-
含高浓度磷酸盐的废水排放到地表水中,可造成各种水体污染问题,如水体富营养化[1]。水中磷酸盐、硝酸盐等无机阴离子刺激蓝藻过度生长,破坏生物多样性[2]。如果大量含氮、磷的废水排入湖泊、河口、水库等缓流水体,这些营养物质会使藻类等水生生物过量繁殖,进而导致水体透明度下降、水质恶化等一系列问题[3]。水体富营养化给人们的生产生活带已成为迫切需要解决的问题[4]。为了控制水体富营养化、进一步减少排入水体中的磷,自从上个世纪年代开始,研发人员对水体中磷的去除技术进行了大量的探索[5]。
吸附法作为一种成本低、操作简便、效率高的方法受到了不同研究者的广泛关注[6]。层状双金属氢氧化物(layered double hydroxides, LDHs)是一种层状材料,俗称水滑石,属于阴离子型层状化合物,由带正电荷的金属氢氧化物层和带负电荷的层间阴离子以及水分子构成[7-8],基于LDHs的无机吸附剂可以从水中去除氧化阴离子,如砷酸盐、铬酸盐和磷酸盐[9]。由于其特殊的层状结构和高比表面积,LDHs比大多数常见的氧阴离子吸附剂具有更强的吸附能力[10]。与其他吸附剂相比,LDHs的合成工艺相对简单,成本较低。但一般的二元LDHs材料的吸附能力相对较低,作为除磷吸附剂仍存在元素组成需进一步优化、吸附速率和吸附选择性需进一步提高等问题[11],而三元、四元LDHs材料与二元LDHs材料有类似的结构,却有更优越的物化性质和吸附能力,对于作为吸附法除磷的除磷剂有很大的应用前景[12]。JIAN等[13]用氧化共沉淀法制备了Fe、Al、Mn摩尔比为3∶3∶1的纳米铁铝锰三元金属氧化物吸附剂,该吸附剂对磷酸盐的最大吸附容量约为48.3 mg·g−1,高于文献报道的单组分氧化物铁铝锰三金属氧化物。
本研究对三元(Mg-Al-Zn)LDHs和四元(Mg-Al-Zn-Fe)LDHs吸附剂进行了制备,考察了LDHs基吸附剂对磷酸盐的吸附性能,探讨了吸附机理,利用响应曲面法(RSM)数学模型优化了吸附过程的相关参数。
-
六水合氯化镁(MgCl2·6H2O),六水合氯化铝(AlCl3·6H2O),氯化锌(ZnCl2),氯化铁(FeCl3),磷酸二氢钾(KH2PO4),氢氧化钠(NaOH),盐酸(HCl),均为分析纯,以上药剂均购于国药集团化学试剂有限公司。
-
本实验采用水热法制备多元LDHs材料[14],首先制备10%(质量分数)的NaOH溶液标记为溶液A,按照Mg∶Zn∶Al(∶Fe)摩尔比为1∶3∶1(∶0.5),称取一定量的AlCl3·6H2O、MgCl2·6H2O、ZnCl2(和FeCl3)于烧杯中,加20 mL去离子水溶解,记为B溶液。在转速300 r·min−1,温度80 ℃下将A、B溶液同时缓慢加入含20 mL纯水的烧杯中,并控制其pH在11左右,反应0.5 h,然后80 ℃陈化过夜,将得到的陈化产物离心、洗涤至中性、干燥、研磨,即得到MgZnAl-LDHs和MgZnAlFe-LDHs。
-
本实验中所使用的磷酸盐按照国标(GB 11893-1989)配制,吸附实验操作步骤:取(0.030 g±0.005) g的样品于150 mL锥形瓶中,加入30 mL 100 mg·L−1的磷酸盐溶液(pH=2),25 ℃,160 r·min−1振荡6 h,过滤后根据国标(GB 11893-1989)进行测定剩余磷酸盐的浓度(以P计),并根据式(1)计算平衡吸附量qe。
式中:qe为平衡吸附容量,mg·g−1;C0为溶液起始质量浓度,mg·L−1;Ce为溶液平衡质量浓度,mg·L−1;V为吸附溶液体积,L;m为吸附剂质量,g。
1)吸附等温线。分别称量(0.030±0.005) g的MgZnAl-LDHs和MgZnAlFe-LDHs加入到30 mL不同质量浓度(60~400 mg·L−1)的磷酸盐溶液中,调节pH=2,在25 ℃,160 r·min−1的条件下,于旋转式恒温振荡器中振荡6 h,吸附完成后,分别测定磷酸盐溶液浓度并计算磷酸盐的平衡吸附量。采用Langmuir(式(2))、Freundlich(式(3))和 Sips吸附等温模型(式(4))对吸附数据进行拟合。
式中:qe、qm分别为平衡吸附容量、吸附剂的吸附容量,mg·g−1;KL为Langmuir 吸附常数,L·mg−1;Ce为溶液平衡质量浓度,mg·L−1;KF为Freundlich 等温吸附常数,(mg·g−1) ·(mg·L−1)−n;n为吸附常数;qms为拟合最大平衡吸附量,mg·g−1;Ks为Sips吸附参数,(L·mg−1)ms;ms为非均一系数。
2)吸附动力学。分别称量(0.030±0.005) g的MgZnAl-LDHs和MgZnAlFe-LDHs加入锥形瓶中,加入30 mL 100 mg·L−1的磷酸盐溶液(pH=2),在25 ℃,160 r·min−1的条件下,于旋转式恒温振荡器中振荡,在0~300 min内测定磷酸盐浓度,并计算磷酸盐的吸附量。采用准一级(式(5))、准二级(式(6))和Elovich吸附动力学模型(式(7))对此动力学过程进行拟合。
式中:qe为平衡吸附容量,mg·g−1;qt为时间为t时的吸附容量,mg·g−1;t为吸附时间,min;k1为准一级动力学方程常数,min−1;k2为准一级动力学方程常数,g·(mg·min)−1;α为代表初始吸附速率的常数,g·(g·min)−1;β为解吸速率平衡常数,mg·g−1。
3) pH对吸附性能的影响。分别称量(0.030±0.005) g的MgZnAl-LDHs和MgZnAlFe-LDHs加入到30 mL 100 mg·L−1磷酸盐溶液中,用NaOH和HCl调节pH为2、3、4、5、6、7、8、9,在25 ℃,160 r·min−1的条件下,于旋转式恒温振荡器中振荡6 h,吸附完成后分别测定磷酸盐溶液浓度并计算磷酸盐的平衡吸附量。
4)共存离子对吸附性能影响。称量(0.030±0.005) g的MgZnAl-LDHs和MgZnAlFe-LDHs加入到30 mL 100 mg·L−1磷酸盐溶液中,分别加入0.1 mol·L−1的Cl−、
SO2−4 、NO−3 和HCO−3 ,并设无背景离子磷酸盐溶液为对照组,调节pH为2,在25 ℃,160 r·min−1的条件下,于旋转式恒温振荡器中振荡6 h,吸附完成后分别测定磷酸盐溶液浓度并计算磷酸盐的平衡吸附量。5)响应面法优化实验设计。在上述实验的基础上,跟据Box-Behnken Design的原理,以pH(X1)、温度(X2)、初始质量浓度(X3) 3个因子为自变量, 以磷酸盐平衡吸附量(Y)为响应值,设计17组优化实验,对MgZnAlFe-LDHs的吸附实验建立响应回归模型,并采用Design Expert 12软件对实验结果进行统计分析,分析各影响因子间的交互作用及对响应值的影响。
-
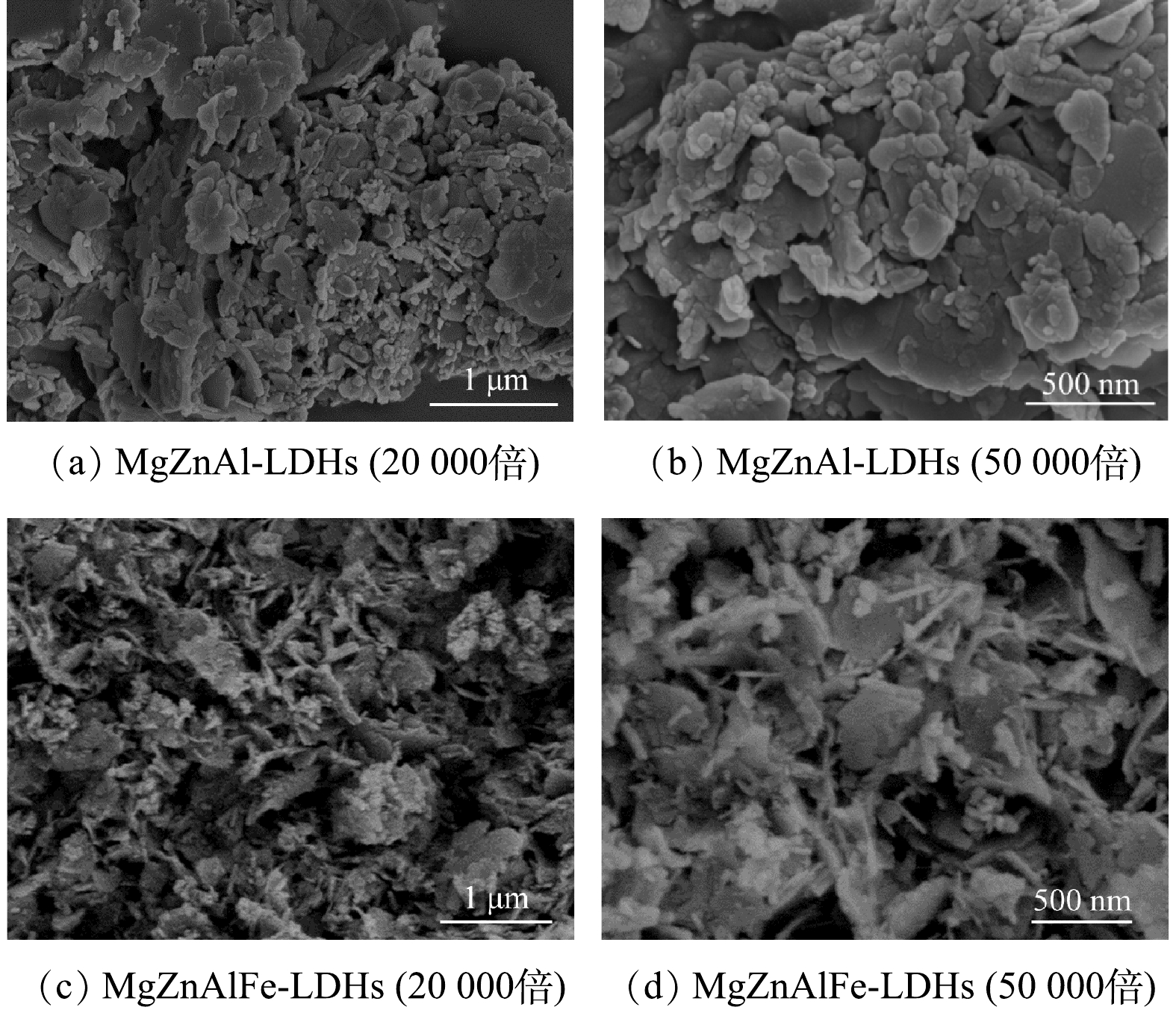
1) SEM分析。多元LDHs的SEM图如图1所示。图1(a)、图1(b)为MgZnAl-LDHs的SEM图,图1(c)、图1(d)为MgZnAlFe-LDHs的SEM表征图。由图1可以看出,MgZnAl-LDHs和MgZnAlFe-LDHs材料均具有清晰的片层结构。图1(a)、图1(b)显示MgZnAl-LDHs表面较为光滑,并有较多的孔隙,结构较疏松,而图1(c)、图1(d)显示的MgZnAlFe-LDHs较MgZnAl-LDHs而言有更多的孔隙,结构变得更加紧密。
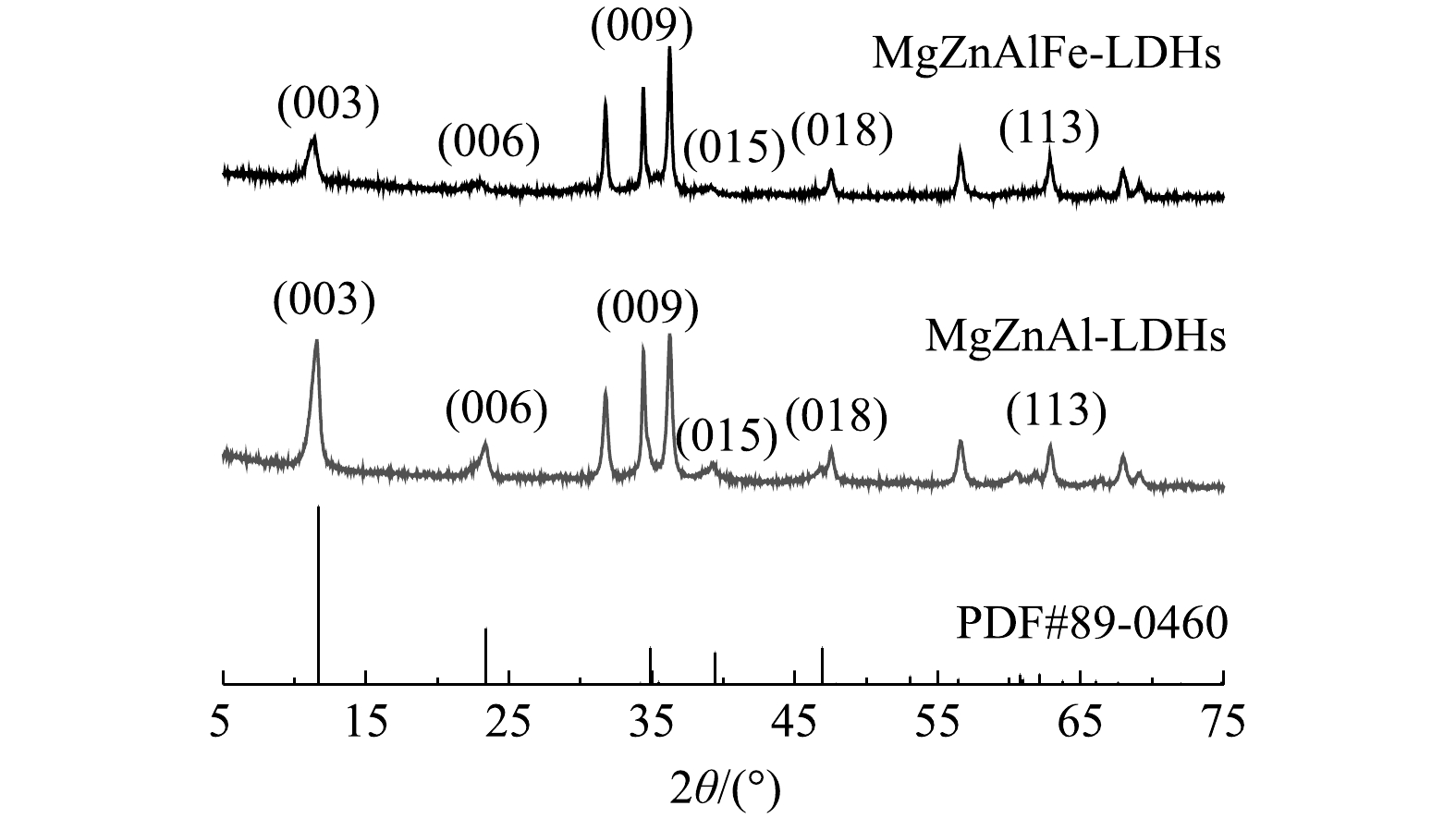
2) XRD分析。采用XRD对制备的吸附剂的晶体结构进行了研究。图2为MgZnAl-LDHs和MgZnAlFe-LDHs的XRD图谱。根据无机晶体结构数据库(ICSD)[15],图中XRD光谱均是典型的LDH相(与LDHs标准卡片PDF#89-0460高度符合),在(003)、(006)和(009)平面上有强烈的反射峰,在(015)、(018)和(113)平面上有宽的不对称峰,表明本实验制备的材料具有LDHs的结构特征。由图2可以看出,制备的MgZnAlFe-LDHs的峰与MgZnAl-LDHs相比发生了轻微的偏移,2θ分别为11.36°和11.55°,根据布拉格公式[16]计算层间距分别为0.778 nm和0.765 nm,可见四元LDHs相比三元LDHs层间通道有所增大。
-
1)吸附等温线。图3为MgZnAl-LDHs和MgZnAlFe-LDHs的吸附平衡等温线拟合结果,拟合参数如表1所示。由表1可知,MgZnAl-LDHs和MgZnAlFe-LDHs对磷酸盐的吸附等温模型符合Sips等温模型和Freundlich等温吸附模型,R2均大于0.9,这表明Sips模型和Freundlich等温吸附模型能够很好的解释MgZnAl-LDHs和MgZnAlFe-LDHs的等温吸附行为。MgZnAl-LDHs和MgZnAlFe-LDHs对磷酸盐的吸附符合Freundlich等温吸附模型方程说明两者可能是多层化学吸附[17],且其1/n值均小于1,说明两者均易吸附磷酸盐;KF值代表吸附剂的吸附能力,KF值越大,表明吸附剂对磷酸盐的吸附容量越大[18],MgZnAlFe-LDHs的KF值大于MgZnAl-LDHs,说明MgZnAlFe-LDHs较MgZnAl-LDHs吸附容量更大。同时,Sips拟合模型显示MgZnAl-LDHs和MgZnAlFe-LDHs对磷酸盐的最大拟合吸附量分别可达172.00 mg·g−1和189.62 mg·g−1。与MgAl-LDHs(76.8 mg·g−1)[19]相比,吸附量增大和吸附性能有所提高,同时MgZnAlFe-LDHs较MgZnAl-LDHs对磷酸盐的吸附量有所提高,这可能由于四元LDHs的层间通道宽于三元LDHs,同时吸附位点增多,使得磷酸盐吸附量增加。
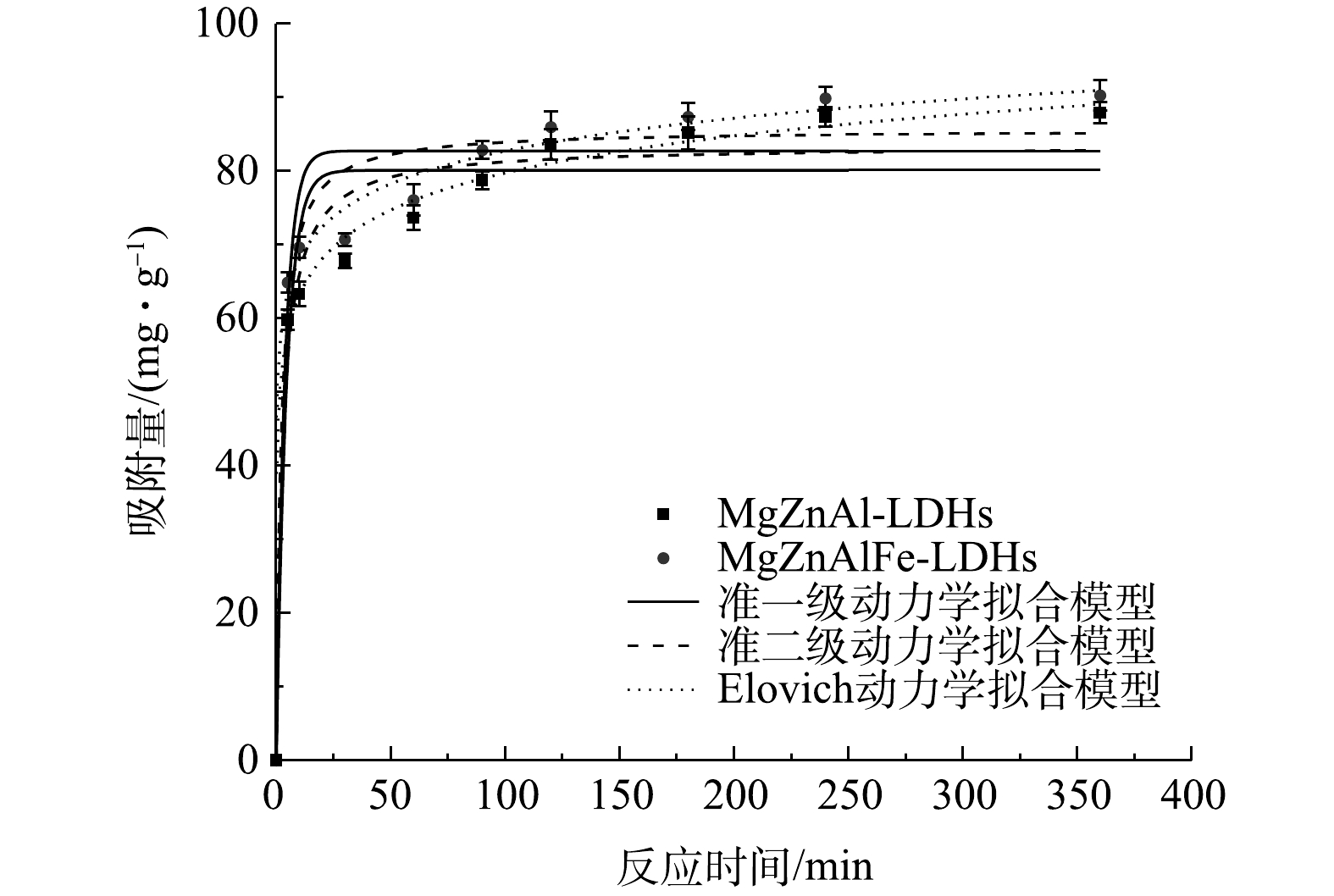
2)吸附动力学。如图4所示,本实验使用准一级动力学、准二级动力学和Elovich模型对吸附过程进行拟合,结果如表2所示。结果表明,MgZnAl-LDHs和MgZnAlFe-LDHs对磷酸盐的吸附动力学拟合符合准二级动力学吸附模型,拟合系数R2分别为0.968和0.963,且其计算吸附容量与实际吸附容量更加接近。同时,Elovich模型拟合系数R2均在0.99以上,说明MgZnAl-LDHs和MgZnAlFe-LDHs对磷酸盐的吸附动力学拟合也符合Elovich模型,吸附过程以化学吸附为主[20]。准二级动力学方程常数k2以及Elovich方程的α值反映了反应速率[21]。由表2可知,MgZnAlFe-LDHs对磷酸盐的吸附速率大于MgZnAl-LDHs的吸附速率,这可能是因为四元LDHs比三元的LDHs层间通道大,在吸附过程中磷酸盐可以更加快速地进入层间,这与XRD分析结果相一致。
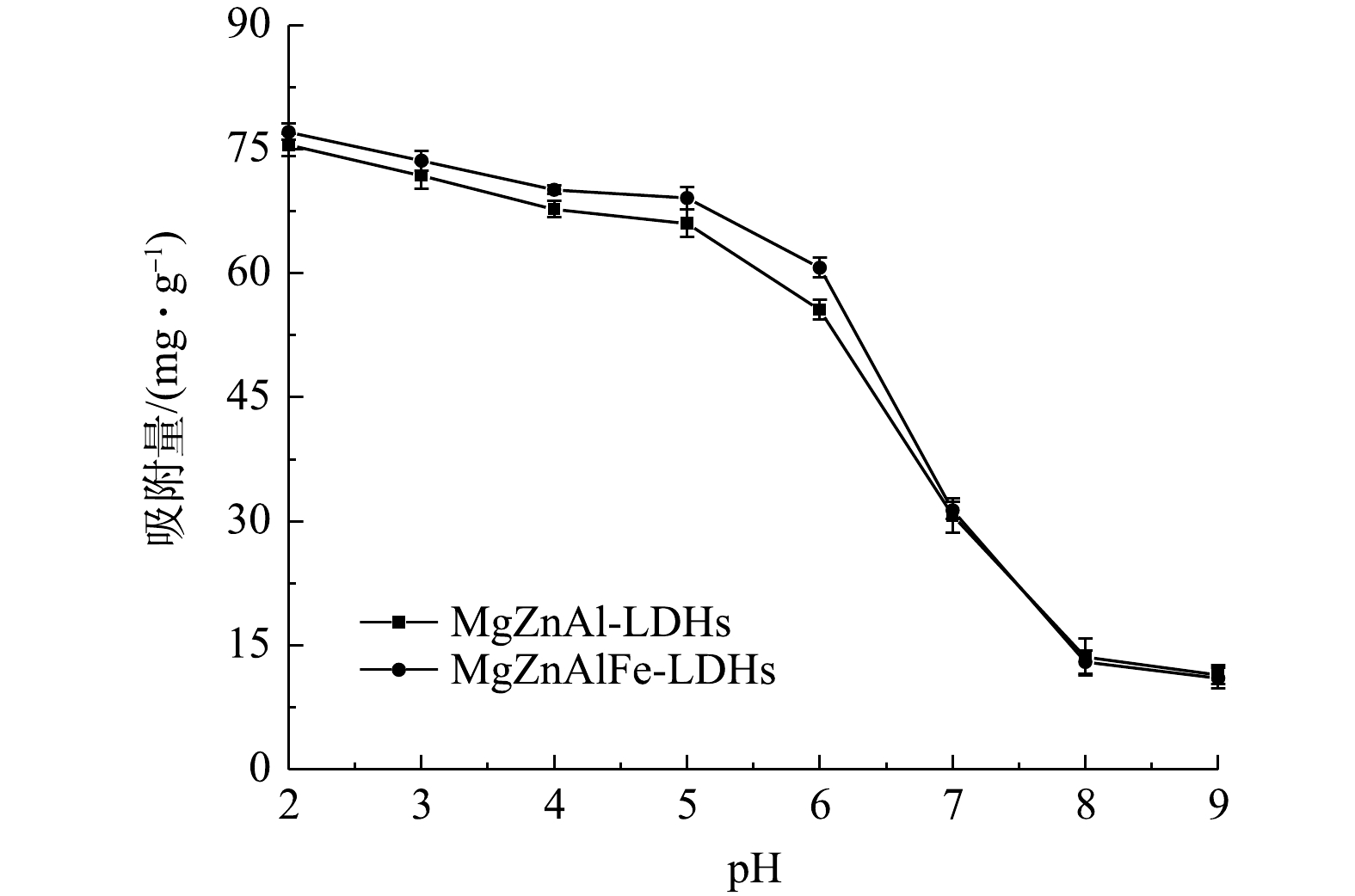
3) pH对吸附的影响。磷酸根在水溶液中的存在形式主要由pH决定[9],在酸性条件下,P离子主要以
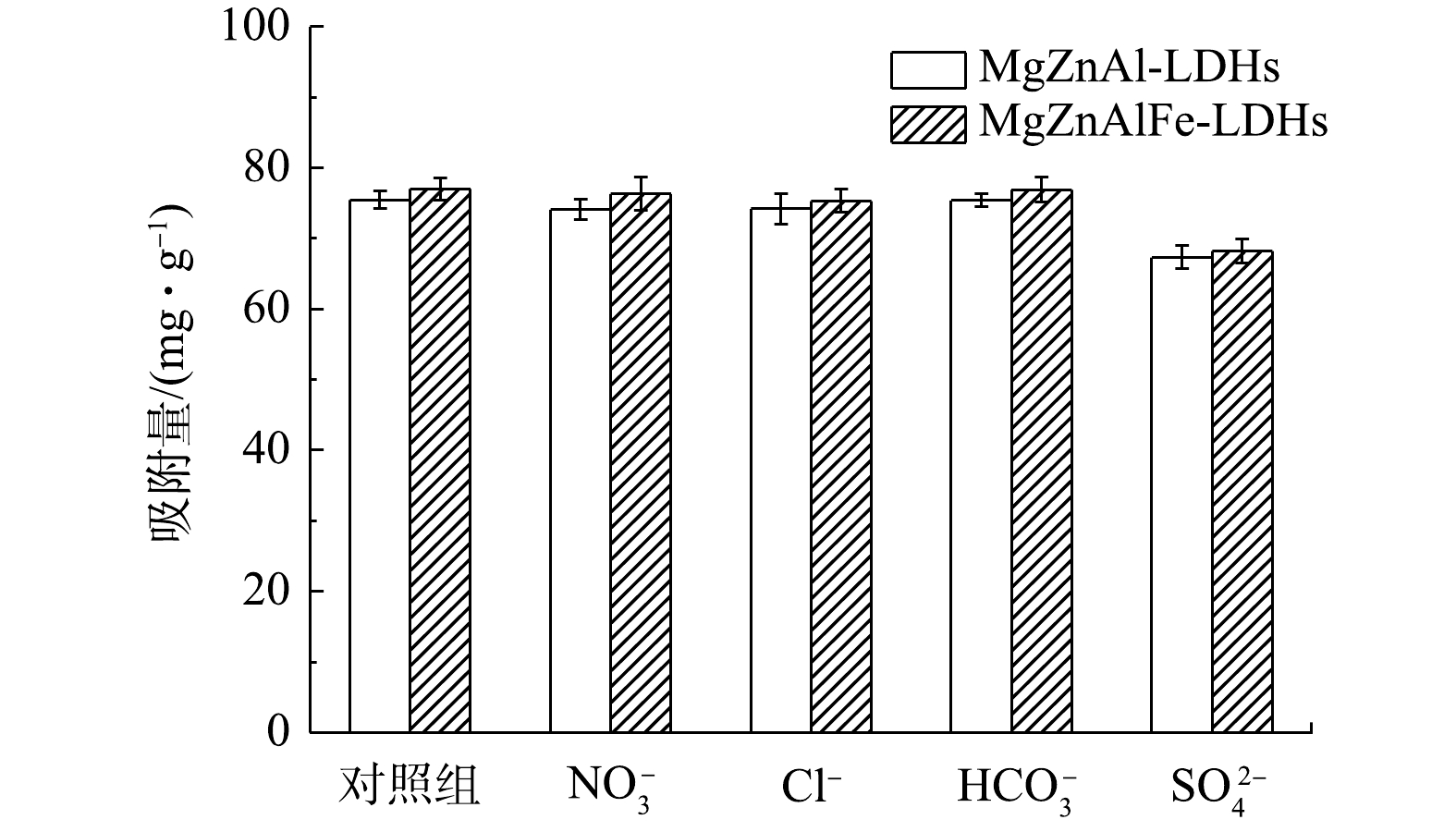
H2PO−4 的形式存在,随着pH的增加,阴离子逐渐转化为HPO2−4 和PO3−4 。与HPO2−4 和PO3−4 相比,H2PO−4 更容易与Cl−在LDH层之间进行离子交换[15]。因此,吸附实验在酸性条件进行可以更好地吸附磷酸盐。由图5可知,MgZnAl-LDHs和MgZnAlFe-LDHs对磷酸盐的吸附随pH的增大而减小,在pH=2的水中对磷酸盐的吸附能力最好,与理论相符。同时,随着pH的降低,吸附剂表面质子化,带更多正电荷,有利于对磷酸盐阴离子的吸附[22]。而随着pH的升高,表面吸附位点开始带负电荷与磷酸盐离子之间的静电斥力增加,表面的羟基离子也会与磷酸盐发生竞争[23],从而导致磷酸盐的吸附降低。4)共存离子对吸附性能影响。图6为常见的阴离子对MgZnAl-LDHs和MgZnAlFe-LDHs吸附磷酸盐的影响。可以看出,
HCO−3 对吸附影响较小,主要是由于在酸性条件下HCO−3 不能稳定存在,所以影响较小。Cl−和NO−3 对吸附影响不大,SO2−4 相较于Cl−和NO−3 而言对吸附略有较大影响,其主要是由于SO2−4 更容易进入LDHs层间,占据吸附位点,导致LDHs的吸附量略有下降。 -
本实验通过设计软件 Design-Expert 12进行响应面分析实验设计及分析,采用Box-Behnken (BBD)方法,实验设计见表3。由表3可得,MgZnAlFe-LDHs响应面编码形式二次回归方程如式(8)所示。
由式(8)可以看出,pH、反应温度以及初始质量浓度这3个影响因子对于磷酸盐的吸附性能的影响不是简单的线性关系,而是交互影响的关系,因此,简单的单因素的影响分析会对结果造成误差。表4为对MgZnAlFe-LDHs二次模型的方差分析(ANONA)。由表4可知,在对MgZnAlFe-LDHs的响应面分析实验中,模型F值为74.07,P<0.000 1,说明回归模型拟合是显著的[24];模型失拟项P=0.060 9>0.05,说明失拟项不显著,无失拟因素存在;模型的校正决定系数
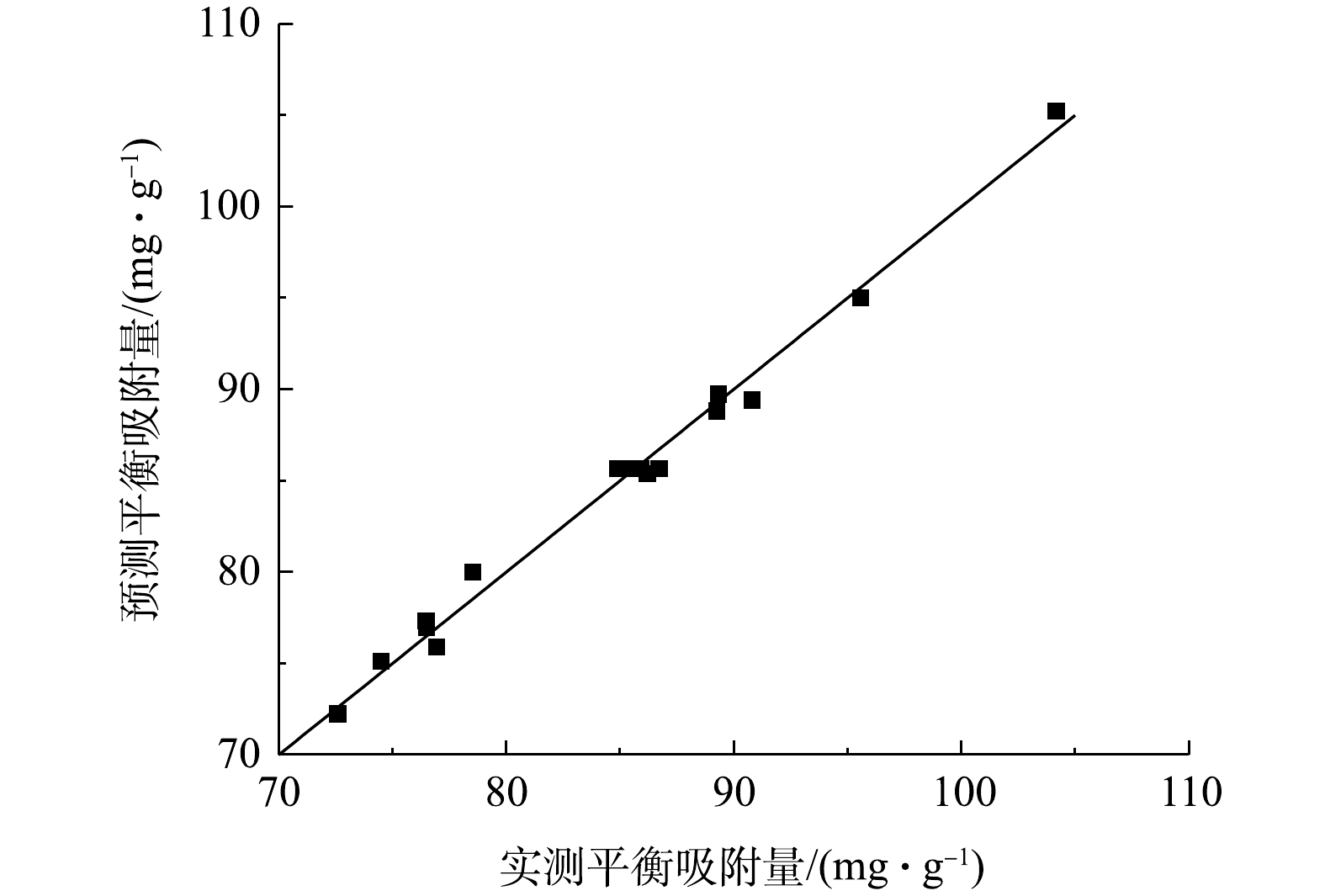
R2adj=0.9962 ,表示在响应面二次模型中有99.62%以上的响应值可以用模型解释[25],说明回归方程模型拟合度好。由表4可知,pH和初始质量浓度的F值分别为142.33、440.23,P <0.000 1,表明pH和初始质量浓度对响应值的影响为显著,影响因子中温度的F值为1.84,P=0.216 6>0.05,表明温度对响应值的影响不显著;在交互项中,pH与初始质量浓度的交互项以及温度与初始质量浓度的交互项对响应值影响均为显著(P<0.05)。图7为平衡吸附量预测值与实测值对比。可以看出,直线斜率接近于1,说明该模型预测结果较好。
响应面图可以反映出3个因素交互作用对响应值的影响[26]。图8为MgZnAlFe-LDHs吸附磷酸盐过程中各因素的交互作用。当初始质量浓度在中心值(100 mg·L−1)时(图8(a)),响应曲面坡度较缓,pH与温度对平衡吸附量的交互作用不显著,平衡吸附量随着温度的增加呈先增大后较小趋势,随着pH的增大呈减小趋势;当温度在中心值(30 ℃)时(图8(b)),pH与初始质量浓度对平衡吸附量的交互作用显著,响应曲面坡度较大,平衡吸附量随着初始质量浓度的增加而增大,随着pH的增加而减小;当pH在中心值(pH=3)时(图8(c)),温度与初始质量浓度对平衡吸附量的交互作用显著,从响应曲面可以看出,响应曲面坡度较大,平衡吸附量随着初始质量浓度的增加而增大,随着温度的增加而呈先增大后减小的趋势。
通过模型拟合可知,最佳平衡吸附量的条件为:pH为2,反应温度为30 ℃,初始质量浓度为120 mg·L−1,此条件下平衡吸附量为104.18 mg·g−1,与预测值105.23 mg·g−1基本一致,说明该模型能够用来预测吸附效果,对实际应用于酸性高磷废水的吸附中有一定的指导作用。
-
1) MgZnAlFe-LDHs较MgZnAl-LDHs在结构上层间距增大,孔隙增多,吸附位点增加,更有利于磷酸盐的吸附。
2) MgZnAl-LDHs和MgZnAlFe-LDHs符合Sips等温吸附模型,最大拟合吸附量分别可达172.00 mg·g−1和189.62 mg·g−1,可见MgZnAlFe-LDHs较MgZnAl-LDHs对磷酸盐的吸附量有所提高。MgZnAl-LDHs和MgZnAlFe-LDHs对磷酸盐的吸附动力学拟合符合准二级动力学吸附模型及Elovich模型,以化学吸附为主,且MgZnAlFe-LDHs对磷酸盐的吸附速率大于MgZnAl-LDHs的吸附速率。
3)通过Box-Behnken响应面分析法建立的回归模型显著,失拟项不明显,99.62%以上的响应值可以用模型解释,说明模型较为准确可靠,可以用于磷酸盐吸附条件优化。
MgZnAl-LDHs和MgZnAlFe-LDHs对磷酸盐的吸附
Adsorption of phosphate by MgZnAl-LDHs and MgZnAlFe-LDHs
-
摘要: 为研究多元层状氢氧化物(LDHs)对磷酸盐的吸附性能及其机理,本实验用水热法制备三元LDHs(MgZnAl-LDHs)及四元LDHs(MgZnAlFe-LDHs)吸附剂,利用扫描电子显微镜(SEM)和X射线衍射分析(XRD)对多元水滑石进行了表征;通过吸附等温模型和吸附动力学模型拟合MgZnAl-LDHs和MgZnAlFe-LDHs对磷酸盐的吸附行为,分析其吸附机理,并通过Box-Behnken响应面分析法(BBD)建立pH、温度和初始质量浓度影响磷酸盐平衡吸附量的模型,对吸附条件进行优化。结果表明,MgZnAlFe-LDHs吸附磷酸盐效果优于MgZnAl-LDHs,MgZnAl-LDHs、MgZnAlFe-LDHs对磷酸盐的吸附均符合Sips等温吸附模型和准二级动力学、Elovich动力学。响应面分析结果显示,通过Box-Behnken响应面分析法建立的回归模型显著(P<0.05),失拟项不明显,99.62%以上的响应值可以用模型解释,最佳吸附条件下平衡吸附量为104.18 mg·g−1,与预测值105.23 mg·g−1基本一致,回归模型可以较好地预测平衡吸附量。本研究可为多元LDHs应用于去除磷酸盐提供参考。Abstract: In this study, ternary LDHs (MgZnAl-LDHs) and quaternary LDHs(MgZnAlFe-LDHs) adsorbents were prepared by hydrothermal method in order to study the adsorption performance and mechanism of phosphate on multi-layered hydroxides(LDHs). The polyhydrotalcite was characterized by scanning electron microscopy(SEM) and X-ray diffraction(XRD) analysis. The adsorption behavior of MgZnAl-LDHs and MgZnAlFe-LDHs on phosphate was fitted by adsorption isotherm model and adsorption kinetics model, and the adsorption mechanism was also analyzed. Box-Behnken response surface analysis(BBD) was used to establish a model of the effects of pH, temperature and initial mass concentration on the phosphate equilibrium adsorption capacity, then the adsorption conditions were optimized. The results show that the adsorption effect of MgZnAlFe-LDHs towards phosphate was better than that of MgZnAl-LDHs. The adsorption of MgZnAl-LDHs and MgZnAlFe-LDHs on phosphate accorded with the Sips isothermal adsorption model, the quasi-second-order kinetics and Elovich kinetics. The response surface results showed that the regression model established by Box-Behnken response surface analysis method was significant(P<0.05), and the loss of fit term was not obvious. The response value of higher than 99.62% could be explained by the model. Under the optimal adsorption conditions, the equilibrium adsorption capacity was 104.18 mg·g−1, which was basically consistent with the predicted value of 105.23 mg·g−1. The regression model could predict the equilibrium adsorption capacity well. This study provides a reference for the application of multiple LDHs in phosphate removal.
-
Key words:
- multi-LDHs /
- phosphate /
- adsorption /
- response surface method
-
随着污水厂尾水排放标准的不断提高,化学辅助除磷、介质过滤、臭氧氧化等[1-15]技术被用在深度处理中,以进一步削减污染物,降低出水水质指标。在城镇污水处理厂提标改造新标准要求下,氨氮和总氮的削减成为重点,强化脱氮技术也备受重视。目前普遍采取以下2种措施:一是改造生物反应池强化除氮,再增加深度处理工艺进一步降低氨氮、总氮等指标;二是依靠深度处理技术改造脱氮工艺[14-20]。而针对生物反应池不同改造工艺运行情况开展现场对比实验的研究报道较少,以不同工艺并联运行,进行同时间、同规模现场生产实验的研究几乎未见报道。
本研究在西安市第五污水处理厂进行。在该厂未满负荷运行、先后具备多种工艺的有利条件下,分批次开展了同时间、同规模、不同工艺的现场生产性实验,对几种工艺的运行情况进行了对比研究。西安市第五污水处理厂总设计污水处理规模为40×104 m3·d−1,设计出水水质执行《城镇污水处理厂污染物排放标准》(GB18918-2002)[21]中的一级A标准,采用“预处理+AAO(A系列生物池采用MBBR投加填料)+纤维转盘滤池+次氯酸钠消毒处理”工艺,现状处理水量约30×104 m3·d−1,全厂分为A、B、C、D四个系列生物反应池并联运行,单系列设计规模10×104 m3·d−1。2018年进行了B系列AAO工艺与A系列“AAO+MBBR”工艺的生产性对比实验,2019年A、B系列Ⅳ类提改造后进行了C、D系列AAO工艺与B系列五段式Bardenpho工艺的生产性对比实验。分析比选了不同工艺在强化除氮方面的优缺点,以期为国内城镇污水处理厂准Ⅳ类水质提标改造工程应用提供参考。
1. 生产性实验方案
1.1 污水处理厂工艺概况
该厂一期工程(A、B系列)AAO工艺于2010年建成投产,于2013年进行了生物池一级A水质标准提标改造。改造后A系列生物反应池采用“AAO+MBBR工艺”,即在原AAO工艺的基础上,在好氧区中后段增加MBBR悬浮填料区,投加MBBR悬浮填料比例约17%,并增设填料专用推进器、拦网等辅助设施设备;B系列生物池沿用原有AAO工艺,并未进行改造。该厂二期工程(C、D系列)AAO工艺于2018年建成投产。A、B、C、D四个系列AAO工艺生物反应池的水力停留时间均为16.59 h。
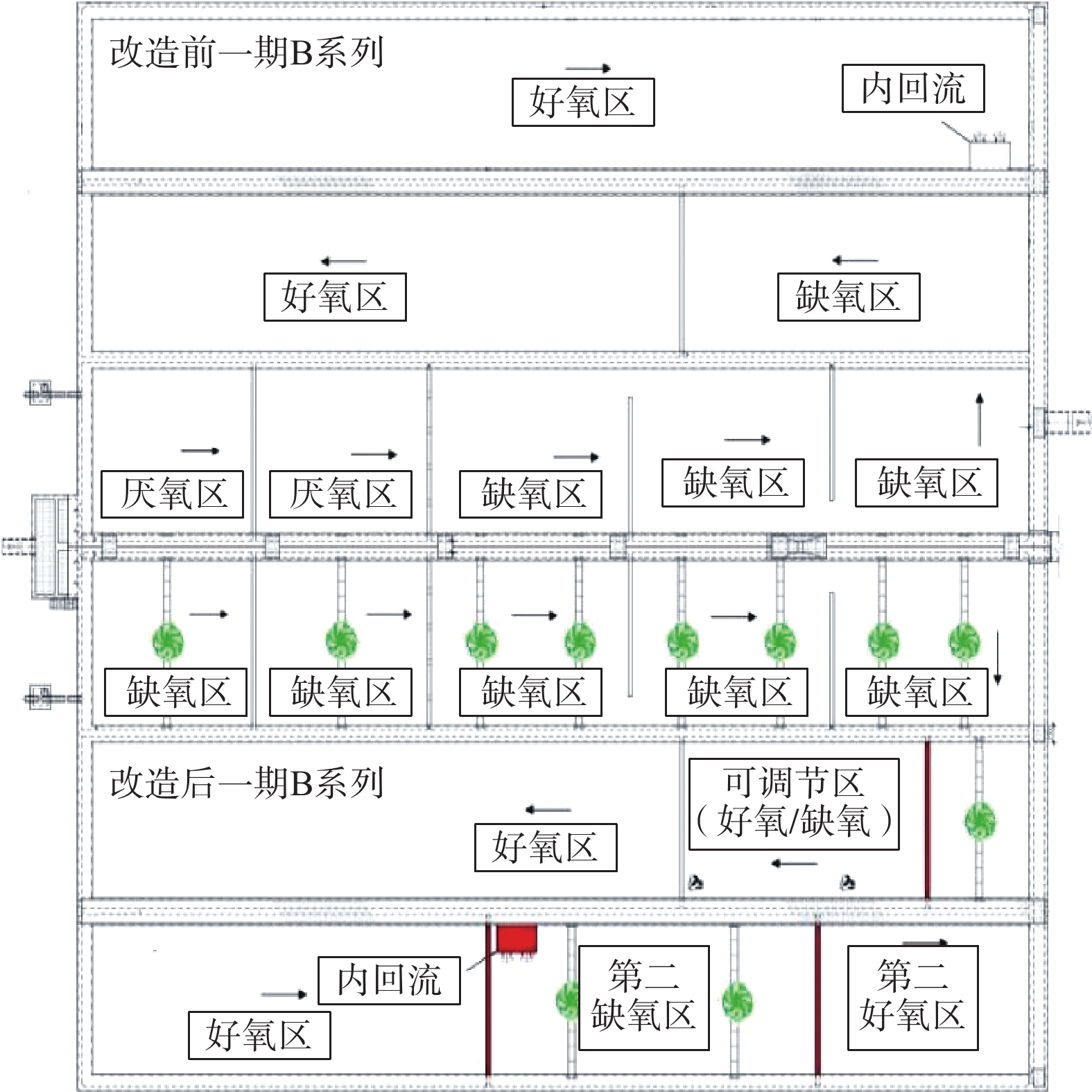
2019年,该厂以准Ⅳ类水质标准提标改造为目的,改造为多模式五段式Bardenpho工艺。具体实施内容包括:对A、B系列生物反应池先后进行了工程改造,同时去除A系列MBBR填料;在生物池缺氧区中后段增加隔墙,在好氧区增加2道隔墙;将A、B系列生物反应池均改造为5个区域,即厌氧区、缺氧区、好氧区、第二缺氧区、第二好氧区;C、D系列未进行改造。
1.2 水样的采集
1)实验进水。即厂区进水原水。进水采样使用自动采样器(sigma900型,哈希公司),每2 h进行自动取样。取样点位于第五污水处理厂进厂市政管网末端10#井。
2)实验出水。即对应实验系列二沉池出水汇流井内或总出水口水样。采样使用取样桶(1 000 mL塑料桶)。取样点位于水下30 cm。每日上午10点取样。
3) MBBR工艺实验用水。该组实验的样品包括A、B系列生物池厌氧区进、出口处混合液,A、B系列生物池缺氧区进、出口处混合液,A、B系列生物池好氧区中段处混合液,以及A、B系列生物池好氧区出口处混合液。采样使用取样桶(1 000 mL塑料桶),取样点位于液面下方1 m处,每日上午10点取样。所有过程样品均使用定量滤纸过滤后,取清液进行检测。
4) Bardenpho工艺实验用水。该组实验的样品包括B系列生物池厌氧区进、出口处混合液,B系列生物池缺氧区进、出口处混合液,B系列生物池好氧区出口处混合液,B系列生物池第二缺氧区出口处混合液,以及B系列生物池第二好氧区出口处混合液。采样使用取样桶(1 000 mL塑料桶),取样点位于液面下方1 m处,每日上午10点取样。所有过程样品均使用定量滤纸过滤后,取清液进行检测。C、D系列未取过程样。
5)分析指标:COD(HJ 828-2017重铬酸钾法)、NH3-N(HJ 535-2009纳氏试剂分光光度法)、
NO−3 PO3−4 1.3 运行方案及参数
1.3.1 MBBR生产性实验运行方案及参数
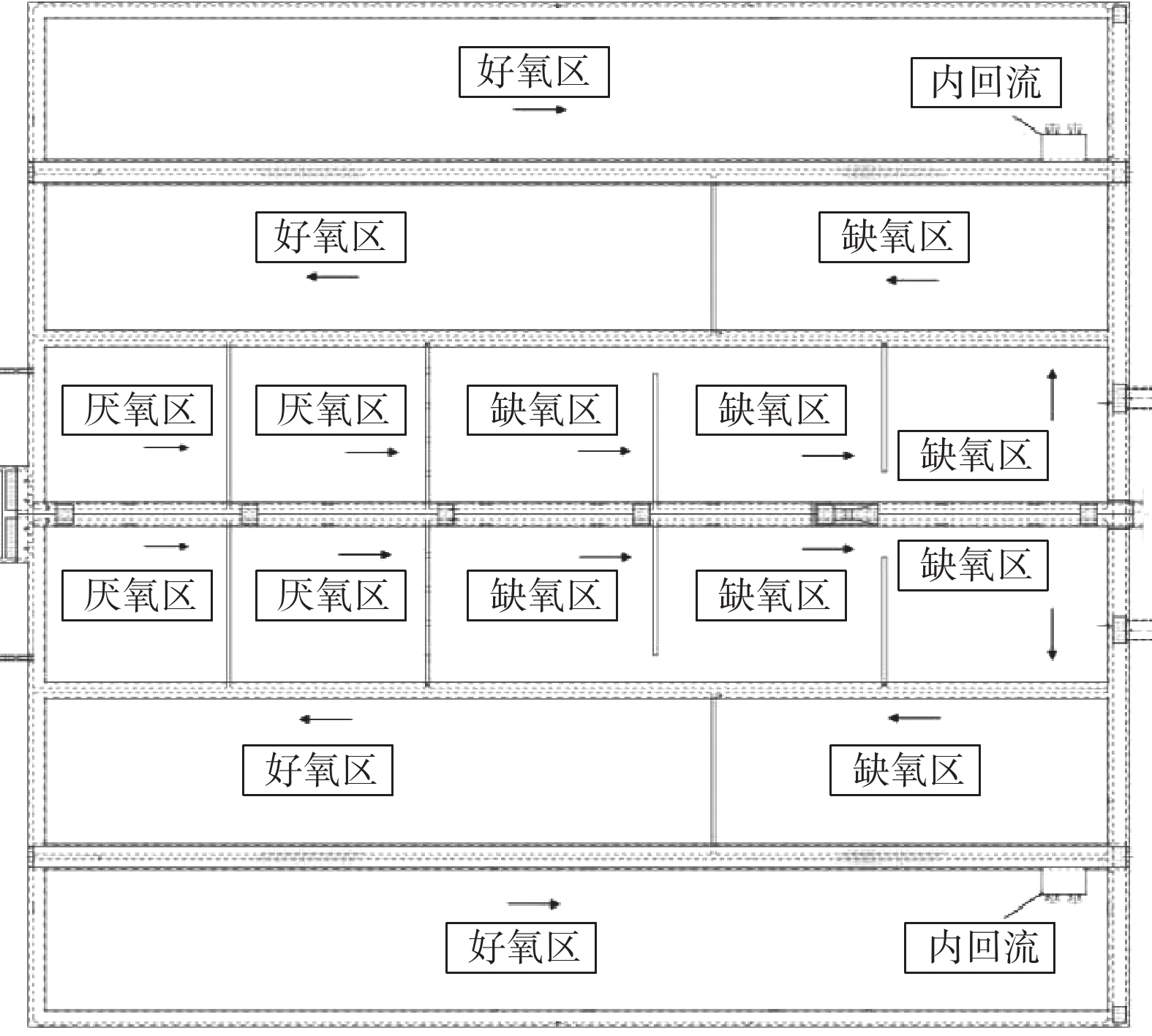
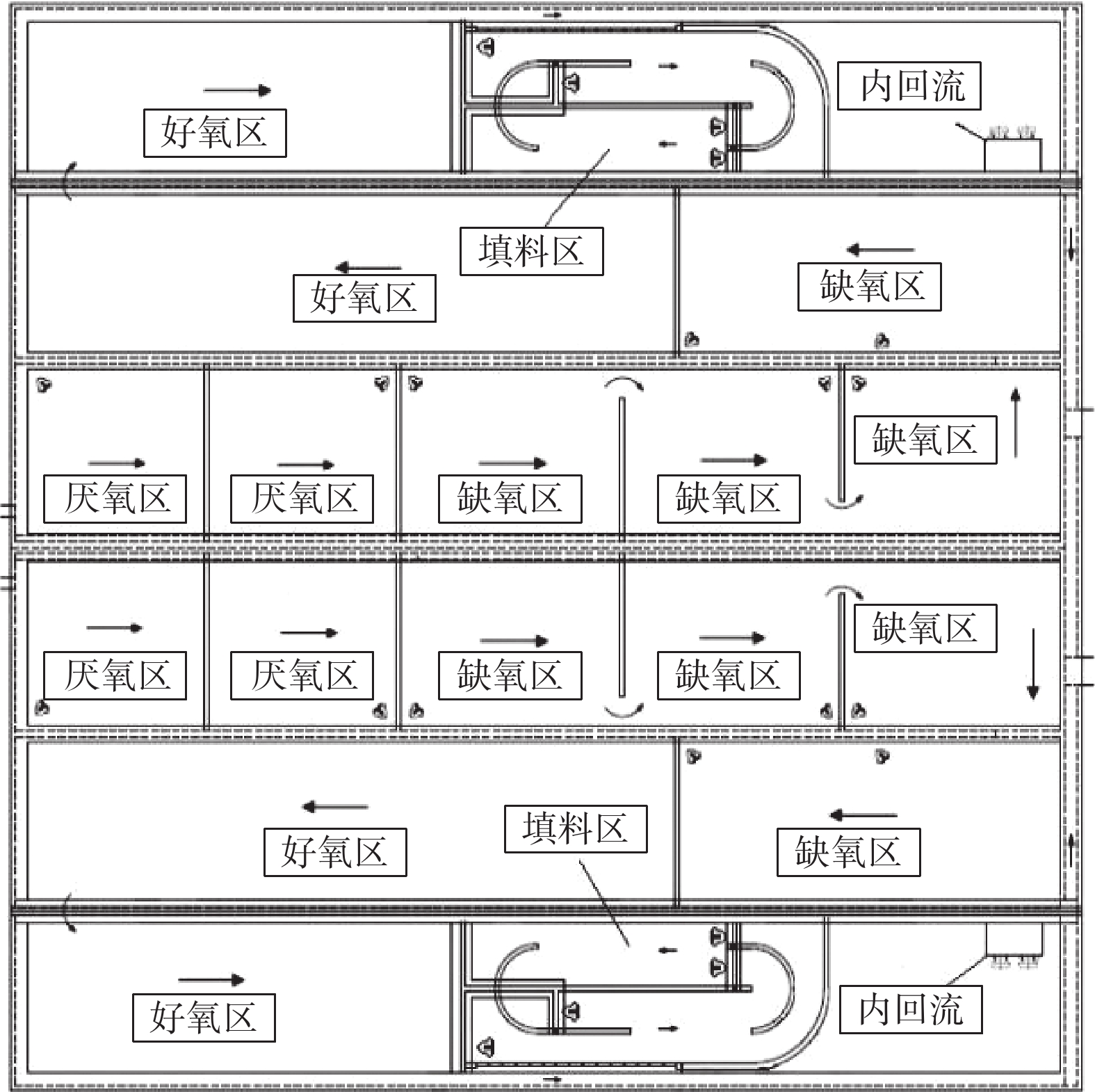
MBBR生产性比选实验在A、B系列同等工况下进行。不投加碳源,在生物池混合液出水口投加除磷药剂。实验的检测日期安排在2018年6月至10月。在这段时间内,随机抽取连续稳定运行11 d的数据。B系列工艺流程图如图1和2所示。实验期间运行水量均为7.5×104 m3·d−1。A、B系列生物池的MLSS控制在5~6 g·L−1;生物池末端DO远程控制在1.0 mg·L−1;污泥回流比为80%;内回流比为200%;除磷药剂的投加量为100 mg·L−1。
A系列生物反应池为“MBBR+AAO工艺”,总水力停留时间16.59 h。其中,厌氧区2.02 h,缺氧区5.53 h,好氧区9.04 h。好氧区MBBR填料区位于好氧段中后段,为循环跑道式填料。填料区的停留时间1.74 h,由4台潜水推流搅拌器进行搅拌及循环推流。B系列生物反应池为AAO工艺,总水力停留时间16.59 h,其中厌氧区2.02 h,缺氧区5.53 h,好氧区9.04 h。
1.3.2 Bardenpho生产性实验运行方案及参数
2019年,该厂进行准Ⅳ类提标改造,在A、B系列生物反应池中开展Bardenpho工艺的生产性实验,对比B系Bardenpho生物反应池与C、D系列生物反应池的污水处理情况。实验在同等工况下进行,不投加碳源,在生物池混合液出水口投加除磷药剂。实验的检测日期安排在2019年11月至12月。在这段时间内,随机抽取连续稳定运行9 d的数据进行分析。实验期间,B、C、D系列均保持连续稳定运行,工艺流程如图3所示。
本次改造后,B生物池系统总停留时间为16.59 h。其中,厌氧区2.02 h,缺氧区4.10 h,好氧区7.30 h(可调节区好氧运行),第二缺氧区1.92 h,第二好氧区1.25 h。为增强搅拌效果,加强反硝化速率,基于原有搅拌器,在厌氧区、缺氧区、第二缺氧区增加双曲面搅拌器。另外,将生物池混合液回流泵由原好氧区末端移动至第二缺氧区前端,新增回流泵安装隔墙,走道板、回流管道等。
Bardenpho生产性比选实验在B、C、D系列同等工况下运行,不投加碳源,期间一期A系列全部停运。B系列按照Bardenpho模式运行,以区别于C、D系列原有AAO工艺的出水水质。实验期间,B系列与C、D系列均满负荷运行,即B系列运行水量为10×104 m3·d−1,C、D系列运行水量为20×104 m3·d−1。B系列及C、D系列生物池的污泥浓度均控制在5~6 g·L−1;生物池末端的DO远程控制在1.0 mg·L−1;外回流比为70%;内回流比为200%;使用厂家专利除磷药剂,投加量稳定为100 mg·L−1。同时,加强对好氧段末端DO的控制,确保第二缺氧段缺氧状态。
2. 结果与讨论
2.1 MBBR工艺生产实验结果
实验中进出水水质见表1和2。A系列的出水平均COD较B系列略低,平均值相差不大。计算COD数据的方差,得到A系列方差为6.611,B系列方差为14.049,说明A系列出水的COD指标更稳定。A系列出水平均NH3-N较B系列略高。除10月19日,由于当天控制DO偏低,导致NH3-N升高,其余样品的出水NH3-N均稳定在1.5 mg·L−1以下。计算NH3-N数据的方差,得到A系列方差为0.252,B系列方差为0.772,说明A系列出水的NH3-N指标更稳定。A系列的出水平均TN较B系列低,A系列的TN去除率略高于B系列,去除效果较好。计算TN数据的方差,得到A系列方差为5.796,B系列方差为6.651,说明A系列出水的TN指标更稳定。在除磷药剂投加量相同的情况下,A系列的出水平均
PO3−4 PO3−4 表 1 MBBR实验进水水质指标Table 1. Influent quality of the MBBR experimentmg·L−1 采样日期 COD NH3-N TN PO3−4-P 2018-06-24 370 36.35 54.8 4.66 2018-06-25 609 39.55 60.8 3.42 2018-06-26 555 42.88 55.6 4.34 2018-06-27 455 31.22 55.5 4.76 2018-07-19 369 32.76 42.5 4.30 2018-07-24 551 40.06 54.1 7.87 2018-08-26 250 33.27 43.9 4.38 2018-09-23 438 37.49 47.9 5.06 2018-10-17 562 35.01 41.9 4.20 2018-10-18 757 27.72 72.7 7.80 2018-10-19 761 29.88 54.5 6.50 平均值 516.09 35.11 53.11 5.21 表 2 MBBR实验出水水质指标Table 2. Comparison of effluent quality of the MBBR experiment of A and B seriesmg·L−1 取样日期 A系列 B系列 COD NH3-N TN PO3−4-P COD NH3-N TN PO3−4-P 2018-06-24 18 0.847 7.505 1.75 19 0.708 3.450 0.600 2018-06-25 16 1.35 2.758 1.10 17 0.431 4.711 0.430 2018-06-26 21 0.458 5.991 1.25 22 0.347 5.380 0.890 2018-06-27 20 0.347 5.964 3.03 21 0.722 9.172 2.02 2018-07-19 21 0.236 3.394 1.65 19 0.458 5.616 1.71 2018-07-24 18 0.747 8.447 2.48 17 0.347 7.047 0.090 2018-08-26 18 0.458 10.958 4.08 18 0.458 4.825 1.13 2018-09-23 21 0.553 2.973 0.219 27 0.220 8.070 0.108 2018-10-17 15 0.980 6.988 0.044 15 0.520 7.916 0.137 2018-10-18 24 0.270 6.727 0.209 27 0.270 5.788 0.034 2018-10-19 22 1.97 8.06 0.310 22 3.46 13.18 0.024 平均值 19.45 0.747 6.342 1.466 20.36 0.722 6.832 0.652 2.2 MBBR生产实验中
NO−3 由于A、B系列工艺的除氮效果存在差异,因此,通过A、B系列
NO−3 NO−3 NO−3 NO−3 NO−3 2.3 Bardenpho生产实验的结果
Bardenpho生产实验的进出水水质见表3和4。B系列的出水平均COD较C、D系列略低,去除率相近。计算COD数据的方差,得到B系列方差为8.599,C、D系列方差为22.122,说明Bardenpho工艺中B系列出水的COD指标更稳定。B系列的出水平均NH3-N较C、D系列略高,但B系列NH3-N去除率较低。计算NH3-N数据的方差,得到B系列方差为0.1647,C、D系列方差为0.043 7。就去除效果而言,C、D系列具有微弱优势,且出水NH3-N指标更稳定,但B系列出水NH3-N稳定低于1.5 mg·L−1。B系列的出水平均TN与C、D系列相比略低,且B系列TN去除率较高。计算TN数据的方差,得到B系列方差为1.369,C、D系列方差为0.599。就去除效果而言,B系列具有一定优势,但C、D系列出水的TN指标更稳定。在除磷药剂投加量相同的情况下,B系列出水的平均
PO3−4 PO3−4 PO3−4 表 3 Bardenpho实验进水水质Table 3. Influent quality of the Bardenpho experimentalmg·L−1 取样日期 COD NH3-N TN PO3−4-P 2019-11-19 387 40.42 48.0 6.37 2019-11-20 372 42.00 47.1 8.52 2019-11-21 399 57.79 67.9 10.9 2019-11-26 466 43.58 56.3 5.38 2019-11-27 477 42.79 54.2 9.99 2019-11-28 823 33.97 48.4 7.53 2019-12-05 259 40.55 50.9 5.61 2019-12-17 323 40.82 49.2 6.77 2019-12-20 253 45.55 55.3 5.21 平均值 417.7 43.05 53.03 7.36 2.4 Bardenpho生产实验中脱氮过程分析
由于Bardenpho工艺第二缺氧区停留时间较短,B系列好氧区与第二好氧区的曝气会影响第二缺氧区的缺氧状态,进而影响第二缺氧区的反硝化效果。为确保实验效果,Bardenpho生产性实验中加强了DO控制,以确保第二缺氧段处于缺氧状态。结果亦表明污水处理过程中TN明显降低。在前文关于MBBR实验部分,已对原有AAO工艺中NH3-N、
NO−3 NO−3 NO−3 分析Bardenpho生产性实验期间B系列生物池过程样的检测结果,发现厌氧区进口至厌氧区出口水样指标NH3-N的平均值下降了2.078 mg·L−1;缺氧区进口至缺氧区出口(剔除11月19日的异常数据)NH3-N的平均值下降了0.327 mg·L−1;缺氧区进口至缺氧区出口
NO−3 NO−3 NO−3 NO−3 好氧区出口至第二缺氧区出口平均
NO−3 NO−3 NO−3 2.5 Bardenpho生产实验运行参数分析
与原有C、D系列AAO工艺相比,B系列Bardenpho工艺的NH3-N去除效果略差,去除率略降低。进一步分析设计参数后发现,B系列Bardenpho工艺好氧区总水力停留时间较C、D系列AAO工艺减少了0.49 h;同时,由于B系列一段、二段好氧区末端的DO明显低于C、D系列AAO工艺好氧区末端的DO,因此,DO可能对出水的NH3-N指标产生较大影响,具体数据如表4所示。
对比原有AAO工艺与Bardenpho工艺,结合实验数据和过程分析,采用硝化、反硝化动力学计算缺氧区容积计算公式(1)进行计算,以核算AAO工艺与Bardenpho工艺的反硝化速率是否符合设计要求。
Vn=0.001QΔN−0.12ΔXvKdeX (1) 式中:Vn为缺氧区(池)容积,m3;Q为生物反应池设计流量,m3·d−1;X为生物反应池内混合液悬浮固体平均浓度,g·L−1;△Xv为排除生物反应池系统的微生物量,kg·d−1;Kde为脱氮速率,kg· (kg·d) −1,根据实验资料确定。无实验资料时,一般20 ℃时Kde经验值为0.03~0.06 kg· (kg·d) −1,并按相关公式进行温度修正,若温差不大可忽略。
Bardenpho生产性实验中C、D系列具体相关数据及计算结果见表5和6。
表 5 Bardenpho生产性实验中各好氧区末端的DOTable 5. Measured values of dissolved oxygen at the end of aerobic zone of the Bardenpho production experimentmg·L−1 采样日期 B系列一段好氧区 B系列二段好氧区 C好氧区 D好氧区 2019-11-19 0.900 0.370 3.615 2.315 2019-11-20 0.710 2.020 3.595 2.985 2019-11-21 3.240 1.930 4.275 2.895 2019-11-26 1.180 1.100 3.725 1.560 2019-11-27 0.720 0.280 3.935 4.360 2019-11-28 1.200 0.420 4.390 2.720 2019-12-05 2.190 0.180 3.255 1.420 2019-12-17 — — 4.190 1.940 2019-12-20 1.960 0.130 3.770 2.275 表 6 Bardenpho生产性实验C、D系列相关参数Table 6. Experimental parameters of C and D series Bardenpho in-process experiments采样日期 进水温度/℃ VN/m3 进水TN/(mg·L−1) 出水TN/(mg·L−1) △Xv/(kg·d−1) VSS比例 X/(g·L−1) Kde/(kg·(kg·d)−1) 2019-11-19 19.9 23 041 48 7.00 2 413 0.5 5 458 0.021 2019-11-20 19.9 23 041 47.1 6.85 2 445 0.5 5 885 0.019 2019-11-21 19.5 23 041 67.9 7.45 2 493 0.5 6 090 0.032 2019-11-26 18.9 23 041 56.3 6.27 1 972 0.5 6 445 0.026 2019-11-27 18.8 23 041 54.2 7.69 2 774 0.5 6 465 0.020 2019-11-28 18.8 23 041 48.4 6.61 3 593 0.5 6 380 0.014 2019-12-05 18.4 23 041 50.9 5.28 3 748 0.5 6 190 0.016 2019-12-17 18.1 23 041 49.2 5.19 1 963 0.5 6 140 0.023 2019-12-20 18.0 23 041 55.3 7.21 1 990 0.5 5 920 0.027 在计算中,C、D系列AAO工艺的反硝化速率以全段水力停留时间计算,B系列五段式Bardenpho工艺的反硝化速率以前3段AAO水力停留时间计算。由于Bardenpho工艺第一缺氧区水力停留时间小于原有AAO工艺缺氧区停留时间,表现为计算中VN不同。另外,Bardenpho工艺的TN取样点在一段好氧区出口,AAO工艺在好氧区出口。根据表6和7,Bardenpho工艺有2 d高于设计下限,AAO工艺有1 d高于设计下限,2种工艺反硝化速率平均值的区别较小,且均低于理论设计负荷0.03~0.06 kg· (kg·d)−1。分析2种工艺的结构,由于Bardenpho工艺有第二缺氧区和第二好氧区进行二次反硝化,且该区域无混合液回流影响,故若进一步增加实际反硝化水力停留时间,可进一步降低出水水样中的TN、NH3-N。
表 4 Bardenpho实验出水水质情况对比表Table 4. Comparison of effluent quality of Bardenpho experimentmg·L−1 取样日期 B系列 C、D系列 COD NH3-N TN PO3−4-P COD NH3-N TN PO3−4-P 2019-11-19 14 0.137 3.56 0.084 16 0.111 7.00 0.132 2019-11-20 21 0.768 4.55 0.044 33 0.400 6.85 0.132 2019-11-21 16 0.347 4.47 0.264 17 0.268 7.45 0.100 2019-11-26 22 1.321 5.75 0.070 19 0.821 6.27 0.132 2019-11-27 21 0.584 5.49 0.120 20 0.295 7.69 0.153 2019-11-28 25 1.421 7.34 0.264 15 0.216 6.61 0.111 2019-12-05 19 0.611 3.90 0.224 21 0.716 5.28 0.163 2019-12-17 19 0.216 6.93 0.064 20 0.295 5.19 0.163 2019-12-20 15 1.350 7.30 0.244 22 0.189 7.21 0.163 平均值 19.11 0.751 5.477 0.153 20.33 0.368 6.617 0.139 最大值 25 1.421 7.340 0.264 33 0.821 7.690 0.163 最小值 15 0.216 3.900 0.044 15 0.189 5.190 0.100 表 7 Bardenpho实验B系列相关参数Table 7. Experimental parameters of B series Bardenpho in-process experiments采样日期 进水温度/℃ VN/m3 进水TN/(mg·L−1) 出水TN/(mg·L−1) △Xv/(kg·d−1) VSS比例 X/(g·L−1) Kde/(kg·(kg·d)−1) 2019-11-19 19.9 17 083 48 5.530 2 268 0.5 5 995 0.028 2019-11-20 19.9 17 083 47.1 4.870 4 460 0.5 6 160 0.015 2019-11-21 19.5 17 083 67.9 7.840 4 463 0.5 5 695 0.034 2019-11-26 18.9 17 083 56.3 11.010 2 163 0.5 5 995 0.032 2019-11-27 18.8 17 083 54.2 15.160 2 879 0.5 6 135 0.021 2019-11-28 18.8 17 083 48.4 8.820 3 363 0.5 6 355 0.018 2019-12-05 18.4 17 083 50.9 2.560 3 906 0.5 6 490 0.022 2019-12-17 18.1 17 083 49.2 14.750 4 260 0.5 6 740 0.008 2019-12-20 18.0 17 083 55.3 7.690 3 657 0.5 6 875 0.022 3. 结论与建议
1)在同工况、不投加碳源情况下,“AAO+MBBR”工艺相较原有AAO工艺对系统NH3-N的去除效果无明显提升,但对TN、
NO−3 PO3−4 2)在同工况、不投加碳源情况下,五段式Bardenpho工艺较原有AAO工艺对TN、
NO−3 3)在Bardenpho工艺生产实验期间,DO数据不稳定,多次出现第二缺氧段无法稳定处于缺氧状态的情况,在一定程度上影响了出水水质指标。建议使用精确曝气系统代替人工远程调控以加强生物池中DO的控制,以进一步确保NH3-N、TN的出水稳定性。五段式Bardenpho工艺可加强除氮效果,保障出水水质指标NH3-N、TN的稳定达标,在第二缺氧段投加碳源还可加强反硝化效果。
-
表 1 吸附等温模型拟合参数
Table 1. Fitting parameters of adsorption isothermal model
多元LDHs Langmuir Freundlich Sips qm /(mg·g−1) KL /(L·mg−1) R2 1/n KF R2 qm /(mg·g−1) Ks /(L·mg−1)ms ms R2 MgZnAl-LDHs 123.012 0.314 0.887 0.150 59.259 0.971 171.995 0.411 0.377 0.996 MgZnAlFe-LDHs 127.569 0.332 0.863 0.153 60.977 0.954 189.623 0.388 0.350 0.988 表 2 动力学拟合参数
Table 2. Kinetic fitting parameters
多元LDHs qe(exp)/(mg·g−1) 准一级动力学 准二级动力学 Elovich qe(cal)/(mg·g−1) k1/(min−1) R2 qe(cal)/(mg·g−1) k2 /(g·(mg·min)−1) R2 α β R2 MgZnAl-LDHs 87.890 80.073 0.225 0.928 83.395 0.004 0.964 4.290 0.138 0.995 MgZnAlFe-LDHs 90.230 82.713 0.266 0.938 85.614 0.006 0.967 24.892 0.156 0.993 表 3 响应面分析方案及实验结果
Table 3. Experimental design and results of RSM
序号 pH 温度/℃ 初始质量浓度/(mg·L−1) 吸附量/(mg·g−1) 1 2 25 100 89.26 2 3 25 120 95.59 3 3 30 100 85.13 4 3 30 100 86.73 5 4 35 100 76.51 6 3 30 100 85.59 7 3 35 120 89.33 8 2 30 120 104.18 9 4 30 120 86.21 10 3 30 100 85.93 11 2 35 100 90.82 12 4 25 100 78.55 13 4 30 80 76.95 14 3 30 100 84.91 15 3 25 80 72.60 16 2 30 80 76.48 17 3 35 80 74.50 表 4 MgZnAlFe-LDHs响应面二次模型的方差分析
Table 4. ANOVA of MgZnAlFe-LDHs for response surface quadratic model
来源 平方和 自由度 均方 F值 P值 模型 1 058.55 9 117.62 74.07 <0.000 1 X1 225.99 1 225.99 142.33 <0.000 1 X2 2.93 1 2.93 1.84 0.216 6 X3 699.01 1 699.01 440.23 <0.000 1 X1X2 3.24 1 3.24 2.04 0.196 2 X1X3 85.01 1 85.01 53.54 0.000 2 X2X3 16.65 1 16.65 10.48 0.014 3 X21 1.22 1 1.22 0.77 0.410 4 X22 24.51 1 24.51 15.43 0.005 7 X23 0.25 1 0.25 0.16 0.704 7 残差 11.11 7 1.59 失拟项 9.04 3 3.01 5.82 0.060 9 注:P<0.05 表示显著,P>0.05 表示不显著。 -
[1] 朱颖, 马骏, 陈睿哲. 富营养化的湖泊[J]. 生态经济, 2018, 34(12): 6-9. [2] PERUMAL K, SANKARAN M. Enhanced removal of phosphate and nitrate ions by a novel ZnFe LDHs-activated carbon composite[J]. Sustainable Materials and Technologies, 2020, 25: 2-7. [3] 赵华, 张先智, 肖娴. 氮磷营养盐控制与湖泊蓝藻水华治理研究进展[J]. 环境科学导刊, 2021, 40(3): 12-15. [4] KOILRAJ P, ANTONYRAJ C A, GUPTA V, et al. Novel approach for selective phosphate removal using colloidal layered double hydroxide nanosheets and use of residue as fertilizer[J]. Applied Clay Science, 2013, 86: 111-118. doi: 10.1016/j.clay.2013.07.004 [5] YONG M L, WEI C, DUN H L, et al. Cyanobacteria-/cyanotoxin-contaminations and eutrophication status before Wuxi Drinking Water Crisis in Lake Taihu, China[J]. Journal of Environmental Sciences, 2011, 23(4): 575-581. doi: 10.1016/S1001-0742(10)60450-0 [6] BACELO H, PINTOR A M A, SANTOS S C R, et al. Performance and prospects of different adsorbents for phosphorus uptake and recovery from water[J]. Chemical Engineering Journal, 2020, 381: 122566. doi: 10.1016/j.cej.2019.122566 [7] 胡锋平, 罗文栋, 彭小明, 等. 层状双金属氢氧化物去除水中污染物研究进展[J]. 水处理技术, 2019, 45(1): 17-22. [8] 韩芸, 胡玉洁, 连洁, 等. 铝污泥酸化提取液改性沸石的除磷特性及机制[J]. 环境科学, 2019, 40(8): 3660-3667. [9] 何天旭. 铁改性镁铝水滑石吸附剂制备及其对水中磷的吸附研究[D]. 武汉: 华中科技大学, 2019 [10] GOH K H, LIM T T, DONG Z. Application of layered double hydroxides for removal of oxyanions: A review[J]. Water Research, 2008, 42(6/7): 1343-1356. [11] FEI H L, JIE J, ZI Y S, et a1. Removal and recovery of phosphate and fluoride from water with reusable mesoporous Fe3O4@mSiO2@mLDH composites as sorbents[J]. Journal of Hazardous Materials, 2020, 388: 121734. doi: 10.1016/j.jhazmat.2019.121734 [12] FREDERICK L T, GODWIN A A, RAY L F. Synthesis of layered double hydroxides containing Mg2+, Zn2+, Ca2+ and Al3+ layer cations by co-precipitation methods-a review[J]. Applied Surface Science, 2016, 383: 200-213. doi: 10.1016/j.apsusc.2016.04.150 [13] JIAN B L, HUI J L, RUI P L. Adsorptive removal of phosphate by a nanostructured Fe-Al-Mn trimetal oxide adsorbent[J]. Powder Technology:An International Journal on the Science and Technology of Wet and Dry Particulate Systems, 2013, 233: 146-154. [14] YANG Y, PAUL C J. Key factors for optimum performance in phosphate removal from contaminated water by a Fe-Mg-La trimetal composite sorbent[J]. Journal of Colloid and Interface Science, 2015, 445: 303-311. doi: 10.1016/j.jcis.2014.12.056 [15] ASHEKUZZAMAN S M, JIA Q J. Strategic phosphate removal/recovery by a reusable Mg-Fe-Cl layered double hydroxide[J]. Process Safety and Environmental Protection, 2017, 107: 454-462. doi: 10.1016/j.psep.2017.03.009 [16] WEI C Q, HAN B, TIAN H T, et al. Recovery and utilization of phosphorus in wastewater by magnetic Fe3O4/Zn-Al-Fe-La layered double hydroxides(LDHs)[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2019, 577: 118-128. [17] 马培根, 张海涛, 丁文明. 颗粒改性活性氧化铝吸附除氟动力学和热力学的研究[J]. 北京化工大学学报: 自然科学版, 2019, 46(3): 1-6. [18] 何剑伟, 王蒙, 王婷婷, 等. 酸预活化对负载膨润土吸附磷酸盐的影响及响应优化研究[J]. 环境科学与技术, 2020, 43(9): 40-46. [19] 张倩. 层状双金属氢氧化物除磷材料及氨基酸插层改性性能研究[D]. 重庆: 重庆大学, 2018 [20] WU F C, TSENG R L, JUANG R S. Characteristics of elovich equation used for the analysis of adsorption kinetics in dyechitosan systems[J]. Chemical Engineering Journal, 2009, 150(2/3): 366-373. [21] KYUNG W J, TAE U J, MIN J H, et al. Phosphate adsorption ability of biochar/Mg–Al assembled nanocomposites prepared by aluminum-electrode based electro-assisted modification method with MgCl2 as electrolyte[J]. Bioresource Technology, 2015, 198: 603-610. doi: 10.1016/j.biortech.2015.09.068 [22] ZHI Q J, SHUANG H, XIAO Y L. Exfoliated Mg-Al-Fe layered double hydroxides/polyether sulfone mixed matrix membranes for adsorption of phosphate and fluoride from aqueous solutions[J]. Journal of Environmental Sciences, 2018, 70(8): 63-73. [23] 刘晨, 张美一, 潘纲. 超薄水滑石纳米片除磷效果与机理[J]. 环境工程学报, 2018, 12(9): 2446-2456. doi: 10.12030/j.cjee.201803195 [24] 马宏林, 贺涛, 洪雷, 等. 响应面分析法优化给水污泥吸附除磷工艺[J]. 环境工程学报, 2015, 9(2): 546-552. doi: 10.12030/j.cjee.20150208 [25] MALEKI S, KARIMI J A. Optimization of Ni(II) adsorption onto cloisite Na+ clay using response surface methodology[J]. Chemosphere, 2020, 246: 125710. doi: 10.1016/j.chemosphere.2019.125710 [26] 黄雪琴, 李天勇, 郭诗, 等. 油菜秸秆对多元离子水体系中pb(Ⅱ)的吸附作用与机理[J]. 中国环境科学, 2017, 37(9): 3363-3370. doi: 10.3969/j.issn.1000-6923.2017.09.020 -








 下载:
下载: