-
随着航空航天和深空探测的发展,保障长期载人航天任务及空间站的运行成为关键,环境控制生命保障系统(environmental control and life support system, ECLSS)因其可实现物质循环和食物的自给而得到广泛的研究,其中的水处理与循环系统是重要的保障之一。ECLSS中水处理与循环系统主要包括卫生废水、冷凝水、相变水和尿液等废水的处理及回用,其中尿液成分复杂,性质不稳定,盐类、营养元素(如N、P、K)及有机物的浓度高[1]。同时尿液中存在多种微量污染物和致病微生物,传统污水处理方法难以有效去除,对人类和环境具有显著的潜在风险[2]。因此,尿液的处理与回收是载人航天生命保障系统发展的关键[3]。
目前,ECLSS中尿液的处理与回收主要应用膜分离技术[4],但其存在严重的盐结垢及有机物膜污染,成为ECLSS中水循环利用系统研究的主要攻关难点[5]。吸附是一种广泛应用的净水技术,已广泛应用于膜过程的预处理中。SOLANKI等[6]研究表明,吸附可有效去除尿液中药物污染物和有机物,也可实现尿液中N、P的去除与回收[7-8]。活性炭是目前应用最广泛的经济型吸附剂,其巨大的比表面积及表面非极性和极性位点可有效吸附营养元素及有机物[9]。XU等[10]研究表明,采用改性碳基吸附材料对尿液中N和P的吸附能力可达48 mg·g−1和116 mg·g−1。由于铁元素具有多种形态和价态而表现出不同的性质和性能[11-13],近年来铁氧化物作为吸附剂在水处理中受到广泛研究与应用[14]。相关研究[15-16]表明,铁氧化物可有效去除水中N、P等污染物。JIA等[17]研究发现,α-Fe2O3、γ-Fe2O3、Fe3O4等铁氧化物具有较高的比表面积及丰富的吸附位点,且其多孔结构有利于污染物的快速扩散,是废水中污染物的优良吸附剂。同时,与其他吸附剂如活性炭相比,其纳米结构因在低温下通过催化燃烧得到,因此,具有更好的再生性能[18]。但将铁氧化物与活性炭结合应用于ECLSS中的尿液处理鲜见报道,相关的吸附性能及机理也有待进一步探究。同时,ECLSS中尿液通常需要进行酸预处理以保障其性质稳定,酸预处理尿液对吸附性能的影响也是评估尿液处理性能的关键。
本研究针对ECLSS中尿液特点,考察了椰壳、果壳、木质3种活性炭对尿液的吸附性能,考察了不同铁氧化物对活性炭吸附尿液的影响,研究了酸预处理对铁氧化物强化活性炭吸附过程的影响,并对吸附机理进行分析,以期为尿液处理提供一种参考。
全文HTML
-
实验尿液为收集的健康男性未稀释的新鲜尿液,其中
${\rm{NH}}_4^ + $ -N、TN、${\rm{PO}}_4^{3 - } $ -P、TP、TOC浓度分别为(520.0±21.0)、(4 157.0±43.0)、(159.0±3.0)、(204.5±8.5)、(4 967.9±349.5) mg·L−1,pH为6.8±0.2,电导率为(18.4±1.8) mS·cm−1。 -
实验材料为椰壳、果壳和木质活性炭(河南环盛炭业有限公司),其比表面积及孔容积见表1。实验试剂包括Fe2O3(分析纯,上海阿拉丁试剂有限公司)、γ-Fe2O3(10 nm,上海阿拉丁试剂有限公司)、α-Fe2O3(30 nm,上海阿拉丁试剂有限公司)、Fe3O4(99%,上海阿拉丁试剂有限公司)、纳米Fe3O4(20~30 nm,上海阿拉丁试剂有限公司)、零价铁(100目,上海阿拉丁试剂有限公司)、抗坏血酸(分析纯,国药集团化学试剂有限公司)、钼酸胺(分析纯,国药集团化学试剂有限公司)、过硫酸钾(分析纯,上海优耐德引用发剂有限公司)、纳氏试剂(哈希中国)、浓硫酸(优级纯,国药集团化学试剂有限公司)、酒石酸钾钠(分析纯,国药集团化学试剂有限公司)。
-
取25 mL新鲜尿液于50 mL锥形瓶中,根据计算药剂投加量,投加不同吸附剂和铁氧化物,放入振荡培养箱(MQD-S3R,上海旻泉仪器有限公司)中进行吸附,吸附温度为25 ℃,转速为150 r·min−1,吸附时间为24 h,以实现吸附平衡,取上清液过0.45 μm膜并进行检测分析。每组实验进行3次平行实验。
在进行活性炭优选实验时,准备3个装有25 mL新鲜尿液的锥形瓶,分别投加1 g椰壳活性炭、果壳活性炭、木质活性炭进行吸附。
在进行铁氧化物强化活性炭吸附实验时,为考察铁氧化物的吸附效果,准备6个装有25 mL新鲜尿液的锥形瓶,分别投加0.2 g的Fe2O3、γ- Fe2O3、α-Fe2O3、Fe3O4、纳米Fe3O4、零价铁进行吸附。为考察铁氧化物强化吸附效果,准备6个装有25 mL新鲜尿液的锥形瓶,向每个锥形瓶中投加1 g活性炭,再分别投加0.2 g的Fe2O3、γ-Fe2O3、α-Fe2O3、Fe3O4、纳米Fe3O4、还原铁粉零价铁进行吸附。为比较铁氧化物投加对污染物去除的影响,计算铁氧化物投加对污染物去除的贡献率(式(1))。
式中:η为贡献率;a为椰壳炭对污染物的去除率;b为铁系材料与椰壳炭对污染物的总去除率。
在预处理强化铁氧化物-活性炭吸附实验时,向新鲜尿液中滴加浓硫酸至pH为2进行酸化预处理,量取25 mL预处理尿液和1 g活性炭分别投入2个锥形瓶中,再分别投加0.2 g的α-Fe2O3、纳米Fe3O4进行吸附。
-
电导率和pH采用便携式多参数水质分析仪(Multi3420,德国WTW)测定;TOC浓度采用TOC分析仪 (TOC-L CPH,日本岛津)测定;
${\rm{NH}}_4^ + $ -N、TN、${\rm{PO}}_4^{3 - } $ -P、TP浓度采用双光束紫外分光光度计(TU-1901,北京普析通用仪器有限公司)测定,其中${\rm{NH}}_4^ + $ -N采用纳氏试剂比色法(HJ 535-2009),TN浓度采用碱性过硫酸钾紫外分光光度法(GB 11894-1989),${\rm{PO}}_4^{3 - } $ -P和TP采用钼酸铵分光光度法(GB 11893-1989)。采用比表面积及孔径分析仪(BET,Micromeritics ASAP2460)检测活性炭比表面积及孔容。
1.1. 实验原料
1.2. 实验材料
1.3. 吸附实验
1.4. 检测方法
-
尿液中污染物浓度较高,TN、
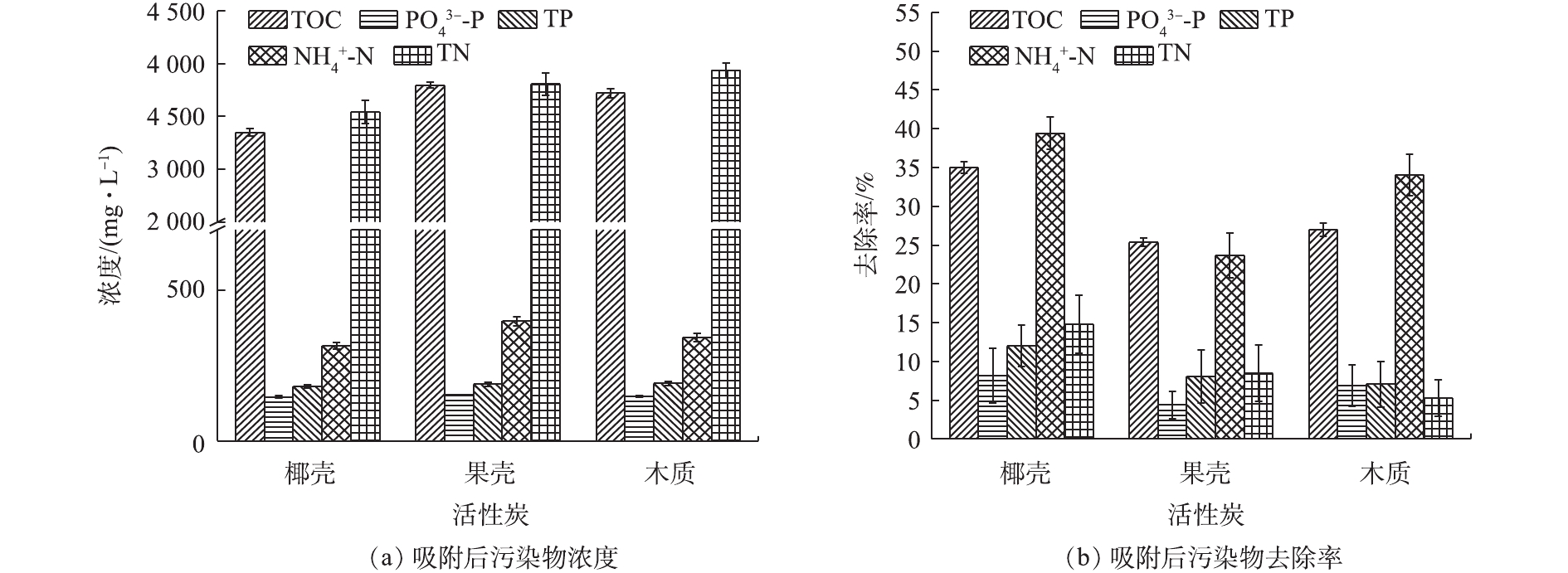
${\rm{NH}}_4^ + $ -N、TOC、TP、${\rm{PO}}_4^{3 - } $ -P分别高达4 157、520、6 000、204.5、159 mg·L−1,电导率为18.4 mS·cm−1,盐浓度较高。因此,采用微生物法进行尿液处理效果不稳定,而采用膜技术处理存在膜污染问题。由图1可知,活性炭对TOC、${\rm{NH}}_4^ + $ -N有较高的去除率,TN的去除率较低,其中椰壳、果壳、木质活性炭对TOC去除率分别为35.02%、25.36%和27.00%,对${\rm{NH}}_4^ + $ -N的去除率分别为39.42%、23.65%和34.03%,但对TN的去除率分别为14.79%、8.44%和5.27%。椰壳活性炭对TOC、TN和${\rm{NH}}_4^ + $ -N的去除效果明显优于木质活性炭和果壳活性炭。由表1可知,椰壳活性炭有最高的比表面积,其总孔容积为0.294 4 cm3·g−1,但微孔容积占比为82.81%,明显高于果壳和木质活性炭,这是椰壳炭吸附性能较高的关键原因。本研究的结果与之前的研究结果[19]一致。果壳和木质活性炭的比表面积分别为516.312 4 m2·g−1和310.526 0 m2·g−1,总孔容积分别为0.267 5 cm3·g−1和0.337 3 cm3·g−1。虽然木质活性炭的总孔容积最高,但其微孔容积显著低于其他2种活性炭,且其比表面积较低,因此,其对TOC的吸附效果与果壳活性炭相似。有机物去除主要是由于活性炭孔道表面对有机物的吸附作用所致;同时,尿素在酶和微生物的作用下会发生分解[20](式(2)),也是有机物削减的另一个重要原因。尿素分解后形成的OH−可引起尿液pH升高,同时游离铵浓度增加可导致${\rm{NH}}_4^ + $ 、磷酸盐与镁离子结合形成磷酸铵镁沉淀(鸟粪石),这是尿液中N、P浓度下降的主要原因[21-22](式(3))。此外,当pH较高时,水中存在的${\rm{NH}}_4^ + $ 易以NH3的形式逸出[23],故${\rm{NH}}_4^ + $ -N浓度降低(式(4))。整体而言,活性炭对P的去除作用较弱,椰壳、果壳、木质活性炭对${\rm{PO}}_4^{3 - } $ -P的去除率分别为8.18%、4.4%和6.92%,其对TP的去除率分别为11.98%、8.07%和7.09%。综合比较,椰壳活性炭具有最佳的尿液处理效果,处理后的TOC、
${\rm{PO}}_4^{3 - } $ -P、TP、${\rm{NH}}_4^ + $ -N、TN浓度分别降为3 350、146、180、315、3 542 mg·L−1。P是膜污染过程中的关键无机结垢物质,提升P的去除效果对膜深度处理尿液具有重要意义。因此,本研究进一步采用铁氧化物和椰壳活性炭结合进行尿液的吸附处理。 -
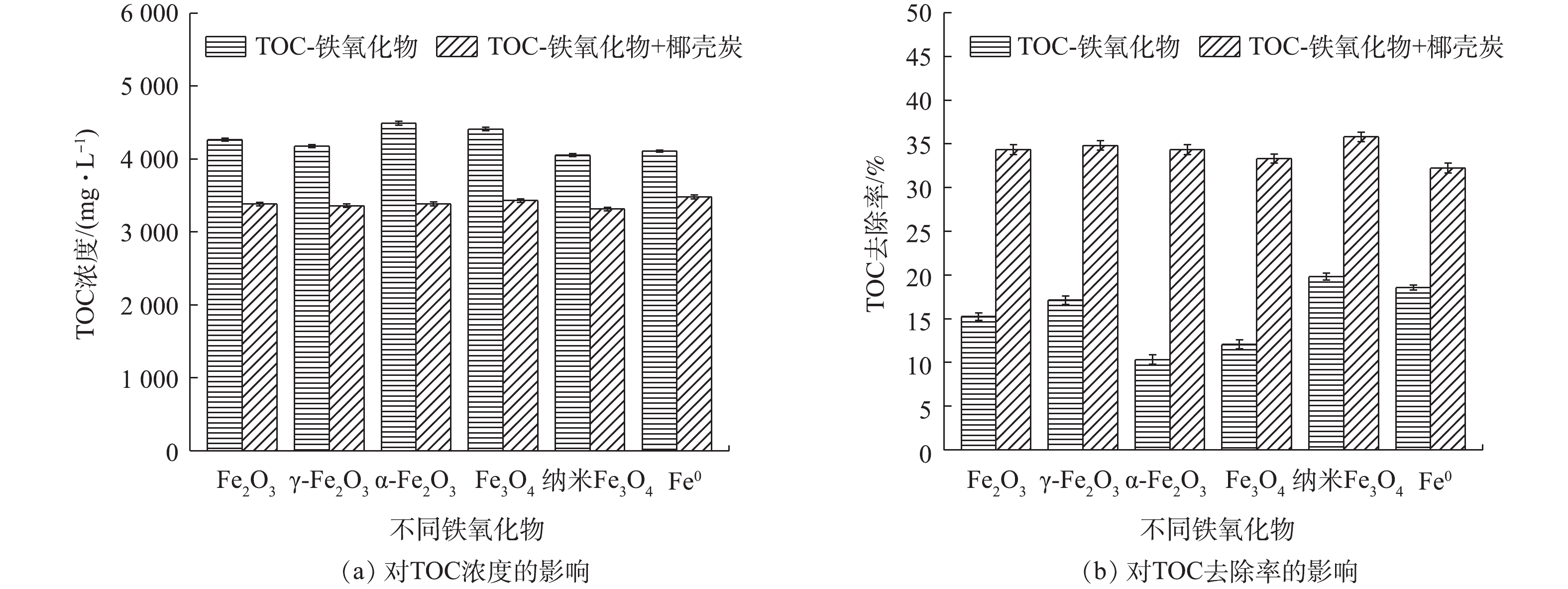
如图2所示,投加8 g·L−1不同铁氧化物和零价铁于尿液中,吸附24 h后TOC的浓度从5 350 mg·L−1降至4 000~4 500 mg·L−1,纳米Fe3O4和零价铁对TOC的去除效果最好,去除率分别为19.82%和18.56%。其原因是纳米Fe3O4具有较大的比表面积,同时可有效降解有机污染物[24];而零价铁因其在水体中易被氧化为Fe2+和Fe3+,生成的多羟基聚合物通过电中和、絮凝作用、吸附架桥的作用能够去除部分有机物[25],同时因为零价铁具有较强的还原性和电化学特性,故可实现有机物的氧化去除[26]。但对比单独椰壳炭的吸附效果可发现,铁氧化物和零价铁的投加对尿液中有机物的去除影响较小。在投加铁氧化物后,TOC的去除率为32.23%~35.81%,而单独活性炭对TOC去除率为35.02%,因此,尿液中TOC的去除主要依靠于椰壳活性炭的吸附作用,铁氧化物对TOC的去除并无显著的促进作用。
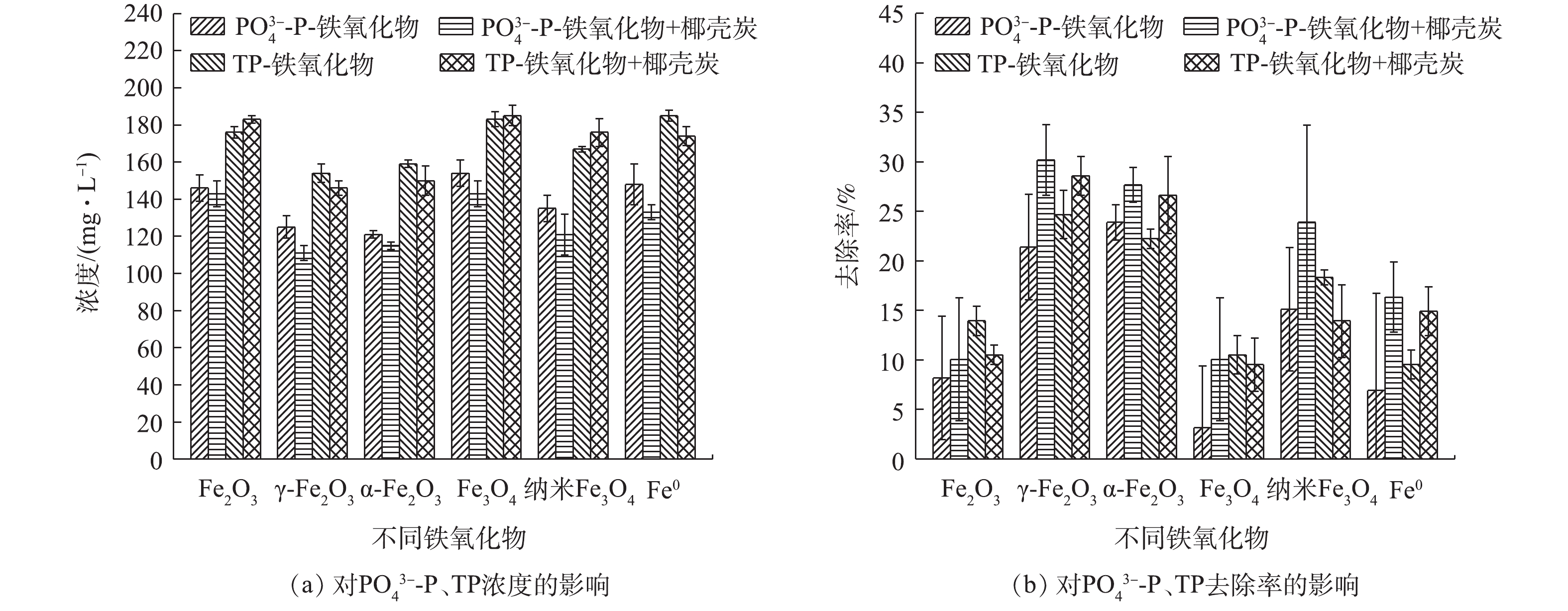
如图3所示,铁氧化物的投加显著增强了尿液中P的去除效果,且总磷和磷酸盐的增强效果基本一致,主要原因是尿液中的
${\rm{PO}}_4^{3 - } $ -P对TP的占例可达80%以上,是尿液中P的主要形态。γ-Fe2O3和α-Fe2O3具有最佳的强化除P效果,投加后,${\rm{PO}}_4^{3 - } $ -P的去除率分别达30.19%和27.67%,去除率提升了22.01%和19.49%;TP的去除率分别达到28.61%和26.65%,分别提升了16.63%和14.67%。进一步分析发现,γ-Fe2O3和α-Fe2O3对${\rm{PO}}_4^{3 - } $ -P去除的贡献率分别为72.90%和70.43%,对TP去除的贡献率分别为58.12%和55.05%。因此,铁氧化物对P的去除具有显著的强化作用,主要原因是铁氧化物具有丰富的羟基,可以与${\rm{PO}}_4^{3 - } $ -P结合[27]。零价铁对${\rm{PO}}_4^{3 - }$ -P和TP的去除率分别为6.92%和9.54%,其与椰壳炭联合对${\rm{PO}}_4^{3 - }$ -P和TP的去除率分别为16.35%和14.91%,相应的去除率分别提升了8.17%和2.93%。其去除机理可能为材料表面基团对${\rm{PO}}_4^{3 - }$ 的吸附作用、产生的Fe2+/Fe3+对${\rm{PO}}_4^{3 - }$ 的化学沉淀作用和Fe2+/Fe3+水解形成氢氧化物对${\rm{PO}}_4^{3 - }$ 的吸附作用[28-30]。另外,吸附过程中的尿素水解导致pH升高,磷酸盐与尿液中的${\rm{NH}}_4^ + $ 、Mg2+、Ca2+等离子共沉淀形成磷酸铵镁结晶[31]及磷酸钙等沉淀[21](式(5))也是尿液中的P去除主要原因。综上所述,铁氧化物特别是γ-Fe2O3和α-Fe2O3,可显著增强椰壳活性炭对P的吸附效果,且铁氧化物是P削减的关键,其主要机制为铁氧化物的吸附作用及其共沉淀作用。
由图4可看出,铁氧化物对N的去除率并不高,Fe2O3、γ-Fe2O3、α-Fe2O3、Fe3O4、纳米Fe3O4、零价铁对
${\rm{NH}}_4^ + $ -N的去除率分别为23.65%、7.88%、10.96%、13.07%、6.73%、7.88%,对TN的去除率均低于4%。与活性炭共同投加后,对${\rm{NH}}_4^ + $ -N和TN的去除有一定的强化作用,Fe2O3、γ-Fe2O3、α-Fe2O3、Fe3O4对尿液中${\rm{NH}}_4^ + $ -N的去除率可提高6%~11%,去除率分别达45.58%、50.77%、46.54%、47.69%。但是吸附后的尿液中TN浓度依然较高,从原尿液的4 157 mg·L−1降低至3 600~4 000 mg·L−1。综上所述,铁氧化物与活性炭联合吸附可以显著强化尿液中P的吸附去除,对N的去除影响较小,对
${\rm{NH}}_4^ + $ -N去除率略提高,对有机物去除基本无影响。考虑到尿液的膜处理过程关键在于预处理过程对有机物和结垢物质的去除,铁氧化物强化活性炭吸附过程将成为一种有效的膜预处理工艺。 -
在ECLSS中通常在尿液中加酸进行预处理,以抑制细菌的滋生和尿液的水解[32],而pH将影响污染物和吸附材料的化学性质,对吸附剂的表面电荷、污染物的电离度以及污染物的分子结构具有较大的影响[18],因此,考察预处理条件下的吸附性能对尿液处理具有重要意义。铁氧化物和零价铁的吸附实验结果表明,γ-Fe2O3、α-Fe2O3和纳米Fe3O4具有较好的强化吸附效果,而α-Fe2O3和纳米Fe3O4具有更稳定的吸附性能,因此,选取α-Fe2O3、纳米Fe3O4考察酸预处理对尿液中污染物的影响。
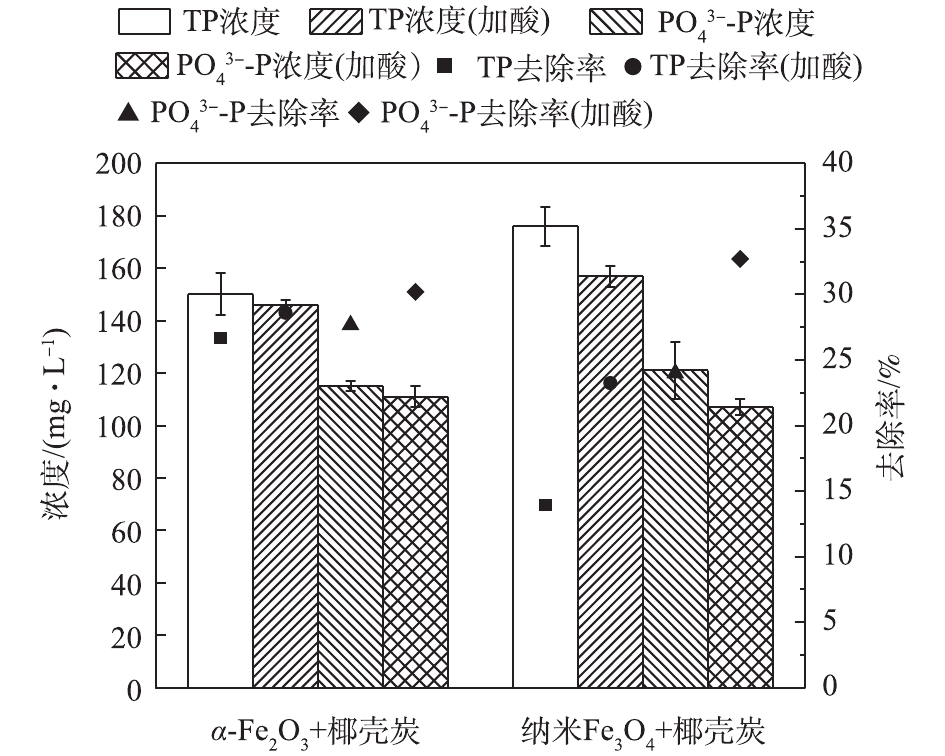
由图5可知,尿液预处理后,TOC的去除效果降低,投加α-Fe2O3和纳米Fe3O4组中TOC浓度均高于未经预处理组的浓度,TOC去除率分别为27.88%和25.87%,相比未经预处理的尿液分别下降了6.5%和9.94%。其原因可能为:一是酸性条件下活性炭表面产生基团质子化,与有机物中氨基正离子产生静电排斥作用[33],对有机物的吸附性能下降;二是酸性条件抑制了尿液中尿素的水解,因此,预处理后尿液中的TOC比未预处理尿液中的去除率更低。
针对经过酸预处理的尿液,由图6可知,
${\rm{PO}}_4^{3 - } $ -P和TP浓度均低于未经预处理组的浓度,这表明尿液预处理进一步提升了铁氧化物-椰壳炭对P的吸附。α-Fe2O3和纳米Fe3O4投加组对${\rm{PO}}_4^{3 - } $ -P的去除率分别为30.19%和32.7%,对TP的去除率分别为28.60%和23.23%;对${\rm{PO}}_4^{3 - } $ -P去除率分别提高了2.51%和8.8%,对TP的去除率分别提高了1.96%和9.29%。铁氧化物对磷酸盐的吸附机理主要为磷酸盐与氧化铁表面羟基结合,但其受pH的影响:对吸附剂铁氧化物而言,当pH较低时,铁氧化物表面羟基发生质子化,即表面正电位增加(式(6)),与带负电的阴离子之间的结合力增强,对${\rm{PO}}_4^{3 - }$ 的吸附能力增加[34];而碱性条件下,金属氧化物表面发生去质子化过程,负电荷增加(式(7)),使得${\rm{PO}}_4^{3 - }$ 与氧化物之间产生排斥作用[35],不利于金属氧化物与${\rm{PO}}_4^{3 - }$ 相互作用;对磷酸盐而言,不同pH条件下磷酸盐发生扩散平衡[36] (式(8)~式(10)),随pH的增加,${\rm{PO}}_4^{3 - }$ 负电性越强,因此,在低pH中${\rm{PO}}_4^{3 - }$ 更易与金属化合物结合。综上所述,在酸性条件下,铁氧化物与${\rm{PO}}_4^{3 - }$ 的质子化过程强化了两者之间的结合(式(11)),磷酸盐主要以质子化合物的形式被吸附[27] (式(11)~式(13))。在铁氧化物-椰壳炭吸附尿液过程中,
${\rm{NH}}_4^ + $ -N的去除主要依靠活性炭的吸附及鸟粪石沉淀作用,溶液中pH的变化对${\rm{NH}}_4^ + $ -N去除有重要影响。由图7可知,在投加纳米Fe3O4和α-Fe2O3后,其对预处理尿液的${\rm{NH}}_4^ + $ -N的去除率分别为36.15%和33.08%,比对未经预处理的尿液下降了13.46%和2.13%。主要原因归为2点:一方面,酸性条件下H+与游离氨反应,使得N元素以${\rm{NH}}_4^ + $ 的形式被固定,减少了氨气的挥发[32];另一方面,当pH较低时,有较多的H3O+与${\rm{NH}}_4^ + $ -N竞争活性炭上的离子交换位点,从而抑制${\rm{NH}}_4^ + $ -N的去除,且pH越低,${\rm{NH}}_4^ + $ -N的去除效果越差[23]。此外,酸性条件不利于磷酸铵镁结晶,也是${\rm{NH}}_4^ + $ -N去除率下降的一个重要原因。但酸预处理对TN去除基本无影响,投加纳米Fe3O4和α-Fe2O3的实验组中TN的去除率分别为10.99%和13.00%,比未经预处理组分别提高了1.63%和1.62%。综上所述,尿液预处理后可显著抑制鸟粪石结晶的形成和尿素分解,故导致有机物和
${\rm{NH}}_4^ + $ -N的吸附效果略有下降。但酸性条件下铁氧化物表面羟基和${\rm{PO}}_4^{3 - }$ 的均发生了质子化作用,强化了两者的结合,因此,增强了P的去除效果。
2.1. 活性炭优选
2.2. 铁氧化物对活性炭处理尿液的影响
2.3. 尿液预处理对铁氧化物-活性炭处理尿液的影响
-
1)活性炭可有效去除尿液中TOC和
${\rm{NH}}_4^ + $ -N,对TN、${\rm{PO}}_4^{3 - } $ -P、TP的去除作用较弱。活性炭处理尿液的吸附性能椰壳活性炭>木质活性炭>果壳活性炭,椰壳活性炭对TOC、${\rm{PO}}_4^{3 - }$ -P、TP、${\rm{NH}}_4^ + $ -N、TN的去除率分别为35.02%、8.17%、11.98%、39.42%、14.79%。2)铁氧化物和零价铁对活性炭吸附TOC的影响较小,有机物去除主要机理是活性炭吸附和尿素分解;对
${\rm{NH}}_4^ + $ -N的去除略有提高,Fe3O4增强${\rm{NH}}_4^ + $ -N去除率的效果最佳,最高增强了11%,主要去除机制为吸附和鸟粪石沉淀;对${\rm{PO}}_4^{3 - } $ -P、TP的去除有明显强化作用,其中,γ-Fe2O3和α-Fe2O3对${\rm{PO}}_4^{3 - } $ -P、TP的去除强化作用最为显著,γ-Fe2O3对${\rm{PO}}_4^{3 - } $ -P和TP的去除率分别提高了22.01%和16.63%,α-Fe2O3对${\rm{PO}}_4^{3 - } $ -P、TP的去除率分别提高了19.49%和14.67%;P去除的主要机制为铁氧化物表面的羟基对${\rm{PO}}_4^{3 - } $ -P的吸附及鸟粪石沉淀。3)尿液酸预处理将降低铁氧化物-椰壳炭对TOC和
${\rm{NH}}_4^ + $ -N的吸附作用,同时提升对P的吸附,且纳米Fe3O4投加对P的吸附效果高于α-Fe2O3,其对${\rm{PO}}_4^{3 - } $ -P和TP的去除率分别提高了8.8%、9.29%,主要吸附机理为酸性条件促进了铁氧化物表面羟基及${\rm{PO}}_4^{3 - }$ 的质子化作用,强化了两者的结合。4)铁氧化物-活性炭处理尿液可有效实现有机物和P的去除,且尿液酸化处理可进一步提升P去除效果并防止结垢,是一种有效的预处理技术,为尿液的处理和回用提供了一种新的思路。










 下载:
下载: