-
盐酸四环素(tetracycline,TC)是抗生素中比较典型的一种[1],四环素类药物在经生物代谢后,大部分的四环素随排泄物仍以原始形式排出,导致四环素在环境中大量蓄积残留[2-4]。长期低浓度的药物残留会危害人类健康,对胃肠、肝肠都有损害。因此,如何有效去除废水中的四环素至关重要[5-6]。目前,在对抗生素的处理方法中,主要有离子交换法[7]、吸附法[8]、絮凝[9]、活性污泥法[10-11]、光催化氧化[12-13], 但这些传统的处理方法存在处理周期长、降解不彻底、容易造成二次污染的问题[14]。
近年来,超声波技术在水处理领域中取得了较大的进展[15]。超声波处理水是基于水分子分解产生的羟基自由基的氧化过程。超声波产生的声空化泡溃灭后可在极短时间内生成高温高压,这种极端环境会伴随放电、发光及射流等,从而使水中污染物得以去除[16-17]。有研究[18]表明,在利用超声波处理三氯乙烯和四氯化碳的过程中,发现三氯乙烯是由于超声空化技术产生的·OH被四氯化碳捕获而降解的。超声波与臭氧的协同作用主要归因于超声波的机械效应,机械效应增强了溶液中臭氧的质量传递[19]。H2O2是一种易获得、成本低的强氧化剂,能有效去除生物毒素等有机污染物[20]。在超声波处理偶氮染料废水的研究[21]中,将超声波与H2O2耦合,结果表明,酸性黑210去除率有了显著提高。然而,将超声协同H2O2降解抗生素的研究却少有报道,2种方法的结合既能发挥超声波技术的优势,又能利用H2O2的强氧化性,从而使得抗生素得以完全降解和矿化。
本研究将超声波和H2O2相结合,分别考察了H2O2投加量、超声功率、TC初始浓度和pH对盐酸四环素去除率的影响;在此基础上,通过液相色谱质谱联用(LC-MS)以及电子顺磁共振(electron spin-resonance spectroscopy, ESR)分析了盐酸四环素在超声波/H2O2体系下可能的降解途径,以期为制药废水的处理方法提供参考。
全文HTML
-
盐酸四环素(C22H25ClN2O8)购自上海阿拉丁生化科技股份有限公司;30% H2O2购自重庆市川东化工有限公司;浓盐酸(HCl)购自成都市科龙化工试剂厂;氢氧化钠(NaOH)购自重庆市川东化工有限公司;5,5-二甲基-1-吡咯啉-N-氧化物(DMPO)购自阿达玛斯试剂有限公司。实验室用水为超纯水。
-
TC降解实验。实验装置如图1所示,所用的仪器为超声波清洗器(HS-3120,天津市恒奥科技发展有限公司)。配制10 mg·L−1 TC溶液80 mL,用1 mol·L−1 NaOH和HCl溶液调节pH,加入一定量的H2O2,调节超声波清洗器的功率,每隔30 min取样置于紫外分光光度计(UV1102 Ⅱ,上海天美科学仪器有限公司),在特征吸收波长358 nm处测定TC的吸光度。
EPR实验。采用德国Bruker公司生产的EMX nano台式波谱仪(EPR)测定自由基。样品加入50 mmol·L−1的DMPO作为电子捕获剂,置于石英毛细管中。EPR测试参数为:微波衰减为19 dB、扫描时间为30.0 s、扫描次数为8、g因子为2.000 0。
-
根据式(1)计算TC的去除率。液体产生空化的最低声强或声压幅值——空化阈
Pc [22]由式(2)表示。式中:R为TC的去除率;C0为TC溶液的初始浓度, mg·L−1;Ct为反应t时刻TC溶液浓度, mg·L−1。
式中:
Pc 为空化阈,Pa;R0 为气泡核的半径,m;P0 为液体的静压力,Pa;Pv 为气泡内的蒸汽压,Pa;σ 为液体的表面张力,N·m−1。LC-MS测定实验。采用液相色谱-质谱联用仪(Afilent1260-6240)对TC降解过程中120 min的产物进行分析测定。液质联用色谱条件:流动相为35%去离子水和65%甲醇的混合物,流速为
1.0mL⋅min−1 ,柱温为30 ℃,进样量为10 μL,使用电喷雾离子源(ESI),在负离子模式下检测,毛细管温度为300 ℃,碰撞电压为135.0 V,全扫描范围为200~500。
1.1. 实验药品
1.2. 实验装置与方法
1.3. 分析方法
-
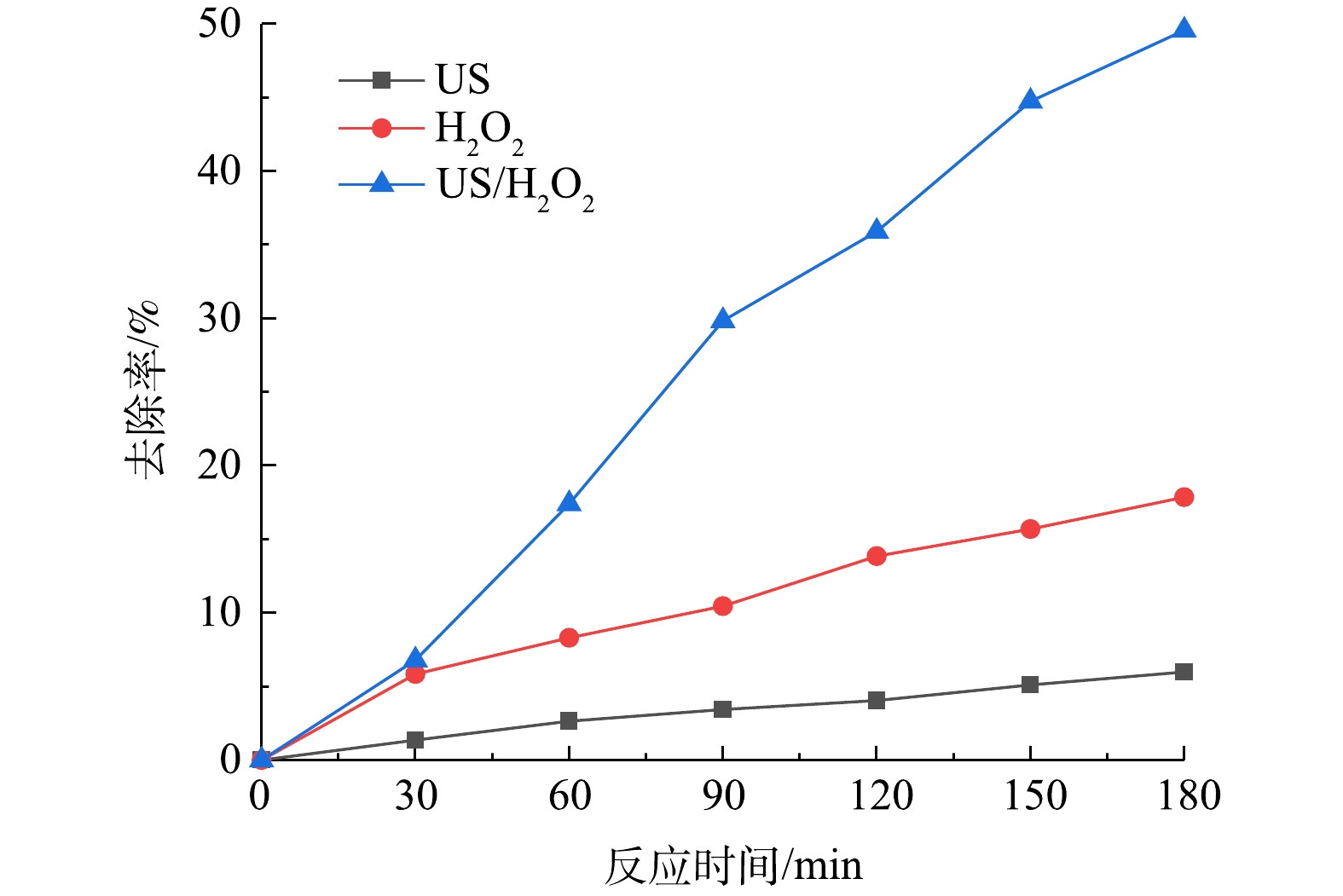
如图2所示,控制TC溶液pH为5,反应3 h后,单独采用超声处理10 mg·L−1 TC溶液,其去除率仅为1.72%;单独使用50 mmol·L−1 H2O2时TC去除率也仅为17.85%;而在超声/H2O2体系中,30 min后TC去除率快速增加,3 h后去除率可达到49.56%。由此可见,单独采用超声或H2O2对TC的去除效果远低于超声波协同H2O2体系处理的效果,这说明超声波对H2O2降解TC有明显的强化作用。
-
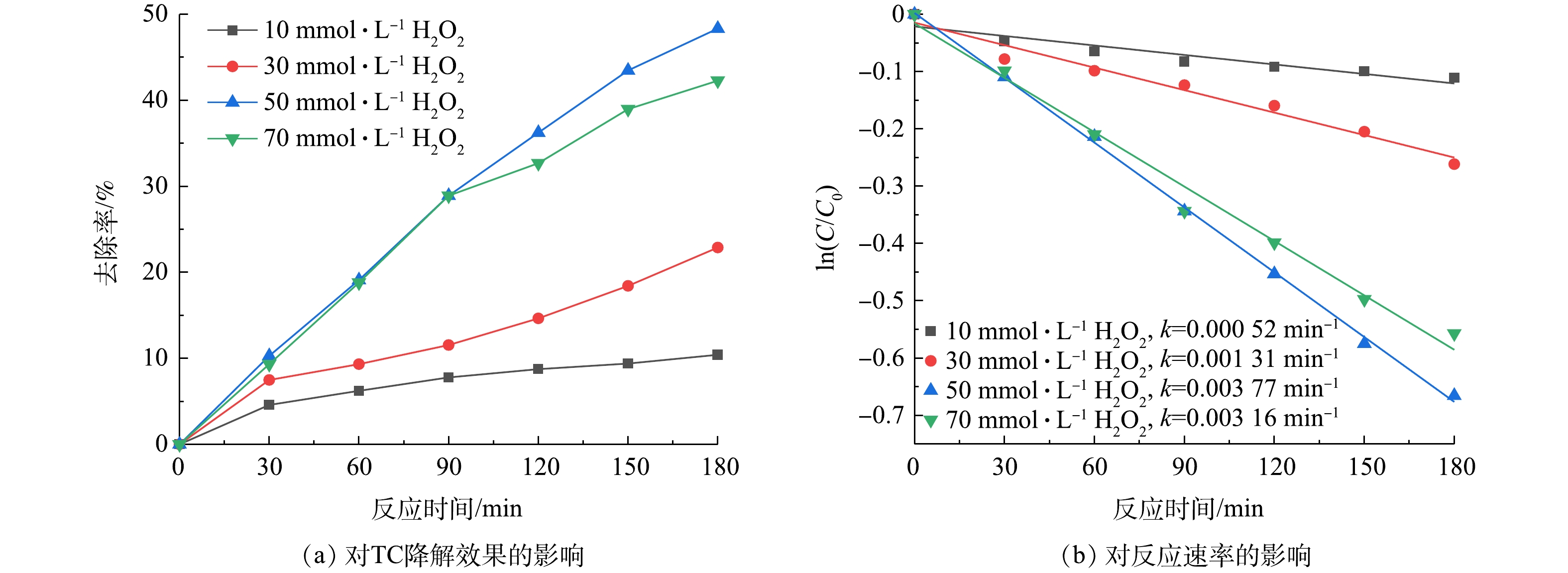
如图3(a)所示,当溶液TC浓度为10 mg·L−1、pH=5、超声功率为120 W时,在超声3 h后,在体系中投加10、30、50、70 mmol·L−1的H2O2后TC的去除率分别为10.42%、19.48%、48.33%、42.27%,TC去除率随H2O2浓度的增加先增大后减小,当 H2O2为50 mmol·L−1 时TC的去除率最高,达到48.33%。由图3(b)可知,ln(C/C0)与t基本呈线性关系。在一定的H2O2投加量内,TC去除率随H2O2的增加而升高,但H2O2投加量过多时,TC去除率反而下降,K先由0.000 52 min−1上升到0.003 77 min−1,再降到0.003 16 min−1。这是由于在超声波作用下,加入适量的H2O2可激发·OH的生成,使废水中·OH浓度升高,从而提高四环素的去除率。但是,过量的H2O2会与·OH发生反应生成氧化能力弱于·OH的氢过氧自由基HO2·[23](式(3))。因此,H2O2反而变成了·OH清除剂,导致·OH自由基浓度降低,故导致TC去除率有所下降。
-
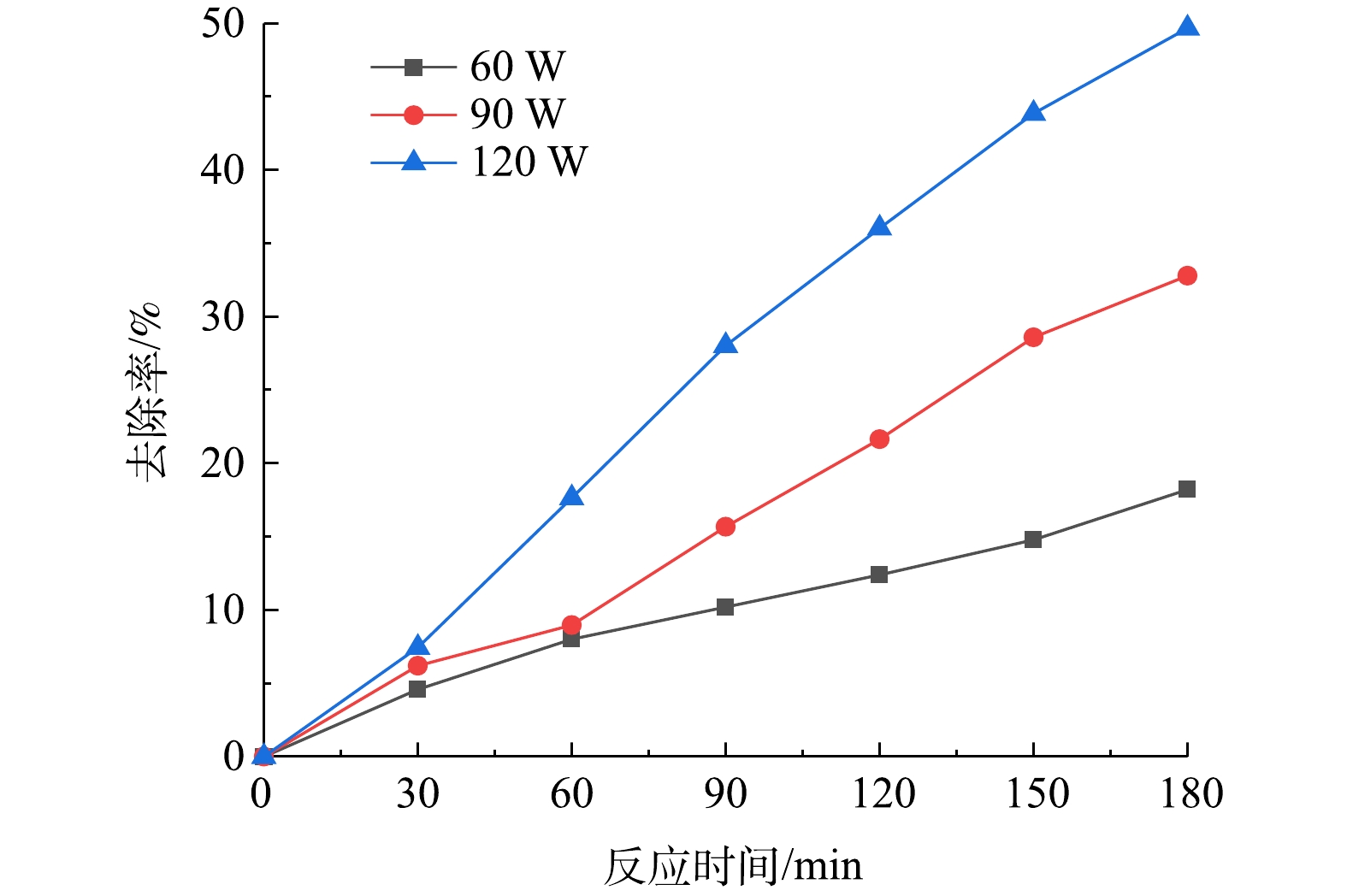
如图4所示,在溶液TC浓度为10 mg·L−1、pH为5、投加50 mmol·L−1H2O2、超声辐照3 h后,TC去除率分别为18.21%、32.79%、49.68%,可见,去除率随超声功率的增大而提高,在120 W时去除率最高,达到50%。超声空化阈值(式(2))与液体的温度、压力状态、含气量和气泡核的大小、分布有关。对于同一液体,影响空化阈值的主要因素是超声的强度。在超声功率较高的情况下,相应的声强度也较高,可以突破空化阈值,发生超声空化[22]。当超声空化发生时,通常伴随着声化学效应和机械效应。增加声功率可以提高输入到体系中的能量,提高声化学效应,从而产生更多的·OH。此外,超声波在高功率下会带来更强烈的机械效应,推动涡流效应产生的传质和传热过程,从而提高TC的去除率[24-25]。有研究[26]表明,当超过一定功率后,因为超声波强度太高,空化泡过多,声波向容器壁散射或反射回发射器,造成气屏效应,从而减弱去除效果。爱本研究中,TC的去除率随超声功率的增大而增大,这说明声功率在60~120 W内没有产生负面影响。
-
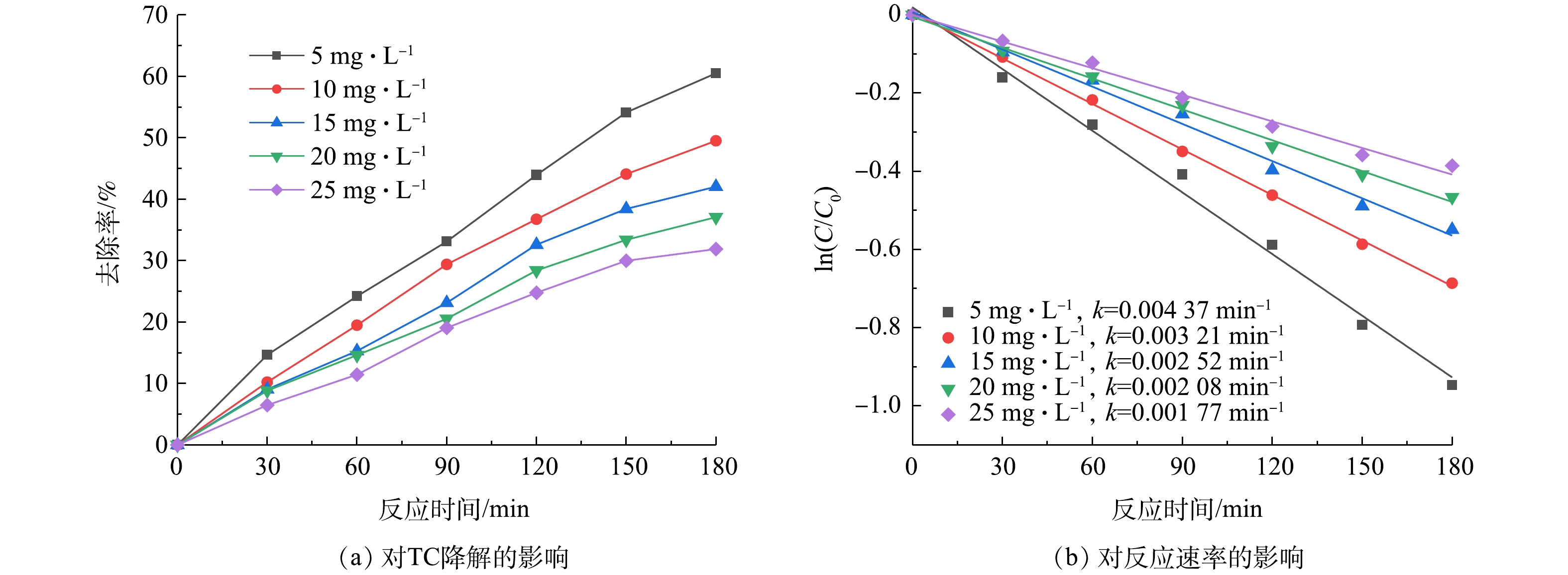
由图5可知,在投加50 mmol·L−1 H2O2、pH=5、超声功率为120 W反应3 h后,TC的去除率按TC初始浓度5、10、15、20、25 mg·L−1由低到高分别为60.51%、49.52%、42.05%、37.08%、31.89%,可见TC去除率随溶液浓度的增大反而降低。当TC浓度由5 mg·L−1上升为25 mg·L−1时,k由0.004 37 min−1 降为0.001 77 min−1,速率常数随溶液浓度的升高而减小,可见初始浓度高的盐酸四环素溶液降解速率最慢。这是由于在超声功率和H2O2投加量一定时,反应产生的自由基总量是一定的,在初始浓度较低时,TC可充分与·OH反应;但在高浓度下,一方面液体的黏度增加,空化点随着溶液浓度升高而趋向于饱和, 使得反应程度降低[27]。另一方面,降解过程中会产生更多的中间产物,这些中间产物会吸收或碰撞一部分超声能量,使得声空化泡崩溃时的能量不足,从而弱化声空化效应。由于·OH没有选择性,这些中间产物还会与TC争夺·OH,导致TC与自由基反应的概率降低,致使TC降解效果变差[28]。
-
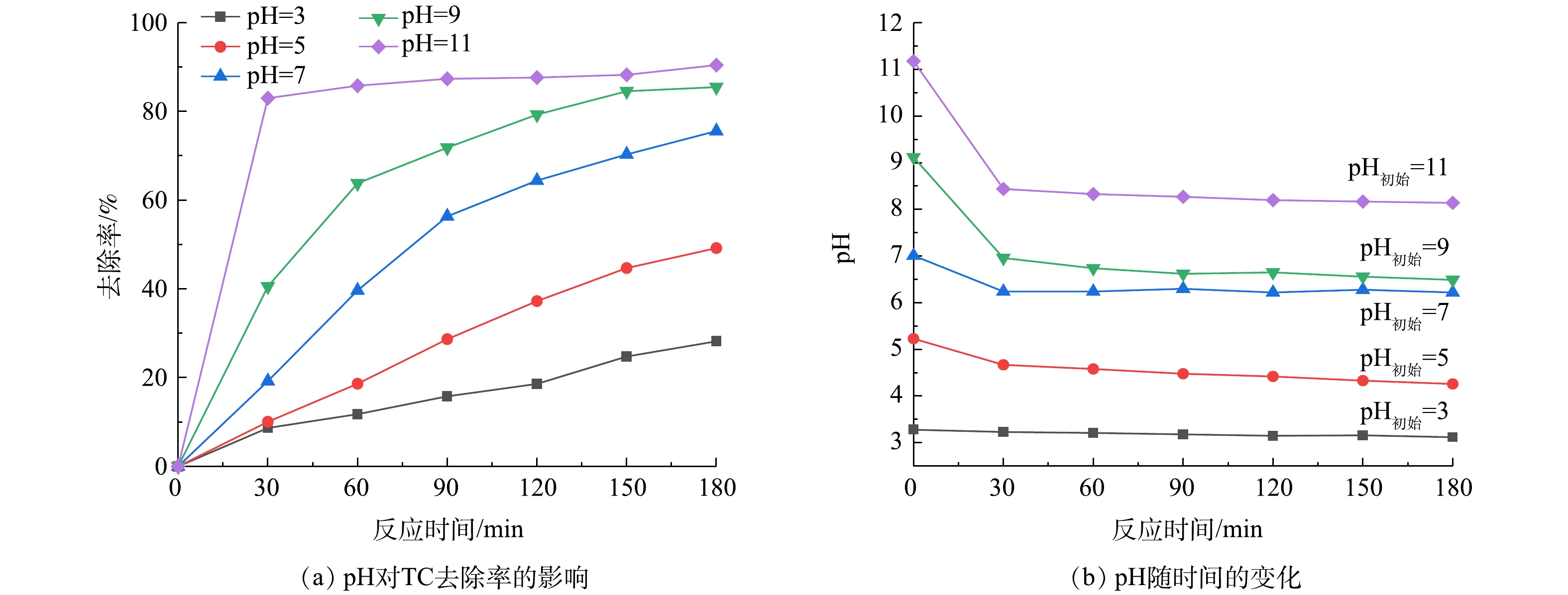
由图6(a)可知,pH对超声降解TC有很大的影响,碱性条件下的TC去除率明显高于酸性条件。在初始浓度为10 mg·L−1、投加50 mmol·L−1H2O2、超声功率120 W、溶液pH为11的条件下,反应30 min后TC去除率达到82.97%,这说明TC溶液在前30 min已基本降解完成。由图6(b)可以看出,反应过程中溶液的pH都呈下降的趋势,这是由于·OH的生成会消耗溶液中的OH−,从而使pH降低[29],溶液初始pH接近碱性时反应前后pH的变化最大。盐酸四环素的水溶液pH在5左右,反应3 h后的pH降为4,TC去除率为49.18%,而pH为11时的TC去除率高达90.4%,为不调节pH的1.8倍。这是因为在碱性条件下,TC离解程度高[29],以离子形式存在的TC溶液经去质子化后主要带负电荷,更容易被·OH氧化降解[30-31]。在碱性条件下,TC在环结构中能表现出较高的电子密度,有利于·OH的进攻[32]。
-
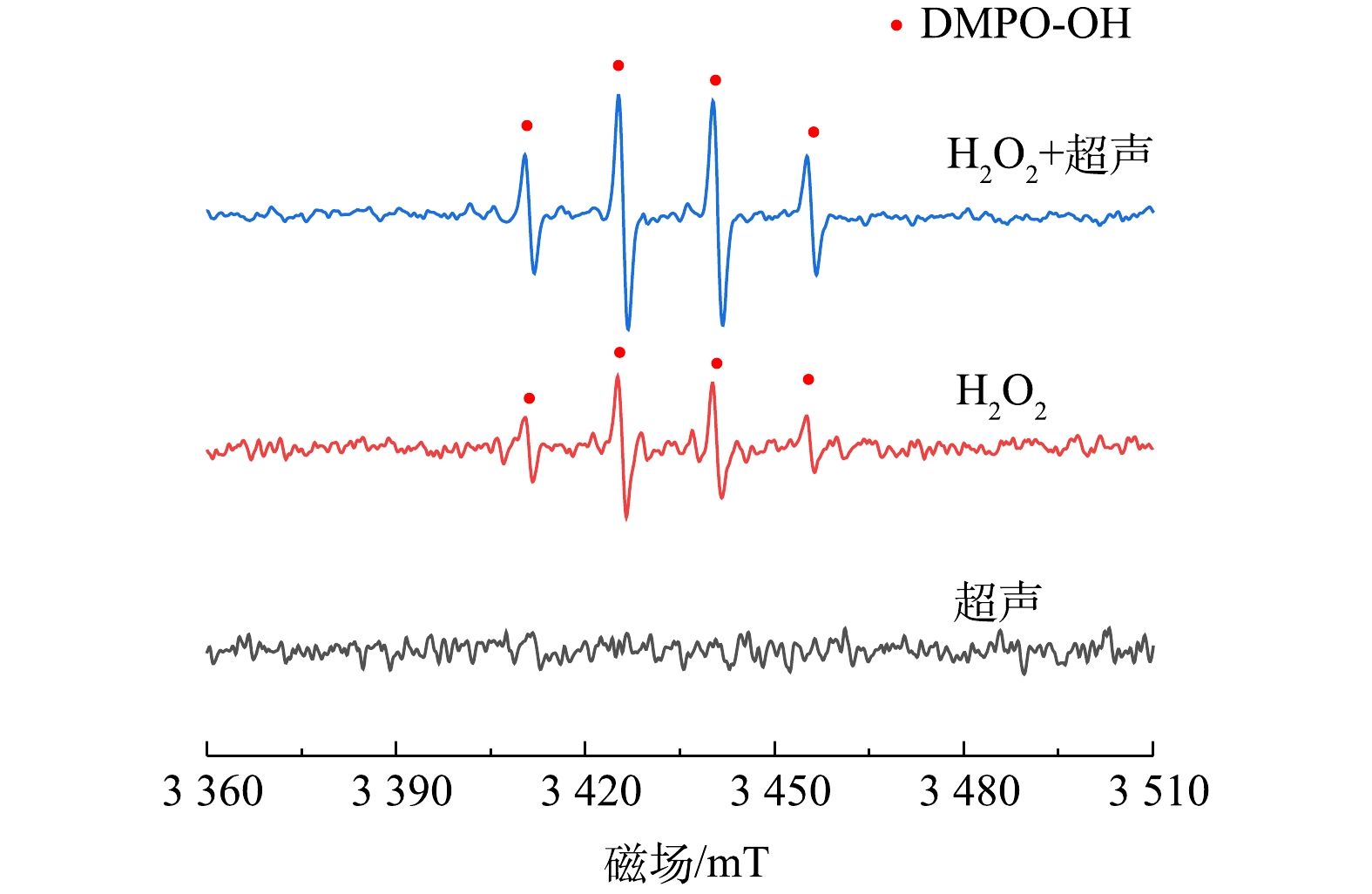
1)活性自由基的测定。本研究采用EPR对单独超声、单独加H2O2和超声/H2O2 3个体系中的活性物质进行了检测。由图7可以看出,单独超声无明显的信号峰,这说明单独超声几乎不产生·OH;在单独H2O2和超声/H2O2体系中,均检测到了强度比为1∶2∶2∶1的4重峰,该特征峰为DMPO捕获·OH形成的加合产物DMPO-OH的信号,说明这2个体系中产生了·OH,且·OH为主要的反应活性物质。超声/H2O2体系中DMPO-OH的4重信号峰更为明显清晰,说明相比于单独H2O2,超声/H2O2体系中·OH的浓度明显增加。
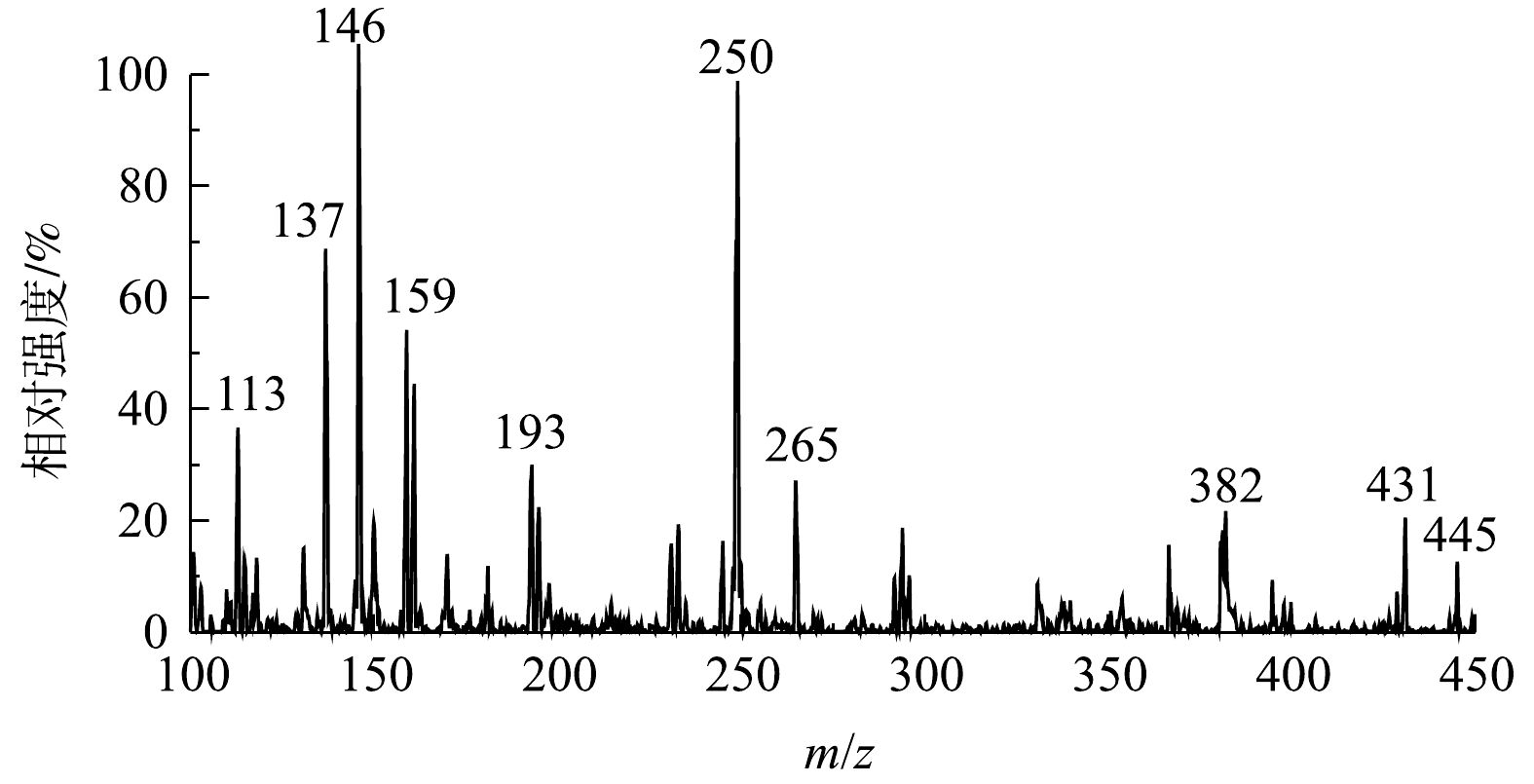
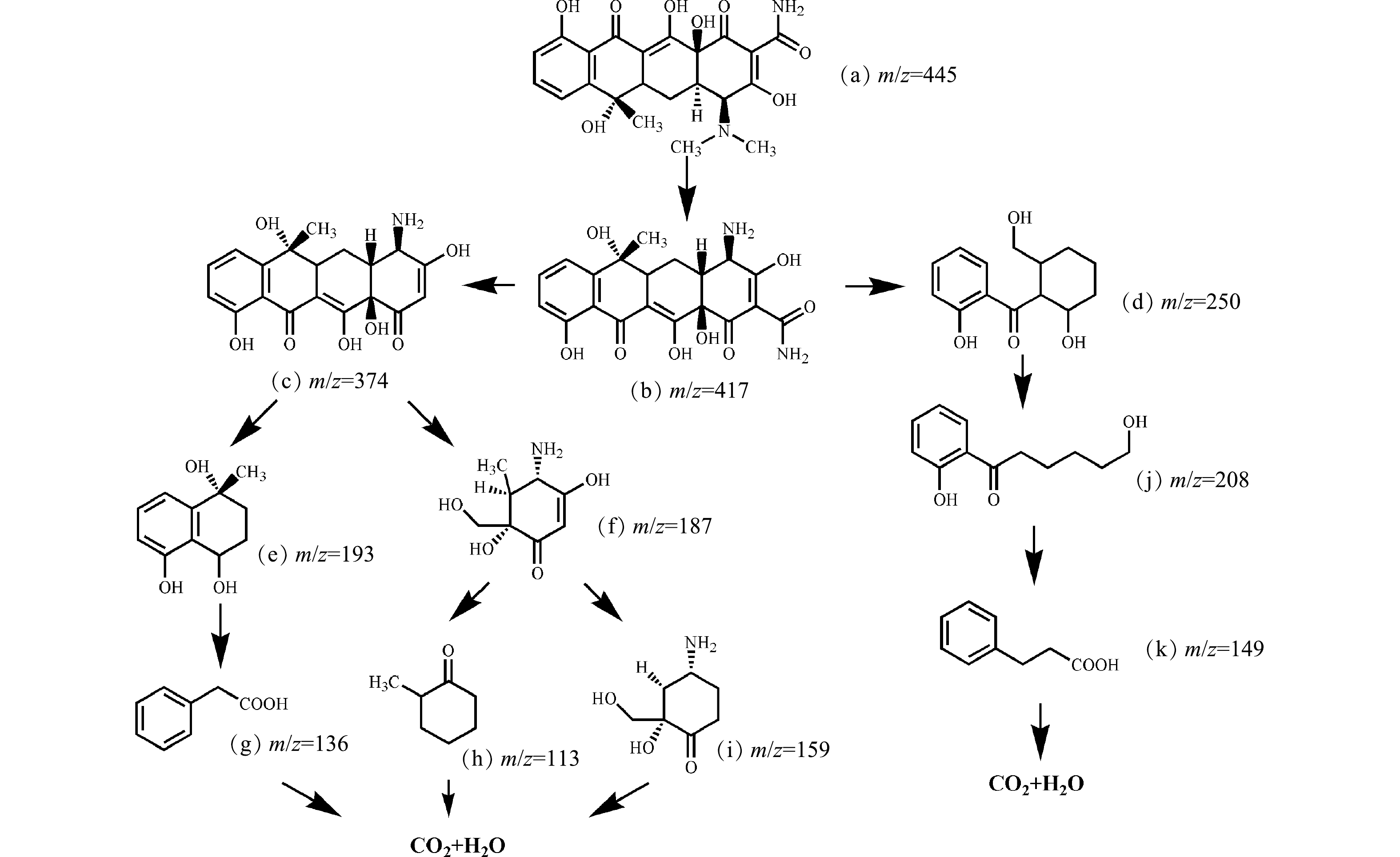
2)降解产物和可能的降解路径。通过LC-MS检测到超声/H2O2体系中TC的主要降解产物,结果如图8所示, TC可能的降解途径如图9所示。由图9可见,TC对应的a(m/z=445)在降解后首先分解成b(m/z=417),通过去甲基化和碳-碳单键断裂分解生成d(m/z=250),最后分解为k(m/z=149)、i(m/z=159)和g(m/z=136)。适量投加的H2O2可加速在空化泡溃灭时·OH的大量生成,·OH与[H]、O·等活性物质相结合可以破坏TC的碳链,加上H2O2的强氧化作用可导致TC的结构发生变化,被氧化分解为小的中间体和副产物,最后分解为CO2和H2O。
2.1. 不同体系下TC的降解效果
2.2. H2O2投加量对TC降解的影响
2.3. 超声功率对TC降解的影响
2.4. TC初始浓度对TC降解的影响
2.5. pH对TC降解的影响
2.6. 超声降解TC的可能机理
-
1)超声波协同H2O2对四环素的去除率远远高于采用超声波或H2O2单独作用的去除率,且超声/H2O2对四环素的降解符合拟一级反应动力学模型。
2) H2O2投加量、超声功率、TC初始浓度和溶液pH对TC的降解有较大影响。当H2O2投加量为50 mmol·L−1、TC为10 mg·L−1、超声功率为120 W、溶液pH为11、超声时间为3 h时,超声波/H2O2对四环素的去除率能够达到90.4%。
3)采用EPR检测到超声/H2O2体系产生的主要活性自由基是·OH,LC-MS测定分析结果显示,TC经过脱烷基、脱胺和开环等方式转化降解为m/z=250、m/z=159、m/z=136等10种中间产物,最后氧化分解为CO2和H2O。



 下载:
下载: