-
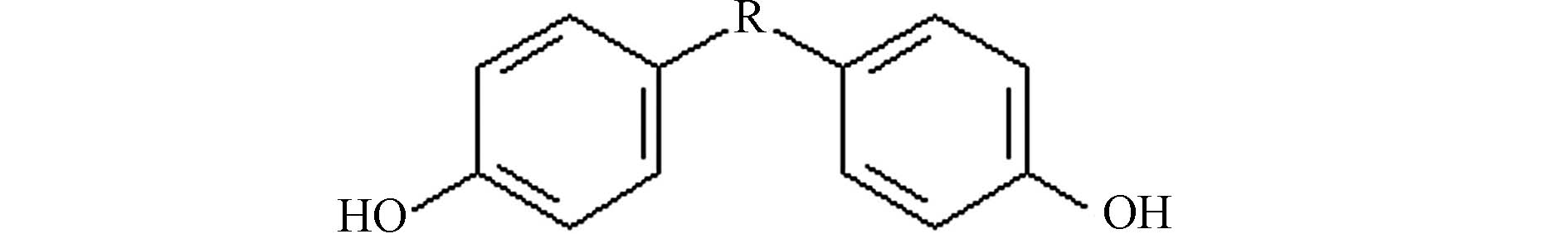
双酚类化合物(bisphenols,简称BPs)是一系列由碳或硫原子桥连两个对羟苯基形成的化合物,其结构示意图如图1所示. 这些化合物被广泛应用于聚碳酸酯和环氧树脂的生产,存在于婴儿奶瓶、塑料水瓶、食品储存容器、牙齿填充物、热敏纸和压敏纸、纸币、收据和玩具等物品中. 此外,它们还被用于隐形眼镜、新生儿培养箱和雾化器等医疗设备和医疗保健服务中[1-2]. 通过食用聚碳酸酯容器或涂有环氧树脂的易拉罐容器等包装的食物,人们会面临直接的BPs暴露风险. 其中,双酚A(bisphenol A,简称BPA)是最早生产使用的一种BPs. 自从1938年Dodds等首次报道了BPA对大鼠具有雌激素效应以来[3],大量研究发现BPA与肥胖、糖尿病、乳腺癌、心血管疾病、肾脏疾病、慢性呼吸道疾病、牙齿发育障碍、行为障碍和生殖障碍等一系列健康问题有关[4-5]. BPA对中枢神经系统、心血管系统、免疫系统、呼吸系统和肾脏系统都有不利影响,而且能穿透胎盘屏障直接影响胎儿[6].
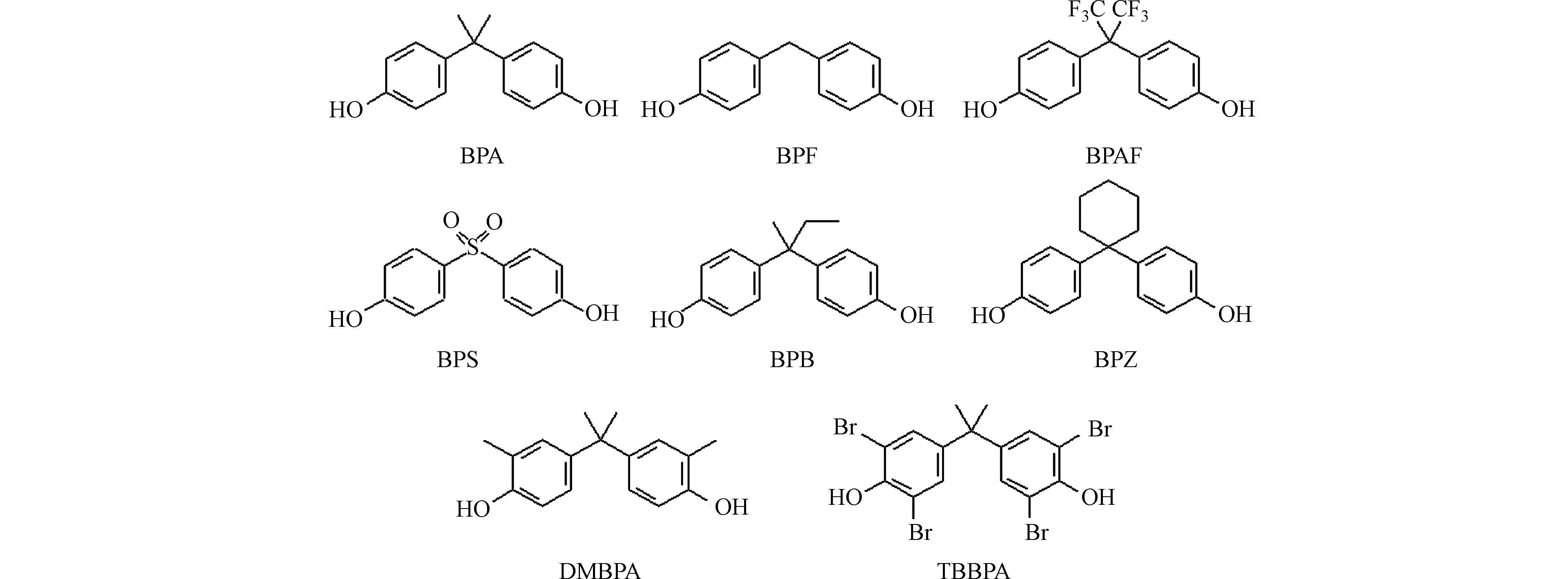
随着人们对BPA毒性的深入了解,多个国家陆续出台了限制或禁止BPA生产和使用的政策. 进而,越来越多的新型BPs被用作BPA的替代品投入生产和使用. 目前,主要的BPA替代品包括双酚F(bisphenol F,BPF)、双酚AF(bisphenol AF,BPAF)、双酚S(bisphenol S,BPS)、双酚B(bisphenol B,BPB)、双酚Z(bisphenol Z,BPZ)、二甲基双酚A(dimethylbisphenol A,DMBPA)等,本文亦将四溴双酚A(tetrabromobisphenol A,TBBPA)与常见BPA替代品一并归入BPs进行讨论分析,具体化学结构见图2. 其中,BPF、BPS和BPAF是聚碳酸酯和环氧树脂制造中最主要的BPA替代品,应用非常广泛[2]. 例如,BPF可用于清漆、衬垫、黏合剂、塑料水管以及牙科密封剂、口腔修复装置、组织替代品和食品包装涂层的生产[7];BPS可用于环氧胶、罐头涂料、热敏纸的生产,以及染料和鞣剂的添加剂[8];BPAF被用作氟橡胶、电子和光纤中的交联剂,以及聚酰亚胺、聚碳酸酯和其他特种聚合物的高性能单体等[9-10]. BPs在环境水体、饮用水及生物体中的检出率和浓度也越来越高[2,11],因此人类面临着日益升高的BPs暴露风险. 需要注意的是,这些BPs也并非完全安全. 例如,BPS具有复杂的内分泌干扰效应,而BPF则存在轻度到中度急性毒性和弱雌激素活性[12-13]. 更值得关注的是,BPs进入人体后会经历一系列生物代谢转化,形成多种代谢产物,产生未知毒性效应,因而对人类生命健康的影响不容小觑[14]. 因此,对新型BPs生物代谢机理开展研究,进一步评估其代谢转化的安全性,有助于减小BPs对人体健康造成的可能危害,寻找最佳BPA替代产品. 本文对BPA及其替代品等BPs的实验和计算代谢研究方法、代谢产物鉴定方法及反应分子机制理论解析等方面的研究进展进行了回顾总结和展望.
-
双酚类物质代谢实验研究可分为体内(in vivo)和体外(in vitro)两种,采用的生物系统如表1所示. 体内实验研究最常采用动物模型,直接获得动物或人类的相关代谢信息;体外实验研究常用的生物系统包括微粒体、S9系统等亚细胞和细胞部分,以及各种复杂的组织. 本部分将讨论BPs体内和体外代谢实验研究最常用生物系统的优缺点.
-
模式动物是研究外源性物质在生物体内不利影响的一种重要研究体系. 通常采用诸如啮齿动物、斑马鱼、果蝇和线虫等动物模型,通过分析受试动物体内BPs水平随时间变化情况、代谢产物鉴定等,直接获得动物或人类体内相关的代谢历程信息[15]. 然而,这一实验方法也存在着一定的局限性. 例如,由于人类生理机能、代谢酶亚型和代谢过程与动物模型不完全相同,难以将动物模型的研究结果直接外推应用到人类身上. 此外,使用哺乳动物等模式生物的实验会涉及到伦理和道德问题. 另外,体内实验研究会消耗巨大的物质和时间资源,不适用于大批量物质的代谢研究.
-
(1)人肝微粒体
人肝微粒体(human liver microsomes,简称HLMs)因为具有成本相对较低、商业可获得性较高以及易于制备处理等优点,是研究外源性物质代谢最常用的工具[16]. HLMs是对人源肝脏组织匀浆采用差速离心方法制备而成,其中富含Ⅰ相细胞色素P450酶(cytochrome P450,简称CYP450)和Ⅱ相尿苷二磷酸葡糖醛酸转移酶(uridine-diphosphate-glucuronosyl transferases,简称UGTs). 在其对外源物质代谢孵育过程中需要补充所需的酶辅因子,如补充磷酸腺苷二磷酸酯(the reduced form of nicotinamide adenine dinucleotide phosphate,简称NADPH)可以激活CYP450. HLMs包含了所有的CYP450亚型,例如HLMs中已知57种CYP450亚型,可较为真实地还原动物肝脏体系,开展BPs体外代谢实验研究;然而使用HLMs难以判断具体哪种CYP450亚型对代谢反应起到主导作用.
(2)肝S9系统
肝S9系统是一种亚细胞体系,由肝脏匀浆在7400 r·min−1转速的离心机中离心分离得到. 该组分含有包括硫酸转移酶(sulfotransferases,简称SULTs)和羧酸酯酶(carboxylesterases,简称CES)等的细胞质酶以及包括CYP450和UGT等的微粒体酶,可以提供更全面的代谢信息,对研究外源物质的生物代谢具有重要意义. 与肝微粒体相比,S9系统所涉及的代谢过程更加复杂,可以通过添加各种辅酶因子激活其Ⅰ相和Ⅱ相代谢功能,如添加尿苷二磷酸葡萄糖酸(uridine diphosphate gluconic acid,简称UDPGA)可激活UGT,添加3′-磷酸腺苷-5′-磷酰硫酸(3′-phosphoadenosine-5′-phosphosulfate,简称PAPS)可激活SULTs,添加乙酰辅酶A(acetyl coenzyme A,简称CoA)则可激活乙酰转移酶(acetyltransferase,简称AT). 然而,与HLMs相比,S9系统包含的CYP450活性通常较低,当分析设备的灵敏度较低时,会限制它们在某些体外实验代谢研究中的应用.
(3)重组酶
使用肝脏的分离部分进行代谢孵育实验,可以有效评估外源物质代谢程度,并鉴定在体内可能生成的代谢产物. 然而,由于HLMs、S9系统或其他肝细胞分离物中均含有多种酶,难以确定具体哪些酶参与了代谢. CYP450重组酶是通过基因工程和细胞工程技术,将某个CYP450亚型cDNA序列整合至异源表达载体上并转入异源表达系统内,利用异源宿主大量表达,再经分离纯化制备而成的[17]. 因此,使用单个重组酶可以确定具体参与代谢外源性物质的酶亚型,并能够表征反应速率. 虽然重组酶技术可以消除由于体内组分的复杂性而引入的误差,然而由于它们是在异源系统中表达的,存在一定程度的变异和不确定性,这可能导致其在代谢方面的准确性受到影响. 此外,重组酶需要耗费大量的时间和资源进行表达和纯化,同样难以适用于大量外源性物质的高通量筛查.
-
常见的体外代谢实验研究流程包括将BPs与混合的CYP450(如HLMs和S9系统)或特定的CYP450(如重组酶)以及辅因子NADPH共同孵育,经一定时间后测量代谢产物. 体内代谢实验研究方法则主要包括用含BPs食物饲喂实验动物,喂养一段时间后获取生物样品并检测代谢产物. 其中,液相色谱-质谱联用技术(liquid chromatography-mass spectrometry,简称LC-MS)是目前最为常用的体外代谢产物检测与分析方法[18]. 在LC-MS中,最为关键的信息是与分子量相关的分子离子峰,最常见的是正离子模式下的M+1峰和负离子模式下的M-1峰. 基于所执行的质谱检测类型,可以进行一系列基于质谱的实验来表征代谢产物,包括MS/MS、MSn、MSe、中性损失等. 通过分析质谱数据,还可以直接推断一些代谢物的结构,例如芳香环增加15.99个质量单位,则表明形成了羟基化代谢产物. 此外,通过分析代谢产物的谱图数据,可以基于常见的分子碎片数据对代谢产物进行推断. 然而,过度依赖常见碎片经验可能导致重要代谢产物的遗漏.
尽管一些外源性物质的代谢反应仅涉及简单的羟基化和环氧化代谢,然而外源性物质在CYP450的介导下仍有可能会发生复杂的反应,可导致复杂代谢产物的生成[19]. 例如,(1)再氧化作用;(2)中间体的重排,特别是高自旋状态下发生的重排反应;(3)酶催化反应后反应性代谢物的进一步反应. 在代谢产物的检测中,一方面在生成代谢产物的质谱图之后,可以通过比较疑似代谢物峰的质谱图与母体化合物的质谱图,检查碎片模式,并结合最有可能形成的代谢物结构来表征代谢产物,也可通过生成的代谢产物质谱图中定制软件包进行筛查. 另一方面,也可以在进行基于质谱的检测分析之前,基于对代谢反应化学知识的了解,预判可能发生的代谢反应. 例如,通过MetaSite[20]或SMARTCyp[21]等代谢预测软件,可对CYP450介导外源物质代谢产物进行预筛选. 然而,基于质谱的检测分析技术存在无法确定确切代谢位点的限制,在这种情况下,可以采用代谢物分离后的结构鉴定技术进一步研究,例如核磁共振技术(nuclear magnetic resonance,简称NMR)[22-23].
-
理论计算化学是一种通过计算机模拟解决化学问题的方法,力学基础主要源自分子力学和量子力学. 对于具有高度结构可塑性和复杂活性位点的酶蛋白而言[24-25],基于分子力学的分子动力学模拟等方法在模拟酶的动态过程中扮演着重要角色,可用于确定底物和酶的结合模式[26-30]. 而量子化学方法则可详细描述代谢转化核外电子扮演的角色及其对反应的影响,特别是涉及传统化学键断裂和生成等关键基元反应过程,接下来就其进行详细介绍.
-
量子化学是应用量子力学的规律和方法来研究化学问题的一门学科,其研究范围包括分子的结构、性质和相互反应机理等. 量子化学的基本假设是微观粒子或体系的性质完全由状态波函数
Ψ 描述,遵从薛定谔方程(Schrödinger equation). 因此,量化计算实质就是求解薛定谔方程,以获得原子和分子的核外电子运动情况,并解析分子的空间结构、电荷分布,原子间结合能和结构与性质的关系甚至反应途径(核运动规律)[31]. 但由于多电子体系的哈密顿算符较为复杂,需要引入各种近似方法来求解多电子体系薛定谔方程. 其中,Hartee-Fock(HF)方法是一种基于波函数的量子化学方法,其核心思想是假设多电子体系的波函数可以表示为多个单电子波函数的乘积[32],可以精确处理小分子. 但随着分子增大,HF计算的复杂性急剧增加,难以处理大分子体系. 此外,HF方法忽略了电子的强关联效应,对分子性质的计算精度有很大影响.相比之下,DFT方法不是基于波函数,而是基于电子密度,而电子密度可以通过实验测量得到[33]. DFT方法考虑了相对论和自旋极化效应的影响,对于重元素和自旋极化强的分子比HF方法有较高精度,在预测分子性质和反应路径方面展现了强大的作用. 譬如2022年Science报道的全氟烷基羧酸在极性非质子化溶剂二甲亚砜与极性质子化溶剂水以体积比8∶1共存的碱性条件下(污染物与氢氧化钠物质的量比是30∶1),能在相对较低温度下通过脱羧作用生成活泼的碳负离子而快速分解乃至最终矿化就充分证实了这一点[34]. 此外,DFT方法计算量小,计算速度相对较快,对于CYP450催化转化污染物这样的体系具有较大优势[35]. 然而,DFT方法中不同的密度泛函对于不同的体系有不同的计算精度,因此选择合适的泛函对于DFT方法的计算结果至关重要. 对于酶催化转化污染物计算研究来说,从简单的局部密度近似方法(local density approximation,简称LDA)到广义梯度近似方法(generalized gradient approximation,简称GGA),以及meta-GGA泛函方法等都有所应用. 但在实践中,首选的是混合泛函,如B3LYP泛函. 一般来说,B3LYP在许多特性上表现良好,即使与最近提出的高度参数化的泛函方法如M06系列泛函相比,在各种特性尤其是相对能量和反应势垒方面也表现更为出色. 另一方面,B3LYP可以很好地再现含血红素体系在不同自旋态下的能量,而血红素酶CYP450是非常重要的外源物质Ⅰ相代谢酶,所以B3LYP在BPs生物代谢转化机理计算研究中被广泛应用. 但B3LYP方法已经提出近30年,在准确度和精确度方面有所不足,虽然目前仍占主导地位,但可以期待未来越来越多更先进的密度泛函方法将会被运用于BPs生物代谢机理的理论计算研究中[36]. 此外,大多数DFT方法不包含重要的色散相互作用,因此近年来通过将经验色散效应包含到现有的泛函中(如B3LYP-D)进行补偿,对计算精度已有了巨大改善[37-40].
-
过渡态理论(transition state theory,简称TST)是一种广泛用于化学反应动力学速率常数研究的统计力学理论[41]. 其核心假设是化学反应的速率由一个过渡态决定. TST利用势能面(potential energy surface,简称PES)的概念,将连接两个极小值的最小能量路径的最高点与过渡态联系起来. 以CYP450催化反应为例,底物分子首先与酶反应活性中心化合物Ⅰ(compound Ⅰ,简称Cpd Ⅰ)的铁氧基团相互作用形成反应物复合物,底物分子的一些活性位点被催化激活,发生键的断裂和形成,从而发生代谢反应,代谢产物最后从CYP450的活性中心解离. 反应活化能是反应物复合物变化到过渡态对应的激活能,它在Born−Oppenheimer自由能面的鞍点处计算,因为在反应坐标上是一个极大值,所以由一个虚频表征. 从PES上可以探测不同的代谢反应机理,并为每个机理关联反应坐标图. 然而,TST无法处理长距离和非键合电子转移机制,这可以通过Marcus理论解决. 感兴趣的读者可以参考相关文献[42-44].
-
量子化学计算的准确性不仅取决于计算方法,还取决于所采用的化学模型. 理想的化学模型应该在考虑计算成本的同时也要考察酶所有关键位点,以便能够准确地模拟酶的催化性能[45-46]. 由于完整的酶蛋白质含有数以万计的原子,无法直接用量子化学方法高效描述. 为了使用DFT等量子化学方法研究代谢机制,通常需要分割出酶的活性位点或者团簇模型,以量子化学方法处理模型中的所有原子. 所选择的活性位点往往是酶参与催化反应的最小区域.
由于BPs在以CYP450为代表的Ⅰ相代谢酶的催化下,会被引入羟基、羧基等极性集团,BPs反应活性增强,易生成高毒性的中间体或产物,是其产生潜在健康风险的主要代谢途径,故接下来以CYP450为例,具体介绍酶的量子化学计算模型. CYP450是一类以铁卟啉结构为催化活性中心的血红素蛋白酶的统称,其活性位点由原卟啉环外接四个甲基、两个乙烯基、两个电离的丙酸基,以及来自半胱氨酸残基的硫醇基和氧基这两个轴向配体组成. 使用DFT方法模拟CYP450的常规做法是采用简缩的活性中间体模型,即Cpd I模型. 简化做法是去除卟啉环连接的基团,同时轴向的氨基酸配体用巯基(—SH),甲基硫酸盐(—SCH3)或者半胱氨酸(—SCys)替代. 对外部酶蛋白环境采用溶剂化模型,如极化连续介质模型(polarizable continuum model,简称PCM)模拟处理.
-
本文进行了全面的文献综述,旨在找到描述包括双酚A及其替代化学品(图2)在内的BPs代谢反应过程的研究. 文献搜索包括2023年3月之前发表的所有文章,在PubMed数据库中进行电子搜索. 用于识别描述每种双酚类物质代谢研究的搜索策略见表2. 本文只选择原创研究文章,排除综述文章. 在接下来的步骤中,仔细检查部分选定研究的全文版本,并将相关研究的数据呈现在本综述中. 双酚类物质代谢实验研究分为体内和体外两种,生物代谢类型可分为Ⅰ相和Ⅱ相反应两种类型. 其中,BPs在CYP450等的催化下发生Ⅰ相反应后,可能会生成一些高毒性的中间体或产物,是BPs存在健康风险的一个主要原因. 而Ⅱ相反应则涉及BPs与葡萄糖醛酸等内源物质的结合,由UGTs、SULTs等催化完成,可以降低BPs的生物毒性,起到脱毒的作用,因此,BPs的Ⅰ相代谢反应引起了人们的极大重视. 但从检测结果看,Ⅱ相反应的产物检出较多.
双酚类物质的葡萄糖苷酸和硫酸盐共轭复合物是体内Ⅱ相代谢的两种主要产物. 以BPA为例,在人体中,BPA能迅速葡萄糖醛酸化并通过尿液排出体外,而在大鼠中BPA则经历肠肝循环[47]. 肝脏中的UGT2B15和UGT1A9是催化BPA葡萄糖醛酸化的两种主要UGTs酶亚型[48]. 此外,呼吸系统、肠道和乳腺组织中的其他UGTs也对BPA的代谢有影响[49-50]. BPs的硫酸化复合物是双酚类物质的另一个重要代谢产物,但是其代谢仅占较小的比例[51]. 例如,在30名志愿者中检测到的BPA代谢产物主要是BPA葡萄糖醛酸化产物,其次是BPA硫酸化产物和非结合态的BPA. 硫酸转移酶SULT1A1是负责BPs硫酸化的主要SULTs亚型,其活性最高,其次是SULT1E1和SULT2A1[52-53].
体内研究检测到双酚类Ⅰ相氧化代谢产物的情况很少,而且代谢产物也会进一步发生结合反应,最终以葡萄糖醛酸结合物等结合形式出现[54-55]. BPA甲基和芳环羟基化合物2,2-二(4-羟基苯基)-1-丙醇和2-(3,4-二羟基苯基)-2-(4-羟基苯基)丙烷的葡萄糖醛酸结合物在尿液中被检测到[54-55]. 非结合态的BPA邻位羟基产物也在粪便中被检测到,但是这很可能是结合产物在肠道细菌作用下脱结合所导致的结果[55-56]. 此外,BPs的邻醌以DNA加合物的形式在CD1雄性大鼠代谢研究中被检测到[57]. 这种BPs邻醌形成的机制被认为是通过CYP450介导苯酚环的羟基化作用形成BPs邻位羟基化合物,继而再被氧化成反应性的BPs邻醌,并进一步与谷胱甘肽结合而形成[58].
双酚类化合物的体外Ⅰ相代谢研究主要使用肝细胞、肝或肠微粒体、S9系统或重组酶进行. 将NADPH添加到如微粒体、S9系统等酶源,检出邻位羟基化BPA是BPA的主要代谢产物[59-60]. 已有研究指出CYP450中的CYP2A1和CYP2C9是参与BPA酚环羟基化的2种主要亚型[59]. 尽管在体外代谢实验中检测到对苯二酚(hydroquinone,简称HQ)、对异丙烯基苯酚(4-isopropenylphenol,简称IPP)、对异丙醇苯酚(4-hydroxycumyl alcohol,简称HCA)和4-甲基-2,4-二对羟基苯基-1-戊烯(4-methyl-2,4-bis(p-hydroxyphenyl)pent-1-ene,简称MBP)等其他氧化代谢产物的浓度较低,但已有证据表明代谢物HCA的雌激素活性大约是BPA的100倍,而二聚体MBP则表现出更高的雌激素活性,约为BPA的1000倍[61-62]. Nakamura等提出HQ、IPP和HCA是通过BPA的原位(ipso)加成-取代反应形成的[61]. 这一结果表明BPA的原位(ipso)加成-取代路径虽然不是CYP450主要催化转化方式,但是其代谢产物的强内分泌干扰效应提示该路径在BPA的系统毒理学机制中扮演着一定角色,特别是在一些葡萄糖醛酸酶缺乏的情况下,这一代谢途径所导致的毒性作用不容忽视.
相应地,其他BPs的体内及体外代谢产物亦有报道. 除了葡萄糖苷酸和硫酸盐共轭复合物外,BPB、BPF、BPAF、BPS等的体外氧化代谢过程主要产生羟基化产物. 如在BPF、BPAF、BPZ和DMBPA与人肝微粒体孵育试验中,均检测到了BPF、BPAF、BPZ和DMBPA对应的邻位羟基化代谢产物[60]. 甚至在雄性大鼠肝微粒体孵育实验中,还检测到了二邻位羟基化BPF[23]. 在BPS的人肝微粒体孵育体外代谢试验中,也检测到了其邻位羟基化代谢产物[63]. 在DMBPA与S9组分和NADPH共孵育代谢实验中,也发现了DMBPA邻位羟基化代谢产物[59]. 此外,BPAF与人肝微粒体在NADPH存在下共孵育试验中,还检测到分解产物4-(1,1,1,3,3,3-六氟-2-羟基异丙基)苯酚和4-羟甲基苯酚,表明BPAF类似于BPA可发生ipso取代反应[62]. 采用大鼠肝S9系统对BPB的Ⅰ相转化过程开展研究,Yoshihara等采用液相色谱-串联质谱技术鉴定了BPB的羟基化及裂解产物,包括BPB的邻位羟基化物和邻苯醌[62]. 此外,两种低极性的二聚化代谢产物包括5-乙基-3,5-二对羟基苯基-3-己烯和5-乙基-3,5-二对羟基苯基-2-己烯[62]亦有检出. 这两种化合物被认为是BPB的烷基和苯环C—C键断裂后进一步发生异丁烯酚自由基二聚化形成的[59-62].
事实上,除了上述羟基化、裂解产物及二聚化物质外,Ashrap等报道了一类普遍存在于酚类污染物的新型代谢产物,即醚类代谢物[64]. 通过酚类污染物三氯生(triclosan,简称TCS)、BPA及氯酚等分别在鲈鱼、鹌鹑和人肝微粒体的体外代谢以及大鼠活体代谢研究,发现这些酚类物质可形成TCS-O-TCS、BPA-O-BPA等脂溶性更强的醚类代谢产物. 并且,这类醚类代谢物在普通人群的尿液中也被高频检出. 由于具有较高的脂溶性,这类新型代谢产物与组成型雄烷受体(constitutive androstane receptor,简称CAR)等受体具有更高的结合活性,如TCS-O-TCS与CAR的结合活性是其母体化合物TCS的7.2倍. 而除了外源性酚类物质自身结合成醚类物质外,Liu等报道BPA等酚类污染物甚至可以与维他命E及雌二醇等人体内源性物质相互结合,致使维他命E浓度降低而增加体内活性氧物质的浓度,从而影响人体正常生理功能[65].
-
与大量BPs类物质代谢转化的实验研究相比,相关的理论计算研究数量有限,多集中于BPA代谢转化的分子机制研究. 本论文将重点从理论计算研究的角度讨论BPs经CYP450介导的氧化转化反应分子机制. BPs可能的反应包括:(1)脂肪链羟基化反应;(2)芳环羟基化反应;(3)酚羟基活化及后续裂解或氧中心自由基偶联反应. 此处以BPA反应为例,逐一详述反应过程.
脂肪族和芳香族羟基化反应途径已在CYP450催化多种替代底物中被广泛研究,通常表现为分步反应[66-68]. BPA脂肪链羟基化机制始于Cpd I提取底物脂肪烃基上的氢原子,通过“双态反应”过程[69],即在低自旋二重态和高自旋四重态两个自旋势能面上进行. 通常情况下,脂肪链基团上的H抽提后形成自由基中间体,之后经过反弹过渡态形成醇类产物. Dias等人运用包含底物结合口袋部分的222个原子较大活性中心模型,开展了BPA脂肪烃基羟基化反应过程的DFT计算,发现BPA分子可以在酶口袋中紧密结合,然而底物分子却难以在结合区域充分旋转使其甲基指向血红素,从而在对构型不加限制或固定部分残基原子进行限制时分别导致模型崩溃或得到高能路径. 因此,Dias等分析由于底物分子的体积和形状以及CYP450底物结合口袋大小的限制,认为CYP2C9催化BPA分子中甲基基团羟基化这一反应路径可以排除[70]. 然而,尽管目前关于BPA与CYP2C9的实验研究未有检测到BPA甲基的羟基化产物,这一产物在Zalko等关于怀孕CD1小鼠体内BPA代谢研究中曾有报道[55],说明CYP450的其它亚型可能会催化BPA发生甲基羟基化反应.
与Cpd Ⅰ催化的脂肪链羟基化不同,在芳环羟基化过程中存在亲电和自由基这2种途径的相互作用,以酶进攻苯环大π体系形成σ-络合物起始. 底物首先和铁氧基阳离子自由基经亲电加成生成C—O键并形成复合中间体[71]. Cpd Ⅰ对芳环的亲电加成导致迈森海默复合物(meisenheimer complex)的形成,该复合物可以是阳离子型或自由基型中间体. 现有研究表明,阳离子和自由基型中间体的能量差距仅有几千卡路里,并且它们的稳定性排序和相对能量大小易受外部扰动的影响[24,72]. 在迈森海默复合物形成后,ipso-C上的质子转移到最近的吡咯氮原子上,形成质子化的卟啉中间体. 而这一N质子化中间体可以中继H,从而协助质子穿梭到底物的氧原子上,形成芳环的羟基化产物. 此外,Yadav等认为Cpd Ⅰ亲电加成形成的迈森海默复合物可在含有羟基的碳原子处发生闭环过程,形成环氧化物[73]. 事实上,Dias等的研究认为BPA的环氧化过程是个高能垒高吸热过程,而Ji等基于Cpd Ⅰ模型开展的苯环亲电加成路径研究同样发现这一过程需要跨越较高的反应能垒[35]. 因此,形成环氧化物的路径可被排除. 而在实验研究中,也从未有检测到BPA环氧化物代谢产物的研究报道.
关于酚羟基的活化历程,Ji等研究认为,Cpd Ⅰ抽提BPA酚羟基的氢原子后,会生成含Fe—OH基团的化合物Ⅱ(compound Ⅱ,简称Cpd Ⅱ)和BPA酚氧自由基中间体,Fe—OH基团的·OH自由基反弹至BPA酚氧自由基苯环的原位(ipso)、邻位(ortho)和间位(meta)上,并生成相应的醌醇中间产物. 羟基自由基的反弹过程在低自旋二重态下是无能垒进行的,其中反弹到ipso-C和ortho-C位点的反应均是强放热反应,在热力学上可自发进行;而反弹到meta-C位点的反应则是吸热反应,该过程在热力学上不能自发进行. 这一结果合理地解释了CYP450催化BPA代谢转化实验中没有检测到间位羟基化代谢产物的原因[59-61]. 邻位反弹醌醇中间产物会在环境水分子的协助下,实现氢转移从而生成邻位羟基化产物;原位反弹醌醇中间产物会进一步发生C—C键的裂解,在水分子等的协助下形成HCA、IPP和HQ. 计算所得的代谢产物与实验检测结果相一致[35].
如前所述,Ashrap等[64]发现酚类污染物经CYP450介导可形成偶联的醚类代谢产物,然而目前仍缺乏对这一过程的理论认识. Guo等采用DFT计算,通过探索涉及双自由基偶联、自由基加成和电子转移的可能的偶联机制,揭示了TCS到TCS-O-TCS的代谢途径. 所得结果表明酚偶联过程可通过CYP450的活性中心Cpd Ⅰ和Cpd Ⅱ经低能垒的双自由基机制而非自由基加成机制进行. 当高自旋四重态下的羟基反弹势垒较高时,酚氧自由基能够具有足够长的寿命实现双自由基偶联[74]. 这一理论认识提示我们,可将这一双自由基偶联途径推广到包括BHPF在内的其他外源性乃至内源性酚类物质的代谢性能评价中.
-
双酚类化合物在环境介质、食品、消费品甚至人体中的大量检出,表明BPs存在着全球性大规模的污染趋势. 尽管最具代表性的BPA已被各国政府禁止或限制生产使用,但越来越多的BPA替代品涌入人们的生产生活中,而这些替代品并非完全安全. 特别是BPs在体内的转化产物可能具有比其母体更复杂的内分泌干扰效应和毒性效应,对于人类生命健康的影响不容忽视. 本综述从BPs在体内和体外代谢研究方法、代谢机制理论计算研究方法,及在实验和计算方面已取得的研究成果进行了回顾和总结. 尽管过去数十年间毒理及环境科研工作者对BPs的代谢过程、代谢产物及其可能毒性效应开展了大量体内和体外的研究工作,并达成一定共识,但仍有一些问题或方法需要进一步解决优化. 第一,分析技术的进步将促进BPs研究在人体暴露、代谢和毒性等领域的发展,因此,仍需强大的分析检测方法来鉴定潜在的环境和代谢转化产物. 第二,以往的代谢研究仅局限于少数选定的BPs化合物,而对大多数类似物特别是近年来新使用的BPA替代品的代谢途径和产物仍未完全确定. 类似地,对于BPs衍生物的代谢研究也很少. 例如,在环境基质和人体样本中发现了BPA的卤代衍生物如氯代双酚A等,与BPA相类似,具有激活人体雌激素受体的完全激动活性,除此以外,BPA的三氯化物还可以激活人体过氧化物增殖激活受体(peroxysome proliferator–activated receptors,简称PPARs)[75]. 此外,BPS的二甲基化衍生物被报道在彗星实验中显示出显著的DNA损伤[76]. 因此,亟需研究阐明更多双酚类似物及其衍生物的代谢途径、代谢产物以及代谢修饰对毒性的影响. 第三,与大量BPs代谢转化的实验研究相比,相关的理论计算化学研究数量有限,且多集中于BPA代谢转化的分子机制研究. 事实上,理论计算化学方法在解析酶促反应的分子机制、探讨可行反应路径及鉴定代谢产物方面具有巨大优势. 以CYP450的代谢机制为例,“双态反应”理论的提出很好地阐述了氧反弹机制,合理解释了许多实验中存在重排产物的现象. 而BPs代谢分子机制的理论计算研究也同样合理阐明了实验观测到的代谢产物及其产物分配比例问题. 因此,加强BPs代谢机制的理论计算与实验研究相结合的综合研究,一方面能够节省大量人力、物力和财力,另一方面能突破实验技术的限制,提供实验难以观测的信息,相信二者结合比任何一种孤立方法都能提供深入的洞察力.
双酚类化合物的生物代谢机理研究进展
Research progresses of biotransformation mechanisms of bisphenols
-
摘要: 以双酚A(bisphenol A,简称BPA)及其替代物为代表的双酚类化合物(bisphenols,简称BPs)在各种环境介质、食品、消费品以及人类和动物体中被广泛检出,表明BPs存在全球性大规模污染趋势. 除了BPs自身毒性外,其在生物体内的转化代谢产物可能具有比母体化合物更复杂的内分泌干扰效应和毒性效应,对于人体生命健康的影响不容小觑. 本文就BPs生物代谢的实验和理论计算研究方法及其取得的研究成果等方面进行回顾总结,在分析现有研究存在的问题和不足上对未来的研究重点提出了展望.Abstract: Bisphenol A (BPA) and its substitutes, as representatives of phenolic compounds (BPs), have been widely detected in various environmental media, foods, consumer products, and human and animal bodies, indicating a pervasive global contamination trend. Beyond their inherent toxicity, BPs’ transformation metabolites within organisms may exhibit more complex endocrine-disrupting and toxic effects than the parent compounds, with significant implications for human health. This paper presents a comprehensive review and summary of experimental and theoretical research methods employed in studying the biotransformation of BPs, along with the corresponding research findings. Furthermore, by examining the existing limitations and deficiencies in current studies, the paper provide insight into future research priorities. The elucidation of BPs' biological metabolism and its implications contributes to a better understanding of their potential risks and aids in the development of effective strategies for mitigating their adverse effects.
-
Key words:
- bisphenol pollutants /
- metabolic transformation /
- experimental study /
- theoretical study.
-
表 1 代谢研究生物系统分类
Table 1. Categorization of biological systems employed in studying xenobiotic metabolism
体外实验in vitro 体内实验in vivo 亚细胞部分Subcellular fractions 细胞部分Cellular fractions 组织Tissues 微粒体、S9系统、血清和血浆、有机大分子 离体肝细胞、原代肝细胞培养物、细胞系 组织切片,离体灌注肝脏 细菌菌株多细胞生物、实验动物 表 2 用于识别描述某些双酚类化合物代谢产物研究的搜索逻辑及文献数目统计
Table 2. Search logic applied to identify the researches about metabolites of BPs and the number of corresponding researches
双酚类物质BPs 运用的搜索逻辑The applied search logic 研究文献数目Number of researches BPA (bisphenol A OR 4,4’-(propane-2,2-diyl)diphenol) AND (metabolism OR biotransformation OR metabolites) 5925 BPAF (bisphenol AF OR hexafluorobisphenol A OR BPAF) AND (metabolism OR biotransformation OR metabolites) 221 BPF (bisphenol F OR Bis(4-hydroxyphenyl)methane OR BPF) AND (metabolism OR biotransformation OR metabolites) 495 BPS bisphenol S OR Bis(4-hydroxyphenyl)sulfone OR BPS) AND (metabolism OR biotransformation OR metabolites 2964 BPB (bisphenol B OR 2,2-Bis(4-hydroxyphenyl)butane OR BPB) AND (metabolism OR biotransformation OR metabolites) 711 TBBPA (Tetrabromobisphenol A OR TBBPA) AND (metabolism OR biotransformation OR metabolites) 584 -
[1] VANDENBERG L N, HAUSER R, MARCUS M, et al. Human exposure to bisphenol A (BPA) [J]. Reproductive Toxicology, 2007, 24(2): 139-177. doi: 10.1016/j.reprotox.2007.07.010 [2] CHEN D, KANNAN K, TAN H L, et al. Bisphenol analogues other than BPA: Environmental occurrence, human exposure, and toxicity—a review [J]. Environmental Science & Technology, 2016, 50(11): 5438-5453. [3] DODDS E C, LAWSON W. Molecular structure in relation to oestrogenic activity. Compounds without a phenanthrene nucleus [J]. Proceedings of the Royal Society of London Series B - Biological Sciences, 1938, 125(839): 222-232. [4] ROCHESTER J R. Bisphenol A and human health: A review of the literature [J]. Reproductive Toxicology, 2013, 42: 132-155. doi: 10.1016/j.reprotox.2013.08.008 [5] BEN-JONATHAN N. Endocrine disrupting chemicals and breast cancer: The saga of bisphenol A[M]. Estrogen Receptor and Breast Cancer. Cham: Humana Press, 2019: 343-377. [6] CORBEL T, GAYRARD V, PUEL S, et al. Bidirectional placental transfer of Bisphenol A and its main metabolite, Bisphenol A-Glucuronide, in the isolated perfused human placenta [J]. Reproductive Toxicology, 2014, 47: 51-58. doi: 10.1016/j.reprotox.2014.06.001 [7] CABATON N, DUMONT C, SEVERIN I, et al. Genotoxic and endocrine activities of bis(hydroxyphenyl)methane (bisphenol F) and its derivatives in the HepG2 cell line [J]. Toxicology, 2009, 255(1-2): 15-24. doi: 10.1016/j.tox.2008.09.024 [8] NADERI M, WONG M Y L, GHOLAMI F. Developmental exposure of zebrafish (Danio rerio) to bisphenol-S impairs subsequent reproduction potential and hormonal balance in adults [J]. Aquatic Toxicology, 2014, 148: 195-203. doi: 10.1016/j.aquatox.2014.01.009 [9] KONNO Y, SUZUKI H, KUDO H, et al. Synthesis and properties of fluorine-containing poly(ether)s with pendant hydroxyl groups by the polyaddition of bis(oxetane)s and bisphenol AF [J]. Polymer Journal, 2004, 36(2): 114-122. doi: 10.1295/polymj.36.114 [10] MATSUSHIMA A, LIU X H, OKADA H, et al. Bisphenol AF is a full agonist for the estrogen receptor ERα but a highly specific antagonist for ERβ [J]. Environmental Health Perspectives, 2010, 118(9): 1267-1272. doi: 10.1289/ehp.0901819 [11] LIU K, LI J, YAN S J, et al. A review of status of tetrabromobisphenol A (TBBPA) in China [J]. Chemosphere, 2016, 148: 8-20. doi: 10.1016/j.chemosphere.2016.01.023 [12] ELADAK S, GRISIN T, MOISON D, et al. A new chapter in the bisphenol A story: Bisphenol S and bisphenol F are not safe alternatives to this compound [J]. Fertility and Sterility, 2015, 103(1): 11-21. doi: 10.1016/j.fertnstert.2014.11.005 [13] ZHANG Y F, REN X M, LI Y Y, et al. Bisphenol A alternatives bisphenol S and bisphenol F interfere with thyroid hormone signaling pathway in vitro and in vivo [J]. Environmental Pollution, 2018, 237: 1072-1079. doi: 10.1016/j.envpol.2017.11.027 [14] GRAMEC SKLEDAR D, PETERLIN MAŠIČ L. Bisphenol A and its analogs: Do their metabolites have endocrine activity? [J]. Environmental Toxicology and Pharmacology, 2016, 47: 182-199. doi: 10.1016/j.etap.2016.09.014 [15] GHOSH P, ROY S S, BEGUM M, et al. Bisphenol A: Understanding its health effects from the studies performed on model organisms[M]// Bisphenol A Exposure and Health Risks. London: InTech, 2017: 2-26. [16] ASHA S, VIDYAVATHI M. Role of human liver microsomes in in vitro metabolism of drugs—a review [J]. Applied Biochemistry and Biotechnology, 2010, 160(6): 1699-1722. doi: 10.1007/s12010-009-8689-6 [17] TANG W, WANG R, LU A. Utility of recombinant cytochrome P450 enzymes: A drug metabolism perspective [J]. Current Drug Metabolism, 2005, 6(5): 503-517. doi: 10.2174/138920005774330602 [18] YOUDIM K A, SAUNDERS K C. A review of LC-MS techniques and high-throughput approaches used to investigate drug metabolism by cytochrome P450s [J]. Journal of Chromatography B, 2010, 878(17-18): 1326-1336. doi: 10.1016/j.jchromb.2010.02.013 [19] GUENGERICH F P. Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity [J]. Chemical Research in Toxicology, 2001, 14(6): 611-650. doi: 10.1021/tx0002583 [20] CRUCIANI G, CAROSATI E, DE BOECK B, et al. MetaSite: Understanding metabolism in human cytochromes from the perspective of the chemist [J]. Journal of Medicinal Chemistry, 2005, 48(22): 6970-6979. doi: 10.1021/jm050529c [21] RYDBERG P, GLORIAM D E, ZARETZKI J, et al. SMARTCyp: A 2D method for prediction of cytochrome P450-mediated drug metabolism [J]. ACS Medicinal Chemistry Letters, 2010, 1(3): 96-100. doi: 10.1021/ml100016x [22] ZÜHLKE M K, SCHLÜTER R, HENNING A K, et al. A novel mechanism of conjugate formation of bisphenol A and its analogues by Bacillus amyloliquefaciens: Detoxification and reduction of estrogenicity of bisphenols [J]. International Biodeterioration & Biodegradation, 2016, 109: 165-173. [23] CABATON N, ZALKO D, RATHAHAO E, et al. Biotransformation of bisphenol F by human and rat liver subcellular fractions [J]. Toxicology in Vitro, 2008, 22(7): 1697-1704. doi: 10.1016/j.tiv.2008.07.004 [24] SHAIK S, DE VISSER S P, KUMAR D. External electric field will control the selectivity of enzymatic-like bond activations [J]. Journal of the American Chemical Society, 2004, 126(37): 11746-11749. doi: 10.1021/ja047432k [25] JACOBSON M P, KALYANARAMAN C, ZHAO S W, et al. Leveraging structure for enzyme function prediction: Methods, opportunities, and challenges [J]. Trends in Biochemical Sciences, 2014, 39(8): 363-371. [26] FU T T, ZHENG Q C, ZHANG H X. Investigation of the molecular and mechanistic basis for the regioselective metabolism of midazolam by cytochrome P450 3A4 [J]. Physical Chemistry Chemical Physics, 2022, 24(14): 8104-8112. doi: 10.1039/D2CP00232A [27] HUI C G, SINGH W, QUINN D, et al. Regio- and stereoselectivity in the CYP450BM3-catalyzed hydroxylation of complex terpenoids: A QM/MM study [J]. Physical Chemistry Chemical Physics, 2020, 22(38): 21696-21706. doi: 10.1039/D0CP03083J [28] CHAI L H, ZHANG H N, GUO F J, et al. Computational investigation of the bisphenolic drug metabolism by cytochrome P450: What factors favor intramolecular phenol coupling [J]. Chemical Research in Toxicology, 2022, 35(3): 440-449. doi: 10.1021/acs.chemrestox.1c00350 [29] ZHU L D, ZHOU J, ZHANG Q Z, et al. Computational study on the metabolic activation mechanism of PeCDD by Cytochrome P450 1A1 [J]. Journal of Hazardous Materials, 2021, 405: 124276. doi: 10.1016/j.jhazmat.2020.124276 [30] HE L, HE F, BI H C, et al. Isoform-selective inhibition of chrysin towards human cytochrome P450 1A2. Kinetics analysis, molecular docking, and molecular dynamics simulations [J]. Bioorganic & Medicinal Chemistry Letters, 2010, 20(20): 6008-6012. [31] SCHRÖDINGER E. An undulatory theory of the mechanics of atoms and molecules [J]. Physical Review, 1926, 28(6): 1049-1070. doi: 10.1103/PhysRev.28.1049 [32] HARTREE D R. The wave mechanics of an atom with a non-coulomb central field. part Ⅰ. theory and methods [J]. Mathematical Proceedings of the Cambridge Philosophical Society, 1928, 24(1): 89-110. doi: 10.1017/S0305004100011919 [33] KOHN W, SHAM L J. Self-consistent equations including exchange and correlation effects [J]. Physical Review, 1965, 140(4A): A1133-A1138. doi: 10.1103/PhysRev.140.A1133 [34] TRANG B, LI Y L, XUE X S, et al. Low-temperature mineralization of perfluorocarboxylic acids [J]. Science, 2022, 377(6608): 839-845. doi: 10.1126/science.abm8868 [35] JI L, JI S J, WANG C C, et al. Molecular mechanism of alternative P450-catalyzed metabolism of environmental phenolic endocrine-disrupting chemicals [J]. Environmental Science & Technology, 2018, 52(7): 4422-4431. [36] SHAIK S, COHEN S, WANG Y, et al. P450 enzymes: Their structure, reactivity, and selectivity-Modeled by QM/MM calculations [J]. Chemical Reviews, 2010, 110(2): 949-1017. doi: 10.1021/cr900121s [37] GUIDEZ E B, GORDON M S. Dispersion correction derived from first principles for density functional theory and Hartree-Fock theory [J]. The Journal of Physical Chemistry A, 2015, 119(10): 2161-2168. doi: 10.1021/acs.jpca.5b00379 [38] GRIMME S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction [J]. Journal of Computational Chemistry, 2006, 27(15): 1787-1799. doi: 10.1002/jcc.20495 [39] RILEY K E, VONDRÁŠEK J, HOBZA P. Performance of the DFT-D method, paired with the PCM implicit solvation model, for the computation of interaction energies of solvated complexes of biological interest [J]. Physical Chemistry Chemical Physics, 2007, 9(41): 5555-5560. doi: 10.1039/b708089a [40] WANG M S, MO F, LI H B, et al. Adsorption based on weak interaction between phenolic hydroxyl, carboxyl groups and silver nanoparticles in aqueous environment: Experimental and DFT-D3 exploration [J]. Journal of Environmental Chemical Engineering, 2021, 9(6): 106816. doi: 10.1016/j.jece.2021.106816 [41] LAIDLER K J, KING M C. Development of transition-state theory [J]. The Journal of Physical Chemistry, 1983, 87(15): 2657-2664. doi: 10.1021/j100238a002 [42] MARCUS R A. Electron transfer reactions in chemistry: Theory and experiment (Nobel lecture) [J]. Angewandte Chemie International Edition in English, 1993, 32(8): 1111-1121. doi: 10.1002/anie.199311113 [43] JI L, ZHANG J, LIU W P, et al. Metabolism of halogenated alkanes by cytochrome P450 enzymes. Aerobic oxidation versus anaerobic reduction [J]. Chemistry - An Asian Journal, 2014, 9(4): 1175-1182. doi: 10.1002/asia.201301608 [44] JI L, WANG C C, JI S J, et al. Mechanism of cobalamin-mediated reductive dehalogenation of chloroethylenes [J]. ACS Catalysis, 2017, 7(8): 5294-5307. doi: 10.1021/acscatal.7b00540 [45] HIMO F. Recent trends in quantum chemical modeling of enzymatic reactions [J]. Journal of the American Chemical Society, 2017, 139(20): 6780-6786. doi: 10.1021/jacs.7b02671 [46] DENISOV I G, MAKRIS T M, SLIGAR S G, et al. Structure and chemistry of cytochrome P450 [J]. Chemical Reviews, 2005, 105(6): 2253-2278. doi: 10.1021/cr0307143 [47] VÖLKEL W, COLNOT T, CSANÁDY G A, et al. Metabolism and kinetics of bisphenol A in humans at low doses following oral administration [J]. Chemical Research in Toxicology, 2002, 15(10): 1281-1287. doi: 10.1021/tx025548t [48] HANIOKA N, NAITO T, NARIMATSU S. Human UDP-glucuronosyltransferase isoforms involved in bisphenol A glucuronidation [J]. Chemosphere, 2008, 74(1): 33-36. doi: 10.1016/j.chemosphere.2008.09.053 [49] GRAMEC SKLEDAR D, TROBERG J, LAVDAS J, et al. Differences in the glucuronidation of bisphenols F and S between two homologous human UGT enzymes, 1A9 and 1A10 [J]. Xenobiotica, 2015, 45(6): 511-519. doi: 10.3109/00498254.2014.999140 [50] STREET C M, ZHU Z H, FINEL M, et al. Bisphenol-A glucuronidation in human liver and breast: Identification of UDP-glucuronosyltransferases (UGTs) and influence of genetic polymorphisms [J]. Xenobiotica, 2017, 47(1): 1-10. doi: 10.3109/00498254.2016.1156784 [51] YE X Y, KUKLENYIK Z, NEEDHAM L L, et al. Quantification of urinary conjugates of bisphenol A, 2,5-dichlorophenol, and 2-hydroxy-4-methoxybenzophenone in humans by online solid phase extraction-high performance liquid chromatography-tandem mass spectrometry [J]. Analytical and Bioanalytical Chemistry, 2005, 383(4): 638-644. doi: 10.1007/s00216-005-0019-4 [52] SUIKO M, SAKAKIBARA Y, LIU M C. Sulfation of environmental estrogen-like chemicals by human cytosolic sulfotransferases [J]. Biochemical and Biophysical Research Communications, 2000, 267(1): 80-84. doi: 10.1006/bbrc.1999.1935 [53] NISHIYAMA T, OGURA K, NAKANO H, et al. Sulfation of environmental estrogens by cytosolic human sulfotransferases [J]. Drug Metabolism and Pharmacokinetics, 2002, 17(3): 221-228. doi: 10.2133/dmpk.17.221 [54] YE X Y, ZHOU X L, NEEDHAM L L, et al. In-vitro oxidation of bisphenol A: Is bisphenol A catechol a suitable biomarker for human exposure to bisphenol A? [J]. Analytical and Bioanalytical Chemistry, 2011, 399(3): 1071-1079. doi: 10.1007/s00216-010-4344-x [55] ZALKO D, SOTO A M, DOLO L, et al. Biotransformations of bisphenol A in a mammalian model: Answers and new questions raised by low-dose metabolic fate studies in pregnant CD1 mice [J]. Environmental Health Perspectives, 2003, 111(3): 309-319. doi: 10.1289/ehp.5603 [56] KNAAK J B, SULLIVAN L J. Metabolism of bisphenol A in the rat [J]. Toxicology and Applied Pharmacology, 1966, 8(2): 175-184. doi: 10.1016/S0041-008X(66)80001-7 [57] ATKINSON A, ROY D. In vivo DNA adduct formation by bisphenol A [J]. Environmental and Molecular Mutagenesis, 1995, 26(1): 60-66. doi: 10.1002/em.2850260109 [58] JAEG J P, PERDU E, DOLO L, et al. Characterization of new bisphenol A metabolites produced by CD1 mice liver microsomes and S9 fractions [J]. Journal of Agricultural and Food Chemistry, 2004, 52(15): 4935-4942. doi: 10.1021/jf049762u [59] OKUDA K, FUKUUCHI T, TAKIGUCHI M, et al. Novel pathway of metabolic activation of bisphenol A-related compounds for estrogenic activity [J]. Drug Metabolism and Disposition, 2011, 39(9): 1696-1703. doi: 10.1124/dmd.111.040121 [60] SCHMIDT J, KOTNIK P, TRONTELJ J, et al. Bioactivation of bisphenol A and its analogs (BPF, BPAF, BPZ and DMBPA) in human liver microsomes [J]. Toxicology in Vitro, 2013, 27(4): 1267-1276. doi: 10.1016/j.tiv.2013.02.016 [61] NAKAMURA S, TEZUKA Y, USHIYAMA A, et al. Ipso substitution of bisphenol A catalyzed by microsomal cytochrome P450 and enhancement of estrogenic activity [J]. Toxicology Letters, 2011, 203(1): 92-95. doi: 10.1016/j.toxlet.2011.03.010 [62] YOSHIHARA S, MIZUTARE T, MAKISHIMA M, et al. Potent estrogenic metabolites of bisphenol A and bisphenol B formed by rat liver S9 fraction: Their structures and estrogenic potency [J]. Toxicological Sciences, 2004, 78(1): 50-59. doi: 10.1093/toxsci/kfh047 [63] SKLEDAR D G, SCHMIDT J, FIC A, et al. Influence of metabolism on endocrine activities of bisphenol S [J]. Chemosphere, 2016, 157: 152-159. doi: 10.1016/j.chemosphere.2016.05.027 [64] ASHRAP P, ZHENG G M, WAN Y, et al. Discovery of a widespread metabolic pathway within and among phenolic xenobiotics [J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(23): 6062-6067. doi: 10.1073/pnas.1700558114 [65] LIU L, CUI H Y, HUANG Y X, et al. Enzyme-mediated reactions of phenolic pollutants and endogenous metabolites as an overlooked metabolic disruption pathway [J]. Environmental Science & Technology, 2022, 56(6): 3634-3644. [66] SHAIK S, KUMAR D, DE VISSER S P, et al. Theoretical perspective on the structure and mechanism of cytochrome P450 enzymes [J]. Chemical Reviews, 2005, 105(6): 2279-2328. doi: 10.1021/cr030722j [67] ASAKA M, FUJII H. Participation of electron transfer process in rate-limiting step of aromatic hydroxylation reactions by compound I models of heme enzymes [J]. Journal of the American Chemical Society, 2016, 138(26): 8048-8051. doi: 10.1021/jacs.6b03223 [68] CANTÚ REINHARD F G, SAINNA M A, UPADHYAY P, et al. A systematic account on aromatic hydroxylation by a cytochrome P450 model Compound Ⅰ: A low-pressure mass spectrometry and computational study [J]. Chemistry - A European Journal, 2016, 22(51): 18608-18619. doi: 10.1002/chem.201604361 [69] SHAIK S, FILATOV M, SCHRÖDER D, et al. Electronic structure makes a difference: Cytochrome P-450 mediated hydroxylations of hydrocarbons as a two-state reactivity paradigm [J]. Chemistry - A European Journal, 1998, 4(2): 193-199. doi: 10.1002/(SICI)1521-3765(19980210)4:2<193::AID-CHEM193>3.0.CO;2-Q [70] DIAS A H S, YADAV R, MOKKAWES T, et al. Biotransformation of bisphenol by human cytochrome P450 2C9 enzymes: A density functional theory study [J]. Inorganic Chemistry, 2023, 62(5): 2244-2256. doi: 10.1021/acs.inorgchem.2c03984 [71] DE VISSER S P, SHAIK S. A proton-shuttle mechanism mediated by the porphyrin in benzene hydroxylation by cytochrome P450 enzymes [J]. Journal of the American Chemical Society, 2003, 125(24): 7413-7424. doi: 10.1021/ja034142f [72] DE VISSER S P, OGLIARO F, SHARMA P K, et al. What factors affect the regioselectivity of oxidation by cytochrome P450? A DFT study of allylic hydroxylation and double bond epoxidation in a model reaction [J]. Journal of the American Chemical Society, 2002, 124(39): 11809-11826. doi: 10.1021/ja026872d [73] YADAV R, AWASTHI N, KUMAR D. Biotransformation of BPA via epoxidation catalyzed by Cytochrome P450 [J]. Inorganic Chemistry Communications, 2022, 139: 109321. doi: 10.1016/j.inoche.2022.109321 [74] GUO F J, CHAI L H, ZHANG S B, et al. Computational biotransformation profile of emerging phenolic pollutants by cytochromes P450: Phenol-coupling mechanism [J]. Environmental Science & Technology, 2020, 54(5): 2902-2912. [75] RIU A, LE MAIRE A, GRIMALDI M, et al. Characterization of novel ligands of ERα, Erβ, and PPARγ: The case of halogenated bisphenol A and their conjugated metabolites [J]. Toxicological Sciences, 2011, 122(2): 372-382. doi: 10.1093/toxsci/kfr132 [76] FIC A, ŽEGURA B, SOLLNER DOLENC M, et al. Mutagenicity and DNA damage of bisphenol A and its structural analogues in HepG2 cells [J]. Archives of Industrial Hygiene and Toxicology, 2013, 64(2): 189-200. doi: 10.2478/10004-1254-64-2013-2319 -




 下载:
下载: