-
自然界中砷经常与贵金属(如Au)或有色金属(如Cu和Pb)矿物伴生,因此在选矿、金属冶炼及硫酸制备的过程中会产生大量酸性高浓度的含砷废水[1-3],根据我国各类工业水污染排放标准的相关规定,这些废水必须进行有效处理,达到排放标准后才能排放,否则会对环境及人类的健康带来很大的危害,其中,硫化物沉淀法是常用的方法之一,但该方法会产生大量的硫化砷渣[4],砷渣中的砷大多以三价的形式存在,而且As(Ⅲ)的毒性是As(Ⅴ)的20—60倍[5],另外,这些硫化砷渣在自然环境中易受温度、pH值、微生物和共存离子等环境因素的影响[6],从而导致硫化砷渣中的砷重新释放到环境中,引起严重的环境砷污染。因此,硫化砷渣的有效处理对于降低其环境风险具有重要的意义。
将砷渣进行有效的固定是降低其潜在风险的方法之一,常用的固定方法是将砷渣利用惰性材料包裹然后进行卫生填埋[7],然而这种方法对于稳定性差的砷渣来讲,不能从根本上解决其二次溶出砷元素的风险,且由于在处理过程加入大量的惰性材料,导致处理后的砷渣体积成倍增加,从而存在占用大量土地的缺点;从另一方面来讲,如果将砷渣中的砷转化成一种结构和化学性能稳定的物质,那么就会大大降低其溶出的二次风险,这种思路常用于废水中As(Ⅲ)和As(Ⅴ)的去除,如Sunyer等[8-10]将水中的As(Ⅴ)有效地固定在钾明矾石的晶格里,从而达到将As(Ⅴ)稳定化的目的,然而此类明矾石的稳定性虽然较好,但其固砷的效率较低,有研究表明类明矾石中只有15%的硫酸根能被As(Ⅴ)替代[10],而臭葱石作为一种砷酸盐矿物,具有溶解度低、稳定性高和固砷率高等优点[11],因此被认为是一种安全的储砷材料,如Fujita等[12-13]将硫酸亚铁与As(Ⅴ)溶液在50—95℃下反应生成臭葱石,且生成的臭葱石在酸性条件下性能稳定;Gonzalez-Contreras等[14]利用生物反应器将溶液中的五价砷转化成大颗粒的臭葱石;Gomez等[15]利用水热法将Fe2(SO4)3-As2O5-H2SO4体系转化成臭葱石晶体;门玉等[16]通过形成臭葱石和铁素体来去除水中的As(Ⅴ);由此可见,通过生成臭葱石去除As(Ⅴ)和As(Ⅲ)的研究主要集中于含砷废水的研究,较少地应用于砷渣的研究;目前,砷渣的研究依然是借鉴含砷废水的处理思路,即首先将砷渣中的砷通过酸或者碱液浸渍,然后再将浸液中的As(Ⅲ)氧化并转换成臭葱石,如Min等[17]利用NaOH浸出阳极泥中的砷,然后再加入Fe2(SO4)3在80—95℃下氧化生成臭葱石;Ma等[11]将硫化砷渣碱浸后加双氧水氧化成As(Ⅴ),然后再引入Fe2(SO4)3和H2SO4,常压下加热到95 ℃形成臭葱石,由此可见将生成臭葱石的方法借鉴到含砷固废的处理中,通常涉及浸出-氧化-固定等一系列的化学过程,实验操作流程较为复杂;因此寻找一种简便、高效的稳定化处理砷渣的方法具有重要的实际意义。
在本工作中提出了一种简便、高效的硫化砷渣稳定化处理的方法,通过添加适量的Fe2(SO4)3和H2O2,在水热条件下通过一步反应直接将硫化砷渣转化成大颗粒的臭葱石,同时硫化砷渣中的硫离子被氧化成硫单质。本文主要优化了反应温度、时间、pH值以及Fe/As摩尔比等实验条件对臭葱石的形成过程的影响,分析了臭葱石的相组成和形貌,最后对臭葱石的短期稳定性及长期稳定性进行了评价,并将该处理方法用于实际砷渣的稳定化处理,结果表明该方法是一种简单可行的硫化砷渣稳定化方法,为砷渣的稳定化处理提供了一种新思路。
-
亚砷酸钠(NaAsO2)纯度99%、九水合硫化钠(Na2S·9H2O)纯度99%、浓硫酸(H2SO4)、硫酸铁(Fe2(SO4)3),以上所有化学试剂均购自国药控股化学试剂有限公司。
-
配置80 mL 0.4 mol·L−1的NaAsO2溶液和110 mL 0.42 mol·L−1的Na2S·9H2O溶液,用46%的H2SO4将NaAsO2溶液调至pH1,在室温条件下,边搅拌边滴加Na2S溶液。反应0.5 h后通过离心获得沉淀,然后用蒸馏水洗涤5次后于60 ℃下干燥过夜,得到黄色固体样品,即为模拟的无定形硫化砷。
-
称取0.1 g模拟硫化砷于反应釜中,加入10 mL 10%的H2O2(H2O2与As(Ⅲ)的摩尔比为41:1)和5 mL(浓度:0.0813—0.146 mol·L−1,通过浓度调控Fe/As摩尔比)的Fe2(SO4)3溶液,然后用1 mol·L−1的H2SO4或者1 mol·L−1的NaOH调节混合液的pH值至1—3,搅拌混合均匀,最终在100—160 ℃下反应数小时,最终分别利用离心和过滤获得沉淀产物和漂浮物,然后利用蒸馏水洗涤5次,得到的固体在60 ℃下干燥24 h,获得的固体分别为Fe-As-O的含砷矿物和硫单质;上清液中残余的砷浓度CAs用ICP测定,最终我们将砷的转化率定义为η。
其中,mAs(mg)为硫化砷渣中砷的质量;CAs(mg·L−1)为反应后上清液中残余的砷浓度;V(mL)为反应釜中液体的总体积。
-
采用Bruker Axs X射线衍射仪进行样品的物相分析,其光源产生条件为40 kV,30 mA,测量时的扫描速度为0.01(°)·s−1,CuKα为激发源,X射线波长为0.154 nm;利用Quanta200型的扫描电镜观察样品的微观形貌和颗粒大小,测试条件为电子加速电压为5—30 kV,真空度为8×10−5 Mba;采用美国尼高力仪器公司生产的FT-IR AVATAR-360型傅里叶变换光谱仪获取样品的红外谱图信息,扫描范围为500—3800 cm−1,扫描次数是32次,分辨率是4 cm−1;采用电感耦合等离子体质谱(ICP-MS)仪测定溶液中砷的浓度。
-
选取醋酸缓冲溶液作为毒性实验的浸出液,浸出液的配置流程如下:将5.7 mL的冰醋酸溶入500 mL去离子水中,再加入64.3 mL 1 mol·L−1的NaOH,最后定容至1 L,用1 mol·L1的HNO3或 1 mol·L−1NaOH调节pH值,使其保持在4.9左右。臭葱石的短期及长期毒性浸出实验的具体步骤如下:在烧杯中加入0.5g固体样品,然后加入10 mL浸出液,使液固比(L/S)为20 mg·g−1,在25 ℃恒温振荡器中振荡1、10、30、60 d,转速为(30±2)r·min−1,然后利用0.22 μm的过滤膜过滤收集上清液,通过上清液中砷浓度的大小评估形成的臭葱石的短期和长期稳定性。
-
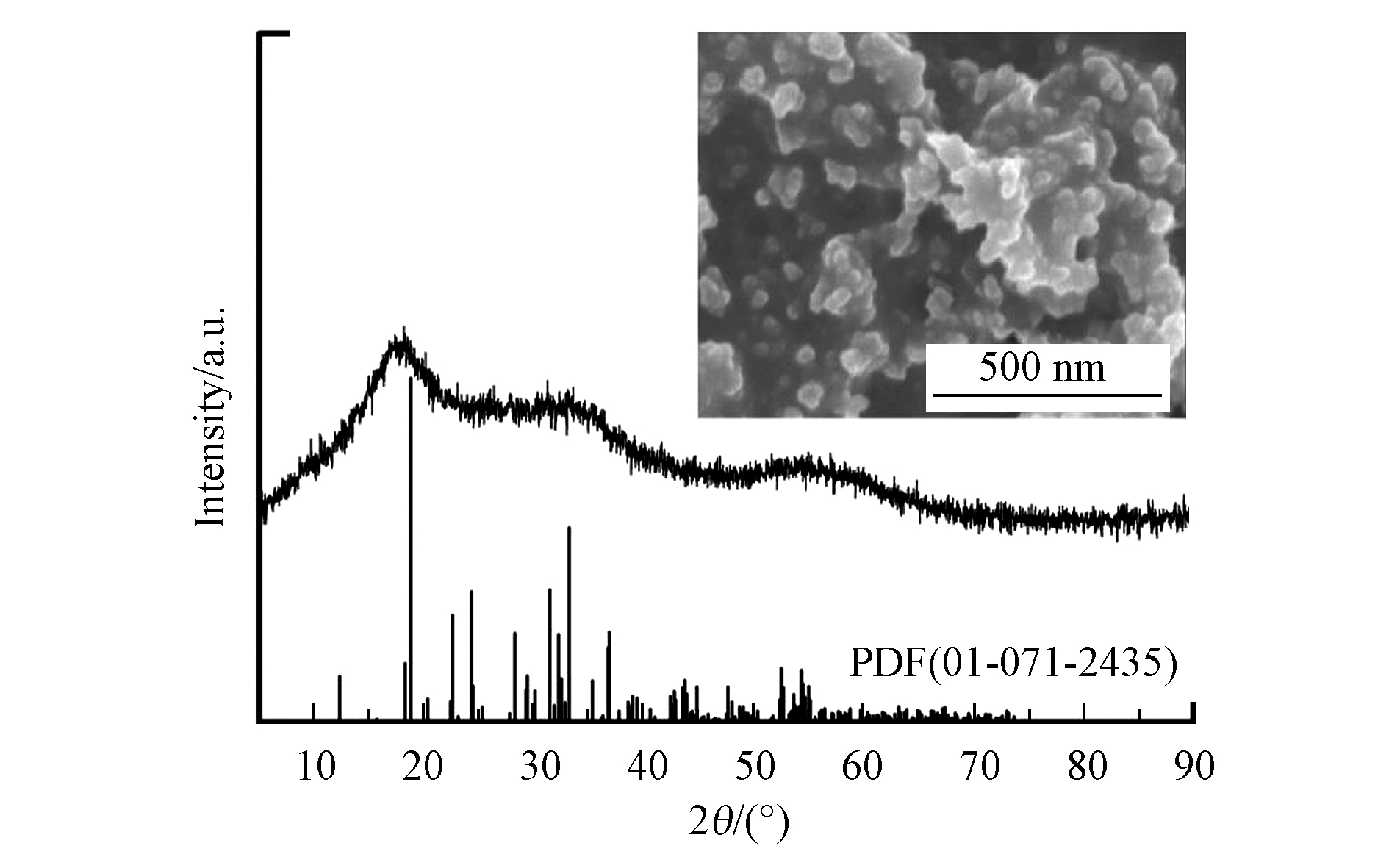
图1给出了合成的硫化砷的XRD及SEM图。由图1可以看出,2θ在18.392o、33.153o和54.582o处有衍射峰,峰位与标准PDF(01-071-2435)卡片大致相符,说明合成的样品确为硫化砷,另外低的衍射强度说明所合成的硫化砷为无定形;由图1的插图可以看出,合成的硫化砷为纳米颗粒的团聚体。另外,合成的硫化砷的浸出实验表明砷的浸出浓度高达500 mg·L−1,远超过《危险废物鉴别标准 浸出毒性鉴别》中的限值(5 mg·L−1)。通常工业上处理含砷废水也是通过硫化物沉淀法去除废水中的As(Ⅲ)或As(Ⅴ),因此我们选取所合成的硫化砷作为稳定化实验的研究对象,进行实验条件的优化,最终利用实际砷渣来验证该方法的有效性和普适性。
-
图2给出了不同水热温度下产物的XRD和SEM图。由图2a可以看出,当水热温度在100—160 ℃范围内,XRD的衍射峰2θ在19.713o、27.858o和29.063o处与臭葱石的标准PDF(00-037-0468)卡片相匹配,基本无其它杂峰,说明水热产物为臭葱石;随着水热温度的升高,XRD峰型变得更加光滑且尖锐,由此可以看出提高反应温度有利于获得结晶度较高的臭葱石。图2b-e给出了不同温度下水热产物的SEM图。由图2可以看出,当反应温度为100 ℃时,产物的形貌为粒径大小不均的微米级颗粒聚集体(图2b);当水热温度升高到120 ℃时,产物为20 μm左右的球体(图2c);继续升高水热温度到140 ℃或者160 ℃时,产物的形貌依然为大颗粒的球体(图2d和图2e),但球体上出现许多小孔;由图2f可以看出,当温度升高至200 ℃时,水热产物中除了臭葱石外,开始出现正砷酸铁(FeAsO4·0.75H2O)的衍射峰,且随着反应时间的延长,FeAsO4·0.75H2O的峰位越来越明显,峰强越来越高,而当反应时间延长至24 h时,产物的衍射峰与标准PDF(01-081-1923)卡片一致,说明此时水热产物以FeAsO4·0.75H2O为主要物相。
对100—160 ℃下的水热产物进行了毒性浸出实验,砷的浸出浓度分别为2.38、0.70、0.68、0.69 mg·L−1,结果表明所有产物中砷的浸出浓度均低于5 mg·L−1 (《危险废物鉴别标准 浸出毒性鉴别》中的限值),这与Gonzalez-Contreras等[14]的研究一致,Majzlan等[18]的研究表明正砷酸铁的溶解度小于臭葱石,为此进行了FeAsO4·0.75H2O和臭葱石的短期毒性浸出实验,结果表明正砷酸铁中砷的浸出浓度为0.61 mg·L−1,臭葱石中砷的浸出浓度为0.8 mg·L−1,均小于上述标准中的限值,但考虑到节能环保的问题,实验温度选定120 ℃。
-
根据上述温度筛选实验的结果,将水热温度设定为120 ℃,改变水热转化时间为2、4、6、8、10 h,研究水热时间对硫化砷稳定化的影响。图3给出了不同水热转化时间下产物的XRD和SEM图。
由图3a中水热产物的XRD可以看出,当水热反应为2 h时,产物的XRD衍射峰强度较弱,与标准PDF卡片比对后,推断水热产物可能是臭葱石和碱式硫酸铁(Fe(OH)SO4,PDF(00-021-0428))的混合物相,如图3c所示,水热转化2h后,产物的形貌为小颗粒聚集体(如图3c中的圆圈2所示),但也存在微米级的球状物(如图3c中的圆圈1所示),根据下文的分析,推断该球状物可能是臭葱石;随着反应时间的延长(4—10 h),如图3a所示,XRD中Fe(OH)SO4的衍射峰峰强先增大后减少,而臭葱石的衍射峰越来越明显;当反应时间为10 h时,水热产物的XRD衍射峰与臭葱石的标准PDF(00-037-0468)卡片完全匹配,说明水热产物以臭葱石为主要物相;由图3d、3e、3f和3g可以看出,不论产物是臭葱石还是臭葱石与Fe(OH)SO4的混合相,其形貌均为约1 μm的球体,因此从形貌上无法区分这两个物相。由图3g和3h可以看出,大颗粒的臭葱石是由八面体的棱柱聚集成的球状。
-
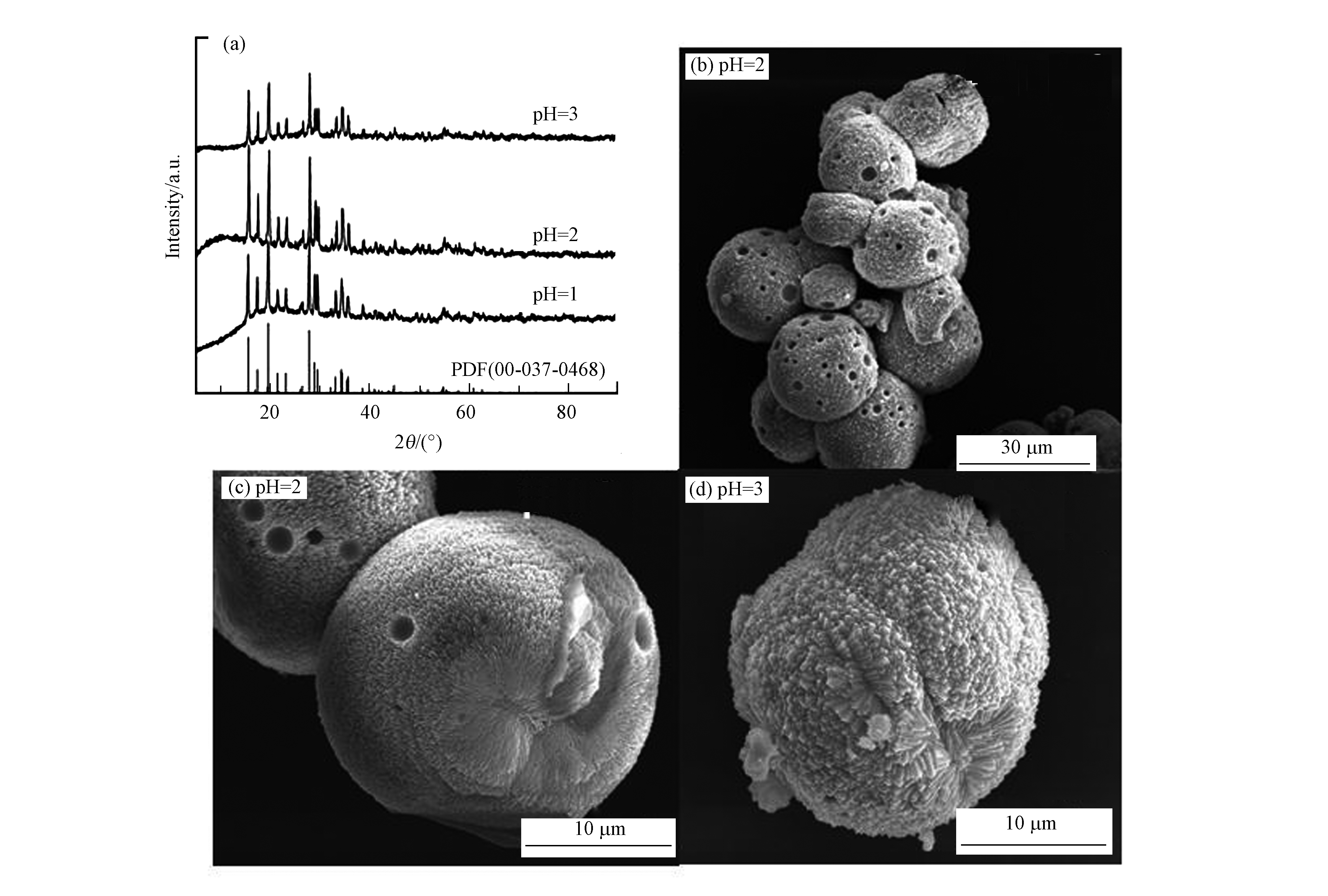
根据上述实验结果,将水热温度和水热转化时间分别设定为120 ℃和10 h,观察反应溶液的pH值对硫化砷稳定化的影响。图4为不同pH值条件下水热产物的XRD和SEM图。由图4a中XRD图可以看出,当反应溶液的pH值为1、2、3时水热产物均为臭葱石物相。图4b—d为在不同pH值(pH=1、2、3)下水热产物臭葱石的微观形貌。由图4可以看出,不同pH值下的臭葱石均为20 μm左右的球状大颗粒,对3种pH值下产生的臭葱石进行了短期毒性浸出试验,砷的浸出浓度分别为0.85、0.81、0.76 mg·L−1,均小于《危险废物鉴别标准 浸出毒性鉴别》标准中的限值(5 mg·L−1),说明反应溶液的pH值在1—3范围内对水热产物的物相和砷的浸出浓度都没有明显的影响,考虑酸性对处理设备的影响,选取pH3进行下一步的实验。
-
根据上述实验结果,将水热温度、水热转化时间和反应溶液pH值分别设定为120 ℃,10 h和3,研究不同Fe/As摩尔比对硫化砷稳定化的影响。图5为在不同Fe/As摩尔比下获得的水热产物的XRD和SEM图。由图5a可以看出,反应体系中的Fe/As摩尔比影响最终水热产物的物相,当Fe/As摩尔比为1:1—1.2:1时,XRD的衍射峰与臭葱石的标准PDF卡片完全一致,当Fe/As摩尔比增大到1.4:1—1.8:1时,水热产物的衍射峰中出现了Fe(OH)SO4的衍射峰,并且随着铁量的增大,Fe(OH)SO4的衍射峰越来越明显。图5b给出了As(Ⅴ)的转化率和Fe3+的沉淀率随Fe/As摩尔比的变化关系图,由图可以看出随着Fe3+浓度的增大,Fe3+的沉淀率基本不变,而As(Ⅴ)的转化率先增大后减小,在Fe/As摩尔比为1.4:1时砷的转化率达到最大,为95.3%,随后砷的转化率又开始逐步降低。当Fe/As摩尔比在1:1—1.4:1之间,随着体系中Fe3+浓度的增大,Fe3+与溶液中的As(Ⅴ)结合生成臭葱石,所以As(Ⅴ)的转化率逐步升高,剩余的少量Fe3+以Fe(OH)SO4形式被沉淀下来,因此Fe3+的沉淀率依然保持在100%附近,当Fe/As摩尔比在1.4:1—1.8:1之间时,其远大于臭葱石中的Fe/As理论摩尔比(1:1),此时体系中的Fe3+大大过量,因此两种水热产物Fe(OH)SO4与臭葱石之间形成竞争,最终导致溶液中As(Ⅴ)浓度逐渐增加,进而As(Ⅴ)的转化率有所降低;图5c-f分别是Fe/As摩尔比为1:1、1.2:1、1.4:1、1.6:1条件下水热产物的SEM图。由图5可以看出,当Fe/As摩尔比小于1.2:1时,水热产物为较为均一的球形聚集体;而当Fe/As摩尔比为1.4:1时,水热产物的形貌为球形聚集体和爆米花状的聚集体两种形式并存,当Fe/As摩尔比为1.6:1时,水热产物的形貌基本全部为爆米花状聚集体,根据XRD分析结果可知水热产物是臭葱石和Fe(OH)SO4(图5e、f)的混合物相。最终我们对水热产物的稳定性进行了评价,Fe/As摩尔比为1.2:1时的产物的砷浸出浓度小于1 mg·L−1,且Fe/As=1.2:1时As(Ⅴ)的转化率达到90%以上,同时考虑到Fe3+用量的经济问题,最终选择Fe/As摩尔比为1.2:1。
-
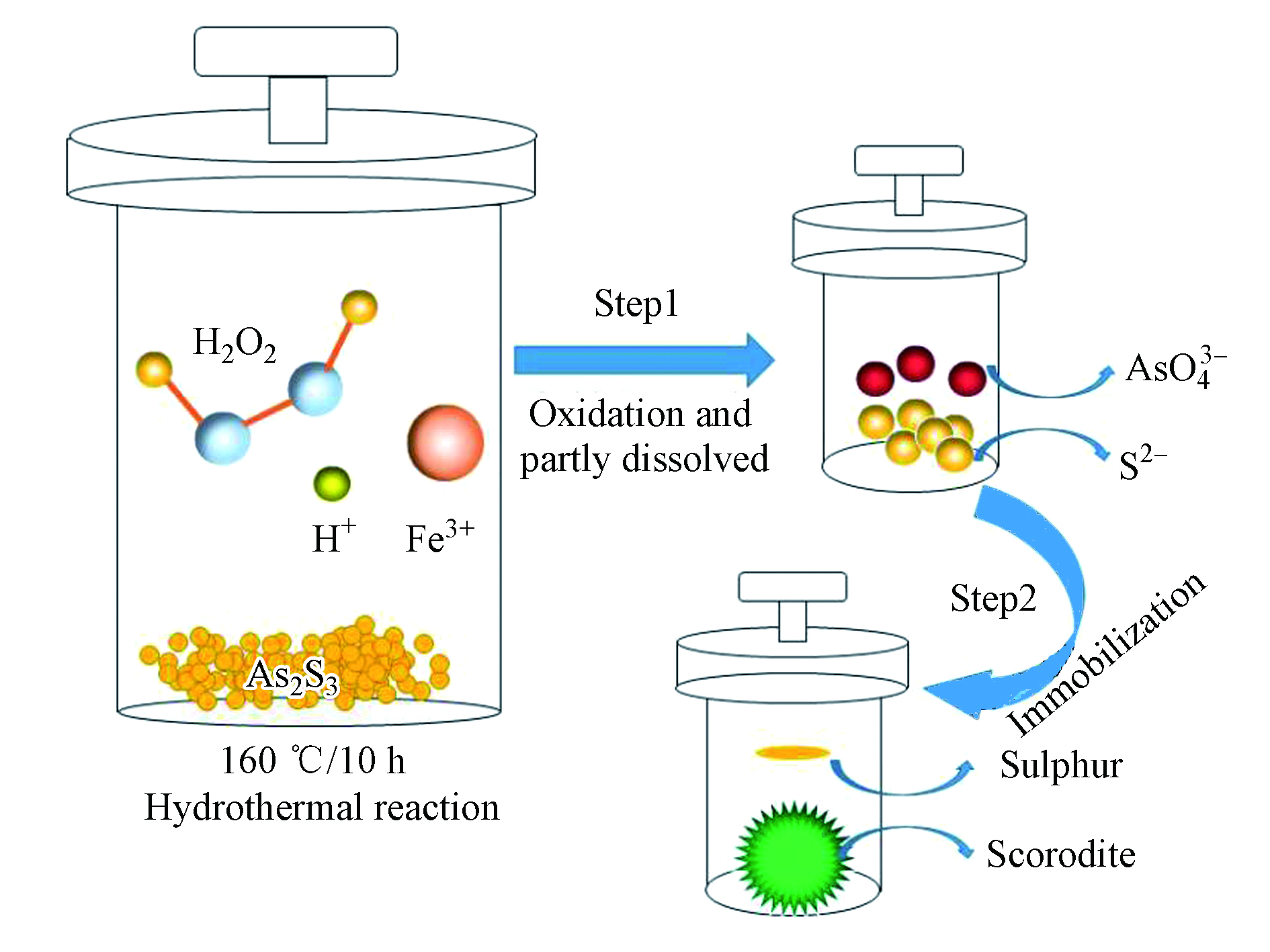
硫化砷在高温高压的水热条件下,在H2O2的氧化作用及弱酸性的水溶液中,硫化砷被逐步溶解,与此同时硫和砷两种元素分别被氧化成单质硫和As(Ⅴ),涉及的反应方程式见(1);然后溶液中的As(Ⅴ)与添加的Fe3+反应生成臭葱石,并在水热条件下不断晶化,涉及的反应方程式见(2)。在硫化砷溶解的过程中,由于硫化砷的氧化溶解需要在一段时间内才能完成,所以在反应的初始阶段,会出现过量的Fe3+生成Fe(OH)SO4的现象(见反应式3),但随着硫化砷的氧化溶解,溶液中As(Ⅴ)的浓度增加,因此Fe(OH)SO4会重新溶解释放出Fe3+(见反应式4),然后溶液中的As(Ⅴ)又与Fe3+结合生成臭葱石(见反应式5);硫化砷中的硫离子被氧化成单质硫,由于其具有疏水性,因此漂浮在溶液上方,易于回收分离(图6)。
-
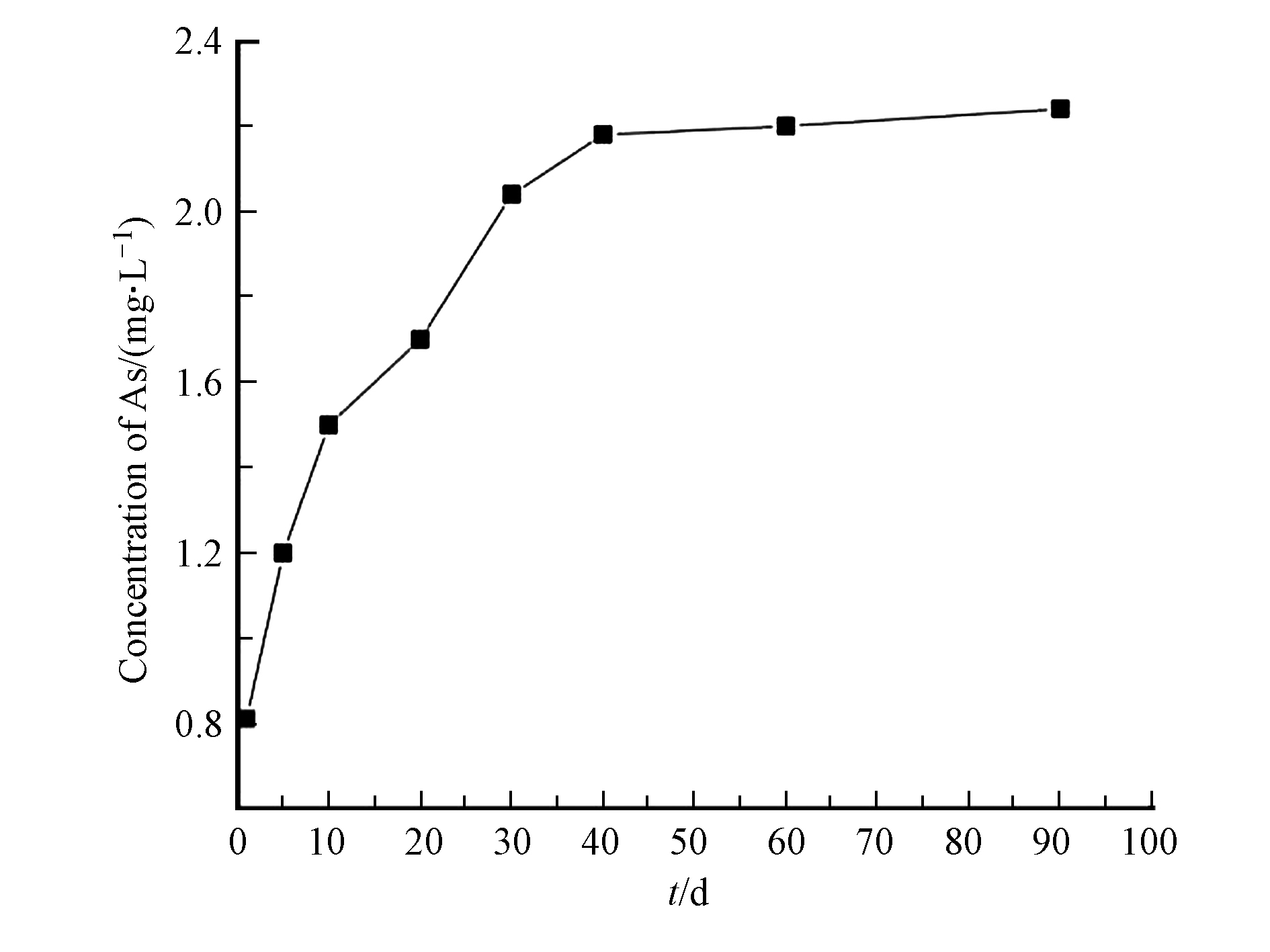
图7给出了臭葱石的长期毒性浸出实验结果。浸出结果表明臭葱石浸渍一个月时,砷的浸出浓度随着时间的延长逐步增大,之后趋于稳定,浸渍90 d后臭葱石中砷的浸出浓度为2.2 mg·L−1,由此说明本方法能够有效地稳定化硫化砷。
-
利用实际砷渣评估此方法的适用性,实际砷渣(HN-ASR)的物理化学性质以及处理前后的毒性浸出情况列于表1中。详细的实验过程与上述实验过程类似,通过XRD和SEM图表征可以发现,实际砷渣水热处理后转化为大颗粒状的臭葱石;这与模拟硫化砷的稳定化实验结果相似;从浸出实验的结果(表1)可以看出实际砷渣中砷的浸出浓度均大于400 mg·L−1,而水热处理后的实际砷渣中砷的浸出浓度小于1 mg·L−1;由此说明该方法可以有效的稳定化处理实际砷渣,而且该方法既实现了砷渣的稳定化还获得了单质硫,为硫化砷渣的稳定化及资源化提供一种可行的方法。
-
目前含砷固废的处理一般是通过酸溶液或者碱溶液浸渍的方法,将砷元素转移到溶液中,然后再借鉴废水中砷的去除方法将其转化成化学性质稳定的物质。本工作以典型含砷固废—硫化砷渣作为研究对象,研究其稳定化的过程。实验结果表明硫化砷在水热条件以及H2O2的氧化作用下逐步氧化溶解成单质硫和As(Ⅴ),然后As(Ⅴ)与溶液中的Fe3+一步转化成臭葱石,该方法将硫化砷的溶解-再沉淀过程合二为一,简化了常规的含砷固废的处理流程,并可获得硫单质进行资源回收。稳定化处理的结果表明如果水热转化时间较短或者Fe/As摩尔比大于等于1.4:1时,容易生成Fe(OH)SO4,因此筛选出的最佳实验条件是水热温度和时间分别为120 ℃和10 h,pH值为3,Fe/As摩尔比为1.2:1,在上述条件下,砷的转化率可达90%以上;臭葱石的长期(90 d)毒性浸出实验结果为砷的浸出浓度是2.2 mg·L−1,由此表明形成的臭葱石具有优越的稳定性;利用该稳定化方法处理实际砷渣,结果表明处理后的实际砷渣中砷的浸出浓度低于1 mg·L−1,因此该方法是一种简单有效的稳定化砷渣的方法,同时可以回收单质硫,该方法为砷渣的稳定化和资源化提供借鉴意义。
硫化砷渣一步水热矿化成臭葱石和单质硫的研究
One-step hydrothermal transformation of arsenic sulfide residues to scorodite and sulfur
-
摘要: 硫化物沉淀法是最常用的处理含砷废水的方法之一,但该方法会产生大量的无定形硫化砷渣,砷渣中的砷元素在微生物和pH值等环境因素的影响下,容易重新释放到环境中引起二次污染。因此本文选取硫化砷作为研究对象,借鉴含砷废水的处理思路,将硫化砷在氧化性条件下转化成化学性质和结构稳定的臭葱石,同时回收单质硫,单质硫的回收率为90%左右,最终实现无定形硫化砷的稳定化处理。实验结果表明,最优的实验条件是水热温度和时间分别为120 ℃和10 h,最佳pH值为3,最佳的Fe/As摩尔比为1.2:1;在上述实验条件下,硫化砷能够一步直接转化成臭葱石,转化率达90%以上。该方法有效地减化了常见的硫化砷渣处理所涉及的溶解-调节-再处理的分步处理过程,且生成的臭葱石浸渍90 d后砷的浸出浓度为2.2 mg·L-1,小于国家的《危险废物鉴别标准浸出毒性鉴别》中规定的限值(5 mg·L-1);最终,将该方法应用于实际砷渣的处理,砷渣处理后砷的浸出浓度小于1 mg·L-1,由此说明该方法是一种简单高效地稳定硫化砷渣和回收硫资源的方法。Abstract: Sulfide precipitation is one of methods used to dispose arsenic-containing wastewater. Notably, the above process can produce many amorphous arsenic sulfide residues. The arsenic sulfide residues are highly toxic and might cause secondary pollution because of the leaching of arsenic element. In this work, the synthesized arsenic sulfide was adopted to study its stabilization based on the protocol of treatment of arsenic-containing wastewater. Under the oxidizing conditions, the arsenic sulfide can be transformed to scorodite and elemental sulfur. The recovery rate of elemental sulfur is about 90%. The optimal conditions are shown as following, hydrothermal temperature is 120 ℃, hydrothermal time is 10 h, pH value is 2 and Fe/As=1.2:1. Under the optimal condition, the conversion rate of arsenic sulfide can reach more than 90%. This method greatly simplifies the conventional treatment of arsenic sulfide residues, which means the process of dissolving-reprecipitation can be accomplished by one step in this work. In addition, the leaching concentration of arsenics is 2.2 mg·L-1 for the treated arsenic sulfide after impregnating 90 days, which is lower than the limiting value (5 mg·L-1 originated from identification standards for hazardous wastes-identification for extraction toxicity). For actual arsenic sulfide, the leaching concentration of arsenics decreased from 425 mg·L-1 to 0.98 mg·L-1 by above transformation treatment. Therefore, this transformation protocol is a promising method for the stabilization of arsenic sulfide residues.
-
Key words:
- arsenic sulfide residues /
- stabilization /
- scorodite /
- sulfur
-
自然界中砷经常与贵金属(如Au)或有色金属(如Cu和Pb)矿物伴生,因此在选矿、金属冶炼及硫酸制备的过程中会产生大量酸性高浓度的含砷废水[1-3],根据我国各类工业水污染排放标准的相关规定,这些废水必须进行有效处理,达到排放标准后才能排放,否则会对环境及人类的健康带来很大的危害,其中,硫化物沉淀法是常用的方法之一,但该方法会产生大量的硫化砷渣[4],砷渣中的砷大多以三价的形式存在,而且As(Ⅲ)的毒性是As(Ⅴ)的20—60倍[5],另外,这些硫化砷渣在自然环境中易受温度、pH值、微生物和共存离子等环境因素的影响[6],从而导致硫化砷渣中的砷重新释放到环境中,引起严重的环境砷污染。因此,硫化砷渣的有效处理对于降低其环境风险具有重要的意义。
将砷渣进行有效的固定是降低其潜在风险的方法之一,常用的固定方法是将砷渣利用惰性材料包裹然后进行卫生填埋[7],然而这种方法对于稳定性差的砷渣来讲,不能从根本上解决其二次溶出砷元素的风险,且由于在处理过程加入大量的惰性材料,导致处理后的砷渣体积成倍增加,从而存在占用大量土地的缺点;从另一方面来讲,如果将砷渣中的砷转化成一种结构和化学性能稳定的物质,那么就会大大降低其溶出的二次风险,这种思路常用于废水中As(Ⅲ)和As(Ⅴ)的去除,如Sunyer等[8-10]将水中的As(Ⅴ)有效地固定在钾明矾石的晶格里,从而达到将As(Ⅴ)稳定化的目的,然而此类明矾石的稳定性虽然较好,但其固砷的效率较低,有研究表明类明矾石中只有15%的硫酸根能被As(Ⅴ)替代[10],而臭葱石作为一种砷酸盐矿物,具有溶解度低、稳定性高和固砷率高等优点[11],因此被认为是一种安全的储砷材料,如Fujita等[12-13]将硫酸亚铁与As(Ⅴ)溶液在50—95℃下反应生成臭葱石,且生成的臭葱石在酸性条件下性能稳定;Gonzalez-Contreras等[14]利用生物反应器将溶液中的五价砷转化成大颗粒的臭葱石;Gomez等[15]利用水热法将Fe2(SO4)3-As2O5-H2SO4体系转化成臭葱石晶体;门玉等[16]通过形成臭葱石和铁素体来去除水中的As(Ⅴ);由此可见,通过生成臭葱石去除As(Ⅴ)和As(Ⅲ)的研究主要集中于含砷废水的研究,较少地应用于砷渣的研究;目前,砷渣的研究依然是借鉴含砷废水的处理思路,即首先将砷渣中的砷通过酸或者碱液浸渍,然后再将浸液中的As(Ⅲ)氧化并转换成臭葱石,如Min等[17]利用NaOH浸出阳极泥中的砷,然后再加入Fe2(SO4)3在80—95℃下氧化生成臭葱石;Ma等[11]将硫化砷渣碱浸后加双氧水氧化成As(Ⅴ),然后再引入Fe2(SO4)3和H2SO4,常压下加热到95 ℃形成臭葱石,由此可见将生成臭葱石的方法借鉴到含砷固废的处理中,通常涉及浸出-氧化-固定等一系列的化学过程,实验操作流程较为复杂;因此寻找一种简便、高效的稳定化处理砷渣的方法具有重要的实际意义。
在本工作中提出了一种简便、高效的硫化砷渣稳定化处理的方法,通过添加适量的Fe2(SO4)3和H2O2,在水热条件下通过一步反应直接将硫化砷渣转化成大颗粒的臭葱石,同时硫化砷渣中的硫离子被氧化成硫单质。本文主要优化了反应温度、时间、pH值以及Fe/As摩尔比等实验条件对臭葱石的形成过程的影响,分析了臭葱石的相组成和形貌,最后对臭葱石的短期稳定性及长期稳定性进行了评价,并将该处理方法用于实际砷渣的稳定化处理,结果表明该方法是一种简单可行的硫化砷渣稳定化方法,为砷渣的稳定化处理提供了一种新思路。
1. 材料与方法(Materials and methods)
1.1 实验材料
亚砷酸钠(NaAsO2)纯度99%、九水合硫化钠(Na2S·9H2O)纯度99%、浓硫酸(H2SO4)、硫酸铁(Fe2(SO4)3),以上所有化学试剂均购自国药控股化学试剂有限公司。
1.2 硫化砷的合成
配置80 mL 0.4 mol·L−1的NaAsO2溶液和110 mL 0.42 mol·L−1的Na2S·9H2O溶液,用46%的H2SO4将NaAsO2溶液调至pH1,在室温条件下,边搅拌边滴加Na2S溶液。反应0.5 h后通过离心获得沉淀,然后用蒸馏水洗涤5次后于60 ℃下干燥过夜,得到黄色固体样品,即为模拟的无定形硫化砷。
1.3 硫化砷的稳定化
称取0.1 g模拟硫化砷于反应釜中,加入10 mL 10%的H2O2(H2O2与As(Ⅲ)的摩尔比为41:1)和5 mL(浓度:0.0813—0.146 mol·L−1,通过浓度调控Fe/As摩尔比)的Fe2(SO4)3溶液,然后用1 mol·L−1的H2SO4或者1 mol·L−1的NaOH调节混合液的pH值至1—3,搅拌混合均匀,最终在100—160 ℃下反应数小时,最终分别利用离心和过滤获得沉淀产物和漂浮物,然后利用蒸馏水洗涤5次,得到的固体在60 ℃下干燥24 h,获得的固体分别为Fe-As-O的含砷矿物和硫单质;上清液中残余的砷浓度CAs用ICP测定,最终我们将砷的转化率定义为η。
η=(mAs−CAsV)/mAs 其中,mAs(mg)为硫化砷渣中砷的质量;CAs(mg·L−1)为反应后上清液中残余的砷浓度;V(mL)为反应釜中液体的总体积。
1.4 固相表征及液相分析
采用Bruker Axs X射线衍射仪进行样品的物相分析,其光源产生条件为40 kV,30 mA,测量时的扫描速度为0.01(°)·s−1,CuKα为激发源,X射线波长为0.154 nm;利用Quanta200型的扫描电镜观察样品的微观形貌和颗粒大小,测试条件为电子加速电压为5—30 kV,真空度为8×10−5 Mba;采用美国尼高力仪器公司生产的FT-IR AVATAR-360型傅里叶变换光谱仪获取样品的红外谱图信息,扫描范围为500—3800 cm−1,扫描次数是32次,分辨率是4 cm−1;采用电感耦合等离子体质谱(ICP-MS)仪测定溶液中砷的浓度。
1.5 毒性浸出试验
选取醋酸缓冲溶液作为毒性实验的浸出液,浸出液的配置流程如下:将5.7 mL的冰醋酸溶入500 mL去离子水中,再加入64.3 mL 1 mol·L−1的NaOH,最后定容至1 L,用1 mol·L1的HNO3或 1 mol·L−1NaOH调节pH值,使其保持在4.9左右。臭葱石的短期及长期毒性浸出实验的具体步骤如下:在烧杯中加入0.5g固体样品,然后加入10 mL浸出液,使液固比(L/S)为20 mg·g−1,在25 ℃恒温振荡器中振荡1、10、30、60 d,转速为(30±2)r·min−1,然后利用0.22 μm的过滤膜过滤收集上清液,通过上清液中砷浓度的大小评估形成的臭葱石的短期和长期稳定性。
2. 结果与讨论 (Results and discussion)
2.1 硫化砷的表征
图1给出了合成的硫化砷的XRD及SEM图。由图1可以看出,2θ在18.392o、33.153o和54.582o处有衍射峰,峰位与标准PDF(01-071-2435)卡片大致相符,说明合成的样品确为硫化砷,另外低的衍射强度说明所合成的硫化砷为无定形;由图1的插图可以看出,合成的硫化砷为纳米颗粒的团聚体。另外,合成的硫化砷的浸出实验表明砷的浸出浓度高达500 mg·L−1,远超过《危险废物鉴别标准 浸出毒性鉴别》中的限值(5 mg·L−1)。通常工业上处理含砷废水也是通过硫化物沉淀法去除废水中的As(Ⅲ)或As(Ⅴ),因此我们选取所合成的硫化砷作为稳定化实验的研究对象,进行实验条件的优化,最终利用实际砷渣来验证该方法的有效性和普适性。
2.2 温度对硫化砷稳定化的影响
图2给出了不同水热温度下产物的XRD和SEM图。由图2a可以看出,当水热温度在100—160 ℃范围内,XRD的衍射峰2θ在19.713o、27.858o和29.063o处与臭葱石的标准PDF(00-037-0468)卡片相匹配,基本无其它杂峰,说明水热产物为臭葱石;随着水热温度的升高,XRD峰型变得更加光滑且尖锐,由此可以看出提高反应温度有利于获得结晶度较高的臭葱石。图2b-e给出了不同温度下水热产物的SEM图。由图2可以看出,当反应温度为100 ℃时,产物的形貌为粒径大小不均的微米级颗粒聚集体(图2b);当水热温度升高到120 ℃时,产物为20 μm左右的球体(图2c);继续升高水热温度到140 ℃或者160 ℃时,产物的形貌依然为大颗粒的球体(图2d和图2e),但球体上出现许多小孔;由图2f可以看出,当温度升高至200 ℃时,水热产物中除了臭葱石外,开始出现正砷酸铁(FeAsO4·0.75H2O)的衍射峰,且随着反应时间的延长,FeAsO4·0.75H2O的峰位越来越明显,峰强越来越高,而当反应时间延长至24 h时,产物的衍射峰与标准PDF(01-081-1923)卡片一致,说明此时水热产物以FeAsO4·0.75H2O为主要物相。
对100—160 ℃下的水热产物进行了毒性浸出实验,砷的浸出浓度分别为2.38、0.70、0.68、0.69 mg·L−1,结果表明所有产物中砷的浸出浓度均低于5 mg·L−1 (《危险废物鉴别标准 浸出毒性鉴别》中的限值),这与Gonzalez-Contreras等[14]的研究一致,Majzlan等[18]的研究表明正砷酸铁的溶解度小于臭葱石,为此进行了FeAsO4·0.75H2O和臭葱石的短期毒性浸出实验,结果表明正砷酸铁中砷的浸出浓度为0.61 mg·L−1,臭葱石中砷的浸出浓度为0.8 mg·L−1,均小于上述标准中的限值,但考虑到节能环保的问题,实验温度选定120 ℃。
2.3 水热时间对硫化砷稳定化的影响
根据上述温度筛选实验的结果,将水热温度设定为120 ℃,改变水热转化时间为2、4、6、8、10 h,研究水热时间对硫化砷稳定化的影响。图3给出了不同水热转化时间下产物的XRD和SEM图。
 图 3 不同水热时间下水热产物的XRD图(a)和SEM图(c,d,e,f,g); g图的局部放大图(h); 砷的转化率和铁的沉淀率随水热时间的变化关系图(b)Figure 3. XRD patterns(a) and SEM images (c,d,e,f,g,h) of the product at the given time; (b) the conversion rate of As elementand the precipitation rate of Fe3+ at the given time(实验条件:反应温度:120 ℃,Fe/As=1.2:1,pH=3)(Temperature: 120 ℃, Fe/As=1.2:1, pH=3)
图 3 不同水热时间下水热产物的XRD图(a)和SEM图(c,d,e,f,g); g图的局部放大图(h); 砷的转化率和铁的沉淀率随水热时间的变化关系图(b)Figure 3. XRD patterns(a) and SEM images (c,d,e,f,g,h) of the product at the given time; (b) the conversion rate of As elementand the precipitation rate of Fe3+ at the given time(实验条件:反应温度:120 ℃,Fe/As=1.2:1,pH=3)(Temperature: 120 ℃, Fe/As=1.2:1, pH=3)由图3a中水热产物的XRD可以看出,当水热反应为2 h时,产物的XRD衍射峰强度较弱,与标准PDF卡片比对后,推断水热产物可能是臭葱石和碱式硫酸铁(Fe(OH)SO4,PDF(00-021-0428))的混合物相,如图3c所示,水热转化2h后,产物的形貌为小颗粒聚集体(如图3c中的圆圈2所示),但也存在微米级的球状物(如图3c中的圆圈1所示),根据下文的分析,推断该球状物可能是臭葱石;随着反应时间的延长(4—10 h),如图3a所示,XRD中Fe(OH)SO4的衍射峰峰强先增大后减少,而臭葱石的衍射峰越来越明显;当反应时间为10 h时,水热产物的XRD衍射峰与臭葱石的标准PDF(00-037-0468)卡片完全匹配,说明水热产物以臭葱石为主要物相;由图3d、3e、3f和3g可以看出,不论产物是臭葱石还是臭葱石与Fe(OH)SO4的混合相,其形貌均为约1 μm的球体,因此从形貌上无法区分这两个物相。由图3g和3h可以看出,大颗粒的臭葱石是由八面体的棱柱聚集成的球状。
2.4 pH值对硫化砷稳定化的影响
根据上述实验结果,将水热温度和水热转化时间分别设定为120 ℃和10 h,观察反应溶液的pH值对硫化砷稳定化的影响。图4为不同pH值条件下水热产物的XRD和SEM图。由图4a中XRD图可以看出,当反应溶液的pH值为1、2、3时水热产物均为臭葱石物相。图4b—d为在不同pH值(pH=1、2、3)下水热产物臭葱石的微观形貌。由图4可以看出,不同pH值下的臭葱石均为20 μm左右的球状大颗粒,对3种pH值下产生的臭葱石进行了短期毒性浸出试验,砷的浸出浓度分别为0.85、0.81、0.76 mg·L−1,均小于《危险废物鉴别标准 浸出毒性鉴别》标准中的限值(5 mg·L−1),说明反应溶液的pH值在1—3范围内对水热产物的物相和砷的浸出浓度都没有明显的影响,考虑酸性对处理设备的影响,选取pH3进行下一步的实验。
2.5 Fe/As摩尔比对硫化砷稳定化的影响
根据上述实验结果,将水热温度、水热转化时间和反应溶液pH值分别设定为120 ℃,10 h和3,研究不同Fe/As摩尔比对硫化砷稳定化的影响。图5为在不同Fe/As摩尔比下获得的水热产物的XRD和SEM图。由图5a可以看出,反应体系中的Fe/As摩尔比影响最终水热产物的物相,当Fe/As摩尔比为1:1—1.2:1时,XRD的衍射峰与臭葱石的标准PDF卡片完全一致,当Fe/As摩尔比增大到1.4:1—1.8:1时,水热产物的衍射峰中出现了Fe(OH)SO4的衍射峰,并且随着铁量的增大,Fe(OH)SO4的衍射峰越来越明显。图5b给出了As(Ⅴ)的转化率和Fe3+的沉淀率随Fe/As摩尔比的变化关系图,由图可以看出随着Fe3+浓度的增大,Fe3+的沉淀率基本不变,而As(Ⅴ)的转化率先增大后减小,在Fe/As摩尔比为1.4:1时砷的转化率达到最大,为95.3%,随后砷的转化率又开始逐步降低。当Fe/As摩尔比在1:1—1.4:1之间,随着体系中Fe3+浓度的增大,Fe3+与溶液中的As(Ⅴ)结合生成臭葱石,所以As(Ⅴ)的转化率逐步升高,剩余的少量Fe3+以Fe(OH)SO4形式被沉淀下来,因此Fe3+的沉淀率依然保持在100%附近,当Fe/As摩尔比在1.4:1—1.8:1之间时,其远大于臭葱石中的Fe/As理论摩尔比(1:1),此时体系中的Fe3+大大过量,因此两种水热产物Fe(OH)SO4与臭葱石之间形成竞争,最终导致溶液中As(Ⅴ)浓度逐渐增加,进而As(Ⅴ)的转化率有所降低;图5c-f分别是Fe/As摩尔比为1:1、1.2:1、1.4:1、1.6:1条件下水热产物的SEM图。由图5可以看出,当Fe/As摩尔比小于1.2:1时,水热产物为较为均一的球形聚集体;而当Fe/As摩尔比为1.4:1时,水热产物的形貌为球形聚集体和爆米花状的聚集体两种形式并存,当Fe/As摩尔比为1.6:1时,水热产物的形貌基本全部为爆米花状聚集体,根据XRD分析结果可知水热产物是臭葱石和Fe(OH)SO4(图5e、f)的混合物相。最终我们对水热产物的稳定性进行了评价,Fe/As摩尔比为1.2:1时的产物的砷浸出浓度小于1 mg·L−1,且Fe/As=1.2:1时As(Ⅴ)的转化率达到90%以上,同时考虑到Fe3+用量的经济问题,最终选择Fe/As摩尔比为1.2:1。
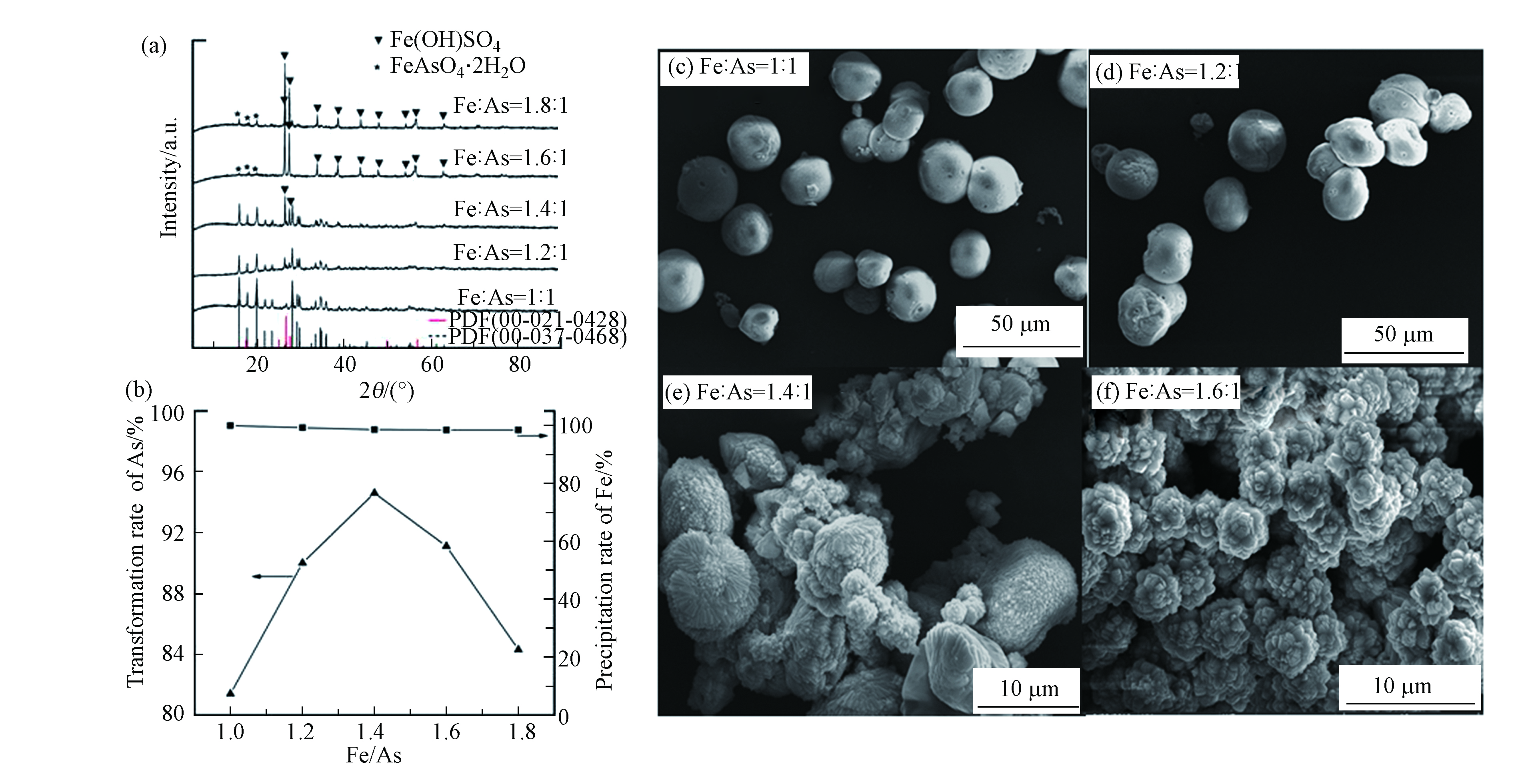 图 5 不同Fe/As摩尔比下水热产物的XRD图(a)和SEM图(c, d, e, f)以及As(Ⅴ)的转化率和Fe3+的沉淀率随Fe/As摩尔比的变化关系图(b)Figure 5. XRD patterns(a) and SEM images(c, d, e, f) of the product for the different Fe/As ratio; (b) the conversion rate of As element and the deposition rate of Fe3+ at the given Fe/As ratio(实验条件:反应温度:120 ℃,pH=3,反应时间:10 h)(Temperature: 120 ℃, pH=3, Time: 10 h)
图 5 不同Fe/As摩尔比下水热产物的XRD图(a)和SEM图(c, d, e, f)以及As(Ⅴ)的转化率和Fe3+的沉淀率随Fe/As摩尔比的变化关系图(b)Figure 5. XRD patterns(a) and SEM images(c, d, e, f) of the product for the different Fe/As ratio; (b) the conversion rate of As element and the deposition rate of Fe3+ at the given Fe/As ratio(实验条件:反应温度:120 ℃,pH=3,反应时间:10 h)(Temperature: 120 ℃, pH=3, Time: 10 h)2.6 稳定化机理
硫化砷在高温高压的水热条件下,在H2O2的氧化作用及弱酸性的水溶液中,硫化砷被逐步溶解,与此同时硫和砷两种元素分别被氧化成单质硫和As(Ⅴ),涉及的反应方程式见(1);然后溶液中的As(Ⅴ)与添加的Fe3+反应生成臭葱石,并在水热条件下不断晶化,涉及的反应方程式见(2)。在硫化砷溶解的过程中,由于硫化砷的氧化溶解需要在一段时间内才能完成,所以在反应的初始阶段,会出现过量的Fe3+生成Fe(OH)SO4的现象(见反应式3),但随着硫化砷的氧化溶解,溶液中As(Ⅴ)的浓度增加,因此Fe(OH)SO4会重新溶解释放出Fe3+(见反应式4),然后溶液中的As(Ⅴ)又与Fe3+结合生成臭葱石(见反应式5);硫化砷中的硫离子被氧化成单质硫,由于其具有疏水性,因此漂浮在溶液上方,易于回收分离(图6)。
As2S3+H2O2高温高压→AsO43−+S↓+H2O (1) AsO43−+Fe3++H+高温高压→FeAsO4⋅2H2O+H2O (2) Fe3++SO42−+H2O高温高压→Fe(OH)SO4↓+H+ (3) Fe(OH)SO4+H+高温高压→Fe3++SO42−+H2O (4) AsO43−+Fe3++H+高温高压→FeAsO4⋅2H2O+H2O (5) 2.7 毒性浸出试验
图7给出了臭葱石的长期毒性浸出实验结果。浸出结果表明臭葱石浸渍一个月时,砷的浸出浓度随着时间的延长逐步增大,之后趋于稳定,浸渍90 d后臭葱石中砷的浸出浓度为2.2 mg·L−1,由此说明本方法能够有效地稳定化硫化砷。
2.8 实例
利用实际砷渣评估此方法的适用性,实际砷渣(HN-ASR)的物理化学性质以及处理前后的毒性浸出情况列于表1中。详细的实验过程与上述实验过程类似,通过XRD和SEM图表征可以发现,实际砷渣水热处理后转化为大颗粒状的臭葱石;这与模拟硫化砷的稳定化实验结果相似;从浸出实验的结果(表1)可以看出实际砷渣中砷的浸出浓度均大于400 mg·L−1,而水热处理后的实际砷渣中砷的浸出浓度小于1 mg·L−1;由此说明该方法可以有效的稳定化处理实际砷渣,而且该方法既实现了砷渣的稳定化还获得了单质硫,为硫化砷渣的稳定化及资源化提供一种可行的方法。
表 1 实际砷渣性质及水热处理前后的砷浸出浓度Table 1. The properties of actual arsenic sulfide residues and the leaching concentration of As before and after treatment砷渣类型Arsenic sulfide residues 来源Place 元素组成/% Composition of element 砷的浸出浓度(TCLP)/(mg·L-1)The leaching concentration of As As S O Cu Al 处理前Before treatment 处理后After treatment 硫化砷渣 河南某铜矿 21.4 37.9 36.1 3.5 1.1 425 0.98 3. 结论(Conclusion)
目前含砷固废的处理一般是通过酸溶液或者碱溶液浸渍的方法,将砷元素转移到溶液中,然后再借鉴废水中砷的去除方法将其转化成化学性质稳定的物质。本工作以典型含砷固废—硫化砷渣作为研究对象,研究其稳定化的过程。实验结果表明硫化砷在水热条件以及H2O2的氧化作用下逐步氧化溶解成单质硫和As(Ⅴ),然后As(Ⅴ)与溶液中的Fe3+一步转化成臭葱石,该方法将硫化砷的溶解-再沉淀过程合二为一,简化了常规的含砷固废的处理流程,并可获得硫单质进行资源回收。稳定化处理的结果表明如果水热转化时间较短或者Fe/As摩尔比大于等于1.4:1时,容易生成Fe(OH)SO4,因此筛选出的最佳实验条件是水热温度和时间分别为120 ℃和10 h,pH值为3,Fe/As摩尔比为1.2:1,在上述条件下,砷的转化率可达90%以上;臭葱石的长期(90 d)毒性浸出实验结果为砷的浸出浓度是2.2 mg·L−1,由此表明形成的臭葱石具有优越的稳定性;利用该稳定化方法处理实际砷渣,结果表明处理后的实际砷渣中砷的浸出浓度低于1 mg·L−1,因此该方法是一种简单有效的稳定化砷渣的方法,同时可以回收单质硫,该方法为砷渣的稳定化和资源化提供借鉴意义。
-
表 1 实际砷渣性质及水热处理前后的砷浸出浓度
Table 1. The properties of actual arsenic sulfide residues and the leaching concentration of As before and after treatment
砷渣类型Arsenic sulfide residues 来源Place 元素组成/% Composition of element 砷的浸出浓度(TCLP)/(mg·L-1)The leaching concentration of As As S O Cu Al 处理前Before treatment 处理后After treatment 硫化砷渣 河南某铜矿 21.4 37.9 36.1 3.5 1.1 425 0.98 -
[1] ZHANG Q L, GAO N Y, LIN Y C, et al. Removal of arsenic(Ⅴ) from aqueous solutions using iron-oxide-coated modified activated carbon [J]. Water Environment Research:A Research Publication of the Water Environment Federation, 2007, 79(8): 931-936. doi: 10.2175/106143007X156727 [2] PENG Y L, ZHENG Y J, CHEN W M, et al. The oxidation of arsenic from As(Ⅲ) to As(Ⅴ) during copper electrorefining [J]. Hydrometallurgy, 2012, 129/130: 156-160. doi: 10.1016/j.hydromet.2012.06.009 [3] ZHENG Y J, PENG Y L, KE L, et al. Separation and recovery of Cu and As from copper electrolyte through electrowinning and SO2 reduction [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(7): 2166-2173. doi: 10.1016/S1003-6326(13)62713-2 [4] XU H, MIN X B, CHAI L Y, et al. Stabilization of arsenic sulfide sludge by hydrothermal treatment [J]. Hydrometallurgy, 2020, 191: 105229. doi: 10.1016/j.hydromet.2019.105229 [5] SMEDLEY P L, NICOLLIHB, MACDONALD D M J, et al. Hydrogeochemistry of arsenic and other inorganic constituents in groundwaters from La Pampa, Argentina [J]. Applied Geochemistry, 2002, 17(3): 259-284. doi: 10.1016/S0883-2927(01)00082-8 [6] 朱义年, 张学洪, 解庆林, 等. 砷酸盐的溶解度及其稳定性随pH值的变化 [J]. 环境化学, 2003, 22(5): 478-484. doi: 10.3321/j.issn:0254-6108.2003.05.013 ZHU Y N, ZHANG X H, XIE Q L, et al. Dependence of arsenate solublility and stability on pH value [J]. Environmental Chemistry, 2003, 22(5): 478-484(in Chinese). doi: 10.3321/j.issn:0254-6108.2003.05.013
[7] SINGH T S, PANT K K, Solidification/stabilization of arsenic containing solid wastes using portland cement, fly ash and polymeric materials[J]. Journal of Hazardous Materials, 2006, 131(1-3): 29-36. [8] SUNYER A, VINALS J, Arsenate substitution in natroalunite:A potential medium for arsenic immobilization. Part 1: Synthesis and compositions [J]. Hydrometallurgy, 2011, 109(1/2): 54-64. [9] SUNYER A, VINAL S J, Arsenate substitution in natroalunite:A potential medium for arsenic immobilization. Part 2: Cell parameters and stability tests [J]. Hydrometallurgy, 2011, 109(1/2): 106-115. [10] SUNYER A, CURRUBI M, VINALS J, Arsenic immobilization as alunite-type phases: the arsenate substitution in alunite and hydronium alunite [J]. Journal of Hazardous Materials, 2013, 261: 559-569. [11] MA X, GOMEZ M A, YUAN Z D, et al. A novel method for preparing an As(Ⅴ) solution for scorodite synthesis from an arsenic sulphide residue in a Pb refinery [J]. Hydrometallurgy, 2019, 183: 1-8. doi: 10.1016/j.hydromet.2018.11.003 [12] FUJITA T, FUJIEDA S, SHINODA K, et al. Environmental leaching characteristics of scorodite synthesized with Fe(II) ions [J]. Hydrometallurgy, 2012, 111(1): 87-102. [13] FUJITA T, TAGUCHI R, ABUMIYA M, et al. Effect of pH on atmospheric scorodite synthesis by oxidation of ferrous ions: Physical properties and stability of the scorodite [J]. Hydrometallurgy, 2009, 96(3): 189-198. doi: 10.1016/j.hydromet.2008.10.003 [14] GONZALEZ-CONTRERAS P, WEIJMA J, VAN DER WEIJDEN R, et al. Biogenic scorodite crystallization by acidianus sulfidivorans for arsenic removal [J]. Environmental science and Technology, 2010, 44(2): 675-680. doi: 10.1021/es902063t [15] GOMEZ M A, BECZE L, CUTLER J N, et al. Hydrothermal reaction chemistry and characterization of ferric arsenate phases precipitated from Fe2(SO4)3-As2O5-H2SO4 solutions [J]. Hydrometallurgy, 2011, 107: 74-90. doi: 10.1016/j.hydromet.2011.01.007 [16] 门玉, 李洪枚, OTGON N, 等. 多级沉淀法处理含砷废水 [J]. 过程工程学报, 2017, 17(2): 57-60. MEN Y, LI H M, OTGON N, et al. Removal of arsenic from wastewater by multi-stage precipitation [J]. The Chinese Journal of Process Engineering, 2017, 17(2): 57-60(in Chinese).
[17] MIN X B, LIAO Y P, CHAI L Y, et al. Removal and stabilization of arsenic from anode slime by forming crystal scorodite [J]. Trans. Nonferrous Met. Soc. China, 2015, 25(4): 1298-1306. doi: 10.1016/S1003-6326(15)63728-1 [18] MAJZLAN J, DRAHOTA P, FILIPPI M, et al. Thermodynamic properties of scorodite and parascorodite (FeAsO4·2H2O), kaňkite (FeAsO4·3.5H2O), and FeAsO4 [J]. Hydrometallurgy, 2012, 117: 47-56. -




 下载:
下载: