-
由于人口的增加、供水质量的下降、气候变化等原因,全球淡水需求急剧增长,水环境形势越发严峻[1]。中国也面临水资源短缺的重大挑战,2016年我国工业用水量达130.8×109 m3,约占总供水量的21.66%[2]。作为补充水资源的有效方式,工业废水的回收利用尤为重要。2005年《中国节水技术政策大纲》首次提出发展外排废水回用技术和零排放(zero liquid discharge, ZLD)技术[3],目前典型工业废水零排放系统一般由深度预处理单元、超滤-反渗透低盐废水脱盐单元、高盐废水脱盐单元、蒸发结晶单元组成。而工业废水零排放的技术关键,在于低盐废水脱盐单元所产生的膜滤浓缩液的进一步高效低耗脱盐及蒸发结晶[4]。相比而言,脱盐技术研究备受关注,但结晶技术进展缓慢。目前,实现结晶的方式主要有自然蒸发法、热法与冷冻法结晶器结晶法和膜蒸馏结晶法[1]。其中自然蒸发法容量有限,土地使用成本高,太阳能效率低;热法与冷冻法结晶器结晶法能耗高,设备成本高昂,反应器易腐蚀;膜蒸馏结晶法存在膜润湿、膜污染和膜结垢问题,这制约了膜蒸馏结晶技术的发展,因此,开发新的结晶技术意义重大[1, 5]。
2018年,澳大利亚昆士兰大学COSTA团队[6]报道了一种新型蒸发结晶工艺,即真空膜渗透结晶。这是一种发生在真空条件下的渗透蒸发结晶过程,在膜内外压力差的作用下,溶液渗透至无机陶瓷膜外表面形成一层液膜,随后溶剂在真空环境中瞬间蒸发,溶质在膜表面结晶并生长,直至自动脱落从而得到回收。
相比于传统的结晶-过滤-干燥流程,VMPC工艺最大的优势是实现了同步蒸发浓缩与结晶,即在同一反应器内实现了盐和水的同步分离和回收,无需后续步骤,简化了工艺流程,节省了设备规模和成本投资[6]。且针对蒸发池和结晶器能耗高、效率低等问题,VMPC工艺通过引入膜界面和真空条件,显著提高了生产能力和能量利用率;更为重要的是,针对现有膜蒸馏结晶技术因膜结垢和膜润湿导致的通量衰减和截留率下降等问题,VMPC工艺采用膜渗透过程,无需考虑膜润湿的影响,同时将膜结垢(膜结晶)作为生产目的,促进膜结晶的发生,且因为结晶体的自动脱离机制,有效地规避了膜堵塞问题。通过技术经济层面的分析可见,VMPC工艺具有良好的技术优势和广阔的应用前景,未来必会成为分离与纯化领域的研究与应用热点。迄今为止,COSTA团队[6-9]通过在无机膜上负载碳化蔗糖分离层,主要探究了膜性能对VMPC过程的影响,具体包括:无机膜如何应用于结晶过程以及何种性质的无机膜能够应用于VMPC过程;膜的化学性质和制备条件对NaCl结晶形态的影响;无机碳膜对不同溶质结晶形态的影响。
总体而言,目前VMPC工艺尚处于初步探索阶段,高性能功能化渗透结晶膜材料与工艺原理需系统研究;关键工艺参数及交叉影响因素需进一步明确、优化;工艺过程模拟和技术经济分析亟待推进。在应用前景方面,尤其是在典型分离与纯化及废水零排放领域,VMPC技术可与其他技术耦合(膜蒸馏-真空膜渗透结晶),实现优势互补,拓展与应用空间巨大。在蒸发结晶领域,VMPC技术有望实现短流程、提质、增效、降耗、资源化等多重目标,因此,有必要推进VMPC工艺的系统性研究。本研究以浓盐水蒸发结晶工艺创新为目标,以丰富VMPC技术体系,促进该技术进步为目的,开展了对VMPC工艺的初步探究,通过自制的乙酸酐改性淀粉纳米晶(modified starch nanocrystals with acetic anhydride, AA-SNC)无机陶瓷膜,阐述了VMPC发生的现象、过程和原理,并分别考察了进料液温度、浓度和操作压力对VMPC工艺的产能影响。
全文HTML
-
实验材料包括:自制AA-SNC管式无机陶瓷膜,平均孔径为50 nm,长度为10 cm,外径为1 cm,内径为0.6 cm;玉米淀粉(试剂级);氯化钠、浓硫酸、乙酸酐、十二烷基磺酸钠和氯仿,以上均为分析纯级别试剂,所有试剂均购自于国药集团化学试剂有限公司。
-
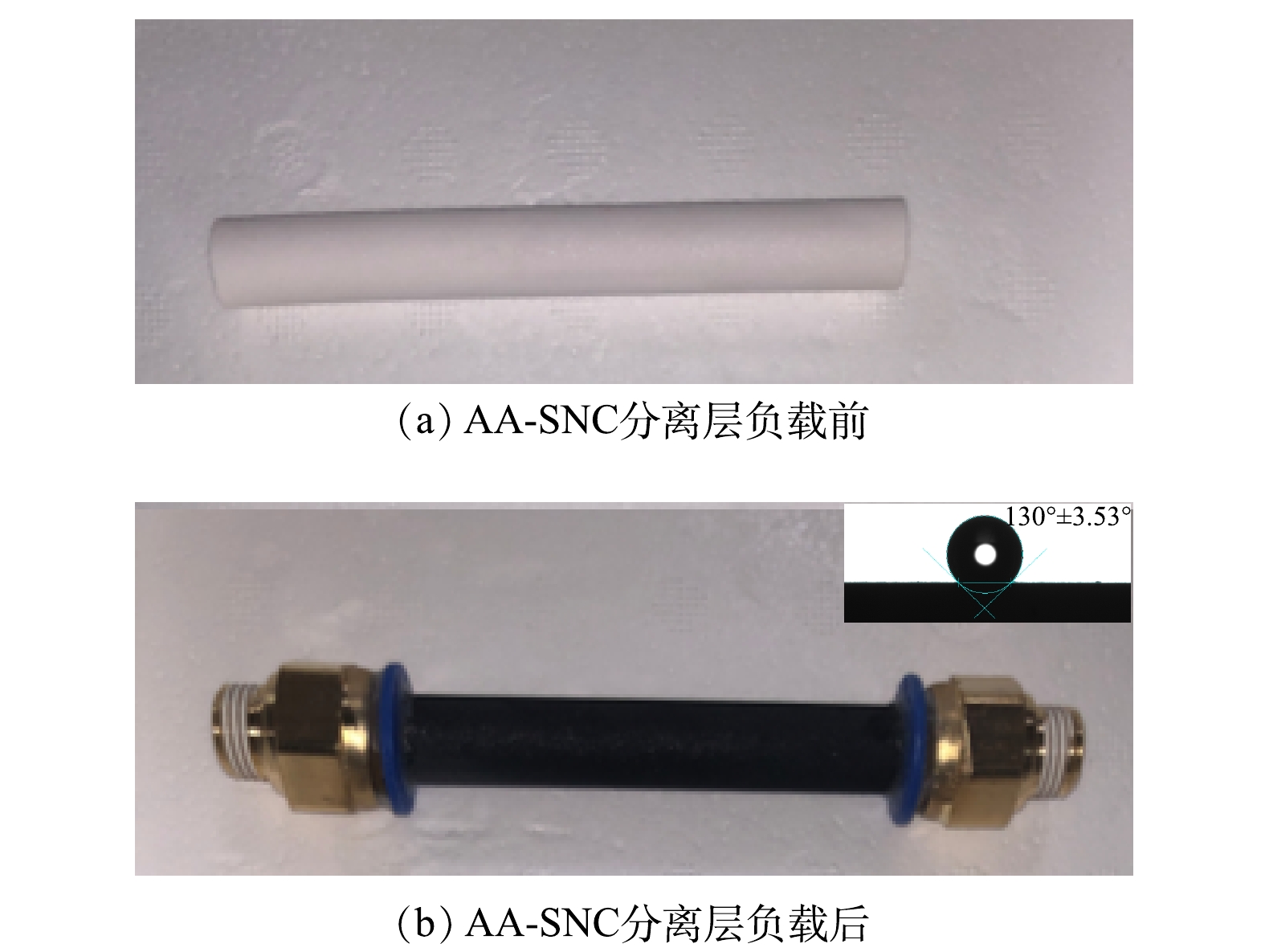
通过在管式α-Al2O3无机陶瓷膜外表面负载AA-SNC碳分离层制得AA-SNC无机陶瓷膜[10-13]。如图1所示,负载碳分离层后的膜外表面均匀致密,呈现典型的石墨亮黑色,其表面静态接触角为130°,其疏水表面有助于缓解由吸附水合离子导致的严重膜污染[14-16],在长期实验过程中未发现膜发生性能变化。孔径主要分布在10~30 nm。对AA-SNC膜进行渗透性能测试:在0.2 MPa压力下,AA-SNC膜的纯水通量为76.5 L·(m2·h)−1,质量分数为15%的NaCl溶液通量为67.6 L·(m2·h)−1。
-
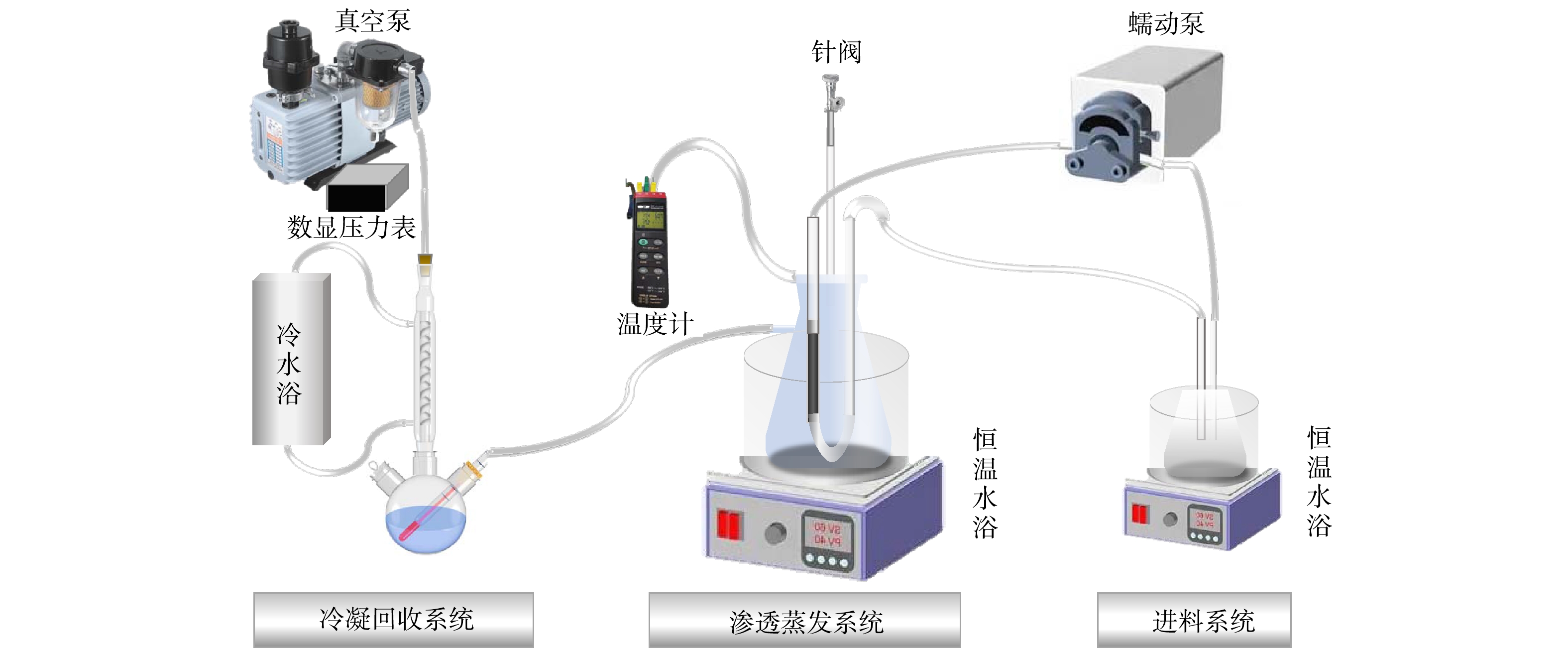
VMPC实验装置如图2所示。实验系统主要分为进料系统、渗透蒸发系统和冷凝回收系统。进料系统料液瓶置于恒温水浴中,将AA-SNC膜连入管路,进料液以错流方式流经管程,进料液流速为400 mL·min−1。渗透蒸发系统主要包括蒸发室,真空泵,压力调节装置和温度测量装置。其中真空泵为系统的核心部件,选用临海市永昊真空设备有限公司生产的DVP-12型直连高速旋片式真空泵,极限压力为10−4 Pa。将AA-SNC膜放置在2 000 mL密封抽滤瓶中,抽滤瓶与真空泵连接作为蒸发室。蒸发室置于恒温水浴中,热电偶探入到AA-SNC膜膜面附近以测量蒸发温度,数显压力表显示蒸发室内绝对压力,控制真空泵阀门(粗调)和抽滤装置针阀(细调)开度调节真空室内压力。冷凝回收系统将系统产生的水蒸汽进行冷凝回收。进料温度、蒸发温度、冷凝温度分别由3个水浴进行控制。实验过程中,所有压力均为实际压力(绝对压力)。压力的考察范围设置为0~100 kPa;温度的考察范围设置为10~35 ℃。
-
VMPC过程的传质通量指标为NaCl通量和蒸发冷凝水通量。通过称量实验前后减少的进料液质量,减去产生结晶盐的质量,计算所得的数值即为实验过程中纯水的真实产量,通过测量每次实验前后蒸发室的质量变化来表征结晶盐的产量。水通量和NaCl通量的计算方法分别如式(1)和式(2)所示。
式中:
JH2O 为蒸发冷凝水通量,L·(m2·h)−1;JNaCl 为NaCl通量,kg·(m2·h)−1;V为渗透液溶液体积,L;M为生成干燥结晶质量,kg;A为膜的有效面积,m2;t为运行时间,h。为保证实验结果的准确性,减小误差,每次实验的测试时间不少于3 h,其中包括压力调节至稳定的时间。在不涉及浓度变化的实验中,使用过饱和溶液以保证实验过程中溶液浓度稳定。
1.1. 实验材料
1.2. AA-SNC无机陶瓷膜的性质
1.3. 实验装置
1.4. 实验方法
-
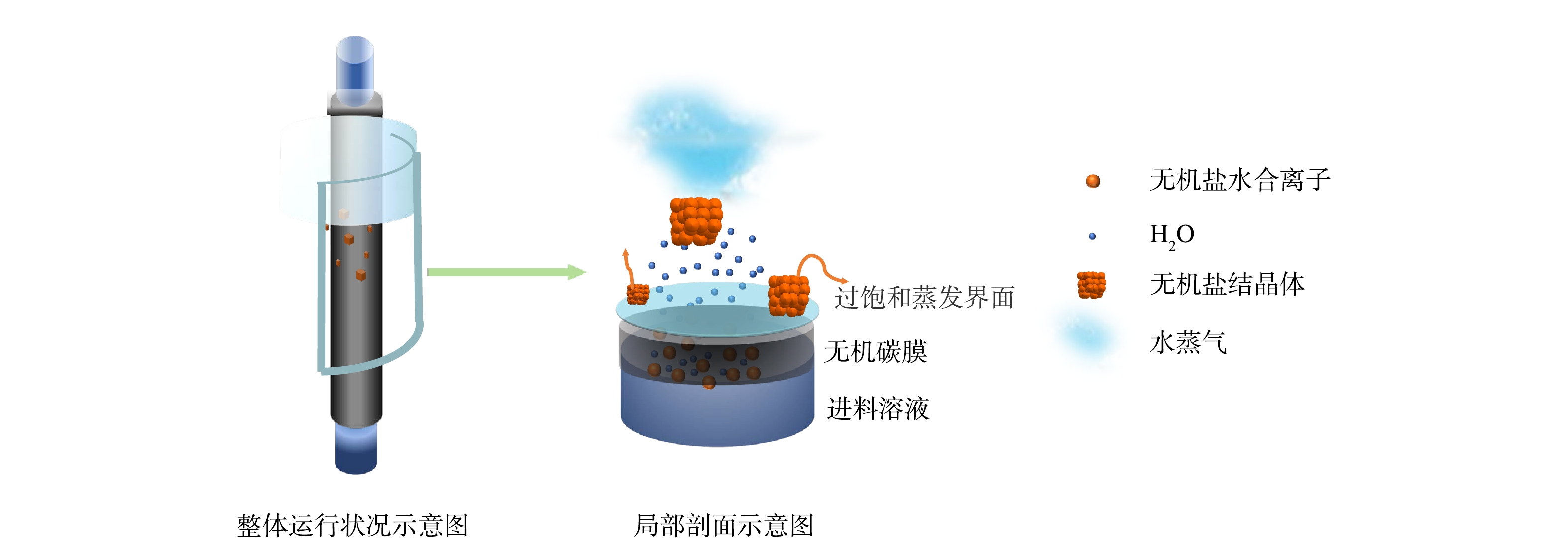
本研究中所检测的瞬时和连续渗透结晶过程的典型现象如图3所示,VMPC过程的原理如图4所示。真空条件可使膜两侧产生压力差,因此,无机盐水合离子和水分子渗透穿过膜孔,在膜界面处由于压力突变发生闪蒸,液体由过冷态转为过热态,液态水迅速相变为水蒸气,同时带走大量热量[17]。当渗透和蒸发达到平衡时,由于表面张力的作用,渗透液在膜表面形成一层液膜。这层液膜即蒸发结晶界面,液膜呈过饱和状态,晶体在此逐渐成核并生长至临界尺寸,最终在复杂力场合力作用下从膜表面脱落,让出成核位点。与此同时,溶液源源不断地通过碳膜扩散,补充液膜蒸发的水分,新的晶核继续生长[18]。VMPC过程仅在一定压力范围内发生,且发生条件随进料液温度、浓度、蒸发温度等影响因素的变化而变化,当操作压力超过临界压力时,由于溶液的渗透速率高于液膜的水分蒸发速率,将发生渗漏现象,此时VMPC过程不能正常进行。
根据以上VMPC过程的原理分析,可以认为,VMPC过程是膜渗透和真空压差闪蒸结晶的协同发展过程。对于某一确定压力点P,首先发生的是溶液的渗透过程,其推动力为膜两侧压力差
ΔP 。在渗透形成的液膜界面上,溶质发生结晶,推动力为过饱和度S;对于溶剂,当压力从大气压到压力点P的下降过程中,发生动态闪蒸,推动力为过热度ΔT∗ ,当到达动态平衡后,进入稳定蒸发阶段,推动力与此时的压力P和溶液的实际温度Tb有关,Tb是由蒸发过程、外界至膜表面的传热、膜表面至料液传热等过程共同作用下决定的平衡温度。渗透过程推动力表达式如式(3)所示。结晶过程的主要推动力过饱和度S的表达式如式(4)所示[19]。动态闪蒸过程中,过热度的定义为液膜初始温度与理论平衡温度之差[20],纯水系统过热度
ΔT 表达式[20]如式(5)所示,NaCl溶液过热度ΔT∗ 由纯水系统而来,其可根据式(6)进行计算[21-22]。闪蒸平衡后,稳定蒸发阶段的蒸发速率v根据式(7)进行计算[23-24]。式中:
P0 为膜内侧压力,为大气压,数值为101.3 kPa;P为绝对压力,为设定值,kPa。式中:C为溶液中溶质实际质量浓度,g·L−1;C*为饱和溶液中溶质的平衡质量浓度,g·L−1。
式中:
T0 为液膜初始温度,℃;Te 为平衡时刻闪蒸腔压力对应的纯水饱和温度,℃;TBs 为平衡时刻闪蒸腔压力对应的NaCl溶液饱和温度,℃;θ 为平衡时刻NaCl溶液相对于纯水的沸点升高量,℃。式中:v为蒸发面的蒸发速率,m·s−1;C为经验常数,通过实际测量可得;E为平衡温度Tb下溶液的饱和蒸气压,E∝Tb,kPa;e为该条件下的实际蒸气压,kPa。
-
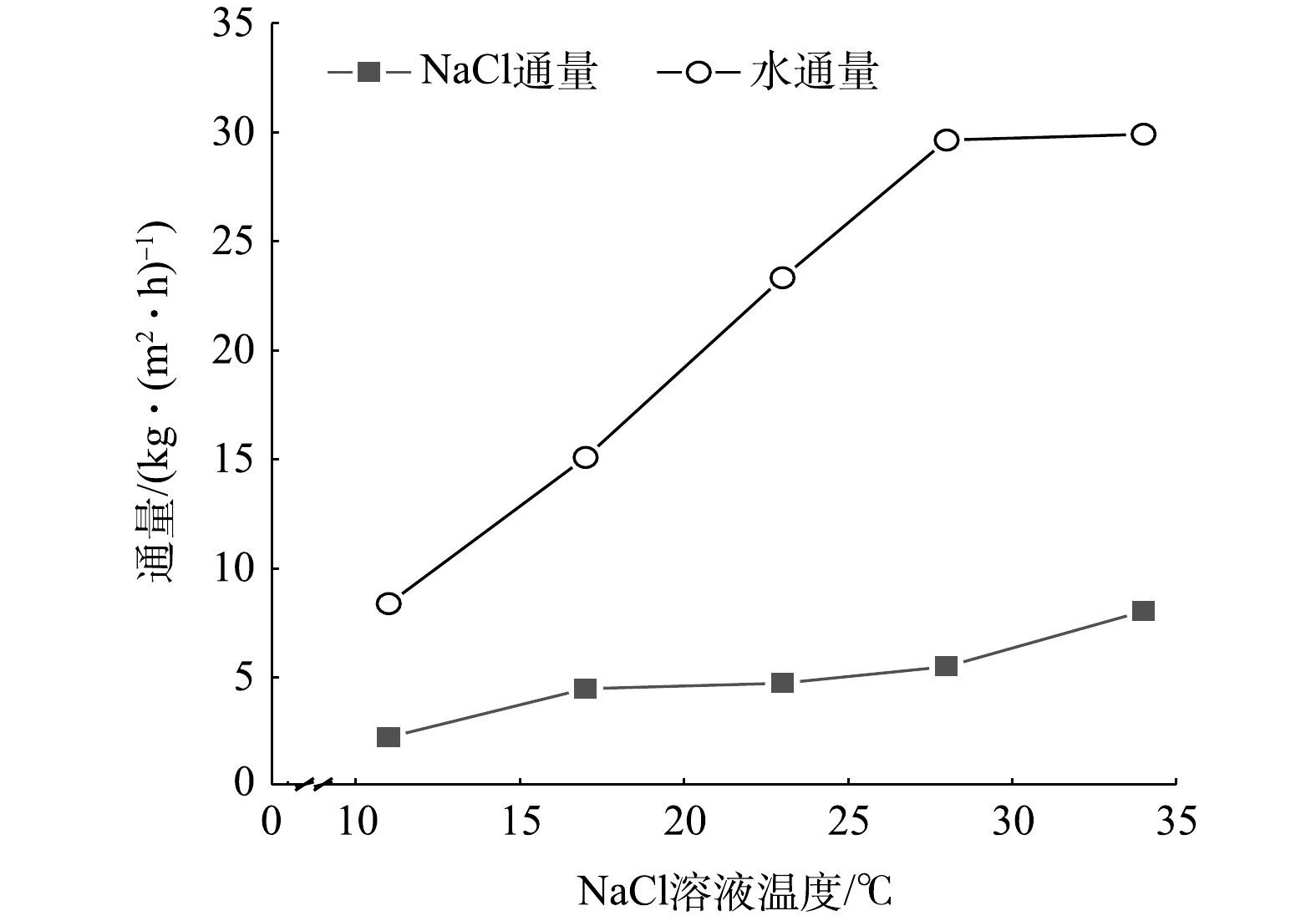
在进料液质量分数为25%、蒸发室水浴温度为75 ℃、绝对压力为0.5 kPa的条件下,工艺产能随进料液温度的变化情况如图5所示。图5表明,结晶盐通量和水通量均随进料温度的升高而增大。一方面,随着进料液温度增加,渗透速率升高,单位时间内通过的溶剂和溶质总量增加。另一方面,进料液温度的增加使液膜初始温度提升,
ΔT∗ 和Tb增加,蒸发推动力加大,动态闪蒸阶段和稳定蒸发阶段的蒸发速率均提高,因此,水通量增大;同时由于过饱和度S提高,结晶推动力加大,导致盐通量增加。在本研究中,当最高测试温度为34 ℃时,获得最高盐通量,为8.04 kg·(m2·h)−1,这明显高于太阳能驱动膜结晶技术的盐通量(0.1~0.6 kg·(m2·h)−1)[25]。优势更为明显的是,VMPC工艺还同步获得了约30 L·(m2·h)−1的纯水。与反渗透工艺(RO)相比,陶氏化学公司生产的FILMTEC系列RO膜[26]的建议运行通量为20~30 L·(m2·h)−1,可耐受NaCl的质量浓度为50 000 mg·L−1,且RO需要克服渗透压实现分离,所以需要的压力较大,推荐运行压力为1~3 MPa。而VMPC过程不截留盐离子,无需克服渗透压,因此,无需高压设备。VMPC工艺水回收能力与RO相当,且还能同步获得结晶盐。
-
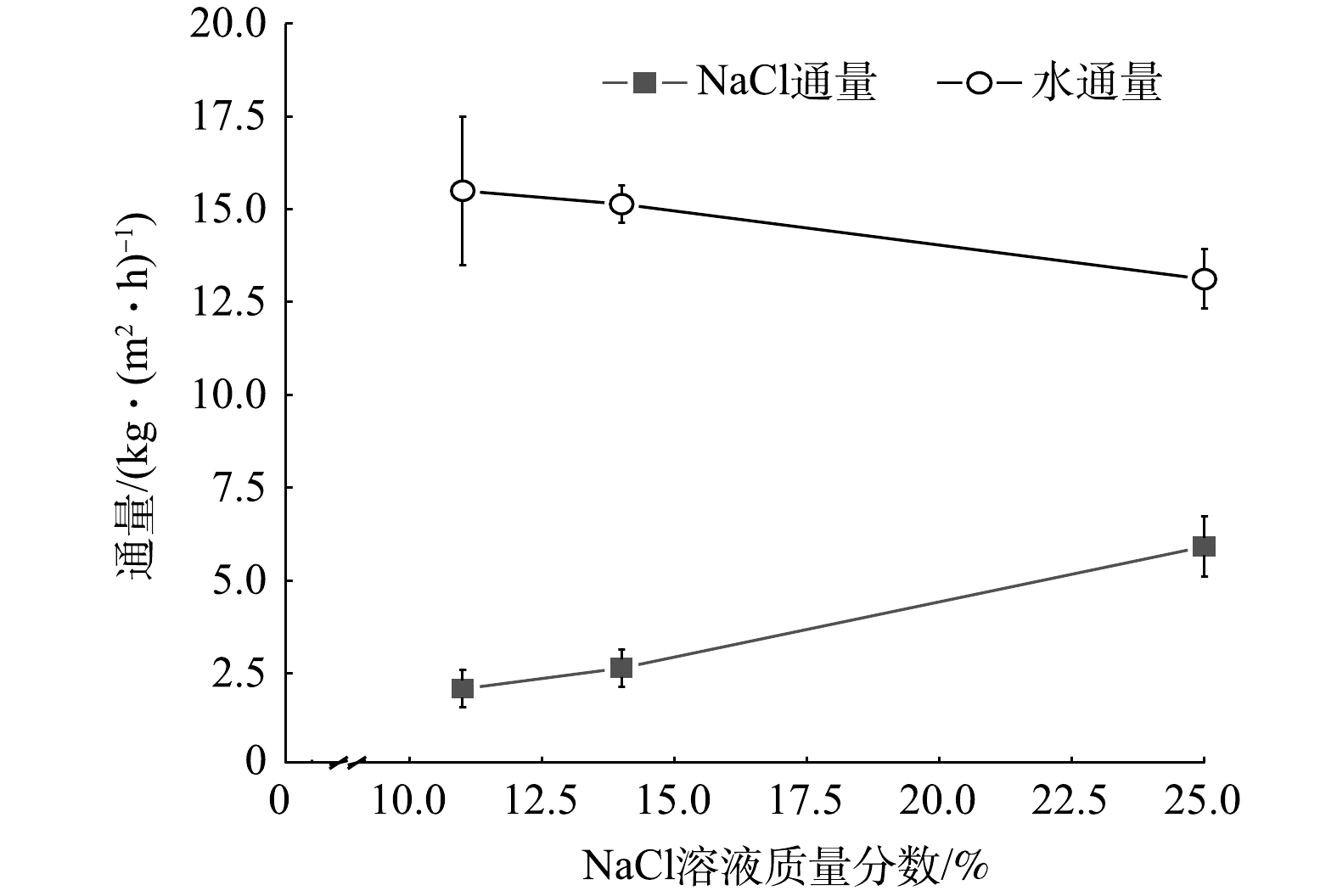
在蒸发室水浴温度为75 ℃,进料液温度为19 ℃,绝对压力为0.3 kPa的条件下,工艺产能随进料液浓度的变化情况如图6所示。图6表明,随着进料液浓度的增大,结晶盐通量随之增大,水通量减小。当进料液质量分数为25%时,盐通量最大,为5.92 kg·(m2·h)−1,此时水通量为13.12 L·(m2·h)−1。这是因为,随进料液浓度增加,蒸发结晶界面的过饱和度有所提高,结晶速率增加,导致盐通量增加;蒸发界面溶液浓度升高,导致沸点降低,过热度减小,平衡温度降低,动态闪蒸过程驱动力变弱[27],蒸发速率降低。此外,渗透速率的下降,使得可供蒸发的水量下降,因此导致水通量降低。
-
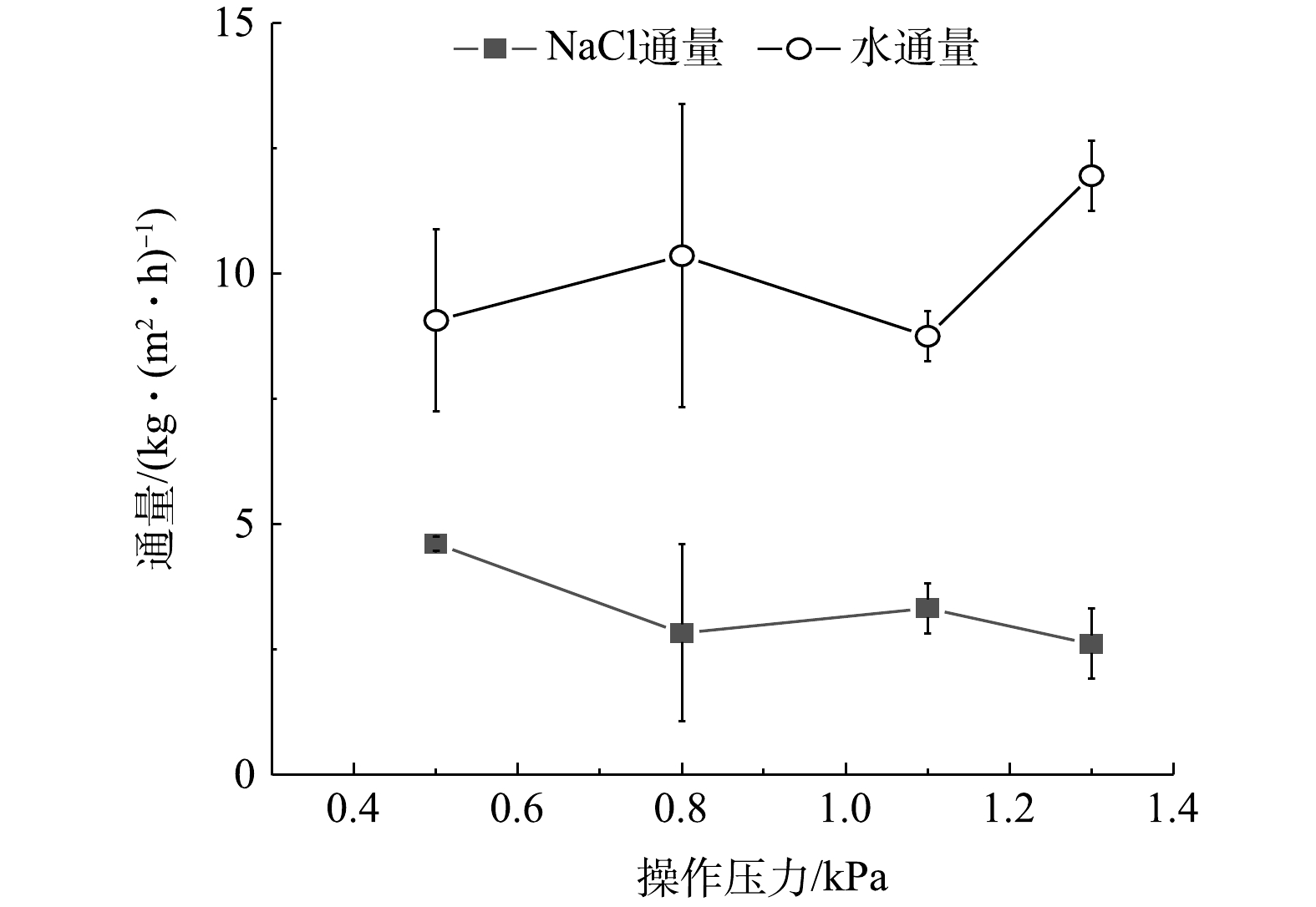
在蒸发室水浴温度为70 ℃,进料液温度为21 ℃,进料液质量分数为25%的条件下,工艺产能随操作压力的变化情况如图7所示。图7表明,操作压力的调节对生成的盐通量和水通量影响程度相对较小,但对形成晶体的尺寸和晶型影响很大。随绝对压力的增大,膜内外压力差减小,渗透通过的溶液通量降低,导致生成的结晶量减少,盐通量降低。但是,由于平稳蒸发过程的蒸发速率
v 随Tb升高而升高,且随P升高而降低,所以,当P增大时,蒸发动力减小,蒸发速率降低,蒸发消耗热量减少,导致Tb有一定程度的升高,这又反过来促进蒸发速率的提升,2方面同时作用再加上实验误差的影响,导致实验结果中水通量变化明显,其波动范围为7~15 L·(m2·h)−1。晶体尺寸和形貌与渗透侧真空环境的驱动力有关[28-29],其随操作压力的变化情况如图8所示。随着绝对压力的降低,颗粒粒径明显减小,获得的晶体尺寸更细。这是因为,绝对压力越小,渗透速率和蒸发速率越大,膜渗透面上液膜蒸发越薄,NaCl成长至临界尺寸晶体的时间越短,临界尺寸也越小。因此,较低的压力往往产生较小的颗粒,而绝对压力越大,晶体聚集并形成较大的颗粒的时间越宽裕,生成颗粒越大,形貌越接近于规则的正方体晶型。由此可见,通过调控操作压力可获得理想形貌的结晶体。
2.1. VMPC过程的原理分析
2.2. 进料液温度对工艺产能的影响
2.3. 进料液浓度对工艺产能的影响
2.4. 操作压力对工艺产能的影响
-
1) VMPC过程是膜渗透和真空压差闪蒸结晶的协同发展过程。其中,渗透过程的推动力为膜内外压力差,蒸发过程推动力为过热度,结晶过程推动力为过饱和度。
2)随进料液温度的升高,结晶盐通量和水通量均增大,盐通量和水通量最高可达8.04 kg·(m2·h)−1和30 L·(m2·h)−1;随进料液浓度的升高,结晶盐通量随之增大,水通量降低,其最高盐通量为5.92 kg·(m2·h)−1,最低水通量为13.12 L·(m2·h)−1;随着绝对压力的升高,结晶盐通量略微减小。
3)操作压力对生成晶体的尺寸和形貌影响显著,可通过控制操作压力获得理想形貌晶体。绝对压力越小,生成结晶粒径越小,获得的晶体尺寸更细;绝对压力越大,生成结晶粒径越大,形貌越接近于规则的正方体晶型。



 DownLoad:
DownLoad: