-
偶氮染料是纺织业中使用最多的一类染料 [1],具有难降解的特性。常见的处理方法包括物化法、化学法和生物法。物化法不能彻底降解污染物,化学法成本过高,生物法降解缓慢[2],因此限制了这些方法的应用。
次氯酸钠氧化法广泛用于降解废水中的污染物,并以污染物去除效率高、设备简单、经济性等优点而受到人们的重视。NaClO溶液的稳定性较差,大大降低了其氧化性的利用率。此外,采用NaClO直接氧化废水,会产生具有强致癌性的氯代有机物。增强NaClO的氧化性和消除氯代有机物对拓宽其在水处理中的应用极为重要。镍基催化剂能促进NaClO溶液的分解增强其氧化性,且以催化效果好、便宜等优点而备受关注[3]。NaClO在氧化镍的作用下生成具有极强活性的原子氧,迅速氧化废水中的污染物,从而达到去除COD和脱色的目的[4-5]。本课题组研发的NaClO催化氧化法能有效降解活性艳红K-2BP、酸性大红3R等偶氮染料,活性艳红K-2BP的脱色率在120 min达到94%以上[6],而酸性大红3R的脱色率在35 min达到90%以上。原子氧在脱色降解过程起了主导作用,因此未在染料降解产物中发现氯代有机物。
研究染料的分子结构与脱色性能之间的关系对废水的处理至关重要。染料种类繁多,结构各异,可以采用QSPR (quantitative molecular structure-properties relationships)法研究分子结构和脱色性能的关系。该方法简单易行,了解了分子结构信息,就可以分析和预测染料的理化性质或环境行为[7]。
本研究分别采用NaClO催化氧化法和NaClO氧化法脱色降解活性黄X-R废水,考察比较两种方法的脱色效果;比较催化氧化法对活性黄X-R和另外两种偶氮染料的脱色效果,在此基础上采用QSPR分析该方法处理不同染料效果存在差异的原因,进而指出其适用范围。本研究选择适当的染料分子结构描述符,通过线性回归法研究上述目标染料分子结构与NaClO催化氧化法对其脱色效果之间的关系。研究结果将为推广应用NaClO催化氧化法提供理论依据。
-
催化剂载体选用水处理用耐酸碱的γ-Al2O3小球,参数如下:直径为 2−3 mm,Al2O3 > 92%,孔容为0.35 mL·g−1,比表面积大于300 m2·g−1。Na2S2O8 (99%)、NAOH (99%)、Ni(NO3)2·6H2O (99%)、H2SO4 (98%)、呋喃甲醇(99.2%)和叔丁醇(99.9%)均为分析纯,NaClO溶液有效氯含量大于10%。
仪器包括:ZYL便携式余氯分析仪、MIT-3F型COD检测仪、723N扫模型可见光分光光度计、Rigaku Smartlab9 x射线衍射仪(日本理学)、EscaLab 250Xi型X射线光电子能谱仪,Al-Kα射线单色源(美国赛默飞)、扫描电镜SEM(美国FEI Inspect F50)。
-
催化剂制备步骤如下:先将γ-Al2O3小球浸泡在摩尔浓度为0.6 mol·L−1 Na2S2O8和0.5 mol·L−1 NaOH的混合液里,在振荡器上以100 r·min−1的频率振荡2 h,将得到的Na2S2O8-NaOH/Al2O3小球滤出后烘干。然后将Na2S2O8-NaOH/Al2O3小球浸泡在含有0.6 mol·L−1 Ni2+的溶液里发生反应,在γ-Al2O3表面形成一层镍氧化物/镍氢氧化物沉淀,将制得的小球滤出后烘干,得到镍基Al2O3小球。将得到的镍基Al2O3小球再次浸泡在Na2S2O8-NaOH混合液里,在振荡器上以100 r·min−1的频率振荡2 h后将催化剂小球滤出,用蒸馏水清洗。最后将洗净的催化剂置于马弗炉中低温焙烧2 h,制得催化氧化实验所需的催化剂样品。
Ni(OH)2为还原性氢氧化物,能和强氧化剂反应生成羟基氧化镍NiOOH。通过Ni2+在碱性溶液中被Na2S2O8氧化的反应来合成NiOOH(见式(1)和式(2)),从中可以看出,碱性条件促进Ni(OH)2和NiOOH的生成。
在NiOOH存在的情况下,NaClO的分解反应2NaClO→2NaCl+O2↑可以看作是反应(3)和反应(4)的总和:
产生的氧分子能被物理吸附在镍基催化层上(式5),捕获电子后转化为化学吸附氧
O−2 和O−以及原子氧O (式6−7)。物理吸附的氧分子也可以直接分解成原子氧(式8) [8]。其中,O2(g)为气态氧,S为表面吸附位置,O2为表面物理吸附的氧,*和e−代表导带底部空位和导带电子,ΔG为反应能。反应产生的原子氧O非常活跃,可以快速氧化和分解有机物。羟基氧化镍可以长时间用作催化剂,因为它可以通过反应(3)和(4)进行再生利用。
-
染料废水在波长为390 nm处有最大特征吸收峰,在390 nm检测不同浓度废水的吸光度,建立质量浓度和吸光度的标准曲线方程y =96.877 x –1.1831,R2 = 0.9987。
-
配制一定浓度的活性黄X-R溶液,加入一定量的NaClO溶液,充分搅拌,调节pH至所需值,得到废水/NaClO混合液。取50 mL混合液放入100 mL锥形瓶内,把一定量催化剂样品快速放入溶液中。在振荡器上以100 r·min−1的速度振荡120 min。分别在第10、30、50、70、90、120 min时取3 mL左右上清液,直接测其吸光度,利用标准曲线方程求得染料的浓度,按公式(9)计算其脱色率。测好吸光度后将染料溶液倒回锥形瓶中继续反应。
式中,C0 和Ct 分别为初始染料浓度和t时刻染料浓度,mg·L−1。
-
配制质量浓度为200 mg·L−1的活性黄X-R溶液,加入120 mg·L−1的NaClO溶液,充分搅拌,调节到pH 9,得到废水/NaClO混合液。在两个相同组分的混合液中分别加入20 mmol·L−1的叔丁醇和呋喃甲醇,与催化剂一起放入100 mL锥形瓶内,在振荡器上以100 r·min−1的速度振荡反应,检测染料的脱色率变化,并以此来判断在反应中起主导作用的自由基。叔丁醇是一种良好的OH·和
SO−4⋅ 清除剂[9];呋喃甲醇能快速地捕获溶液中的原子氧,常被用作原子氧的指示剂[10]。 -
检测了当pH 为9、有效氯浓度为120 mg·L、催化剂投加量为240 g·L−1、初始染料浓度为100、200、300、400 mg·L−1时的脱色率,结果如图1所示。前期的研究结果表明[11],有催化剂时,弱碱性环境pH 9有利于反应(1)和反应(2)的发生,促进活性氧的产生,增大NaClO的转化率。当催化剂存在时,脱色率随着初始染料浓度的增加而增加:当染料浓度为100、200、300、400 mg·L−1时,脱色率在120 min时分别为41.1%、41.21%、42.47%和50.03%。没有催化剂时,当初始染料浓度为100、200、300、400 mg·L−1时,脱色率在反应120 min时分别为43.7%、42.67%、41.78%和40.17%。加入催化剂后,初始染料浓度为100、200、300 mg·L−1溶液在120 min时的脱色率基本没有发生变化,可能是因为NaClO的直接氧化起了主导作用。当初始染料浓度为400 mg·L−1时,催化剂的加入使脱色率增加到50.03%,催化剂的吸附和活性氧的氧化作用对脱色率的增量做了一定的贡献,吸附量随着染料浓度的增加而增大,进而促进了活性氧与之发生反应。
催化氧化法对活性黄X-R的脱色效果远差于其对活性艳红K-2BP和酸性大红3R的脱色效果,脱色率随浓度变化的趋势也和后面两种染料的相反,活性艳红K-2BP和酸性大红3R的脱色率随着染料初始浓度的增加而减小(图2),因为反应的速率受到活性氧产量的限制,高浓度溶液需要更多的活性氧才能保持同样的降解速率。
以上实验结果表明,催化氧化体系降解活性黄X-R的机理可能有别于活性艳红K-2BP和酸性大红3R的降解机理,NaClO更倾向于直接氧化活性黄X-R,因此抑制了其本身的催化分解反应,减少了活性氧的产量;活性黄X-R分子也可能不易被具有亲电子性的活性氧攻击,使NaClO的直接氧化作用在脱色反应中占据了主导地位。因此,当NaClO有效氯浓度足够高的时候,染料的分解速率随着染料浓度的增加而增大,从表观上看,脱色率增大。
-
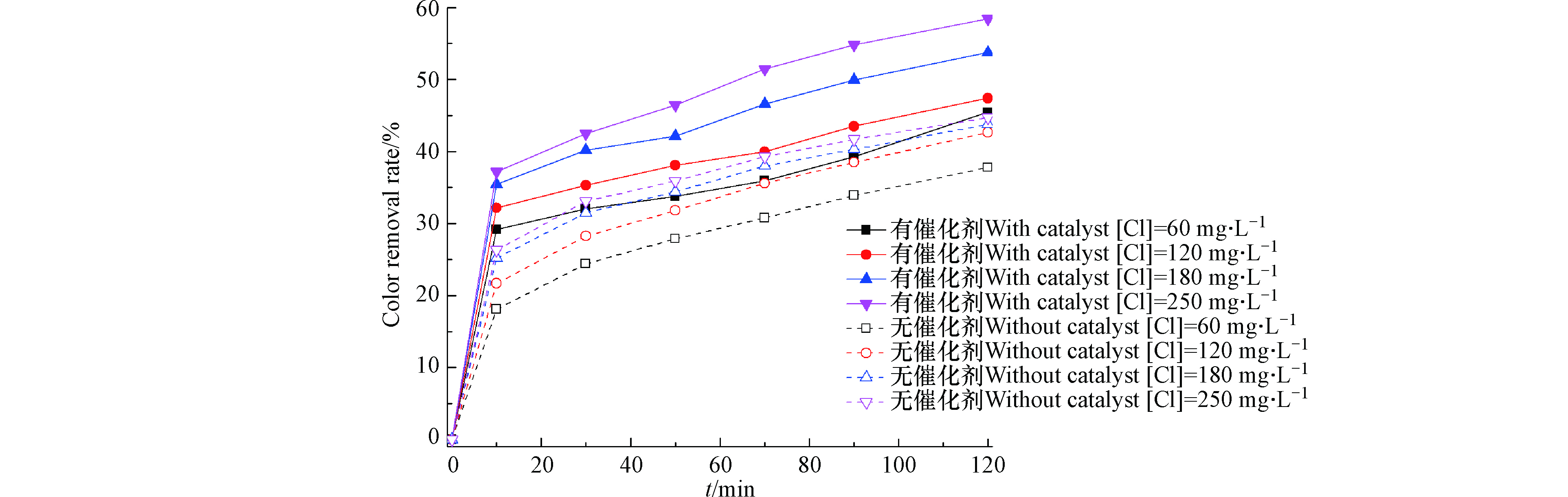
检测了当pH 9、染料浓度为200 mg·L−1、催化剂投加量为240 g·L−1、 有效氯为60、120、180 、250 mg·L−1时的脱色率,结果如图3所示。脱色率随着有效氯浓度的增加而增大。有催化剂存在时,当有效氯浓度从60 mg·L−1增加到250 mg·L−1,120 min时染料的脱色率从45%提升到了58%,增幅有限,主要是因为NaClO的直接氧化起了主导作用。NaClO在pH 9时的氧化性较弱,脱色效果差,单独氧化时NaClO的利用率较低[12]。有效氯浓度的增大也促进了NaClO直接氧化染料反应的进行,改善了活性黄X-R的脱色效果:当初始有效氯浓度从60 mg·L−1增加到250 mg·L−1,染料的脱色率从37.8%提升到了44.7%,比催化剂存在时的脱色率低6.3%–13.3%,增值是由催化剂的吸附和产生的活性氧作用所导致的。
-
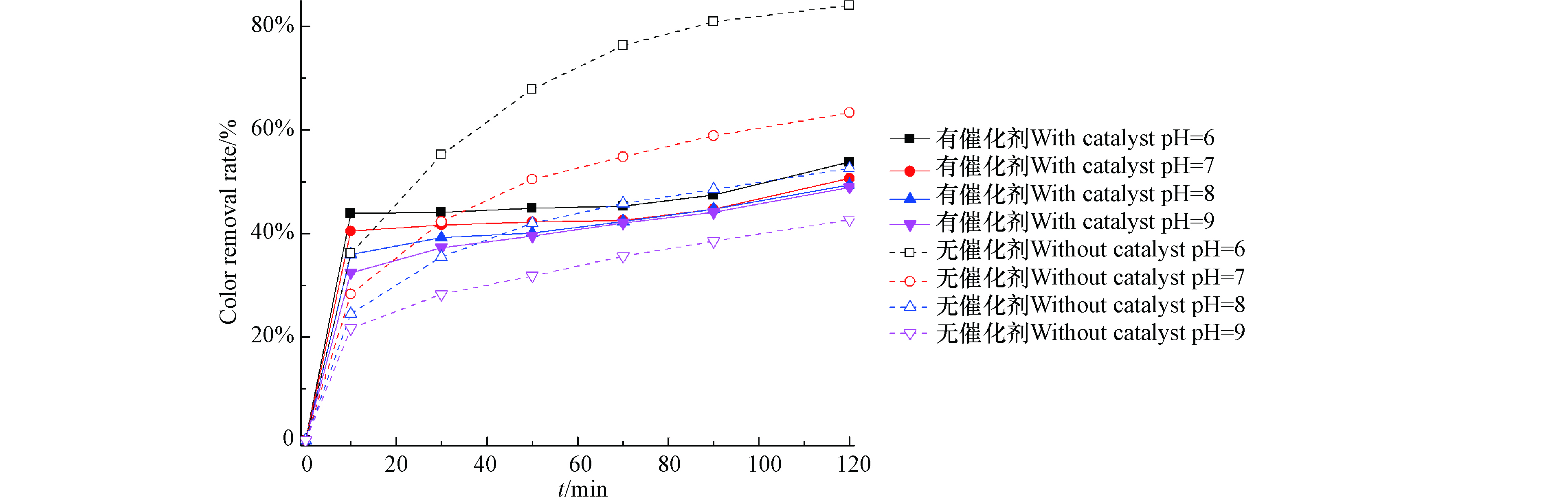
检测了当有效氯为120 mg·L−1、染料浓度为200 mg·L−1、催化剂投加量为240 g·L−1、初始pH值为6、7、8、9时的脱色率,结果如图4所示。NaClO催化氧化法的脱色率随着pH的提升而减小,pH 6、7时的脱色效果略胜于pH 8、9时的脱色效果,初始pH为6时的脱色效果最好,当反应时间为120 min,脱色率达到53.8%,远低于弱碱性条件下NaClO催化氧化降解活性艳红K-2BP和酸性大红3R溶液的脱色率(图5),催化作用明显受到了抑制,这是因为反应机理不同而导致的。原子氧在活性艳红K-2BP和酸性大红3R的脱色中起了主导作用,NaClO的直接氧化在活性黄X-R的脱色中起了主导作用。当初始pH为9,加催化剂反应120 min,活性黄X-R的脱色率达到49%,无催化剂时脱色率为42.7%(图4)。NaClO法降解活性黄X-R的脱色率随着pH的增加明显降低,当pH值从6增加到9,脱色率从84.1%降到42.7%,这是因为NaClO在酸性条件下会以游离氯存在,氧化性能大大提升。
当pH值为6、7、8时,加入催化剂反而导致活性黄X-R的脱色效果变差(图4)。当pH 6时,NaClO主要以HClO的形式存在。随着pH值的提升,ClO−的量增多,pH 9时NaClO主要以ClO−的形式存在。催化剂表面含有大量Ni(OH)2,带有正电荷[13]。当pH 6时,催化剂与次氯酸电荷相斥,不利于其在催化剂表面的有效吸附;当pH值为8、9,催化剂与ClO−存在电荷相吸作用,有利于NaClO在催化剂表面的有效吸附。催化剂表面和含有磺酸根离子的染料分子之间的静电吸引可能有利于染料在其表面的有效吸附。因此,当pH 6时,催化剂的存在降低了HClO与染料分子之间的反应几率,从而降低了脱色率,虽然此时的溶液具有较强的氧化性。当pH值为8、9,NaClO和染料分子在催化剂表面的有效吸附使两者之间的反应几率增大,从而使脱色率和酸性条件下的相差不大。催化剂存在时,弱碱性条件促进了活性氧的产生,但对染料的脱色率基本没有产生影响,可能是活性氧与染料分子之间的反应受到了阻碍。
采用NaClO催化氧化法降解活性艳红K-2BP和酸性大红3R,初始pH 9时的脱色效果最好(图6),弱碱性条件有利于NiOOH的生成和催化层的固定(式(1)和式(2)),进而促进原子氧的产生(式(3)—(8));同时,弱碱性条件还能抵消由于降解过程产生有机酸而导致的pH值的下降,起到平衡pH值的作用。
-
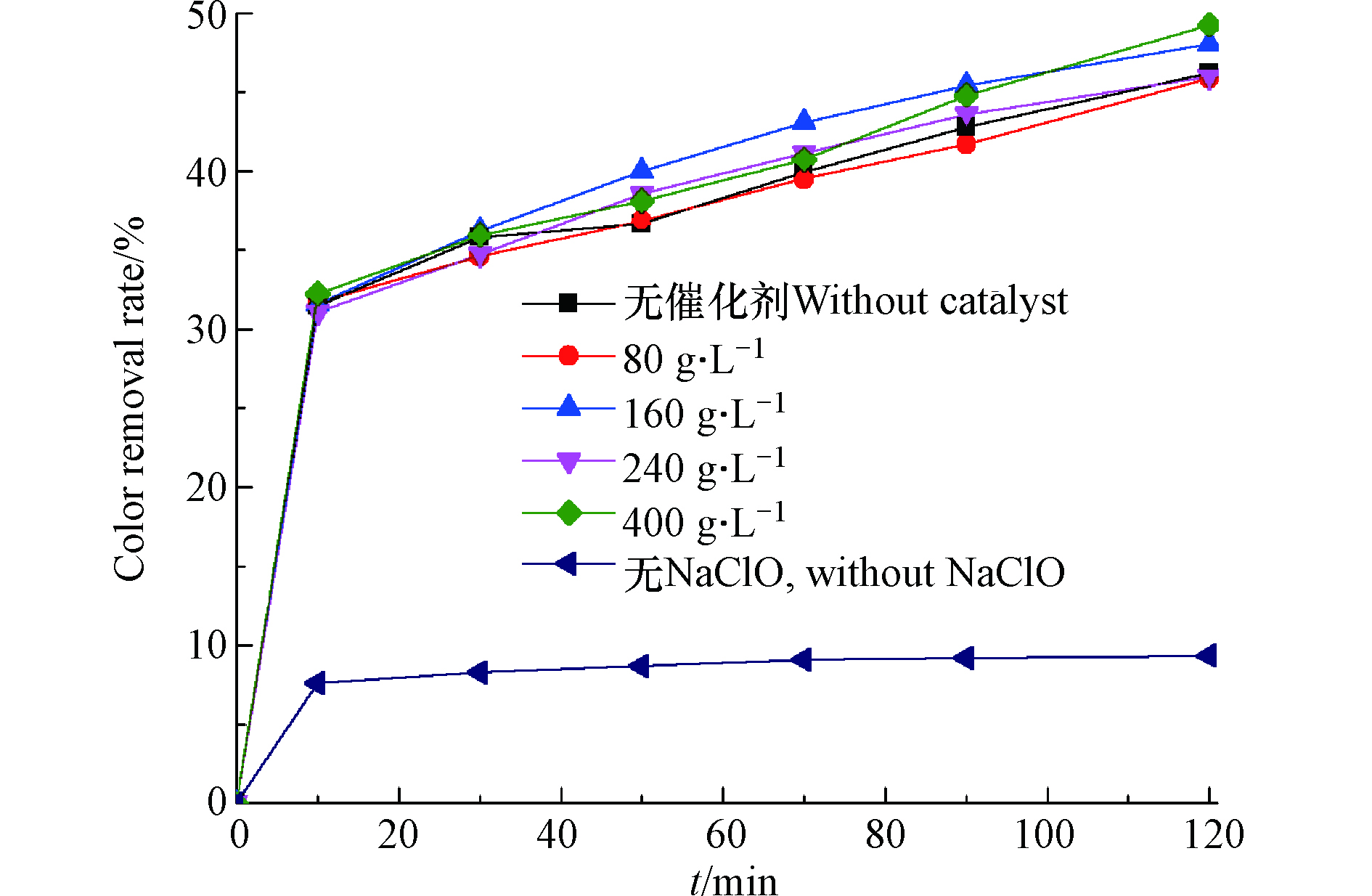
检测了当有效氯浓度为120 mg·L−1、染料浓度为200 mg·L−1、初始pH为9、催化剂投加量为80、160、240、400 g·L−1时的脱色率,结果如图7所示。催化剂投加量的增加对染料脱色率的影响很小,当催化剂投加量从80 g增加到400 g·L−1,脱色率在反应120 min时只提升了3.4%。单独使用NaClO时的脱色率与催化剂存在时的基本相同。单独使用400 g·L−1新制备的催化剂处理废水120 min,仅有7.6%–9.4%的脱色率,随着反应时间的延长,脱色率变化幅度很小,说明这部分脱色率主要是由吸附导致的,其贡献较小。实验结果再次验证了NaClO的氧化在脱色反应中的主导作用,当初始pH为9时,催化剂对脱色反应的影响甚微,催化作用受到了抑制。因此,催化氧化法不太适用于脱色降解活性黄X-R废水。
2.1.3节的研究结果表明,当pH为6,200 mg·L−1活性黄X-R废水中有效氯浓度达到120 mg·L−1,反应 120 min后脱色率达到84.1%。继续调低pH能进一步提升脱色率,但是NaClO氧化法会导致出水中产生毒性较强的氯代有机物。王元刚等[14]采用Mg(OH)2混凝脱色降解浓度为100 mg·L−1的活性黄X-R废水,当pH 值为12、Mg2+投加量为216 mg·L−1时,脱色率达到90%以上。王昶等[15]采用蛭石类芬顿降解活性黄X-R染料,结果表明,把蛭石粉碎到7.5 μm的颗粒,其投加量为1.0 g·L−1、pH 3、H2O2用量 2.94 mmol·L−1时,反应180 min 后,50—300 mg·L−1活性黄X-R 脱色率均能达到95%以上。后面两种方法的运行成本偏高。
-
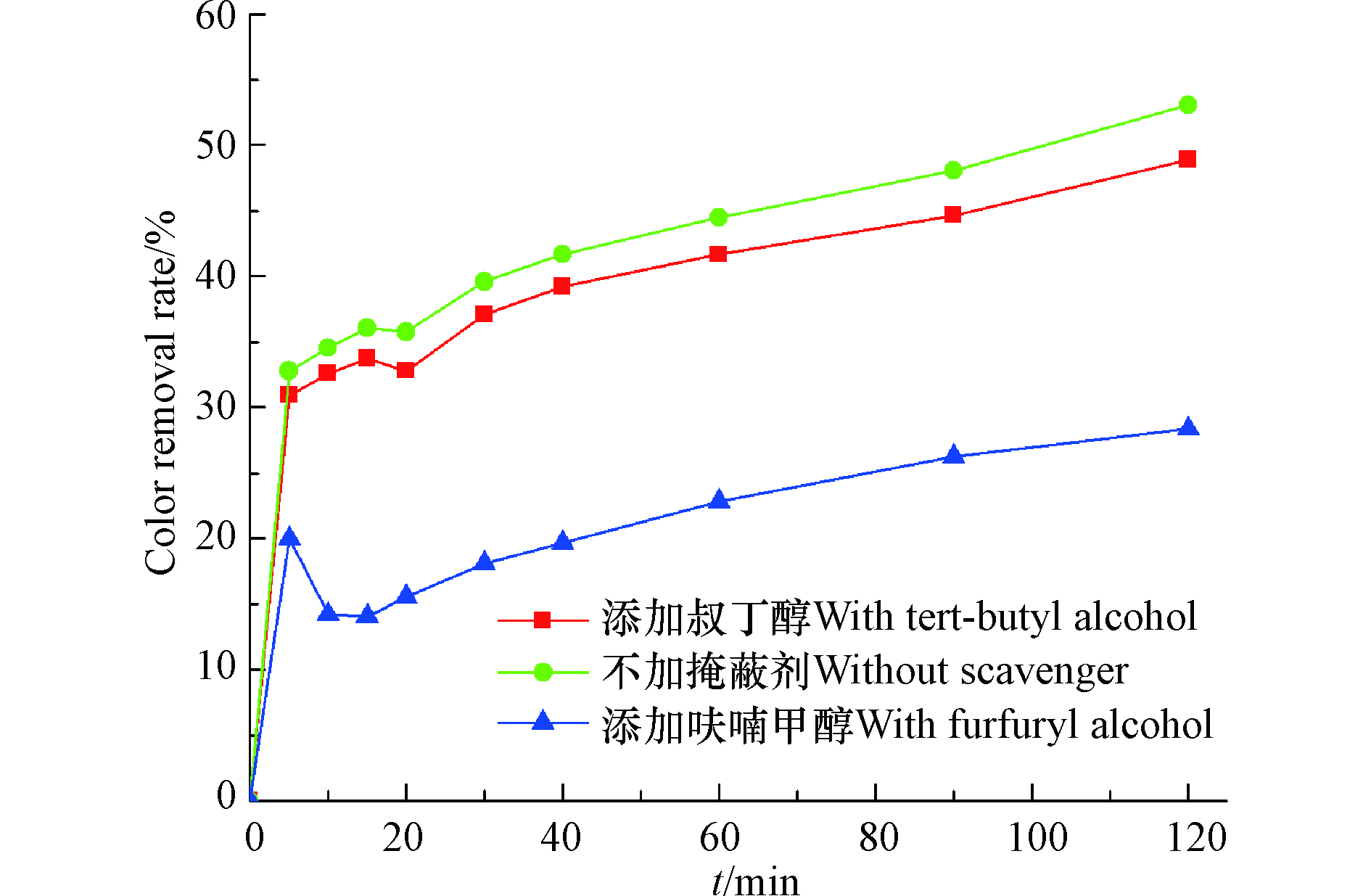
叔丁醇的加入对活性黄X-R的降解过程几乎没有影响,而呋喃甲醇对脱色具有一定的抑制作用,两者之间的脱色率差值在12%―24%之间(图8),因此可以断定原子氧在染料脱色过程中起了一定的作用,OH·和
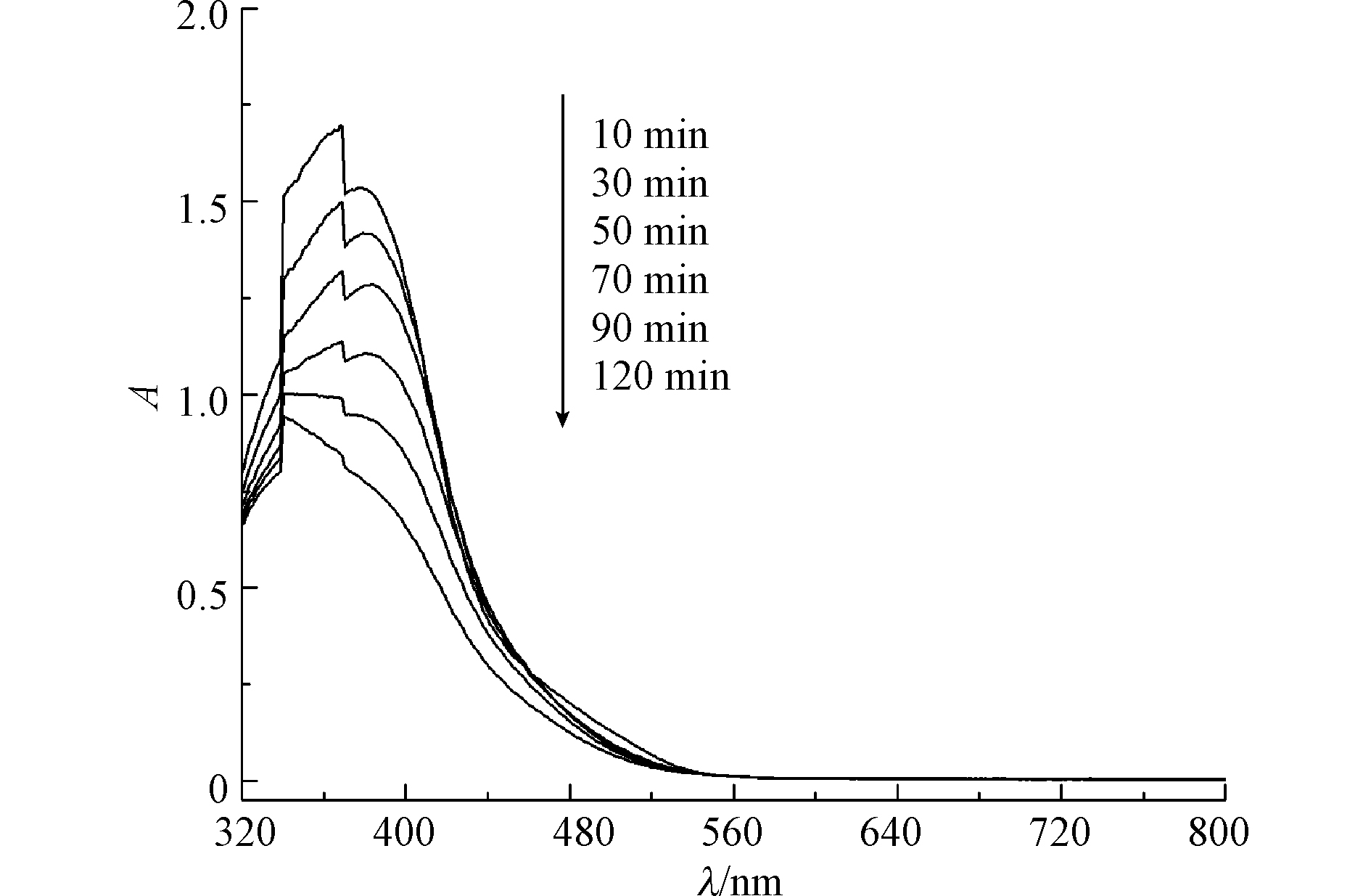
SO−4⋅ 在脱色反应中起的作用很小。原子氧能与染料分子中的偶氮键发生加成反应并使之断裂产生氨基[16]。活性黄X-R在390 nm的最大吸收峰对应偶氮键(图9),该峰随着反应的进行逐渐减小,但没有消失,可见偶氮键并未被完全破坏。367―370 nm之间为萘环的吸收峰,峰面积随着反应的进行减小,但反应120 min后该特征吸收峰还在,萘环没有完全打开。
-
根据染料的分子结构特征及脱色率,选择磺酸基(
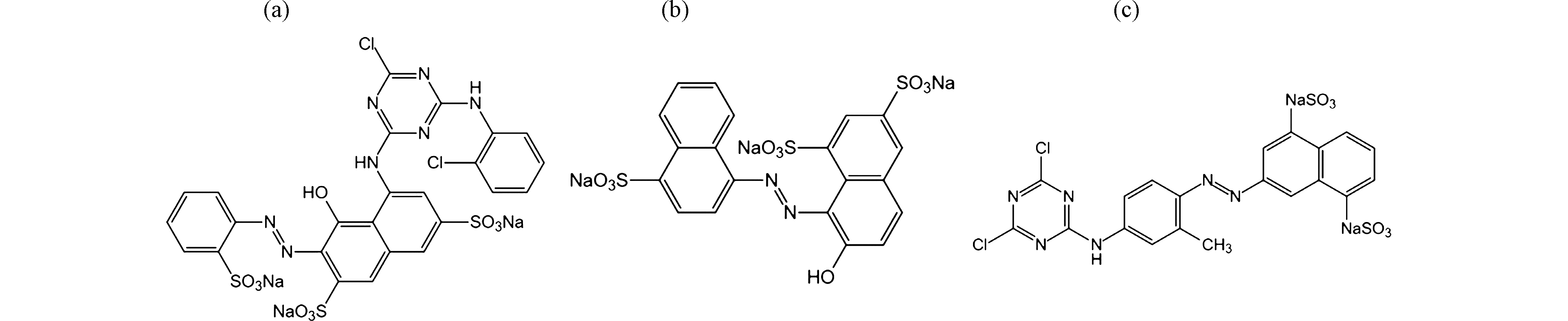
SO−3 )的数目A、无机性值与有机性值的比值B(I/O)和芳香环的数目(No-Ar) C 3个参数作为分子结构描述符,其中B值依据染料分子结构通过文献[17]给定的方法计算得到。分别配制浓度为200 mg·L−1的活性艳红K-2BP、酸性大红3R和活性黄X-R溶液,往里投加NaClO,使混合液中有效氯浓度达到120 mg·L−1,调节废水/NaClO混合液的pH值到9,投加催化剂240 g·L−1,在振荡器上反应90 min后取样,于染料的最大吸收波长下测定溶液的吸光度,换算成浓度,计算其脱色率。3种染料都属于偶氮染料,它们的分子结构见图10,它们的去除率和分子结构描述符见表1.
对表1中的分子结构描述符与脱色率进行线性回归分析,其线性回归方程和参数如表2所示。A、B与脱色率的线性回归数值比较高,说明目标染料的去除率与染料分子结构中磺酸基数目和I/O值之间具有较强的线性相关性,其中磺酸基数目A对去除率的影响尤为突出。染料分子的A值升高,其脱色率增加,这是因为磺酸基数目的增加使染料分子的电子云密度增加,原子氧具有较强的亲电子性,易与偶氮键发生加成反应。染料分子的B值升高,其无机性增强,电离度也随之增大,溶液中磺酸根离子的量增加,原子氧与染料分子之间的反应几率也因此增大,染料的脱色效率提高。C值增大,脱色率也随之增加,但去除率和C值的相关性较差,R2=0.1481,染料分子中C值一般小于10。因此,C值对脱色率的影响很小。
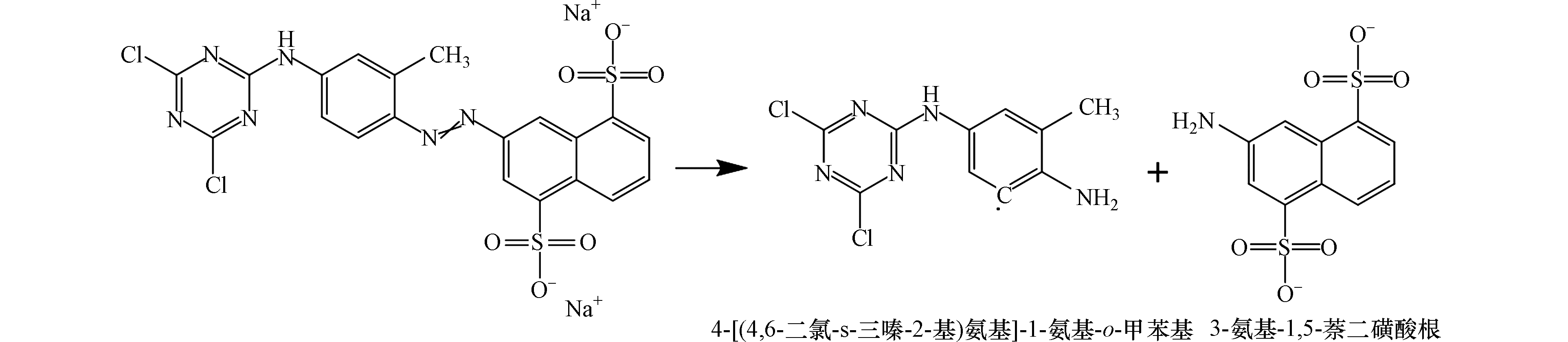
偶氮键断开后,活性黄X-R分子被分解成2个芳胺:3-氨基-1,5-萘二磺酸根和4-[(4,6-二氯-s-三嗪-2-基)氨基]-1-氨基-o-甲苯基 (图11)。3-氨基-1,5-萘二磺酸根包含一个萘环,萘环上引入了两个磺酸基,其耐氯牢度大大提高,也就是说OCl−的进攻能力减弱;引入磺酸基的数目越多,耐氯牢度提升也越大[18]。活性黄X-R分子含有二氯均三嗪活性基,由于两个氯原子的吸电子诱导效应,环上的电子云密度较低,故其活性较高,水解率高,固色效果差,容易被浓度高氧化能力弱一些的OCl−进攻。活性艳红K-2BP的分子包含1个偶氮基、一氯均三嗪基、2个苯环和1个萘环(图10)。一氯均三嗪环上的电子云密度高,稳定性强,固色效果好,不易被OCl−进攻[19],却能被氧化性极强的原子氧脱色降解。董亚荣等[20]曾对10种染料的生物降解脱色性能进行了研究,结果表明,X型染料最易降解,K型染料次之。实验结果与该结论吻合。酸性大红3R分子包含1个偶氮基、1个羟基和2个萘环;萘环上有3个磺酸基,其中1个磺酸基位于偶氮基的邻位(图10),其耐氯稳定性好[19],降解过程不易产生有机氯产物。
综上,活性黄X-R分子的耐氯牢度较低,NaClO倾向将其直接氧化,从而抑制了NaClO的催化分解和原子氧的产生,降低了原子氧对脱色降解过程的贡献度。活性艳红K-2BP和酸性大红3R的耐氯稳定性好。NaClO催化氧化法更适合降解耐氯性强的K型活性偶氮染料和含有磺酸钠盐的酸性偶氮染料,不太适合于降解X型活性偶氮染料。苯环染料比萘环染料耐氯性好,这也是吴正雷等[5]用NaClO/镍基催化剂处理甲基橙(对二甲氮基偶苯磺酸钠)废水,没有发现有机氯产物的原因。
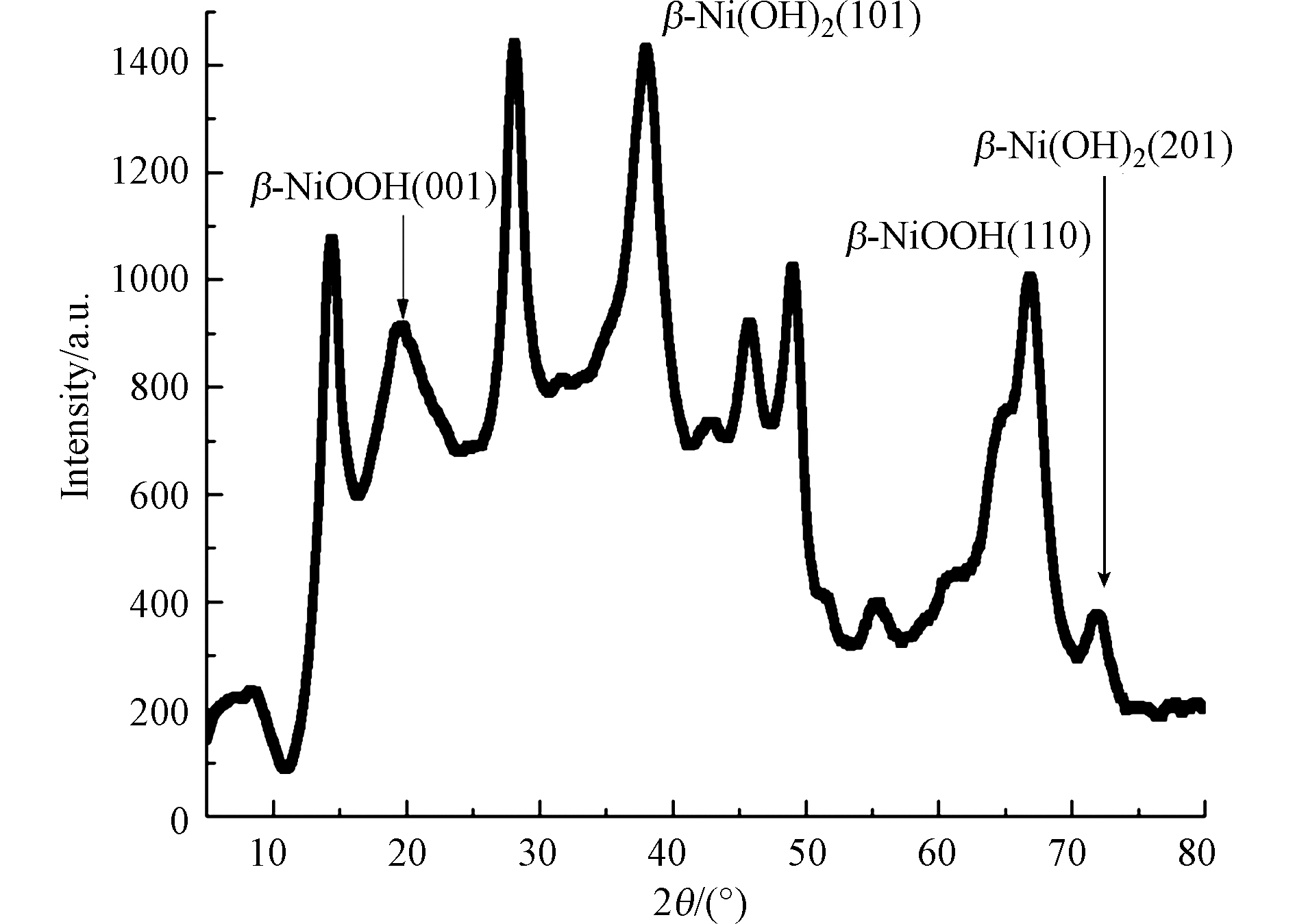
采用XRD对催化剂表层进行物相分析。样品在19.2°和66.7°附近有强的衍射峰(图12),与标准卡(卡号60141)对比可知,样品里含有β-NiOOH,两个衍射峰分别为001和110晶面。样品在38.5°和72.2°处出现两个衍射峰,与标准卡(卡号14117)对比可知,样品里含有β-Ni(OH)2,两个衍射峰分别为101和201晶面。
采用XPS分析法检测催化剂表面的元素及其相对含量。选取新制备的催化剂和分别处理过3000 min不同偶氮染料的三个催化剂小球作为检测样品。样品中氧元素的含量在78%以上(表3)。531.46 eV左右的峰为化学吸附氧引起的[21]。处理过3000 min染料的催化剂样品中氧元素的含量有不同程度的减小,主要是因为产生的原子氧参与降解染料。855.79―856.14 eV的峰是由Ni(Ⅱ)引起的,861.59―861.95 eV的峰是由Ni(Ⅲ)引起的[22]。新制的催化剂样品的Ni(Ⅲ)/Ni(Ⅱ)比值为0.62,处理过3000 min三种不同染料的催化剂样品中Ni(Ⅲ)/Ni(Ⅱ)比值分别为0.46、0.44和0.42,Ni(Ⅲ)的相对含量减少,催化性能略微降低。
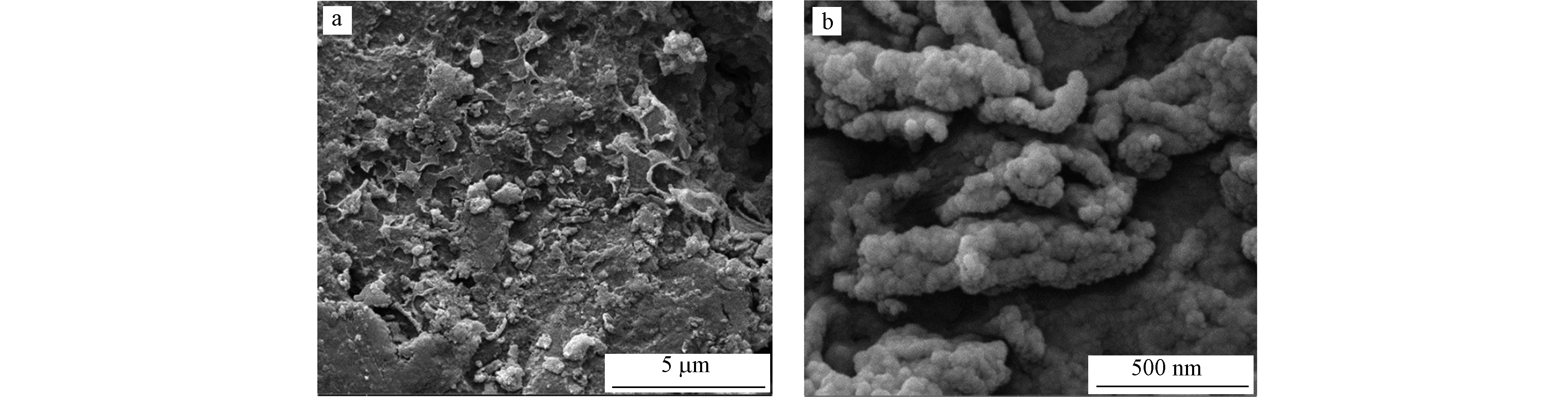
图13为新制备的催化剂的SEM照片。由图13可见,催化剂的表面比较平整,镍的沉淀物较为均匀且致密地包裹在氧化铝小球表面,因此,Ni2+不容易渗透到Al2O3小球里。β-NiOOH为球形粒子,β-Ni(OH)2具有六方晶系结构[23],两者紧密缠绕。
-
(1) NaClO催化氧化法对活性黄X-R废水的脱色效果欠佳,催化剂对脱色率的影响甚微。NaClO的直接氧化在脱色过程起了主导作用,原子氧的作用次之。
(2) NaClO催化氧化法更加适合于降解具有较多磺酸基和较高I/O值的偶氮染料。该工艺更加适合于降解稳定性强、耐氯性好的K型活性染料、含有磺酸钠盐的酸性染料和苯环染料。可以采用Mg(OH)2混凝法和Fenton法降解活性黄X-R染料。
(3) XPS分析表明,与用过3000 min的催化剂比较,新制备催化剂表面化学吸附氧占比略高;用过催化剂表面的Ni(Ⅲ)的相对含量减少,催化剂的性能略有降低。化学吸附氧消耗在染料的脱色降解。
NaClO催化氧化法降解活性黄X-R废水和该方法的适用范围
Degradation of reactive yellow X-R wastewater by NaClO catalytic oxidation method and its application scope
-
摘要: 采用NaClO催化氧化法对活性黄X-R废水进行了脱色降解的系统研究,探讨了反应条件对脱色效果的影响,分析了染料的降解机理。结果表明,该方法对染料的脱色率不到50%,脱色率随着染料和有效氯浓度的增加而略微增大,随着初始pH的上升而减小,催化剂的投加对脱色率的影响甚微。NaClO氧化法的对比实验结果表明,NaClO的直接氧化在催化氧化降解过程中起了主导作用,原子氧的作用次之。自由基清除剂实验结果也证明了这一点。定量分析了活性黄X-R和另外2种偶氮染料的分子结构与NaClO催化氧化法对其脱色效果的关系(QSPR分析),结果表明,染料分子结构中磺酸基数目和无机性值与有机性值的比值对脱色率有较强的影响,磺酸基数目的影响尤为突出。NaClO催化氧化法更加适合于降解K型活性染料、含有磺酸钠盐的酸性染料和苯环染料,因为它们具有较强的耐氯牢度。采用XPS、XRD和SEM法对催化剂进行表征,结果发现其表层化学吸附氧的比例随着催化剂使用时间的延长略微减小,表层里含有β-NiOOH和β-Ni(OH)2,且牢牢附着在氧化铝小球表面,不易脱落造成二次污染。Abstract: The decolorization and degradation of reactive yellow X-R wastewater were systematically studied by NaClO catalytic oxidation method. The effects of reaction conditions on decolorization were discussed, and the degradation mechanism of the dye was analyzed. The results showed that the decolorization rate of the dye by this method was lower than 50%. The decolorization rate increased slightly with dye and available chlorine concentration, and decreases with initial pH. The addition of the catalyst had little influence on the decolorization rate. The comparative experiment results of NaClO oxidation showed that the direct oxidation of NaClO played a leading role, followed by atomic oxygen in the catalytic oxidation degradation process. The experimental results of free radical scavengers also confirmed this point. The relationship between the molecular structure of reactive yellow X-R and other two azo dyes and the decolorization effect of NaClO catalytic oxidation method (QSPR analysis) was quantitatively analyzed. The results showed that the number of sulfonic acid groups in dye molecular structure and the ratio of inorganic value to organic value had great influence on the decolorization rate, especially the number of sulfonic acid groups. NaClO catalytic oxidation is more suitable for degradation of K reactive dyes, acid dyes containing sodium sulfonate and benzene ring dyes, because they have stronger chlorine fastness. The catalyst was characterized by XPS, XRD and SEM. The results showed that the proportion of chemisorbed oxygen in the surface layer decreased slightly with the prolonging of catalyst service time, and β-NiOOH and β-Ni(OH)2 were contained in the surface layer, which was firmly attached to the surface of alumina beads and was not easy to fall off and cause secondary pollution.
-
Key words:
- nickel oxyhydroxide /
- sodium hypochlorite /
- catalytic mechanism /
- reactive yellow X-R /
- QSPR analysis
-
偶氮染料是纺织业中使用最多的一类染料 [1],具有难降解的特性。常见的处理方法包括物化法、化学法和生物法。物化法不能彻底降解污染物,化学法成本过高,生物法降解缓慢[2],因此限制了这些方法的应用。
次氯酸钠氧化法广泛用于降解废水中的污染物,并以污染物去除效率高、设备简单、经济性等优点而受到人们的重视。NaClO溶液的稳定性较差,大大降低了其氧化性的利用率。此外,采用NaClO直接氧化废水,会产生具有强致癌性的氯代有机物。增强NaClO的氧化性和消除氯代有机物对拓宽其在水处理中的应用极为重要。镍基催化剂能促进NaClO溶液的分解增强其氧化性,且以催化效果好、便宜等优点而备受关注[3]。NaClO在氧化镍的作用下生成具有极强活性的原子氧,迅速氧化废水中的污染物,从而达到去除COD和脱色的目的[4-5]。本课题组研发的NaClO催化氧化法能有效降解活性艳红K-2BP、酸性大红3R等偶氮染料,活性艳红K-2BP的脱色率在120 min达到94%以上[6],而酸性大红3R的脱色率在35 min达到90%以上。原子氧在脱色降解过程起了主导作用,因此未在染料降解产物中发现氯代有机物。
研究染料的分子结构与脱色性能之间的关系对废水的处理至关重要。染料种类繁多,结构各异,可以采用QSPR (quantitative molecular structure-properties relationships)法研究分子结构和脱色性能的关系。该方法简单易行,了解了分子结构信息,就可以分析和预测染料的理化性质或环境行为[7]。
本研究分别采用NaClO催化氧化法和NaClO氧化法脱色降解活性黄X-R废水,考察比较两种方法的脱色效果;比较催化氧化法对活性黄X-R和另外两种偶氮染料的脱色效果,在此基础上采用QSPR分析该方法处理不同染料效果存在差异的原因,进而指出其适用范围。本研究选择适当的染料分子结构描述符,通过线性回归法研究上述目标染料分子结构与NaClO催化氧化法对其脱色效果之间的关系。研究结果将为推广应用NaClO催化氧化法提供理论依据。
1. 材料与方法 (Materials and methods)
1.1 实验材料
催化剂载体选用水处理用耐酸碱的γ-Al2O3小球,参数如下:直径为 2−3 mm,Al2O3 > 92%,孔容为0.35 mL·g−1,比表面积大于300 m2·g−1。Na2S2O8 (99%)、NAOH (99%)、Ni(NO3)2·6H2O (99%)、H2SO4 (98%)、呋喃甲醇(99.2%)和叔丁醇(99.9%)均为分析纯,NaClO溶液有效氯含量大于10%。
仪器包括:ZYL便携式余氯分析仪、MIT-3F型COD检测仪、723N扫模型可见光分光光度计、Rigaku Smartlab9 x射线衍射仪(日本理学)、EscaLab 250Xi型X射线光电子能谱仪,Al-Kα射线单色源(美国赛默飞)、扫描电镜SEM(美国FEI Inspect F50)。
1.2 催化剂制备
催化剂制备步骤如下:先将γ-Al2O3小球浸泡在摩尔浓度为0.6 mol·L−1 Na2S2O8和0.5 mol·L−1 NaOH的混合液里,在振荡器上以100 r·min−1的频率振荡2 h,将得到的Na2S2O8-NaOH/Al2O3小球滤出后烘干。然后将Na2S2O8-NaOH/Al2O3小球浸泡在含有0.6 mol·L−1 Ni2+的溶液里发生反应,在γ-Al2O3表面形成一层镍氧化物/镍氢氧化物沉淀,将制得的小球滤出后烘干,得到镍基Al2O3小球。将得到的镍基Al2O3小球再次浸泡在Na2S2O8-NaOH混合液里,在振荡器上以100 r·min−1的频率振荡2 h后将催化剂小球滤出,用蒸馏水清洗。最后将洗净的催化剂置于马弗炉中低温焙烧2 h,制得催化氧化实验所需的催化剂样品。
Ni(OH)2为还原性氢氧化物,能和强氧化剂反应生成羟基氧化镍NiOOH。通过Ni2+在碱性溶液中被Na2S2O8氧化的反应来合成NiOOH(见式(1)和式(2)),从中可以看出,碱性条件促进Ni(OH)2和NiOOH的生成。
Ni2++2OH−⟶Ni(OH)2 (1) 2Ni(OH)2+S2O2−8+2OH−⟶2NiOOH+2SO2−4+2H2O (2) 在NiOOH存在的情况下,NaClO的分解反应2NaClO→2NaCl+O2↑可以看作是反应(3)和反应(4)的总和:
2NiOOH+NaClO⟶NaCl+2NiO2+H2O (3) 2NiO2+NaClO+H2O⟶2NiOOH+NaCl+O2 (4) 产生的氧分子能被物理吸附在镍基催化层上(式5),捕获电子后转化为化学吸附氧
O−2 O2(g)+S↔O2 (5) O2+e−↔O−2+∗ (6) O−2\xLeftrightarrowΔG1O−+O (7) O2\xLeftrightarrowΔG22O (8) 其中,O2(g)为气态氧,S为表面吸附位置,O2为表面物理吸附的氧,*和e−代表导带底部空位和导带电子,ΔG为反应能。反应产生的原子氧O非常活跃,可以快速氧化和分解有机物。羟基氧化镍可以长时间用作催化剂,因为它可以通过反应(3)和(4)进行再生利用。
1.3 活性黄X-R浓度的检测
染料废水在波长为390 nm处有最大特征吸收峰,在390 nm检测不同浓度废水的吸光度,建立质量浓度和吸光度的标准曲线方程y =96.877 x –1.1831,R2 = 0.9987。
1.4 染料脱色率分析
配制一定浓度的活性黄X-R溶液,加入一定量的NaClO溶液,充分搅拌,调节pH至所需值,得到废水/NaClO混合液。取50 mL混合液放入100 mL锥形瓶内,把一定量催化剂样品快速放入溶液中。在振荡器上以100 r·min−1的速度振荡120 min。分别在第10、30、50、70、90、120 min时取3 mL左右上清液,直接测其吸光度,利用标准曲线方程求得染料的浓度,按公式(9)计算其脱色率。测好吸光度后将染料溶液倒回锥形瓶中继续反应。
脱色率=C0−CtC0×100(%) (9) 式中,C0 和Ct 分别为初始染料浓度和t时刻染料浓度,mg·L−1。
1.5 自由基清除剂实验
配制质量浓度为200 mg·L−1的活性黄X-R溶液,加入120 mg·L−1的NaClO溶液,充分搅拌,调节到pH 9,得到废水/NaClO混合液。在两个相同组分的混合液中分别加入20 mmol·L−1的叔丁醇和呋喃甲醇,与催化剂一起放入100 mL锥形瓶内,在振荡器上以100 r·min−1的速度振荡反应,检测染料的脱色率变化,并以此来判断在反应中起主导作用的自由基。叔丁醇是一种良好的OH·和
SO−4⋅ 2. 结果与讨论 (Results and discussion)
2.1 反应条件对活性黄X-R溶液脱色率的影响
2.1.1 染料浓度对脱色率的影响
检测了当pH 为9、有效氯浓度为120 mg·L、催化剂投加量为240 g·L−1、初始染料浓度为100、200、300、400 mg·L−1时的脱色率,结果如图1所示。前期的研究结果表明[11],有催化剂时,弱碱性环境pH 9有利于反应(1)和反应(2)的发生,促进活性氧的产生,增大NaClO的转化率。当催化剂存在时,脱色率随着初始染料浓度的增加而增加:当染料浓度为100、200、300、400 mg·L−1时,脱色率在120 min时分别为41.1%、41.21%、42.47%和50.03%。没有催化剂时,当初始染料浓度为100、200、300、400 mg·L−1时,脱色率在反应120 min时分别为43.7%、42.67%、41.78%和40.17%。加入催化剂后,初始染料浓度为100、200、300 mg·L−1溶液在120 min时的脱色率基本没有发生变化,可能是因为NaClO的直接氧化起了主导作用。当初始染料浓度为400 mg·L−1时,催化剂的加入使脱色率增加到50.03%,催化剂的吸附和活性氧的氧化作用对脱色率的增量做了一定的贡献,吸附量随着染料浓度的增加而增大,进而促进了活性氧与之发生反应。
催化氧化法对活性黄X-R的脱色效果远差于其对活性艳红K-2BP和酸性大红3R的脱色效果,脱色率随浓度变化的趋势也和后面两种染料的相反,活性艳红K-2BP和酸性大红3R的脱色率随着染料初始浓度的增加而减小(图2),因为反应的速率受到活性氧产量的限制,高浓度溶液需要更多的活性氧才能保持同样的降解速率。
 图 2 初始染料浓度对脱色率的影响Figure 2. Effect of initial dye concentration on color removal ratepH为9,催化剂投加量为240 g·L−1和 (a) 活性艳红K-2BP,有效氯为120 mg·L−1 (b) 酸性大红3R,有效氯为60 mg·L−1pH 9, catalyst dosage 240 g·L−1 and (a) reactive brilliant red K-2BP, available chlorine 120 mg·L−1 (b) acid red 3R, available chlorine 60 mg·L−1
图 2 初始染料浓度对脱色率的影响Figure 2. Effect of initial dye concentration on color removal ratepH为9,催化剂投加量为240 g·L−1和 (a) 活性艳红K-2BP,有效氯为120 mg·L−1 (b) 酸性大红3R,有效氯为60 mg·L−1pH 9, catalyst dosage 240 g·L−1 and (a) reactive brilliant red K-2BP, available chlorine 120 mg·L−1 (b) acid red 3R, available chlorine 60 mg·L−1以上实验结果表明,催化氧化体系降解活性黄X-R的机理可能有别于活性艳红K-2BP和酸性大红3R的降解机理,NaClO更倾向于直接氧化活性黄X-R,因此抑制了其本身的催化分解反应,减少了活性氧的产量;活性黄X-R分子也可能不易被具有亲电子性的活性氧攻击,使NaClO的直接氧化作用在脱色反应中占据了主导地位。因此,当NaClO有效氯浓度足够高的时候,染料的分解速率随着染料浓度的增加而增大,从表观上看,脱色率增大。
2.1.2 有效氯浓度对脱色率的影响
检测了当pH 9、染料浓度为200 mg·L−1、催化剂投加量为240 g·L−1、 有效氯为60、120、180 、250 mg·L−1时的脱色率,结果如图3所示。脱色率随着有效氯浓度的增加而增大。有催化剂存在时,当有效氯浓度从60 mg·L−1增加到250 mg·L−1,120 min时染料的脱色率从45%提升到了58%,增幅有限,主要是因为NaClO的直接氧化起了主导作用。NaClO在pH 9时的氧化性较弱,脱色效果差,单独氧化时NaClO的利用率较低[12]。有效氯浓度的增大也促进了NaClO直接氧化染料反应的进行,改善了活性黄X-R的脱色效果:当初始有效氯浓度从60 mg·L−1增加到250 mg·L−1,染料的脱色率从37.8%提升到了44.7%,比催化剂存在时的脱色率低6.3%–13.3%,增值是由催化剂的吸附和产生的活性氧作用所导致的。
2.1.3 pH对脱色率的影响
检测了当有效氯为120 mg·L−1、染料浓度为200 mg·L−1、催化剂投加量为240 g·L−1、初始pH值为6、7、8、9时的脱色率,结果如图4所示。NaClO催化氧化法的脱色率随着pH的提升而减小,pH 6、7时的脱色效果略胜于pH 8、9时的脱色效果,初始pH为6时的脱色效果最好,当反应时间为120 min,脱色率达到53.8%,远低于弱碱性条件下NaClO催化氧化降解活性艳红K-2BP和酸性大红3R溶液的脱色率(图5),催化作用明显受到了抑制,这是因为反应机理不同而导致的。原子氧在活性艳红K-2BP和酸性大红3R的脱色中起了主导作用,NaClO的直接氧化在活性黄X-R的脱色中起了主导作用。当初始pH为9,加催化剂反应120 min,活性黄X-R的脱色率达到49%,无催化剂时脱色率为42.7%(图4)。NaClO法降解活性黄X-R的脱色率随着pH的增加明显降低,当pH值从6增加到9,脱色率从84.1%降到42.7%,这是因为NaClO在酸性条件下会以游离氯存在,氧化性能大大提升。
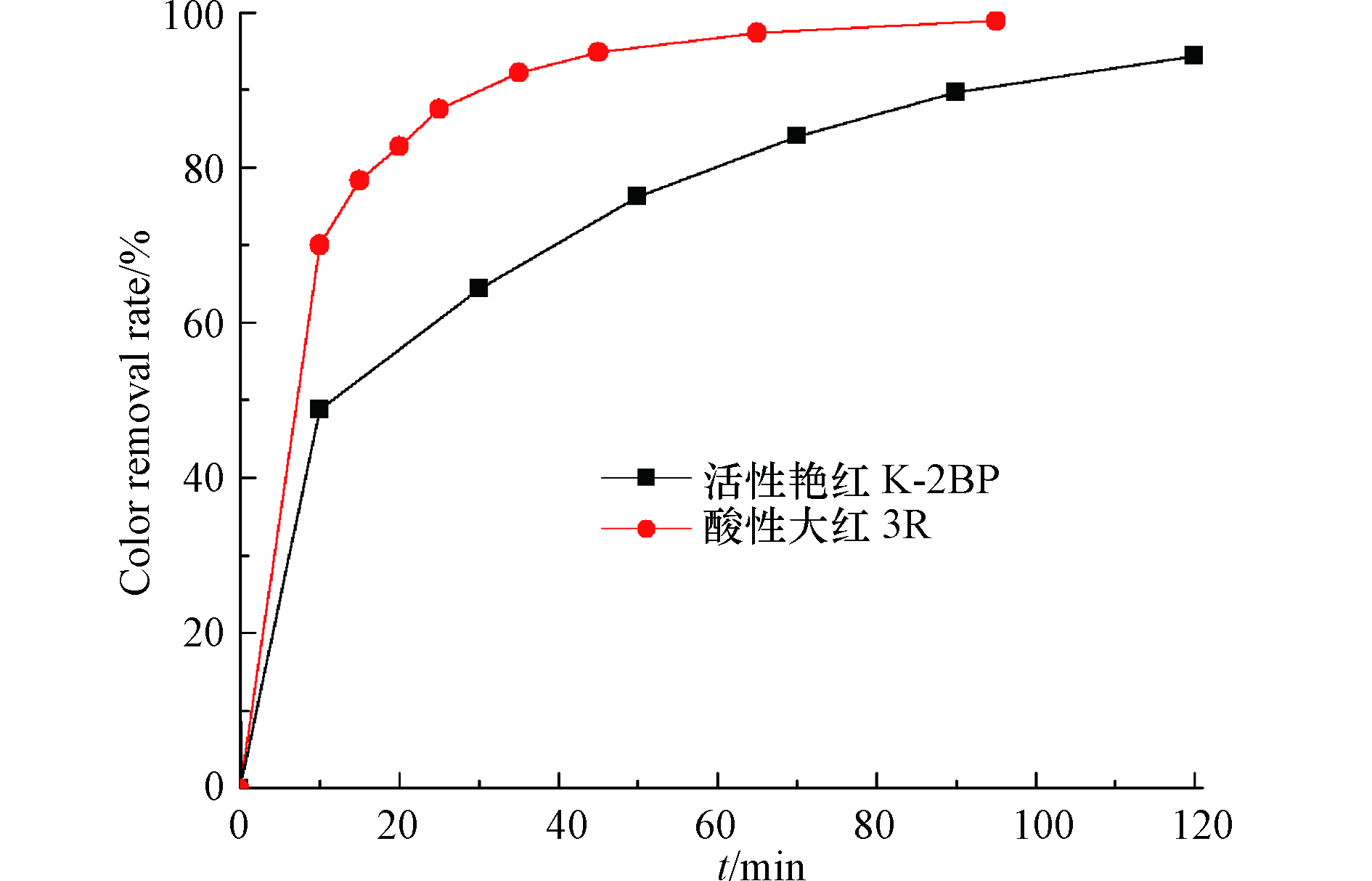 图 5 不同偶氮染料对脱色率的影响Figure 5. Effect of different azo-dyes on color removal rate初始pH 9,催化剂投加量240 g·L−1,活性艳红K-2BP和有效氯浓度为200 mg·L−1和120 mg·L−1,酸性大红3R和有效氯浓度为200 mg·L−1和60 mg·L−1initial pH 9, catalyst dosage 240 g·L−1, the concentrations of reactive brilliant red K-2BP and available chlorine are 200 mg·L−1 and 120 mg·L−1, while the concentrations of acid red and available chlorine are 200 mg·L−1 and 60 mg·L−1
图 5 不同偶氮染料对脱色率的影响Figure 5. Effect of different azo-dyes on color removal rate初始pH 9,催化剂投加量240 g·L−1,活性艳红K-2BP和有效氯浓度为200 mg·L−1和120 mg·L−1,酸性大红3R和有效氯浓度为200 mg·L−1和60 mg·L−1initial pH 9, catalyst dosage 240 g·L−1, the concentrations of reactive brilliant red K-2BP and available chlorine are 200 mg·L−1 and 120 mg·L−1, while the concentrations of acid red and available chlorine are 200 mg·L−1 and 60 mg·L−1当pH值为6、7、8时,加入催化剂反而导致活性黄X-R的脱色效果变差(图4)。当pH 6时,NaClO主要以HClO的形式存在。随着pH值的提升,ClO−的量增多,pH 9时NaClO主要以ClO−的形式存在。催化剂表面含有大量Ni(OH)2,带有正电荷[13]。当pH 6时,催化剂与次氯酸电荷相斥,不利于其在催化剂表面的有效吸附;当pH值为8、9,催化剂与ClO−存在电荷相吸作用,有利于NaClO在催化剂表面的有效吸附。催化剂表面和含有磺酸根离子的染料分子之间的静电吸引可能有利于染料在其表面的有效吸附。因此,当pH 6时,催化剂的存在降低了HClO与染料分子之间的反应几率,从而降低了脱色率,虽然此时的溶液具有较强的氧化性。当pH值为8、9,NaClO和染料分子在催化剂表面的有效吸附使两者之间的反应几率增大,从而使脱色率和酸性条件下的相差不大。催化剂存在时,弱碱性条件促进了活性氧的产生,但对染料的脱色率基本没有产生影响,可能是活性氧与染料分子之间的反应受到了阻碍。
采用NaClO催化氧化法降解活性艳红K-2BP和酸性大红3R,初始pH 9时的脱色效果最好(图6),弱碱性条件有利于NiOOH的生成和催化层的固定(式(1)和式(2)),进而促进原子氧的产生(式(3)—(8));同时,弱碱性条件还能抵消由于降解过程产生有机酸而导致的pH值的下降,起到平衡pH值的作用。
 图 6 pH对不同偶氮染料脱色率的影响Figure 6. Effect of different azo-dyes on color removal ratepH 9,催化剂投加量240 g·L−1,活性艳红K-2BP和有效氯浓度为200 mg·L−1和120 mg·L−1,酸性大红3R和有效氯浓度为200 mg·L−1和60 mg·L−1pH 9, catalyst dosage 240 g·L−1, the concentrations of reactive brilliant K-2BP and available chlorine are 200 mg·L−1 and 120 mg·L−1, while the concentrations of acid red 3R and available chlorine are 200 mg·L−1 and 60 mg·L−1
图 6 pH对不同偶氮染料脱色率的影响Figure 6. Effect of different azo-dyes on color removal ratepH 9,催化剂投加量240 g·L−1,活性艳红K-2BP和有效氯浓度为200 mg·L−1和120 mg·L−1,酸性大红3R和有效氯浓度为200 mg·L−1和60 mg·L−1pH 9, catalyst dosage 240 g·L−1, the concentrations of reactive brilliant K-2BP and available chlorine are 200 mg·L−1 and 120 mg·L−1, while the concentrations of acid red 3R and available chlorine are 200 mg·L−1 and 60 mg·L−12.1.4 催化剂对脱色率的影响
检测了当有效氯浓度为120 mg·L−1、染料浓度为200 mg·L−1、初始pH为9、催化剂投加量为80、160、240、400 g·L−1时的脱色率,结果如图7所示。催化剂投加量的增加对染料脱色率的影响很小,当催化剂投加量从80 g增加到400 g·L−1,脱色率在反应120 min时只提升了3.4%。单独使用NaClO时的脱色率与催化剂存在时的基本相同。单独使用400 g·L−1新制备的催化剂处理废水120 min,仅有7.6%–9.4%的脱色率,随着反应时间的延长,脱色率变化幅度很小,说明这部分脱色率主要是由吸附导致的,其贡献较小。实验结果再次验证了NaClO的氧化在脱色反应中的主导作用,当初始pH为9时,催化剂对脱色反应的影响甚微,催化作用受到了抑制。因此,催化氧化法不太适用于脱色降解活性黄X-R废水。
2.1.3节的研究结果表明,当pH为6,200 mg·L−1活性黄X-R废水中有效氯浓度达到120 mg·L−1,反应 120 min后脱色率达到84.1%。继续调低pH能进一步提升脱色率,但是NaClO氧化法会导致出水中产生毒性较强的氯代有机物。王元刚等[14]采用Mg(OH)2混凝脱色降解浓度为100 mg·L−1的活性黄X-R废水,当pH 值为12、Mg2+投加量为216 mg·L−1时,脱色率达到90%以上。王昶等[15]采用蛭石类芬顿降解活性黄X-R染料,结果表明,把蛭石粉碎到7.5 μm的颗粒,其投加量为1.0 g·L−1、pH 3、H2O2用量 2.94 mmol·L−1时,反应180 min 后,50—300 mg·L−1活性黄X-R 脱色率均能达到95%以上。后面两种方法的运行成本偏高。
2.2 机理研究
叔丁醇的加入对活性黄X-R的降解过程几乎没有影响,而呋喃甲醇对脱色具有一定的抑制作用,两者之间的脱色率差值在12%―24%之间(图8),因此可以断定原子氧在染料脱色过程中起了一定的作用,OH·和
SO−4⋅ 活性黄X-R在390 nm的最大吸收峰对应偶氮键(图9),该峰随着反应的进行逐渐减小,但没有消失,可见偶氮键并未被完全破坏。367―370 nm之间为萘环的吸收峰,峰面积随着反应的进行减小,但反应120 min后该特征吸收峰还在,萘环没有完全打开。
2.3 染料分子结构与脱色率的关系
根据染料的分子结构特征及脱色率,选择磺酸基(
SO−3 分别配制浓度为200 mg·L−1的活性艳红K-2BP、酸性大红3R和活性黄X-R溶液,往里投加NaClO,使混合液中有效氯浓度达到120 mg·L−1,调节废水/NaClO混合液的pH值到9,投加催化剂240 g·L−1,在振荡器上反应90 min后取样,于染料的最大吸收波长下测定溶液的吸光度,换算成浓度,计算其脱色率。3种染料都属于偶氮染料,它们的分子结构见图10,它们的去除率和分子结构描述符见表1.
表 1 偶氮染料的去除率和分子结构描述符Table 1. Removal rate and molecular structure descriptor of the azo dyes染料名称Dye name 分子结构描述符Molecular structure descriptor 脱色率/%Color removal rate A B C 活性艳红K-2BP 3 3.95 5 91.3 酸性大红3R 3 6.25 4 99.69 活性黄 X-R 2 3.14 4 39.3 对表1中的分子结构描述符与脱色率进行线性回归分析,其线性回归方程和参数如表2所示。A、B与脱色率的线性回归数值比较高,说明目标染料的去除率与染料分子结构中磺酸基数目和I/O值之间具有较强的线性相关性,其中磺酸基数目A对去除率的影响尤为突出。染料分子的A值升高,其脱色率增加,这是因为磺酸基数目的增加使染料分子的电子云密度增加,原子氧具有较强的亲电子性,易与偶氮键发生加成反应。染料分子的B值升高,其无机性增强,电离度也随之增大,溶液中磺酸根离子的量增加,原子氧与染料分子之间的反应几率也因此增大,染料的脱色效率提高。C值增大,脱色率也随之增加,但去除率和C值的相关性较差,R2=0.1481,染料分子中C值一般小于10。因此,C值对脱色率的影响很小。
表 2 分子结构描述符与去除率的线性回归方程Table 2. Linear regression equation between molecular structure descriptor and removal rate分子结构描述符Molecular structure descriptor 回归方程Regression equation R2 F A ω 0.9836 59.815 B ω 0.6194 2.627 C ω 0.1481 0.174 偶氮键断开后,活性黄X-R分子被分解成2个芳胺:3-氨基-1,5-萘二磺酸根和4-[(4,6-二氯-s-三嗪-2-基)氨基]-1-氨基-o-甲苯基 (图11)。3-氨基-1,5-萘二磺酸根包含一个萘环,萘环上引入了两个磺酸基,其耐氯牢度大大提高,也就是说OCl−的进攻能力减弱;引入磺酸基的数目越多,耐氯牢度提升也越大[18]。活性黄X-R分子含有二氯均三嗪活性基,由于两个氯原子的吸电子诱导效应,环上的电子云密度较低,故其活性较高,水解率高,固色效果差,容易被浓度高氧化能力弱一些的OCl−进攻。活性艳红K-2BP的分子包含1个偶氮基、一氯均三嗪基、2个苯环和1个萘环(图10)。一氯均三嗪环上的电子云密度高,稳定性强,固色效果好,不易被OCl−进攻[19],却能被氧化性极强的原子氧脱色降解。董亚荣等[20]曾对10种染料的生物降解脱色性能进行了研究,结果表明,X型染料最易降解,K型染料次之。实验结果与该结论吻合。酸性大红3R分子包含1个偶氮基、1个羟基和2个萘环;萘环上有3个磺酸基,其中1个磺酸基位于偶氮基的邻位(图10),其耐氯稳定性好[19],降解过程不易产生有机氯产物。
综上,活性黄X-R分子的耐氯牢度较低,NaClO倾向将其直接氧化,从而抑制了NaClO的催化分解和原子氧的产生,降低了原子氧对脱色降解过程的贡献度。活性艳红K-2BP和酸性大红3R的耐氯稳定性好。NaClO催化氧化法更适合降解耐氯性强的K型活性偶氮染料和含有磺酸钠盐的酸性偶氮染料,不太适合于降解X型活性偶氮染料。苯环染料比萘环染料耐氯性好,这也是吴正雷等[5]用NaClO/镍基催化剂处理甲基橙(对二甲氮基偶苯磺酸钠)废水,没有发现有机氯产物的原因。
采用XRD对催化剂表层进行物相分析。样品在19.2°和66.7°附近有强的衍射峰(图12),与标准卡(卡号60141)对比可知,样品里含有β-NiOOH,两个衍射峰分别为001和110晶面。样品在38.5°和72.2°处出现两个衍射峰,与标准卡(卡号14117)对比可知,样品里含有β-Ni(OH)2,两个衍射峰分别为101和201晶面。
采用XPS分析法检测催化剂表面的元素及其相对含量。选取新制备的催化剂和分别处理过3000 min不同偶氮染料的三个催化剂小球作为检测样品。样品中氧元素的含量在78%以上(表3)。531.46 eV左右的峰为化学吸附氧引起的[21]。处理过3000 min染料的催化剂样品中氧元素的含量有不同程度的减小,主要是因为产生的原子氧参与降解染料。855.79―856.14 eV的峰是由Ni(Ⅱ)引起的,861.59―861.95 eV的峰是由Ni(Ⅲ)引起的[22]。新制的催化剂样品的Ni(Ⅲ)/Ni(Ⅱ)比值为0.62,处理过3000 min三种不同染料的催化剂样品中Ni(Ⅲ)/Ni(Ⅱ)比值分别为0.46、0.44和0.42,Ni(Ⅲ)的相对含量减少,催化性能略微降低。
表 3 催化剂表面元素的相对占比Table 3. Relative proportion of catalyst surface elements元素Elements 峰位置Position 结合能/eVBinding energy 相对含量/% Relative content 新制备的催化剂New catalyst 处理过3000 min活性艳红K-2BP的催化剂Catalyst of K-2BP treated for 3000 min 处理过3000 min酸性大红3R的催化剂Catalyst of 3R treated for 3000 min 处理过3000 min活性黄X-R的催化剂Catalyst of X-R treated for 3000 min Ni Ni(OH)2,2p3/2 855.98 6.34 10.61 13.46 10.50 Ni Ni(OH)2,2p1/2 873.61 Ni NiOOH,2p3/2 861.86 3.92 4.84 5.95 4.43 Ni NiOOH,2p1/2 879.81 O O1s 531.40 87.67 83.08 78.46 83.83 S S2p 168.67 2.07 1.47 2.13 1.24 图13为新制备的催化剂的SEM照片。由图13可见,催化剂的表面比较平整,镍的沉淀物较为均匀且致密地包裹在氧化铝小球表面,因此,Ni2+不容易渗透到Al2O3小球里。β-NiOOH为球形粒子,β-Ni(OH)2具有六方晶系结构[23],两者紧密缠绕。
3. 结论 (Conclusion)
(1) NaClO催化氧化法对活性黄X-R废水的脱色效果欠佳,催化剂对脱色率的影响甚微。NaClO的直接氧化在脱色过程起了主导作用,原子氧的作用次之。
(2) NaClO催化氧化法更加适合于降解具有较多磺酸基和较高I/O值的偶氮染料。该工艺更加适合于降解稳定性强、耐氯性好的K型活性染料、含有磺酸钠盐的酸性染料和苯环染料。可以采用Mg(OH)2混凝法和Fenton法降解活性黄X-R染料。
(3) XPS分析表明,与用过3000 min的催化剂比较,新制备催化剂表面化学吸附氧占比略高;用过催化剂表面的Ni(Ⅲ)的相对含量减少,催化剂的性能略有降低。化学吸附氧消耗在染料的脱色降解。
-
表 1 偶氮染料的去除率和分子结构描述符
Table 1. Removal rate and molecular structure descriptor of the azo dyes
染料名称Dye name 分子结构描述符Molecular structure descriptor 脱色率/%Color removal rate A B C 活性艳红K-2BP 3 3.95 5 91.3 酸性大红3R 3 6.25 4 99.69 活性黄 X-R 2 3.14 4 39.3 表 2 分子结构描述符与去除率的线性回归方程
Table 2. Linear regression equation between molecular structure descriptor and removal rate
分子结构描述符Molecular structure descriptor 回归方程Regression equation R2 F A ω 0.9836 59.815 B ω 0.6194 2.627 C ω 0.1481 0.174 表 3 催化剂表面元素的相对占比
Table 3. Relative proportion of catalyst surface elements
元素Elements 峰位置Position 结合能/eVBinding energy 相对含量/% Relative content 新制备的催化剂New catalyst 处理过3000 min活性艳红K-2BP的催化剂Catalyst of K-2BP treated for 3000 min 处理过3000 min酸性大红3R的催化剂Catalyst of 3R treated for 3000 min 处理过3000 min活性黄X-R的催化剂Catalyst of X-R treated for 3000 min Ni Ni(OH)2,2p3/2 855.98 6.34 10.61 13.46 10.50 Ni Ni(OH)2,2p1/2 873.61 Ni NiOOH,2p3/2 861.86 3.92 4.84 5.95 4.43 Ni NiOOH,2p1/2 879.81 O O1s 531.40 87.67 83.08 78.46 83.83 S S2p 168.67 2.07 1.47 2.13 1.24 -
[1] 周宁, 宇秉勇, 宋红, 等. 染料工业废水产污情况分析 [J]. 染料与染色, 2018, 55(1): 54-61. ZHOU N, YU B Y, SONG H, et al. Analysis on the pollution of dye industrial wastewater [J]. Dyestuffs and Coloration, 2018, 55(1): 54-61(in Chinese).
[2] 武俐, 邰超, 王晴晴, 等. 负载型纳米Fe-Pd降解水溶性偶氮染料 [J]. 环境化学, 2012, 31(8): 1125-1130. WU L, TAI C, WANG Q Q, et al. Degradation of azo dye by resin-supported nano-iron/palladium [J]. Environmental Chemistry, 2012, 31(8): 1125-1130(in Chinese).
[3] 申晨, 梅华, 石晓鹏, 等. 镍基催化剂的改性及其提高NaClO氧化性能 [J]. 化学反应工程与工艺, 2010, 26(1): 47-51. doi: 10.3969/j.issn.1001-7631.2010.01.009 SHEN C, MEI H, SHI X P, et al. Improving the oxidation of NaClO by modified Ni-based catalysts prepared through immerse precipitation method [J]. Chemical Reaction Engineering and Technology, 2010, 26(1): 47-51(in Chinese). doi: 10.3969/j.issn.1001-7631.2010.01.009
[4] 吴正雷, 彭文博, 董凯, 等. 催化氧化-BAF工艺处理乙基麦芽酚废水研究 [J]. 广东化工, 2020, 47(11): 165-166,164. doi: 10.3969/j.issn.1007-1865.2020.11.069 WU Z L, PENG W B, DONG K, et al. The treatment of ethyl maltol wastewater by catalytic oxidation and biological aerated filter technology [J]. Guangdong Chemical Industry, 2020, 47(11): 165-166,164(in Chinese). doi: 10.3969/j.issn.1007-1865.2020.11.069
[5] 吴正雷, 彭文博, 董凯, 等. Ni基催化剂处理甲基橙废水动力学及机理研究 [J]. 当代化工, 2020, 49(5): 850-854. doi: 10.3969/j.issn.1671-0460.2020.05.024 WU Z L, PENG W B, DONG K, et al. Research on kinetics and mechanism of degradation of methyl orange wastewater catalyzed by Ni-based catalyst [J]. Contemporary chemical industry, 2020, 49(5): 850-854(in Chinese). doi: 10.3969/j.issn.1671-0460.2020.05.024
[6] 徐文英, 高浩阳. NiOx(OH)y/NaClO催化氧化体系对模拟印染废水中活性艳红K-2BP的降解脱色效果[J]. 环境工程学报, 2021, 15(3): 835-836. XU WY, GAO H Y. Degradation and decolorization of reactive brilliant red K-2BP in simulated printing and dying wasterwater by NiOx(OH)y/NaClO catalytic oxidation system[J]. Chinese Journal of Environmental Engineering, 2021, 15(3): 835-846 (in Chinese)
[7] 田长顺, 刘祖文. 偶氮染料分子结构与脱色性能的定量关系研究 [J]. 工业用水与废水, 2008, 39(4): 10-12. doi: 10.3969/j.issn.1009-2455.2008.04.003 TIAN C S, LIU Z W. Quantitative relationship between molecular structure of azo dyes and their decolorization performance [J]. Industrial Water & Wastewater, 2008, 39(4): 10-12(in Chinese). doi: 10.3969/j.issn.1009-2455.2008.04.003
[8] 李蕾, 夏思淝, 刘文利, 等. 气敏金属氧化物吸附氧负离子O2-, O-的研究[J]. 山东工业大学学报, 1994, 24(3): 287-290. LI L, XIA S F, LIU W L, et al. Investigation of adsorbed O2- and O- on the surface of gas sensing metal oxides[J]. Journal of Shandong University of Technology, 1994, 24(3): 287-290 (in Chinese).
[9] 唐玉朝, 尹汉雄, 黄健, 等. 零价铁活化过硫酸钠对偶氮染料4BS的脱色机理 [J]. 环境化学, 2018, 37(5): 1071-1078. TANG Y C, YIN H X, HUANG J, et al. Decoloration mechanism of azo dye 4BS by zero valent iron activated sodium persulfate [J]. Environmental Chemistry, 2018, 37(5): 1071-1078(in Chinese).
[10] 陈柏言. 冰中黑炭来源单线态氧的光化学生成[D]. 长春: 吉林大学, 2017. CHEN B Y. Photochemical production of singlet oxygen from black carbon in ice[D]. Changchun: Jilin University, 2017 (in Chinese).
[11] XU W Y, GAO H Y. Decomposition performance of hypochlorite on bead-type NiOx(OH)y catalyst: Improving applicability of catalysts [J]. Water Science and Technology, 2020, 82(5): 967-983. doi: 10.2166/wst.2020.402 [12] ZENG Q F, FU J, ZHOU Y, et al. Photooxidation degradation of reactive brilliant red K-2BP in aqueous solution by ultraviolet radiation/ sodium hypochlorite [J]. Clean, 2009, 37(8): 574-580. [13] 姬学敏. 花状氢氧化镍微球、二氧化钛及其复合物的制备、吸附与光催化性能研究[D]. 杭州: 浙江工业大学, 2016. JI X M. Preparation, adsorption and photocatalytic performance of flower-like Ni(OH)2 microspheres, TiO2 and its composite[D]. Hangzhou: Zhejiang University, 2016 (in Chinese).
[14] 王元刚, 刘诗雨, 赵建海, 等. 氢氧化镁混凝过程应用于活性黄X-R废水脱色研究 [J]. 环境工程, 2015, 33(7): 61-65. WANG Y G, LIU S Y, ZHAO J H, et al. Removal of color from reactive yellow X-R wastewater by magnesium hydroxide coagulation process [J]. Environmental Engineering, 2015, 33(7): 61-65(in Chinese).
[15] 王昶, 邱鸿雨, 李荣, 等. 蛭石类芬顿降解偶氮活性黄X-R染料的研究 [J]. 天津科技大学学报, 2019, 34(3): 49-54. WANG C, QIU H Y, LI R, et al. Degradation of azo dye reactive yellow X-R by using vermiculite as a Fenton-like catalyst [J]. Journal of Tianjing University of Science & Technology, 2019, 34(3): 49-54(in Chinese).
[16] DEROSA M C, CRUTCHLEY R J. Photosensitized singlet oxygen and its applications [J]. Coordination Chemistry Reviews, 2002, 233-234: 351-371. doi: 10.1016/S0010-8545(02)00034-6 [17] 黑木宣彦. 染色理论化学(陈水林译)[M]. 北京, 纺织工业出版社, 1957, 85-87. NOBUHIKO KUROKI. Dyeing theoretical chemistry (translated by CHEN S L) [M].Beijing:Textile Industry Press, 1957, 85-87.
[18] 陈荣圻. 活性染料染色牢度对策和固色剂的发展(二) [J]. 印染, 2016, 42(3): 51-54. CHEN R Q. Countermeasures for dyeing fastness of reactive dyes and development of fixing agents (2) [J]. Dyeing and Finishing, 2016, 42(3): 51-54(in Chinese).
[19] 李锦簇. 国内活性染料发展概况 [J]. 染料工业, 1985, 1: 1-7. LI J C. Development of reactive dyes in China [J]. Dyestuff Industry, 1985, 1: 1-7(in Chinese).
[20] 董亚荣, 王立栋, 金泥沙. 含活性染料印染废水生物降解脱色性能研究 [J]. 染整技术, 2016, 38(2): 54-57. doi: 10.3969/j.issn.1005-9350.2016.02.016 DONG Y R, WANG L D, JIN N S. Research on biodegradation and capability of decolorization of reactive dyeing wastewater [J]. Textile Dyeing and Finishing Journal, 2016, 38(2): 54-57(in Chinese). doi: 10.3969/j.issn.1005-9350.2016.02.016
[21] 徐向荣, 王文华. Fenton试剂与染料溶液的反应 [J]. 环境科学, 1999, 20(3): 72-74. doi: 10.3321/j.issn:0250-3301.1999.03.019 XU X R, WANG W H. The reaction of Fenton’s reagent and dye solution [J]. Chinese Journal of Environmental Science, 1999, 20(3): 72-74(in Chinese). doi: 10.3321/j.issn:0250-3301.1999.03.019
[22] YANG S X, FENG Y J, WAN J F, et al. Effect of CeO2 addition on the structure and activity of RuO2/γ-Al2O3 catalyst [J]. Applied Surface Science, 2005, 246(1-3): 222-228. doi: 10.1016/j.apsusc.2004.11.013 [23] 刘正. 绒球状β-氢氧化镍微米球的可控合成及其电化学性能 [J]. 合成化学, 2016, 24(10): 861-865. LIU Z. Synthesis and electrochemical properties of pompon-like β-Ni(OH)2 microspheres [J]. Chinese journal of synthetic chemistry, 2016, 24(10): 861-865(in Chinese).
-







 下载:
下载: