-
砷(As)主要伴生在硫铁矿和有色金属矿中[1]。长期以来,我国以硫铁矿生产硫酸和有色冶炼烟气制酸为主,在制酸废水处理过程中产生了大量高As污泥,且As以溶解态As(Ⅲ)为主[2]。高As污泥的随意堆放对周边环境及人群健康危害性极大。近年来,对其进行固化后安全填埋,已成为重要处理途径之一[3]。一般的材料很难使高As污泥中As浸出浓度满足《危险废物填埋污染控制标准》(GB 18598-2001) [4]填埋入场要求,因此,固As材料的选择成为关键。
无机硫化物、含钙(石灰、石灰石等)材料、含铁锰或铝材料(Fe0、亚铁盐、铁盐、铁锰铝氧化物、铁的氢氧化物等)和水泥等[5-9]对As的固定机制不同,目前,用于高As污泥方面的研究较少。其中,无机硫化物主要通过与As形成螯合物,或与Fe、As形成共沉淀物质,如三硫化二砷(As2S3)或硫铁化砷(AsFeS) [10-11],但无机硫化物的实际固As效果须进一步验证。含钙材料(如CaO等)主要与As形成CaHAsO4和Ca3(AsO4)2) 沉淀[12],但有研究[13-14]认为,CaO固As效果不佳,As经CaO固定后,在高pH条件和酸性浸出条件下容易活化。含铁材料对As主要进行化学专性吸附并将其固定到氧化物晶格层间,可生成FeAsO4和FeAsO4·2H2O等[15]。但不同的含铁材料对As的固定效果具有明显的差异[16-18]。水泥是国内外处理危险废物最常用的也最廉价的固化材料,其通过水化过程的吸附、物理包裹、晶格化等作用抑制As和重金属的渗滤扩散[19-23]。有研究[19, 24-25]表明,在水泥固化前,添加其他固As材料,可通过吸附和共沉淀等作用进一步降低As的释放风险。然而,采用固定化技术处理高As污泥的可行性仍不确定,须根据其污染特性进行固As材料的筛选。
本研究以南方某硫酸厂产生的高浓度含As污泥为处理对象,选用硫化物、含钙、含铁或铝共10种固As材料,采用3种毒性浸出法评估了材料的固As效果,考察了各材料处理对污泥中As结合态和价态的影响,最终筛选出了固As效果最佳的材料,并与水泥进行了联合固化研究,为高As污泥的安全处理提供参考。
-
供试污泥取自南方某硫酸厂工业废水处理后的蓄泥池,为灰黑色。将污泥采集后,自然风干、除杂、混匀、磨碎,过2 mm尼龙筛后,用于固As实验,过0.16 mm筛后,测As含量和重金属总量。供试污泥As含量最高,达到27.33%,其次是Zn(3.63%)和Cu(3.08%),Pb、Cd、Fe、Mn含量依次为0.66%、0.46%、0.17%、0.01%,pH为2.50。
供试固As材料分3类共10种(表1),均为分析纯,包括硫化物(Na2S·9H2O)、含钙材料(CaO)、含铁或铝材料(Fe0、亚铁盐(FeSO4·7H2O)、铁盐(Fe(NO3)3·9H2O、FeCl3、Fe2(SO4)3、Fe(OH)3)、Fe2O3、Al2O3)。供试水泥为工业级PO 425#。
-
1)固As材料的筛选实验。称量20.00 g污泥置于200 mL三角瓶中,按表1的设计方法,投加各种材料,充分搅拌混匀后,加去离子水,保持含水率为25%,室温养护7 d后,进行样品pH、As毒性浸出、As结合态、As价态分析,设置空白对照(CK),每种处理设3个重复。硫化物、CaO、含铁或铝材料中有效元素与污泥总As的理论摩尔比分别按反应产物As2S3、Ca3(AsO4)2、FeAsO4·2H2O、AlAsO4计算,如S∶As=3∶2、Ca∶As=3∶2、Fe∶As=1∶1,因污泥总As含量很高(表1),材料投加量初按理论摩尔数的30%计算。
2) FeCl3与水泥配伍(复配)实验。将FeCl3按与污泥干重质量比为50∶100、100∶100、150∶100、200∶100、250∶100进行固定化处理,将水泥分别按25%、50%、75%、100%、125%投加比进行固化处理,并将不同比例的水泥与250% FeCl3进行配伍处理(见表2),处理和养护过程同上。
-
污泥pH测定采用《土壤pH的测定》(NY/T 1121.2-2006)中的方法[26],使用酸度计(pHs-3C型,上海仪电科学仪器股份有限公司)测定;As浸出采用TCLP法[27](L/S=1∶20)、H2SO4-HNO3法[28](L/S=1∶10)、H2O浸法[29](L/S=1∶10)、SBET法[30](L/S=1∶100);As结合态前处理采用WENZEL(2001)化学连续浸提法[13];污泥中As和重金属含量以及As结合态中的残渣态测试的前处理均采用HNO3-HF-HClO4消解法[31];消解液中重金属含量采用电感耦合等离子体发射光谱仪(ICP-MS 7500,美国Agilent公司)测定。浸出As含量、结合态As含量和消解液中的As含量均采用原子荧光分光光度计(AFS-9120,北京吉天仪器有限公司)测定;污泥As价态采用1.0 mol·L−1 H3PO4+0.1 mol·L−1抗坏血酸[32]提取,采用液相-原子荧光联用仪(LC-AFS(SA-20),北京吉天仪器有限公司)测定。
修复效果评估根据式(1)进行计算。
式中:η为As固定率;C0为废渣在处理前的As浸出浓度,mg·L−1;Ct为处理后As的浸出浓度,mg·L−1。
-
污泥中As浸出特性见表3。可以看出,污泥As浸出浓度均很高,依次为H2SO4-HNO3>H2O>TCLP>SBET。其中,TCLP和H2SO4-HNO3浸提As浓度分别可高达10 634.05 mg·L−1和14 961.25 mg·L−1,超出《危险废物鉴别标准浸出毒性鉴别》(GB 5085.3-2007)标准值(5 mg·L−1)的2 125.81倍和2 991.25倍,各种方法浸提出的As总量占污泥总As的比例均很高,为44.93%~98.01%,大小顺序依次为SBET(98.01%)>TCLP(77.83%)>H2SO4-HNO3(54.75%)>H2O(44.93%)。
污泥中As结合态分布见表4。可以看出,污泥中As主要以F1非专性吸附态和F2专性吸附态为主,占总As的比例达56.09%,与H2SO4-HNO3浸出As水平相当,残渣态占比不到20%,因此,As泥的毒性和危害性极大。
-
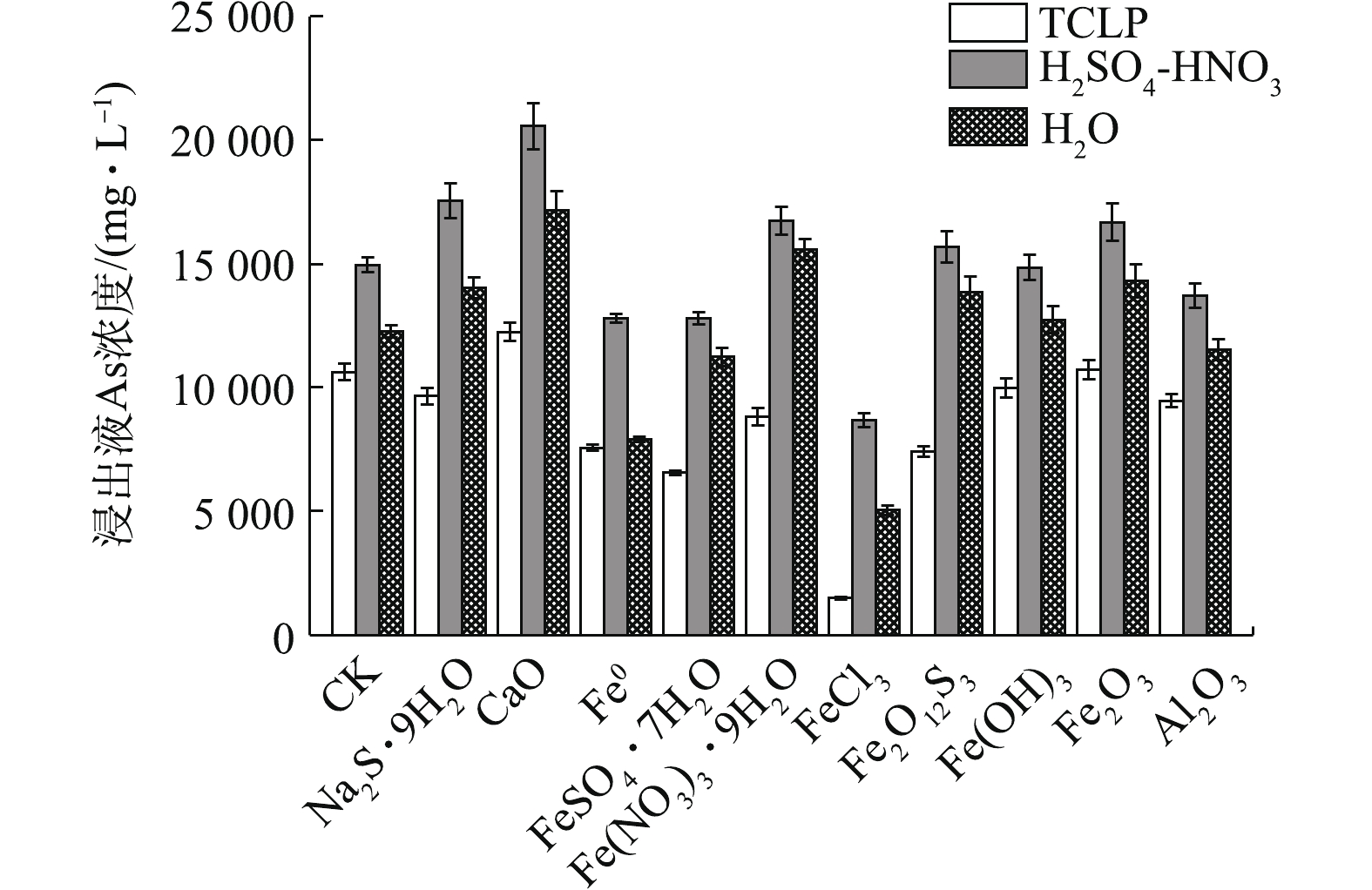
TCLP法模拟了污泥在生活垃圾填埋场中有机弱酸浸提条件下的环境风险。如图1所示,除CaO和Fe2O3外,各材料均可明显降低污泥As的浸出浓度,固As能力依次为FeCl3>FeSO4·7H2O>Fe2O12S3>Fe0>Fe(NO3)3·9H2O>Al2O3>Na2S·9H2O>Fe(OH)3。其中,FeCl3处理使As浸出由10 634.05 mg·L−1降至1 487.66 mg·L−1,固砷率η高达86.01%;CaO和Fe2O3效果最差,CaO甚至活化As,使As浸出增加了15.16%。在10种材料中,FeCl3在应对有机弱酸浸出方面表现出了最强的固As能力。
H2SO4-HNO3浸提法模拟了污泥在强酸降雨情境下As的淋滤风险。结果表明,材料固As能力依次为FeCl3>FeSO4·7H2O≈Fe0>Al2O3,其他材料均无效甚至活化As。其中,FeCl3效果最好,使As浸出从14 961.25 mg·L−1降至8 674.35 mg·L−1,η可达42.02%;其次是FeSO4·7H2O和Fe0。CaO、Na2S·9H2O、Fe2O3、Fe(NO3)3·9H2O、Fe2O12S3 等5种处理材料均对As产生了活化效果。其中,以CaO和Na2S·9H2O最为明显,相应的As浸出率分别增加了37.44%和17.42%。
H2O浸提法模拟了污泥在自然情景下中性H2O对As的浸沥风险。结果表明,FeCl3效果最好,使As浸出从12 276.50 mg·L−1降至5 049.40 mg·L−1,η为58.87%;其次是Fe0,η为35.52%。CaO、Fe(NO3)3·9H2O、Fe2O3、Na2S·9H2O、Fe2O12S3则明显增加了As的浸出,Fe(OH)3对As的浸出影响不明显。
FeCl3均明显降低了污泥的3种As浸出浓度,在各种材料中,FeCl3固As效果均最优,应对有机弱酸淋滤风险的能力最强,其次是H2O和强酸,这可能与FeCl3可促进污泥As向固定态转化有关。FeCl3表面的双配位基对As有很强的吸附能力[33],除提供Fe3+与砷酸根生成砷酸铁外,还可同时产生氢氧化铁胶体,与As发生吸附共沉淀作用。Fe(OH)3对As的浸出影响不大,这可能是因Fe主要以胶体形式存在,不易直接与As结合,其对As的吸附能力主要与pH有关,在中性和酸性条件下,As可能只以双配位表面络合的质子化的FeO2As(O)(OH)−和非质子化的≡FeO2As(O)2−形态存在于Fe(OH)3表面;在弱酸性和弱碱性条件下,吸附As的能力可能变强[34]。Fe2O3和Al2O3对As主要以专性吸附作用为主,与Fe0、亚铁盐和大多三价铁盐一样,与As(Ⅲ)不易形成固定化合物,可能更适用于弱酸性条件下含As(V)为主的污泥固定化处理[35-36]。
CaO在3种浸提条件下固As效果均最差,单一CaO并不适用于高As污泥处理。在还原条件下,污泥中As主要以还原态As(Ⅲ)或三元含氧酸H3AsO3形式存在,pH>12.13时,方可形成溶解度较低的As-Ca化合物[37];而在氧化条件下,pH为中性或为4.5~8.5,As-Ca才较易形成[10]。受污泥中pH和As(Ⅲ)含量的影响,可能须先对污泥中As(Ⅲ)进行预氧化,才利于形成砷酸钙盐及其水合化合物[38]。但也有研究[13-14]表明,As-Ca并不稳定,易被酸性浸提液和水提取出来,单独的CaO处理效果并不佳,须与其他固定化材料联合处理,才可能达到有效固As的目的[39]。
虽然Na2S·9H2O在一定程度上降低了污泥TCLP浸出As,但明显增加了H2SO4-HNO3和H2O浸出As浓度,这说明对于高As污泥,S2−与As形成的溶度积较小的硫化物沉淀或螯合物,在应对有机弱酸浸滤风险方面有一定效果,但应对强酸和H2O浸滤风险的能力并不强,这也可能与S2−在反应水解过程中会产生OH−、不利于As的固定有关[40]。
综上所述,处理以As(Ⅲ)为主的高As污泥时,FeCl3为首选固定化剂。采用TCLP法评估的固As率优于H2SO4-HNO3和H2O的原因还须进一步研究。
-
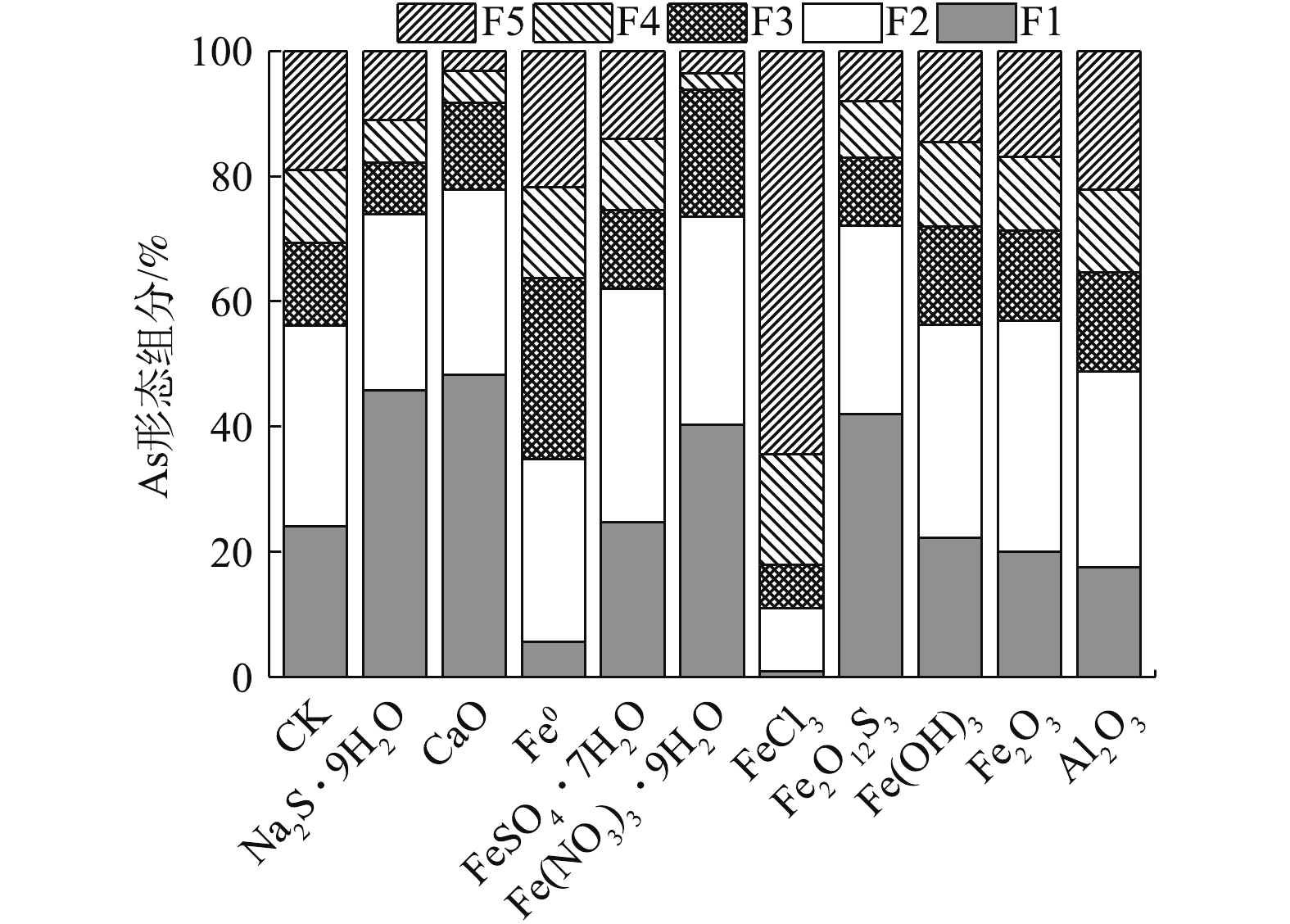
10种材料处理后,污泥As结合态分布变化情况见图2。结果表明,污泥F1~F5态As占总As的比例分别为0.84%~48.26%、10.04%~37.41%、7.03%~28.96%、2.58%~17.66%、3.16%~64.42%。F1和F2结合态As与介质结合弱,迁移能力较强,对环境风险较大。FeCl3、Fe0和Al2O3处理均可降低F1态和F2态占比,与CK相比,分别降低了80.60%、38.13%和13.15%。环境释放风险最大的F1态分别降低了96.51%、76.69%和27.07%;CaO、Na2S·9H2O、Fe(NO3)3·9H2O和Fe2O12S3的F1态和F2态占比均增加>28%。其中CaO和Na2S·9H2O的F1态和F2态占比分别增加38.71%和31.72%。
由图1和图2可知,FeCl3固As效果突出主要与促进污泥As从非专性/专性吸附态向结晶铁锰或铁铝水化氧化物结合态和残渣态转化有关。与CK相比,F4和F5占比分别增加了50.84%和239%;Fe0固As主要与F1向F3、F4转化有关,F3和F4分别增加119%和24.82%,F5仅增加14.40%;Al2O3促进部分F1和F2态转为固定态,F3、F4和F5态占比分别增加了20.29%、12.50%、17.02%。有研究[41]表明,Al2O3八面体的表层与As可形成双齿单核、单齿单核和双齿双核络合物,与Fe2O3一样,对As均有较好的吸附作用。但本研究中Al2O3固As效果有限,稍优于Fe2O3的原因可能与其和As可形成结构更为固定的双齿双核配合物有关[42]。综上所述,各材料可有效固As与促进As从非专性和专性吸附态向稳定态转化密切相关。
-
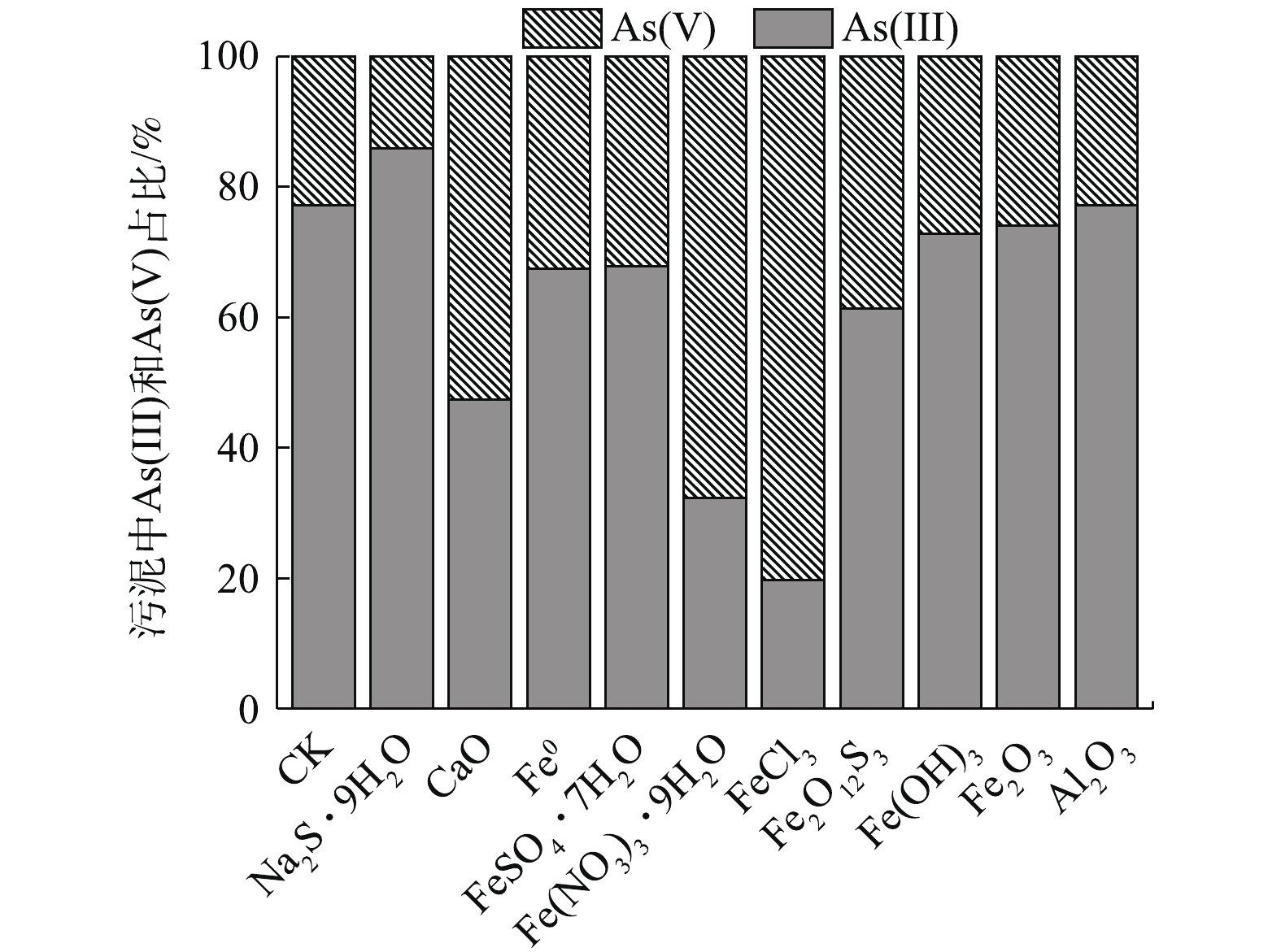
原污泥中的As主要以As(Ⅲ)形态存在,As(Ⅲ)占比高达77.14%(图3)。由图3可知,除Na2S·9H2O外,各材料处理后,污泥As(Ⅲ)比例均有所降低,降低幅度依次为FeCl3>Fe(NO3)3·9H2O>CaO>Fe2O12S3>Fe0≈FeSO4·7H2O。这些材料在高As污泥固定化过程中表现出一定的氧化特性,Fe(OH)3、Fe2O3和Al2O3处理对As(Ⅲ)比例分布的影响则不明显,Na2S·9H2O本身还原性强,与污泥反应后,产生H2S,处理后As(Ⅲ)占比升至85.84%,与CK相比,增加了45.85%,As(V)占比降低了2.7%。
经FeCl3、Fe(NO3)3·9H2O和CaO处理后,As(Ⅲ)占比分别降至19.72%、32.28%、47.38%。尤其经FeCl3处理后,与CK相比,污泥As(Ⅲ)占比降低了74.44%,As(V)则增加了2.51倍。这说明FeCl3处理不仅有效降低了As的总浸出浓度(图1),且能有效降低污泥As(Ⅲ)的相对毒性。铁基材料表现出的氧化性与铁的价态有关[43-44],Fe(NO3)3·9H2O与污泥反应产生的HNO3氧化性强,FeCl3的致酸性和腐蚀性比较强,两者和CaO处理高As污泥时发生放热反应,这可能会促进污泥As(Ⅲ)向As(Ⅴ)的转化。Fe0吸附污泥中的As(Ⅲ)后,向Fe(Ⅱ)氧化物缓慢转化[45],Fe0和Fe(Ⅱ)的耦合也可将部分高溶性As(Ⅲ)氧化为不溶性As(Ⅴ),最终也可能会形成As(Ⅲ)-Fe(Ⅲ)和As(Ⅴ)-Fe(Ⅲ)沉淀[44]。
-
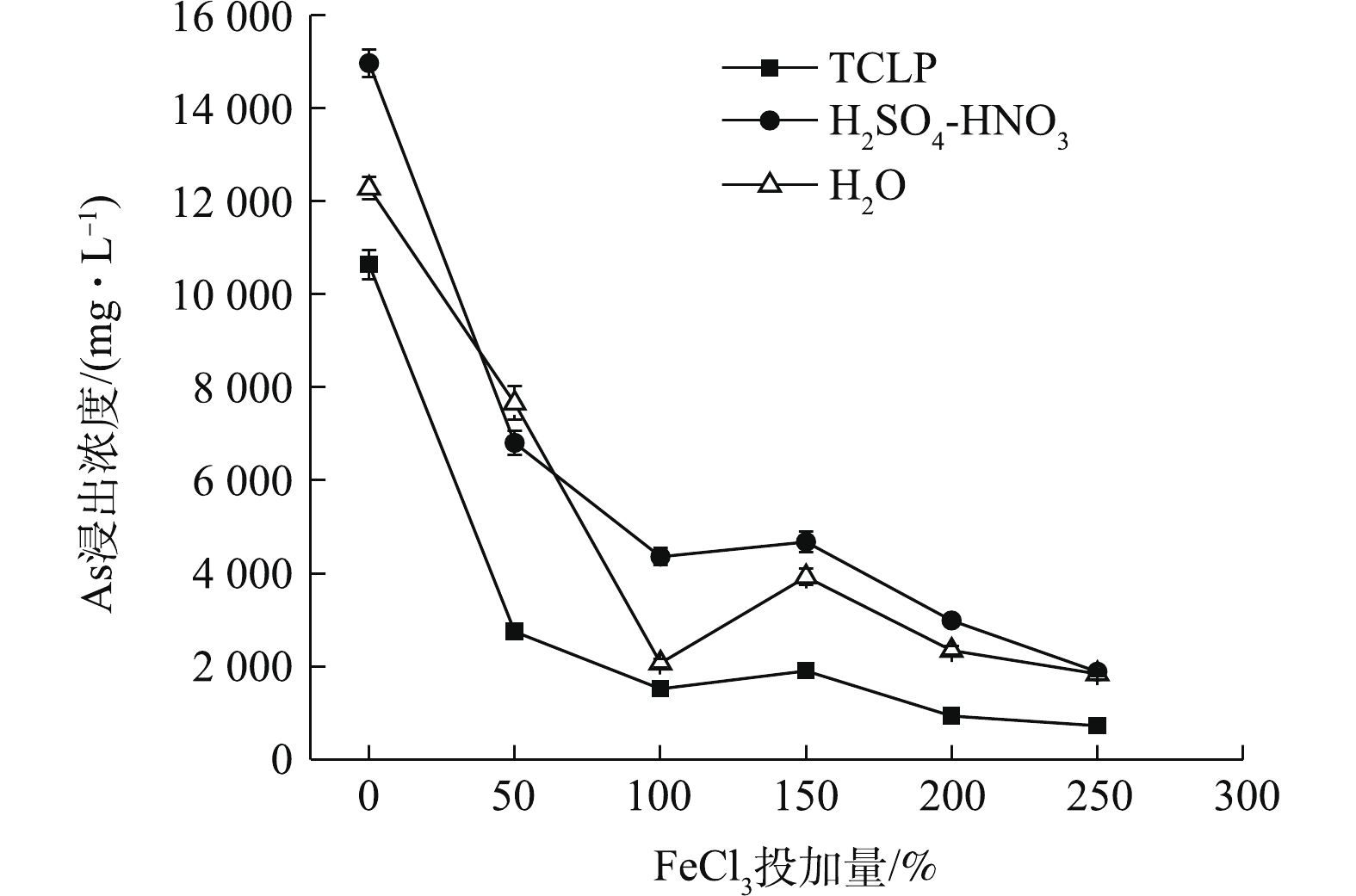
FeCl3处理可使污泥TCLP、H2SO4-HNO3和H2O 浸出As浓度均明显降低(图4),随FeCl3投加量的增加,3种As浸出浓度均呈先降低,后稍升,后继续降低的趋势。在最低投加量为50% FeCl3处理后,As浸出浓度均急剧下降,分别降至2 746.18、6 805.90、7 656.85 mg·L−1,与CK相比,η分别达到74.18%、54.51%、37.63%。其中,TCLP浸出As降幅最大。3种As浸出浓度在100% FeCl3时,已降至较低水平,之后降幅变化趋于平缓。250% FeCl3处理后,污泥TCLP-As降至最低,为727.03 mg·L−1,η最高可达到93.16%,H2SO4-HNO3和H2O浸出As此时趋于接近,分别降至1 889.94 mg·L−1和1 840.63 mg·L−1,η均高于85%,但As浸出仍未达到危险废物填埋入场要求。
-
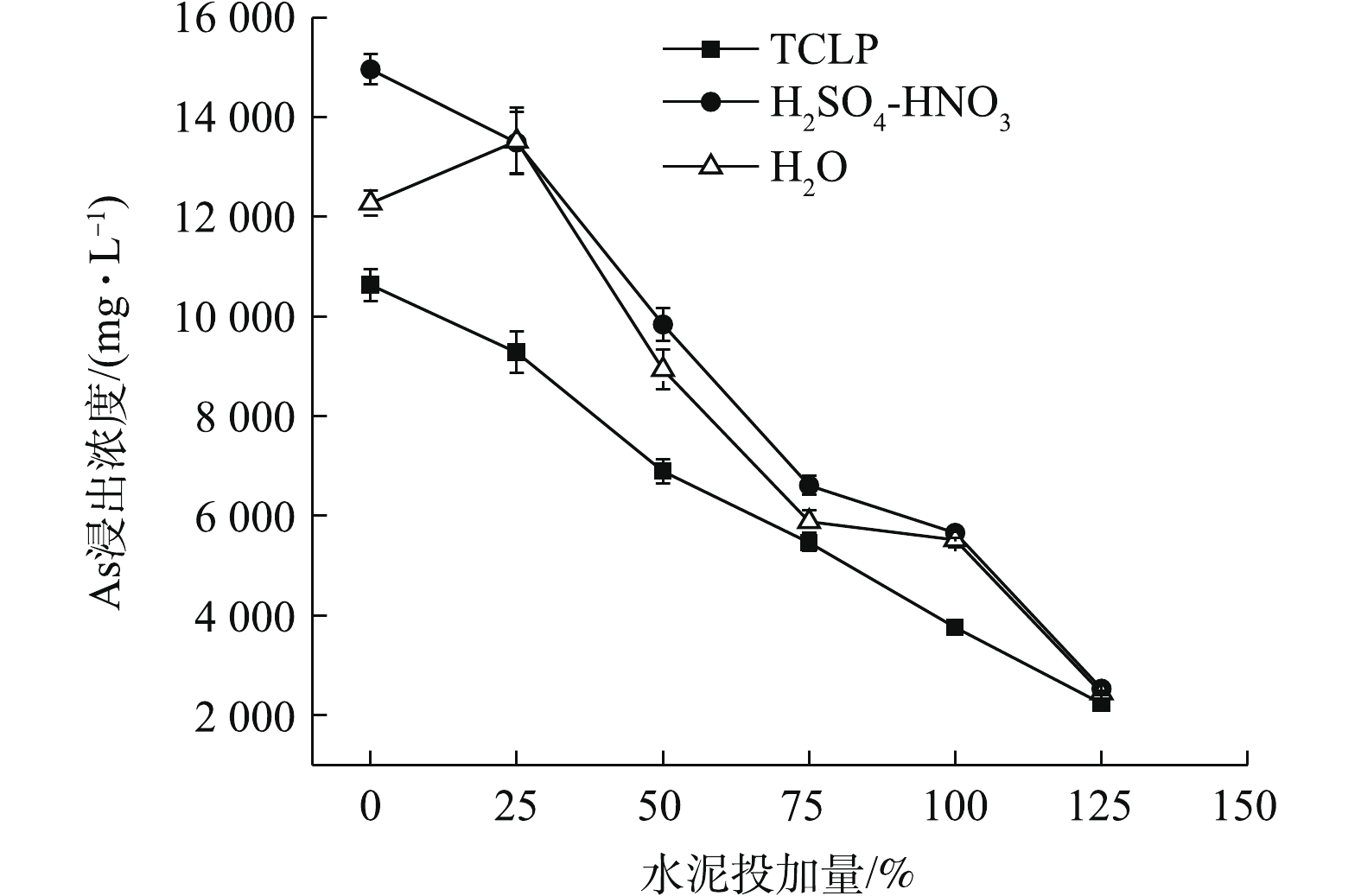
如图5所示,单独添加水泥可大大降低污泥As浸出毒性,随着水泥添加量的增加,污泥TCLP和H2SO4-HNO3浸出As浓度均呈下降的趋势,但25%的水泥投加量的固As效果并不明显,As浸出仅分别降至9 282.36、13 492.20 mg·L−1,η仅为12.71%、9.82%。污泥H2O浸出As呈先升高后降低的趋势,25%水泥投加处理,使污泥pH明显升高,增加了污泥颗粒表面负电荷,降低了污泥中带正电荷胶体对亚砷酸和砷酸根的吸附,导致H2O浸出As浓度升至13 521.95 mg·L−1,接近于H2SO4-HNO3浸出As值。与CK相比,H2O浸出As浓度被活化了10.14%,之后,随水泥投加量的增加,As浸出不断下降。3种方法浸提后,As浸出浓度均在水泥最高投加量125%时降至最低,且数值趋于2 200~2 600 mg·L−1,η均在80%左右。在同等投加量时,水泥固As效果明显弱于FeCl3。
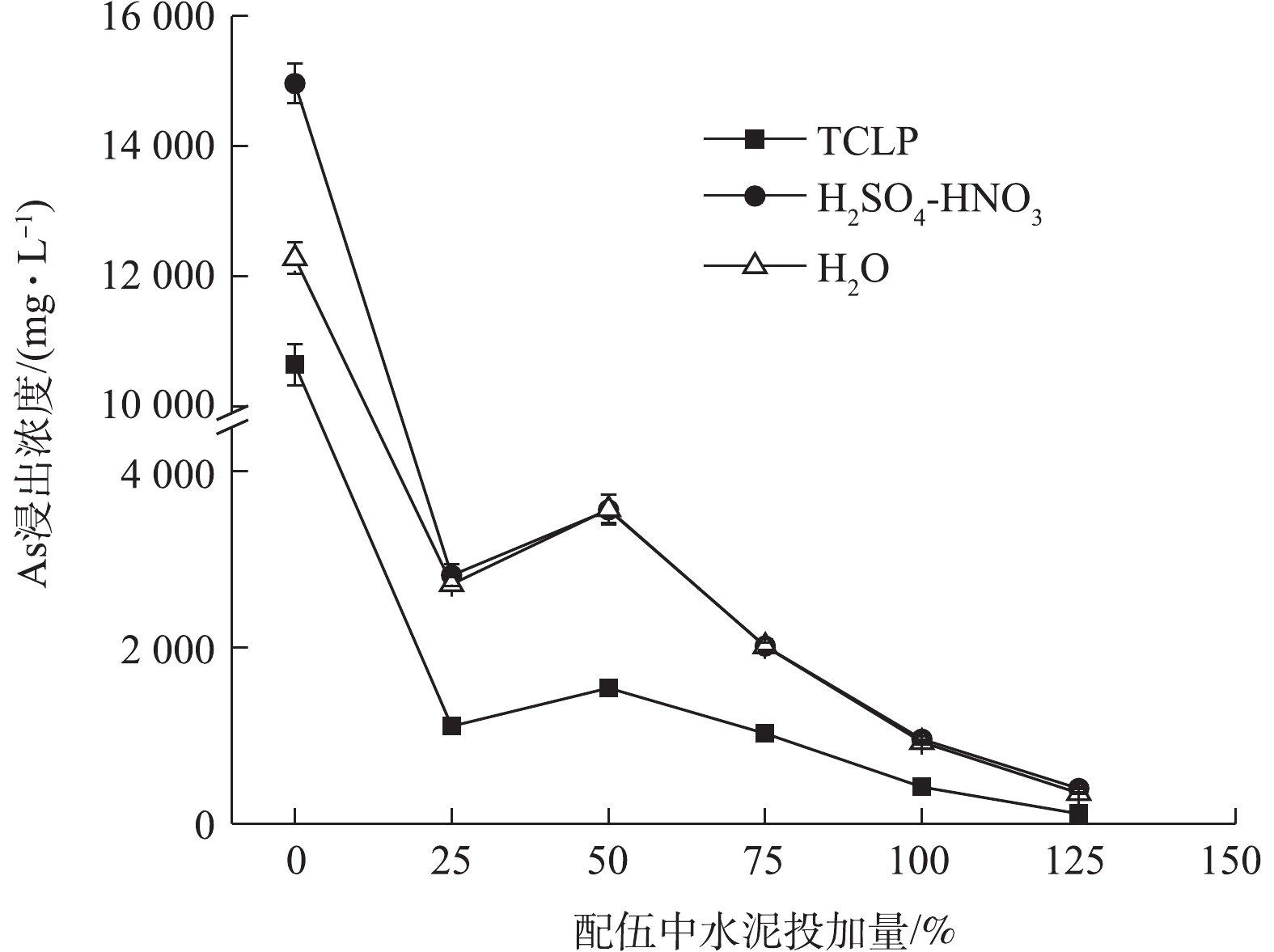
250% FeCl3与水泥复配结果如图6所示,3种As浸出变化规律相似,As浸出浓度均先急剧下降,然后上升,后再下降,H2SO4-HNO3和H2O浸出As浓度和变化基本趋于一致,固As效果均弱于TCLP。水泥最低复配比为25%时,3种As浸出浓度可分别降至1 106.15、2 816.99、2 716.97 mg·L−1,η分别达到89.60%、81.17%、77.87%。与单一水泥相比,各配伍处理的固As效果均明显提高。与单一250% FeCl3处理相比,水泥复配比<100%时,配伍固As效果有所下降,这说明低量水泥的添加对FeCl3固As产生了拮抗作用,水泥易升高污泥pH,FeCl3则更易降低pH,在FeCl3与水泥的交互影响下,FeCl3虽使污泥正电荷增加,仍能维持固As效果较好的酸性条件[46],但配伍并未达到协同增效的目的。在水泥复配比≥100%后,配伍固As效果占优;在水泥复配比125%条件下,3种As浸出均降至最低,分别为113.81、399.28、347.27 mg·L−1,η均高于97%,但材料高投加量仍未使As浸出达到危险废物填埋控制标准。
铁盐和水泥虽是处理含As固废常用材料[47-48],但本研究结果表明,针对高As污泥,FeCl3和水泥复配不一定优于单一材料,这与材料种类、特性和投加量等有关。材料的高投加量虽可使固As率有所提高,但仍难达标,且存在增容比高、FeCl3强腐蚀性等问题。因此,须进一步研发更为高效的固As材料,可考虑联合其他技术或措施(如淋洗后再固定化固化、烧结固化等),以尽量降低As释放风险,满足危险废物填埋入场要求。
-
1)固As材料筛选结果表明,FeCl3固As效果最好。TCLP、H2SO4-HNO3、H2O浸提法评估的固砷率分别为86.01%、42.02%、58.87%,均强于其他9种材料,且明显促进了As的非专性/专性吸附态向结晶铁锰或铁铝水化氧化物结合态和残渣态转化,非专性和专性吸附态As占比降低了80.60%,非专性态降低96.51%。
2)在10种材料处理中,有6种材料对As(Ⅲ)具一定的氧化作用。其中,FeCl3处理对As(Ⅲ)的氧化作用最强,使As(Ⅲ)占比由77.14%降至19.72%。Fe(OH)3、Fe2O3和Al2O3处理对As(Ⅲ)的氧化性不明显,Na2S·9H2O处理使As(Ⅲ)占比升至85.84%。
3) FeCl3联合水泥固化结果表明,单一FeCl3和FeCl3与水泥配伍的固As效果均优于单一水泥。水泥复配比≥100%时,配伍处理的固As效果优于单一FeCl3;在250% FeCl3+125%水泥条件下,TCLP、H2SO4-HNO3和H2O浸出As浓度降至最低,分别为113.81、399.28、347.27 mg·L−1,η均高于97%。高投加量并未使As浸出达到危险废物填埋控制标准。
不同材料对高As污泥中As的固定效果
Immobilization effect of different materials on high As-bearing sludge
-
摘要: 针对制酸行业产生的高砷(As)污泥浸出毒性过高且难处理的问题,选择典型无机硫化物、含钙材料、含铁或铝材料(Fe0、铁盐、Fe2O3/Al2O3) 对高As污泥进行固定化,采用醋酸(TCLP)、H2SO4-HNO3和H2O 3种浸提法评估了各材料对As的固定效果;考察了固定化对污泥中As结合态和价态分布的影响,筛选最佳固定化材料,进行联合水泥固化。结果表明,FeCl3固化As的效果最好,3种浸提法评估的固砷率分别为86.01%、42.02%、58.87%。FeCl3和Fe0处理能够促进污泥As向固定态转化,非专性和专性吸附态占比分别降低了80.60%和38.13%。FeCl3促进非专性和专性吸附态向结晶铁锰或铁铝水化氧化物结合态和残渣态转化。CaO、Fe0、FeSO4·7H2O、Fe(NO3)3·9H2O、FeCl3和Fe2O12S3处理对As(Ⅲ)具有一定的氧化作用。FeCl3处理后,As(Ⅲ)占比由77.14%降为19.72%。Fe(OH)3、Fe2O3和Al2O3处理对As(Ⅲ)的氧化性不明显,Na2S·9H2O处理使As(Ⅲ)占比升至85.84%。随FeCl3、水泥和两者配伍材料投加量的增加,在3种方法中,污泥中As的浸出量均明显降低,单一FeCl3和配伍固砷效果均优于水泥;水泥配伍比≥100%时的固砷效果优于单一FeCl3;250% FeCl3+125%水泥可使3种方法中浸出砷浓度降至最低,分别为113.81、399.28、347.27 mg·L−1,固砷率均高于97%。本研究结果可为高As污泥的固定化提供参考。供参考。Abstract: The high arsenic (As)-bearing sludge produced from acid industry has too high leaching toxicity to dispose. In this study, three kinds of typical materials: inorganic sulfide, calcium-based material and several iron-aluminum-based materials (Fe0, ferric salt, Fe2O3/Al2O3), were used to immobilize the high As-bearing sludge. Their As immobilization effects were assessed by three leaching methods of TCLP(toxicity characteristic leaching procedure), H2SO4-HNO3 and H2O. The effect of immobilization on the valence and binding form distribution of arsenic in sludge was investigated. Then the optimal material for As immobilization was determined, which was used to conduct the subsequent joint solidification with cement. The screening result of materials showed that FeCl3 had the best As immobilization effect, and its As immobilization efficiencies assessed by above three leaching methods were 86.01%, 42.02%, 58.87%, respectively. The FeCl3 or Fe0 treatment could promote the As transformation to stable speciation, and the proportions of non-specific and specific bound As fractions decreased by 80.60% and 38.13%, respectively. In which FeCl3 treatment promoted the transformation from non-specific and specific bound As fractions to crystalline hydrous Fe(Mn, Al)oxide fraction and residual fraction. CaO, Fe0, FeSO4·7H2O, Fe(NO3)3·9H2O, FeCl3 and Fe2O12S3 could oxide As(Ⅲ) to As(V). Among them, the proportion of As(Ⅲ) in FeCl3 treated sludge decreased from 77.14% to 19.72%, and no obvious oxidation of As(Ⅲ) occurred in Fe(OH)3, Fe2O3 and Al2O3 treated sludge. However, due to the strong reducibility of Na2S·9H2O, the proportion of As(Ⅲ) in Na2S·9H2O treated sludge increased to 85.84%. With the increase of the dosage of FeCl3, cement or composite materials, the leaching amount of As in sludge decreased significantly. The As immobilization effect of FeCl3 alone or FeCl3+cement was better than that of cement alone. When the cement ratio was set to above 100%, FeCl3-cement As immobilization effect was better than that of FeCl3 alone. The As leaching concentrations of TCLP, H2SO4-HNO3 and H2O in 250% FeCl3+125% cement treatment were reduced to the lowest values of 113.81, 399.28 and 347.27 mg·L−1, respectively, and As immobilization efficiency reached above 97%. This study can provide reference for the immobilization of high As-bearing sludge.
-
人工湿地由于运行简单,维护方便等特点,被广泛应用于养殖废水、农村生活污水、富营养化水体等方面的治理,但传统人工湿地存在的DO分布不均匀,扩散速率慢等问题,限制了污染物的高效去除。近年来的研究发现,通过改变湿地填料、增加曝气等,可以改善湿地内部环境,提高DO浓度,从而有效提升污染物的去除效果。LIU等[1]以红砖、粉煤灰作为填料,通过改变填料构成和配比,实现了污染物的高效去除;王宁等[2]在传统潜流湿地中增加间歇曝气,在强化氮素污染物去除的同时,能够实现氧化亚氮减排。生物炭作为一种性能优良的吸附剂[3],具有疏松多孔的特点,逐渐成为水体污染物去除的重要材料。通过投加生物炭,可以显著提升潜流湿地中氮素污染物的去除效果[4]。SUN等[5]将生物炭应用于间歇曝气湿地流湿地,使TN去除率提高了13.5%。生物炭还可以通过改变湿地环境,改善微生物菌落,促进污染物的去除[6]。DENG等[7]发现,生物炭添加能改变潜流湿地中微生物群落分布特征,增加Thauera、Candidatus Competibacter、Dechloromonas、Desulfobulbus、Chlorobium和Thiobacillus等功能菌属的相对丰度,提高对有机物和氮素污染物的降解能力。尽管已有研究关注了生物炭对潜流湿地污染物去除和微生物群落多样性的影响,但生物炭在间歇曝气湿地中对污染物和功能微生物等作用的研究尚显不足。本研究通过构建室内间歇曝气生物炭湿地,探究湿地中污染物的去除效果,分析微生物群落结构,揭示生物炭对间歇曝气湿地的影响特征,以期为生物炭在湿地工程中的应用提供参考。
1. 材料与方法
1.1 湿地系统构建
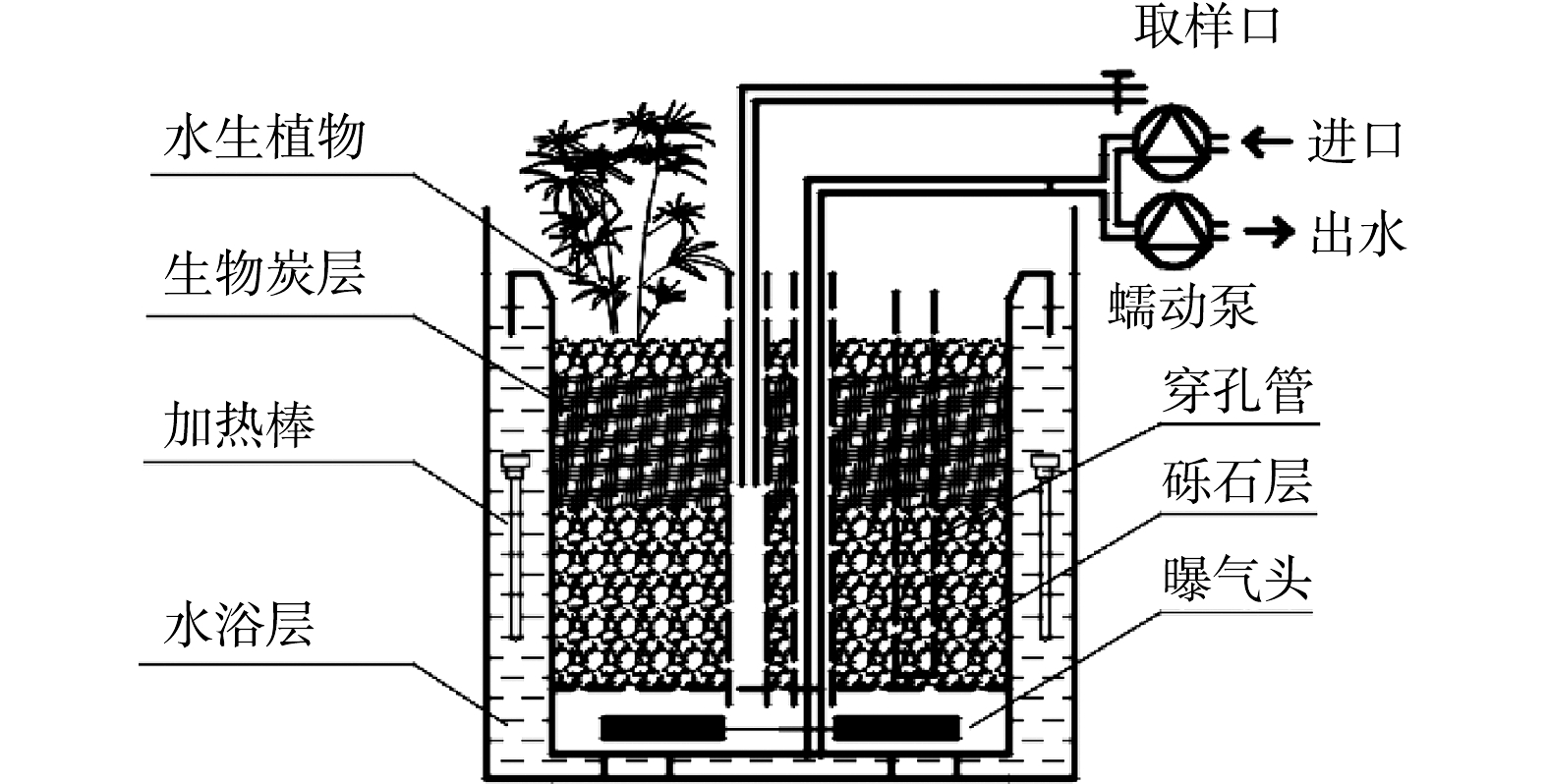
实验湿地装置采用圆柱形聚乙烯容器,每个装置底面积为0.1 m2,深为35 cm。装置内基质填充总高度为33 cm,下部填充粒径为1~3 cm的碎石,上部按0、10%、30%、40%比例填充生物炭,表层覆盖3 cm厚碎石层防止生物炭上浮,并依次命名为CW、BW1、BW2、BW3,每组设置1组平行,共计8个湿地反应器。反应器的结构如图1所示。实验所采用的生物炭参考HUANG等[8]的研究制备,制得的生物炭比表面积为345.92 m2·g‒1,孔径为1.95 nm,孔容为0.246 7 cm3·g‒1。湿地植物选用美人蕉(Canna indica L.),经驯化培养后移栽至反应器内,种植密度30 株·m−2。湿地污泥取自重庆市某生活污水处理厂,在经过人工配水驯化后一次性接种至湿地系统。
反应器中央设有1根直径为3 cm的穿孔管,用于虹吸排水、水样采集和物理化学相关参数的测定。另外布置3根穿孔管,其内部密封并装有与湿地等高的填料(下称填料柱),用于湿地微生物的采集。穿孔管孔径为0.5 cm,孔密度为500~600 个·m−2。反应器外部设有水浴层,控制湿地系统温度为(27±1) ℃,以保障湿地植物和微生物的稳定生长。容器底部设有微型曝气头进行间歇曝气,曝气量采用流量控制器(AST10-DX,阿斯特,北京)控制,曝气时间采用时间开关控制。
1.2 系统运行
本实验进水采用人工配水,每升水里包含0.235 g葡萄糖、0.114 g蔗糖、6.3 mg蛋白胨、0.2 g NaHCO3、0.16 g NH4Cl、0.5 g MgSO4·7H2O、0.44 g KH2PO4、0.72 g K2HPO4·3H2O、0.6 g FeSO4·7H2O、0.38 g CaCl2和微量元素液。每升微量元素液成分含量为0.15 g H3BO3、0.03 g CuSO4·5H2O、0.18 g KI、0.12 g MnCl2·4H2O、0.06 g Na2MoO4·2H2O、0.12 g ZnSO4·7H2O、0.15 g CoCl2·6H2O、10 g EDTA-2Na。经多次测定,进水水质为
NH+4 1.3 样品采集与测定
1)水质测定分析。本研究历时180 d,稳定运行后选取连续60 d对水质进行测定,每2 d测定1次,其中COD、
NH+4 NO−3 NO−2 2)微生物采集与测定。反应器稳定期间,从填料柱中采集样品,用200 mL进水将填料上的污泥全部洗出,收集泥水混合液,混匀后置于25 mL灭菌离心管中,在10 000 r·min−1转速下离心10 min,用磷酸缓冲液洗涤2次后,取沉淀样品置于−80 ℃下保存。本实验中微生物样品由上海美吉生物医药科技有限公司进行Illumina Miseq高通量测序。细菌所测区间为16S rRNA V3-V4可变区,测序引物为338F-806R。
1.4 数据分析
实验数据通过Origin 8.5整理作图,并由SPSS21.0进行数据分析。所有实验数据均采用平均值加减标准差表示。对象之间的差异性分析采用One-way ANOVA(水平包括P<0.05和P<0.01)。
2. 结果与讨论
2.1 生物炭添加量对湿地中DO、pH的影响
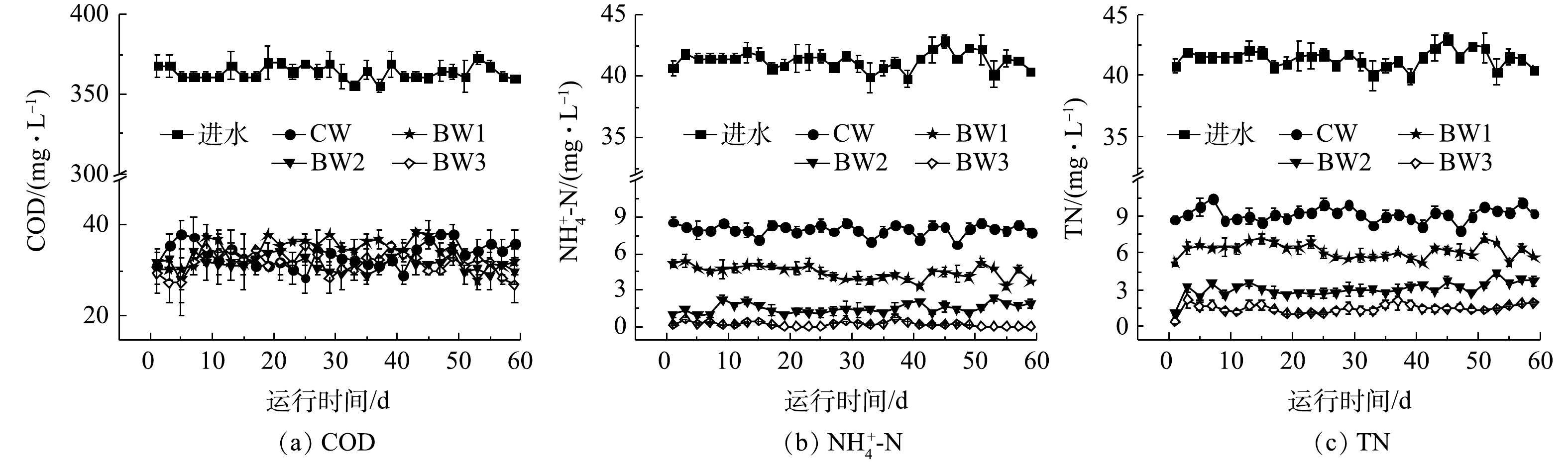
在曝气段,随着生物炭投加比例增加,湿地水体中平均DO浓度也随之显著增加(P<0.05)。BW3水体中平均DO质量浓度为2.5 mg·L−1,与CW相比,曝气段的平均DO质量浓度提高了13.6%(图2(a))。生物炭的多孔结构改善了湿地内部条件[10],有利于氧气的扩散,可帮助提高曝气段水体中DO质量浓度。在非曝气段,尽管生物炭能够提高水体中的DO质量浓度,但影响不显著(P>0.05)。湿地系统出水平均pH为7.15~7.35,呈中性(图2(b)),除BW1出水pH高于CW,BW2~BW3出水pH均低于CW。本研究添加的生物炭为碱性[8],可使湿地出水pH有所升高。但随着生物炭的投加比例的增加,湿地中硝化作用得到增强[11],使硝化过程消耗的碱度大大提升,从而导致湿地系统出水pH有所降低。
2.2 生物炭添加量对有机物去除及脱氮效果的促进
曝气湿地系统对有机物均表现出较高的去除效果(图3(a)),这与ZHOU等[2,4]的研究结果一致。CW~BW3系统中出水平均COD分别为(34.0±2.0)、(33.0±2.0)、(32.0±2.0)和(32.0±2.0) mg·L−1,去除率均已达到90%以上;BW2和BW3中出水COD显著低于CW。人工湿地中有机物的去除主要依赖于微生物的氧化分解,生物炭的添加增加了曝气段DO质量浓度,利于好氧微生物对有机物的代谢利用。此外,生物炭表面上含有不同功能基团,如羧基、羟基、羰基等[12-14],能够吸附各种有机物,从而降低其迁移率和提高生物利用率[15-17],进而利于有机物的进一步去除。
随着生物炭添加量的增加,各系统
NH+4 NH+4 NH+4 NH+4 NH+4 实验湿地中TN去除趋势与
NH+4 NO−3 NO−2 2.3 生物炭添加对湿地微生物群落结构特征的改变
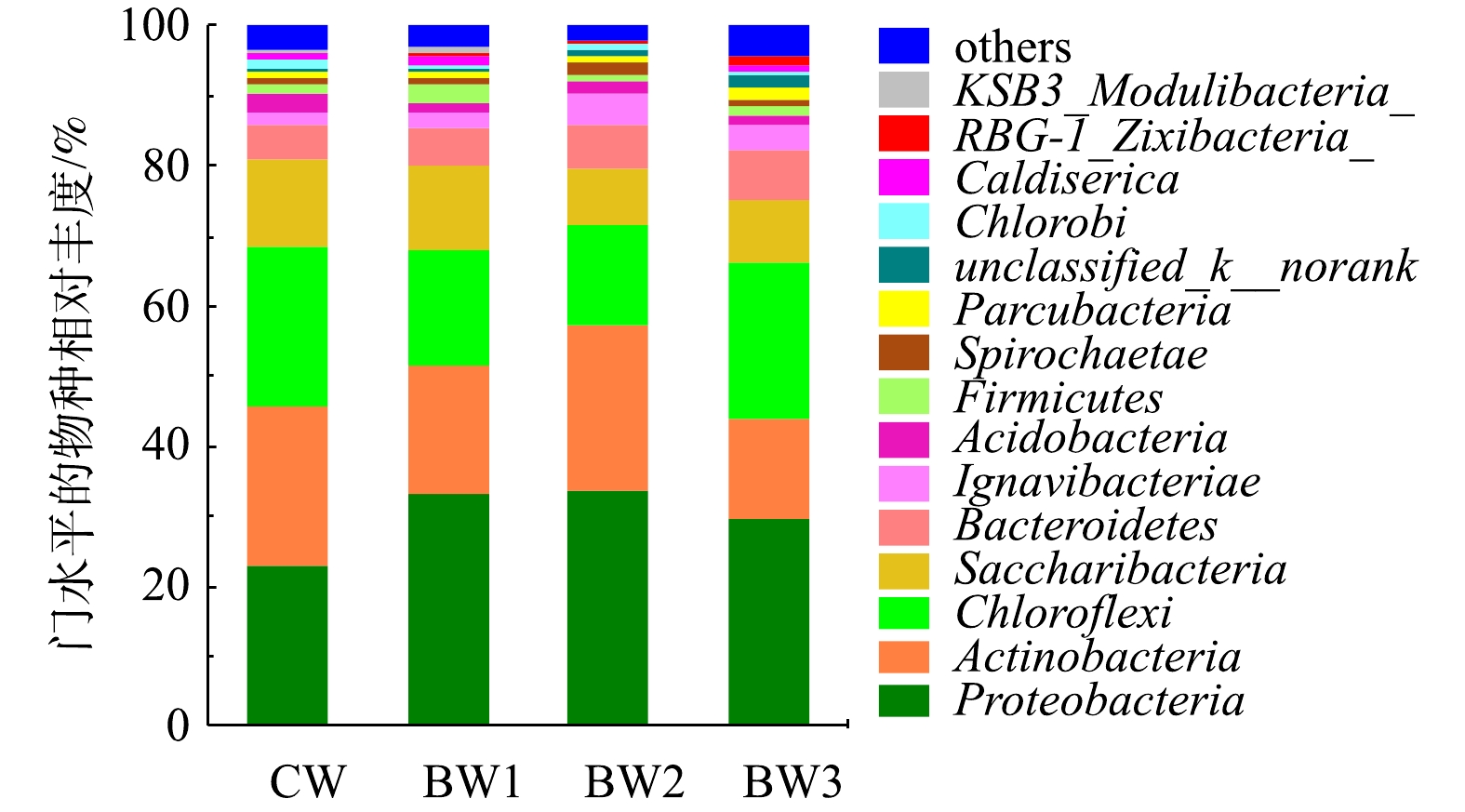
本研究采用Illumina Miseq高通量测序,分析了各反应系统微生物群落结构的特征(图4)。在4个湿地系统中均检测出13种菌门,包括变形菌门(Proteobacteria)、放线菌门(Actinobacteria)、绿弯菌门(Chloroflexi)、螺旋体菌门(Saccharibacteria)、拟杆菌门(Bacteroidetess)、Ignavibacteria、酸杆菌门(Acidobacteria)、厚壁菌门(Firmicutes)、螺旋体菌门(Spirochaetae)、Parcubacteria、绿菌门(Chlorobi)、嗜热丝菌门(Caldiserica)和芽单胞菌门(Gemmatimonadetes),其中Proteobacteria、Actinobacteria、Chloroflexi和Saccharibacteria的丰度相对较高。Proteobacteria包括β-变形菌亚门(Betaproteobacteria)和γ-变形菌亚门(Gammaproteobacteria),其在生物脱氮除磷[23]和有机物降解中[24]起核心作用。大量的氨氧化菌(AOB)、亚硝酸氧化菌(NOB)和反硝化菌均属于β-变形菌亚门和γ-变形菌亚门[25],间歇曝气能显著提升AOB和NOB的数量[26],生物炭的投加能提高DO质量浓度可增加Proteobacteria丰度。Actinobacteria是1种偏碱性菌[27],添加生物炭降低了出水pH,因此,Actinobacteria数量在生物炭系统中略有降低。整体来看,生物炭添加增加了Bacteroidetess和Proteobacteria的数量,降低了Actinobacteria、Chloroflexi和Saccharibacteria的相对丰度。
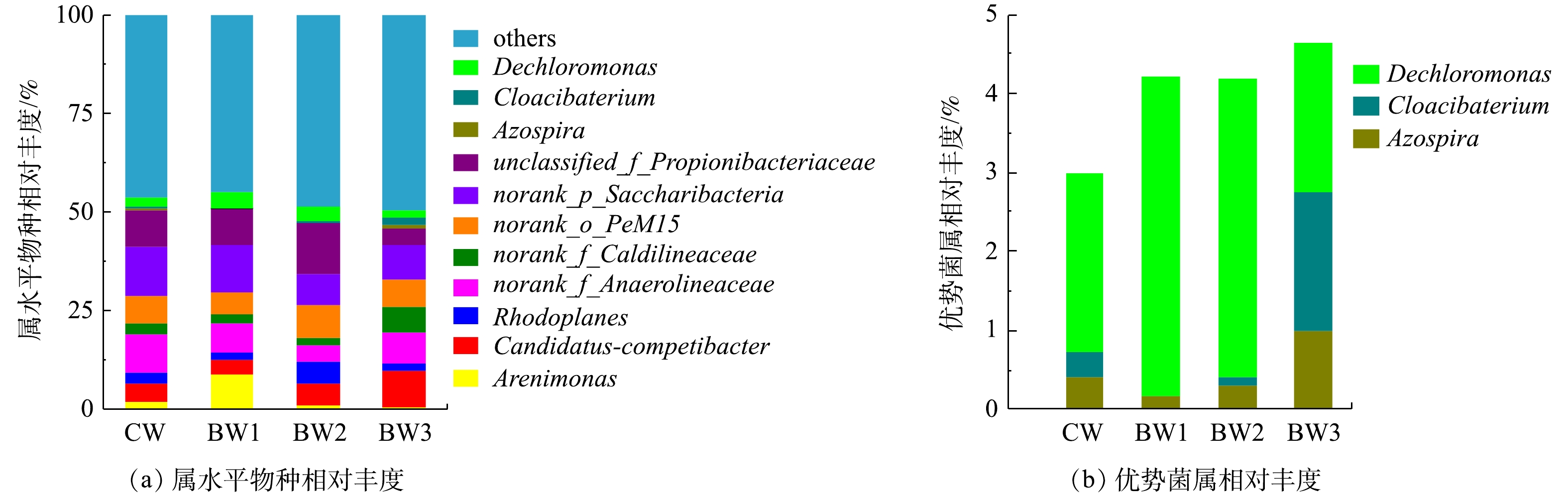
为了进一步研究生物炭添加比例对属水平微生物种群结构的影响,取相对丰度占比超过5%的前9个属,其他菌属归于Others(图5)。结果表明,湿地系统中主要的优势菌属有Candidatus_Competibacter属、红游动菌属(Rhodoplanes)、丙酸杆菌科(Propionibacteriaceae)中的1个未知属等,生物炭添加改变了湿地系统中微生物属水平的种群结构。
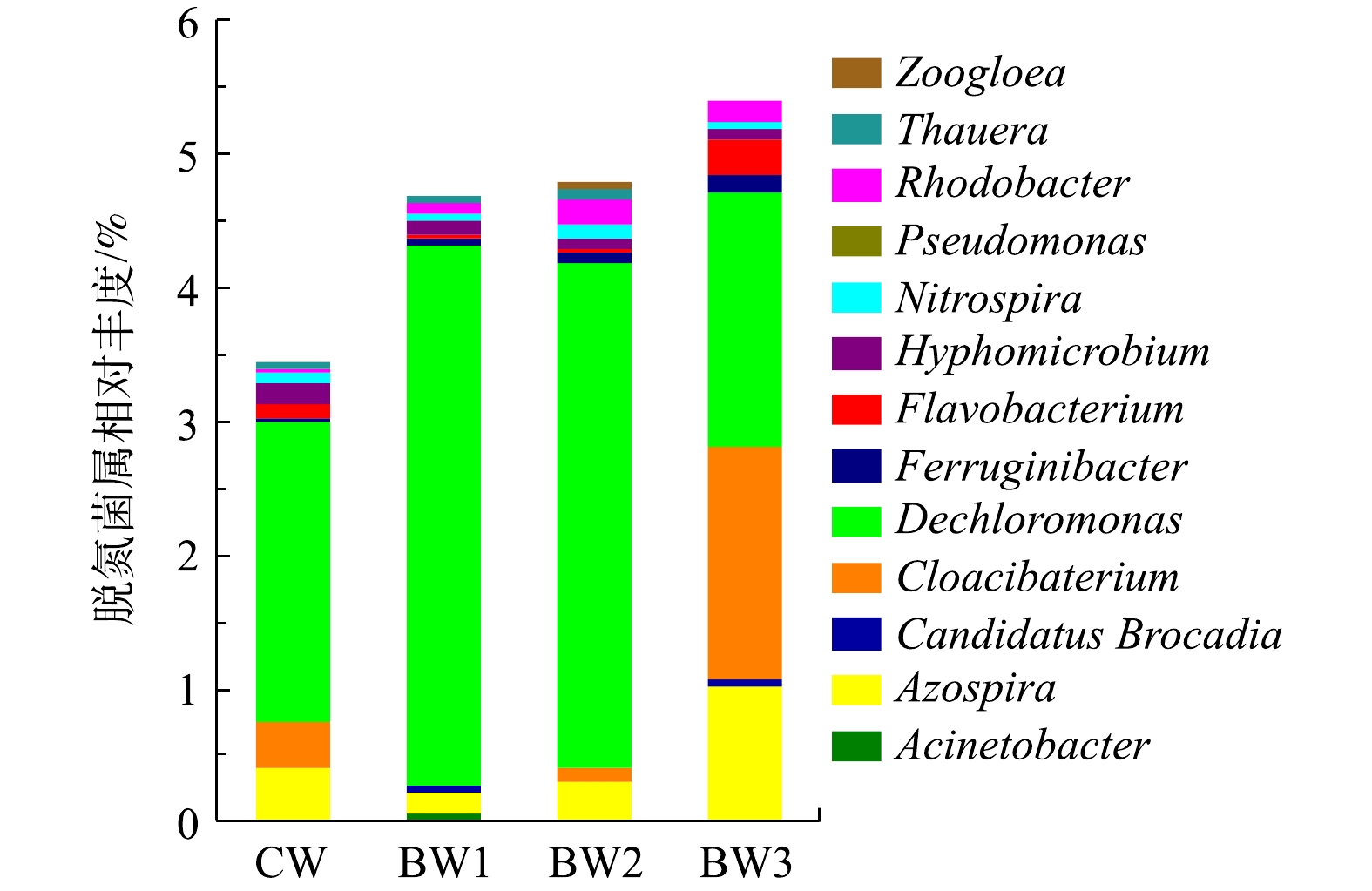
各湿地系统中检出与脱氮相关的菌属共13种(图6),检测到的硝化菌属是硝化螺旋菌属(Nitrospira)。Nitrospira在好氧条件下将
NO−2 NO−3 NO−3 NO−2 3. 结论
1)在间歇曝气潜流湿地中,投加生物炭可以显著增加曝气段DO质量浓度,但对非曝气段DO质量浓度影响较小。湿地系统出水呈中性,生物炭的投加未对出水pH造成显著影响。
2)生物炭对湿地系统中有机物去除影响不显著,但投加生物炭可提高
NH+4 NO−3 NO−2 3)生物炭改变了湿地系统中微生物种群的相对丰度,可以增加Nitrospira、Thauera、Rhodobacter和Pseudomonas等10余种与脱氮相关的菌属丰度,从而提高脱氮效率。
-
表 1 固定化材料筛选实验设计
Table 1. Design of As immobilization materias screening experiment
实验处理 固定化材料名称 材料投加量/g 摩尔比 1 Na2S·9H2O 7.88 S/As=0.5∶1 2 CaO 1.84 Ca/As=0.5∶1 3 Fe0 1.22 Fe/As=0.3∶1 4 FeSO4·7H2O 6.08 Fe/As=0.3∶1 5 Fe(NO3)3·9H2O 8.84 Fe/As=0.3∶1 6 FeCl3 3.55 Fe/As=0.3∶1 7 Fe2O12S3 4.37 Fe/As=0.3∶1 8 Fe(OH)3 2.34 Fe/As=0.3∶1 9 Fe2O3 1.75 Fe/As=0.3∶1 10 Al2O3 1.11 Al/As=0.3∶1 空白对照 — — — 表 2 FeCl3与水泥配伍实验设计
Table 2. Design of the composite experiments of FeCl3 and cement
实验处理 砷泥/g FeCl3/g 水泥/g 1 20 10 — 2 20 20 — 3 20 30 — 4 20 40 — 5 20 50 — 6 20 — 5 7 20 — 10 8 20 — 15 9 20 — 20 10 20 — 25 11 20 50 5 12 20 50 10 13 20 50 15 14 20 50 20 15 20 50 25 空白对照 20 — — 注:—表示未添加,药剂添加顺序为先加FeCl3,再加水泥。 表 3 污泥中As浸出浓度和浸出量
Table 3. As leaching concentration and quantity of tested sludge
浸出方法 浸出浓度/(mg·L−1) 浸出量/(mg·kg−1) TCLP 10 634.05 21 2681.00 H2SO4-HNO3 14 961.25 149 612.50 H2O 12 276.50 122 765.00 SBET 2 678.14 26 7814.00 表 4 污泥中As结合态分布
Table 4. Distribution of As binding form in tested sludge
As结合态 含量/(mg·kg−1) 占总As百分比/% F1非专性吸附态 99 264.50±3 176.46 24.04 F2专性吸附态 132 332.50±5 425.63 32.05 F3无定形和弱结晶铁铝或铁锰水化氧化物结合态 54 535.50±2 399.56 13.21 F4结晶铁锰或铁铝水化氧化物结合态 48 348.25±1 257.05 11.71 F5残渣态 78 369.00±2 899.65 18.98 -
[1] RIVEROS P A, DUTRIZAC J E, SPENCER P, et al. Arsenic disposal practices in the metallurgical industry[J]. Canadian Metallurgical Quarterly, 2001, 40: 395-420. doi: 10.1179/cmq.2001.40.4.395 [2] CLANCY T M, HAYES K F, RASKIN L. Arsenic waste management: A critical review of testing and disposal of arsenic-bearing solid wastes generated during arsenic removal from drinking water[J]. Environmental Science & Technology, 2013, 47(19): 10799-10812. [3] KAMESWARI K S B, BHOLE A G, PARAMASIVAM R, et al. Evaluation of solidification/stabilization(S/S) process for the disposal of arsenic-bearing sludges in landfill sites[J]. Environmental Engineering Science, 2002, 18(3): 167-176. [4] 国家环境保护总局, 国家质量监督检验检疫总局. 危险废物填埋污染控制标准: GB 18598-2001[S]. 北京: 中国环境科学出版社, 2001. [5] MOORE T J, RIGHTMIRE C M, VEMPATI R K, et al. Ferrous iron treatment of soils contaminated with arsenic containing wood-preserving solution[J]. Soil and Sediment Contamination, 2000, 9(4): 375-405. doi: 10.1080/10588330091134310 [6] KUMPIENE J, LAGERKVIST A, MAURICE C. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments: A review[J]. Waste Management, 2008, 28: 215-225. doi: 10.1016/j.wasman.2006.12.012 [7] KOMÁREK M, VANEK A, ETTLER V. Chemical stabilization of metals and arsenic in contaminated soils using oxides: A review[J]. Environmental Pollution, 2013, 172(172C): 9-22. [8] 纪冬丽, 孟凡生, 薛浩, 等. 国内外土壤砷污染及其修复技术现状与展望[J]. 环境工程技术学报, 2016, 6(1): 90-99. doi: 10.3969/j.issn.1674-991X.2016.01.014 [9] NAZARI A M, RADZINSKI R, GHAHREMAN A. Review of arsenic metallurgy: Treatment of arsenical minerals and the immobilization of arsenic[J]. Hydrometallurgy, 2017, 174: 258-281. doi: 10.1016/j.hydromet.2016.10.011 [10] PORTER S K, SCHECKEL K G, IMPELLITTERI C A, et al. Toxic metals in the environment: Thermodynamic considerations for possible immobilisation strategies for Pb, Cd, As, and Hg[J]. Critical Reviews in Environmental Science & Technology, 2004, 34(6): 495-604. [11] 高峰, 贾永忠, 孙进贺, 等. 锌冶炼废渣浸出液硫化法除砷的研究[J]. 环境工程学报, 2011, 5(4): 812-814. [12] MOON D H, DERMANTAS D, MENOUNON N. Arsenic immobilization by calcium-arsenic precipitates in lime treated soil[J]. Science of the Total Environment, 2004, 330(1/2/3): 171-185. [13] WENZEL W W, KIRCHBAUMER N, PROHASKA T, et al. Arsenic fractionation in soils using an improved sequential extraction procedure[J]. Analytica Chimica Acta, 2001, 436(2): 309-323. doi: 10.1016/S0003-2670(01)00924-2 [14] SEAMAN J C, HUTCHISON J M, JACKSON B P, et al. In situ treatment of metals in contaminated soils with phytate[J]. Journal of Environmental Quality, 2003, 32: 153-161. doi: 10.2134/jeq2003.1530 [15] CARLSON L, BIGHAM J M, SCHWERTMANN U, et al. Scavenging of As from acid mine drainage by schwertmannite and ferrihydrite: A comparison with synthetic analogues[J]. Environmental Science & Technology, 2002, 36: 1712-1719. [16] HARTLEY W, EDWARDS R, LEPP N W. Arsenic and heavy mental mobilization in iron oxide-amended contaminated soils as evaluated by short and long-term leaching tests[J]. Environmental Pollution, 2004, 131(3): 495-504. doi: 10.1016/j.envpol.2004.02.017 [17] 胡立琼, 曾敏, 雷鸣, 等. 含铁材料对污染水稻土中砷的固定化效果[J]. 环境工程学报, 2014, 8(4): 13-18. [18] 黄玲, 雷鸣, 胡立琼, 等. 2种含铁物质对底泥中砷的固化效果[J]. 水土保持学报, 2014, 28(1): 253-256. doi: 10.3969/j.issn.1009-2242.2014.01.048 [19] DERMATAS D, MENG X. Utilization of fly ash for stabilization/solidification of heavy metal contaminated soils[J]. Engineering Geology, 2003, 70: 377-394. doi: 10.1016/S0013-7952(03)00105-4 [20] COUSSY S, PAKTUNC D, ROSE J, et al. Arsenic speciation in cemented paste backfills and synthetic calcium-silicate-hydrates[J]. Minerals Engineering, 2012, 39(10): 51-61. [21] VOGLARA G E, LESTAN D. Efficiency modeling of solidification/stabilization of multi-metal contaminated industrial soil using cement and additives[J]. Journal of Hazardous Materials, 2011, 192: 753-762. doi: 10.1016/j.jhazmat.2011.05.089 [22] VINTER S, MONTANES M T, BEDNARIK V, et al. Stabilization/solidification of hot dip galvanizing ash using different binders[J]. Journal of Hazardous Materials, 2016, 320: 105-113. doi: 10.1016/j.jhazmat.2016.08.023 [23] LIU D G, MIN X B, KE Y, et al. Cotreatment of flotation waste, neutralization sludge, and arsenic-containing gypsum sludge from copper smelting: Solidification/stabilization of arsenic and heavy metals with minimal cement clinker[J]. Environment Science Pollution, 2017, 25(8): 7600-7607. [24] KOGBARA R B. A review of the mechanical and leaching performance of stabilized/solidified contaminated soils[J]. Environment Reviews, 2013, 22: 66-86. [25] LI J S, WANG L, CUI J L, et al. Effects of low-alkalinity binders on stabilization/solidification of geogenic As-containing soils: Spectroscopic investigation and leaching tests[J]. Science of the Total Environment, 2018, 631-632: 1486-1494. doi: 10.1016/j.scitotenv.2018.02.247 [26] 中华人民共和国农业部. 土壤检测 第2部分: 土壤pH的测定: NY/T 1121.2-2006[S]. 北京: 中国农业出版社, 2006. [27] US EPA. Test methods for evaluating solid waste, physical/chemical methods[DB/OL]. [2019-05-01]. http://www.epa.gov/SW-846/main.htm. [28] 国家环境保护总局. 固体废物浸出毒性浸出方法 硫酸硝酸法: HJ/T 299-2007[S]. 北京: 中国环境科学出版社, 2007. [29] 中华人民共和国环境保护部. 固体废物浸出毒性浸出方法 水平振荡法: HJ 557-2010[S]. 北京: 中国环境科学出版社, 2010. [30] RAURET G, LOPEZ-SANCHEZ J F, SAHUQUILLO A, et al. Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials[J]. Journal of Environmental Monitoring, 1999, 1(1): 57-61. doi: 10.1039/a807854h [31] BI C, ZHOU Y, CHEN Z, et al. Heavy metals and lead isotopes in soils, road dust and leafy vegetables and health risks via vegetable consumption in the industrial areas of Shanghai, China[J]. Science of the Total Environment, 2018, 619-620: 1349-1357. doi: 10.1016/j.scitotenv.2017.11.177 [32] GARCIA-MANYES S, JIMENEZ G, PADRO A, et al. Arsenic speciation in contaminated soils[J]. Talanta, 2002, 58(1): 97-109. doi: 10.1016/S0039-9140(02)00259-X [33] MARTA I, LITTER, MARIA E, et al. Possible treatments for arsenic removal in Latin American waters for human consumption[J]. Environmental Pollution, 2010, 158(5): 1105-1118. doi: 10.1016/j.envpol.2010.01.028 [34] 刘辉利, 梁美娜, 朱义年, 等. 氢氧化铁对砷的吸附与沉淀机理[J]. 环境科学学报, 2009, 29(5): 1011-1020. doi: 10.3321/j.issn:0253-2468.2009.05.019 [35] BASKAN M B, PALA A. Determination of arsenic removal efficiency by ferric ions using response surface methodology[J]. Journal of Hazardous Materials, 2009, 166(2/3): 796-801. [36] GUTIERREZ C, HANSEN H K, NUNEZ P, et al. Electrochemical peroxidation using iron nanoparticles to remove arsenic from copper smelter wastewater[J]. Electrochimica Acta, 2015, 181: 228-232. doi: 10.1016/j.electacta.2015.01.070 [37] YOKOYAMA Y, TANAKA K, TAKAHASHI Y. Differences in the immobilization of arsenite and arsenate by calcite[J]. Geochimica et Cosmochimica Acta, 2012, 91: 202-219. doi: 10.1016/j.gca.2012.05.022 [38] 张淑媛, 童宏祥, 徐诗琦, 等. 次氯酸钙/氧化钙对高砷污泥的氧化固定化处理[J]. 环境工程学报, 2018, 12(2): 625-629. doi: 10.12030/j.cjee.201706216 [39] WARREN G P, ALLOWAY B J. Reduction of arsenic uptake by lettuce with ferrous sulfate applied to contaminated soil[J]. Journal of Environmental Quality, 2003, 32(3): 767-772. doi: 10.2134/jeq2003.7670 [40] 曹宇, 陈明, 张佳文, 等. 硫化钠对土壤中铅镉的固定效果[J]. 环境工程学报, 2014, 8(2): 782-788. [41] LADEIRA A C Q, CIMINELLI V S T, DUARTE H A, et al. Mechanism of anion retention from EXAFS and density functional calculations: Arsenic(V) adsorbed on gibbsite[J]. Geochimica Et Cosmochimica Acta, 2001, 65: 1211-1217. doi: 10.1016/S0016-7037(00)00581-0 [42] WANG Y N, ZENG X B, LU Y H, et al. Effect of aging on the bioavailability and fractionation of arsenic in soils derived from five parent materials in a red soil region of Southern China[J]. Environmental Pollution, 2015, 207: 79-87. doi: 10.1016/j.envpol.2015.08.033 [43] 王强, 锦春, 魏世强. 赤铁矿对砷的吸附解吸及氧化特征[J]. 环境科学学报, 2008, 28(8): 1612-1617. doi: 10.3321/j.issn:0253-2468.2008.08.018 [44] RAMOS M A V, YAN W, LI X, et al. Simultaneous oxidation and reduction of arsenic by zero-valent iron nanoparticles: understanding the significance of the core-shell structure[J]. Journal of Physical Chemistry C, 2009, 113(33): 14591-14594. doi: 10.1021/jp9051837 [45] LEUPIN O X, HUG S J. Oxidation and removal of arsenic(III) from aerated groundwater by filtration through sand and zero-valent iron[J]. Water Research, 2005, 39(9): 1729-1740. doi: 10.1016/j.watres.2005.02.012 [46] 雷鸣, 曾敏, 胡立琼, 等. 3种含铁材料对重金属和砷复合污染底泥固定化处理[J]. 环境工程学报, 2014, 8(9): 3983-3988. [47] KIM J Y, DAVIS A P, KIM K W. Stabilization of available arsenic in highly contaminated mine tailings using iron[J]. Environmental Science & Technology, 2003, 37(1): 189-195. [48] 熊正为, 朱雷, 杨博豪, 等. 水泥回转窑共处置含砷污泥[J]. 环境工程学报, 2016, 10(1): 301-305. doi: 10.12030/j.cjee.20160149 -




 下载:
下载: