-
城市排水系统是指收集和处理城市污水和雨水的市政设施,主要由排水管网和污水处理厂组成。随着城市化进程的推进,城市排水系统规模不断扩大。截至2019年底,我国城市排水管道总长度达74.4×104km,建成区排水管道密度为9.27 km·km−2,城市污水日处理能力为19 171×104 m3[1]。加之水环境问题日益严峻,水行业中各种标准也随之提高,城市排水系统的运行面临“体量大、标准严”的挑战,随之造成排水管网运行维护成本的增加、污水处理环节药耗加大等问题。
混凝是饮用水处理中的重要环节[2]。硫酸盐系絮凝剂是全球饮用水处理领域主要使用的药剂[3]。饮用水生产中使用硫酸铝为絮凝剂,会导致饮用水中含有较高浓度的
SO2−4 ,并间接造成生活污水中SO2−4 浓度的升高[3]。而城市排水管道属于相对封闭的空间,易形成厌氧环境。在厌氧条件下,管壁生物膜和管道沉积物中的硫酸盐还原菌(sulfate-reducing bacteria,SRB)可将SO2−4 还原成S2−,进而水解形成HS−和H2S[4-7]。气相H2S扩散到排水管道壁面后会被硫化物氧化菌(sulfide-oxidizing bacteria,SOB)氧化成H2SO4,最终导致管道腐蚀[8-10]。化学药剂的投加是控制排水管道内硫化物的常用方法,但普遍药耗量巨大,且控制效果不一[11-12]。仅在美国,每年因硫化物引起排水管道腐蚀而投入的维护费用就高达上百亿美元[13]。污水处理厂作为城市排水系统末端的设施,日渐严格的排放标准造成厂内化学药剂使用量陡增,从而极大地增加了运行成本。铁盐作为一种常见的絮凝剂可用于排水管道腐蚀和异味管理[11, 14]、污水处理厂化学除磷[15-17],以及污泥厌氧消化中H2S的去除[18]。以前针对铁盐絮凝剂在城市排水系统中的应用及研究主要集中于优化投加点处目标污染物的去除效率,并同时减少投加量,其研究范围仅局限在孤立单元中,并未综合考虑投加的絮凝剂对整个系统造成的影响。随着厂网一体化运营理念的推广,部分学者研究了铁盐在城市排水系统中的迁移转化路径及其给下游单元带来的影响和二次效益。
本文综述了铁盐在城市排水系统中综合使用的研究进展,介绍了一种既减少用量又实现多种管理效益的铁盐使用策略,重点阐述了铁盐在各个阶段的作用机制和末端铁盐的回收利用,最后根据目前的研究现状,总结了铁盐综合使用中存在的不足和可进一步优化的方向。
-
城市排水系统中铁盐的综合使用是指将铁盐投加到排水管网中,以控制硫化物的生成,解决管道异味和腐蚀问题,并在下游的污水处理厂内继续实现化学除磷和消除沼气中H2S的作用。而在饮用水处理的混凝沉淀段(混凝剂为铁盐)产生的含铁沉淀物(后简称饮用水混凝沉淀)中的有机物含量较低而铁含量较高[19],故将其投加到排水管网中可实现与常规铁盐投加相当的效益[20]。
铁盐在城市排水系统中的迁移转化路径见图1。不管是铁盐还是含铁的饮用水混凝沉淀,投加到排水管道内的铁最终会与硫化物形成FeS沉淀,然后随污水一起被送入污水处理厂。FeS在曝气池中被氧化,再生出的Fe3+可沉淀磷酸盐,可明显提高污水处理厂的除磷效果。Fe-P沉淀进一步随剩余污泥一同排入厌氧消化池中,在厌氧条件下,沉淀中的Fe3+会被还原成Fe2+。部分Fe2+可与环境中的硫化物结合,从而减少沼气中的H2S含量,而剩余的Fe2+以蓝铁矿的形式进行回收利用。通过“一铁多用”的综合使用策略,最大限度地发挥了铁盐的效益,极大地减少了化学药剂的使用量,为城市排水系统的管理节省了巨大成本。
-
排水管网是现代城市最重要的基础设施之一,承担着污水输送的作用,但异味和腐蚀一直是其面临的两大问题,这主要与管道内产生的H2S气体有关[3, 11, 21]。H2S气体在体积分数超过0.5×10−6时,会产生难闻的气味;超过10×10−6会引起刺激和恶心;超过50×10−6会导致呼吸困难和眼睛损伤;超过700×10−6则会致命[22]。对于腐蚀问题,总硫化物质量浓度在0.1~0.5 mg·L−1时会引发小范围的混凝土管道腐蚀,质量浓度达到2.0 mg·L−1时,会发生严重的混凝土管道腐蚀[23]。铁盐作为一种常用的化学药剂被投加到排水管道中控制硫化物,可达到缓解异味和减少管道腐蚀的目的[14, 24-25]。
-
排水管道中硫的转化主要包括以下4个过程[9]:1)硫化物的形成;2)H2S的挥发;3)硫化物的化学和生物氧化;4)硫化物的沉淀。
生活污水中硫的主要来源是
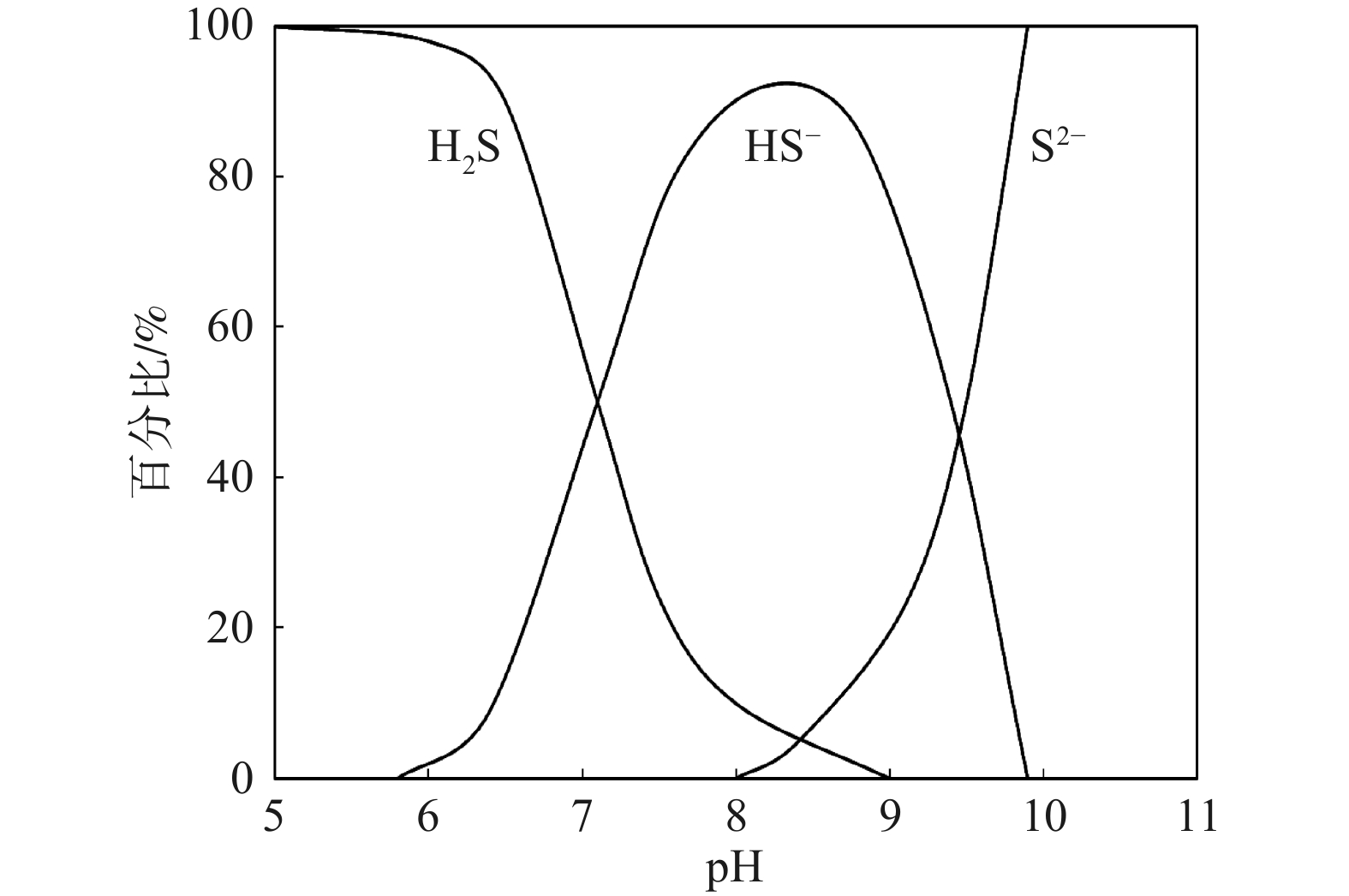
SO2−4 ,饮用水处理过程中使用的硫酸盐系絮凝剂会间接增加污水中SO2−4 的浓度[3]。在厌氧条件下,管壁生物膜和管道沉积物中的SRB可将SO2−4 还原成硫化物[26-27]。硫化物的存在形式受酸碱度影响(见图2)[28]。由于生活污水的pH约为7.0,故硫化物以H2S(aq)和HS−的形式存在于污水中[29]。H2S(aq)可通过气-液界面转移到气相中,气相中的H2S扩散到管壁后可被生物膜中的SOB氧化为硫酸,从而侵蚀排水管道及其结构[8-10]。液相中硫化物的转化分为2个方向:一部分通过化学和生物氧化转化成单质硫或硫酸盐[30-31];另一部分与污水中的铁、锌、铜等金属结合形成金属硫化物沉淀。 -
目前主要从以下3个方向进行硫化物的控制:1)向排水管道注入氧气[32]或投加硝酸盐[11]提高氧化还原电位,改变管道内的厌氧条件,从而抑制SRB还原硫酸盐;2)投加氢氧化钠[33]或氢氧化镁[34]等碱性物质提高污水的酸碱度,降低总硫化物中H2S(aq)的占比,从而减少H2S的挥发;3)投加金属盐与硫化物形成沉淀或投加氧化剂将硫化物氧化,从而减少已生成的硫化物总量。
铁盐可氧化(式(1))和沉淀(式(2))污水中的硫化物,已被广泛用于控制排水管网中的硫化物[11]。理论上,Fe3+在氧化硫化物的同时被还原成了Fe2+,并与硫化物继续形成沉淀,因此,Fe3+比Fe2+具有更强的硫化物控制能力。但式(1)的反应速率相对较慢,故当反应时间较短时,纯Fe3+的控制效率并不高[35],反而是Fe2+和Fe3+混合投加具有更好的控制效率[36]。另外,还有2点值得注意:1)形成的硫化物沉淀是FeS,而非其他形式(如Fe3S4)[14, 35];2)污水中分子态硫化物H2S不会与铁盐反应,且投加铁盐会水解降低污水酸碱度,造成H2S含量升高,不利于硫化物的沉淀去除[36-37]。
SUN等[20]发现,污水中总溶解性硫的质量浓度为20~25 mg·L−1时,按[Fe]∶[S]为1∶1的比例向实验室模拟排水管道中投加含铁饮用水混凝沉淀,能将出水硫化物质量浓度控制在0.7~2.3 mg·L−1。实验中Fe-S反应动力学表明,延长反应时间可进一步降低硫化物质量浓度。FIRER等[14]的研究表明,为了将污水中的硫化物降低到0.1 mg·L−1以下,需至少按[Fe]∶[S]=1.3∶1投加亚铁盐,或按[Fe]∶[S]=0.9∶1投加混合铁盐。
另外,ZHANG等[38]的实验室研究表明,与未投加Fe3+的对照组相比,长期投加Fe3+的实验组中硫酸盐还原率最多降低了60%,且硫酸盐还原的最终产物也发生了改变,硫化物仅占硫酸盐还原产物的54%。ZHANG等[39]的另一项研究发现,投加Fe2+后管壁生物膜中硫元素含量明显增高,表明SRB还原产物也发生改变。随后的研究表明[40],长期投加Fe3+的反应器中,管壁生物膜内的挥发性固体与总固体之比(VS∕TS)大幅降低,且表征显示生物膜中铁和硫含量较高,表明沉积在生物膜内的FeS可能是抑制SRB活性的原因。停止投加Fe3+后,SRB的还原活性能在数周内缓慢恢复。
酸碱度对铁盐控制效率的影响是巨大的,比如pH从7.0降到6.5时,为使硫化物质量浓度同样控制到0.1 mg·L−1,铁盐投加量增加了2倍[14]。针对此问题,有研究者利用电化学氧化铁的方法[41-42],在污水中原位生成Fe2+/ Fe3+和OH−,增加了污水的酸碱度,从而提高了铁对硫化物的控制效率,同时也极大地减少了H2S的挥发。另有研究者通过投加高铁酸盐(Fe(IV))[43],实现了对排水管壁生物膜中微生物的灭活,大幅降低了SRB的相对丰度,从源头上减少了硫化物产生,且此方法不受污水酸碱度的影响。
铁盐除了能较好地控制硫化物以外,对排水管道中的甲烷[38, 40, 42-43]和有机微污染物[44-45]也有一定控制效果。与其他几种控制硫化物的方法相比,在达到相当的控制效果的同时,铁相关的控制方法在成本上具有不错的优势(相关文献的结果见表1)。若用含铁的饮用水混凝沉淀替代常规铁盐投加属于废物再利用,仅会产生一部分运输成本。
-
向排水管网投加化学药剂导致的污水成分变化会对下游污水处理厂的运行产生多重影响[11]。其中,上游排水管网投加的铁盐更能在污水处理厂中被再次利用,可分别在污水处理中实现化学除磷[46]和污泥处理中控制H2S [47]。
-
磷是引起水体富营养化的物质之一。为达到严格的排放标准,污水处理中常通过投加金属盐来降低出水磷浓度。前文提到,在污水输送过程中为控制硫化物而投加的铁盐,最终会形成FeS沉淀并随污水进入污水处理厂。在曝气池中,FeS会被再次氧化而生成Fe(OH)3(反应见式(3))[48]。Fe(OH)3随后可与磷酸盐结合形成羟基磷酸铁沉淀(反应见式(4)),用FerPO4(OH)3r-3的通式表示[49],从而降低出水磷酸盐的浓度,实现化学除磷。
GUTIERREZ等[46]发现,向排水管道投加Fe3+或Fe2+以控制硫化物,可明显改善下游污水处理厂的除磷效果,除磷率(以
PO3−4 质量计)分别为每毫克Fe3+或Fe2+可分别去除0.44 mg或0.37 mg的P。REBOSURA等[50-51]用同一套实验装置证明,向排水管道反应器投加FeCl3或含Fe3+的饮用水混凝沉淀使污水中铁浓度达到10 mg·L−1时,既可有效降低排水管道中硫化物的浓度,也有助于下游污水处理过程除磷,同时还发现污泥的沉降性和脱水性有所增强。现场试验结果表明[52-53],排水管网中投加的FeCl2,在污水处理厂内被有效再利用于化学除磷,其除磷效率与厂内直接投加明矾时相当。 -
剩余污泥作为污水处理的副产品,在外运处置之前通常需要在厂内进行厌氧消化处理。这一过程可减少污泥的质量和体积,并能产生沼气这类有价值的能源。沼气主要由甲烷和二氧化碳组成,其中也混杂着具有异味和腐蚀性的H2S气体。随剩余污泥一同进入厌氧消化池的羟基磷酸铁沉淀,在厌氧条件下可被还原生成Fe2+。Fe2+主要以蓝铁矿(Fe3(PO4)2·8H2O)的形式存在于消化污泥中[54-55]。由于FeS的溶解度比蓝铁矿更低,部分Fe2+能从蓝铁矿中释放出来与环境中的硫化物结合(反应见式(5)),从而减少H2S气体的生成。
GE等[47]的研究表明,在排水管道中投加FeCl3并使铁的质量浓度(以铁元素质量计)达到5~20 mg·L−1时,可实现下游所产沼气中H2S的完全控制。另一项研究通过投加含铁饮用水混凝沉淀使排水管道中铁的质量浓度(以铁元素质量计)达到10 mg·L−1,并发现下游厌氧消化池中的可溶性硫化物质量浓度(以硫元素质量计)降低了(15.9±0.9) mg·L−1[51]。现场试验也进一步证明了排水管道中投加的铁盐能被再次利用于沼气中H2S的控制[52-53]。与此同时,几乎所有的研究都表明铁盐或含铁混凝沉淀的投加不会对沼气的生产过程产生不利影响。
-
综合使用策略大大减少了铁盐用量。为进一步优化铁盐的使用,使其成为可循环的过程,就必须实现城市排水系统末端铁盐的回收。
有研究表明,无论是投加氯化铁还是含铁的饮用水混凝沉淀,都有超过90%的铁结合在消化污泥的蓝铁矿中[56]。在厌氧消化过程中,SRB还原硫酸盐形成的硫化物会导致蓝铁矿中Fe2+的释放,但消化池中硫酸盐的浓度是有限的,并不会消耗过多的蓝铁矿[57]。REBOSURA等[50-51]的研究也证明了这一点。从实验过程中铁的质量平衡(见表2)可以看出,不管哪种铁盐投加方式,最终用于去除硫化物的铁都少于消化污泥中总铁的5%。这意味着绝大部分铁并未在污水和污泥处理过程中流失,其回收前景是非常可观的。
以往针对蓝铁矿的研究大部分与磷回收相关,回收的技术路线也是更侧重于磷的回收[58]。而关于从蓝铁矿中回收铁的研究,尚处于起步阶段。磁性分离技术(磁选)作为一种重要的物理选矿方法,可利用不同矿物之间的磁性差异实现矿物质分离。由于蓝铁矿本身具有一定的顺磁性[59],理论上可通过磁选将其从消化污泥中分离出来。
SALEHIN等[56]通过插入钕磁铁(neodymium magnet)的方式,回收了(11±0.2)%和(15.3±0.08)%的消化污泥中的蓝铁矿。回收的蓝铁矿纯度为(70±5)%和(49±3)%,其分别对应于FeCl3和含铁饮用水混凝沉淀这2种不同投加物的处理。对回收的蓝铁矿再进行碱处理,可从蓝铁矿中接近完全地(即(98±0.3)%)分离出水铁矿(ferrihydrite)形式的铁。随后直接利用回收的水铁矿进行硫化物控制,其非常有效地将污水中可溶性硫化物浓度控制在远小于1 mg·L−1的水平。这种简单磁选的铁回收效率比较有限,但将回收的铁再利用时,其脱硫效率相当高,这可看作是城市排水系统铁盐回收再利用的开端。
-
1)基于污水输送过程中铁盐控制硫化物的机制,反应时间越长,FeS沉淀生成得越彻底,且铁盐对SRB的抑制作用越强。因此,建议铁盐投加点选在排水管网上游。这将延长铁盐在管道内的停留时间,充分发挥对硫化物的控制作用,进一步减少药剂使用量。
2)投加含铁饮用水混凝沉淀可能会改变排水管道的水流条件,甚至造成低流速段管道的堵塞,也会显著增加进入厂内污水的化学需氧量和固体浓度,因此,应根据厂网的实际条件,合理使用饮用水混凝沉淀这种投加方式。
3)有实验室试验结果表明,初沉池的存在会截留一部分进入生物池及后续单元的铁盐,影响铁盐的综合使用效率[60]。未来的研究需在现场试验中权衡初沉池的各项利弊,给出具有实际指导意义的建议。
4)已有研究者开发了基于排水管网中硫化物控制的铁盐自动加药方法[61],但为实现厂网一体化管理,完善铁盐综合使用策略,还需进一步探明铁盐投加后在管网中及下游污水处理厂内的反应机理,不断改进厂网集成模型,从而实现高精度、高稳定、高效率的一体化铁盐自动加药控制。
5)关于城市排水系统末端铁盐的回收和利用的研究尚处于起步阶段,未来还需继续加大这方面的研究力度,以进一步提高铁盐的回收率,并用现场试验证明回收铁盐的再利用效果。
铁盐在城市排水系统中的综合使用研究进展
Research progress of comprehensive use of iron salt in urban drainage system
-
摘要: 对排水管网和污水处理厂的一体化管理,可极大提升城市排水系统运行的高效性和安全性。作为厂网一体化运营理念的一部分,铁盐的综合使用愈发受到关注。在城市排水系统中,铁盐可在污水输送、污水处理及污泥处理过程中发挥作用。围绕铁盐的迁移转化路径、铁盐在污水输送和污水处理厂中的作用机制,以及系统末端铁盐的回收这4个方面,综述了城市排水系统中铁盐综合使用的研究进展。最后根据当前的研究现状,总结了铁盐综合使用中面临的挑战并给出了相关建议,并从厂网一体化铁盐自动加药控制和末端铁盐的进一步回收两方面进行了展望。Abstract: The integrated management of drainage network and sewage treatment plant has greatly improved the efficiency and safety of urban drainage system. As a part of plant-network integrated operation concept, the comprehensive use of iron salt has attracted more and more attention. In urban drainage systems, iron salt can play a role in sewage conveyance, sewage treatment and sludge treatment. This paper summarizes the research progress of comprehensive use of iron salt in urban drainage system from four aspects: the migration and transformation path of iron salt, the action mechanism of iron salt in sewage conveyance and sewage treatment plant, and the recovery of iron salt at the end of the system. Finally, according to the current research status, the challenges faced in the comprehensive use of iron salt are summarized, and the usage suggestions are given,and the research prospects are put forward on plant-network integrated automatic dosing control and terminal further recovery of iron salt.
-
Key words:
- iron salt /
- comprehensive use /
- plant-network integration /
- sewage conveyance /
- sewage treatment /
- sludge treatment
-
人工湿地微生物燃料电池(constructed wetland - microbial fuel cell, CW-MFC)是一种结合人工湿地(CW)和微生物燃料电池(MFC)的新兴技术,该技术能在对污水净化的同时高效产电,具有广阔的应用前景和实际价值,在近些年得到广泛关注 [1]。目前,大量的研究集中在上流式CW-MFC方面,但最大输出功率密度通常仅为10~30 mW·m−2 [2-3]。上流式CW-MFC可能由于长时间的运行,从而形成污泥堵塞效应,导致系统的内阻变大,影响电子量转移,进而引起产电性能下降 [4-5]。DOHERTY通过下流式与上流式协同作用将系统的最大输出功率密度由单一上流式的16.8 mW·m−2提高至27.6 mW·m−2 [6],由此可知,下流式作用是不可忽视的。然而,关于一体化下流式CW-MFC产电性能的研究鲜有报道。
CW-MFC运行时通常采用葡萄糖或乙酸钠作为基质碳源 [7-8],然而不同基质碳源(乙酸钠、葡萄糖和丙酸)会影响阳极生物的形成和系统内阻 [9],同时会影响系统的产电性能。当以葡萄糖和乙酸钠为下流式CW-MFC的基质碳源时,对系统启动时间及电化学性能的影响如何,目前尚未见报道。为了降低植物对实验的影响及复杂性,在设计构建的下流式CW-MFC系统中通常不种植植物。为了解基质碳源对下流式CW-MFC的电化学行为影响,本研究分析了其产电性能和电化学行为,并通过微生物群落结构分析及FAPROTAX功能预测从微生物学角度探究葡萄糖和乙酸钠作为基质碳源下的功能菌群,以期为一体化下流式CW-MFC在实现同时高效产电与治理污染水体的研究中提供参考。
1. 材料和方法
1.1 集电体及电极的制作
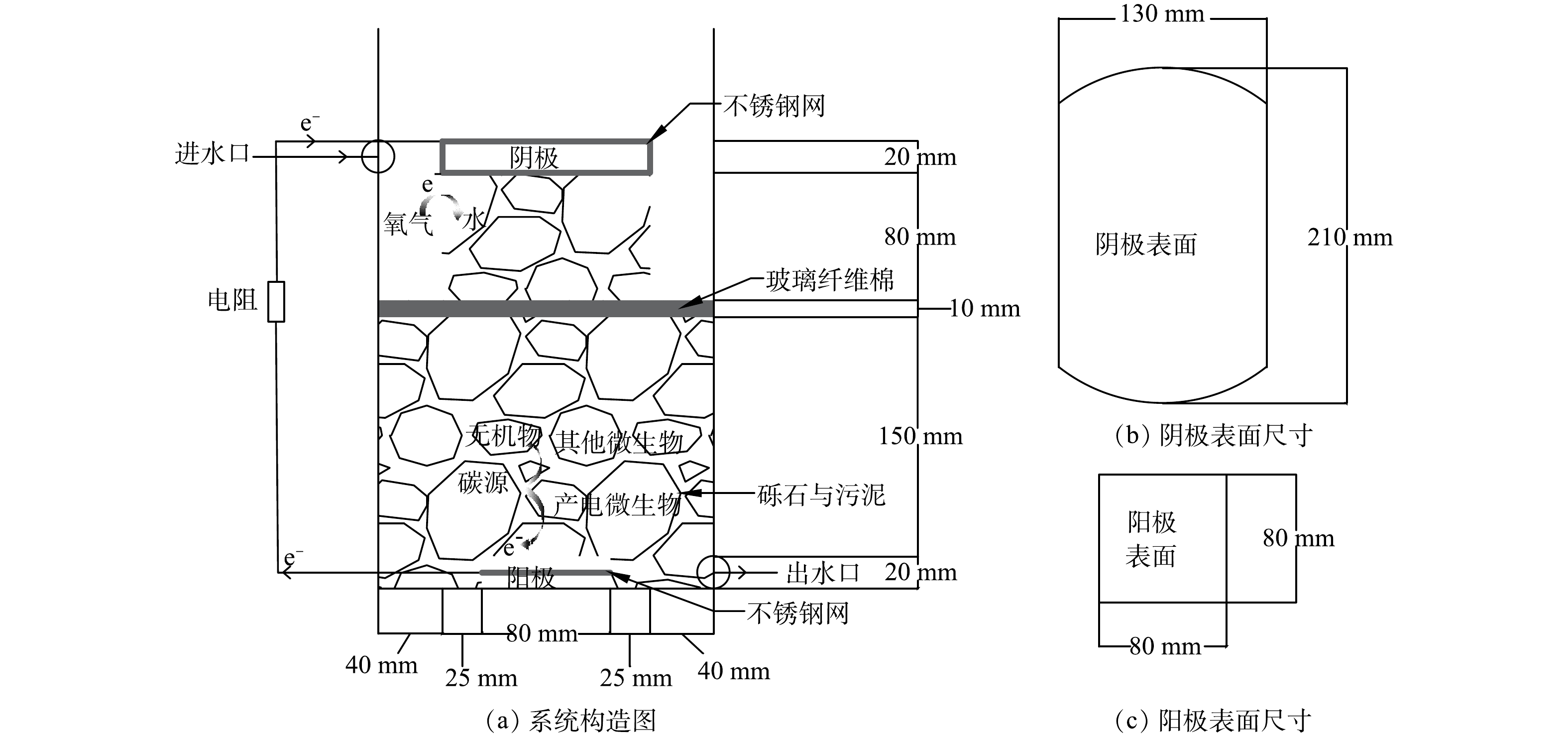
本研究所用的电极材料采用石墨毡(北京晶龙特碳石墨厂),制成厚度为2 cm表面为256 cm2的阴极以及表面为64 cm2的阳极(图1),并浸泡在0.1 mol·L−1 HCl中24 h进行预处理,以去除其表面的油渍和金属离子污染物,再用蒸馏水洗涤干净后备用 [6]。采用具有价格低廉的不锈钢丝网(5目304不锈钢丝网,钢丝丝粗直径为0.6 mm)作为集电体,有助于增强电子的传递,提高导电性,避免出现集电死区 [10]。将不锈钢丝网在1 mol·L−1 H2SO4溶液中浸泡4 h以去除表面的钝化层增加表面粗糙度,这有助于微生物富集和降低电荷转移内阻 [11]。将其制成边长为8 cm正方形埋入阳极中,以及制成能够包裹阴极的尺寸包裹阴极形成一个整体,至此电极构建完成。
1.2 系统的设计与构建
本研究构建了下流式未种植植物CW-MFC实验装置,其构造见图1。反应器使用的是直径21 cm高42 cm的圆柱桶,有效高度为28 cm。填充直径为0.3~0.6 cm的砾石共(12.15±0.1) kg作为支撑层,以改善废水在湿地中的分布并起到承托的作用 [12]。整个反应器的总有效液体体积为(2.92±0.08) L,反应器中距底部18~28 cm的区域作为阴极区,中间隔层采用厚度为15 mm压缩至10 mm且直径为21 cm的玻璃纤维棉,这样可以防止阴极区的氧气向阳极扩散,有助于降低氧气传质系数,对电子的逆向流动起缓解作用,有助于提高产电性能 [13-15]。在阴极区左右两侧空出的区域用于测定时放置参比电极。由直径为1 mm的铜导线连接阳极、阴极和外接可变电阻箱电阻(2 000 Ω)形成闭合回路 [16],并使液面处于阴极下表面在构成闭合回路的同时形成空气阴极,加入适量蒸馏水至原水位以防止水分的正常蒸发造成液面下降,从而影响反应器的运行[17]。
1.3 CW-MFC的接种和启动
污泥取自中国广西桂林市七星区七里店污水处理厂(公共自行车污水净化厂站旁)的厌氧池。污泥在投入反应器之前先进行厌氧培养(每天采用浓缩溶液更换厌氧培养液体0.6 L,1 L浓缩溶液的基本成分包括9.38 g葡萄糖、0.75 g NH4Cl、0.65 g KCl、6.8 g Na2HPO4·12H2O、0.95 g NaH2PO4·2H2O以及5 mL微量营养液),共培养60 d。厌氧培养后的混合液悬浮固体质量浓度(MLSS)为6.89 g·L−1,为确保污泥来源一致,将培养后的污泥稀释1倍并同步从各反应器的底部通入直到充满至阴极下表面以进行接种,静置1 d后形成生物膜。静置完成后,人工配置溶液以1.5 d的水力停留时间并在(30±2) °C下通过蠕动泵(雷弗BT101L DG10-2,保定,中国)从顶部连续进水,底部出水(图1)。1 L人工配置溶液的基本成分包括5 mmol·L−1磷酸盐缓冲液(0.5 g NaH2PO4·2H2O和0.64 g Na2HPO4·12H2O)、0.281 5 g葡萄糖(S.G.)或者0.384 5 g乙酸钠(S.S.)(理论COD值均为300 mg·L−1)、0.15 g NH4Cl、0.13 g KCl以及1 mL微量营养液。微量营养液的配制参考LIU等[2]的方法。并通过NaH2PO4·2H2O或Na2HPO4·12H2O调节进水溶液pH为6.5 [18]。系统运行至电压稳定时,则视为驯化成功 [19]。
1.4 电化学性能分析
1)电压、电势和功率密度曲线。采用联想扬天V110-15电脑连通LabQuest mini主机并连接电压传感器DVP-BTA(威尼尔软件与技术公司,美国),每隔30 min采集1次外接电阻的两端电压 [18]。当系统输出稳定后,开路电压达到最大值时,接入电阻箱,并采用恒阻放电法测定极化曲线。通过调节外接电阻箱的不同电阻值,稳定30 min后,读取电压并绘制极化曲线 [2,20]。用万用表(福禄克F18B,淘宝,中国)测量阴极和阳极电势。通过功率(P=V2·R−1)除以阳极表面(64 cm2)计算出功率密度,绘制功率密度曲线 [15,21]。
2)电化学测定。循环伏安法(CV)曲线、线性扫描伏安法(LSV)曲线、塔菲尔(TAFEL)曲线和电化学阻抗(EIS)测量使用CHI604E电化学工作站(上海辰华仪器有限公司,中国),并采用三电极系统即工作电极、对电极和参比电极(Ag/AgCl)电极 [22-23]。CV扫描范围为−2.0~1.8 V,扫描速度为30 mV·s−1。LSV扫描范围为0~1.0 V,扫描速度为30 mV·s−1 [24]。电容C根据CV曲线和式(1)进行计算。Tafel测试的扫描电压为−2.0~2.0 V并以10 mV·s−1的扫描速度进行扫描,记录工作电极的Tafel图 [25-26]。EIS测量在0.01~1 mHz,使用5 mV的交流电信号。通过测量EIS来拟合等效电路R(Q(RW)),可以得到电化学欧姆电阻、电荷传递电阻和传质电阻等重要参数。用Nova 2.1.4软件分析阻抗谱(Nyquist plot) [25,27]。
C=SvΔV (1) 式中:S为循环伏安环的积分面积,V·A;ν为扫描速率,V·s−1;ΔV为扫描电位区间,V。
1.5 出水COD的测定
在完成各系统电化学性能指标测定后,对各反应器的出水口取约10 mL水样,取3次。耗氧有机污染物浓度(以COD计)的测定采用哈希COD消解仪(DRB200,HACH,美国)进行快速消解,并利用COD测定仪(DR1010,HACH,美国)进行测定。采用SPSS 24.0软件进行差异显著性分析。
1.6 阳极微生物群落样品
实验结束后(第15天),取出阳极置于pH=7.0的20 mmol·L−1磷酸盐缓冲液中,用UC-6超声波振荡器(上海泰坦科技有限公司35 kHz)处理10 min,并摇匀溶液 [27]。最后,用0.45 μm滤膜过滤溶液。将过滤膜上附着的微生物样品送至苏州帕诺米克生物医药科技有限公司(BioNovoGene)进行高通量焦磷酸测序分析,以研究微生物群落结构。采用十六烷基三甲基溴化铵(CTAB)/十二烷基硫酸钠(SDS)法提取样品DNA[28]。引物515F/806R用于PCR扩增细菌V3~V4区16S rRNA基因。配对末端rRNA测序在Illumina NovaSeq6000平台(Illumina,圣迭戈,美国)上进行。通过SILVA138的SSUrRNA数据库(http://www.arb-silva.de/),进行物种注释分析(阈值为0.8~1),获得分类学信息,并分别在门和属水平上统计每个样本的群落组成。所有样本归一化后,使用R软件(Version 4.0.4)绘制维恩图,分析不同样本之间的共同和独特的OTU。使用Qiime软件(Version 1.9.1)计算有效OTUs、Chao1、Shannon、Simpson和ace指数。根据扩增子物种注释结果,进行FAPROTAX [29]环境功能预测分析。
2. 结果与讨论
2.1 产电性能与水体COD
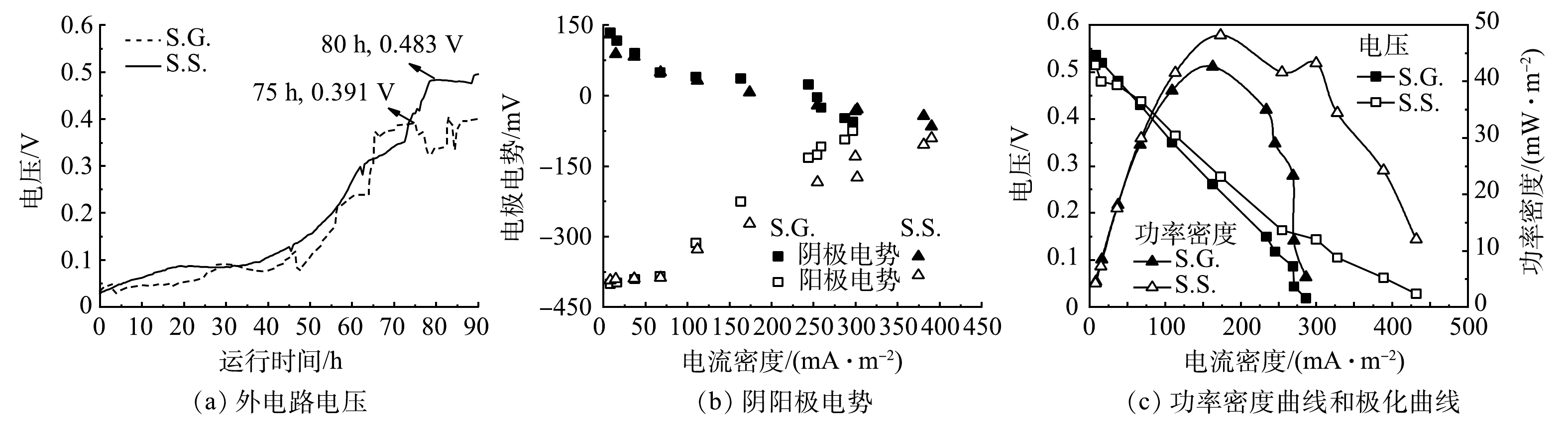
图2表明,S.S.的产电性能优于S.G.,S.G.和S.S.从开始启动至稳定所需时间分别为75 h和80 h(图2(a)),电压可分别达到0.391 V和0.483 V。以乙酸钠和葡萄糖为基质碳源的阳极反应分别见式(2)和式(3)[30]。由阴阳极电势(图2(b))可知,S.S.和S.G.的阳极电势差异均比阴极大,表明阴极发生更多的还原反应,但由于电阻的减少造成电子流出阳极的速度较大,从而正电荷的累积使得阳极电势向正方向移动。当电流密度由0 mA·m−2增加到300 mA·m−2时,S.S.和S.G.的阳极电势分别提高了55.5%和81.3%,S.S.的阳极电势随着电流密度增加的变化幅度较小,说明S.S.的阳极性能更好,生物膜稳定性更高[31]。在390 mA·m−2的电流密度下,S.S.可以维持−66 mV的阴极电势以及−91 mV的阳极电势,从而在高电流密度下S.S.依然可以保持更高的外电压进而增加功率的输出。S.S.和S.G.的最大输出功率密度分别为48.14 mW·m−2和42.61 mW·m−2(图2(c))。S.G.的电压降比S.S.更为明显,这主要由于欧姆极化和传质损耗,更高的欧姆损耗是由膜的电阻引起的,而传质损耗是由于还原化合物的有限放电能力或向电极供应氧化化合物有限所导致的[24]。S.G.具有更大内阻在EIS结果中被证实。
CH3COO−+4H2O→2HCO−3+9H++8e− (2) C6H12O6+12H2O→6HCO−3+30H++24e− (3) 本研究所采用的下流式未种植植物CW-MFC与其他已报道CW-MFC发电性能的比较见表1。可以看出,本研究构建的下流式CW-MFC在发电性能上优于其他CW-MFC系统,所产生的输出功率密度提高了74.4%~187%。因此,尽管上流式是目前主流形式,但下流式CW-MFC应得到更多关注。
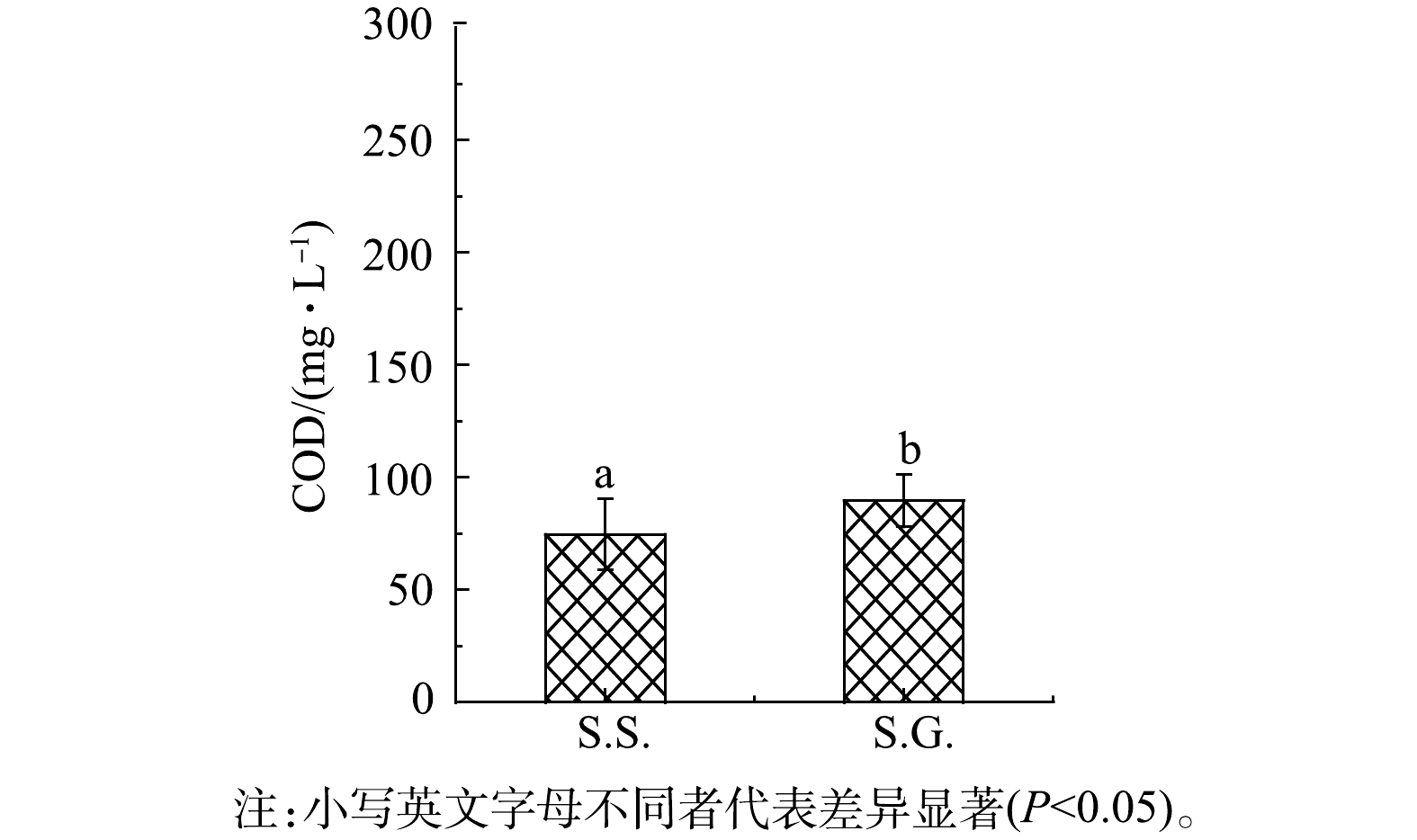
表 1 与其他已报道CW-MFC在发电性能上的比较Table 1. Compared with other reported CW-MFC on the power generation performanceS.S.出水口COD为74.7 mg·L−1,去除率达到75.1%,而S.G.的COD去除率仅为70.1%(图3)。S.S.表现出更高的水体COD去除效果,可能是由于S.S.基质碳源更容易被微生物所利用。
2.2 电化学行为
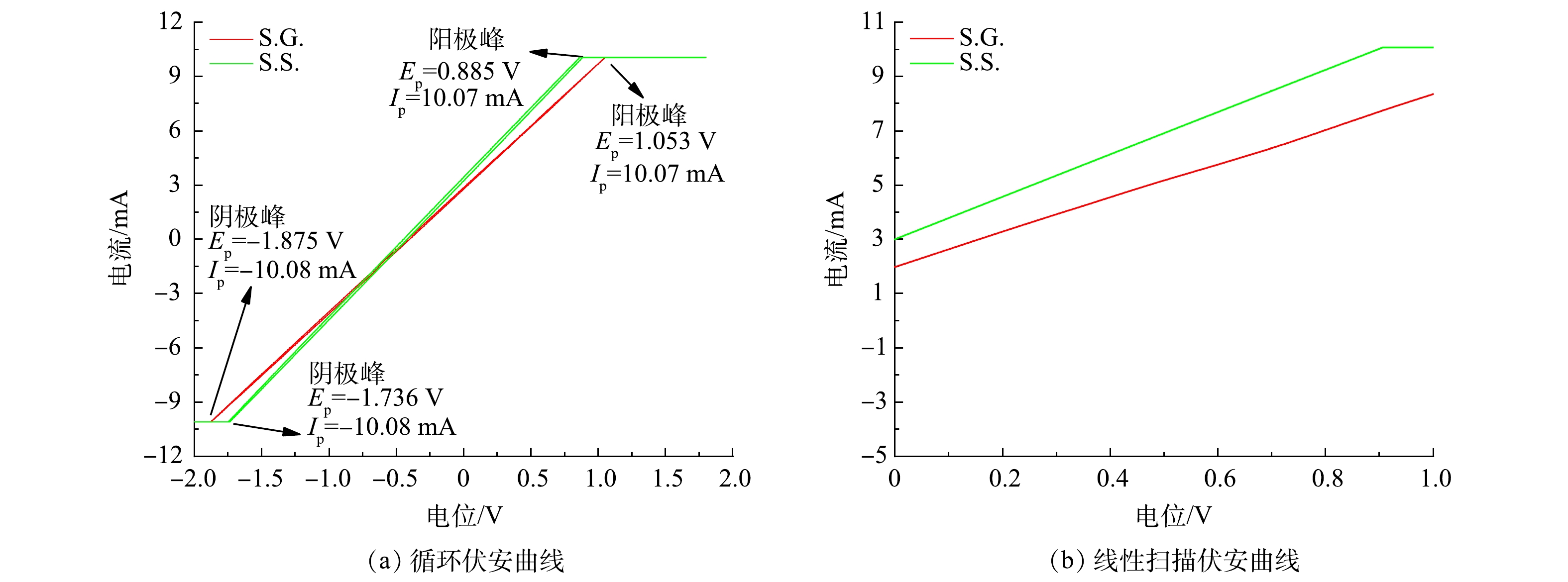
通过电化学实验考察了S.G.和S.S.阳极的生物电化学活性。CV通常用于分析基于生物膜的胞外电子传递机制 [35]。S.S.与S.G.的氧化电流同为10.07 mA(图4(a)),其还原电流/氧化电流为1.001,基本接近1,代表S.S.与S.G.均具有高可逆程度。S.G.的氧化与还原峰的峰电位差ΔE大于S.S.的ΔE,由于ΔE与转移的电子数n呈反比例关系[36],可见S.S.有着更强的产电能力。这清楚地表明S.S.具有更高的微生物活性和更快的电子转移,这可能与阳极中富集的氧化还原介质或大量参与电子传递的胞外膜蛋白覆盖在阳极有关。在JADHAV、PAREEK等的研究中 [37-38]也出现类似的原位CV扫描图。由图4(a)中可以看出,S.S.比S.G.更接近超级电容器。这是因为超级电容器的理想形状是矩形的[39]。S.S.与S.G.的电容分别为440 F和412 F,鉴于两者采用的阳极材料一致,因此S.S.的比电容比S.G.高6.7%。这是由于醋酸盐溶液的介电常数大于葡萄糖导致溶液电容的增大,并受限于位于电极及砾石上附着的生物膜自身膜电容会小于溶液电容[40]。S.S.的阳极比S.G.表现出更高的电流响应(图4(b)),表明微生物对乙酸钠的利用优于葡萄糖,并表明S.S.具有更强的氧化能力进而有助于电子转移。
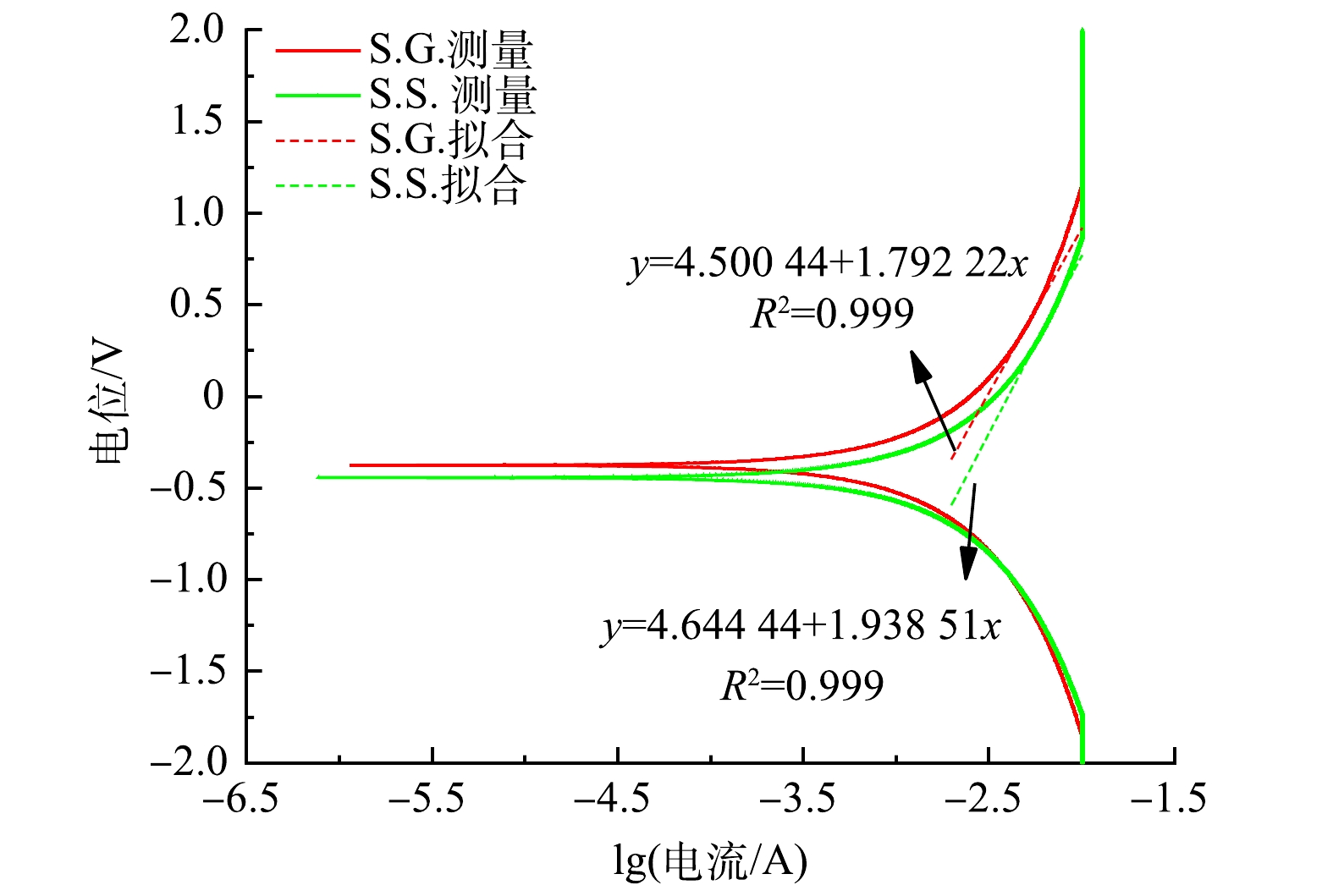
图5显示了S.G.和S.S.的阳极Tafel曲线和在0.3~0.5 V过电位区间的线性拟合曲线。将Tafel曲线外推至0 V过电位,计算出S.G.和S.S.交换电流(i0)分别为3.083×10−3 A和4.019×10−3 A。S.S.阳极的i0比S.G.高30.38%,表明S.S.阳极具有更高的动力学活性,更快的氧化还原速率,更小的活化损失。这与CV测量的电子传递速度较快的结果一致。结合LSV(图4(b))曲线结果可知,S.S.的阳极反应更容易发生,并具有更强的去极化作用。这进一步说明S.S.具有更高的功率密度输出。
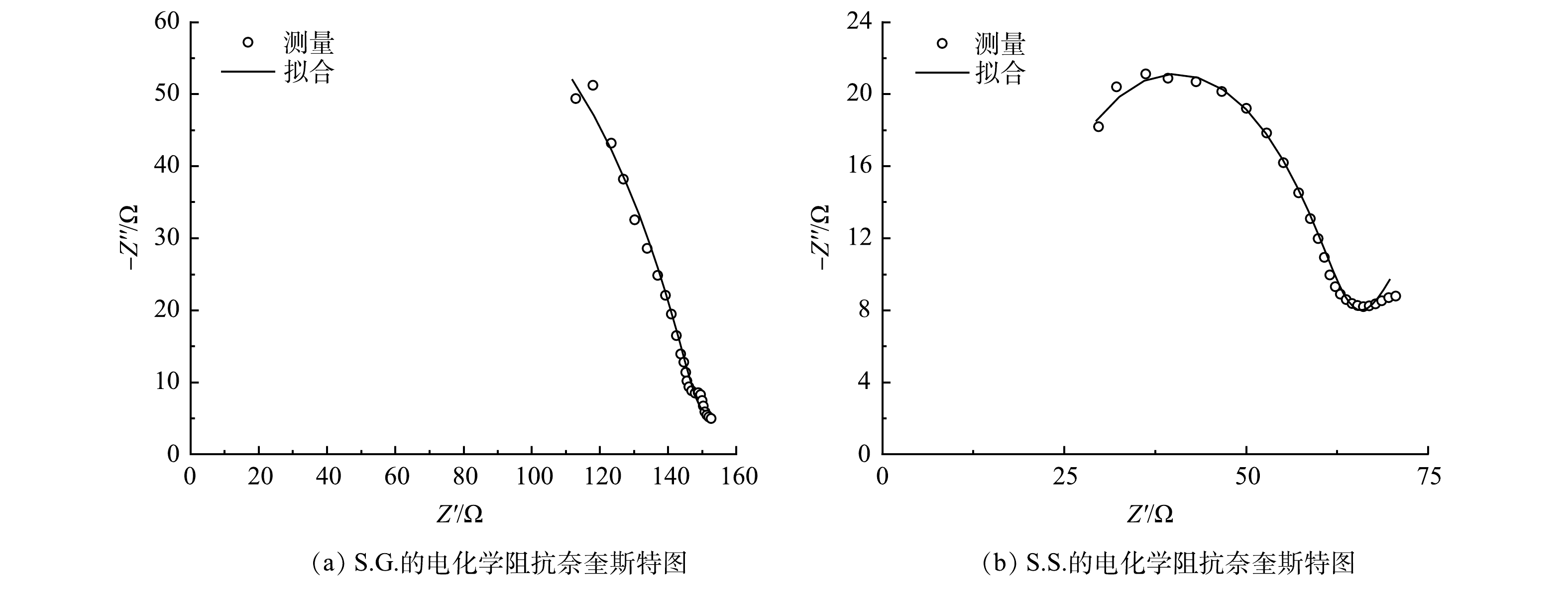
在EIS分析中,奈奎斯特图表明系统的内阻决定了不同基质碳源下系统的性能。其中,高频区域的半圆与阳极生物膜的电性能有关。测量阻抗谱通常是由极化电阻(电荷转移电阻,Rp) [41]、溶液电阻(欧姆电阻,Rs)、韦伯阻抗扩散元件(W)与常相角元件(CPE)组合而成。S.S.和S.G.的Rp分别为45.2 Ω和197 Ω (图6),由于Rp与i0成反比,故此结果进一步表明S.S.阳极生物膜促进了电子从乙酸钠分子向阳极进行转移,提高了乙酸钠的氧化速率 [37],亦说明S.S.有更强的阳极电荷转移能力。之所以没有观察到完整的半圆电弧,是因为仪器的最大频率限制。同时,S.G.拟合的Rs为负值的原因可能是由于在电子学中某些电路和器件的特性,例如在BOINOVICH等的研究中也未显示出Rs的结果 [42]。EIS分析结果还显示,S.S.的韦伯阻抗电阻为3.36×10−4 Ω,小于S.G.(6.35×10−4 Ω),从而使S.S.形成的离子向电极界面的扩散能力更强,并有助于提高电荷传递能力 [43]。葡萄糖分子较乙酸钠分子大,会导致形成的离子半径大,进而在运动时存在的空间位阻就越大,而且其黏度大 [44-45]易造成局部运动能力下降,使得离子扩散受阻,这可能是S.G.内阻较大的原因。S.S.的界面电容达到1.5×10−8 F,远大于S.G.的6.18×10−9 F,这意味着S.S.能够传递更多的电流。S.S.的N值为0.939,比S.G.的0.807更接近于1,说明S.S.离理想电容的偏离度更小。这些结果均与本节所述CV、LSV和TAFEL结果一致。本研究所构建的一体化下流式CW-MFC,无论是以乙酸钠还是葡萄糖作为基质碳源所产生的内阻均远低于YE等[46]的研究结果。同时考虑到S.G.和S.S.两者的设计和运行条件都是相同的,唯独基质碳源不一样,故氧化还原活性的变化还可能与微生物代谢基质碳源有关。综上所述,乙酸钠作为基质碳源有助于在阳极上形成有效地电活性生物膜并促进电子的转移,能够实现高电压及功率密度输出,并有助于水体COD的去除。
2.3 微生物群落结构及功能预测
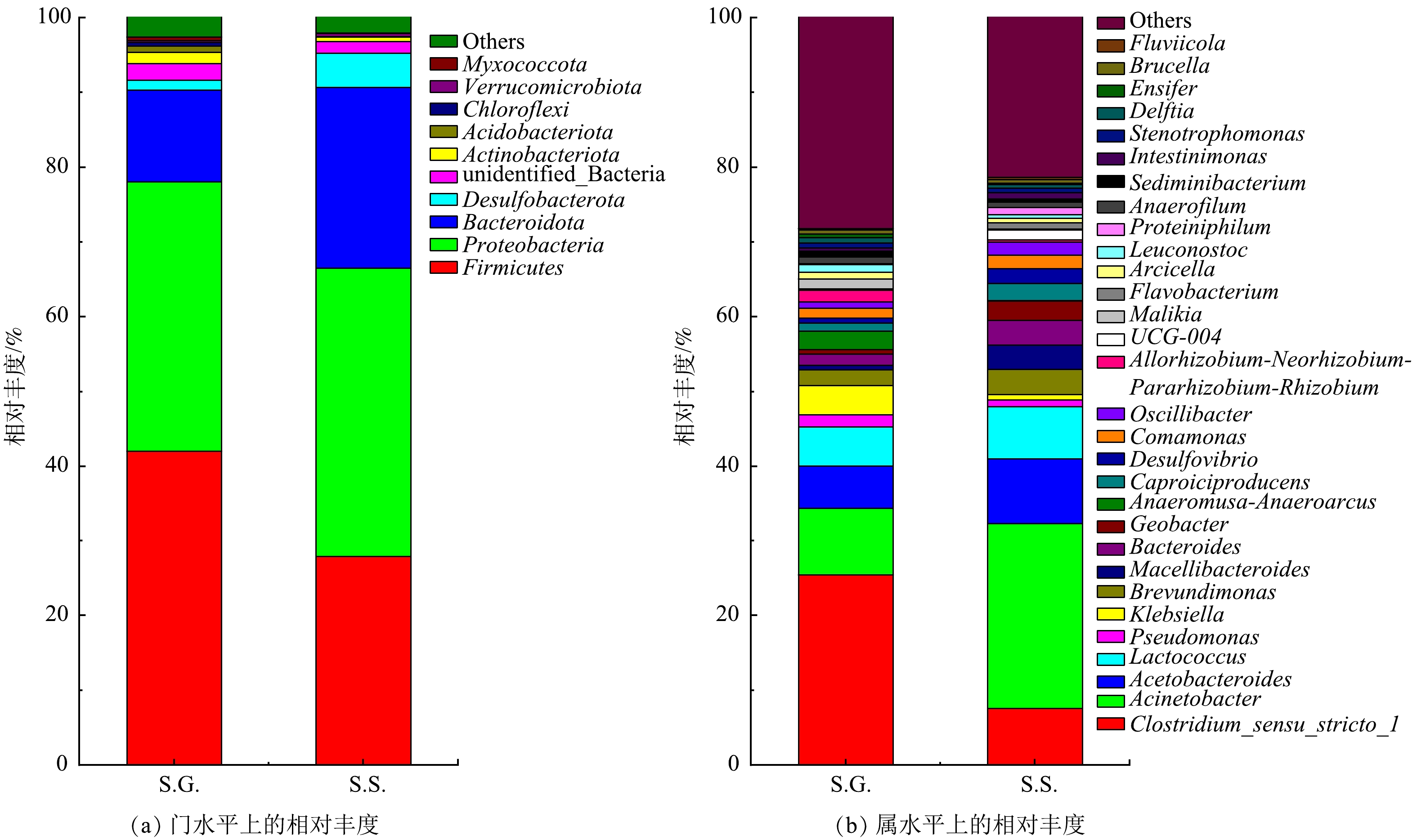
为了证明S.S.系统的发电性能优于S.G.,对S.G.和S.S.阳极中的微生物群落进行了结构分析及FAPROTAX功能预测。S.G.和S.S.的前4种优势门均被定义为MFC系统中主要的活性细菌,属于传统厌氧消化器中的关键门[47-49]。S.G.的前4种门的相对丰度之和为91.6%,其由 Firmicutes (42.0%)、Proteobacteria (36.0%)、Bacteroidota (12.3%)和 Desulfobacterota (1.3%)组成;S.S.的前4种优势门的相对丰度之和为 95.3%,其由 Firmicutes (27.9%)、Proteobacteria (38.6%)、Bacteroidota (24.2%)和 Desulfobacterota (4.6%)组成(图7(a))。
基于ACE和Chao指数的alpha多样性分析结果见表2。由于S.S.具有较高的选择性,故其微生物物种多样性更低。在属水平上(图7(b)),与S.G.相比,在S.S.中观察到 Clostridium_sensu_stricto_1(梭状芽胞杆菌)相对丰度的降低,以及 Acinetobacter(不动杆菌属)、Acetobacteroides(类醋酸杆菌属)和 Lactococcus(乳球菌属)相对丰度的升高。Clostridium_sensu_stricto_1 和 Proteiniphilum 主要是沼液中的细菌,其能将有机质分解成小分子糖和小分子酸,可为产电微生物提供直接的分解基质碳源[50]。Acetobacteroides 由S.G.的5.707%增加至S.S.中的8.671%,该菌是一种产生H2和乙酸的细菌,可以降解细菌代谢的分泌物(细胞外聚合物)和死亡细菌,并能增加和丰富电子供体的数量和种类,有利于其他细菌的生长 [51-52]。众所周知,电活性微生物 Geobacter(地杆菌属)的首选电子供体是醋酸盐 [53],其相对丰度由S.G.的0.621%增加至S.S.的2.584%。Pseudomonas(假单胞菌属)可以通过电子穿梭传递电子。与其他研究 [48]结果相同, Pseudomonas 的相对丰度与 Geobacter相反,该菌由S.G.的1.533% 降低至S.S.的0.884%。Acinetobacter 由S.G.的8.879%大幅增至S.S.的24.718%,这进一步说明 Acinetobacter在发电过程中起着重要作用。SCIARRIA等[54]也得出了类似的结果。Lactococcus 从S.G.的5.259%增至S.S.的6.972%,其可以介导电子向细胞外电子受体的转移,并进行细胞外电子向阳极的转移,同时与其他微生物一起负责更高的功率密度输出[55]。本研究发现了一个有趣的现象,Brevundimonas(短波单胞菌属)由S.G.的2.052%增至S.S.的3.366%,能将硝酸盐还原为亚硝酸盐[56]。这进一步说明电子除供给给阴极以形成高电压外,还可以供给于反硝化作用。总之,S.S.系统中存在着更多的电活性微生物,但这些微生物共存对发电性能的影响还有待进一步的研究。
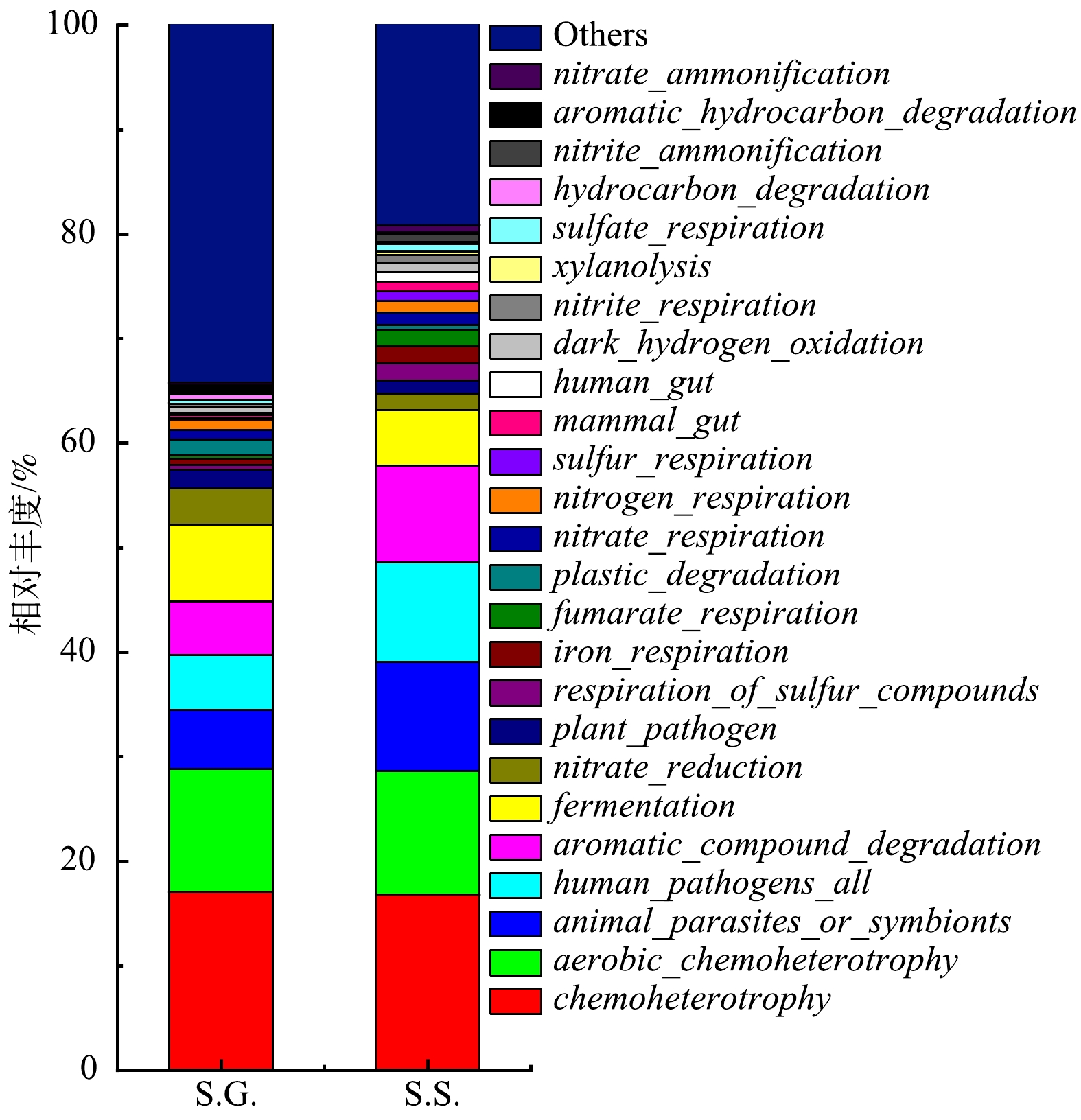
表 2 Alpha指数统计表Table 2. Alpha index statistical table基质碳源 有效OTUs Chao1 Shannon Simpson Ace S.G. 1 136 1 140.042 6.017 0.922 1 150.820 S.S. 729 777.030 5.676 0.943 783.491 根据FAPROTAX功能预测结果(图8)可知,S.G.的 fermentation 的相对丰度比S.S.高2.047%,表明S.G.系统有着更多的细菌(Clostridium_sensu_stricto_1)代谢葡萄糖。乙酸钠有助于细菌生长和繁殖,因此S.S.的呼吸细菌(iron_respiration、nitrogen_respiration、sulfur_respiration 和sulfate_respiration)相对丰度比S.G.高。由于呼吸细菌在电子传递过程中起着重要的作用,故可以氧化电子供体并伴随其他物质的还原,同时为生命活动储存能量。呼吸细菌自身还可以作为电极催化剂,促进电子从基质碳源向电极转移 [57],S.S.的呼吸细菌相对丰度更高进一步表明S.S.能够具有更强的产电性能和电化学性能。S.S.比S.G.有更高的产电性能还可能是由于 aromatic_compound_degradation 和 xylanolysis 细菌能够降解有机物,S.S.中的 aromatic_compound_degradation 和 xylanolysis 分别比在S.G.高出4.1%和0.34%。此外,以牺牲另一有机体(宿主)为代价生长的 animal_parasites_or_symbionts 从S.G.的5.6426%增至S.S.的10.4624%,其变化趋势与 Acetobacteroides 相似。在S.S.中, dark_hydrogen_oxidation 比在S.G.高0.3262%,其属于无机自养细菌可以利用H2作为电子供体并同化CO2,因此,在S.S.中可以进一步产生更多的电子。同时还观察到, nitrite_ammonification 在S.G.和S.S.的相对丰度分别为0.272%和0.709%,nitrate_ammonification 在S.G.和S.S.的相对丰度分别为0.262%和0.698%。这种氨化菌可以以氨化合物为基质进行除氨并产电 [58]。因此,与产电有关的一些细菌的相对丰度在S.S.中高于在S.G.中。
3. 结论
1)乙酸钠相比葡萄糖更适合作为CW-MFC的基质碳源,所设计的下流式未种植植物CW-MFC可产生483 mV输出电压并具有高功率密度输出(48.14 mW·m−2)。
2) S.S.具有更强的去极化能力和电荷转移能力,以及更低的Rp(45.2 Ω),并具有更大的界面电容(1.5×10−8 F)。
3)基于相同的体系在不同的基质碳源下观察到微生物群落结构及FAPROTAX功能预测结果,证实了与发电相关的细菌的相对丰度在S.S.中高于S.G.。
-
表 1 不同化学药剂的硫化物控制成本
Table 1. Cost of s ulfide control by different chemicals
化学药剂 剂量水平 药剂单价 质量分数 控制成本/(10−6澳元·L−1) 参考文献 氧气 15.8~91.5 mg·L−1 1.15澳元·m−3 0.995 12.8~74.0 [33] 硝酸盐 1.33~15.5 mg·L−1 1 010澳元·m−3 0.5 41.3~483.6 [33] 氢氧化钠 11.4~28.6 μL·L−1 3.46澳元·L−1 0.5 39.6~99.1 [33] 氢氧化镁 47.9~156.6 mg·L−1 590澳元·t−1 0.58 48.7~159.3 [33] 铁盐 3~47 mg·L−1 526澳元·t−1 0.42 10.9~170.6 [33] 电化学氧化铁 (40~82) × 10−6 kWh·L−1 0.15澳元·(kWh)−1(电极成本19~37澳元·(kWh)−1) − 25.0~49.3 [42] 高铁酸盐 180 mg·L−1(实验室反应器试验,每隔4.5 d投加一次) 4澳元·kg−1 0.99 20 [43] 表 2 实验室模拟城市排水系统中各种反应器内铁的质量平衡
Table 2. Iron mass balance in the laboratory-scale urban drainage system
-
[1] 中华人民共和国国家统计局. 2020年中国统计年鉴[M]. 北京: 中国统计出版社, 2020. [2] MATILAINEN A, VEPSÄLÄINEN M, SILLANPÄÄ M. Natural organic matter removal by coagulation during drinking water treatment: A review[J]. Advances in Colloid and Interface Science, 2010, 159(2): 189-197. doi: 10.1016/j.cis.2010.06.007 [3] PIKAAR I, SHARMA K R, HU S, et al. Reducing sewer corrosion through integrated urban water management[J]. Science, 2014, 345(6198): 812-814. doi: 10.1126/science.1251418 [4] SHARMA K R, YUAN Z, DE HAAS D, et al. Dynamics and dynamic modelling of H2S production in sewer systems[J]. Water Research, 2008, 42(10/11): 2527-2538. [5] GUISASOLA A, DE HAAS D, KELLER J, et al. Methane formation in sewer systems[J]. Water Research, 2008, 42(6/7): 1421-1430. [6] LIU Y, SHARMA K R, NI B, et al. Effects of nitrate dosing on sulfidogenic and methanogenic activities in sewer sediment[J]. Water Research, 2015, 74: 155-165. doi: 10.1016/j.watres.2015.02.017 [7] AI T, HE Q, XU J, et al. A conceptual method to simultaneously inhibit methane and hydrogen sulfide production in sewers: The carbon metabolic pathway and microbial community shift[J]. Journal of Environmental Management, 2019, 246: 119-127. [8] WIENER M S, SALAS B V, QUINTERO-NÚÑEZ M, et al. Effect of H2S on corrosion in polluted waters: A review[J]. Corrosion Engineering, Science, and Technology, 2006, 41(3): 221-227. doi: 10.1179/174327806X132204 [9] ZHANG L, DE SCHRYVER P, DE GUSSEME B, et al. Chemical and biological technologies for hydrogen sulfide emission control in sewer systems: A review[J]. Water Research, 2008, 42(1/2): 1-12. [10] YOUSEFI A, ALLAHVERDI A, HEJAZI P. Accelerated biodegradation of cured cement paste by Thiobacillus species under simulation condition[J]. International Biodeterioration & Biodegradation, 2014, 86: 317-326. [11] HVITVED-JACOBSEN T, VOLLERTSEN J, NIELSEN A H. Sewer Processes: Microbial and Chemical Process Engineering of Sewer Networks[M]. Los Angeles: CRC Press, 2013. [12] JIANG G, SUN J, SHARMA K R, et al. Corrosion and odor management in sewer systems[J]. Current Opinion in Biotechnology, 2015, 37(4): 33. [13] EPA. Hydrogen Sulfide Corrosion in Wastewater Collection and Treatment Systems[M]. Washington, DC: U. S. Environmental Protection Agency, 1991. [14] FIRER D, FRIEDLER E, LAHAV O. Control of sulfide in sewer systems by dosage of iron salts: Comparison between theoretical and experimental results, and practical implications[J]. Science of the Total Environment, 2008, 392(1): 145-156. doi: 10.1016/j.scitotenv.2007.11.008 [15] 季斌, 秦慧, 陈威, 等. 铁盐应用于污水协同除磷研究进展[J]. 水处理技术, 2018, 44(2): 11-14. [16] LI R, WANG W, LI B, et al. Acidogenic phosphorus recovery from the wastewater sludge of the membrane bioreactor systems with different iron-dosing modes[J]. Bioresource Technology, 2019, 280: 360-370. doi: 10.1016/j.biortech.2019.02.060 [17] 董建威, 何强, 司马卫平. 含盐废水生物/化学除磷模型及除磷剂的强化效果[J]. 中国给水排水, 2014, 30(21): 106-109. [18] CHARLES W, CORD-RUWISCH R, HO G, et al. Solutions to a combined problem of excessive hydrogen sulfide in biogas and struvite scaling[J]. Water Science and Technology, 2006, 53(6): 203-211. doi: 10.2166/wst.2006.198 [19] BABATUNDE A O, ZHAO Y Q. Constructive approaches toward water treatment works sludge management: An international review of beneficial reuses[J]. Critical Reviews in Environmental Science and Technology, 2007, 37(2): 129-164. doi: 10.1080/10643380600776239 [20] SUN J, PIKAAR I, SHARMA K R, et al. Feasibility of sulfide control in sewers by reuse of iron rich drinking water treatment sludge[J]. Water Research, 2015, 71: 150-159. doi: 10.1016/j.watres.2014.12.044 [21] WANG B, SIVRET E C, PARCSI G, et al. Reduced sulfur compounds in the atmosphere of sewer networks in Australia: Geographic (and seasonal) variations[J]. Water Science and Technology, 2014, 69(6): 1167-1173. doi: 10.2166/wst.2013.798 [22] PARK K, LEE H, PHELAN S, et al. Mitigation strategies of hydrogen sulphide emission in sewer networks: A review[J]. International Biodeterioration & Biodegradation, 2014, 95: 251-261. [23] HVITVED-JACOBSEN T, VOLLERTSEN J, MATOS J S. The sewer as a bioreactor: A dry weather approach[J]. Water Science and Technology, 2002, 45(3): 11-24. [24] PADIVAL N A, WEISS J S, ARNOLD R G. Control of Thiobacillus by means of microbial competition: Implications for corrosion of concrete sewers[J]. Water Environment Research, 1995, 67(2): 201-205. doi: 10.2175/106143095X131358 [25] NIELSEN A H, HVITVED-JACOBSEN T, VOLLERTSEN J. Kinetics and stoichiometry of sulfide oxidation by sewer biofilms[J]. Water Research, 2005, 39(17): 4119-4125. doi: 10.1016/j.watres.2005.07.031 [26] SUN J, HU S, SHARMA K R, et al. Stratified microbial structure and activity in sulfide-and methane-producing anaerobic sewer biofilms[J]. Applied and Environmental Microbiology, 2014, 80(22): 7042-7052. doi: 10.1128/AEM.02146-14 [27] BACHE D H, PAPAVASILOPOULOS E N. Viscous behaviour of sludge centrate in response to polymer conditioning[J]. Water Research, 2000, 34(1): 354-358. doi: 10.1016/S0043-1354(99)00143-8 [28] CHURCHILL P, ELMER D. Hydrogen sulfide odor control in wastewater collection systems[J]. Journal of New England Water Environment Association, 1999, 33(1): 57-63. [29] YANG W, VOLLERTSEN J, HVITVED-JACOBSEN T. Anoxic sulfide oxidation in wastewater of sewer networks[J]. Water Science and Technology, 2005, 52(3): 191. doi: 10.2166/wst.2005.0076 [30] GADEKAR S, NEMATI M, HILL G A. Batch and continuous biooxidation of sulphide by Thiomicrospira sp. CVO: Reaction kinetics and stoichiometry[J]. Water Research, 2006, 40(12): 2436-2446. doi: 10.1016/j.watres.2006.04.007 [31] NIELSEN A H, VOLLERTSEN J, HVITVED-JACOBSEN T. Determination of kinetics and stoichiometry of chemical sulfide oxidation in wastewater of sewer networks[J]. Environmental Science & Technology, 2003, 37(17): 3853-3858. [32] GUTIERREZ O, MOHANAKRISHNAN J, SHARMA K R, et al. Evaluation of oxygen injection as a means of controlling sulfide production in a sewer system[J]. Water Research, 2008, 42(17): 4549-4561. doi: 10.1016/j.watres.2008.07.042 [33] GANIGUE R, GUTIERREZ O, ROOTSEY R, et al. Chemical dosing for sulfide control in Australia: An industry survey[J]. Water Research, 2011, 45(19): 6564-6574. doi: 10.1016/j.watres.2011.09.054 [34] GUTIERREZ O, PARK D, SHARMA K R, et al. Effects of long-term pH elevation on the sulfate-reducing and methanogenic activities of anaerobic sewer biofilms[J]. Water Research, 2009, 43(9): 2549-2557. doi: 10.1016/j.watres.2009.03.008 [35] NIELSEN A H, LENS P, VOLLERTSEN J, et al. Sulfide-iron interactions in domestic wastewater from a gravity sewer[J]. Water Research, 2005, 39(12): 2747-2755. doi: 10.1016/j.watres.2005.04.048 [36] NIELSEN A H, HVITVED-JACOBSEN T, VOLLERTSEN J. Effects of pH and iron concentrations on sulfide precipitation in wastewater collection systems[J]. Water Environment Research, 2008, 80(4): 380-384. doi: 10.2175/106143007X221328 [37] ZHANG L, VERSTRAETE W, DE LOURDES MENDOZA M, et al. Decrease of dissolved sulfide in sewage by powdered natural magnetite and hematite[J]. Science of the Total Environment, 2016, 573: 1070-1078. doi: 10.1016/j.scitotenv.2016.08.206 [38] ZHANG L, KELLER J, YUAN Z. Inhibition of sulfate-reducing and methanogenic activities of anaerobic sewer biofilms by ferric iron dosing[J]. Water Research, 2009, 43(17): 4123-4132. doi: 10.1016/j.watres.2009.06.013 [39] ZHANG L, KELLER J, YUAN Z. Ferrous salt demand for sulfide control in rising main sewers: Tests on a laboratory-scale sewer system[J]. Journal of Environmental Engineering, 2010, 136(10): 1180-1187. doi: 10.1061/(ASCE)EE.1943-7870.0000258 [40] ZHANG L, DERLON N, KELLER J, et al. Dynamic response of sulfate-reducing and methanogenic activities of anaerobic sewer biofilms to ferric dosing[J]. Journal of Environmental Engineering, 2012, 138(4): 510-517. doi: 10.1061/(ASCE)EE.1943-7870.0000481 [41] LIN H, KUSTERMANS C, VAIOPOULOU E, et al. Electrochemical oxidation of iron and alkalinity generation for efficient sulfide control in sewers[J]. Water Research, 2017, 118: 114-120. doi: 10.1016/j.watres.2017.02.069 [42] PIKAAR I, FLUGEN M, LIN H, et al. Full-scale investigation of in-situ iron and alkalinity generation for efficient sulfide control[J]. Water Research, 2019, 167: 115032. doi: 10.1016/j.watres.2019.115032 [43] YAN X, SUN J, KENJIAHAN A, et al. Rapid and strong biocidal effect of ferrate on sulfidogenic and methanogenic sewer biofilms[J]. Water Research, 2020, 169: 115208. doi: 10.1016/j.watres.2019.115208 [44] KULANDAIVELU J, CHOI P M, SHRESTHA S, et al. Assessing the removal of organic micropollutants from wastewater by discharging drinking water sludge to sewers[J]. Water Research, 2020, 181: 115945. doi: 10.1016/j.watres.2020.115945 [45] KULANDAIVELU J, GAO J, SONG Y, et al. Removal of pharmaceuticals and illicit drugs from wastewater due to ferric dosing in sewers[J]. Environmental Science & Technology, 2019, 53(11): 6245-6254. [46] GUTIERREZ O, PARK D, SHARMA K R, et al. Iron salts dosage for sulfide control in sewers induces chemical phosphorus removal during wastewater treatment[J]. Water Research, 2010, 44(11): 3467-3475. doi: 10.1016/j.watres.2010.03.023 [47] GE H, ZHANG L, BATSTONE D J, et al. Impact of iron salt dosage to sewers on downstream anaerobic sludge digesters: Sulfide control and methane production[J]. Journal of Environmental Engineering, 2013, 139(4): 594-601. doi: 10.1061/(ASCE)EE.1943-7870.0000650 [48] SCHIPPERS A, JRGENSEN B B. Biogeochemistry of pyrite and iron sulfide oxidation in marine sediments[J]. Geochimica Et Cosmochimica Acta, 2002, 66(1): 85-92. doi: 10.1016/S0016-7037(01)00745-1 [49] LUEDECKE C, HERMANOWICZ S W, JENKINS D. Precipitation of ferric phosphate in activated sludge: A chemical model and its verification[J]. Water Pollution Research & Control Brighton, 1988, 21(4/5): 325-337. [50] REBOSURA M, SALEHIN S, PIKAAR I, et al. A comprehensive laboratory assessment of the effects of sewer-dosed iron salts on wastewater treatment processes[J]. Water Research, 2018, 146: 109-117. doi: 10.1016/j.watres.2018.09.021 [51] REBOSURA M, SALEHIN S, PIKAAR I, et al. Effects of in-sewer dosing of iron-rich drinking water sludge on wastewater collection and treatment systems[J]. Water Research, 2020, 171: 115396. doi: 10.1016/j.watres.2019.115396 [52] SALEHIN S, KULANDAIVELU J, REBOSURA M, et al. Opportunities for reducing coagulants usage in urban water management: The oxley creek sewage collection and treatment system as an example[J]. Water Research, 2019, 165: 114996. doi: 10.1016/j.watres.2019.114996 [53] KULANDAIVELU J, SHRESTHA S, KHAN W, et al. Full-scale investigation of ferrous dosing in sewers and a wastewater treatment plant for multiple benefits[J]. Chemosphere, 2020, 250: 126221. doi: 10.1016/j.chemosphere.2020.126221 [54] WILFERT P, MANDALIDIS A, DUGULAN A I, et al. Vivianite as an important iron phosphate precipitate in sewage treatment plants[J]. Water Research, 2016, 104: 449-460. doi: 10.1016/j.watres.2016.08.032 [55] WILFERT P, DUGULAN A I, GOUBITZ K, et al. Vivianite as the main phosphate mineral in digested sewage sludge and its role for phosphate recovery[J]. Water Research, 2018, 144: 312-321. doi: 10.1016/j.watres.2018.07.020 [56] SALEHIN S, REBOSURA M, KELLER J, et al. Recovery of in-sewer dosed iron from digested sludge at downstream treatment plants and its reuse potential[J]. Water Research, 2020, 174: 115627. doi: 10.1016/j.watres.2020.115627 [57] CHEN Y, CHENG J J, CREAMER K S. Inhibition of anaerobic digestion process: A review[J]. Bioresource Technology, 2008, 99(10): 4044-4064. doi: 10.1016/j.biortech.2007.01.057 [58] WU Y, LUO J, ZHANG Q, et al. Potentials and challenges of phosphorus recovery as vivianite from wastewater: A review[J]. Chemosphere, 2019, 226: 246-258. doi: 10.1016/j.chemosphere.2019.03.138 [59] FREDERICHS T, VON DOBENECK T, BLEIL U, et al. Towards the identification of siderite, rhodochrosite, and vivianite in sediments by their low-temperature magnetic properties[J]. Physics and Chemistry of the Earth, Parts A/B/C, 2003, 28(16/17/18/19): 669-679. [60] REBOSURA M, SALEHIN S, PIKAAR I, et al. The impact of primary sedimentation on the use of iron-rich drinking water sludge on the urban wastewater system[J]. Journal of Hazardous Materials, 2021, 402(2): 124051. [61] GANIGUÉ R, JIANG G, LIU Y, et al. Improved sulfide mitigation in sewers through on-line control of ferrous salt dosing[J]. Water Research, 2018, 135: 302-310. doi: 10.1016/j.watres.2018.02.022 -




 下载:
下载: