-
随着我国高等教育的快速发展,在校学生数量急剧扩张,原有的校园已不能满足高校教学需求。2000年后,我国大部分高校在城郊或城市临近区域新建了一大批新型校园。随着学生人数的增加,高校的新校区逐渐成为用水和污水排放大户。由于这些校园往往远离城市,园区生活污水无法就近接入城市排水管网,因此,这些校园一般自身配套规划建设了校园污水处理站,处理后的污水部分回用,剩余部分就近排入地表水体[1-2]。目前,我国对校园污水的研究主要集中在处理工艺的选择与优化和对出水水质的进一步强化[3-5],对工艺运行策略的调整与优化的研究相对较少。
与传统城镇污水处理厂不同,校园污水处理站的处理水量由于学校的寒暑假而呈现周期性变化的特征。在假期,由于园区人员大幅减少,造成进入校园污水处理站的污水水量也大幅减小,进水中的营养物质不足以维持生物反应池中的微生物正常代谢,此时生物池内微生物进行内源代谢,功能微生物将会衰减,其衰减量随进水水量及负荷的减少而加快。当假期结束后,学生又在较短时间内集中入学,造成短期内污水处理站进水水量急剧增加,处理系统又必须在短期内迅速恢复对污染物的处理能力,否则将造成出水水质超标。
针对校园污水处理面临的这一突出问题,本研究提出了低水量期间的维护策略及低水量期结束后的重新启动策略并获得了较好的效果,为校园污水的处理提供参考,并可进一步推广至旅游型城市及其他水量呈周期性变化的污水处理系统。
全文HTML
-
西安市某大学污水处理站处理规模为每天2 500 t,处理工艺为间歇式A2/O工艺,设计水力停留时间为14 h(厌氧+缺氧=4.6 h,好氧=9.4 h),(厌氧+缺氧)∶好氧=1∶2,设计污泥停留时间为15 d。污水处理站投入运营后,各项出水水质指标满足《城镇污水处理厂污染物排放标准》(GB 18918-2002)一级A标准。
A2/O工艺活性污泥中的功能微生物可分为聚磷菌、硝化菌、其他异氧菌(包括反硝化菌)3类。聚磷菌(PAOs)负责除磷,硝化菌(包括AOB和NOB)负责硝化,其他异氧菌包括反硝化菌(将硝氮还原为氮气)则氧化各类有机物。在这3类微生物中,硝化菌增殖速率最低,聚磷菌次之,其他异氧菌增殖最快[6]。因此,当污水处理站进水营养物不足时,应优先维持增殖缓慢的硝化菌的活性和数量,其次是聚磷菌。而硝化菌和聚磷菌的衰减和外界环境密切相关,现有的研究[7-8]和实际运行结果表明,两者的衰减速率在厌氧条件下最小,缺氧次之,好氧最快。基于此,将假期污水处理站的(厌氧+缺氧)∶好氧时间调整为2∶1,即对污水处理站运行周期中厌氧段进行延长,缩短好氧时间,以减小其衰减速率。
运行策略调整后,虽能减缓硝化菌和聚磷菌的衰减,但是不可避免地仍有一部分聚磷菌进行内源呼吸衰减,这些聚磷菌衰减时会释放出大量的磷,这是一种不可逆的释磷过程,即释放出的磷在好氧段无法吸收。因此,假期生物反应池出水磷浓度有可能大幅度升高。针对这一情况,可依据生物池出水磷浓度变化,增加化学辅助除磷,以保证处理系统的除磷效果。假期结束后,将运行周期的(厌氧+缺氧)∶好氧时间恢复至放假前的正常状态(1∶2)。假期前后及期间的(厌氧+缺氧)∶好氧时间的变化见表1。
-
COD、
NH+4 -N、PO3−4 -P、TP、MLSS和MLVSS的测定参照《水和废水监测分析方法(第4版)》推荐的标准方法[9]。其中NH+4 -N采用纳氏试剂分光光度法;PO3−4 -P采用钼锑抗分光光度法;TP采用5% 过硫酸钾消解法;COD采用化学滴定法;MLSS和MLVSS采用重量法。 -
活性污泥的硝化活性采用氧吸收速率法进行表征。具体测定方法如下:取1 000 mL活性污泥,经淘洗后充分曝气2 h,除去污泥中残留的有机物。曝气结束后,将其分置于2个500 mL广口瓶中搅拌并记录DO随时间的变化情况,由此得到微生物内源呼吸速率(r);然后向一个广口瓶中加入NH4Cl和NaClO3(20 mg·L−1
NH+4 -N,0.02 mol·L−1 NaClO3),并记录DO变化;向另一广口瓶中加入NaNO2(20 mg·L−1NO−2 -N),并记录DO变化。依据式(1)和式(2)计算硝化菌的活性。式中:μmax-AOB为AOB的活性,mg·(g·h)−1;μmax-NOB为NOB的活性,mg·(g·h)−1;r为微生物内源呼吸的耗氧速率,mg·h−1;r1为AOB的耗氧速率,mg·h−1;r2为NOB的耗氧速率,mg·h−1;X1为测定AOB活性时的污泥浓度,g;X2为测定NOB活性时的污泥浓度,g。
-
活性污泥中的聚磷释磷活性测定采用间歇实验法,参照常蝶等采用的方法[10]进行测定。取适量的污泥,加入乙酸和氨氮,浓度分别为50 mg·L−1(以COD计)和15 mg·L−1。设计反应总时间为5 h,其中厌氧时间为1.5 h,好氧时间为3.5 h,以保证活性污泥在厌氧阶段能够充分释磷,在好氧阶段充分吸磷。在不同的时间点进行取样,并测定对应的乙酸浓度和磷酸盐浓度。实验结束后,测定污泥的悬浮固体(SS)和挥发性悬浮固体(VSS)浓度,并绘制出磷酸盐浓度和乙酸浓度随时间的变化关系曲线,从而计算出活性污泥的最大厌氧释磷速率、最大好氧吸磷速率、最大乙酸吸收速率和吸收单位乙酸的释磷量。
-
FISH分析采用AMANN等[11-12]使用的方法。杂交实验中使用的生物探针(生工生物工程(上海)股份有限公司)的16s rRNA序列及针对的目标微生物见表2。杂交完成后利用激光共聚焦显微镜(徕卡,SP8)确定目标微生物与总微生物的百分比。杂交后的污泥样品经激光共聚焦显微镜(100倍物镜)进行观察并采集图像。每个污泥样品随机采集50张图像,利用Image-Pro Plus软件对采集的图像进行处理,统计目标微生物与总细菌的数量,以此获得相应的占比。
1.1. 污水处理站简介及假期维护策略
1.2. 常规水质分析
1.3. 硝化活性测定
1.4. 聚磷释磷活性测定
1.5. FISH分析
-
图1为该污水处理站假期前后进水流量变化情况。正常运行期间,该污水处理站的处理水量为1 200~1 400 m3·d−1。随着假期的临近,部分专业学生开始校外实习,进水量逐渐减少,在开始放假时,进水量已减至约800 m3·d−1;在进入假期后,即在第Ⅱ阶段内进水水量显著降低,由800 m3·d−1降至50~100 m3·d−1,约为第Ⅰ阶段的5%~10%;当第Ⅱ阶段结束再进入第Ⅲ阶段后,此时学生已返校,污水处理站进水水量则迅速回升,一段时间后进水水量可达1 500 m3·d−1。
-
与进水水量剧烈变化相比,假期中污水处理站进水水质与假期前相比变化较小,具体测定结果见图2。
可以看出,在第Ⅰ阶段,污水处理站日平均进水COD约为400 mg·L−1,日平均进水氨氮浓度约为70 mg·L−1,日平均进水总磷浓度约为8 mg·L−1,污水处理系统正常运行;进入假期后,即进入第Ⅱ阶段后,由于学生离校,进水COD由400 mg·L−1降至约300 mg·L−1,氨氮由70 mg·L−1降至约40 mg·L−1,总磷降至约4 mg·L−1。在进入第Ⅱ阶段后,污水处理站改变运行策略。在第Ⅱ阶段初期的出水COD和氨氮均可稳定达标,此时出水总磷浓度开始升高;在进入第Ⅱ阶段后期,此时出水COD值逐渐升高,这是由于进水碳源不足导致细胞内源呼吸产生了一定量的代谢产物,而这一部分代谢产物不能再次被微生物所利用转化,成为无法被生物降解的耗氧有机物(以COD计),导致出水COD有所升高;但同期出水氨氮依然稳定达标;同时由于聚磷菌的衰减,导致反应池内发生了不可逆的释磷过程,反应池出水总磷浓度迅速升高,最高可达14 mg·L−1。针对这一现象,在后续处理中,加入化学除磷药剂(PAC),保证出水总磷能达到标准。进入第Ⅲ阶段后,污水处理站进水逐渐恢复正常,其中日平均进水COD已恢复至400 mg·L−1,日平均进水氨氮浓度也逐渐恢复至50 mg·L−1以上,日平均进水总磷浓度也恢复至8 mg·L−1。反应池出水COD和氨氮依然保持稳定达标,出水总磷于第90天后可恢复至达标水准。
-
污水处理站反应池假期前后污泥浓度的变化情况如图3所示。由图3可见,在第Ⅰ阶段,污水处理站依然保持较为稳定的进水水量,污泥浓度稳定维持在4 500~5 000 mg·L−1。当进入假期后,即污水处理站运行状态进入第Ⅱ阶段时,结合图1和图2可知,无论进水水量还是进水水质相较正常运行状态下均有显著下降,此时调整污水处理站的运行策略至假期维护策略,即以厌氧运行为主,适时曝气以保证出水达标。厌氧运行期间,反应池内的活性污泥进行内源呼吸(即衰减),随着内源呼吸的持续进行,反应池内污泥浓度开始逐渐降低,由正常水平的4 500 mg·L−1持续降低至约3 200 mg·L−1后,维持稳定,污泥浓度降低约1/3。在进入第Ⅲ阶段后,由于进水水量的恢复,基质逐渐增多,活性污泥可以进行正常的能量摄取,以代替内源呼吸,同时系统关闭排泥,此时污泥浓度开始升高,经一段时间后,污泥浓度恢复至4 600 mg·L−1,与第Ⅰ阶段污泥浓度近似相同。
对假期前后污水处理站反应池中活性污泥的硝化活性进行测定,结果见图4。由图4可知:在第Ⅰ阶段内,AOB活性保持在1.5~2.0 mg·(g·h)−1,NOB活性则为0.75 mg·(g·h)−1,AOB活性约为NOB活性的2倍;进入第Ⅱ阶段后,由于进水水量的减少,污水处理站运行策略改变,硝化菌进入厌氧衰减阶段,AOB和NOB活性均开始逐渐降低,AOB活性降至0.55 mg·(g·h)−1,活性仅为策略调整前的36%,NOB活性降至0.5 mg·(g·h)−1,为策略调整前的66%。AOB活性降低较NOB更为显著,这说明在厌氧衰减期内AOB的衰减速率相较NOB更大。在进入第Ⅲ阶段后,结束假期策略,AOB活性增长较快,约14 d后即恢复至2.0 mg·(g·h)−1,已达到第Ⅰ阶段的活性水平,NOB活性则由0.5 mg·(g·h)−1升至1.2 mg·(g·h)−1。
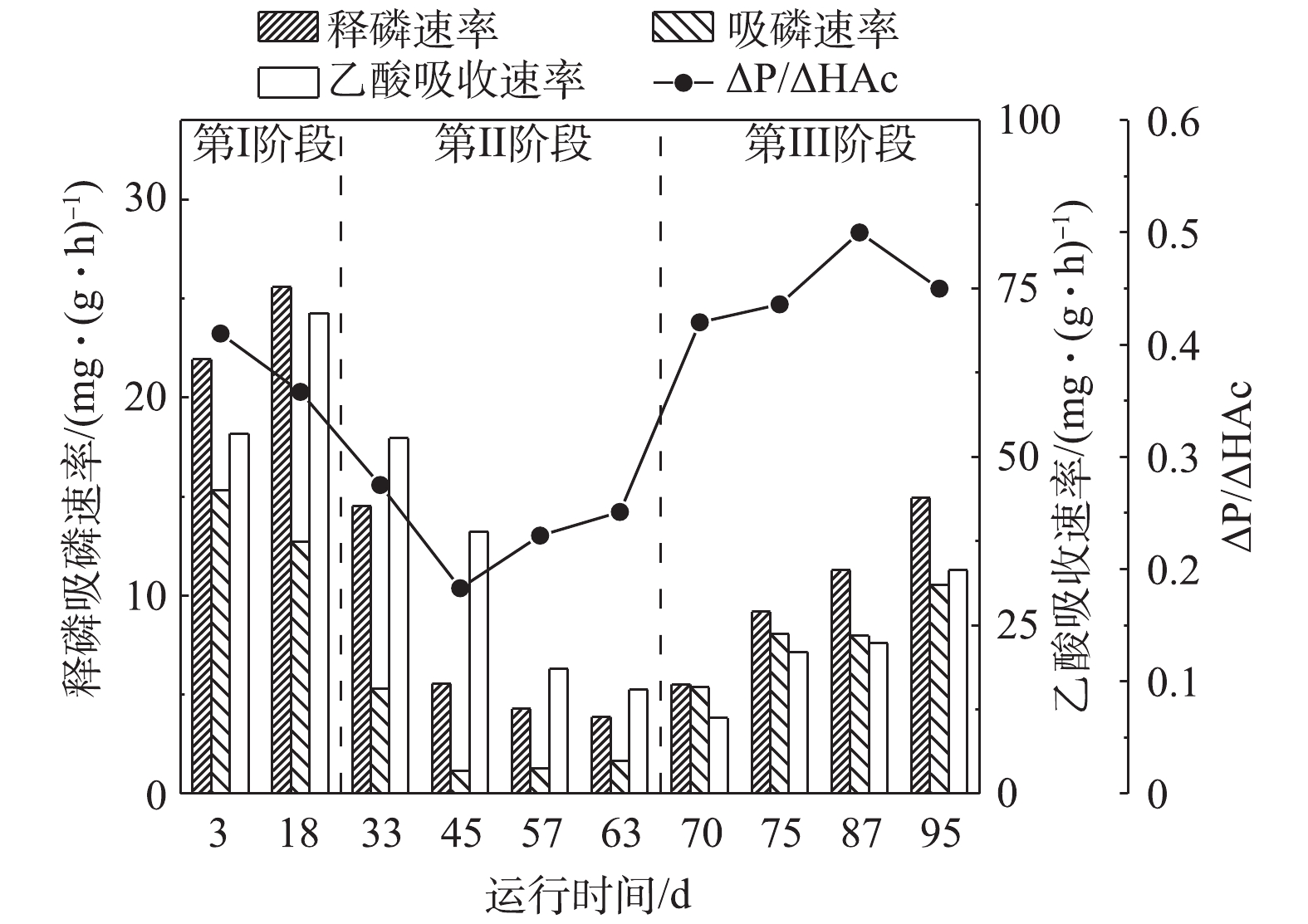
图5为假期前后活性污泥释磷速率、吸磷速率、乙酸吸收速率及ΔP/ΔHAc的变化情况。由图5可知,在第Ⅰ阶段内,处理系统稳定运行,活性污泥的释磷、吸磷活性及乙酸利用速率均维持在较高的水平,释磷速率可达26 mg·(g·h)−1,吸磷速率可维持在15 mg·(g·h)−1。进入第Ⅱ阶段后,调整污水处理站运行策略,此时污泥聚磷释磷活性逐渐降低,释磷速率由18 mg·(g·h)−1逐渐降至5 mg·(g·h)−1,吸磷速率则由12 mg·(g·h)−1降至2 mg·(g·h)−1。在这一阶段中,活性污泥由于基质的不足逐渐转为内源呼吸阶段,此时聚磷菌衰减,从而导致细胞受损造成不可逆的释磷过程,造成出水磷浓度较高,此时应辅以化学除磷以保证出水达标。此期间释磷活性仅为第Ⅰ阶段的23%,吸磷活性为第Ⅰ阶段的13.3%。此外,乙酸吸收速率降至15 mg·(g·h)−1,降低了77%,表征聚磷菌吸收单位乙酸的释磷量的ΔP/ΔHAc值也随释磷活性的降低而降低,由0.4降至0.2。而进入第Ⅲ阶段后,结束假期运行策略,实行重启策略,此时活性污泥的释磷吸磷活性逐渐开始恢复。释磷速率由5 mg·(g·h)−1逐渐回升至15 mg·(g·h)−1,吸磷速率则由2 mg·(g·h)−1升至10 mg·(g·h)−1,乙酸吸收速率升至30 mg·(g·h)−1,ΔP/ΔHAc上升至0.45。
在进行微生物种群结构对比时,取污水处理站第I、II、III阶段污泥样品进行预处理,其中在第Ⅱ阶段的前期和后期分别取样。经预处理后进行荧光原位杂交,通过激光共聚焦显微镜进行拍摄,聚磷菌FISH杂交结果见图6。通过对杂交图像进行统计分析,得到目标菌占总菌(以EUBmix表示)的占比,从而获得活性污泥中PAOs和GAOs份额在各个阶段的占比并展现其历时变化,结果见表3。
由图6和表3的结果可知,污泥中的PAOs以菌胶团的形式存在,且分布较多,而GAOs总体占比很低。在第Ⅰ阶段,污泥中PAOs占总菌份额的22.15%,而GAOs的份额仅占2.21%;在第Ⅱ阶段的前期(40 d),污泥中PAOs所占份额相较第Ⅰ阶段有所下降,为总菌份额的17.89%,而GAOs占比则为总菌份额的 1.37%,又经过25 d后,在第Ⅱ阶段后期(65 d),PAOs所占份额进一步下降至 12.24%;进入第Ⅲ阶段后,污泥中PAOs占比相较第Ⅱ阶段有所回升,占总菌份额的15.60%。结合图5可知,微生物种群数量的变化与活性的变化相一致,即实行假期策略过程中活性污泥中的聚磷菌处于衰减状态。
取污水处理站第I、II、III阶段的活性污泥进行预处理,进行荧光原位杂交,通过激光共聚焦显微镜进行拍摄,硝化菌杂交结果见图7。通过对杂交图像进行统计分析,得到硝化菌占总菌(以EUBmix表示)的百分比,从而获得活性污泥中AOBs和NOBs份额的历时变化,结果见表4。由图7和表4的结果可得出,污泥中的AOBs相对较高而NOBs份额始终较低。在第Ⅰ阶段,AOB占总菌份额的4.56%,而NOB的份额仅占1.75%;在进入第Ⅱ阶段后,污泥中AOB占总菌份额略微下降至3.24%,而NOB则未在此次检测中检出,相隔25 d后再次测定污泥中AOB占比,略微下降至 3.02%,NOB则占 1.21%;进入第Ⅲ阶段后,AOB占比为4.33%,NOB则为2.07%。由图4可知,在第Ⅱ阶段期间,AOB的活性基本维持不变,与同时期AOB占比相对应,而进入第Ⅲ阶段后,AOB活性有显著上升趋势,并已达到第Ⅰ阶段的活性,此时AOB占比也已基本恢复至第Ⅰ阶段同一水平;NOB活性相较于第Ⅰ阶段及第Ⅱ阶段均有提高。结合种群结构变化及活性变化,可以看出,在第Ⅱ阶段,内源呼吸阶段AOB和NOB都在进行衰减,但NOB的衰减量较AOB更小,而硝化菌总体衰减量显著低于聚磷菌。
-
校园污水的显著特征是水质水量变化巨大,同时在假期结束后要求校园污水处理系统在短时间内快速恢复其处理能力。针对该特征重新接种污泥往往是一种较为简单易行的方案。而本研究考虑调整污水处理系统在假期期间的运行方式,以降低活性污泥中功能菌的衰减速率来达到保存功能微生物的目的,使污水处理站在经历低水量运行期后可迅速恢复处理能力。同时,可针对不同季节可作出以下推论:在夏季假期,由于水温高,功能菌衰减较快,且衰减量大,但在进入恢复期时,水温依然保持较高,因此恢复得也快;而在冬季假期,恰好相反,低水温条件下会有较低的衰减速率及较慢的恢复时间;2种情况下总体恢复的时间大致相同。综合污泥浓度变化及各功能菌活性变化,计算出在厌氧运行策略期间各种功能菌的衰减速率及其活性变化,计算结果见表5。
活性污泥系统中功能微生物的衰减一直都是环境工程领域内研究的一个重点,在国际水协会(IWA)提出的ASM系列模型中对各种功能微生物的衰减速率进行了测定,给出了参考数值,同时一些研究学者也对具体的功能微生物的衰减速率进行了研究,综合各种衰减速率与本研究中的衰减速率进行对比,结果见表6。由表6可知,相较于LOPEZ等[8]的研究,本研究中PAO在厌氧条件下衰减速率为0.1 d−1,而AOB的衰减速率基本与SALEM等[18]的研究一致,为0.06 d−1,NOB衰减速率仅为0.03 d−1。因此,结合表5中对应活性的降低可知,本研究在假期中采用的延长污水处理系统厌氧+缺氧时间的策略可以较为有效地将活性污泥的衰减速率控制在较低的水平下,这有利于假期中功能微生物的保存及假期结束后污水处理系统处理能力的恢复。
此外,目前已知的针对假期后污水处理站污泥活性恢复的手段大多为投加污泥(属于生物添加技术),以加快恢复过程。本研究通过调整运行策略使污水处理系统在无需投加污泥的条件下即可恢复活性。假期的厌氧运行策略可使硝化菌维持在较低的衰减速率下,更利于污水处理系统在重新启动阶段快速恢复活性,较杜兴治等[20]的研究能更为快速地恢复系统的处理效果,同时较投加污泥的方法更为经济,且能更好地维持活性污泥中的生物群落。
2.1. 假期前后污水处理站水量的变化
2.2. 假期前后进出水水质
2.3. 污泥浓度及活性的变化
2.4. 关于衰减速率的讨论
-
1)校园污水处理站假期中较正常运行时水量变化较为剧烈,假期水量仅为正常运行期间水量的5%~10%。
2)污水处理站在低水量条件下运行时,污染物负荷较低,可调整运行策略,使活性污泥处于厌氧衰减状态,以维持功能微生物的活性及数量,此时污泥浓度由4 500 mg·L−1下降至3 200 mg·L−1,污泥的释磷吸磷活性降至5 mg·(g·h)−1和2 mg·(g·h)−1,分别降低了77%和13.3%;AOB活性降至0.55 mg·(g·h)−1,NOB活性降至约0.5 mg·(g·h)−1,分别降低了64%和34%,荧光原位分析结果与活性变化相一致。
3)实行假期运行策略期间,活性污泥聚磷释磷活性降低较为显著,AOB活性和NOB活性变化相对较小。通过计算其衰减速率,实行假期策略,即调整厌氧+缺氧时间与好氧时间的比值可以使硝化菌的衰减速率维持在较低的水平,当假期结束后,污水处理站进水水量逐渐恢复,活性污泥的硝化活性可在较短时间内恢复至放假前的水平。





 下载:
下载: