-
抗生素应用于多个领域,主要涉及医药和畜牧饲料行业。由于抗生素的滥用,导致环境中抗生素污染问题普遍存在[1-3],目前,在水环境[4-6]、土壤[7-9]、水产动物[10]和植物[11]中均检测到了多种抗生素。青霉素G(PCN)是由青霉菌产生的一种β-内酰胺类水溶性抗生素[12],其可阻止肽聚糖的产生从而破坏细菌细胞壁的合成[13],是最具抗菌活性的抗生素,现已被广泛用于治疗人类和动物的疾病中[14]。PCN具有难以降解且含有生物毒性的特性,传统水处理方法难以完全对其产生作用,如果直接将其排放到水环境中,将会对生态环境以及人类构成较大威胁[15-16],因此,探索去除水环境中PCN的新方法十分必要。
O3氧化是一种清洁的水处理技术,且具有无二次污染和经济可行等特点[17],可作为强氧化剂,对污水中的难降解有机物进行降解[18]。有学者用O3氧化降解垃圾渗滤液[19]、有机氯农药[20]和布洛芬[21]等难降解有机物,结果表明,降解效果均十分明显。有研究[22-24]表明,将H2O2与O3联合时,H2O2会促进HO·的产生,从而使O3的利用率以及降解效果均可得到显著提升。陈炜鸣等[23]在采用O3降解垃圾渗滤液浓缩液的过程中,发现添加0.13 mol·L−1 H2O2能显著提升有机物的去除效果,且O3利用率提升了22.29%,同时废水可生化性得到了明显改善,BOD5/COD值由0.01提高到0.43。LI等[25]采用O3预处理氢化可的松制药废水,在H2O2/O3的摩尔比为0.3的条件下,反应15 min后,COD去除率可达67%,COD去除率相对于单一O3氧化体系提升了23%,证明添加适量H2O2可显著提高降解效果。虽然众多研究已经证明了O3和O3/H2O2法对难降解有机物的降解效果显著,但目前许多研究倾向于对工艺条件的优化,而对降解过程中的中间产物分析和降解规律的研究却相对较少。
基于此,本研究以难降解有机物PCN为目标,对其在O3/H2O2体系中的降解规律及其相关的机理进行研究,对降解过程中的中间产物及可能的降解路径进行探讨,并根据实验数据对降解动力学过程进行分析,为利用该方法处理水中PCN的工程应用提供参考。
全文HTML
-
实验试剂包括PCN(1 650 U·mg−1,阿拉丁)、H2O2(分析纯)、淀粉(分析纯)、甲酸(色谱级)、乙腈(色谱级)、NaOH(分析纯)、Na2S2O3(分析纯)、KI(分析纯)。
-
自制反应器、微波快速消解COD测定仪(GZ-WXJ-Ⅲ)、pH计(pHS-3C)、液相色谱仪(Agilent-1200,美国Agilent公司)、液质联用色谱仪(WATERS TQD,美国waters公司)、精密分析天平(FA1004)、傅里叶红外光谱仪器(Nicolet Nexus 410,美国Nicolet公司)、真空冷冻干燥机(LFD-56D10S)等。
自制有机玻璃材质反应器高度为200 mm,内径为90 mm,O3由臭氧发生器(JZ110B-SJG)供给,采用微孔石英砂芯底层曝气,通过转子流量计控制流量,同时O3产量使用碘量法进行测量。利用2个串联的吸收瓶组成尾气收集装置,对溢出尾气进行收集,定时在反应器中部取样。
-
将PCN溶液加入反应器中均匀混合,在通入O3前,加入适量H2O2并控制反应温度和pH,待臭氧发生器稳定工作后,调节气体流量为1.2 L·min−1(臭氧产量为492 mg·h−1),反应开始后按时取样,然后用Na2S2O3终止反应。样品经0.22 μm滤膜过滤后,测定其COD和ρ(PCN)。每次均设计重复实验,每个样品都进行平行测定,然后取其均值。
-
使用HPLC对PCN的降解产物进行检测。具体实验条件为:Hypersil BDS C18色谱柱;流动相为超纯水(含0.1%甲酸)∶乙腈=50∶50(体积比);流速为1.0 mL·min−1;柱温为30 ℃;进样量为20 μL[26]。质谱检测采用电喷雾电离源,在负离子模式下进行检测,扫描的质荷比m/z为100~700。O3气相浓度采用碘量法(CJ/T 3028.2-1994)测定,COD采用重铬酸钾快速消解法进行测定。
1.1. 实验试剂
1.2. 实验设备与仪器
1.3. 实验方法
1.4. 分析方法
-
在温度为20 ℃、ρ(PCN)为25 mg·L−1、O3气体流量为1.2 L·min−1、H2O2投加量为7.84 mmol·L−1的反应条件下,考察pH对PCN和COD去除效果的影响,结果如图1所示。由图1可知,在不同pH下,COD和PCN的去除效果差异明显,在酸性和中性条件下,COD去除效果相对较差,PCN去除速率缓慢,当pH升高时,反应去除速率也相应加快;在碱性反应环境下,去除效果显著提升,在反应5 min后,PCN去除率为92.5%,在反应3 h后,COD去除率为71.9%。这是因为pH会影响O3/H2O2体系中HO·的产生效率,在酸性条件下,主要是O3分子的氧化,而在碱性情况下,溶液中OH-会促进HO·的生成,此时主要以HO·氧化为主,反应速率得到了提升,具体反应如式(1)~式(3)所示。
此外,在碱性环境中,H2O2更容易离解生成
HO−2 ,而HO−2 又是HO·的诱发剂,所以可促进HO·的生成,进而加快氧化速率[23]。 -
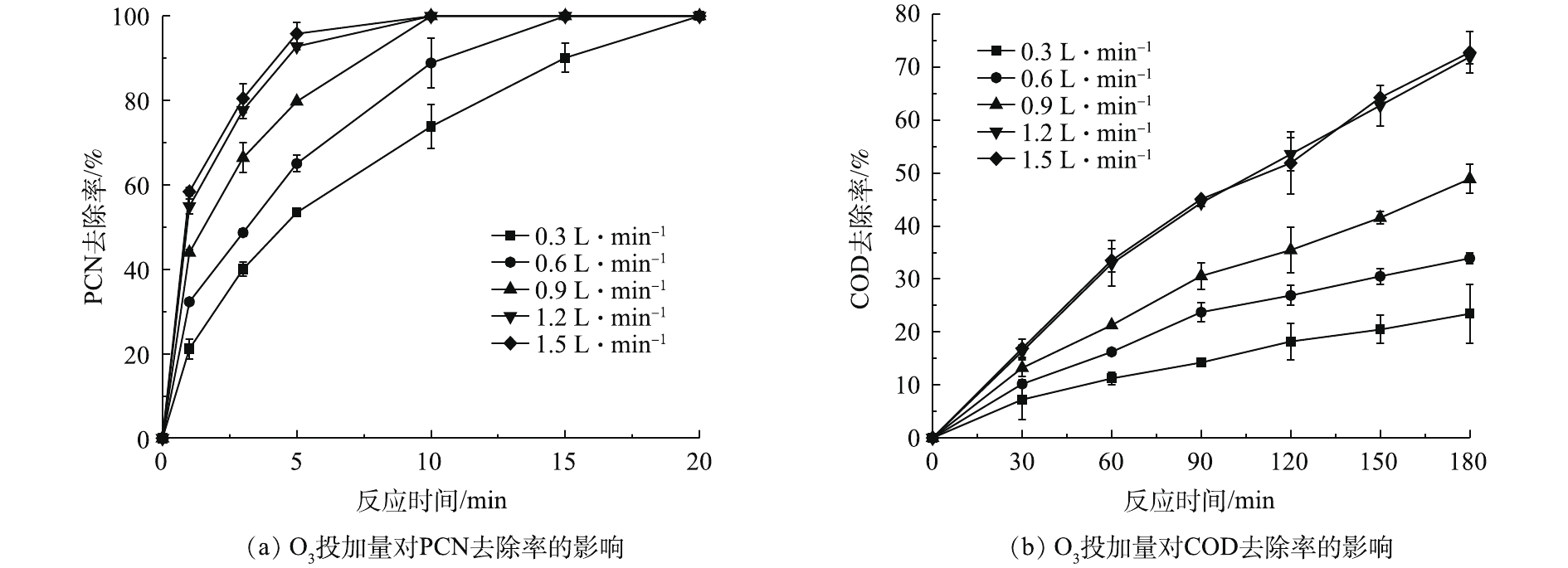
在温度为20 ℃、ρ(PCN)为25 mg·L−1、pH=10,H2O2投加量为7.84 mmol·L−1的反应条件下,考察O3投加量对PCN和COD去除效果的影响,结果如图2所示。由图2可知,O3投加量对去除PCN和COD的影响较大,当流量由0.3 L·min−1(O3产量为123 mg·h−1)升至1.5 L·min−1 (O3产量为615 mg·h−1)时,随着O3投加量的不断增加,PCN和COD的去除率也不断提升,当O3流量为1.5 L·min−1时,PCN和COD去除效果达到最佳。在反应5 min后,PCN去除率为95.83%,在反应3 h后,COD去除率为72.8%。由图2还可看出,在1.2 L·min−1(O3产量为492 mg·h−1)和1.5 L·min−1反应条件下,PCN和COD的去除效果无明显差异,PCN和COD的去除率增幅明显降低,原因可能是,当水中O3溶解度达到最大时,O3的利用率将会降低,未参加反应的O3分子将会直接从液相转移至气相中,故导致无法继续提高降解效能。所以本实验最佳O3流量设定为1.2 L·min−1,以避免造成O3的浪费。
-
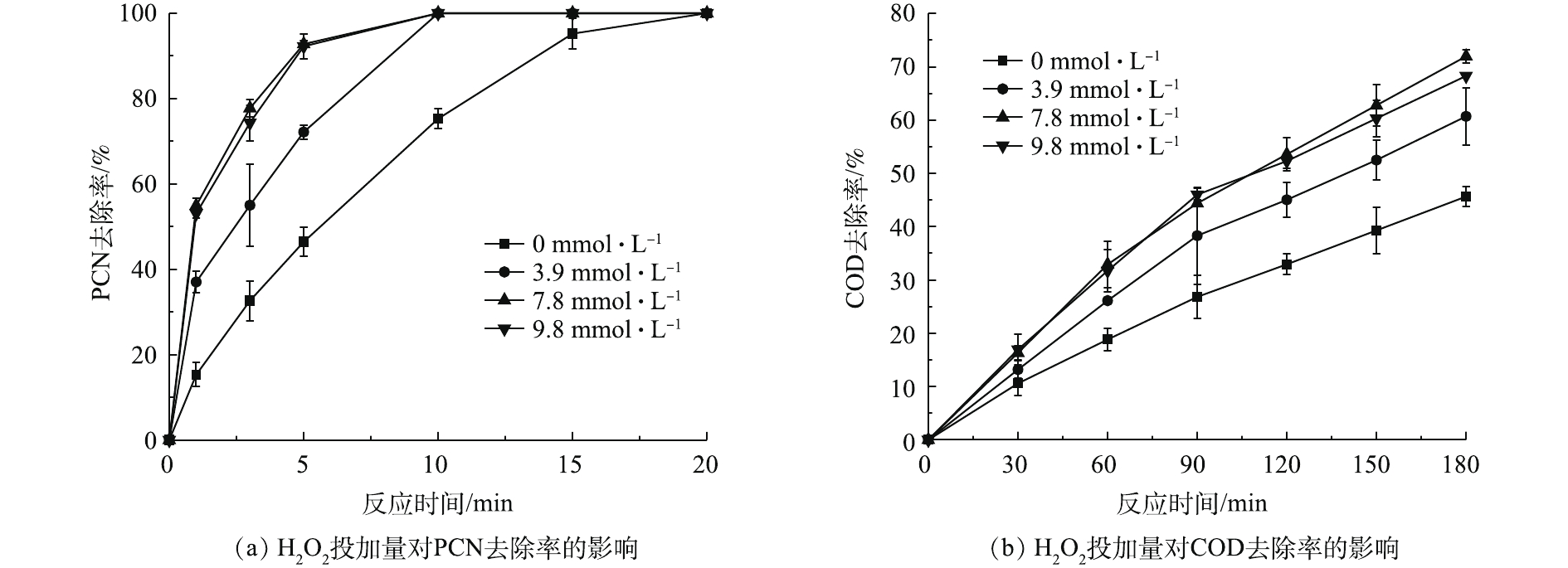
在温度为20 ℃、ρ(PCN)为25 mg·L−1、pH=10、O3气体流量为1.2 L·min−1的条件下,考察H2O2投加量对PCN和COD去除效果的影响,结果如图3所示。由图3可知,在O3/H2O2体系氧化PCN的过程中,PCN能在较短时间内快速被氧化成中间产物,而中间产物的氧化速率则较为缓慢,但H2O2的促进效果明显。当H2O2的投加量由0升至7.84 mmol·L−1时,PCN和COD的去除率也相应随之升高。在反应5 min后,PCN去除率为100%,增幅为37.4%;在反应3 h后,COD去除率为71.9%,增幅为26.3%。相比于单独的O3体系,添加双氧水能显著提升COD和PCN的去除率,这是由于O3和H2O2之间存在协同机制,适量双氧水可促进氧化过程中HO·的生成,从而提升反应效果[3]。具体反应如式(4)所示。
由图3看出,当H2O2投加量大于7.84 mmol·L−1时,COD去除率略微下降。这可能是由于反应体系中多余的H2O2成为了HO·的捕获剂,从而降低了HO·氧化有机物的效率[22-23]。具体反应机理如式(5)所示。
-
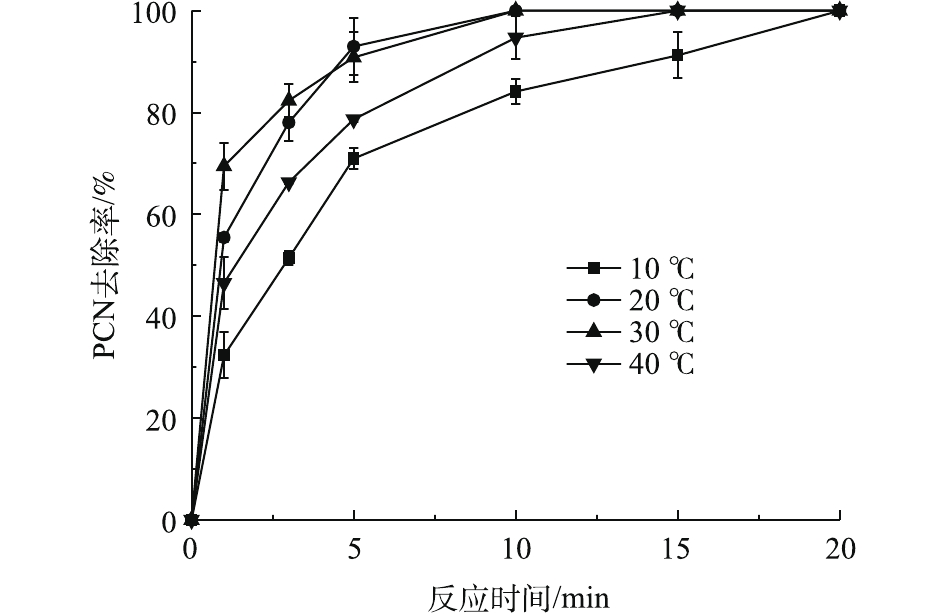
在ρ(PCN)为25 mg·L−1、pH为10、H2O2投加量为7.84 mmol·L−1、O3气体流量为1.2 L·min−1的条件下,考察温度对PCN去除效果的影响,结果如图4所示。由图4可知:在10~30 ℃时,随着温度的上升,PCN去除速率也逐渐加快,去除速率由8.11 mg·(L·min)−1增至17.34 mg·(L·min)−1;但当温度为40 ℃时,PCN去除速率明显降低。原因可能是:当反应温度升高时,加快了分子之间的运动,加速了HO·的生成和O3在水中的扩散速率,从而提升了PCN去除速率。但当温度继续升高时,H2O2的自分解效果加剧,且O3在溶液中的溶解度也有所降低,导致去除速率明显减缓。
-
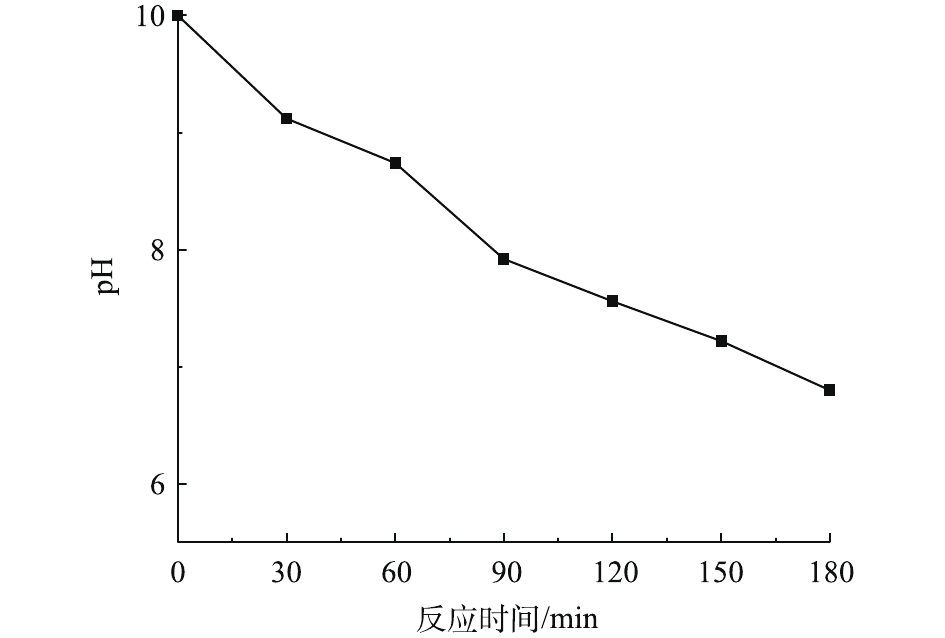
在温度为20 ℃、ρ(PCN)为25 mg·L−1、pH为10,O3气体流量为1.2 L·min−1、H2O2投加量为7.84 mmol·L−1的条件下,考察O3/H2O2反应体系中pH的变化趋势,结果如图5所示。由图5可知,在O3氧化PCN的过程中,反应体系pH随反应时间的延长呈下降趋势,在反应3 h后,pH由10下降至6.8,最终反应溶液呈弱酸性,这可能是由于在降解过程中产生了酸性中间产物,从而导致pH的下降。这与红外光谱和LC-MS的分析结论相一致。体系pH的降低不利于反应的进行,这可能也是反应过程中反应速率均呈先快后慢的变化趋势的原因。
-
通过大量的实验得出最优pH和温度,研究O3、H2O2和PCN初始浓度对氧化过程中PCN浓度衰减的影响,结果如表1所示,降解动力学方程见式(6)。
式中:α、β、γ分别为PCN、O3、H2O2的反应级数;CPCN、
CO3 、CH2O2 分别为PCN、O3、H2O2的初始浓度,mol·L−1;Ea为反应活化能,kJ·mol−1;k0为指前因子,mol·(L·s)−1;R为气体常数,取值8.314 J·(mol·K)−1;T为热力学温度,K。根据表1的数据并结合表观动力学计算原理,可计算出PCN、O3和H2O2反应物的反应级数,其数值分别为α=0.367、β=0.697 3、γ=0.323 3。
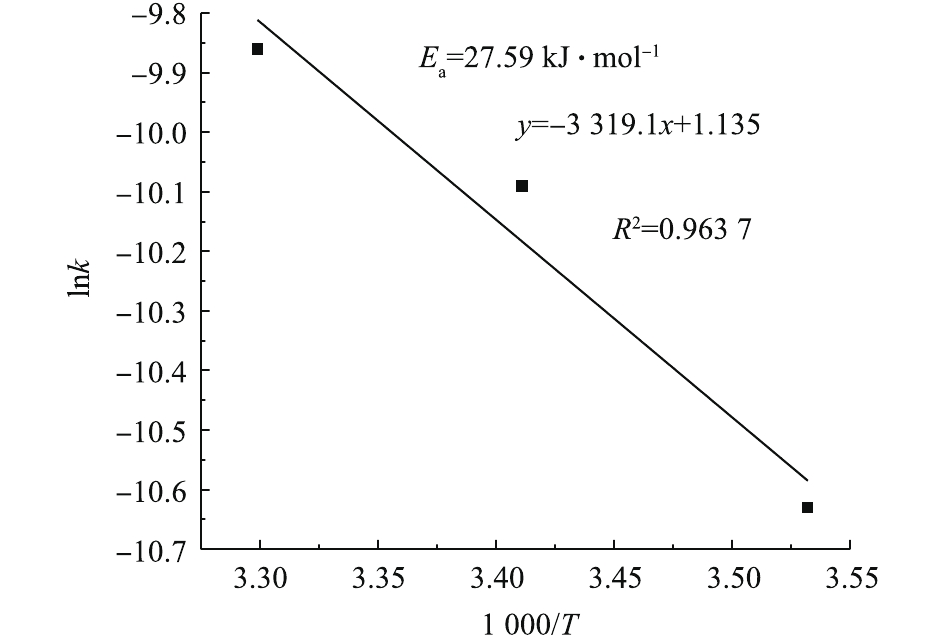
由于总反应速率常数k=k0exp(−Ea/RT),两侧一起取对数可得式(7)。据T和k相应值可得图6。计算得到Ea=27.59 kJ·mol−1,k0=0.052 mol·(L·s)−1,因此,得出总动力学方程,见式(8)。
本动力学模型是依据反应物初始浓度对降解速率的影响而建立的,对于整个降解过程而言,模型可能会高估反应速率。由式(8)可知,O3的反应级数为0.697 9,高于PCN (0.358 9)和H2O2 (0.335 4)的反应级数,说明降解过程中O3初始浓度对反应速率的影响最大。原因可能是,在O3氧化降解PCN的过程中,存在O3分子直接氧化和HO·氧化2种氧化方式,反应过程中只要有O3就能氧化有机物,而H2O2与O3反应只能加快HO·的生成。此外,反应活化能 (Ea=27.59 kJ·mol−1)较低,说明该反应容易发生。
-
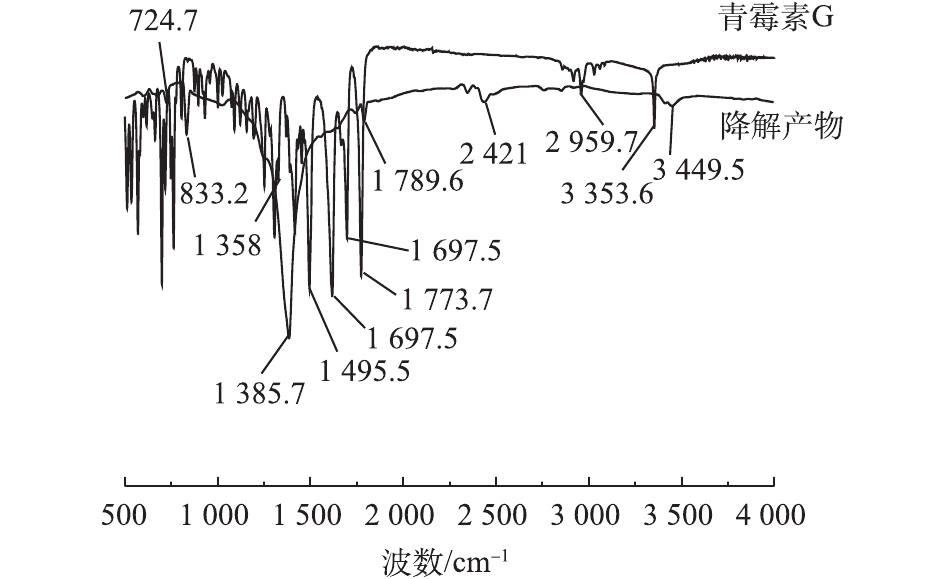
将PCN溶液及其氧化降解的最终产物进行冷冻干燥后进行红外光谱检测,结果如图7所示。在PCN红外光谱图中,1 773.7 cm−1为—COOH中的C=O的伸缩振动峰,3 353.6 cm−1为—COOH中的O—H的伸缩振动峰;1 495.5、1 617.9和2 959.7 cm−1为苯环结构对应的吸收峰,650~1 000 cm−1为苯环上的C—H取代伸缩振动峰;而1 697.5 cm−1为酰胺结构的C=O的伸缩振动;1 307 cm−1处为—(CH3)2的吸收峰。
由图7可知,PCN在氧化前后的谱图有着明显差异,在1 450~1 620 cm−1和3 000 cm−1处苯环骨架吸收峰消失,这说明氧化破坏了PCN的苯环结构。在2 421 cm−1处出现了新的吸收峰,这说明在氧化过程中可能有含叁键或者累积双键的物质产生。在1 697.5 cm−1处的酰胺结构吸收峰消失不见,说明氧化反应破坏了PCN的抑菌结构β-内酰胺环,从而使PCN的抑菌性减弱[27-28]。在1 385.7 cm−1处的峰强度有明显增大,这说明原—(CH3)2结构仍存在,吸收峰在3 449.5 cm−1处出现,有可能是伯胺官能团的不对称伸缩振动与—COOH上O—H的伸缩振动,说明最终产物中可能含有胺类化合物。在1 789.5 cm−1和833.2 cm−1处分别出现羧酸的C=O的伸缩振动和O—H的弯曲振动,这表明最终产物中可能含有酸类化合物,这是导致反应中pH下降的原因。
-
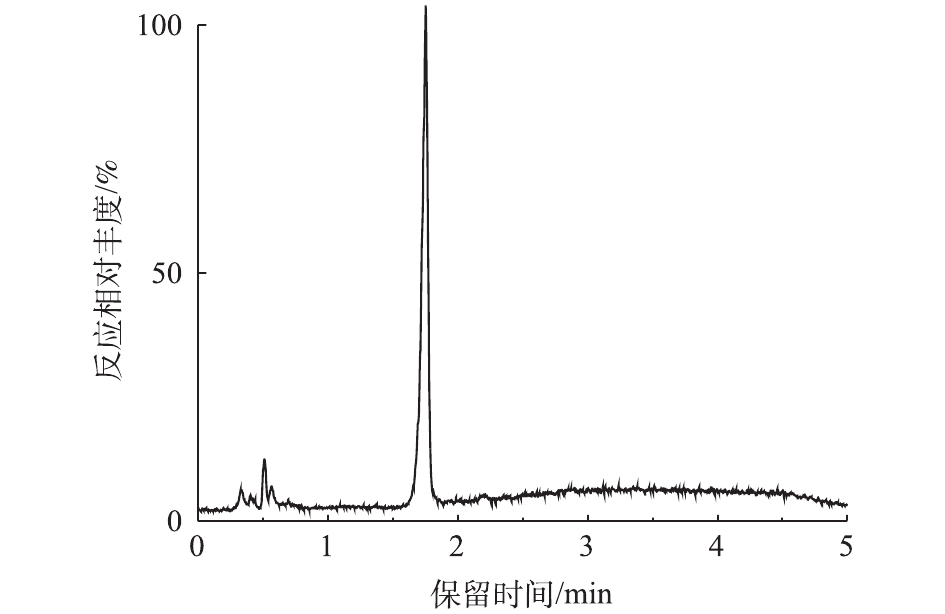
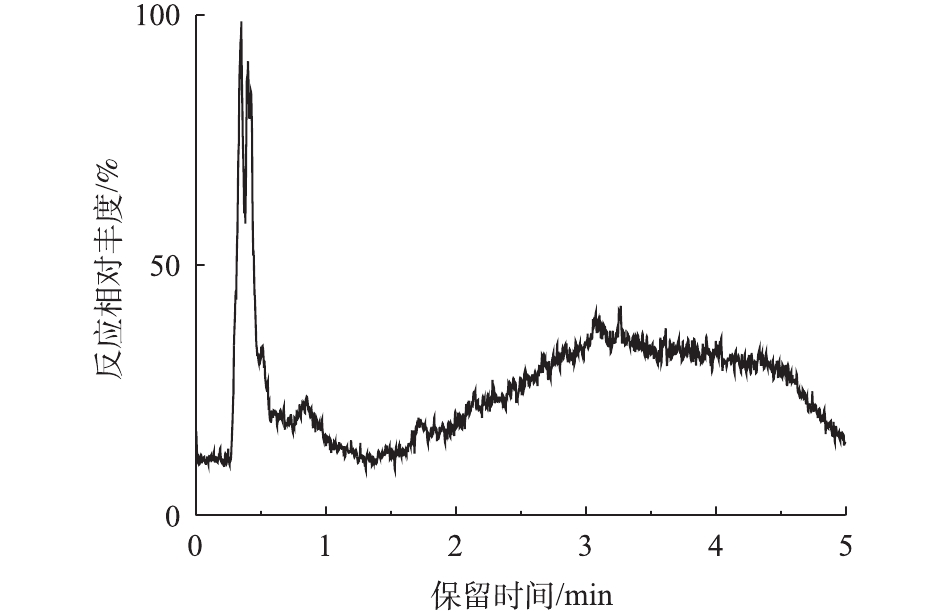
对PCN的降解产物进行LC-MS检测,PCN及其降解产物的总离子流图如图8和图9所示。结果表明,PCN及其降解产物得到了较好的分离,降解后没有检测到PCN的出峰,说明PCN已被完全降解,离子流图显示了PCN降解产物的变化;综合FT-IR的表征结果,对降解产物进行了质谱分析,推测出PCN降解产物可能的分子结构(表2)。
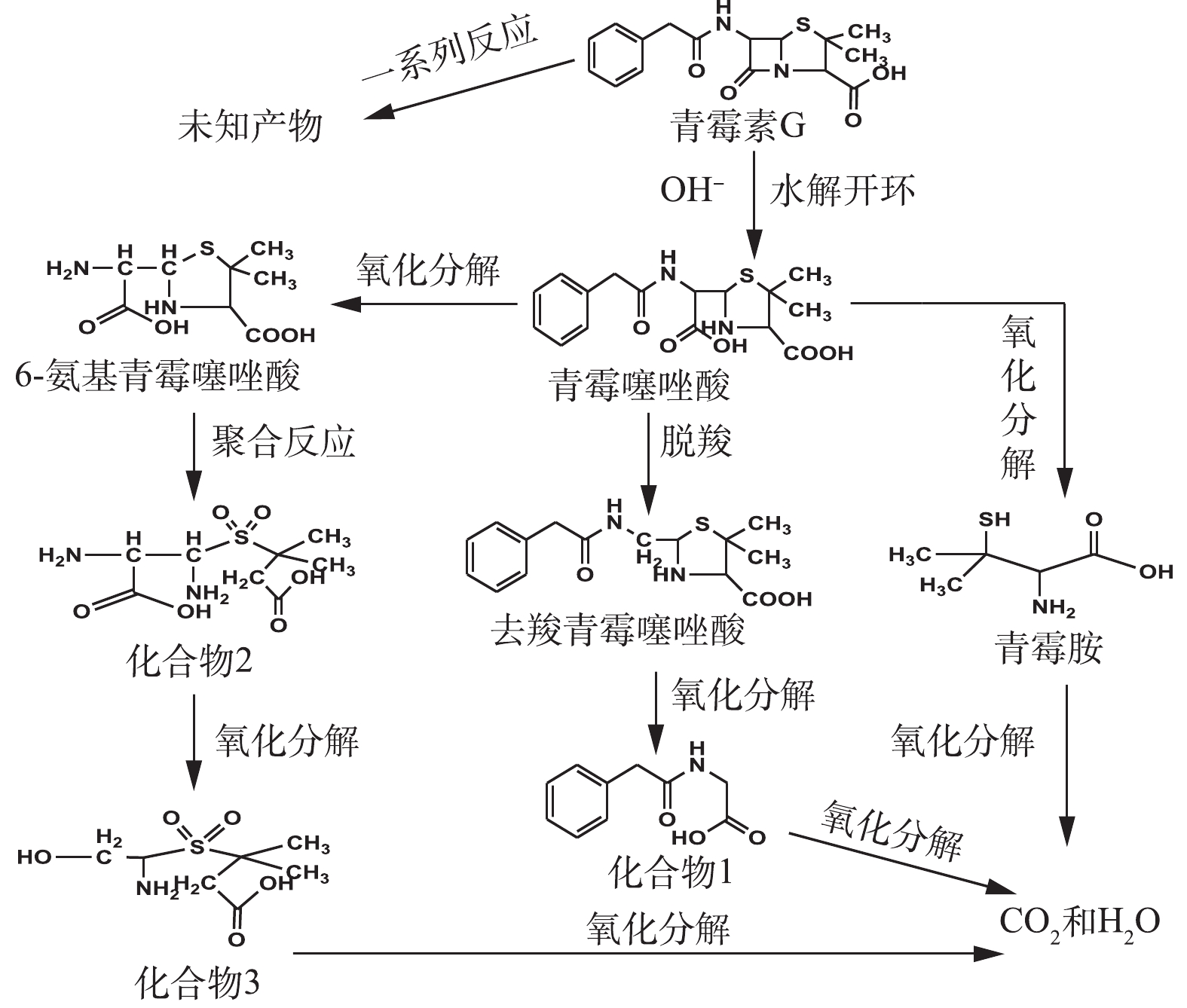
在O3降解PCN的过程中,可能有HO·氧化以及水解等非常复杂的反应存在。在碱性条件下,PCN的β-内酰胺环容易水解打开生成青霉噻唑酸;经脱酸反应后,可能生成去羧青霉噻唑酸;同时在HO·的强氧化能力下,青霉噻唑酸可能进一步被氧化降解成6-氨基青霉噻唑酸、青霉胺和其他未知产物;中间产物也可能最终矿化成为CO2和H2O。根据中间产物分析,推测PCN可能的降解路径如图10所示。
根据LC-MS对产物的分析结果,并结合红外光谱表征结果可知,PCN降解前后的官能团结构发生了较大的变化,氧化使PCN的β-酰胺环被破坏,这也解释了PCN及其降解产物的抑菌性消失或者减弱的原因。
2.1. pH对PCN和COD去除率的影响
2.2. O3投加量对PCN和COD去除率的影响
2.3. H2O2投加量对PCN和COD去除率的影响
2.4. 温度对PCN去除率的影响
2.5. 反应体系中pH的变化
2.6. 表观动力学方程的建立
2.7. PCN降解前后红外光谱分析
2.8. PCN降解产物分析
-
1) O3和H2O2有显著的协同作用,能明显加快反应速率,显著提升COD和PCN的去除率。在初始ρ(PCN)为25 mg·L−1、pH=10、O3投加量为1.48 g·L−1、H2O2投加量为7.84 mmol·L−1、温度为20 ℃的条件下,反应10 min后,PCN被完全去除,反应3 h后,COD去除率为71.9%。这说明O3/H2O2体系能有效氧化降解PCN和降解过程中产生的中间产物。
2)通过数据的拟合,得到了O3/H2O2降解PCN的反应动力学方程,O3的反应级数为0.697 3,高于PCN(0.367)和H2O2(0.323 3)的反应级数,说明在降解过程中,O3初始浓度对反应速率的影响最大;此反应的活化能(Ea=27.59 kJ·mol−1)较低,说明此反应容易发生。
3)根据LC-MS和红外光谱检测结果得出,PCN分子结构在降解前后发生了明显变化,PCN的抑菌结构β-内酰胺环被破坏。此外,降解产物中含有酸性物质,这会导致反应体系pH下降,从而不利于O3反应的进行。





 下载:
下载: