-
在饮用水处理工艺中,超滤(utrafiltration,UF)技术因其优异的固液分离性能和出水生物安全性,已成为第三代净水工艺的核心技术[1-3],但超滤对有机物的低去除能力使其必须和其他净水技术联合使用才能保证出水水质[4-6]。吸附和预氧化是应用最广泛的膜前预处理技术[7-9]。一方面,在众多吸附剂中,磁性阴离子交换树脂(magnetic anion exchange resin,MIEX)是近年来为针对水中天然有机物(natural organic matter,NOM)去除而专门开发的一种新型树脂[10]。MIEX树脂颗粒的粒径约为150~180 μm,是传统树脂粒径的1/5~1/2倍,因而具有更大的比表面积和更低的固相传质阻力,可以提供更多的吸附活性点位[11]。与水处理中最常用的吸附剂粉末活性炭(powder activated carbon,PAC)相比,MIEX对有机物的去除速率是PAC的40倍,而且去除的有机物分子质量范围也较PAC更广泛[12]。已有大量的研究证明,MIEX对NOM、消毒副产物以及离子型污染物都有很好的去除效果[13-15]。作为一种新兴的吸附净水技术,MIEX正在受到越来越多的关注[16-17]。另一方面,在众多的预氧化技术中,高铁酸盐(
FeO2−4 )作为一种新型绿色高效氧化剂[18-19],集氧化、吸附和絮凝于一体,能够有效消减水中各类有机污染物[20-21]。高铁酸盐具有比传统氧化剂更高的氧化电势(在酸性条件下为E0=2.20 V,在碱性条件下为E0=0.72 V),而且没有消毒副产物生成[22]。同时高铁酸盐还具有微絮凝效应,其氧化有机物后会在水中形成新生态氢氧化铁胶体,能够进一步去除水中的颗粒态和胶体态污染物[23]。目前,高铁酸盐已被广泛用于污水、废水和给水的水质净化等领域[24-27]。众多周知,氧化法能够破坏大分子的结构,进而将其转化为小分子化合物,吸附则优先去除小分子有机物。因此,将氧化和吸附耦合作为UF的预处理工艺,可使得两者在净水方面存在较好的协同效果。但是,高铁酸盐预氧化和MIEX吸附在净水方面的性能差异对比,以及二者耦合是否存在明显的协同净水效应,还未见有研究报道。基于此,本研究以地表水模拟微污染水源水,系统地对比分析了MIEX吸附和高铁酸钾(K2FeO4)预氧化在有机物去除性能方面的差异,探讨了两者在净水性能方面存在协同效应的可能性,以期从水质净化角度为UF预处理工艺的筛选优化提供参考。
全文HTML
-
实验所用MIEX由澳大利亚ORICA公司生产并提供,平均粒径为180 μm。为保持性能,MIEX在使用前放置在饱和NaCl浓盐水中避光保存。每次使用时,取出适量MIEX置于烧杯中,用超纯水多次清洗至上清液电导率和纯水基本相同后备用[28]。树脂投加量按充分沉降后的湿体积计算。实验所用K2FeO4购自天津三江科技有限公司(纯度为99%,主要杂质为NaCl≤0.46%、Pb≤0.001%、As≤0.000 1%、水不溶物≤0.2%、干燥失重(150 ℃)≤1.0%)。在本实验中,高铁酸钾的投加方式为固体粉末投加。
实验中使用的原水来自于天津工业大学泮湖,使用前将湖水静置24 h去除较大颗粒物和表层悬浮物。稀释1倍后作为本实验进水,以模拟微污染水源水。实验进水综合水质特征为:实验原水的pH为8.31~8.37、浊度为1.93~1.96 NTU、UV254为0.187 1~0.190 0 cm−1、DOC为8.51~8.55 mg·L−1、电导率为1 716~1 718 mS·cm−1、
SO2−4 为140.2~143.4 mg·L−1。 -
K2FeO4预氧化实验和MIEX吸附实验均在ZR4-6型混凝实验搅拌仪中进行。搅拌速度为100 r·min−1,吸附和预氧化时间均为60 min。反应结束后沉淀30 min,取上清液经0.45 µm滤膜过滤后,再进行水质测定分析。
-
pH使用HACH 2100AN型测定仪测定;浊度采用HACH 2100Q型便携式浊度检测仪测定;UV254采用Agilent Craay60 UV-Vis紫外分光光度计进行测定;DOC采用岛津TOC-Vcph测定仪进行测定;有机物分子质量分布采用高效凝胶色谱分析,使用美国Waters公司生产的Waters 1515-2414型高效凝胶色谱仪,检测器为Waters 2414示差折光检测器,标准物质为聚乙二醇。实验进水中亲、疏水NOM组分的制备根据沈兆欢[29]提供的方法进行。
水样的三维荧光光谱(three-dimensional fluorescence spectrum, 3D-EEM)分析采用Perkin Elmer LS50B型荧光分光光度计(Perkin Elmer, New Jersey, USA)在室温(25 ℃)下进行。激发波长(excitation wavelength, Ex)为200~500 nm,扫描步长5 nm,发射波长(emission wavelength, Em)为200~600 nm,扫描步长为1 nm。激发和发射单色仪的狭缝宽度均设定为5 nm,扫描速度为1200 nm·min−1。采用荧光区域整合(fluorescence regional integration, FRI)技术对3D-EEM光谱数据进行分析。该方法先对3D-EEM图像及数据进行分区,激发-发射荧光光谱响应值矩阵分区与有机物种类对应关系[30]如表1所示。根据每个分区面积占总面积的比例计算每个区域的乘法因子Fi,再根据式(1)和式(2)计算标准化3D-EEM区域响应体积Φi,n,得出有机物构成情况[31]。
式中:I(λexλem)为三维荧光各点响应值总和;Fi为3D-EEM每个区域的乘法因子;Δλem为发射光谱扫描步长,nm;Δλex为激发光谱扫描步长,nm;Φi,n为3D-EEM区域响应体积。
-
为评价K2FeO4预氧化和MIEX吸附两者之间的协同效应,采用协同效应系数评估两者对各项指标的协同去除情况,协同效应系数[32]根据式(3)进行计算。
式中:S为协同效应系数;ηA为MIEX对有机物的去除率;ηB为K2FeO4对有机物的去除率;ηAB为MIEX和K2FeO4联合对有机物的去除率。当S>1时,表明两者有协同作用。
1.1. 实验材料
1.2. 实验装置
1.3. 分析方法
1.4. 协同效应系数
-
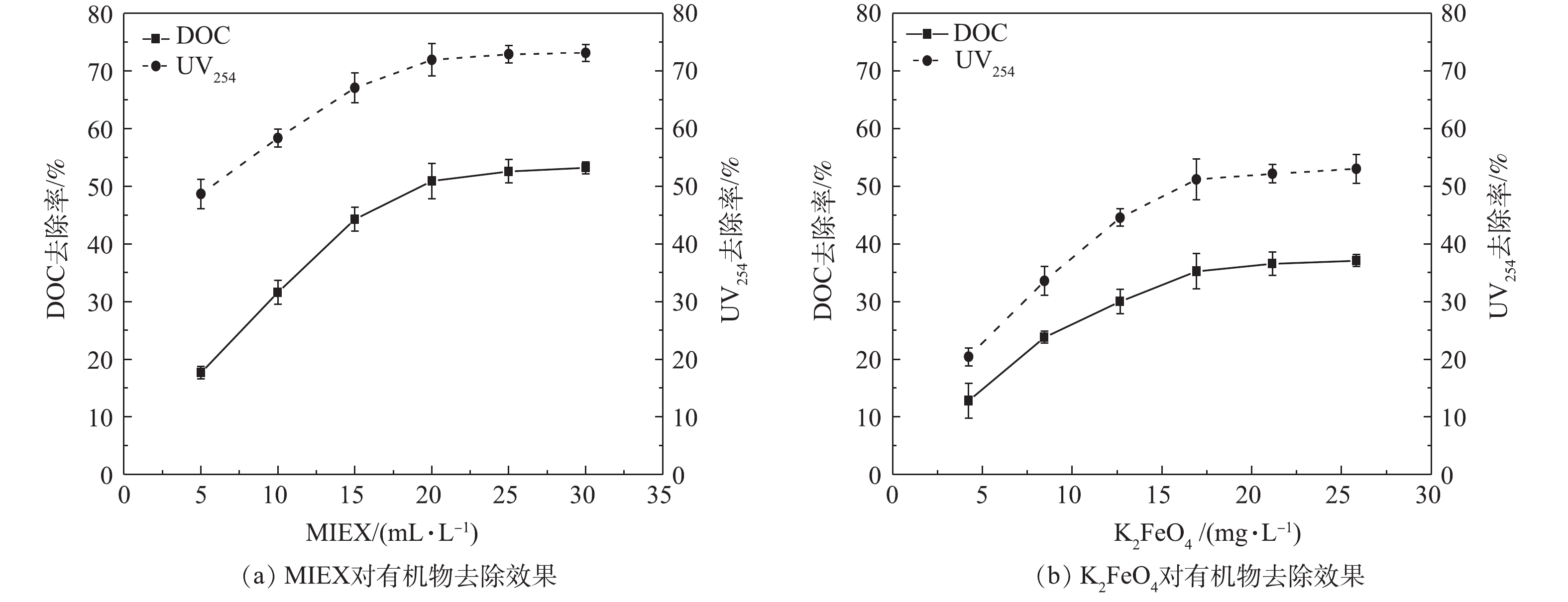
1) MIEX和K2FeO4对有机物总量的去除。图1(a)和图1(b)分别为MIEX和K2FeO4投加量对DOC和UV254去除效果的影响规律,两者的去除率随MIEX和K2FeO4投加剂量的变化趋势相同。当MIEX的投加量由5 mL·L−1增至20 mL·L−1时,MIEX对DOC的去除率由17.7%增至50.9%,之后DOC的去除率明显有所减缓。MIEX规律在UV254的去除率方面表现更加明显,去除率由5 mL·L−1投加量下的48.7%增至20 mL·L−1投加量下的71.9%,之后去除率不再有明显变化。当K2FeO4投加量(以Fe计)由4.23 mg·L−1增加至16.92 mg·L−1时,DOC的去除率由12.8%增至35.3%,UV254的去除率由14.4%增至53.0%。此后,DOC和UV254的去除率增长幅度均随K2FeO4投加量的进一步增加而明显减缓。在MIEX和K2FeO4的实验投加量范围内,MIEX对DOC和UV254的去除率均明显高于K2FeO4预氧化,这说明MIEX对总有机物和芳香族类有机物的去除效果均优于K2FeO4预氧化工艺。
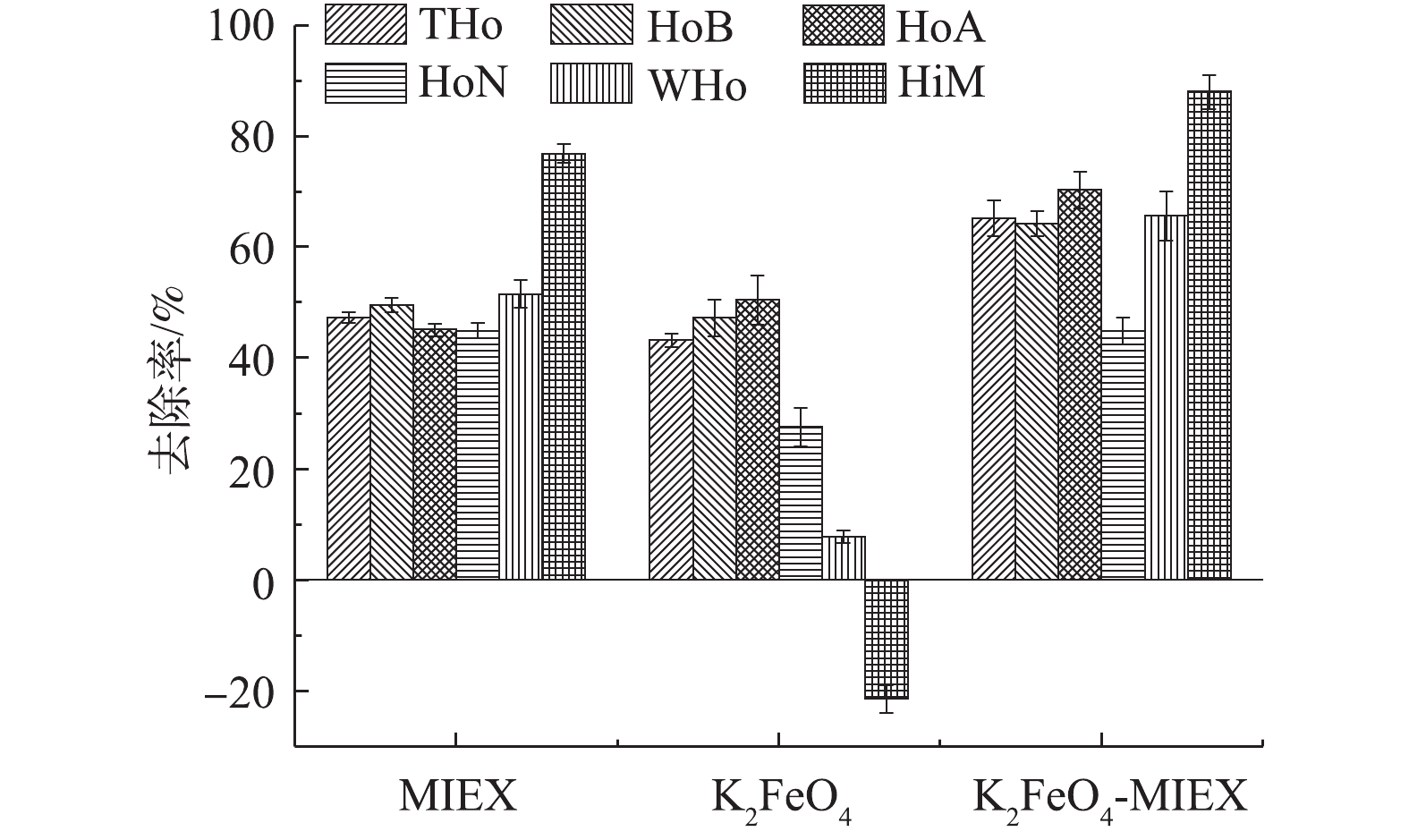
2) MIEX和K2FeO4对不太亲疏水组分有机物的去除。图2为MIEX和K2FeO4预氧化对不同亲疏水组分有机物的去除效果。需要特别指出的是,基于图1所得到的实验结果,在图2及后续实验中,K2FeO4和MIEX的投加量分别设定在16.92 mg·L−1和20 mL·L−1。在本实验中,通过树脂分级制备方法将原水中NOM组分分成疏水酸性组分(HoA)、疏水碱性组分(HoB)、疏水中性组分(HoN)、弱疏水组分(WHo)和亲水性组分(HiM),原水中总疏水总分(THo)是各疏水组分含量之和。对比MIEX吸附和K2FeO4预氧化对这些组分的去除率可以发现,MIEX吸附对每种单一疏水组分和疏水性NOM总量(THo)的去除率均相差不大,为44.9%~51.6%,但对亲水性组分的去除率明显高于疏水性组分,去除率可高达76.9%。相比之下,K2FeO4预氧化只对疏水酸性和疏水碱性组分的去除率较高,去除率分别为47.3%和50.5%,与MIEX的去除率相差不大,对疏水酸性组分的去除率甚至略高于MIEX。但K2FeO4预氧化对疏水中性组分的去除率仅为27.5%,对弱疏水组分几乎没有去除(去除率仅为7.8%),这些均远远弱于MIEX的去除效果。K2FeO4预氧化对亲水性组分不但没有去除,预氧化出水中亲水性组分反而增加了21.5%,同时考虑到预氧化对总NOM的去除率为33.8%,这再次证实了预氧化能够促使某些疏水性NOM组分向亲水性NOM转化[33]。
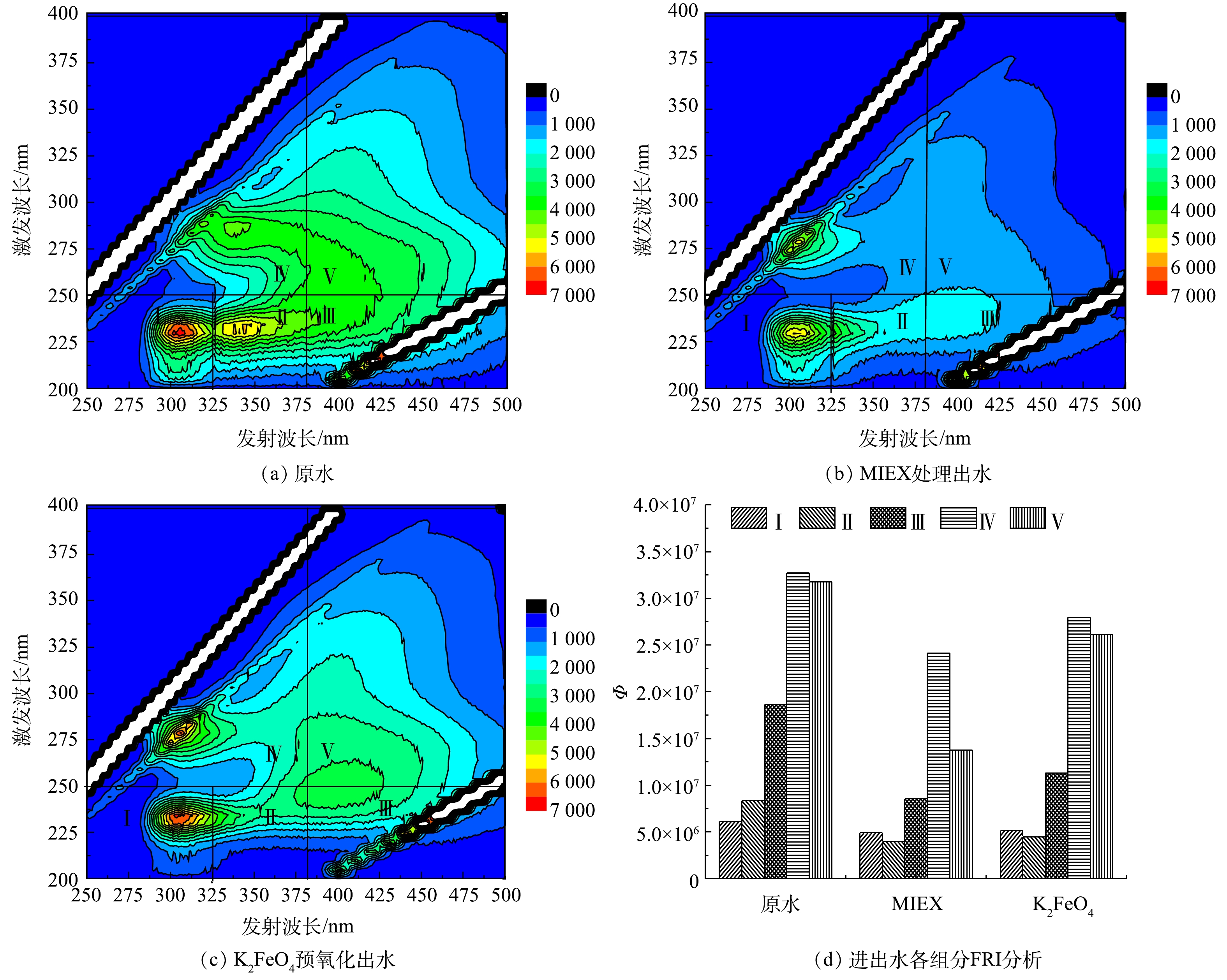
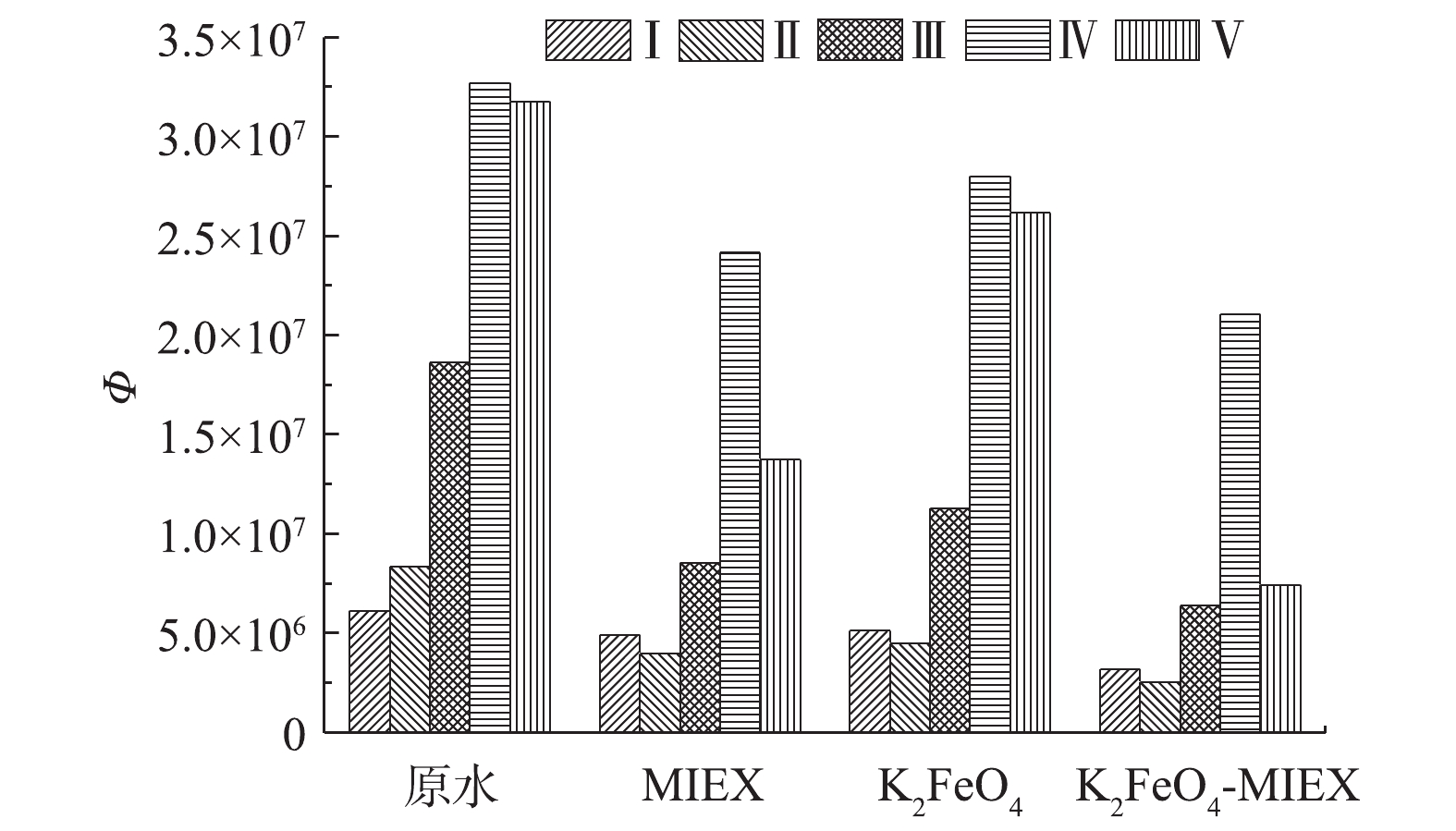
3) MIEX和K2FeO4对不同种类有机物的去除效果分析。采用3D-EEM荧光光谱考察了MIEX吸附和K2FeO4预氧化对水中不同类型有机物的去除效果。图3为三维荧光光谱分区及FRI分析结果。图3(a)为原水的三维荧光光谱,图3(b)和图3(c)分别为MIEX吸附和K2FeO4预氧化出水的三维荧光光谱。原水在经上述2种预处理后,各组分有机物的荧光强度均有显著降低,其中,MIEX吸附对各组分有机物的消减幅度比K2FeO4预氧化更加明显。通过对三维荧光光谱进行荧光区域整合分析可以看出,原水中5种有机物质所占比例分别为类酪氨酸类蛋白质(Ⅰ)6.30%、类色氨酸类蛋白质(Ⅱ)8.56%、类富里酸(Ⅲ)19.08%、可溶微生物代谢产物(Ⅳ)33.51%和类腐殖酸(Ⅴ)32.55%。显然原水中IV和V类有机物占据主要成分,总占比为66.06%。MIEX对Ⅰ和Ⅳ类有机物去除率较低,仅为20.0%和26.2%;但对Ⅱ、Ⅲ和Ⅴ类有机物的去除率均较高,分别为52.5%、54.0%和56.7%。K2FeO4对Ⅰ、Ⅳ和Ⅴ类有机物的去除率均较低,去除率分别为16.6%、14.4%和17.7%;对Ⅱ类去除率较高,为46.2%,其次为Ⅲ类物质,去除率为39.3%。上述结果表明,MIEX对水中的Ⅱ、Ⅲ、Ⅴ类有机物有着较高的去除率,而K2FeO4优先去除水中II类有机物。两者对Ⅲ类物质均有较高的去除率,但对Ⅰ类物质的去除率均较低。
4) MIEX和K2FeO4对不同相对分子质量有机物的去除效果。对实验原水进行高效凝胶色谱分析发现,原水中有机物主要是中、小分子质量的有机物,其主要集中在10~15、1.7~2.7和0.2~1 kDa(图4)。通过对这3个分子质量区间对应的峰面积进行积分计算可知,MIEX对这3个分子质量的有机物均有很好的吸附和去除,由分子质量由高到低的3个峰所对应的去除率依次为60.7%、65.7%、74.9%。显然,MIEX对分子质量为0.2~1 kDa有机物的去除率最高,这与YU等[7]的研究结论是一致的。
K2FeO4预氧化对3个分子质量有机物的去除率由分子质量由高到低依次为31.3%、74.4%和34.8%。从去除率来看,K2FeO4预氧化对分子质量为1.7~2.7 kDa有机物的去除率最高,优于MIEX。对10~15 kDa有机物去除能力弱于MIEX。理论上,K2FeO4预氧化对分子质量为10~15 kDa有机物的去除率应该优于1.7~2.7 kDa的有机物,但测定结果却表明,预氧化对这一小分子有机物的去除率很低,这应该是因为高铁酸钾对更高分子质量的有机物去除并没有达到完全矿化,而是将大分子有机物氧化分解成小分子有机物,最终导致预氧化出水小分子有机物的去除率并不高[34]。
-
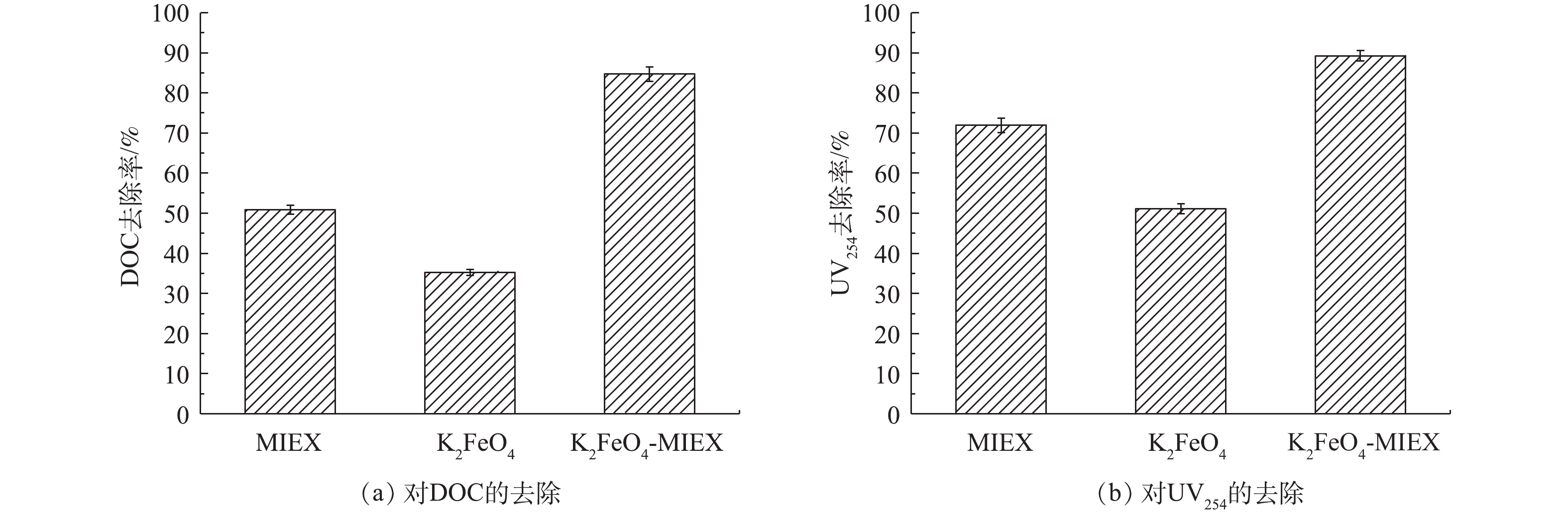
1) K2FeO4和MIEX在有机物总量去除方面的协同效应分析。在对比了K2FeO4预氧化和MIEX吸附净水效果的差异之后,实验进一步分析了K2FeO4预氧化耦合MIEX吸附在净水性能方面的协同效应。图5反映了两者在有机物总量去除方面的协同效应。K2FeO4-MIEX联合处理对DOC的去除率可达84.7%,比单独MIEX吸附提高了33.8%,比单独K2FeO4预氧化提高了49.4%,两者对DOC去除的协同系数为2.08,这说明两者在有机物总量消减方面具有显著的协同去除效应。在对芳香族化合物(UV254)的去除方面,K2FeO4-MIEX联合处理对UV254的去除率为89.2%,比单独MIEX提高了17.3%,比单独K2FeO4预氧化提高了36.2%,两者对UV254去除的协同系数为1.22,也表现出一定的协同去除效应。
2) K2FeO4和MIEX在亲疏水组分去除方面的协同性。图6反映了K2FeO4-MIEX联合预处理对水中亲疏水有机物组分的协同去除率。经过K2FeO4预氧化协同MIEX吸附处理以后,对每一种单一疏水组分、总疏水性有机物以及亲水性有机物的去除效果均有明显的提升,最明显的是对亲水性有机物的去除效果。联合处理去除率为87.6%,比单独MIEX吸附提高11.1%,比单独K2FeO4预氧化提高109.1%,对应的协同效应系数高达2.34。这是因为MIEX对亲水组分的去除率明显高于对疏水组分的去除率,而K2FeO4预氧化则能促使一部分疏水组分转化为亲水组分(图2),更有利于MIEX的吸附去除。此外,联合预处理对弱疏水性有机物也表现出一定的协同去除效应,对应的协同效应系数为1.30,这可能是K2FeO4预氧化促使一部分疏水性组分向弱疏水性组分转化,而MIEX对弱疏水性组分的去除率又远高于K2FeO4的结果。
3) K2FeO4和MIEX在不同种类有机物去除方面的协同性。如图7所示,三维荧光光谱分析结果表明,K2FeO4-MIEX联合处理对五类有机物质的去除效果都较单独处理有一定改善,其中是对原水中主要成分Ⅴ类有机物的联合去除率改善最为明显,联合去除率高达77.3%,比单独MIEX吸附提高20.6%,比单独K2FeO4预氧化提高59.6%,对应的协同效应系数高达1.57。其次,联合预处理对MIEX和K2FeO4都不擅长去除的I类有机物也表现出一定的协同去除效应,协同效应系数为1.29。MIEX和K2FeO4对Ⅴ类和Ⅰ类有机物都表现出协同去除效应的原因可能有2点。一方面,类腐殖质和类酪氨酸类蛋白质都属于大分子质量有机物,MIEX对中小分子质量有机物去除率较高,但对大分子质量有机物去除率较差[7];而K2FeO4预氧化可以把大分子质量有机物分解成为小分子质量的有机物,但很难完全矿化,所以去除率并不高[35];但将两者联合时,K2FeO4可以先通过预氧化将这些大分子质量物质分解为中小分子质量的化合物,然后其再被MIEX吸附去除,所以联合预处理的效果较2种单独预处理方法均有明显改善。另一方面,K2FeO4预氧化的副产物会产生氢氧化铁,有微絮凝效应,但形成的絮体尺度很小,扫捕能力较弱[23]。当预氧化出水进入膜反应器与MIEX混合后,氢氧化铁微絮凝体在MIEX的微磁场作用下会发生磁絮凝效应,矾花尺度明显变大,这会使絮凝体对大分子物质的扫捕沉淀效果进一步加强[36]。
4) K2FeO4和MIEX对不同分子质量有机物的协同去除效应。图8反映了K2FeO4和MIEX联合预处理对不同分子质量有机物的去除效果。联合预处理对3个分子质量的有机物均较单独预处理有明显提高,联合去除率随分子质量由高到低依次达到了72.0%、90.2%和86.2%。协同效应结果表明,K2FeO4和MIEX在去除分子质量为0.2~1 kDa有机物时表现出了明显的协同效应,对应的协同效应系数为1.20。根据图4的结果可知,K2FeO4预氧化将大分子质量有机物转化为0.2~1 kDa的小分子有机物,而MIEX对分子质量0.2~1 kDa的有机物的去除效率最高,因此,K2FeO4和MIEX对这一分子质量的有机物表现出较好的协同效应。本实验所用原水中分子质量高于15 kDa的有机物几乎没有,进水中有机物分子质量分布整体偏低,这致使K2FeO4预氧化将大分子质量有机物转化为中小分子质量有机物的效果不是特别明显,导致K2FeO4和MIEX之间的协同效应系数比预期偏低,但这种协同去除效应的存在是毋庸置疑的。
2.1. MIEX和K2FeO4净水效果对比分析
2.2. K2FeO4和MIEX的协同净水效果
-
1)在微污染水源水净化实验中,MIEX吸附工艺对总有机物和芳香族类有机物的去除效果均好于K2FeO4预氧化工艺, K2FeO4-MIEX联合处理工艺效果较单独MIEX吸附工艺提高了33.8%,较单独K2FeO4预氧化工艺提高了49.4%,两种工艺在有机物总体去除方面表现出很好的协同效应。
2) K2FeO4-MIEX联合处理工艺对类色氨酸类蛋白质有机物的去除效果均较单独处理有明显改善,其中,对腐殖酸类有机物的协同去除效果最为明显,协同去除效应系数高达1.57。此外,二者在类酪氨酸类蛋白质的去除方面也表现出较好的协同去除能力,在一定程度上弥补了两种单独工艺对类酪氨酸类蛋白质去除能力较弱的缺陷。
3) K2FeO4预氧化工艺对疏水酸性和疏水碱性组分的去除率较高,同时增加了出水中的亲水性有机物含量;MIEX吸附工艺对亲水性组分的去除率明显高于疏水性组分,这使得K2FeO4-MIEX联合预处理工艺对亲水组分去除具有显著的协同效应,协同去除系数高达2.34。此外,二者在弱疏水性有机物的去除方面也有一定的协同去除效应,对应的协同效应系数为1.30。
4)凝胶色谱结果表明,MIEX吸附工艺对小分子质量的有机物去除率最高,K2FeO4预氧化工艺在有效去除中等分子质量有机物的同时,又促进了原水中大分子质量有机物向小分子质量有机物的转化,使得K2FeO4-MIEX联合预处理工艺对分子质量为0.2~1 kDa的有机物表现出明显的协同去除效应。



 下载:
下载: