-
随着我国城镇化水平的不断提高,污水处理设施建设高速发展。有机污染物综合指标(COD、TN等)已无法准确反映水质污染的严重性。许多新兴有机微污染物(如酚类、苯系物等),虽然在环境中残留水平很低,对综合水质指标BOD、COD和TOC等的贡献极小,但其环境危害却很大[1-5]。为了进一步削减污水中痕量有机污染物的排放通量,目前已经有许多污水深度处理技术方面的研究,主要包括膜分离技术、高级氧化技术、电化学氧化技术和吸附技术等[6]。膜技术在再生水处理中应用广泛,但因膜污染严重,制约了其规模化应用[7]。氧化技术存在产生有毒副产物、催化剂的高度选择性以及难分离回收、处理成本高等问题[8-9],导致目前实际应用并不多。活性炭是一种多孔性物质,且易于自动控制,对水量、水质、水温变化适应性强[10-11]。因此,活性炭吸附法是一种具有广阔应用前景的污水深度处理技术。
污泥基生物炭(sludge-derived carbons,SC)是一种以城市污泥为原料制备的高含碳物质,具有高的孔隙率、发达的空隙结构和大的比表面积、丰富的表面活性结合位点,是一种新型廉价高效的吸附剂[12],对重金属、染料中间体、抗生素及内分泌干扰物具有较强的吸附性能[13]。有研究表明,污泥热解条件(温度、终温停留时间、升温速率)是影响污泥炭的微观形貌和表面化学性质的重要因素,不同的表面理化性质对有机物吸附能力各有不同。为了使污泥炭具有一定的应用性能,众多学者深入研究了污泥炭热解条件对污泥炭性质影响,分析了吸附过程的作用机制。YUN等[14]发现,在高温下,热解污泥会得到更大的比表面,同时炭基材料的芳香化程度更高。SEREDYCH等[15]研究指出,热解温度与终温停留时间和升温速率相比,是影响污泥微观形貌更为重要的因素。DING等[16]指出,在400 ℃条件下制备的污泥炭的微孔比例并不高,但其表面化学特性可提高其对抗生素的吸附能力。
本研究基于前人研究的基础,旨在通过分析热解温度对污泥炭基材料表面理化性质、微观形貌的调控,探究污泥炭表面理化性质对污水厂深度处理过程中有机物的吸附效能的影响,深入分析污泥炭表面的理化性质与吸附有机物种类的匹配机制,为污泥炭用于污水深度处理过程中有机物的吸附提供参考。
-
实验用污泥取自北京市高碑店再生水厂脱水污泥,置于105 ℃鼓风烘干箱中,烘干至恒重,然后研磨粉碎,过100目筛,保存于干燥器中。污水取自其再生水厂二沉池,置于4 ℃冷藏室。污泥性质如下:有机质含量54%,比表面11.64 m2·g−1,孔容0.060 m3·g−1,平均孔径20.69 nm。
-
1)污泥碳的制备。污泥热解装置采用开启式管式炉(合肥科晶材料技术有限公司,OTF-1200X)。取烘干过筛后污泥10 g,置干污泥放入瓷舟中,用瓷质盖板将瓷舟盖住,防止通气导致的材料损失。首先以1 L·min−1向水平石英管内通入氮气20 min,再以10 ℃·min−1升至所选定的峰值温度(400、500、600、700和800 ℃)。在设定的5个温度梯度下,分别热解30 min后,冷却至室温,热解过程中氮气流量为0.5 L·min−1,冷却过程继续通氮气,直至石英管冷却至室温。待样品冷却后,研磨过100目筛并密封保存。将在400、500、600、700和800 ℃制备的污泥基生物炭分别记为SC400、SC500、SC600、SC700和SC800,原污泥记为SC。
2)等温吸附实验。取一定量的不同热解温度下的污泥炭粉末(0.075 g)加入装有150 mL二沉池污水的250 mL锥形瓶中,将其置于恒温振荡箱中,140 r·min−1摇振8 h,反应后样品过0.45 μm滤膜,测定吸附后剩余溶液的COD及三维荧光。
3)吸附动力学实验。在500 mL锥形瓶中加入250 mL二沉池污水,加入0.1 g污泥炭样品。在298.15 K恒温条件下,用磁力搅拌器搅拌,并在10、20、30、45、60、120、240、360、480 min取样,过0.45 μm滤膜,测定吸附后剩余溶液的COD及三维荧光。
-
1)污泥炭基材料表征。采用全自动比表面积与孔径分析仪(美国麦克仪器公司,Micromeritics ASAP2020HD88)对污泥炭的比表面积、孔径结构及分布进行分析。采用X-射线光电子能谱仪(美国赛默飞世尔科技公司,ESCALAB250Xi)对污泥炭表面元素及结合能的变化进行分析。采用红外光谱(美国赛默飞世尔科技公司,Nicolet 8700)对污泥炭表面官能团进行分析。采用场发射扫描电镜(HITACHI SU-8020)对污泥活性炭表面形貌进行分析。
2)吸附实验分析方法。COD测定参照《城镇污水处理厂污染物排放标准》(GB 18918-2002)。污水中有机物组成采用三维荧光光谱仪(日立F-7000)进行分析,该方法广泛应用于天然水体中有机物的测定,并且具有高度的识别性和灵敏度[17]。
污泥基生物炭对COD的平衡吸附量Qe的计算方法见式(1)。
式中:Qe为平衡吸附量,mg·g−1;C0为初始溶液中COD的质量浓度,mg·L−1;Ce为吸附平衡后溶液中COD的质量浓度,mg·L−1;V为溶液体积,L;m为投加生物炭质量,g。所有检测数据采用ORIGIN进行数据运算及作图。
-
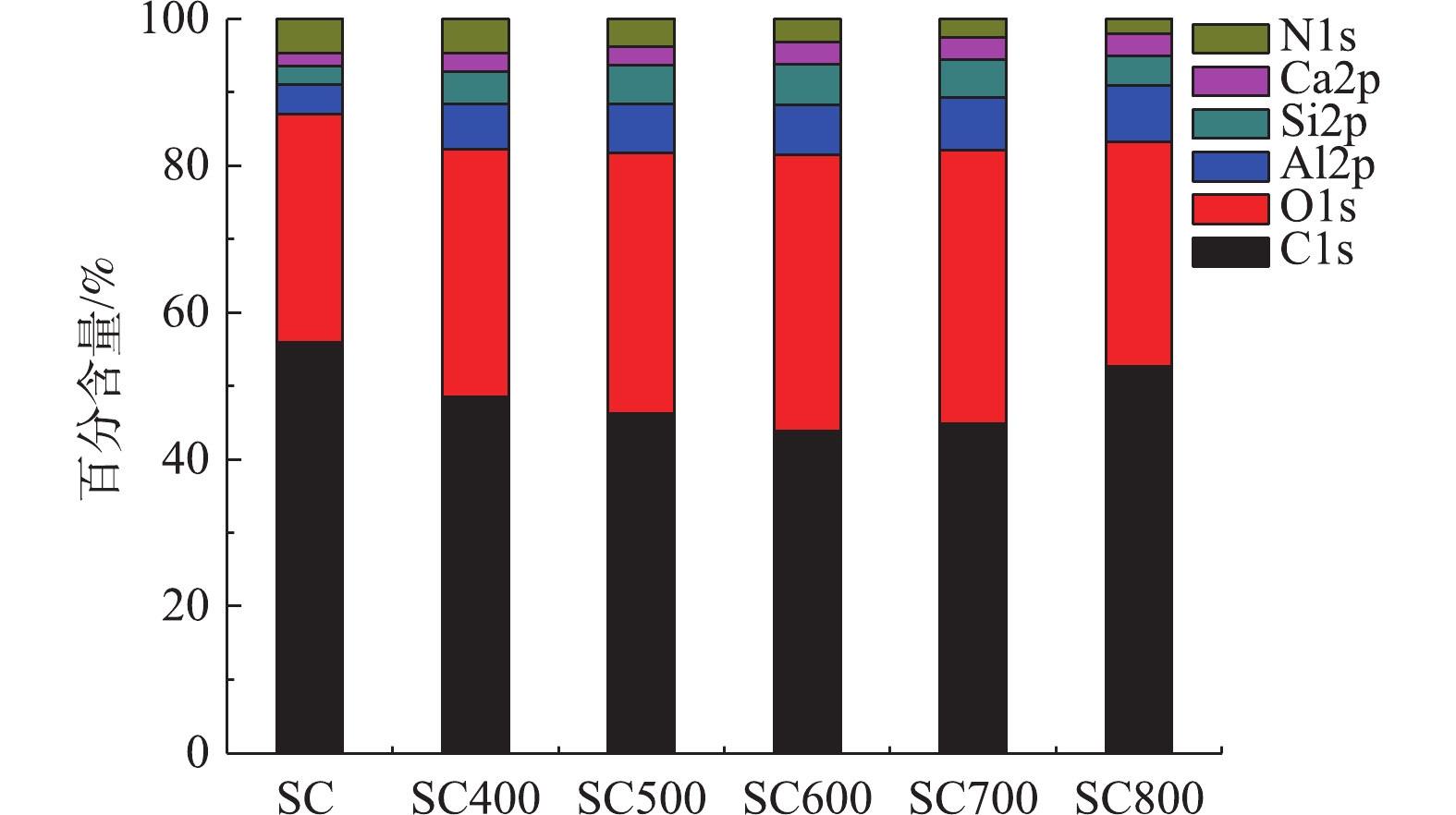
1)污泥炭表面元素组成分析。图1为不同热解温度下污泥炭及污泥表面元素组成、含量及变化情况。由图1可知,热解前污泥主要由C、O、N等有机元素和Al、Ca、Si等无机元素构成,其中C含量为55.96%,较高的炭含量使其较易转化为含炭吸附剂。从图1中可以发现,污泥热解前后污泥炭表面元素含量均发生变化,随着热解温度的升高,C含量呈现先下降后升高趋势,O含量呈现先升高后降低趋势,N含量逐渐降低。这可能是由于在低温热解时,污泥中易挥发物质(脂肪族和糖类化合物)优先发生热分解反应,释放出HCN、CO、CH4等气态组分[18];而热解温度较高时,污泥中的不饱和烃和芳香类物质发生稠环化裂解反应[19],C元素以气体形式释放比例较少,使得污泥中碳含量升高。这说明较高的热解温度有利于得到碳含量高且性质稳定的污泥炭。Si、Al、Ca等无机成分由于C、O等元素的烧失,相对含量上升。
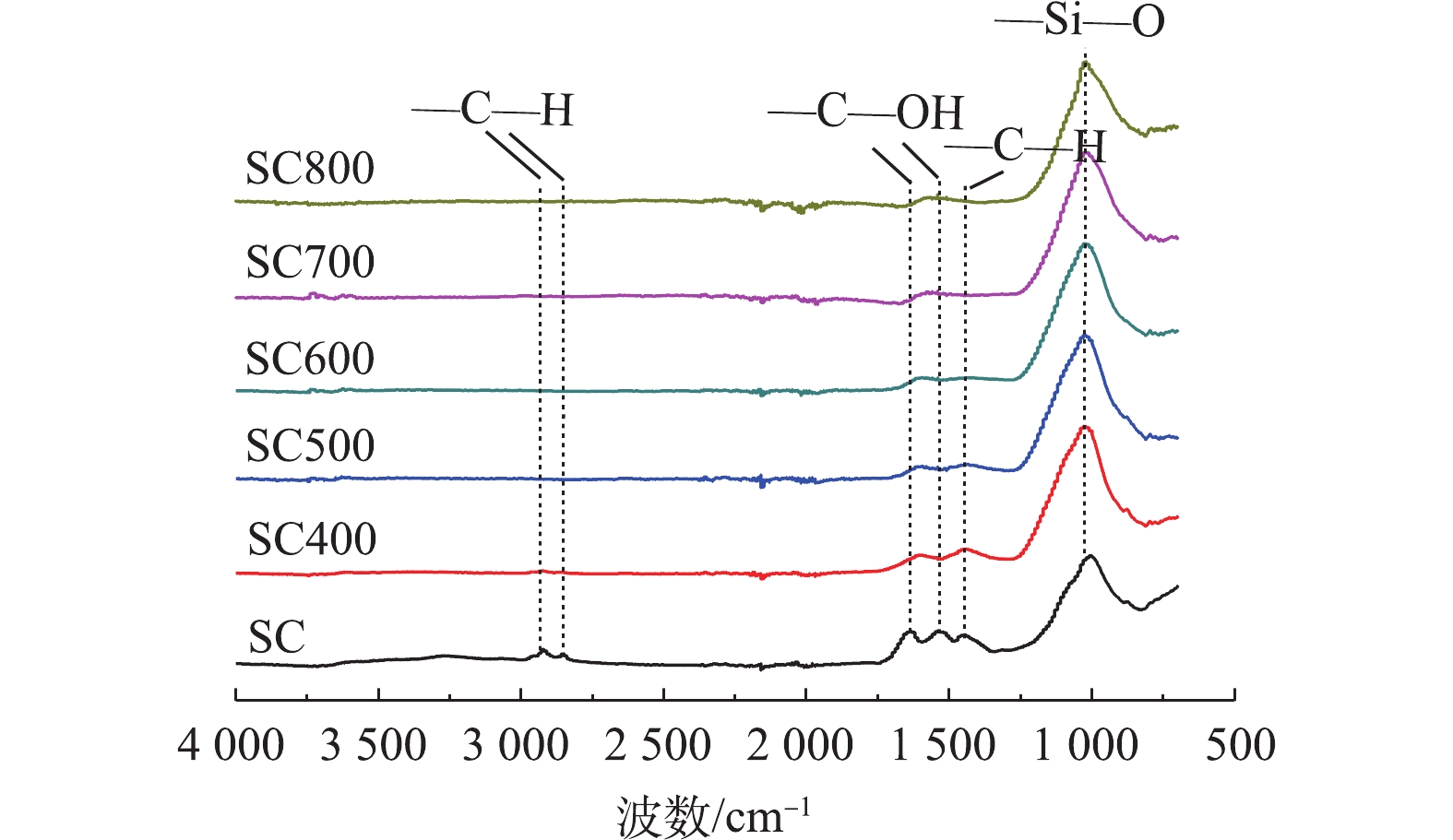
2)污泥炭表面官能团组成分析。图2为不同热解温度下制得的污泥炭及污泥的FT-IR谱图。由图2可知,2 800~3 000 cm−1的吸收峰以及1 460 cm−1的吸收峰为脂肪碳的—CHx的拉伸、收缩和弯曲振动峰[20]。热解温度小于600 ℃时,2 800~3 000 cm−1的吸收峰以及1 460 cm−1的吸收峰明显减弱。温度≥600 ℃时,污泥炭中的—CHx吸收峰趋近于0,表明温度超过600 ℃时,污泥中的—CHx在热解后基本完全分解。1 540 cm−1为羧基的—C=O不对称拉伸振动峰,1 645 cm−1为酮类和酰胺基的—C=O伸缩振动峰[21]。污泥炭的—C=O吸收峰皆明显弱于污泥,是由于热解过程中发生了强烈的脱羧反应,—C=O发生分解。污泥炭中无机组分均高于污泥(图1),因此,污泥炭的Si—O伸缩振动峰均有所加强。
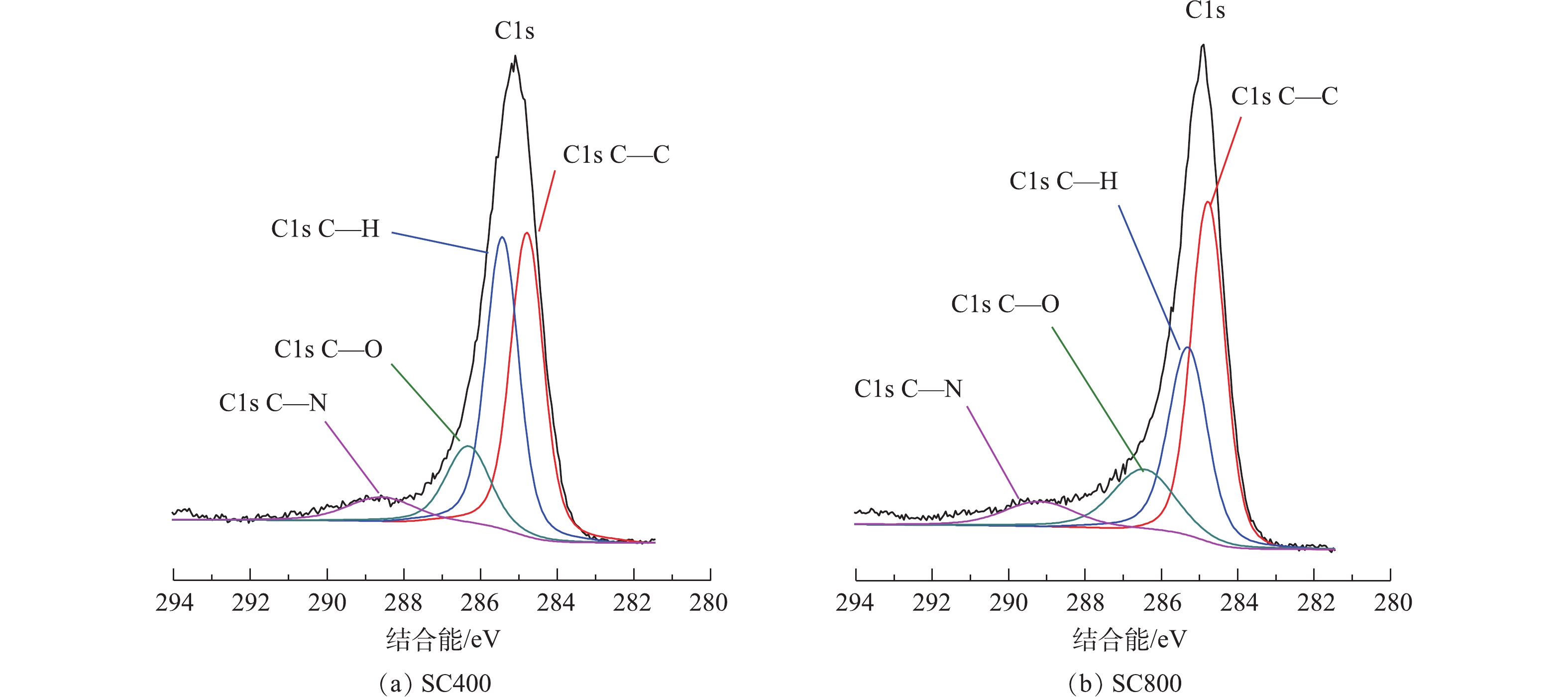
表1为XPS分析拟合得到的不同热解温度下污泥炭及污泥表面官能团的相对含量。由表1可知,随着热解温度的升高,C—C结合比例呈现整体升高趋势,C—N和O—C=O结合比例呈现整体降低趋势,C—OH/C—OR结合比例呈现先降低后小幅升高趋势。图3为SC400和SC800的C1s的XPS谱图,拟合得到4条曲线,分别代表石墨炭(284.5 eV)、羟基或醚基官能团中的炭(286.1 eV)、氨基官能团中的炭(285.5 eV)、羰基或醌基官能团中的炭(287.3 eV)[22]。由图3可以看出,SC800相比于SC400,C—C相对含量明显上升,C—H比例明显降低。这表明较高热解温度下制备的污泥炭芳香化程度增强,生成了稳定性更好的含碳官能团,同时保留了丰富的含氧官能团(如酯、酚和羰基)。污泥炭表面的含氮官能团可能是因为污泥中微生物释放的含氮有机物热解过程中暴露在污泥炭表面。
3)污泥炭比表面积和孔容分析。表2为不同热解温度下污泥炭及污泥的比表面积、孔容的变化情况。由表2可知,随着热解温度的升高,污泥炭的比表面积呈现阶跃升高趋势。热解前的比表面积仅为11.64 m2·g−1,当热解温度升高至500 ℃时,比表面积有显著提升,500~700 ℃趋于稳定,700~800 ℃又出现快速升高,最终比表面积为115.3 m2·g−1;而孔容也存在相同的变化趋势,随热解温度升至800 ℃,由0.060 m3·g−1增加至0.174 m3·g−1。
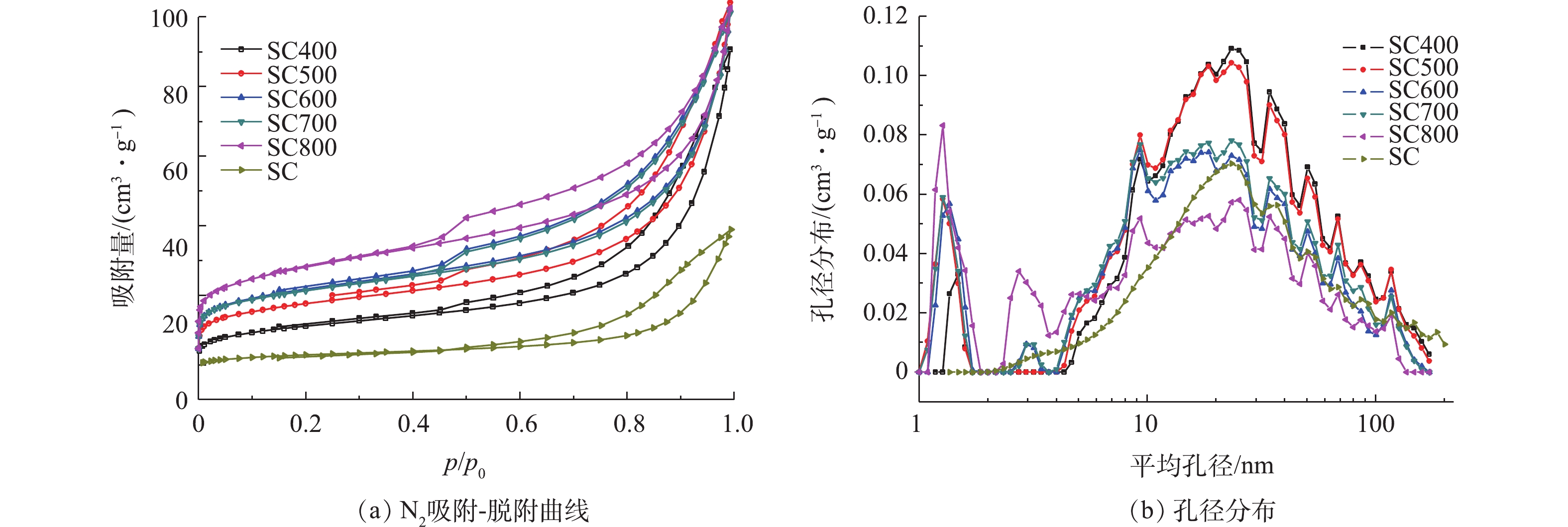
图4为不同热解温度下污泥炭及污泥的吸附、脱附曲线及孔径分布。由图4可知,不同热解温度下得到相似的等温吸附曲线,而且存在明显的滞回环。这表明污泥炭微观结构中不仅存在比例较大的介孔结构,同时不同温度下获得的污泥炭具有不同的孔隙结构和表面。由孔径分布可知,污泥未热解时主要以20 nm左右的介孔为主,随着热解温度的升高,出现了微孔,且微孔数量不断增多。结合材料的比表面积和孔容的变化趋势,推断该材料在500 ℃前发生了一次热解,导致热解时比表面积明显提升,而温度为500~700 ℃时,第1阶段炭化反应趋于稳定,比表面积无明显提升;而当热解温度提升至800 ℃时,材料又发生了第2次热解反应,改变了炭材料的表面结构和性质,使其比表面积、微孔数量再次提升。
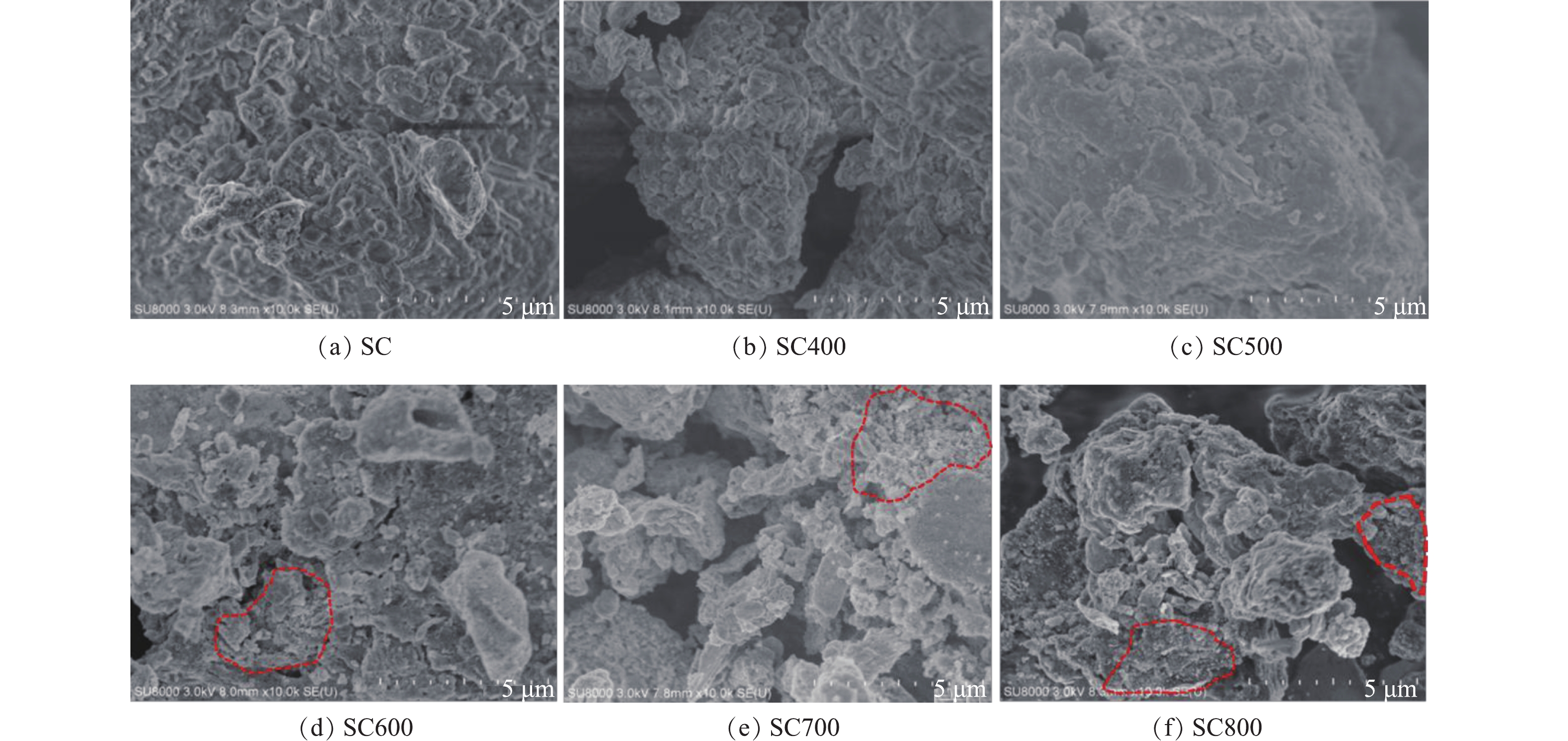
4)污泥炭微观形貌(SEM)分析。由图5可知,未热解的材料和400 ℃下制备的材料表面几乎无明显的孔隙,覆盖在材料表面的颗粒粒径较大,颗粒大多成块状或椭圆形,无明显片状颗粒,表面相对平整。而500~800 ℃条件下的炭材料随热解温度的升高,表面孔隙逐渐明显,颗粒也从块状逐渐变为片状,颗粒粒径也逐渐变小。说明热解温度的升高有利于孔径的生长,使材料表面的孔隙逐渐增多,从而增加了材料的比表面积和孔容。
-
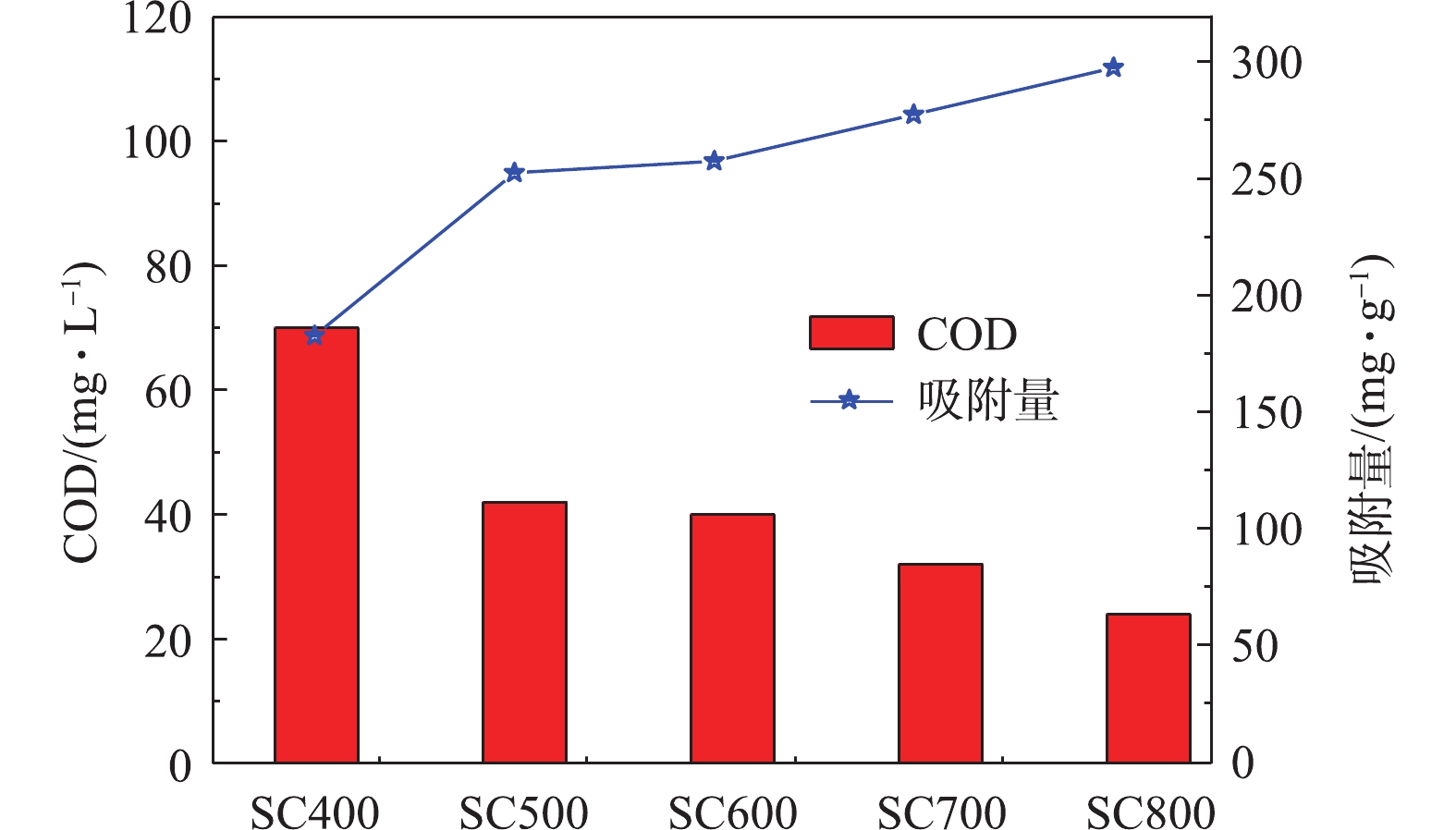
1)不同热解温度下的污泥炭对COD吸附性能。图6为不同热解温度下的炭基材料对COD吸附变化情况。可以看出,随着热解温度的升高,吸附后剩余液中COD逐渐降低,吸附容量逐渐升高。热解温度为从400 ℃升高至800 ℃时,污泥炭的COD去除率可由46.20%上升至75.31%,吸附容量由182.5 mg·g−1上升至297.5 mg·g−1。这是由于随着热解温度的升高,污泥炭比表面增大,1~2 nm微孔比例升高,3 nm左右的介孔增多,使得污染物与污泥炭的接触面积增大。同时随着热解温度的升高,污泥炭表面形成了丰富的活性吸附结合位点,有助于吸附水体中溶解性有机物。
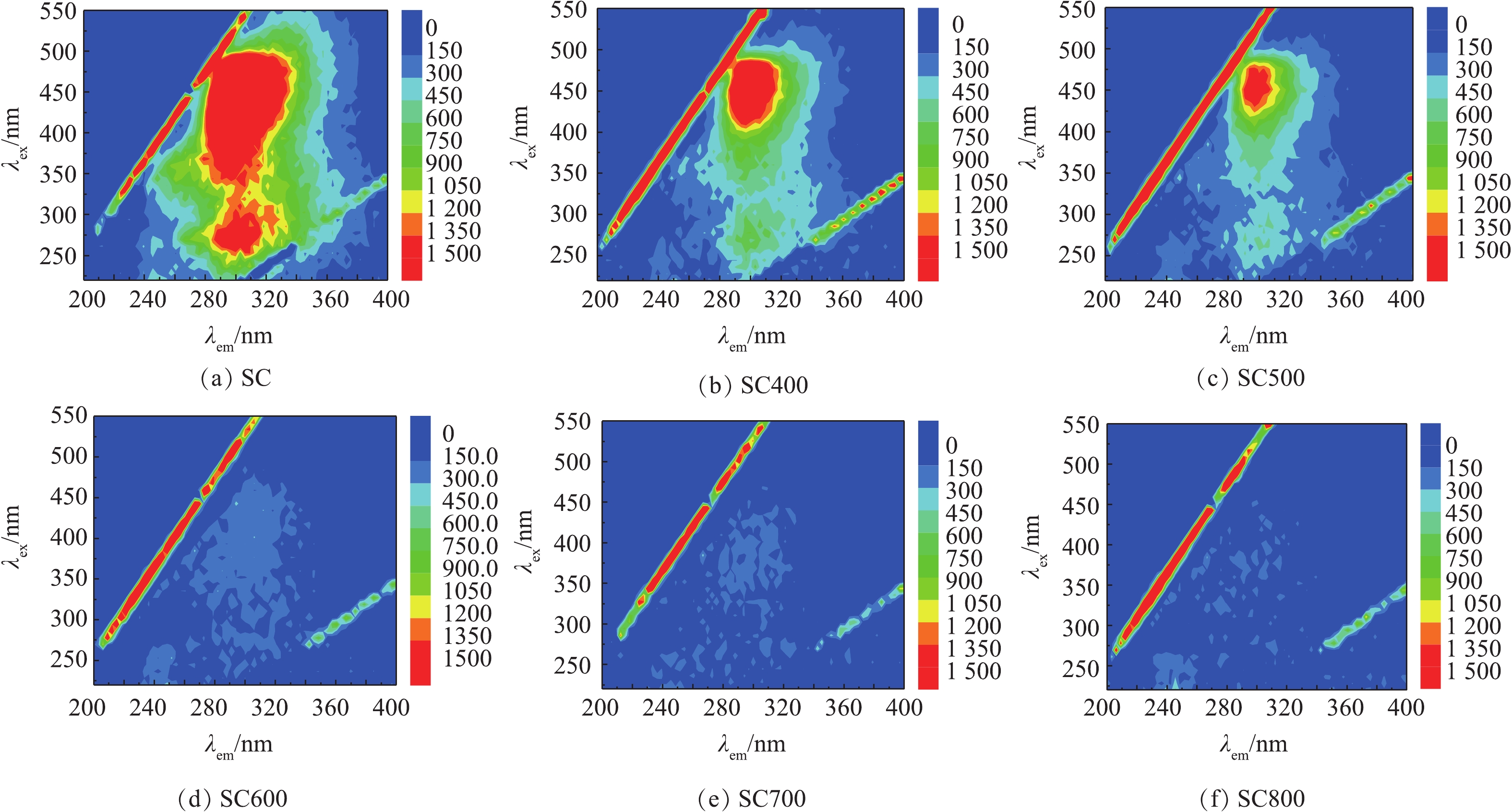
2)三维荧光分析。不同热解温度下污泥炭吸附后污水的三维荧光谱图如图7所示。污水的谱图中明显存在2个特征峰,分别为A峰(λex/em=295/270)—腐殖酸和B峰(λex/em=380/340)—富里酸。从表3和图7中可以看出,污泥炭对2类物质的吸附效果明显。随着热解温度的升高,污泥炭对污水中腐殖酸和富里酸的吸附逐渐增强,荧光强度逐渐减弱,2种物质的荧光强度分别由最初的2 004、4 609下降至164.6、177.9。这说明高温条件下制备的污泥炭对水体中的腐殖酸、富里酸等微生物代谢产物具有较好的吸附效能。实验条件下,污泥炭表面带负电荷与污水中带负电荷的腐殖酸、富里酸具有相同的电性,不利于以静电引力作用的吸附反应发生,而高温下制得的污泥炭对腐殖酸、富里酸具有较大的吸附量,说明高温制备的污泥炭可能存在表面官能团/原子与吸附分子发生了电子的交换或共有,形成比较稳定的化学键[23],而较大的比表面积可提供更多的反应活性位点,进而增强对腐殖酸、富里酸等微生物代谢产物的吸附性能。因此,吸附过程是表面官能团/原子和孔隙结构共同作用的结果。
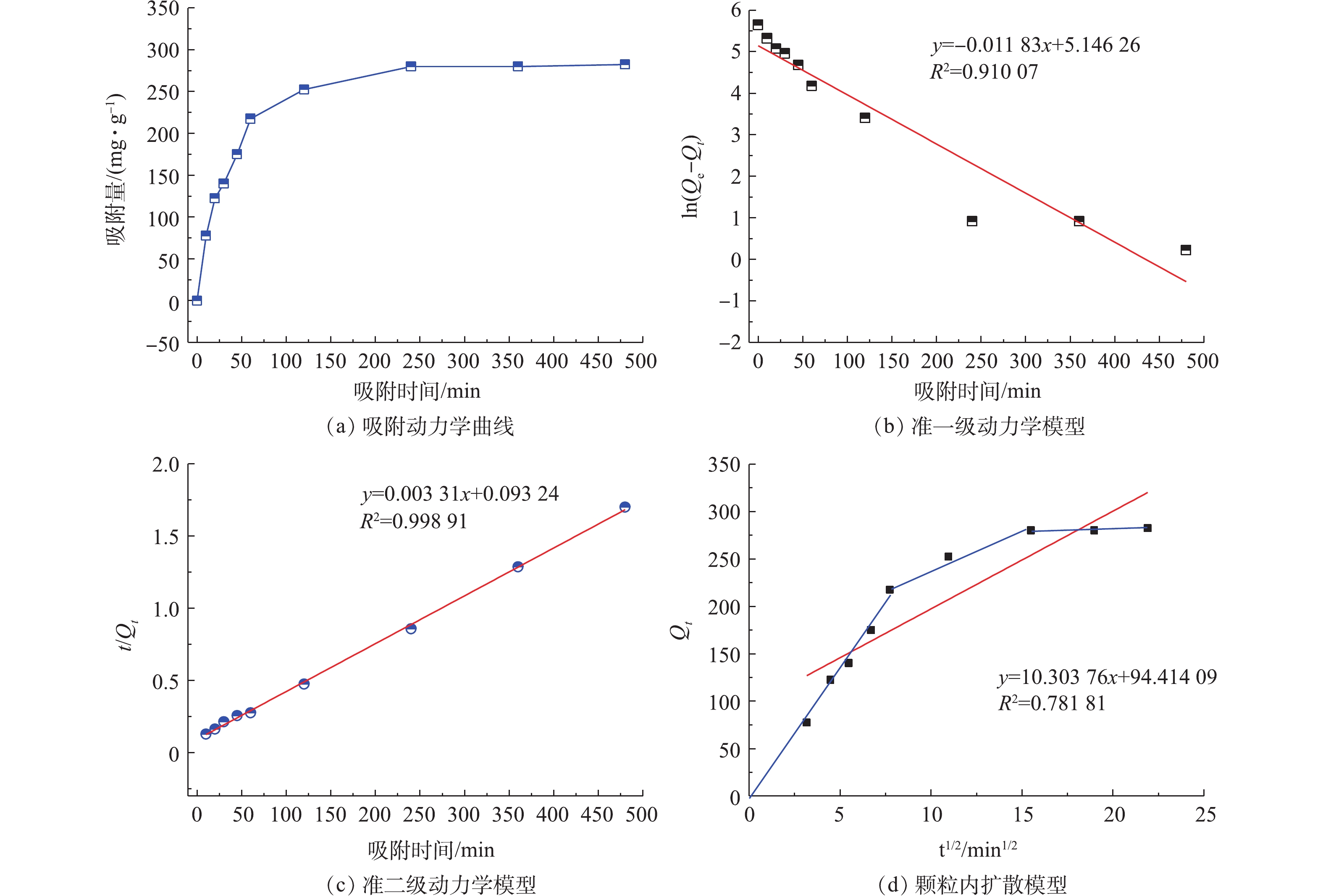
3)吸附动力学实验。由图8可知,随着吸附时间的延长,COD吸附量明显上升。为了更好地了解有机物在污泥炭吸附下随时间的变化情况,本研究采用准一级动力学、准二级动力学及颗粒内扩散模型对实验数据进行拟合。
准一级动力学方程见式(2),准二级动力学方程见式(3),颗粒内扩散方程见式(4)。
式中:Qe为平衡吸附量,mg·g−1;Qt为不同时间的吸附量,mg·g−1;k1为准一级动力学吸附方程吸附速率常数;k2为准二级动力学吸附方程吸附速率常数;k3为颗粒内扩散速率常数;t为吸附时间,min。
颗粒内扩散模型可以用于吸附扩散过程限速步骤的判定,通过以Qt对t0.5作图,直线部分的斜率即为内扩散速率常数。WEBER等[24]认为,如果吸附量Qt与t0.5呈线性关系并通过原点,则表明吸附由内扩散控制,若不通过原点,截距b越大,膜扩散在速率控制步骤中的影响也越大。
由图8(a)可知,吸附实验前120 min,生物炭对COD的吸附表现为快速吸附过程,占饱和吸附量的71.12%。120~240 min后为慢吸附过程,在360 min时吸附基本达到平衡。吸附过程呈快反应、慢反应2个阶段的现象,主要是由于污泥基生物炭表面活性点位随时间被覆盖饱和造成的。前120 min,生物炭上的吸附作用主要发生在外表面,随着表面活性点位的饱和,逐渐由吸附剂表面深入至介孔和微孔中,传质速度减慢。吸附动力学回归参数见表4,3种动力学模型曲线见图8。由拟合曲线的相关系数可知,准二级动力学方程相比准一级反应具有更高的拟合度(R2=0.998 9),且准一级方程计算的平衡吸附量与实际吸附平衡量相差较大,而准二级吸附方程平衡吸附量计算值与实际吸附量基本吻合。这是由于准二级动力学模型是建立在整个吸附平衡时间范围内,基于吸附过程的电子共享或得失的化学吸附的基础上,涉及吸附的整个过程,包括外部液膜扩散、表面吸附和颗粒内扩散等,往往能更好地揭示吸附机理[25]。实验结果表明,准二级吸附动力学模型能够更好地描述该吸附过程,证明该过程以化学吸附为主。
为了进一步确定吸附过程的限速控制步骤,利用颗粒内扩散模型对污泥炭吸附COD的动力学数据进行分析。由图8(b)可以看出,内扩散图曲线由3段构成。第1阶段,0~60 min,吸附反应速度很快。在边界层扩散作用下,吸附质从液相主体中迁移到污泥炭样品的表面,这一过程为表面传质。第2段和第3段直线部分反映了颗粒内扩散过程,第2段表示吸附质在大孔内的扩散,第3段表示吸附质在中孔和微孔内的扩散[26]。污泥炭表面的活性点位有限,在表面结合位点吸附作用后,不能吸附所有的有机物分子。这时液相主体和颗粒内表面存在浓度梯度,在浓度差的推动作用下,污泥基活性炭继续吸附有机物分子。随着吸附时间的增加,液相主体中吸附质浓度变化逐渐减小,达到吸附平衡。这说明,在0~60 min时,吸附速率控制步骤为颗粒内扩散控制,在60 min以后,膜扩散和颗粒内扩散共同控制。
-
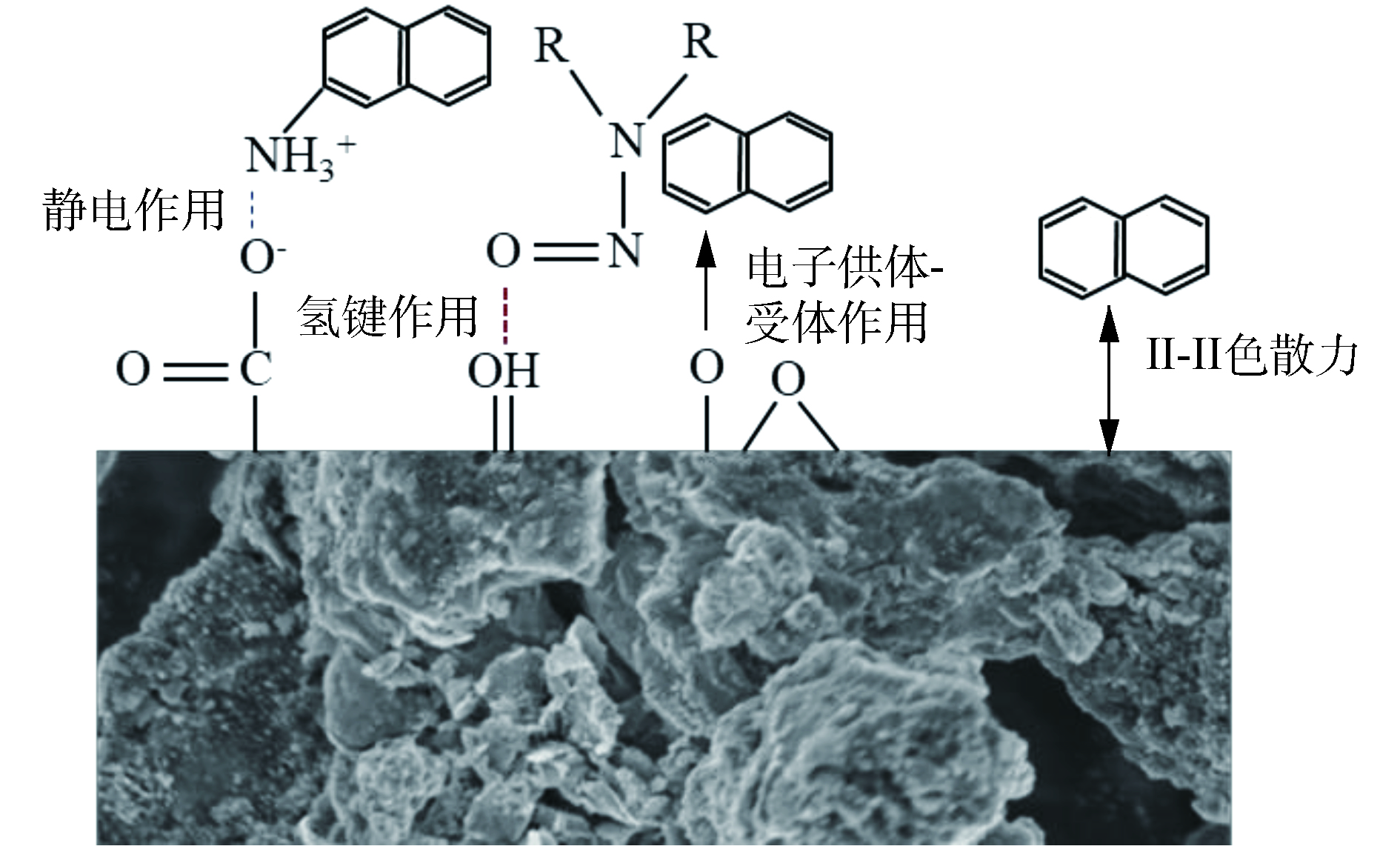
污泥炭处理二沉池污水的吸附过程主要由化学吸附主导。因此,污泥炭表面的官能团性质就起到了重要作用,而污泥炭表面官能团复杂多样,致使污泥碳具有两性性质。本实验过程中处理污水的pH为7.67,污泥炭的零电点位5.47,当溶液pH大于5.47,活性炭表面负电荷数增大。因此,污泥炭表面带负电荷官能团(如羧酸基)无法有效通过静电引力作用(electrostatic interaction)吸附水体中的腐殖酸、富里酸等有机物,氢键作用成为了吸附过程主要作用力。羟基官能团与腐殖酸上的N能形成氢键,成为潜在的吸附位,羰基可与腐殖酸分子上芳环的氮以电子供体-受体作用成键[23]。污泥炭中的芳香环结构上自由π电子与腐殖酸、富里酸芳香环之间存在π-π色散力,也可能在污泥炭吸附过程发挥作用。综上分析,污泥炭吸附二沉池污水中的有机物机理可用图9所示。
-
1)通过比表面积、X-射线光电子能谱及红外光谱测试分析,热解温度从400 ℃升高至800 ℃,污泥发生了2次热解反应。即在较低热解温度条件下(<600 ℃),污泥发生一次热解反应,污泥炭20 nm左右介孔比例增多,并出现少量微孔,比表面积、孔容相比于热解前污泥明显提升,脂肪类和糖类等物质中碳以气态组分逸散;而在500~700 ℃,比表面及孔容无明显提升,表面元素比例趋于稳定。当热解温度升高至800 ℃,发生了深度裂解炭化,1~2 nm微孔增多,介孔向微孔发展趋势明显,芳香化程度增加,且保留了丰富的含氧官能团。
2)不同热解温度下制备的污泥炭对污水中COD都具有一定的吸附效果,而800 ℃下制备的污泥炭吸附效果尤为突出,吸附容量可达297.5 mg·g−1,COD去除率可达75.31%;吸附过程符合准二级动力学模型,且以化学吸附为主。
3)污泥炭对水体中腐殖酸和富里酸有较强的吸附作用。这主要是由于污泥炭表面官能团/原子与吸附分子发生了电子的交换或共有的化学键缔合和π-π共轭作用,形成比较稳定的化学键,而较大的比表面积可提供更多的反应活性位点,进而增强对腐植酸、富里酸等微生物代谢产物的吸附性能。
热解温度对污泥碳基材料表面性质及吸附性能的影响
Effect of pyrolysis temperature on the surface properties and adsorption performance of sludge biochar
-
摘要: 以市政污泥为原料,在300、400、500、600、700和800 ℃无氧气氛下,热解制备了污泥基生物炭。采用BET、SEM、XPS、FT-IR对不同热解温度下污泥炭进行了表征分析;研究了不同热解温度下污泥炭对污水中有机物的吸附效果和动力学;探究了热解温度对污泥炭微观调控下吸附实际水体中有机物的匹配机质。结果表明,随热解温度的升高,C—H、C—C结合比例降低,C=C、C—O=C比例升高,芳香化程度增加,且比表面积、孔容及表面粗超度均有所增加,1~2 nm微孔比例增多,介孔向微孔发展趋势逐渐明显。800 ℃热解温度条件下制备的污泥炭对二沉池出水中有机物的吸附效果优于其他温度下制备的污泥炭。吸附温度为298.15 K时,最大吸附容量为282.5 mg·g−1,且符合准二级吸附动力学。高温下制备的污泥炭对水体中腐殖酸和富里酸具有较强的吸附效能。这主要是由于表面丰富的含氧官能团、芳香键与腐殖酸和富里酸发生了氢键、化学键缔合作用和π-π共轭作用,同时污泥碳表面发达的孔隙结构和较大的比表面积也提供了更多的活性结合位点,促进了污染物的吸附。Abstract: Biochar was derived from municipal sewage sludge at five different temperatures (400, 500, 600, 700 and 800 ℃) in an anaerobic environment, which was characterized by using BET, SEM, XPS and FT-IR. The adsorption effect and kinetics of organic matter in sewage on sludge biochar at different pyrolysis temperatures were studied. The matching mechanism of organic matter adsorption in actual water on micro-controlled biochar by pyrolysis temperature was also discussed. The results showed that with the increase of the pyrolysis temperature, the ratios of C—H, C—C decreased, while the ratios of C=C, C—O=C increased, the aromatization degree, specific surface area, pore volume, surface roughness and micro-pore ratio of 1~2 nm increased, the transformation trend from meso-pores to micro-pores was gradually obvious. The adsorption capability of organics in the effluent of secondary tank on sludge biochar prepared at pyrolysis temperature of 800 ℃ was better than that prepared at other temperatures.The corresponding maximum adsorption capacity was 282.5 mg·g−1 at adsorption temperature of 298.15 K, and the adsorption kinetics conformed to the quasi-second-order adsorption kinetics equation model. Biochar had strong adsorption efficiency towards humic acid and fulvic acid in water. The main reason was the hydrogen bonds, chemical bond association and π-π conjugation functions occurred between the abundant oxygen-containing functional groups, aromatic bonds on the surface of sludge biochar and humic acid or fulvic acid. Meanwhile, the developed pore structure and large specific surface area of sludge biochar provided lots of active binding sites and promoted the adsorption of pollutants.
-
Key words:
- sludge biochar /
- pyrolysis temperature /
- adsorption /
- organic matter
-

-
表 1 C1s XPS拟合得到的表面官能团相对含量(峰面积的百分比)
Table 1. Relative contents of functional groups in C1s XPS fitting (percent of total peak area)
% 样品名称 C—C C—OH/C—OR C—N O—C=O SC 21.11 18.76 10.15 5.94 SC400 18.22 22.34 5.76 2.26 SC500 22.02 16.15 5.2 2.81 SC600 19.75 17.38 4.3 2.45 SC700 21.37 15.48 5.63 2.4 SC800 27.84 19.24 3.52 2.05 表 2 不同热解温度下污泥炭及污泥的比表面积、孔容
Table 2. BET surface areas and porosity of sludge and biochar prepared at different pyrolysis temperatures
样品名称 比表面积/(m2·g−1) 孔容/(m3·g−1) SC 11.64 0.06 SC400 51.13 0.14 SC500 73.09 0.161 SC600 87.89 0.153 SC700 86.6 0.155 SC800 110.7 0.169 表 3 不同热解温度下污泥炭吸附后的有机物荧光强度
Table 3. Fluorescence intensity of organic matter adsorbed by biochar preparedat different pyrolysis temperatures
样品名称 腐殖酸荧光强度 富里酸荧光强度 SC 2 004 4 609 SC400 853.3 2 697 SC500 518.7 1 699 SC600 306.6 196.1 SC700 134.8 163.2 SC800 164.6 177.9 表 4 动力学拟合结果
Table 4. Kinetics fitting results
样品名称 Qe,exp/(mg·g−1) 准一级吸附模型 准二级吸附模型 颗粒内扩散模型 Qe,cal/(mg·g−1) k1 R2 Qe,cal/(mg·g−1) k2 R2 k3 C R2 SC800 282.5 171.79 0.011 8 0.910 1 302.11 0.000 12 0.998 9 10.304 94.41 0.781 8 -
[1] KASPRZYK-HORDERN B, DINSDALE R M, GUWY A J. The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters[J]. Water Research, 2009, 43(2): 363-380. doi: 10.1016/j.watres.2008.10.047 [2] ARCHER E, PETRIE B, KASPRZYK-HORDERN B, et al. The fate of pharmaceuticals and personal care products (PPCPs), endocrine disrupting contaminants (EDCs), metabolites and illicit drugs in a WWTW and environmental waters[J]. Chemosphere, 2017, 174: 437-446. doi: 10.1016/j.chemosphere.2017.01.101 [3] YANG Y, OK Y S, KIM K H, et al. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review[J]. Science of the Total Environment, 2017, 596: 303-320. [4] LIN T, YU S, CHEN W. Occurrence, removal and risk assessment of pharmaceutical and personal care products (PPCPs) in an advanced drinking water treatment plant (ADWTP) around Taihu Lake in China[J]. Chemosphere, 2016, 152: 1-9. doi: 10.1016/j.chemosphere.2016.02.109 [5] YIN L, WANG B, YUAN H, et al. Pay special attention to the transformation products of PPCPs in environment[J]. Emerging Contaminants, 2017, 3(2): 69-75. doi: 10.1016/j.emcon.2017.04.001 [6] KRISTIANA I, JOLL C, HEITZ A. Powdered activated carbon coupled with enhanced coagulation for natural organic matter removal and disinfection by-product control: Application in a Western Australian water treatment plant[J]. Chemosphere, 2011, 83(5): 661-667. doi: 10.1016/j.chemosphere.2011.02.017 [7] CUI Y, LIU X Y, CHUNG T S, et al. Removal of organic micro-pollutants (phenol, aniline and nitrobenzene) via forward osmosis (FO) process: Evaluation of FO as an alternative method to reverse osmosis (RO)[J]. Water Research, 2016, 91: 104-114. doi: 10.1016/j.watres.2016.01.001 [8] DODD M C, RENTSCH D, SINGER H P, et al. Transformation of β-lactam antibacterial agents during aqueous ozonation: Reaction pathways and quantitative bioassay of biologically-active oxidation products[J]. Environmental Science & Technology, 2010, 44(15): 5940-5948. [9] STALTER D, MAGDEBURG A, OEHLMANN J. Comparative toxicity assessment of ozone and activated carbon treated sewage effluents using an in vivo test battery[J]. Water Research, 2010, 44(8): 2610-2620. doi: 10.1016/j.watres.2010.01.023 [10] GIBERT O, LEFÈVRE B, FERNÁNDEZ M, et al. Fractionation and removal of dissolved organic carbon in a full-scale granular activated carbon filter used for drinking water production[J]. Water Research, 2013, 47(8): 2821-2829. doi: 10.1016/j.watres.2013.02.028 [11] KIM J, KANG B. DBPs removal in GAC filter-adsorber[J]. Water Research, 2008, 42(1/2): 145-152. [12] AHMED M B, ZHOU J L, NGO H H, et al. Progress in the preparation and application of modified biochar for improved contaminant removal from water and wastewater[J]. Bioresource Technology, 2016, 214: 836-851. doi: 10.1016/j.biortech.2016.05.057 [13] SMITH K M, FOWLER G D, PULLKET S, et al. Sewage sludge-based adsorbents: A review of their production, properties and use in water treatment applications[J]. Water Research, 2009, 43(10): 2569-2594. doi: 10.1016/j.watres.2009.02.038 [14] YUN BO Z, XIAN XUN W, GUANG MING Z. Effect of pyrolysis temperature and hold time on the characteristic parameters of adsorbent derived from sewage sludge[J]. Journal of Environmental Sciences, 2004, 16(4): 683-686. [15] SEREDYCH M, BANDOSZ T J. Sewage sludge as a single precursor for development of composite adsorbents/catalysts[J]. Chemical Engineering Journal, 2007, 128(1): 59-67. doi: 10.1016/j.cej.2006.06.025 [16] DING R, ZHANG P, SEREDYCH M, et al. Removal of antibiotics from water using sewage sludge-and waste oil sludge-derived adsorbents[J]. Water Research, 2012, 46(13): 4081-4090. doi: 10.1016/j.watres.2012.05.013 [17] HENDERSON R K, BAKER A, MURPHY K R, et al. Fluorescence as a potential monitoring tool for recycled water systems: A review[J]. Water Research, 2009, 43(4): 863-881. doi: 10.1016/j.watres.2008.11.027 [18] 王兴栋, 张斌, 余广炜, 等. 不同粒径污泥热解制备生物炭及其特性分析[J]. 化工学报, 2016, 67(11): 4808-4816. [19] CASAJUS C, ABREGO J, MARIAS F, et al. Product distribution and kinetic scheme for the fixed bed thermal decomposition of sewage sludge[J]. Chemical Engineering Journal, 2009, 145(3): 412-419. doi: 10.1016/j.cej.2008.08.033 [20] SUN Y, LIU L, WANG Q, et al. Pyrolysis products from industrial waste biomass based on a neural network model[J]. Journal of Analytical and Applied Pyrolysis, 2016, 120: 94-102. doi: 10.1016/j.jaap.2016.04.013 [21] DE OLIVEIRA S J, RODRIGUES F G, DA SILVA M C, et al. Thermal analysis and FTIR studies of sewage sludge produced in treatment plants. The case of sludge in the city of Uberlândia-MG, Brazil[J]. Thermochimica Acta, 2012, 528: 72-75. doi: 10.1016/j.tca.2011.11.010 [22] ROZADA F, OTERO M, PARRA J B, et al. Producing adsorbents from sewage sludge and discarded tyres: Characterization and utilization for the removal of pollutants from water[J]. Chemical Engineering Journal, 2005, 114(1/2/3): 161-169. [23] INYANG M, DICKENSON E. The potential role of biochar in the removal of organic and microbial contaminants from potable and reuse water: A review[J]. Chemosphere, 2015, 134: 232-240. doi: 10.1016/j.chemosphere.2015.03.072 [24] WEBER W J, MORRIS J C. Kinetics of adsorption on carbon from solution[J]. Journal of the Sanitary Engineering Division, 1963, 89(2): 31-60. [25] CHANG M Y, JUANG R S. Adsorption of tannic acid, humic acid, and dyes from water using the composite of chitosan and activated clay[J]. Journal of Colloid and Interface Science, 2004, 278(1): 18-25. doi: 10.1016/j.jcis.2004.05.029 [26] FENG Y, ZHOU H, LIU G, et al. Methylene blue adsorption onto swede rape straw (Brassica napus L.) modified by tartaric acid: Equilibrium, kinetic and adsorption mechanisms[J]. Bioresource Technology, 2012, 125: 138-144. doi: 10.1016/j.biortech.2012.08.128 -



 下载:
下载: