-
人工湿地(constructed wetlands,CWs)被认为是处理废水的低成本技术,其较低的建设和运营成本、便利的管理和高效的去除氮磷效率适合低浓度水平污染物的去除,能够较好地削减水体的污染物负荷,同时还具有良好的生态效应[1-2],已经广泛应用于污水处理。在人工湿地中,氮磷平衡是分析氮磷去除途径和评估各自去除贡献的常用方法[3]。人工湿地氮磷的去除途径主要是通过植物、基质和微生物的物理、化学和生物作用完成的[4]。WU等[5]发现,表面流人工湿地中植物同化吸收氮、磷的能力分别为8.46%~30.98%和0.46%~2.13%。LU等[6]指出,表面流人工湿地基质填料的吸附、截留、交换等作用去除水中磷的比例大约为26%。ZHANG等[7]发现,微生物的硝化反硝化作用是人工湿地去除废水中TN(66.9%~80.5%)的主要途径。
人工湿地植物不仅具有美学价值,还显示出对污染物的去除作用,它通过提供理想的附着位点来调节微生物群落,且根系分泌物可加速异养反硝化细菌的生长,增加微生物群落丰富度和多样性[8],有利于水中氮磷去除。DU等[9]发现,植物的种植增加了微生物的丰富度和生物多样性;同时,相关的反硝化属假单胞菌、不动杆菌、根瘤菌、芽孢杆菌和红假单胞菌丰度的增加,增强了微生物对氮的去除作用。HE等[10]发现,γ-变形菌、α-变形菌和β-变形菌是人工湿地基质中的主要细菌,并在减少硝酸盐和亚硝酸盐的功能上发挥了重要作用。DU等[11]发现,植物的种植改变了垂直流人工湿地微生物的组成,并指出假单胞菌属可能是微生物除磷吸附的主要参与者。但是,现有的研究缺少了人工湿地污染物去除途径和微生物群落相结合的分析,以及植物的种植对微生物群落以及微生物氮磷净化作用的影响分析。因此,为了系统地研究人工湿地净化机制和人工湿地处理效果与微生物群落之间的关系,采用5种人工湿地系统种植不同植被用于处理低浓度污水,通过人工湿地的氮磷平衡和高通量测序相结合的手段,研究种植不同植物的人工湿地系统氮磷净化效果差异、污染物的去除途径以及微生物群落的变化,并探讨了植物的种植对微生物群落和微生物氮磷净化作用的影响,以期为提高人工湿地对污水的净化效果提供参考。
全文HTML
-
人工湿地装置位于重庆大学校内,采用100 L的LLDPE桶(上口直径520 mm,下口直径430 mm,高度610 mm),湿地填料为沸石(粒径为2~5 mm),铺设高度为30 cm,湿地水深为20 cm。设置5组人工湿地实验,分别为无植物组(Ⅰ组)、黄菖蒲组(Ⅱ组)、水生美人蕉组(Ⅲ组)、梭鱼草组(Ⅳ组)和风车草组(Ⅴ组),植物的种植密度为30株·m-2,每组设置3个平行实验。
正式实验日期为2018-07-03—2018-09-30,水温维持在23.4~32.2 ℃。实验分为2个阶段:第1阶段(2018-07-03—2018-09-14),为低负荷阶段(COD=(45±5) mg·L−1、
NH+4 -N=(3.56±0.48) mg·L−1、TN=(5.18±0.30) mg·L−1、TP=(0.425±0.044) mg·L-1);第2阶段(2018-09-14—2018-09-30),为高负荷阶段(COD=(45±5) mg·L−1、NH+4 -N=(6.06±0.16) mg·L−1、TN=(8.15±0.18) mg·L−1、TP=(0.806±0.035) mg·L−1)。采用间歇性进水的方式,水力停留时间3 d后,收集水样用于后续的分析并排出湿地的污水,通入新配制的污水。实验中及时对实验植物进行维护,对死亡的植物用生长良好的备用植物替换。 -
在实验期间,每3 d对人工湿地的进、出水进行取样分析。其中,温度、pH、DO通过溶解氧仪(520M-01A,Thermo,USA)和pH计(8107UWMMD,Thermo,USA)测量。
NH+4 -N采用纳氏试剂分光光度法测定,TN采用过硫酸钾氧化-紫外分光光度法测定,TP采用钼酸铵分光光度法测定。 -
在实验开始和结束阶段,采用梅花取样法分3层采样,然后将收集的各层基质混匀,采用H2SO4-H2O2溶液消解,采用半微量凯氏法测定全氮,用钼锑抗比色法测定全磷。在低负荷实验结束阶段,收集植物根系附近的基质样品,并置于无菌密封袋中,基质样品用于分析微生物群落结构,并储存在−80 ℃,直至DNA提取。在实验的开始和结束时测定湿地中植物的湿重,挑选代表性的植株样品在105 ℃下干燥10 min,以灭活植物中酶的活性,然后在70 ℃下干燥12 h,测定干重。将植物粉碎成粉末,混合均匀并密封保存,采用H2SO4-H2O2溶液消解,采用半微量凯氏法测定全氮,用钼锑抗比色法测定全磷。
-
通过OMEGA Soil DNA试剂盒提取根系基质样品中微生物DNA。使用通用细菌引物16S rRNA的5′-ACTCCTACGGGAGGCAGCAG-3′和5′-GGACTACHVGGGTWTCTAAT-3′(V3~V4区)对提取的DNA进行PCR扩增。PCR扩增条件:98 ℃(3 min)初始变性,然后进行25个循环,98 ℃变性30 s,50 ℃退火30 s,72 ℃延伸30 s,最后5 min延伸至72 ℃。将所有序列读数聚类到操作分类单位(OTU)(相似性阈值为97%)。高通量测序服务由上海美吉生物平台提供(上海,中国)。
-
氮的去除是由植物吸收、基质吸附、微生物硝化-反硝化作用和NH3的挥发决定的,因为进水中氨氮相对较低并且pH为中性,NH3的挥发可忽略不计[12]。磷的去除由植物吸收、基质吸附和微生物作用决定。
基质总氮和总磷积累速率的计算见式(1)。
式中:ηSR为基质的总氮和总磷积累速率,mg·(m2·d)−1;CSC0和CSC1为实验开始和结束时基质的总氮和总磷含量,mg·kg−1;MS为基质的质量,kg;A为湿地面积,m2;t为实验总运行时间,d。
植物总氮和总磷吸收速率的计算见式(2)。
式中:ηPR为植物的总氮和总磷吸收速率,mg·(m2·d)−1;CPC0和CPC1为实验开始和结束时植物的总氮和总磷含量,mg·kg−1;M1,0和M1, 1为实验开始和结束时植物的干重,kg;A为湿地面积,m2;t为实验总运行时间,d。
微生物总氮和总磷去除速率的计算见式(3)。
式中:ηMR、ηSR和ηPR为微生物、基质和植物的总氮和总磷去除速率,mg·(m2·d)−1;Ci, 0和Ci, 1为每次进水和出水总氮和总磷浓度,mg·L−1;V为每次进水的水量,L;A为湿地面积,m2;t为实验总运行时间,d。
-
用单因素方差分析(One-way ANOVA)进行差异性分析,检验数据间的差异性。用Pearson检验方法进行相关性分析,检验数据间的相关水平。所有统计分析均使用SPSS 22.0版软件进行,并且在P<0.05水平时被认为是显著的。
1.1. 人工湿地装置与运行
1.2. 水质指标分析方法
1.3. 基质和植物相关指标分析方法
1.4. 高通量测序分析方法
1.5. 人工湿地氮磷平衡计算方法
1.6. 统计分析
-
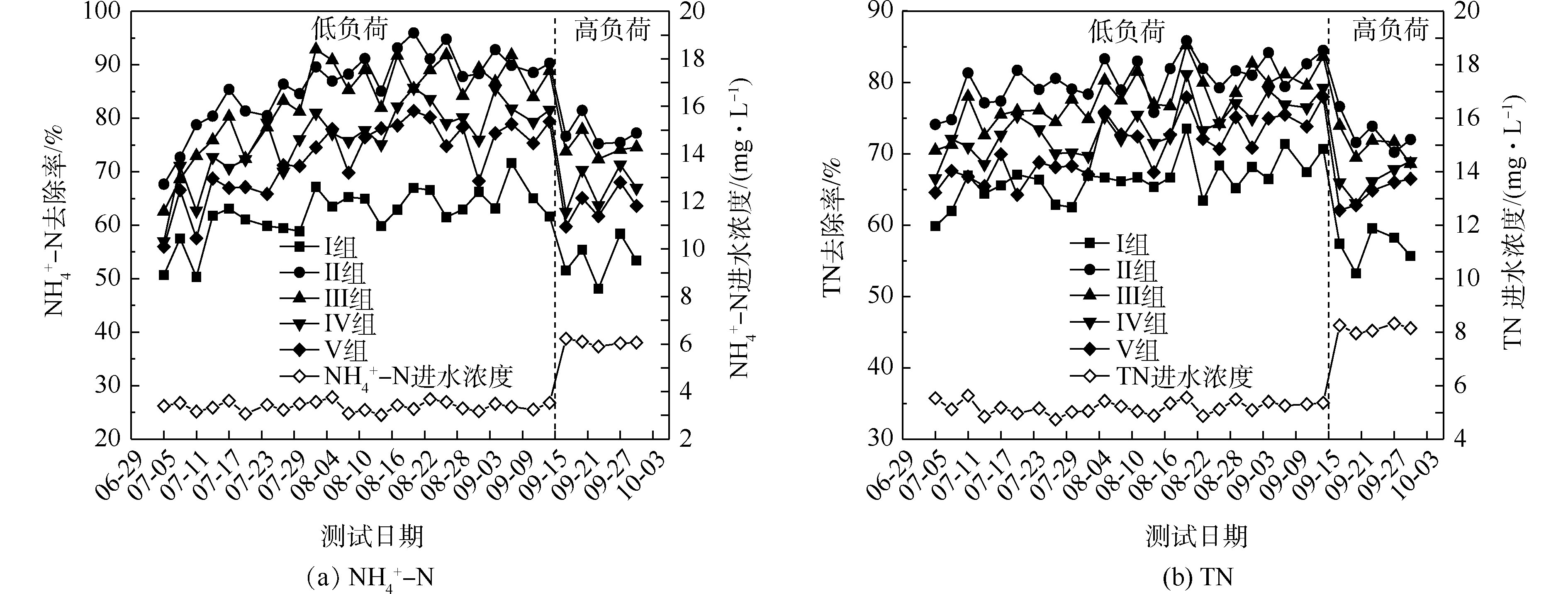
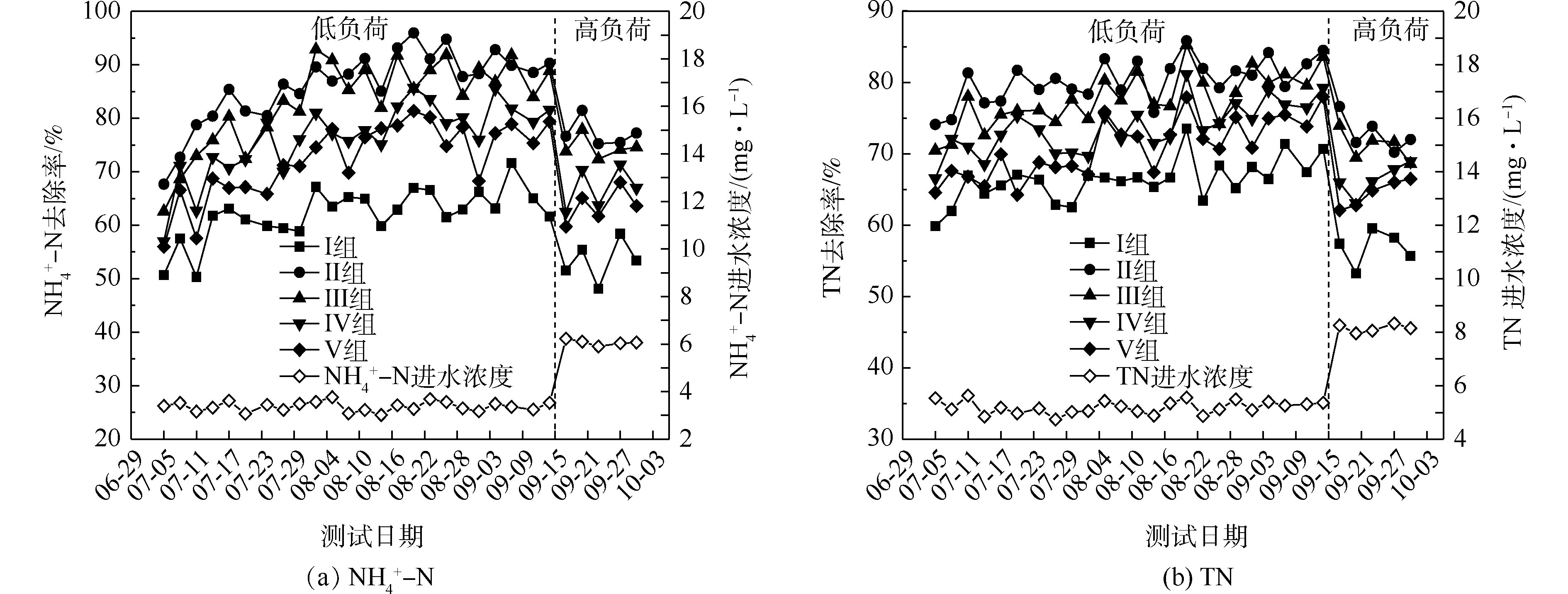
氮的去除率随取样时间的变化情况如图1所示。5组人工湿地系统都能有效地去除水中氨氮和总氮。在低负荷测试期间,
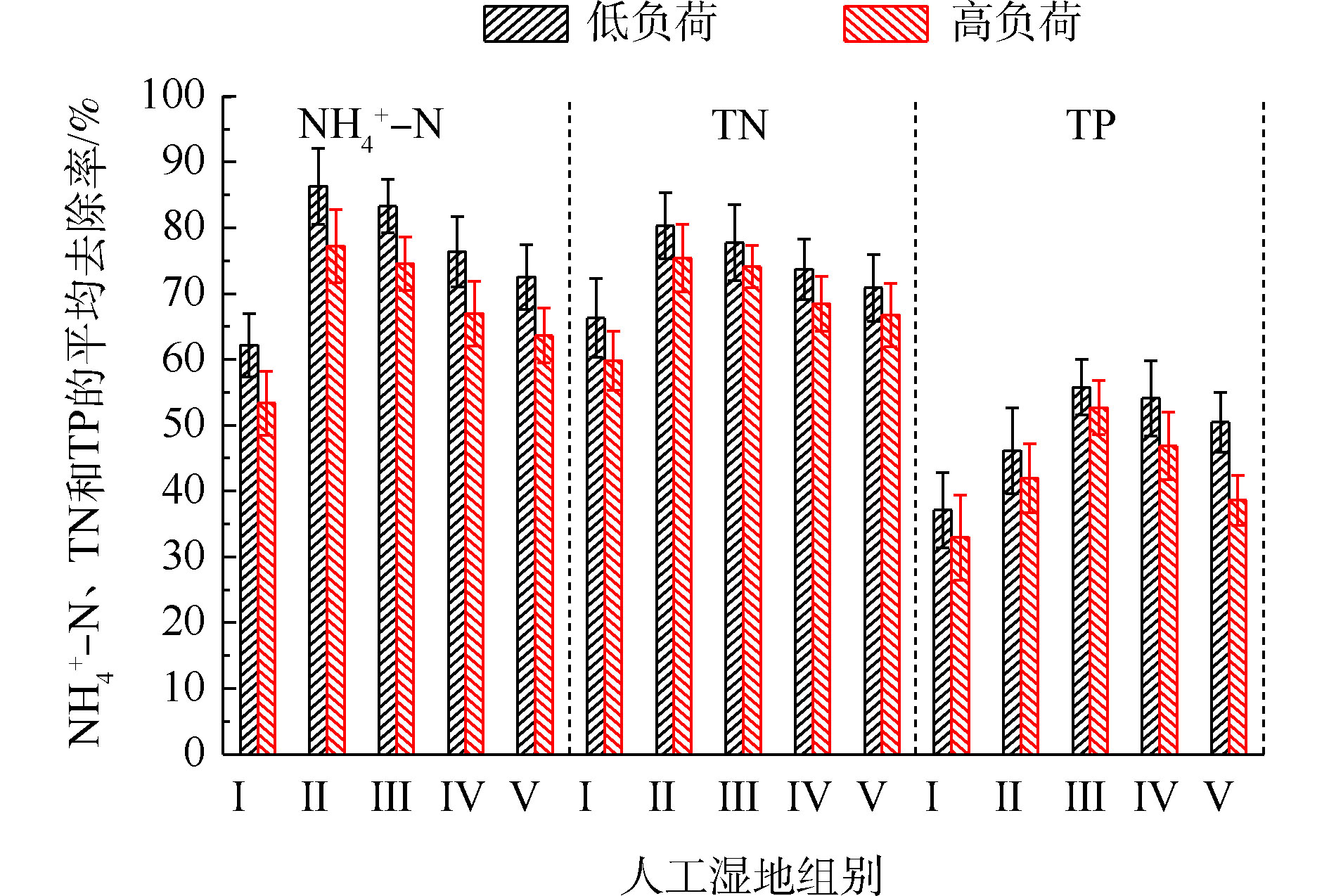
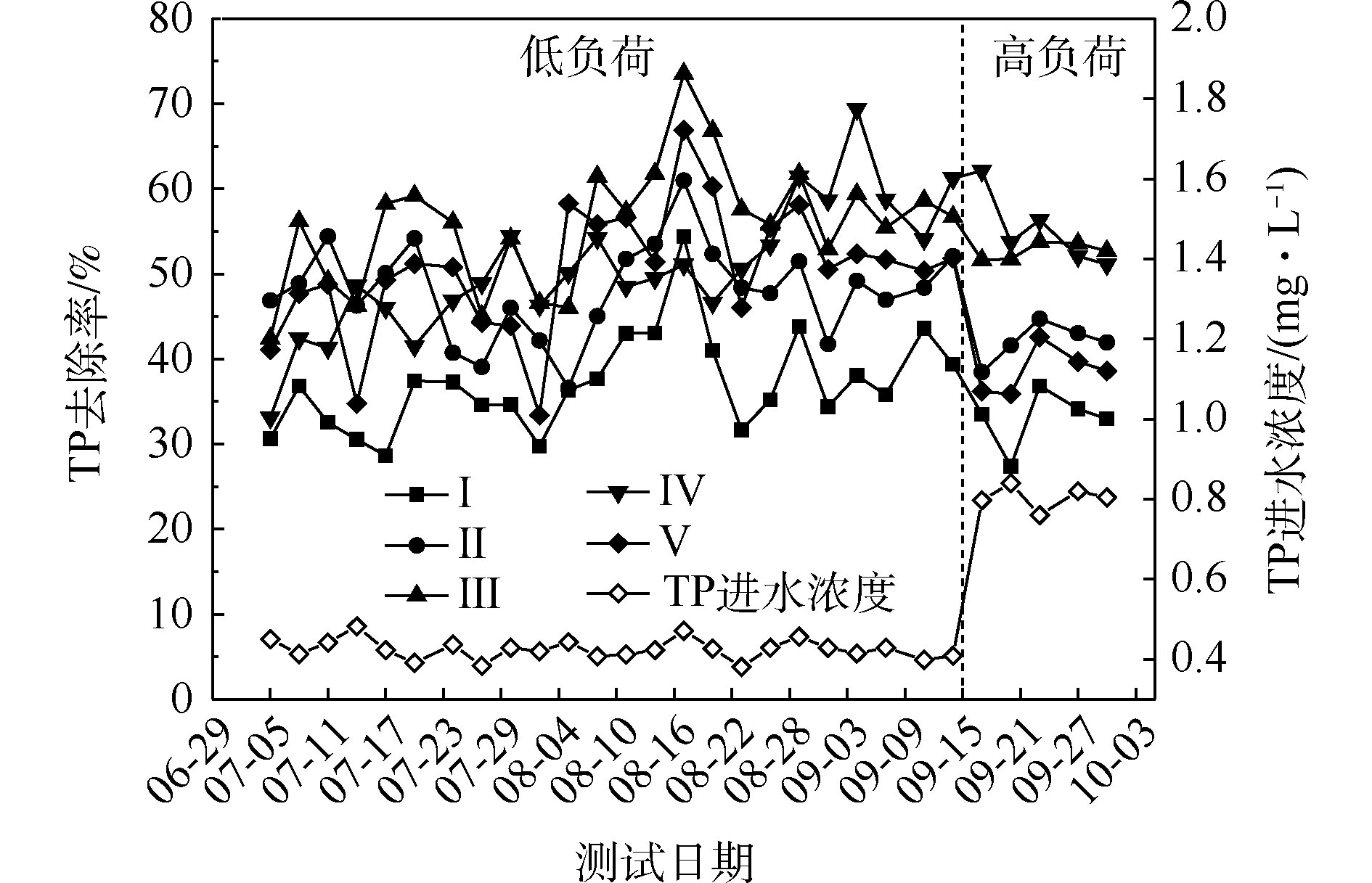
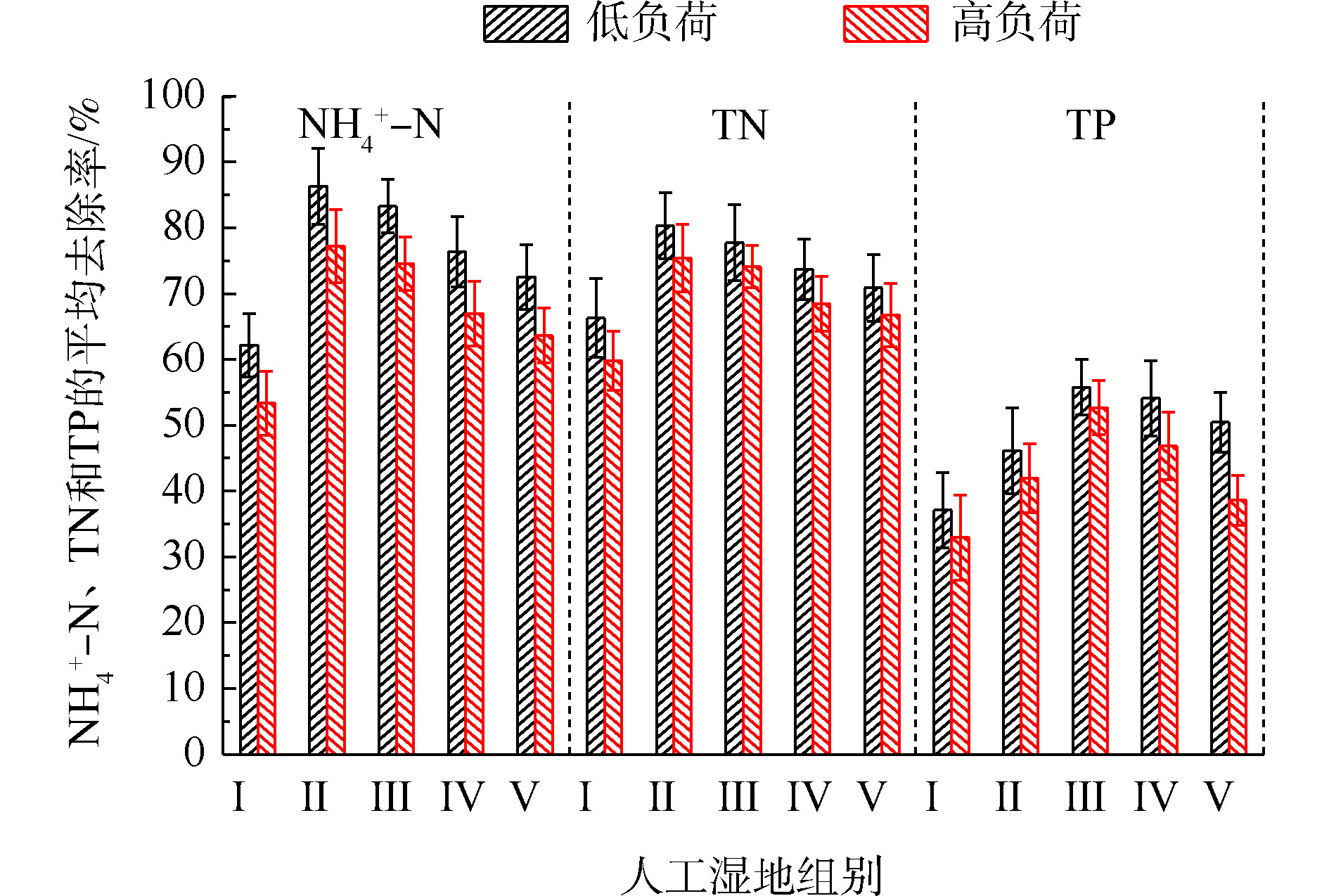
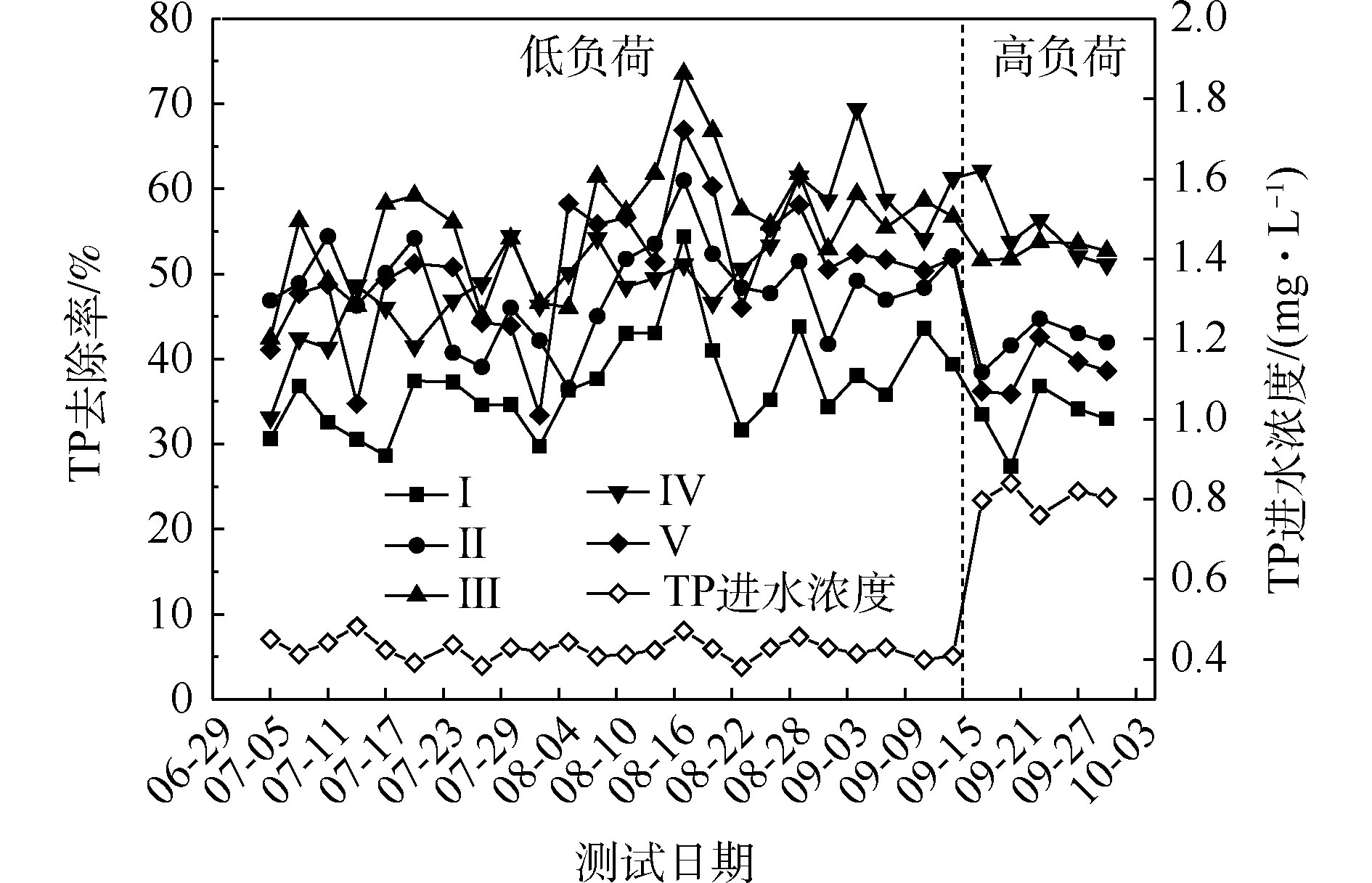
NH+4 -N的去除率为50%~94%,Ⅱ组(黄菖蒲)的去除率最高。如图2所示,Ⅰ组、Ⅱ组、Ⅲ组、Ⅳ组和Ⅴ组的平均去除率分别为62.16%、86.31%、83.28%、76.38%和72.51%。在低负荷测试期间,TN的去除率为59%~85%,Ⅱ组(黄菖蒲)表现出最高的去除性能。如图2所示,Ⅰ组、Ⅱ组、Ⅲ组、Ⅳ组和Ⅴ组的平均去除率分别为66.29%、80.30%、77.71%、73.68%和70.89%。夏季的人工湿地有高NH+4 -N和TN去除率,这与ZHANG等[13]的研究结论相似。在高负荷期间,各组NH+4 -N去除率均降低了9%~11%,TN去除率均降低了3%~8%,说明随着氮浓度的升高,氮的去除率会降低。NH+4 -N和TN表现出相似的去除趋势,这是因为NH+4 -N是进水中氮的主要来源,NH+4 -N经过硝化-反硝化作用去除,说明人工湿地有较强的反硝化作用。通过种植植物组与Ⅰ组比较发现,对NH+4 -N和TN去除有显著差异(P<0.05)。这是因为NH+4 -N容易被植物吸收和利用[14]以及植物的种植改变了基质微生物群落。磷的去除率随取样时间的变化情况如图3所示。5组人工湿地系统都能有效地去除水中总磷。在低负荷测试期间,TP的去除率为28%~73%,种植Ⅲ组(美人蕉)表现出最高的去除性能。如图2所示,Ⅰ组、Ⅱ组、Ⅲ组、Ⅳ组和Ⅴ组的平均去除率分别为37.09%、46.12%、55.77%、54.07%和50.45%。在高负荷期间,各组TP去除率均降低了3%~12%,说明随着TP浓度的升高,TP的去除率降低。通过种植植物组与Ⅰ组比较发现,TP的去除率显著提高(P<0.05)。这是因为植物生长过程中能吸收和利用磷酸盐,提高了TP的去除率[15]。
实验期间,进水和出水的pH、DO和水温T的平均值如表1所示。与进水DO相比,各组出水DO浓度都下降了1.80~2.64 mg·L−1。这是因为微生物去除COD和氨氧化过程中消耗了水体中的DO。另外,种植植物组比Ⅰ组DO要高,这是由于植物根系有泌氧功能,从而导致水体中DO浓度的增加。Pearson检验表明,DO与pH呈显著正相关(相关系数为0.923,P<0.05)。这是因为植物的光合作用会影响水体的碳酸电离平衡,导致氢氧根离子的浓度增加[16]。DO与
NH+4 -N去除率不存在相关性(P>0.05)。这可能与各组植物吸收作用和根系富集微生物群落不同有关。 -
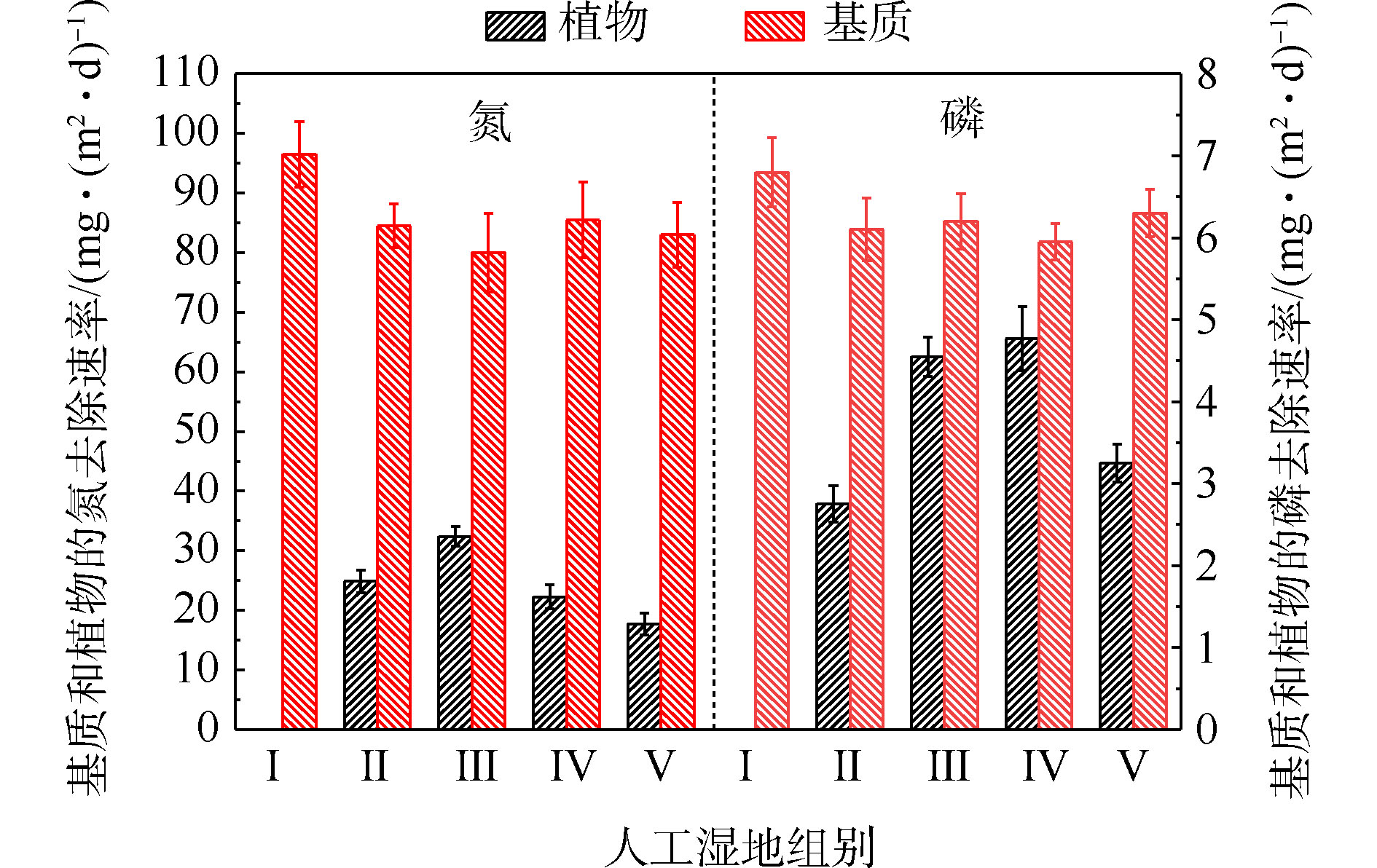
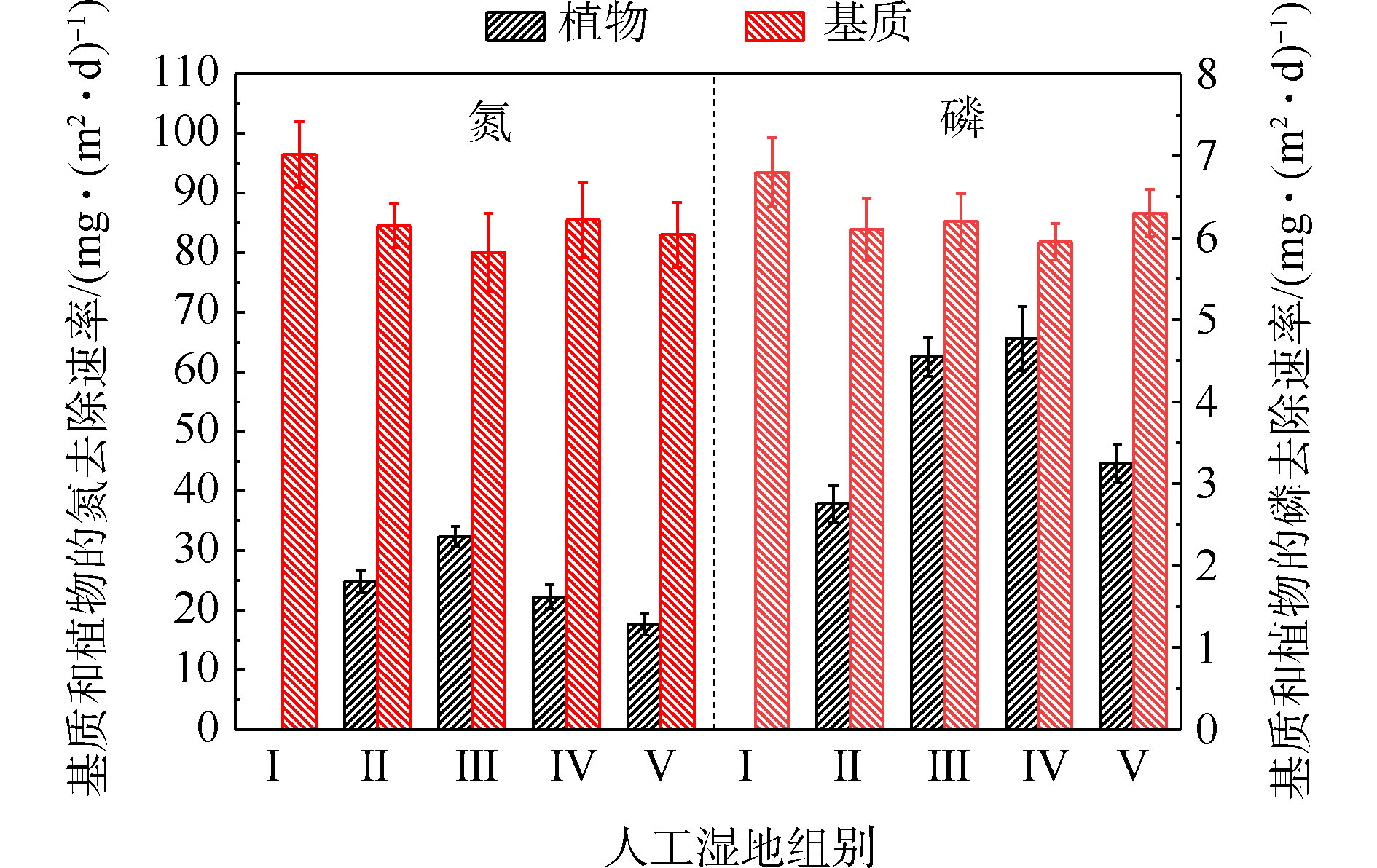
基质的氮磷平均积累速率如图4所示。由图4可知,Ⅱ组(84.46 mg·(m2·d)−1)、Ⅲ组(80.11 mg·(m2·d)−1)、Ⅳ组(85.53 mg·(m2·d)−1)和Ⅴ组(83.04 mg·(m2·d)−1)平均TN积累速率显著低于Ⅰ组(96.52 mg·(m2·d)−1)(P<0.05)。Ⅱ组(6.10 mg·(m2·d)−1)、Ⅲ组(6.21 mg·(m2·d)−1)、Ⅳ组(5.95 mg·(m2·d)−1)和Ⅴ组(6.42 mg·(m2·d)−1)平均TP积累速率低于Ⅰ组(6.81 mg·(m2·d)−1)( P <0.05)。这说明人工湿地中种植植物会降低基质中氮磷的积累速率,并与植物吸收和基质吸附2个氮磷积累过程存在竞争关系有关。种植植物组间的基质氮磷积累效果差异,与不同的植物具有不同生长状况有关[8]。
植物的氮磷平均吸收速率如图4所示。可以看出,平均氮吸收速率Ⅲ组(32.41 mg·(m2·d)−1)>Ⅱ组(24.87 mg·(m2·d)−1)>Ⅳ组(22.24 mg·(m2·d)−1)>Ⅴ组(17.73 mg·(m2·d)−1),平均磷吸收速率Ⅳ组(4.77 mg·(m2·d)−1)>Ⅲ组(4.55 mg·(m2·d)−1)>Ⅴ组(3.25 mg·(m2·d)−1)>Ⅱ组(2.75 mg·(m2·d)−1)。结果表明,水生美人蕉的氮吸收速率最高,梭鱼草的磷吸收速率最高。植物的氮磷平均吸收速率不同,与植物干重增加量、植物体内氮磷含量不同有关。
-
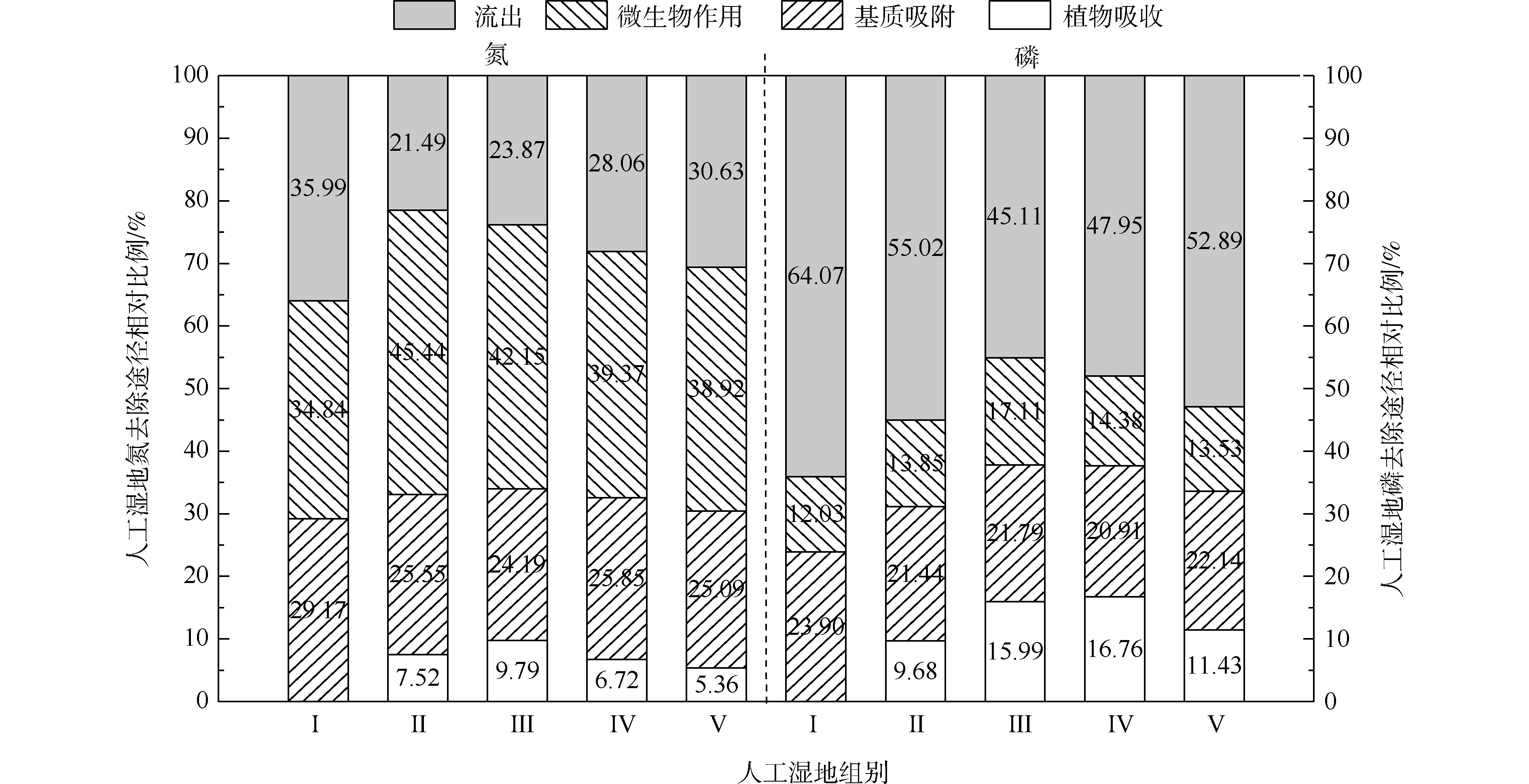
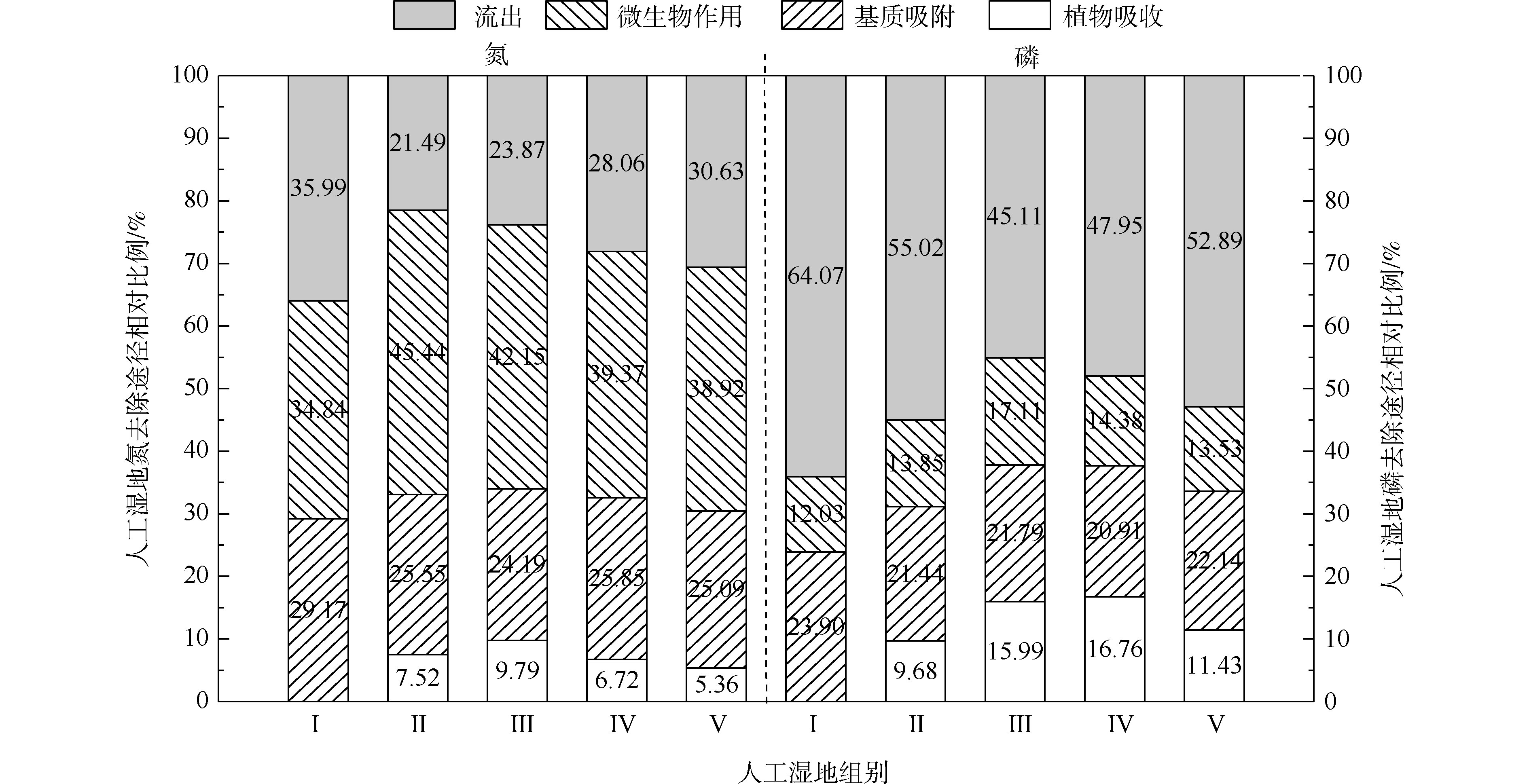
人工湿地水体氮磷的去除主要通过基质、植物、微生物和排水4种途径,各组人工湿地的基质、植物、微生物和排水的氮磷去除率占比如图5所示。在5个不同的实验组中,系统排水中的氮含量占总负荷的23.87%~35.99%,植物吸收的氮约占5.36%~9.79%,基质吸附氮约占24.19%~29.17%,系统微生物去除氮约占34.84%~45.44%。在氮的去除方面,微生物去除>基质吸附>植物吸收的作用。植物吸收氮仅占5.36%~9.79%,说明植物对水中氮直接吸收作用很小。郑于聪[17]研究了中试规模的人工湿地处理污水发现,植物对水中氮的去除贡献仅占5.80%~13.90%,即植物对污水中氮的去除贡献很小,与本实验的结果相似。微生物去除氮约占34.84%~45.44%,说明微生物作用是人工湿地氮去除的最主要的途径。通过与Ⅰ组比较,发现种植植物组微生物氮去除率明显增加(P<0.05)。这可能与植物根系分泌物能改善微生物生长环境,有利于反硝化微生物的附着生长有关。植物的种植不仅对氮有吸收作用(5.36%~9.79%),还增强了微生物对氮的去除作用(增加了4.08%~10.60%)。
在5个不同的实验组中,系统排水中的磷含量占总负荷的45.10%~64.07%,植物吸收的磷约占9.68%~16.76%,基质吸附磷约占20.91%~23.90%,系统微生物去除磷约占12.73%~17.11%。在磷的去除方面,排水中的磷含量达到45.10%~64.07%,说明人工湿地对总磷去除效果较差。这可能与TP浓度低和运行条件不利于人工湿地对其去除有关。基质吸附比植物吸收和微生物作用对磷去除作用强,说明了基质吸附是人工湿地中磷去除的主要途径。植物吸收磷仅占9.68%~16.76%,说明植物对水中磷直接吸收作用很小。吴海明[18]研究中试湿地处理污水发现,植物吸收的磷仅占湿地中磷总去除量的4.81%~22.23%。沈莹等[19]研究中试潜流湿地处理污染河水发现,植物的磷吸收量占湿地磷去除量的8.80%。以上研究均发现,植物对湿地中磷去除的贡献较小,与本实验有相似的结论。通过与Ⅰ组比较,种植植物组微生物磷去除率出现增加现象,Ⅲ组与各组有显著差异。这是因为植物根系分泌物的不同影响了基质中微生物群落分布[20]。植物的种植不仅对磷(9.68%~16.76%)有吸收作用,还增强了微生物对磷(增加了1.82%~5.08%)的去除作用。
-
微生物多样性和丰富度如表2所示。各组湿地微生物测序中样本文库覆盖率(coverage)均大于0.98,说明测序结果可以很好地代表样本的真实情况[21]。Ⅰ组、Ⅱ组、Ⅲ组、Ⅳ组和Ⅴ组检测到的OTU数目分别为1 441、1 736、1 594、1 464和1 478,Ace分别为1 770.18、2 004.13、1 747.37、1 754.82和1 672.91,Chao分别为1 754.28、2 063.44、1 772.99、1 806.11和1 705.38,发现了Ⅱ组与其他组有明显差异(P<0.05),说明黄菖蒲的种植改善了微生物丰富度。Ⅰ组、Ⅱ组、Ⅲ组、Ⅳ组和Ⅴ组的Shannon指数为4.99、6.38、6.01、5.79和5.59,Simpson指数为0.024 5、0.003 3、0.006 7、0.011 0和0.020 8,植物组与Ⅰ组有明显差异(P<0.05)。其中Ⅱ组和Ⅲ组的多样性最高,说明种植植物能有效地改善群落多样性。植物种植改善了微生物群落多样性和丰富度,这与植物根系泌氧和根系分泌的碳源有利于微生物富集生长有关[22]。另外,通过逐步线性回归分析发现,Shannon和Sobs决定系数分别为0.828和0.208(P<0.05)。说明微生物丰富度和多样性的改善有利于微生物对氮的去除作用。
-
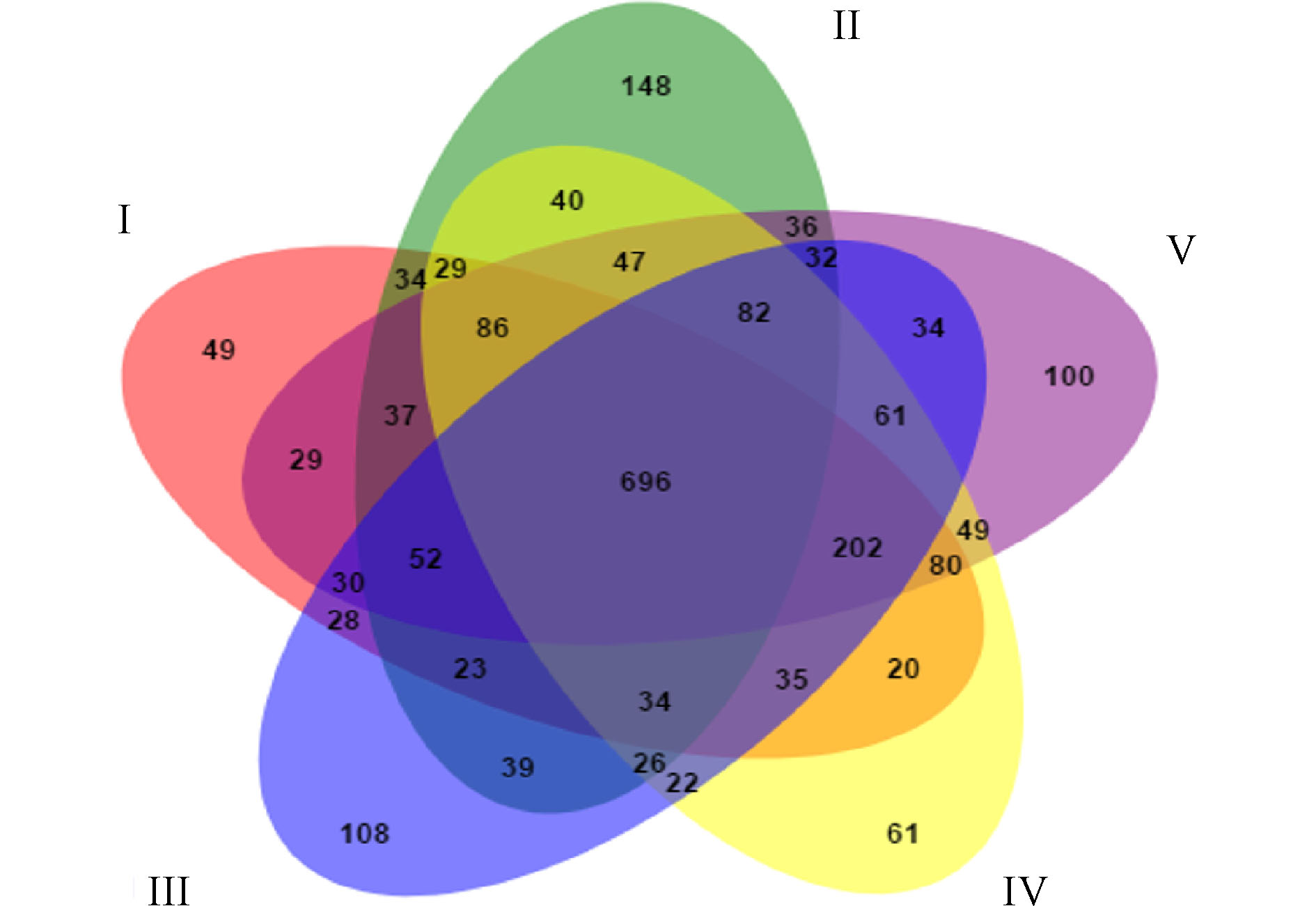
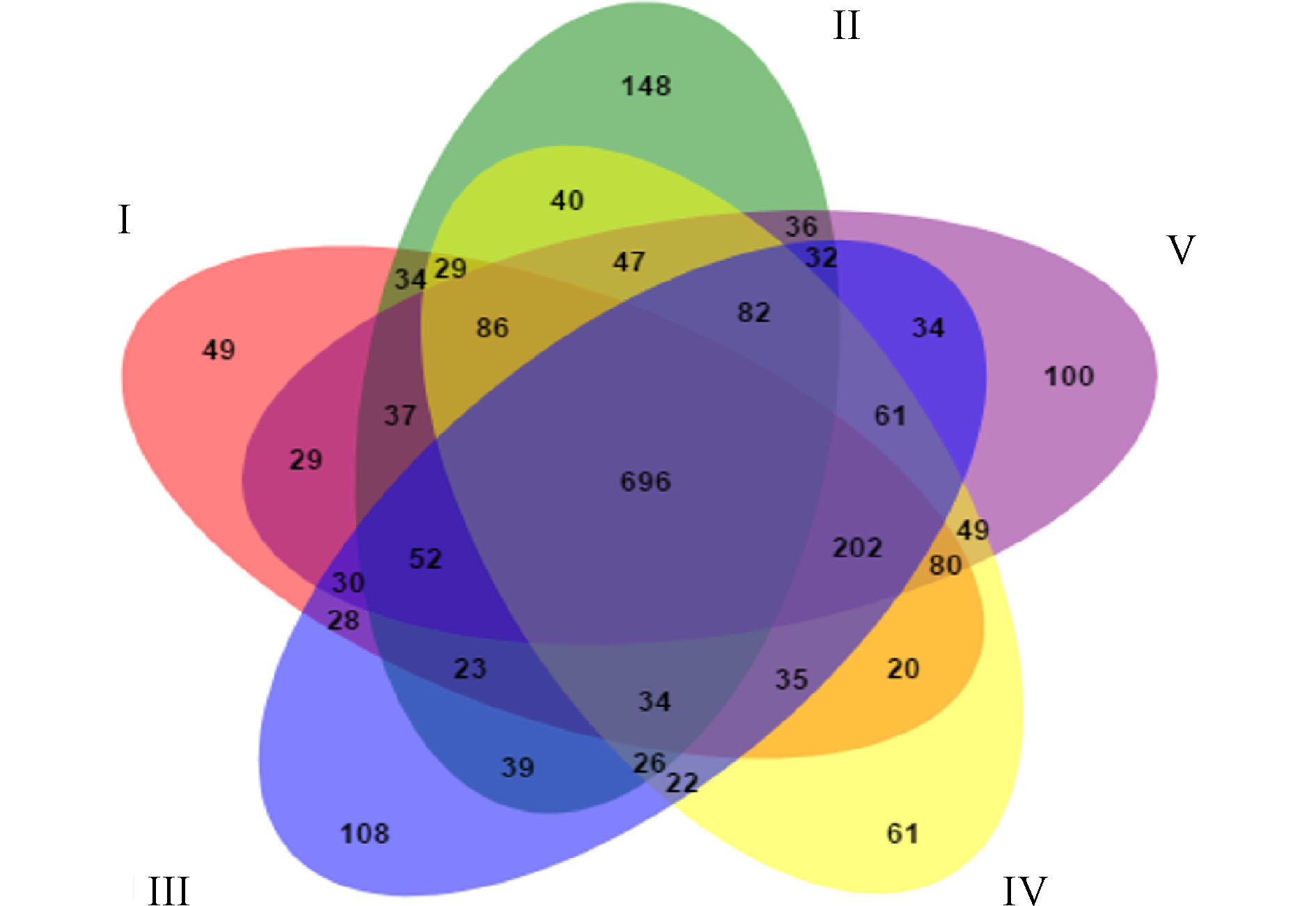
图6展示了人工湿地各组微生物独有和共有的OTU数目。5组中共有的OTU数目为696,分别占Ⅰ组、Ⅱ组、Ⅲ组、Ⅳ组和Ⅴ组检测到的OTU数目的48.33%、40.09%、43.66%、47.54%和47.09%,说明人工湿地基质微生物群落出现了显著的变化。这是因为植物根系改变了微生物生长的环境,使得微生物群落出现差异。
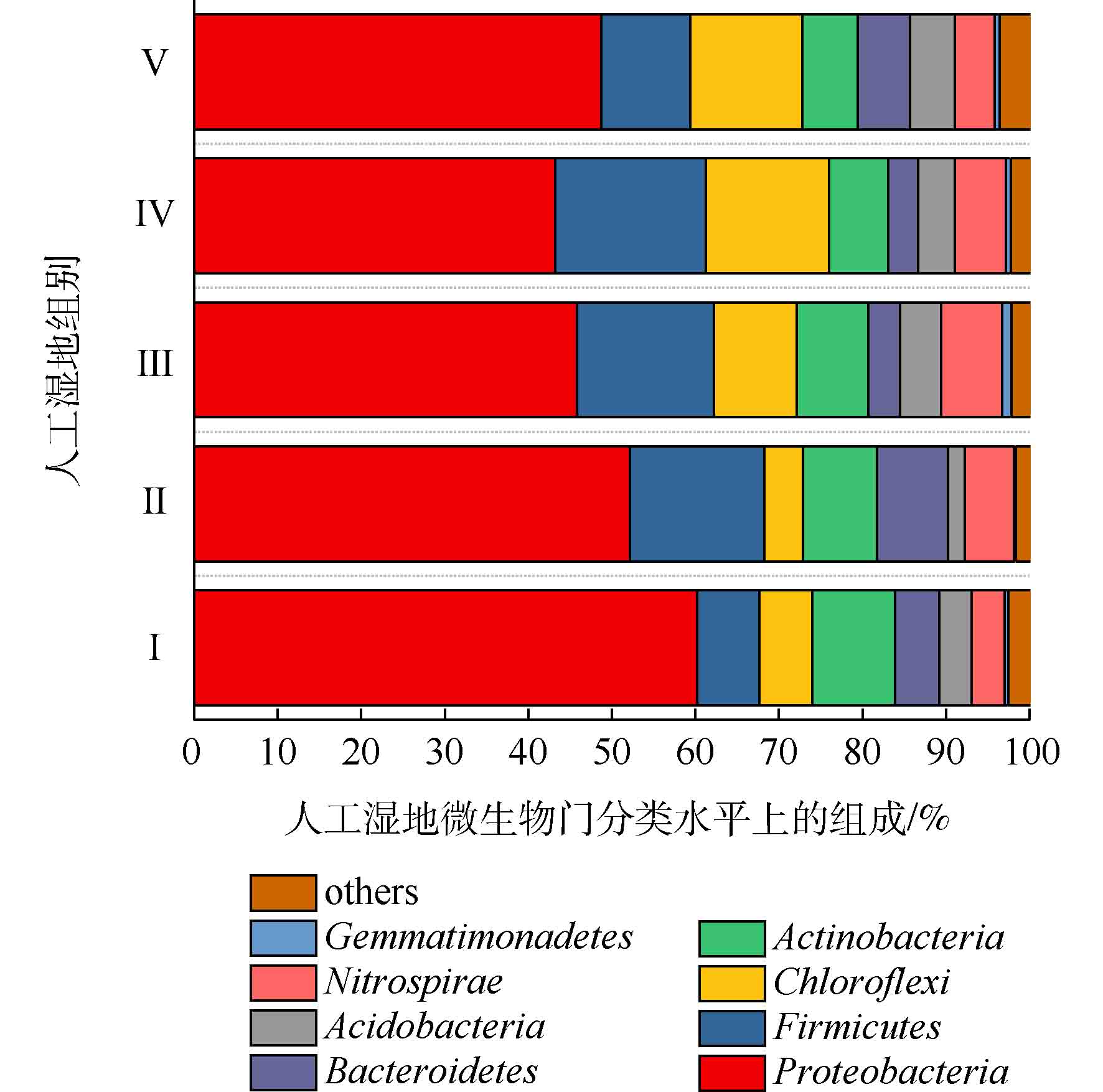
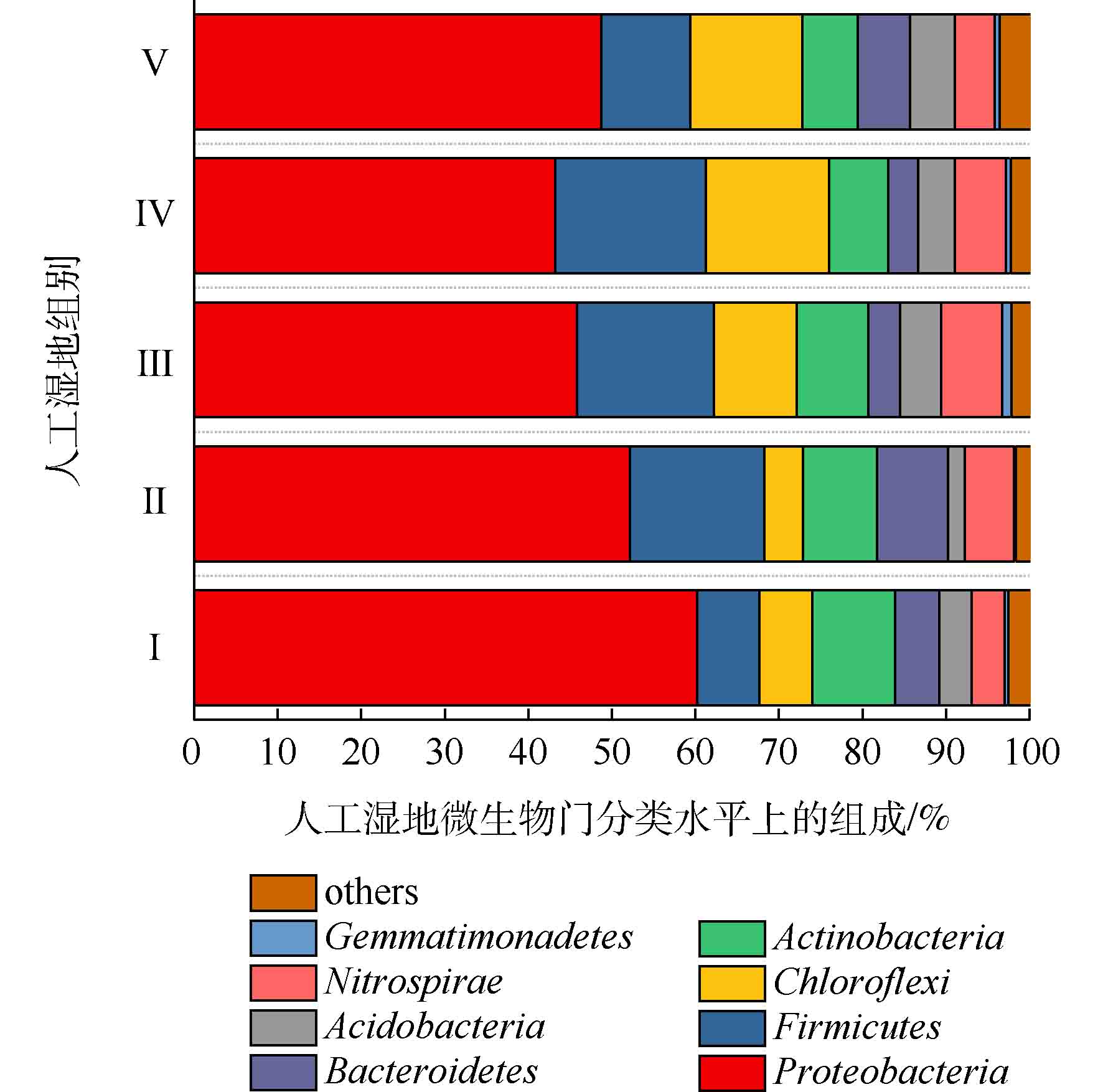
人工湿地各组微生物门水平组成如图7所示。可将微生物检测频率>1%的菌门做为主要的菌门[23]。共发现8个菌门,分别为变形菌门(Proteobacteria)、厚壁菌门(Firmicutes)、绿弯菌门(Chloroflexi)、放线菌门(Actinobacteria)、硝化螺旋菌门(Nitrospirae)、拟杆菌门(Bacteroidetes)、酸杆菌门(Acidobacteria)和芽单胞菌门(Gemmatimonadetes)。其中5大优势菌门分别为变形菌门、厚壁菌门、绿弯菌门、放线菌门和硝化螺旋菌门。ZHANG等[24]研究三亚河红树林湿地发现,湿地基质的优势菌门为变形菌、拟杆菌、放线菌和厚壁菌。LI等[25]研究发现,多级表面流湿地基质的优势菌门为变形菌、厚壁菌、绿弯菌和放线菌,其中变形菌为最主要的菌门。以上研究中人工湿地的优势菌门,与本实验的优势菌门相似,说明了人工湿地基质有相似的优势菌门。变形菌为最主要菌门,Ⅰ组、Ⅱ组、Ⅲ组、Ⅳ组和Ⅴ组的相对丰度为60.23%、52.15%、45.80%、43.19%和48.70%,其次是厚壁菌门(分别为7.39%、16.08%、16.42%、18.06%和10.64%),绿弯菌门(分别为6.39%、4.64%、9.91%、14.72%和13.46%),放线菌门(分别为9.88%、8.84%、8.53%、7.06%和6.59%)和硝化螺旋菌门(分别为3.94%、5.89%、7.31%、6.09%和4.74%)。相对于Ⅰ组,植物组变形菌门的相对丰度降低,这是因为植物的种植改善了微生物多样性。据相关研究,变形菌门和厚壁菌门对反硝化有至关重要的作用[26],硝化螺旋菌门含有丰富的硝化功能的菌属[27]。相对于Ⅰ组,植物组的厚壁菌门和硝化螺旋菌门相对丰度增加,这可能是植物组微生物有较高的氮去除率的原因。
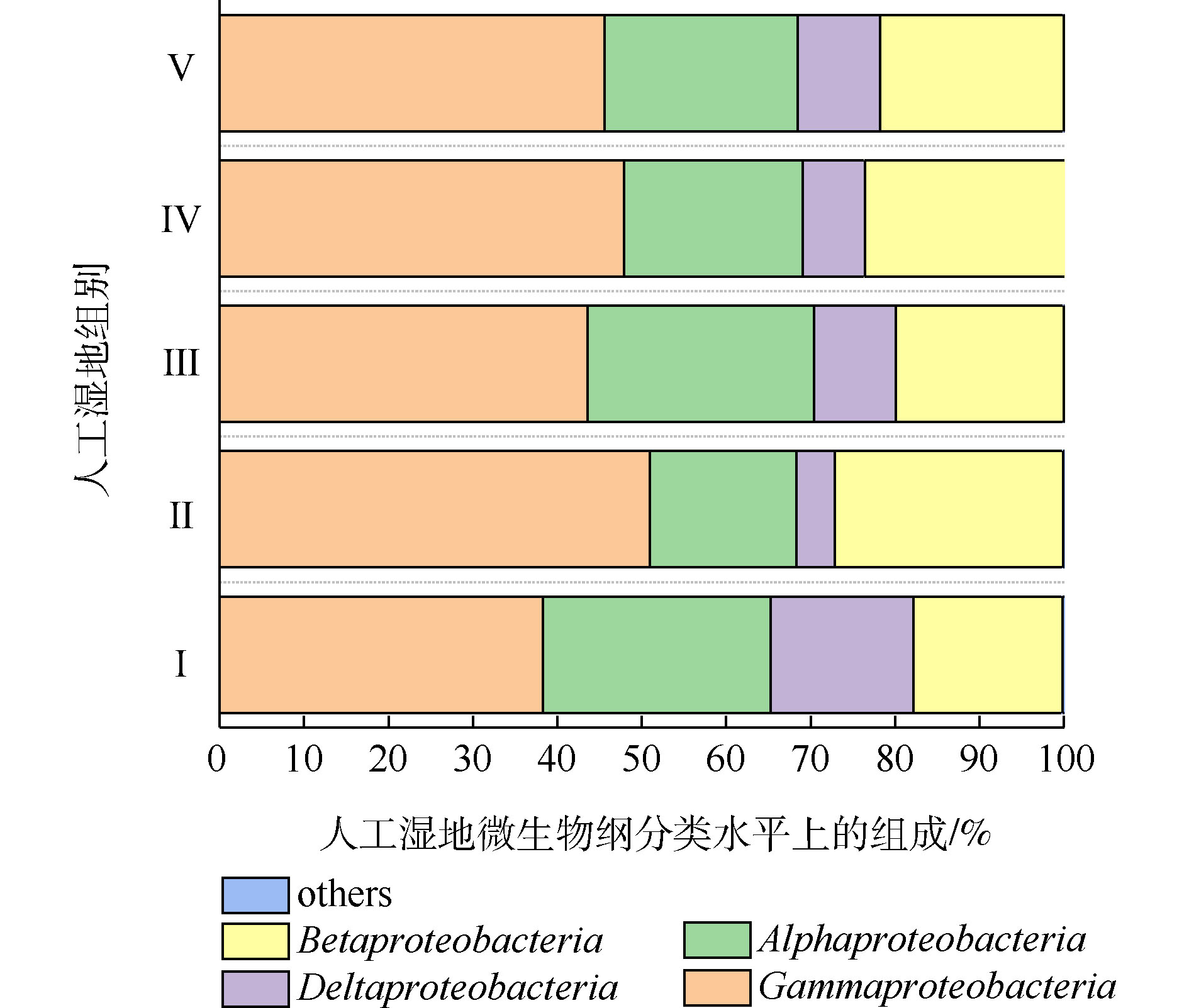
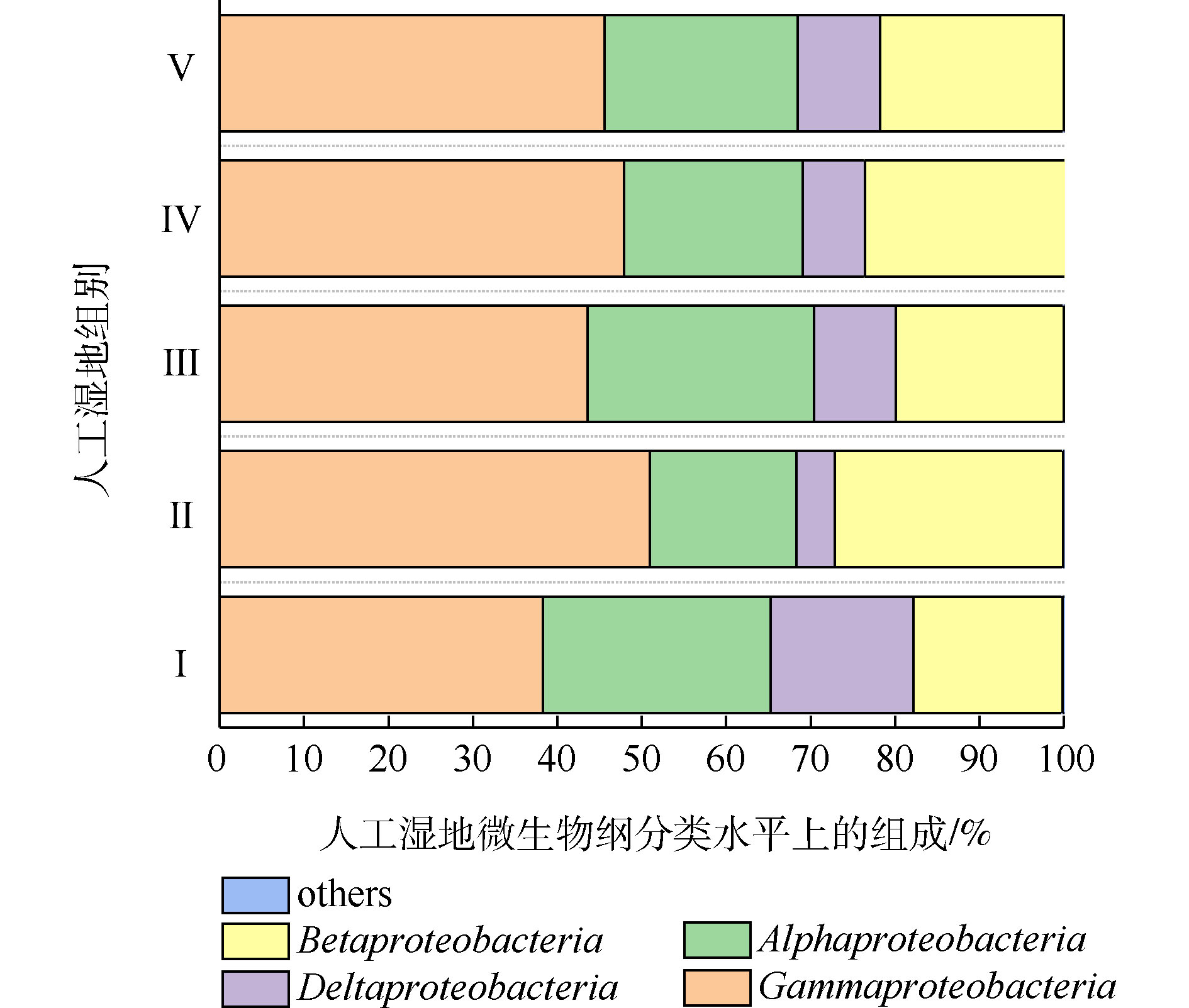
变形菌为最主要菌门。据相关研究,变形菌在人工湿地微生物氮磷去除中起到主要作用[28]。人工湿地各组变形菌的纲水平组成如图8所示。可将微生物检测频率>1%的菌纲做为主要的菌纲。共发现4个菌纲,分别为γ-变形菌纲(Gammaproteobacteria)、β-变形菌纲(Betaproteobacteria)、α-变形菌纲(Alphaproteobacteria)和δ-变形菌纲(Deltaproteobacteria),其中γ-变形菌纲、α-变形菌纲和β-变形菌纲是主要的菌纲。4个菌纲都属于革兰氏阴性菌,说明了人工湿地基质富集革兰氏阴性菌。这有利于污染物的生物降解。LI等[29-30]发现β-变形菌和γ-变形菌在去除硝酸盐和亚硝酸盐的生态功能方面发挥重要作用。ZHONG等[31]发现人工湿地中大多数可能的异养反硝化菌属于β-变形菌。LIU等[32]发现γ-变形菌纲中高丰度的假单胞菌属和动杆菌属细菌包含能发生高效聚磷的细菌。如图8所示,相比于Ⅰ组,植物组的β-变形菌和γ-变形菌的丰度均高于Ⅰ组,说明了植物根系富集β-变形菌和γ-变形菌。这可能是植物组微生物氮磷去除率高于Ⅰ组的原因。
-
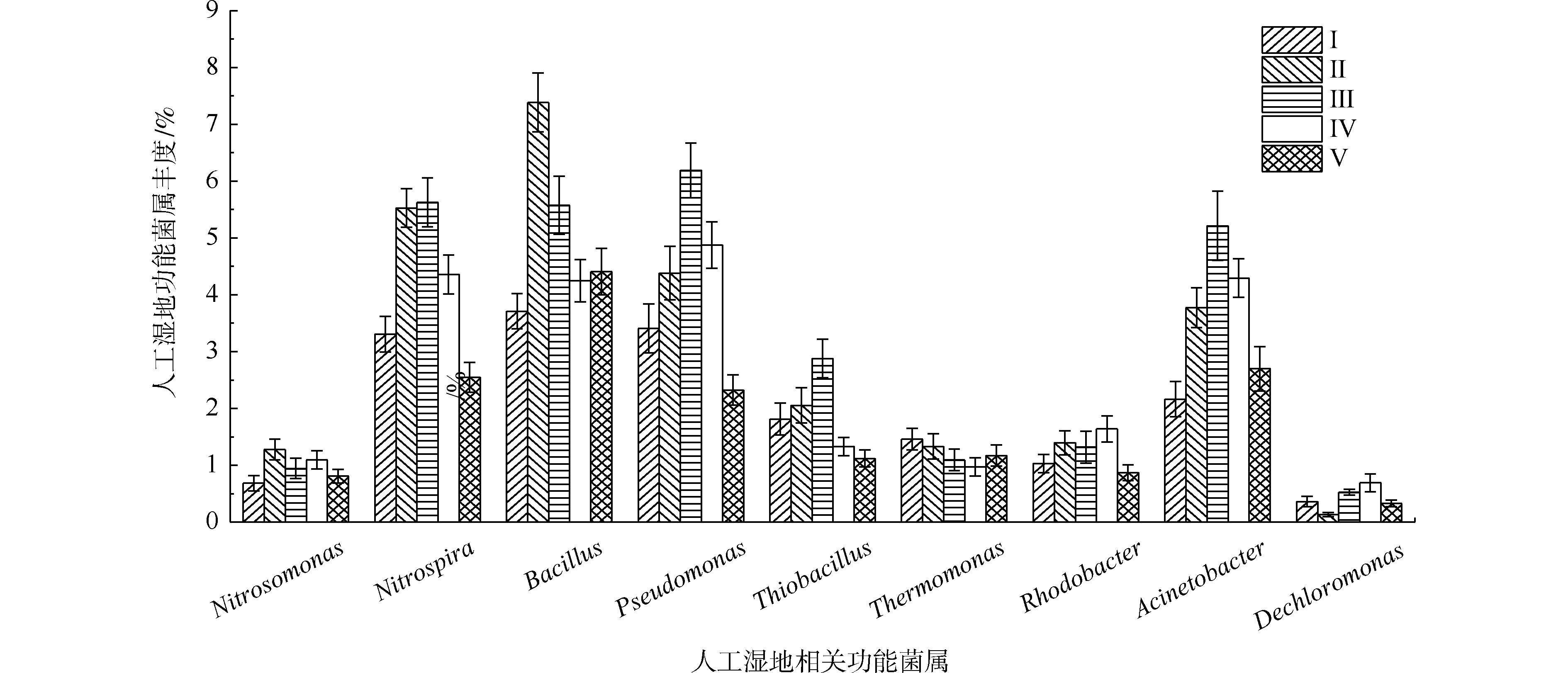
据有关研究,不动杆菌属(Acinetobacter)、假单胞菌属(Pseudomonas)、芽孢杆菌属(Bacillus)、硫杆菌属(Thiobacillus)、脱氯单胞菌属(Dechloromonas)、罗思河小杆菌属(Rhodanobacter)、热单胞菌属(Thermomonas)和索氏菌属(Thauera)等具有反硝化的作用,参与氮的转化[33-35]。另据有关研究,硝化杆菌属(Nitrobacter)和硝化螺菌属(Nitrospira)具有硝化功能[36],氨基杆菌属(Aminobacterium)、亚硝化单胞菌属(Nitrosomonas)、亚硝化螺菌属(Nitrosospira)和亚硝化球菌属(Nitrosococcus)具有氨氧化功能[23,37]。另外,YAO等[38]发现不动杆菌可以通过异养来转化氮硝化和好氧反硝化。DU等[11]认为不动杆菌或假单胞菌可能是主要的在人工湿地中负责TP去除的属。STREICHAN等[39]发现假单胞菌具有高磷的PAO清除能力。
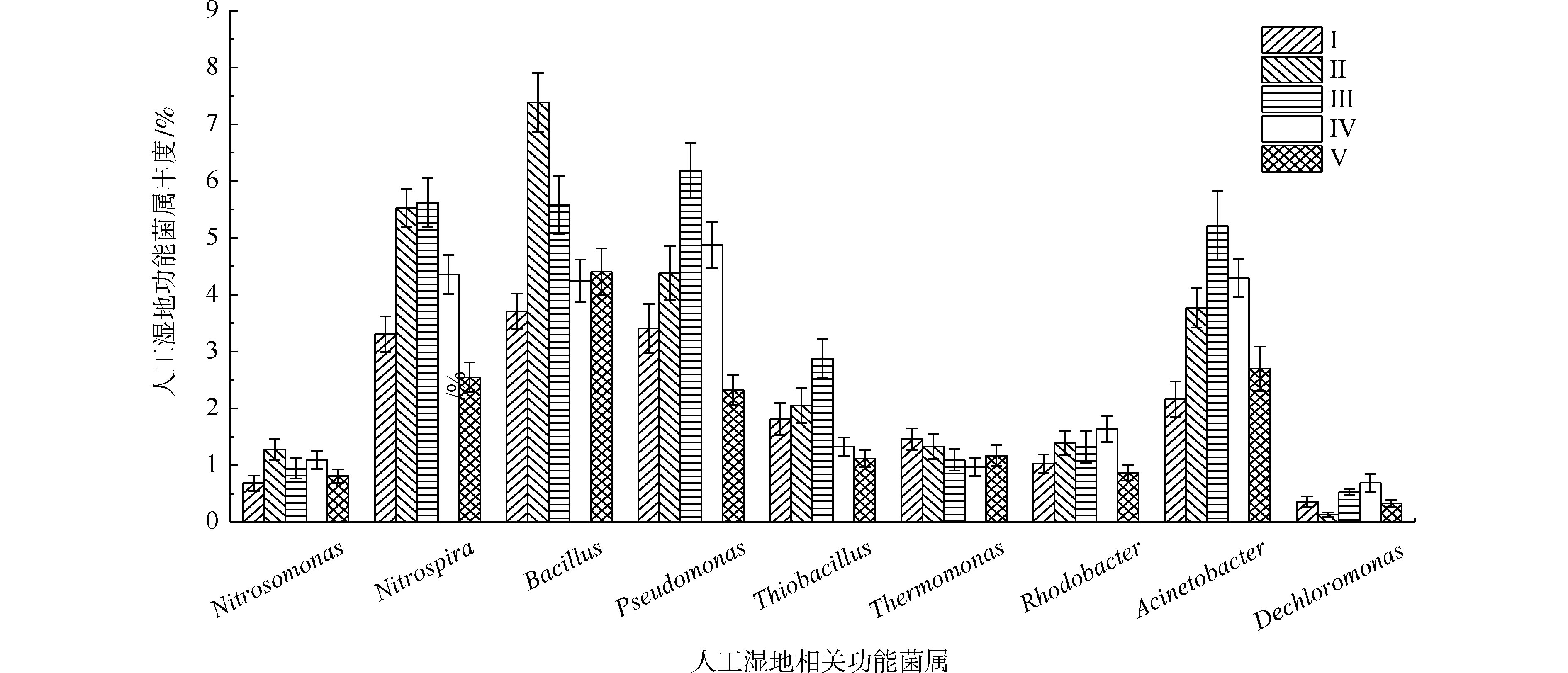
如图9所示,在人工湿地中检测出相对丰度大于0.5%的9种脱氮除磷功能菌属,分别是不动杆菌属、假单胞菌属、芽孢杆菌属、硫杆菌属、脱氯单胞菌属、红杆菌属、热单胞菌属、硝化螺菌属和亚硝化单胞菌属,发现了具有氨氧化细菌亚硝化单胞菌属丰度(0.68%~1.27%)远低于硝化作用硝化螺菌属(2.54%~5.62%)。据相关研究,某些硝化螺菌属能将
NH+4 -N完全氧化成NO−3 -N[40],这可能是人工湿地各组均有较好氨氮去除效果的原因。自养反硝化的硫杆菌的丰度(1.12%~2.87%)明显低于异养反硝化的不动杆菌属、假单胞菌属、芽孢杆菌属、热单胞菌属、红杆菌属和脱氯单胞菌属的总丰度(12.13%~19.91%),说明人工湿地易于富集异养反硝化菌。这是因为实验有较高的C/N(7.8~10.2),同时植物根系分泌碳源,可加速异养反硝化细菌的生长。不动杆菌属、假单胞菌属和芽孢杆菌属是丰度最高的异养反硝化菌属,占异养反硝化菌属总丰度的66.5%~75.9%,故认为这3个菌属在TN去除过程中起主要作用。植物组的不动杆菌属、假单胞菌属、芽孢杆菌属和硝化螺菌属丰度明显高于Ⅰ组,在Ⅱ组和Ⅲ组(最高微生物氮去除率的2组)丰度最高,表明不动杆菌、假单胞菌和芽孢杆菌丰度与人工湿地微生物氮去除率有关,其丰度的增加是植物组微生物氮去除率较高的原因。不动杆菌属、假单胞菌属具有高效聚磷作用和分泌有机酸和磷酸酶将难溶性磷转化可以被植物吸收利用的
H2PO−4 和HPO2−4 形态[41]。通过植物组与Ⅰ组比较发现,植物的种植提高了除磷功能菌属(不动杆菌属和假单胞菌属)的丰度,增强了微生物磷去除率,其中Ⅲ组(最高微生物磷去除率的组)丰度最高,表明不动杆菌和假单胞菌的丰度与人工湿地微生物磷去除率有关,其丰度的增加是植物组微生物磷去除率较高的原因。
2.1. 人工湿地对污水中氮磷的净化效果
2.2. 人工湿地氮磷平衡分析
2.2.1. 基质和植物的氮磷去除速率
2.2.2. 人工湿地氮磷平衡
2.3. 人工湿地微生物群落分析
2.3.1. 微生物序列和多样性分析
2.3.2. 微生物门、纲分类水平组成差异分析
2.3.3. 功能微生物差异分析
-
1)夏季人工湿地各组可有效地去除污水中
NH+4 -N、TN和TP污染物。低负荷下,NH+4 -N、TN和TP的平均去除率为51.48%~80.95%、62.43%~80.30%和37.09%~55.77%。高负荷下,NH+4 -N、TN和TP的平均去除率为45.53%~75.14%、53.67%~71.11%和32.97%~52.69%。种植植物的人工湿地比未种植人工湿地,在去除NH+4 -N、TN和TP污染物方面具有更好的性能。其中Ⅱ组(黄菖蒲)对氮的去除效果最好,Ⅲ组(美人蕉)对磷的去除效果最好。2)微生物作用(34.84%~45.44%)是人工湿地氮去除的主要途径,基质吸附(20.90%~23.91%)是人工湿地磷去除的主要途径。植物的种植不仅对氮(5.36%~9.79%)和磷(9.68%~16.76%)有吸收作用,还增强了微生物对氮(4.08%~10.60%)和磷(1.82%~5.08%)的去除作用。
3)植物的种植改善了基质微生物的丰富度和生物多样性,并发现微生物丰富度和多样性的改善有利于微生物对氮的去除作用。植物的种植提高了脱氮除磷功能微生物的丰度,增强了微生物对氮磷去除作用。人工湿地富集了假单胞菌属、不动杆菌属、芽孢杆菌属和硝化螺菌属等优势硝化和反硝化菌属,其中假单胞菌属、不动杆菌属、芽孢杆菌属和硝化螺菌属是人工湿地中主要的脱氮菌属,其丰度的增加是种植植物的人工湿地实验组微生物氮去除率较高的原因。人工湿地富集了优势聚磷菌属(不动杆菌和假单胞菌),不动杆菌和假单胞菌丰度增加是植物组微生物磷去除率较高的原因。





 下载:
下载: