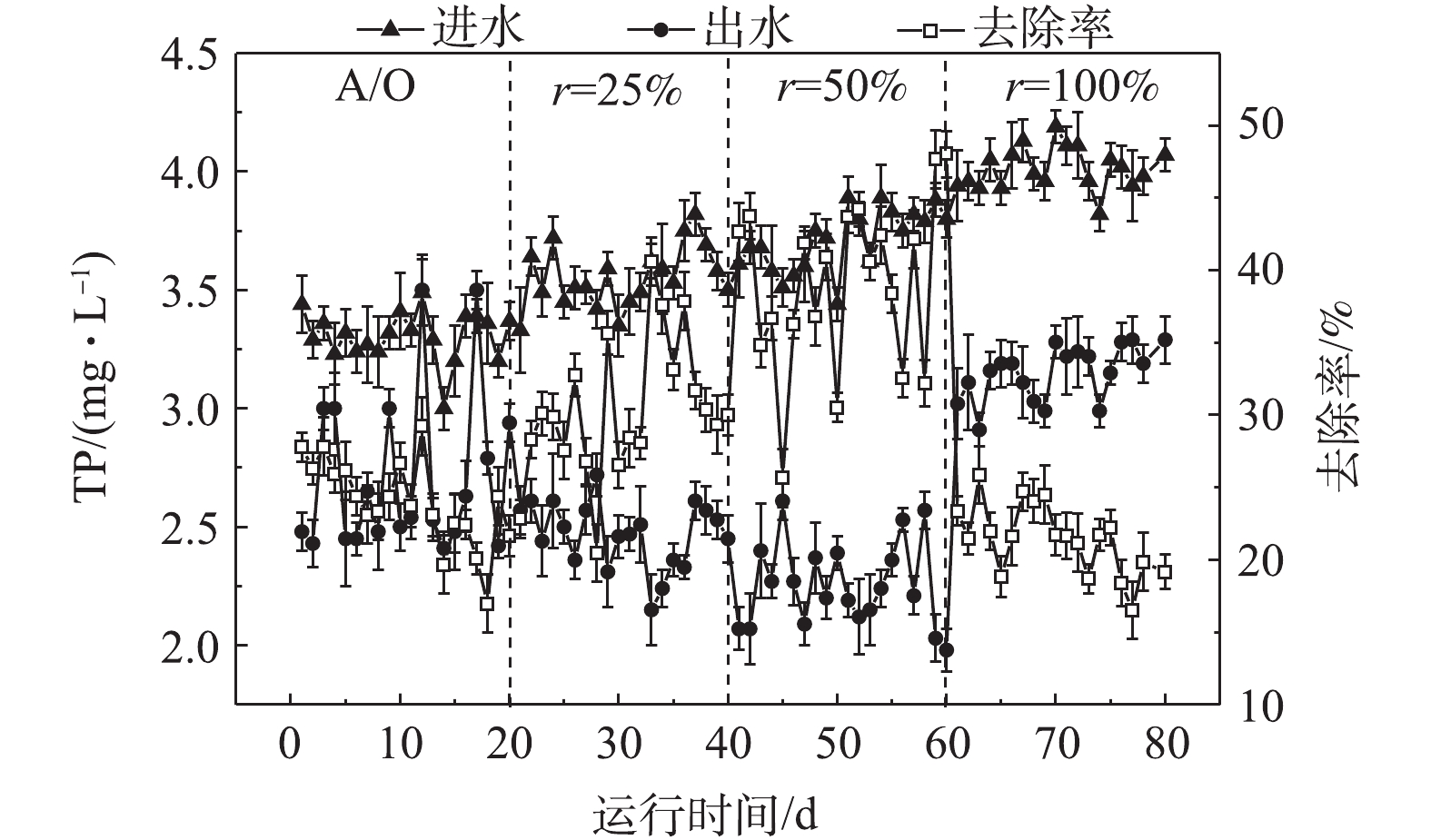
-
随着人们对环境保护的重视,有机废气排放标准日益严格,对挥发性有机化合物(VOCs)的减排治理工作一直是国内外研究热点[1-3]。特别是近几年来,VOCs的持续减排成为我国“十三五”大气污染防控的重点工作[4]。油气回收技术能有效减少石油生产运输过程中的有机气体排放。随着国家对石化产业油气排放指标的精细化具体化,油气回收技术也有了新的挑战[5]。吸附法是油气回收的主要工艺之一[6],由于各种吸附剂的吸附容量有限,因此,油气吸附回收装置须频繁吸附-解吸再生吸附剂,才能节省成本,达到最高的使用价值。
如何高效、经济地再生吸附剂,是油气吸附回收技术中必须重点解决的问题,同时亦是国内外研发的热点。目前常见的解吸方法有热解吸、真空解吸、超声波解吸等。SALVADOR等[7]通过热解吸和吹扫,分析了碳质吸附剂的热再生解吸过程。LASHAKI等[8]研究了解吸吹扫中氧杂质对活性炭性能的影响。LEMUS等[9]在常温、常压条件下实现了活性炭的再生。ZHANG等[10]对多孔矿物吸附剂进行了热解吸分析。蔡道飞等[11]和王英霞等[12]用微波加热、微波真空等方法对吸附剂的解吸再生进行了分析。胡克伟[13]、范春辉等[14]和周超[15]对吸附剂解吸过程进行了动力学拟合,得到了不同方程的解吸拟合效果,但并未涉及吸附剂对有机废气的解吸动力学情况。
在现有研究中,吸附剂多是处在静态吸附或微小流量下的吸附-解吸再生过程,与工业应用中的动态吸附-解吸过程有一定差距。而超声波解吸在工业应用中的成本较高,工业化应用尚存在一定困难[16]。为了进一步研究工业应用中,在高浓度有机气体的低排放国家标准背景下[17]不同解吸方式对吸附剂的解吸效果与影响机理,本研究搭建中型实验平台,以炭基吸附剂A-1、硅基吸附剂S-1为研究对象,对多种解吸方式下吸附剂解吸效果进行研究,以期为工业应用中吸附剂的解吸方法提供参考。
全文HTML
-
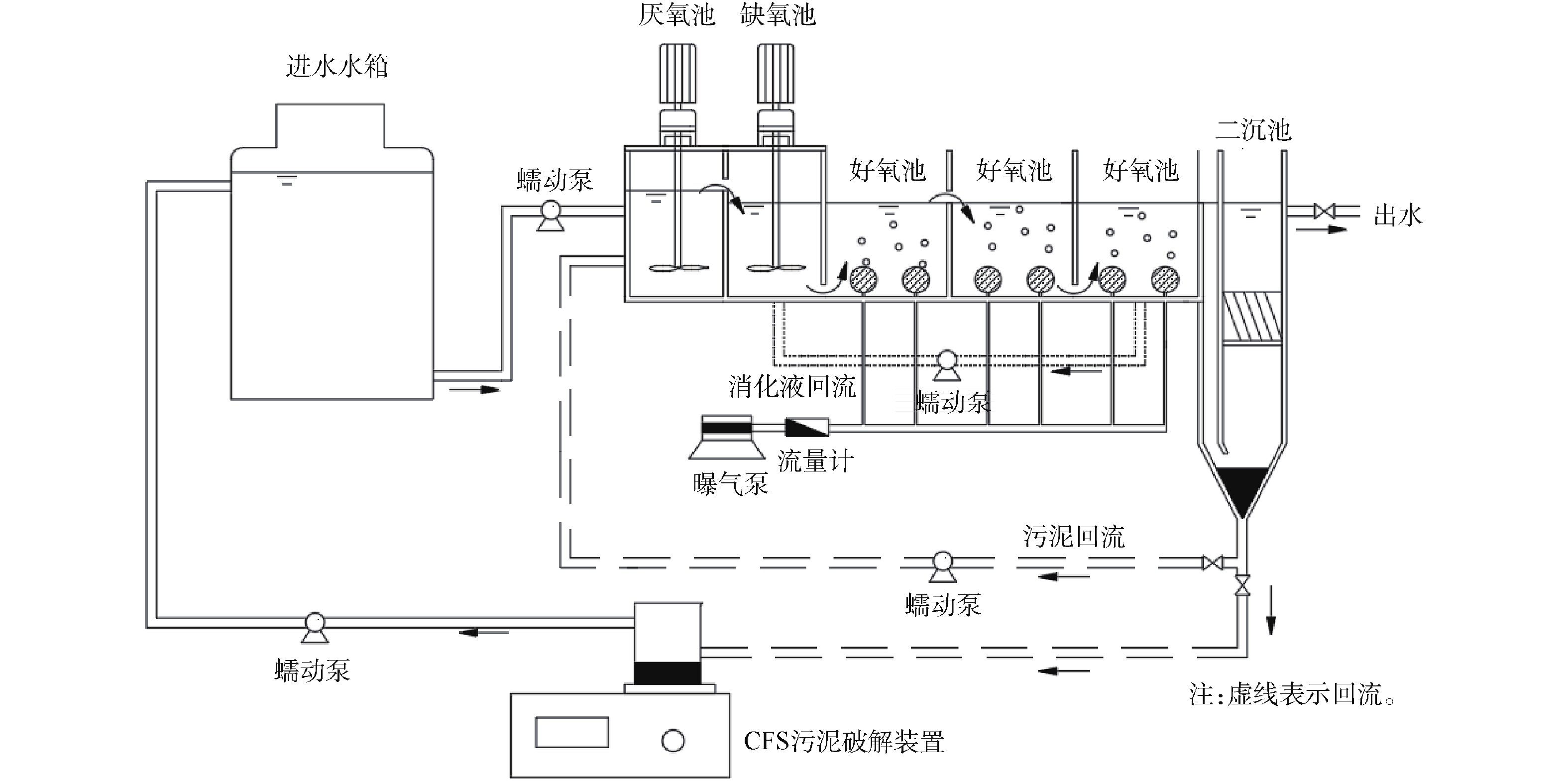
实验样品为正己烷(工业纯);吸附剂为自制的炭基吸附剂A-1和改性疏水硅基吸附剂S-1。实验装置为多功能循环吸附解吸实验平台(如图1所示),该平台主要由气体发生模块、吸附模块、多功能解吸模块以及检测模块组成。气体发生模块由辅助配气罐、有机气体发生罐和缓冲罐组成。吸附模块由多功能吸附解吸塔组成。多功能解吸模块由塔内盘管、干式真空泵(阿法帕DPSH020)、热气发生器组成。提供的解吸方式包括:单纯抽真空的解吸方式(真空解吸);抽真空同时使用盘管加热(盘管温度95 ℃)的解吸方式(真空加热解吸);真空泵工作的同时打开塔上方阀门部分开度,使塔内存在小流量与部分真空度(负压30、53、77 kPa)的解吸方式(负压+补气解吸);利用空气泵与热气发生器进行大流量((50±1) m3·h−1)热空气((85±3) ℃)吹扫的解吸方式(热吹扫解吸)等。检测模块由多路温度巡检仪(安柏AT4320,精度0.1 ℃)、压力表(测量范围−0.1~0.1 MPa,精度0.1 kPa)、气相色谱仪(岛津GC2010 Plus)组成,通过控制系统可每间隔120 s记录1次炭层温度变化(温度探头距离吸附床底部位置分别是:T1为135 mm,T2为265 mm,T3为395 mm,T4为525 mm)。多功能吸附解吸塔配备有取样口,用于收集气体,进行进出口气体浓度检测,实验步骤如下。
1)吸附剂的准备。吸附剂装填完毕后(装填高度550 mm,A-1装填时总质量25 kg,S-1装填时总质量49.53 kg),将实验平台切换为解吸通路状态,先对塔内吸附剂进行8 h热吹扫,以保持吸附剂初始活性;再对吸附塔抽真空,并密封12 h,进行气密性检测,若真空压力降低在10%内,则认为吸附塔气密性合格。
2)动态吸附实验。保证气体发生罐内正己烷含量充足,打开气泵,调整气体发生模块进气阀门开度,先测量缓冲罐内气体浓度,当进气浓度、压力、温度、流量达到设定值时,再打开吸附解吸塔进口阀门,将气体引入吸附解吸塔。每隔5 min,采集1次吸附塔进、出口处气体样本,通过气相色谱仪测定塔内进出口浓度。当吸附剂吸附开始穿透后(出口浓度开始迅速上升,并超过80 mg·m−3),停止吸附实验。吸附剂对正己烷蒸气的吸附量按式(1)计算。
式中:q为1 g吸附剂吸附的质量,g;t为时间,min;k为色谱图峰面积与油气浓度的换算系数;S0为吸附柱进口气体浓度对应的峰面积;S为吸附柱出口气体浓度对应的峰面积;Q为混合气体流量,m3·min−1;m为吸附剂的质量,g。
3)解吸实验。吸附实验完成后,关闭气体发生模块阀门,利用真空泵、热气解热器、塔内分布盘管,改变塔内解吸环境,进行不同解吸方式下的解吸实验。解吸过程采用干式真空泵,可在真空泵出口处每隔5 min采集1次气体样本,测量解吸出口气体浓度和出口流量。解吸时间根据不同解吸方式、不同吸附剂而有所调整。吸附剂对正己烷气体的解吸量按式(2)计算。
式中:φt为t时刻解吸量,g;t为时间,min;k为色谱图峰面积与油气浓度的换算系数;δ为解吸出口气体浓度对应的峰面积;H为混合气体解吸流量,m3·min−1。
由于不同吸附剂吸附量差异大,因此,解吸效果应按照解吸率进行比较,解吸率计算方法如式(3)所示。
式中:ηt为解吸率;φt为t时刻解吸量;n为前期吸附实验初始吸附量,g。
使用气相色谱仪(岛津GC2010 Plus,毛细管色谱柱:Rtx-1,30 m × 0.25 mm × 0.25 μm,FID检测器)进行气体浓度分析,载气为氮气。使用物理吸附仪(美国康塔Autosorb-IQ2)测定A-1、S-1的比表面积、孔径、孔容。
利用解吸动力学来描述解吸过程中解吸量随时间的变化。通过准一级动力学模型(式(4))、准二级动力学模型(式(5))、班厄姆(Bangham)动力学模型(式(6)),对解吸数据进行拟合,得到相关参数,找到最合适的动力学模型[18-20]。
式中:t为解吸时间,min;φe、φt分别为平衡时刻和t时刻的解吸量,g;k1为准一级解吸速率常数,min−1;k2为准二级解吸速率常数,g·(g·min)−1;k为脱附速率常数,min−2;z为常数。
-
由表1可以看出,炭基吸附剂A-1比表面积高于硅基吸附剂S-1。但比较两者孔径,中孔孔径硅基吸附剂S-1>A-1,并且S-1不含微孔。
-
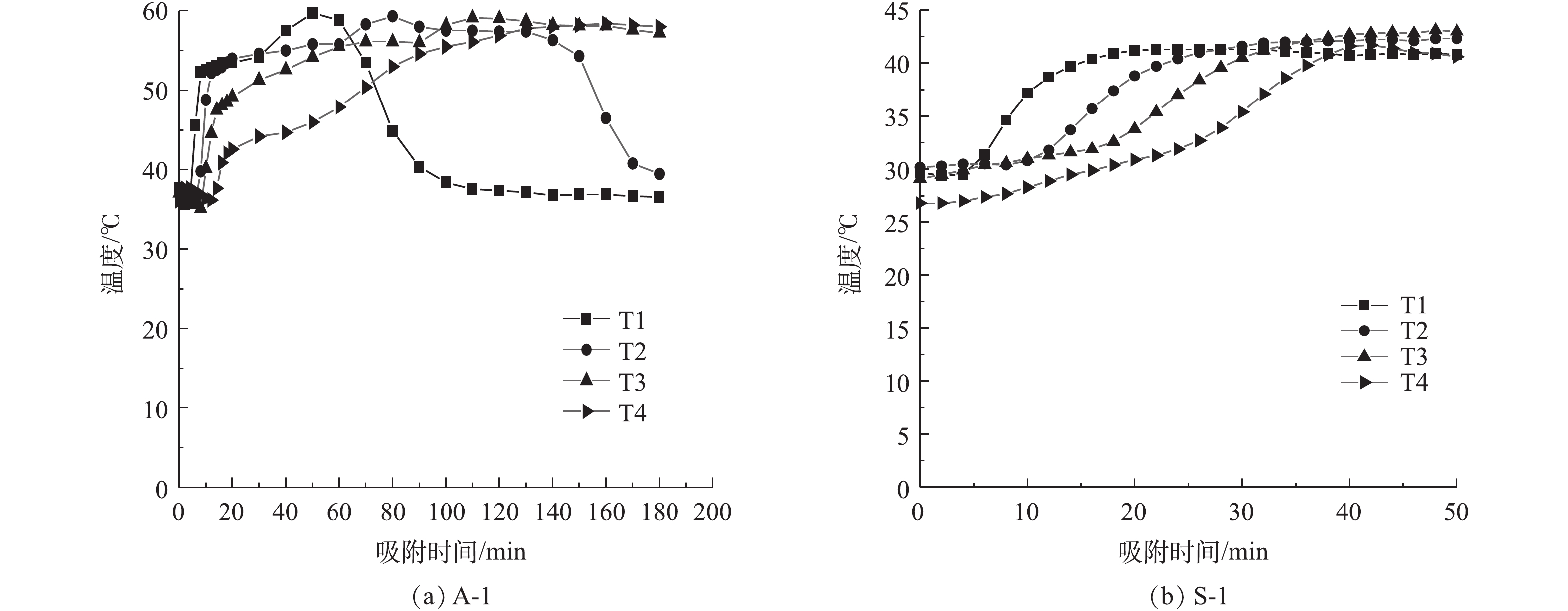
通过前期吸附实验得到吸附剂吸附过程温升曲线(图2)。由图2可以看出,2种吸附剂温度变化趋势相同,在吸附过程前期,均为吸附层从低到高逐渐吸附放热升温。2种吸附剂的区别在于:A-1吸附剂由于穿透时间晚,持续吸附时间长,下方吸附层在吸附放热后逐渐失去吸附能力,在动态气体流动中,热量流失,吸附层逐渐降温;而S-1吸附剂穿透时间早,下方吸附层还未开始降温,吸附过程就已穿透并停止实验。
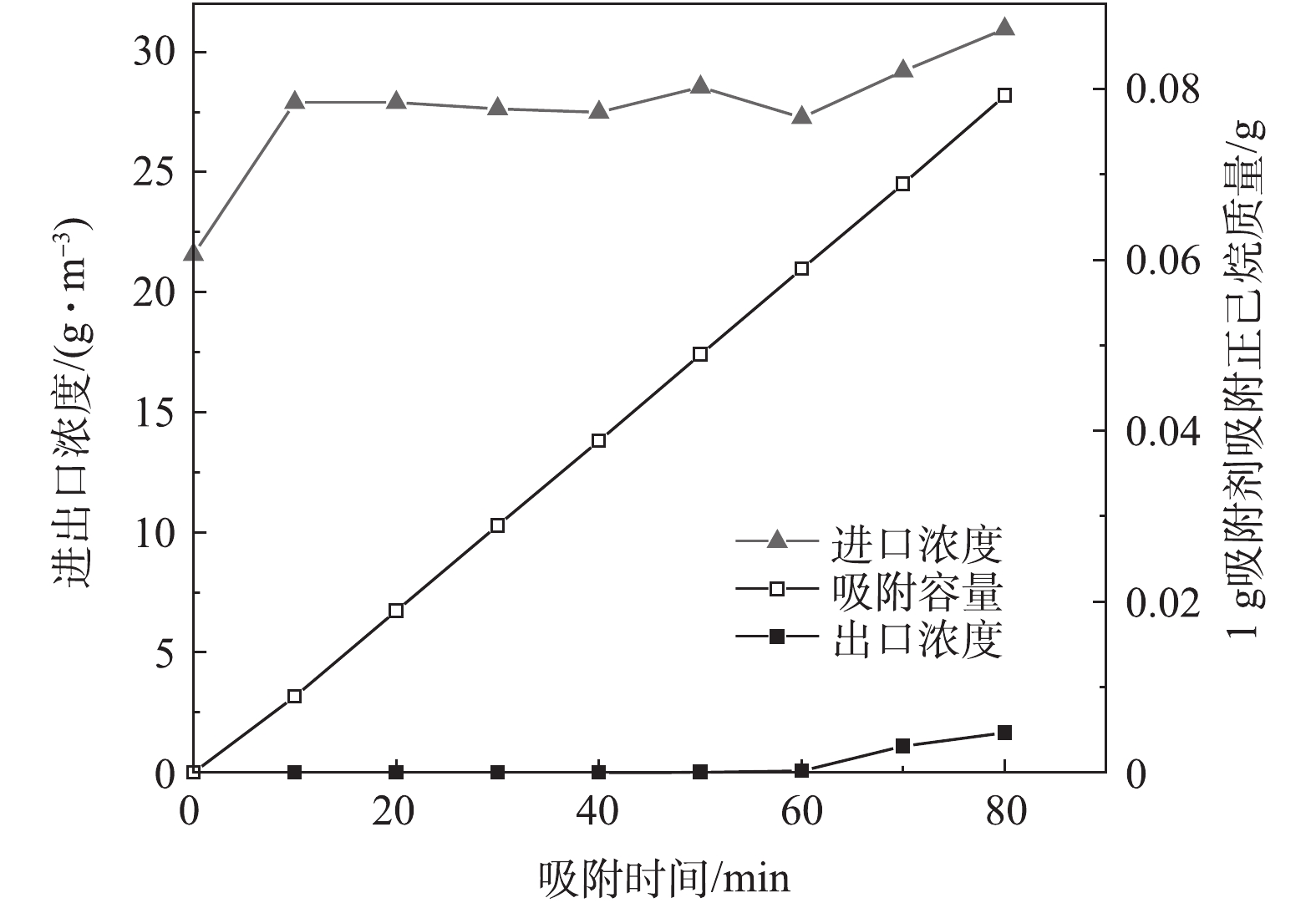
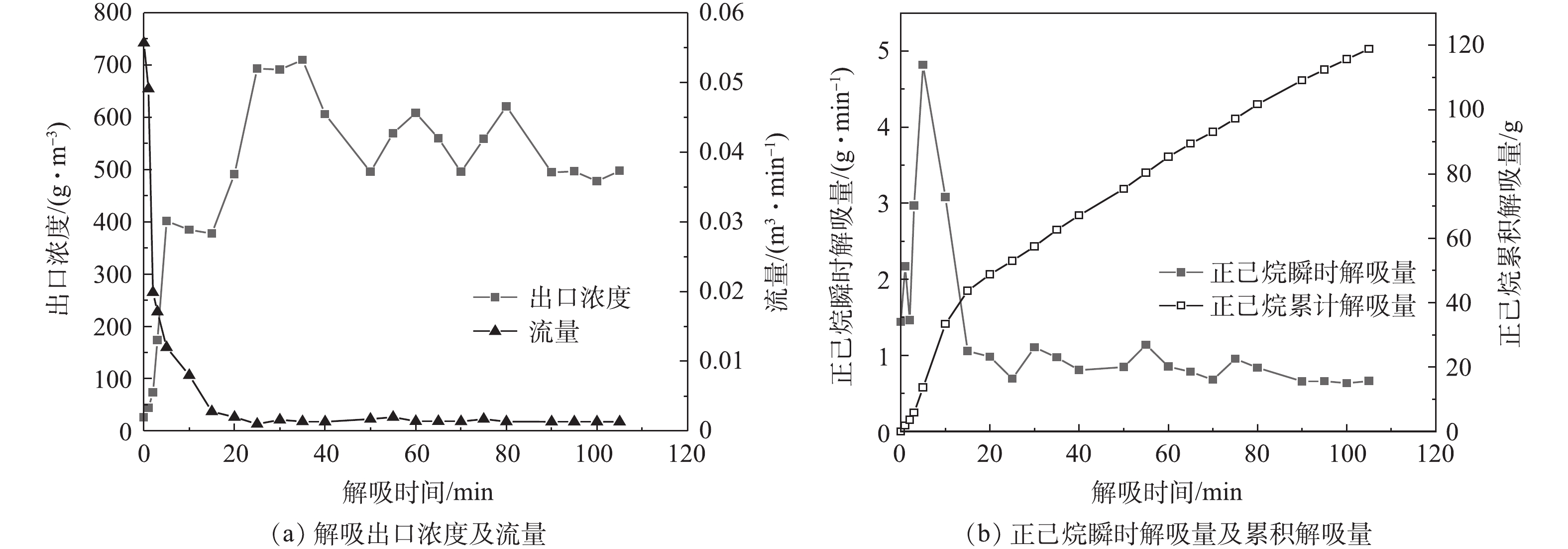
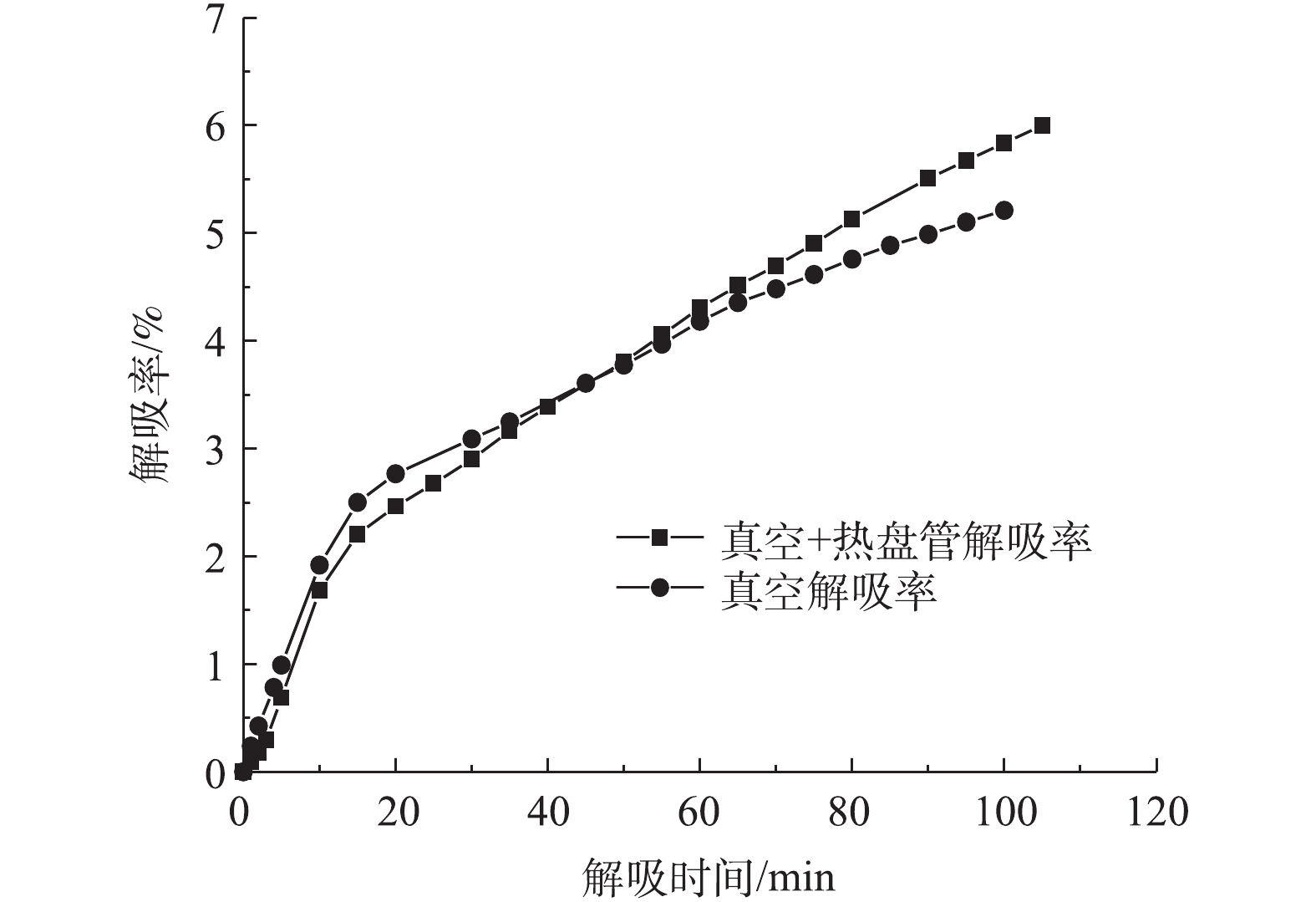
以吸附剂A-1真空加热吸附/解吸实验为例,考察吸附过程中进、出口浓度与时间的关系和解吸过程中出口浓度、流量与时间的关系,结果如图3和图4所示。可以看出,在动态吸附实验阶段,1 g吸附剂的吸附质量为0.079 g(也即初始吸附1 981 g正己烷),在后续真空加热解吸阶段,解吸100 min后,解吸量为118.92 g,解吸率为6%。在静态吸附实验的理想条件下,对于炭基吸附剂,1 g吸附剂的吸附质量通常可达到0.3~0.4 g。而本研究为了满足工业应用要求,出口浓度超过80 mg·m−3即停止实验,事实上,此时吸附剂远未达到饱和吸附状态。因此,在动态吸附实验中,吸附剂的利用率较低,只使用了吸附剂部分吸附能力。
经过多次吸附解吸实验发现,以吸附穿透作为参考点进行动态吸附实验,即使经过不同解吸方式(真空、真空加热、负压补气、热吹扫)的解吸实验,吸附剂在整个动态吸附-解吸-再吸附过程的有效吸附总质量也会维持在一定范围内;对于A-1,1 g吸附剂的吸附质量其为0.068~0.084 g;对于S-1,1 g吸附剂的吸附质量为0.001 5~0.002 g。经过多次吸附/解吸实验,其吸附剂解吸寿命保持稳定。以此为依据,对不同解吸方式下吸附剂解吸效果进行了汇总与分析。
-
图5为同种解吸方式下A-1和S-1的解吸率曲线。由图5可见,在相同解吸方式下,S-1的解吸效率都高于A-1。在解吸100 min后,S-1在真空解吸、真空加热解吸、负压+补气解吸、热吹扫解吸的解吸率分别比A-1提升了83.11%、99.56%、331.53%和85.35%。
根据开尔文(Kelvin)方程[21],可以看出,多孔结构材料在吸附、解吸过程中,会伴随着毛细管凝聚现象,计算方法见式(7)。
式中:p为曲面上的蒸汽压;p0为平面上的蒸汽压,即常规饱和蒸气压;σ为液体的表面张力,取值10−5 N·cm−1;V为吸附质液体的摩尔体积,mL·mol−1;θ为液面与毛细管壁的接触角,对于多孔材料油气体系,简化为cosθ=1[21];r为毛细管半径,nm,R为气体常数;T为热力学温度,K。
由式(7)可知,曲面上饱和蒸气压小于平面饱和蒸气压,且r越小,p/p0越小。因此,在真空解吸过程中,正己烷在吸附剂毛细管内的凹液面上已经处于饱和状态或过饱和状态,发生了毛细凝聚,造成毛细管表面到塔内空间之间的浓度梯度增高,产生浓差极化边界层,增加了有效扩散的阻力[22]。
由于A-1的孔径分布中有大量的微孔结构,毛细管半径小,而S-1的孔径分布中主要为中孔与大孔结构,毛细管半径较大。因此,在解吸过程中,A-1产生的毛细管凝聚与浓差极化现象更加严重,影响了解吸率。另一方面,S-1的有效吸附容量小于A-1,这导致了在解吸过程中S-1所需解吸的吸附质总量相对较少,因此,间接提升了S-1的解吸率。
本实验结果进一步印证了本研究团队此前提出的观点,即非封闭型的中孔结构吸附剂在脱附再生以及长期使用方面更有优势[5]。在未来吸附剂的有效解吸再生手段的研究中,应充分考虑以穿透点为参考的有效吸附容量的稳定解吸,以保证吸附剂的解吸寿命。
-
由图6可知,不同解吸方式对吸附剂解吸效率差别很大,由于2种吸附剂解吸方式对解吸效率的趋势一样,本研究主要分析不同解吸方式对A-1的影响。
图7为A-1真空解吸下2种解吸方式的解吸曲线。可以看出,同样为抽绝对真空解吸,当炭层内部加入盘管加热后,在解吸时间为100 min时,A-1抽真空、真空加热解吸的解吸率分别为5.2%、6%,与传统真空解吸相比,真空加热解吸方式的解吸率提升了15.16%。这是由于在解吸过程中,环境温度与解吸量成正相关[23],加入热盘管加热后,炭层通过热盘管持续增加炭层温度传递能量,从而改变解吸环境温度,提高炭层解吸率。
吸附剂受到毛细管凝聚现象的影响,气体有效扩散阻力较大。因此,在真空解吸后期吸附塔内没有气体流动的情况下,很难破坏浓差极化边界层,故真空加热解吸的提高效果不明显。
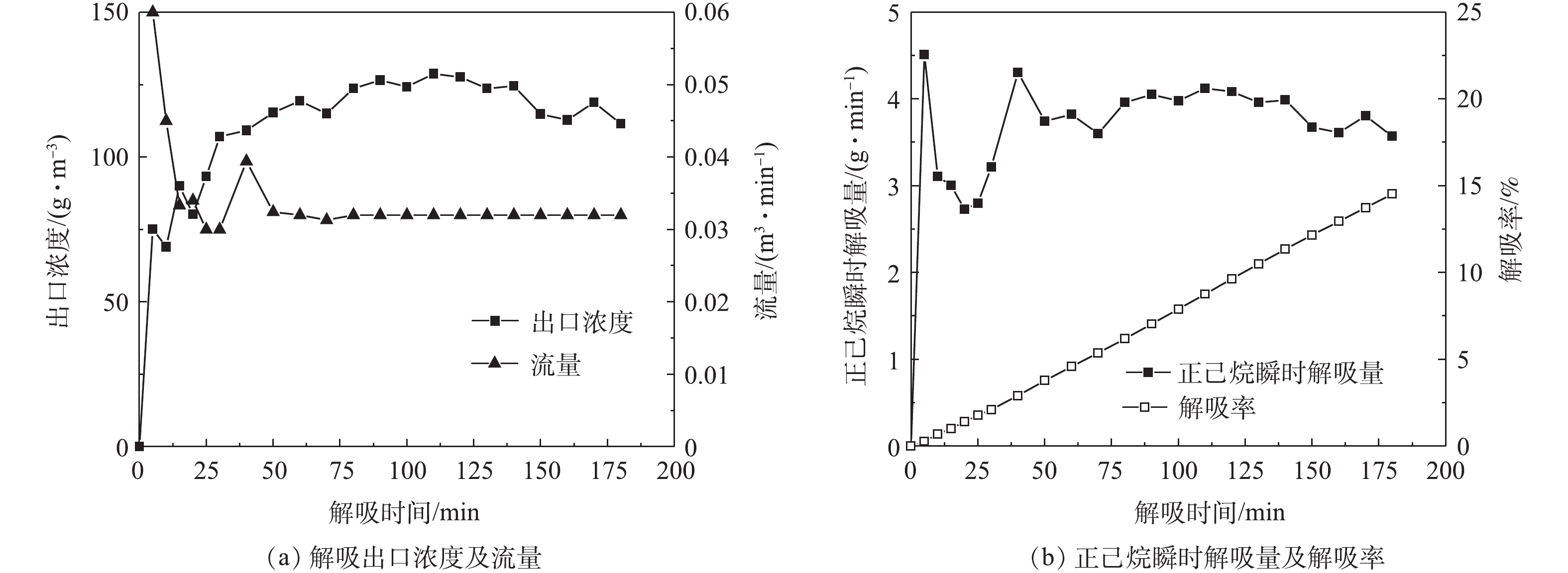
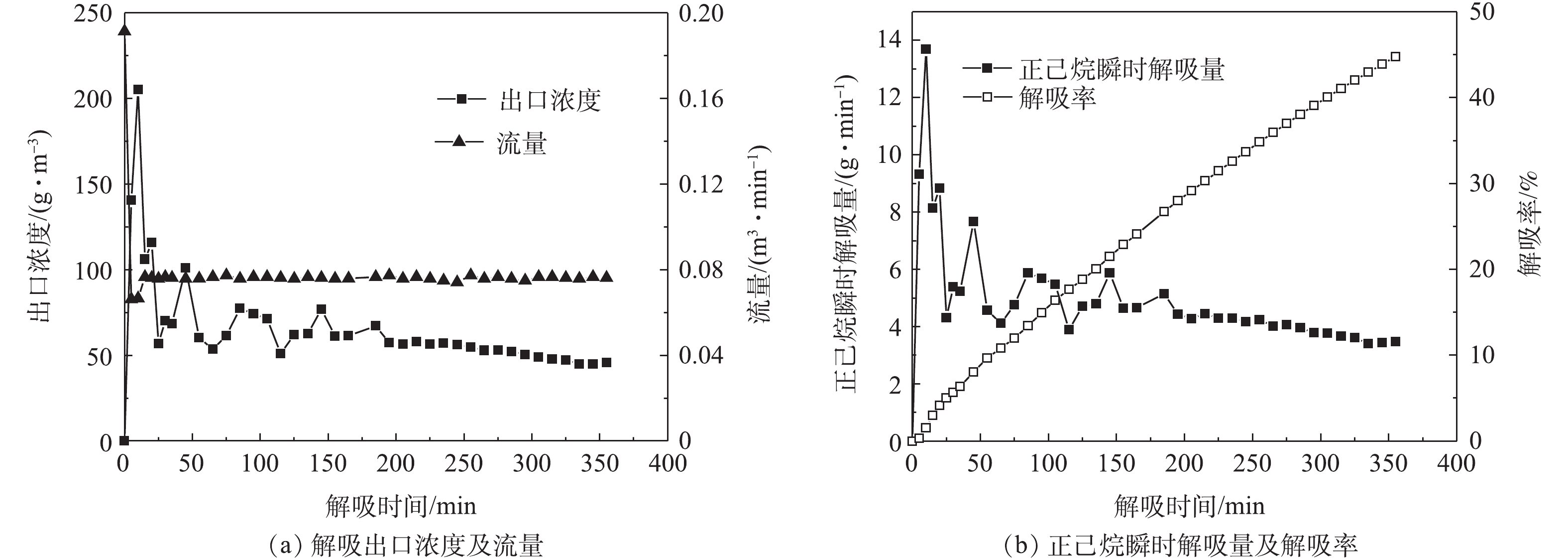
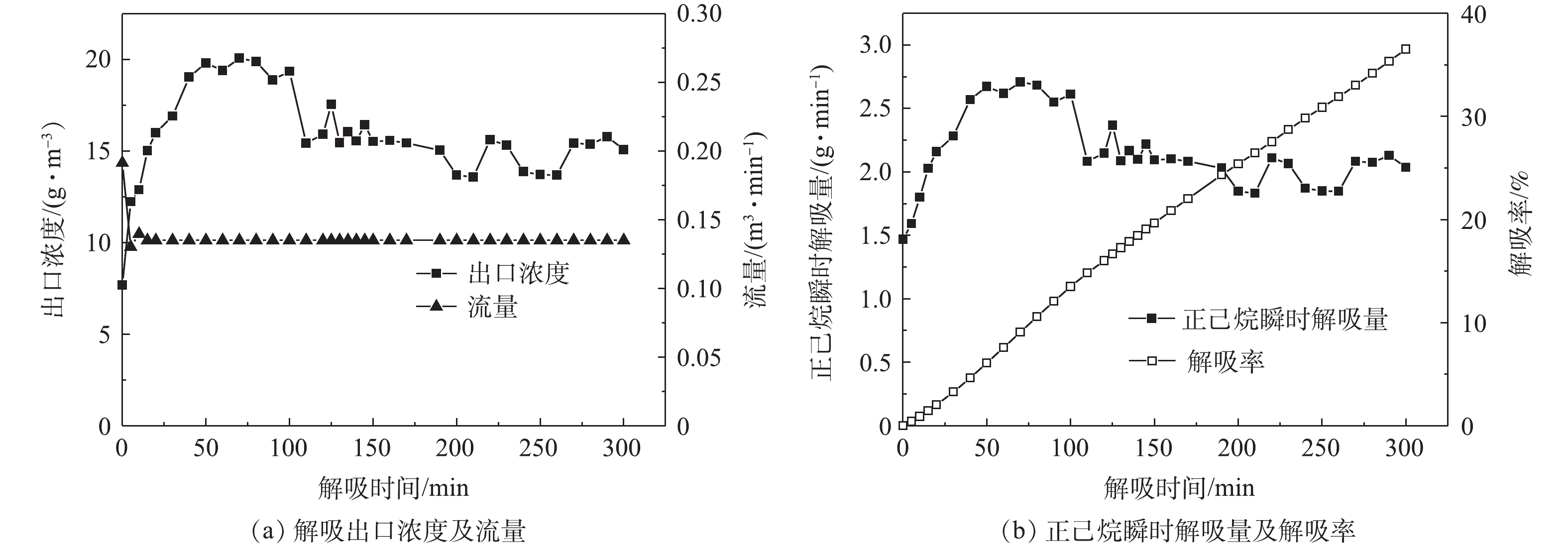
在吸附塔内无气体流动的情况下,解吸过程很难破坏浓差极化边界区。因此,须改善解吸条件,采用负压+补气的方式来破真空解吸,通过增加塔内流量,破坏浓差极化边界区来增加解吸效率。图8~图11为不同压力状态下“负压+补气”解吸过程A-1的出口浓度、流量、解吸率随时间的变化情况。
在解吸100 min后,A-1负压(77 kPa)、负压(53 kPa)、负压(30 kPa)、热吹扫解吸的解吸率分别为7.8%、16.4%、13.51%、53.95%。以抽真空解吸为初始解吸手段进行比较,则负压(77 kPa)、负压(53 kPa)、负压(30 kPa)、热吹扫解吸的解吸率分别在此基础上提升了51.24%、215.35%、159.3%、935.5%。可以看出,随着解吸工艺的改进,活性炭吸附剂的解吸率也在增加。
由图8~图11可知,随着解吸过程真空度的降低,其出口浓度不断减小,而出口流量不断增大,因此,吸附剂解吸率受到解吸浓度和解吸流量双重因素的影响。在压力与流量的耦合效应影响下,吸附剂的解吸率也发生了变化。在负压53 kPa状态和负压30 kPa状态的2次解吸实验中,随着真空度的降低,解吸浓度的减少幅度超过了流量增加的幅度。因此,解吸浓度变化成为影响解吸率的主要因素,从而导致在主要因素影响下,负压53 kPa状态的解吸率高于负压30 kPa状态。
从理论上讲,热吹扫解吸与其他解吸方式相比,解吸率最高,是最佳的一种解吸方式。然而热吹扫解吸耗能大,同时出口浓度过低,不利于后续尾气处理与环境保护,因此,热吹扫解吸更应该用于处理已完全失去吸附能力的吸附剂,而不是循环吸附解吸工艺领域。
与热吹扫解吸相比,负压+补气解吸的解吸出口浓度更高,易于后续尾气回收处理;与传统真空解吸相比,负压+补气解吸的解吸率又有很大提升,正成为工业应用中较为合适的解吸方式。同时,在负压+补气解吸下,存在某个最佳压力范围,使吸附剂得到最佳解吸效率。
-
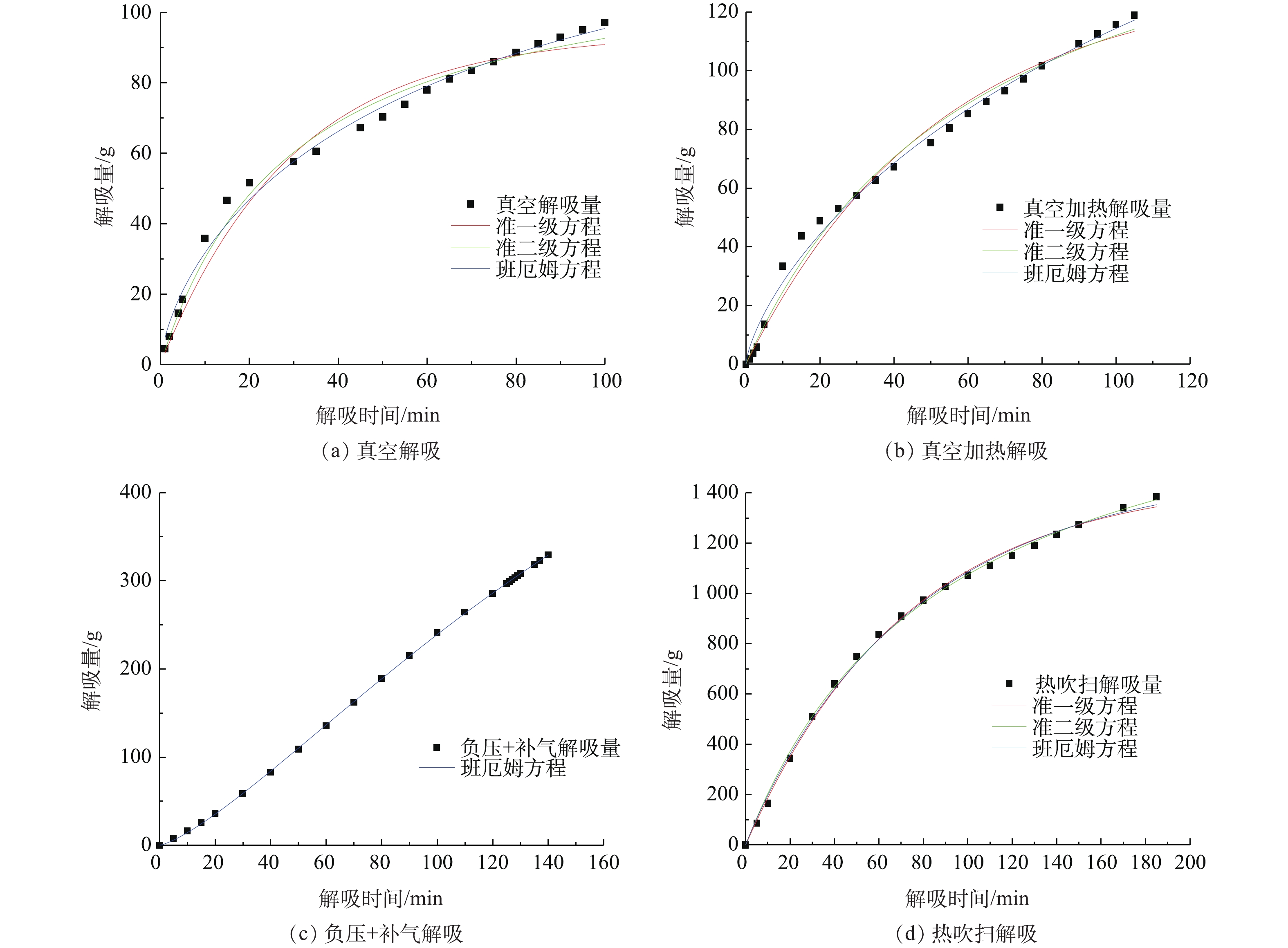
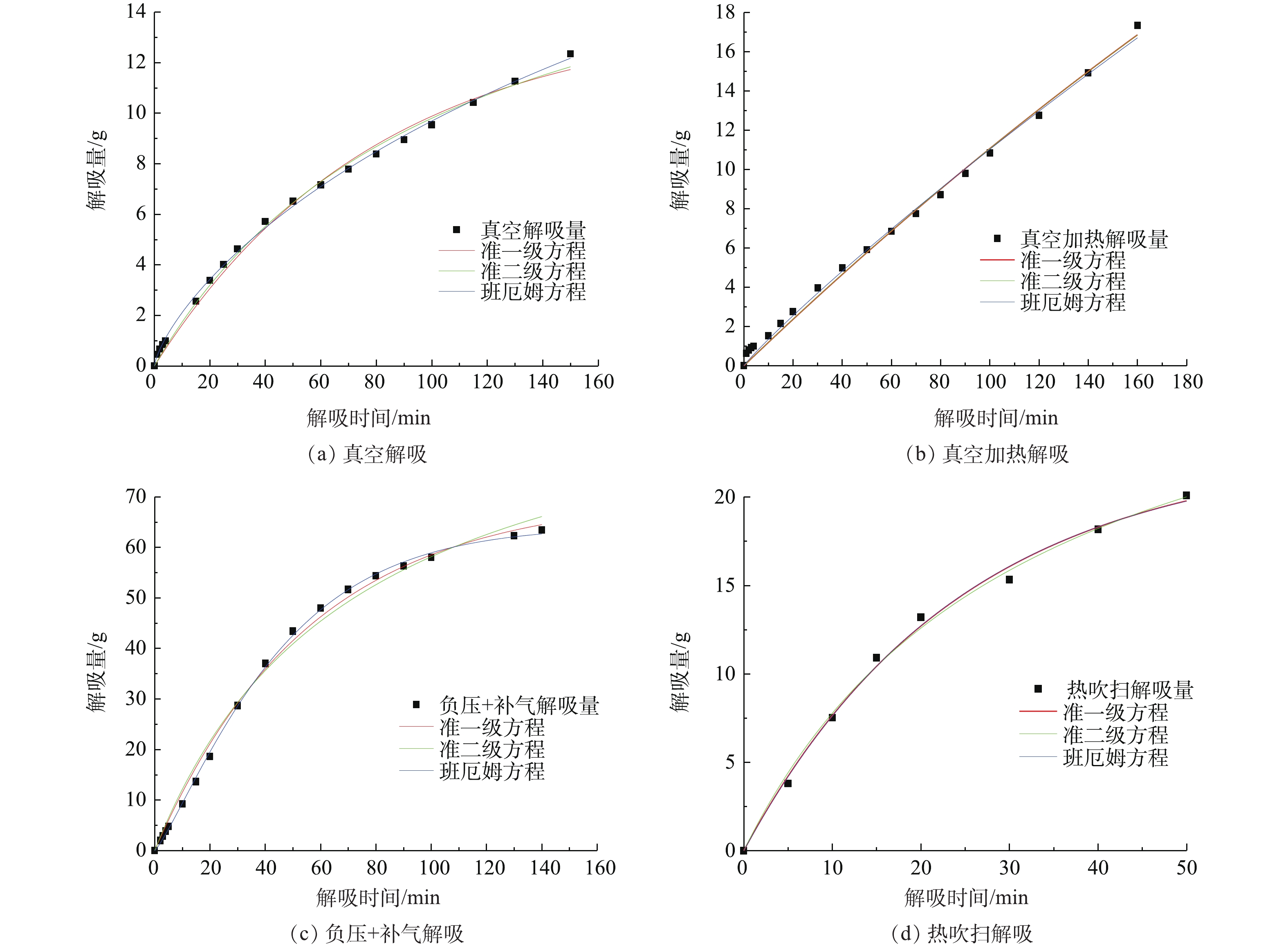
本研究对2种吸附剂解吸正己烷的动力学进行探讨。选用准一级、准二级、班厄姆(Bangham)3种动力学模型,对不同解吸方式下的解吸数据进行Origin非线性拟合,研究2种吸附剂对正己烷的解吸动力学行为,实验结果如图12、图13所示,拟合计算结果见表2所示。
由图12、图13以及表2数据可知:从横向看,描述内部孔道扩散机理的班厄姆(Bangham)动力学模型在不同条件下可决系数均高于0.99,且模拟得到的φe数值与真实数据最相近,可以描述动力学解吸过程;从纵向看,真空解吸下,准一级动力学模型拟合效果较差,说明在真空解吸下,内部传质阻力较大,解吸受控过程受内部扩散的影响更大;负压+补气解吸下,解吸环境真空度降低,解吸流量增加,准一级动力学模型、准二级动力学模型不收敛,模型不成立,说明解吸过程中影响因素较多;热吹扫解吸下,解吸过程处在常压状态,各模型拟合效果均良好,说明增加流量破除了浓差极化层,内部传质阻力减小,进而表现为外扩散传质影响增加,同时基于Langmuir方程的准二级动力学模型,也随着热吹扫解吸中热传递过程增加而表现出良好的拟合效果。从吸附剂角度看,孔径结构较大的S-1各个模型都表现出更好的拟合效果,说明影响内部传质阻力的因素为孔径结构,S-1的孔径较大,因而毛细管凝聚现象更小,其内扩散阻力更小,从而使准一级、准二级模型表现出更好的拟合效果。
综上所述,吸附剂解吸过程受多个扩散步骤控制,Bangham动力学模型能很好地描述吸附剂解吸动力学过程。
2.1. 吸附剂结构表征
2.2. 吸附剂的吸附/解吸
2.3. 不同吸附剂的解吸情况
2.4. 解吸方式对解吸效率的影响
2.5. 解吸动力学分析
-
1)在基于吸附穿透为参考点的动态吸附过程中,经过不同解吸方式(真空、真空加热、负压补气、热吹扫)的解吸实验发现,吸附剂在整个动态吸附-解吸-再吸附过程的有效吸附总容量保持稳定。
2)孔径结构对吸附剂解吸效率有很大影响,微孔结构造成的毛细管凝聚现象阻碍了吸附剂的解吸过程。
3)通过减少解吸过程真空度,进行负压+补气解吸,能有效破坏毛细管凝聚造成的浓差极化边界层,减轻了微孔结构对解吸速率的影响,进而提高了解吸效率。
4)对解吸过程进行了准一级动力学模型、准二级动力学模型、Bangham动力学模型拟合分析。拟合结果表明,3种动力学模型中Bangham动力学模型的拟合效果最好,与真实数据最相近,最能描述解吸动力学过程。





 下载:
下载:






 百度学术
百度学术


