-
抗生素在医疗、畜牧、养殖等行业广泛应用。由于不正当地使用和无组织排放,致使其大量流入环境介质中,近年来,国内外在水环境[1]、土壤[2]、动植物组织[3]等环境介质中均有抗生素的检出报道[4-5]。畜禽养殖业中抗生素的不合理使用引发了一系列潜在危害,主要体现在产生抗药基因、破坏生物体内的微环境、人畜共患病增多等方面。动物源性食品安全成为了重大问题,为了保证人类健康,世界卫生组织(WHO)和联合国粮食及农业组织(FAO)曾共同建立了农药和兽药的最大残留限量(MRL)标准来对其进行控制。
检测食品中微量或痕量级别抗生素最常采用仪器法,有高效液相色谱法(HPLC)[6]、高效液相色谱-串联质谱法(HPLC-MS/MS)[7-9]、液相色谱-紫外-质谱法(LC-UV-MS)[10]、液相色谱-荧光检测方法[11]等。仪器检测法的灵敏度高,检测数据准确,但由于其前处理复杂、耗时长等劣势,限制了其在原位快速检测中的应用。生物检测技术因其高灵敏度、高选择性被广泛用于动物源性食品中的抗生素残留量检测研究。基于抗体的生物免疫检测技术最为成熟,研究应用也比较广泛。而稳定性好、适用范围广的核酸适配体与响应快、操作简便的传感器相结合成为了生物检测研究的热门发展方向。基于此,本文综述了以抗体和核酸适配体为生物识别元件的生物检测技术特点及其在动物源性食品中抗生素残留量检测的研究进展,并在归纳总结生物检测技术检测抗生素的研究基础上,预测抗生素检测技术未来的发展趋势。
-
免疫分析技术是基于抗体、抗原之间特异性结合的一种生物化学技术,被广泛应用于环境和食品行业中药物残留等有害物质的定性和定量。
-
ELISA是通过被酶标记的抗体对抗生素的竞争,结合到固相包被抗原上,洗涤后加入酶底物,固定在载体(酶标板)上的酶催化底物出现显色反应,最后通过分光光度法(酶标仪)对待测物质进行定量分析。近年来,双斑点法、直接(图1A)和间接性竞争ELISA在检测抗生素残留研究方面皆有开发。
直接性ELISA和间接性ELISA有着不同的优势,Ashu等[12]比较了两种ELISA测定抗生素的灵敏性,找到了一种特异性强、灵敏度高的多克隆抗体As172,采用直接竞争ELISA法建立了用于饲料中氟喹诺酮类抗生素的检测方法,其中该抗体对恩诺沙星的检测能力达20 ng·g−1,对8种氟喹诺酮类抗生素有高达42%的交叉反应率,可实现多种抗生素残留物的同时测定。为了使ELISA更加简便且节省抗原,Wei等[13]制备出6H3D5单克隆抗体,研究出了一种双斑点ELISA可同时检测卡那霉素(KANA)和链霉素,该方法的固相载体为硝酸纤维素膜(NCM),经酶促反应后从视觉上可观测到显色情况,其检测限分别为0.09 ng·mL−1和1.37 ng·mL−1。
酶联免疫的方法操作简单,普适性强,但在其方法的特异性和灵敏度上,对抗体的要求比较高,因此,采用其他标记物的新方法也被研究。
-
RIA的检测原理为被放射性同位素标记的抗原与待测抗原竞争性结合有限的特异性抗体的位点(图1B),洗涤后加入闪烁液(示踪剂),经Charm闪烁计数器分析后,闪烁计数值越高,样品中待测物质浓度越低,常用于抗生素的定性与半定量检测。
Meyer等[14]在购置的检测限为1 μg·L−1左右的Charm Ⅱ RIA的基础上加以优化,用于检测样品中残留的四环素类抗生素。结果表明,该RIA的灵敏度低于传统的LC/MS法,有待于开发灵敏度更高的RIA。为了拓宽RIA的适用范围,Yang和Carlson[15]将样品先经固相萃取(SPE)技术纯化后,再对四环素和磺酰胺进行半定量分析,该方法的检测限低至0.05 μg·L−1。Al-Mazeedi等[16]利用Charm Ⅱ RIA筛选出阳性和疑似阳性样品,而后通过LC/MS-MS进行确认,完成了1517份乳制品中四环素残留的调查。
虽然放射免疫分析法无法对待测样品进行准确定量,但它适用于大批量样品筛查,针对RIA的假阳性结果,可对样品进一步浓缩纯化来改善解决。不过由于该方法中使用到的放射性物质对人类健康和生态环境有一定的危害,现如今已多被ELISA所取代。
-
荧光免疫分析通常采用荧光物质进行标记(图1C),有荧光色素、镧系螯合物以及荧光酶等化合物。FIA具有高特异性、高灵敏度、检测速度快等特点,但由于一般的荧光标记物产生荧光测定本底值较高。为了减少误差,开发出了时间分辨荧光免疫分析法(TRFIA),利用了镧系螯合物的量子产率高、荧光寿命长等特性,通过时间分辨技术来测定荧光强度。
Le等[17-18]利用Sm3+和Eu3+标记不同的抗体,将双标记TRFIA用于检测金霉素和强力霉素、磺胺二甲嘧啶和磺胺喹喔啉,其检测限在0.02—0.04 ng·mL−1。Ashuo等[19]利用生物素和链霉亲和素的高亲和度制备出了Eu3+微球-mAb荧光探针,建立了一种基于TRFIA的侧向免疫测定法。该试纸条可通过扫描QR码获取相应信息,搭配荧光读取器,可实现10 min左右完成牛奶样品中3种抗生素的实时检测,检测限分别为0.10、0.06、0.27 ng·mL−1。一些自身具备荧光特性的纳米材料(如上转换纳米颗粒(UCNPs))以及不具荧光特性的磁性纳米粒子(MNPs)也被广泛应用于检测环境样品中的抗生素残留(表1)。
FIA是一种可靠且强大的筛选方法,其信号强度大,但量子点的最终荧光发射易受干扰,所以需对样品进行脱色,以达最佳效果。
-
CLIA是将高灵敏度的化学发光技术和高特异性的免疫反应结合起来的一种操作简便、特异性强、灵敏度高、分析速度快、线性范围宽的超微量检测技术。它通过添加氧化剂或酶底物,使体系中化学发光物质被氧化至不稳定的激发态中间体,而后回到稳定的基态并发射光子,利用发光信号测量仪进行定量。CLIA有两大类型,一种是发光物质直接对抗体进行标记(图1D),加入发光启动剂即可;另一种是采用酶(如HRP)标记抗体,然后加入相应的发光酶底物(如鲁米诺Luminol)。
Li等[20]制备出了一种能够同时识别32种磺酰胺的mAb,采用化学发光直接竞争酶免疫测定法(CL-dcELISA)对鸡肉进行检测。Yu等[21]采用CL-dcELISA,以单链可变片段(scFvs)代替抗体提高了与FQs的交叉反应性(CR),用于检测鱼虾类20种氟喹诺酮(FQs),其中诺氟沙星(NOR)的检出限为0.017 μg·kg−1,线性范围在0.04—1.08 μg·kg−1。不少学者将微流控芯片技术和纳米材料引入CLIA用于检测动物源性食品中抗生素的残留(表1),显著减少了试剂和样品用量,增强了发光系统。
限制CLIA的有化合物的溶解度以及闪光的即时测量等因素,但是该方法的灵敏度远高于ELISA、FIA等,反应时间优于TRFIA,在免疫检测市场上,有着无可替代的地位。
-
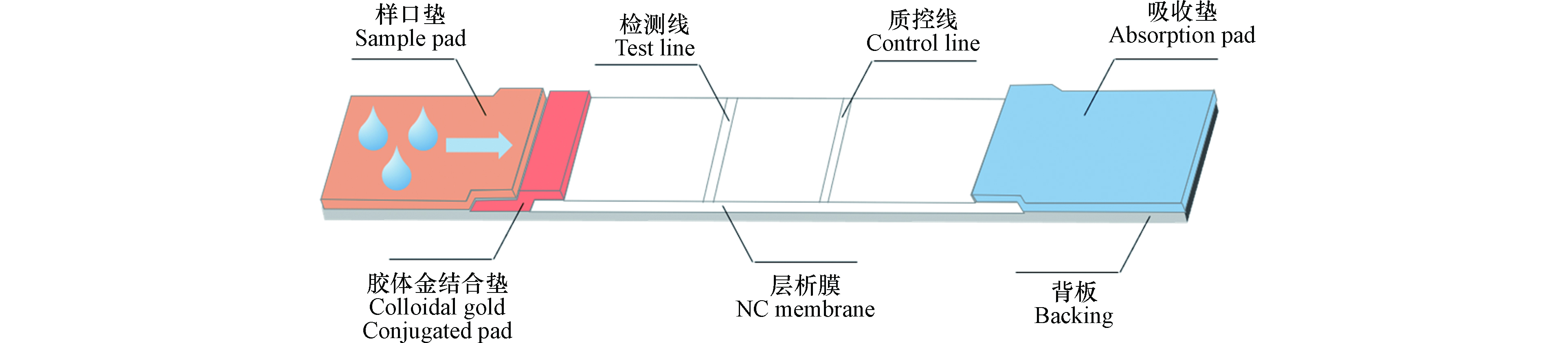
胶体金通常是氯金酸(HAuCl4)在还原剂的作用下,聚合形成粒径均匀的金粒子,粒子间的静电作用使其形成了稳定的胶体状态。由于AuNPs表面的强吸附作用,可以很好地同蛋白质结合进行标记。当大量标记物聚集时,视觉上呈红色,在不使用仪器的情况下即可通过肉眼比色来完成检测物的定性与半定量。CGIA是在硝酸纤维素膜上的检测线和质控线内固定好特异性抗原或抗体,样品加在样品垫,借助毛细作用向另一端流动(图2),到达T或C线相应的抗体或抗原被截留,实现分离,整个检测过程在10 min左右。
Luo等[22]使用对马波沙星(MBF)有高灵敏度的M4E3 mAb开发了一种基于视觉的CGIA,检测限在1 ng·mL−1,还可灵敏地检测6种氟喹诺酮类似物,交叉率超过20%。Guo等[23]开发了一种用于筛选鸡胸肉中卡巴氧(CBX)和喹赛多(CYA)残留物的CGIA。该方法以(E)-2-(hydr甲基)喹喔啉1,4-二氧化物作半抗原制备了mAb,在最佳条件下,视觉检测限达8 μg·kg−1,利用条带扫描仪定量检出限为2.92 μg·kg−1、2.68 μg·kg−1。Xu等[24]则建立了一种无需进行样品前处理的CGIA,用于检测牛奶中的氯霉素(CAP)。与SERS技术结合,可极大提高检测的灵敏度,对新霉素的检测限低至0.216 pg·mL−1,证实该方法具有较高的重现性和稳定性[25]。
CGIA无法进行多种抗生素的同时检测,但基于CGIA开发的试纸条与微流控芯片检测技术结合,经小型仪器扫描将色度值转化为电信号,在8 min内即可完成现场快速检测,具有良好的稳定性和准确性[26]。
-
电化学免疫传感器(EIS)是由生物感受器和换能器组成的一种小型分析设备。其原理为生物受体识别目标分析物会产生特定的生物信号,此信号通过换能器转换,成为可测量的电信号,分析物的浓度与电信号存在相对应的关系。利用高灵敏度、低成本的EIS可对痕量待测物完成准确定量。根据检测的信号类型不同,通常将EIS分为电流型、电容型、电位型和电导型4类。
不同的纳米材料可不同程度地提升传感器的电化学性能(表1)。纳米酶是一种具有酶活性的纳米材料,其特点是成本低,稳定性强,在EIS的信号放大方面起着极其重要的作用。Xiao等[27]将二维的Cu-TCPP(Fe)用作纳米酶修饰于电极上,基于Cu2+和聚乙烯亚胺(PEI)的强亲和力致使酶活性降低,进而引起电化学电流下降的原理,开发了一种新型电化学免疫传感器,用于测定磺酰胺,检测限达0.395 ng·mL−1。但电极传感器的制造需耗费大量时间,可以通过批量生产来用于现场检测。
抗体作为识别元件时,生物蛋白的特异性受制诸多因素制约,如pH、温度、存放时间等。而核酸适配体作为一种“化学抗体”,它具有良好的稳定性、可修饰性以及宽适用范围等特性,因而近年来被广泛应用于动物源性食品中抗生素的残留检测。
-
核酸适配体是通过SELEX,即指数富集的配体系统进化技术筛选出的单链DNA或RNA寡核苷酸序列。适配体有着复杂多样的二级结构,能够形成凸环、发夹、G-四联体等立体结构,这些结构特性使之被广泛应用于生物传感器的开发。它可通过范德华力、氢键、静电相互作用与靶分子相结合[37-38],具有高度的亲和性和特异性。相对于抗体来说,适配体通过化学合成,减少了批次间的差异,缩短了生产时间,成本相对较低;良好的热稳定性,使其更易储存;经过化学修饰和标记后仍具有良好的特异性;适用范围更广泛,从离子到细胞的任何靶点,包括非免疫或有毒的靶点[39-40] 。由于适配体的优良特性,目前在分析动物源性食品中抗生素的残留检测应用中有着良好的前景。与基于抗体的酶联免疫吸附法相比,酶联适体法具有更高的灵敏度和回收率。一些基于适配体的生物传感器,如电化学适配体传感器、光学适配体传感器等[41],不断被设计开发应用。适配体的可扩增性使得利用PCR技术实现信号放大成为可能。
-
核酸适配体生物传感器(aptamer biosensor,简称AB)是一种由生物识别元件和换能器组成的装置,其生物识别元件通常以核酸适配体为主。适配体具有易修饰性,与纳米材料结合可增强传感系统的灵敏度,提高传感器的性能。AB法通常会引入一定的物质用于放大信号系统,进一步提升传感器的灵敏度。当目标分析物接触识别元件时,若有目标物的存在,适配体则会适应型折叠形成特殊的空间结构与目标物特异性结合,换能器将结合瞬间的信号转换为物理信号,最后经电子系统放大并显示。对物理信号进行分析,就可以完成对于样品中目标物的定性与定量。
-
光学生物传感器的换能器通常利用光的分光、荧光、折射等性质来对目标物进行定量检测。
光学核酸适配体传感器中最常采用的是基于比色的检测,目前开发的比色核酸适配体传感器大都基于胶体的测定,最常用的就是AuNPs,由于AuNPs具有独特的光学性质和高消光系数,研究者们利用这些优势开发出来许多应用于抗生素检测的AB。也有一些研究者建立了一种酶联适体测定法[42-43],通过测定吸光度来完成检测抗生素,具有较宽的线性检测范围和较低的检测限。
较短的单链DNA(ssDNA)其表面密度更高,与载体有更强的亲和力、更好的动力学。Wu等[44]基于ssDNA这一特性,利用长链(CAP适配体)、短链(TET适配体)偶联于AuNPs表面造成密度差异这一原理引发的AuNPs溶液颜色变化,开发了一种无标记超灵敏比色适体传感器。Abedalwafa等[45]和 Liu等[46]都开发了一种便携式比色传感器条用于动物源性食品中KANA的检测。前者是通过谷氨酸(GA)接枝到乙酸纤维素(CA)来制备带有羧基的电纺纳米纤维膜(NFM),将KANA适体标记在NFM上,适体与cDNA@AuNPs偶联,以此获得比色生物传感器条,当KANA存在时,传感条由粉色变为白色,检测限低至2.5 nmol·L−1;后者是基于AuNPs-apt作探针和AgNPs-cDNA作为信号放大元件开发了检测KANA的试纸条传感器,通过仪器扫描可实现1—30 nmol·L−1的线性检测范围和0.0778 nmol·L−1的检测限。Emrani等[47]设计了一种荧光猝灭和比色适配体传感器,传感器基于AuNPs、双链DNA(dsDNA)和FAM标记的cDNA(互补链DNA)。当链霉素不存在时,溶液由红变蓝,有强烈的荧光;反之,溶液呈红色,荧光被AuNPs猝灭。THMS(三螺旋结构)的结构比dsDNA更加坚固,能够保护AuNPs免受盐诱导的聚集,利用这一特点,Ramezani等[48]设计了一种比色适配体传感器,对四环素的检测限为266 pmol·L−1。
另一种最常采用的检测信号就是荧光强度,荧光信号一般是由荧光染料或是具有荧光性质的纳米材料发出的,适体与靶标特异性结合后,一些物质可通过荧光能量共振转移对荧光进行猝灭或使荧光信号得以释放,辅以一定手段对荧光信号进行放大,通过测定即可完成分析物的定量。
MNPs、UCNPs[49-50]、GO[51]等纳米材料和噻唑橙[52]等荧光染料常被用于光学核酸适配体传感器,灵敏、快速的检测抗生素。 Yang等[53]使用典型的MB-SELEX技术进行磺胺二甲嘧啶(SMZ)适体的选择,并引入裸羧基MB和SMR-MB用于反选择,提高SMZ适体的特异性。筛选得到的SA07适体用于开发化学发光适配体传感器,与27种磺酰胺类抗生素几乎无交叉反应性,检测限为0.92 ng·mL−1。Liu等[54]开发了一种比例荧光传感器,其中适配体标记上荧光染料,锆卟啉MOF(PCN-222)作为荧光猝灭剂,PCN-222与apt通过π-π堆积、静电等相互作用吸附结合,由FRET发生荧光猝灭。该传感器可在26 min内完成残留于牛奶和虾中CAP的检测,检测范围0.1 pg·mL−1—10 ng·mL−1,检测限低至0.08 pg·mL−1。Rouhbakhsh等[55]使用成本低的液晶(LC)设计了一种无标记向列型LC适体传感器,其原理是利用靶标诱导适体构象转换进而改变LC的垂直取向,可通过偏振光学显微镜计算出图像的灰度强度来定量牛奶中四环素的浓度。
表面等离子共振(SPR)的量子力学现象是SPR生物传感器的基本原理,平行于入射平面的偏振光撞击导电薄膜表面,在两种介质间产生的表面等离子激元被完全反射,当适配体结合分析物时,薄膜的折射率及共振角发生改变,反射光强度随结合分析物的质量呈比例变化。SPR生物传感器对于无标记、未经预处理的浑浊的样品也可完成高灵敏、高通量的检测。Wang等[56]在金膜表面引入了DNA四面体以纳米级距离定向的方式固定四环素适配体,提升了对TC的捕获效率,从而提高了SPR适体传感器的灵敏度,在蜂蜜样品中TC的回收率可达80.2%—114.3%,检测限为0.0069 μg·kg−1,较未引入DNA四面体的适体传感器低至10倍。
表面增强拉曼散射(SERS)是一种高度灵敏的光学测量方法,它基于光在纳米级粗糙贵金属表面附近原子或分子上的增强的非弹性散射(拉曼散射)提供信号。SERS适体传感器有着极高的灵敏度,成本低、响应速度快等优势,使用各种纳米材料能够不同程度的提升SERS适体传感器的灵敏度。Nguyen等[57]开发了一种基于GO/AuNPs的SERS传感器用于检测牛奶中的KANA,该纳米复合材料有较高的热稳定性和低热辐射,降低了微流体检测系统中的闪烁效应,获得了1—100 nmol·L−1的线性范围,0.75 nmol·L−1的检测限。Jiang等[58]通过监测4-巯基苯甲酸(4-MBA)的特征峰强度变化来定量牛奶中KANA的浓度,4-MBA修饰于Au@AgNPs表面,适体加入时削弱了4-MBA的拉曼强度,当KANA存在时,恢复了对4-MBA的表面增强作用。Meng等在适配体连接的两种大小的AuNPs之间构建了拉曼热点,当鱼粉中存在OTC时,系统拉曼强度增加,其检测限低至4.35
× 10−3 fg·mL−1,回收率在91.29%—110.98%之间。 -
固定于电极上的适配体与目标分析物特异性结合,这一反应会被电极表面的电活性物质所识别,从而引起可测量的电性能,如电流、电势、电容,发生变化,由此可完成目标分析物灵敏的定量检测。为了增强电化学性能,经常采用导电纳米材料或化学物质(如β-CD(环糊精)[59])来修饰电极,以放大信号,提高传感器的灵敏度。
Chen等[60]基于场效应晶体管(FET)开发了一种可集成的小型电子传感器,用于检测妥布霉素。还原氧化石墨烯(rGO)纳米片用作通道材料,适体RNA作为探针偶联于rGO,并采用了MCH和PBA对未结合适体的位点进行封闭,降低特异性结合引起的误差,传感器可在5 s内做出反应,0.3 nmol·L−1的检测限。有人在笔状石墨电极(PGE)上依次固定了rGO和AuNPs,巯基化适配体偶联于AuNPs表面,组建了电化学适体传感器,成功应用于肉类及牛奶样品中磺胺二甲氧嘧啶[61]和四环素[62]的检测,在最佳条件下,检测范围宽至1×10−16—1×10−6 mol·L−1,检测限低至3×10−17 mol·L−1。石墨相氮化碳(g-C3N4)是一种2D半导体纳米材料,具有独特的光学、电子、机械和化学特性,广泛应用于生物传感器。Li等[63]将g-C3N4修饰于钒酸铋(BiVO4)电极表面,g-C3N4通过π-π共轭吸附固定DNA适配体,开发出了一种通用的光电化学(PEC)传感器,改变DNA适体即可实现对于抗生素的灵敏性检测。有人将适体探针和微芯片电泳(MCE)平台相结合,根据apt-分析物和dsDNA产生的峰位置及强度不同,对牛奶及鱼肉中的抗生素进行定量检测[64]。Huang等[65]将四环素的短适体(Apt40)和长适体(Apt76)混合物固定在金丝网印刷电极表面上,使二者有良好的空间序列可与含TC的蜂蜜样品有较大的接触面积,识别能力远高于使用单个适体,检测限为0.0073 ng·mL−1,96.45%—114.6%良好的回收率。
基于核酸适配体的传感器有着高灵敏度、强选择性、响应速度快、成本低等特点,微量样品即可完成检测,被广泛应用于动物源性食品中抗生素的残留检测。而核酸适配体本身作为一段核苷酸序列是可以在体外利用聚合酶链式反应(PCR)技术进行扩增的,利用这一特性,不少学者开发出了基于适配体的新型抗生素生物检测方法。
-
PCR技术能够实现在体外快速扩增目的DNA序列,早期应用于转基因和克隆技术,现被成功引入到环境监测领域。核酸适配体或互补寡核苷酸链通过标记生物素和链霉亲和素来与纳米磁珠载体进行结合,在外磁场作用下,可高效分离目标扩增DNA,减少后续PCR中的非特异性扩增,提高方法的特异性。
Tao等[66]将抗体和DNA结合设计了一种用于检测牛奶中CAP的方法,把报告基因DNA连接在CAP抗体上,而后通过EDC/NHS交联法将其固定于磁珠表面,以此获得免疫磁珠。牛奶样品中的CAP结合到一部分免疫磁珠后,剩余的免疫磁珠被固定于表面有CAP的PCR管中,经洗涤后对报告基因进行实时荧光定量PCR(qPCR),样品中CAP的浓度与阈值循环(Ct)值呈正比,有0.0008 μg·L−1的检测限,回收率在75%—155%之间。与免疫磁分离(IMS)相比,适体磁捕获(AMC)的捕获效率随着靶标数量减少而提高[67],与PCR信号放大技术的成功联用,实现了微量样品的高灵敏检测。Duan等[68]将互补链固定于磁珠上,适体与互补链杂交,当氯霉素(CAP)加入后,适体从磁珠上解离,收集含有适体的上清液进行qPCR,在最佳实验条件下,基于AMC的qPCR检测限为0.1 ng·mL−1,有0.1—20 ng·mL−1的线性检测范围。在AMC和PCR的基础上,Zhou等[69]结合微芯片电泳(MCE)阵列开发了一种可同时检测牛奶和鱼肉中KANA和CAP的传感器。适体固定于磁性金粒子上,与其互补链杂交,当待测物存在时,互补链被解离释放至上清液,将互补链与内标链共同扩增20个循环,产物通过MCE确定不同位置的电流强度,检测限分别达0.0025 nmol·L−1和0.006 nmol·L−1。除了单独利用PCR增强检测信号外,Ma等[70]设计了一种基于PCR和链霉亲和素(SA)双重放大的荧光偏振(FP)适体传感器,最佳条件下,对CAP的线性检测范围宽至0.001—200 nmol·L−1。适体和CAP孵育一段时间后,利用氧化石墨烯(GO)除去未结合适体,适体-CAP复合物作为模板,加入FAM标记的正向引物和生物素标记的反向引物后进行PCR,在产物中加入SA以增大分子量,该方法中FP与CAP的浓度呈正比。
qPCR是用于基因序列快速测定、精准定量的重要技术。通过加入各种类型的荧光标记物,在扩增过程中,荧光信号随着PCR产物的增加而增强,从而完成分析物的高灵敏度测定。
-
综上所述,在对低浓度抗生素检测方法的研究中,基于抗体的免疫检测技术是高通量、高灵敏度、强特异性分析方法中不可或缺的一项,其应用领域及技术已经相当成熟。但RIA由于放射性元素危害人体健康,限制了其发展,逐渐被其他免疫技术所取代。基于CGIA的免疫试纸条技术可在十几分钟内完成样品的半定量测定,非常适宜于对抗生素的快速筛查,而荧光、化学发光和电化学免疫检测技术是提高检测技术灵敏度的良好选择,其最低检测限可达pg·mL−1,线性范围跨越2个数量级。与抗体识别元件相比更为稳定的适配体具有特异性强、热稳定性、成本低、易于修饰等特点,以其为识别元件的生物传感器检测方法研究发展迅速,广泛应用于动物源性食品中抗生素的检测。随着适配体筛选技术不断改进,越来越多的高特异性适配体被选出,进一步扩大了适配体识别元件的应用范围。PCR技术能够指数级地扩增模板信号,加上后续对产物进行凝胶电泳来定量或是加入荧光基团或染料可以进行全过程荧光定量,可实现对动物源性食品中痕量抗生素的检测。
未来动物源性食品中抗生素的检测技术发展趋向于简便、高灵敏、高通量的快速原位检测。对于新的生物检测技术的开发,可从以下几个方面着手:
(1)提高技术的选择性能:传统的生物抗体识别可被性能更加优越的核酸适配体所替代,因此,筛选出高特异性适配体以及提升适配体与靶物质的亲和度,提升技术抗扰能力是未来建立单一靶物质测定的研究方向之一。
(2)降低技术的操作成本:目前食源性食品中抗生素的残留趋向于一大类抗生素乃至多种类抗生素残留,因此建立能够同时对多种抗生素产生反应的检测方法,可以提高其在实际样品中的实用性。例如可将不同抗生素的核酸适配体同时固定于丝网印刷电极或磁性粒子上。
(3)研发微型智能化技术:计算机技术和微电子技术发展迅猛,设计开发适合原位快速检测的便携技术,借助计算机网络实现数据的实时在线监测分析,改善在市场应用的可行性。
动物源性食品中抗生素类污染物生物检测技术研究进展
Research progress of bioassay technology for antibiotic pollutants in animal-derived foods
-
摘要: 抗生素类污染物在动物源性食品中的残留,因其对人类健康有着潜在危害而受到了广泛的关注,因此迫切需要建立快速、灵敏、高效、便捷的检测方法以便对其及时管理和防治。生物检测技术在实现对抗生素高灵敏度、高特异性、低成本、快速的检测方面具有很强的优势,主要包括微生物检测法、免疫分析法和生物传感器法等。本文基于免疫抗体和核酸适配体两种生物识别元件的检测原理和特点,对近年来采用这两种识别方式建立起的生物检测方法在动物源性食品中抗生素残留检测中的应用进行了综述。同时,对目前检测方法研究中存在的问题进行了综合评价,并对抗生素生物检测技术的未来发展做出展望。Abstract: The residues of antibiotic contaminants in animal-derived foods have received widespread attention due to their potential harm to human health. Thus, it is urgent to establish fast, sensitive, efficient and convenient detection method to manage and prevent antibiotic residues in time. Bioassay technology which mainly include microbiological assay, immunoassay and biosensor can achieve high sensitivity, high specificity, low cost and rapid detection of antibiotics. Based on detection principles and characteristics of antibodies and aptamers, this paper reviews the application of the bioassay technology established in recent years using these two biorecognition elements in the detection of antibiotic residues in animal-derived foods. In addition, this paper comprehensively evaluates the shortcomings in the current research and prospects the future development of antibiotic bioassay technology.
-
Key words:
- antibiotic /
- antibody /
- nucleic acid aptamer /
- immunoassay /
- biosensor /
- PCR
-
抗生素在医疗、畜牧、养殖等行业广泛应用。由于不正当地使用和无组织排放,致使其大量流入环境介质中,近年来,国内外在水环境[1]、土壤[2]、动植物组织[3]等环境介质中均有抗生素的检出报道[4-5]。畜禽养殖业中抗生素的不合理使用引发了一系列潜在危害,主要体现在产生抗药基因、破坏生物体内的微环境、人畜共患病增多等方面。动物源性食品安全成为了重大问题,为了保证人类健康,世界卫生组织(WHO)和联合国粮食及农业组织(FAO)曾共同建立了农药和兽药的最大残留限量(MRL)标准来对其进行控制。
检测食品中微量或痕量级别抗生素最常采用仪器法,有高效液相色谱法(HPLC)[6]、高效液相色谱-串联质谱法(HPLC-MS/MS)[7-9]、液相色谱-紫外-质谱法(LC-UV-MS)[10]、液相色谱-荧光检测方法[11]等。仪器检测法的灵敏度高,检测数据准确,但由于其前处理复杂、耗时长等劣势,限制了其在原位快速检测中的应用。生物检测技术因其高灵敏度、高选择性被广泛用于动物源性食品中的抗生素残留量检测研究。基于抗体的生物免疫检测技术最为成熟,研究应用也比较广泛。而稳定性好、适用范围广的核酸适配体与响应快、操作简便的传感器相结合成为了生物检测研究的热门发展方向。基于此,本文综述了以抗体和核酸适配体为生物识别元件的生物检测技术特点及其在动物源性食品中抗生素残留量检测的研究进展,并在归纳总结生物检测技术检测抗生素的研究基础上,预测抗生素检测技术未来的发展趋势。
1. 基于抗体的免疫检测技术(Antibody-based immunoassay technology)
免疫分析技术是基于抗体、抗原之间特异性结合的一种生物化学技术,被广泛应用于环境和食品行业中药物残留等有害物质的定性和定量。
1.1 酶联免疫吸附分析法(ELISA)
ELISA是通过被酶标记的抗体对抗生素的竞争,结合到固相包被抗原上,洗涤后加入酶底物,固定在载体(酶标板)上的酶催化底物出现显色反应,最后通过分光光度法(酶标仪)对待测物质进行定量分析。近年来,双斑点法、直接(图1A)和间接性竞争ELISA在检测抗生素残留研究方面皆有开发。
直接性ELISA和间接性ELISA有着不同的优势,Ashu等[12]比较了两种ELISA测定抗生素的灵敏性,找到了一种特异性强、灵敏度高的多克隆抗体As172,采用直接竞争ELISA法建立了用于饲料中氟喹诺酮类抗生素的检测方法,其中该抗体对恩诺沙星的检测能力达20 ng·g−1,对8种氟喹诺酮类抗生素有高达42%的交叉反应率,可实现多种抗生素残留物的同时测定。为了使ELISA更加简便且节省抗原,Wei等[13]制备出6H3D5单克隆抗体,研究出了一种双斑点ELISA可同时检测卡那霉素(KANA)和链霉素,该方法的固相载体为硝酸纤维素膜(NCM),经酶促反应后从视觉上可观测到显色情况,其检测限分别为0.09 ng·mL−1和1.37 ng·mL−1。
酶联免疫的方法操作简单,普适性强,但在其方法的特异性和灵敏度上,对抗体的要求比较高,因此,采用其他标记物的新方法也被研究。
1.2 放射免疫分析法(RIA)
RIA的检测原理为被放射性同位素标记的抗原与待测抗原竞争性结合有限的特异性抗体的位点(图1B),洗涤后加入闪烁液(示踪剂),经Charm闪烁计数器分析后,闪烁计数值越高,样品中待测物质浓度越低,常用于抗生素的定性与半定量检测。
Meyer等[14]在购置的检测限为1 μg·L−1左右的Charm Ⅱ RIA的基础上加以优化,用于检测样品中残留的四环素类抗生素。结果表明,该RIA的灵敏度低于传统的LC/MS法,有待于开发灵敏度更高的RIA。为了拓宽RIA的适用范围,Yang和Carlson[15]将样品先经固相萃取(SPE)技术纯化后,再对四环素和磺酰胺进行半定量分析,该方法的检测限低至0.05 μg·L−1。Al-Mazeedi等[16]利用Charm Ⅱ RIA筛选出阳性和疑似阳性样品,而后通过LC/MS-MS进行确认,完成了1517份乳制品中四环素残留的调查。
虽然放射免疫分析法无法对待测样品进行准确定量,但它适用于大批量样品筛查,针对RIA的假阳性结果,可对样品进一步浓缩纯化来改善解决。不过由于该方法中使用到的放射性物质对人类健康和生态环境有一定的危害,现如今已多被ELISA所取代。
1.3 荧光免疫分析法(FIA)
荧光免疫分析通常采用荧光物质进行标记(图1C),有荧光色素、镧系螯合物以及荧光酶等化合物。FIA具有高特异性、高灵敏度、检测速度快等特点,但由于一般的荧光标记物产生荧光测定本底值较高。为了减少误差,开发出了时间分辨荧光免疫分析法(TRFIA),利用了镧系螯合物的量子产率高、荧光寿命长等特性,通过时间分辨技术来测定荧光强度。
Le等[17-18]利用Sm3+和Eu3+标记不同的抗体,将双标记TRFIA用于检测金霉素和强力霉素、磺胺二甲嘧啶和磺胺喹喔啉,其检测限在0.02—0.04 ng·mL−1。Ashuo等[19]利用生物素和链霉亲和素的高亲和度制备出了Eu3+微球-mAb荧光探针,建立了一种基于TRFIA的侧向免疫测定法。该试纸条可通过扫描QR码获取相应信息,搭配荧光读取器,可实现10 min左右完成牛奶样品中3种抗生素的实时检测,检测限分别为0.10、0.06、0.27 ng·mL−1。一些自身具备荧光特性的纳米材料(如上转换纳米颗粒(UCNPs))以及不具荧光特性的磁性纳米粒子(MNPs)也被广泛应用于检测环境样品中的抗生素残留(表1)。
FIA是一种可靠且强大的筛选方法,其信号强度大,但量子点的最终荧光发射易受干扰,所以需对样品进行脱色,以达最佳效果。
1.4 化学发光免疫分析法(CLIA)
CLIA是将高灵敏度的化学发光技术和高特异性的免疫反应结合起来的一种操作简便、特异性强、灵敏度高、分析速度快、线性范围宽的超微量检测技术。它通过添加氧化剂或酶底物,使体系中化学发光物质被氧化至不稳定的激发态中间体,而后回到稳定的基态并发射光子,利用发光信号测量仪进行定量。CLIA有两大类型,一种是发光物质直接对抗体进行标记(图1D),加入发光启动剂即可;另一种是采用酶(如HRP)标记抗体,然后加入相应的发光酶底物(如鲁米诺Luminol)。
Li等[20]制备出了一种能够同时识别32种磺酰胺的mAb,采用化学发光直接竞争酶免疫测定法(CL-dcELISA)对鸡肉进行检测。Yu等[21]采用CL-dcELISA,以单链可变片段(scFvs)代替抗体提高了与FQs的交叉反应性(CR),用于检测鱼虾类20种氟喹诺酮(FQs),其中诺氟沙星(NOR)的检出限为0.017 μg·kg−1,线性范围在0.04—1.08 μg·kg−1。不少学者将微流控芯片技术和纳米材料引入CLIA用于检测动物源性食品中抗生素的残留(表1),显著减少了试剂和样品用量,增强了发光系统。
限制CLIA的有化合物的溶解度以及闪光的即时测量等因素,但是该方法的灵敏度远高于ELISA、FIA等,反应时间优于TRFIA,在免疫检测市场上,有着无可替代的地位。
表 1 基于不同纳米材料用于检测动物源性食品抗生素残留的免疫技术Table 1. Immunoassay based on different nanomaterials for the screening of antibiotics in animal-derived foods检测类型 Assay mode 纳米材料Nanomaterials 抗生素 Antibiotics 样品 Sample 检测限/ (ng·mL−1)LOD 回收率/% Recovery 参考文献 Reference FIA MNPs 环丙沙星 牛奶 8 90—100 [28] QDs 链霉素 牛奶 0.005 80.21—108.3 [29] 四环素 80.5—109.2 [29] 青霉素 82.4—101.4 [29] NaYF4∶Yb, Er 诺氟沙星 牛奶 0.01 82.37—132.22 [30] CLIA MNPs 氯霉素 食品 2 × [31] AuNPs 虾、蜂蜜 3.3 × [32] SiO2 虾、蜂蜜 3.3 × 83.7—115.1 [33] EIA MOFs/AuPt 马杜霉素 鸡蛋 0.045 96.4—106 [34] PAMAM-Au 诺氟沙星 牛奶、鸡蛋、猪肉 0.3837 91.6—106.1 [35] PEDOT/MWCNT 瘦肉精 牛肉 4.66 85—111 [36] 1.5 胶体金免疫层析法(CGIA)
胶体金通常是氯金酸(HAuCl4)在还原剂的作用下,聚合形成粒径均匀的金粒子,粒子间的静电作用使其形成了稳定的胶体状态。由于AuNPs表面的强吸附作用,可以很好地同蛋白质结合进行标记。当大量标记物聚集时,视觉上呈红色,在不使用仪器的情况下即可通过肉眼比色来完成检测物的定性与半定量。CGIA是在硝酸纤维素膜上的检测线和质控线内固定好特异性抗原或抗体,样品加在样品垫,借助毛细作用向另一端流动(图2),到达T或C线相应的抗体或抗原被截留,实现分离,整个检测过程在10 min左右。
Luo等[22]使用对马波沙星(MBF)有高灵敏度的M4E3 mAb开发了一种基于视觉的CGIA,检测限在1 ng·mL−1,还可灵敏地检测6种氟喹诺酮类似物,交叉率超过20%。Guo等[23]开发了一种用于筛选鸡胸肉中卡巴氧(CBX)和喹赛多(CYA)残留物的CGIA。该方法以(E)-2-(hydr甲基)喹喔啉1,4-二氧化物作半抗原制备了mAb,在最佳条件下,视觉检测限达8 μg·kg−1,利用条带扫描仪定量检出限为2.92 μg·kg−1、2.68 μg·kg−1。Xu等[24]则建立了一种无需进行样品前处理的CGIA,用于检测牛奶中的氯霉素(CAP)。与SERS技术结合,可极大提高检测的灵敏度,对新霉素的检测限低至0.216 pg·mL−1,证实该方法具有较高的重现性和稳定性[25]。
CGIA无法进行多种抗生素的同时检测,但基于CGIA开发的试纸条与微流控芯片检测技术结合,经小型仪器扫描将色度值转化为电信号,在8 min内即可完成现场快速检测,具有良好的稳定性和准确性[26]。
1.6 电化学免疫分析法(EIA)
电化学免疫传感器(EIS)是由生物感受器和换能器组成的一种小型分析设备。其原理为生物受体识别目标分析物会产生特定的生物信号,此信号通过换能器转换,成为可测量的电信号,分析物的浓度与电信号存在相对应的关系。利用高灵敏度、低成本的EIS可对痕量待测物完成准确定量。根据检测的信号类型不同,通常将EIS分为电流型、电容型、电位型和电导型4类。
不同的纳米材料可不同程度地提升传感器的电化学性能(表1)。纳米酶是一种具有酶活性的纳米材料,其特点是成本低,稳定性强,在EIS的信号放大方面起着极其重要的作用。Xiao等[27]将二维的Cu-TCPP(Fe)用作纳米酶修饰于电极上,基于Cu2+和聚乙烯亚胺(PEI)的强亲和力致使酶活性降低,进而引起电化学电流下降的原理,开发了一种新型电化学免疫传感器,用于测定磺酰胺,检测限达0.395 ng·mL−1。但电极传感器的制造需耗费大量时间,可以通过批量生产来用于现场检测。
抗体作为识别元件时,生物蛋白的特异性受制诸多因素制约,如pH、温度、存放时间等。而核酸适配体作为一种“化学抗体”,它具有良好的稳定性、可修饰性以及宽适用范围等特性,因而近年来被广泛应用于动物源性食品中抗生素的残留检测。
2. 基于核酸适配体的生物检测技术(Aptamer-based bioassay technology)
核酸适配体是通过SELEX,即指数富集的配体系统进化技术筛选出的单链DNA或RNA寡核苷酸序列。适配体有着复杂多样的二级结构,能够形成凸环、发夹、G-四联体等立体结构,这些结构特性使之被广泛应用于生物传感器的开发。它可通过范德华力、氢键、静电相互作用与靶分子相结合[37-38],具有高度的亲和性和特异性。相对于抗体来说,适配体通过化学合成,减少了批次间的差异,缩短了生产时间,成本相对较低;良好的热稳定性,使其更易储存;经过化学修饰和标记后仍具有良好的特异性;适用范围更广泛,从离子到细胞的任何靶点,包括非免疫或有毒的靶点[39-40] 。由于适配体的优良特性,目前在分析动物源性食品中抗生素的残留检测应用中有着良好的前景。与基于抗体的酶联免疫吸附法相比,酶联适体法具有更高的灵敏度和回收率。一些基于适配体的生物传感器,如电化学适配体传感器、光学适配体传感器等[41],不断被设计开发应用。适配体的可扩增性使得利用PCR技术实现信号放大成为可能。
2.1 基于核酸适配体的生物传感器抗生素检测技术
核酸适配体生物传感器(aptamer biosensor,简称AB)是一种由生物识别元件和换能器组成的装置,其生物识别元件通常以核酸适配体为主。适配体具有易修饰性,与纳米材料结合可增强传感系统的灵敏度,提高传感器的性能。AB法通常会引入一定的物质用于放大信号系统,进一步提升传感器的灵敏度。当目标分析物接触识别元件时,若有目标物的存在,适配体则会适应型折叠形成特殊的空间结构与目标物特异性结合,换能器将结合瞬间的信号转换为物理信号,最后经电子系统放大并显示。对物理信号进行分析,就可以完成对于样品中目标物的定性与定量。
2.1.1 光学核酸适配体传感器
光学生物传感器的换能器通常利用光的分光、荧光、折射等性质来对目标物进行定量检测。
光学核酸适配体传感器中最常采用的是基于比色的检测,目前开发的比色核酸适配体传感器大都基于胶体的测定,最常用的就是AuNPs,由于AuNPs具有独特的光学性质和高消光系数,研究者们利用这些优势开发出来许多应用于抗生素检测的AB。也有一些研究者建立了一种酶联适体测定法[42-43],通过测定吸光度来完成检测抗生素,具有较宽的线性检测范围和较低的检测限。
较短的单链DNA(ssDNA)其表面密度更高,与载体有更强的亲和力、更好的动力学。Wu等[44]基于ssDNA这一特性,利用长链(CAP适配体)、短链(TET适配体)偶联于AuNPs表面造成密度差异这一原理引发的AuNPs溶液颜色变化,开发了一种无标记超灵敏比色适体传感器。Abedalwafa等[45]和 Liu等[46]都开发了一种便携式比色传感器条用于动物源性食品中KANA的检测。前者是通过谷氨酸(GA)接枝到乙酸纤维素(CA)来制备带有羧基的电纺纳米纤维膜(NFM),将KANA适体标记在NFM上,适体与cDNA@AuNPs偶联,以此获得比色生物传感器条,当KANA存在时,传感条由粉色变为白色,检测限低至2.5 nmol·L−1;后者是基于AuNPs-apt作探针和AgNPs-cDNA作为信号放大元件开发了检测KANA的试纸条传感器,通过仪器扫描可实现1—30 nmol·L−1的线性检测范围和0.0778 nmol·L−1的检测限。Emrani等[47]设计了一种荧光猝灭和比色适配体传感器,传感器基于AuNPs、双链DNA(dsDNA)和FAM标记的cDNA(互补链DNA)。当链霉素不存在时,溶液由红变蓝,有强烈的荧光;反之,溶液呈红色,荧光被AuNPs猝灭。THMS(三螺旋结构)的结构比dsDNA更加坚固,能够保护AuNPs免受盐诱导的聚集,利用这一特点,Ramezani等[48]设计了一种比色适配体传感器,对四环素的检测限为266 pmol·L−1。
另一种最常采用的检测信号就是荧光强度,荧光信号一般是由荧光染料或是具有荧光性质的纳米材料发出的,适体与靶标特异性结合后,一些物质可通过荧光能量共振转移对荧光进行猝灭或使荧光信号得以释放,辅以一定手段对荧光信号进行放大,通过测定即可完成分析物的定量。
MNPs、UCNPs[49-50]、GO[51]等纳米材料和噻唑橙[52]等荧光染料常被用于光学核酸适配体传感器,灵敏、快速的检测抗生素。 Yang等[53]使用典型的MB-SELEX技术进行磺胺二甲嘧啶(SMZ)适体的选择,并引入裸羧基MB和SMR-MB用于反选择,提高SMZ适体的特异性。筛选得到的SA07适体用于开发化学发光适配体传感器,与27种磺酰胺类抗生素几乎无交叉反应性,检测限为0.92 ng·mL−1。Liu等[54]开发了一种比例荧光传感器,其中适配体标记上荧光染料,锆卟啉MOF(PCN-222)作为荧光猝灭剂,PCN-222与apt通过π-π堆积、静电等相互作用吸附结合,由FRET发生荧光猝灭。该传感器可在26 min内完成残留于牛奶和虾中CAP的检测,检测范围0.1 pg·mL−1—10 ng·mL−1,检测限低至0.08 pg·mL−1。Rouhbakhsh等[55]使用成本低的液晶(LC)设计了一种无标记向列型LC适体传感器,其原理是利用靶标诱导适体构象转换进而改变LC的垂直取向,可通过偏振光学显微镜计算出图像的灰度强度来定量牛奶中四环素的浓度。
表面等离子共振(SPR)的量子力学现象是SPR生物传感器的基本原理,平行于入射平面的偏振光撞击导电薄膜表面,在两种介质间产生的表面等离子激元被完全反射,当适配体结合分析物时,薄膜的折射率及共振角发生改变,反射光强度随结合分析物的质量呈比例变化。SPR生物传感器对于无标记、未经预处理的浑浊的样品也可完成高灵敏、高通量的检测。Wang等[56]在金膜表面引入了DNA四面体以纳米级距离定向的方式固定四环素适配体,提升了对TC的捕获效率,从而提高了SPR适体传感器的灵敏度,在蜂蜜样品中TC的回收率可达80.2%—114.3%,检测限为0.0069 μg·kg−1,较未引入DNA四面体的适体传感器低至10倍。
表面增强拉曼散射(SERS)是一种高度灵敏的光学测量方法,它基于光在纳米级粗糙贵金属表面附近原子或分子上的增强的非弹性散射(拉曼散射)提供信号。SERS适体传感器有着极高的灵敏度,成本低、响应速度快等优势,使用各种纳米材料能够不同程度的提升SERS适体传感器的灵敏度。Nguyen等[57]开发了一种基于GO/AuNPs的SERS传感器用于检测牛奶中的KANA,该纳米复合材料有较高的热稳定性和低热辐射,降低了微流体检测系统中的闪烁效应,获得了1—100 nmol·L−1的线性范围,0.75 nmol·L−1的检测限。Jiang等[58]通过监测4-巯基苯甲酸(4-MBA)的特征峰强度变化来定量牛奶中KANA的浓度,4-MBA修饰于Au@AgNPs表面,适体加入时削弱了4-MBA的拉曼强度,当KANA存在时,恢复了对4-MBA的表面增强作用。Meng等在适配体连接的两种大小的AuNPs之间构建了拉曼热点,当鱼粉中存在OTC时,系统拉曼强度增加,其检测限低至4.35
× 2.1.2 电化学核酸适配体传感器
固定于电极上的适配体与目标分析物特异性结合,这一反应会被电极表面的电活性物质所识别,从而引起可测量的电性能,如电流、电势、电容,发生变化,由此可完成目标分析物灵敏的定量检测。为了增强电化学性能,经常采用导电纳米材料或化学物质(如β-CD(环糊精)[59])来修饰电极,以放大信号,提高传感器的灵敏度。
Chen等[60]基于场效应晶体管(FET)开发了一种可集成的小型电子传感器,用于检测妥布霉素。还原氧化石墨烯(rGO)纳米片用作通道材料,适体RNA作为探针偶联于rGO,并采用了MCH和PBA对未结合适体的位点进行封闭,降低特异性结合引起的误差,传感器可在5 s内做出反应,0.3 nmol·L−1的检测限。有人在笔状石墨电极(PGE)上依次固定了rGO和AuNPs,巯基化适配体偶联于AuNPs表面,组建了电化学适体传感器,成功应用于肉类及牛奶样品中磺胺二甲氧嘧啶[61]和四环素[62]的检测,在最佳条件下,检测范围宽至1×10−16—1×10−6 mol·L−1,检测限低至3×10−17 mol·L−1。石墨相氮化碳(g-C3N4)是一种2D半导体纳米材料,具有独特的光学、电子、机械和化学特性,广泛应用于生物传感器。Li等[63]将g-C3N4修饰于钒酸铋(BiVO4)电极表面,g-C3N4通过π-π共轭吸附固定DNA适配体,开发出了一种通用的光电化学(PEC)传感器,改变DNA适体即可实现对于抗生素的灵敏性检测。有人将适体探针和微芯片电泳(MCE)平台相结合,根据apt-分析物和dsDNA产生的峰位置及强度不同,对牛奶及鱼肉中的抗生素进行定量检测[64]。Huang等[65]将四环素的短适体(Apt40)和长适体(Apt76)混合物固定在金丝网印刷电极表面上,使二者有良好的空间序列可与含TC的蜂蜜样品有较大的接触面积,识别能力远高于使用单个适体,检测限为0.0073 ng·mL−1,96.45%—114.6%良好的回收率。
基于核酸适配体的传感器有着高灵敏度、强选择性、响应速度快、成本低等特点,微量样品即可完成检测,被广泛应用于动物源性食品中抗生素的残留检测。而核酸适配体本身作为一段核苷酸序列是可以在体外利用聚合酶链式反应(PCR)技术进行扩增的,利用这一特性,不少学者开发出了基于适配体的新型抗生素生物检测方法。
2.2 基于聚合酶链式反应(PCR)扩增信号的抗生素测定技术
PCR技术能够实现在体外快速扩增目的DNA序列,早期应用于转基因和克隆技术,现被成功引入到环境监测领域。核酸适配体或互补寡核苷酸链通过标记生物素和链霉亲和素来与纳米磁珠载体进行结合,在外磁场作用下,可高效分离目标扩增DNA,减少后续PCR中的非特异性扩增,提高方法的特异性。
Tao等[66]将抗体和DNA结合设计了一种用于检测牛奶中CAP的方法,把报告基因DNA连接在CAP抗体上,而后通过EDC/NHS交联法将其固定于磁珠表面,以此获得免疫磁珠。牛奶样品中的CAP结合到一部分免疫磁珠后,剩余的免疫磁珠被固定于表面有CAP的PCR管中,经洗涤后对报告基因进行实时荧光定量PCR(qPCR),样品中CAP的浓度与阈值循环(Ct)值呈正比,有0.0008 μg·L−1的检测限,回收率在75%—155%之间。与免疫磁分离(IMS)相比,适体磁捕获(AMC)的捕获效率随着靶标数量减少而提高[67],与PCR信号放大技术的成功联用,实现了微量样品的高灵敏检测。Duan等[68]将互补链固定于磁珠上,适体与互补链杂交,当氯霉素(CAP)加入后,适体从磁珠上解离,收集含有适体的上清液进行qPCR,在最佳实验条件下,基于AMC的qPCR检测限为0.1 ng·mL−1,有0.1—20 ng·mL−1的线性检测范围。在AMC和PCR的基础上,Zhou等[69]结合微芯片电泳(MCE)阵列开发了一种可同时检测牛奶和鱼肉中KANA和CAP的传感器。适体固定于磁性金粒子上,与其互补链杂交,当待测物存在时,互补链被解离释放至上清液,将互补链与内标链共同扩增20个循环,产物通过MCE确定不同位置的电流强度,检测限分别达0.0025 nmol·L−1和0.006 nmol·L−1。除了单独利用PCR增强检测信号外,Ma等[70]设计了一种基于PCR和链霉亲和素(SA)双重放大的荧光偏振(FP)适体传感器,最佳条件下,对CAP的线性检测范围宽至0.001—200 nmol·L−1。适体和CAP孵育一段时间后,利用氧化石墨烯(GO)除去未结合适体,适体-CAP复合物作为模板,加入FAM标记的正向引物和生物素标记的反向引物后进行PCR,在产物中加入SA以增大分子量,该方法中FP与CAP的浓度呈正比。
qPCR是用于基因序列快速测定、精准定量的重要技术。通过加入各种类型的荧光标记物,在扩增过程中,荧光信号随着PCR产物的增加而增强,从而完成分析物的高灵敏度测定。
3. 结论与展望(Conclusion and perspective)
综上所述,在对低浓度抗生素检测方法的研究中,基于抗体的免疫检测技术是高通量、高灵敏度、强特异性分析方法中不可或缺的一项,其应用领域及技术已经相当成熟。但RIA由于放射性元素危害人体健康,限制了其发展,逐渐被其他免疫技术所取代。基于CGIA的免疫试纸条技术可在十几分钟内完成样品的半定量测定,非常适宜于对抗生素的快速筛查,而荧光、化学发光和电化学免疫检测技术是提高检测技术灵敏度的良好选择,其最低检测限可达pg·mL−1,线性范围跨越2个数量级。与抗体识别元件相比更为稳定的适配体具有特异性强、热稳定性、成本低、易于修饰等特点,以其为识别元件的生物传感器检测方法研究发展迅速,广泛应用于动物源性食品中抗生素的检测。随着适配体筛选技术不断改进,越来越多的高特异性适配体被选出,进一步扩大了适配体识别元件的应用范围。PCR技术能够指数级地扩增模板信号,加上后续对产物进行凝胶电泳来定量或是加入荧光基团或染料可以进行全过程荧光定量,可实现对动物源性食品中痕量抗生素的检测。
未来动物源性食品中抗生素的检测技术发展趋向于简便、高灵敏、高通量的快速原位检测。对于新的生物检测技术的开发,可从以下几个方面着手:
(1)提高技术的选择性能:传统的生物抗体识别可被性能更加优越的核酸适配体所替代,因此,筛选出高特异性适配体以及提升适配体与靶物质的亲和度,提升技术抗扰能力是未来建立单一靶物质测定的研究方向之一。
(2)降低技术的操作成本:目前食源性食品中抗生素的残留趋向于一大类抗生素乃至多种类抗生素残留,因此建立能够同时对多种抗生素产生反应的检测方法,可以提高其在实际样品中的实用性。例如可将不同抗生素的核酸适配体同时固定于丝网印刷电极或磁性粒子上。
(3)研发微型智能化技术:计算机技术和微电子技术发展迅猛,设计开发适合原位快速检测的便携技术,借助计算机网络实现数据的实时在线监测分析,改善在市场应用的可行性。
-
表 1 基于不同纳米材料用于检测动物源性食品抗生素残留的免疫技术
Table 1. Immunoassay based on different nanomaterials for the screening of antibiotics in animal-derived foods
检测类型 Assay mode 纳米材料Nanomaterials 抗生素 Antibiotics 样品 Sample 检测限/ (ng·mL−1)LOD 回收率/% Recovery 参考文献 Reference FIA MNPs 环丙沙星 牛奶 8 90—100 [28] QDs 链霉素 牛奶 0.005 80.21—108.3 [29] 四环素 80.5—109.2 [29] 青霉素 82.4—101.4 [29] NaYF4∶Yb, Er 诺氟沙星 牛奶 0.01 82.37—132.22 [30] CLIA MNPs 氯霉素 食品 2 × [31] AuNPs 虾、蜂蜜 3.3 × [32] SiO2 虾、蜂蜜 3.3 × 83.7—115.1 [33] EIA MOFs/AuPt 马杜霉素 鸡蛋 0.045 96.4—106 [34] PAMAM-Au 诺氟沙星 牛奶、鸡蛋、猪肉 0.3837 91.6—106.1 [35] PEDOT/MWCNT 瘦肉精 牛肉 4.66 85—111 [36] -
[1] 吕凯, 刘晓薇, 邓呈逊, 等. SPE-RRLC-MS/MS测定河水和沉积物中14种典型抗生素 [J]. 环境化学, 2019, 38(11): 2415-2424. LV K, LIU X W, DENG C X, et al. SPE-RRLC-MS/MS determination of 14 typical antibiotics in river water and sediments [J]. Environmental Chemistry, 2019, 38(11): 2415-2424(in Chinese).
[2] 赵文, 潘运舟, 兰天, 等. 海南商品有机肥中重金属和抗生素含量状况与分析 [J]. 环境化学, 2017, 36(2): 408-419. doi: 10.7524/j.issn.0254-6108.2017.02.2016051803 ZHAO W, PAN Y Z, LAN T, et al. Status and analysis of heavy metals and antibiotics in commercial organic fertilizers in Hainan [J]. Environmental Chemistry, 2017, 36(2): 408-419(in Chinese). doi: 10.7524/j.issn.0254-6108.2017.02.2016051803
[3] 潘寻, 韩哲, 李浩. 抗生素在畜禽养殖业中的应用、潜在危害及去除 [J]. 农业环境与发展, 2012, 29(5): 1-6. PAN X, HAN Z, LI H. Application, potential harm and removal of antibiotics in livestock and poultry breeding [J]. Agricultural Environment and Development, 2012, 29(5): 1-6(in Chinese).
[4] 陈强, 邴乃慈, 谢洪勇, 等. 不同环境介质中抗生素的污染现状及其检测方法研究进展 [J]. 环境监控与预警, 2017, 9(5): 24-31. doi: 10.3969/j.issn.1674-6732.2017.05.007 CHEN Q, BING N C, XIE H Y, et al. Contamination status of antibiotics in different environmental media and research progress in detection methods [J]. Environmental Monitoring and Early Warning, 2017, 9(5): 24-31(in Chinese). doi: 10.3969/j.issn.1674-6732.2017.05.007
[5] 周志洪, 吴清柱, 王秀娟, 等. 环境中抗生素污染现状及检测技术 [J]. 分析仪器, 2016(6): 1-8. ZHOU Z H, WU Q Z, WANG X J, et al. Antibiotic pollution in the environment and its detection technology [J]. Analytical Instruments, 2016(6): 1-8(in Chinese).
[6] MOUDGIL P, BEDI J S, AULAKH R S, et al. Validation of HPLC multi-residue method for determination of fluoroquinolones, tetracycline, sulphonamides and chloramphenicol residues in bovine milk [J]. Food Analytical Methods, 2019, 12(2): 338-346. doi: 10.1007/s12161-018-1365-0 [7] DU J, ZHAO H, LIU S, et al. Antibiotics in the coastal water of the South Yellow Sea in China: Occurrence, distribution and ecological risks [J]. Science of the Total Environment, 2017, 595: 521-527. doi: 10.1016/j.scitotenv.2017.03.281 [8] GROS M, RODRIGUEZ-MOZAZ S, BARCELO D. Fast and comprehensive multi-residue analysis of a broad range of human and veterinary pharmaceuticals and some of their metabolites in surface and treated waters by ultra-high-performance liquid chromatography coupled to quadrupole-linear ion trap tandem mass spectrometry [J]. Journal of Chromatography A, 2012, 1248: 104-121. doi: 10.1016/j.chroma.2012.05.084 [9] XU X, XU X Y, HAN M, et al. Development of a modified QuEChERS method based on magnetic multiwalled carbon nanotubes for the simultaneous determination of veterinary drugs, pesticides and mycotoxins in eggs by UPLC-MS/MS [J]. Food Chemistry, 2019, 276: 419-426. doi: 10.1016/j.foodchem.2018.10.051 [10] BAILAC S, BARRON D, BARBOSA J. New extraction procedure to improve the determination of quinolones in poultry muscle by liquid chromatography with ultraviolet and mass spectrometric detection [J]. Analytica Chimica Acta, 2006, 580(2): 163-169. doi: 10.1016/j.aca.2006.07.064 [11] POSYNIAK A, MITROWSKA K. Analytical procedure for the determination of fluoroquinolones in animal muscle [J]. Bulletin of the Veterinary Institute in Pulawy, 2008, 52(3): 427-430. [12] ASHU TUFA R, PINACHO D G, PASCUAL N, et al. Development and validation of an enzyme linked immunosorbent assay for fluoroquinolones in animal feeds [J]. Food Control, 2015, 57: 195-201. doi: 10.1016/j.foodcont.2015.04.015 [13] WEI D, MENG H, ZENG K, et al. Visual dual dot immunoassay for the simultaneous detection of kanamycin and streptomycin in milk [J]. Analytical Methods, 2019, 11(1): 70-77. doi: 10.1039/C8AY02006J [14] MEYER M T, BUMGARNER J E, VARNS J L, et al. Use of radioimmunoassay as a screen for antibiotics in confined animal feeding operations and confirmation by liquid chromatography/mass spectrometry [J]. The Science of the total environment, 2000, 248(2-3): 181-187. doi: 10.1016/S0048-9697(99)00541-0 [15] YANG S, CARLSON K. Routine monitoring of antibiotics in water and wastewater with a radioimmunoassay technique [J]. Water Research, 2004, 38(14-15): 3155-3166. doi: 10.1016/j.watres.2004.04.028 [16] AL-MAZEEDI H M, ABBAS A B, ALOMIRAH H F, et al. Screening for tetracycline residues in food products of animal origin in the State of Kuwait using charm Ⅱ radio-immunoassay and LC/MS/MS methods [J]. Food Additives and Contaminants Part a-Chemistry Analysis Control Exposure & Risk Assessment, 2010, 27(3): 291-301. [17] LE T, YI S, WEI S, et al. A competitive dual-label time-resolved fluoroimmunoassay for the simultaneous detection of chlortetracycline and doxycycline in animal edible tissues [J]. Food and Agricultural Immunology, 2015, 26(6): 804-812. doi: 10.1080/09540105.2015.1036355 [18] LE T, YAN P, LIU J, et al. Simultaneous detection of sulfamethazine and sulfaquinoxaline using a dual-label time-resolved fluorescence immunoassay [J]. Food Additives and Contaminants Part a-Chemistry Analysis Control Exposure & Risk Assessment, 2013, 30(7): 1264-1269. [19] ASHUO A, ZOU W, FU J, et al. High throughput detection of antibiotic residues in milk by time-resolved fluorescence immunochromatography based on QR code [J]. Food Additives and Contaminants Part a-Chemistry Analysis Control Exposure & Risk Assessment, 2020, 37(9): 1481-1490. [20] LI Z B, CUI P L, LIU J, et al. Production of generic monoclonal antibody and development of chemiluminescence immunoassay for determination of 32 sulfonamides in chicken muscle [J]. Food Chemistry, 2020, 311: 125966. doi: 10.1016/j.foodchem.2019.125966 [21] YU X, TAO X, SHEN J, et al. A one-step chemiluminescence immunoassay for 20 fluoroquinolone residues in fish and shrimp based on a single chain Fv-alkaline phosphatase fusion protein [J]. Analytical Methods, 2015, 7(21): 9032-9039. doi: 10.1039/C5AY01410G [22] LUO M, XING K, GUO Z, et al. Sensitive immunoassays based on a monoclonal antibody for detection of marbofloxacin in milk [J]. Journal of Dairy Science, 2020, 103(9): 7791-7800. doi: 10.3168/jds.2019-18108 [23] GUO L, WU X, CUI G, et al. Colloidal gold immunochromatographic assay for rapid detection of carbadox and cyadox in chicken breast [J]. Acs Omega, 2020, 5(3): 1422-1429. doi: 10.1021/acsomega.9b02931 [24] XU N, XU L, MA W, et al. An ultrasensitive immunochromatographic assay for non-pretreatment monitoring of chloramphenicol in raw milk [J]. Food and Agricultural Immunology, 2015, 26(5): 635-644. doi: 10.1080/09540105.2014.998640 [25] SHI Q, HUANG J, SUN Y, et al. Utilization of a lateral flow colloidal gold immunoassay strip based on surface-enhanced Raman spectroscopy for ultrasensitive detection of antibiotics in milk [J]. Spectrochimica Acta Part a-Molecular and Biomolecular Spectroscopy, 2018, 197: 107-113. doi: 10.1016/j.saa.2017.11.045 [26] YANG N, XIE L L, PAN C, et al. A novel on-chip solution enabling rapid analysis of melamine and chloramphenicol in milk by smartphones [J]. Journal of Food Process Engineering, 2019, 42(2): e12976. doi: 10.1111/jfpe.12976 [27] XIAO J, HU X, WANG K, et al. A novel signal amplification strategy based on the competitive reaction between 2D Cu-TCPP(Fe) and polyethyleneimine (PEI) in the application of an enzyme-free and ultrasensitive electrochemical immunosensor for sulfonamide detection [J]. Biosensors & Bioelectronics, 2020, 150: 111883. [28] KERGARAVAT S V, NAGEL O G, ALTHAUS R L, et al. Detection of quinolones in milk and groundwater samples using an indirect immunofluorescent magneto assay [J]. International Journal of Environmental Analytical Chemistry, 2020: 1-18. [29] SONG E, YU M, WANG Y, et al. Multi-color quantum dot-based fluorescence immunoassay array for simultaneous visual detection of multiple antibiotic residues in milk [J]. Biosensors & Bioelectronics, 2015, 72: 320-325. [30] HU G, SHENG W, ZHANG Y, et al. A novel and sensitive fluorescence immunoassay for the detection of fluoroquinolones in animal-derived foods using upconversion nanoparticles as labels [J]. Analytical and Bioanalytical Chemistry, 2015, 407(28): 8487-8496. doi: 10.1007/s00216-015-8996-4 [31] WANG L, YAO M, FANG C, et al. A highly sensitive detection of chloramphenicol based on chemiluminescence immunoassays with the cheap functionalized Fe3O4@SiO2 magnetic nanoparticles [J]. Luminescence, 2017, 32(6): 1039-1044. doi: 10.1002/bio.3288 [32] LUO L, ZHOU X, PAN Y, et al. A simple and sensitive flow injection chemiluminescence immunoassay for chloramphenicol based on gold nanoparticle-loaded enzyme [J]. Luminescence:the journal of biological and chemical luminescence, 2020, 35(6): 877-884. doi: 10.1002/bio.3795 [33] ZHOU X, SHI J, ZHANG J, et al. Multiple signal amplification chemiluminescence immunoassay for chloramphenicol using functionalized SiO2 nanoparticles as probes and resin beads as carriers [J]. Spectrochimica Acta Part a-Molecular and Biomolecular Spectroscopy, 2019, 222: 117177. doi: 10.1016/j.saa.2019.117177 [34] HU M, WANG Y, YANG J, et al. Competitive electrochemical immunosensor for maduramicin detection by multiple signal amplification strategy via hemin@Fe-MIL-88NH2/AuPt [J]. Biosensors & Bioelectronics, 2019, 142: 111554. [35] LIU B, LI M, ZHAO Y, et al. A sensitive electrochemical immunosensor based on PAMAM dendrimer-encapsulated Au for detection of norfloxacin in animal-derived foods [J]. Sensors, 2018, 18(6): 1946. doi: 10.3390/s18061946 [36] TALIB N A A, SALAM F, YUSOF N A, et al. Enhancing a clenbuterol immunosensor based on poly (3, 4-ethylenedioxythiophene)/multi-walled carbon nanotube performance using response surface methodology [J]. Rsc Advances, 2018, 8(28): 15522-15532. doi: 10.1039/C8RA00109J [37] SUN H, ZU Y. A highlight of recent advances in aptamer technology and its application [J]. Molecules, 2015, 20(7): 11959-11980. doi: 10.3390/molecules200711959 [38] KIM Y S, GU M B. Advances in aptamer screening and small molecule aptasensors [M]//GU M B and KIM H S. Biosensors based on aptamers and enzymes. 2014: 29-67. [39] KUDLAK B, WIECZERZAK M. Aptamer based tools for environmental and therapeutic monitoring: A review of developments, applications, future perspectives [J]. Critical Reviews in Environmental Science and Technology, 2020, 50(8): 816-867. doi: 10.1080/10643389.2019.1634457 [40] CHEN A, YANG S. Replacing antibodies with aptamers in lateral flow immunoassay [J]. Biosensors & Bioelectronics, 2015, 71: 230-242. [41] MEHLHORN A, RAHIMI P, JOSEPH Y. Aptamer-based biosensors for antibiotic detection: A review [J]. Biosensors-Basel, 2018, 8(2): 54. doi: 10.3390/bios8020054 [42] WANG S, LIU J, YONG W, et al. A direct competitive assay-based aptasensor for sensitive determination of tetracycline residue in honey [J]. Talanta, 2015, 131: 562-569. doi: 10.1016/j.talanta.2014.08.028 [43] KIM C H, LEE L P, MIN J R, et al. An indirect competitive assay-based aptasensor for detection of oxytetracycline in milk [J]. Biosensors & Bioelectronics, 2014, 51: 426-430. [44] WU Y Y, HUANG P C, WU F Y. A label-free colorimetric aptasensor based on controllable aggregation of AuNPs for the detection of multiplex antibiotics [J]. Food Chemistry, 2020, 304: 125377. doi: 10.1016/j.foodchem.2019.125377 [45] ABEDALWAFA M A, TANG Z, QIAO Y, et al. An aptasensor strip-based colorimetric determination method for kanamycin using cellulose acetate nanofibers decorated DNA-gold nanoparticle bioconjugates [J]. Microchimica Acta, 2020, 187(6): 1-9. [46] LIU J, ZENG J, TIAN Y, et al. An aptamer and functionalized nanoparticle-based strip biosensor for on-site detection of kanamycin in food samples [J]. Analyst, 2018, 143(1): 182-189. doi: 10.1039/C7AN01476G [47] EMRANI A S, DANESH N M, LAVAEE P, et al. Colorimetric and fluorescence quenching aptasensors for detection of streptomycin in blood serum and milk based on double-stranded DNA and gold nanoparticles [J]. Food Chemistry, 2016, 190: 115-121. doi: 10.1016/j.foodchem.2015.05.079 [48] RAMEZANI M, DANESH N M, LAVAEE P, et al. A novel colorimetric triple-helix molecular switch aptasensor for ultrasensitive detection of tetracycline [J]. Biosensors & Bioelectronics, 2015, 70: 181-187. [49] OUYANG Q, LIU Y, CHEN Q, et al. Rapid and specific sensing of tetracycline in food using a novel upconversion aptasensor [J]. Food Control, 2017, 81: 156-163. doi: 10.1016/j.foodcont.2017.06.004 [50] WU S, ZHANG H, SHI Z, et al. Aptamer-based fluorescence biosensor for chloramphenicol determination using upconversion nanoparticles [J]. Food Control, 2015, 50: 597-604. doi: 10.1016/j.foodcont.2014.10.003 [51] TAN B, ZHAO H, DU L, et al. A versatile fluorescent biosensor based on target-responsive graphene oxide hydrogel for antibiotic detection [J]. Biosensors & Bioelectronics, 2016, 83: 267-273. [52] SUN C, SU R, BIE J, et al. Label-free fluorescent sensor based on aptamer and thiazole orange for the detection of tetracycline [J]. Dyes and Pigments, 2018, 149: 867-875. doi: 10.1016/j.dyepig.2017.11.031 [53] YANG L, NI H, LI C, et al. Development of a highly specific chemiluminescence aptasensor for sulfamethazine detection in milk based on in vitro selected aptamers [J]. Sensors and Actuators B-Chemical, 2019, 281: 801-811. doi: 10.1016/j.snb.2018.10.143 [54] LIU S, BAI J, HUO Y, et al. A zirconium-porphyrin MOF-based ratiometric fluorescent biosensor for rapid and ultrasensitive detection of chloramphenicol [J]. Biosensors & Bioelectronics, 2020, 149: 111801. [55] ROUHBAKHSH Z, VERDIAN A, RAJABZADEH G. Design of a liquid crystal-based aptasensing platform for ultrasensitive detection of tetracycline [J]. Talanta, 2020, 206: 120246. doi: 10.1016/j.talanta.2019.120246 [56] WANG S, DONG Y, LIANG X. Development of a SPR aptasensor containing oriented aptamer for direct capture and detection of tetracycline in multiple honey samples [J]. Biosensors & Bioelectronics, 2018, 109: 1-7. [57] NGUYEN A H, MA X, PARK H G, et al. Low-blinking SERS substrate for switchable detection of kanamycin [J]. Sensors and Actuators B-Chemical, 2019, 282: 765-773. doi: 10.1016/j.snb.2018.11.037 [58] JIANG Y, SUN D W, PU H, et al. A simple and sensitive aptasensor based on SERS for trace analysis of kanamycin in milk [J]. Journal of Food Measurement and Characterization, 2020: 1-10. [59] WU Y H, BI H, NING G, et al. Cyclodextrin subject-object recognition-based aptamer sensor for sensitive and selective detection of tetracycline [J]. Journal of Solid State Electrochemistry, 2020: 1-8. [60] CHEN X, LIU Y, FANG X, et al. Ultratrace antibiotic sensing using aptamer/graphene-based field-effect transistors [J]. Biosensors & Bioelectronics, 2019, 126: 664-671. [61] MOHAMMAD-RAZDARI A, GHASEMI-VARNAMKHASTI M, IZADI Z, et al. Detection of sulfadimethoxine in meat samples using a novel electrochemical biosensor as a rapid analysis method [J]. Journal of Food Composition and Analysis, 2019, 82: 103252. doi: 10.1016/j.jfca.2019.103252 [62] MOHAMMAD-RAZDARI A, GHASEMI-VARNAMKHASTI M, ROSTAMI S, et al. Development of an electrochemical biosensor for impedimetric detection of tetracycline in milk[J]. Journal of Food Science and Technology-Mysore, 2020. [63] LI Y, BU Y, JIANG F, et al. Fabrication of ultra-sensitive photoelectrochemical aptamer biosensor: Based on semiconductor/DNA interfacial multifunctional reconciliation via 2D-C3N4 [J]. Biosensors & Bioelectronics, 2020, 150: 1-9. [64] ZHOU L, GAN N, ZHOU Y, et al. A label-free and universal platform for antibiotics detection based on microchip electrophoresis using aptamer probes [J]. Talanta, 2017, 167: 544-549. doi: 10.1016/j.talanta.2017.02.061 [65] HUANG Y, YAN X, ZHAO L, et al. An aptamer cocktail-based electrochemical aptasensor for direct capture and rapid detection of tetracycline in honey [J]. Microchemical Journal, 2019, 150: 104179. doi: 10.1016/j.microc.2019.104179 [66] TAO X, HE Z, CAO X, et al. Development of a highly sensitive real-time immuno-PCR for the measurement of chloramphenicol in milk based on magnetic bead capturing [J]. Analytical Methods, 2014, 6(23): 9340-9347. doi: 10.1039/C4AY02158D [67] SUH S H, DWIVEDI H P, JAYKUS L-A. Development and evaluation of aptamer magnetic capture assay in conjunction with real-time PCR for detection of Campylobacter jejuni [J]. Lwt-Food Science and Technology, 2014, 56(2): 256-260. doi: 10.1016/j.lwt.2013.12.012 [68] DUAN Y, WANG L, GAO Z, et al. An aptamer-based effective method for highly sensitive detection of chloramphenicol residues in animal-sourced food using real-time fluorescent quantitative PCR [J]. Talanta, 2017, 165: 671-676. doi: 10.1016/j.talanta.2016.12.090 [69] ZHOU L, GAN N, HU F, et al. Microchip electrophoresis array-based aptasensor for multiplex antibiotic detection using functionalized magnetic beads and polymerase chain reaction amplification [J]. Sensors and Actuators B-Chemical, 2018, 263: 568-574. doi: 10.1016/j.snb.2018.02.136 [70] MA P, YE H, DENG J, et al. A fluorescence polarization aptasensor coupled with polymerase chain reaction and streptavidin for chloramphenicol detection [J]. Talanta, 2019, 205: 120119. doi: 10.1016/j.talanta.2019.120119 -







 下载:
下载: