-
三氯乙烯(TCE)作为氯代烃的一种,具有水溶性差、易吸附于土壤以及化学结构稳定等特点,因此,其很难从低渗透土壤介质中被去除。长期存在于土壤介质中的TCE作为污染源,会持续污染土壤和地下水,并严重威胁着人类健康[1-2]。近年来,表面活性剂由于对氯代烃污染物具有增溶作用,故其受到了越来越多的关注。表面活性剂可分为阳离子型表面活性剂、阴离子型表面活性剂和非离子型表面活性剂。大量研究[3-6]表明,阳离子型表面活性剂较容易吸附到土壤颗粒上,而阴离子型表面活性剂容易与土壤中的阳离子共沉淀,相比于阳离子型和阴离子型表面活性剂,非离子型表面活性剂由于其临界胶束浓度低,具有更好的增溶能力和经济效益。作为非离子型表面活性剂,吐温-80(Tween-80)具有非离子型表面活性剂的许多优点,如水溶性好、稳定性高、受电解质和酸碱等外部因素影响较小等。此外,与其他非离子型表面活性剂相比,Tween-80具有成本低、对土壤和地下水中微生物的毒性弱等优点[7-8]。因此,Tween-80已广泛应用于土壤和地下水中氯代烃污染物的增溶过程中。然而,为了防止二次污染,Tween-80增溶后的TCE溶液需要进一步处理,但含有表面活性剂的TCE会被Tween-80形成的胶束包裹,从而影响TCE的处理效果[9]。因此,如何高效去除含表面活性剂Tween-80的水相中的TCE是当前亟待解决的一个技术难题。
高级氧化技术(AOPs)因具有高效快速的特性,常被用于处理含有表面活性剂水溶液中的污染物[10-11]。在高级氧化技术中,常用的氧化剂主要有臭氧[12]、过氧化氢[13-14]、过硫酸盐[15-16]和过碳酸钠[17-18]等。过碳酸钠(Na2CO3·1.5H2O2,SPC)被称为固体过氧化氢(H2O2),是一种实用价值较高的绿色氧化剂。作为H2O2的载体,SPC与水混合时可以释放出H2O2[19]。其分解产物通常为水、二氧化碳和碳酸钠,故其具有无毒性及无二次污染的优势。与传统的H2O2相比,SPC具有价格低廉、操作安全、方便储存运输和适用pH范围广等优点[20]。与过硫酸盐相比,SPC可以通过引入碳酸根,以保证水环境pH不致过低,进而降低氧化过程酸性条件对水环境中微生物的影响[21]。基于上述优点,近年来SPC在污染场地修复中受到了越来越多的关注[22-23]。
与传统Fenton反应[24]相似,Fe(Ⅱ)可以活化SPC用于降解各种污染物。然而,该体系反应速度过快,Fe(Ⅱ)迅速转化为Fe(Ⅲ),不能持续降解污染物,制约了其在实际中的应用。纳米零价铁(nZVI)作为一种环境友好型催化材料,与普通铁粉相比,具有更强的还原能力和更好的迁移能力,近年来常用于污染场地修复[25-26]。但是该过程耗时长、成本高。采用nZVI活化SPC需要降低溶液的pH,因为只有在酸性条件下nZVI表面才被腐蚀且释放出Fe(Ⅱ)。
迄今为止,将nZVI与Fe(Ⅱ)协同活化SPC应用于降解有机污染物(含表面活性剂)的研究尚鲜有报道。本研究基于Fe(Ⅱ)与SPC释放的H2O2反应产生强氧化性的羟基自由基(·OH)的过程中,也同时降低溶液pH的特点,通过nZVI自身腐蚀逐步释放Fe(Ⅱ),及时地将体系中产生的Fe(III)转化为Fe(Ⅱ),以期达到持续强化的协同效果。nZVI协同Fe(Ⅱ)活化SPC体系既能克服单独的Fe(Ⅱ)活化SPC时不能持续高效降解TCE的缺点,也能克服单独的nZVI不能活化SPC的不足,具有较高的潜在应用价值。鉴于此,本研究以含Tween-80的水相中TCE为研究对象,SPC/Fe(Ⅱ)/nZVI为反应体系,主要探究了在Tween-80存在下该反应体系降解TCE的有效性;分别考察了Tween-80浓度、溶液初始pH以及无机阴离子对TCE降解效果的影响;最后考察了该体系中活性氧自由基的类型及其对TCE降解的作用机制。
-
试剂:SPC购于Acros Organics上海公司;TCE、七水合硫酸亚铁(FeSO4·7H2O)、硫氰酸铵和硝酸钴购于阿拉丁试剂(上海)有限公司;Tween-80、正己烷、氢氧化钠(NaOH)、碳酸氢钠(NaHCO3)和氯化钠(NaCl)购于上海泰坦科技股份有限公司;硝基苯(NB)、四氯化碳(CT)、异丙醇(IPA)、氯仿(CF)和硫酸(H2SO4)购于上海凌峰化学试剂有限公司。以上试剂均为分析纯,直接使用。纳米零价铁(纯度>99%,平均粒径50 nm)购于上海超威纳米科技有限公司。所有实验用水均为超纯水。
实验仪器:Agilent 7890A型气相色谱仪(安捷伦科技有限公司);LC-20AT型高效液相色谱仪(日本岛津公司);85-2型恒温磁力搅拌器(上海闵行虹浦仪器厂);SDC-6型低温恒温槽(宁波新芝生物科技股份有限公司);DR-6000型紫外分光光度计(哈希水质分析仪器有限公司);P8-10型pH测定仪(德国Sartorius公司);XW-80A型涡旋振荡器(上海青浦沪西仪器厂);Classic UV MK2型超纯水机(英国ELGA公司);AL204型电子分析天平(瑞士Metter-Toledo集团);SK2200LH型超声波清洗器(上海科导超声仪器有限公司)。
-
首先用超纯水配制TCE和Tween-80混合母液,之后将母液稀释至所需浓度(TCE=0.15 mmol·L−1,Tween-80=13 mg·L−1),置于带夹层的实验反应器中(有效体积250 mL,内径6 cm,高度9 cm),采用低温恒温槽使反应温度控制在20 ℃。为确保反应溶液混合均匀,将反应器置于磁力搅拌器上(转速为600 r·min−1)。实验过程中依次加入所需量的FeSO4·7H2O、nZVI和SPC,开始反应计时,在即定时间点取样,并与正己烷快速混合,以终止反应,样品通过气相色谱仪分析。除考察溶液初始pH的影响外,其他实验均不调整溶液pH。所有实验设2个平行样,结果取平均值。
-
溶液中TCE和CT浓度均采用气相色谱仪分析测定。其中TCE分析条件:电子俘获检测器(ECD),自动进样器(Agilent 7693),DB-VRX色谱柱(长60 m、内径250 μm、膜厚1.4 μm),进样口和检测器温度分别为240 ℃和260 ℃,色谱柱温度为75 ℃,载气为氮气,进样量为1.0 µL,分流比20∶1。在分析CT时,除色谱柱温度为100 ℃外,其余条件与TCE一致。
溶液中NB浓度采用高效液相色谱仪分析测定。分析条件:紫外可见光检测器(UV),C18反相色谱柱(250 mm×4.6 mm,5 µm),流动相由40%超纯水和60%色谱级甲醇组成,进样量为10 µL,检测器温度为35 ℃,流量为1.0 mL·min−1。
溶液中Tween-80浓度采用紫外分光光度计(波长623 nm,显色剂(NH4)2Co(SCN)4)测定[27]。
-
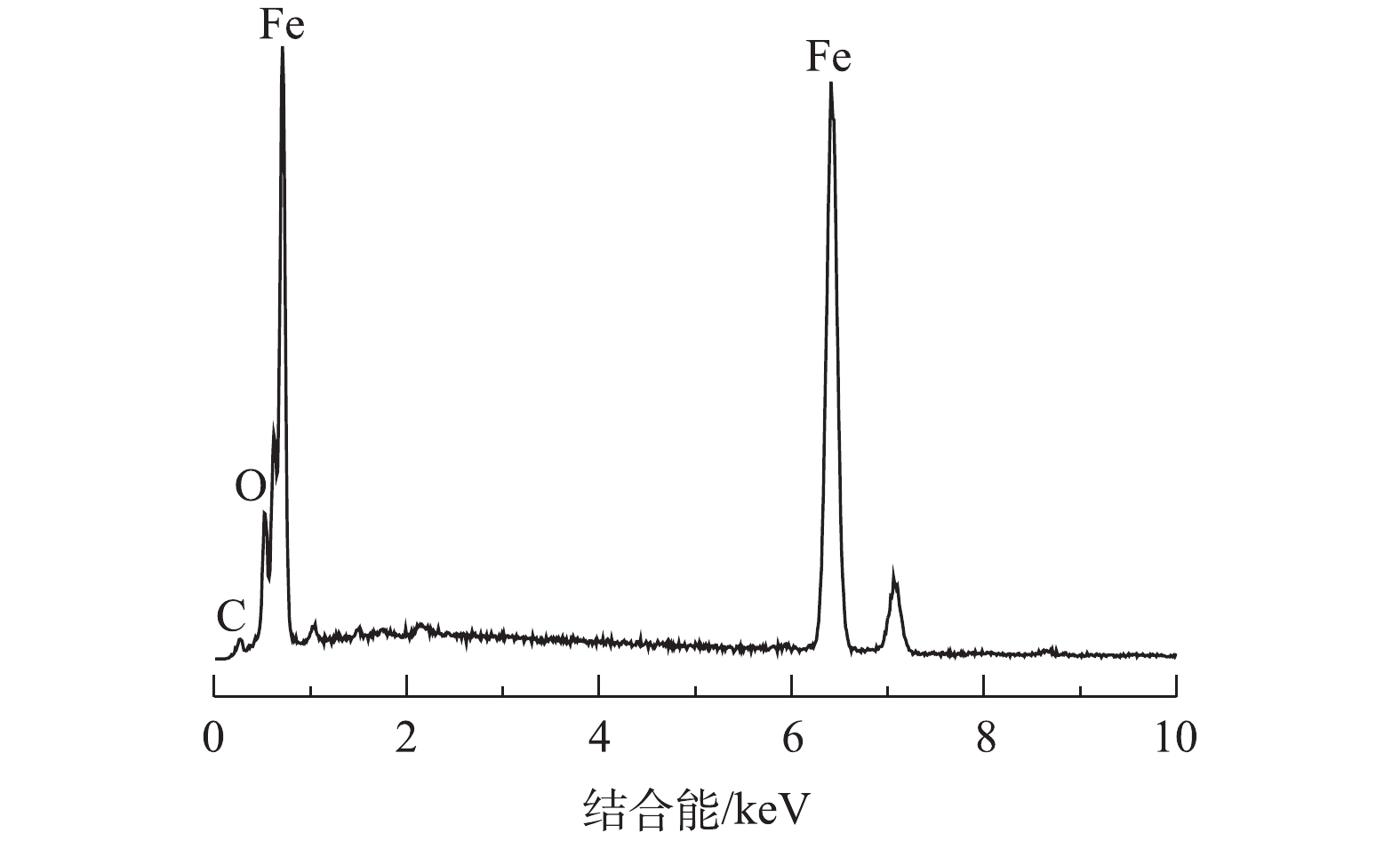
nZVI的TEM结果如图1所示,单一的nZVI颗粒具有典型的核壳结构,内部为新鲜的纳米零价铁,外部为氧化物薄膜,颗粒的平均粒径为50~100 nm。颗粒与颗粒之间有团聚现象,呈球形链状排列聚集在一起,与已有研究报道的典型纳米零价铁的形貌特征[28]一致。nZVI的XRD特征图谱如图2所示。与JCPDS粉末衍射标准卡数据[JCPDS 06-0696]比对后可以看出,在2θ=44.6°处有很强的α-Fe0特征衍射峰。为了分析nZVI材料的化学元素组成,本研究对nZVI进行了EDS分析,结果如图3所示。EDS分析显示,nZVI材料中C、O和Fe元素的质量分数分别为1.21%、2.48%和92.59%,表明在nZVI表面形成了铁氧化物,这与nZVI的TEM分析得出的铁的氧化物外壳组成结果相符合。
-
图4为TCE分别在nZVI、SPC/nZVI、Fe(Ⅱ)/nZVI、SPC/Fe(Ⅱ)和SPC/Fe(Ⅱ)/nZVI等体系中的降解效果。其中,SPC、Fe(Ⅱ)和TCE初始浓度分别为0.6、0.6和0.15 mmol·L−1,nZVI和Tween-80初始浓度分别为25 mg·L−1和13 mg·L−1。空白组的实验结果表明,当体系中未投加任何药剂时,60 min内,因挥发损失的TCE量小于5%,可以忽略不计。虽然有研究[29]报道,nZVI可以通过还原作用有效去除TCE,但是通常需要较大的nZVI投加量和较长的反应时间,且在单独投加nZVI时,TCE在60 min内的去除率仅为8.5%,因此,在本研究中少量的nZVI在短时间内无法有效去除TCE。在Fe(Ⅱ)/nZVI体系中,TCE的去除率也仅有7.7%,这与单独投加nZVI时所得到的结果相似。在SPC/nZVI体系中,TCE的去除率为8.3%,这说明单独的nZVI不能有效活化SPC。这是因为在SPC/nZVI体系中,反应过程中溶液的pH始终维持在10左右,在此条件下,nZVI难以腐蚀释放Fe(Ⅱ),进而阻碍了活性氧自由基的产生,且少部分腐蚀产生的Fe(Ⅱ)也会因为pH升高导致Fe(Ⅱ)沉淀,从而失去催化活性。而在SPC/Fe(Ⅱ)体系中,虽然TCE的降解率达到64.5%,但反应过于迅速,在3 min时,反应即终止,不能持续高效降解TCE。ZANG等[30-31]探究了SPC/Fe(Ⅱ)体系对水溶液中以及泥浆系统中TCE的降解效果,结果显示TCE最终去除率均可达到95%以上,然而SPC/Fe(Ⅱ)体系的反应过于迅速,导致SPC和Fe(Ⅱ)的利用率降低,不能持续降解污染物。臧学轲[32]还探究了羟胺促进柠檬酸-Fe(Ⅱ)活化过碳酸钠降解TCE,发现盐酸羟胺能有效地将Fe(Ⅲ)还原为Fe(Ⅱ),强化降解TCE,然而该体系中加入的药剂种类较多,且加入盐酸羟胺以及柠檬酸会导致地下水中化学需氧量的升高,从而容易导致二次污染和修复成本的增高。而在SPC/Fe(Ⅱ)/nZVI体系中,3 min时TCE的降解率为73.9%,60 min时TCE基本完全去除(97.3%),既克服了单独的Fe(Ⅱ)活化SPC时不能持续高效降解TCE的缺点,也克服了单独的nZVI不能活化SPC的不足,表明Fe(Ⅱ)与nZVI起到了良好的协同活化SPC的作用,达到了预期的目的。
nZVI和Fe(Ⅱ)协同活化SPC能够持续高效降解TCE。这主要是因为:在该体系中,Fe(Ⅱ)先活化SPC释放的H2O2,产生羟基自由基(·OH)(如式(1)和式(2)所示),TCE被快速氧化降解;同时Fe(Ⅱ)会被快速氧化成Fe(Ⅲ),投加的Fe(Ⅱ)被迅速消耗完,经检测,反应3 min时Fe(Ⅱ)的量与最初投加的0.6 mmol·L−1的Fe(Ⅱ)量相比较,Fe(Ⅱ)量迅速下降为0.03 mmol·L−1。而此后TCE仍继续缓慢降解,一方面这是由于Fe(Ⅱ)活化SPC降解TCE后溶液的pH降低(pH由5.20降为3.74),促使nZVI腐蚀释放Fe(Ⅱ)[33](如式(3)所示),在nZVI表面产生活性氧自由基,TCE被进一步缓慢降解;另一方面,nZVI也可以将体系中产生的Fe(III)部分还原为Fe(Ⅱ)(如式(4)所示)。综上可知,nZVI和Fe(Ⅱ)可以协同活化SPC降解TCE(含Tween-80)。
-
为了探究Tween-80浓度对TCE降解效果的影响,固定SPC、Fe(Ⅱ)、nZVI和TCE的初始浓度分别为0.6 mmol·L−1、0.6 mmol·L−1、25 mg·L−1和0.15 mmol·L−1,分别投加不同浓度的Tween-80(0、7、13、130、260 mg·L−1),结果如图5所示。在SPC/Fe(Ⅱ)/nZVI体系中,TCE的降解率分别为98.6%、98.5%、97.3%、50.2%和19.4%。这表明随着Tween-80浓度的增加,TCE的降解率逐渐降低,Tween-80的加入会抑制TCE的降解,Tween-80浓度越高,抑制效果越明显。这是因为当表面活性剂Tween-80达到临界胶束浓度(CMC,13 mg·L−1)时,Tween-80分子会形成胶束,将TCE包裹在内,由于该体系中产生的·OH不具有选择性,·OH只有先破坏包覆TCE的Tween-80胶束后[34],才能与TCE进行反应。综上可知,SPC/Fe(Ⅱ)/nZVI体系中过多的Tween-80会与TCE竞争·OH,进而不利于TCE的降解。
为了验证Tween-80是否会在反应过程中与TCE竞争·OH,表1呈现了初始浓度为260 mg·L−1的Tween-80在SPC/Fe(Ⅱ)/nZVI和SPC/Fe(Ⅱ)/nZVI/TCE 2体系中浓度的变化结果。结果表明,在60 min内,2个体系中Tween-80的浓度分别下降至205 mg·L−1和199 mg·L−1,下降幅度基本相同,说明在SPC/Fe(Ⅱ)/nZVI体系中(无论有无TCE),Tween-80均会被部分去除。该结果进一步证实了Tween-80可以与·OH发生反应,Tween-80和TCE在反应过程中均会被降解,因此,Tween-80的存在会影响TCE的去除。
-
目前已经有许多研究证实,在Fenton反应过程中,起主要作用的是·OH,而
O−2 ·也参与部分反应[35]。为了考察在Tween-80存在的情况下SPC/Fe(Ⅱ)/nZVI体系中活性氧自由基的类型,实验中选择NB和CT分别作为·OH和O−2 ·的化学探针[36-37],研究在不同Tween-80浓度(0、13、130 mg·L−1)下探针化合物的降解情况。在本次实验中,SPC、Fe(Ⅱ)、nZVI的投加量分别为0.6 mmol·L−1、0.6 mmol·L−1、25 mg·L−1,NB和CT的浓度均为0.15 mmol·L−1。由图6(a)可知,因NB挥发产生的损失较小(< 5%)。当Tween-80的浓度从0 mg·L−1分别增加到13 mg·L−1和130 mg·L−1时,在60 min时NB的降解率由42.2%分别降低至39.2%和19.3%。这说明在SPC/Fe(Ⅱ)/nZVI体系中产生了·OH,但Tween-80的存在会消耗部分·OH,Tween-80浓度越高,消耗的·OH越多。由图6(b)可知,因CT挥发产生的损失也比较小(< 4%),不同Tween-80浓度下CT的降解率分别为12.7%(0 mg·L−1)、11.8%(13 mg·L−1)、13.1%(130 mg·L−1),此结果说明在Tween-80存在下,该体系中也产生了O−2 ·。化学探针实验已经证实了SPC/Fe(Ⅱ)/nZVI体系(Tween-80存在的情况下)中·OH和
O−2 ·的存在。为了进一步确定该体系中TCE降解起主导作用的自由基,实验选用IPA和CF分别作为·OH和O−2 ·的淬灭剂[38]。在本次实验中,SPC、Fe(Ⅱ)、nZVI、TCE和Tween-80的投加量分别为0.6 mmol·L−1、0.6 mmol·L−1、25 mg·L−1、0.15 mmol·L−1和13 mg·L−1,淬灭剂IPA和CF的浓度均为50 mmol·L−1,在设定时间点测定TCE的降解率,结果如图7所示。当反应体系中没有淬灭剂时,在60 min时TCE的降解率为97.3%。加入过量IPA后,TCE降解率仅有14.4%,这说明·OH在TCE降解过程中起主导作用;加入过量CF后,TCE的降解率降低到58.3%,与IPA相比,CF对TCE降解的影响较小,O−2 ·主要是将Fe(Ⅲ)还原为Fe(Ⅱ)[39](如式(5)所示),进而产生更多的·OH,间接促进溶液中TCE的去除。综上可知,在SPC/Fe(Ⅱ)/nZVI体系(Tween-80存在的情况下)中,·OH和O−2 ·对TCE的降解均起到一定的作用,其中起主导作用的是·OH,但也不能忽视O−2 ·的作用。 -
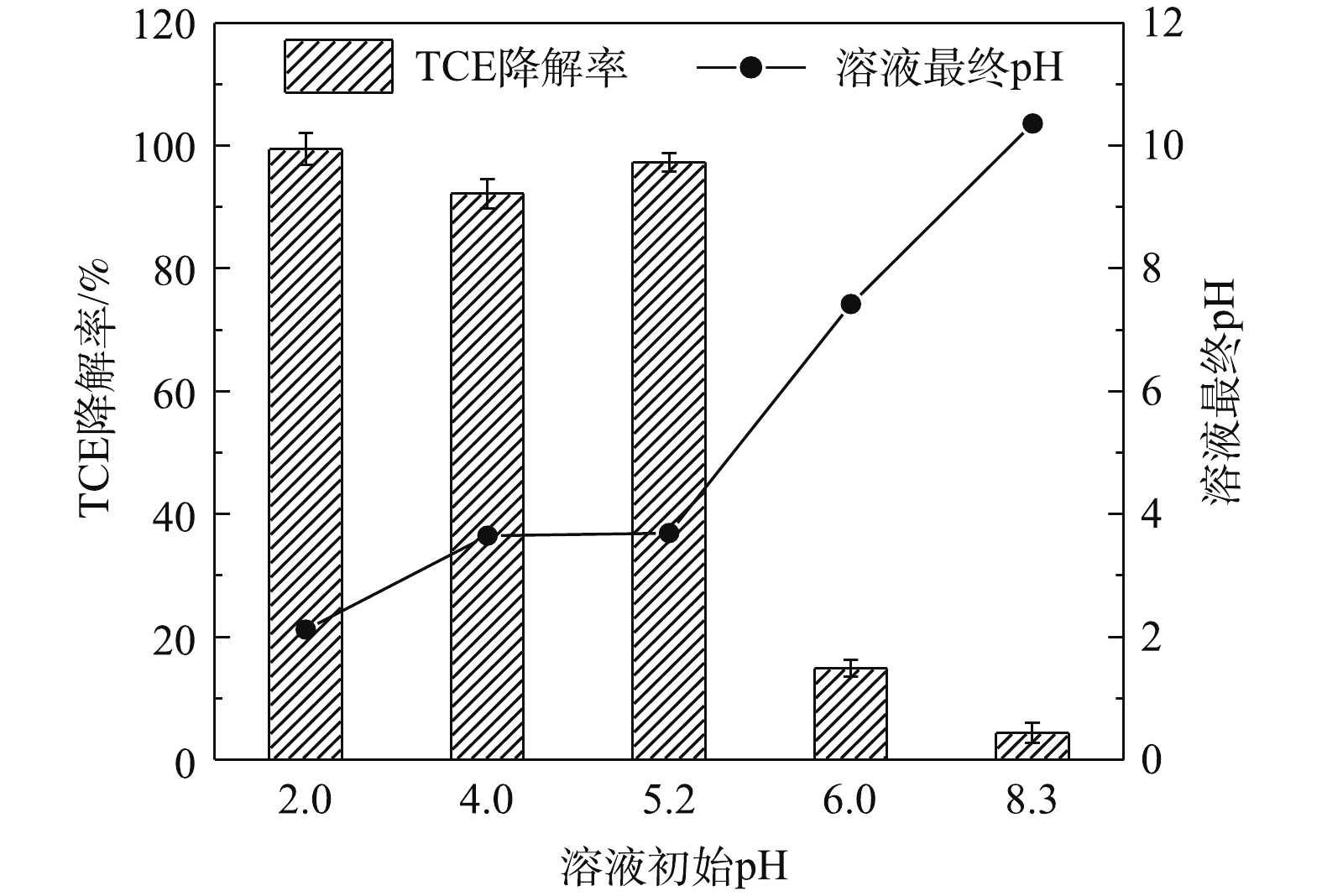
为探究在Tween-80存在下溶液初始pH对SPC/Fe(Ⅱ)/nZVI体系降解TCE的影响,实验选用0.1 mol·L−1的NaOH和H2SO4溶液将溶液初始pH调整为2.0、4.0、5.20(未调整)、6.0和8.3。在本次实验中,SPC、Fe(Ⅱ)、nZVI、TCE、Tween-80的投加量分别为0.6 mmol·L−1、0.6 mmol·L−1、25 mg·L−1、0.15 mmol·L−1、13 mg·L−1。由图8可知,反应溶液在未调整时的初始pH为5.20,60 min时溶液的pH为3.69,TCE的降解率为97.3%。用H2SO4调整溶液初始pH至2.0和4.0时,对TCE的降解效果影响比较小,且反应后溶液的最终pH分别为2.12和3.65。用NaOH调整初始pH至6.0和8.3时,体系中TCE的降解率有明显的下降,60 min时TCE的降解率分别为14.9%和4.4%,最终溶液pH分别升高至7.42和10.36,以上结果表明弱酸性或碱性条件不利于该体系中TCE的去除。由于溶液pH对Fenton反应的影响很大,通常取最佳pH为3左右[40],在此条件下·OH的产生量最高。溶液初始pH的提高,会导致Fe(Ⅱ)沉淀,减少了Fe(Ⅱ)的有效浓度,影响体系中·OH的产生。而溶液初始pH为2.0、4.0和5.2时,其相应的终点pH分别为2.12、3.65和3.69,这说明在溶液初始pH为2.0~5.2时,不存在由于反应过程中pH升高导致Fe(Ⅱ)沉淀而失去催化活性的情况。但是,在碱性条件下,nZVI不易转化为Fe(Ⅱ),·OH的氧化能力也比较弱[41],因此,TCE的降解率明显下降。综上可知,在Tween-80存在下,溶液初始pH为2.0~5.2时,SPC/Fe(Ⅱ)/nZVI体系可有效去除TCE。
-
为探究在Tween-80存在下无机阴离子对SPC/Fe(Ⅱ)/nZVI体系降解TCE的影响,实验选用水中最常见的Cl−和
HCO−3 作为阴离子的代表。在本次实验中,SPC、Fe(Ⅱ)、nZVI、TCE、Tween-80的投加量分别为0.6 mmol·L−1、0.6 mmol·L−1、25 mg·L−1、0.15 mmol·L−1、13 mg·L−1,投加不同浓度的NaCl和NaHCO3(0、1、10、100 mmol·L−1)。如图9所示,当Cl−浓度为0、1和10 mmol·L−1时,该体系中TCE在60 min内的降解率分别为97.3%、92.2%、92.7%,这说明低浓度的Cl−对TCE的降解影响并不明显。然而当Cl−浓度增至100 mmol·L−1时,TCE的降解率可降低至86.5%,这是因为Cl−可以淬灭体系中产生的·OH[42],生成比·OH活性低的自由基(如式(6)~式(8)所示),不利于TCE的降解;此外,由于Fe(Ⅱ)与Cl−络合降低了活性,因此,减少了体系中·OH的产生,降低TCE的去除率[43]。与Cl−相比,HCO−3 (浓度相同时)对TCE的抑制作用更明显。未加HCO−3 时TCE的降解率为97.3%,增加HCO−3 浓度至1、10、100 mmol·L−1时,TCE在60 min内的降解率分别降至17.7%、9.0%、3.8%。这是因为HCO−3 会使溶液的初始pH升高(由5.20分别升高至6.33、7.22、8.27),并使反应溶液具有一定的缓冲能力,促进了Fe(Ⅱ)形成沉淀,不利于nZVI转化为Fe(Ⅱ),影响体系中·OH的产生,最终导致TCE降解率的下降;此外,HCO−3 可与·OH反应生成HCO3·(如式(9)所示),与TCE竞争·OH,降低了体系对TCE的氧化能力[44]。虽然过碳酸钠在水中分解释放H2O2时产生的碳酸根离子(CO2−3 )可以与·OH反应生成碳酸根自由基(CO−3 ·)(如式(10)所示),从而影响TCE的降解效率,但幸运的是,·OH与目标污染物TCE的化学反应速率为4.0×109 L·(mol·s)−1,远高于碳酸根离子(CO2−3 )与·OH反应生成碳酸根自由基(CO−3 ·)的化学反应速率(3.0×108 L·(mol·s)−1)[45]。因此,在这个体系中,过碳酸根分解产生的碳酸根不会影响TCE的降解。 -
1)在Tween-80存在下,nZVI与Fe(Ⅱ)协同活化SPC能够持续高效降解TCE,在TCE和Tween-80初始浓度分别为0.15 mmol·L−1和13 mg·L−1的溶液中,SPC、Fe(Ⅱ)和nZVI的投加量分别为0.6 mmol·L−1、0.6 mmol·L−1和25 mg·L−1时,在60 min内,TCE的降解率可达97.3%。
2)在SPC/Fe(Ⅱ)/nZVI体系中,Tween-80的存在会抑制TCE的降解,Tween-80浓度越高,抑制效果越明显。
3)在SPC/Fe(Ⅱ)/nZVI体系(Tween-80存在的情况下)中,产生·OH和
O−2 ·自由基,其中起主导作用的是·OH,但也不能忽视O−2 ·的作用。4)在Tween-80存在下,溶液初始pH为2.0~5.2时,SPC/Fe(Ⅱ)/nZVI体系可有效去除TCE;溶液中Cl−的存在对TCE的抑制效果不明显,而
HCO−3 的抑制效果较为明显。
纳米零价铁协同Fe(Ⅱ)活化过碳酸钠降解含吐温-80水体中的三氯乙烯
Degradation of trichloroethylene in aqueous solution containing surfactant Tween-80 by nanoscale zero-valent iron and Fe(Ⅱ) synergistically activating sodium percarbonate
-
摘要: 在表面活性剂吐温-80(Tween-80)存在下,采用纳米零价铁(nZVI)协同Fe(Ⅱ)共同活化过碳酸钠(SPC)体系去除污染物场地水相中的三氯乙烯(TCE),验证了SPC/Fe(Ⅱ)/nZVI体系降解TCE的有效性,探究了Tween-80浓度、无机阴离子以及溶液初始pH对TCE降解效果的影响,并确定了该体系中活性氧自由基的类型。结果表明:nZVI协同Fe(Ⅱ)共同活化SPC能够高效持续降解TCE,在TCE和Tween-80初始浓度分别为0.15 mmol·L−1和13 mg·L−1的溶液中,SPC、Fe(Ⅱ)和nZVI的投加量分别为0.6 mmol·L−1、0.6 mmol·L−1和25 mg·L−1时,在60 min内,TCE的降解率可达97.3%;Tween-80的存在会抑制TCE的降解,Tween-80浓度越高,抑制效果越明显;溶液中Cl−的存在对TCE的影响不明显,而
HCO−3 的抑制效果较明显;当溶液初始pH为2.0~5.2时,SPC/Fe(Ⅱ)/nZVI体系可有效去除TCE;化学探针实验证明体系中产生了·OH和O−2 ·,自由基淬灭实验证实了降解TCE起主导作用的是·OH。综上所述,SPC/Fe(Ⅱ)/nZVI体系可以有效地去除含吐温-80的水相中的TCE,本研究成果可为污染土壤场地修复工程提供参考。Abstract: In the presence of surfactant Tween-80, the system of nanoscale zero-valent iron (nZVI) and Fe(Ⅱ) synergistically activating sodium percarbonate (SPC) was used to remove trichloroethene (TCE) in aqueous phase of contaminated sites. The effectiveness of TCE degradation by SPC/Fe(Ⅱ)/nZVI system was demonstrated. The effects of Tween-80 concentration, inorganic anions concentration and initial solution pH on TCE degradation were explored. The generation of the reactive oxygen species (ROSs) was confirmed. The experimental results showed that TCE could be degraded continuously and effectively by the nZVI and Fe(Ⅱ) synergistically activated SPC system. In the aqueous solution with 0.15 mmol·L−1 TCE and 13 mg·L−1 Tween-80, 97.3% TCE could be degraded within 60 min by SPC/Fe(Ⅱ)/nZVI system at the dosages of 0.6 mmol·L−1 SPC, 0.6 mmol·L−1 Fe(Ⅱ) and 25 mg·L−1 nZVI. The presence of Tween-80 could inhibit TCE degradation, and the inhibitive effect increased with the increase of Tween-80 concentration. The presence of Cl− in solution had no obvious effect on TCE removal, while the presence ofHCO−3 in solution had significant inhibitive effect. TCE removal performed well at the initial solution pH range of 2.0~5.2 in SPC/Fe(Ⅱ)/nZVI system. The chemical probe tests confirmed that ·OH andO−2 · were generated in the system, and radical scavenging tests suggested that the major ROSs responsible for TCE degradation was ·OH. The above results strongly confirmed that TCE could be efficiently removed from water phase containing Tween-80 by SPC/Fe(Ⅱ)/nZVI system, and it provides a theoretical basis for practical application in TCE contaminated site remediation. -
表 1 Tween-80(初始浓度260 mg·L−1)在有无TCE体系中浓度的变化
Table 1. Changes of concentration of Tween-80 (the initial concentration 260 mg·L−1) in systems with or without TCE
反应时间/min SPC/Fe(Ⅱ)/nZVI Tween-80浓度/(mg·L−1) SPC/Fe(Ⅱ)/nZVI/TCE Tween-80浓度/(mg·L−1) 0 260 260 3 239 233 15 207 228 30 208 218 60 205 199 -
[1] 张凤君, 王斯佳, 马慧, 等. 三氯乙烯和四氯乙烯在土壤和地下水中的污染及修复技术[J]. 科技导报, 2012, 30(18): 65-72. doi: 10.3981/j.issn.1000-7857.2012.18.010 [2] CLEWELL H J, GENTRY P R, GEARHART J M, et al. Considering pharmacokinetic and mechanistic information in cancer risk assessments for environmental contaminants: Examples with vinyl chloride and trichloroethylene[J]. Chemosphere, 1995, 31(1): 2561. doi: 10.1016/0045-6535(95)00124-Q [3] 李爽, 胡晓钧, 李玉双, 等. 表面活性剂对多环芳烃的淋洗修复[J]. 环境工程学报, 2017, 11(3): 1899-1905. doi: 10.12030/j.cjee.201512026 [4] SINGH S K, JOHN S. Surfactant-enhanced remediation of soils contaminated with petroleum hydrocarbons[J]. International Journal of Environment and Waste Management, 2013, 11(2): 178. doi: 10.1504/IJEWM.2013.051843 [5] 李果, 毛华军, 巩宗强, 等. 几种表面活性剂对柴油及多环芳烃的增溶作用[J]. 环境科学研究, 2011, 24(7): 775-780. [6] 马浩, 刘元元, 肖文燕, 等. 表面活性剂CMC对石油烃污染土壤的增溶[J]. 环境工程学报, 2016, 10(12): 7333-7338. doi: 10.12030/j.cjee.201507133 [7] 王欢, 王瑶, 肖鹏飞, 等. 非-阴离子表面活性剂对PAHs的增溶作用及无机盐的强化效果研究[J]. 环境科学与管理, 2014, 39(10): 96-100. doi: 10.3969/j.issn.1673-1212.2014.10.021 [8] BAI X X, WANG Y, ZHENG X, et al. Remediation of phenanthrene contaminated soil by coupling soil washing with Tween 80, oxidation using the UV/S2O2−8 process and recycling of the surfactant[J]. Chemical Engineering Journal, 2019, 369: 1014-1023. doi: 10.1016/j.cej.2019.03.116[9] MA X H, ZHAO L, LIN Z R, et al. Soil washing in combination with homogeneous Fenton-like oxidation for the removal of 2,4,4′-trichlorodiphenyl from soil contaminated with capacitor oil[J]. Environmental Science and Pollution Research, 2016, 23(8): 7890-7898. doi: 10.1007/s11356-016-6037-2 [10] HUGUENOT D, MOUSSET E, VAN HULLEBUSCH E D, et al. Combination of surfactant enhanced soil washing and electro-Fenton process for the treatment of soils contaminated by petroleum hydrocarbons[J]. Journal of Environmental Management, 2015, 153: 40-47. [11] MOUSSET E, OTURAN N, VAN HULLEBUSCH E D, et al. Influence of solubilizing agents (cyclodextrin or surfactant) on phenanthrene degradation by electro-Fenton process: Study of soil washing recycling possibilities and environmental impact[J]. Water Research, 2014, 48: 306-316. doi: 10.1016/j.watres.2013.09.044 [12] SUNDER M, HEMPEL D C. Oxidation of tri- and perchloroethene in aqueous solution with ozone and hydrogen peroxide in a tube reactor[J]. Water Research, 1997, 31(1): 33-40. doi: 10.1016/S0043-1354(96)00218-7 [13] CHE H, BAE S, LEE W. Degradation of trichloroethylene by Fenton reaction in pyrite suspension[J]. Journal of Hazardous Materials, 2011, 185(2/3): 1355-1361. [14] 黄伟英, 刘菲, 鲁安怀, 等. 过氧化氢与过硫酸钠去除有机污染物的进展[J]. 环境科学与技术, 2013, 36(9): 88-95. doi: 10.3969/j.issn.1003-6504.2013.09.018 [15] 李永涛, 岳东, 熊鑫高原, 等. 零价铁活化过硫酸钠降解含油废水[J]. 环境工程学报, 2016, 10(8): 4239-4243. doi: 10.12030/j.cjee.201503147 [16] 王继鹏, 胡林潮, 杨彦, 等. Fe2+活化过硫酸钠降解1,2-二氯苯[J]. 环境工程学报, 2014, 8(9): 3767-3772. [17] MIAO Z W, GU X G, LU S G, et al. Perchloroethylene (PCE) oxidation by percarbonate in Fe2+-catalyzed aqueous solution: PCE performance and its removal mechanism[J]. Chemosphere, 2015, 119: 1120-1125. doi: 10.1016/j.chemosphere.2014.09.065 [18] 崔航, 傅晓日, 顾小钢, 等. 二价铁催化过碳酸钠处理水中乙苯[J]. 中国环境科学, 2016, 36(5): 1449-1455. doi: 10.3969/j.issn.1000-6923.2016.05.024 [19] LA CALLE R G, GIMENO O, RIVAS J, et al. Percarbonate as a hydrogen peroxide carrier in soil remediation processes[J]. Environmental Engineering Science, 2012, 29(10): 951-956. doi: 10.1089/ees.2011.0237 [20] ZHANG Y H, XUE C M, GUO C H. Application sodium percarbonate to oxidative degradation trichloroethylene contamination in groundwater[J]. Procedia Environmental Sciences, 2011, 10: 1668-1673. doi: 10.1016/j.proenv.2011.09.262 [21] SINDELAR H R, BROWN M T, BOYER T H. Evaluating UV/H2O2, UV/percarbonate, and UV/perborate for natural organic matter reduction from alternative water sources[J]. Chemosphere, 2014, 105: 112-118. doi: 10.1016/j.chemosphere.2013.12.040 [22] FU X R, GU X G, LU S G, et al. Benzene depletion by Fe(II)-catalyzed sodium percarbonate in aqueous solution[J]. Chemical Engineering Journal, 2015, 267: 25-33. doi: 10.1016/j.cej.2014.12.104 [23] TANG P, JIANG W C, LU S G, et al. Enhanced degradation of carbon tetrachloride by sodium percarbonate activated with ferrous ion in the presence of ethyl alcohol[J]. Environmental Technology, 2019, 40(3): 356-364. doi: 10.1080/09593330.2017.1393012 [24] YAP C L, GAN S Y, NG H K. Fenton based remediation of polycyclic aromatic hydrocarbons-contaminated soils[J]. Chemosphere, 2011, 83(11): 1414-1430. doi: 10.1016/j.chemosphere.2011.01.026 [25] 朱雪强, 韩宝平. 零价铁修复受三氯乙烯污染地下水的实验研究[J]. 环境工程学报, 2012, 6(1): 94-98. [26] LEFEVRE E, BOSSA N, WIESNER M R, et al. A review of the environmental implications of in situ remediation by nanoscale zero valent iron (nZVI): Behavior, transport and impacts on microbial communities[J]. Science of the Total Environment, 2015, 565: 889-901. [27] 许卉, 杨昕, 杨倩文, 等. 一种快速高效测定吐温80含量的比色方法及其应用: CN107300555A[P]. 2020-01-07. [28] YAN W, HERZING A A, KIELY C J, et al. Nanoscale zero-valent iron (nZVI): Aspects of the core-shell structure and reactions with inorganic species in water[J]. Journal of Contaminant Hydrology, 2010, 118(3): 96-104. [29] HAN Y, YAN W L. Reductive dechlorination of trichloroethene by zero-valent iron nanoparticles: Reactivity enhancement through sulfidation treatment[J]. Environmental Science and Technology, 2016, 50(23): 12992-13001. doi: 10.1021/acs.est.6b03997 [30] ZANG X K, GU X G, LU S G, et al. Trichloroethylene oxidation performance in sodium percarbonate (SPC)/Fe2+ system[J]. Environmental Technology, 2014, 35(5): 791-798. [31] 臧学轲, 吕树光, 顾小钢, 等. 泥浆系统中Fe2+活化过碳酸钠降解三氯乙烯[J]. 环境工程学报, 2015, 9(8): 4042-4046. doi: 10.12030/j.cjee.20150873 [32] 臧学轲. 羟胺促进柠檬酸-Fe2+活化过碳酸钠降解三氯乙烯[J]. 华东理工大学学报(自然科学版), 2018, 44(3): 454-462. [33] 贾汉忠, 宋存义, 李晖. 纳米零价铁处理地下水污染技术研究进展[J]. 化工进展, 2009, 28(11): 2028-2034. [34] CHENG M, ZENG G, HUANG D, et al. Advantages and challenges of Tween 80 surfactant-enhanced technologies for the remediation of soils contaminated with hydrophobic organic compounds[J]. Chemical Engineering Journal, 2017, 314: 98-113. doi: 10.1016/j.cej.2016.12.135 [35] ANIPSITAKIS G P, DIONYSIOU D D. Radical generation by the interaction of transition metals with common oxidants[J]. Environmental Science Technology, 2004, 38(13): 3705-3712. doi: 10.1021/es035121o [36] SMITH B A, TEEL A L, WATTS R J. Identification of the reactive oxygen species responsible for carbon tetrachloride degradation in modified Fenton’s systems[J]. Environmental Science Technology, 2004, 38(20): 5465-5469. doi: 10.1021/es0352754 [37] BUXTON G V, GREENSTOCK C L, HELMAN W P, et al. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O−) in aqueous solution[J]. Journal Physical and Chemical Reference Data, 1988, 17(2): 513-886. doi: 10.1063/1.555805 [38] TEEL A L, WATTS R J. Degradation of carbon tetrachloride by modified Fenton's reagent[J]. Journal of Hazardous Materials, 2002, 94(2): 179-189. doi: 10.1016/S0304-3894(02)00068-7 [39] ZHANG X, GU X G, LU S G, et al. Degradation of trichloroethylene in aqueous solution by calcium peroxide activated with ferrous ion[J]. Journal of Hazardous Materials, 2015, 284: 253-260. doi: 10.1016/j.jhazmat.2014.11.030 [40] PIGNATELLO J J, OLIVEROS E, MACKAY A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry[J]. Critical Reviews in Environmental Science and Technology, 2006, 36(1): 1-84. doi: 10.1080/10643380500326564 [41] KHAN J A, HE X X, KHAN H M, et al. Oxidative degradation of atrazine in aqueous solution by UV/H2O2/Fe2+, UV/S2O2−8 /Fe2+ andUV/HSO−5 /Fe2+ processes: A comparative study[J]. Chemical Engineering Journal, 2013, 218: 376-383. doi: 10.1016/j.cej.2012.12.055[42] LIPCZYNSKA E, SPRAH G, HARMS S. Influence of some groundwater and surface waters constituents on the degradation of 4-chlorophenol by the Fenton reaction[J]. Chemosphere, 1995, 30(1): 9-20. doi: 10.1016/0045-6535(94)00371-Z [43] SIEDLECKA E M, WIECKOWSKA A, STEPNOWSKI P. Influence of inorganic ions on MTBE degradation by Fenton’s reagent[J]. Journal of Hazardous Materials, 2007, 147(1/2): 497-502. [44] GREBEL J E, PIGNATELLO J J, MITCH W A. Effect of halide ions and carbonates on organic contaminant degradation by hydroxyl radical-based advanced oxidation processes in saline waters[J]. Environmental Science and Technology, 2010, 44(17): 6822. doi: 10.1021/es1010225 [45] BUXTON G V, ELLIOT A J. Rate constant for reaction of hydroxyl radicals with bicarbonate ions[J]. International Journal of Radiation Applications & Instrumentation. Part C. Radiation Physics & Chemistry, 1986, 27(3): 241-243. -









 下载:
下载: