-
垃圾在堆放和填埋过程中经历发酵、雨水冲刷、淋溶和地表水及地下水浸泡等过程,会产生大量的垃圾渗滤液[1],其氨氮浓度会随填埋龄延长而逐年增高。老龄化垃圾渗滤液为典型的高氨氮、低C/N比废水[2-3],对其进行高效脱氮处理是亟需攻克的难题。
对氨氮废水的处理,一般需要物化法与生物法[4]的紧密结合。常见的物化脱氮方法主要有吸附法[5]、离子交换法[6]、折点氯化法[7]、化学沉淀法(又称磷酸铵镁(magnesium ammonium phosphate)沉淀法,简称MAP法)[8]等。这些方法大多只适合中低浓度氨氮废水的处理,对高氨氮垃圾渗滤液而言,即使加大吸附剂、交换树脂使用剂量或化学药剂投加量,也难以达到理想效果,出水氨氮仍然很高,导致后续生物脱氮压力极大,无法解决脱氮难题。传统吹脱法由于水气界面作用不充分,氨逸出速率低及设备易结垢的问题,导致吹脱效率不高,分离效果差[9-10]。目前,在实践中广泛采用的“双膜”[11]处理工艺,由于维护费用和浓缩液处理的问题,受到业界诸多诟病。
动力波(dynawave)洗涤装置是美国杜邦公司于20世纪70年代开发的一种高效分离专利技术,主要应用于废酸回收、冶炼烟气处理、硫酸净化、粉煤锅炉尾气处理等40多个不同生产领域[12]。本课题组以老龄化垃圾渗滤液为处理对象,研究利用动力波泡沫区气液两相接触面积大且接触表面不断迅速更新的极大优势,对垃圾渗滤液进行氨分离,为后续生化处理创造有利条件。本研究探讨吹脱时间、pH、气液比、温度、进水氨氮浓度等多种因素对氨氮吹脱效能的影响,在单因素实验的基础上,通过正交实验考察最优工艺条件,为垃圾渗滤液中氨氮的高效分离提供创新方法。
全文HTML
-
实验用水取自福州市红庙岭垃圾渗滤液厂均衡池,具体水质情况:pH为7.8~8.7,温度10~30 ℃,总氮浓度为800~1 200 mg·L−1,其中氨氮的浓度为500~1 000 mg·L−1、BOD5和COD的浓度分别为300~600、2 000~4 000 mg·L−1。该渗滤液氨氮占总氮60%~85%,高效分离氨氮可大大降低总氮含量,为后续生物处理创造良好条件并节约脱氮成本。
-
实验所用药剂有氢氧化钠(NaOH)、酒石酸钾钠(KNaC4H4O6·4H2O)、碘化汞(HgI)、碘化钾(KI)、碱性过硫酸钾(K2O8S2)、盐酸(HCl)。主要仪器有高压蒸汽灭菌锅、雷磁pHS-3C型pH计、HACH COD消解器、HACH便携式多参数比色计、HACH生化需氧量分析仪等。
-
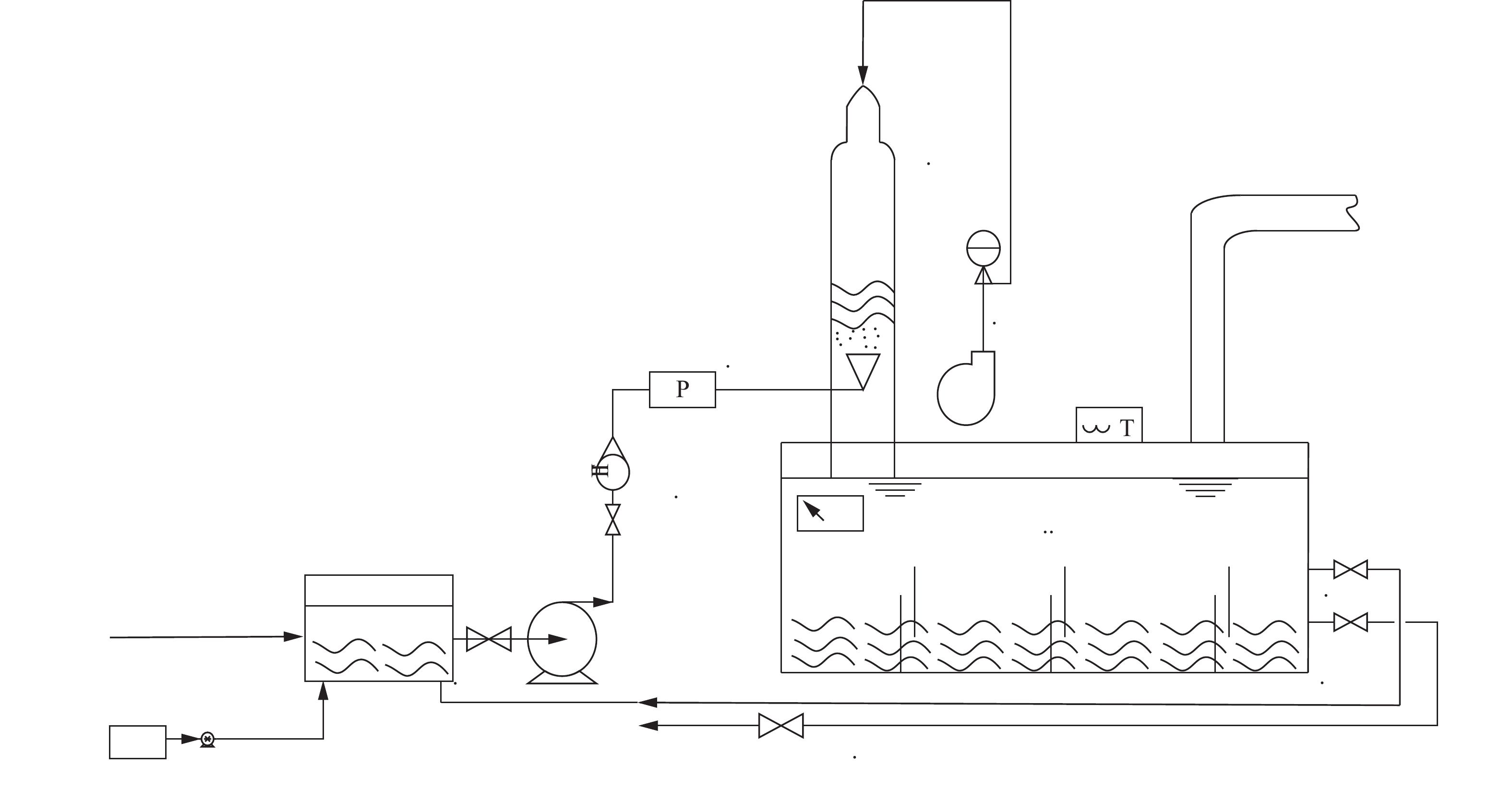
动力波吹脱中试实验装置如图1所示。其中,调节池(尺寸:50 cm×20 cm×40 cm=40 L)、反应循环箱体(尺寸:90 cm×30 cm×40 cm=108 L),箱体采用PP材质,一次吹脱处理水量为42 L。
中试实验流程:1)将渗滤液加入调节池内,调节pH和温度;2)开启可调速风机,改变风机气量,控制气液比,并启动污水离心泵,即可进行吹脱实验;3)风机将空气自动力波反应器的上方鼓入,垃圾渗滤液由离心泵经转子流量计自下而上,由特制的喷头呈幅射状旋流射入动力波吹脱装置的波管中;4)动力波吹脱后的液体流入反应循环箱体,在箱体中安装隔板,强化水力搅拌;5)在箱体中强化搅拌后的液体经循环管道再次由污水离心泵送至动力波装置进行循环吹脱;6)实验中,定时从排水管取样检测,以研究不同因素对吹脱效果的影响。
-
pH采用雷磁pHS-3C型pH计现场检测,温度在监测控制装置上直接读取。COD采用快速消解分光光度法[13],用HACH DR900仪器测量;BOD5采用稀释与接种法[14]用HACH BODTrakTMⅡ生化需氧量分析仪测量;TN和氨氮浓度按照国标法[15-16]进行检测。
1.1. 废水来源与水质
1.2. 实验试剂与仪器
1.3. 实验装置
1.4. 分析方法
-
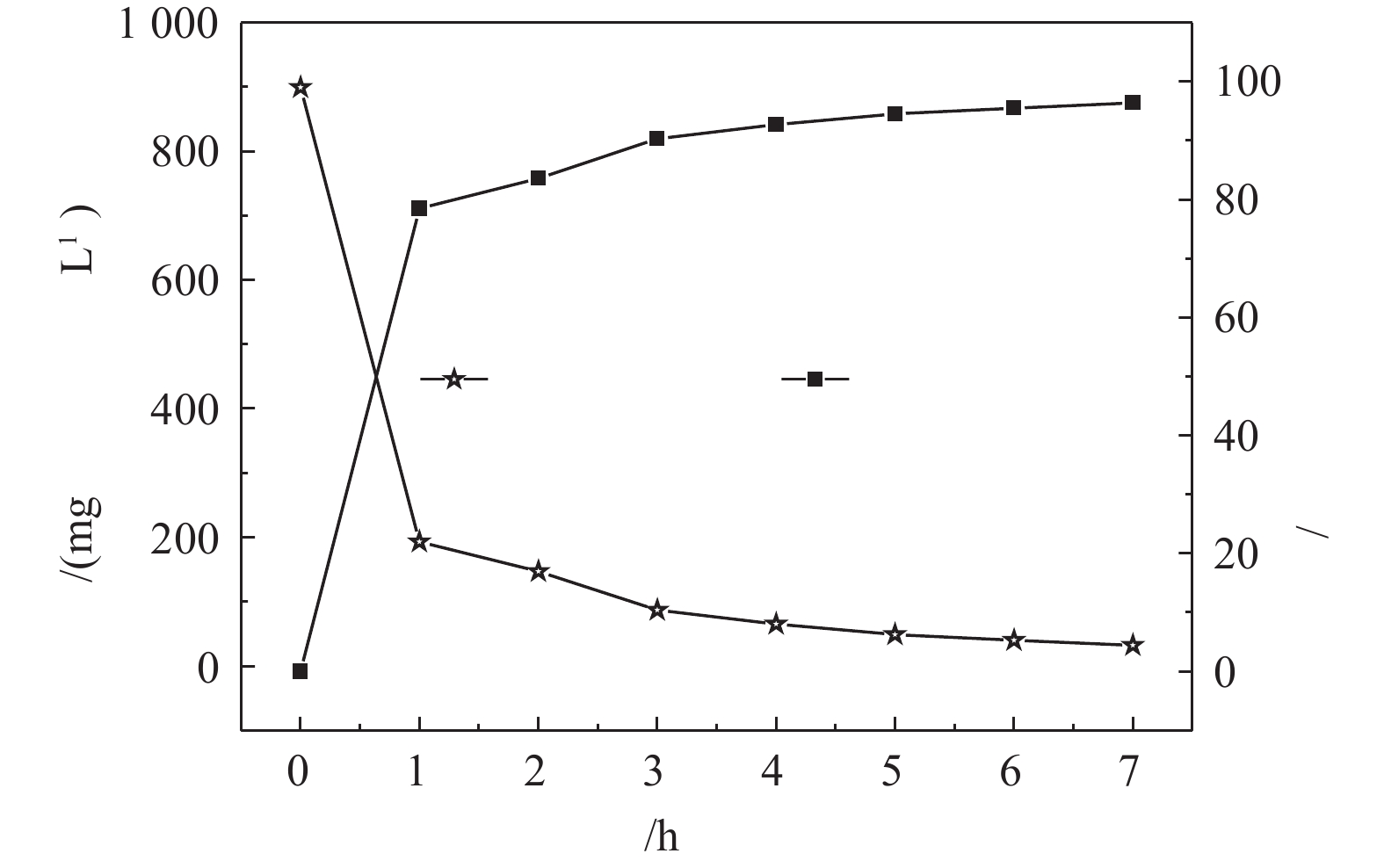
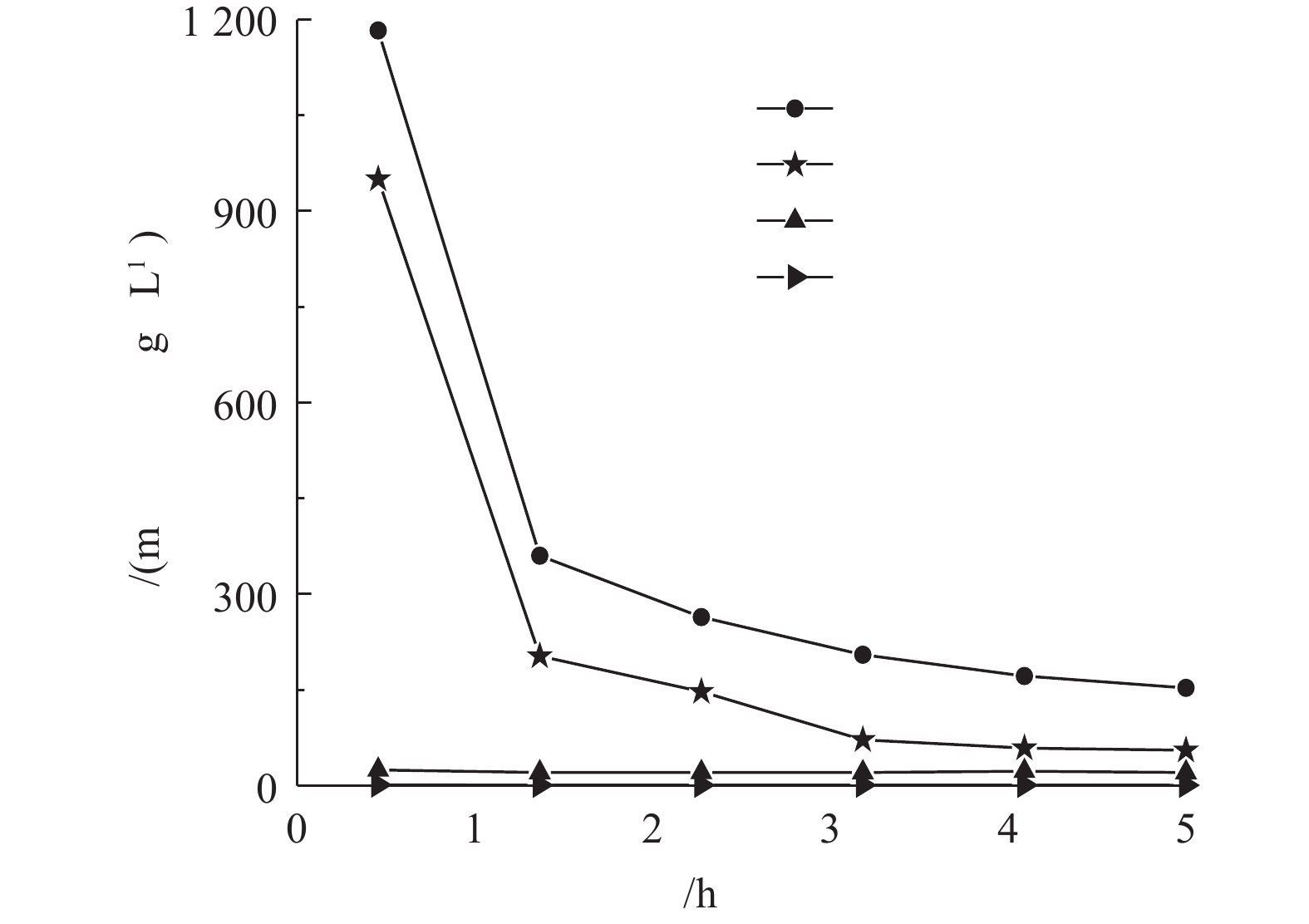
调节废水温度为30 ℃,气液比为129∶1,pH为10.5,在不同的时间间隔取样,检测氨氮的浓度,结果见图2。垃圾渗滤液氨氮浓度随吹脱时间的增加而降低,前3 h为吹脱的高效段,氨氮去除率高达90%以上,当吹脱时间到5 h时,氨氮浓度由950.10 mg·L−1降至55.60 mg·L−1,吹脱7 h后,氨氮浓度为42.63 mg·L−1。到吹脱后期,随着时间的延长,氨氮的脱除效果并无显著变化。这是由于此时废水中氨氮浓度已经非常低,要将离子态的铵(
NH+4 )转换为游离态的氨(NH3),需要更为理想的条件,但这会导致吹脱成本增加。综合考虑,动力波吹脱时间控制在5 h。 -
调节废水温度为25 ℃,气液比为129∶1,废水pH分别为8.0、9.0、10.0、10.5、11.0、12.0时,测定吹脱5 h后废水氨氮的浓度,结果如图3所示。氨氮吹脱效率随pH增大而升高,是因废水中NH3-N的吹脱去除效果与废水中游离氨NH3和铵离子
NH+4 有关,pH增加使得NH3的含量越高,吹脱效果越好。废水中NH3和NH+4 间的平衡关系为:NH+4 +OH−=NH3+H2O,保持其他条件不变,当渗滤液的初始pH≤9.0时,渗滤液含有大量的NH+4 ,NH+4 有较强的缓冲作用,吹脱时氨逸出量很小,此时吹脱效果不理想,pH=8.0时吹脱去除率仅为70%左右,pH=9.0时吹脱去除率也只有80%左右;当pH>9.0时,氨的电离平衡被打破,平衡向着转化为游离氨方向变动,NH3浓度增加,吹脱过程容易进行[17],吹脱去除率达90%以上。虽然废水pH升高平衡向右移动,氨氮吹脱效果越好,但调节pH大于10.5,污水中的氨氮以NH3形式为主,此时,提高pH仅增加少量NH3。考虑到碱投加量会导致成本升高,在实际工程应用中,pH调节为10.5左右最为经济。 -
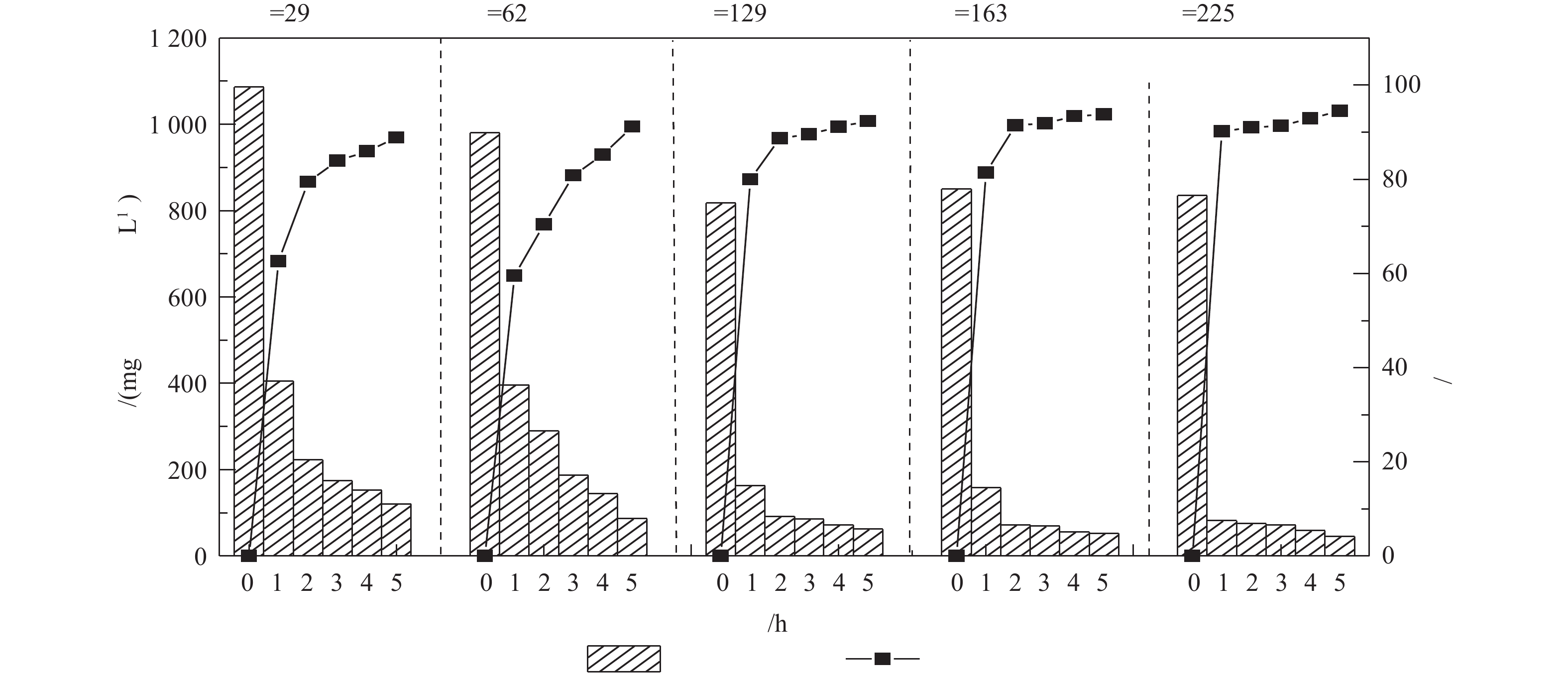
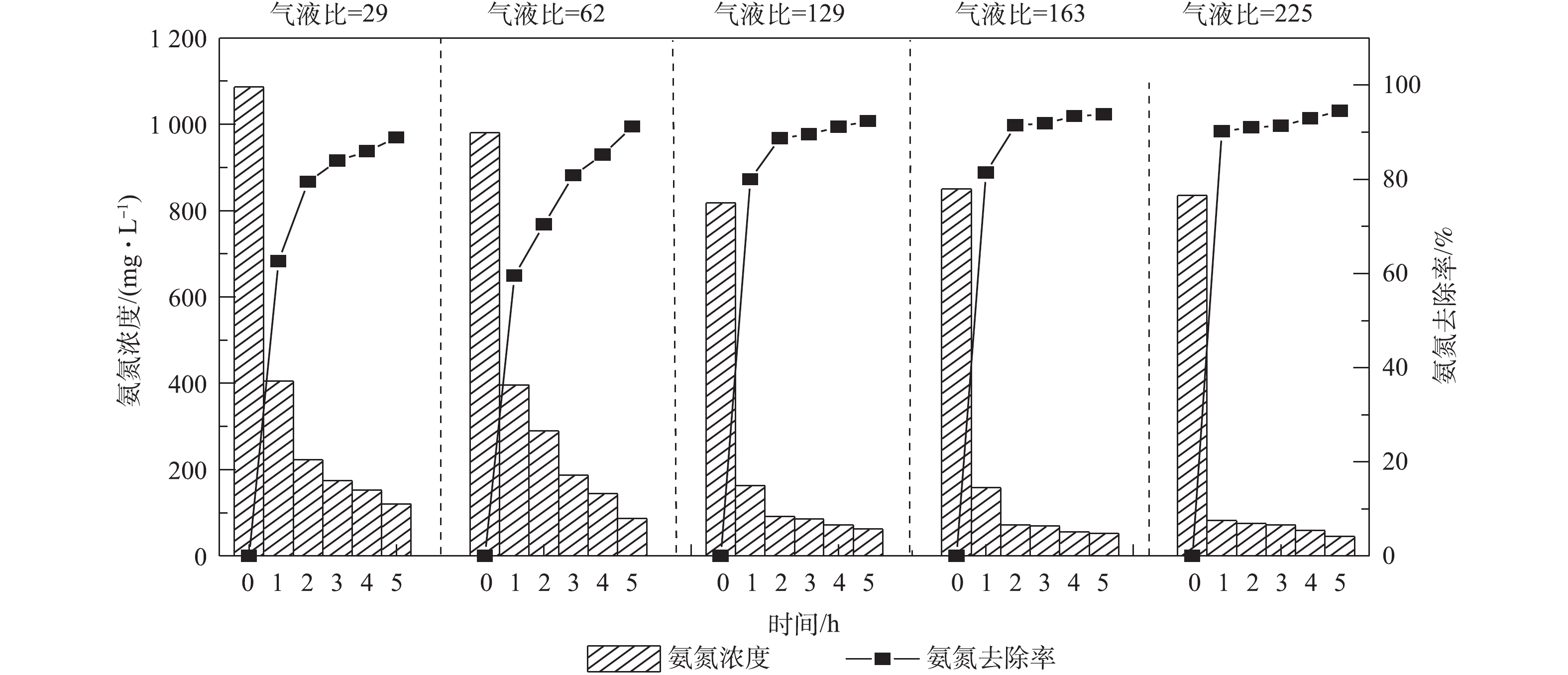
调节废水温度为25 ℃,pH为10.5,进水量为1.2 m3·h−1,控制气量分别为35、75、155、195和270 m3·h−1(即对应气液比为29、62、129、163和225),检测吹脱5 h后出水氨氮的浓度,实验结果如图4所示。垃圾渗滤液氨氮浓度随气液比的增大而下降,当气液比为129,吹脱5 h后,氨氮浓度由817.84 mg·L−1降低至62.23 mg·L−1,氨氮脱除率达92.34%,若继续增加气液比至225,相较于气液比为129时去除率仅增加2.18%。表明气液比高于129时,氨氮的去除率呈缓慢增长趋势,这是因为在气液比为129左右时,动力波吹脱洗涤管中即可形成稳定泡沫区,在泡沫区,空气与水的比表面积增加,气泡的破裂和更新导致了气相界面面积的增加。动力波吹脱过程中所形成泡沫区气泡的大小会影响NH3的转移速率,NH3在较大气泡中的传质速度较慢,气液比低于129时,动力波吹脱泡沫区气泡较大,吹脱去除率较低;气液比大于或等于129时,形成较为理想的泡沫区,有利于NH3在液相和气相之间的传质[18]。考虑到实际应用时的能耗问题,气液比控制在129较为理想。
-
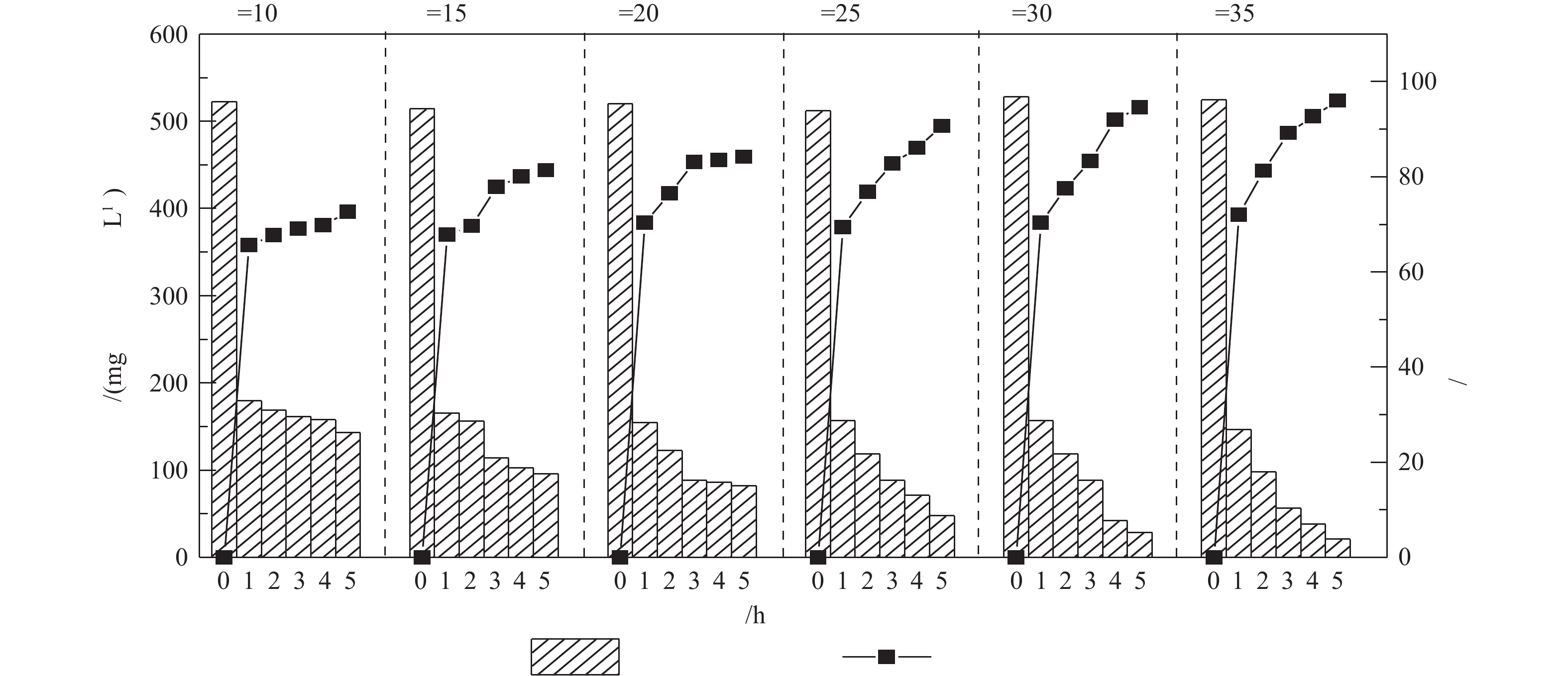
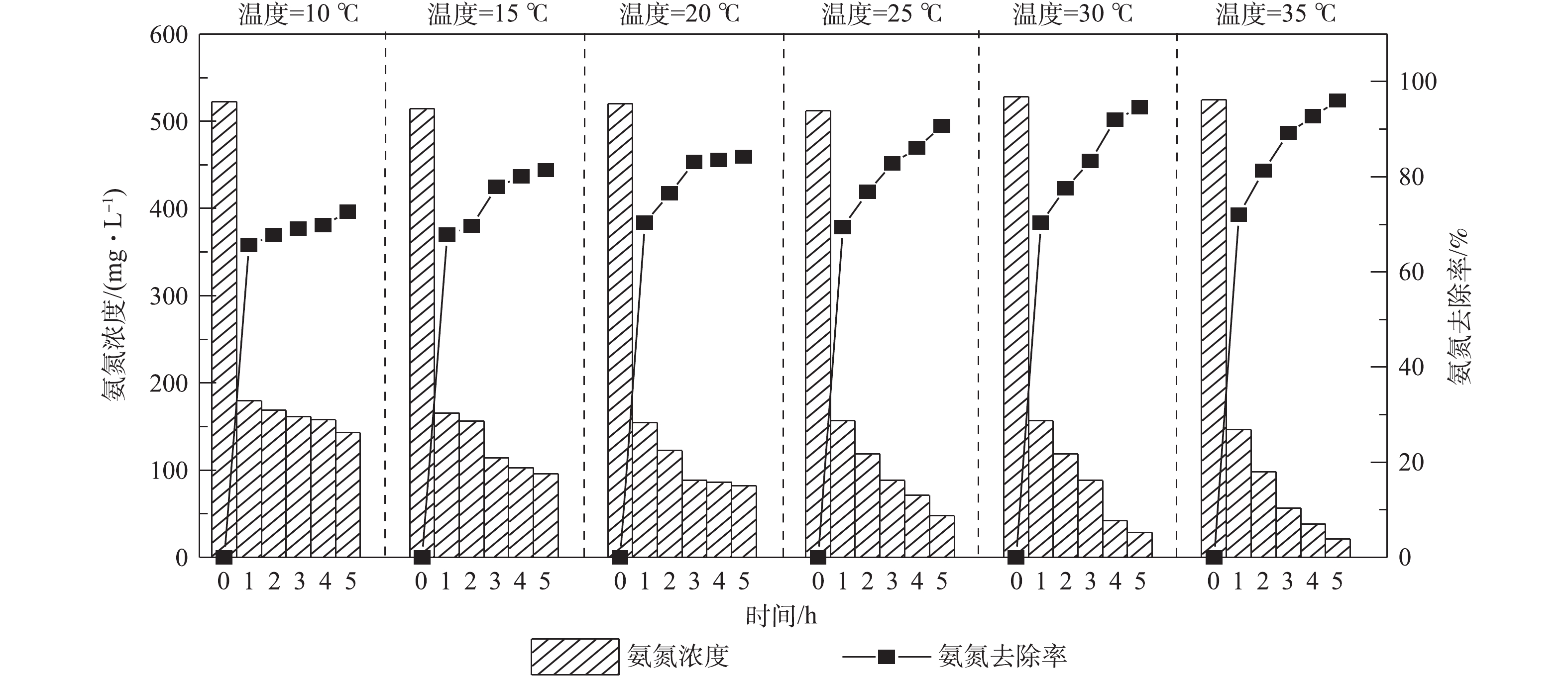
调节废水pH为10.5,液气比为129,进水量为1.2 m3·h−1,在温度分别为10、15、20、25、30和35 ℃时,检测吹脱5 h后出水氨氮的浓度,结果见图5。由图5可知,垃圾渗滤液中氨氮的去除率随温度的升高而持续升高。当温度为10、25和35 ℃时,废水的氨氮吹脱去除率分别为72.62%,91.25%和96.03%,可见废水温度对氨氮的脱除影响大。这是因为当温度升高时,游离氨的含量也不断增大,分子扩散快,传质系数增大[19];另外,在一定压力下,温度升高使氨的平衡分压增加,降低了氨在废水中的溶解度,有利于氨气的析出。从节约成本的角度综合考虑,吹脱温度为25 ℃较为理想。在实际工程应用中,因冬季气温比较低,往往吹脱效果不佳,一般会在吹脱处理前对垃圾渗滤液进行加热升温。根据本研究的结果,采用动力波吹脱装置预处理脱氨,即使在低温条件下也能实现较高的氨氮去除率。废水温度为10 ℃时,吹脱去除率高达70%以上,相比于传统的吹脱方式具有较大优势。
-
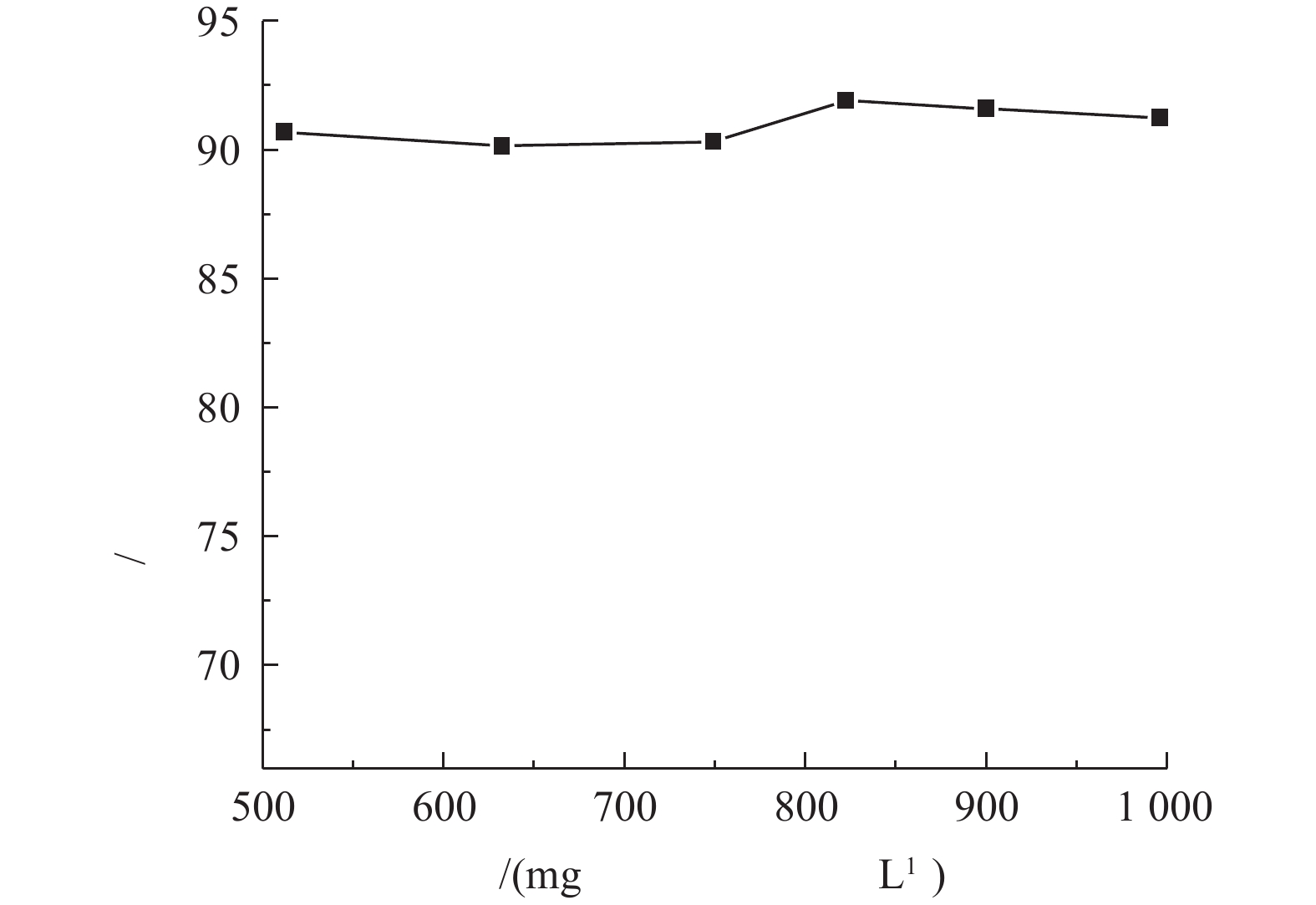
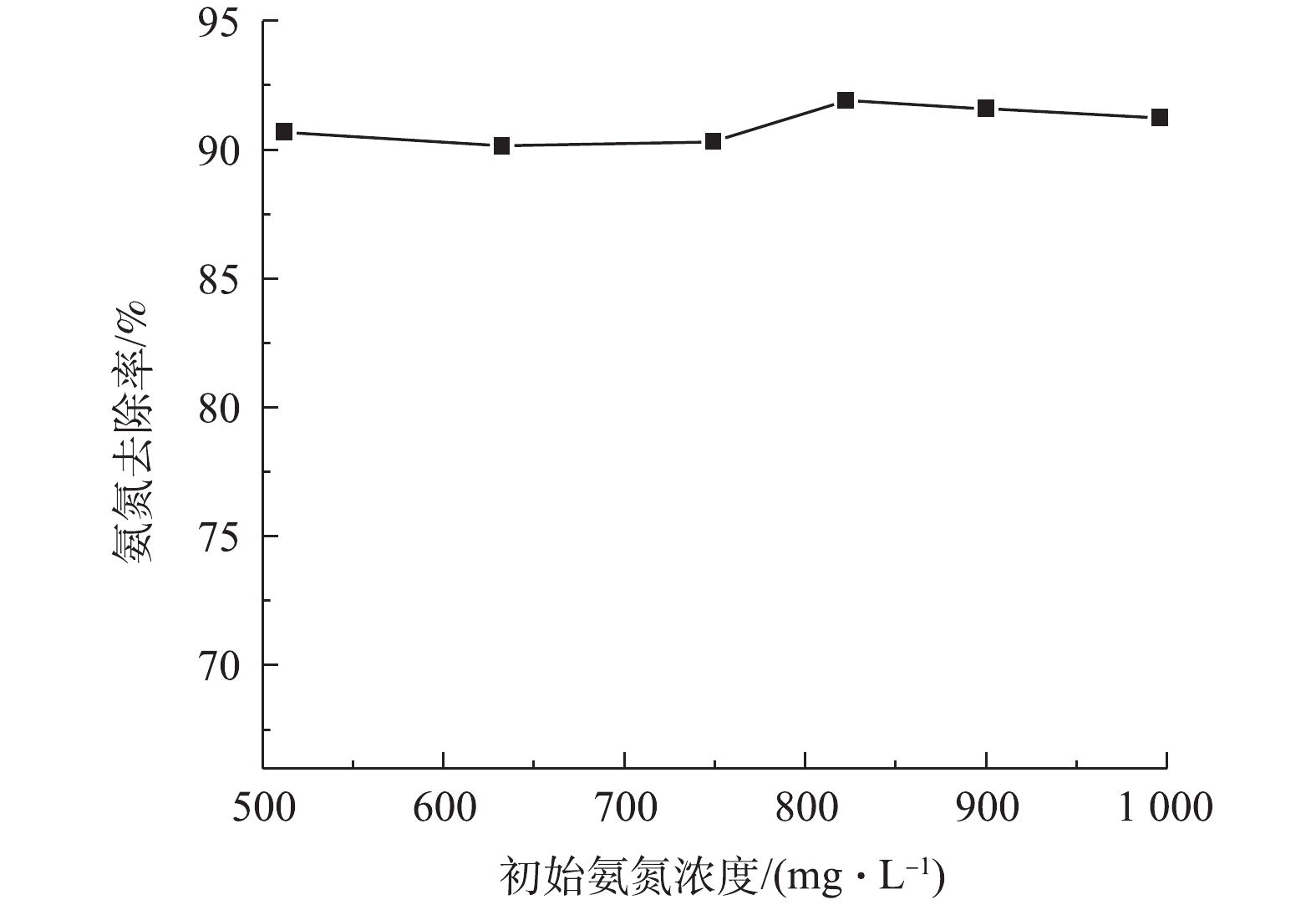
调节废水温度为25 ℃,pH为10.5,液气比为129,进水量为1.2 m3·h−1时,在渗滤液初始浓度(由于渗滤液水质变化大,不同批次的水样氨氮浓度不同,实验中将不同批次水样进行混合的方式得到不同氨氮浓度的渗滤液)分别为512.13、632.46、749.21、822.30、900.10和995.97 mg·L−1时,检测吹脱5 h后出水氨氮的浓度,实验结果如图6所示。由图6可知,在初始氨氮浓度为500~1 000 mg·L−1的情况下,保持温度、pH、气液比和进水量不变,吹脱5 h后氨氮的去除率均为90%~92%,这表明氨氮吹脱效率与垃圾渗滤液中初始氨氮浓度关系不大。
-
为比较pH、温度、气液比和进水氨氮浓度4个因素对吹脱效果影响的大小,进行了正交实验分析,每个因素选用3个水平,实验设计见表1。
以垃圾渗滤液氨氮去除率为指标,按L9(34)安排实验,对试验数据分别进行直观分析和方差分析,实验结果见表2~表4。由表2可知,R2>R1>R3>R4,KA3>KA2>KA1,KB3>KB2>KB1,KC3>KC2>KC1表明氨氮的吹脱效果在一定条件下是随着pH、温度和吹脱时间的增大而变大,与氨氮进水浓度关系不大。为进一步确定不同因素对氨氮去除率影响的显著程度,对实验数据进行了方差分析。因F检验需要计算误差偏差平方和以及自由度,在进行方差分析时所选正交表应留出一定空列。本实验为4因素3水平实验无空列,采用重复实验来估计实验误差,实验方案及实验结果见表3。对实验结果进行方差分析,列于表4。F检验结果表明,pH(A)、温度(B)、气液比(C)3因素对氨氮去除率都有极显著影响;进水氨氮浓度(D)相对其他3因素对氨氮去除率影响不显著,区组间差异不显著,且温度(B)影响显著性>气液比(C)>pH(A)。综合直观分析和方差分析得出,此实验设计的最优组合为A3B3C3。而实际工程中氨氮吹脱往往受到运行成本、投药量、环境等因素的影响,限制了最优吹脱条件的选择。若将原水氨氮浓度降低到150 mg·L−1以下,再进入后续生化处理系统,对于生化处理系统中的菌群来讲,此浓度既不超过菌群自身的处理能力,反之还可为反硝化过程提供一定的氮源。综合考虑,垃圾渗滤液吹脱较适宜的条件为pH=10.5,温度为25 ℃,气液比为129。由表3可知,在此条件下,氨氮吹脱率介于84.17%~95.81%,而对应的剩余氨氮浓度为20.95~142.47 mg·L−1。由此可知,对垃圾渗滤液进行吹脱处理,可以实现高效生物脱氮,大幅度减轻后续生物处理负担。
-
由图7可知,垃圾渗滤液中氨氮占TN 80%左右,硝态氮占TN 2.09%、亚硝态氮占0.11%;在动力波吹脱5 h后,氨氮的浓度显著下降,而NO3−-N和NO2−-N的浓度基本不发生变化,说明吹脱过程主要是去除氨氮,对硝态氮和亚硝态氮的去除效果不明显。
-
由表5可知,在氨氮去除率达到90%以上时,传统吹脱所耗时间久、气液体积比大、pH一般控制在11左右,且处理水量较小。而本研究所采用的动力波吹脱技术,在pH为10.5,温度为25 ℃,气液比为129时,一次吹脱42 L的废水,可达到91.25%,表现出极大的优势。
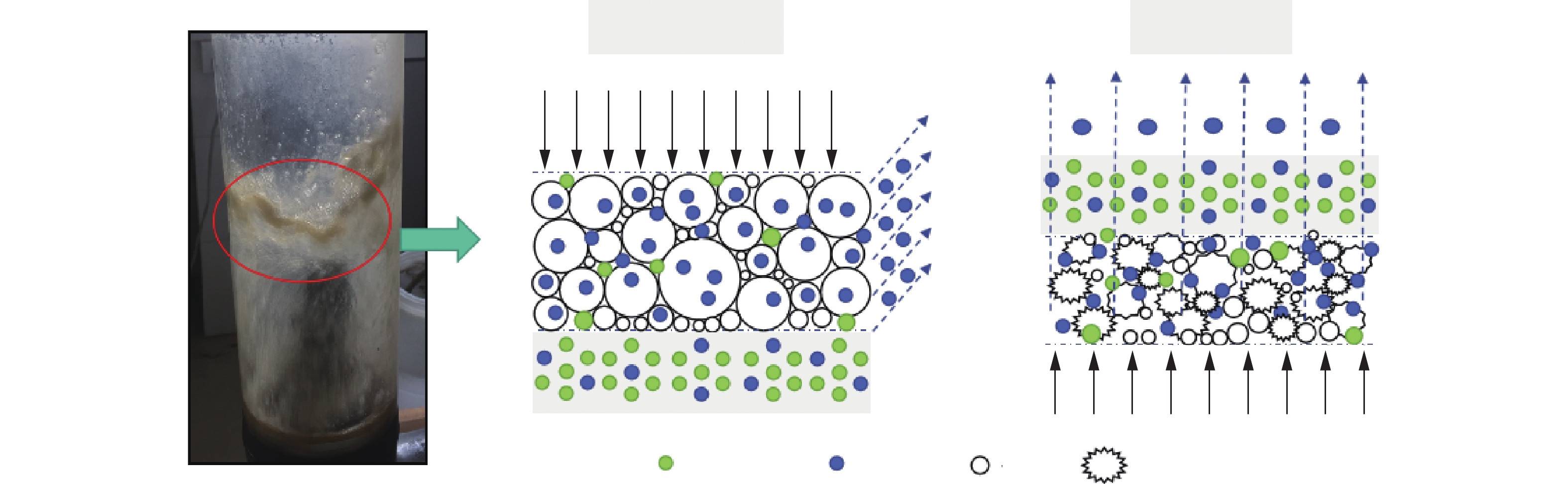
对传统吹脱和动力波吹脱进行对比分析,结果见图8。已有研究[32]表明,吹脱过程中形成气泡尺寸、上升速度和局部含气量等都会影响液相与气相之间的传质,从而响应氨分离效率。对传统吹脱而言,废水一般从上部进入,空气由底部鼓入,塔内可添加填料,最终吹脱出的气体从塔的顶部排出,从吹脱塔的底部往上,气泡的大小迅速增加,此时空气与水比表面积随气泡尺寸的增大而减小,因存在从小到大气泡的压差,当气泡到达液相表面时,它们会因为内部压差而破裂,无法进一步随气流上升排出游离氨,这也是为什么NH3在较大气泡中的传质速度较慢的原因。对于动力波吹脱而言,装置由上而下进气,液相由下往上射出,装置中特制喷头起到关键作用,喷头逆向喷射出污水与气相高速逆向对撞,当气相与液相达到动量平衡时,动力波吹脱波管中会形成一个高度湍动的泡沫区,在该区域内气液两相呈高速湍流接触,一方面泡沫层的大量气泡可增加气液两相的接触,另一方面高速流动的气液两相相互挤压使气泡破裂,使气液两相得以分离,由此在泡沫层有极高的更新频率,极大强化了气液两相间的反应,从而使动力波吹脱装置具有良好的传质和分离效率。垃圾渗滤液中存在大量的悬浮物,废水在传统吹脱池和填料塔中的流速较慢,随着时间的推移易容易停滞积累结垢,出现堵塔现象,而动力波吹脱高速流动的气液两相碰撞形成泡沫区,气液两相不断迅速更新,不易发生结垢现象。
2.1. 吹脱时间对氨氮吹脱效能的影响
2.2. pH对氨氮吹脱效能的影响
2.3. 气液比对氨氮吹脱效能的影响
2.4. 温度对氨氮吹脱效能的影响
2.5. 进水氨氮浓度对吹脱效能的影响
2.6. 正交分析
2.7. 吹脱过程氮素的变化
2.8. 动力波吹脱氨氮实验处理效率与传统吹脱法对比及机理分析
-
1)中试实验结果表明,前3 h为吹脱的高效段,故吹脱5 h比较经济合理。pH增加对吹脱有利,而pH为10.5左右时最经济。本研究采用的动力波吹脱技术适用温度范围广,即使在低温下(10 ℃)也能有较高的吹脱去除率。气液比增加能提高吹脱效率,从节省能耗的角度考虑,气液比为129比较合理;氨氮浓度对吹脱去除率的影响较小。
2)正交实验结果表明,在动力波吹脱过程中不同因素的影响不同(温度>气液比>pH),垃圾渗滤液初始氨氮浓度对吹脱效率影响不大。经正交实验优化得到最佳工艺条件为:温度25 ℃,pH=10.5,气液比129,吹脱时间5 h。此时可达较高的去除率,约91.25%~94.15%,同时也能降低投药量。
3)动力波吹脱所产生的泡沫区,气液接触界面不断迅速更新,克服了传统吹脱法受温度和结垢等多种不利因素的限制,实现了高效脱氨。
4)本研究采用的动力波吹脱技术,相较于传统的吹脱法具有占地面积小、投药量更低、不易结垢、低温也能稳定运行等明显的优势,能为老龄化填埋场设计改造提供指导和参考,具有十分广阔的应用前景。



 下载:
下载: