-
药用活性化合物(PhACs)包括抗生素、消炎止痛药、β-阻滞剂、抗菌药和雌激素类,广泛用于疾病治疗,常通过固体废物和废水进入环境中,成为一类新兴环境污染物[1]。卡马西平(carbamazepine,CBZ)是一种常见药用活性化合物,主要用于治疗癫痫、精神运动性发作和三叉神经痛等疾病[2]。据报道全球CBZ年均消耗量高达1014t[3],但人体对CBZ的吸收只有78%[4],剩余部分则直接排出体外,从而导致自然环境中存在CBZ药物残留,在污水处理厂出水、地表水、地下水、土壤和污泥中广泛检出[5]。当前传统水处理工艺很难去除CBZ[6],使得CBZ可能对生态系统和人类健康构成潜在威胁,因此需要一种有效去除水中CBZ的方法。
已有研究者使用O3[7]、UV/TiO2[8]、UV/H2O2[9]和UV/H2O2/Fe2+[10]等高级氧化工艺(AOPs)进行卡马西平的控制与去除。UV/氯是一种新型的高级氧化工艺,它通过紫外线激发游离氯(HClO/ClOˉ)产生高氧化能力的羟基自由基·OH(2.8 V)和氯自由基·Cl(2.4 V)[11],能有效去除水中有机污染物和病原微生物,应用前景广阔。研究表明,UV/氯工艺对布洛芬、双氯芬酸和莠去津等有良好的去除效果[12-13]。然而UV/氯降解过程中可能会生成含氯副产物[14],表现出比母物质更强的潜在毒性。因此UV/氯工艺降解副产物的潜在危害亟待引起重视。
本研究以CBZ为目标污染物,考察UV光强、余氯初始浓度、溶液初始pH值和氨氮浓度等因素对UV/氯工艺降解效果的影响。利用高分辨率质谱HRMS Orbitrap(Q-E Plus)对CBZ降解中间产物进行鉴定,探讨CBZ降解机制,提出降解路径,评估降解过程中的毒性变化,为水中PhACs新兴污染物的去除与控制、水质安全保障提供理论依据和技术支撑。
-
CBZ(纯度>98%)、硝基苯和次氯酸钠NaClO(含14%活性氯)购自于阿拉丁试剂(上海)。衍生剂N,O-双(三甲基硅)三氟乙酰胺(含三甲基氯硅烷)(98% BSTFA+1% TMCS)购自于阿拉丁试剂(上海)。甲醇(HPLC级)购自于Sigma公司(美国)。二氯甲烷(CH2Cl2)购自于永华化学(江苏)。其他分析纯试剂(NH4)2SO4、NaOH、HCl、Na2HPO4·12H2O、Na2HPO4、Na2SO3和NaCl购自于上海国药集团,试剂用水均采用超纯水(电导率18.3 MΩ·cm)。
-
UV/氯降解反应在50 mL玻璃表面皿反应器中进行,表面皿上面悬挂波长254 nm UV汞灯(上海飞利浦),通过调节灯管与反应液表面的距离来改变UV强度,使用磁力搅拌器(HJ-6,江苏金怡)确保反应均匀,使用紫外辐照计(UV-B,北师大光电仪器厂)测定紫外光强。
-
取50 mL一定浓度CBZ溶液于玻璃反应器中,用0.2 mol·L−1磷酸盐缓冲液、氢氧化钠和稀盐酸调节溶液初始pH值。然后投加一定浓度的NaClO溶液,打开UV汞灯,开始降解反应。在不同反应时间取样,立即投加过量Na2SO3溶液(40 mmol·L−1)终止反应,用HPLC-MS/MS测定剩余CBZ浓度。用DPD分光光度法[15]测定游离余氯含量。考察单一UV、单一氯和UV/氯工艺对CBZ的降解效果的影响,探究不同因素(UV光强、余氯初始浓度、溶液初始pH值和氨氮浓度)对CBZ降解的影响。用准一级反应动力学方程拟合实验数据,见式(1)和(2)。半衰期计算见式(3)。所有反应均进行3次平行实验,取平均值。
式中,[CBZ]t表示t时刻反应体系中CBZ浓度,mmol·L−1;[CBZ]0表示CBZ初始浓度,mmol·L−1;
$k_{{\text{app}}}'$ 为准一级表观动力学速率常数,min−1。式中,C0为反应物初始浓度;k表示反应速率常数;b为截距。
-
使用HPLC-MS/MS(Thermo TSQ Quantum Access Max)测定剩余CBZ浓度,色谱条件:Thermo Accucore C18色谱柱(3.0 mm×50.0 mm,2.6 μm);采用梯度洗脱,流动相为水和甲醇,流速1.0 mL·min−1;柱温25 ℃。质谱条件:SRM扫描,正离子模式,CBZ子母离子对为237.0/193.9,碎裂电压41 V。
CBZ降解中间产物的反应液制备方法:按照1.3节的方法进行CBZ降解反应,分别在1、5、20、120 min取样,对应降解初期、中期和末期等反应阶段,降解液混合后进行浓缩和脱盐处理,同时取0 min样品作为空白对照。CBZ降解反应溶液均使用超纯水配置。
使用HRMS Orbitrap(Q-E Plus)鉴定降解中间产物,进样前需对反应液进行预处理。根据美国EPA 1694的标准预处理方法[16],通过全自动固相萃取仪SPE432(北京普立泰科)对CBZ反应液进行萃取、浓缩和脱盐。色谱条件为:Waters HSS T3色谱柱(2.1 mm×50.0 mm,1.7 μm);流动相为甲醇和水。正离子扫描。离子源参数设定为:鞘气流速12 mL·min−1,毛细管温度320 ℃,喷雾电压4 kV。其他MS条件:扫描范围为m/z 70—600,动态排除5 s,HCD碰撞能量值(CE%)35.0,质量分辨率为70000。使用Xcalibur 4.1软件进行数据分析。
使用GC-MS(Thermo ISQ)鉴别CBZ小分子降解产物。样品前预处理方法为:将反应液通过旋转蒸发器(XD-5000ADQ上海贤德)蒸干,加入BSTFA/TMCS硅烷化试剂进行硅烷化反应。GC-MS分析条件为:Thermo TG-624色谱柱(30.0 m×0.25 mm×1.4 μm),载气流量1.0 mL·min−1,进样口温度280.0 ℃,无分流进样。全扫模式,范围为m/z 50—500。升温程序设置如下:在50 ℃保持3 min,以20.0 ℃·min−1的速率上升到150.0 ℃,以10.0 ℃·min−1的速率上升到280.0 ℃并保持3 min。
-
选用费氏弧菌Vibrio fischeri作指示细菌,考察CBZ降解反应液的毒性变化。菌种冻干粉由北京金达清创提供。采用生物毒性分析仪(ATD-P1,北京金达清创)测定发光细菌与CBZ降解液接触前后的发光强度,利用样品对发光细菌的相对抑制率评估急性毒性,每个样品测定3组平行数据,以NaCl(2%)溶液为空白对照,前后各设置两组。由公式(4)计算发光细菌发光强度的相对抑制率(I):
式中,I为相对抑制率;Lt为样品发光强度;L0为阴性对照发光强度。
-
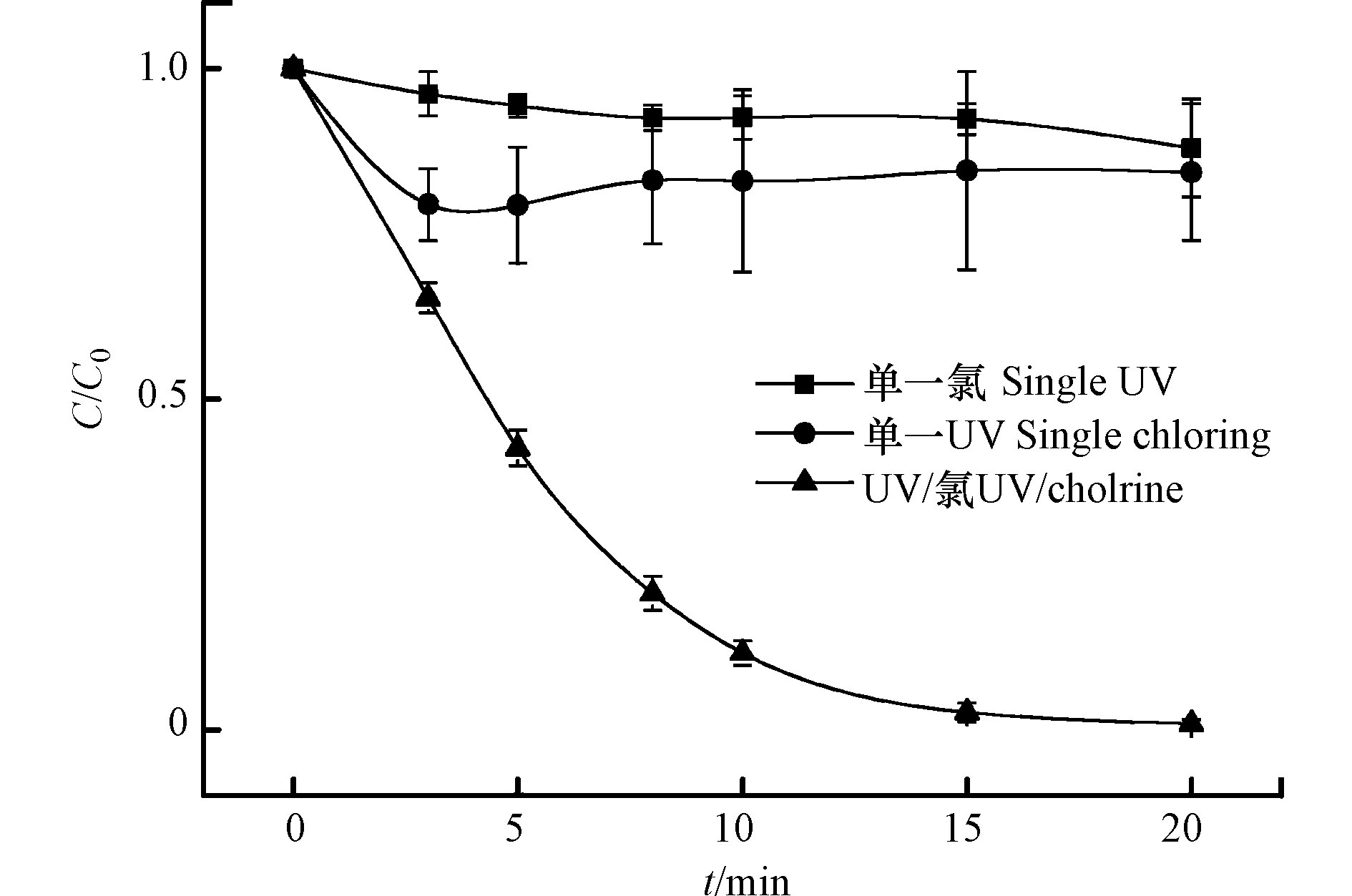
在光强1000 μW·cm−2,CBZ初始浓度0.017 mmol·L−1,余氯初始浓度0.340 mmol L−1和溶液初始pH值为7的条件下,考察单一UV、单一氯和UV/氯组合工艺条件对CBZ降解的影响。结果见图1和表1。
由表1和图1可知,单一氯条件下对CBZ几乎没有降解效果,20 min内降解率仅为12.0%。这主要因为HClO的氧化电位仅为1.61 V,氧化能力较低,不足以破坏CBZ的化学键而使其得到降解,这与Wang等[17]观察结果一致。而单一UV也不能有效地降解CBZ,20 min内仅有16.0%的CBZ被降解,这是因为CBZ结构式中存在酰胺键,对紫外光具有较强的抵抗性[18]。相比之下,CBZ在UV/氯工艺中迅速降解,反应20 min降解率可达99.0%,准一级反应速率常数为0.2601 min−1。由此可知UV/氯工艺的降解效果远远大于单一UV和单一氯。
由式(5)—(7)可知,NaClO在水溶液中以HClO和ClOˉ两种形式存在。在UV照射下,HClO和ClOˉ光解产生·OH和·Cl等具有强氧化能力的自由基,氧化电位分别为2.8 V和2.4 V[19],从而加速了CBZ降解过程。
-
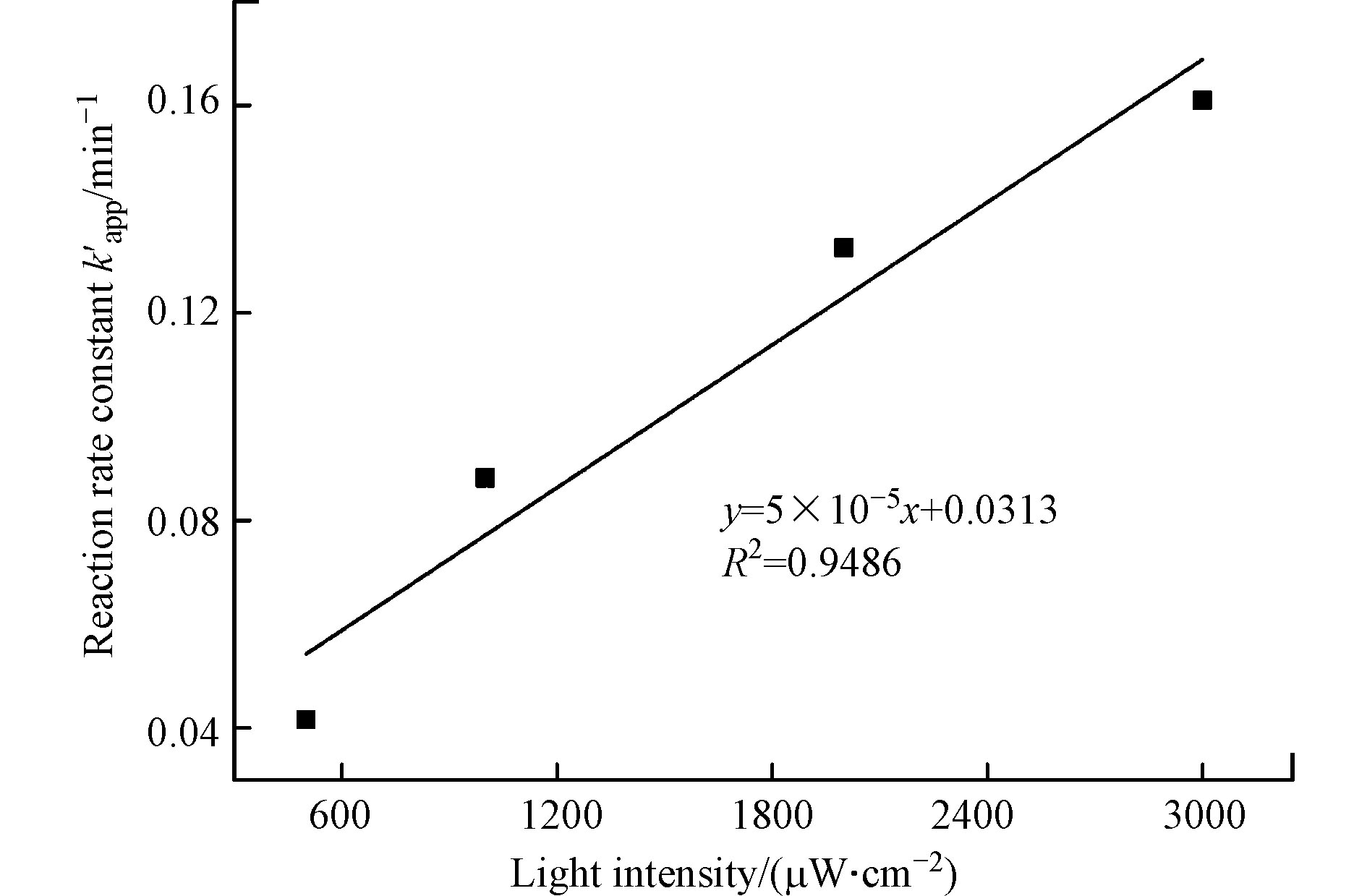
考察CBZ初始浓度0.017 mmol·L−1,余氯初始浓度0.170 mmol·L−1,溶液初始pH值为7的条件下,不同光强对CBZ降解的影响,结果见图2和表2。
图2和表2结果表明,提高UV光强能显著加速CBZ降解过程。当UV光强为500 μW·cm−2时,20 min内CBZ的降解率为52.0%;而当UV光强增大到3000 μW·cm−2时,降解率达到95.0%;且当光强从500 μW·cm−2增加到3000 μW·cm−2时,反应速率常数由0.0416 min−1升高为0.1609 min−1,半衰期也由19.9 min下降到3.4 min。从图3可以发现,反应速率常数和光强具有明显的线性相关性(
$k_{{\text{app}}}'$ =5×10−5,R2=0.9486)。这主要是因为UV光强在自由基形成过程中起着重要作用。UV光强增大提高了单位体积内的光子流量,使得单位时间内产生的自由基数量增多[20]。因此在余氯初始浓度一定的条件下,单位时间内光强越大,产生的活性自由基越多,从而加快CBZ的降解速率。 -
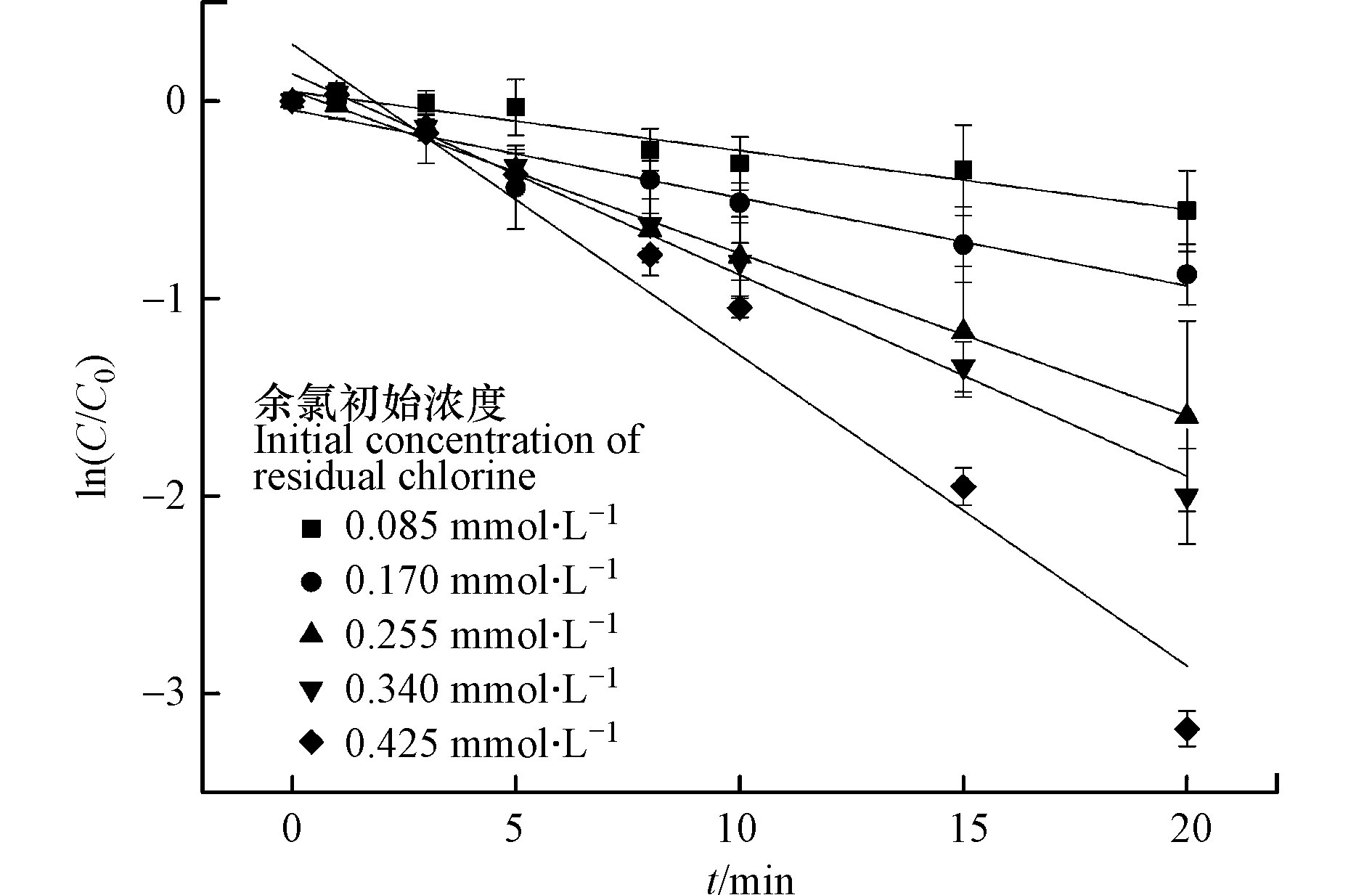
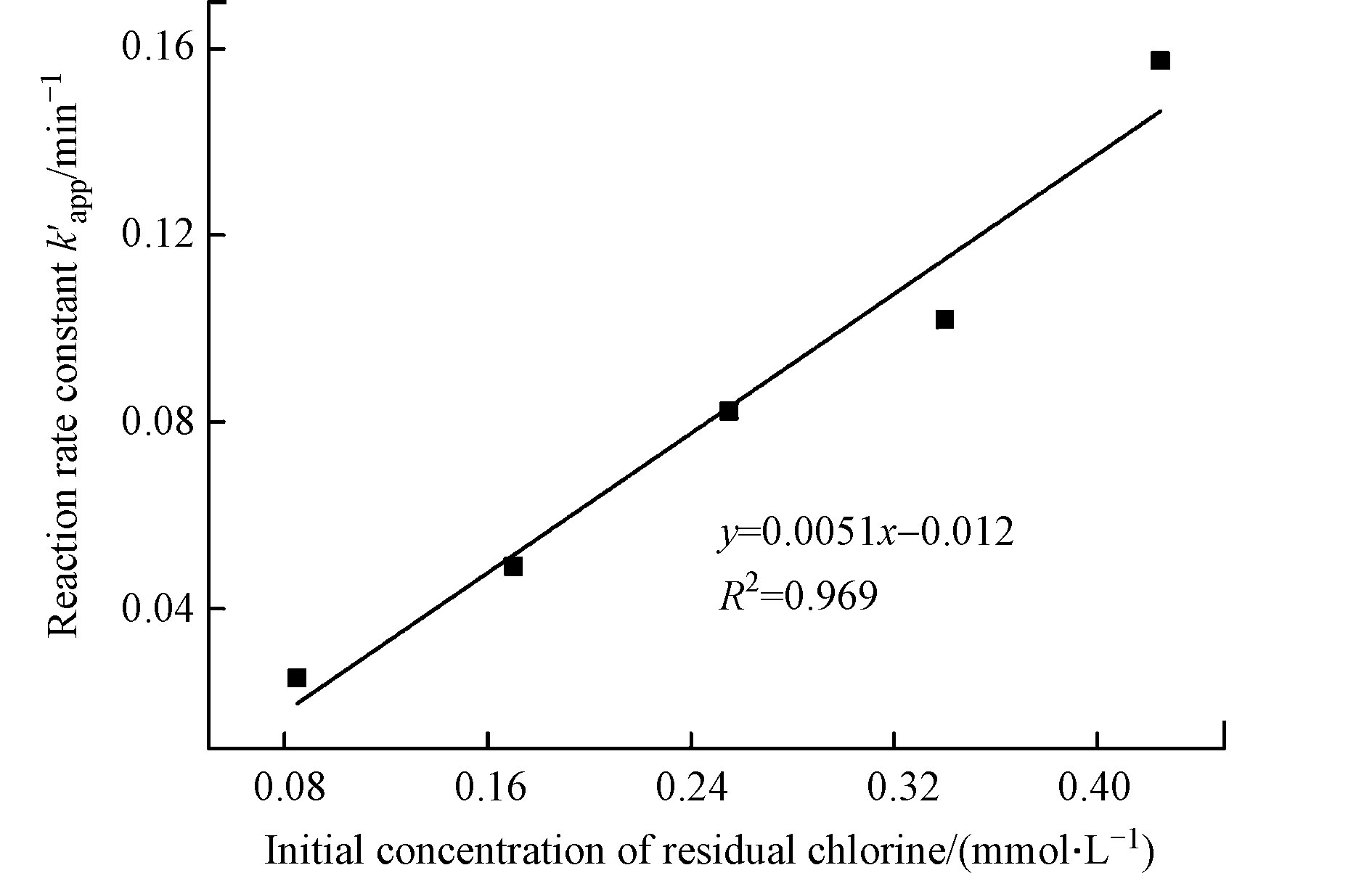
改变余氯初始浓度,考察其对CBZ降解的影响,其他反应条件包括:CBZ初始浓度0.017 mmol·L−1,光强500 μW·cm−2,溶液初始pH值7,结果见图4和表3。
从图4和表3可知,余氯初始浓度增加,CBZ降解反应速率常数逐渐增大。当余氯初始浓度从0.085 mmol·L−1提高到0.425 mmol·L−1时,反应速率常数由0.0252 min−1增加到0.1574 min−1,半衰期也由29.0 min减小到6.2 min。余氯初始浓度和反应速率常数
$k_{{\text{app}}}'$ 的拟合相关系数为0.969,呈现明显的线性关系(图5),这在Wang等[17]和Zhou等[21]的研究中亦有相似发现。说明余氯初始浓度增加对CBZ降解呈显著促进作用,主要原因是增加余氯初始浓度可以产生更多有效的·OH和·Cl自由基来攻击卡马西平分子,从而加速CBZ的降解速率。 -
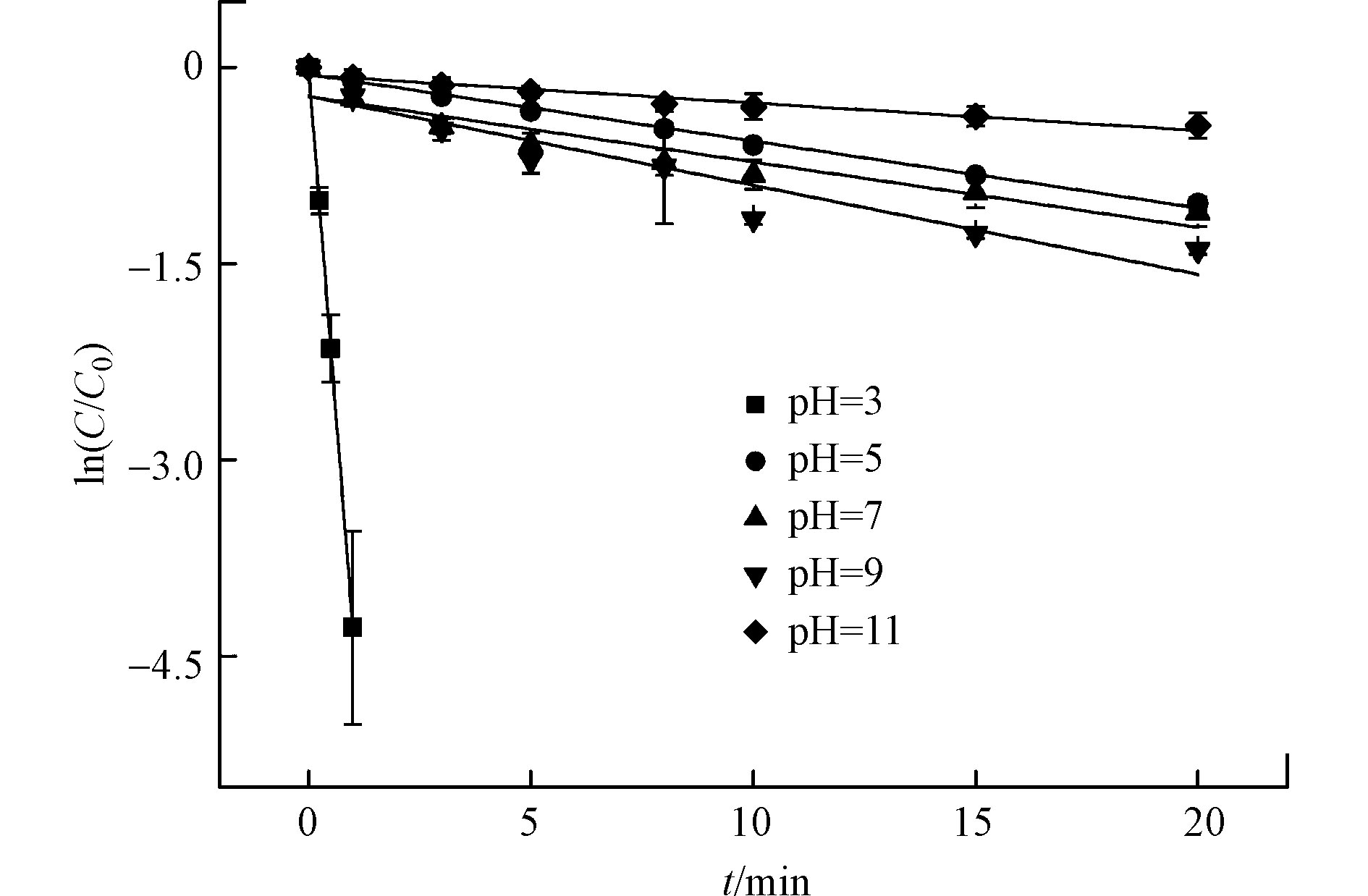
在光强500 μW·cm−2,CBZ初始浓度0.017 mmol·L−1,余氯初始浓度0.255 mmol·L−1的条件下,用氢氧化钠和盐酸调节pH值,考察不同溶液初始pH值对反应的影响。结果见图6和表4。
由图6和表4可知,溶液不同初始pH值对CBZ降解有明显影响。强酸条件下的降解效果远远大于碱性和弱酸条件。当溶液pH值从11.0减小到3.0时,反应速率常数由0.0210 min−1升高到4.2991 min−1,此时降解率可达98.0%。
HClO是一种弱酸,因此pH值对酸(HClO)及其共轭碱(ClOˉ)的分布有显著影响。根据HClO的pka(式(8)),当pH<7.5时,HClO是主要的活性氯成分;当pH>7.5时,ClOˉ是主要的活性氯成分[22]。溶液偏碱性时ClOˉ比例更高,由于ClOˉ被认为是一种自由基捕获剂,会消耗水中活性自由基,从而抑制反应过程,见式(9)。在酸性条件下HClO比例高,从而在紫外激发下产生更多·OH和·Cl自由基,加速反应过程。此外CBZ分子中含有氨基,在酸性条件下以离子态为主。研究表明与分子态物质相比,离子态物质更容易被降解[23]。因此UV/氯工艺中酸性条件比碱性条件更利于CBZ的降解。
-
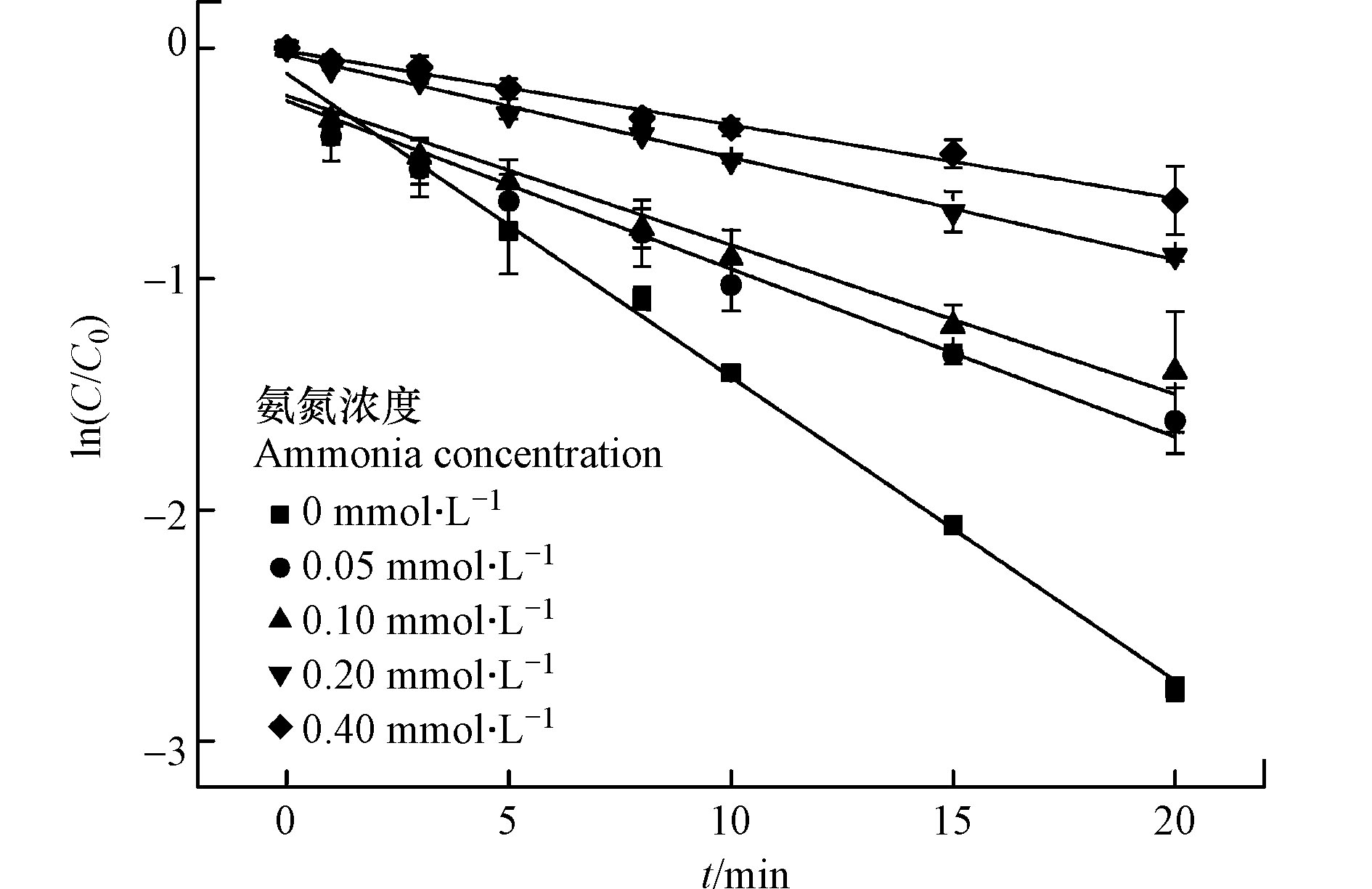
在长三角等较为发达的区域,氨氮经常是水环境和饮用水源地水质超标的指标之一。检测发现黄浦江支流水源氨氮指标合格率仅有53%(氨氮限值1.0 mg·L−1)[24-25]。此外水处理过程中氨氮会与氯消毒剂反应从而影响消毒效率。因此考察氨氮浓度对UV/氯工艺降解CBZ的影响十分必要。考察光强500 μW·cm−2,CBZ初始浓度0.017 mmol·L−1,余氯初始浓度0.340 mmol·L−1,溶液初始pH值为7时,氨氮浓度对降解的影响。结果见图7和表5。
由图7和表5可知,随着氨氮浓度增大,CBZ降解反应速率常数逐渐降低。当氨氮浓度从0升高到0.4 mmol·L−1,反应速率常数从0.1315 min−1减少至0.0319 min−1,CBZ的降解率从94.0%下降至47.7%。这说明氨氮对CBZ的降解有明显抑制作用,主要原因是氨氮会和HClO快速反应生成氯胺[26],降低了HClO的浓度,进而减少活性自由基的产生[14]。
-
UV/氯降解工艺过程中存在多种活性自由基,其中以·OH和·Cl为主。作为·OH捕获剂,硝基苯选择性地和·OH反应,而不会和·Cl发生反应。以硝基苯为自由基探针,进行UV/氯和UV/H2O2工艺降解硝基苯和CBZ的动力学实验,来定量分析不同活性自由基的相对贡献。基于自由基稳态模型,·OH和·Cl的相对贡献可以由式(10)—(12)确定[27-28]。
式中,
$ k'{_{{\rm{NB}}}} $ 和$ k'{_{{\rm{CBZ}}}} $ 分别代表UV/氯工艺中硝基苯和CBZ的准一级反应速率常数(图8a);[·OH]ss代表·OH的稳态浓度;$ k''{_{ \cdot {\rm{OH}} - {\rm{NB}}}} $ 为硝基苯和·OH的二级反应速率常数,为3.90×109(mol·L−1)−1s−1。$ k''{_{ \cdot {\rm{OH}} - {\rm{CBZ}}}} $ 为CBZ与·OH的二级反应速率常数,为5.0×109(mol·L−1)−1s−1(图8b);$ k'{_{{\rm{chlorine}}}}_{ - {\rm{CBZ}}} $ 、$ k'{_{{\rm{UV}} - {\rm{CBZ}}}} $ 和$ k'{_{{\rm{other}}\; {\rm{radicals}} - {\rm{CBZ}}}} $ 分别为单一氯、单一UV和其他活性自由基降解CBZ的准一级反应速率常数,通常认为是次要降解因素而忽略不计[29]。UV/氯工艺降解硝基苯和CBZ的反应动力学结果见图8a,UV/H2O2工艺降解硝基苯和CBZ的反应动力学结果见图8b。基于降解反应结果,结合动力学公式(10)—(12),最终得到硝基苯和CBZ在UV/氯中的准一级反应动力学常数分别为0.0598和0.1208。因此·Cl和·OH对CBZ降解的相对贡献分别为31.6%和68.4%。这表明,·OH对CBZ降解的贡献远远大于·Cl,·OH在UV/氯工艺去除CBZ中起到主要作用。 -
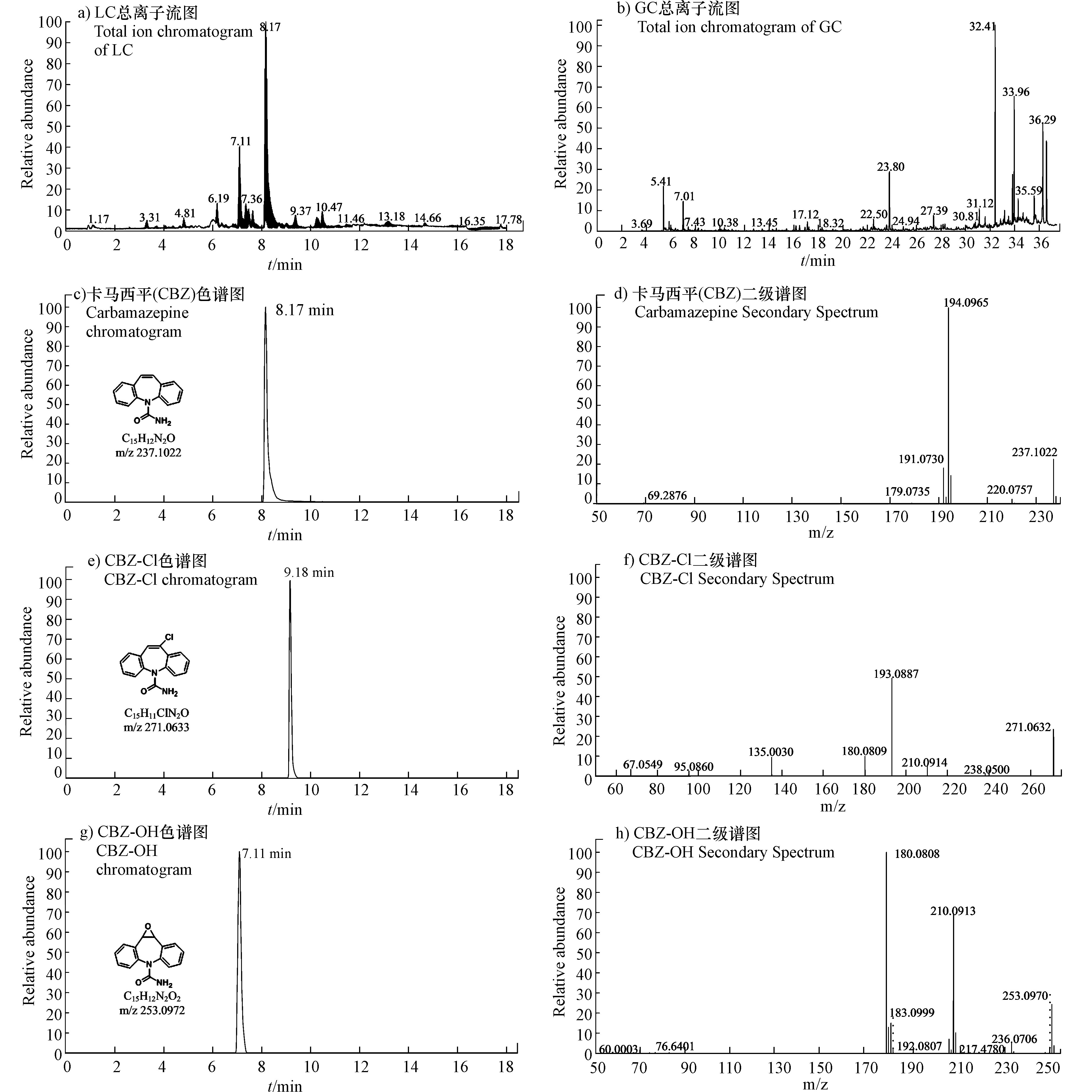
在CBZ降解过程中总共鉴定出10种中间产物,其中高分辨液质Orbitrap解析出5种产物(表6),GC-MS鉴定出5种小分子酸产物(表7)。CBZ及典型中间产物的总离子流图、色谱图和质谱图见图9。从图9a、图9c和图9d可以看出,CBZ出峰时间为8.17 min,分子离子质荷比为m/z 237.1022,有1个主要碎片离子m/z 194.0965。
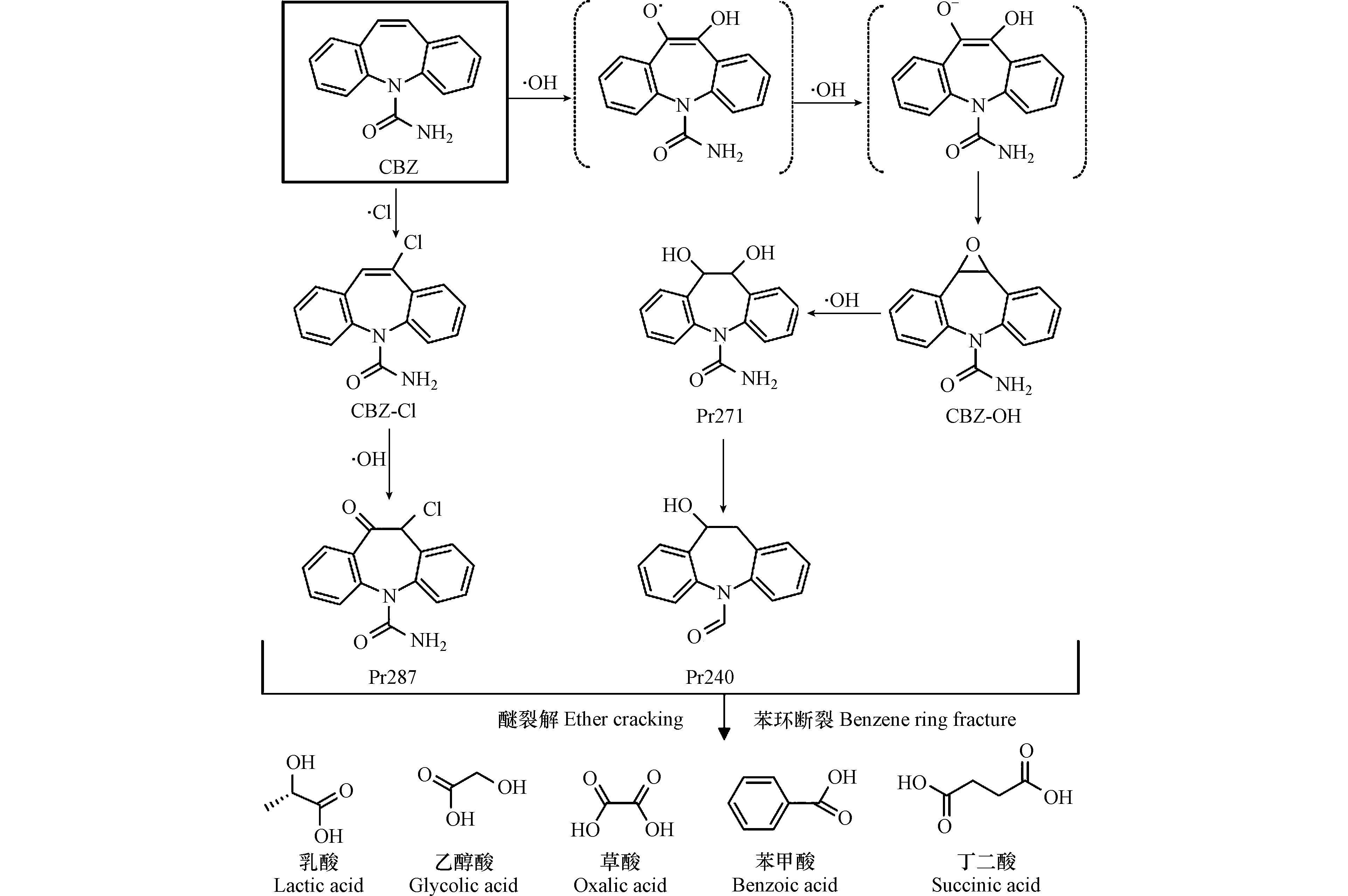
UV/氯反应体系受到大量产生的·Cl和·OH自由基所驱动,推动CBZ降解过程。CBZ在·Cl自由基攻击下发生氯取代反应,生成氯代产物CBZ-Cl(m/z 271.0633,图9e和图9f),出峰时间为9.18 min,二级质谱中有1个主要碎片m/z 193.0887,氯取代反应主要发生卡马西平的杂环结构上,氯代产物CBZ-Cl是主要的降解中间产物,该物质在前人研究中亦多次被检出[30]。
溶液中存在的大量·OH自由基也会参与CBZ降解过程。CBZ杂环分子结构中的双键具有较高的反应活性[30],·OH攻击杂环双建生成产物CBZ-OH(m/z 253.0972,见图9g和图9h)。随后通过产生杂环自由基和电子转移反应,形成含有两个羟基的产物Pr271(m/z 271.1077),并且在过量·OH持续攻击下进一步脱除(-HNO)生成Pr240(m/z 240.1019)。此外在·OH和·Cl的共同作用下,产物CBZ-Cl通过羟基化反应生成Pr287(m/z 287.0582)。在降解末期CBZ分子结构进一步醚键和苯环碎裂生成小分子有机酸,包括乳酸、乙醇酸、草酸、苯甲酸和丁二酸等(表7)。在UV/氯降解CBZ过程中主要发生羟基化、氯取代和电子转移等反应,基于以上分析最终提出CBZ降解反应路径(图10)。
-
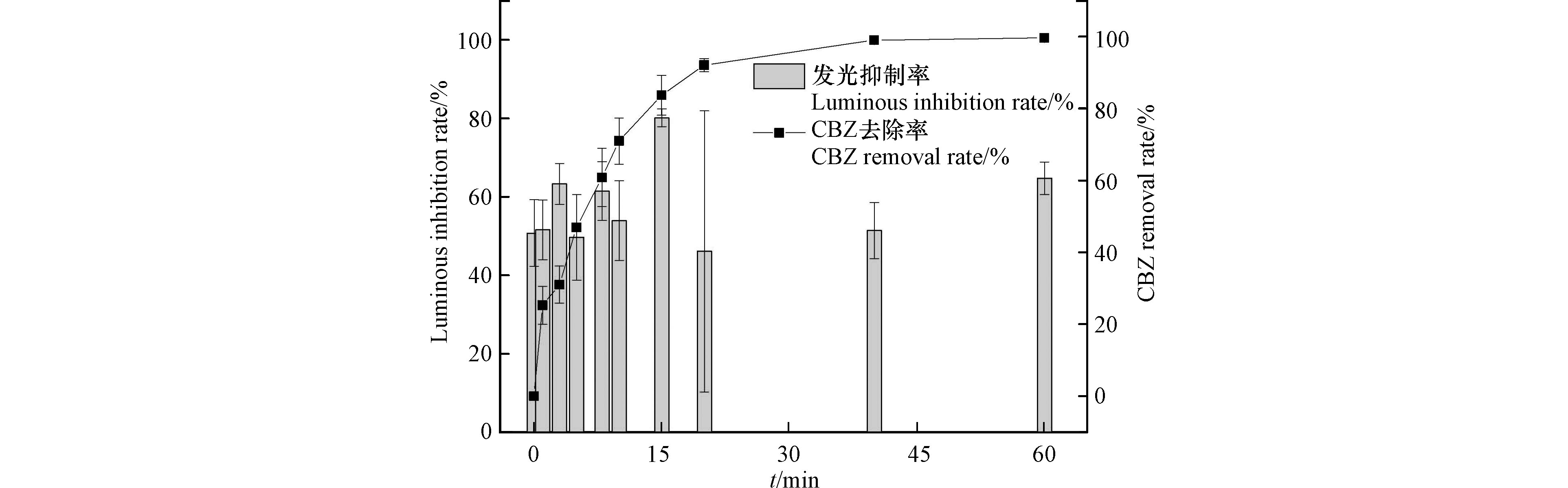
通过费氏弧菌发光抑制率(%)来评估反应过程中溶液的急性毒性变化,结果见图11。降解反应条件与2.1节反应条件相同。CBZ空白溶液对发光细菌的相对抑制率为50.7%。随着降解反应发光抑制率反而增大。降解15 min时CBZ去除率为83.6%,但发光抑制率升高到80.1%;降解20 min时CBZ已基本去除(去除率91.8%),反应液发光抑制率降低为46.1%;延长反应时间至60 min,发光抑制率逐渐升高,达到64.8%。在降解过程中CBZ溶液的发光抑制率没有随CBZ去除同步下降,反而逐步升高,这表明CBZ在降解过程中可能产生了毒性高于母物质的中间产物,CBZ的去除并不代表生态风险的降低。
使用ECOSAR(美国EPA)软件对CBZ及其中间产物的水生生物(鱼类、水蚤和绿藻)急性毒性进行预测[31],结果见表8。除CBZ-OH和Pr287外,所有其它产物的LC50(鱼96 h)值均低于CBZ,其中产物Pr271的LC50(鱼96 h)为0.24 mg·L−1,明显低于母物质LC50(鱼96 h)3个数量级。此外LC50(水蚤48 h)和EC50(绿藻96 h)这两个指标也有相似的特征分布,表明部分CBZ降解中间产物对水生生物的急性毒性明显高于母物质,这与发光细菌毒性实验结论相似。因此CBZ在UV/氯过程中生成毒性副产物的潜在危害亟待引起关注,以确保水质安全。
-
(1)UV/氯对CBZ的降解符合准一级反应动力学,反应20 min降解率达99.0%。
(2)降解速率常数随UV光强和余氯初始浓度增加而增加,随氨氮浓度增大而减小。酸性溶液中CBZ降解率显著增加。
(3)使用HRMS Orbitrap和GC-MS共解析出10种中间产物。UV/氯降解CBZ主要通过羟基化、氯取代和电子转移等反应来实现。
(4)发光细菌急性毒性实验和ECOSAR软件预测表明,UV/氯工艺中产生了毒性高于母物质的中间产物,对水质安全存在潜在威胁。
卡马西平在UV/氯高级氧化工艺中的去除、转化与毒性评价
Removal, transformation and toxicity evaluation of carbamazepine by the UV/chlorine advanced oxidation process
-
摘要: UV/氯作为一种新型高级氧化工艺在新兴污染物控制领域引起了广泛关注。采用UV/氯工艺对典型抗癫痫药物卡马西平(CBZ)进行降解研究。比较单一UV、单一氯和UV/氯对CBZ的降解效果,考察了UV光强、余氯初始浓度、溶液初始pH值和氨氮浓度等因素的影响,解析CBZ在降解过程中的中间产物,提出降解机理,并评估生态风险。结果表明,UV/氯工艺的降解效果明显优于单一UV和单一氯。降解过程遵循准一级反应动力学。降解速率常数随UV光强和余氯初始浓度增大而增大,随氨氮浓度增加而减小,酸性条件更有利于降解过程。采用HRMS Orbitrap和GC-MS鉴定出10种CBZ降解中间产物,CBZ降解主要通过羟基化、氯取代和电子转移等反应实现。发光细菌毒性实验和ECOSAR预测均表明,CBZ在UV/氯工艺中会产生毒性高于母体的中间产物,对水质安全保障造成潜在风险。Abstract: The UV/chlorine process has attracted wide attention as a new advanced oxidation process for emerging pollutant control. In this study, the degradation of the typical anti-epileptic drug carbamazepine (CBZ) was investigated by UV/chlorine process. The degradation of CBZ by single UV, single chlorine and UV/chlorine process was compared. Besides, the effects of UV light intensity, concentration of residual chlorine, pH of solution and ammonia nitrogen concentration on the degradation were also investigated. Built on the identification of degradation products, the degradation mechanism of carbamazepine (CBZ) in the UV/chlorine process was proposed. The ecological risk during the process was also evaluated. The results showed that the UV/chlorine process was more efficient than single UV and single chlorine. The whole process followed pseudo-first-order reaction kinetics. The degradation rate constants increased with the increase of UV light intensity and residual chlorine concentration, while decreased with the increasing ammonia nitrogen concentration. The acidic condition was in favor of the degradation process. Ten CBZ degradation intermediates were identified by HRMS Orbitrap and GC-MS. CBZ degradation was mainly achieved through hydroxylation, chlorine substitution and electron transfer. Both luminescent bacteria toxicity test and ECOSAR prediction indicated that the intermediates with higher toxicity than the parent could be generated during the UV/chlorine process, which may process the potential ecological risk for the water quality security.
-

-
表 1 不同工况下CBZ的准一级反应动力学模型参数
Table 1. Parameters of pseudo-first-order kinetics at different conditions of CBZ degradation
工况 Operating condition $k_{{\text{app}}}'$ R2 t1/2/min 单一UV 0.0084 0.7320 77.8 单一氯 0.0056 0.8794 154.3 UV/氯 0.2601 0.9898 3.8 表 2 不同UV光强下CBZ的准一级反应动力学模型参数
Table 2. Parameters of pseudo-first-order kinetics of CBZ degradation at different UV intensities
UV光强/(μW·cm-2) UV intensity $k_{{\text{app}}}'$ R2 t1/2/min 500 0.0416 0.9518 19.9 1000 0.0882 0.9956 8.4 2000 0.1326 0.9899 4.7 3000 0.1609 0.9757 3.4 表 3 不同余氯初始浓度下准一级反应动力学模型参数
Table 3. Parameters of pseudo-first-order kinetics of CBZ degradation at various initial residual chlorine dose
余氯初始浓度 /(mmol·L−1)
Initial concentration of residual chlorine$k_{{\text{app}}}'$ R2 t1/2/min 0.085 0.0252 0.9768 29.0 0.170 0.0491 0.9872 14.4 0.255 0.0823 0.9957 9.0 0.340 0.1019 0.9866 8.2 0.425 0.1574 0.9639 6.2 表 4 溶液不同初始pH值下CBZ的准一级反应动力学模型参数
Table 4. Parameters of pseudo-first-order kinetics of CBZ degradation at different pH values
pH $k_{{\text{app}}}'$ R2 t1/2/min 3 4.2991 0.9998 0.16 5 0.0510 0.9934 12.5 7 0.0501 0.8919 9.4 9 0.0681 0.8990 6.9 11 0.0210 0.9411 29.9 表 5 不同氨氮浓度下CBZ的准一级反应动力学参数
Table 5. Parameters of pseudo-first-order kinetics of CBZ degradation at different ammonia concentrations
氨氮浓度(/mmol·L−1 ) Ammonia concentration $k_{{\text{app}}}'$ R2 t1/2/min 0 0.1315 0.9932 4.4 0.05 0.0728 0.9581 6.4 0.10 0.0647 0.9548 7.5 0.20 0.0444 0.9945 14.9 0.40 0.0319 0.9900 21.4 表 6 通过HRMS检出的CBZ及中间产物
Table 6. CBZ and its intermediates detected by HRMS
序号
Serial
number化合物
Compound分子式
Molecular
formula结构式
Structural
formula保留时间/min
Retention
time[M+H]+ 理论质荷比
Theoretical mass-
charge ratio(m/z)实际质荷比
Actual mass-
charge ratio(m/z)Δ(×10−6) 1 CBZ C15H12N2O 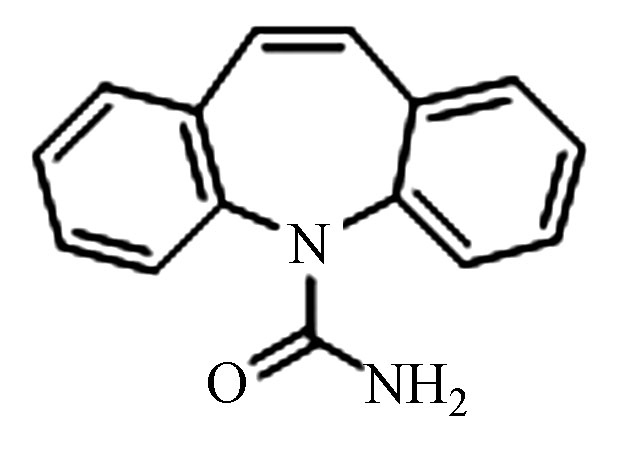
8.17 237.1022 237.1022 0 2 Pr287 C15H11ClN2O2 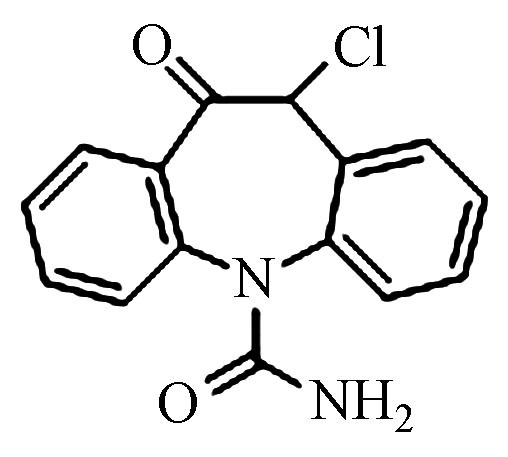
7.75 287.0582 287.0587 1.7 3 Pr271 C15H14N2O3 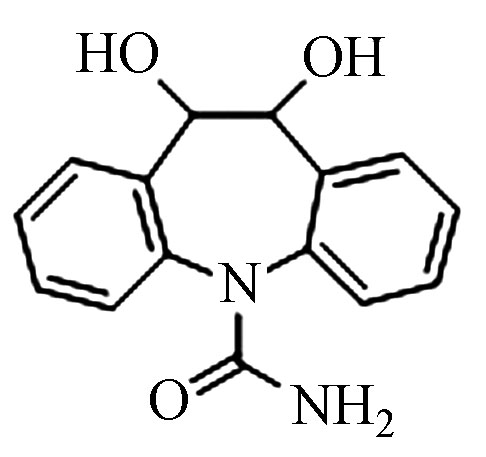
4.83 271.1077 271.1080 1.1 4 CBZ-Cl C15H11ClN2O 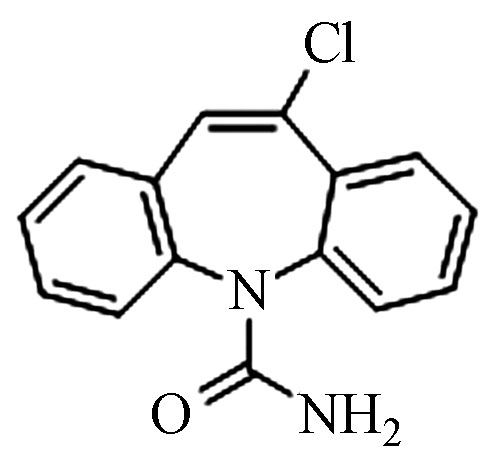
9.18 271.0633 271.0632 −0.3 5 CBZ-OH C15H12N2O2 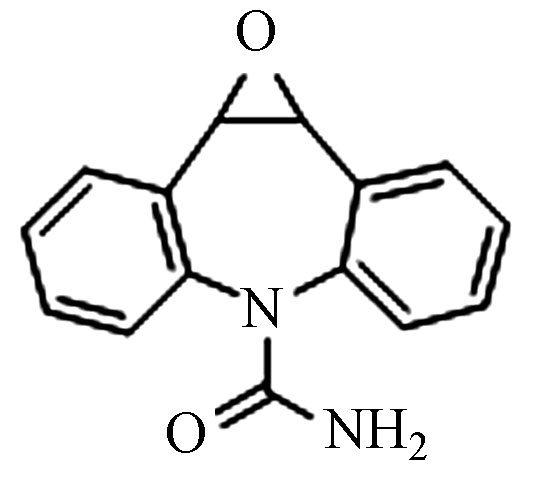
7.11 253.0972 253.0970 −0.8 6 Pr240 C15H13NO2 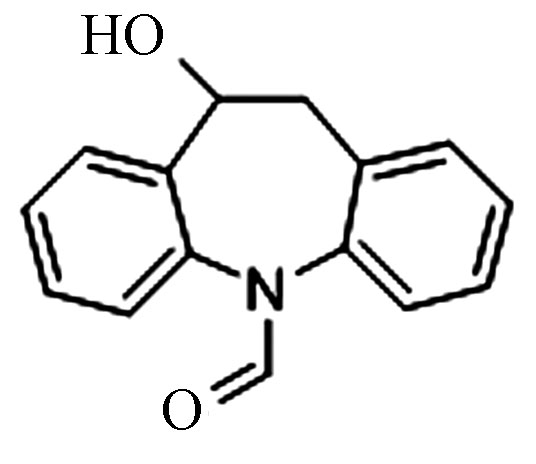
7.36 240.1019 240.1021 0.8 表 7 通过GC-MS检出的降解产物
Table 7. Degradation products detected by GC-MS
序号
Serial
number产物
Product分子量
Molecular
weightCAS号
CAS
number保留时间/min
Retention
time匹配度/%
Degree of
match分子式
Molecular
formula分子结构
Molecular
structure1 乳酸(2TMS) 90 79-33-4 10.6 87.2 C3H6O3 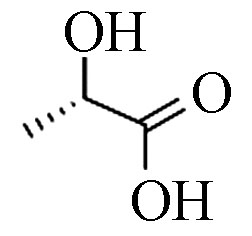
2 乙醇酸(2TMS) 76 79-14-1 11.1 83.4 C2H4O3 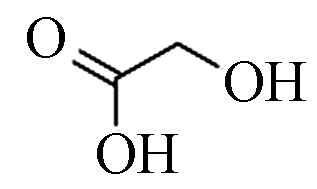
3 草酸(2TMS) 89 144-62-7 12.9 80.5 C2H2O4 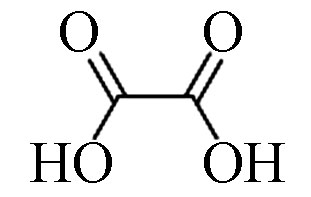
4 苯甲酸(TMS) 122 65-85-0 15.9 88.2 C7H6O2 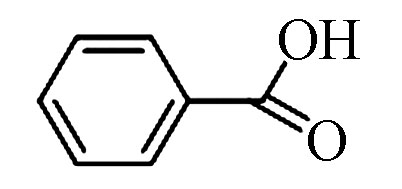
5 丁二酸(2TMS) 118 110-15-6 18.5 83.1 C4H6O4 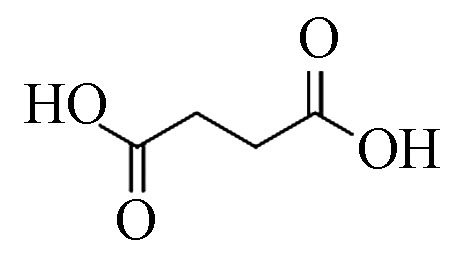
注:TMS表明为小分子酸硅烷化物质.
Note: TMS stands for silylated small molecule acid.表 8 利用ECOSAR预测CBZ及其中间产物的急性毒性(mg·L−1)
Table 8. Estimated acute toxicity of CBZ and its intermediates by ECOSAR software
鱼 Fish 水蚤 Daphnid 绿藻 Green Algae 96 h-LC50 48 h-LC50 96 h-EC50 CBZ 116.13 64.40 59.82 CBZ-Cl 36.59 22.84 21.29 CBZ-OH 1317.45 588.22 542.75 Pr271 0.24 0.24 0.22 Pr240 43.68 26.53 24.71 Pr287 120.87 67.99 63.17 -
[1] 吕小明. 典型新兴环境污染物的研究进展 [J]. 中国环境监测, 2012, 28(4): 118-123. doi: 10.3969/j.issn.1002-6002.2012.04.029 LV X M. Research progress of endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products(PPCPs) [J]. Environmental Monitoring In China, 2012, 28(4): 118-123(in Chinese). doi: 10.3969/j.issn.1002-6002.2012.04.029
[2] 李怡帆, 孙剑辉, 孙胜鹏. Mn2+协同Fe3+-EDTA在中性pH条件下催化类Fenton降解水中卡马西平 [J]. 环境化学, 2017, 36(11): 2319-2324. doi: 10.7524/j.issn.0254-6108.2017040502 LI Y F, SUN J H, SUN S P, el al. Degradation of carbamazepine in aqueous solutions by Mn2+-mediated Fenton-like reaction of Fe3+-EDTA complex at neutral pH [J]. Environmental Chemistry, 2017, 36(11): 2319-2324(in Chinese). doi: 10.7524/j.issn.0254-6108.2017040502
[3] CALISTO V, DOMINGUES M R M, ERNY G L, et al. Direct photodegradation of carbamazepine followed by micellar electrokinetic chromatography and mass spectrometry [J]. Water Research, 2011, 45(3): 1095-1104. doi: 10.1016/j.watres.2010.10.037 [4] 谭娜, 卜龙利, 高波, 等. ZnIn2S4光催化降解水中痕量药物卡马西平的特性 [J]. 环境工程学报, 2017, 11(1): 223-229. doi: 10.12030/j.cjee.201508197 TAN N, BO L L, GAO B, el al. Characteristics of photocatalytic degradation by ZnIn2S4 for trace pharmaceutical carbamazepine in aqueous solution [J]. Chinese Journal of Environmental Engineering, 2017, 11(1): 223-229(in Chinese). doi: 10.12030/j.cjee.201508197
[5] 郭倩, 唐光贝, 彭稳, 等. 卡马西平及其衍生物的环境行为及其去除研究进展 [J]. 环境化学, 2019, 38(8): 1708-1715. doi: 10.7524/j.issn.0254-6108.2018102701 GUO Q, TANG G B, PENG W, el al. Environmental fate and removal of carbamazepine and its derivatives [J]. Environmental Chemistry, 2019, 38(8): 1708-1715(in Chinese). doi: 10.7524/j.issn.0254-6108.2018102701
[6] 陈建, 王朋, 曹艳贝, 等. 生物炭的制备温度及酸处理对卡马西平的吸附动力学影响 [J]. 环境化学, 2016, 35(7): 1461-1467. doi: 10.7524/j.issn.0254-6108.2016.07.2015112401 CHEN J, WANG P, CAO Y B, el al. Impact of pyrolytic temperature and acid wash on adsorption kinetics of carbamazepine on biochar [J]. Environmental Chemistry, 2016, 35(7): 1461-1467(in Chinese). doi: 10.7524/j.issn.0254-6108.2016.07.2015112401
[7] 姬小平, 缪恒锋, 任洪艳, 等. 饮用水中卡马西平的臭氧和二氧化氯降解研究 [J]. 安全与环境学报, 2015, 15(4): 261-267. JI X P, MIAO H F, REN H Y, el al. On the approach to degrading the carbamazepine in drinking water via ozone and chlorine dioxide [J]. Journal of Safety and Environment, 2015, 15(4): 261-267(in Chinese).
[8] 方远航. 紫外LED协同TiO2光催化降解阿替洛尔及卡马西平研究 [D]. 沈阳: 沈阳建筑大学, 2018. FANG Y H. Degradation of atenolol and carbamazepine by ultraviolet light emitting diode combined with TiO2 photocatalytic[D]. Shenyang: JianZhu University, 2018 (in Chinese).
[9] 邓靖, 邵益生, 高乃云, 等. UV/H2O2工艺对水中典型药物卡马西平的光化学降解研究 [J]. 中南大学学报(自然科学版), 2013, 44(9): 3933-3939. DENG J, SHAO Y S, GAO N Y, el al. Photochemical degradation of typical pharmaceutical carbamazepine in water by UV/H2O2 process [J]. Journal of Central South University (Science and Technology), 2013, 44(9): 3933-3939(in Chinese).
[10] ALIA F, KHAN J A, SHAH N S, et al. Carbamazepine degradation by UV and UV-assisted AOPs: Kinetics, mechanism and toxicity investigations [J]. Process Safety and Environmental Protection, 2018, 117(Part B): 307-314. [11] FANG J, FU Y, SHANG C. The roles of reactive species in micropollutant degradation in the UV/free chlorine system [J]. Environmental Science & Technology, 2014, 48(3): 1859-1868. [12] KONG X J, JIANG J, MA J, et al. Degradation of atrazine by UV/chlorine: Efficiency, influencing factors, and products [J]. Water Research, 2016, 90: 15-23. doi: 10.1016/j.watres.2015.11.068 [13] XIANG Y, FANG J, SHANG C. Kinetics and pathways of ibuprofen degradation by the UV/chlorine advanced oxidation process [J]. Water Research, 2016, 90: 301-308. doi: 10.1016/j.watres.2015.11.069 [14] LI J Q, ZHOU S Q, LI M, et al. Mechanism insight of acetaminophen degradation by the UV/chlorine process: kinetics, intermediates, and toxicity assessment [J]. Environmental science and pollution research international, 2019, 26(24): 25012-25025. doi: 10.1007/s11356-019-05747-1 [15] GB/T 5750.11—2006, 生活饮用水标准检验方法 消毒剂指标 [S]. 北京: 中国标准出版社, 2007. GB/T 5750.11—2006, Standard examination methods for drinking water-Disinfectants parameters[S]. Beijing: Standards Press of China, 2007 (in Chinese).
[16] SAPOZHNIKOVA Y, HEDGESPETH M, WIRTH E, et al. Analysis of selected natural and synthetic hormones by LC-MS-MS using the US EPA method 1694 [J]. Analytical Methods, 2011, 3(5): 1079-1086. doi: 10.1039/c0ay00748j [17] WANG W L, WU Q Y, HUANG N, et al. Synergistic effect between UV and chlorine (UV/chlorine) on the degradation of carbamazepine: Influence factors and Radical Species [J]. Water Research, 2016, 98: 190-198. doi: 10.1016/j.watres.2016.04.015 [18] KIM I, TANAKA H. Photodegradation characteristics of PPCPs in water with UV treatment [J]. Environment International, 2009, 35(5): 793-802. doi: 10.1016/j.envint.2009.01.003 [19] PAN Y, CHENG S, YANG X, et al. UV/chlorine treatment of carbamazepine: Transformation products and their formation kinetics [J]. Water Research, 2017, 116: 254-265. doi: 10.1016/j.watres.2017.03.033 [20] 马艳, 高乃云, 郑琪, 等. UV-C辐照降解水中2, 4, 6-三氯酚 [J]. 华中科技大学学报(自然科学版), 2012, 40(6): 128-132. MA Y, GAO N Y, ZHENG Q, el al. Degradation of 2, 4, 6-trichlorophenol in water by UV-C irradiation [J]. J. Huazhong Unvi of Sci& Tech (Natural Science Edition), 2012, 40(6): 128-132(in Chinese).
[21] ZHOU S, XIA Y, LI T, et al. Degradation of carbamazepine by UV/chlorine advanced oxidation process and formation of disinfection by-products [J]. Environmental Science & Pollution Research, 2016, 23(16): 16448-16455. [22] FENG Y, SMITH D W, BOLTON. J R Photolysis of aqueous free chlorine species (HOCl and OCl-) with 254 nm ultraviolet light1 [J]. Journal of Environmental Engineering and Science, 2007, 6(3): 277-284. doi: 10.1139/s06-052 [23] GALLARD H, VON G U. Chlorination of phenols: kinetics and formation of chloroform [J]. Environmental Science and Technology, 2002, 36(5): 884-890. doi: 10.1021/es010076a [24] 孙中兴, 陈鑫, 姜永根, 等. 2006-2013年黄浦江支流水源水氨氮及高锰酸盐指数检测分析 [J]. 上海预防医学, 2016, 28(2): 119-121. SUN Z X, CHEN X, JIANG Y G, el al. Determination and analysis of ammonia nitrogen and permanganate index in source water of Huangpu River branch in 2006-2013 [J]. Shanghai Journal of Preventive Medicine, 2016, 28(2): 119-121(in Chinese).
[25] 张欣然, 李伟光, 公绪金, 等. 紫外/氯耦合处理饮用水中氨氮的响应面优化 [J]. 化工学报, 2014, 65(3): 1049-1055. doi: 10.3969/j.issn.0438-1157.2014.03.039 ZHANG X R, LI W G, GONG X J, el al. Optimization on combined UV/chlorine process for removal of ammonia in drinking water [J]. CIESC Journal, 2014, 65(3): 1049-1055(in Chinese). doi: 10.3969/j.issn.0438-1157.2014.03.039
[26] 李佳琦, 杜尔登, 樊鑫鑫, 等. 氯消毒中有机防晒剂BP9的去除转化与风险评价 [J]. 中国环境科学, 2018, 38(3): 968-976. doi: 10.3969/j.issn.1000-6923.2018.03.021 LI J Q, DU E D, FAN X X, el al. Removal, transformation and risk assessment of UV-filter BP9 during chlorination disinfection [J]. China Environmental Science, 2018, 38(3): 968-976(in Chinese). doi: 10.3969/j.issn.1000-6923.2018.03.021
[27] XIANG H, SHAO Y, GAO N, et al. Degradation of diuron by chlorination and UV/chlorine process: Degradation kinetics and the formation of disinfection by-products [J]. Separation and Purification Technology, 2018, 202: 365-372. doi: 10.1016/j.seppur.2018.03.073 [28] YIN K, HE Q, LIU C, et al. Prednisolone degradation by UV/chlorine process: Influence factors, transformation products and mechanism [J]. Chemosphere, 2018, 212: 55-66. [29] GUO K, WU Z, SHANG C, et al. Radical chemistry and structural relationships of PPCP degradation by UV/chlorine treatment in simulated drinking water [J]. Environmental Science & Technology, 2017, 51(18): 10431-10439. [30] BU L, ZHOU S, ZHU S, et al. Insight into carbamazepine degradation by UV/monochloramine: Reaction mechanism, oxidation products, and DBPs formation [J]. Water Research, 2018, 146: 288-297. doi: 10.1016/j.watres.2018.09.036 [31] YAN C, YANG Y, ZHOU J, et al. Antibiotics in the surface water of the Yangtze Estuary: Occurrence, distribution and risk assessment [J]. Environmental Pollution, 2013, 175: 22-29. doi: 10.1016/j.envpol.2012.12.008 -



 下载:
下载: