eV校正结合能,采用XPSPEAK4.1软件分析结果。在制备XRD样品时,与批处理反应条件完全一样,而且反应后,无需任何处理,直接将铁粉分离,然后在高纯氮气气流下干燥,用于XRD分析(日本理学,Ultima IV)。
-
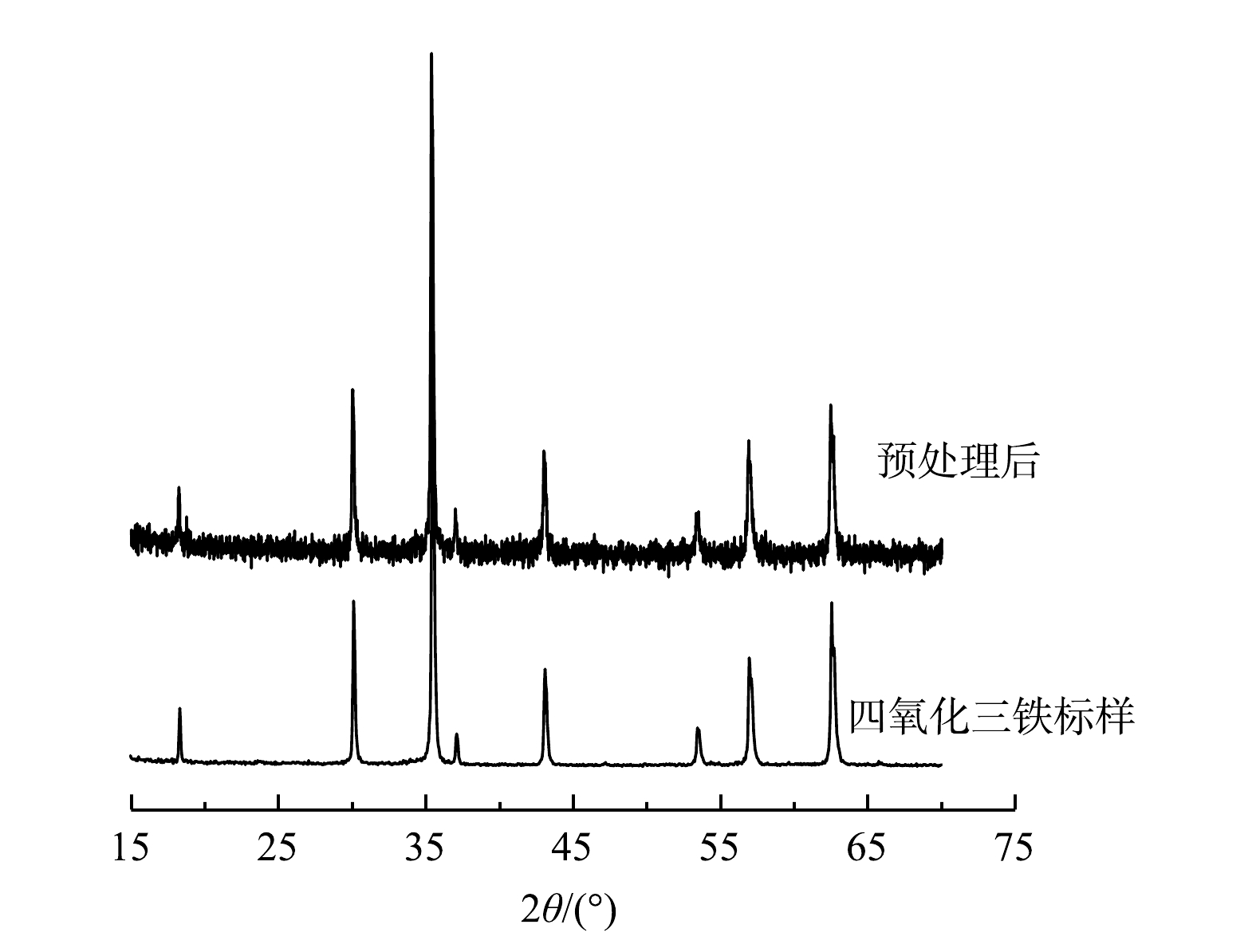
由图2可看出,复合体系的谱图与Fe3O4标样完全一致。这与SU等[23]制成的纳米Fe3O4峰形一致,表明预处理后,在铁表面形成了晶型完好且纯度很高的Fe3O4,没有其它晶态铁氧化物或氢氧化物峰。这说明,该预处理方法可以在Fe0表面生成了一层晶相单一的Fe3O4,形成了Fe0/Fe3O4复合体系。由图3可看出,反应前的铁粉表面光滑,铁粉颗粒不规则(图3(a))。这与前人的研究观察现象一致[24]。在厌氧条件下反应后,铁表面由于发生反应而变得粗糙,而且有一些白色的小点,可能是沉淀的重金属离子。由于没有强氧化剂的参与,铁腐蚀反应很慢,产生的腐蚀产物也很少(图3(b))。如图3(c)所示,在预处理后复合体系中铁腐蚀产物的表面有很多颗粒状和片状的晶体。这些晶体的形貌与Fe3O4(图3(d))的晶体形态非常相似[21]。WU等[25]经过酸预处理,反应后的铁腐蚀物主要是棒状和片状。这也再次证明腐蚀产物主要是Fe3O4,与图2中的XRD的结果吻合。由此可见,通过预处理可在Fe0表面形成一层晶型完好的Fe3O4。
-
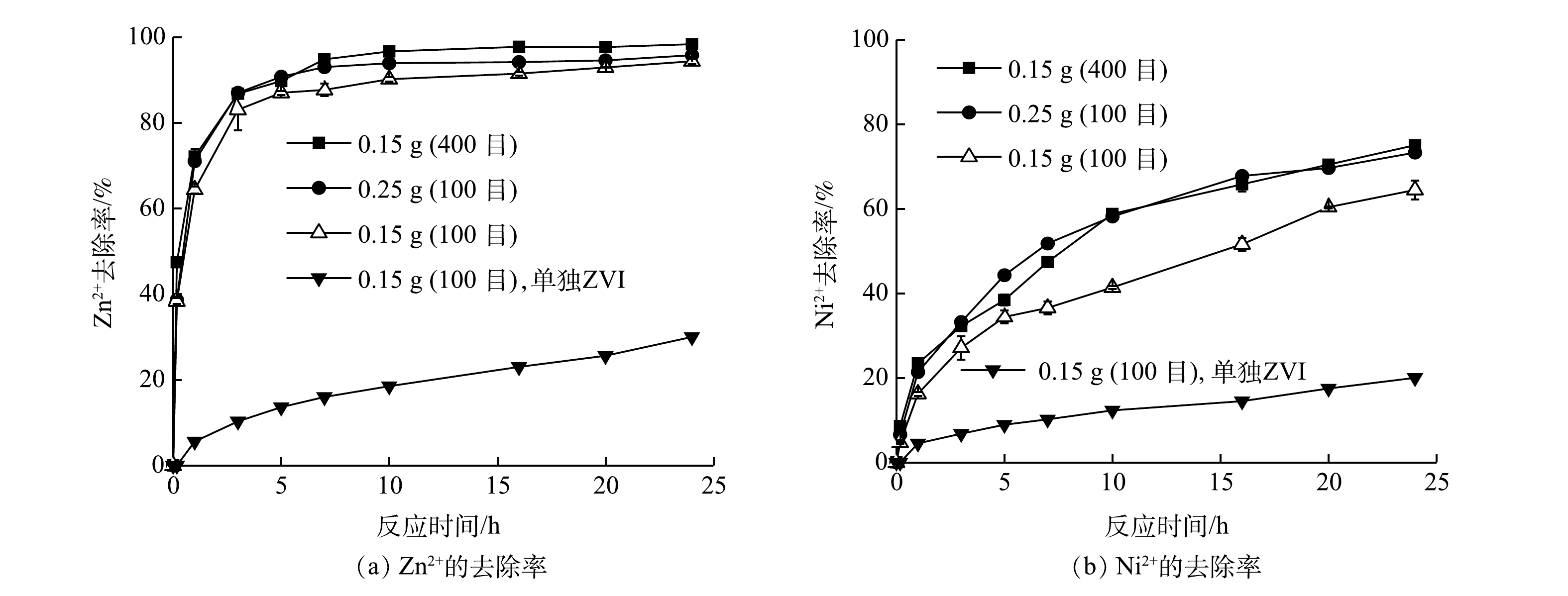
1)不同铁粉用量和不同铁粉粒径的影响。为了评估Fe0/Fe3O4的活性,对Fe0/Fe3O4与Fe0处理Zn2+和Ni2+的效果进行了比较,结果见图4。显然,Fe0/Fe3O4的活性远高于Fe0。在相同铁粉用量下,反应24 h后,Fe0对Zn2+和Ni2+的去除率分别只有31%和20%;而Fe0/Fe3O4对Zn2+和Ni2+的去除率分别为93%和62%。因此,后续的实验只考察Fe0/Fe3O4在不同条件下对复合重金属离子的去除效果。
通常,铁粉粒径越小或铁粉用量越大,均会促进Fe0对污染物的去除[26]。由图4可以看出,随着铁粉粒径减小或铁粉用量的增加,Fe0/Fe3O4去除Ni2+和Zn2+的效率均升高。当加入0.15 g 100目的铁粉时,反应24 h以后,Zn2+的去除率为93%,而Ni2+去除率为62%。当铁粉用量增加到0.25 g或铁粉用量不变而粒径减小为400目时,Zn2+的去除率分别提高到95%和98%,而Ni2+去除率均约为70%。增加铁粉用量或减小铁粉粒径均可增加铁粉表面活性位点[24],从而提高反应速率。在相同条件下,Zn2+的去除率明显高于Ni2+,说明Zn2+更容易被吸附。由于Zn的标准电位(-0.76 V)比Fe(-0.44 V)低,Fe0不可能将Zn2+还原,只能通过铁腐蚀产物的吸附和共沉淀来去除。前人在研究微米或纳米Fe0处理Zn2+的过程中,也认为吸附和共沉淀是其去除机理[27-28]。由于Fe3O4的导电性,不会钝化铁表面,从而使铁腐蚀反应能够持续进行,不断产生新的铁腐蚀产物,从而能够持续地去除Ni2+和Zn2+。
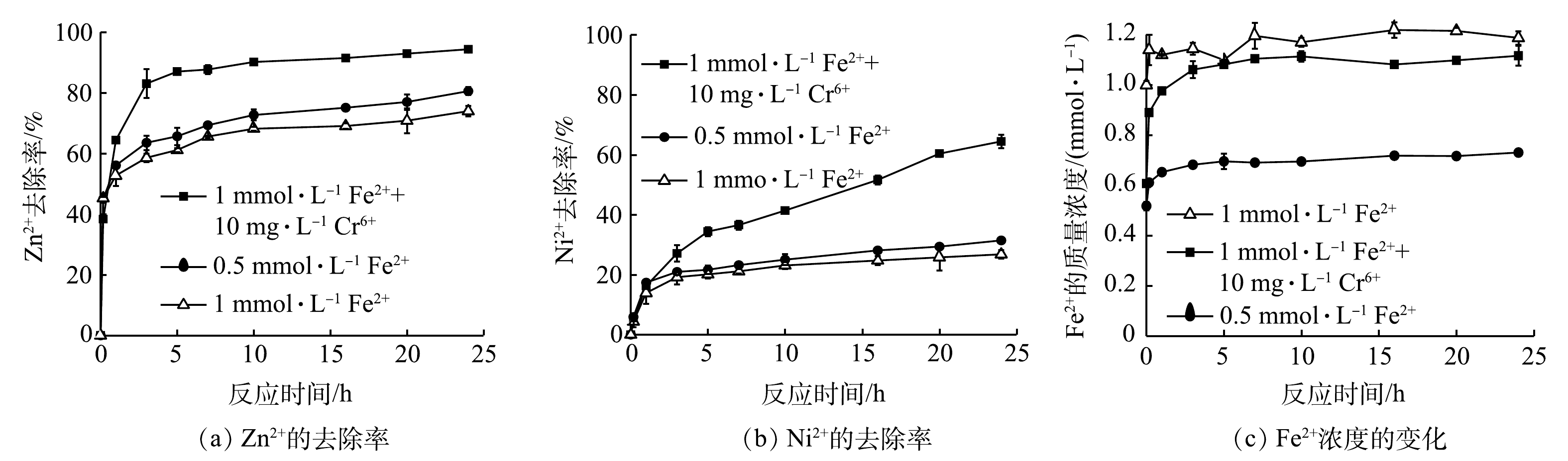
2)预处理时,不同Fe2+浓度对重金属去除的影响。在Fe0预处理的时候,利用反应瓶溶液中的溶解氧(来自瓶中顶部空气中的氧气)作为氧化剂,借助溶液中的Fe2+,通过一系列化学反应在铁表面形成一层Fe3O4膜,从而得到Fe0/Fe3O4复合体系。因此,在预处理的时候,氧气和Fe2+的量都可能改变复合体系中Fe3O4的量,从而改变后续的反应速率。同时,Fe2+与Zn2+和Ni2+的价态一样、离子半径也相似,可能会对其吸附产生抑制作用。因此,预处理的时候,分别加入0.5、1.0和2.0 mmol·L−1Fe2+,考察其对不同重金属去除的影响。由图5(a)可知,预处理以后,溶液中还保留了大量的溶解态Fe2+。起始加入0.5、1.0和2.0 mmol·L−1 Fe2+,预处理后,分别检测到0.37、0.71和1.8 mmol·L−1溶解态的Fe2+。这表明在预处理阶段,只要少量的Fe2+就可以完成预处理。与重金属混合液反应后,Fe2+浓度逐渐升高,在前2 h内增加最快,随后逐渐减慢并趋于稳定。这说明,在反应过程中,伴随重金属的去除,可以释放出Fe2+。其中,Zn2+不可能与Fe0发生置换反应,只有Ni2+有可能被Fe0还原,释放等量的Fe2+[29-30]。但也有研究认为,当Zn2+吸附到铁腐蚀产物表面后,会通过水解过程释放等量的H+,这些H+再与Fe0反应,释放出与Zn2+等量的Fe2+[31]。由图5(b)、图5(c)可知,体系中过多的Fe2+会抑制Fe0/Fe3O4对Zn2+和Ni2+的去除,尤其对Ni2+的去除抑制作用更明显。当Fe2+起始浓度为0.5 mmol·L−1和1.0 mmol·L−1时,对Zn2+的去除影响不大,反应24 h以后,去除率均在90%左右;但当Fe2+起始浓度增加到2.0 mmol·L−1,在反应前8 h内,Zn2+去除速率最慢,但在24 h以后,去除率与其它Fe2+浓度的相当。这说明Fe2+对Zn2+去除的影响比较小,对于低浓度的Fe2+(≤2.0 mmol·L−1),可以忽略其影响。但对于Ni2+的去除,Fe2+的抑制作用非常明显,随着其浓度的增加,其抑制作用则增强。当预处理Fe2+为0.5 mmol·L−1时,Ni2+的去除效果最高,可以达到77%;但当Fe2+初始浓度分别为1.0 mmol·L−1和2.0 mmol·L−1时,Ni2+去除率分别只有65.48%和48.85%。这可能归因于以下2点:一是由于过多的Fe2+会抑制Fe0与Ni2+的置换反应(Fe0 + Ni2+→ Fe2+ + Ni0),从而不利于Ni2+的去除;二是由于Ni2+在铁氧化物表面的吸附能力比Zn2+和Fe2+差。
3)不同Cr(Ⅵ)浓度对Zn2+和Ni2+去除的影响。Cr(Ⅵ)是环境中很常见的重金属污染物,常与Zn2+和Ni2+等重金属共存于污染水体中。Cr(Ⅵ)的水溶性好,迁移能力很强,容易被生物利用。图6是不同浓度的Cr(Ⅵ)对Zn2+和Ni2+去除的影响。相比于不加Cr(Ⅵ)的体系,低浓度(10 mg·L−1)的Cr(Ⅵ)会显著促进Ni2+和Zn2+的去除,反应24 h后,Zn2+的去除率达到92%,Ni2+的去除率达到67.5%。但随Cr(Ⅵ)浓度的升高,其促进作用不明显,甚至在反应开始后的最初几个小时,反而抑制了Ni2+和Zn2+的去除,直到24 h后,Cr(Ⅵ)对Ni2+和Zn2+的去除最终表现为促进作用,且促进作用较小。Cr(Ⅵ)影响Zn2+和Ni2+去除的原因主要有2点:一是由于Cr(Ⅵ)的还原也会消耗Fe2+,从而减少了Fe2+的竞争吸附作用,有助于Zn2+和Ni2+的去除;二是由于Cr(Ⅵ)被还原为Cr(Ⅲ)后,与Fe(Ⅲ)形成共沉淀FexCr1-x(OH)3,附在Fe0表面,抑制了电子传递和Fe0的腐蚀反应,从而不利于Ni2+和Zn2+的去除[32-33]。相比于Zn2+和Ni2+,Cr(Ⅵ)在很短的时间内就被快速去除(图6(c))。这说明Fe0/Fe3O4可以快速去除Cr(Ⅵ)。对于相对低浓度的Cr(Ⅵ)与Zn2+和Ni2+共存时,Fe0/Fe3O4可以同时快速地去除3种重金属,但当Cr(Ⅵ)浓度过高时,只能有效地去除Cr(Ⅵ),而对Zn2+和Ni2+的去除效果并不理想。
4)不同Fe2+预处理及其与Cr(Ⅵ)共存时对重金属去除的影响。根据以上结果,在预处理中,过高浓度的Fe2+不利于后续对Zn2+和Ni2+的去除。而低浓度的Cr(Ⅵ)会显著促进对Zn2+和Ni2+的去除。因此,单独考察了在预处理过程中加入不同初始浓度的Fe2+以及同时加入Fe2+和Cr(Ⅵ)对Fe0/Fe3O4去除Zn2+和Ni2+的影响。如图7所示,不加Cr(Ⅵ)时,预处理Fe2+浓度升高,不利于后续Zn2+和Ni2+的去除。这与前面的结果一致。在相同条件下,加入10 mg·L−1Cr(Ⅵ)后, Zn2+的去除率由74%提高到94%。Fe2+增加不利于Ni2+的去除,但在加入Cr(Ⅵ)后,Ni2+的去除率由26%提高到65%。加入Cr(Ⅵ)会消耗部分Fe2+,这也是其促进作用的原因之一。此外,有研究[34]表明,Fe(Ⅲ)与Cr(Ⅵ)还原产生的Cr(Ⅲ)形成CrxFe1-x(OH)3,该产物具有很好的混凝沉淀效果,能够吸附去除重金属离子。但是,产生的CrxFe1-x(OH)3覆盖在铁表面,由于其导电性差,不利于后续铁腐蚀反应的进行,因而对Ni2+和Zn2+的去除产生不利的影响。因此,Cr(Ⅵ)对Ni2+和Zn2+去除会受到Cr(Ⅵ)浓度变化的影响。
-
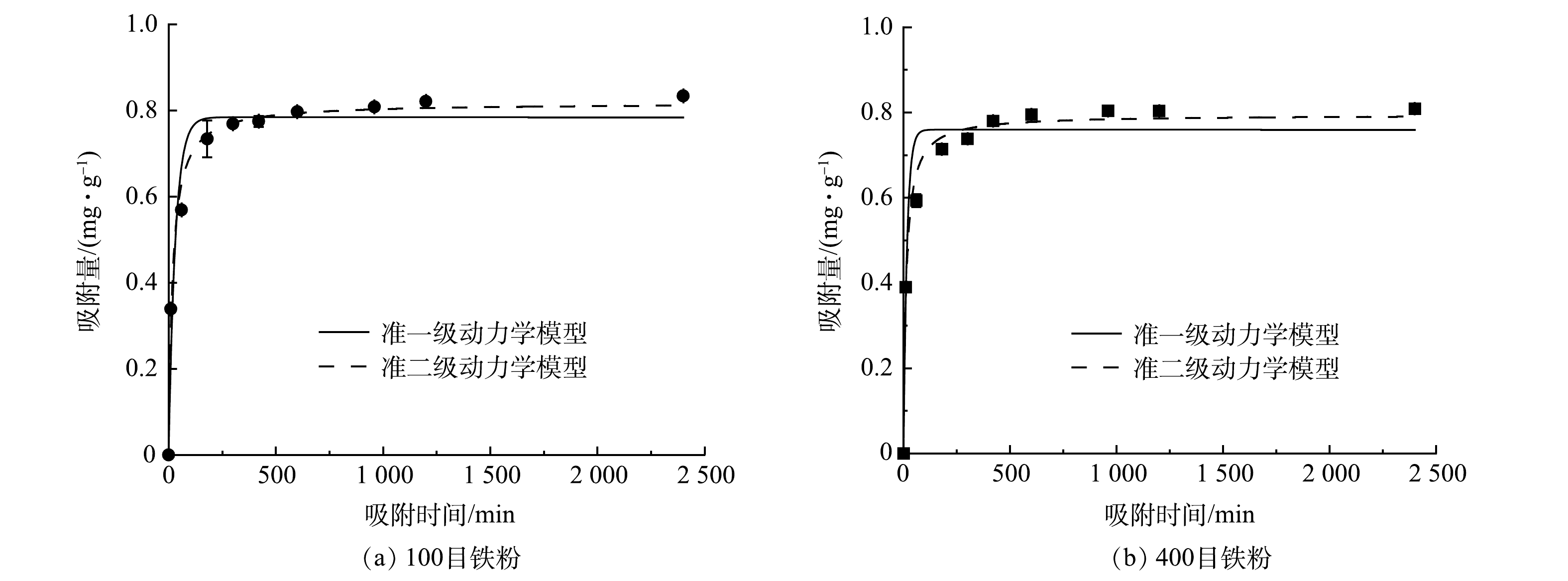
对所获得的实验结果分别用准一级动力学和准二级动力学模型进行了拟合。由图8可看出,不论哪种粒径的铁粉,对于Zn2+的去除,反应前10 min均非常迅速,很快就达到平衡。如图9所示,Ni2+的去除速率在反应初期慢很多,平衡吸附量也比Zn2+低。这说明Fe0对Zn2+的吸附性能要明显优于Ni2+。由表2可见,准二级动力学模型的可决系数R2要稍微优于准一级动力学模型,说明Fe0/Fe3O4对Zn2+和Ni2+的吸附更符合准二级动力学模型。这也表明在整个吸附过程中,化学吸附占主导地位。这与Fe0去除Zn2+和Ni2+的结论一致[35- 36]。
-
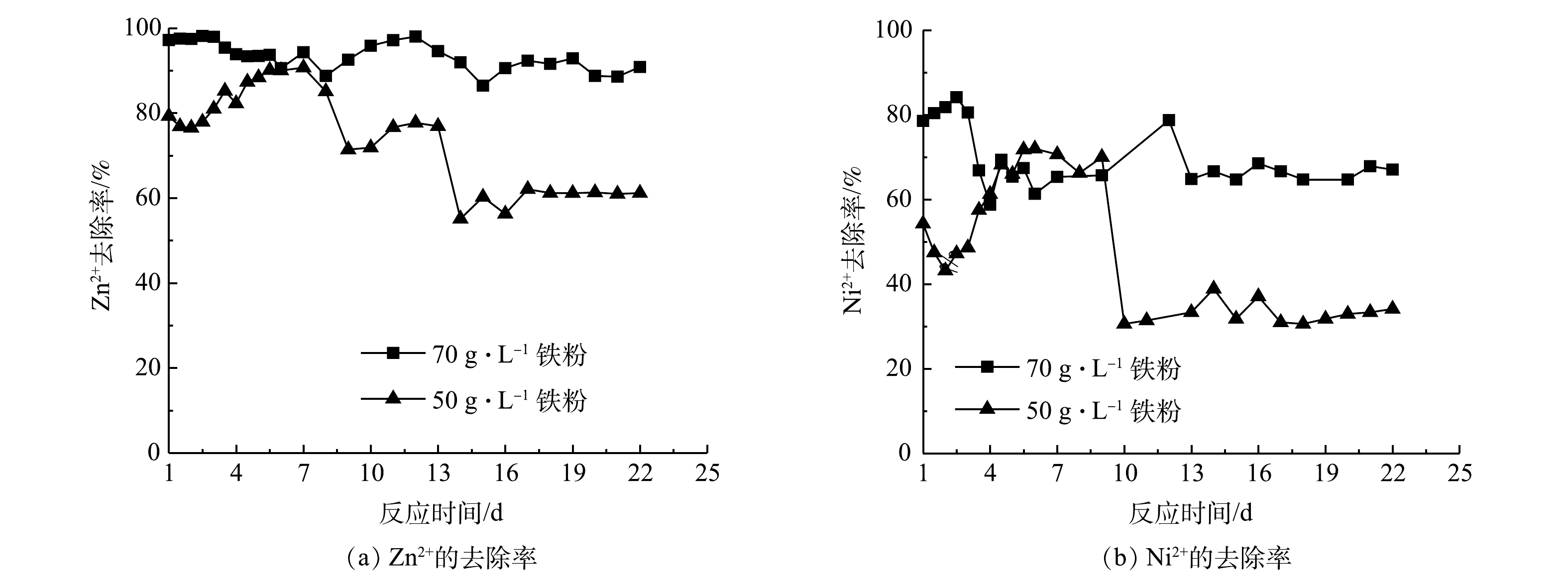
1)不同铁粉浓度对复合重金属去除的影响。从前面的批处理实验结果可知,Fe0/Fe3O4是一个高活性的系统,可以持续有效地去除Zn2+、Ni2+。由图10可见,以K2Cr2O7作为预处理剂,当铁粉质量浓度从50 g·L−1提升到70 g·L−1时,Zn2+和Ni2+的去除率随之升高。当铁粉质量浓度70 g·L−1时,在22 d的运行时间内,Zn2+的去除率基本维持在90%左右,而Ni2+的去除率虽然波动较大,仍可维持在68%左右。但当铁粉质量浓度降到50 g·L−1时,在前7 d,Zn2+的去除率在80%~90%,然后逐渐降低,15 d以后,维持在62%;在第6~9天,Ni2+的去除率保持在70%左右,随后快速降低到30%左右,并一直保持到第22天。增加铁粉用量,单位体积内有更多的铁表面活性点位吸附Ni2+和Zn2+,其去除效果更好。这也与前面的批处理实验结果一致。对于50 g·L−1的铁粉用量,在起始阶段,其去除率就比70 g·L−1的铁粉用量下低,虽然随后差距缩小,但十几天以后,去除率远低于70 g·L−1。这可能是由于:在初始阶段,铁粉腐蚀产物的不断增加,减小了低浓度铁粉带来的影响;但是随着铁粉的不断消耗,产生的腐蚀产物越来越少,其处理效率也很快降低,但由于Zn2+和Ni2+的浓度不高(10 mg·L−1),因而依然可以保持相对稳定的去除效果。这说明50 g·L−1的铁粉用量过少,难以维持持续、有效的处理效果,最好要保持70 g·L−1或更高的铁粉质量浓度。对于Cr(Ⅵ)的处理效果,与前面批处理的结果一致,很快就被去除完全,因此,这里没有给出其结果。
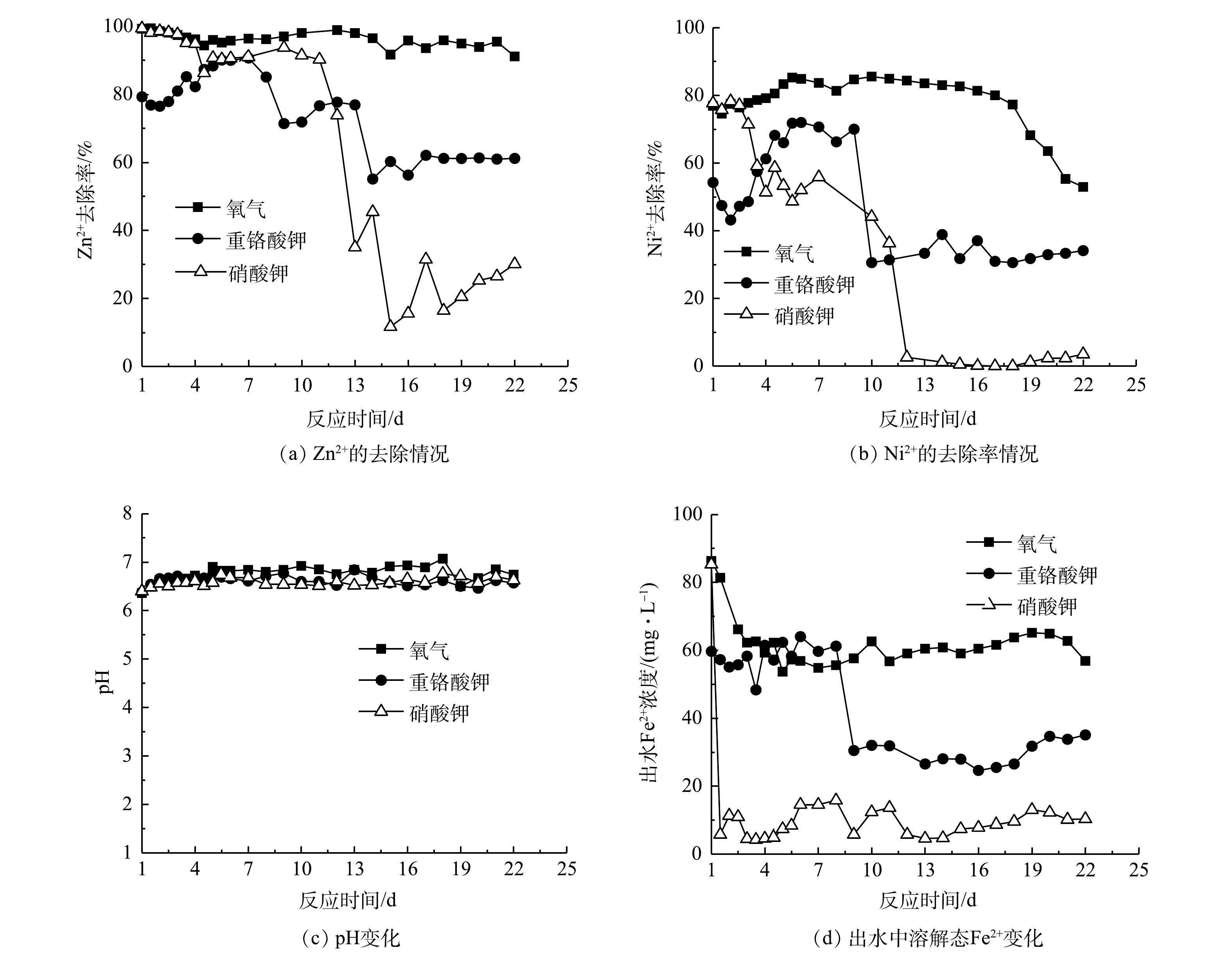
2)不同氧化剂对复合重金属去除的影响。在预处理阶段,加入氧化剂才能生成复合体系。因此,比较了不同氧化剂(氧气、硝酸盐和重铬酸钾)对复合体系活性的影响,结果如图11所示。以氧气(空气)预处理制备的复合体系对Zn2+和Ni2+的去除率最高。连续运行22 d,Zn2+的去除效果一直可以保持在90%以上,而Ni2+的去除率在前18 d可稳定在在80%左右,随后去除率逐渐降低,到第22天的时候,只有50%。以重铬酸钾预处理时,在第一周内,Zn2+和Ni2+的去除效果持续升高,最高分别达到91%和72%,但随后又不断下降,尤其是Ni2+,在第10天的时候,去除率快速下降到~30%。以硝酸钾作为预处理剂,Ni2+和Zn2+的处理效果最差。在前10 d,对Zn2+的去除效果保持在90%左右,随后去除效果快速降低,而且不稳定。而对于Ni2+的去除,从一开始就持续下降,12 d以后,几乎没有去除。从3种体系中的pH来看,并没有明显差别(图11(c))。在3种氧化剂预处理体系中,Fe2+的浓度差别较大(图11(d))。在氧气体系中,Ni2+和Zn2+的去除效果最好,所以Fe2+的浓度一直最高;而另外2个反应器中的Fe2+最低,尤其是硝酸钾预处理的反应器,出水中的Fe2+仅有5 mg·L−1左右。与批处理实验的结果相似,出水中都没有检测到溶解态的Cr(Ⅵ)。这说明复合体系对Cr(Ⅵ)的处理效果有保障。总体而言,以氧气作为预处理剂的效果最好,其次是用重铬酸钾和硝酸钾的预处理。
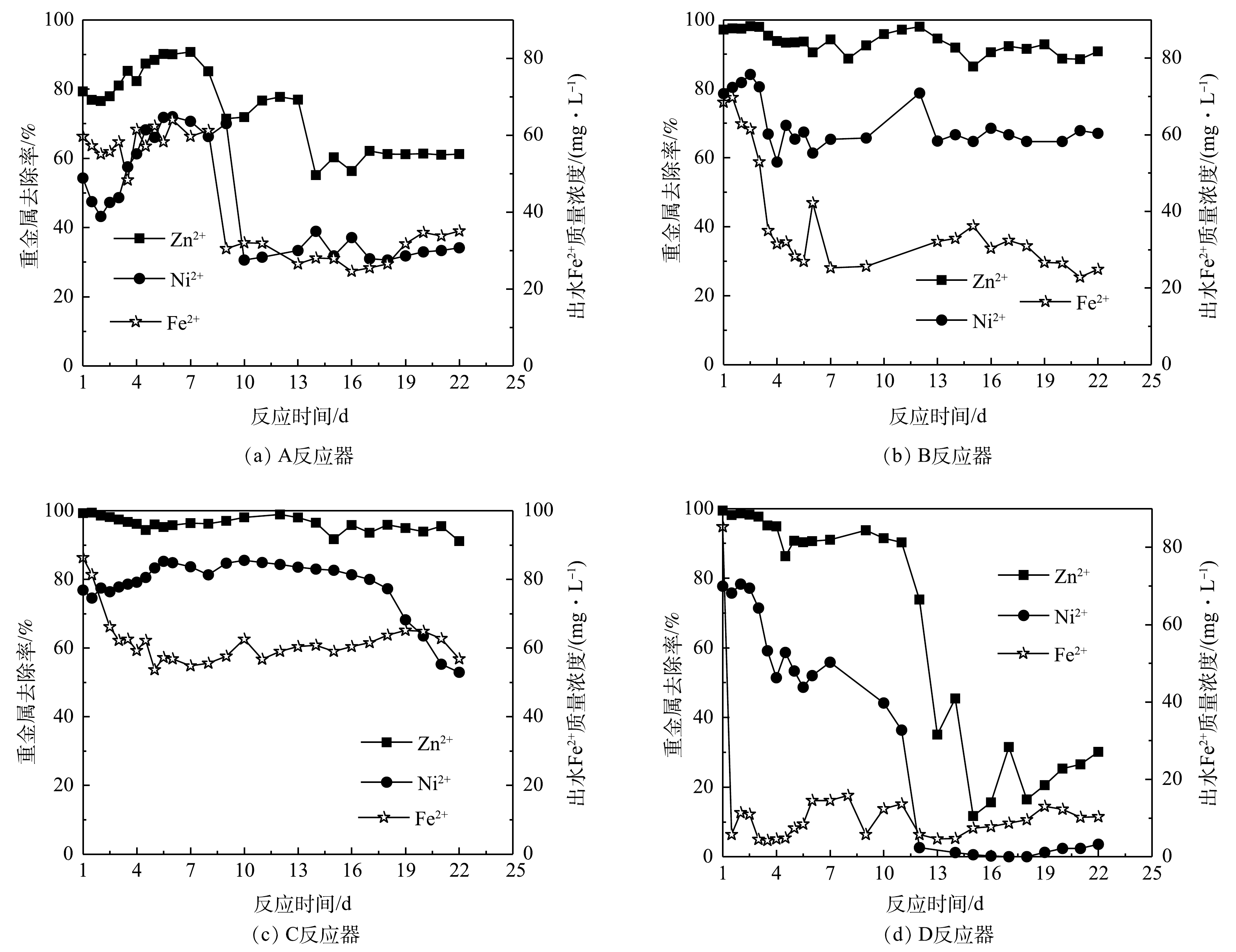
3)4个反应器去除复合重金属的效果比较。图12展示了4个流化床反应器对Zn2+和Ni2+的处理效果。各流化床反应器的操作条件见表1。其中,由于4个反应器的出水中都未检测到溶解态的Cr(Ⅵ),故未提供其数据。由图12可看出,反应器C对Zn2+和Ni2+的处理效果最好,Zn2+的去除率都维持在90%以上;Ni2+在前17 d的去除率一直保持在80%左右,随后不断下降,到第22天,去除率降到50%。其次是反应器B,对Zn2+的去除效果基本保持在90%左右,尽管Ni2+的去除率在开始阶段波动很大,但运行7 d后也可稳定在68%左右。C反应器出水中的Fe2+浓度最高,这说明在Zn2+和Ni2+去除的同时释放出Fe2+,与前文的实验结果一致。尽管C反应器(50 g·L−1)的铁粉质量浓度比B反应器(70 g·L−1)低,但C反应器的处理效果最好,这说明以氧气作为预处理剂制备的复合体系活性最强。A反应器在前9 d对Ni2+和Zn2+的去除率较高,但随后快速下降,Fe2+浓度的变化趋势类似。D反应器的处理效果最差,不管是Zn2+还是Ni2+,去除率很快下降。综上所述,复合体系对Zn2+的去除效果要远远好于Ni2+,这与批处理实验的结果一致。4个反应器的Fe2+出水浓度与Ni2+和Zn2+的变化趋势一致。出水中的Fe2+浓度越高,Ni2+和Zn2+的去除效果也越好。这说明Ni2+和Zn2+的去除过程中,会直接或间接影响产生Fe2+。
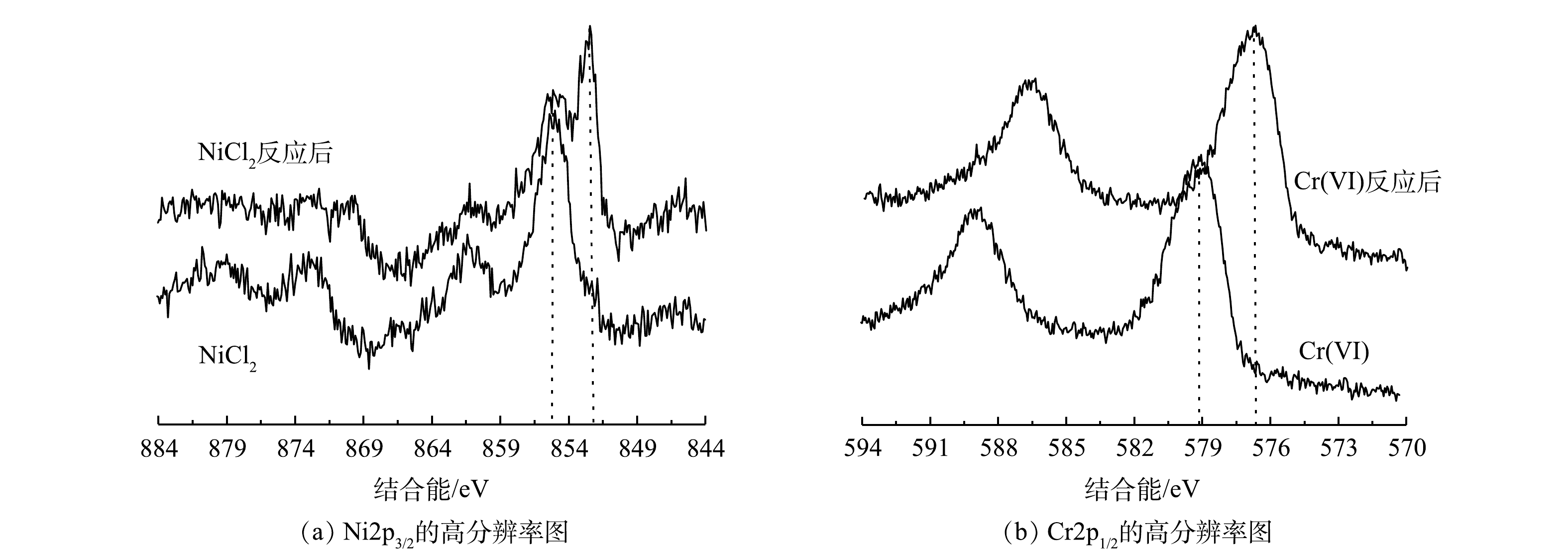
4)重金属去除机理分析。批处理和流化床实验的结果均说明Fe0/Fe3O4具有很强的活性,可以持续地去除Cr(Ⅵ)、Zn2+和Ni2+。对三者的处理速率为Cr(Ⅵ)>Zn2+>Ni2+。在很短的时间内Cr(Ⅵ)就可以被快速去除。根据3种重金属和Fe0的标准电位,可以从理论上判断不同重金属的去除机理。根据Zn和Ni的标准电位,Fe0不可能还原Zn2+,只能通过铁腐蚀产物的吸附和共沉淀去除。Ni2+的标准电位稍微高于Fe0,其氧化还原反应的驱动力很小,还原反应的速率很慢,也可能不会发生氧化还原反应。Cr(Ⅵ)的标准电位为1.33V,远高于Fe0,因此,很容易被Fe2+和Fe0还原。为确定Cr(Ⅵ)和Ni2+的去除机理,通过XPS对反应前后Cr和Ni的价态进行了分析,结果如图13所示。反应前,Ni2+的2p3/2结合能在856.5 eV(图13(a)),反应后在852.6 eV处出现1个尖峰,归属于单质镍,856.5 eV处的峰变弱。这说明部分Ni2+被还原为Ni0,部分Ni(Ⅱ)可能是被吸附或共沉淀。在纳米Fe0去除Ni2+的过程中,也存在部分还原和部分吸附的现象[37-38]。由图13(b)可见,反应后Cr(Ⅵ)的2p1/2峰(579.2 eV)消失,而在577.1 eV处出现1个几乎相同的峰,可归于Cr(Ⅲ),说明Cr(Ⅵ)全部被还原为Cr(Ⅲ)。已有的研究[39-40]也表明Fe0可以有效地还原Cr(Ⅵ)为Cr(Ⅲ)。
-
1)经过预处理后,在Fe0表面生成一层晶型完好、晶相单一的Fe3O4。复合体系的活性远高于单独的Fe0,显著提高其对Zn2+、Ni2+和Cr(Ⅵ)的去除率。
2)增加铁粉用量或减小铁粉粒径均可提高对Zn2+和Ni2+的去除效果。Fe2+浓度升高对Fe0/Fe3O4去除Zn2+的影响不大,但会明显抑制对Ni2+的去除。10 mg·L−1Cr(Ⅵ)会促进Fe0/Fe3O4对Zn2+和Ni2+的去除,但过多的Cr(Ⅵ)由于与Fe(Ⅲ)共沉淀覆盖在Fe0表面,不利于铁腐蚀反应的进行,从而大大降低其促进作用。无论是改变铁粉用量、铁粉粒径、Fe2+或Cr(Ⅵ)浓度,还是预处理氧化剂的不同,对Cr(Ⅵ)的处理效果最好,其次是Zn2+和Ni2+。
3)在流化床反应器中,Fe0/Fe3O4可有效去除Zn2+、Ni2+和Cr(Ⅵ),增加铁粉用量可提高Zn2+和Ni2+的去除效果。相比于重铬酸钾和硝酸钾,以空气作为预处理辅助剂,制备的复合体系活性最强,能持续、有效地去除Zn2+和Ni2+。
4)在Fe0/Fe3O4中,Ni2+的去除机理为部分还原为单质镍、部分吸附或共沉淀,而Cr(Ⅵ)全部被还原为Cr(Ⅲ)。
Fe0/Fe3O4复合材料同时去除水中多种重金属的效果
Simultaneous removal of multiple heavy metals from water using Fe0/Fe3O4 composite materials
-
摘要: 零价铁(Fe0)具有中等还原性,可通过还原反应或通过铁腐蚀产物的吸附和共沉淀过程去除水中重金属。但由于铁表面易钝化,导致铁腐蚀反应难以持续,这是限制Fe0应用的关键。因此,本研究通过原位制备零价铁复合体系(Fe0/Fe3O4),并采用批处理和连续流的流化床反应器考察了该复合体系同时去除水中Zn2+、Ni2+和Cr(VI)的效果。XRD和SEM的结果表明,预处理可以在Fe0表面形成一层晶型完好的Fe3O4。相比Fe0,Fe0/Fe3O4体系可显著促进Zn2+、Ni2+和Cr(Ⅵ)的去除。在不同条件下,复合体系均能在几分钟内高效去除Cr(Ⅵ)(>99%),Zn2+的去除率较好(65%~93%),但Ni2+的去除率较差(25%~77%)。Fe0粒径越小、用量越多,重金属去除速率越快。在预处理制备复合体系时,Fe2+含量越高,残留的溶解性Fe2+也越高,由于竞争吸附作用,这会抑制Zn2+和Ni2+的吸附。当3种重金属共存时,少量(10 mg·L−1)的Cr(Ⅵ)会明显促进Zn2+和Ni2+的去除;但过多的Cr(Ⅵ),其促进作用有所减弱。流化床反应器的结果也证明了Fe0/Fe3O4能有效地同时去除Zn2+、Ni2+和Cr(Ⅵ),三者的去除速率为Cr(VI) > Zn2+> Ni2+,与批处理结果一致。Fe0用量越大,处理效果越好。不同氧化剂作为预处理剂时,氧气(曝气)的效果最好,其次是重铬酸钾和硝酸钾。XPS结果表明,Cr(Ⅵ)可全部被还原为Cr(Ⅲ),Ni2+部分被还原为Ni0,部分以Ni(Ⅱ)的形式通过吸附或沉淀的方式被固定。本研究提出了一种有效解决Fe0表面钝化的方法,为Fe0用于水体重金属污染的修复方法提供参考。Abstract: Zero-valent iron (Fe0) is a type of metal with moderate reducibility, it can be used to remove heavy metals from water through Fe0 reduction, adsorption and/or co-precipitation by iron corrosion products. However, Fe0 corrosion reaction is not continuously occurred due to its surface passivation, which is the key step to limit the application of Fe0. In this study, Fe0/Fe3O4 hybrid system was prepared in situ by simple chemical method. Then simultaneous removal of Zn2+, Ni2+ and Cr(Ⅵ) from water by this hybrid system was investigated using batch experiment and fluidized bed reactor with continuous flow. The results showed that pretreatment could lead the coating of Fe3O4 with good crystal structure on Fe0 surface, which was identified by SEM and XRD. Compared with Fe0 alone, Fe0/Fe3O4 system significantly improved the removal efficiencies of Zn2+, Ni2+ and Cr(Ⅵ). Under different conditions, Cr(Ⅵ) could be almost completely (>99%) removed by the hybrid system in a few minutes, Zn2+ could be well removed with the efficiency of 65%~93%, while Ni2+ showed a low removal efficiency of 25%~77%. The smaller particle size and more dosage of Fe0 promoted heavy metals removal. During pretreatment, the higher Fe2+ concentration, the higher dissolved Fe2+, which could inhibit the adsorption of Zn2+ and Ni2+ due to competitive effect. The presence of low concentration of Cr(Ⅵ) (10 mg·L−1) could significantly promote the removal of Zn2+ and Ni2+ in the co-presence of these three heavy metals, but its promotion effect decreased in the presence of excessive Cr(Ⅵ). The results of fluidized bed reactor also proved that Fe0/Fe3O4 could effectively remove Zn2+, Ni2+ and Cr(Ⅵ) simultaneously. The order of removal efficiencies of three heavy metals was Cr(Ⅵ) > Zn2+> Ni2+, which was consistent with the results of batch experiment. The higher Fe0 dosage, the higher removal efficiencies of heavy metals. Oxygen (aeration) exhibited the best performance as a preconditioning agent during pretreatment, potassium dichromate and potassium nitrate followed. The results of XPS showed that Cr(Ⅵ) was totally reduced to Cr(Ⅲ), Ni2+ was partially reduced to Ni0, and a partial Ni2+ was absorbed or co-precipitated in the form of Ni(Ⅱ). This study presents a novel method to effectively overcome the iron surface passivation, and provides a theoretical reference for the application of zero-valent iron-based technology for heavy metal-polluted water treatment.
-
Key words:
- zero-valent iron /
- heavy metals /
- hybrid system /
- fluidized bed reactor /
- reduction
-
如果大量含重金属的废水、固废等未经妥善处理直接排入环境,会造成地表水重金属污染[1]。同时,多种重金属复合污染的问题也越来越普遍[2]。重金属进入环境后,会随食物链不断富集,最后通过直接或间接摄入的方式进入人体,引起多种疾病,因而一直受到环保学者的广泛关注[3-4]。重金属不能被降解,只能通过改变其形态或价态的方式来降低其迁移能力和生物有效性。通常,重金属污染水体的治理方法主要有化学沉淀法、氧化还原、生物处理和吸附法[5-7]。随着环保标准越来越严,急切需要更有效的方法来处理重金属复合污染。锌(Zn)、镍(Ni)和铬(Cr)是水体中很常见的重金属污染物。其中,Zn和Ni主要以阳离子的形式存在于水体中,而Cr主要以Cr2O72−、CrO42−等阴离子的形式存在于水体中。对于这些以不同离子形态共存的重金属复合污染水体,其处理难度更大。
零价铁(Fe0或ZVI)具有来源广泛、价格低廉、生态风险小和中等还原性(E0= -0.44V)的特点,已成为环境污染控制和修复的重要材料之一[8-10]。通过还原反应,Fe0可以处理标准电位比其高的重金属,例如Cr(Ⅵ)、Cu(Ⅱ)、Hg(Ⅱ)、Ag(Ⅰ)、As(Ⅴ)和Se(Ⅵ)等[11-14],但对于标准电位与其非常接近的重金属元素(Ni(Ⅱ)或Zn(Ⅱ)),则主要依靠铁腐蚀产物的吸附和共沉淀作用,处理效果都不够理想[9,15]。不管是通过Fe0的直接还原,还是通过铁腐蚀产物的吸附和共沉淀,都必须保证Fe0腐蚀反应的持续进行,从而不断释放电子并且产生新的腐蚀产物。常规的Fe0体系,由于产生的三价铁腐蚀产物覆盖在Fe0表面,抑制了Fe0腐蚀反应的持续进行,从而容易导致Fe0表面钝化,这是限制Fe0技术被广泛应用的关键瓶颈[16]。为了延缓Fe0表面钝化,很多学者提出了不同的方法,如酸洗[17]、超声处理[18]、弱磁场[19]、双金属体系[20]等。TANG等[21-22]通过直接加入或反应生成Fe3O4,形成复合体系Fe0/Fe3O4,可显著提高Fe0对Se(Ⅵ)的还原速率。由于Fe3O4是半导体,不会阻碍电子传递,从而可以持续保持Fe0的活性。同时,利用Fe3O4的磁性,能实现固-液的快速分离。目前,大多数的研究是直接加入Fe3O4或利用化学反应生成Fe3O4。这样做操作比较麻烦,且难以保证只生成Fe3O4,往往是不同铁氧化物/氢氧化物的混合物。如果能通过简单的方法,在Fe0表面只生成Fe3O4,则在实际应用中将更方便。基于上述研究,本研究通过简便方法,原位制备了Fe0/Fe3O4复合体系,并且考察了该体系同时去除Zn2+、Ni2+和Cr(Ⅵ) 3种重金属的效果和机理;通过批处理实验和连续进出水的流化床反应器考察了不同反应条件对复合体系去除这3种重金属的影响;并借助XRD、SEM和XPS,探讨了对不同金属的去除机理。
1. 材料与方法
1.1 实验试剂
还原铁粉(98%)(100目和400目)、锌标样、镍标样、铬标样、乙酸铵、乙酸、氯化锌(ZnCl2)、氯化镍、重铬酸钾、氯化亚铁、盐酸、硝酸、邻菲罗啉等试剂均为分析纯;溶液配制及材料制备均采用去离子水。
1.2 实验方法
1)批处理实验。称取0.15 g和0.25 g的铁粉置于20 mL血清瓶中,再加入14 mL去离子水和1 mL FeCl2储备液,此时反应瓶内顶部还有一定体积的空气,再密封后将反应瓶置于360°旋转的木箱中,以30 r·min−1的转速开始反应一定时间。预处理完成后,再用微量注射器加入300 μL的Zn2+、Ni2+和Cr(Ⅵ)的混合储备液,得到10 mg·L−1的Ni2+和Zn2+以及不同浓度的Cr(Ⅵ),再将反应瓶放入旋转箱中反应并开始计时,在预定时间每次取出2个反应瓶作为重复,打开塞子后先测定溶液pH,再经0.45 μm滤膜过滤,收集滤液测定Fe2+和重金属离子的浓度。在Fe2+的样品中加入2滴3 mol·L−1的HCl酸化保存待测。其余滤液加入1滴3 mol·L−1HNO3保存,待测金属离子。采用准一级(式(1))、准二级(式(2))动力学方程拟合各重金属去除情况。
ln(qe−qt)=lnqe−k1t (1) tqt=1k2qe2+1qet (2) 式中:
qe qt k1 k2 2)流化床反应器实验。使用纯水配制50 mmol·L−1FeCl2和30 mmol·L−1HCl混合液,进水中Zn、Ni和Cr的质量浓度均为10 mg·L−1。4个流化床反应器的操作条件见表1,流化床反应器的示意图见图1。通过可调式电子机械搅拌器搅动溶液,使铁粉悬浮于溶液中,防止铁粉沉积到反应器底部。反应器的有效体积为11.5 L。与批处理实验类似,流化床反应器也需经过预处理,先制备Fe0/Fe3O4复合体系。预处理过程中,只添加不同的氧化剂,反应2~3 d完成预处理,后再通入重金属模拟废水。模拟废水用自来水配置,为防止重金属沉淀,先用HCl将溶液pH调为7.0,再加入重金属试剂,采用连续进出水的方式运行。在反应区反应后,通过溢流口排出,固体会自动下沉流入反应区,从而保证出水所含悬浮物少,减少铁粉的流失。
表 1 不同流化床反应器的操作参数Table 1. Operation parameters of different fluidized bed reactors条件反应器 铁粉粒径/目 铁粉浓度/(g·L−1) 预处理氧化剂 预处理氧化剂浓度/(mg·L−1) 水力停留时间/h 搅拌器转速/(r·min−1) A 400 50 K2Cr2O7 20 5 700 B 400 70 K2Cr2O7 20 5 700 C 400 50 DO(曝气) / 5 700 D 400 50 KNO3 20 5 700 1.3 分析方法
采用原子吸收光谱(PerkinElmer 900T)测定滤液中的Zn2+、Ni2+和总Cr;采用二苯碳酰二肼分光光度法测定Cr(Ⅵ);采用邻菲啰啉分光光度法(紫外可见光度计,TU-1810,北京普析)在波长510 nm测定Fe2+浓度。文中的结果均为2个平行样。
在制备SEM样品时,按照批处理实验的条件,反应后分离固液,将固体在高纯氮气保护下干燥,再用SEM(日本电子,JSM-7500F)分析。在制备XPS样品时,为了增大待测元素的含量,所加入的各重金属浓度比实验高1倍,其它反应物均相应提高1倍。反应一定时间后,通过超声处理将铁腐蚀物从铁粉表面剥落下来,再过滤分离腐蚀物,通过连续吹入高纯氮气风干样品用于XPS(日本岛津,AXIS Supra)分析。XPS采用单色铝Kα X射线(hv=1 486.6 eV),管电压15 kV,管电流12 mA,功率180 W,步长为0.05 eV,分析Ni和Cr反应前后的价态变化,以C1s 284.5 eV校正结合能,采用XPSPEAK4.1软件分析结果。在制备XRD样品时,与批处理反应条件完全一样,而且反应后,无需任何处理,直接将铁粉分离,然后在高纯氮气气流下干燥,用于XRD分析(日本理学,Ultima IV)。
2. 结果与分析
2.1 材料表征
由图2可看出,复合体系的谱图与Fe3O4标样完全一致。这与SU等[23]制成的纳米Fe3O4峰形一致,表明预处理后,在铁表面形成了晶型完好且纯度很高的Fe3O4,没有其它晶态铁氧化物或氢氧化物峰。这说明,该预处理方法可以在Fe0表面生成了一层晶相单一的Fe3O4,形成了Fe0/Fe3O4复合体系。由图3可看出,反应前的铁粉表面光滑,铁粉颗粒不规则(图3(a))。这与前人的研究观察现象一致[24]。在厌氧条件下反应后,铁表面由于发生反应而变得粗糙,而且有一些白色的小点,可能是沉淀的重金属离子。由于没有强氧化剂的参与,铁腐蚀反应很慢,产生的腐蚀产物也很少(图3(b))。如图3(c)所示,在预处理后复合体系中铁腐蚀产物的表面有很多颗粒状和片状的晶体。这些晶体的形貌与Fe3O4(图3(d))的晶体形态非常相似[21]。WU等[25]经过酸预处理,反应后的铁腐蚀物主要是棒状和片状。这也再次证明腐蚀产物主要是Fe3O4,与图2中的XRD的结果吻合。由此可见,通过预处理可在Fe0表面形成一层晶型完好的Fe3O4。
2.2 Fe0/Fe3O4去除复合重金属的批处理实验
1)不同铁粉用量和不同铁粉粒径的影响。为了评估Fe0/Fe3O4的活性,对Fe0/Fe3O4与Fe0处理Zn2+和Ni2+的效果进行了比较,结果见图4。显然,Fe0/Fe3O4的活性远高于Fe0。在相同铁粉用量下,反应24 h后,Fe0对Zn2+和Ni2+的去除率分别只有31%和20%;而Fe0/Fe3O4对Zn2+和Ni2+的去除率分别为93%和62%。因此,后续的实验只考察Fe0/Fe3O4在不同条件下对复合重金属离子的去除效果。
通常,铁粉粒径越小或铁粉用量越大,均会促进Fe0对污染物的去除[26]。由图4可以看出,随着铁粉粒径减小或铁粉用量的增加,Fe0/Fe3O4去除Ni2+和Zn2+的效率均升高。当加入0.15 g 100目的铁粉时,反应24 h以后,Zn2+的去除率为93%,而Ni2+去除率为62%。当铁粉用量增加到0.25 g或铁粉用量不变而粒径减小为400目时,Zn2+的去除率分别提高到95%和98%,而Ni2+去除率均约为70%。增加铁粉用量或减小铁粉粒径均可增加铁粉表面活性位点[24],从而提高反应速率。在相同条件下,Zn2+的去除率明显高于Ni2+,说明Zn2+更容易被吸附。由于Zn的标准电位(-0.76 V)比Fe(-0.44 V)低,Fe0不可能将Zn2+还原,只能通过铁腐蚀产物的吸附和共沉淀来去除。前人在研究微米或纳米Fe0处理Zn2+的过程中,也认为吸附和共沉淀是其去除机理[27-28]。由于Fe3O4的导电性,不会钝化铁表面,从而使铁腐蚀反应能够持续进行,不断产生新的铁腐蚀产物,从而能够持续地去除Ni2+和Zn2+。
2)预处理时,不同Fe2+浓度对重金属去除的影响。在Fe0预处理的时候,利用反应瓶溶液中的溶解氧(来自瓶中顶部空气中的氧气)作为氧化剂,借助溶液中的Fe2+,通过一系列化学反应在铁表面形成一层Fe3O4膜,从而得到Fe0/Fe3O4复合体系。因此,在预处理的时候,氧气和Fe2+的量都可能改变复合体系中Fe3O4的量,从而改变后续的反应速率。同时,Fe2+与Zn2+和Ni2+的价态一样、离子半径也相似,可能会对其吸附产生抑制作用。因此,预处理的时候,分别加入0.5、1.0和2.0 mmol·L−1Fe2+,考察其对不同重金属去除的影响。由图5(a)可知,预处理以后,溶液中还保留了大量的溶解态Fe2+。起始加入0.5、1.0和2.0 mmol·L−1 Fe2+,预处理后,分别检测到0.37、0.71和1.8 mmol·L−1溶解态的Fe2+。这表明在预处理阶段,只要少量的Fe2+就可以完成预处理。与重金属混合液反应后,Fe2+浓度逐渐升高,在前2 h内增加最快,随后逐渐减慢并趋于稳定。这说明,在反应过程中,伴随重金属的去除,可以释放出Fe2+。其中,Zn2+不可能与Fe0发生置换反应,只有Ni2+有可能被Fe0还原,释放等量的Fe2+[29-30]。但也有研究认为,当Zn2+吸附到铁腐蚀产物表面后,会通过水解过程释放等量的H+,这些H+再与Fe0反应,释放出与Zn2+等量的Fe2+[31]。由图5(b)、图5(c)可知,体系中过多的Fe2+会抑制Fe0/Fe3O4对Zn2+和Ni2+的去除,尤其对Ni2+的去除抑制作用更明显。当Fe2+起始浓度为0.5 mmol·L−1和1.0 mmol·L−1时,对Zn2+的去除影响不大,反应24 h以后,去除率均在90%左右;但当Fe2+起始浓度增加到2.0 mmol·L−1,在反应前8 h内,Zn2+去除速率最慢,但在24 h以后,去除率与其它Fe2+浓度的相当。这说明Fe2+对Zn2+去除的影响比较小,对于低浓度的Fe2+(≤2.0 mmol·L−1),可以忽略其影响。但对于Ni2+的去除,Fe2+的抑制作用非常明显,随着其浓度的增加,其抑制作用则增强。当预处理Fe2+为0.5 mmol·L−1时,Ni2+的去除效果最高,可以达到77%;但当Fe2+初始浓度分别为1.0 mmol·L−1和2.0 mmol·L−1时,Ni2+去除率分别只有65.48%和48.85%。这可能归因于以下2点:一是由于过多的Fe2+会抑制Fe0与Ni2+的置换反应(Fe0 + Ni2+→ Fe2+ + Ni0),从而不利于Ni2+的去除;二是由于Ni2+在铁氧化物表面的吸附能力比Zn2+和Fe2+差。
3)不同Cr(Ⅵ)浓度对Zn2+和Ni2+去除的影响。Cr(Ⅵ)是环境中很常见的重金属污染物,常与Zn2+和Ni2+等重金属共存于污染水体中。Cr(Ⅵ)的水溶性好,迁移能力很强,容易被生物利用。图6是不同浓度的Cr(Ⅵ)对Zn2+和Ni2+去除的影响。相比于不加Cr(Ⅵ)的体系,低浓度(10 mg·L−1)的Cr(Ⅵ)会显著促进Ni2+和Zn2+的去除,反应24 h后,Zn2+的去除率达到92%,Ni2+的去除率达到67.5%。但随Cr(Ⅵ)浓度的升高,其促进作用不明显,甚至在反应开始后的最初几个小时,反而抑制了Ni2+和Zn2+的去除,直到24 h后,Cr(Ⅵ)对Ni2+和Zn2+的去除最终表现为促进作用,且促进作用较小。Cr(Ⅵ)影响Zn2+和Ni2+去除的原因主要有2点:一是由于Cr(Ⅵ)的还原也会消耗Fe2+,从而减少了Fe2+的竞争吸附作用,有助于Zn2+和Ni2+的去除;二是由于Cr(Ⅵ)被还原为Cr(Ⅲ)后,与Fe(Ⅲ)形成共沉淀FexCr1-x(OH)3,附在Fe0表面,抑制了电子传递和Fe0的腐蚀反应,从而不利于Ni2+和Zn2+的去除[32-33]。相比于Zn2+和Ni2+,Cr(Ⅵ)在很短的时间内就被快速去除(图6(c))。这说明Fe0/Fe3O4可以快速去除Cr(Ⅵ)。对于相对低浓度的Cr(Ⅵ)与Zn2+和Ni2+共存时,Fe0/Fe3O4可以同时快速地去除3种重金属,但当Cr(Ⅵ)浓度过高时,只能有效地去除Cr(Ⅵ),而对Zn2+和Ni2+的去除效果并不理想。
4)不同Fe2+预处理及其与Cr(Ⅵ)共存时对重金属去除的影响。根据以上结果,在预处理中,过高浓度的Fe2+不利于后续对Zn2+和Ni2+的去除。而低浓度的Cr(Ⅵ)会显著促进对Zn2+和Ni2+的去除。因此,单独考察了在预处理过程中加入不同初始浓度的Fe2+以及同时加入Fe2+和Cr(Ⅵ)对Fe0/Fe3O4去除Zn2+和Ni2+的影响。如图7所示,不加Cr(Ⅵ)时,预处理Fe2+浓度升高,不利于后续Zn2+和Ni2+的去除。这与前面的结果一致。在相同条件下,加入10 mg·L−1Cr(Ⅵ)后, Zn2+的去除率由74%提高到94%。Fe2+增加不利于Ni2+的去除,但在加入Cr(Ⅵ)后,Ni2+的去除率由26%提高到65%。加入Cr(Ⅵ)会消耗部分Fe2+,这也是其促进作用的原因之一。此外,有研究[34]表明,Fe(Ⅲ)与Cr(Ⅵ)还原产生的Cr(Ⅲ)形成CrxFe1-x(OH)3,该产物具有很好的混凝沉淀效果,能够吸附去除重金属离子。但是,产生的CrxFe1-x(OH)3覆盖在铁表面,由于其导电性差,不利于后续铁腐蚀反应的进行,因而对Ni2+和Zn2+的去除产生不利的影响。因此,Cr(Ⅵ)对Ni2+和Zn2+去除会受到Cr(Ⅵ)浓度变化的影响。
2.3 吸附动力学
对所获得的实验结果分别用准一级动力学和准二级动力学模型进行了拟合。由图8可看出,不论哪种粒径的铁粉,对于Zn2+的去除,反应前10 min均非常迅速,很快就达到平衡。如图9所示,Ni2+的去除速率在反应初期慢很多,平衡吸附量也比Zn2+低。这说明Fe0对Zn2+的吸附性能要明显优于Ni2+。由表2可见,准二级动力学模型的可决系数R2要稍微优于准一级动力学模型,说明Fe0/Fe3O4对Zn2+和Ni2+的吸附更符合准二级动力学模型。这也表明在整个吸附过程中,化学吸附占主导地位。这与Fe0去除Zn2+和Ni2+的结论一致[35- 36]。
表 2 吸附动力学拟合参数Table 2. Adsorption kinetics fitting parameters重金属 铁粉粒径/目 准一级动力学 准二级动力学 qe/(mg·g−1) K1/(min−1) R2 qe/(mg·g−1) K2/(mg·(g·min)−1) R2 Zn 100 0.784 0.031 0.951 0.817 0.067 0.989 400 0.759 0.061 0.944 0.794 0.098 0.986 Ni 100 0.570 0.002 0.959 0.673 0.004 0.978 400 0.694 0.002 0.962 0.807 0.004 0.975 2.4 Fe0/Fe3O4流化床反应器去除复合重金属
1)不同铁粉浓度对复合重金属去除的影响。从前面的批处理实验结果可知,Fe0/Fe3O4是一个高活性的系统,可以持续有效地去除Zn2+、Ni2+。由图10可见,以K2Cr2O7作为预处理剂,当铁粉质量浓度从50 g·L−1提升到70 g·L−1时,Zn2+和Ni2+的去除率随之升高。当铁粉质量浓度70 g·L−1时,在22 d的运行时间内,Zn2+的去除率基本维持在90%左右,而Ni2+的去除率虽然波动较大,仍可维持在68%左右。但当铁粉质量浓度降到50 g·L−1时,在前7 d,Zn2+的去除率在80%~90%,然后逐渐降低,15 d以后,维持在62%;在第6~9天,Ni2+的去除率保持在70%左右,随后快速降低到30%左右,并一直保持到第22天。增加铁粉用量,单位体积内有更多的铁表面活性点位吸附Ni2+和Zn2+,其去除效果更好。这也与前面的批处理实验结果一致。对于50 g·L−1的铁粉用量,在起始阶段,其去除率就比70 g·L−1的铁粉用量下低,虽然随后差距缩小,但十几天以后,去除率远低于70 g·L−1。这可能是由于:在初始阶段,铁粉腐蚀产物的不断增加,减小了低浓度铁粉带来的影响;但是随着铁粉的不断消耗,产生的腐蚀产物越来越少,其处理效率也很快降低,但由于Zn2+和Ni2+的浓度不高(10 mg·L−1),因而依然可以保持相对稳定的去除效果。这说明50 g·L−1的铁粉用量过少,难以维持持续、有效的处理效果,最好要保持70 g·L−1或更高的铁粉质量浓度。对于Cr(Ⅵ)的处理效果,与前面批处理的结果一致,很快就被去除完全,因此,这里没有给出其结果。
2)不同氧化剂对复合重金属去除的影响。在预处理阶段,加入氧化剂才能生成复合体系。因此,比较了不同氧化剂(氧气、硝酸盐和重铬酸钾)对复合体系活性的影响,结果如图11所示。以氧气(空气)预处理制备的复合体系对Zn2+和Ni2+的去除率最高。连续运行22 d,Zn2+的去除效果一直可以保持在90%以上,而Ni2+的去除率在前18 d可稳定在在80%左右,随后去除率逐渐降低,到第22天的时候,只有50%。以重铬酸钾预处理时,在第一周内,Zn2+和Ni2+的去除效果持续升高,最高分别达到91%和72%,但随后又不断下降,尤其是Ni2+,在第10天的时候,去除率快速下降到~30%。以硝酸钾作为预处理剂,Ni2+和Zn2+的处理效果最差。在前10 d,对Zn2+的去除效果保持在90%左右,随后去除效果快速降低,而且不稳定。而对于Ni2+的去除,从一开始就持续下降,12 d以后,几乎没有去除。从3种体系中的pH来看,并没有明显差别(图11(c))。在3种氧化剂预处理体系中,Fe2+的浓度差别较大(图11(d))。在氧气体系中,Ni2+和Zn2+的去除效果最好,所以Fe2+的浓度一直最高;而另外2个反应器中的Fe2+最低,尤其是硝酸钾预处理的反应器,出水中的Fe2+仅有5 mg·L−1左右。与批处理实验的结果相似,出水中都没有检测到溶解态的Cr(Ⅵ)。这说明复合体系对Cr(Ⅵ)的处理效果有保障。总体而言,以氧气作为预处理剂的效果最好,其次是用重铬酸钾和硝酸钾的预处理。
3)4个反应器去除复合重金属的效果比较。图12展示了4个流化床反应器对Zn2+和Ni2+的处理效果。各流化床反应器的操作条件见表1。其中,由于4个反应器的出水中都未检测到溶解态的Cr(Ⅵ),故未提供其数据。由图12可看出,反应器C对Zn2+和Ni2+的处理效果最好,Zn2+的去除率都维持在90%以上;Ni2+在前17 d的去除率一直保持在80%左右,随后不断下降,到第22天,去除率降到50%。其次是反应器B,对Zn2+的去除效果基本保持在90%左右,尽管Ni2+的去除率在开始阶段波动很大,但运行7 d后也可稳定在68%左右。C反应器出水中的Fe2+浓度最高,这说明在Zn2+和Ni2+去除的同时释放出Fe2+,与前文的实验结果一致。尽管C反应器(50 g·L−1)的铁粉质量浓度比B反应器(70 g·L−1)低,但C反应器的处理效果最好,这说明以氧气作为预处理剂制备的复合体系活性最强。A反应器在前9 d对Ni2+和Zn2+的去除率较高,但随后快速下降,Fe2+浓度的变化趋势类似。D反应器的处理效果最差,不管是Zn2+还是Ni2+,去除率很快下降。综上所述,复合体系对Zn2+的去除效果要远远好于Ni2+,这与批处理实验的结果一致。4个反应器的Fe2+出水浓度与Ni2+和Zn2+的变化趋势一致。出水中的Fe2+浓度越高,Ni2+和Zn2+的去除效果也越好。这说明Ni2+和Zn2+的去除过程中,会直接或间接影响产生Fe2+。
4)重金属去除机理分析。批处理和流化床实验的结果均说明Fe0/Fe3O4具有很强的活性,可以持续地去除Cr(Ⅵ)、Zn2+和Ni2+。对三者的处理速率为Cr(Ⅵ)>Zn2+>Ni2+。在很短的时间内Cr(Ⅵ)就可以被快速去除。根据3种重金属和Fe0的标准电位,可以从理论上判断不同重金属的去除机理。根据Zn和Ni的标准电位,Fe0不可能还原Zn2+,只能通过铁腐蚀产物的吸附和共沉淀去除。Ni2+的标准电位稍微高于Fe0,其氧化还原反应的驱动力很小,还原反应的速率很慢,也可能不会发生氧化还原反应。Cr(Ⅵ)的标准电位为1.33V,远高于Fe0,因此,很容易被Fe2+和Fe0还原。为确定Cr(Ⅵ)和Ni2+的去除机理,通过XPS对反应前后Cr和Ni的价态进行了分析,结果如图13所示。反应前,Ni2+的2p3/2结合能在856.5 eV(图13(a)),反应后在852.6 eV处出现1个尖峰,归属于单质镍,856.5 eV处的峰变弱。这说明部分Ni2+被还原为Ni0,部分Ni(Ⅱ)可能是被吸附或共沉淀。在纳米Fe0去除Ni2+的过程中,也存在部分还原和部分吸附的现象[37-38]。由图13(b)可见,反应后Cr(Ⅵ)的2p1/2峰(579.2 eV)消失,而在577.1 eV处出现1个几乎相同的峰,可归于Cr(Ⅲ),说明Cr(Ⅵ)全部被还原为Cr(Ⅲ)。已有的研究[39-40]也表明Fe0可以有效地还原Cr(Ⅵ)为Cr(Ⅲ)。
3. 结论
1)经过预处理后,在Fe0表面生成一层晶型完好、晶相单一的Fe3O4。复合体系的活性远高于单独的Fe0,显著提高其对Zn2+、Ni2+和Cr(Ⅵ)的去除率。
2)增加铁粉用量或减小铁粉粒径均可提高对Zn2+和Ni2+的去除效果。Fe2+浓度升高对Fe0/Fe3O4去除Zn2+的影响不大,但会明显抑制对Ni2+的去除。10 mg·L−1Cr(Ⅵ)会促进Fe0/Fe3O4对Zn2+和Ni2+的去除,但过多的Cr(Ⅵ)由于与Fe(Ⅲ)共沉淀覆盖在Fe0表面,不利于铁腐蚀反应的进行,从而大大降低其促进作用。无论是改变铁粉用量、铁粉粒径、Fe2+或Cr(Ⅵ)浓度,还是预处理氧化剂的不同,对Cr(Ⅵ)的处理效果最好,其次是Zn2+和Ni2+。
3)在流化床反应器中,Fe0/Fe3O4可有效去除Zn2+、Ni2+和Cr(Ⅵ),增加铁粉用量可提高Zn2+和Ni2+的去除效果。相比于重铬酸钾和硝酸钾,以空气作为预处理辅助剂,制备的复合体系活性最强,能持续、有效地去除Zn2+和Ni2+。
4)在Fe0/Fe3O4中,Ni2+的去除机理为部分还原为单质镍、部分吸附或共沉淀,而Cr(Ⅵ)全部被还原为Cr(Ⅲ)。
-
表 1 不同流化床反应器的操作参数
Table 1. Operation parameters of different fluidized bed reactors
条件反应器 铁粉粒径/目 铁粉浓度/(g·L−1) 预处理氧化剂 预处理氧化剂浓度/(mg·L−1) 水力停留时间/h 搅拌器转速/(r·min−1) A 400 50 K2Cr2O7 20 5 700 B 400 70 K2Cr2O7 20 5 700 C 400 50 DO(曝气) / 5 700 D 400 50 KNO3 20 5 700 表 2 吸附动力学拟合参数
Table 2. Adsorption kinetics fitting parameters
重金属 铁粉粒径/目 准一级动力学 准二级动力学 qe/(mg·g−1) K1/(min−1) R2 qe/(mg·g−1) K2/(mg·(g·min)−1) R2 Zn 100 0.784 0.031 0.951 0.817 0.067 0.989 400 0.759 0.061 0.944 0.794 0.098 0.986 Ni 100 0.570 0.002 0.959 0.673 0.004 0.978 400 0.694 0.002 0.962 0.807 0.004 0.975 -
[1] WANG P, HU Y, CHENG H F. Municipal solid waste (MSW) incineration fly ash as an important source of heavy metal pollution in China[J]. Environmental Pollution, 2019, 252: 461-475. doi: 10.1016/j.envpol.2019.04.082 [2] ZHOU Q Q, YANG N, LI Y Z, et al. Total concentrations and sources of heavy metal pollution in global river and lake water bodies from 1972 to 2017[J]. Global Ecology and Conservation, 2020, 22: e925. [3] LIU X P, JIANG J, YAN Y, et al. Distribution and risk assessment of metals in water, sediments, and wild fish from Jinjiang River in Chengdu, China[J]. Chemosphere, 2018, 196: 45-52. doi: 10.1016/j.chemosphere.2017.12.135 [4] HUANG Y, CHEN Q Q, DENG M H, et al. Heavy metal pollution and health risk assessment of agricultural soils in a typical peri-urban area in southeast China[J]. Journal of Environmental Management, 2018, 207: 159-168. [5] 李钰婷, 张亚雷, 代朝猛, 等. 纳米零价铁颗粒去除水中重金属的研究进展[J]. 环境化学, 2012, 31(9): 1349-1354. [6] 杨世迎, 任腾飞, 张艺萱, 等. 水环境中ZVI/氧化剂体系及其电子迁移作用机制[J]. 化学进展, 2017, 29(4): 388-399. doi: 10.7536/PC170133 [7] LINGAMDINNE L P, CHANG Y, YANG J, et al. Biogenic reductive preparation of magnetic inverse spinel iron oxide nanoparticles for the adsorption removal of heavy metals[J]. Chemical Engineering Journal, 2017, 307: 74-84. doi: 10.1016/j.cej.2016.08.067 [8] FU F L, DIONYSIOU D D, LIU H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review[J]. Journal of Hazardous Materials, 2014, 267: 194-205. doi: 10.1016/j.jhazmat.2013.12.062 [9] ZOU Y D, WANG X X, KHAN A, et al. Environmental remediation and application of nanoscale zero-valent iron and its composites for the removal of heavy metal ions: A review[J]. Environmental Science & Technology, 2016, 50(14): 7290-7304. [10] SUN Y L, LI J X, HUANG T L, et al. The influences of iron characteristics, operating conditions and solution chemistry on contaminants removal by zero-valent iron: A review[J]. Water Research, 2016, 100: 277-295. doi: 10.1016/j.watres.2016.05.031 [11] LEWIS A S, HUNTINGTON T G, MARVIN-DIPASQUALE M C, et al. Mercury remediation in wetland sediment using zero-valent iron and granular activated carbon[J]. Environmental Pollution, 2016, 212: 366-373. doi: 10.1016/j.envpol.2015.11.047 [12] 席冬冬, 李晓敏, 熊子璇, 等. 生物炭负载纳米零价铁对污染土壤中铜钴镍铬的协同去除[J]. 环境工程, 2020, 38(6): 58-66. [13] 张守秋, 岑洁, 吕德义, 等. 纳米零价铁去除水中重金属铅、铬离子的研究[J]. 高校化学工程学报, 2019, 33(3): 524-532. doi: 10.3969/j.issn.1003-9015.2019.03.003 [14] WU Y X, WANG Y, HUANG X F, et al. Zerovalent iron in conjunction with surfactants to remediate sediments contaminated by polychlorinated biphenyls and nickel[J]. Chemosphere, 2017, 189: 479-488. doi: 10.1016/j.chemosphere.2017.09.038 [15] LING L, HUANG X Y, LI M R, et al. Mapping the reactions in a single zero-valent iron nanoparticle[J]. Environmental Science & Technology, 2017, 51(24): 14293-14300. [16] GUAN X H, SUN Y K, QIN H J, et al. The limitations of applying zero-valent iron technology in contaminants sequestration and the corresponding countermeasures: The development in zero-valent iron technology in the last two decades (1994-2014)[J]. Water Research, 2015, 75: 224-248. doi: 10.1016/j.watres.2015.02.034 [17] HAN W J, FU F L, CHENG Z H, et al. Studies on the optimum conditions using acid-washed zero-valent iron/aluminum mixtures in permeable reactive barriers for the removal of different heavy metal ions from wastewater[J]. Journal of Hazardous Materials, 2016, 302: 437-446. doi: 10.1016/j.jhazmat.2015.09.041 [18] CHEN L, CHEN Z H C, CHEN D, et al. Removal of hexavalent chromium from contaminated waters by ultrasound-assisted aqueous solution ball milling[J]. Journal of Environmental Science, 2017, 52: 276-283. doi: 10.1016/j.jes.2016.04.006 [19] LEONEL A G, MANSUR A A P, MANSUR H S. Advanced functional nanostructures based on magnetic iron oxide nanomaterials for water remediation: A review[J]. Water Research, 2021, 190: 116693. doi: 10.1016/j.watres.2020.116693 [20] ELJAMAL O, THOMPSON I P, MAAMOUN I, et al. Investigating the design parameters for a permeable reactive barrier consisting of nanoscale zero-valent iron and bimetallic iron/copper for phosphate removal[J]. Journal of Molecular Liquids, 2020, 299: 112144. doi: 10.1016/j.molliq.2019.112144 [21] TANG C L, HUANG Y H, ZENG H, et al. Reductive removal of selenate by zero-valent iron: The roles of aqueous Fe2+ and corrosion products, and selenate removal mechanisms[J]. Water Research, 2014, 67: 166-174. doi: 10.1016/j.watres.2014.09.016 [22] TANG C L, HUANG Y H, ZHANG Z, et al. Rapid removal of selenate in a zero-valent iron/Fe3O4/Fe2+ synergetic system[J]. Applied Catalysis B:Environmental, 2016, 184: 320-327. doi: 10.1016/j.apcatb.2015.11.045 [23] SU J J, CHEN H, WANG J L, et al. Enhanced dechlorination of carbon tetrachloride by Ni-doped zero-valent iron nanoparticles@magnetic Fe3O4(Ni4/Fe@Fe3O4) nanocomposites[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2021, 623: 126691. doi: 10.1016/j.colsurfa.2021.126691 [24] YANG Z, MA X H, SHAN C, et al. Activation of zero-valent iron through ball-milling synthesis of hybrid Fe0/Fe3O4/FeCl2 microcomposite for enhanced nitrobenzene reduction[J]. Journal of Hazardous Materials, 2019, 368: 698-704. doi: 10.1016/j.jhazmat.2019.01.105 [25] WU B, JIA H C, YANG Z, et al. Enhanced removal of selenate from mining effluent by H2O2/HCl-pretreated zero-valentiron[J]. Water Science & Technology, 2018: 526/514878. [26] 秦泽敏, 董黎明, 刘平, 等. 零价纳米铁吸附去除水中六价铬的研究[J]. 中国环境科学, 2014, 34(12): 3106-3111. [27] LIANG W, DAI C M, ZHOU X F, et al. Application of zero-valent iron nanoparticles for the removal of aqueous zinc ions under various experimental conditions[J]. PloS One, 2014, 9(1): e85686. doi: 10.1371/journal.pone.0085686 [28] LI X Q, ZHANG W X. Sequestration of metal cations with zerovalent iron nanoparticles a study with high resolution X-ray photoelectron spectroscopy (HR-XPS)[J]. The Journal of Physical Chemistry C, 2007, 111(19): 6939-6946. doi: 10.1021/jp0702189 [29] 桑丽. 鼠李糖脂改性纳米零价铁对镍污染土壤修复及机理研究[D]. 上海: 华东理工大学, 2021. [30] SANG L, WANG G H, LIU L, et al. Immobilization of Ni (II) at three levels of contaminated soil by rhamnolipids modified nano zero valent iron (RL@nZVI): Effects and mechanisms[J]. Chemosphere, 2021, 276: 130139. doi: 10.1016/j.chemosphere.2021.130139 [31] Musić, S RistićM. Adsorption of trace elements or radionuclides on hydrous iron oxides[J]. Journal of Radioanalytical and Nuclear Chemistry, 1988, 120(2): 289-304. doi: 10.1007/BF02037344 [32] MA L Y, DU Y G, CHEN S H, et al. Highly efficient removal of Cr(VI) from aqueous solution by pinecone biochar supported nanoscale zero-valent iron coupling with Shewanella oneidensis MR-1[J]. Chemosphere, 2022, 287: 132184. doi: 10.1016/j.chemosphere.2021.132184 [33] MIN X B, LI Q, ZHANG X M, et al. Characteristics, kinetics, thermodynamics and long-term effects of zerovalent iron/pyrite in remediation of Cr(VI)-contaminated soil[J]. Environmental Pollution, 2021, 289: 117830. doi: 10.1016/j.envpol.2021.117830 [34] 吕晓书. 稳定化纳来级零价铁的制备及对水中Cr(Ⅵ)的去除机制研究[D]. 杭州: 浙江大学, 2015. [35] ARECO M M, SALEH-MEDINA L, TRINELLI M A, et al. Adsorption of Cu(II), Zn(II), Cd(II) and Pb(II) by dead Avena fatua biomass and the effect of these metals on their growth[J]. Colloids and Surfaces B:Biointerfaces, 2013, 110: 305-312. doi: 10.1016/j.colsurfb.2013.04.035 [36] ARGUN M E, DURSUN S, OZDEMIR C, et al. Heavy metal adsorption by modified oak sawdust: Thermodynamics and kinetics[J]. Journal of Hazardous Materials, 2007, 141(1): 77-85. doi: 10.1016/j.jhazmat.2006.06.095 [37] MAHDY A M, ZHANG T Q, LIN Z, et al. Zero-valent iron nanoparticles remediate nickel-contaminated aqueous solutions and biosolids-amended agricultural soil[J]. Materials, 2021, 14(10): 2655. doi: 10.3390/ma14102655 [38] LI Z, DONG H, ZHANG Y, et al. Enhanced removal of Ni(II) by nanoscale zero valent iron supported on Na-saturated bentonite[J]. Journal of Colloid and Interface Science, 2017, 497: 43-49. doi: 10.1016/j.jcis.2017.02.058 [39] XIE Y Y, LU G T, TAO X Q, et al. A collaborative strategy for elevated reduction and immobilization of Cr(VI) using nano zero valent iron assisted by schwertmannite: Removal performance and mechanism[J]. Journal of Hazardous Materials, 2022, 422: 126952. doi: 10.1016/j.jhazmat.2021.126952 [40] FU F L, HAN W J, TANG B, et al. Insights into environmental remediation of heavy metal and organic pollutants: Simultaneous removal of hexavalent chromium and dye from wastewater by zero-valent iron with ligand-enhanced reactivity[J]. Chemical Engineering Journal, 2013, 232: 534-540. doi: 10.1016/j.cej.2013.08.014 -










 DownLoad:
DownLoad: