-
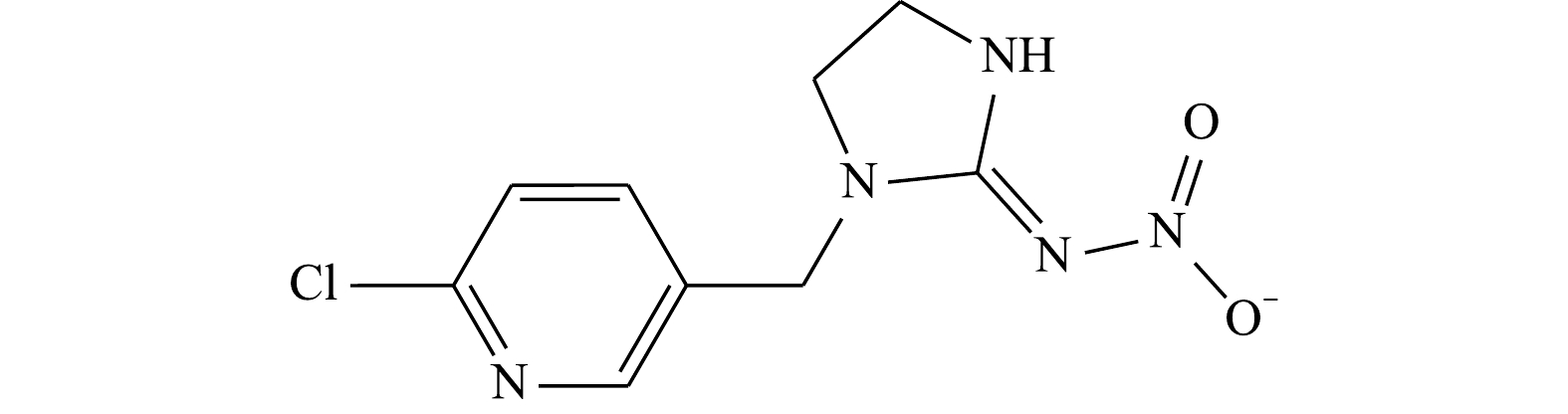
吡虫啉(imidacloprid,IMI)是一种典型的新烟碱类杀虫剂,分子式为C9H10ClN5O2,化学名称为1-(6-氯-3-吡啶基甲基)-N-硝基亚咪唑-2-基胺,化学结构如图1所示。吡虫啉属于硝基亚甲基类内吸性低毒杀虫剂,通过作用于昆虫的神经系统致死[1]。吡虫啉不仅对目标害虫有高效致死性,对非目标生物也会造成严重威胁[2-3]。随着使用量的不断增加,吡虫啉在不同的环境介质中均有检出[4],开发切实有效的吡虫啉等新烟碱类杀虫剂的控制技术[5-6],减少其对人体的暴露非常必要。目前,水解[7]、光解[8]、生物降解[9]、氧化降解[10-11]等技术均已被应用于吡虫啉的降解研究中。其中,高级氧化技术通过产生强氧化性的自由基降解污染物[12],其具有反应迅速、降解速率快等优势,故显示出广阔的应用前景。
高级氧化方法中常用的氧化剂主要有H2O2、O3、过硫酸盐等。其中,过硫酸盐是近年来新兴的高效氧化剂[13],其在环境温度下稳定、成本低且效率高,并且是无害的。过硫酸盐包括过氧单硫酸盐(PMS)和过二硫酸盐(PDS),在光、热、电及过渡金属等的活化下,会发生分解而产生
SO⋅−4 [14]。SO⋅−4 的氧化还原电位(E0=2.5~3.1 V)高于·OH (E0=1.8~2.7 V)[15],且SO⋅−4 在水溶液中寿命可达4 s左右(·OH的寿命一般小于10−4 s)。因此,SO⋅−4 的氧化能力更强,可氧化大部分有机化合物。过硫酸盐在水中具有较大的溶解性,且在宽pH范围内都有适用性,因此,在实际应用中可行性较高。近几年,基于过硫酸盐的高级氧化技术开始被应用于农药及杀虫剂废水处理中。HAYAT等[16]制备了CuO、CuO/BC、PyR活化过硫酸钠降解IMI,均可以实现有效降解,其中PyR/PS体系对IMI降解率最高,在180 min内降解率可达86.9%。WANG等[17]使用UV/PS和UV/PMS 2个体系去除IMI,结果表明,仅使用氧化剂无法有效去除IMI,而UV/PS和UV/PMS体系对IMI的降解率分别可达94.3%和86.7%。实践证明,基于过硫酸盐的高级氧化技术对IMI有非常好的去除效果。
过硫酸盐的活化方式较多,其中金属离子活化由于效率高、反应条件易控制等优点被广泛使用。但均相金属离子应用时容易造成二次污染,因此,研究人员逐渐将目光转移至非均相复合金属氧化物上来。根据现有报道,在过渡金属活化PMS的研究中,Co表现出优异的活化性能[18-19]。结合Co的非均相体系活化PMS既可以实现高效率,又可以减少金属离子溶出对环境的影响,因此,制备钴基双金属氧化物活化PMS降解有机污染物具有潜在的应用前景。CuCo2O4[20]、NiCo2O4[21]、ZnCo2O4[22]等被报道具有高的电子电导率、出色的催化性能以及较好的稳定性,并且在活化过硫酸盐降解不同种类有机污染物的研究中均有较高的降解效果[20-22]。Fe作为一种环境友好型的过渡金属,其活化效果在常用的过渡金属中仅次于Co。MnFe2O4[23]、NiFe2O4[24]等尖晶石型铁氧体具有较高的过电势和丰富的表面羟基,在催化过程中表现出优异的性能,同时,铁氧体易磁性分离的优点也使其受到广泛关注[23-24]。此外,ZnO作为一种常用的光催化材料,被报道与其他金属氧化物复配时可以有助于加快电子传递速率、增加活性位点,从而提高有机物去除效果,如CuO-ZnO等[25]。上述双金属氧化物在活化PMS降解有机污染物方面均有报道,但在氧化降解IMI方面研究较少,因此,对比几种双金属氧化物催化剂活化PMS降解IMI具有较高可行性。
基于上述研究,本研究以IMI为目标污染物,制备了6种双金属氧化物;根据IMI降解率及离子溶出情况,选取最优双金属氧化物催化剂,并考察了其活化PMS降解IMI的效果,探究了IMI初始浓度、溶液初始pH、催化剂投加量、PMS浓度及水中常见阴离子对IMI降解率的影响;表征了降解过程中产生的活性自由基,并分析了其可能的产生机理,应用GC-MS测定了降解产物并推测了IMI降解途径,以期为双金属氧化物活化PMS修复IMI污染水体提供技术参考。
全文HTML
-
吡虫啉(IMI,纯度≥98%,阿拉丁试剂有限公司),过一硫酸氢钾(PMS,纯度≥98.0%,国药集团化学试剂有限公司),甲醇(MeOH)、叔丁醇(TBA)、六水合硝酸钴(Co(NO3)2·6H2O)、六水合硝酸锌(Zn(NO3)2·6H2O)、六水合硝酸镍(Ni(NO3)2·6H2O)、三水合硝酸铜(Cu(NO3)2·3H2O)、九水合硝酸铁(Fe(NO3)3·9H2O)、硝酸锰(Mn(NO3)2)、柠檬酸(CA,C6H8O7·H2O)、氨水、盐酸、氢氧化钠,均购自上海生工试剂有限公司。实验中所用化学试剂均为分析纯,实验用水均采用超纯水机出水。
-
液相色谱仪(Ultimate 3000,美国);气相色谱质谱联用仪(GCMS-QP2010U1tra,美国);扫描电子显微镜(S-4800,日本);X射线衍射仪(D/max-2550VB+/PC,日本);傅里叶红外光谱分析仪(NEXUS-670,美国);X射线光电子能谱分析仪(Escalab 250Xi,中国);电子自旋共振(Bruker A300,德国)。
-
1) 6种双金属氧化物及ZnO、Co3O4的制备。6种双金属氧化物的制备采用凝胶法[26],以ZnCo2O4为例:按n(Co2+)∶n(Zn2+)=1∶1称取一定量的Co(NO3)2·6H2O和Zn(NO3)2·6H2O,溶于适量去离子水中,搅拌10 min使其完全溶解,形成紫色的透明溶液;按n(CA)∶n(M2+)=1∶1称取一定量的柠檬酸,溶于适量去离子水中。将配制好的柠檬酸溶液加入金属离子溶液中,放于90 ℃水浴锅中加热搅拌,使其充分混合,同时用氨水调节其pH为9左右,加热搅拌至形成紫色的黏稠凝胶;将凝胶转至110 ℃的烘箱中干燥12 h得到干凝胶。将干凝胶研磨后转移至坩埚中,于600 ℃的马弗炉中煅烧2 h,得到ZnCo2O4,研磨待用。
ZnO[27]、Co3O4[28]的制备。分别称取一定量的Co(NO3)2·6H2O和Zn(NO3)2·6H2O,各自溶于适量去离子水中形成溶液,按n(CA)∶n(M2+)=1∶1称取一定量的柠檬酸溶于上述溶液;用氨水调节溶液pH为9左右,于90 ℃水浴锅中加热搅拌至形成粘稠凝胶;将凝胶转至110 ℃的烘箱中干燥12 h得到干凝胶,干凝胶研磨后于600 ℃的马弗炉中煅烧2 h,分别得到ZnO、Co3O4,研磨待用。
2)降解实验。取一定量IMI储备液于100 mL锥形瓶中,加入适量去离子水稀释为20 mg·L−1的IMI溶液,向溶液中加入一定量的PMS和ZnCo2O4催化剂,放于室温的水浴恒温振荡器中开始反应并计时。在预定时间内取样,立即加入同等体积的甲醇进行淬灭,每个实验设置3个平行。将混合溶液用0.22 μm滤膜过滤后转移至液相色谱进样瓶中,通过HPLC分析样品中IMI浓度。
-
1)催化剂表征。通过Quanta200, pHilips 2500扫描电镜(SEM)观察ZnCo2O4的微观形貌;采用D/max-2550VB+/PC X射线衍射仪(XRD)对ZnCo2O4进行物相分析;使用NEXUS-670傅里叶红外光谱分析仪(FTIR)对ZnCo2O4的结构进行分析;利用Escalab 250Xi X射线光电子能谱仪(XPS)分析ZnCo2O4表面的成分及化合态。
2)吡虫啉检测。通过安捷伦高效液相色谱仪(HPLC,美国)对吡虫啉含量进行测定。检测条件:流动相为甲醇∶水(v∶v)=45∶55,流速为1.0 mL·min−1。色谱柱为Diomand C18(5 μm×200 mm×4.6 mm,赛默飞世尔),柱温 30 ℃,进样量为10 μL,检测波长为270 nm。吡虫啉在高效液相色谱中的保留时间为6.4 min。
1.1. 材料与试剂
1.2. 主要仪器
1.3. 实验方法
1.4. 分析方法
-
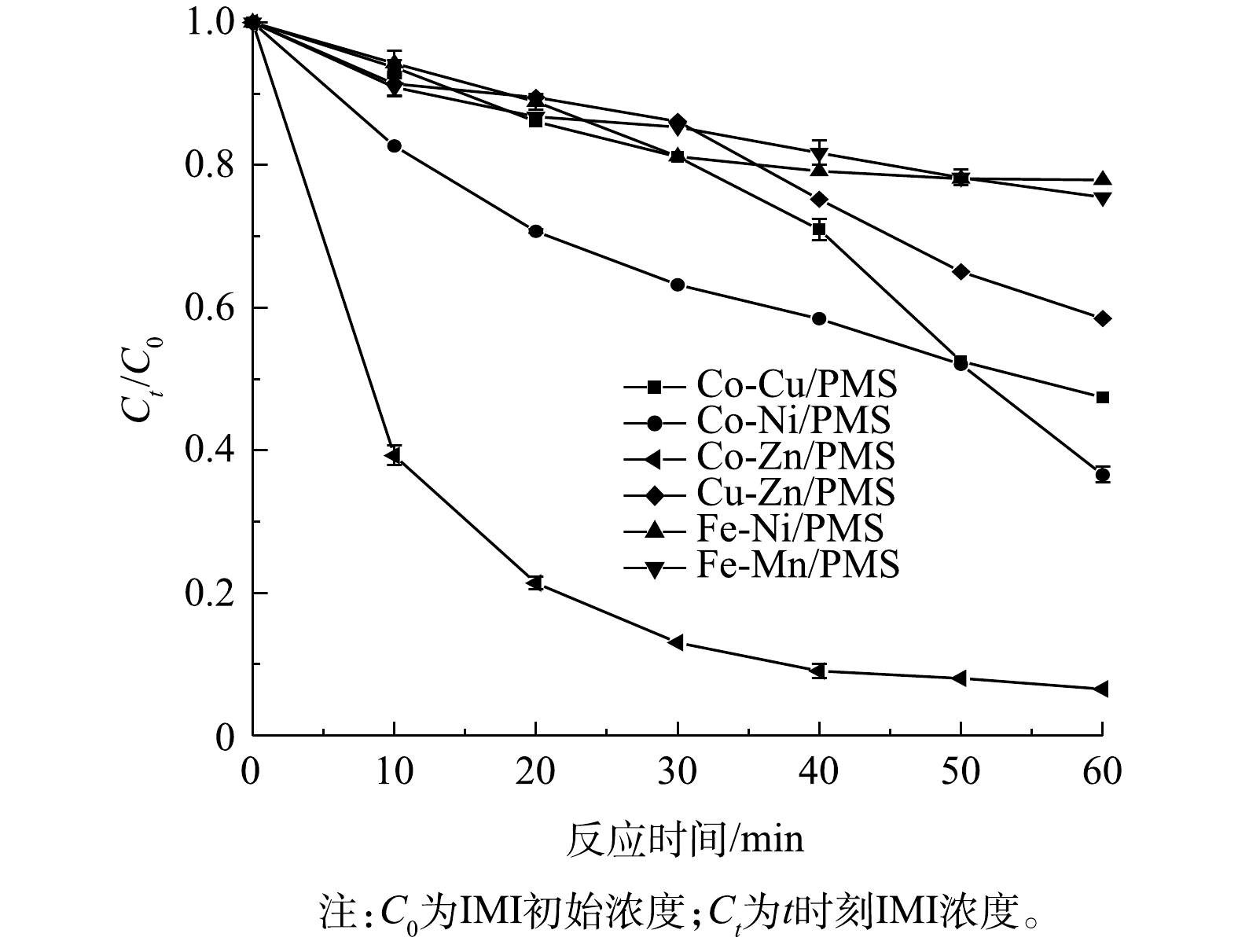
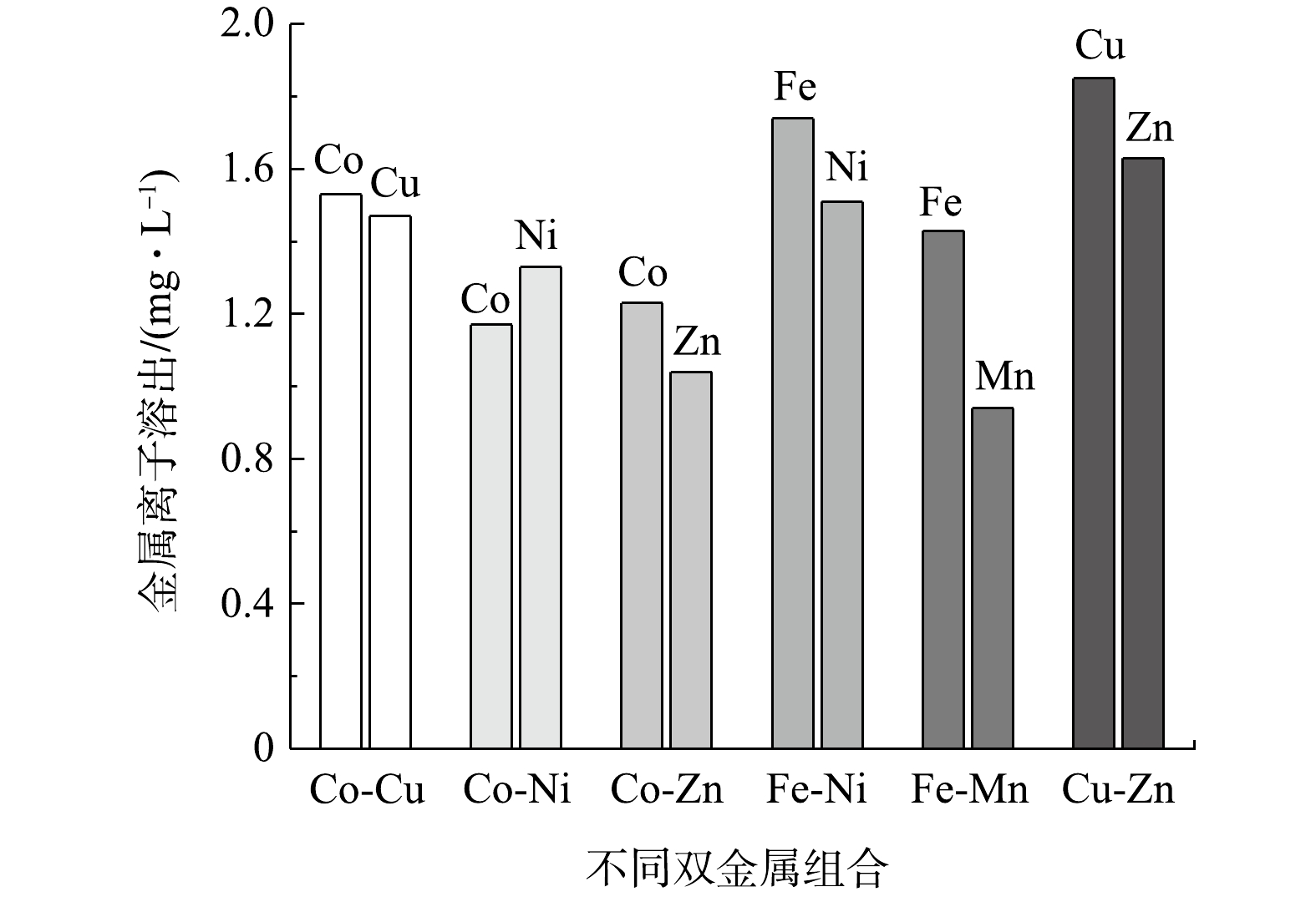
使用凝胶法制备了6种不同双金属氧化物催化剂,分别为Co-Cu、Co-Ni、Co-Zn、Fe-Mn、Fe-Ni、Cu-Zn氧化物。在IMI初始浓度为20 mg·L−1,PMS用量为15 mmol·L−1,催化剂用量为0.4 g·L−1的条件下,考察了6种催化剂对IMI的降解效果及金属离子溶出情况,结果分别如图2和图3所示。由图2可以看到,Co-Zn/PMS体系在60 min对IMI的降解率最高,明显优于其他5种组合体系;Co-Ni/PMS、Co-Cu/PMS体系对IMI的降解效果相比于其他3种非钴基金属氧化物体系而言偏好。从整体上来看,钴基金属氧化物在活化PMS降解IMI体系中表现出较好的催化活性,其中Co-Zn双金属氧化物催化活性最高。结合图3可见,Co-Zn双金属氧化物在实现最高降解率的同时离子溶出较少,因此,后续表征和降解实验主要围绕Co-Zn双金属氧化物展开。
-
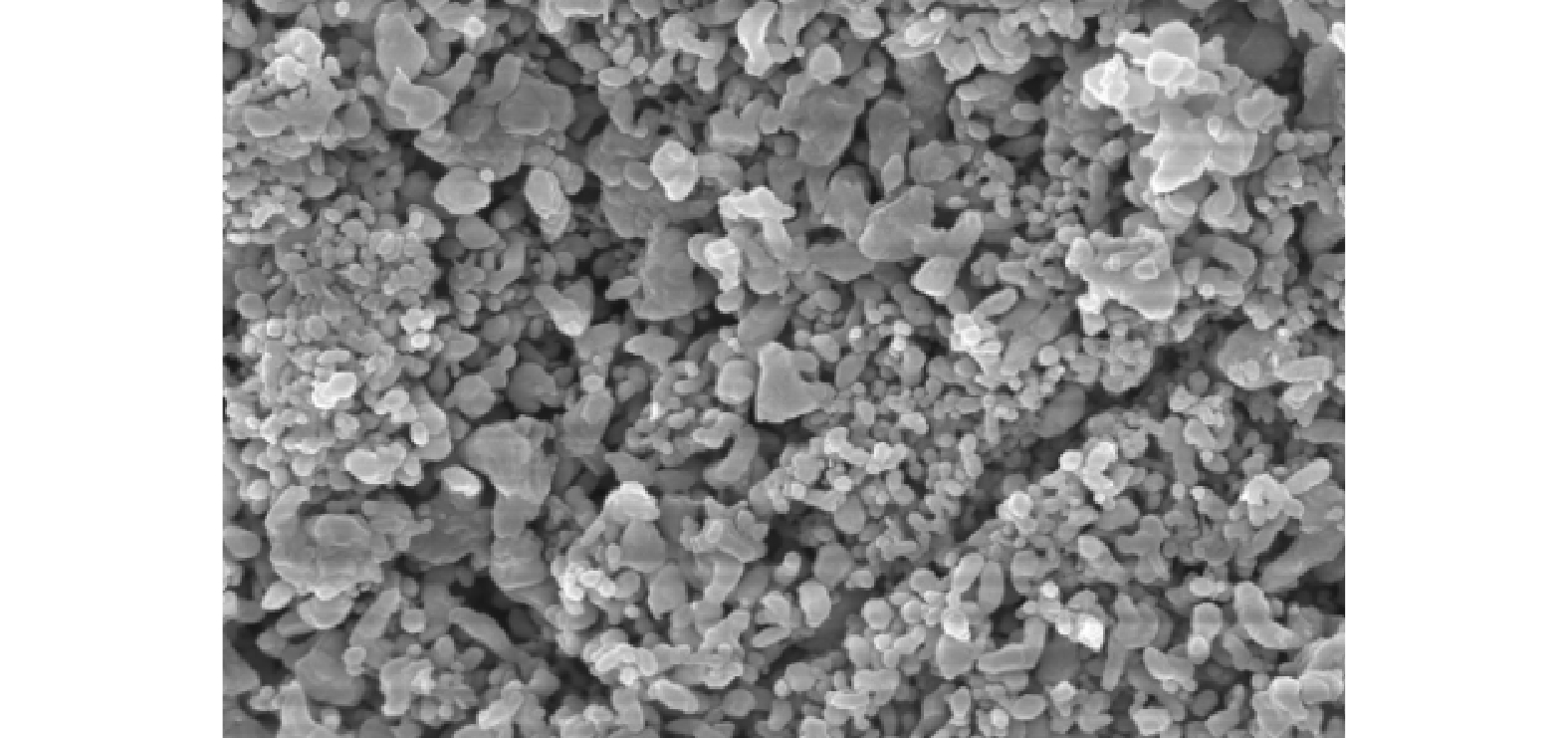
1) SEM表征。在非均相催化反应中,反应过程通常发生在固体催化剂的表面,因此,催化剂的结构对其效果有一定的影响。为研究催化剂的形貌和结构,采用扫描电镜对样品进行了分析,图4为放大50 000倍的SEM图。可以看到,催化剂粉末颗粒细小,分散均匀,晶粒致密。催化剂内部呈多孔结构,有利于增大其比表面积,从而为活化反应提供更多的活性位点,加快反应速率。在SHIH等[29]对ZnCo2O4/G的SEM表征中也看到相似的晶体结构。
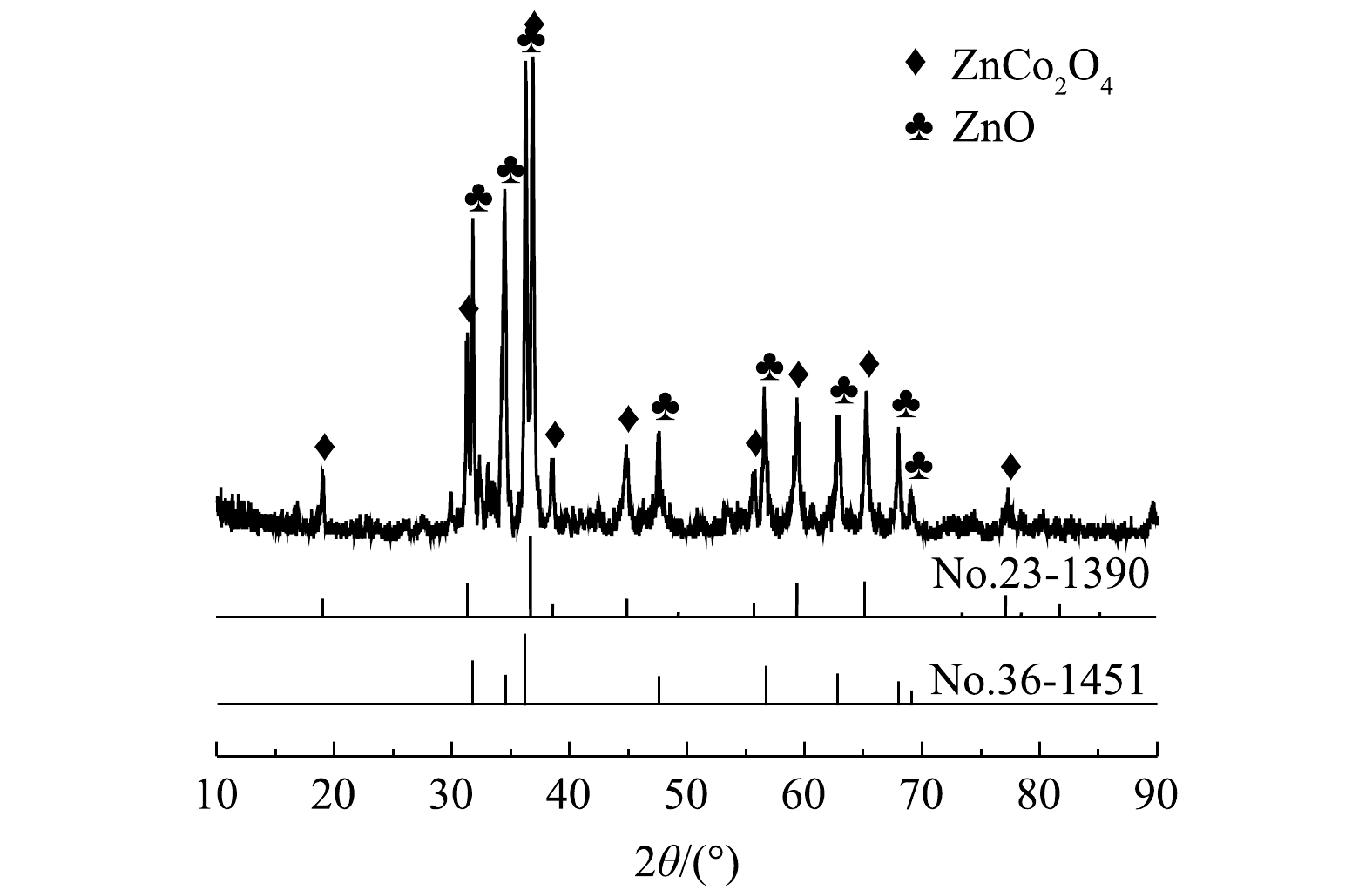
2)XRD表征。为确定催化剂的物质组成与晶型,对催化剂进行XRD表征,结果如图5所示。19.00°、31.22°、36.81°、44.74°、55.57°、59.28°、66.15°对应ZnCo2O4的衍射峰,相对应的miller指数分别为(111)、(220)、(311)、(400)、(422)、(511)、(440),均符合立方晶型的ZnCo2O4的标准卡片(NO.23-1390)。此外,在31.73°、36.21°、47.49°、56.52°、62.80°、68.99°、69.10°等处也有较强衍射峰,经对比符合ZnO的标准卡片(NO.36-1451),这说明制备ZnCo2O4时有少量ZnO生成。KATHALINGAM等[30]和XIE等[31]的研究均表明,在制备氮掺杂ZnCo2O4/石墨烯/聚苯胺复合材料及CoxZnyAlOz催化剂时均伴有ZnO生成。ZnO作为一种有许多自由移动电子的半导体材料,其掺入可以加快电子的传递速率[32],在一定程度上也可以活化PMS[33-34],从而促进反应的进行。但ZnO在酸性体系中会发生溶解,对催化剂的稳定性有一定影响,在今后的研究中需进一步优化,以避免ZnO的生成。
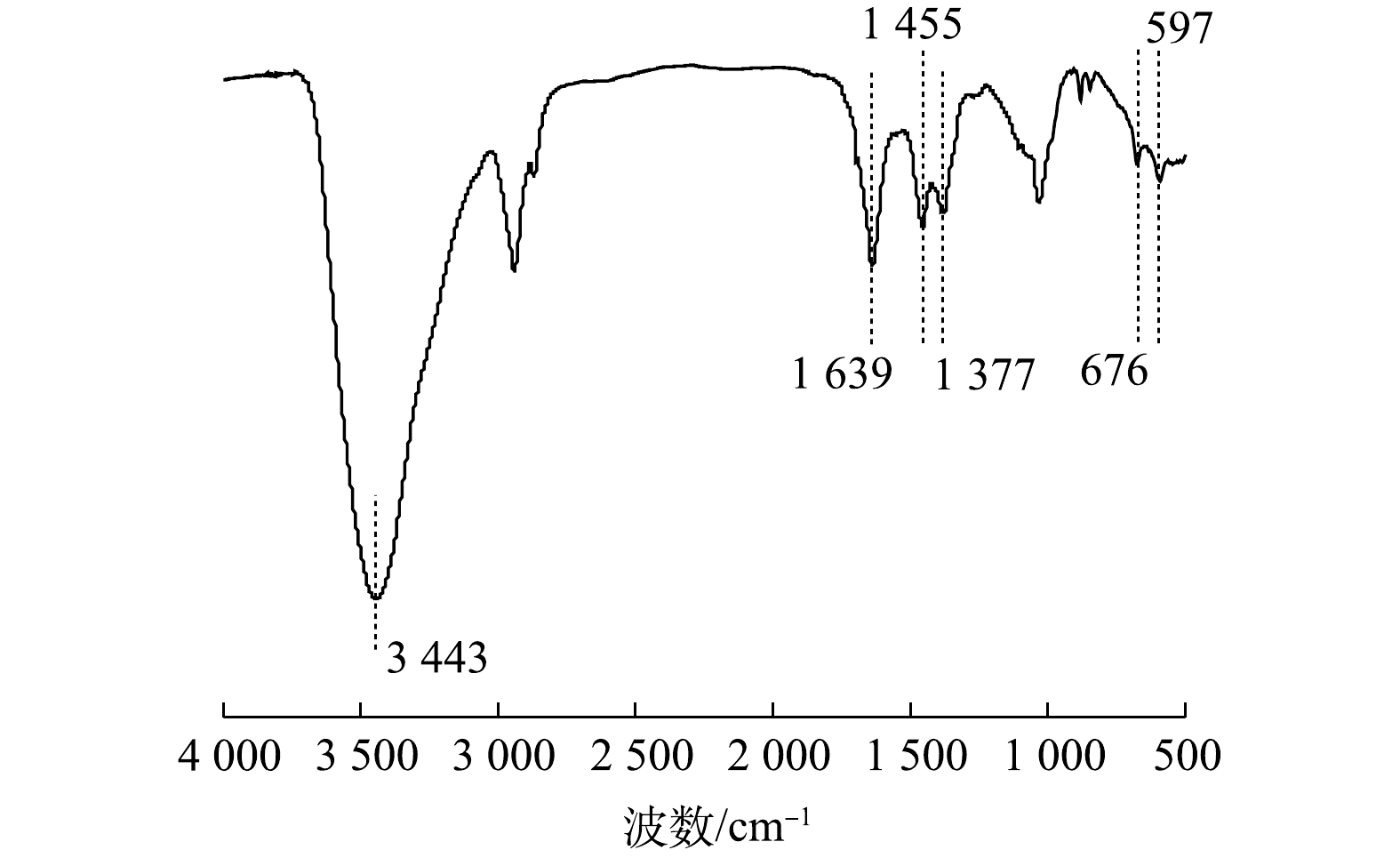
3) FTIR表征。对催化剂的红外光谱分析结果如图6所示。由图6可见,3 443 cm−1处的吸收峰为O—H的特征吸收峰[35],这可能是由于其表面吸附水分所致;1 639 cm−1处的吸收峰是由C=O(羰基)的伸缩振动产生;1 455 cm−1和1 377 cm−1处的吸收峰为—COOM(羧酸盐,M为金属离子)耦合反对称和对称伸缩振动吸收峰,该峰的存在表明作为络合剂的柠檬酸通过共价键与金属离子相连,有助于形成交联的配位结构。参考LI等[36]及MANDAL等[37]的报道,676 cm−1处的峰值归因于四面体配位金属氧化物Zn—O键的拉伸振动,597 cm−1处的吸收峰归因于八面体配位金属氧化物Co—O键的拉伸振动。
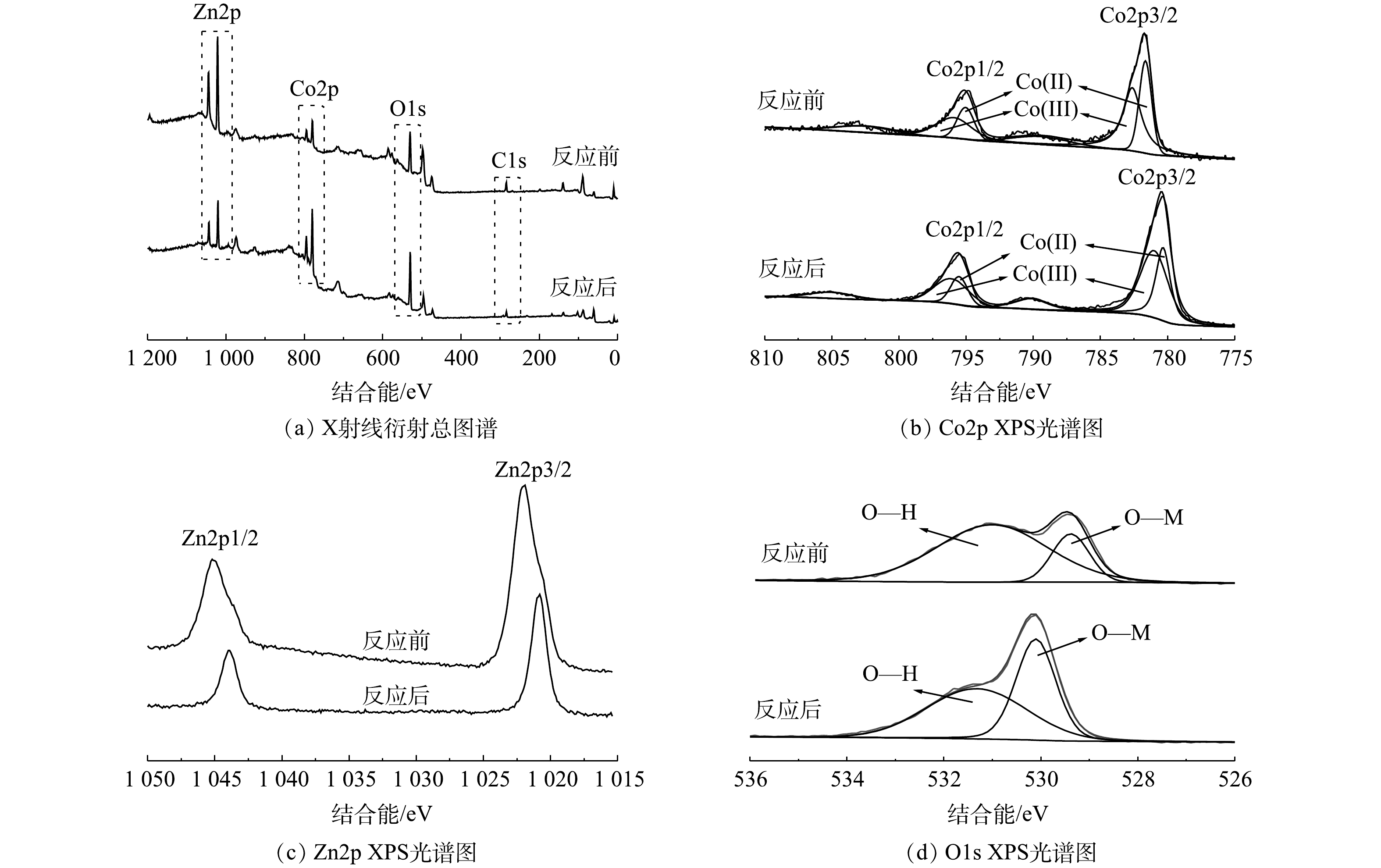
4) XPS表征。通过XPS表征可以进一步理解催化剂表面的成分与化合态,图7为ZnCo2O4反应前后各元素的状态。图7(a)所示为反应前后ZnCo2O4的总谱图,反应前后Zn、Co、O均有较强的特征峰,C元素有微弱吸收峰,其存在可能因柠檬酸残留或吸附杂质所致。图7(b)为反应前后Co2p谱图,反应前在779.6 eV和794.9 eV处及反应后在780.3 eV和795.5 eV均有典型的Co2p峰,分别对应Co2p3/2和Co2p1/2轨道,反应后Co的结合能略有上升;每个特征峰又对应了Co(Ⅱ)和Co(Ⅲ)的特征峰,经峰面积计算,反应后Co(Ⅱ)的含量从40.2%降至38.8%,而Co(Ⅲ)的含量从59.8%升至61.2%。这说明Co(Ⅱ)活化PMS后自身被氧化至Co(Ⅲ),实验前后2种价态钴离子的含量变化较小,也说明催化剂的重复性较好。催化剂反应前后的Zn2p谱图如图7(c)所示。可以看到,反应前在1 021.9 eV和1 045.2 eV处以及反应后在1 021.2 eV和1 044.3 eV处均有2个强峰,分别对应了Zn2p3/2和Zn2p1/2,反应后Zn的结合能略有下降。图7(d)为O1s的XPS谱图,由该图可见,反应前在529.6 eV和531.2 eV处有较强吸收峰,分别代表了O—M(Zn和Co)和O—H[38]。反应后O—M峰强度增加,可能为部分游离金属离子被氧化所致。
-
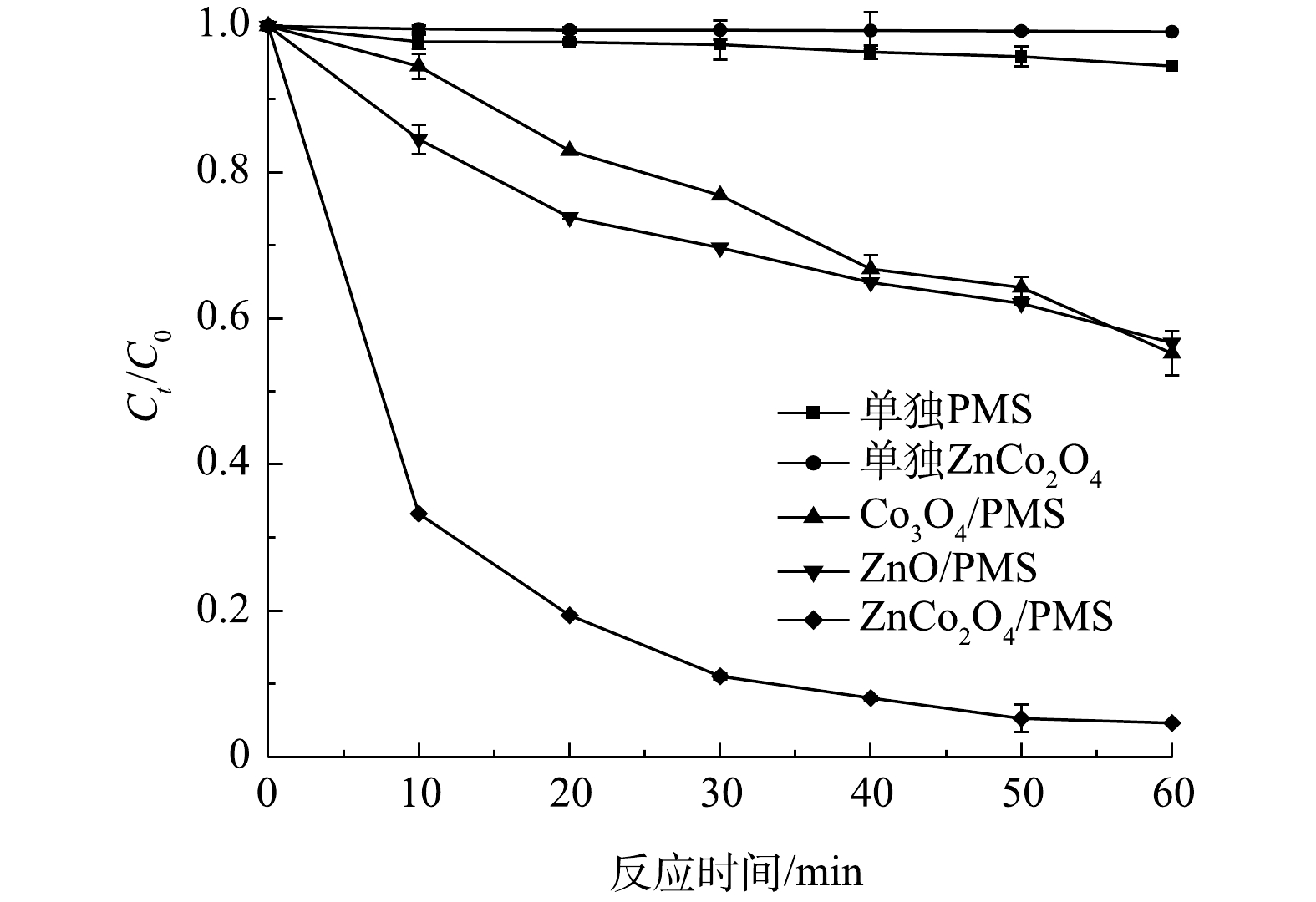
选取IMI的初始浓度为20 mg·L−1,PMS投加量为15 mmol·L−1,ZnO、Co3O4、ZnCo2O4投加量均为0.4 g·L−1,考察单独PMS、单独ZnCo2O4、ZnO/PMS、Co3O4/PMS、ZnCo2O4/PMS 5种不同体系中IMI的降解情况,实验结果如图8所示。由图8可见,单独投加PMS作用下,60 min后IMI的降解率仅为5.0%左右;ZnCo2O4单独作用时,在60 min内对IMI几乎不能实现降解。可以推测,ZnCo2O4催化剂对IMI几乎没有吸附作用,单独PMS对IMI的氧化作用很弱。根据图8所示,单独ZnO活化PMS降解IMI的效率仅为43.3%。依据SHUKLA等[39]的研究结果,ZnO可引发PMS生成·OH和
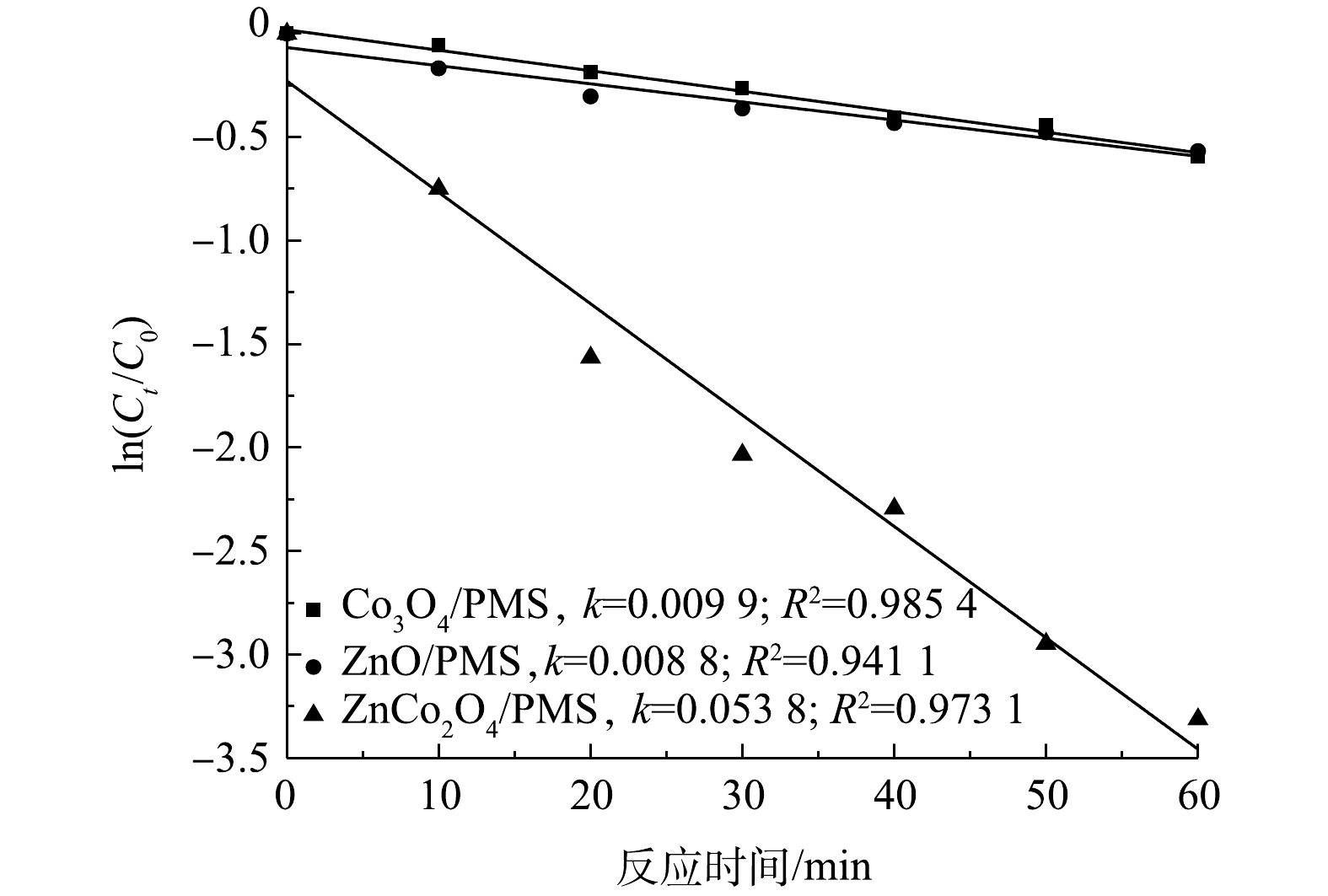
SO⋅−4 [34](式(1)),因而对有机污染物有一定降解效果。此外,单独Co3O4活化PMS降解IMI的效率仅为44.8%。根据已有研究[40-41]可知,PMS的活化反应通过固体催化剂表面的Co2+及水溶液中的Co2+共同实现(式(2)~式(5))。但ZnO、Co3O4在反应中易发生金属离子流失,因而催化效果较差。相比之下,在ZnCo2O4催化作用下,PMS对IMI的降解效果十分显著,可以在60 min内对IMI实现95.3%的降解,这表明Co、Zn在一定程度上有协同作用,可共同促进降解。ZnO/PMS、Co3O4/PMS、ZnCo2O4/PMS 3个体系的动力学拟合结果如图9所示。3个体系下IMI的降解均符合拟一级降解动力学模型(R2>0.94)。其中,ZnCo2O4/PMS体系在60 min内降解速率最快,其一级动力学常数k值为0.053 8 min−1,远大于ZnO/PMS(k=0.008 8 min−1)和Co3O4/PMS (k=0.009 9 min−1)体系。
-
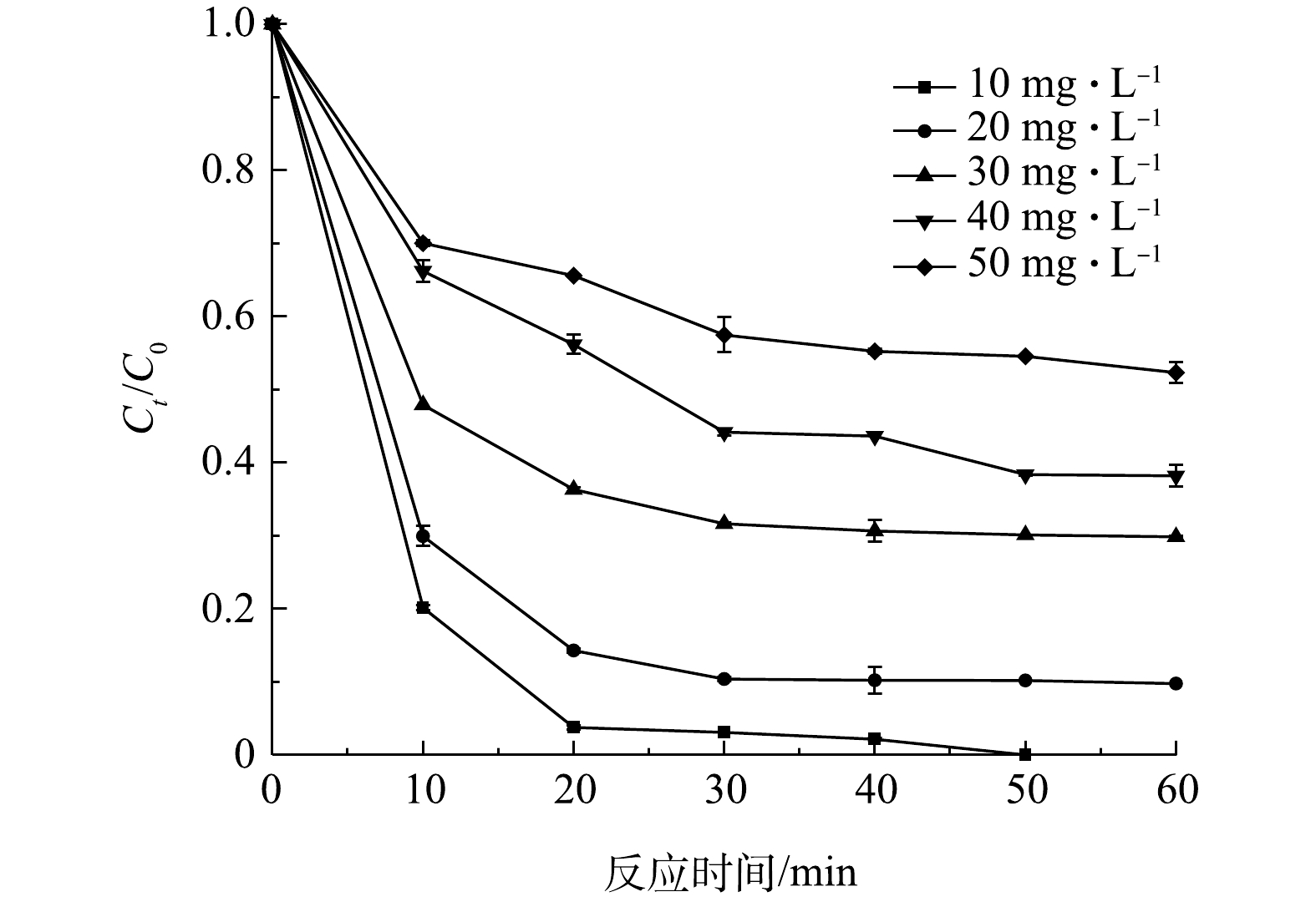
1) IMI初始浓度的影响。选取PMS用量为15 mmol·L−1,ZnCo2O4用量为0.4 g·L−1,考察IMI初始浓度(10、20、30、40、50 mg·L−1)对降解效果的影响,结果如图10所示。由图10可见,IMI浓度为10 mg·L−1时,降解20 min后可达98.1%左右,50 min即可实现完全降解;增大IMI浓度至20、30、40、50 mg·L−1,反应60 min后其降解率分别为91.3%、70.2%、61.9%、47.6%。这说明,随着IMI浓度增大,其降解率随之降低。这是因为,在其他反应条件相同的情况下,体系中产生的活性自由基数量一定,提高IMI的初始浓度会使过量的IMI分子无法与自由基接触,从而降低了IMI降解率。在SHAH等[42]的研究中还提到,污染物与其降解产物对自由基的竞争也可能会随污染物初始浓度的增加而增加,这也是导致IMI去除率随初始浓度增加反而降低的原因之一。
2) pH的影响。反应溶液的pH是影响PMS氧化降解有机污染物的重要参数。选取IMI的初始浓度为20 mg·L−1,PMS用量为15 mmol· L−1,ZnCo2O4用量为0.4 g·L−1,考察初始pH(3.0、5.0、7.0、9.0、11.0)对降解效果的影响,结果如图11所示。由图11可见,在不同的pH下,60 min内IMI的降解率分别为88.6%、79.5%、77.9%、69.8%、43.3%。这表明,随着pH的增加,IMI降解率降低,酸性条件下PMS的活性更高。这与HAYAT等[43]用纳米Fe0活化PMS降解IMI结论一致,体系在宽pH范围内均可以有效降解,酸性条件下降解率最高。这是因为,pH对体系中的自由基种类有一定的影响,酸性条件下,PMS产生的主要自由基是
SO⋅−4 ,其对IMI的氧化能力强;当pH增大时,大量的SO⋅−4 与OH−发生反应转化为·OH(式(6)),生成的·OH氧化还原电位和半衰期均低于SO⋅−4 [42],体系反应活性降低,导致IMI降解率的下降。同时,PMS在碱性条件下会以非自由基的形式自分解(式(7)),从而降低了ZnCo2O4/PMS体系的氧化能力,这也是导致IMI降解率随pH的增大而降低的原因之一。YI等[44]研究发现,加入PMS后可以使体系的pH降低到3.1~3.5,这与本研究结果相一致。向不同pH(3.0~11.0)的反应体系中加入PMS后,反应最终pH为2.5~2.8(图12),这是由于
HSO−5 被活化后生成SO⋅−4 和H+,造成体系pH的下降(式(8))。调节不同pH时,酸性环境是通过盐酸进行调节,本节中pH为3时IMI降解率最高,但仍低于考察其他影响因素时得到的IMI最高降解率,这也验证了Cl−对体系的抑制作用,与后文探究Cl−对IMI降解率影响的结果吻合。3) ZnCo2O4投加量对降解率的影响。选取IMI的初始浓度为20 mg·L−1,PMS用量为15 mmol·L−1,考察ZnCo2O4投加量(0.1、0.2、0.4、0.8、1.0 g·L−1)对降解效果的影响,结果如图13所示。由图13可见,当催化剂用量为0.1 g·L−1时,IMI降解率较低,60 min只有58.6%;随着催化剂用量的增大,IMI的降解率随之增大。当催化剂用量为0.4 g·L−1时,60 min后对IMI的降解率达92.3%;继续增加催化剂用量至0.8、1.0 g·L−1,IMI降解率增加不明显。这是因为,当PMS的量一定,即使再增加催化剂ZnCo2O4的量,并不能使体系中自由基数量增多。同时,过量的ZnCo2O4还会增大体系中金属离子的溶出。为节约催化剂用量成本,后续实验选用ZnCo2O4的投加量为0.4 g·L−1。
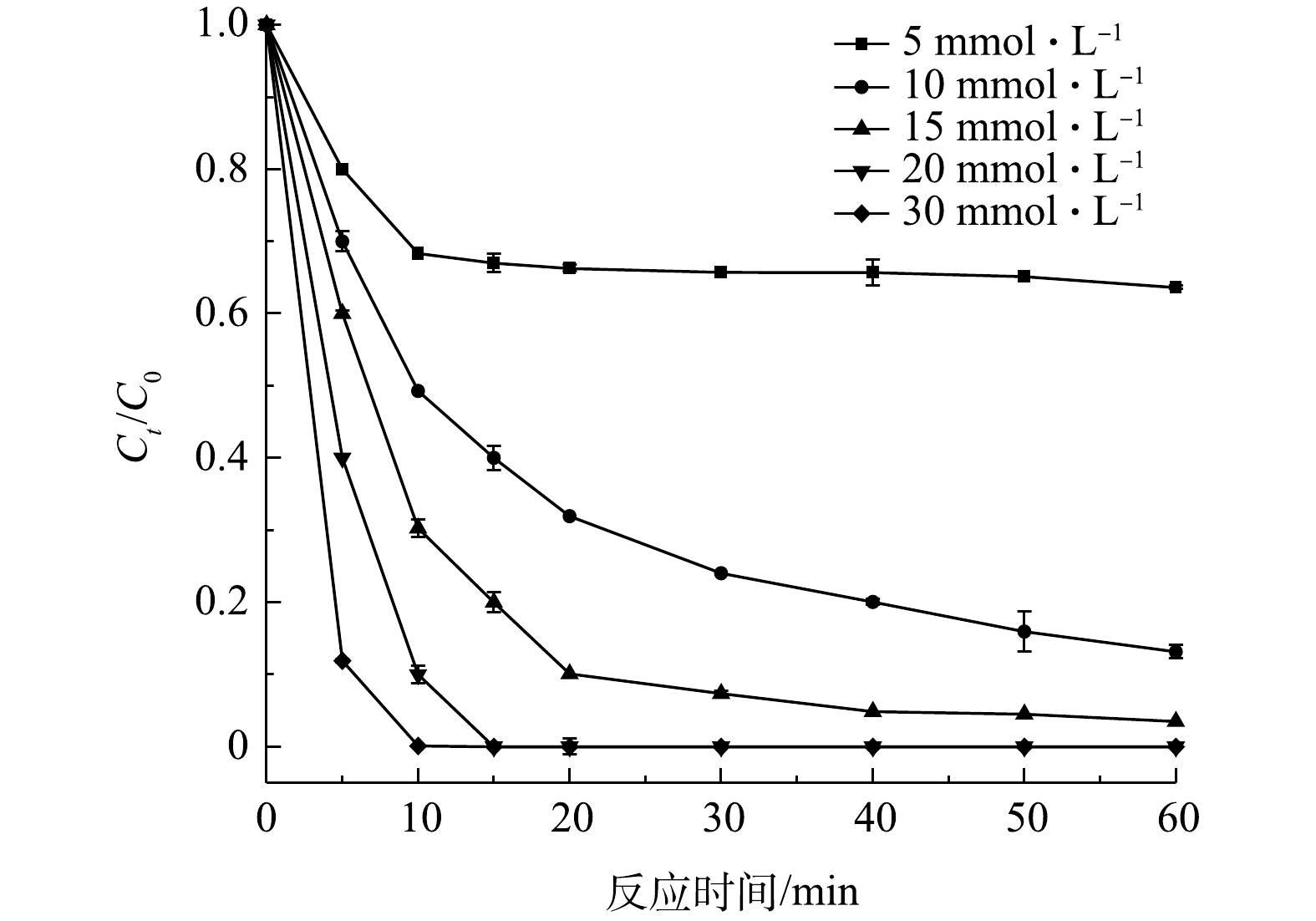
4)PMS浓度的影响。PMS作为
SO⋅−4 的前驱体,其浓度对IMI的降解效果有很大影响。选取IMI的初始浓度为20 mg·L−1、ZnCo2O4投加量为0.4 g·L−1,考察PMS浓度(5、10、15、20、30 mmol·L−1)对IMI降解率的影响,结果如图14所示。由图14可见,随PMS用量增大,IMI降解率随之增大。当PMS浓度为5、10 mmol·L−1时,反应60 min后IMI的降解率分别为36.4%和86.8%;当PMS浓度增大到15 mmol·L−1时,反应20 min时IMI降解率达90.1%,随着反应继续进行,60 min时降解率可达97.8%;继续增大PMS浓度至20 mmol·L−1和30 mmol·L−1时,分别在15 min和10 min内可实现对IMI的完全降解。这是因为,增大PMS的浓度使体系中产生更多的SO⋅−4 [45],加快了氧化速率,从而提高了IMI的降解率。5)常见阴离子对降解率的影响。选取IMI的初始浓度为20 mg·L−1,ZnCo2O4用量为0.4 g·L−1,PMS用量为15 mmol·L−1,向体系中添加不同浓度的无机阴离子(Cl−、
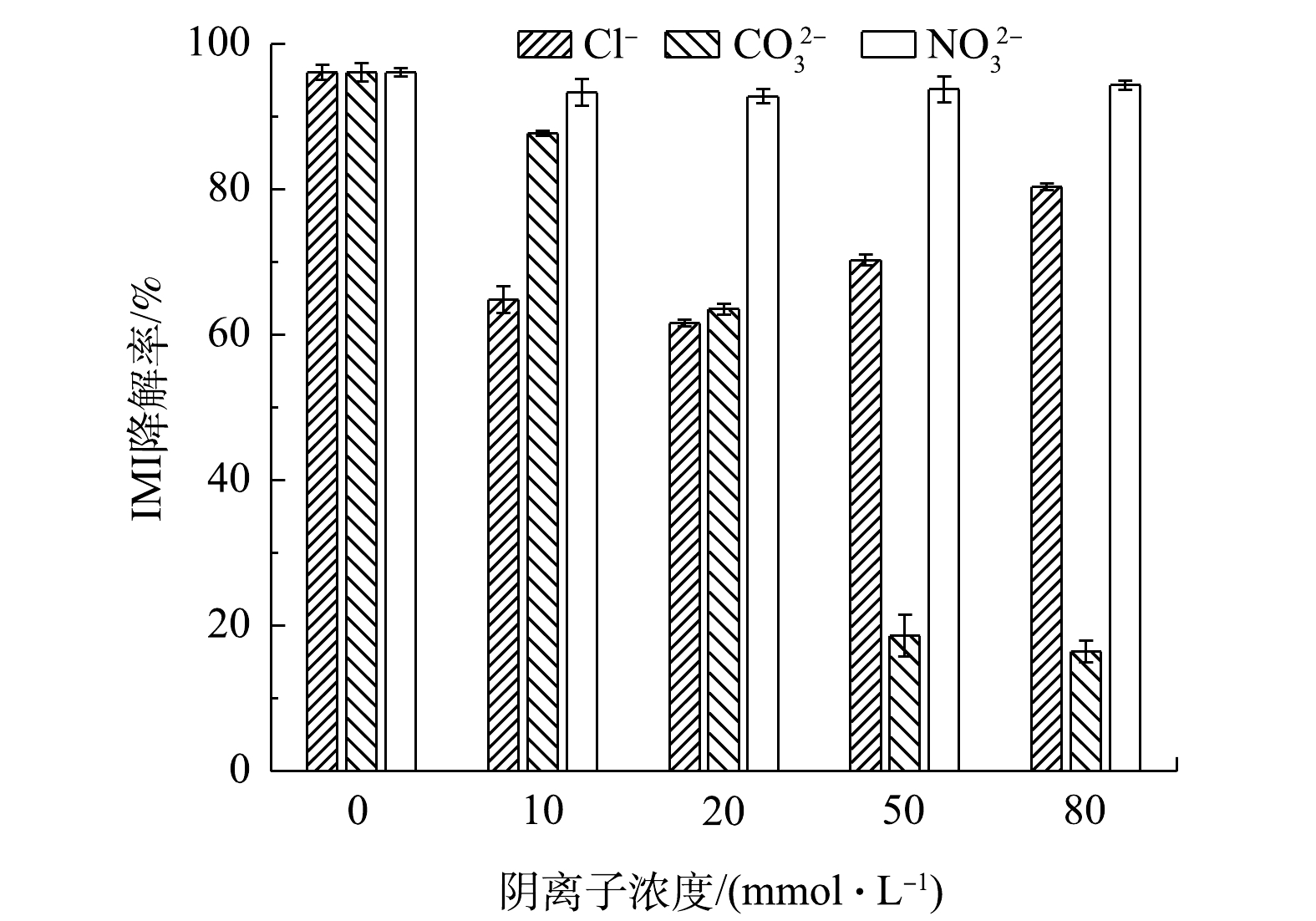
CO2−3 、NO−3 ),考察了3种常见阴离子对降解效果的影响,结果如图15所示。由图15可以看出,Cl−的加入对IMI在60 min内的降解率整体上呈抑制的趋势。造成该现象的原因有2个方面:一是加入体系中的Cl−会和·OH、SO⋅−4 发生反应生成氧化性较低的Cl·和Cl⋅−2 [17](式(9)~式(11));二是加入体系中的Cl−会和HSO−5 发生反应,消耗HSO−5 [46](式(12)和式(13))。这2个途径均会使强氧化性的·OH、SO⋅−4 数量减少,导致体系的氧化能力降低,降解率下降。如图15所示,
CO2−3 的加入也会对体系的氧化降解能力造成抑制,且随着CO2−3 浓度的增加,抑制作用也增强。这是因为,CO2−3 会与SO⋅−4 发生反应生成CO⋅−3 (式(14)),CO⋅−3 的氧化能力很弱,因而导致IMI的降解率下降。在XU等[14]、王琰涤等[47]关于CO2−3 对PMS活化体系的影响中也得到类似的结论。由图15可以看出,不同浓度的NO−3 对ZnCo2O4/PMS体系的降解效率无明显的影响,在郑立庆等[48]通过热活化PMS降解IMI的研究中也有相同的结果。 -
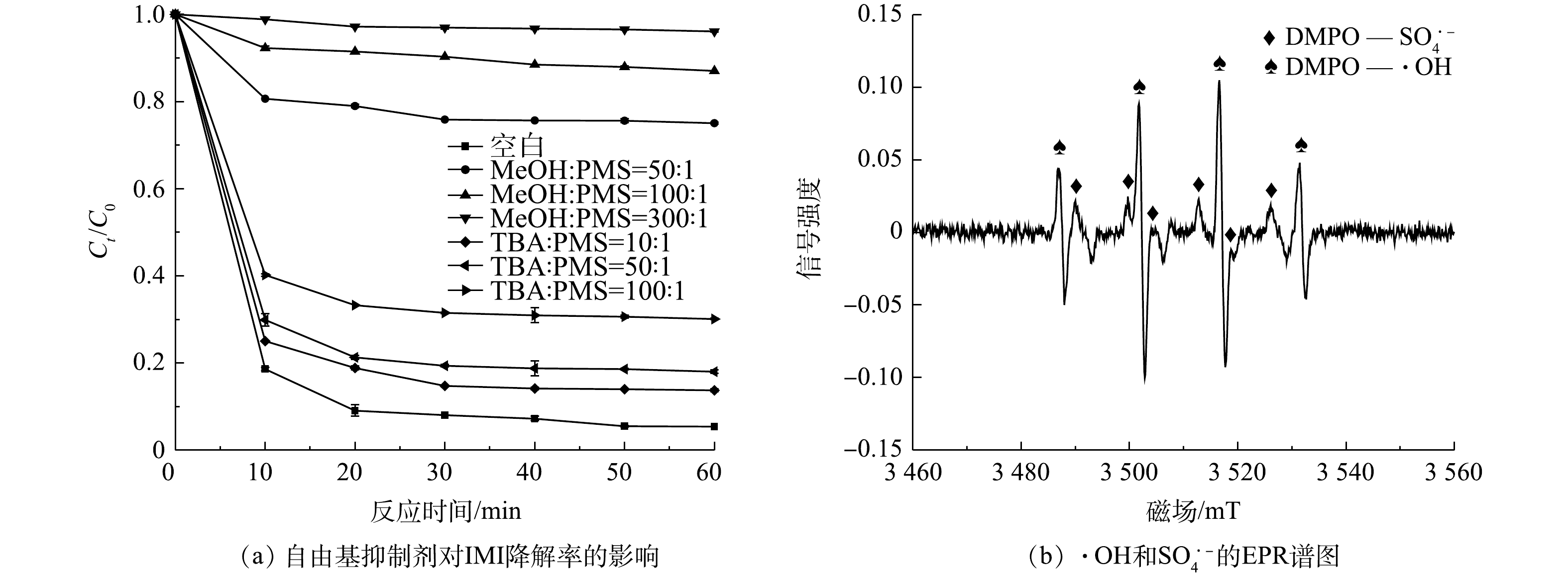
为确定ZnCo2O4/PMS体系中的主要活性自由基种类,选用甲醇(MeOH)和叔丁醇(TBA)进行淬灭实验。其中,MeOH与
SO⋅−4 和·OH之间均有较快的反应速率[32];而TBA仅与·OH反应速率较快[49-50]。由图16(a)可见,未添加MeOH和TBA的空白组对IMI的降解率达95.1%;当添加的TBA与PMS的摩尔比为10∶1和50∶1时,IMI的降解率分别保持在86.3%和81.2%,当摩尔比增加至100∶1时,降解率降至69.0%,说明TBA对体系的降解存在抑制效果,但并不明显,即氧化体系中存在·OH,但其对体系的降解贡献较小;而MeOH对体系的抑制效果就显著得多,可以看到当添加的MeOH与PMS的摩尔比为50∶1就能使IMI的降解率降至23.8%,继续增大摩尔比至100∶1和300∶1时,IMI的降解率仅有9.6%和3.9%。以上结果说明SO⋅−4 是体系中参与反应的主要活性自由基。为进一步确定体系中产生的活性自由基,用DMPO作为捕获剂,使用电子顺磁共振技术(EPR)进行检测。如图16(b)所示,·OH、
SO⋅−4 均被检测到,这与淬灭实验的结果一致。推测ZnCo2O4/PMS体系中自由基产生机理如下:首先,表面结合的Co2+可直接与HSO−5 反应生成SO⋅−4 (式(2));同时,部分Co2+发生溶出,在水溶液中形成CoOH+中间体,CoOH+与PMS反应生成SO⋅−4 [51-52](式(3)~式(4));SO⋅−4 与H2O反应可生成·OH(式(5));催化剂表面及水溶液中的Co3+向Co2+的还原通过Co3+与HSO−5 反应生成Co2+和SO⋅−5 以实现(式(15)~式(17));此外,部分SO⋅−5 也可生成同等数量的SO⋅−4 [53](式(18))。 -
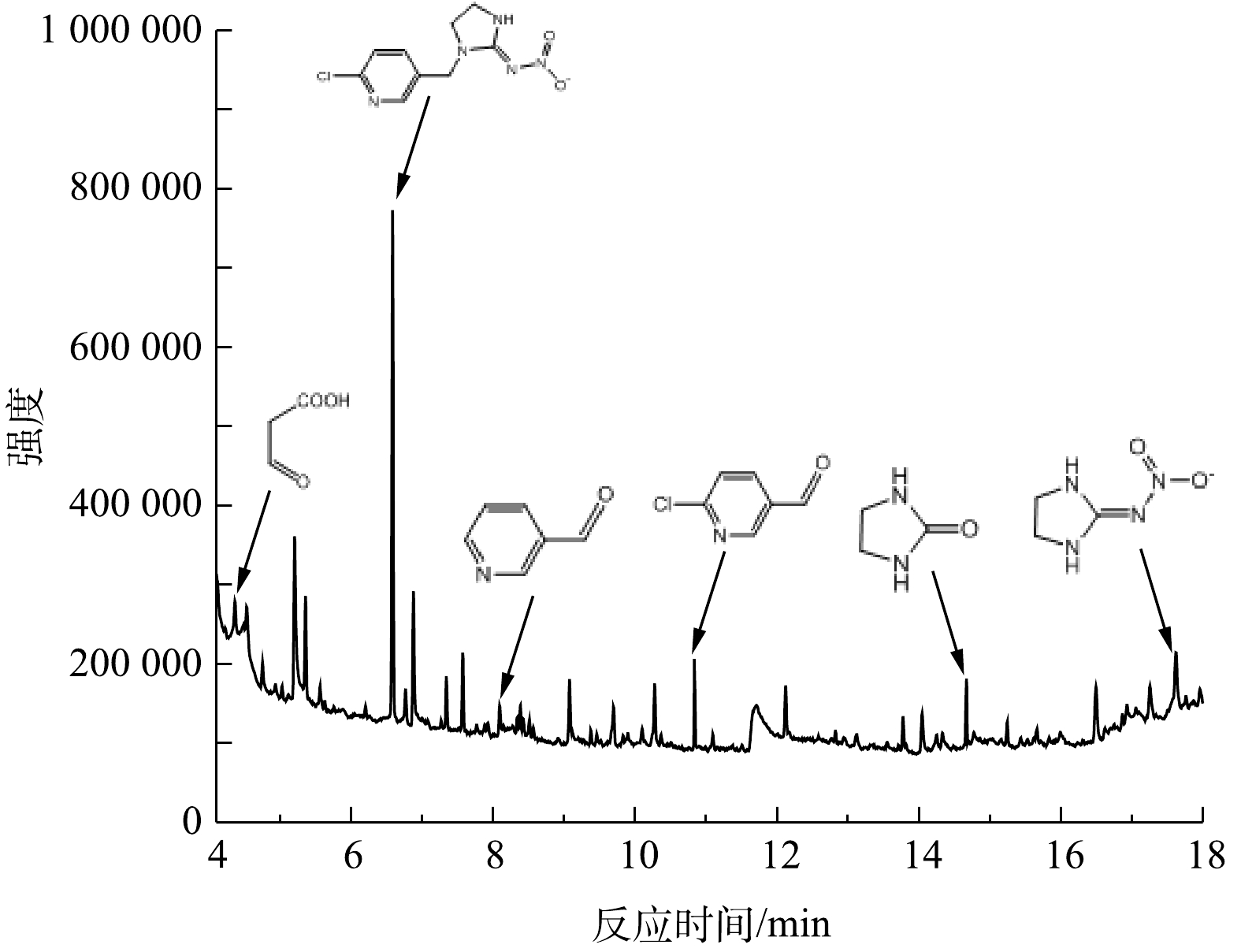
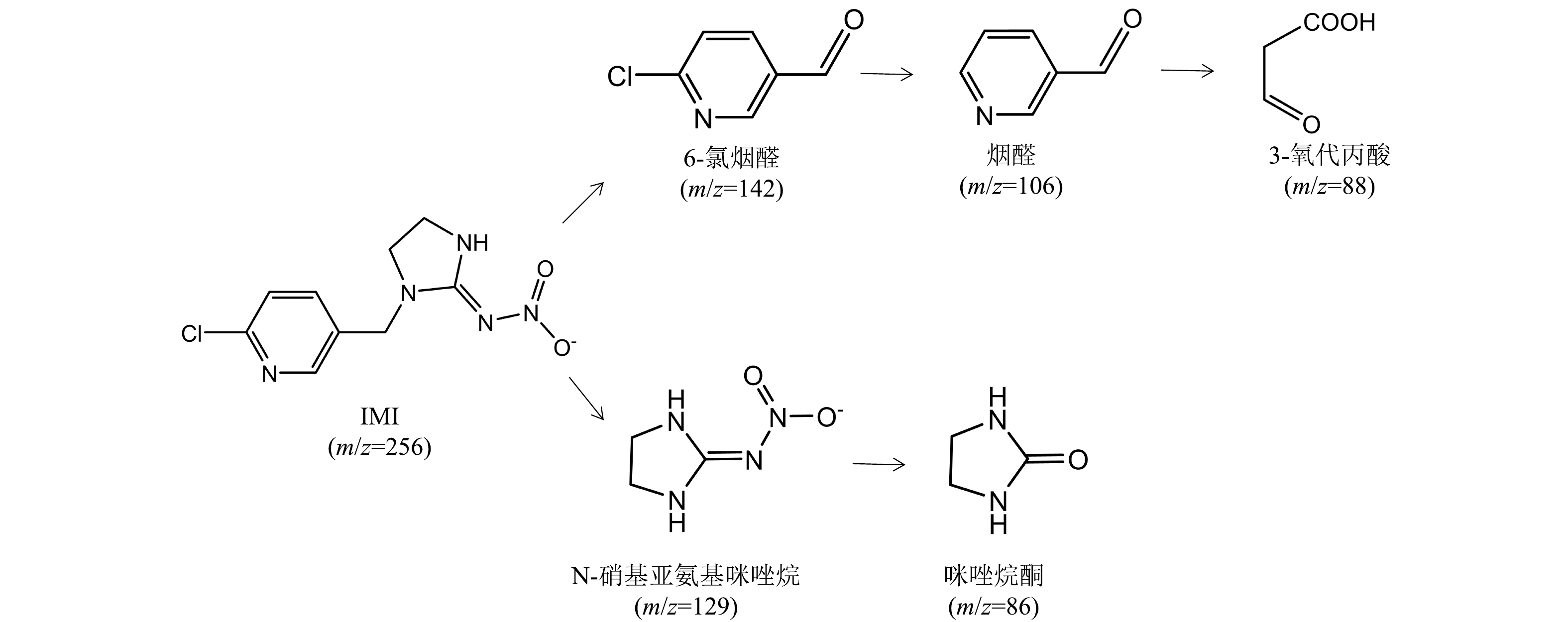
在ZnCo2O4/PMS体系中,IMI在
SO⋅−4 、·OH的攻击下会发生电子转移、脱氯反应等以被去除。利用GCMS对IMI降解产物进行鉴定,结果如图17所示。共检测到5种中间产物,分别为6-氯烟醛(m/z=142)、烟醛(m/z=106)、3-氧代丙酸(m/z=88)、N-硝基亚氨基咪唑烷(m/z=129)、咪唑烷酮(m/z=86)。根据这些中间产物进一步推测IMI的降解途径,结果如图18所示。首先,SO⋅−4 、·OH攻击IMI中连接吡啶环与咪唑烷的C—N键,使IMI分子裂解为6-氯烟醛和N-硝基亚氨基咪唑烷,6-氯烟醛分子的C—Cl键易受攻击而断裂发生脱氯反应生成烟醛,随后烟醛中的吡啶环被氧化分解为3-氧代乙酸[54],咪唑烷上的—NO2是强吸电子基团,在自由基攻击下发生电子转移生成咪唑烷酮[55]。 -
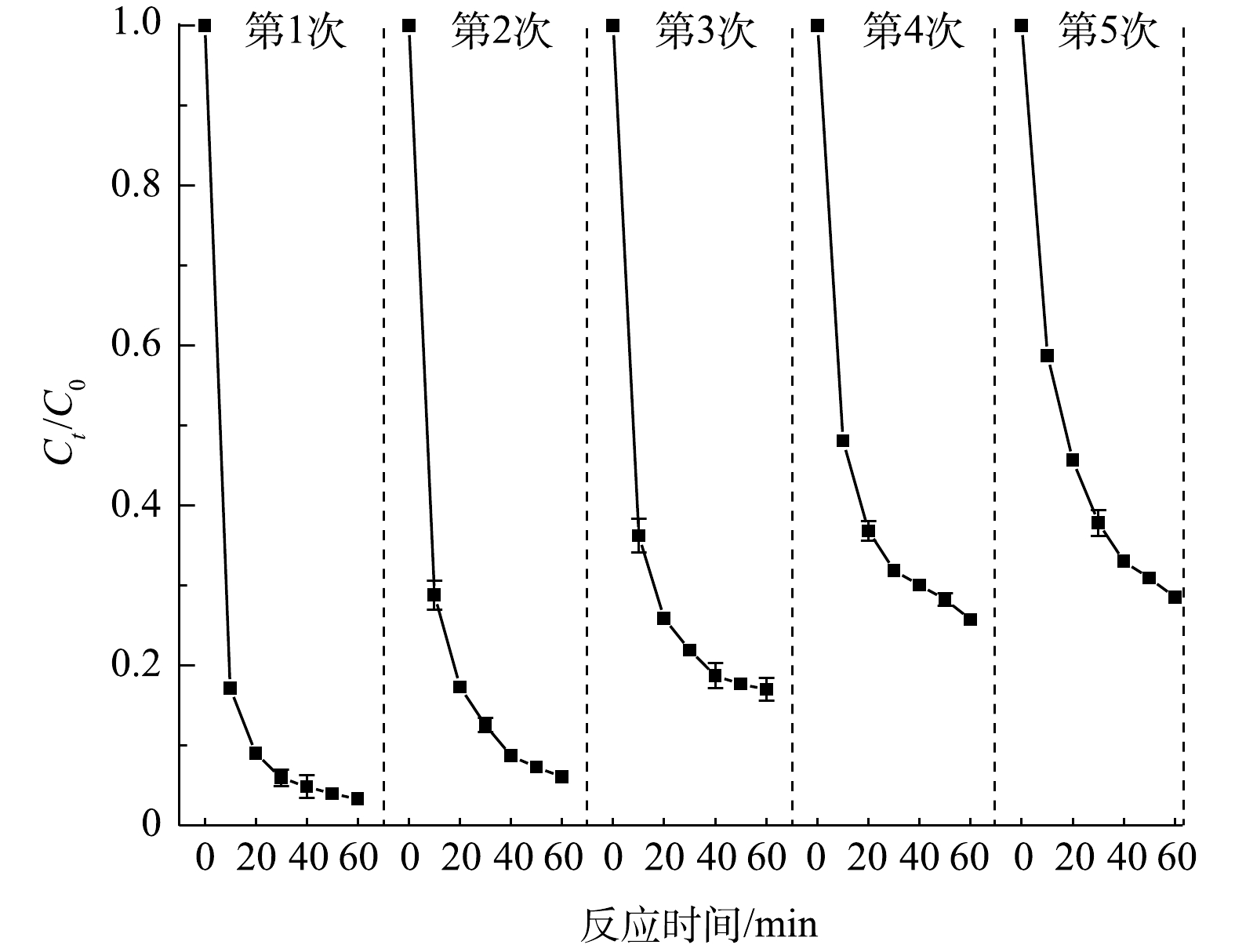
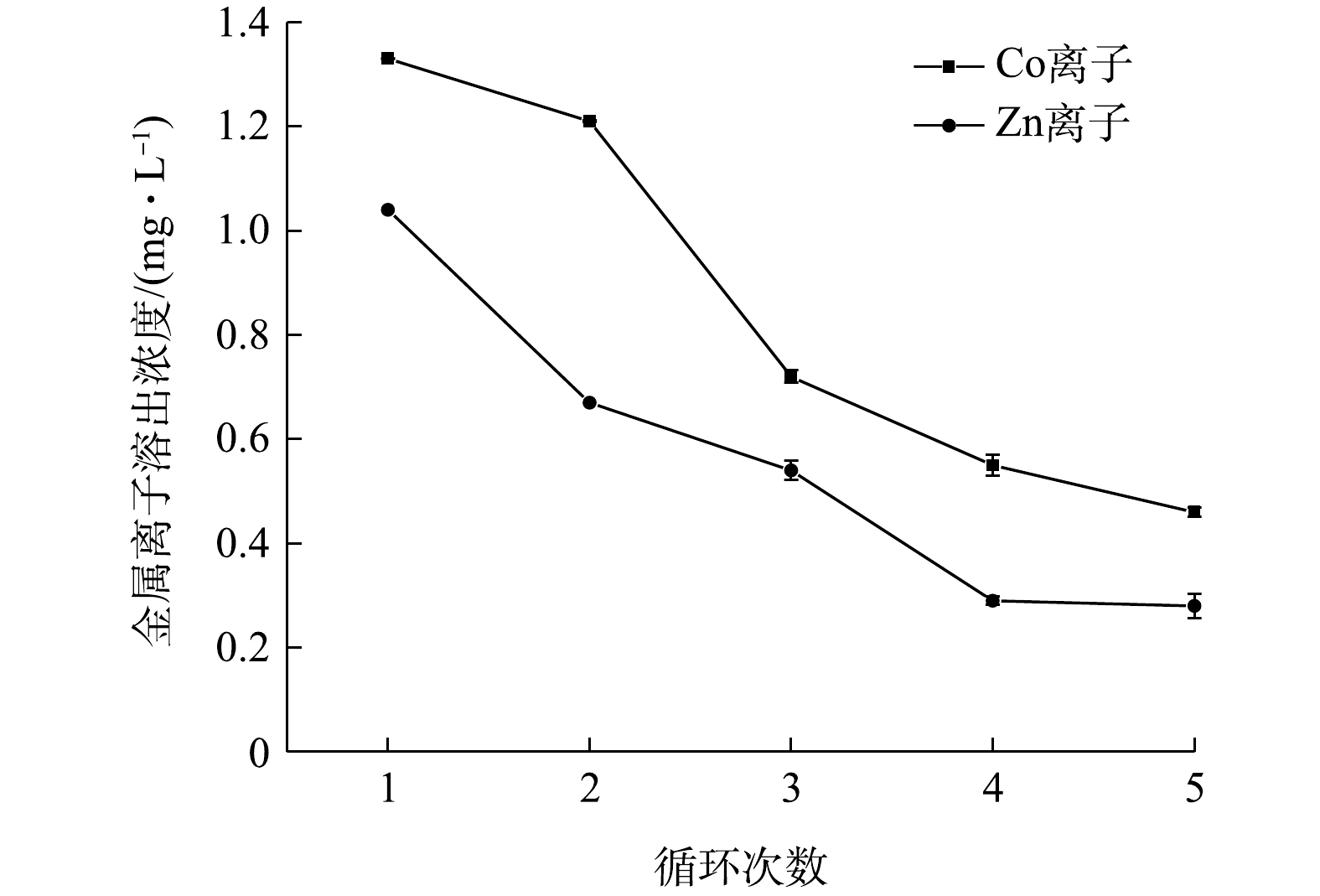
为了进一步了解ZnCo2O4在体系中的使用性能,探讨了在相同实验条件下5次循环使用ZnCo2O4对IMI的降解情况,结果如图19所示。由图19可见,前2次使用对IMI降解率无太大影响,均可达96%以上;第3次循环使用时降解率为82%左右;第4、5次放入后降解率略有降低,这与金属离子的氧化和溶出有关。对5次循环的反应溶液进行Co、Zn的溶出进行检测,结果如图20所示。由图20可见,随着循环次数的增加,Co、Zn离子的溶出浓度逐渐降低,后续可通过负载等改进手段以减少金属离子的溶出。对于含较高Co离子的酸性废水,可以通过添加氧化钙等低成本碱性物质,以生成氢氧化钴沉淀降低Co离子向环境中的排放。综上所述,经5次循环后反应体系仍可维持较高的催化活性。
2.1. 6种不同双金属氧化物活化PMS降解IMI效果对比
2.2. 催化剂的表征
2.3. ZnCo2O4活化PMS降解IMI的效果
2.4. 不同因素对IMI降解率的影响
2.5. 活性自由基鉴定
2.6. 降解产物及途径预测
2.7. 催化剂的循环使用
-
1)以硝酸盐及柠檬酸为原料,采用凝胶法成功合成6种双金属氧化物,依据IMI降解率及金属离子溶出情况确定ZnCo2O4双金属氧化物为最优氧化剂。通过SEM观测、XRD分析、FTIR图谱等分析表明ZnCo2O4具有尖晶石结构。
2) IMI浓度、PMS浓度、ZnCo2O4投加量、初始pH以及常见阴离子等因素对IMI的降解均有影响。在初始IMI浓度为20 mg·L−1、ZnCo2O4投加量为0.4 g·L−1、PMS浓度为15 mmol·L−1的条件下,60 min内IMI的降解率达95.8%,PMS浓度的增加可缩短达到完全降解的时间,初始污染物浓度增加、pH的增大及Cl−、
CO2−3 对IMI降解均有抑制作用。3) ZnCo2O4/PMS体系中产生的自由基有·OH和
SO⋅−4 ,且SO⋅−4 为主要起作用的活性自由基;通过GCMS检测到5种降解中间产物,并推测IMI的降解包括电子转移、脱氯、吡啶环裂解等过程;ZnCo2O4重复使用3次仍可达到80%以上的降解率,重复性能良好。















 下载:
下载: