磷在磁铁矿-针铁矿混合相上的吸附
Adsorption of phosphorus from aqueous solution on magnetite-goethite mix phase
-
摘要: 首次采用使用后贴式取暖物(商业名:热力贴)为原料制备吸附剂,元素分析、XRD、红外图谱综合鉴定为磁铁矿-针铁矿混合相(magnetite-goethite mix phase,MGM),其比表面积为98.3 m2/g,平均粒径为510 nm,零电荷点在pH=5.7附近;磷在MGM上的吸附等温线符合Langmuir方程,其吸附历时曲线遵循准二级动力学模型,升温能促进磷的吸附;在实际废水磷浓度为51.8 mg/L、初始溶液pH=2的条件下,当MGM投加量为10 g/L时,磷的去除率为94.16%。Abstract: In this study, an adsorbent was first prepared from used hot pack. The adsorbent was confirmed to be magnetite-goethite mix phase (MGM) by XRD, FTIR and elemental analysis. BET surface area, average diameter and point of zero charge were 98.3 m2/g, 510 nm, and pH 5.7, respectively. The adsorption isotherms of phosphorus fit Langmuir equation well, and the time-resolved curves of phosphorus adsorption on MGM follows pseudo-second-order kinetic model. With the increase of temperature, the adsorption of phosphorus was promoted. A removal rate of 94.16% was observed at the dosage of 10 g/L when the initial phosphate concentration in sewage (pH 2.0) was 51.8 mg/L.
-
Key words:
- magnetite /
- goethite /
- phosphorus /
- adsorption
-
在2014—2018年,我国年产酚醛树脂量由8.970×105 t增加到1.303×106 t,年复合增长率为9.78%。随着酚醛树脂产量的增加,其相应生产废水的量也在逐年增加。据统计,每生产1 t热固性酚醛树脂约产生0.90 m3 的高浓度有机废水[1]。酚醛废水中含有大量挥发酚、苯酚和游离甲醛,还含有部分甲醇和少量低分子树脂等有毒物质,其中,甲醛>1 000 mg·L−1、苯酚>5 000 mg·L−1、COD>10 000 mg·L−1。
目前,通常采用多种工艺联合的方法来处理酚醛废水,以化学-生物联用法较为普遍[2-6]。然而,化学法的预处理需要添加药剂,不仅提高了运行成本,而且残留的药剂还会影响后续生化反应。例如,在微电解+Fenton氧化预处理过程中,H2O2的残留会对后续生化反应造成不利影响,同时氧化剂的大量消耗会导致运行成本升高[2]。因此,本课题组前期开发了微生物辅助铁炭微电解的预处理工艺,实现了酚醛废水中有机物低成本的有效去除,且提供了废水的可生化性。厌氧处理工艺和好氧处理工艺的联合是当前处理酚醛废水的常用生物处理方法[3-5],然而,微生物对苯酚和甲醛浓度较为敏感,苯酚对大部分微生物有毒,是生物转化过程中的抑制性底物。但在低浓度时,苯酚可以被生物降解[4-5]。甲醛具有抗微生物性质,能与细胞内蛋白质结合,使细胞失去活力。2.40 mol·L−1的甲醛对厌氧生物过程造成50%的抑制[7]。有研究[8]还表明,甲醛的生物降解需要将甲醛浓度控制在100 mg·L−1以下。相对于好氧菌,厌氧菌对苯酚和甲醛的浓度更为敏感[6]。然而,对于高浓度有机废水,厌氧生物处理具有产能和治污相结合的优点,是目前污染物资源化最有效最直接的方式[9]。且本课题组前期研究结果也表明,传统的厌氧生物技术对高浓度苯酚的降解具有一定的限制性。因此,如何实现厌氧工艺处理对高浓度苯酚废水的高效处理是本研究要解决的问题。
虽然传统的厌氧技术对苯酚和甲醛的降解能力有限,但近年来兴起的微生物电化学系统(bio-electrochemical system, BES)为厌氧系统中乙酸的高效利用和加快有机物梯级降解提供了新的研究思路和技术创新路线[10]。作为BES的一种,微生物电解池(microbial electrolysis cell, MEC)的产甲烷过程能在无质子膜的单室MEC中轻易实现,这样的MEC可以看成是耦合了微生物电化学催化的厌氧反应器,只需要在厌氧系统中插入电极并施加一定的电压即可实现在阳极氧化有机物和在阴极产甲烷。此外,产甲烷MEC的电极制作还无需贵金属催化剂从而大大降低了运行成本并提高了可操作性[11]。因此,MEC在实际应用中更受青睐[12-13]。有研究[14]表明,厌氧折流板反应器耦合微生物电解池体系(anaerobic baffled reactor, ABR-MECs)对高浓度难降解有机废水的去除具有优势。同时还发现中温的ABR-MECs中试系统实现了对污水中有机能源的高效挖掘,微生物电化学强化对厌氧系统的产甲烷增益作用远大于其增加的能耗[15]。
基于以上研究结果,本研究在构建酚醛废水处理组合工艺过程中,在借鉴常用的预处理-厌氧-好氧的工艺联合的基础上,进行了新的技术探究,引入ABR-MECs作为酚醛废水处理组合工艺的厌氧段工艺,且采用前期探究中获得的高效低成本的微生物辅助铁炭微电解为预处理工艺,好氧段选择运行高效稳定且操作简单序批式活性污泥反应器(sequencing batch reactor, SBR),并针对新构建的组合工艺进行了酚醛废水的处理性能分析和技术经济分析。
1. 实验材料与方法
1.1 实验材料
预处理实验选用的铁碳填料购买于南京宝热化工公司,其密度约为1 200 kg·m−3,比表面积约为1.20 m2·g−1, 空隙率>65%,规格直径为2.50 cm,物理强度≥1 000 kg·cm−2。铁碳填料纯度为87%,其中,含铁量为75%,碳含量为12%,金属催化剂(铜和锰)含量为5%,其他部分为高黏土。首次使用铁碳填料前,使用1%~2%稀盐酸搅拌清洗3~5 min以进行激活。为了保证污泥种群多样性,体系中接种了2种不同来源的厌氧活性污泥,即柠檬酸废水和淀粉废水处理的厌氧污泥,2种污泥混合后的污泥VSS为22.63 g·L−1、VSS/TSS为0.65。
好氧段SBR反应接种污泥来自厦门市集美污水厂的二沉池污泥,取回污泥后,过筛去除杂物和沙子,以乙酸纳为碳源,对污泥进行间歇曝气培养,每天曝气2 h,曝气培养1周。本实验中污泥的MLSS为4 g·L−1,按照SBR的工作体积(2.50 L)进行污泥接种,比例为20%。
1.2 组合工艺流程
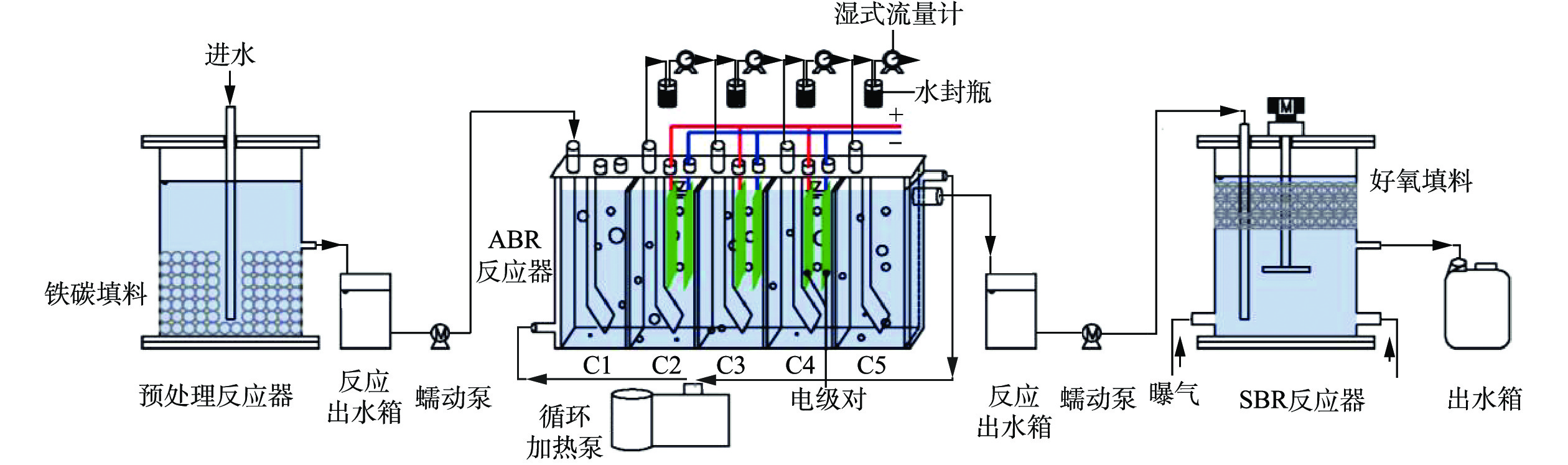
预处理反应器为直径为14 cm,高度为18 cm的有机玻璃圆柱体,反应器中间设置排水口,排水口以下体积为1 L。ABR反应器为5格室(C1~C5)有机玻璃结构,有效体积2.50 L,单格有效体积0.50 L。下流区和上流区体积比1:3。通过加热恒温循环槽实现控温,温度为(37±1.50) ℃。每一格室都有1个出水口和1个排气口,气体通过5 L的铝箔气袋收集。电极对安置于ABR中C2、C3和C4的上流区,阴阳电极的间距为15 mm,通过内置隔离棒隔离,电极的输入电压为0.90 V,由稳压电源提供。SBR反应器选用直径14 cm,高度18 cm的圆柱体,底部有2个曝气口,反应器工作体积为2.50 L,其中每个周期排水1.50 L,反应体中间设置排水口,排水口以下体积为1 L。反应器上部接电动磁力搅拌器(100 W),每个运行周期以200 r·min−1搅拌1 h。预处理-厌氧-好氧的联合的组合工艺示意图见图1。
1.3 实验方法
1)厌氧ABR-MECs系统对苯酚的降解实验。采用中温ABR-MECs系统,水力停留时间为24 h。以葡萄糖和苯酚为底物,逐步提高苯酚的浓度,对ABR-MECs体系降解苯酚的性能进行研究。实验分为5个阶段(表1)。由于实验室在前期的研究中发现,C1~C4已经基本可以完成有机物的梯级降解,因此本研究主要在C1~C4进行。
表 1 ABR-MECs工艺处理苯酚废水的实验阶段Table 1. Experimental stages in the treatment of phenol wastewater by ABR-MECs process运行阶段 运行时间/d 进水COD/(mg·L−1) HRT/d 苯酚浓度/(mg·L−1) 进水碱度/(mg·L−1) 第1阶段 1~4 5 000 1 0 2 155 第2阶段 4~8 5 000 1 100 2 155 第3阶段 8~10 5 000 1 200 2 155 第4阶段 10~16 5 000 1 300 2 155 第5阶段 16~22 5 000 1 400 2 155 2)组合工艺的启动运行。预处理反应器的工作体积为2.50 L,每次排水1.50 L,系统运行工艺参数如下:反应温度为室温 (25 ℃左右),水力停留时间为12 h。铁碳填料添加量1 400 g·L−1,污泥接种量为10%,进水负荷为16.56 kg·(m3·d)−1(以COD计),未曝气。反应进水管插入底部,由蠕动泵将稀释5倍的酚醛生产废水打入反应器中,反应进行12 h后从出水口排出,出水口设置定时排水阀门,可实现对反应器出水的自动控制运行。预处理出水进行3倍(2倍)稀释后,由蠕动泵将稀释后的预处理出水打入ABR-MECs反应器中进行厌氧反应,水力停留时间为24 h。SBR进行一个反应周期的时间为12 h,分别包括进水1 h、厌氧搅拌1 h、曝气8 h、沉降2 h、排水(小于1 min),采用定时器实现每个过程的自动控制。
1.4 分析方法
pH、SS、VSS、BOD5和COD测定采用《水和废水监测分析方法》[16]中的标准方法;CH4和H2的测定采用福立GC9790Ⅱ型气相色谱仪来测量,具体方法见PAN等[17]的研究。
苯酚和甲醛的测定采用高效液相色谱(HPLC)(日立L-2000),液相色谱条件如下:C18色谱柱(250 mm×4.60 mm,粒径为5 μm);柱温为35 ℃;流动相A为甲醇、流动相B为超纯水,流动A和B比例为50:50(v:v),流速为1 mL·min−1;检测器为紫外检测器,检测波长分别为340 nm(甲醛)和278 nm(苯酚)。
2, 4 -二硝基苯脐溶液:称取2, 4 -二硝基苯肼1 g放入100 mL容量瓶中,加乙睛溶解并稀释至刻度,摇匀后置于暗处待用。甲醛(10 mg·mL−1)和苯酚(分析标准品≥99.50%)标准品购置于阿拉丁。以甲醇/水(1∶1, v∶v)配制标准储备液,制备200 mg·L−1的甲醛的标准储备液和1 000 mg·L−1的苯酚标准储备液。实验用水为重蒸馏水。
甲醛标液的样品准备:取配制的甲醛溶液(200 mg·L−1)2 mL,置于10 mL容量瓶中,加入4 mL 2,4-二硝基苯脐溶液,用蒸馏水稀释,置于40 ℃水浴锅中避光放置12 h,经0.45 μm的微孔滤膜过滤。将滤液逐步稀释,配制成40、20、10、5、1和0.10 mg·L−1不同质量浓度的甲醛标准溶液(现配现用)。然后,用取1 mL滤液到棕色色谱瓶中,待测。苯酚测试的样品制备:直接将1 000 mg·L−1的苯酚标准储备液用蒸馏水进行梯度稀释配制成50、25、10、5、1和0.1 mg·L−1的苯酚标准溶液(现配现用)。取1 mL滤液到棕色色谱瓶中待测。样品溶液与甲醛和苯酚的标准溶液用相同的处理方法处理后进样,绘制工作曲线,使用外标法进行定量检测。
2. 结果与讨论
2.1 ABR-MECs工艺对苯酚的降解性能
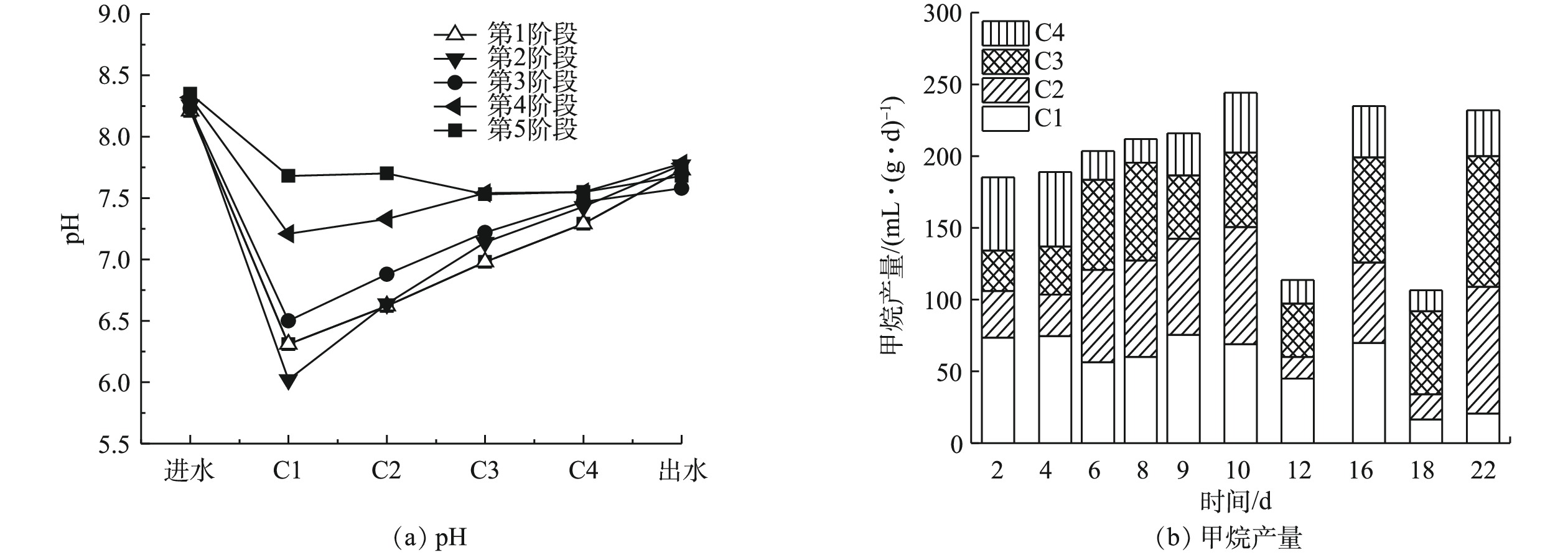
虽然在不同的反应阶段,C1和C2中的pH存在差异,但最终出水的pH基本保持一致(图2(a)),这说明ABR-MECs可以通过不同格室的反应来缓冲外界有机负荷的变化,从而最终达到对有机物进行有效稳定去除的效果。产气结果表明(图2(b)),在ABR-MECs进水中苯酚的浓度提高到300 mg·L−1(第12天)和400 mg·L−1(第18天)的初期中,产气量会受到苯酚冲击负荷的影响而下降;然而,稳定运行2个周期后,系统的产气性能得到恢复。由C1~C4对产气量的贡献占比来看,苯酚浓度越高,C3和C4的产气量占比越大。这说明ABR-MECs体系可以通过各个格室的作用调节来实现对难降解有机物的去除[14-15]。这一结果也与pH的数据相吻合。同时,苯酚浓度的提高并未对反应器的运行造成不利影响,反而随着反应的进行,COD的去除率略有升高,由94.50%升高至99.60%(表2)。
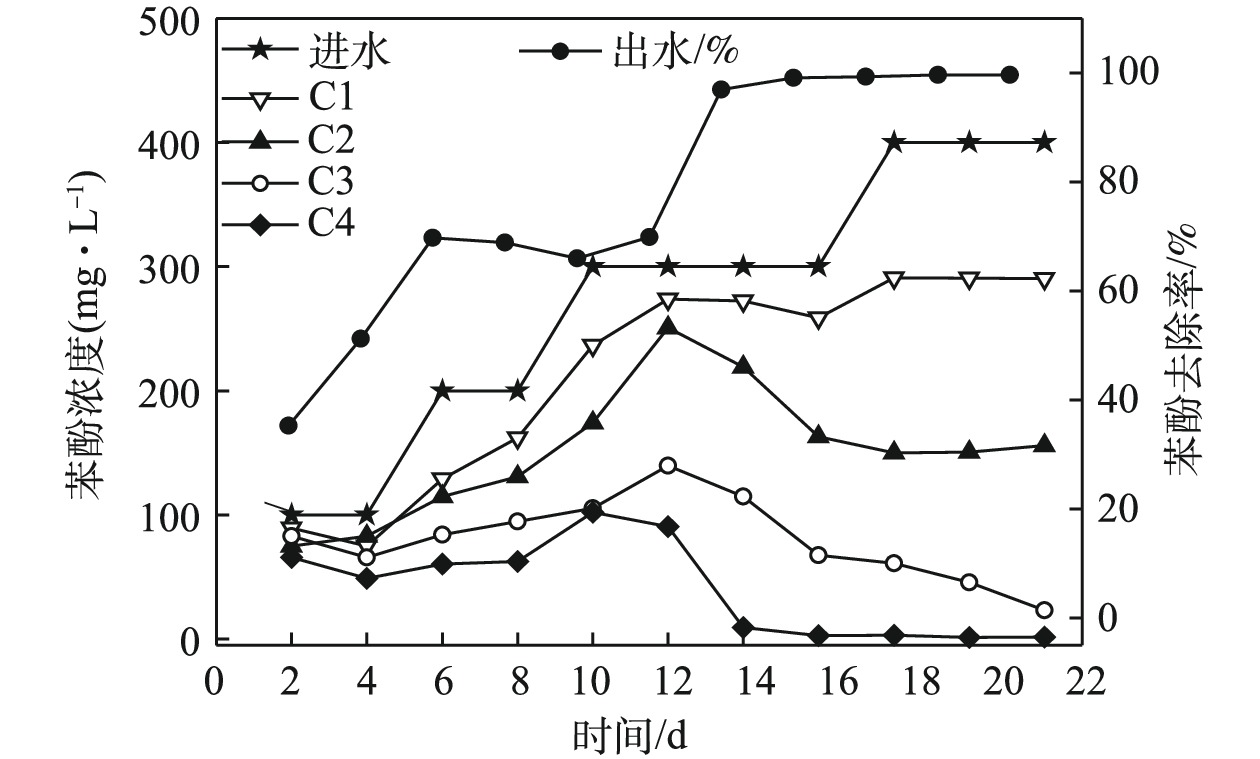
表 2 各个运行阶段末期ABR-MECs中COD变化情况Table 2. Changes of COD in ABR-MECs at the end of each stage运行阶段 进水/(mg·L−1) C1/(mg·L−1) C2/(mg·L−1) C3/(mg·L−1) C4/(mg·L−1) 出水/(mg·L−1) 去除率/% 第1阶段 5 000 978 1 347 1 076 730 346.15 95.31 第2阶段 5 200 3 138 2 197 955.50 511.50 285.88 94.50 第3阶段 5 400 1 091 842.50 413.85 218.20 301 94.44 第4阶段 7 016.25 1 128.50 672.70 495.10 61.70 52.70 99.24 第5阶段 7 025.60 451.50 916.50 500.20 45.15 30.25 99.60 随着苯酚浓度的提高,微生物对苯酚的去除效果也有明显增强(图3)。在400 mg·L−1下,ABR-MECs对苯酚的去除率达到98%以上。而有研究[18]表明,当苯酚浓度达到200 mg·L−1时,苯酚对厌氧微生物出现抑制作用。从反应器的C1~C4中苯酚的浓度来看,前期反应(苯酚浓度低于400 mg·L−1),C1对苯酚的降解作用不大,发挥作用的主要是C2和C3。由图3可见,在ABR反应器C2~C4内增加了MEC。由此可以推测,ABR-MECs对高浓度苯酚的高效去除可能得益于MEC的添加。但值得注意的是,在苯酚浓度为400 mg·L−1时,ABR-MECs系统中C1表现出对苯酚一定程度的去除:一方面可能是因为,随着苯酚浓度逐渐提高,系统中微生物培养出对环境的适应性(比如冲击负荷),从而逐渐发挥其生物学活性;此外,高浓度可以满足所有的微生物生长代谢,包括C1的产酸过程主导的系统。
2.2 组合工艺的运行性能分析
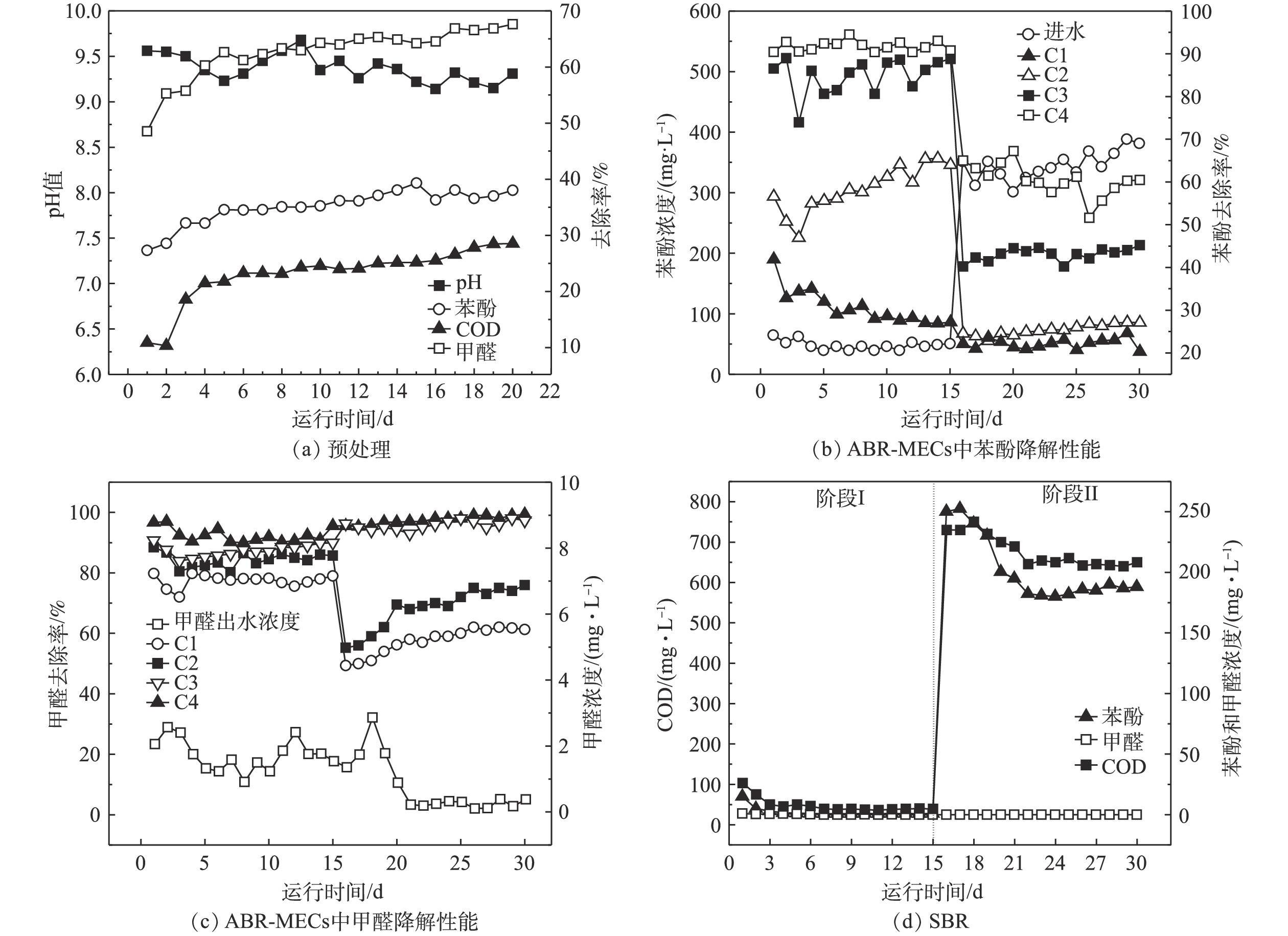
1)预处理运行性能。由于实际酚醛废水的有机物浓度过高,需要对原水进行稀释,根据前期的探究,稀释倍数为5倍。预处理阶段进水中COD、苯酚和甲醛的浓度分别为13.80、3.10和0.60 g·L−1。由图4(a)可知,预处理反应后,出水偏碱性,pH稳定在9~10。其可能的原因是,微生物产酸发酵过程产生的H+被铁碳填料中的Fe所消耗,促进了铁炭微电解反应。通过对预处理工艺的运行,BOD5/COD也从0.11升高到了0.50以上,废水的生化性能得到大幅度的提高,有助于进行后续的厌氧生物处理。随着反应的进行,苯酚、甲醛和COD的去除率均有所上升,然后趋于稳定,在稳定期苯酚、甲醛和COD的降解率分别在34%~36%、64%~66%和22%~25%。COD去除率比其他使用铁碳微电解预处理的系统略低[6],主要是本研究目的在于开发低成本处理工艺,因此,预处理阶段并未进行曝气处理。预处理后废水的可生化性的提高和部分COD的去除,主要是通过微生物进行的产酸发酵和铁炭微电解共同作用来实现的。铁炭微电解发挥作用时,发生如式(1)和式(2)的反应。
阳极(Fe):Fe−2e−→Fe2+ (1) 阴极(C):2H++2e−→2[H]→H2 (2) 反应中产生的Fe2+和原子[H]具有较高的化学活性,能改变废水中许多有机物的结构和特性,使有机物发生断链、开环等作用。产酸菌降解有机物产生挥发酸,为铁炭微电解提供H+。此外,有研究[19]还表明,铁炭微电解会通过促进乙酸激酶、磷酸转乙酰、丁酸激酶和磷酸转丁酰酶等挥发酸合成过程中关键酶实现对产酸发酵的强化。后续的生物处理阶段会进行剩余COD的去除。由于预处理提高了废水的可生化性,为后续微生物提供可利用的底物,且通过对苯酚和甲醛的去除,可减少高浓度酚醛废水对厌氧生物的毒害作用。采用预处理稳定期的出水作为后续厌氧反应的进水,即COD、苯酚和甲醛的浓度分别在10 350、1 984和264 mg·L−1左右。虽然ABR-MECs可以进行高浓度有机废水的处理,但是考虑到厌氧微生物对苯酚和甲醛的耐受性,对预处理的出水进行2~3倍稀释。
2)ABR-MECs的运行性能。为了探究ABR-MECs作为厌氧阶段进行酚醛废水处理的降解性能,首先将预处理的出水进行3倍稀释(COD、苯酚和甲醛分别为3 450、661和88 mg·L−1),待系统稳定后,保持其他运行参数不变的前提下,提高进水负荷,进水设置为2倍稀释的预处理出水(COD、苯酚和甲醛分别在5 175、991.50和132 mg·L−1左右)。实验结果表明:将出水进行3倍稀释时,系统的平均甲烷产量为181 mL·(g·d)−1;而将出水进行2倍稀释时,系统的平均甲烷产率降低至110 mL·g−1。如图4(b)所示,当ABR-MECs的进水为3倍稀释的预处理出水时,苯酚的出水浓度为39.66~64.77 mg·L−1,去除率达到90%以上。随着反应的进行,ABR-MECs不同格室发挥着不同的作用,C1的作用逐渐减弱,C2和C3对苯酚的去除率在逐渐升高。其可能的原因是,酚醛废水经过ABR-MECs反应器的处理过程中,首先进入C1,此时体系中的微生物因前期进行的400 mg·L−1苯酚的降解实验,具有很高的生物活性,所以在反应开始前几天,具有较高的去除率(35%~40%)。而随着持续高浓度的酚醛废水的暴露,部分微生物的活性受到高浓度苯酚的抑制,从而导致后期C1中苯酚的去除率下降至27%左右。虽然C1中的苯酚的浓度会随进水浓度的升高而升高,但未被降解的苯酚被后续格式中的微生物所降解,体现了厌氧折流板反应器的优势[15]。由于有C1的部分降解作用,进入C2的有机废水中苯酚和甲醛的浓度已经有所降低,加上C2中有MEC的促进作用,使C2对苯酚的去除率具有很大的贡献,同样C3中也有MEC的作用,同时进入C3的有机废水中苯酚浓度已经降低至约为100 mg·L−1,可以被微生物很快地降解。进入C4的有机物的含量已经相对较少,故C4发挥作用的空间有限。由此可见,ABR-MECs对苯酚具有很高的去除率,达到90.20%~94%,且其中主要起作用的是前3个格室。由甲醛的降解特征来看,当进水中甲醛的浓度为88 mg·L−1时,主要是C1发挥作用,其去除率达到70%以上,反应器最终去除率在97%以上,厌氧反应器出水中甲醛的浓度为0.91~2.56 mg·L−1。
为了使ABR-MECs的各个格室都能充分发挥作用,将进水的负荷提高,采用2倍稀释的预处理出水作为厌氧反应的进水,图4(b)和4(c)中第16~30天的数据为这一阶段反应的苯酚和甲醛去除特征。在高浓度的苯酚进水条件下,C1和C2都暴露在很高的苯酚浓度下,分别为990 mg·L−1和770 mg·L−1左右,导致ABR反应器的C1和C2中苯酚的降解率下降。而此时,C3和C4对苯酚的降解速率较前一阶段明显提高,特别是C4充分地发挥了作用。然而由于负荷的提高超出系统降解能力,且高浓度的苯酚对厌氧微生物的生长有抑制作用[18],因此,最终苯酚的降解率为51%~66%,ABR-MECs出水中苯酚的浓度为301.26~388 mg·L−1。虽然,系统中苯酚的降解率出现大幅下降,但甲醛的降解并未受到影响,反而随着反应器的运行,系统对甲醛的降解效能逐渐上升,最终的去除率达到99%以上,厌氧出水中甲醛的浓度极低,为0.10~1.35 mg·L−1,且各格室均发挥了重要作用。有研究[20]表明,高温UASB反应器(55 ℃)对含酚废水进行处理时,苯酚的去除率达到99%,这与本研究的结果相当,但后者采用中温厌氧处理体系,较高温体系更具有成本优势。
3)SBR的运行性能。SBR阶段的进水完全来自于ABR-MECs的出水,因此,SBR阶段的运行也分为2个阶段:即阶段I (1~15 d)为ABR-MECs进水为3倍稀释的预处理出水时,进水COD为530~549 mg·L−1、苯酚为39.66~64.77 mg·L−1、甲醛为0.91~2.56 mg·L−1;阶段II(15~30 d)即为ABR-MECs进水为2倍稀释的预处理出水时,进水COD为1 820~2 000 mg·L−1、苯酚为301.26~388 mg·L−1、甲醛为0.10~1.35 mg·L−1。
如图4(d)所示,阶段I中SBR运行过程较为稳定,随着运行时间的增加,出水苯酚和COD均有所降低,体系中苯酚的浓度由反应初期(1~3 d)的15 mg·L−1降低至后期的(10~15 d)的0.85 mg·L−1,COD由103.20 mg·L−1降低至39 mg·L−1左右,而甲醛由于进水浓度低,故被SBR降解完全,已经无法检测甲醛的浓度。根据《中华人民共和国污水综合排放标准》(GB 8978-1996)[21],本研究的酚醛废水处理组合工艺达到GB 8978-1996的三级排放标准。然而在第II阶段,由于进水浓度的大幅提高,导致系统运行不稳定。虽然SBR出水中的pH和甲醛的浓度满足污水的排放标准,但是COD和苯酚的浓度达到600 mg·L−1和180 mg·L−1,均远远超过出水排放标准。这说明阶段II中SBR的进水已经超过了SBR系统的降解能力。
2.3 组合技术经济分析
根据组合工艺的运行过程,组合工艺支出主要包括电耗、填料的费用和稀释水的费用等。其中,电费参考厦门市工业用电平、峰值,统一以1元·kWh−1进行计算;水费以2.20元·t−1进行计算。具体能耗分析如下。
1)预处理的成本主要由铁碳填料消耗、稀释用水的费用和进料泵电耗3部分组成。其中,预处理反应器添加铁碳填料1.40 kg,单价为6元·kg−1;每吨水损失20%铁碳填料,每吨酚醛废水铁碳填料支出为1.68元;每升酚醛废水用水稀释5倍, 稀释每吨酚醛废水增加的用水支出为8.80元;进料泵的提升水头为1 m(含估算的沿程和局部水头损失),按照进料泵的电机效率按60%计算,需要消耗的电功为16.33 kJ,进料泵的电耗支出为0.004 5元·t−1。因此,预处理部分的成本为10.48元·t−1。
2)ABR-MECs反应阶段主要的成本来自稀释用水支出和电耗(进料泵、水热循环泵、电化学组件)。由于每吨酚醛废水需要稀释3倍,处理每吨废水稀释用水支出为4.40元。电耗的计算中,电化学组件电耗参考汪涛[22]的计算,电化学组件运行一天消耗的电能为0.036 kWh,本工艺处理1 t废水需要16.67 d,电化学组件的电耗支出为0.60元·t−1;水热循环泵电耗按平均水温由室温(25 ℃)提高10 ℃计算(厦门,运行温度35 ℃),提升1 t水需要的热量为4.20 J·(kg·℃)−1×1 000 kg×10 ℃=42 000 kJ,按照电能利用率按60%计算,此部分需要的电耗为19.44元·t−1;根据预处理中计算获得进料泵的电耗支出为0.004 5元·t−1。此外,ABR-MECs进行厌氧酚醛废水处理时,会产生可再生能源物质甲烷,本研究中甲烷的产率为181 mL·g−1(以COD计),厌氧处理的进水有机物浓度为34 500 g·m−3,平均COD去除率为90.70%,可算出平均产甲烷率为0.56。采用单位体积甲烷发电量经验值3.59 kWh·m−3计算[14],ABR-MECs系统产甲烷可回收电能为2.01 kWh·m−3,甲烷回收电能为2.01元·t−1。因此,厌氧反应阶段总成本为(电化学组件+进料泵电耗+水热循环泵) 22.35元·t−1。
3) SBR反应过程的成本主要包括进料泵的电耗(0.004 5元·t−1)、曝气和电器搅拌支出。然而,由于本实验为小试,反应器较小,会导致单位体积的曝气支出和电器搅拌支出的计算结果会远远超出实际工程中的数值。因此,这部分计算的意义不大。
因此,本研究中构建的组合工艺以微生物辅助铁炭微电解作为预处理反应,相较于其他化学反应可大大降低药剂添加的费用(表3)。常规的工艺预处理费用为35元·t−1,铁碳微电解+Fenton预处理技术处理酚醛废水的成本为28.92元·t−1[6],其中药剂费为主要成本(20.07元·t−1),而本工艺无需添加药剂,预处理成本仅为10.48元·t−1。电化学氧化与生物流化床组合的处理成本为103.66元·t−1[3],而本研究中处理预处理和厌氧段合计费为32.83元·t−1,一般好氧工艺的成本小于20元·t−1,无论从预处理还是组合工艺来说,都具有一定的成本优势。虽然预处理阶段和厌氧阶段的进水都进行了稀释,但稀释的目的是降低酚醛废水中的苯酚的浓度,从而减少对微生物的毒害。因此,该技术还可以考虑采用其他需要处理的有机废水对酚醛废水进行稀释,既降低了稀释的成本,同时可实现不同种类的有机废水的同步处理。
表 3 不同酚醛废水处理组合工艺成本对比Table 3. The cost comparison of different combined processes treating phenolic wastewater3. 结论
1)相比传统的厌氧工艺,ABR-MECs对处理高浓度含苯酚的废水具有优势。
2)构建的微生物辅助铁炭微电解-ABR-MECs-SBR组合工艺可以实现酚醛生产废水的高效去除,且最终SBR出水达到GB 8978-1996的三级排放标准。
3)本研究中的组合工艺具有一定的经济优势,预处理成本大约为10.48元·t−1,厌氧处理的成本大约为22.35元·t−1。
-
[1] Montgomery M.A.,Elimelech M.Water and sanitation in developing countries:Including health in the equation. Environ.Sci.Technol.,2007,41(1):17-24 [2] Conley D.J.,Paerl H.W., Howarth R.W.,et al. Controlling eutrophication: Nitrogen and phosphorus. Science,2009,323(5917):1014-1015 [3] Bingjun Pan, Jun Wu, Bingcai Pan,et al. Development of polymer-based nanosized hydrated ferric oxides (HFOs) for enhanced phosphate removal from waste effluents.Water Res.,2009,43(17):4421-4429 [4] Rahman S., Mukhtar S. Efficacy of microbial treatment to reduce phosphorus and other substances from dairy lagoon effluent.Applied Engineering in Agriculture,2008,24(6):809-819 [5] Suzuki K.,Tanaka Y.,Kuroda K.,et al. Removal and recovery of phosphorous from swine wastewater by demonstration crystallization reactor and struvite accumulation device.Bioresour.Technol.,2007,98(8):1573-1578 [6] Li J.,Xing X.H.,Wang B.Z.Characteristics of phosphorous removal from wastewater by biofilm sequencing batch reactor (SBR).Biochem. Eng. J.,2003,16(3):279-285 [7] Oehmen A., Lemos P.C., Carvalho G.,et al.Advances in enhanced biological phosphorus removal: From micro to macro scale.Water Res.,2007,41(11):2271-2300 [8] Jae W. C., Seung Y.L., Ki Y.P.,et al.Investigation of phosphorous removal from wastewater through ion exchange of mesostructure based on inorganic material.Desalination,2011,266(1-3):281-285 [9] Onyango M.S., Kuchar D., Kubota M.,et al.Adsorptive removal of phosphate ions from aqueous solution using synthetic zeolite.Industrial and Engineering Chemistry Research,2007,46(3):894-900 [10] Zhao D., Sengupta A.K.Ultimate removal of phosphate from wastewater using a new class of polymeric ion exchangers.Water Res.,1998,32(5):1613-1625 [11] 刘可.KMnO4与FeSO4联用去除水中磷的效能与机理.哈尔滨:哈尔滨工业大学博士学位论文,2010.8-9 Liu K. Efficiency and mechanism of phosphate removal by combined use of KMnO4 and FeSO4. Harbin: Doctor Dissertation of Harbin Institute of Technology,2010.8-9 (in Chinese) [12] Cheng X.,Huang X., Wang X.,et al.Phosphate adsorption from sewage sludge filtrate using zinc-aluminum layered double hydroxides.J.Hazard.Mater.,2009,169(1-3):958-964 [13] 刘勇,李晖,张永丽,等. 羟基铝柱撑蛭石的制备及其吸附磷酸根性能研究. 四川大学学报(工程科学版),2008,40(4):77-82 Liu Y., Li H., Zhang Y.L., et al. The preparation and application of Al-pillared veimiculite in phosphate adsorption. Journal of Sichuan University(Engineering Science), 2008,40(4):77-82(in Chinese) [14] 赵冰清,程翔,孙德智 磁性类水滑石吸附水中磷的研究. 哈尔滨工业大学学报,2008,40(12):1962-1964 Zhang B.Q., Cheng X., Sun D.Z. Adsorption of phosphate by magnetic hydrotalcite-like compounds in aqueous solution. Journal of Harbin Institute of Technology, 2008,40(12):1962-1964(in Chinese) [15] Zeng L., Li X., Liu J.Adsorptive removal of phosphate from aqueous solutions using iron oxide tailings.Water Res.,2004,38(5):1318-1326 [16] 李延波,邱立平,王广伟,等. 水热改性颗粒钢渣的除磷效能.中国给水排水,2011,27(9):74-77 Li Y.B., Qiu L.P., Wang G.P., et al. Phosphorus removal efficiency of hydrothermally modified granular steel slag. China Water and Wastewater, 2011,27(9):74-77(in Chinese) [17] Cheung K.C., Venkitachalam T.H. Improving phosphate removal of sand infiltration system using alkaline fly ash.Chemosphere,2000,41(1-2):243-252 [18] 郭照冰,陈天,陈天蕾.铁盐改性废弃蛋壳对水中磷的吸附特征研究. 中国环境科学,2011,31(4):611-615 Guo Z.B., Chen T., Chen T.L. Phosphorus adsorption behaviors on iron-coated wasted eggshell in aqueous solution. China Environmental Science, 2011,31(4):611-615(in Chinese) [19] 李新勇,李树本.纳米半导体研究进展.化学进展,1996,8(3):231-239 Li X.Y., Li S.B. Progress in nanometer semiconductor materials. Progress in Chemistry,1996, 8(3?挺漲洳瀱漭甲渳搹猨?灮愠牃瑨????摥猩漼牢灲琾楛漲渰???捡瑮慧??桨楡浮楧捨慵?匬楗湡楮捧愠???ふち????????????????楦湦??桴椠湯敦猠敬??戠牭?孬??嵵??湲愠??????匠潯牲灧瑡楮潩湣?潡晣?慤湳琠楯浮漠湰票?潳湰瑨潯?桵祳搠牡潤硳祯慲灰慴瑩楯瑮攠??渠癦楥牲潲湩?匭捡楬?呭攠捷桡湴潥汲????ちぴ?????????????????Hazard.Mater., 2012,203-204:145-150 [20] 刘承帅.铁锰氧化物/水界面有机污染物的氧化降解研究.广州:中国科学院研究生院博士学位论文,2007.11-11 Liu C.S. Oxidative degradation of organic pollutants in the interface of iron oxides/water and interface of manganese oxides/water. Guangzhou: Doctor Dissertation of the Graduate School of the Chinese Academy of Sciences,2007.11-11 (in Chinese) [21] 郑怀礼,邓琳莉,吉方英,等. 高铁酸钾制备新方法与光谱表征. 光谱学与光谱分析,2010,33(10):2646-2649 Zheng H.L., Deng L.L., Ji F.Y., et al. A new method for the preparation of potassium ferrate and spectroscopic characterization. Spectroscopy and Spectral Analysis,2010,33(10):2646-2649(in Chinese) [22] 曹建劲 改性丝光沸石结构研究. 光谱学与光谱分析,2004,24(2):251-254 Cao J.J. Study on crystal structure of modified mordenite. Spectroscopy and Spectral Analysis,2004,24(2):251-254(in Chinese) [23] 胡英,吕瑞东,刘国杰,等. 物理化学.北京:高等教育出版社,2007.549-549 [24] Yuh S. H. Review of second-order models for adsorption systems. J. Hazard.Mater.,2006, 136(3):681-689 [25] 丁葵英,朱茂旭,卞永荣.聚合羟基铁铝蒙脱石复合体对磷的吸附行为及其动力学. 矿物学报,2009,29 (1): 19-25 Ding K.Y., Zhu M.X., Bian Y.R. Behavior and kinetics of phosphate adsorption on hydrosyaluminum/hydroxyiron-montmorillonite complexes.Acta Mineralogica Sinica, 2009,29 (1): 19-25 (in Chinese) [26] Ning P., Bark H. J., Li B., et al. Phosphate removal from wastewater by model-La(Ⅲ) zeolite adsorbents. Journal of Environmental Science,2008,20(6):670-674 [27] Daou T. J., Begin C. S., Greneche J. M., et al. Phosphate adsorption properties of magnetite-based nanoparticles. Chem.Mater, 2007,19(18):4494-4505 [28] Luengo C., Brigante M., Avena M. Adsorption kinetics of phosphate and arsenate on goethite:A comparative study. J. Colloid Interface Sci., 2007,311(2):354-360 [29] 臧运波,侯万国,王文兴. Cr(VI)在Mg-Al型类水滑石上的吸附-脱附性研究 I.吸附性. 化学学报,2007,65(9):773-778 Zang Y.B., Hou W.G., Wang W.X. Adsorption-desorption of chromium(Ⅵ) on Mg-AI hydrotalcite-like -

 点击查看大图
点击查看大图
计量
- 文章访问数: 1838
- HTML全文浏览数: 1061
- PDF下载数: 949
- 施引文献: 0



 下载:
下载: