-
近年来,我国污水处理行业发展迅速,城镇污水处理厂的数量不断增加。但是,污水处理厂在处理污水的过程中,也会产生大量的剩余污泥,这些污泥含水率高、有机质多、稳定性差,且含有重金属、致癌有机物、病原微生物等有毒有害物质[1],处理不当将会造成二次污染。因此,目前主流的污泥处理处置方法之一是资源化利用,如好氧堆肥、制作建筑材料、制备生物炭等[2]。其中,通过剩余污泥热解制备得到的污泥质生物炭具有比表面积大、离子交换性强、孔隙度高以及表面官能团丰富等特点,对污水中的重金属离子[3]、有机污染物[4]、氮磷营养物[5]等有较好的吸附去除效果,因此被广泛应用于污水处理领域。此外,生物炭制备过程产生的裂解气和生物油可以直接用作能源[6],进一步提高了污泥的附加值和资源化效率。
研究表明,生物炭对有机污染物的去除能力主要取决于其理化性质[7]。由于污泥自身的特性及热解过程中可能伴随产生的副产物,生物炭实际的比表面积、孔隙结构与表面官能团的丰富度会有所减少;同时,生物炭在水环境中也存在固液分离困难等缺点。因此,目前部分学者通过利用物理、化学等方法将生物炭与其他材料组合使用,来改善原生物炭的理化特性,使其具有更多的表面活性位点,进而提升对污染物的吸附选择性和吸附容量[8],且磁性材料的添加还能增强生物炭的回收利用率[9]。
近年来生物炭材料得到快速发展与应用,然而,针对以污泥为原料制备生物炭复合材料的研究较少,且其对有机污染物的去除效果及机理分析缺少系统性阐述。因此,本文总结了污泥质生物炭的改性方法,系统分析了污泥质生物炭对有机污染物吸附过程中的主要去除机理,并归纳了近年来污泥质生物炭的应用,以期推动生物炭技术在污水处理领域的发展。
-
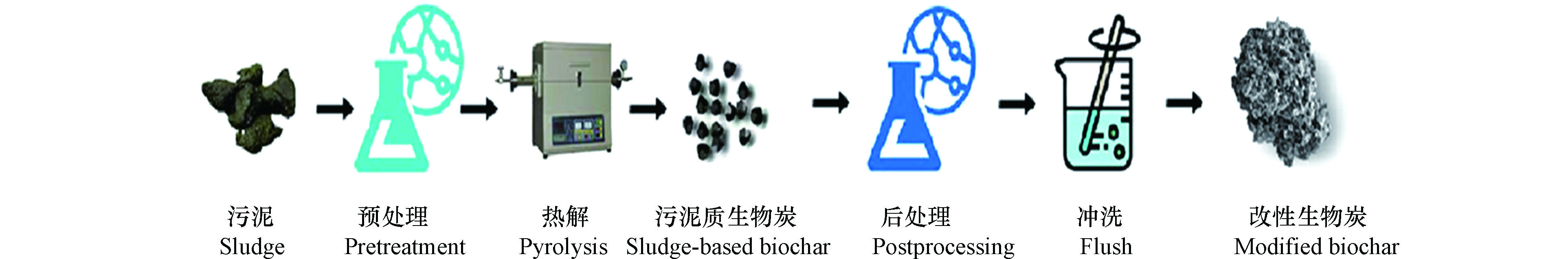
目前,污泥质生物炭常用的改性方法是化学改性法,包括酸碱改性法、磁性材料改性、纳米材料改性和有机材料改性等[10],由此得到不同性质的生物炭(表1)。改性污泥质生物炭的制备过程见图1。
-
酸碱改性是用酸(如HCl、H2SO4、H3PO4、HNO3)或碱(如NaOH、KOH、氨水、尿素)来处理污泥质生物炭。经酸改性后,污泥质生物炭的总孔容积有所提升,孔隙结构更为明显。由于污泥质生物炭本身的高灰分会掩盖其活性吸附位点,削弱对有机污染物的吸附能力[11],因此,可以对生物炭进行酸洗处理,使其灰分、H/C和O/C含量均减少,同时增强污泥质生物炭的疏水性和芳香性能[11]。此外,具有强氧化性的酸能够活化生物炭表面的含氧官能团,使羟基、羧基和酯基的含量提高,这些含氧官能团产生的表面负电荷使污泥质生物炭具有阳离子交换能力,有利于吸附阳离子有机污染物[12]。
与酸改性相比,碱改性能显著提高污泥质生物炭的比表面积。例如,经KOH处理后的污泥质生物炭,其比表面积由原来的小于10 m2·g−1提升到783 m2·g−1[13];相反,经HNO3处理后的污泥质生物炭,其比表面积减少26%—35%[14]。污泥质生物炭碱活化后,也能增加表面含氧官能团的数量。Gómez-Pacheco等[15]发现,NaOH活化制备的污泥质生物炭对亚甲基蓝最大吸附量为518 mg·g−1,主要是由其表面羰基含量提高的缘故。此外,碱改性能够以化学键的方式提高材料的热稳定性,促进污泥质生物炭孔隙结构的形成[12]。Martin等[16]认为由于污泥质生物炭的介孔性质,碱改性制备的吸附剂对大分子有机化合物的去除效率更高。因此使用碱改性得到的生物炭,其吸附性能通常较高。
-
由于粉末生物炭较难从污水处理中分离回收,极大限制了其实际应用。因此,不少学者通过添加磁性材料的方法,使生物炭改性后具有磁性,以提高分离再生能力。
对于磁性污泥质生物炭,目前常用的制备方法是在污泥热解反应前,通过化学沉淀法将Fe3+/Fe2+沉积在生物质上,在热解过程中原位生成磁性颗粒[17]。该制备过程较简便、易操作,所得材料具有强磁力性与稳定性,且在强酸性环境下铁元素的浸出率低。同时,磁性颗粒的加入还能改善污泥质生物炭表面的负电性,提升污泥质生物炭对阴离子污染物的吸附能力[18]。此外,也有学者通过后续添加磁性材料将污泥质生物炭磁化,如Devi等[9]将造纸污泥在700 ℃下裂解制成生物炭后,与FeSO4·7H2O和NaBH4混合溶液充分反应,烘干制得零价铁磁性生物炭(ZVI-MBC),其比表面积由67 m2·g−1提高至101 m2·g−1,孔径和官能团数量均有所增加,ZVI-MBC对五氯苯酚的吸附效率显著提高至100%;此外,ZVI-MBC的饱和磁化强度接近纯γ-Fe2O3颗粒,利用磁铁即可进行固液分离。
-
一般来说,将金属纳米颗粒或层状纳米材料引入生物炭中可以改变其比表面积、孔隙度、表面官能团以及其他功能(如磁性、粒子分散等),有利于水中有机污染物的去除[7]。负载纳米金属氧化物改性主要是利用负载的金属元素与有机物之间的结合力来提高吸附效果,通常采用湿法浸渍法将纳米材料的前驱体直接接枝固定到生物炭上[19]。例如,Song等[20]将热解后的污泥质生物炭浸泡于KMnO4溶液混合制得MnOx-生物炭复合材料(MBC),其孔径增加了5倍,MBC的O/C从0.04升高到0.53,对盐酸西环素的吸附能力较原生物炭提高了2倍。此外,还可以通过将金属氧化物纳米颗粒直接沉积到生物炭上来制备生物炭纳米复合材料[21]。以纺织工业污水处理厂污泥为原料,将氧化铁作为前驱体合成污泥质生物炭纳米颗粒,其比表面积为91 m2·g−1,中孔数量增多(占81.21%),该复合生物炭对氧氟沙星的吸附率为96%,最大吸附量为19.74 mg·g−1[22]。
不同的纳米材料具有不同的理化特性,因此所得改性污泥质生物炭性能亦不同。如石墨化氮化碳(g-C3N4)和纳米零价铁(nZVI)等功能性纳米颗粒具有催化氧化还原的性质,可同时吸附并降解苯酚等酸性有机污染物[23-24]。而纳米零价铁生物炭则具有良好的阳离子可交换能力,因此对阳离子有机物的吸附效率高。例如,Ahmad等[25]将nZVI负载到生物炭表面,不仅抑制了nZVI粒子的团聚,还赋予了生物炭高效的吸附性能,其阳离子交换量可达(72.55±3.95)cmol·kg−1,对四环素的最大吸附量为89.05 mg·g−1。此外,采用浸涂法制备的碳纳米管/生物炭可以增大生物炭的热稳定性、孔隙体积和表面电荷,其比表面积由9 m2·g−1提高至390 m2·g−1,羟基、酯基、羧基和含氟基团等官能团数量有所增加,使得复合材料对亚甲基蓝的吸附容量提升了2.56倍[19]。
-
有机物改性主要是在生物质/炭悬浮液中加入有机胺、有机酸盐、螯合剂和表面活性剂以及醇类等有机化合物,活化污泥质生物炭表面羧基、羰基、酯基和醚基等含氧官能团,使其与POPs、PPCPs等有机物间形成多种化学键,进而增强污泥质生物炭的吸附能力。
污泥质生物炭有机物改性的方法有两种,一种是生物质预处理,如污泥在乙醇-水混合溶剂中(体积比1∶1)液化后制备产生生物炭,生物炭上羧基、内酯基、酚类和羰基等酸性含氧基团数量的增加提高了其C、O元素含量,其中含量占比较大的含氧官能团羧基(59.78%)可提高生物炭与亚甲基蓝离子的络合能力[2]。另一种是对热解获得的生物炭通过浸渍、共沉淀等方式活化得到复合体。Kong等[26]利用柠檬酸和ZnCl2对市政污泥热解所得的生物炭进行改性,比表面积提升至792.4 m2·g−1,且有机酸使生物炭表面引入大量羧基,提供了有机物吸附的活性位点,其对苯酚、4-对氯苯酚和苯甲酸的最大吸附容量分别达2.1、2.9、3.49 mmol·g−1。可见,污泥质生物炭与有机材料复合后,一方面为生物炭表面增加了与污染物作用的基团;另一方面,生物炭为有机基团提供了附着位点,有利于增大与有机污染物的作用能力。
-
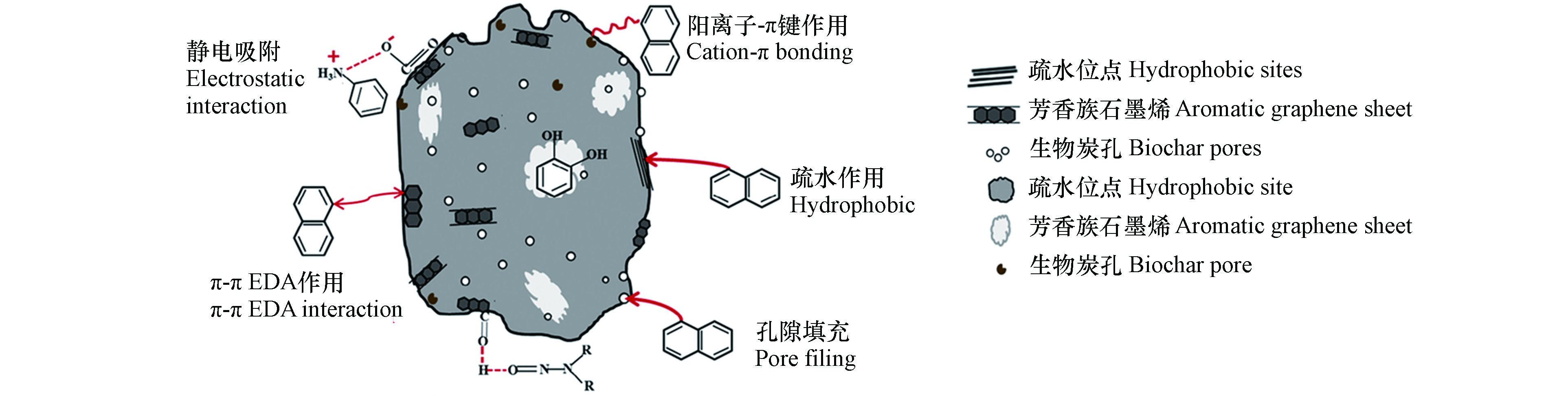
改性污泥质生物炭吸附有机污染物由孔隙填充、分配作用、疏水作用、π-π EDA电子供体-受体作用、静电吸附作用和氢键等微观吸附机制共同作用,主要取决于污染物类型、负载材料以及吸附剂表面的化学性质。改性污泥质生物炭吸附有机污染物的作用机制见图2。
-
生物炭的孔隙结构主要由小于2 nm的微孔、2—50 nm的中孔和大于50 nm的大孔组成。而微孔和中小孔(2—20 nm)为污泥质生物炭主要部分,在吸附有机污染物时发挥着重要作用[28]。通常,分子粒径较小的有机污染物可以通过孔隙填充被吸附到生物炭内部。因此,在吸附质浓度相对较低或污泥质生物炭的挥发性物质含量较低的吸附过程中,生物炭对有机污染物的吸附机理主要由孔隙填充决定[29]。大多数情况下,孔隙填充过程的吸附速率较快,并且符合Langmuir吸附等温线模型[30],这表明改性污泥质生物炭对有机污染物的吸附为单分子层吸附过程。
-
分配作用通常发生在高溶质浓度下或富含挥发性物质的生物炭中,被吸附的有机化合物溶解在有机质基质中,从而扩散到生物炭非碳化部分的有机质基质,进而增强生物炭的吸附性能[30-31]。富含芳香族或脂肪族化合物(如吡咯、酚、酮和糖等)的生物炭,其非晶碳相也可发生分配作用[32]。但由于生物炭颗粒具有多孔性,可能不利于对大粒径有机物的吸附,特别是表面涂有有机质涂层的陈化生物炭[30],因此通过分配作用对有机化合物的吸附速率较慢;而含有疏水性分子的有机化合物则易被扩散[33]。
-
疏水作用是生物炭疏水表面与非极性分子(疏水基团)之间一种非共价的作用,或有机分子中存在极性基团(亲水基团)时与亲水性生物炭材料之间存在相互吸引的作用[34]。以全氟辛烷磺酸盐[35]为例,其吸附主要受生物炭疏水位点与C—F链间的疏水相互作用影响。而氧化铁生物炭纳米颗粒对氧氟沙星的吸附,则是由于生物炭表面存在亲水性较强的羟基和羧基等极性基团,因此能与氧氟沙星分子之间发生疏水作用[22]。与分配作用相比,疏水吸附通常发生在水化能较低的生物炭表面,这是由于吸附的非极性分子与水分子之间产生了直接竞争[34, 36]。
-
π-π EDA电子作用是一种特殊的、非共价键的吸引力,主要存在于电子供体物质和电子受体物质之间。电子由供体物质能量最高的分子轨道即最高占有轨道,转移到受体能量最低的分子轨道即最低未占轨道[37]。该作用一般发生在石墨烯类污泥质生物炭表面与芳香族化合物之间[38]。污泥质生物炭的石墨化发生在热解温度1100 ℃以上[39],然而当温度高于500 ℃时,生物炭芳香环内的电荷分布不均匀,会增加或降低其电子密度,从而形成富π电子或缺π电子体系[32]。低温处理的污泥质生物炭(<500 ℃)中,吸电子基团可作为π电子受体[40];而高温处理的污泥质生物炭(>1100 ℃)中,聚合芳香环或负载石墨烯片层可作为π电子供体[41]。
例如,阿特拉津含有吸电子基团的氯取代基,可作为强电子受体与污泥质生物炭表面的芳香环产生π-π EDA作用[42]。而磺胺类抗生素和多环芳烃这类含多苯环的有机污染物,由于2个芳香环的π系统产生电子缺陷的结构性质,可作为强电子受体。改性污泥质生物炭表面上含氧或含氮基团由于含高电负性的孤电子对,因此可作为强电子供体,与上述污染物发生强烈的π-π EDA作用[41]。此外,在低pH条件下,表面富含π电子的石墨烯类污泥质生物炭可以与磺胺甲恶唑中苯胺环发生质子化作用使其带正电荷,这种发生在质子化的有机分子和供电子的苯环之间的反应被称为π+-π EDA作用。
-
静电吸附作用是生物炭吸附离子和可电离有机化合物的主要机制[41],通过官能团得失电子后形成带电基团,如两者之间带电性相反,则为静电吸引,反之则为静电排斥。污泥质生物炭可以通过静电吸附来结合阳离子染料,如亚甲基蓝[43]和甲基紫[44]。具有苯酚和羧基等含氧官能团的低温生物炭,其在溶液中去质子化后可以吸附阳离子有机物[45]。由于溶液pH会直接影响生物炭表面的电荷性质,当溶液pH高于生物炭零电荷点(pHPZC)时,生物炭表面的含氧官能团发生去质子化作用带负电荷,从而促进污泥质生物炭与阳离子染料之间的静电作用[46]。
此外,阳离子的浓度也会影响静电作用。当静电吸附作用为静电吸引力时,提高金属盐溶液的离子浓度,会降低其对有机物的吸附。因此,当溶液中Na+浓度从0.01 mol·L−1 Na+增加到0.1 mol·L−1 Na+时,改性污泥质生物炭对亚甲基蓝的吸附量从4.5 mg·g−1下降到3 mg·g−1,这可能是Na+会占据生物炭表面单独的吸附位点[47]。而当静电吸附作用为静电排斥力时,增加金属盐溶液的离子浓度会提高生物炭对有机污染物的吸附容量,如Cu2+、Al3+[48]。
-
氢键作用是生物炭对极性有机化合物的可能吸附机制[49-50],这是因为生物炭上丰富的极性基团能促进其与含电负性元素的有机化合物之间以氢键结合[50]。如改性污泥质生物炭对邻苯二甲酸二丁酯的吸附,主要是通过生物炭上的氢原子与邻苯二甲酸二丁酯上酯基的氧原子之间以氢键结合的[51]。此外,在含氮原子的污泥质生物炭中,N-二甲基亚硝胺的—N=O基团与生物炭的酯基之间形成的氢键也促进了N-二甲基亚硝胺从水溶液的去除[49]。
-
改性污泥质生物炭具有比表面积大、含氧官能团丰富、芳香性及电荷密度高等特点,使得其对有机污染物具有良好的吸附效果[52]。但是,污水中有机污染物种类繁多,改性污泥质生物炭对不同类型有机污染物的吸附机理存在差别,从而使得吸附去除效果存在差异。因此,论文以污水中典型污染物-染料、有机酚类、药物活性物质为代表,阐明改性污泥质生物炭在这些典型污染物吸附去除中的应用。
-
染料是最普遍的有机污染物之一,具有化学稳定性强、水溶性好、色度高、芳香族结构复杂、难于自然分解等特点[53-54]。改性污泥质生物炭中存在大量的官能团(羧基、胺基和膦酸基团等)和金属离子(Ca2+、Mg2+、Na+、K+),可为染料分子提供活性位点,并借助静电吸附、离子交换和表面络合等作用提升其对染料的吸附效率[23]。
利用改性污泥质生物炭吸附去除染料的相关研究进展见表2。例如,石墨烯包覆的污泥质生物炭复合材料对亚甲基蓝的饱和吸附量可达174 mg·g−1,远大于纯污泥质生物炭对亚甲基蓝的饱和吸附量(8 mg·g−1)[55],而使用NaOH活化制备的污泥质生物炭对亚甲基蓝最大吸附量更是高达518 mg·g−1[56]。将脱水污泥和ZnCl2混合制备的生物炭,在最佳操作条件下分别对浓度为300 mg·L−1的活性红、活性蓝、弱酸性艳红等溶液进行脱色处理,其吸附效率可达90.7%、94.4%和97.5%[57-58]。Chaukura等[59]将铁负载至污泥质生物炭上作为吸附剂处理废水中的甲基橙,红外光谱分析发现该改性生物炭上存在着大量的羟基和芳香基团,对甲基橙的吸附能力比原生物炭高52.8%。可见,复合材料的添加可增强污泥质生物炭对染料的吸附能力。
但是,目前关于污泥质生物炭吸附染料的研究工作大多采用单一染料,而实际废水中常含有共存污染物,例如多种重金属离子、阳离子染料、阴离子染料等,吸附过程中不同染料之间可能存在竞争吸附。因此,后期还需关注污泥质生物炭对多种复合染料的去除规律。
-
苯酚、双酚A、对硝基苯酚和五氯酚等都是常见的有机酚类污染物,这些有机化合物均含有芳香族和异芳香族分子结构,因此难以被生物降解[75],而利用改性污泥质生物炭可以有效吸附这类物质[76]。这是因为碱性官能团能够促进酚类物质在生物炭表面的化学吸附,且有机酚类能结合生物炭表面基团进入其孔结构中,从而提升生物炭的吸附效果。
利用改性污泥质生物炭吸附去除有机酚类的相关研究见表3。Martin等[16]利用生物污泥质生物炭来去除在活性污泥工艺中的苯酚时,发现生物炭与商业活性炭的吸附性能一致,并且在苯酚初始浓度为100 mg·L−1时,加入污泥质生物炭使苯酚的去除率从58.0%提高到98.7%,且污泥活化与否并不影响其对苯酚的吸附能力[77]。同样,利用市政污泥与尿素共混在550 ℃下热解制备的改性生物炭材料UBC-x,也适用于除水溶液中的苯酚和双酚A等酚类污染物的去除[78]。可见,污泥质生物炭表面丰富的负电荷点和官能团使得生物炭对酚类表现出良好的吸附效果。
然而,水体中有机酚类化学中间体的污染问题日益突出,如4-氨基-5-羟基萘-2,7-二磺酸、2-巯基苯并噻唑(MBT)等生产染料和农药的常用化学试剂,污泥质生物炭针对这类物质的去除研究较少,有待深入研究。
-
农药等药物活性物质作为新型污染物,进入环境中将导致耐药性细菌的产生,从而人体健康构成潜在威胁,而改性污泥质生物炭可依靠π-π EDA电子作用和氢键作用实现对药物活性物质的吸附去除.
改性污泥质生物炭吸附去除药物活性物质的相关研究见表4。研究表明,将污泥质生物炭与金属氧化物/金属纳米颗粒结合使用,对药物活性物质特别是抗生素的去除效率更高[81]。由于改性后,污泥质生物炭表面的羰基吸电子能力提高,有利于吸引药物活性物质结构上的羟基以及相关基团,从而增强其去除效果。郑永昕等[82]合成了氧化石墨烯改性污泥质生物炭GO-SBC,其对培氟沙星的最大吸附容量为137.51 mg·g−1,比原生物炭的吸附容量提高了40.32%。可见,常用酸/碱和金属改性的方式可提高污泥质生物炭表面与PPCPs有机体的特异性亲和力,从而提高其吸附能力。
但是,溶液的pH值会影响污泥质生物炭对药物活性物质的吸附,受生物炭表面静电吸附的影响,药物活性物质会由于溶液的pH值的变化而转化自身在溶液中的存在形式,从而产生不同的吸附情况,如四环素[99]等。也有研究表明,随着溶液温度升高,污泥质生物炭对盐酸四环素吸附量减少[100]。此外,溶液中的其他共存物质(如腐殖酸等)也会影响其对药物活性物质的吸附去除效果[101]。因此在研究污泥质生物炭对药物活性物质的吸附时,还应关注于实验条件对其吸附的影响。
-
若将吸附饱和的污泥质生物炭丢弃,将会造成资源浪费和二次污染等问题,因此,需要对生物炭再生工艺进行研究。生物炭的再生是在保持原结构基础上,通过物理或化学方法,将吸附质从生物炭的表面分解或者脱离,恢复其吸附能力,使之可以多次使用。目前常用的再生方法包括:热再生法、溶剂再生法、微波辐射法和超临界流体萃取法(表5)。
-
热再生法是运用最广泛的一种生物炭再生方法,主要由脱水干燥、碳化、活化、冷却4个步骤完成。通过添加水蒸气、惰性气体、燃烧气体、CO2加热至700—1000 ℃,此时吸附质在高温下碳化分解,变成比生物炭孔径小的分子或者气体逸出,从而恢复生物炭的吸附性能[102]。夏晶晶等[106]对改性污泥质生物炭热再生性能进行研究时发现再生温度、时间和升温速率对再生效果和再生率均有影响,其中温度影响较为明显。在再生温度550 ℃、再生时间60 min、升温速率10 ℃·min−1的条件下,生物炭经多次吸附后阳离子蓝X-GRRL染料吸附量分别从408.22 mg·g−1降至343.78 mg·g−1,再生效率均在80%以上。虽然热再生法对多种有机污染物都具有一定的解吸或者分解作用,但再生损失大、运行条件严格;且对于四环素等沸点较高的有机物,由于其解吸效果较差,在没有充分处理的情况下进行热再生将会造成二次污染[107]。
-
溶剂再生法是利用生物炭、吸附质和溶剂之间的三相平衡状态,通过改变溶剂的温度或pH等条件来打破吸附平衡,从而将吸附质从生物炭表面脱附下来,使得生物炭再生。溶剂再生法包括酸碱再生法和有机溶剂再生法。
酸碱再生法是指用无机酸(如盐酸、硫酸)或碱(氢氧化钠)等化学溶剂来去除吸附质。如利用氢氧化钠溶液洗涤吸附高浓度苯酚的生物炭,可以将生物炭中的苯酚以苯酚钠的形式回收[103]。酸碱再生法的无机试剂成本低,再生条件温和,但处理时间长,再生效率一般小于80%[105]。有机溶剂再生法是利用苯、丙酮和甲醇等有机溶剂来提取污泥质生物炭中的吸附质。如浓度为60%的丙酮用于解吸吸附桃红染料的生物炭,其解吸效率为52%;而对于吸附黄色染料的生物炭,可使用40%异丙醇作为溶剂,解吸效率为54%[108]。
溶剂再生法的再生速率比较快,生物炭损失较少,因此其再生后的吸附速率较高。此方法适用于高浓度、低沸点有机物的解析,但由于污染物质的种类繁多,在溶剂选择上有一定的困难。由于污染物从生物炭表面脱落下来后又转移至溶剂中,因此不能完全去除污染物质,存在着二次污染的可能性。
-
微波辐照再生法是利用微波诱导污泥质生物炭中吸附的极性物质分子产生偶极子极化,电磁能转化为热能,使得生物炭孔隙中的有机污染物均匀受热而解吸或挥发[104]。与传统加热方法相比,微波辐照法能够更好地保存生物炭的多孔结构,且易于控制热量、再生时间短,对节能降耗、降低二次污染风险也具有积极意义。但该方法对设备要求较高,且有机物脱附过程中是否产生其他中间产物尚不明确。
-
超临界流体再生法是利用超临界流体作为萃取剂,通过调节压力从液体或固体中萃取出特定成分,达到分离目的。目前常用的超临界流体是二氧化碳,多作为非极性物质烷烃、中等极性物质如多环芳烃和多氯联苯的良好溶剂。超临界流体再生具有操作周期短、操作温度低、生物炭损失低等优点,并且能在不改变生物炭理化性质的情况下对污染物进行回收。与有机溶剂再生法相比,以二氧化碳和水为流体的超临界再生法不存在溶剂污染环境的问题[105]。但该方法耐压能力强,设备成本高,目前还只处于实验室阶段。
-
经过改性后的污泥质生物炭具有较大的比表面积、高孔隙率和丰富的官能团,通过孔隙填充、分配作用、疏水作用、π-π EDA作用、静电吸附作用和氢键等微观吸附机制共同作用,为有机污染物的结合提供了大量的活性位点,吸附饱和后的污泥质生物炭也可通过热再生法、溶剂再生法、微波辐射法和超临界流体萃取法恢复其吸附能力。对于以污泥为原料制备生物炭材料及其吸附应用,还需要关注以下几个方面:
(1)制备更高效的污泥质生物炭复合材料。目前对生物炭复合材料的研究主要集中于无机材料和碳纳米材料,关于生物炭与其他材料的复合,如与钛基材料、纳米硫化物、纳米高分子材料等其他类型的研究较少。且复合材料的应用研究多局限于实验室对污染物去除效果的对比,缺乏生物基吸附剂的经济成本分析和生命周期评价。
(2)生物炭复合材料对有机污染物的去除往往由多种机理共同作用。目前的研究多从定性的角度分析各种机制的作用,定量化研究复合材料对有机污染物的作用机理较少。今后可以更多的研究机理定量,为其中优势机制的放大提供科学依据。
(3)目前研究周期多为短期,长期试验带来的生物炭老化问题可能会影响有机污染物的吸附效果,今后应多考虑进长期试验。此外,污泥质生物炭及其复合材料对水生生物的影响(如生物毒性)也有待深究。
改性污泥质生物炭吸附污水中有机污染物的研究进展
Research progress on the removal of organic contaminants from wastewater by modified sludge-based biochar
-
摘要: 污泥质生物炭作为一类碳材料,具有优异的理化性质和多样化的功能,在有机污染物处理领域具有巨大的潜力。利用污泥质生物炭自身的吸附性能,将其与其他材料复合制成新材料,可以赋予生物炭新的性能,提高其吸附效率。本文综述了污泥质生物炭的改性方法及其作为吸附剂在去除废水中有机污染物的应用情况,阐明了改性污泥质生物炭对有机污染物的去除机理,由孔隙填充、分配作用、疏水作用、π-π电子供体-受体作用、静电吸附作用和氢键等微观吸附机制共同作用。最后针对污泥质生物炭吸附后的再生方法进行讨论,并在此基础上提出将来研究高效生物炭吸附材料的重点和研究方向,以期为污泥质生物炭在污水处理系统中的应用提供参考。Abstract: Being one of the classes of carbon materials, sludge-based biochar with excellent physicochemical properties and diversified functions presents great potential in the field of organic contaminant treatment. By compounding raw sludge-based biochar and other materials, novel functional properties could be endowed and the adsorption efficiency of biochar can be much improved. This study reviewed the recent progress on the modification approaches of sludge-based biochar and the efficiencies of these modified materials in the removal of organic contaminants from wastewater were summarized. The removal mechanisms of organic contaminants by modified sludge biochar, including pore filling, partition, ion, hydrophobic interaction, π-π EDA interaction, electrostatic interaction and hydrogen bonding etc. have been described. The regeneration methods of sludge-based biochar after adsorption of organic contaminants have also been discussed. Based on the review, the authors have proposed the research direction of high-efficient biochar-based adsorption materials in the future, providing scientific and engineering basis for the potential applications of sludge-based biochar in the field of wastewater treatment.
-
Key words:
- sludge /
- biochar /
- modification /
- organic contaminants /
- adsorption mechanism
-
表 1 污泥质生物炭改性方法及其特性
Table 1. Modified method of sludge-based biochar materials and its properties
改性剂Modified material 常用改性材料Commonly used modified material 改性方法Modified method 理化性质Physicochemical property 吸附优势Adsorption advantage 酸 HCl、H2SO4、H3PO4、HNO3等 ①污泥在溶液中浸渍老化后再热解制备生物炭;②将污泥质生物炭在溶液中浸渍老化后,经化学共沉降等方式充分混合后得到复合材料。 提升生物炭的总孔容积,多孔结构更为显著,表面灰分减少、阳离子数量提升 易于吸附阳离子污染物 碱 NaOH、KOH、氨水、尿素等 显著提高生物炭的比表面积,表面含氧官能团增加,并以化学键的方式提高材料的热稳定性,促进生物炭孔隙结构的形成 易于吸附大分子有机化合物 磁性材料 零价铁、Fe3O4、Fe2O3、FeO、FeCl3、BiFeO3 生物炭改性后具有磁性,以便于分离再生,并且其孔径和官能团数量均有所增加 易于吸附阴离子污染物 纳米材料 石墨烯、碳纳米管、纳米氧化物、纳米零价铁、纳米金属氢氧化物 增大生物炭的热稳定性、孔隙体积和比表面积,羟基、酯基和羧基等官能团数量有所增加 纳米零价铁生物炭复合材料对阳离子染料和抗生素吸附效果好 有机物 乙二胺四乙酸、乙二胺、二甲基甲酰胺、十六烷基三甲基溴化铵、柠檬酸、乙醇、甲醇、乙二醇等 增加生物炭表面羧基、羰基、酯基和醚基等含氧官能团 易于吸附药物和个人护理品(PPCPs)、持久性污染有机物(POPs)等有机物,易吸附阳离子污染物 表 2 改性污泥质生物炭吸附去除染料
Table 2. Dye adsorption by modified sludge-based biochar
污染物种类Contaminant 生物质Biomass 改性方法Modified method 比表面积/ (m2·g−1)Surface area 吸附容量/(mg·g−1)Adsorption capacity 吸附效率/%Adsorption efficiency 参考文献Reference Bomaplex red CR-L 电凝法污泥 NaOH — 192.31 99.9 [60] 刚果红16 金属氢氧化物污泥 nZVI 640 178 — [15] 活性红2 制皮厂污泥 Fe(NO3)3·9H2O 167.4 55.87 — [14] 活性红24 纸浆污泥 HCl 79.65 — — [61] 活性红31 制皮厂污泥 TiO2 167.4 39.37 — [14] 活性红 脱水污泥 ZnCl2 — — 90.7 [56] 弱酸性艳红 脱水污泥 ZnCl2 — — 97.5 [56] 活性蓝 脱水污泥 ZnCl2 — — 94.4 [55] 活性蓝13 厌氧污泥 碳纳米管 — — 73.4 [62] 活性蓝19 金属氢氧化物污泥 CeO2 640 158 — [15] 蓝胭脂红 市政污泥 Fe-Ni纳米材料 70 654.33 98.8 [63] 罗丹明B 市政污泥 FeCl3溶液前处理 69.8 — 98.3 [64] 牛仔蓝 市政污泥 10%HCl改性 70 — 99.9 [65] 偶氮活性红31 制皮厂污泥 HNO3 188.25 39.37 — [14] 偶氮染料 剩余污泥 纳米α-FOOH+碳酸钾 202 98.03 — [66] 普施安红色 市政污泥 F2O3 39.84 25.06 — [67] 普施安红色5B 纸浆污泥 nZVI 49.08 18.83 — [68] 酸性橙2 市政污泥 F2O3 605.2 385 — [67] 酸性橙G 铝盐污泥 碳纳米管 — — 93 [69] 酸性橙G 市政污泥 FeCl3溶液后处理 — — 99.33 [70] 亚甲基蓝 市政污泥 石墨烯 — 174 — [55] 亚甲基蓝 市政污泥 HNO3 250 — 45 [71] 亚甲基蓝 纸浆污泥 Fe2O3+FeCl3 — 11.78 95 [72] 亚甲基蓝 市政污泥 乙醇-水(5∶1)混合处理 — 132.2 — [2] 亚甲基蓝 棕榈油厂污泥 KOH 23.5 23.5 — [43] 亚甲基蓝 棕榈油厂污泥 ZnCl2 29.2 22.4 — [43] 亚甲基蓝 纸浆污泥 HCl 79.65 130.69 — [61] 亚甲基蓝 市政污泥 HNO3 605.2 345 — [73] 亚甲基蓝 剩余污泥 NaOH — 518 — [56] 亚甲基蓝 脱水污泥 TiO2 — 29.85 — [74] 表 3 改性污泥质生物炭吸附去除有机酚类
Table 3. Organic phenol adsorption by modified sludge-based biochar
污染物种类Contaminant 生物质Biomass 改性方法Modified method 比表面积/(m2 ·g−1)Surface area 吸附容量Adsorption capacity 吸附效率/%Adsorption efficiency 参考文献Reference 4-对氯苯酚 市政污泥 柠檬酸+ZnCl2 792.4 2.9 mmol·g−1 — [26] 4-对氯苯酚 干污泥 CO2 1800 3.21 mmol·g−1 85 [79] 4-对氯苯酚 干污泥 空气 1800 2.37 mmol·g−1 — [79] 4-对氯苯酚 干污泥 KOH预处理 1800 2.82 mmol·g−1 — [79] 对硝基苯酚 市政污泥 FeCl3溶液前处理 69.8 — 97 [64] 苯酚 市政污泥 柠檬酸+ZnCl2 792.4 2.1 mmol·g−1 — [26] 苯酚 市政污泥 NaOH 121 96.15 mg·g−1 — [80] 苯酚 市政污泥 ZnCl2 — — 98.7 [16] 苯酚 市政污泥 尿素 — — 96 [78] 苯酚 生物污泥 — — — 98.7 [16] 双酚A 市政污泥 尿素 — — 90 [78] 表 4 改性污泥质生物炭吸附去除药物活性物质
Table 4. Pharmacoactive substance adsorption by modified sludge-based biochar
污染物种类Contaminant 生物质Biomass 改性方法Modified method 比表面积/ (m2·g−1)Surface area 吸附容量/(mg·g−1)Adsorption capacity 吸附效率/%Adsorption efficiency 参考文献Reference 1-重氮基-2-萘酚-4-磺酸 市政污泥 Fe3O4 585 117.8 — [83] 2,4-二氯苯氧基乙酸 高炉瓦斯泥 柠檬酸 380 212 — [84] 2-萘酚 市政污泥 TiO2 321 111.9 — [85] Diazothiophene 市政污泥 KMnO4 629 55.5 — [86] 苯 石化污泥 碳纳米管 624 369 — [87] 苯甲酸 市政污泥 柠檬酸+ZnCl2 792.4 3.49 mmol·g−1 — [26] 丙酮 市政污泥 ZVI — 30 — [26] 草甘膦 铝盐污泥 NaOH — 113.6 91.6 [88] 粗甘油 市政污泥 纳米氧化铝 940 8.88 93 [81] 氟苯氧丙胺 纸浆污泥 KOH 115 191.6 — [89] 氟苯氧丙胺 纸浆污泥 NaOH 60 136.6 — [89] 氟苯氧丙胺 纸浆污泥 ZnCl2 592 28.4 — [89] 甲苯 市政污泥 柠檬酸 117 0.32 — [90] 甲苯 市政污泥 ZnCl2 57.7 0.43 — [90] 甲苯 市政污泥 柠檬酸+ZnCl2 869.5 0.83 — [90] 甲苯 市政污泥 Bi(NO3)3·5H2O 990 350 — [90] 卡巴咪嗪 市政污泥 硝酸铵 67 5.2 — [91] 氯仿 市政污泥 HNO3 757 113 — [92] 氯四环素 市政污泥 ZVI — 309.9 — [92] 柠檬烯 市政污泥 KMnO4 — 640 — [93] 氰化甲烷 市政污泥 碳纳米管 — 150 — [93] 培氟沙星 市政污泥 氧化石墨烯 — 137.51 — [82] 全氟羧酸 市政污泥 石墨烯 — 2.72 — [42] 石油废水 油罐底泥 NaCl 53.8 405 — [94] 四环素 市政污泥 氯化铈预处理 — — 93.4 [95] 四环素 市政污泥 FeCl3·6H2O 139 672 67 [96] 硝基苯 活性污泥 十六烷基三甲基溴化铵 — 40.6 — [97] 有机化合物 生物处理污泥 硝酸铵 89.5 — 66.71 [98] 表 5 污泥质生物炭再生方法的适用性及优缺点比较
Table 5. Comparison of the scope of application and advantages and disadvantages of regeneration methods
再生方法Regeneration method 适用范围Scope of application 优点Advantages 缺点Disadvantages 参考文献Reference 热再生 大规模应用于工业及污水处理厂 工艺成熟,再生时间较短,成本低,经济适用性强 再生损失大,运行条件严格 [102] 溶剂再生 大规模应用于工业 工艺设备简单,使用无机溶剂操作成本低,适用于吸附高浓度、难降解、低沸点有机污染物的脱附 设备易腐蚀,再生液容易造成二次污染 [103] 微波辐照再生 仅处于实验室阶段 再生时间短,易控制加热温度 设备复杂,成本高 [104] 超临界流体再生 仅处于实验室阶段 操作周期短、操作温度低、生物炭的损失低,适用于吸附高挥发性有机物的生物炭 成本高,超临界流体易造成二次污染 [105] -
[1] WEN H, GU L, YU H, et al. Radical assisted iron impregnation on preparing sewage sludge derived Fe/carbon as highly stable catalyst for heterogeneous Fenton reaction [J]. Chemical Engineering Journal, 2018, 352(1): 837-846. [2] 潘紫倩, 黄华军, 何小武, 等. 污泥液化生物炭对亚甲基蓝的吸附特性及机理 [J]. 中国环境科学, 2020, 40(1): 217-226. doi: 10.3969/j.issn.1000-6923.2020.01.024 PAN Z Q, HUANG H J, HE X W, et al. Adsorption characteristics and mechanism of methylene blue by sludge liquefaction biochar [J]. China Environmental Sicence, 2020, 40(1): 217-226(in Chinese). doi: 10.3969/j.issn.1000-6923.2020.01.024
[3] 刘蕾, 付临汝, 杜馨, 等. 联合改性污泥吸附剂去除废水中铬(Ⅵ) [J]. 环境化学, 2018, 37(12): 16-22. LIU L, FU L R, DU X, et al. Removal of Cr (Ⅵ) from wastewater by sludge-based adsorbents with combined modification [J]. Environmental Chemistry, 2018, 37(12): 16-22(in Chinese).
[4] 余丽, 刘允康, ATTIMAMA, 等. CWPO体系中污泥炭催化降解头孢氨苄废水 [J]. 环境化学, 2020, 39(5): 1262-1270. doi: 10.7524/j.issn.0254-6108.2019050602 YU L, LIU Y-K, ATTIMAMA, et al. Catalytic wet peroxide oxidation of wastewater containing cephalexin with sludge derived carbon catalyst [J]. Environmental Chemistry, 2020, 39(5): 1262-1270(in Chinese). doi: 10.7524/j.issn.0254-6108.2019050602
[5] 刘殷迪, 周真明, 张红忠, 等. 煅烧改性净水厂污泥的除磷特性 [J]. 环境化学, 2019, 38(2): 325-333. doi: 10.7524/j.issn.0254-6108.2018030203 LIU Y D, ZHOU Z M, ZHANG H Z, et al. Phosphorus removal characteristics of calcined water treatment plant sludge [J]. Environmental Chemistry, 2019, 38(2): 325-333(in Chinese). doi: 10.7524/j.issn.0254-6108.2018030203
[6] 于颖, 于俊清, 严志宇. 污水污泥微波辅助快速热裂解制生物油和合成气 [J]. 环境化学, 2013, 32(3): 486-491. YU Y, YU J Q, YAN Z Y. Rapid pyrolysis of sewage sludge for the production of bio-oil and syngas under microwave radiation [J]. Environmental Chemistry, 2013, 32(3): 486-491(in Chinese).
[7] WANG X, BAYAN M R, YU M, et al. Atomic layer deposition surface functionalized biochar for adsorption of organic pollutants: improved hydrophilia and adsorption capacity [J]. International Journal of Environmental Science & Technology, 2017, 13762(17): 1300-1208. [8] 任爱玲, 符凤英, 曲一凡, 等. 改性污泥活性炭对苯乙烯的吸附 [J]. 环境化学, 2013, 32(5): 833-838. doi: 10.7524/j.issn.0254-6108.2013.05.016 REN A L, FU F Y, QU Y F, et al. Adsorption properties of modified activated sludge carbon to styrene [J]. Environmental Chemistry, 2013, 32(5): 833-838(in Chinese). doi: 10.7524/j.issn.0254-6108.2013.05.016
[9] DEVI P, SAROHA A K. Synthesis of the magnetic biochar composites for use as an adsorbent for the removal of pentachlorophenol from the effluent [J]. Bioresource Technology, 2014, 169: 525-531. doi: 10.1016/j.biortech.2014.07.062 [10] LI W H, YUE Q Y, GAO B Y, et al. Preparation of sludge-based activated carbon made from paper mill sewage sludge by steam activation for dye wastewater treatment [J]. Desalination, 2011, 278(1/2/3): 179-185. [11] PENG P, LANG Y H, WANG X M. Adsorption behavior and mechanism of pentachlorophenol on reed biochars: pH effect, pyrolysis temperature, hydrochloric acid treatment and isotherms [J]. Ecological Engineering, 2016, 90: 225-233. doi: 10.1016/j.ecoleng.2016.01.039 [12] LAU A Y T, TSANG D C W, GRAHAM N J D, et al. Surface-modified biochar in a bioretention system for Escherichia coli removal from stormwater [J]. Chemosphere, 2017, 169(7): 89-98. [13] AZARGOHAR R, DALAI A K. Steam and KOH activation of biochar: Experimental and modeling studies [J]. Microporous & Mesoporous Materials, 2008, 110(2/3): 413-421. [14] GEETHAKARTHI A, PHANIKUMAR B R. Adsorption of reactive dyes from aqueous solutions by tannery sludge developed activated carbon: Kinetic and equilibrium studies [J]. International Journal of Environmental Science & Technology, 2011, 8(3): 561-570. [15] GóMEZ-PACHECO C V, RIVERA-UTRILLA J, SáNCHEZ-POLO M, et al. Optimization of the preparation process of biological sludge adsorbents for application in water treatment [J]. Journal of Hazardous Materials, 2012, 217/218(5): 76-84. [16] MARTIN. Carbonaceous adsorbents from sewage sludge and their application in a combined activated sludge-powdered activated carbon (AS-PAC) treatment [J]. Carbon, 2004, 42(7): 1389-1394. doi: 10.1016/j.carbon.2004.01.011 [17] 姚娟, 石太宏, 刘金凤, 等. 市政污泥制备铁/碳复合材料的研究与应用 [J]. 环境工程, 2016, 34(S1): 674-678. YAO J, SHI T H, LIU J F, et al. Study and application of iron/carbon complex materials preparated by municipal sludge [J]. Environmental Engineering, 2016, 34(S1): 674-678(in Chinese).
[18] AGRAFIOTI E, KALDERIS D, DIAMADOPOULOS E. Ca and Fe modified biochars as adsorbents of arsenic and chromium in aqueous solutions [J]. Journal of Environmental Management, 2014, 146: 444-450. doi: 10.1016/j.jenvman.2014.07.029 [19] INYANG M, GAO B, ZIMMERMAN A, et al. Sorption and cosorption of lead and sulfapyridine on carbon nanotube-modified biochars [J]. Environmental Science & Pollution Research, 2015, 22(3): 1868-1876. [20] SONG Z, LIAN F, YU Z, et al. Synthesis and characterization of a novel MnOx-loaded biochar and its adsorption properties for Cu2+ in aqueous solution [J]. Chemical Engineering Journal, 2014, 242: 36-42. doi: 10.1016/j.cej.2013.12.061 [21] LING, ZHU, LIHONG, et al. Coupling interaction between porous biochar and nano zero valent iron/nano α-hydroxyl iron oxide improves the remediation efficiency of cadmium in aqueous solution [J]. Chemosphere, 2018, 219: 493-503. [22] SINGH V, SRIVASTAVA V C. Self-engineered iron oxide nanoparticle incorporated on mesoporous biochar derived from textile mill sludge for the removal of an emerging pharmaceutical pollutant [J]. Environmental Pollution, 2020, 259(4): 1-9. [23] ZHI Z, XU T, TIANSHUAI W, et al. Insight into the effect of Co-doped to the photocatalytic performance and electronic structure of g-C3N4 by first principle [J]. Applied Catalysis B:Environmental, 2018, 241(3): 397-412. [24] 李敏, 李海岩, 孙发民, 等. 高比表面积石墨化氮化碳的制备及应用 [J]. 石油学报(石油加工), 2014, 30(1): 158-168. LI M, LI H Y, SU F M, et al. Synthesis and application of high-surface-area graphitic carbon nitride [J]. Acta Petrolei Sinica(Petroleum Processing Section), 2014, 30(1): 158-168(in Chinese).
[25] MUNIR, AHMAD, ADEL, et al. Engineered biochar composites with zeolite, silica, and nano-zerovalent iron for the efficient scavenging of chlortetracycline from aqueous solutions [J]. Environmental Science & Pollution Research International, 2019, 26(15): 15136-15152. [26] KONG L, XIONG Y, SUN L, et al. Sorption performance and mechanism of a sludge-derived char as porous carbon-based hybrid adsorbent for benzene derivatives in aqueous solution [J]. Journal of Hazardous Materials, 2014, 274(15): 205-211. [27] FU Y, QIN L, HUANG D, et al. Chitosan functionalized activated coke for Au nanoparticles anchoring: Green synthesis and catalytic activities in hydrogenation of nitrophenols and azo dyes [J]. Applied Catalysis B:Environmental, 2019, 255: 1-11. [28] PIGNATELLO J, KWON S, LU Y. Effect of natural organic substances on the surface and adsorptive properties of environmental black carbon (char): Attenuation of surface activity by humic and fulvic acids [J]. Environmental Science & Technology, 2016, 40(24): 7757-7763. [29] NGUYEN T H, CHO H H, POSTER D L, et al. Evidence for a pore-filling mechanism in the adsorption of aromatic hydrocarbons to a natural wood char [J]. Environmental Science & Technology, 2007, 41(4): 1212-1217. [30] KASOZI G N, ZIMMERMAN A R, NKEDI-KIZZA P, et al. Catechol and humic acid sorption onto a range of laboratory-produced black carbons (biochars) [J]. Environmental Science & Technology, 2010, 44(16): 6189-6195. [31] CHEN B, ZHOU D, ZHU L. Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures [J]. Environmental Science & Technology, 2008, 42(14): 5137-5143. [32] KEILUWEIT M, NICO P S, JOHNSON M G, et al. Dynamic molecular structure of plant biomass-derived black carbon (biochar) [J]. Environmental Science & Technology, 2010, 44(4): 1247-1253. [33] KLEINEIDAM S, SCHüTH C, GRATHWOHL P. Solubility-normalized combined adsorption-partitioning sorption isotherms for organic pollutants [J]. Environmental Science & Technology, 2002, 36(21): 4689-4697. [34] MURPHY E M, ZACHARA J M, SMITH S C, et al. Interaction of hydrophobic organic compounds with mineral-bound humic substances [J]. Environmental Science & Technology, 1994, 28(7): 1291-1299. [35] CHEN X, XIA X, WANG X, et al. A comparative study on sorption of perfluorooctane sulfonate (PFOS) by chars, ash and carbon nanotubes [J]. Chemosphere, 2011, 83(10): 1313-1319. doi: 10.1016/j.chemosphere.2011.04.018 [36] ZHU D, PIGNATELLO J J. Characterization of aromatic compound sorptive interactions with black carbon (charcoal) assisted by graphite as a model [J]. Environmental Science & Technology, 2005, 39(7): 2033-2041. [37] 李广. 生物炭去除有机污染物的初步探讨 [J]. 山东化工, 2019, 48(20): 260-261. doi: 10.3969/j.issn.1008-021X.2019.20.109 LI G. Study on Removal of organic pollutants by biochar [J]. Shan Dong Chemical Industry, 2019, 48(20): 260-261(in Chinese). doi: 10.3969/j.issn.1008-021X.2019.20.109
[38] ROCHEFORT A, WUEST J D. Interaction of substituted aromatic compounds with graphene [J]. Langmuir, 2009, 25(1): 210-215. doi: 10.1021/la802284j [39] SPOKAS, A K. Review of the stability of biochar in soils: predictability of O: C molar ratios [J]. Carbon Management, 2010, 1(2): 289-303. doi: 10.4155/cmt.10.32 [40] KE S, JIN J, KEILUWEIT M, et al. Polar and aliphatic domains regulate sorption of phthalic acid esters (PAEs) to biochars [J]. Bioresource Technology, 2012, 118: 120-127. doi: 10.1016/j.biortech.2012.05.008 [41] ZHENG H, WANG Z, ZHAO J, et al. Sorption of antibiotic sulfamethoxazole varies with biochars produced at different temperatures [J]. Environmental Pollution, 2013, 181(12): 60-67. [42] ZHANG P, SUN H, YU L, et al. Adsorption and catalytic hydrolysis of carbaryl and atrazine on pig manure-derived biochars: Impact of structural properties of biochars [J]. Journal of Hazardous Materials, 2013, 244-245(4): 217-224. [43] ZAINI M A A, ZAKARIA M, MOHD-SETAPAR S H, et al. Sludge-adsorbents from palm oil mill effluent for methylene blue removal [J]. Journal of Environmental Chemical Engineering, 2013, 1(4): 1091-1098. doi: 10.1016/j.jece.2013.08.026 [44] XU R K, XIAO S C, YUAN J H, et al. Adsorption of methyl violet from aqueous solutions by the biochars derived from crop residues [J]. Bioresource Technology, 2011, 102(22): 10293-10298. doi: 10.1016/j.biortech.2011.08.089 [45] 刘蕊, 罗璇, 刘兴. 不同温度制备的HNO3改性生物炭结构表征研究 [J]. 贵州师范学院学报, 2018, 6: 21-24. doi: 10.3969/j.issn.1674-7798.2018.02.005 LIU R, LUO X, LIU X. Study on characterization of HNO3-modified biochar produced in different temperatures [J]. Journal of Guizhou Educational Institute, 2018, 6: 21-24(in Chinese). doi: 10.3969/j.issn.1674-7798.2018.02.005
[46] MUKHERJEE A, ZIMMERMAN A R, HARRIS W. Surface chemistry variations among a series of laboratory-produced biochars [J]. Geoderma, 2011, 163(3/4): 247-255. [47] ZHANG D, PAN B, WU M, et al. Adsorption of sulfamethoxazole on functionalized carbon nanotubes as affected by cations and anions [J]. Environmental Pollution, 2011, 159(10): 2616-2621. doi: 10.1016/j.envpol.2011.05.036 [48] PEI Z, YANG S, LI L, et al. Effects of copper and aluminum on the adsorption of sulfathiazole and tylosin on peat and soil [J]. Environmental Pollution, 2013, 184(1): 579-585. [49] CHEN C, ZHOU W, LIN D. Sorption characteristics of N-nitrosodimethylamine onto biochar from aqueous solution [J]. Bioresource Technology, 2015, 179: 359-366. doi: 10.1016/j.biortech.2014.12.059 [50] SUN H, PENG X, ZHANG S, et al. Activation of peroxymonosulfate by nitrogen-functionalized sludge carbon for efficient degradation of organic pollutants in water [J]. Bioresource Technology, 2017, 241: 244-251. doi: 10.1016/j.biortech.2017.05.102 [51] KAUR K, JINDAL R. Comparative study on the behaviour of Chitosan-Gelatin based hydrogel and nanocomposite ion exchanger synthesized under microwave conditions towards photocatalytic removal of cationic dyes [J]. Carbohydrate Polymers, 2019, 207: 398-410. doi: 10.1016/j.carbpol.2018.12.002 [52] MéNDEZ A, PAZ-FERREIRO J, GIL E, et al. The effect of paper sludge and biochar addition on brown peat and coir based growing media properties [J]. Scientia Horticulturae, 2015, 193: 225-230. doi: 10.1016/j.scienta.2015.07.032 [53] YAGUB M T, SEN T K, AFROZE S, et al. Dye and its removal from aqueous solution by adsorption: A review [J]. Advances in Colloid & Interface Science, 2018, 209: 172-184. [54] HE K, CHEN G, ZENG G, et al. Three-dimensional graphene supported catalysts for organic dyes degradation [J]. Applied Catalysis B Environmental, 2018, 228: 19-28. doi: 10.1016/j.apcatb.2018.01.061 [55] ZHANG M, GAO B, YAO Y, et al. Synthesis, characterization, and environmental implications of graphene-coated biochar [J]. Science of the Total Environment, 2012, 435/436(3): 567-572. [56] VASQUES A R, SOUZA S M G U D, VALLE J A B, et al. Application of ecological adsorbent in the removal of reactive dyes from textile effluents [J]. Journal of Chemical Technology & Biotechnology, 2009, 84(8): 1146-1155. [57] FAN S S, TANG J, WANG Y, et al. Biochar prepared from co-pyrolysis of municipal sewage sludge and tea waste for the adsorption of methylene blue from aqueous solutions: Kinetics, isotherm, thermodynamic and mechanism [J]. Journal of Molecular Liquids, 2016, 98(7): 145-165. [58] LENG L, YUAN X, HUANG H, et al. Biochar derived from sewage sludge by liquefaction: Characterization and application for dye adsorption [J]. Applied Surface Ence, 2015, 346(15): 223-231. [59] CHAUKURA N, MURIMBA E C, GWENZI W. Synthesis, characterisation and methyl orange adsorption capacity of ferric oxide–biochar nano-composites derived from pulp and paper sludge [J]. Applied Water Science, 2017, 7(4): 2175-2186. [60] YILMAZ A E, BONCUKCUOĞLU R, KOCAKERIM M, et al. Waste utilization: The removal of textile dye (Bomaplex Red CR-L) from aqueous solution on sludge waste from electrocoagulation as adsorbent [J]. Desalination, 2011, 277(1/2/3): 156-163. [61] LI W, YUE Q, PENG T, et al. Adsorption characteristics of dyes in columns of activated carbon prepared from paper mill sewage sludge [J]. Chemical Engineering Journal, 2011, 178: 197-203. doi: 10.1016/j.cej.2011.10.049 [62] LI Z, JIANG N, WU F, et al. Experimental investigation of phosphorus adsorption capacity of the waterworks sludges from five cities in China [J]. Ecological Engineering, 2013, 53: 165-172. doi: 10.1016/j.ecoleng.2012.12.038 [63] TRUJILLO-REYES J, SOLACHE-RIOS M, VUCHIS-NESTOR A R, et al. Fe-Ni nanostructures and C/Fe-Ni composites as adsorbents for the removal of a textile dye from aqueous solution [J]. Water Air & Soil Pollution, 2012, 223(3): 1331-1341. [64] 王文刚, 陶红, 戴晓虎. 脱水污泥基铁炭复合材料用于光Fenton催化降解有机污染物 [J]. 环境工程学报, 2020, 14(8): 2232-2241. doi: 10.12030/j.cjee.201911101 WANG W G, TAO H, DAI X H. Dewatered sludge derived iron-carbon composite as a photo-Fenton catalyst for organic pollutant degradation [J]. Chinese Journal of Environmental Engineering, 2020, 14(8): 2232-2241(in Chinese). doi: 10.12030/j.cjee.201911101
[65] GUTIERREZ-SEGURA E, COLIN-CRUZ A, SOLACHE-RIOS M, et al. Removal of Denim Blue from aqueous solutions by inorganic adsorbents in a fixed-bed column [J]. Water Air & Soil Pollution, 2012, 223(8): 5505-5513. [66] ATHALATHIL S, STüBER F, BENGOA C, et al. Characterization and performance of carbonaceous materials obtained from exhausted sludges for the anaerobic biodecolorization of the azo dye Acid Orange Ⅱ [J]. Journal of Hazardous Materials, 2014, 267(2): 21-30. [67] HU S H, HU S C. Kinetics of ionic dyes adsorption with magnetic-modified sewage sludge [J]. Environmental Progress & Sustainable Energy, 2014, 33(3): 905-912. [68] HU S H, HU S C. Pyrolysis of paper sludge and utilization for ionic dye adsorption [J]. Bioresources, 2013, 8(1): 1-16. [69] TONG D S, LIU M, LI L, et al. Transformation of alunite residuals into layered double hydroxides and oxides for adsorption of acid red G dye [J]. Applied Clay Science, 2012, 70: 1-7. doi: 10.1016/j.clay.2012.08.001 [70] 张倩, 谢陈飞洋, 仇玥, 等. Fe/污泥基生物炭持久活化过硫酸盐降解酸性橙G [J]. 中国环境科学, 2019, 39(9): 3879-3886. doi: 10.3969/j.issn.1000-6923.2019.09.034 ZHANG Q, XIE C F Y, QIU Y, et al. Durable degradation of orange G using persulfate activated by sludge-derived heterogeneous catalyst [J]. China Environmental Sicence, 2019, 39(9): 3879-3886(in Chinese). doi: 10.3969/j.issn.1000-6923.2019.09.034
[71] SMITH K M, FOWLER G D, PULLKET S, et al. The production of attrition resistant, sewage–sludge derived, granular activated carbon [J]. Separation & Purification Technology, 2012, 98: 240-248. [72] ZHU X, LI C, LI J, et al. Thermal treatment of biochar in the air/nitrogen atmosphere for developed mesoporosity and enhanced adsorption to tetracycline [J]. Bioresource Technology, 2018, 263(5): 475-483. [73] KONG L, TIAN S, LUO R, et al. Demineralization of sludge-based adsorbent by post-washing for development of porosity and removal of dyes [J]. Journal of Chemical Technology & Biotechnology, 2013, 88(8): 1473-1480. [74] FAN S, WANG Y, WANG Z, et al. Removal of methylene blue from aqueous solution by sewage sludge-derived biochar: Adsorption kinetics, equilibrium, thermodynamics and mechanism [J]. Journal of Environmental Chemical Engineering, 2017, 5(1): 601-611. doi: 10.1016/j.jece.2016.12.019 [75] CHO D W, KIM S, TSANG Y F, et al. Preparation of nitrogen-doped Cu-biochar and its application into catalytic reduction of p-nitrophenol [J]. Environmental Geochemistry & Health, 2017, 1: 1-9. [76] FITZGERALD S, KOLAR P, CLASSEN J, et al. Swine manure char as an adsorbent for mitigation of p-cresol [J]. Environmental Progress & Sustainable Energy, 2015, 34(1): 125-131. [77] MANFRED C, BIRGIT S, ERNIS S, et al. Adsorption of bisphenol-A, 17β-estradiole and 17α-ethinylestradiole to sewage sludge [J]. Chemosphere, 2004, 56(9): 843-851. doi: 10.1016/j.chemosphere.2004.04.048 [78] WANG J, KOU L, ZHAO L, et al. One-pot fabrication of sludge-derived magnetic Fe, N-codoped carbon catalysts for peroxymonosulfate-induced elimination of phenolic contaminants [J]. Chemosphere, 2020, 248: 126076-126087. doi: 10.1016/j.chemosphere.2020.126076 [79] MONSALVO V M, MOHEDANO A F, RODRIGUEZ J J. Adsorption of 4-chlorophenol by inexpensive sewage sludge-based adsorbents [J]. Chemical Engineering Research & Design, 2012, 90(11): 1807-1814. [80] ZOU J, DAI Y, WANG X, et al. Structure and adsorption properties of sewage sludge-derived carbon with removal of inorganic impurities and high porosity [J]. Bioresource Technology, 2013, 142: 209-217. doi: 10.1016/j.biortech.2013.04.064 [81] HUNSOM M, AUTTHANIT C. Adsorptive purification of crude glycerol by sewage sludge-derived activated carbon prepared by chemical activation with H3PO4, K2CO3 and KOH [J]. Chemical Engineering Journal, 2013, 229(2): 334-343. [82] 郑永昕, 魏东宁, 余学, 等. 氧化石墨烯改性污泥基生物炭对培氟沙星的去除机理研究 [J]. 环境科学研究, 2020, 33(12): 2879-2887. ZHENG Y X, WEI D N, YU X, et al. Removal mechanism of pefloxacin by graphene oxide modified sludge based biochar [J]. Research of Environmental Sciences, 2020, 33(12): 2879-2887(in Chinese).
[83] GU L, WANG Y, ZHU N, et al. Enhanced adsorptive removal of naphthalene intermediates from aqueous solution by introducing reed straw into sewage sludge-based activated carbon [J]. Environmental Science & Pollution Research International, 2014, 21(3): 2043-2053. [84] GUPTA V K, ALI I, SUHAS, et al. Adsorption of 2, 4-D and carbofuran pesticides using fertilizer and steel industry wastes [J]. Journal of Colloid & Interface Science, 2006, 299(2): 556-563. [85] GU L, WANG Y, ZHU N, et al. Preparation of sewage sludge based activated carbon by using Fenton's reagent and their use in 2-naphthol adsorption [J]. Bioresour Technol, 2013, 146(4): 779-784. [86] NUNTHAPRECHACHAN T, PENGPANICH S, HUNSOM M. Adsorptive desulfurization of dibenzothiophene by sewage sludge-derived activated carbon [J]. Chemical Engineering Journal, 2013, 228(28): 263-271. [87] CHIANG H L, LIN K H, CHEN C Y, et al. Adsorption characteristics of benzene on biosolid adsorbent and commercial activated carbons [J]. Journal of the Air & Waste Management Association, 2006, 56(5): 591-600. [88] HU Y S, ZHAO Y Q, SOROHAN B. Removal of glyphosate from aqueous environment by adsorption using water industrial residual [J]. Desalination, 2011, 271(1/2/3): 150-156. [89] JARIA G, CALISTO V, GIL M V, et al. Removal of fluoxetine from water by adsorbent materials produced from paper mill sludge [J]. J Colloid Interface Sci, 2015, 448(7): 32-40. [90] ANFRUS A, CANALS C-B, ROS A, et al. Removal of odour-causing compounds using carbonaceous adsorbents/catalysts prepared from sewage sludge [J]. Water Science & Technology, 2009, 59(7): 1371-1376. [91] NIELSEN L, ZHANG P, BANDOSZ T J. Adsorption of carbamazepine on sludge/fish waste derived adsorbents: Effect of surface chemistry and texture [J]. Chemical Engineering Journal, 2015, 267: 170-181. doi: 10.1016/j.cej.2014.12.113 [92] TSAI J H, CHIANG H M, HUANG G Y, et al. Adsorption characteristics of acetone, chloroform and acetonitrile on sludge-derived adsorbent, commercial granular activated carbon and activated carbon fibers [J]. Journal of Hazardous Materials, 2008, 154(1/2/3): 1183-1191. [93] CHUN Y N, JI D W, YOSHIKAWA K. Pyrolysis and gasification characterization of sewage sludge for high quality gas and char production [J]. Journal of Mechanical Science & Technology, 2013, 27(1): 263-272. [94] SUEYOSHI M, AL-MAAMARI R S, JIBRIL B, et al. Preparation and characterization of adsorbents for treatment of water associated with oil production [J]. Journal of Analytical & Applied Pyrolysis, 2012, 97: 80-87. [95] ZHANG W, TANG M, YANG P, et al. Micro-interfacial mechanisms on sludge dewaterability enhancement using cerium chloride for preparation of carbon-based functional material [J]. Journal of Hazardous Materials, 2020, 386: 121930-121943. doi: 10.1016/j.jhazmat.2019.121930 [96] OH T K, CHOI B, SHINOGI Y, et al. Effect of pH conditions on actual and apparent fluoride adsorption by biochar in aqueous phase [J]. Water Air & Soil Pollution, 2012, 223(7): 3729-3738. [97] PAN J, GUAN B. Adsorption of nitrobenzene from aqueous solution on activated sludge modified by cetyltrimethylammonium bromide [J]. Journal of Hazardous Materials, 2010, 183(1/2/3): 341-346. [98] PAN Z H, TIAN J Y, XU G R, et al. Characteristics of adsorbents made from biological, chemical and hybrid sludges and their effect on organics removal in wastewater treatment [J]. Water Research, 2010, 45(2): 819-827. [99] 王静松, 刘杰, 唐蕾. 污泥基生物质炭在水处理中的应用 [J]. 化工管理, 2020, 7: 113-114. doi: 10.3969/j.issn.1008-4800.2020.15.073 WANG J S, LIU J, TANG L. Application of wasted sludge-based biochar in water treatment [J]. Chemical Enterprise Management, 2020, 7: 113-114(in Chinese). doi: 10.3969/j.issn.1008-4800.2020.15.073
[100] 罗惠莉, 周静如, 冯逸轲, 等. 市政污泥炭对盐酸四环素的吸附净化研究[C]// 2019中国环境科学学会科学技术年会论文集(第四卷), F, 2019. LUO H L, ZHOU J R, FENG Y K, et al. Studies on adsorption and purification of tetracycline hydrochloride from municipal sludge [C]// 2019 Proceedings of the Annual Meeting of Chinese Society for Environmental Science of Science and Technology (Vol. 4), F, 2019(in Chinese).
[101] 汪华, 方程冉, 王群, 等. 腐殖酸对生物炭吸附四环素的影响 [J]. 环境污染与防治, 2018, 40(4): 62-67. WANG H, FANG C R, WANG Q, et al. Effect of humic acid on the adsorption of tetracycline by biochar [J]. Environmental Pollution & Control, 2018, 40(4): 62-67(in Chinese).
[102] GUO D, LI Y, CUI B, et al. Natural adsorption of methylene blue by waste fallen leaves of Magnoliaceae and its repeated thermal regeneration for reuse [J]. Journal of Cleaner Production, 2020, 267: 1-8. [103] 白玉洁, 张爱丽, 周集体. 吸附剂再生技术的研究进展 [J]. 辽宁化工, 2012, 41(1): 21-24. doi: 10.3969/j.issn.1004-0935.2012.01.007 BAI Y J, ZHANG A L, ZHOU J T. Research process in regeneration technologies of sorbents [J]. Liao Ning Chemical Industry, 2012, 41(1): 21-24(in Chinese). doi: 10.3969/j.issn.1004-0935.2012.01.007
[104] LV Y, ZHANG J, ASGODOM M E, et al. Study on the degradation of accumulated bisphenol S and regeneration of magnetic sludge-derived biochar upon microwave irritation in the presence of hydrogen peroxide for application in integrated process [J]. Bioresource Technology, 2019, 293: 1-8. [105] FRANCISCO S, NICOLAS M S, RUTH S H, et al. Regeneration of carbonaceous adsorbents. Part Ⅱ: chemical, microbiological and vacuum regeneration [J]. Microporous and Mesoporous Materials, 2015, 202: 277-296. doi: 10.1016/j.micromeso.2014.08.019 [106] 夏晶晶. 胶乳生产污泥制备吸附剂对染料吸附/再生性能研究 [D]. 青岛: 青岛大学, 2019. XIA J J. Study on dye adsorption/regeneration performance of adsorbent prepared from latex production sludge [D]. Qingdao: Qingdao University, 2019(in Chinese).
[107] 祝林. 生物质和GO/TiO2复合材料对四环素的吸附作用及其再生研究 [D]. 合肥: 合肥工业大学, 2019. ZHU L. Study on the adsorption and regeneration of tetracycline by biomass and GO/TiO2 composite materials [D]. Hefei: Hefei University of Technology, 2019(in Chinese).
[108] LU P J, LIN H C, YU W T, et al. Chemical regeneration of activated carbon used for dye adsorption [J]. Journal of the Taiwan Institute of Chemical Engineers, 2011, 42(2): 305-311. doi: 10.1016/j.jtice.2010.06.001 期刊类型引用(14)
1. 谢谚. 土壤微塑料污染及微生物降解研究进展. 化工环保. 2024(01): 21-27 .  百度学术
百度学术
2. 游洋洋,张涛,梁增强,霍宁. 我国农田土壤中微塑料污染研究进展与环境管理现状. 环境生态学. 2024(02): 101-106 .  百度学术
百度学术
3. 刘贺,宋树贤,孙梅,李双双,于小晶,戴九兰. 土壤和植物中微塑料研究现状分析及检测方法研究进展. 地学前缘. 2024(02): 183-195 .  百度学术
百度学术
4. 吴思怡,谢福武,王少露,何书海,田毓婷,姜晓旭. 基于密度分离-氧化消解法前处理的土壤微塑料检测. 中国无机分析化学. 2024(08): 1091-1098 .  百度学术
百度学术
5. 谢福武,田毓婷,吴思怡,姜晓旭,何书海. 海南乐东农用地土壤微塑料分布状况调查. 中国无机分析化学. 2024(08): 1116-1125 .  百度学术
百度学术
6. 张学军,康梦晨,史增录,鄢金山,刘小鹏,郭磊,王美静. 双滚筒式收后地表残膜回收机设计与试验. 农业工程学报. 2024(14): 1-13 .  百度学术
百度学术
7. 陈方涛,孙鹏,刘国宁,张颖,谭金峰. 渤海表层海水微塑料污染与监测研究. 环境科学与管理. 2024(10): 131-136 .  百度学术
百度学术
8. 陈占,杨旅,刘海龙,王洪涛,李天鹏,崔红标. 土壤中微塑料污染及处理技术综述. 安徽农业科学. 2024(23): 16-19+24 .  百度学术
百度学术
9. 张彦,窦明,郝松泽,李平,王国豪,周雨泽,梁志杰. 中国农田土壤微塑料赋存特征研究进展及展望. 灌溉排水学报. 2024(12): 11-20 .  百度学术
百度学术
10. 朱强,张雨荷. 我国农田土壤微塑料污染的现实困境及防治策略. 辽宁农业科学. 2024(06): 72-77 .  百度学术
百度学术
11. 邓海洋,廖芷安琪,刘沛文,区华丽,曹耀宇,欧桦瑟. 华南地区污水处理厂污泥中微塑料的典型赋存特征. 净水技术. 2024(12): 84-95 .  百度学术
百度学术
12. 游洋洋,张涛,梁增强,霍宁. 新污染物视角下我国土壤环境管理现状分析. 环境生态学. 2023(09): 105-110 .  百度学术
百度学术
13. 高硕欣,刘璐璐,张凯旋,刘东阳,郭中领,常春平,张鉴达. 土壤微塑料的来源及分离鉴定方法. 山东化工. 2023(20): 136-138+142 .  百度学术
百度学术
14. 高洪娥,刘树宝,敖淑楠,刘潇,李玉红,杨军霞,杨彦武. 土壤污染成因及防治策略研究. 农业工程与装备. 2023(04): 11-13 .  百度学术
百度学术
其他类型引用(7)
-






 下载:
下载: